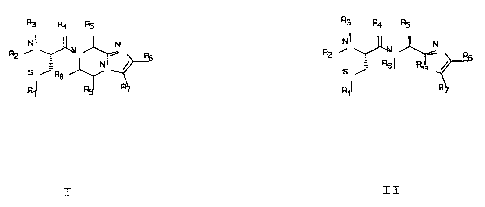Note: Descriptions are shown in the official language in which they were submitted.
CA 02245823 1997-08-10
r
W O 97130053 PCTlUS97/02651
- 1 -
F~RNESYL TRANSFERASE INHIBITORS
~ackaround of the Invention
Ras is a 21,000 molecular weight protein important
in the signal transduction pathway for normal cell
~ 5 growth. The protein is produced in the ribosome,
released into the cytosol, and post-translationally
modified. The first step in the series of post-
translational modifications is the alkylation of Cysl6s
with farnesyl pyrophosphate in a reaction catalyzed by
the enzyme farnesyl transferase (Hancock, JF, et al.,
Cell 57:1167 -1177 (1989)). Subsequently, the three C-
terminal amino acids are cleaved (Gutierrez, L, et al.,
EMBO J. 8:1093-1098 (1989)), and the terminal Cys~~s is
methyl esterified (Clark, S, et al., Proc. Nat'1 Acad.
Sci. (USA) 85:4643-4647 (1988)). Some forms of Ras are
also reversibly palmitoylated on cysteine residues
immediately N-terminal to Cysl6s (Buss, JE, et al., Mol.
Cell. Biol. 6:116-122 (1986)). These modifications
increase the hydrophobicity of the C-terminal region of
2D Vitas, ~at~si~g zt to localize at the surface of the cell
membrane. Localization of Ras to the cell membrane is
necessary for normal function (Willumsen, BM, et al.,
Science 310:583-586 (1984))..
- Oncogenic forms of Ras are observed in a
relatively large number of cancers including over 50
percent of colon cancers, over 30 percent of lung
cancers, and over 90 perceni~ of pancreatic cancers (Bos,
JL, Cancer Research 49:4682--4689 (1989)). These
w- observations suggest that intervention in the function of
Ras mediated signal transduction may be useful in the
treatment of cancer.
Previously, it has been shown that the C-terminal
tetrapeptide of Ras has the °'CAAX" motif (wherein C is
cysteine, A is an aliphatic amino acid, and X is any
amino acid). Tetrapeptides having this structure have
CA 02245823 1997-08-10
WO 97/30053 PCT/US97/02651
- 2 -
been shown to be inhibitors of farnesyl transferase
(Reiss, et al., Cell 62:81-88 (1990)). Poor potency of
these early farnesyl transferase inhibitors has prompted ,
the search for new inhibitors with more favorable
pharmacokinetic behavior (James, GL, et al., Science
260:1937-1942 (1993); Kohl, NE, et al., Proc. Nat'1 Acad.
Sci. (USA) 91:9141-9145 (1994); deSolms, SJ, et al., J.
Med. Chem. 38:3967-3971 (1995}; Nagasu, T, et al., Cancer
Research 55:5310-5314 (1995); Lerner, EC, et al., J.
Biol. Chem. 270:26802-26806 (1995)).
Recently, it has been shown that a farnesyl
transferase inhibitor will block growth of Ras-dependent
tumors in nude mice (Kohl, NE, et al., Proc. Nat'1 Acad.
Sci. (USA} 91:9141-9145 (1994}). In addition, it has
been shown that over 70 percent of a large sampling of
tumor cell lines are inhibited by farnesyl transferase
inhibitors with selectivity over non-transformed
epithelial cells (Sepp-Lorenzino, I, et al., Cancer
Research, 55:5302-5309 (1995}).
Summary of the Invention
In one aspect, the invention features a compound
having the formula ( I ) or formula ( II )
Q3 R4 AS ~ R4 AS
i
S Jp ~ ' S % ~~ Rt7
8
R
I II
CA 02245823 1997-08-10
WO 97/30053 PCTlUS97I0265~
- 3 -
wherein:
R1 is H, lower alkyl,, cycloalkylthio, or lower
~ alkylthio, or, together with R2, form -CH2-, -CO-, or -
C(CH3)2-;
~ 5 each of R2 and R3, independently, is H, lower
alkyl, and cycloalkyl;
R4 is H2 or O;
R5 is H, or substituted or unsubstitute;~ lower
alkyl, lower alkenyl, lower alkynyl, cycloalkyl,
cycloalkyl lower alkyl, cyc7.oalkenyl, cycloalkenyl lower
alkyl, aryl, aryl lower alkyl, heterocyclyl, or
heterocyclyl lower alkyl, wYierein the substituent is
lower alkyl, -O-Rlo, -S(O)mRlo (where m is 0, I, or 2), -
N(R1o) (R11) ~ -N-C(O)-Rlo~ -r~-(S02)-Rlo% -C02-Rlo~ -C(O)-
I5 N(RI~) (Rll) ~ °r (S02)-N(R10) (Rll) r
each of R6 and R7, independently, is H, -C(O)-
NHCHR13C02R14, or substituted or unsubstituted lower
alkyl, cycloalkyl, cycloalkyl lower alkyl, cycloalkenyl,
cycloalkenyl lower alkyl, aryl, aryl lower alkyl,
~t~rocy~lyl,.or heterocyclyl lower alkyl, wherein the
substituent is OH, lower allcyl, lower alkoxy, aryloxy,
aryl lower alkoxy, -N(R10) (R11) , -COOH, -CON(Rlo) (R11) , or
halo, or R6 and R7, together, form aryl or heterocyclyl;
each of Rg and R~, independently, is H, or
25 substituted or unsubstituted lower alkyl, cycloalkyl,
cycloalkyl lower alkyl, cycloalkenyl, cycloalkenyl lower
alkyl, aryl, aryl lower alkyl, heterocyclyl, or
heterocyclyl lower alkyl, wherein the substituent is OH,
- -- lower alkyl, lower alkoxy, -LT (R1o) (R11) , cooH, -c (o) N-
30 (Rlo)(Rll), or halo, or R8 and R9, together, form aryl or
heterocyclyl;
each of R1~ and R11, independently, is H, lower
alkyl, aryl, aryl lower alkyl, cycloaikyl, cycloalkyl
lower alkyl, heterocyclyl, or heterocyclyl lower alkyl;
35 R12 is NR9, S, or O;
CA 02245823 2006-03-16
-
R13 is substituted or unsubstituted lower alkyl
wherein the substituent is lower alkyl, -ORlo, -S(O)mRlO
(wherein m is 0, 1, or 2) or -N(Rlo) (Rll) ; and
R14 is H or lower alkyl; or
a pharmaceutically acceptable salt thereof.
Examples of the present invention include the
following:
7-(2-amino-1-oxo-3-thio-propyl)-8-butyl-2-phenyl-
5,6,7,8-tetrahydro-imidazo-[1,2a]-pyrazine (Compound 1);
7-(2-amino-1-oxo-3-thio-propyl)-8-butyl-2-(4-
fluorophenyl)-5,6,7,8-tetrahydro-imidazo-[1,2a]-pyrazine
(Compound 2);
7-(2-amino-1-oxo-3-thio-propyl)-8-butyl-2-(2-
methoxy-phenyl)-5,6,7,8-tetrahydro-imidazo-[1,2a]-
pyrazine (Compound 3);
7-(2-amino-1-oxo-3-thio-propyl)-8-butyl-2-(3-
methoxy-phenyl)-5,6,7,8-tetrahydro-imidazo-[1,2a]-
pyrazine (Compound 4);
7-(2-amino-1-oxo-3-thio-propyl)-8-butyl-2-(4-
2o methoxy-phenyl)-5,6,7,8-tetrahydro-imidazo-[1,2a]-
pyrazine (Compound 5);
7-(2-amino-1-oxo-3-thio-propyl)-8-(2-hydroxy-
ethyl)-2-phenyl-5,6,7,8-tetrahydro-imidazo-[1,2a]-
pyrazine (Compound 6);
7-(2-amino-3-thio-propyl)-8-butyl-3-phenyl-
5,6,7,8-tetrahydro-imidazo-[1,2a]-pyrazine (Compound 7);
2-(1-(N-(2-amino-1-oxo-3-thiopropyl)-N-methyl)-
amino-pentyl)- 5-phenyl-imidazole (Compound 8);
2-(((2-amino-1-oxo-3-mercapto-propyl)-amino)-
3o methyl)-5-phenyl-thiazole-4-carbonyl-methionine (Compound
9);
7-(2-amino-1-oxo-3-thio-propyl)-2-(2-
methoxyphenyl)-8-(2-methylpropyl)-5,6,7,8-tetrahydro-
imidazo[1,2a]pyrazine (Compound 11);
CA 02245823 2006-03-16
- 5 -
7-(2-amino-1-oxo-3-thio-propyl)-8-butyl-2-(2-
ethoxyphenyl)-5,6,7,8-tetrahydro-imidazo-[1,2a]-pyrazine
(Compound 13);
7-(2-amino-1-oxo-3-thio-propyl)-8-butyl-2-(2-
hydroxyphenyl)-5,6,7,8-tetrahydro-imidazo-[1,2a]-pyrazine
(Compound 14);
2-(1-(N-(2-amino-1-oxo-3-thiopropyl)-N-methyl)-
amino-pentyl-5-(2-methoxyphenyl)-imidazole (Compound 15);
7-(2-amino-1-oxo-3-thiopropyl)-8-(2-
methylpropyl)-2-(1-naphthyl)-5,6,7,8-
tetrahydroimidazo[1,2a]pyrazine (Compound 17);
7-(2-amino-1-oxo-3-thiopropyl)-8-(1-
methylpropyl)-2-(2-methoxyphenyl)-5,6,7,8-
tetrahydroimidazo[1,2a]pyrazine (Compound 18);
S-(dimethylethyl)-s'-[2-amino-3-oxo-3(8-butyl-2-
(2-methoxyphenyl;)-5,6,7,8-
tetrahydroimidazo[1,2a]pyrazin-7-yl)propyl]disulfide
(Compound 21);
7-(2-amino-1-oxo-3-thiopropyl)-8-butyl-2-(2-
methylphenyl)-5,6,7,8-tetrahydroimidazo[1,2a]pyrazine
(Compound 22);
7-(2-amino-1-oxo-3-thiopropyl)-8-(1,1-
dimethylethyl)-2-(2-methoxyphenyl)-5,6,7,8-
tetrahydroimidazo[1,2a]pyrazine (Compound 24);
7-(2-amino-1-oxo-3-thiopropyl)-8-(1-
methylpropyl)-2-(2-(phenylmethoxy)phenyl)-5,6,7,8-
tetrahydroimidazo[1,2a] pyrazine (Compound 25);
7-(2-amino-1-oxo-3-thiopropyl)-8-
(cyclohexylmethyl)-2-(2-methoxyphenyl)-5,6,7,8-
tetrahydroimidazo[1,2a] pyrazine (Compound 26);
7-(2-amino-1-oxo-3-thiopropyl)-2-(2-
methoxyphenyl)-8-(1-methylethyl)-5,6,7,8-
tetrahydroimidazo[1,2a]]pyrazine (Compound 27);
CA 02245823 2006-03-16
- 6 -
7-(2-amino-1-oxo-3-thiopropyl)-8-butyl-2(2-
hydroxy-6-methoxyphenyl)-5,6,7,8-tetrahydro[1,2a]pyrazine
(Compound 29);
2-(2-methoxyphenyl)-8-(1-methylpropyl)-5,6,7,8-
tetrahydro-7-((thiazolidin-4-yl)carbonyl)-
imidazo[1,2a]pyrazine (Compound 31);
7-(2-amino-1-oxo-3-thiopropyl)-3-bromo-8-butyl-2-
(2-methoxyphenyl)-5,6,7,8-tetrahydro-
imidazo[1,2a]pyrazine (Compound 32);
so 7-(2-amino-1-oxo-3-thiopropyl)-8-butyl-2,3-
diphenyl-5,6,7,8-tetrahydroimidazo-[1,2a]pyrazine
(Compound 34);
7-(2-amino-1-oxo-3-thiopropyl)-3-bromo-8-butyl-2
phenyl-5,6,7,8-tetrahydro-imidazo[1,2a]pyrazine (Compound
36) ;
7-(2-amino-1-oxo-3-thiopropyl)-2-cyclohexyl-8-
(cyclohexylmethyl)-5,6,7,8-tetrahydro-
imidazo[1,2a)pyrazine (Compound 37);
7-(2-amino-1-oxo-3-thiopropyl)-8-hexyl-2-(2-
methoxyphenyl)-5,6,7,8-tetrahydro-imidazo[1,2a]pyrazine
(Compound 42);
7-(2-amino-1-oxo-3-thiopropyl)-8-
(cyclohexylethyl)-2-(2-methoxyphenyl)-5,6,7,8-
tetrahydroimidazo[1,2a] pyrazine (Compound 44);
7-(2-amino-1-oxo-3-thiopropyl)-8-(cyclohexyl)-2-
(2-methoxyphenyl)-5,6,7,8-tetrahydroimidazo[1,2a]
pyrazine (Compound 46);
7-(2-amino-1-oxo-3-thiopropyl)-8-(2-(4-
methoxycyclohexyl)-methyl)-2-(2-methoxyphenyl)-5,6,7,8-
3o tetrahydroimidazo[1,2a] pyrazine (Compound 47);
7-(2-amino-1-oxo-3-thiopropyl)-8-
(cyclohexylmethyl)-2-phenyl)-5,6,7,8-
tetrahydroimidazo[1,2a] pyrazine (Compound 49);
CA 02245823 2006-03-16
- 7 -
7-(2-amino-1-oxo-3-thiopropyl)-8-(4-
methoxycyclohexyl)-2-(2-methoxyphenyl)-5,6,7,8-
tetrahydroimidazo[1,2a] pyrazine (Compound 51);
[S-[2-amino-3-oxo-3-(8-cyclohexylmethyl)-2-(2-
methoxyphenyl)-5,6,7,8-tetrahydro-imidazo(1,2a]pyrazin-7-
yl)-
propyl]-S'-cyclohexyl]disulfide (Compound 52);
7-(2-amino-1-oxo-3-thiopropyl)-8-(4-
methoxycyclohexyl) methyl-2-(2-methoxyphenyl)-5,6,7,8-
~o terahydroimidazo[1,2a] pyrazine (cis isomer) (Compound
53);
7-(2-amino-1-oxo-3-thiopropyl)-2-(2-
methoxyphenyl)-8-(4-piperidinylmethyl)-5,6,7,8-
terahydroimidazo[1,2a] pyrazine (Compound 54);
7-(2-amino-1-oxo-3-thiopropyl)-2-(2-
methoxyphenyl)-8-(2-piperidinylmethyl)-5,6,7,8-
terahydroimidazo[1,2a] pyrazine (Compound 55);
7-(2-amino-1-oxo-3-thiopropyl)-2-(2-
methoxyphenyl)-8-(3-piperidinylmethyl)-5,6,7,8-
2o terahydroimidazo[1,2a] pyrazine (Compound 56);
7-(2-amino-1-oxo-3-thiopropyl)-8-
(cyclohexylmethyl)-2-(1-naphthyl)-5,6,7,8-
tetrahydroimidazo[1,2a] pyrazine (Compound 57);
[S-[2-amino-3-oxo-3-(8-cyclohexylmethyl)-2-(2-
methoxyphenyl)-5,6,7,8-tetrahydro-imidazo(1,2a]pyrazin-7-
yl)-propyl]-S'-ethyl]disulfide (Compound 58);
7-(2-amino-1-oxo-3-thiopropyl)-2-(2-
methoxyphenyl)-8-(2-methylthio)-ethyl-5,6,7,8-
tetrahydroimidazo[1,2a] pyrazine (Compound 59);
7-(2-amino-1-oxo-3-thiopropyl)-8-(3-
indolinylmethyl)-2-(2-methoxyphenyl)-8-5,6,7,8-
tetrahydroimidazo[1,2a] pyrazine (Compound 60); and
7-(2-amino-1-oxo-3-thiopropyl)-8-(1-
methylimidazol-3-yl) methyl-2-(2-methoxyphenyl)-8-
5,6,7,8-tetrahydroimidazo (1,2a] pyrazine (Compound 61).
CA 02245823 2006-03-16
- g _
8-(cyclohexylmethyl)-2-(2-methoxyphenyl)-7-(2-
oxo-thiazolidin-4-carbonyl)-5,6,7,8-
tetrahydroimidazo[1,2a]pyrazine (Compounds 62); and
7-(2-amino-1-oxo-3-thiopropyl)-2-(2-
methoxyphenyl)-8-(2-phenoxyethyl)-5,6,7,8-
tetrahydroimidazo[1,2a]pyrazine (Compound 63).
In another aspect, the invention features a
dimeric compound made of two identical or different
compounds (monomers) as described above, or a
1o pharmaceutically acceptable salt thereof. The monomers
are linked to each other to form the dimer via a
disulfide bond. More specifically, R1 in the first
monomer and R1 in the second monomer, in combination,
form a disulfide bond.
z5 Examples of dimers of the invention include:
bis-1,1'-[2-amino-3-(8-butyl-2-(2-methoxyphenyl)-
5,6,7,8-tetrahydro-imidazo[1,2a]piperazine-7-yl)-3-
oxo]propyl disulfide (Compound 10);
bis-1,1'-[2-amino-3-(2-(2-methoxyphenyl)-8-(2-
2o methylpropyl)-5,6,7,8-tetrahydro-imidazo[1,2a]piperazine-
7-yl)-3-oxo]propyl disulfide (Compound 12);
bis-1,1'-[2-(1-(N-(2-amino-1-oxo-3-thiopropyl)-N-
methylamino)-pentyl]-5-(2-methoxyphenyl)imidazole]
disulfide (Compound 16);
25 bis-1,1'-7-(2-amino-1-oxo-3-thiopropyl -(2-(1-
naphthyl)-8-(2-methylpropyl)-5,6,7,8-
tetrahydroimidazo[1,2a]pyrazin-7-yl) disulfide (Compound
19) .
bis-1,1'-[7-(2-amino-1-oxo-3-thiopropyl)-2-
30 (methoxyphenyl)-8-(1-methylpropyl)-5,6,7,8-
tetrahydroimidazo[1,2a]pyrazine] disulfide (Compound 20);
bis-1,1'-[7-(2-amino-1-oxo-3-thiopropyl)-8-butyl-
2-(2-methylphenyl)-5,6,7,8-tetrahydroimidazo[1,2a]
pyrazine~ disulfide (Compound 23).
CA 02245823 1997-08-10
WO 97!30053 PCTIUS97/Q265i
- 9 -
bis-Z,1'-[7-(2-amino--1-oxo-3-thiopropyl)-2-(2-
methoxyphenyl)-8-(1-methylethyl)-5,6,7,8-
tetrahydroimidazo[1,2a]]pyra:.ine] disulfide (Compound
28).
bis-1,1'-[7-(2-amino-1-oxo-3-thiopropyl)-8-(1,1-
dimethylethyl ) -2- { 2-methoxyplzenyl ) -5 , 6 , 7 , 8-
tetrahydroimidazo[1,2a]pyrazine] disulfide {Compound 30);
bis-1,1'-[2-amino-3-(8-butyl-2-cyclohexyl-
5,6,7,8-tetrahydro-imidazo-[1,2a]pyrazin-7-yl)-3-oxo-
propyl]disulfide (Compound 33);
bis-1,1'-[2-amino-3-(3-bromo-8-butyl-2-phenyl-
5,6,7,8-tetrahydro-imidazo[1,2a]-pyrazin-7-yl)-3-oxo-
propyl]disulfide (Compound 35);
bis-1,1'-[7-(2-amino-1-oxo-3-thiopropyl)-8-butyl-
2,3-diphenyl-5,6,7,8-
tetrahydroimidazo[1,2a]pyrazine]disulfide (Compound 38);
his-1,1'-[7-(2-amino-1-oxo-3-thiopropyl)-8-(1-
methylpropyl)-2-(2-(phenylmethoxy)phenyl)-5,6,7,8-
tetrahydroimidazo[1,2a] pyra.zine]disulfide (Compound 39);
Zp ~i~--1, 1' - [ 2-amino-3-~ ( 2-cyclohexyl-8-
(cyclohexy!methyl)-5,6,7,8-tetrahydro-
imidazo[1,2a]pyrazin-7-yl)-3-oxo-propyl]disulfide
(Compound 40);
_ bis-1,1'-[7-{2-amino-1-oxo-3-thiopropyl)-8-
(cyclohexylmethyl)-2-(2-methoxyphenyl)-5,6,7,8-
tetrahydroimidazo[1,2a]pyrazine] disulfide {Compound 41);
bis-1,1'-[7-(2-amino-1-oxo-3-thiopropyl)-8-hexyl-
2-(2-methoxyphenyl)-5,6,7,8-tetrahydro-
_ imidazo[1,2a]pyrazine] disulfide (Compound 43);
bis-2,1'-[7-{2-amino-1-oxo-3-thiopropyl)-8-
(cyclohexylethyl)-2-(2-methoxyphenyl)-5,6,7,8-
- tetrahydroimidazo[1,2a] pyrazine] disulfide (Compound
45) ;
bis-1,1'-[7-{2-aml.nD-1-oxo-3-thiopropyl)-8-
(cyclohexyl)-2-{2-methoxyphenyl)-5,6,7,8-
CA 02245823 2005-07-07
- 10 -
tetrahydroimi~lazo[1,2a] pyrazine ] disulfide (Compound
48); and
bis-1,1'-[7-(2-amino-1-oxo-3-thiopropyl)-8-(2-(4-
methoxycyclohexyl)-methyl)-2-(2-methoxyphenyl)-5,6,7,8-
tetrahydroimidazo[1,2a] pyrazine] disulfide (Compound
50);
bis-1,1'-[2-amino-3-(2-(2-methoxyphnenyl)-8-(2-
phe:~oxyethyl)-5,6,7,8-tetrahydro-imidazo[1,2a]pyrazin-7-
yl)-3-oxopropyl]-disulfide (Compound 64).
The structures of these compounds are listed in
Table I below.
This invention also provides the use of a compound
or salt of this invention for treating tumor or
restenosis in a subject as well as the use of such a
compound or salt for preparation of a medicament for such
treatment. Also provided are pharmaceutical compositions
for use in treating tumor or restenosis in a subject
comprising a compound or salt of this invention and a
pharmaceutically acceptable carrier.
CA 02245823 1997-08-10
WO 97/30453 _ t .~ _ PCT'1US97/02652
Z'ABi.E
cup
0
COMPOUND t H s
as
a
COMPQUND 2
N
'cx;
Ira
COMPaUND 3
s~ ~~' / \
xs j ~x',-r'i
~a,c-o
cn~
0
COMPOUND 4 K=x Y ~ N / \
K: j
o-~x~
c:x,
0
COMPOUND 5 ~ s gar
xsj ~~~/ ov
c~
os
a
COMPaUND 6 err J
_ ~~!"~'r / \
CA 02245823 1997-08-10
WO 97/30053 PCT/US97l02651
- 12 -
cH,
COMPOUND' H=N ~. H ~~
i ~K
Hs
cx.
COMPOUND8 °
~N~,~~x / \
Ks ~
Ht
_H
COMPOUN09 °
N it '~H~ H7f ~j
' f H ~ os
a ° H /
~s~.cx,
H,c
~x l
COMPOUND t0
~~--:~ Is s~ ~~~/
= I/ I
O Y K . !t N-~" d
t~Kt flJf ~ Ci7
O p
- x,c
_~ i
COMPOUND t t
~t N o
a~x ~/ , c~c,
a
~4 Hoc
H,c-j
COMPOUND I2 ~ , x~~ ~s s ,°~ .~\
a ~ :~/ 'x ~ ~ N o
~ ~eae~ H;a~ \rJ 'ac
a c
CA 02245823 1997-08-10
WO 97/30053 PCTlUS97l0265!
- 13 -
cx,
0
COMPQUNO t 3 ~ . ~ ~ /
. N~ Y
KS ~ '1 /
0
COMPaUNO t ~
~N
~ /
ao
.~ :3
0
COMPOUND I5
COMPOtTND :7
0
CA 02245823 1997-08-10
WO 97/30053 PCTlLTS97/0265I
- 14 -
:a V ~ / ,
CCHPOL~1D L9
v= N .~
O
CCtfi'OL~ID L9
a r s
w :~~ ~_ a
GCT'~OL'ND ZD
\ ' a
a'FL;
CCI~iPOtIND Z.
O
CCMPO~ ZZ
O
SUBSTITUTE SHEET (RULE 26)
CA 02245823 1997-08-10
WO 97130053 PCTlUS97/OZ65I
_ ~c~ _
COM7?CtJND Z3
O
corspocrrra z a
cor~ovrra zs
cor~ocrrm z s
COMPOUND Z7
CA 02245823 1997-08-10
WO 97/30053 PCTIU597/02659
_ 76 _
.5
COMPOUND 29
v ,t~ O
0
COMPOUND 29
S
COMPOUND 30
O ~Y
,
COMPOUND 3i
CA 02245823 1997-08-10
WO 97130053 PCTlI7S9710265f
7 _
COI-:POL~rD 32
CCMPOU2ID 33
O
N_
:r J
~s-
COHPOVND 34
COMPOUND 35
O
y
NJ S_
_ O Br
COMPOUND 30
8r
SUBSTITUTE SHEET (RULE 26)
CA 02245823 1997-08-10
WO 97/30Q53 PCT/US97/02651
- 18 -
0
caMaocr.rD m ~'='~~ v
' ~' ~
1
.COMPOUND 39
COMPOUND 35 D
~. ~r
-s% w
COMPOUND 40 ji :: 'Htt H
O
COMPOUND 4I
O
SUBSTITUTE SHEET (RULE 26)
CA 02245823 1997-08-10
WO 97130Q53 ' ~ PCT/US97/02651
_ ~g _
GOHPOL"ND 4 2
~C02iE'CUYD 43 O
~i'rr.
v :~ J ~.S_
0
0
COI'~OVND 4 4
0
GOM'POL~ 45 O
N NFL. F:;N
v
a
- COMPOU21D 4 6
O
SUBSTITUTE SI~fEET (RULE 26)
CA 02245823 1997-08-10
WO 97/30053 PCT/US97102651
- 20 -
COMPOUND 4? cr " I1 / '
COMPOUND 48
COHPOUND 49
COMPOUND 50
COMPOUND 5I
O
O
0
CA 02245823 1997-08-10
WO 97!30053 rca~rrrs9~io26s~
- 21 -
cor~ocrrro s z
a
o
~H
cora?acrcra s a
lSn
cor~aurrn <_ a
TizH
cOMFaUND 5~
O
COMPOCfND 5 n'
O
CA 02245823 1997-08-10
WO 97/30053 PCTlUS97l0z651
- 22 -
cor~ovrrn s~
.COMPOtIL3D 58
COI~OVHD 59
COMPOUND 60
COI~OVI~177 61
O
s O
CA 02245823 1997-08-10
WO 97/30053 PCTlUS97/OZ65I
- 23 -
COM.POUtiD 62
~O
s.GHPOUtiD 6:3 ' ~N~~N
xs j ~-N ~
0
0
cc:.pavriD 59
. ._ =. N yatI=
J '
a
CA 02245823 1997-08-10
WO 97/30053 PCT/US97/02651
- 24 -
The compounds of the present invention may have
asymmetric centers and occur as racemates, racemic
mixtures, and as individual diastereomers, with all
possible isomers, including optical isomers, being
included in the present invention. For simplicity, where
no specific configuration is depicted in the structural
formulae, it is understood that all enantiometric forms
and mixtures thereof are represented.
As used herein, "lower alkyl" is intended to
~10 include both branched and straight-chain saturated
aliphatic hydrocarbon groups having 1-6 carbon atoms.
Examples of lower alkyl groups include methyl, ethyl,
propyl, isopropyl, butyl, t-butyl, isobutyl, sec-butyl,
and the like. "Lower alkenyl" groups include those
groups having 2-6 carbon atoms and having one or several
double bonds. Examples of alkenyl groups include vinyl,
allyl, isopropenyl, butenyl, pentenyl, hexenyl, 1-
propenyl, 2-butenyl, 2-methyl-2-butenyl, isoprenyl, and
the like. "Alkynyl groups" include those groups having
ZD 2-6 card atoms and having one or several triple bonds.
Examples of alkynyl groups include ethynyl, propynyl, 1-
butynyl, 2-butynyl, 3-butynyl, s-butynyl, and the like.
All alkyl, alkenyl, and alkynyl groups are noncyclic.
- As used herein, "cycloalkyl" is intended to
include non-aromatic cyclic hydrocarbon groups having 3-
10 carbon atoms. Examples of cycloalkyl groups include
cyclopropyl, cyclobutyl, cyclopentyl, cyclobenzyl, and
the like. "Cycloalkenyl" is intended to include non-
aromatic hydrocarbon cyclic groups having 3-10 carbon
atoms and having one or several double bonds. Examples
of cycloalkenyl groups include cyclopropenyl,
cyclobutenyl, cyclopentenyl, and cyciohexyl, and the -
like.
As used herein, "aryl" is intended to include any .
stable monocyclic, bicyclic, or tricyclic carbon rings)
CA 02245823 2006-03-16
- 25 -
of up to 7 members in each ring, wherein at least one
ring is aromatic. Examples of aryl groups include
phenyl, naphthyl, anthracenyl, biphenyl,
tetrahydronaphthyl, indanyl, phenanthrenyl, and the like.
The term heterocyclyl, as used herein, represents
a stable 5- to 7-membered monocyclic or stable 8- to 11-
membered bicyclic or stable 11-15 membered tricyclic
heterocyclic ring which is either saturated or
unsaturated, and which consists of carbon atoms and from
one to four heteroatoms selected from the group
consisting of N, 0, and S, and including any bicyclic
group in which any of the above-defined heterocyclic
rings is fused to a benzene ring. The heterocyclic ring
may be attached at any heteroatom or carbon atom which
results in the creation of a stable structure. Examples
of such heterocyclic elements include, but are not
limited to, azepinyl, benzimidazolyl, benzisoxazolyl,
benzofurazanyl, benzopyranyl, benzothiopyranyl,
benzofuryl, benzothiazolyl, benzothienyl, benzoxazolyl,
2o chromanyl, cinnolinyl, dihydrobenzofuryl,
dihydrobenzothienyl, dihydrobenzothiopyranyl,
dihydrobenzothio-pyranyl sulfone, furyl, imidazolidinyl,
imidazolinyl, imidazolyl, indolinyl, indolyl,
isochromanyl, isoindolinyl, isoquinolinyl,
isothiazolidinyl, isothiazolyl, isothiazolidinyl,
morpholinyl, naphthyridinyl, oxadiazolyl, 2-oxoazepinyl,
2-oxopiperazinyl, 2-oxopiperidinyl, 2-oxopyrrolidinyl,
piperidyl, piperazinyl, pyridyl, pyridyl N-oxide,
quinoxalinyl, tetrahydrofuryl, tetrahydroisoquinolinyl,
3o tetrahydro-quinolinyl, thiamorpholinyl, thiamorpholinyl
sulfoxide, thiazolyl, thiazolinyl, thienofuryl,
thienothienyl, thienyl, and the like.
The term halo is meant to include fluoro, chloro,
bromo, and iodo.
CA 02245823 1997-08-10
WO 97/30053 PCTlUS97/02651
- 26 -
The term "substituted" is meant to include the
recited chemical group (e. g., lower alkyl, heterocycle,
aryl, cycloalkyl, etc.) substituted with one to four of .
the recited substituents (e. g., halo, OH, lower alkyl,
etc.). The substituent may be attached to any atom in -
the chemical group.
The compounds of this invention can be
provided in the form of pharmaceutically acceptable
salts. Acceptable salts include, but are not limited to,
acid addition salts of inorganic acids such as
hydrochloride, sulfate, phosphate., diphosphate,
hydrobromide, and nitrate or organic acids such as
acetate, maleate, fumarate, tartrate, succinate, citrate,
lactate, methanesulfonate, p-toluenesulfonate, pamoate,
salicylate, oxalate, and stearate. Also within the scope
of the present invention, where applicable, are salts
formed from bases such as sodium or potassium hydroxide.
For further examples of pharmaceutically acceptable saps
see, "Pharmaceutical Salts," J. Pharm. Sci. 66:1 (1977).
In another aspect, the invention features a
method of inhibiting farnesyl transferase in a patient,
e.g., a mammal such as a human, by administering to a
patient a therapeutically effective amount of a compound
of formula (I) or formula (TI). In particular, the
present invention also covers a method of treating
restenosis or tissue proliferative diseases (i.e., tumor)
in a patient by administering to a patient a
_ therapeutically effective amount of a compound or its
salt. Examples of tissue proliferative disease include
both those associated with benign (e. g., non-malignant)
cell proliferation such as fibrosis, benign prostatic -
hyperplasia, atherosclerosis, and restenosis, and those
associated with malignant cell proliferation, such as
cancer (e. g., tumors expressing farnesyl transferase).
CA 02245823 2006-03-16
- 27 -
Examples of treatable tumors are breast, colon, pancreas,
prostate, lung, ovarian, epidermal, and hematopoietic
cancers (Sepp-Lorenzino, I, et al., Cancer Research
55:5302 (1995)).
A therapeutically effective amount of a compound
of this invention and a pharmaceutically acceptable
carrier substance (e.g., magnesium carbonate, lactose, or
a phospholipid with which the therapeutic compound can
form a micelle) together form a therapeutic composition
(e.g., a pill, tablet, capsule, or liquid) for
administration (e. g., orally, intravenously,
transdermally, or subcutaneously) to a subject in need of
the compound. The pill, tablet, or capsule can be coated
with a substance capable of protecting the composition
from the gastric acid or intestinal enzymes in the
subject's stomach for a period of time sufficient to
allow the composition to pass undigested into the
subject's small intestine.
The dose of a compound of the present invention for
2o treating the above-mentioned diseases or disorders
varies depending upon the manner of administration, the
age and the body weight of the subject, and the condition
of the subject to be treated, and ultimately will be
decided by the attending physician or veterinarian. Such
2s an amount of the compound as determined by the attending
physician or veterinarian is referred to herein as a
"therapeutically effective amount."
Also contemplated within the scope of the
invention is a method of preparing the compound of
3o formula (I) or formula (II) and the novel chemical
intermediates used in these syntheses as described
herein.
Other features and advantages of the present
invention will be apparent from the detailed description
3s of the invention and from the claims.
CA 02245823 2006-03-16
- 28 -
Description of the Invention
It is believed that one skilled in the art can,
based on the description herein, utilize the.present
invention to its fullest extent. The following specific
embodiments are to be construed as merely illustrative,
and not limitative of the remainder of the disclosure in
any way whatsoever.
Unless defined otherwise, all technical and
scientific terms used herein have the same meaning as
i0 commonly understood by one of ordinary skill in the art
to which this invention belongs. Also, all publications,
patent applications, patents, and other references
mentioned herein are incorporated by reference.
Synthesis
The following is a description of the synthesis
of compounds 1 to 31. Other compounds of the invention
can be prepared in an analogous manner by a person of
ordinary skill in the art. As used herein, the term Cbz
2o means carbobenzyloxy; DMF means dimethylformamide; EtOAc
means ethyl acetate, NH40Ac means ammonium acetate; LAH
means lithium aluminum hydride; THF means
tetrahydrofuran; BOC means t-Butoxycarbonyl; Trt means
trityl; Tfa means trifluoroacetic acid; Et20 means ethyl
ether; NMR means nuclear magnetic resonance; mass spec.
means mass spectroscopy; DMSO-d6 means methyl sulfoxide;
DCC means dicyclohexyl carbodiimide; NMM means 4-methyl
morpholine; iPr3SiH means triisopropylsilane, HPLC means
high performance liquid chromatography; DIC means
3o diisopropylcarbodiimide; MeOH means methanol; KOtBu means
potassium tert-butoxide; HOSU means N-hydroxysuccinimide;
and iBuOCOCl means isobutyl chloroformate.
Example 1: 7-(2-amino-1-oxo-3-thio-propyl)-8-butyl-2-
phenyl-5,6,7,8-tetrahydro-imidazo-[1,2a]-
pyrazine (Compound 1)
CA 02245823 1997-08-10
WO 97/34053 ~ PCTlU897I02b5Z
- 29
Compound 1 was synthesized according to synthetic
scheme 1 as shown below:
SCHEI~fE 1
OH '~'
Cbz-h ~
0
:.
i
Cbz_ v ~ / ~ /
H
se
O
0
ld
,_
.--r , v
,.
SUBSTITUTE SHEET (RULE 26)
CA 02245823 2006-03-16
- 30 -
a. 2-[1-(S)-(((Phenylmethoxy)carbonyl)amino)-pentyl]-4-
phenyl-imidazole
Cbz-(L)-Norleucine (10.0 g, 37.7 mmole) and CsaC03
(6.14 g, 18.9 mmole) were combined in 1:1/DMF:H20 (75
ml), and the mixture was swirled until a homogeneous
mixture was obtained. Solvents were removed in vacuo.
The residue was dissolved in DMF (50 ml), and the
solvents were removed in vacuo again to remove any
so residual H20. The residue was dissolved in DMF (50 ml),
and 2-bromoacetophenone (7.5 g, 37.7 mmole) in DMF (25
ml) was added to the solution. The solution was stirred
for 15 min. at room temperature and then concentrated in
vacuo. The resulting keto-ester was dissolved in EtOAc
(75 ml), CsBr was filtered off, and the solution was
concentrated in vacuo. NH40Ac (50.0 g, 0.65 mole) and
xylenes (150 ml) were added to the solution, and the
solution was heated at reflux for 1.5 hr. The solution
was then cooled, and the solvents were removed in vacuo.
2o The residue was dissolved in EtOAc (75 ml) and washed two
times with saturated NaHC03 solution (50 ml). The EtOAc
layer was then dried over MgS04, filtered, and hexanes
were added to turbidity. The resulting crystalline
product was filtered off, and the product was dried to
yield 10.04 g (73~) of product. m.r.= 136-138C, Mass
spec. (MH+364.3) . NMR (300MHz, CD3COzD) 7.7 (3H, m) , 7.4
(3H, m), 7.3 (5H, m), 5.1 (3H, m), 2.1 (2H, m (obscured
by solvent)), 1.4 (4H, M), 0.9 (3H, t).
3o b. Ethyl, 2-[1-(S)-
(((Phenylmethoxy)carbonyl)amino)- pentyl]-4-
phenyl-1-imidazoleacetate
Ethyl bromoacetate (2.64 ml, 24 mmole), K2C03
(1.93 g, 14.0 mmole), and intermediate 1a (4.36 g,
12.0 mmole) were mixed in DMF (25 ml), and the mixture
was heated at 60°C for 4 hr. The mixture was then
concentrated in vacuo and the residue was dissolved in
CA 02245823 2006-03-16
- 31 -
EtOAC (50 ml). The solution was washed with both a
saturated NaHC03 solution (25 ml) and a saturated NaCl
solution (25 ml). The solution was then dried over
MgS04, filtered, and the solvents were removed in vacuo.
s The residue was further purified by flash chromatography
on silica gel using 80:20 / hexanes:EtOAc as an eluant.
Pure product fractions were combined and concentrated in
vacuo to yield an oil which was crystallized. as 3.09 g
(57~) of product. m.r. - 85-87°C, mass spec. 450.2
(MH+), 472.2 (MNa+). NMR (300MHz, CD3COzD) 7.7 (2H, d),
7.5 (1H, s), 7.2-7.45 (8H, m), 5.25 (2H, dd), 5.1 (2H,
dd), 5.1 (1H, m), 2.15 (2H, m), 1.4 (7H, m), 0.9 (3H, t).
c. (S)-8-Butyl-6-oxo-2-phenyl-5,6,7,8-tetrahydro-
imidazo-[1,2a]-pyrazine
Intermediate 1c (2.89 g, 6.44 mmole) was dissolved
in 50 ml acetic acid containing 290 mg of 10~ Pd on
carbon. The mixture was hydrogenated for 8 hrs at room
2o temperature. The catalyst was removed by filtration
through celite. Lactamization was accomplished by
heating at 70°C for 3 hrs. The product was concentrated
under reduced pressure, and the residue was distributed
between EtOAc and a saturated NaHC03 solution. The EtOAc
2s layer was dried over MgS04 and filtered. The product was
crystallized from EtOAc/hexanes to yield 1.37 g (79~) of
product, m.r.=208-211C, mass spec. 270.2 (MH+), 292.2
(MNa+). NMR (300MHz, CD3C02D) 7.75 (2H, d), 7.5 (1H, s),
7.3-7.45 (3H, m), 5.25 (1H, m), 4.95 (2H, s), 2.1 (2H, m
30 (obscured by solvent peak)), 1.4 (4H, m), 0.9 (3H, t).
d. (S)-8-Butyl-2-phenyl-5,6,7,8-tetrahydro-imidazo-
[1,2a]-pyrazine
35 A solution of intermediate 1c (1.25 g, 4.65 mmole)
in 20 ml THF was added dropwise to a stirred solution of
1M LAH in THF (16 ml). The mixture was heated to reflux
for 1 hr, and then stirred at room temperature overnight.
CA 02245823 1997-08-10
WO 97!30053 PCT/US97102651
- 32 -
The mixture was then quenched by the slow addition of a
mixture of 3 g celite and 2 ml of a saturatd K2C03
solution. The mixture was stirred for 1 hr., and
filtered solids were extracted three times with 25 ml
EtOAc. Solvents were removed under reduced pressure, and
the residue was purified by flash chromatography on
silica gel using ethyl acetate: acetic
acid:pyridine:water/900:54:16:30 as an eluant. The
product fractions were concentrated to oil and then taken
up in ethyl acetate. The solution was washed with 25 ml
of saturated NaHC03, dried over MgS04, filtered, and
concentrated in vacuo. The product was dried on a vacuum
pump to yield I90 mg (16~) of product. Mass spec. 256.2
{MH+) , 278.2 (MNa-i-) .
I5 e. 7-[2-(((I,1-dimethylethoxy)carbonyl)amino)-1-
oxo-3-((triphenylmethyl)thio)propyl]-8-Butyl-2-
phenyl-5,6,7,8-tetrahydro-imidazo-[1,2a]-
.pyrazine
Dicyclohexylcarbodiimide (155 mg, 0.75 mmole) and
2D B~c-~s('Trt)-OH (Advanced Chemtech) were dissolved in 8
ml of THF and stirred for 5 min. The resulting
dicyclohexylurea was filtered off, and the filtrate was
added to intermediate ld. The resulting mixture was
- stirred at room temperature for 6 hrs, concentrated to a
25 gum, and purified by flash chromatography on silica gel
using 7:3/hexanes:EtOAc as an eluant. Product fractions
were combined and concentrated to a glass yielding 500 mg
(95~) of product. Mass spec. 701.5 (MH+), 723.5 (MNa+).
NMR (300MHz, CD3C02D), 7.7 {2H, d), 7.5 (1H, s), 7.2-7.45
30 (18H, m), 6.05 (1H, d), 4.6 (1H, t) 4.2 (2H, t), 3.95
(1H, t), 3.8 (1H, m), 2.6 (2H, m), 2.0 (2H,m (obscured by
solvent)), 1.4 (9H, s), 1.2-1.4 (4H, m), 0.9 (3H, t)
f. 7-(2-amino-1-oxo-3-thio-propyl)-8-butyl-2-
phenyl-5,6,7,8-tetrahydro-imidazo-[1,2a]-
35 pyrazine (Compound 1)
CA 02245823 1997-08-10
WO 97!30053 PCT/US97/0265I
- 33 -
Intermediate le (322 mg, 0.46 mmole) was stirred
with 10 ml of Reagent B (Tfa:phenol:(iPr3SiH):H20/8.8:
0.5:0.2:0.5) under nitrogen for 15 min. The solvents
were removed under reduced pressure, and the residue was
taken up in 25 ml H20 and washed two times with 25 ml
Et20. The aqueous layer was purified by reverse phase
column chromatography to provide 9 mg (5~) of compound 1
as a white lyophilized powder. Mass spec: 359.1 (MH+).
Example 2: 7-(2-amino-1-oxo-3-thio-propyl)-8-butyl-2-(4-
f luorophenyl)-5,6,7,8-tetrahydro-imidazo-
[1,2a]-pyrazine (Compound 2)
Compound 2 was prepared by in a manner analogous to
Example 1 except that 2-bromo-4'-fluoro-acetophenone was
used in place of 2-bromoacetophenone in step a. Mass
spec. 377.2 MH+. NMR {300MEtz, CD3COZD), (approximately 2
to 1 mixture of conformers abserved) 7.8-8.0 (2H, m),
7.6-7.8 (1H, s), 7.1-7.3 (12H, m), 5.8-6.3(1H, m), 3.5-
5.3(5H, t), 3.0-3.4 (2H, m), 2.1-2.6 {2H, m), 1.3-1.7
(.~, ,m) ~ ~_B-1. 0 ( 3 H , m ) .
Example 3: 7-(2-amino-1-oxo-3-thio-propyl)-8-butyl-2-(2-
methoxy-phenyl)--5,6,7,8-tetrahydro-imidazo-
[1,2a]-pyrazine (Compound 3)
- Compound 3 was prepared in a manner analogous to
Example 1 except that 2-bromo-2'-methoxy-acetophenone was
used in place of 2-bromoacetophenone in step a. Mass
spec. 389.3 MH+. NMR (300MHz, DMSO-d6), 8.2-8.8 (3H, s),
7.7-8.0 (2H, m), 7.2-7.4 (1:EI, m), 6.8-7.2(2H, m), 5.4-5.8
-- (lH,t), 4.5-4.8 (1H, t), 3.7-4.5 (4H, m), 3.9(3H, s),
2.7-3.1 (2H, m), i.8-2.1 (2H, m) 1.3-1.6 (4H, m), 0.8-3..0
(3H, t).
Example 4: 7-(2-amino-1-oxo-3-thio-propyl)-8-butyl-2-(3-
methoxy-phenyl)-5,6,7,8-tetrahydro-imidazo-
[1,2a]-pyrazine (Compound 4)
CA 02245823 1997-08-10
WO 97/30053 PCT/US97/02651
- 34 -
Compound 4 was prepared in a manner analogous to
Example 1 except that 2-bromo-3'-methoxy-acetophenone was
used in place of 2-bromoacetophenone in step a. Mass
spec. 389.3 MH+. NMR (300MHz, DMSO-d6), ),
(approximately 4 to 1 mixture of conformers observed)
8.2-8.7 (3H, s), 7.7-8.0 (2H, s), 7.2-7.5 (3H, m), 6.8-
7.0 (2H, d), 5.4-5.8 (1H, t), 4.5-4.8 (1H, t), 3.7-4.5
(4H, m), 3.8 (3H, s), 2.7-3.1 (2H, m), 1.8-2.1 (2H, m)
1.2-1.6 (4H, m), 0.8-1.0 (3H, t).
Example 5: 7-(2-amino-1-oxo-3-thio-propyl)-8-butyl-2-(4-
methoxy-phenyl)-5,6,7,8-tetrahydro-imidazo-
[1,2a]-pyrazine (compound 5)
Compound 5 was prepared in a manner analogous to
Example 1 except that 2-bromo-4'-methoxy-acetophenone was
used in place of 2-bromoacetophenone in step a. Mass
spec. 389.2 MH+. NMR (300MHz, DMSO-d6), ),
(approximately 6 to 1 mixture of conformers observed)
8.2-8.8 (3H, s), 7.7-8.0 (1H, s), 7.5-7.8 (2H, d), 6.9-
?-2 (?I3, ~ ~ 5 . 4-5 . 8 ( 1H, t) , 4 . 5-4 . 8 ( 1H, t) , 3 . 7-4 . 5
(4H, m~, 3.8"(3H, s), 2.7-3.2 (2H, m), 1.8-2.1 (2H, m)
1.2-1.6 (4H, m), 0.8-1.0 (3H, t).
Example 6: 7-(2-amino-1-oxo-3-thio-propyl)-8-(2-hydroxy-
- ethyl)-2-phenyl.-5,6,7,8-tetrahydro-imidazo-
[1,2a]-pyrazine (Compound 6)
Compound 6 was prepared in a manner analogous to
Example 1 except that 2-bromo-4'-methoxy-acetophenone in
place of 2-bromoacetophenone in step a. Mass spec. 347.1
- MH-!- .
Example 7: 7-(2-amino-3-thin-propyl)-8-butyl-3-phenyl-
5,6,7,8-tetrahydro-imidazo-[1,2a]-pyrazine ,
(Compound 7)
Compound 7 was synthesized according to synthetic
Scheme 2 as set forth below:
CA 02245823 1997-08-10
WO 97!30053 PCTliTS9710265I
- 35 -
SCF~EME .'.
O
Bocht'H~~;' ~ O
:~I-Cbz
BocNFi~"r~ Bx
,_
O
-..; Chz
NH
Tc
CA 02245823 2006-03-16
- 36 -
a. Ethyl, 2-((2-(((1,1-dimethylethoxy-)carbonyl-)
amino-)ethyl-)amino-)-hexanoate
Boc-NHCH2CH2NHz was prepared as described by A.P.
Krapcho and C.S. Kuell, Syn. Comm. 20(16):2559-2564
(1990). BoC-NHCHzCH2NH2 (5.00 g, 31.25 mmole), ethyl 2-
bromohexanoate (5.71 ml, 31.25 mmole), and K2C03 (4.31 g,
31.25 mmole) were combined in 75m1 DMF and stirred at
40~C for 1.5 hr. Solvents were removed under reduced
1o pressure, and the residue was then distributed between
Et20 and H20. The ether layer was dried over MgS04 and
filtered, and the solvents were removed under reduced
pressure to yield 7.48 g (79~) of an oil. Mass spec.
303.0 MH+, NMR (CDC13), 4.9-5.1 (1H, S br), 4.1-4.4 (2H,
25 M), 3.0-3.6 (2H, M), 2.5-3.0 (2H, M), 1.9-2.2 (1H, S br),
1.3-1.8 (2H, M), 1.5 (9H, S), 1.2-1.5 (7H, M), 0.8-1.0
(3H t) .
b. Ethyl, 2-(N-(2-(((1,1-dimethylethoxy-)carbonyl-)
20 amino-)ethyl-)]-N-[(phenylmethoxy)carbonyl-
]amino- hexanoate
Intermediate 7a (7.40 g, 24.5 mmole) was dissolved
in 40 ml THF, and 10 ml H20 was then added. The mixture
25 was cooled to 5°C, and Cbz-Cl was added in four portions
to the mixture. The pH of the mixture was maintained
between 8-9 by addition of 2.5N NaOH. When the reaction
was completed, the solvents were removed under vacuum,
and the residue was taken up in EtOAc and washed with 5~
30 citric acid solution. The solvents were removed under
reduced pressure. The residue was dissolved in hexanes,
filtered to remove a crystalline impurity, and dried to
yield 7.348 (69~) of an oil. Mass spec. 337.2 (M-Boc)H+,
459.3 M Na+. NMR (CDC13), 7.2-7.6 (5H, M), 5.1-5.4 (3H,
35 M), 3.9-4.4 (3H, M), 3.5-3.8 (1H, M), 3.1-3.5 (3H, M),
1.6-2.2 (2H, M), 1.4-1.5 (9H, S), 1.1-1.5 (7H, M), 0.8-
1.0 (3H t) .
CA 02245823 1997-08-10
WO 97/30053 PC'T/US97/0265I
- - 37 -
c. 3-Butyl-2-oxo-4-((phenylmethoxy)-carbonyl)-
piperazine
Intermediate 7b (7.10 g, 16.3 mmole) was dissolved
in 25 ml of 9:1/Tfa:H20 and stirred for 15 min. under
nitrogen. Solvents were removed under reduced pressure,
and the residue was taken up in EtOAc. The solution was
washed with a saturated NaHC03 solution, dried over Mgs04,
filtered, and solvents were removed under reduced
pressure. 10 ml acetic acid and lOml pyridine was added
l0.to the residue, and it was refluxed for 1 hour under
nitrogen. Solvents were removed under reduced pressure,
and the residue was dissolved in EtOAc and washed two
times with 5~ citric acid. 'rhe solution was dried over
MgS04, filtered, and solvent:a were removed under reduced
pressure. The product was crystallized from a solution
of EtOAc/hexanes to yield 2.40g (51~) of a white powder.
m.r. - 107-108°C. Mass spec. 291.2 MH+, 313.2 M Na+. NMR
(CDClg), 7.3-7.5 (5H, S), 7.0-7.2 (1H, S br), 5.1-5.3
(2H, Q), 4.5-4.8 (1H, S br), 4.1-4.4 (1H, S br), 3.4-3.6
[3ii,, Tj ~ 3.3-3. 4 ( 2H, D) , I . 7-2 . 1 ( 2H, M) , 1. 2-1. 5 ( 4H,
M), 0.8-1.0 (3H S br).
d. 3-Butyl-4-((phenylmethoxy)-carbonyl)-2-thio-
piperazine
Intermediate 7c (2.85 g, 9.83 mmole) and Lawsson's
reagent (2.02 g, 5.00 mmole) were dissolved in 20 ml THF
and heated at reflux under nitrogen for 1.5 hours. The
solution was cooled, and solvents were removed under
reduced pressure. The residue was dissolved in 50 ml
Et20 and washed three times with 25 ml of 1N NaOH. The
solution was dried over MgS04, filtered, and solvents
were removed under reduced pressure. The residue was
purified by flash chromatography on silica gel using
65:35/hexanes:EtOAc as an eluant. The product fractions
were concentrated to yield 2.19 g (73~) of an oil which
crystallized. m.r. - 94-96°C. Mass spec. 307.2 MH+,
CA 02245823 2006-03-16
- 38 -
329.2 M Na+. NMR (CDC13), 8.5-8.8 (1H, S), 7.3-7.5 (5H, S
br), 4.9-5.4 (3H, M), 4.0-4.5 (1H, M), 1.1-3.6 (3H, M),
3.4-3.6 (1H, T), 2.2-2.4 (2H, S br), 1.7-2.0 (2H, M),
1.2-1.6 (4H, S br), 0.7-1.0 (3H S br).
e. 8-Butyl-3-phenyl-7-((phenylmethoxy)-carbonyl)
5,6,7,8-tetrahydro-imidazo-[1,2a]-pyrazine
Intermediate 7d (1.07 g, 3.5 mmole) was dissolved in
Zo 10 ml THF. zodomethane (2.18 ml, 35.0 mmole) was added
and stirred at room temperature for 8 hours. Solvents
were removed under reduced pressure. The residue was
dissolved in 10 ml THF, and 4-methylmorpholine (771 ul,
7.0 mmole) and 2-aminoacetophenone hydrochloride (686 mg,
4.00 mmole) were added to the solution. The solution was
stirred overnight at room temperature and then refluxed
for 2 hours. 15 ml acetic acid was added, and 15 ml
solvent was distilled off. The solution was then
refluxed for 1 hour and concentrated under vacuum. The
2o residue was purified by flash chromatography on silica
gel using 70:30/hexanes:EtOAc as an eluant. The product
fractions were concentrated to yield 0.978 (71~) of an
oil. Mass spec. 390.3 MH+, 412.2 M Na+. NMR (CD3COaD),
(approximately 1:1 mixture of conformers) 7.2-7.6 (11H,
M), 5.6-5.8 (1H, M), 5.0-5.4 (2H, M), 4.4-4.8 (1H, M),
4.1-4.4 (1H, M), 3.9-4.1 (1H, M), 3.3-3.6 (1H, S br),
1.8-2.2 (obscured by solvent)(2H, M), 1.7-2.0 (2H, M),
1.1-1.6 (4H, S br), 0.7-0.9 (3H S br).
3o f. 8-butyl-3-phenyl-5,6,7,8-tetrahydro-imidazo-
[1,2a]-pyrazine
Intermediate 7e (1.08 g, 2.78 mmole) was dissolved
in 4 ml of THF. 10 ml of 4N HC1 was added, and the
mixture was heated at reflux under nitrogen for 4 hours.
Solvents were removed under reduced pressure to yield a
CA 02245823 1997-08-10
W O 97130053 PC~YiJS97/D2652
39 -
solid product which was washed with ether and dried to
740 mg. Mass spec. 256 MH+.
g. 8-butyl-7-(2-((1,1-dimethylethoxy-)carbonyl-
amino-1-oxo-3-(triphenylmethyl-thio)-propyl)-3-
phenyl-5,6,7,8-tetrahydro-imidazo-[1,2a)-
pyrazine
Boc-Cys(Trt)-OH (2.32 g, 5.00 mmole; Advanced
Chemtech) was dissolved in 20 ml of THF. DCC (515 mg,
2.50 mmole) was added to this solution. The solution was
allowed to stand for 15 min., and the DCC was filtered
off. The filtrate was added to a solution of
intermediate 7f (700 mg, 2.4 mmole) and NMM (655 ul, 4.80
mmole) in 20 ml of THF. The solution was stirred for two
hours at room temperature, and solvents were removed
under reduced pressure. The resulting product was
purified by silica gel chromatography using
70:30/hexanes:EtOAc as an eluant. The product fractions
were combined and concentrated to a foam, which was dried
to yield 1.47 g (87.50 of product. Mass spec. 701.4
._2D ...,MFi~-~ ~ f~D3C02D) , 7 . 1-7 . 7 ( 21H, M) , 6 . 0-6 . 2 ( 1H, M) ,
6.5-6.7 jl~I, T), 3.4-4.4 (4H, M), 2.4-2.8 (2H, M), 1.8-
2.4 (2H, M) (partially obscured by solvent signal), 1.4
(9H, S), 1.1-1.4 (4H, M), 0.7-1.0 (3H, M)).
- h. 7-butyl-6-(2-((dimethylethoxy)-carbonyl-)amino-
3-(triphenylmethyl~-thio)propyl-)-3-phenyl-
4,5,6,7-tetrahydro-imidazo-[1,2a]piperazine
Intermediate 7g (350 mg, 0.50 mmole) was dissolved
in THE (3 ml) and iM BH3/THf (7 ml, 7.0 mmole) was added.
- - The reaction was heated at i:eflux for 2 hr under N2. The
solution was cooled to room temperature, and the excess
reagent was destroyed by careful addition of a solution
of MeOH (8 ml) and acetic a<~id (2 ml). The crude product
was
CA 02245823 1997-08-10
WO 97/30053 PCTJUS97/02651
- 40 -
concentrated under reduced pressure and redissoived in
3:1/acetic acid: H2) for 1 hr. After removing solvents
under reduced pressure, the residue was purified by flash
chromatography on silica gel using 1~ HOAc/ETOAc as
eluant. The product fractions were combined,
concentrated, and dried to 100 mg (29~). MH+ 687.5.
i. 7-(2-amino-3-thin-propyl)-8-butyl-3-phenyl-
5,6,7,8-tetrahydro-imidazo-[1,2a~-pyrazine
(Compound 7)
Intermediate 7h (100 mg, 0.146 mmole) was treated
under nitrogen with a mixture of Tfa:H20:iPr3SiH/93:5:2
(10 mI} for 15 min. Solvents were removed under reduced
pressure, and the resulting solids were triturated eight
times with 4 ml of H20 and then filtered off. The
filtrate was purified by prep HPLC to yield 45 mg (74~)
of lyophilized compound 7 which appeared as a 1:1 mixture
of isomers on analytical HPLC. Mass spec. 345.2 MH+, NMP
(CD3C02D), 7.4-7.7 (6H, M), 6.0-6.4 (1H, M}, 3.8-5.3 (5H,
3I) , 3. O-3 _~ (2ii, M) , 2 . O-2 . 4 ( 2H, M) , 1. 2-1. 7 ( 4H, M) ,
0.9-1.0 (3H, M) .
Example 8: 2-(1-(N-(2-amino-1-oxo-3-thiopropyl}-N-
methyl)- amino-pentyl)- 5-phenyl-imidazole
(Compound 8)
Compound 8 was synthesized according to Scheme 3 as
set forth below:
CA 02245823 1997-08-10
WO 97!30053 PCTlUS97/0265I
_ 4:1 -
SCHEME 3
H
f
a
H N
Za to
BOG-NH : I
TrT-S
tb
to
a. 5-(1-(Methylamino)-pentyl)-2-phenyl-imidazole
Intermediate la (1.50 g, 4.10 mmole) and T.AH (50~ in
oil; Alfa Products, Danvers, MA) (1.25 g, 16.4 mmole)
_ 5 were combined in toluene (10 ml) and TFIF (5 ml) under
nitrogen, and the mixture was heated to 55°C for 4 hrs.
The mixture was poured into EtOAc (100 ml), and moist
celite was added to the mixture. Solids were filtered
off, and the filtrate was dried over Na2SO4 and
- 10 concentrated. The crude product was used without further
purification.
CA 02245823 1997-08-10
WO 97/30053 PCT/LTS97/02651
- 42 -
b. 5-((N-(2-(((1,1-dimethylethoxy-)carbonyl-)-
amino)-1-oxo-3-(triphenylmethyl-thio)-propyl)-N-
methyl-amino)-pentyl}- 2-phenyl-imidazole
Boc-Cys(Trt)-OH (3.8 g, 8.2 mmole) and DTC (643 ul,
4.1 mmole) were combined in CH2C12 (50 ml) and stirred for
0.5 hours at room temperature. Intermediate 8a (1.00 g,
4.1 mmole) was added and stirred at room temperature for
1 hour. Solids were filtered off and diluted to 100 ml
with CH2C12. The solution was washed with saturated
NaHC~g (3 x 50 ml) and saturated NaCl (1 x 50 ml), dried
over Na2SO4, filtered, and concentrated. The residue was
purified by flash chromatography on silica gel (120 g)
using first CH2C12, and then 1~ MeOH/CH2C12 as eluants.
Product fractions were combined and concentrated to yield
1.36 g (46~) of product.
c. 2-(1-(N-(2-amino-1-oxo-3-thiopropyl)-N-methyl)-
amino-pentyl)-5-phenyl-imidazole (Compound 8)
Intermediate 8b was dissolved in 1o ml of Reagen*~ B
under nitrogen, and the solution was stirred for 0.5
2D hours.. Solids were filtered off, and solvents were
removed under a stream of nitrogen. The residue was
triturated with ethyl ether and purified by reverse phase
HPLC to yield compound 8 as a white solid after
lyophilization (?4.lmg, 49~). Mass spec: 347.2 MHt.
Example 9: 2-(((2-amino-1-oxo-3-mercapto-propyl)-amino}-
methyl}-5-phenyl-thiazole-4-carbonyl-
methionine (Compound 9)
CA 02245823 1997-08-10
WO 97/30053 ' PCTlUS97/0265~
_ 4~
Compound 9 was synthesized according to Scheme 4 as
set forth below.
_ SCE 4
O/ ----r
::
CbzNF-i
9e
O S / w
0 0.~~~
?d
.mss
BOC-1'~H 'i~
1
E
- Trt-S
. D
~ N~"'~ / '
Ei ~ O
HS 0
OH
...s
SUBSTITUTE SHEET (RULE 26)
CA 02245823 1997-08-10
WO 97/30053 PCTlUS97/02651
- 44 -
a. Methyl, 2-amino-3-oxo-3-phenylpropionate
Schiff base (10.0 g, 39.5 mmole) was prepared as
described in (O'Donnel, et al., J. Org. Chem. 47:2663
(1982). Schiff base was dissolved in THF ( 60 ml) and
added dropwise to a stirred mixture of KOtBu (4.43g, 39.5
mmole) in THF (30 ml) which was cooled to -70°C under
nitrogen. The solution was stirred for l0 min. at -70°C,
and the anion was transferred to a stirred solution of
benzoyl chloride {4.59 ml, 39.5 mmole) in THF (50 ml)
which had also been cooled to -70°C under nitrogen. The
solution was stirred for 45 min. at -70°C and then
quenched by the addition of 4N HC1 (30 ml). THF was
removed under reduced pressure, and the aqueous layer was
washed twice with 50 ml of ethyl ether. The solution was
concentrated to a solid, and the product was dissolved in
MeOH (30 ml), and the KC1 was ffiltered. The product was
crystallized by the addition of ether to the point of
turbidity. The product was filtered off and dried to
. 20 yi~lc3 x.89 'g j32~) of product. Mass spec. 194.1 (MH+)
b. Methyl, 2-({(1,1-dimethylethoxy-)carbonyl-
glycyl)-amino-3-oxo-3-phenylpropionate
_ Boa-Gly-OH (3.15 g, 18.0 mmole) and NMM (1.98 ml,
18.0 mmole) were combined in THF (50 ml) and the solution
was cooled to -20°C. iBuCOCl (2.34 ml, 18.0 mmole) was
added to the solution, and the mixture was stirred for 5
min. at -20°C. Intermediate 9a (4.13 g, 18.0 mmole) and
NMM (1.98 ml, 18.0 mmole) was added to the solution which
was stirred vigorously while returning to room
temperature. The solution was concentrated under reduced
pressure, and the residue was dissolved in EtOAc (50 ml)
and washed once with H20, once with 5~ citric acid
solution, and once with saturated NaCl solution. The ,
solution was dried over MgS04, ffiltered, and concentrated
under reduced pressure. Further purification was
CA 02245823 1997-08-10
W O 9?!30053 PCT/LTS97f02651
- 45 -
accomplished by flash chromatography on silica gel using
1:1/hexanes:EtOAc as an elu:ant. Product fractions were
combined and concentrated to yield 3.28g (52~) of
product. Mass spec. 373.2 MNa+
c. Methyl, 2-(((1,1-dimethylethoxy-)carbonyl-)-
amino)-methyl-5-phenyl-thiazole-4-carboxylate
Intermediate 9b (3.10 g, 8.86 mmole) and Lawesson's
reagent (3.6 g, 8.9 mmole; Aldrich Chem. Co., St. Louis,
MO) were combined in THF (30 ml) and heated to reflex for
1 hour. Solvents were removed under a stream of
nitrogen, and the residue was purified by flash
chromatography on silica gel using 1:1/hexanes:EtOAc as
an eluant. Product fractions were combined and
concentrated under reduced pressure to yield 2.21 g (72~)
of product. Mass spec. 34.0 MH+, 371.2 MNa+.
d. 2-(((1,1-dimethylethoxy-)carbonyl-)-amino)-
methyl-5-phenyl-thiazole-4-carbonyl-methionine
methyl ester
Zntet~ediate 9c was dissolved in methanol (5 ml),
and an aqueous solution of NaOH (344 mg, 8.61 mmole) in
minimum of H20 was added to the solution. The solution
was stirred at 40°C for 1 hour, and the solvents were
- removed under reduced pressure. The residue was
distributed between EtOAc (20 ml) and 5~ citric acid (20
ml). The EtOAc layer was dried over MgS04, filtered, and
concentrated under reduced. pressure. The residue was
dissolved in THF (10 ml), and HOSu (330 mg, 2.87 mmole),
- HC1-Met-OMe (573 mg, 2.87 mmole), NMM (316 mg, 2.87
mmole) and DCC (591 mg, 2.87 mmole) were added to the
solution. The mixture was stirred at room temperature
overnight, filtered, and concentrated under reduced
pressure. The residue was: taken up in EtOAc (25 ml), and
washed once in 5~ citric acid solution and twice in a
saturated NaHC03 solution. The solution was dried over
CA 02245823 1997-08-10
WO 97/30053 PCT/US97/02651
- 46 -
MgSO4, filtered, and concentrated under reduced pressure
to yield 590 mg (43~) of product. Mass spec. 502.2 MNa+,
4 8 0 . 4 MH-t- -
e. 2-(((2-(((1,1-dimethylethoxy)-carbonyl)-amino)- .
1-oxo-3-(triphenylmethyl-thio)-propyl)-amino)-
methyl)-5-phenyl-thiazole-4-carbonyl-methionine
methyl ester
Intermediate 9d (590 mg, 1.23 mmole) was treated
with Reagent B (10 ml) for 15 min at room temperature
under nitrogen. Solvents were removed under reduced
pressure. The residue was triturated twice with 25 mi
Et20 and decanted. The residue was then dissolved in THF
(10 ml) and added to the mixed anhydride generated from
Boc-Cys(Trt)-OH (570 mg, 1.23 mmole), NI~f (135 ul. 1.23
mmole) and iBuOCOCl (160 ul, 1.23 mmole) at -20°C under
nitrogen over 5 min. NMM (135 ul, 1.23 mmole) was added
to the mixture which was then allow~~ ~~ warm to raom
temperature. Solvents were removed under reduced
~ssute_ .~ residue was taken up in EtOAc (25 ml) and
washed with 25 ml of H2O and 25 ml of 5~ citric acid
solution. The solution was dried over Mg504 and
concentrated to yield l.Olg (1000 of a white foam.
f. 2-(((2-amino-1-oxo-3-mercapto-propyl)-amino)-
methyl)-5-phenyl-thiazole-4-carbonyl-methionine
- (Compound 9)
Intermediate 9e (250 mg, 0.30 mmole) was dissolved
in MeOH (2 ml). NaOH (40 mg), dissolved in a minimum of
- H20, was added to the solution. The solution was stirred
overnight at room temperature. Solvents were removed
under reduced pressure and the residue was dissolved in
reagent B (10 ml). The solution was stirred fox 15 min.
at room temperature under nitrogen and then concentrated
under reduced pressure. The residue was purified by
reverse phase HPLC. Product fractions were combined and
CA 02245823 1997-08-10
WO 97!30053 PCT/US97/OZ65I
_. 47 -
lyophilized to yield 32 mg (20~) of compound 9 as a white
solid. Mass spec 469 MH+.
Example 10: bis-1,1'-[2-amino-3-(8-butyl-2-(2-
methoxyphenyi)-5,6,7,8-tetrahydro-
imidazo[1,2a]pyrazine-6-yl)-3-oxo~propyl
disulfide (Compound 10)
Intermediate 3e (300 mg, 0.41 mmole) was dissolved
in methanol, and H20 (0.3 nal) was added. A solution of
iodine (104 mg, 0.41 mmol) in methanol (3 ml) was added,
1.0 and the mixture was stirred for 2 hours. The solvents
were removed under reduced pressure, and the residue was
triturated with hexanes (2 x 5 ml). The residue was then
dissolved in ETOAc (5 ml) .and washed with 5~ Na2S203
solution (i0 ml). The organic layer was dried over
Na2S04, filtered, and concentrated.to a glass.
The glass was treated with 93:5:2/Tfa:H20:iPr3SiH
for 25 minutes under N2. 'The solvents were removed under
reduced pressure, and the residue was purified by RP HPLC
and lyophilized. Yield = 48 mg (25~). Mas spec. 775.4
20' I~tii-t-, 388.5 M2H++ ~ ~ (DMSO-d6) , (approx. 5:1 mixture of
conformers) 8.5-9.0 (3H,S), 8.0-8.2 (lH,d}, 7.5-7.7
(1H,S), 7.1-7.3 (lH,t), 7.0-7.1 (lH,d), 6.9-7.1 (1H, t),
5.2-5.6 (lH,t), 4.8-5.0 (l.H,t), 3.6-4.7 (1H,M), 3.8-4.0
(3H,S), 3.2-3.5 (2H,M), 1.8-2.2 (2H,M), 1.2-1.7 (4H, M),
0.8-1.0 (3H, t).
CA 02245823 1997-08-10
WO 97/30053 PCTIUS97/0265I
- 48 -
Example 11: 7-(2-amino-1-oxo-3-thio-propyl)-2-(2-
methoxyphenyl)-8-(2-methylpropyl)-5,6,7,8-
tetrahydro-imidazo-[1,2a]-pyrazine -(Compound
11) -
Compound 11 was prepared in a manner analogous to
Example 3 except Boc-L-Leucine was used in place of Cbz-
(L)-Noxleucine in step a and the Boc group was cleaved
with a 9:1/Tfa:H2) mixture instead of by catalytic
hydrogenation in step c. Mass spec. 389.1 MH+. NMR (300
MHz, DMSO-d6) 8.6-8.8 (3H, s), 8.1-8.2 (1H, d), 7.9-8.1
(1H, S), 7.3-7.5 (1H, t), 7.1-7.3 (1H, d), 7.0-7.1 (1H,
T), 5.9-6.1 (1H, d), 4.7-4.8 (1H, S), 4.5-4.7 (1H, d),
4.3-4.4 (1H, d), 4.1-4.3 (1H, t), 3.9-4.0 (3H, S), 3.8-
4.0 (1H, T), 3.3-3.5 (1H, t), 2.8-3.1 (2H, M), 1.9-2.2
(2H, M), 1.7-1.8 (1H, M), 1.0-1.2 (3H, t), 0.8-1.0 (3H,
t) .
Example 12: bis-1,1'-[2-amino-3-(2-(2-methoxyphenyl)-8-
(2-methylpropyl)-5,6,7,8-tetrahydro-
imidazo[1,2a] piperazine-7-yl)-3-oxo~propyl
2D r3isulf ide ( Compound 12 )
Compound i2 was prepared in a manner analogous to
Example 10 except intermediate 11e was used in place of
intermediate 3e. Mass. spec 388.5 M2H++, 775.4 MH+. NMR
(300 MHz, DMSO-d6} 8.7-9.2 (3H, S), 8.1-8.2 (1H, d), 7.9-
8.1 (1H, S), 7.3-7.5 (1H, t), 7.1-7.3 (1H, d), ?.0-7.2
(1H, t}, 5.9-6.1 (1H, d), 4.8-5.0 (1H,S), 4.5-4.7 (1H,
d), 4.3-4.5 (1H, d}, 4.1-4.4 (1H, t}, 3.8-4.1 (1H,M),
3.8-4.0 (3H, S), 3.2-3.5 (2H, M), 1.8-2.2 (2H, M), 1.7-
_ 1.9 (1H, M), 1.0-1.2 (3H, d), 0.8-1.0 (3H, d).
CA 02245823 1997-08-10
WO 97/30053 PCT/US97/0265I
- 49 -
Example 13: 7-(2-amino-1-oxo-3-thiopropyl)-8-butyl-2-(2-
ethoxyphenyl)--5,6,7,8-tetrahydroimidazo
[1,2a] pyrazine (Compound 13).
a. Intermediate 3c (2.54 g, 8.50 mMole) was
dissolved in THF (15 ml) and a IM solution of borane in
THF (34.0 ml, 34.0 mMole) was added dropwise over 10
minutes at room temperature. The mixture was refluxed
for 2 hours and allowed to stand at room temperature
overnight. A solution of 4 N HC1 (25 ml) was added
l0 dropwise and the resulting mixture was heated at reflex
for a hour. The mixture was concentrated to H20, made
basic by careful addition of solid NaHC03, and extracted
with EtOAc (2 x 25 ml). The EtOAc layers were dried over
Na2S04, filtered, and concentrated to an oil. A solution
of 5& HC1 (25 ml) was added, and the mixture was
concentrated to a solid. 'rhe solid was recrystallized
from methanol and ethyl etlner to yield 2.72 g (89.50 of
the dihydrochloride salt. Mass spec. 286.2. M.R. - 242-
247°C.
b. Intermediate 13a (850 mg, 2.37 mMole) was
distributed between CH2C12 and saturated NaHCO3 solution,
the CHZC12 layer was dried over Na2S04, and filtered. A
1M solution. of BBr3 in CH2C12 was added and the resulting
mixture was heated at reflex for 1 hour. The reaction
was cooled and poured onto saturated NaHCOg solution (25
ml). The CH2C12 layer was dried over Na2S04 and filtered.
Di-(tert) -butyldicarbonate (523 mg, 2.40 mMole) was
added and the mixture was stirred at room temperature
over the weekend. Solvents were evaporated, and the
resulting oil was purified by column chromatography on
silica gel using 70 . 30 /' hexanes . Ethyl acetate as
eluant. Yield = 700 mg (80~) of a clear oil. Mass spec.
372.2 (MH+). NMR
CA 02245823 1997-08-10
WO 97/30053 PCT/US97102651
- 50 -
c. Protected intermediate (13b) (600 mg, 1.62
mMole) was dissolved in THF (10 ml) and added dropwise to
a solution of NaH (60~ in oil, 120 mg, 3.0 mMole) in THF .
(10 m1) at room temperature under N2. The reaction was
stirred 15 minutes and ethyl iodide (400 ul, 5.00 mMole)
was added. The mixture was stirred overnight at room
temperature then concentrated under reduced pressure.
Saturated NaHC03 solution (10 ml) was added, and the
product was extracted with ethyl ether (2 x 20 ml). The
l0.ether was evaporated, and the residue was purified by
column chromatography on silica gel using 3:1 / hexanes .
ethyl acetate as eluant. Yield = 410mg (64~) of the
ether. Mass spec. 400.3 (MH+). M.R. - 103-109°C.
d. Intermediate (13c) was treated with 90~
TFA/H20 (2 ml) for 0.5 hours and concentrated to remove
the BOC group. Coupling with Boc-(L)-Cys(Trt}-OH and
deprotection were accomplished in a manner analogous to
example le and lf, respectively, to yield compound 13.
lriass spec_ hQ3_2 (MH+). NMR (3OOMHz, DMSO-d6) 8.4-8.7
(3H, broad s), 7.9-8.0 (1H, s), 7.75-7.9 (1H, d}, 7.15-
7.3 (1H, t), 7.0-7.1 (1H, d), 6.85-7.0 (1H, t), 5.7-5.85
(1H, m}, 4.65-4.8 (1H, broad s), 4.45-4.6 (1H, d), 4.3-
4 . 4 ( 1I3, d, d) , 4 . 1-4 . 25 ( l.H, m) , 3 . 75-3 . 95 ( 1H, m) , 3 . 1-
3.3 (1H, m), 2.8-3.1 (2H, m), 1.9-2.15 (2H, m), 1.2-1.5
(4H, m), 0.8-1.0 (3H, t).
Example 14: 7-(2-amino-1-oxo-3-thioprapyl}-8-butyl-2-(2-
hydroxyphenyl)-5,6,7,8-tetrahydroimidazo
- [1,2a]pyrazine (compound 14).
a. Intermediate 13a (179 mg, 0.50 mMole) was
distributed between CH2C12 and saturated NaHC03 solution,
the CHZC12 layer was dried over Na2S04, and filtered. A
1M solution of BBr3 in CH2C12 was added, and the resulting ,
mixture was heated at reflux for 1 hour. The reaction
CA 02245823 1997-08-10
WO 97!30053 PCT1US97/0265I
- - 51 -
was cooled and poured onto saturated NaHC03 solution (25
ml). The CH2C12 layer was dried over Na2S04, filtered,
and stripped to yield crude de-methylated product as a
gum. This material was used without further
purification.
b. Coupling of (14a) with Boc-(L)-Cys(Trt)-OH and
deprotection were accomplished in a manner analogous to
example le and lf, respectively, to yield compound 14.
Mass spec. 403.2 (MH+). NM1~ (300MHz, DMSO-d6) 8.55-8.8
(3H, broad s), 8.1-8.2 (1H, d), 7.85-7.95 (1H, s), 7.35-
7.45 (1H, t), 7.15-7.25 (1H, d), 7.0-7.15 (1H, t), 5.85-
6.0 (1H, d,d), 4.65-4.8 (1H, broad s), 4.55-4.7 (IH,
d,d), 4.15-4.3 (2H, q), 4.1~-4.2 (1H, m), 3.8-3.95 (1H,
m), 3.3-3.5 (1H, t), 2.15-2.3 (1H, m), 1.95-2.15 (1H, m),
1.4-1.5 (3H, t), 1.2-I.5 (4H, m), 0.8-1.0 (3H, t).
Example 15: 2-(1-(N-(2-amino-1-oxo-3-thiopropyl)-N-
Methyl)-amino-pentyl-5-(2-methoxyphenyl)-
imidazole (Compound 15)
~ompo~znd 15 was prepared in a manner analogous to
example 8 except 2-Bromo-2'-methoxyacetophenone was used
in place of 2-Bromoacetophenone in step la. Mass Spec.
377.1 MH+. NMR (300MHz, CD3C02D) 7.8-7.9 (1H, s), 7.65-
7.75 (1H, d,d), 7.4-7.55 (1H, m), 7.14-7.2 (1H, d), 7.05-
7.14 (1H, t), 5.6-5.8 (1H, t), 4.8-4.9 (1H, t), 3.9-4.0
(3H, s), 3.25-3.35 (3H, s), 3.05-3.25 (2H, m), 2.2-2.4
(2H, m), 1.2-1.6 (4H, m), 0~.8-1.0 (3H, t).
CA 02245823 2006-03-16
- 52 -
Example 16: bis-1,1'-[2-(1-(N-(2-amino-1-oxo-3-
thiopropyl)-N-methylamino)-pentyl]-5-(2-
methoxyphenyl) imidazole] disulfide
(Compound 16)
Compound 16 was prepared in a manner analogous to
example 10 except compound 15 in place of intermediate
3e. Mass spec. 751.5 MH+. NMR (300MHz, CD3C02D) 7.75-
7.85 (1H, s), 7.65-7.75 (1H, d,d), 7.35-7.5 (1H, m), 7.1-
7.2 (1H, d), 7.0-7.1 (1H, t), 5.5-5.6 (1H, t), 4.8-4.95
(1H, t), 3.9-4.1 (3H, s), 3.3-3.5 (2H, m), 3.2-3.3 (1H,
s), 2.2-2.4 (2H, m), 2.0-2.2 (acetate signal), 1.2-1.6
(4H, m), 0.8-1.0 (3H, t).
Example 17: 7-(2-amino-1-oxo-3-thiopropyl)-8-(2-
methylpropyl)-2-(1-naphthyl)-5,6,7,8-
tetrahydroimidazo [1,2a]pyrazine (Compound
17) .
2o a. 1'-Acetonaphthone (10.2 g, 60.0 mMole) and 0.1
ml of concentrated HC1 were dissolved in acetic acid (100
ml) and bromine (9.6 g, 60.0 mMole) were added dropwise
with stirring over a three hour period. The reaction was
concentrated under reduced pressure and dried to constant
weight. The product was used without further
purification.
b. Compound 17 was prepared in a manner analogous
to example 1 except Cbz-(L)-Leucine was used in place of
3o Cbz-(L)-Norleucine, intermediate 17a was used in place of
2-Bromoacetophenone in step 1a, and 1M BH3/THF was used
for reduction of lactam intermediate in step d. Mass
spec. 409.2 MH+. NMR (300MHz, DMSO-d6) 8.5-8.9 (3H, s),
8.1-8.25 (1H, d), 7.9-8.15 (3H, m), 7.7-7.8 (1H, d), 7.5-
7.7 (3H, m), 5.8-6.1 (1H, d), 4.7-4.85 (1H, s), 4.55-4.75
(1H, d), 4.2-4.45 (2H, m), 3.85-4.05 (1H, m), 3.0-3.4
(10H, H20), 2.9-3.1 (2H, q), 1.9-2.2 (1H, t), 1.7-1.9
(2H, m), 1.0-1.2 (3H, d), 0.8-1.0 (3H, d).
CA 02245823 2006-03-16
- 53 -
Example 18: 7-(2-amino-1-oxo-3-thiopropyl)-8-(1-
methyl-propyl)-2-(2-methoxyphenyl)-
5,6,7,8-tetrahydroimidazo [1,2a]pyrazine
(Compound 18).
Compound 18 was prepared in a manner analogous to
example 3 except Cbz-(L)-Isoleucine was used in place on
Cbz-(L)-Norleucine in step a. Mass spec. 389.3 MH+. NMR
(300MHz, DMSO-d6) 8.5-8.9 (3H, s), 8.05-8.2 (1H, d), 7.9-
8.05 (1H, s), 7.35-7.5 (1H, t), 7.15-7.25 (1H, d), 7.0-
7.15 (1H, t), 5.65-5.85 (1H, d), 4.65-4.8 (1H, s), 4.5-
4.65 (1H, d,d), 4.3-4.45 (1H, d,d), 3.9-4.0 (3H, s), 3.8-
4.0 (1H, m), 3.2-3.7 (8H, H20), 2.8-3.0 (2H, m), 2.2-2.4
(1H, m), 1.4-1.6 (1H, m), 1.15-1.35 (1H, m), 1.0-1.15
(3H, d), 0.8-0.95 (3H, t).
Example 19: bis-1,1'-7-(2-amino-1-oxo-3-thiopropyl
-(2-(1-naphthyl)-8-(2-methylpropyl)-
5,6,7,8-tetrahydroimidazo [1,2a]pyrazin-7-
2o yl) disulfide (Compound 19).
Compound 19 was prepared in a manner analogous to
example 10 except compound 17 was used in place of
intermediate 3e. Mass spec. 815.5 MH+. NMR (300MHz,
DMSO-d6) 8.7-9.2 (3H, s), 8.15-8.3 (1H, s), 8.0-8.1 (2H,
m), 7.85-8.0 (1H, s), 7.7-7.8 (1H, d), 7.5-7.7 (3H, m),
5.8-6.0 (1H, s), 4.8-5.0 (1H, s), 4.5-4.54 (1H, d), 4.4-
4.5 (1H, d), 4.2-4.4 (1H, t), 3.9-4.1 (1H, t), 3.0-3.9
(12H, m H20 obscures signal), 2.0-2.2 (1H, t), 1.7-2.0
(2H, m), 1.0-1.2 (3H, d), 0.85-1.0 (3H, d).
Example 20: bis-1,1'-[7-(2-amino-1-oxo-3-thiopropyl)-
2-(methoxy-phenyl)-8-(1-methylpropyl)-
5,6,7,8-tetrahydroimidazo [1,2a] pyrazine]
disulfide (Compound 20).
Compound 20 was prepared in a manner analogous to
example 10 except compound 18 was used in place of
intermediate 3e. Mass spec. 775.5 MH+. NMR (300MHz,
4o DMSO-d6) 8.7-9.0 (3H, s), 8.05-8.15 (1H, d), 7.9-8.1 (1H,
s), 7.35-7.5 (1H, t), 7.15-7.25 (1H, d), 7.0-7.15 (1H,
CA 02245823 1997-08-10
WO 97/30053 PCTlUS97/02651
- 54 -
t), 5.65-5.85 (iH, d), 4.8-5.0 (1H, s), 4.45-4.6 (iH, d),
4.35-4.5 (1H, d), 4.2-4.35 (iH, m), 3.8-4.1 (1H, m), 3.8-
3.9 (3H, s), 3.4-3.8 (lOH, H20), 3.2-3.4 (2H, d), 2.2-2.4
(1H, m), 1.4-1.65 (iH, m}, 1.15-1.35 (1H, m), 1.0-1.15
(3H, d), 0.8-0.95 (3H, t).
Example 21: S-(dimethylethyl)-S'-[2-amino-3-oxo-3(8-
butyl-2-(2-methoxyphenyl;}-5,6,7,8-
tetrahydroimidazo [1,2a]pyrazin-7-
yl)propyl]disulfide (Compound 21)
Compound 21 was prepared in a manner analogous to
example 3 except that Fmoc-{L)- Cys(tBuS-)OH was used in
step a and final deprotection was accomplished by
treatment with tris{aminoethyl)amine (1.5m1 per mmole} in
CH2C12 (10 ml per mmole) for 0.5 hour at room temperature.
The product was purified by preparative reverse phase
column chromatography to provide pure compound 21. Mass
spec_ 477.3 MH-E-. NMR (300MHz, DMSO-d5, 90°C) 8.0-8.1 (1H,
d), 7.4-7.5 (iH, s), 7.1-7.3 (1H, t), 7.0-7.1 (1H, d),
6.9-7. 0 (1~3, t) , 5. 4-5 . 55 ( 1H, s) , 4 . 3-4 . 7 ( 1H, m) , 4 . 1-
4.3 (1H, t3), 3.8-4.1 (7H, m), 3.0-3.2 (2H, m + H20), 2.8-
2.9 (iH, d,d), 2.1-2.3 (2H, m), 1.7-2.1 (2H, m), 1.2-1.7
(13H, m}, 0.8-1.0 (3H, t).
Example 22: 7-(2-amino-1-oxo-3-thiopropyl)-8-butyl-2-
(2-methylphenyl)-5,6,7,8-tetrahydroimidazo
[1,2a]pyrazine (Compound 22)
a. 2'-Methylacetophenone (25.Og, 186 mMole) was
dissolved in glacial acetic acid (250 ml) and
- concentrated HC1 (250 uL) was added followed by a
dropwise addition of bromine (9.6 ml, 186 mMole) over 15
minutes. The mixture was stirred 3 hours and then
concentrated under reduced pressure. The residue was
taken up in ethyl ether and washed with saturated NaHC03
solution. The ether Layer was dried over Na2S04, '
filtered, and concentrated to yield 38.Og, (96~} of crude
CA 02245823 1997-08-10
WO 97/30053 PCTlUS97/02651
- 55 -
2-bromo-2'-methylacetophenone which was used without
further purification.
b. Compound 22 was prepared in a manner analogous
to example 3 except 2-bromo-2'-methylacetophenone was
used in place of 2-bromo-2'-methoxyacetophenone in step
a. Mass spec. 373.2 MH+. NMR (300MHz, DMSO-d6) 8.6-8.8
(3H, s), 7.9-8.0 (1H, s), 7.6-7.75 {1H, d), 7.3-7.5 (3H,
m), 5.8-6.0 (1H, d,d), 4.7-4.8 (1H, s), 4.55-4.7 (iH, d),
4.3-4.44 (1H, d,d), 4.1-4.3 (1H, m), 3.8-4.0 (iH, m),
3.4-3.55 {1H, t), 2.85-3.1 (2H, m), 2.4-2.5 {3H, s), 2.0-
2.3 (2H, m), 1.2-1.6 (4H, m.), o.s-i.o {3H, t).
Example 23: bis-1,1'-[7-(2-amino-1-oxo-3-thiopropyl)-
8-butyl-2-(2-methylphenyl)-5,6,7,8-
tetrahydroimidazo[l,2aJ pyrazine]
disulfide (Compound 23)
Compound 23 was prepared in a manner analogous to
example 10 except compound 23 was used in place of
intermediate 3e. Mass spec:. 743.4 MH+. NMR (300MHz,
.~Sp-$.s, ~3D°C) 7.6-7.8 (1H, d), 7.2-7.3 (1H, s), 7.0-7.2
(3H, m), 5.3-5.6 (1H, broad s), 4.3-4.8 (1H, broad s),
3.5-4.2 (4H, m), 3.0-3.3 (:?H, broad s), 2.8-3.0 (1H, m),
2.4-2.5 (3H, s), 2.1-2.4 (2H, broad s), 1.7-2.1 (2H, m),
_ 1.2-1.7 (4H, m) , 0.8-1.0 {:3H, t) .
CA 02245823 1997-08-10
WO 97/30053 PCT/U897/02651
- 56 -
Example 24: 7-(2-amino-1-oxo-3-thiopropyl)-8-(1,1-
dimethylethyl)-2-(2-methoxyphenyl)-
5,6,7,8-tetrahydroimidazo- (1,2a]pyrazine
(Compound 24)
Compound 24 was prepared in a manner analogous to
example 3 except Boc-(L)-t-Leucine was used in place of
Cbz-(L)-Norleucine in step a and deprotection was
accomplished in step c by treatment with trifluoroacetic
acid for 0.5 hours. Mass spec. 389.3 MH+. NMR (300MHz,
DMSO-d6) 8.5-8.8 (3H, broad s), 7.95-8.1 (1H, d), 7.9-8.0
(1H, s), 7.3-7.5 (1H, t), 7.1-7.25 {1H, d), 7.0-'7.15 {1H,
t), 5.55-5.7 (1H, s), 4.65-4.8 (1H, broad s), 4.5-4.6
(1H, m), 4.35-4.5 (1H, m), 4.1-4.3 (1H, m), 3.9-4.1 (1H,
m), 3.85-3.95 (3H, s), 3.3-3.4 (1H, t), 2.7-34.1 (2H, m),
1.0-1.2 (9H, s).
Example 25: 7-(2-amino-1-oxo-3-thiopropyl)-8-(1-
methyl-propyl)-2-{2-
(phenylmethoxy)phenyl)-5,6,7,8-
tetrahydroimidazo [1,2a] pyrazine
(Compound 25)
a. Tntermediate 18d (3.36 g, 11.8 mMole) was
dissolved in lOml CH2C12 and a 1M solution of BBr3 in
CH2CI2 (47 ml) was added dropwise. The mixture was heated
. at reflux for 2 hours, cooled and poured into saturated
NaHC03 solution (25 ml). The aqueous layer was extracted
3 times with CH2CI2 (60 ml), dried over Na2S04, filtered,
and concentrated to 30 ml. Di-{tert) butyldicarbonate
(2.57 g, 11.8 mMole) was added, and the reaction stirred
. _ at room temperature overnight. The crude product was
purified by column chromatography on silica gel using 1:1
ethyl acetate:hexanes as eluant. The yield was 3.318
(75~) of white solid product.
b. Intermediate 25a (850 mg, 2.29 mMole) was ,
dissolved in THF (20 ml) that contained sodium hydride
CA102245823 1997-08-10
WO 97/30053 PCTlUS97/0265I
- 57 -
(96.1 mg, 2.4 mMole) and the. mixture was treated with
benzyl bromide (292 uL, 2.4 mMole} under. N2 at room
temperature. The reaction was stirred overnight at room
temperature and concentrated. The residue was
partitioned between CH2C12 (30 ml) and H20 (15 ml). The
CH2C12 layer was dried over Na2S04, filtered, and
concentrated. Crystallization from ethyl ether and
hexanes yielded 887 mg (83.70 of the product.
c. Intermediate 25b (E387 mg, 1.92 mMole) was
treated with 90~ Tfa/H20 (50 ml) for 15 minutes at room
temperature under N2. Solvents were removed under
reduced pressure and the re:aidue was distributed between
CH2C12 and saturated NaHC03 solution. The CH2C12 layer
was dried over Na2S04 soluta_on, filtered and concentrated.
The crude intermediate was acylated in a manner analogous
to example 1, step le, and 'then deprotected in a manner
analogous to example 1, step f. Mass spec. 465.3 MH+.
NMR (300MHz, DMSO-d6) 8.3-8.8 (3H, brand s), 8.0-8.1 (1H,
$,~,i), '7.8 3.0 (1H, s) , 7.45-7.55 (2H, m) , 7.3-7.45 (4H,
m), 7.15-7.3 (1H, d), 7.0-7.15 (1H, t), 5.6-5.8 (1H, d),
5.3-5.4 (2H, s), 4.65-4.8 (1H, broad s), 4.45-4.6 (1H,
m), 4.25-4.4 (IH, m), 4.1-4.25 (1H, m), 3.75-3.95 (1H,
m), 3.25-3.4 (1H, t), 2.8-3.0 (2H, m), 2.15-2.4 (1H, m),
1.4-1.6 (iH, m), 1.1-1.35 (1H, m), 0.95-1.1(3H, d), 0.8-
1.0 (3H, t).
Example 26: 7-(2-amino-1.-oxo-3-thiopropyl)-8-
._ (cyclohexyl--methyl) -2- ( 2-methoxyphenyl) -
5,6,7,8-tetrahydroimidazo[1,2a] pyrazine
( Compound 2 E> ) .
a. A solution of H-(L)-Phe-OH (10.0 g, 60.6 mMole)
in acetic acid (60.m1) and 5~ aqueous HCl (60 ml) was
hydrogenated over Pt02 (430 mg) until hydrogen was no
longer consumed. Solvents were removed under reduced
pressure, and the residue was dissolved in methanol (50
CA 02245823 1997-08-10
WO 97/30053 PCT/US97102651
- 58 - x
ml) and H20 (20 ml). A 10~ NaOH solution was added with
vigorous stirring to pH = 4.4, the solution was cooled,
and the product was filtered off and washed with H20. .
b. Crude intermediate 26a (60.6 mMole) was
suspended in 100 ml H20 containing K2C03 (8.36 g, 60.6
mMole), and a solution of Cbz-Osu (15.1 g, 60.6 mMole) in
CH3CN (150 ml) was added with vigorous stirring for 45
minutes at room temperature. The CH3CN was distilled off
at reduced pressure and the aqueous layer was washed with
ethyl ether. The aqueous layer was acidified with
concentrated HC1 to pH = 1 and the product extracted with
ethyl acetate (2 X 50 ml). The ethyl acetate layers were
dried over Na2S04, ffiltered, and concentrated under
reduced pressure to yield 17.27 g (93~) of Cbz-(L)-
cyclohexylalanine (26b).
c. Compound 26 was prepared in a manner analogous
to example 3 except Cbz-(L)-Cyclohexylalanine (26b) was
nsgd -~ dace of Cbz-(L)-Norleucine in step a. Mass
spec. 429.3 (MH-f-). NMR (300MHz, DMSO-d6) 8.6-8.9 (3H,
s), 8.1-8.3 (1H, d,d), 7.9-8.1 (1H, s), 7.35-7.5 (1H, m),
7.15-7.25 (1H, d), 7.05-7.15 (1H, t), 6.0-6.1 (1H, t),
_ 4.7-4.8 (1H, m), 4.55-4.7 (1H, m), 4.3-4.45 (1H, m), 4.1-
4.3 (1H, m), 3.9-4.0 (3H, s), 3.8-3.95 (1H, m), 3.35-3.5
(1H, t), 2.8-3.1 (2H, m), 2.05-2.2 (1H, d), 1.9-2.1 (2H,
t), 0.8-1.7 (10H, m).
CA 02245823 1997-08-10
WO 97130053 PCTliJ897/02651
- 59 -
Example 27: 7-(2-amino-1-oxo-3-thioprvpyl)-2-(2-
methoxyphenyl)-B-(1-methylethyl)-5,6,7,8-
tetrahydroimidazo [1,2a] pyrazine {Compound
27 ) .
Compound 27 was prepared in a manner analogous to
- example 3 except Cbz-(L)-Va7.ine was used in place of Cbz-
(L)-Norleucine in step a. Mass spec. 375.1 MH+. NMR
(300MHz, DMSO-d6) 8.6-8.8 (3H, broad s), 8.1-8.3 (1H, d),
8.0-8.1 (1H, s), 7.35-7.5 (J:H, t), ?.15-7.25 (1H, d),
7.05-7.15 (1H, t), 5.6-5.8 (1H, d), 4.65-4.8 (1H, broad
s), 4.5-4.7 (1H, m), 4.3-4.~E5 (1H, m), 4.1-4.3 (IH, m),
3.9-4.0 (3H, s), 3.8-3.95 (1H, m), 3.35-3.5 (1H, t), 2.8-
3.05 (2H, m), 2.5-2.7 (1H, m), 1.1-1.2 (3H, d), 0.9-1.05
(3H, d) .
Example 28: bis-1,1~-[7-(2-amino-1-oxo-3-thiopropyl)-2-
(2-methoxyphenyl)-8-(1-methylethyl)-5,6,7,8-
tetrahydroimida.zo [1,2a]]pyrazine] disulfide
(Compound 28).
Compound 28 was prepared in a manner analogous to
2D example 10 except compound 27 was used in place of
intermediate 3e. Mass spec. 747.4 MH+. NMR (300MHz,
DMSO-d6) 8.8-9.0 (3H, broad s), 8.05-8.2 {1H, d), 7.9-8.1
(IH, s), 7.35-7.5 (1H, t), '7.15-7.25 (1H, d), 7.0-7.15
_ (1H, t), 5.55-5.75 (1H, broad s), 4.8-5.0 {1H, broad s),
4.45-4.65 (1H, m), 4.35-4.5 (1H, m), 4.2-4.35 (1H, m),
3.85-3.95 (3H, s), 3.9-4.05 (1H, m), 3.2-3.4 (2H, d),
2.45-2.65 (1H, m partially obscured by solvent), 1.05-1.2
(3H, d), 0.9-1.05 (3H, d).
Example 29: 7-(2-amino-1-oxo-3-thiopropyl)-8-butyl-2(2-
hydroxy-6-methoxyphenyl)-5,6,7,8-
tetrahydro[1,2a]pyrazine (Compound 29).
a. Bromine (3.19 ml, 61.9 mMole) was added dropwise
to a mixture of 2',6'-dimethoxysacetophenone (11.15 g,
61.9 mMole) and concentrated HC1 (100 uL) in acetic acid
(50 mL) over 20 minutes. The reaction was stirred at
CA 02245823 1997-08-10
WO 97/30053 PCT/US97/02651
- 60 -
room temperature for 2 hours, and the solvents were
evaporated under reduced pressure. The residue was
dissolved in ethyl acetate (100 ml) and washed with ,
saturated NaHC03 solution (100) and with saturated NaCl
solution (100 ml). The ethyl acetate layer was dried
over Na2S04, filtered, and concentrated to an oil (14.9
g). Crystallization from ethyl acetate and hexanes
yields 4.87 g (30~) of 2-bromo-2',6'-
dimethoxyacetophenone (29a).
b. Compound 29 was prepared in a manner analogous
to example 3 except 2-bromo-2',6'-dimethoxyacetophenone
(29a) was used in place of 2-bromoacetophenone in step a.
One methyl ether group is cleaved efficiently during BH3
reduction of lactam 29d. Mass spec. 405.3.
Example 30: bis-1,1!-[7-(2-amino-1-oxo-3-thiopropyl)-8-
(1,1-dimethylethyl)-2-(2-methoxyphenyl)-
5,6,7,8-tetrahydroimidazo [1,2a]pyrazine~
disulfide (Compound 30)
Compound 30 was prepared in a manner analogous to
examgle 10 except compound 24 was used in place of
intermediate 3e. Mass spec. 775.5 (MH+). NMR (300MHz,
DMSO-d6) 8.7-9.1 (3H, broad s), 8.0-8.1 (1H, d), ?.8-8.0
(lFi, S) , 7.3~-7.5 (1H, t) , 7.1-7.2 (1H, d) , 7.0-7.1 (1H,
t), 5.55-5.65 (1H, s), 4.8-5.0 (IH, s), 4.4-4.6 (2H, m),
4.2-4.4 (1H, m), 3.9-4.1 (iH, m), 3.8-4.0 (3H, s), 3.2-
3.4 (2H, d), 1.0-1.2 (9H, s).
CA 02245823 1997-08-10
WO 97!30053 PC~'1iJ897/0265I
- 61 -
Example 31: 2-(2-methoxyphE:nyl)-8-(1-methylpropyl)-
5,6,7,8-tetrahydro-7-((thiazolidin-4-
yl)carbonyl)-imidazo[1,2a]pyrazine (Compound
32)
Compound 31 was prepared in a manner analogous to
- example 18 except Boc-(L)-thiaproline was used for the
coupling in step e. Mass spec. 401.3 (MHt). NMR (300MHz,
DMSO-d6, 90°C) 8.0-8.2 {IH, d), 7.85-8.0 (1H, s), 7.3-7.5
(1H, t), 7.15-7.25 (1H, d), 7.05-7.15 (1H, t), 5.7-5.85
{1H, d), 4.75--5.0 (1H, s), 4.45-4.7 (1H, m), 4.3-4.45
(2H, m), 4.1.5-4.3 (2H, m), 3.9-4.0 (3H, s), 3.8-3.95 (1H,
m), 3.4-3.6 (1H, t), 3.1-3.25 (lI~, m), 2.25-2.45 (iH, m),
1.4-1.6 (1H, m), 1.15-1.4 {1H, m), 1.0-1.23 (3H, t), 0.8-
1.0 3H, t) .
An iproliferati.ve activity of farnesvl-transferase
inhibitors on human tumoral. cells
The assays were performed using either A-427 lung
carcinomas (expressing mutated Ki-ras gene), HT-29 colon
adenocarcinomas (expressing wild type ras gene), Calu-1
lung carcinomas (expressing mutated Ki-ras gene), and
MIA-PaCa pancreatic cancer cells (expressing mutated Ki-
ras gene). These tumoral cells were seeded on 96 well
plates at day 0 and maintained at 37QC in 5~ C02
atmosphere. At day 1, cells were treated with increased
concentrations of test compounds ranging from 0 to 100 ~M
for 96 hrs. At the end of this period, the
quantification of cell proliferation was evaluated by the
colorimetric assay based on the cleavage of the
'-- tetrazolium salt WST-1 by ~nitochondrial dehydrogenases in
viable cells leading to the formazan formation (Cell
Proliferation Reagent WST-1 Kit, Boehringer Mannheim,
Germany). These experiments, done in octuplicate, were
repeated twice. The results, shown in Table I, depict
the concentration range (~:M) of test compound required to
CA 02245823 1997-08-10
WO 97/30053 ' ' PCTJUS97/02651
- 62 -
inhibit proliferation as compared to control cells in
which no test compound was added.
TABLE I
GELI. TYPE
COMPOUND
A-427 HT-29 Calu-1 MIA PaCa-2
3 6.25 - 12.5 12.5 10 - 30 12.5 - 25
4 12.5 - 25 50 - 100 10 - 30
5 6.25 - 25 50 - 100 12.5 - 25
6 50 - 100
8 12.5 - 25 25 . 50 25 - 50
10 3.12 - 125 25 - 50 3 - 10
11 6.25 - 12.2550 - 100 30 - 100
12 3.12 - 6.25 50 10 - 30
13 6.25 - 12.5 25 - 50 12.5 - 50
14 3.12 - 12.5 50 - 100 10 - 30
15 6.25 - 12.5 25 - 50 12.5 - 25
16 3.12 - 12.5 25 - 50 12.5 - 25
17 6.25 - 12.5 6 - 12.5 12.5 - 25
18 6.25 50 - 100 12.5 - 25
19 0.78 - 1.56 6.25 - 12.5
2 20 0.78 50 - 100 6.25 - 12.5 12.5
0
21 12.5 - 25 25 - 50 25 - 50
22 6.25 - 12.5 12.5 - 25
23 3.12 - 6.25 12.5 - 25 6.25 - 12.5
24 6.25 - 12.5 50 - 100 25 - 50
2 25 0.78 - 1.56 12.5 - 25 12.5 - 25 6.25 - 12.5
5
26 0.39 - 1.56 12.5 12.5 - 25 6.25 - 12.5
27 6.25 50 - 100 12.5 - 25
28 12.5 - 25 50 - 100
29 3.12 - 6.25 25 - 50 6.25 - 12.5
3 30 3.12 - 6.25 25 - 50 12.5 - 25
O
31 25 - 50
SUBSTITUTE SHEET (RULE 26)
CA 02245823 1997-08-10
WO 97/30053 PCTlUS97/U265i
- 63 -
Other Embodiments
It is to be understood. that while the invention has
been described in conjunction with the detailed
description thereof, that the foregoing description is
intended to illustrate and not limit the scope of the
invention, which is defined by the scope of the appended
claims. Other aspects, advantages, and modifications are
within the claims.
What is claimed is: