Note: Descriptions are shown in the official language in which they were submitted.
CA 02246870 1998-09-08
BACKGROUND OF THE INVENTION
Field of the Invention
This invention relates to a low-cost method for the production of
engineered materials from salt/polymer aqueous solutions by which method
engineered structures having a broad range of features and properties can be
prepared, which features and properties can be preset for a wide range of
applications. For example, the method of this invention is suitable for producing
continuous thin films which can be utilized as surface protection against harsh
environments (temperature, chemical, friction and grinding, etc.), as an
electrochemical component, such as for solid oxide fuel cells and electroceramic
membranes, porous filters and membranes, and as a surface with desired optical
or decorative properties. The method of this invention may also be used to
produce spherical granules of polycrystalline materials having good flowability and
packing properties, and which are suitable for use as pigments, sorbents, catalysts,
or as powders for efficient pressing and sintpring into dense materials. The method
of this invention may also be used to prepare materials having an engineered pore
structure, for example porous ceramics having wide applications as filters,
membranes, and chemical sorbents or reactants.
High technology ceramics are known for possessing a combination
of good thermal, chemical, mechanical and electronic properties, making them
unique for certain technical applications. Their usefulness, however, depends upon
the manner in which they are produced, including the characteristics of the ceramic
IGT-1353-A 2 10/F
CA 02246870 1998-09-08
powders used as starting powders which are sintered to produce the ceramic
product. In addition, methods for producing such high technology ceramics are
generally of high cost due, in part, to the expense and difficulty associated with
preparing suitable ceramic powders in large quantities.
SUMMARY OF THE INVENTION
Accordingly, it is one object of this invention to provide a method for
producing engineered materials, including thin films, spherical granules, and porous
ceramics, which is considerably less expensive than conventional methods.
It is another object of this invention to provide a method for
producing engineered materials, including high technology ceramics, which
elimin~tes the need for producing ceramic powders used as starting powders.
These and other objects of this invention are achieved by a method
for producing engineered materials from salt/polymer aqueous solutions in which
an aqueous continuous phase comprising at least one metal cation salt is mixed
with a hydrophilic organic polymeric disperse phase, resulting in formation of a
metal cation/polymer gel. The metal cation/polymer gel is then processed in a
manner which produces a structural mass precursor. The structural mass precursor
is then heated, forming a structural mass having predetermined characteristics based
upon the intended application or applications of the structural mass. Structural
masses which can be produced in accordance with the method of this invention
include, but are not limited to, thin films, spherical granules, and porous ceramics.
Thin films produced in accordance with the method of this invention can be used
IGT-1353-A 3 10/F
CA 02246870 1998-09-08
for surface protection against harsh environments, as electrochemical components,
as porous filters and membranes, and as a surface having desired optical or
decorative properties. Spherical granules produced in accordance with the method
of this invention may be used as pigments, sorbents, catalysts, or as powders for
efficient pressing and sintering into dense materials. By the term "spherical
granules," we mean polycrystalline structures having a generally spherical shape.
Porous ceramics produced in accordance with the method of this invention may be
used as filters, membranes, and chemical sorbents or reactants.
BRIEF DESCRIPTION OF THE DRAWINGS
The above-mentioned and other features and objects of this invention
will be better understood from the following detailed description taken in
conjunction with the drawings wherein:
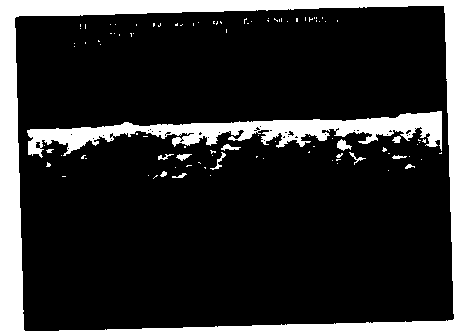
Fig. 1 is an SEM picture of a fractured cross section of a film coated
glass showing a thin iron oxide film of uniform thickness produced in accordance
with one embodiment of the method of this invention; and
Fig. 2 is an SEM picture of a dried material of spherical granules 2-3
microns in diameter of aluminum oxide produced in accordance with one
embodiment of the method of this invention.
DESCRIPTION OF PREFERRED EMBODIMENTS
My earlier filed U.S. patent application, Serial No. 08/406,173 teaches
the pl~a~lion of fine grained high surface area powders starting with an aqueous
salt solution with a hydrophilic organic additive that forms a gelatinous
IGT-1353-A 4 10/F
CA 02246870 1998-09-08
intermediate liquid product which is then dried and calcined to form the powder.
The method of the present invention provides an alternative treatment of the
gelatinous intermediate liquid product to produce materials with structures and
properties which can be engineered for a wide range of applications.
In accordance with each embodiment of the method of this invention,
an aqueous continuous phase comprising at least one metal cation salt is mixed
with a hydrophilic organic polymeric disperse phase, resulting in formation of a
metal cation/polymer gel. The metal cation/polymer gel is then treated so as to
produce a structural mass precursor of the structural mass end product, which
structural mass precursor is then heated to form the end product structural mass
having characteristics predetermined based upon the specific application of the end
product structural mass.
By the term "gel" as used throughout the specification and claims, I
mean a colloid in which a disperse phase is combined with a continuous phase to
produce a viscous gel-like product. In the gel formed in accordance with the
method of this invention, the disperse or colloidal phase is the hydrophilic organic
polymer and the continuous phase is water. The metal cation sait is dissolved in
the water. When the hydrophilic organic polymer is added to the aqueous metal
salt solution, a gel is formed by virtue of the gelling property of the polymer. In
this process, the hydrophilic organic polymer absorbs the liquid onto its structure
due to chemical affinity. The amount and nature of the water absorbed depends
on the chemical composition of the polymer. The hydrophilic absorption of the
IGT-1353-A 5 10/F
CA 02246870 1998-09-08
water causes the polymer to swell. This action is distinguishable from a sponge
which, for example, absorbs water due to capillarity, although it may also absorb
water by chemical absorption as in the method of this invention.
I have determined that hydrophilic organic materials serve as good
media for uniformly absorbing the metal ions of aqueous soluble salts. Hydrophilic
polymers, such as polyethylene glycol and some polyurethanes, have high capacities
for ~ ing water. When a hydrophilic polymer is added to an aqueous metal salt
solution, it swells as it absorbs the solution into its structure. The product is a gel
with the metal salt solution "frozen" within the dispersed polymeric network. If
the metal salt solution is dilute and the polymer added is not enough to gel the
mixture, excess water may be dried off until the mixture is thick enough to form
a gel. All hydrophilic organic material such as carbohydrates (sucrose, starches and
cellulose) and carbohydrate derivatives; hydrophilic homopolymer and copolymers
of ethylene oxide, 2-hydroxethylenemethacrylate, hydroxyalkyl-methacrylates,
hydroxyalkylacrylates, acrylamide, and n-vinylpyrrolidone, hydrophilic polymer
such as polyurethanes, polyurethane-acrylic, and polyurethane-methacrylic
copolymers and interpenetrating polymer networks, and proteins derived from
animal-protein-gelatins, are suitable for use in the method of this invention. In
accordance with a particularly preferred embodiment, said organic material is
polyethylene glycol.
Metal cation salts suitable for use in accordance with the method of
this invention are selected from the group consisting of chlorides, carbonates,
IGT-1353-A 6 10/F
CA 02246870 1998-09-08
hydroxides, isopropoxides, nitrates, acetates, epoxides, oxalates, and mi~Lules
thereof. Metal cations suitable for use in accordance with the method of this
invention are selected from the group consisting of at least one metal from Group
IA, IIA, IIIA, IVA, VA, VIA, IB, IIB, IIIB, IVB, VB, VIB, VIIB, and VIII of the
Periodic Table of the Elements, lanthanides, actinides, and mixtures thereof.
Thin continuous films are produced in accordance with one
embodiment of the method of this invention by applying a metal cation/polymer
gel formed by mixing an aqueous continuous phase comprising at least one metal
cation salt with a hydrophilic organic polymeric disperse phase to a substrate
surface, forming a continuous thin film thereon. Upon drying and heat treatment,
the continuous thin film forms a continuous ceramic layer over the substrate
surface.
EXAMPLE I
A hydrated ferric nitrate salt was dissolved in water to form a
solution. Polyethylene glycol was also dissolved in the solution. The solution was
stirred over a hot plate to thicken, thickening occurring as a result of evaporation
of free water. If the solution is too thick, the resulting continuous film is also thick
and flakes off the substrate surface after drying. I found that a solution containing
6.45 grams of polyethylene glycol, 13.49% Fe(NO3)3.9H20 with the balance being
H20, was thin enough to form a continuous reddish brown film on a glass slide.
After heating to 500~C, the film remained continuous, transparent, and reddish
brown. An SE~I picture of a fractured cross section of the film coated glass is
IGT-1353-A 7 10/F
CA 02246870 1998-09-08
shown in Fig. 1, in which can be seen a thin iron oxide film of substantially
uniform thickness.
In accordance with another preferred embodiment of the method of
this invention, the structural mass precursor formed from the metal cation/polymer
gel is formed by placing the metal cation/polymer gel in a hydrothermal reaction
vessel and increasing the pressure inside the hydrothermal reaction vessel by
heating, thereby forming a colloidal suspension therein. The colloidal suspension
is then removed from the hydrothermal reaction vessel and heated, resulting in
formation of a plurality of substantially spherical granules.
EXAMPLE II
To produce spherical granules in accordance with one embodiment
of the method of this invention, an aluminum nitrate salt was dissolved in water
after which polyethylene glycol was dissolved therein. The clear solution,
containing 10.7% Al(NO3)3.9H2O, 17.9% polyethylene glycol, and 71.4% water
was contained in a Teflon~ cup that had a cover. This, in turn, was placed in a
hydrothermal reaction vessel which was placed in an oven at 150~C for 20 hours.
During this treatment, pressure in the hydrothermal reaction vessel increased due
to the vapors or gaseous products from the thermal process. At the end of the
hydrothermal treatment, the solution remained clear, but a white colloidal
suspension settled at the bottom of the reaction vessel. The white colloidal
suspension was filtered out and dried at about 100~C. Fig. 2 is an SEM picture of
the dried material and shows spherical granules 2-3 microns in diameter of
IGT-1353-A 8 10/F
CA 02246870 1998-09-08
aluminum oxide.
Porous ceramics are produced in accordance with one embodiment of
this invention wherein the structural mass precursor is formed by dissolving the
metal cation/polymer gel in water to form a metal cation/polymer solution. A
porous preform is immersed in the metal cation/polymer solution resulting in
absorption of at least a portion of the metal cation/polymer solution into the porous
preform. The saturated porous preform is then dried after which it is heated to a
temperature suitable for burning out at least a portion of the porous preform,
leaving behind a porous structure having a shape and porosity corresponding to the
porous preform.
The preparation of porous bodies whose pore structures are replicas
of the skeletal structures of porous preforms in accordance with this embodiment
of this invention allows the preparation of a wide range of materials with pre-
designed pore structures. Porous materials such as papers, fabrics, threads, foams
or monoliths, whether natural or synthetic, having random or ordered structure, can
all be replicated by impregn~ting them with the metal cation/polymer solution
which is subsequently converted to an oxide phase. If the preform is organic, it
can be burned off in air, creating a pore structure in the oxide product that is a
replica of its skeletal structure. Some organic preforms may also be pyrolyzed,
leaving a fibrous m~tt ri~l that may act as a reinforcement for the ceramic product.
In accordance with another embodiment, if the porous preform is a metal or
ceramics, upon heat treatment, it may form a compound or a composite with the
IGT- 13 53-A 9 1 O/F
CA 02246870 1998-09-08
salt material in the liquid.
EXAMPLE III
A solution was prepared by dissolving 40 grams of Al(NO3)3.9H2O
in 25 grams of water. One gram of polyethylene glycol was dissolved in the
solution. The solution was stirred over a hot plate during which it was allowed to
thicken and fume. The combined weight reduced to just 30 grams, indicating that
the aluminum salt had lost some of its water of hydration. Ten grams of water
were added to the dried materials which dissolved the fumed solid to a clear
solution again. The final solution, therefore, weighed 40 grams, and contained
0.107 moles of aluminum nitrate and 1 gram of polyethylene glycol. A Fisher
brand filter paper, rated 7-790A, was saturated with this solution and allowed to
dry to a tacky sheet by h~rl~ng it in air. The tacky, saturated sheet was placed on
an alumina plate and heated in air at a rate of 2~C per minute up to a temperature
of about 450~C. The material remained as a continuous sheet, mostly white, with
brown specks. Under a microscope, the brown specks were determined to be
fibers, presumably incompletely burned fibers of the filter material. The white
material appeared as a continuous glassy phase with pores, which presumably were
spaces originally occupied by the filter material that had burned off. There was no
evidence of cracks in the resulting structure. The material was further treated by
heating in air at a rate of about 2~C per minute to 900~C and held at this
temperature for 5 hours. At the conclusion of this period, the sheet structure was
retained, but had turned completely white. The pore openings, as well as its glassy
IGT-1353-A 10 10/F
CA 02246870 1998-09-08
appearance, also remained. The sheet structure was handleable, in spite of being
only 7 mils thick with a density of 0.58 grams/cm3, or 15% of the theoretical
density of alumina.
While in the foregoing specification this invention has been described
in relation to certain preferred embodiments thereof, and many details have been
set forth for purpose of illustration, it will be apparent to those skilled in the art
that the invention is susceptible to additional embodiments and that certain of the
details described herein can be varied considerably without departing from the basic
principles of the invention.
IGT-1353-A 11 10/F