Note: Descriptions are shown in the official language in which they were submitted.
CA 02247268 1998-08-24
WO 97/37719 PCT/LTS96/12891
BIOMEDICAL ELECTRODE WITH LOSSY DIELECTRIC PROPERTIES
COLOR PHOTOGRAPHS
The file of this patent contains at least one drawing executed in
color. Copies of this patent with color drawings will be provided by the
Patent and
Trademark Office upon request and payment of the necessary fee.
TECHNICAL FIELD
The present invention relates generally to biomedical electrodes of
the type that delivers to or receives currents from a body, such as dispersive
electrodes used to return current from a patient's body in electrosurgery or
cardiac
stimulating electrodes used to deliver current to a patient's body.
BACKGROUND
Biomedical electrodes are used in a variety of applications and are
configured to operate according to the size, type, and direction of current
flowing into
or out of a body of a patient.
Dispersive electrodes are used in electrosurgery. In modern surgical
practice there are many times when eleetrosurgery is more preferable than the
use of the
traditional scalpel. In electrosurgery, cutting is performed by an intense
electrical
current passing through a cutting electrode. The surgeon directs this current
to exactly
where cutting is required by wielding the cutting electrode, which because of
its
cylindrical shape and the way it is held in the hand is commonly called an
'blectrosurgical pencil". By activating controls which change the
characteristics of the
electrical current being sent to the pencil by an electrosurgical generator,
the surgeon
can use the pencil either to cut or to coagulate areas of bleeding. This makes
electrosurgery particularly convenient when surgery requiring extra control of
blood loss
is being performed. Because of concerns to minimize the transmissions of blood-
borne
illnesses between health care patients and health care providers, in both
directions,
electrosurgery is becoming increasingly important.
CA 02247268 1998-08-24
WO 97/37719 PCT/US96112891
In electrosurgery, as in all situations where electrical current is flowing,
a complete circuit must be provided to and from the current source. In this
case, the
current that enters the body at the pencil must leave it in another place and
return to the
generator. It will readily be appreciated that when current enough to
deliberately cut is
brought to the body of a patient in one place, great care must be taken that
unintentional damage is not also done to the patient at the location where
that current is
leaving the body. The task of collecting the return current safely is
performed by a
dispersive electrode.
A dispersive electrode performs this task by providing a large surface
area through which the current can pass; the same current which was at cutting
intensity
when focused at the small surface area at the tip of the pencil is relatively
harmless, with
the goal of being painless to the patient, when spread out over the large
surface area of
the dispersive electrode.
There are two major known types of dispersive electrodes, characterized
by the predominant type of electrical phenomenon taking place where the
electrode
contacts the body. Each of the two types has competing advantages and
disadvantages.
The first type is known as the resistive type of dispersive electrode, since
resistance phenomena are responsible for the current transfer between the body
of the
patient and the electrode. A disadvantage of this type of dispersive electrode
is that it
has a so-called 'bdge effect'; the current density is much higher at the
corners and edges
than at the center of the electrode. Thus, the maximum temperature rise in
patient tissue
for this type of electrode is much greater than for a theoretically 'perfect"
resistive
electrode where the current would be evenly distributed over the entire
conductive
surface area. If a theoretically 'perfect"resistive electrtode were to be
constructed that
caused the same maximum temperature rise in patient tissue as a commercially
available
resistive electrode as presently manufactured, the 'perfect" electrode would
be
substantially smaller in size because of its ability to uniformly distribute
the current
flowing from the body of the patient into the conductive surface of the
electrode
contacting the patient.
An advantage of the resistive type of dispersive electrode is that the
adequacy of the contact between the electrode and the patient's body can be
monitored
-2-
CA 02247268 1998-08-24
WO 97/37719 PCT/US96/12891
by contact quality monitoring ('CQM'~ circuitry in the electrosurgical
generator. Many
of the most popular generator systems have safety circuits which can detect
when a
resistive electrode does not have good attachment to the body. If some
mischance in the
surgical suite has caused the electrode to be applied without adequate initial
contact
with the body or some event during surgery has caused the adequate initial
contact to
become inadequate, these safety circuits will detect that problem and refuse
to let any
cutting current be applied or continued.
The second type of dispersive electrode is known as the capacitive type,
since capacitance phenomena are responsible for the current transfer between
the body
of the patient and the electrode. An advantage of this type of dispersive
electrode is that
it does not have the edge effect of concern for resistive type dispersive
electrodes, and in
normal use the current transfer is much more uniform across the surface of the
electrode
compared to resistive types.
A disadvantage of capacitive electrodes is they are not compatible with
1 S the above described CQM circuits, and thus when used do not have this
protection
against inadvertent misapplication.
From the foregoing, it is clear that the art requires a dispersive electrode
which has both a very uniform distribution of the current across its surface
while at the
same time is suitable for being monitored against the hazard of accidental
detachment.
Uniform distribution of current density has been studied with respect to
stimulating electrodes, where electrical energy is being delivered to a
patient via a
biomedical electrode. Representative of these studies is Kim et al.,
'Uniformity of
Current Density Under Stimulating Electrodes" Critical Reviews in Biomedical
Engineering Vol. 17, Issue 6 pp. 585-619 (1990) . Electrodes for stimulation
ofthe
heart, i.e., pacing electrodes have also been concerned with area resistivity.
U.S. Pat.
No. 4,776,350 (Grossman et al.) has disclosed an electrode that has differing
resistivities
between different conductive members on the electrode.
Defibrillation, cardioversion, and pacing electrodes are used to deliver
current to a body of a patient with sufficient power to reach and alter
performance of the
3 0 myocardial muscles of the heart. Distribution of current density for these
types of
-3-
CA 02247268 1998-08-24
WO 97/37719 PCT/US96/12891
cardiac stimulating electrodes is critical to the performance of the
electrodes for their
intended functions: to cause the heart to change its pattern of beating.
SUMMARY OF THE INVENTION
The present invention solves the problems discussed above by
providing a biomedical electrode in which one can control current density at
the
electrode/body interface.
The biomedical electrode of the present invention has a "lossy
dielectric" region over a part of the electrode/body interface.
Unexpectedly, the "lossy dielectric" properties of the biomedical
electrode solves problems that confront both resistive-type and capacitive-
type
dispersive electrodes and cardiac stimulating electrodes such as
defibrillation,
cardioversion, and pacing electrodes. Edge effect common to resistive type
dispersive electrodes and cardiac stimulating electrodes is substantially
reduced.
1 S Yet the electrode of the present invention provides acceptable CQM
performance,
which is unavailable in capacitive type dispersive electrodes with sufficient
accuracy.
For purposes of this invention, "lossy dielectric" properties mean
that biomedical electrodes have a conductor surface that has performance
parameters between the extremes of
(1) a resistive-type dispersive electrode that provides an edge effect
at its periphery of a conductive surface; and
(2) a capacitive-type dispersive electrode that provides only
capacitive current flow between the conductive surface and the body of a
patient,
i.e., providing almost totally uniform current density over the conductive
surface.
Cardiac stimulating electrodes are resistive-type electrodes that
cause an edge effect similar to resistive-type dispersive electrodes.
Avoidance of
edge effect in cardiac stimulating electrodes is an advantage of the present
invention
by helping to avoid needless discomfort for the patient undergoing such
cardiac
stimulation through extracorporally-placed electrodes.
CA 02247268 1998-08-24
WO 97/37719 PCTlLTS96/12891
Nonlimiting examples of performance parameters that can be used to
characterize a "lossy dielectric" biomedical electrode include
(a) the resistive skin contacting area of the conductive surface
(through conductive adhesive) compared with the area of the total conductive
surface of the biomedical electrode;
(b) at specified frequencies, the electrical impedance gradient from
the center of the conductive surface to the edge of the conductive surface;
(c) the maximum impedance at the edge of the conductive surface of
the electrode;
(d) the average current density levels at given levels of amplitude
and frequency of electrical energy to be dispersed;
(e) the resistance component of impedance of the conductive surface
at determined locations on the biomedical electrode;
(f) the reactance component of impedance of the conductive surface
at determined locations on the biomedical electrode; and
(g) the tan 8, that divides the resistance component of impedance by
the reactance component of impedance, at determined locations on the
biomedical
electrode.
"Reactance" is the electrical resistance offered by a capacitor to
current flow at a given frequency.
Therefore, lossy dielectric materials occupy that region between pure
capacitance and pure resistance and have a 8 of > 0° and < 90°.
This invention employs lossy dielectric materials in dispersive
electrodes, a field previously dominated by either resistive-type electrodes
or
capacitive-type electrodes.
This invention also employs lossy dielectric materials in cardiac
stimulating electrodes, such as defibrillation, cardioversion, and pacing
electrodes.
Tan b has also been used to determine the anti-corroding properties
of paint in industrial fields.
Each of the above performance parameters mean little to the patient
unless the biomedical electrode actually minimizes the maximum amount of
-S-
CA 02247268 1998-08-24
WO 97/37719 PCT/U596/12891
temperature rise at a determined locations) on the biomedical electrode when
in
use. However, use of a combination of the above performance parameters can
provide a biomedical electrode of the present invention that nUnimizes the
maximum
amount of temperature rise in tissue of a patient in contact with a biomedical
electrode of the present invention.
One aspect of the invention is a biomedical electrode that has a
maximum rise in temperature of less than 6°C from beginning of usage
with an
electrosurgical generator, when tested according to industry testing standard
"AANII Standard Section 4.2.3.1, Maximum Safe Temperature Rise" (Association
for the Advancement of Medical Instrumentation, 1986).
Another aspect of the invention is a dispersive electrode having a
rise in temperature of the dispersive electrode during usage with an
electrosurgical
generator that is proportional to a maximum current density.
Another aspect of the invention is a biomedical electrode having the
same or lower maximum temperature rise performance as a larger biomedical
electrode.
Another aspect of the invention is a dispersive electrode that has a
more uniform temperature profile, beneficially similar to a capacitive-type
dispersive
electrode, and an ability to respond to CQM monitoring via its non-periphery
skin-
contacting portion, beneficially similar to a resistive-type dispersive
electrode.
Preferably, a biomedical electrode of the present invention has an
electrically non-conductive backing and at least one conductive plate adjacent
to the
electrically non-conductive backing. In many of the most preferred
embodiments,
there will be two conductive plates which lie adjacent to each other with both
adhered to the electrically non-conductive backing. There is a field of lossy
dielectric material between the conductive plate and a surface of the
electrode
contacting a body of a patient. A field of conductive adhesive will be
present, in
contact with both the conductive plates) and the field of lossy dielectric
material.
It is particularly convenient to provide this biomedical electrode so
that the conductive plates have a body contacting portion and an extended tab.
This
arrangement allows for easy connection to conventional electrosurgical
generators.
-6-
CA 02247268 2006-03-17
60557-5913
Most preferably, the field of lossy dielectric material
contacts a region adjacent the exterior edge of the body
contacting portion.
According to one aspect of the present invention,
there is provided a biomedical electrode, comprising: an
electrically non-conductive backing; a conductive plate
contacting the electrically non-conductive backing; a field
of lossy dielectric material between the conductive plate
and a surface of the electrode that is contactable with a
body of a patient, wherein the field of lossy dielectric
material is located in a region adjacent an exterior edge of
a body contacting portion of the conductive plate; and a
field of conductive adhesive in contact with both the
conductive plate and the field of lossy dielectric material.
According to another aspect of the present
invention, there is provided an electrosurgical system,
comprising: an electrosurgical generator and a biomedical
electrode as described herein.
According to yet another aspect of the present
invention, there is provided a method of controlling the
dielectric properties of a biomedical electrode, comprising
the steps of: (a) applying a lossy dielectric material to
at least a portion of the electrode between a conductive
surface and a surface of the electrode that contacts a
patient, wherein the lossy dielectric material is located in
a region adjacent an exterior edge of a body contacting
portion of the conductive surface; (b) assembling remaining
components of the electrode.
Embodiments of the invention are described using
the following drawings.
CA 02247268 2006-03-17
X0557=5913
BRIEF DESCRIPTION OF THE DRAWING
The reference numerals refer to like parts in the several views, and
wherein:
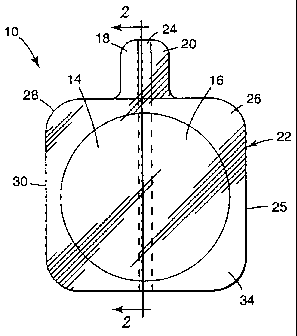
Fig. 1 is bottom perspective view according to one presently
preferred embodiment of the dispersive electrode of the present invention;
Fig. 2 is a cross-section view which is taken along section lines 2-2
in Fig. 1;
Fig. 3 is a graph of impedance in Ohms and frequency in Hertz
plotted for dispersive electrodes of the present invention applied to a human
arm
and tested for CQM acceptance.
Fig. 4 shows comparative subtracted thermograms of a dispersive
electrode of the prior art and a dispersive electrode of the present invention
.
Fig. 5 shows comparative thermograms of dispersive electrodes.
Fig. 6 shows comparative subtracted thermograms of a dispersive
electrode of the prior art and a dispersive electrode of the present
invention.
EMBODIMENTS OF THE INVENTION
Figure 1 shows a bottom perspective view of the dispersive
electrode 10 . The upper surface of the electrode 10, which is on the far side
in this
bottom view, can be a flexible and conformable electrically non-conductive
backing
12. Two conductor plates 14 and 16 are present, adjacent to, and can be
adhered
to, the electrically non-conductive backing 12. Providing two separate
conductor
plates is not a requirement of the invention, but is convenient in many
preferred
embodiments because the impedance between the conductor plates 14 and 16 is
measured by several of the above mentioned CQM circuits. Only dispersive
-7a-
CA 02247268 1998-08-24
WO 97/37719 PCT/US96/12891
electrodes having two conductor plates and being in adequate contact with the
body
of a patient will provide the impedance expected by the CQM circuits.
Each of the two conductor plates 14 and 16 has an extended tab I 8
and 20, respectively, extending away from the body contact portion 22 of the
electrode 10 for the attachment of a cable assembly which connects the
electrode 10
to an electrosurgical generator (not shown). In order to provide more support
for
the conductor plates 14 and 16, and especially for their respective tabs 18
and 20, a
non-conductive support layer 24 can be laminated to the conductor plates.
The region adjacent the exterior edge 25 of the body contact portion
22 of the electrode 10 can be covered with a layer 26 of a lossy dielectric
material.
In the embodiment of Figure 1, the width of the layer 26 of a lossy dielectric
material is widest at the corners 28, and narrowest along the edge 30 midway
between the corners. As presently understood, this arrangement of the layer 26
serves best to reduce edge ei~'ect at the corners 28 of the dispersive
electrode.
Preferably, the entire body contact portion 22 of electrode 10 is
covered with a field 32 of conductive adhesive. Many compositions suitable for
use
for the field 32 of conductive adhesive are transparent, or at least
translucent, and
have been depicted that way in Figure i for convenience in providing an
explanatory drawing. The field 32 of conductive adhesive serves the dual
purposes
of adhering the electrode 10 to the body of the patient and of transferring
the
electrosurgical current between the body of the patient and the electrode for
electrosurgical currents and between the electrode and the body for CQM
monitoring.
Figure 2 shows a cross-section view of the electrode shown in
Figure 1, taken along section lines 2-2. In this view, a release liner 34 is
shown
adhered to the field 32 of conductive adhesive This release liner 34 protects
the
conductive adhesive during shipping and handling and is removed just prior to
use.
In this view a layer of adhesive 36 is seen adhering the support layer 24 to
conductor plate 14 and its extended tab 18. Another layer of adhesive 38 is
provided for adhering the electrically non-conductive backing 12 to the other
side
of the support layer 24.
_g_
CA 02247268 1998-08-24
WO 97/37719 PCT/US96/12891
Electricall3r non-conductive backing
Electrically non-conductive backing 12 can be electrically insulative,
and preferably is very conformable to the human body. Many materials can be
used
for this purpose, as will be apparent to those skilled in the art. In one
presently
preferred embodiment, a closed-cell foam is considered particularly suitable.
_ One
such material is commercially available as Volara brand foam from Voltek, Inc.
of
Massachusetts. The electrically non-conductive backing can have a thickness
ranging from about 0.75 mm (0.03 inch) to about 1.5 mm (0.06 inch), and
preferably 1.0 mm (0.04 inch).
Conductor plates and support layer
The conductor plates 14 and 16 are conveniently made from metal,
preferably in the form of a foil; a metal-containing or graphite-containing
coated ink
or pain, or a vapor coated metal, and most preferably, aluminum foil. If a
support
layer 24 is not being used, a thickness of about 0.08 mm (0.0003 inch) is
considered
preferred. If a support layer 24 is being used, the metal foil or vapor coated
metal
can be thinner because of the support provided by the support layer. A
suitable
support layer 24 can be made from polyethylene terephthalate (PET) film,
conveniently approximately 0.05 mm (0.002 inch) thick. This allows the
aluminum
layer to range in thickness between about 0.0075 mm (0.0003 inch) to about
0.025
mm (0.001 inch) and preferably 0. 012 mm (0.0005 inch) or allows vapored
coated
metal to have a minimum thickness of about 1000 Angstroms. An example of
vapored coated metal on a substrate is found in PCT Publication No. WO
94/26950.
Conductive adhesive
Nonlimiting examples of conductive adhesives useful in connection
with the present invention include those compositions disclosed in U.S. Patent
Nos.
4,524,087 (Engel); 4,539,996 (Engel); 4,848,353 (Engel) and 5,133,356 (Bryan
et
al), ; 5,225,473 (Duan); 5,276,079 (Duan et al); 5,338,490 (Dietz et al);
5,362,420
-9-
CA 02247268 2006-03-17
60557'-5913
(Itoh et a1); 5,385,679 (Uy et al); copending and coassigned applications PCT
Publication Nos. WO 95/20634; WO 94/12585;
WO 97/24378; WO 97/24376 and WO 97/24149.
Release liner
Release Iiner 34 can be any construction suitable for protecting the
conductive adhesive 32 during shipping and handling while still releasing
easily from
the conductive adhesive at the time of use. One suitable liner is a 0.05 mm
(0.002
inch) thick sheet of biaxially oriented polypropylene liner, commercially
available as
Daubert 1642 from Daubert Co. of Dixon, IL.
Adhesive lavers
In, some presently preferred embodiments, adhesive layers 36 and 38
may be used for holding other components of the electrode 10 together.
Nonlimiting examples suitable adhesives 36 and 38 include acrylate ester
adhesives,
and more particularly acrylate ester copolymer adhesives. Such adhesives are
generally described in U.S. Patent Nos. 2,973,826; Re 24,906; Re 33,353;
3,389,827; 4,112,213; 4,310,509; 4,323,557; 4,732,808; 4,917,928; 4,917,929;
and
European Patent Publication 0 051 935.
Optionally,~such adhesives can also be used to provide a border of
pressure sensitive adhesive on the exterior edge 25 of electrode 10, as is
disclosed
in U.S. Pat. Nos. 4,524,087 (Engel); 4,539,996 (Engel); 4,848,353 (Engel).
Lossy dielectric layer
The Layer 26 of lossy dielectric material has the performance
parameters identified above in association with the ultimate performance of
electrode 10 to minimize the maximum rise in temperature of tissue of a
patient
during electrosurgical procedures.
-10-
CA 02247268 1998-08-24
WO 97/37719 PCT/US96/12891
The lossy dielectric layer 26 can occupy an area of the body contact
portion 22 ranging from about S % to about 70 %and preferably from about 40
to about 60%.
The lossy dielectric layer 26 can be made from a material and be
applied in a non-uniform thickness to result in an electrical impedance
gradient from
the center of the body contact portion 22 to the exterior edge 25 ranging from
about 30% to about 90% of the maximum impedance at exterior edge 25, and
preferably from about 50% to about 70% of the maximum impedance at exterior
edge of body contact portion 22.
The layer 26 can have a maximum impedance/area at the exterior
edge 25 of the body contact portion 22 of the electrode 10 ranging from about
0.387 S2/ 129 cm2 to about 20 S2/ 129 cm2 and preferably ranging from about 1
S~/
129 cm2 to about 8 S2/ 129 cmz , as determined by use of a Schlumberger 1260
spectrum impedance analyzer, operating at a frequency of S00 kHz and a
constant
voltage of 60 mV (RMS), and subjected to a nulling file to subtract out the
effects
of leadwires, connecting clips, and test fixture. A dispersive electrode of
approximately 129 cm2 (20 in2 ) is the approximate size of most commercially
available dispersive electrodes.
The layer 26 can have a resistance component per unit area (R/
area) of the complex impedance Z of from about 0.4 S2/ 129 cm2 to about 5 S2/
129
cm2 at exterior edge 25 on the dispersive electrode 10. Preferably, the layer
26 can
have a resistance component per unit area ranging from about 0.5 S2/ 129 cm2
to
about 1.4 S2/ 129 cm2 . These values were determined as done for the maximum
impedance per unit area.
The layer 26 can have a reactance component per unit area (X/ area)
of the complex impedance of from about -0.5 S2/ 129 cm2 to about -16 SZ/ 129
cmz
at exterior edge 25 on the dispersive electrode 10. Preferably, the layer 26
can
have a reactance component per unit area ranging from about -2 S2/ 129 cmz to
about -10 SZ/ 129 cm2, using the same testing method as above for resistance
per
unit area and impedance per unit area.
-11-
CA 02247268 1998-08-24
WO 97/37719 PCT/LTS96/12891
The layer 26 can have a tan b ranging from about 0.14 to about 1.7
at exterior edge 25 on the electrode 10, when measured at 500 kHz and a signal
amplitude of 60 mV (RMS). Desirably, the tan 8 can range from about 0.2 to
about
1.0 at exterior edge 25 on the electrode 10, when measured at 500 Hz and a
signal
S amplitude of 60 mV (RMS). Preferably, the tan 8 ranging from about 0.2 to
about
0.7 at exterior edge 25 on the electrode 10, when measured at 500 Hz and a
signal
amplitude of 60 mV (RMS).
Layer 26 can be made from any lossy dielectric material that can be
applied to body contact portion 22 and provide the performance parameters
identified above for layer 26.
Layer 26 can be formed from an ink or paint on body contact portion
22 according to electrode manufacturing techniques known to those skilled in
the
art. It has been found particularly convenient to provide this material in the
form
of a paint, which can then be screen printed or sprayed in an appropriately
shaped
pattern onto the electrode 10 at the proper time during its fabrication. Oil-
based
enamels, commercially available as Cat. nos. 7776, 7790, 7730, 7727, and 7715
from Rust-oleum Corp. of Vernon Hills, IL are considered particularly
suitable.
Inks such as Summit UVII 300, UVII 800, and UVII 801 white inks, from Summit,
Inc. of North Kansas City, MO can also be used.
Method of Making_Electrodes
Electrode 10 can be made using conventional tab/pad style
electrodes as described in U.S. Pat. Nos. 4,352,359 (Larimore); 4,524,087
(Engel);
4,539,996 (Engel); 4,554,924 (Engel); 4,848,348 (Carim); 4,848,353 (Engel);
5,012,810 (Strand et al.); 5,133,356 (Bryan et al.); 5,215,087 (Anderson et
al.); and
5,296,079 (Duan et al.). Generally, multiple layered electrode 10 can be
assembled
from rolls of starting materials for insulative electrically non-conductive
hacking 12,
upon which is applied conductor plates 14 and 16, upon which is coated paints
or
inks to form lossy dielectric layer 26, upon which is coated or cured field 32
of
ionically conductive pressure sensitive adhesive. Alternatively, a sheet of
lossy
-12-
CA 02247268 1998-08-24
WO 97/37719 PCT/US96112891
dielectric material of a desired geometrical shape can be laminated onto
conductor
plates 14 and 16.
Automated machinery can be employed to make electrode 10. One
skilled in the art of making electrodes can select from a variety of machinery
manufacturers and manufacturing techniques to minimize manufacturing expense
and waste. Some types of machinery are disclosed in U.S. Pat. Nos. 4,715,382
(Strand); 5,133,356 (Bryan et al.); and copending, coassigned PCT patent
application PCT/CTS95/14291 (Yasis et al), and U.S. Pat. No. 5,352,315
(Carrier et
al. ).
Method of Controlling, Lossy Dielectric Properties on Biomedical Electrodes
The choice of lossy dielectric material for a particular biomedical
electrode application can be made based on tan b for a given dielectric
material.
As stated previously, reactance and resistance components of the
dielectric material are compared at a given frequency of interest, to
determine tan b,
which is a dimensionless value independent of size of the biomedical
electrode.
Thus normalized, the determination of tan 8 for any material can be
used to control dielectric properties of the biomedical electrode or any
location or
region of the biomedical electrode where lossy dielectric properties are
desired.
Use of tan 8 is based on the following analysis.
For a sinusoidal signal of frequency "F" Hertz passing through a
capacitor of "C" Farads, the resistance offered to the flow of current is
called the
reactance, measured in units of Ohms. Reactance is a positive value if it is
due to
an inductance; it is a negative value if due to a capacitance. If the
resistance of a
piece of material is "R" Ohms, and if it has a capacitance of "C" Farads, the
reactance due to the capacitance is X~ _ -1/ {2*~*F*C) Ohms.
The impedance is Z = R - jX, where j denotes the imaginary
component X of the complex impedance Z. The real component is R. Tan 8 is the
ratio of R/~X~, where ~X~ denotes the magnitude of -X or +X.
-13-
CA 02247268 1998-08-24
WO 97/37719 PCT/US9G/12891
If measured complex impedance has no capacitance (i.e., pure
resistance) and hence jX = 0, then Z = R and tan 8 = 90°. If measured
complex
impedance has no resistance (i.e., pure capacitance), then R=0 and
8=0°.
By convention known to those skilled in the art, capacitance is
identified by the equation:
C- (Er * Eo )(~d)
where s~ = relative permittivity of the dielectric material being
analyzed between the plates of a capacitor "C", so = permittivity of free
space (8.85
X 10''2 F/m), A = area of plates, and d = distance between plates.
It can be shown that relative permittivity, s~ , can be a complex
quantity such that s~= E' - j8". Then the current, "l" flowing through the
lossy
dielectric material consists of an "in phase" component "iL" through a pure
resistor
"rP" and an imaginary capacitive component current "lc" through, a pure
capacitor
"cp" in parallel to the pure resistor. It can then be shown that the phase
angle, tan
8, between "l" and "lc" is such that
tan b = ~ iL~/ ~ icy = E"/E'
If the dielectric material is loss free, then s" = 0. Otherwise, E" is a
measure of the performance of a dielectric material as an electrical insulator
and is
also known as the dielectric conductivity.
8 = arctan (E"/s').
Any circuit with a resistor rP and a capacitor cp in parallel can be
represented by as an equivalent circuit of a resistor, r$ , in series with a
capacitor, cg
For a parallel circuit,
tan 8 = 1/w * rP * cP
with the equivalent series being,
tan 8 = c~ * rS * cs
where w = 2 * rc * F.
Those skilled in the art are also directed to Anderson, Dielectrics
(Chapman & Hall, London, 1967 pp. 14-28, 39-48).
-14-
CA 02247268 1998-08-24
WO 97/37719 PCT/US96/12891
When a material is characterized by modelling it as a parallel circuit,
then it is usually evaluated in terms of its admittance, Y, defined as the
inverse of its
impedance, Z. Conversely, evaluation of circuit elements in series uses
impedance,
Z.
Dielectric properties discussed above are true for a given frequency,
f, considering e~ is represented by a pure capacitor, cP , in parallel with a
pure
resistor, rp . In practice, however, s" and E' are functions of frequency,
i.e., the
parallel equivalent circuit is only good at one frequency. The frequency
dependence
of e~ is known as "dielectric relaxation."
The simple measurement of impedance, Z, at frequency, f, is
Z = re + ( 1/j * w * ca) = r$ - (j/ c~ * c,)
and
8 = arctan (w * rg * c$)
These two equations are considered good estimates of the real
frequency dependent s~ for the purpose of comparing various lossy dielectric
materials in the biomedical electrodes of the present invention. Thus, one
skilled in
the art can control the lossy dielectric properties of the biomedical
electrode with
this information to determine tan b for that material.
Usefulness of the Invention
Biomedical electrodes can be constructed from a variety of lossy
dielectric materials in a variety of geometries and sizes according to the
desired uses
of the electrodes by those skilled in the art.
The embodiments of Figs. 1 and 2, and the graphs and thermograms
of Figs. 3-6, illustrate how one can use the scope of the present invention to
make a
useful dispersive electrode.
However, the same principles can be used to determine the
appropriate lossy dielectric material, electrode geometry and size, etc., for
the
construction and usage of a cardiac stimulation electrode.
For example, one can provide a differential in distances between
conductor plates 14 and 16 on backing 12. One can create a concave non-
-15-
CA 02247268 1998-08-24
WO 97/37719 PCT/LTS96/12891
conductive area between plates 14 and 16 while still providing cooperation
with
CQM monitoring. Indeed, cooperation with CQM monitoring could be enhanced
by such concave area between plates 14 and 16, for those occasions when
tension
on an extended tab of the electrode 10 causes the electrode to begin to lift
from the
body of the patient, creating an alarm situation during CQM monitoring. The
concave area will cause the electrode to reach the CQM alarm condition during
CQM monitoring with less total separation of the electrode from the body of
the
patient and thus further protect the patient from unacceptable maximum tissue
temperature rise.
In a defibrillation electrode, used where the myocardial muscle in
fibrillation needs an immediate, stabilizing delivery of intense current, one
can
control the current density of any region of the cardiac stimulation electrode
by use
of lossy dielectric material in order to concentrate the delivery of the
current to
penetrate into the body for immediate defibrillation.
In a cardioversion electrode, the amount of current desired for the
procedure may differ from defibrillation current requirements, causing a
desire to
revise the choice of the lossy dielectric material, its geometry or size on
the
electrode, and the like to provide the correct amount of current to the
myocardial
muscle to alter arhythmias.
In a pacing electrode, the amount of current desired for the
procedure may also differ from either the defibrillation electrode or the
cardioversion electrode, causing a desire to revise the choice of the lossy
dielectric
material, its geometry or size on the electrode, and the like to provide the
correct
amount of current to the myocardial muscle to provide efficacious external
pacing.
Thus, one can control the distribution pattern and magnitude of
current density in the tissue volume of the patient's body under an electrode.
Embodiments of the invention are further described in the following
examples.
-16-
CA 02247268 1998-08-24
WO 97/37719 PCT/US96/12891
Example 1
An electrode was constructed from a 129 cm2 cm (20 square inches)
of Aluminum foil with an electrically insulating material, flat black spray
paint (No.
7776 from Rustoleum Corporation) , sprayed from about 20 cm (8 inches) between
spray nozzle and electrode surface, at the edge of the return electrode,
resulting in
the edge between resistive and lossy dielectric regions of the electrode
surface
becoming much less resistively conductive. However, due to the dispersion of
the
spray from the outer edge of the electrode, there was a gradual transition
from
100% electrically insulating coverage at the outer edge of the electrode to no
coverage in the middle of the electrode.
A layer of conductive adhesive was prepared according to the
following procedure. Into a 300 gallon kettle equipped with overhead stirrer
and a
cooling jacket was charged 562.8 pounds (255.5 kg) acrylic acid, 1.4 pounds
(636
grams) 2,2-dimethoxy-2-phenyl acetophenone, 2.8 pounds (1273 grams) 4-(2-
hydroxyethoxy)phenyl-(2-hydroxy-2-methylpropyl)ketone, 1.12 pounds (508
grams) methylene bis(acrylamide), 1251.6 pounds (568.2 kg) glycerin, 2.8
pounds
(1273 grams) guar gum, and 459.6 pounds (208.7 kg) deionized water. To the
well
stirred solution was charged 499.8 pounds (226.9 kg) 50% aqueous NaOH
portionwise maintaining the batch temperture below 38°C. The hydroxide
line was
rinsed into the kettle with an additional 18 pounds (8.2 kg) deionized water
and
stirred for 30 minutes to yield coater-ready precursor. The precursor was
coated
onto the foil side of a polyester/aluminum foil laminate at 23 mil (0.6 mm)
thick,
overlaminated with a siliconized polyester liner, and passed through a curing
chamber consisting of banks of fluorescent "black" lights, exposing the
material to
an intensity of 1.9 mW/sqcm and a total dose of 315 mJ/sqcm.
The layer of conductive adhesive so prepared was then placed over
the whole square of the electrically conductive surface. This electrode was
placed
on an anterior thigh of a human and energized according to the AAMI standard
for
thermography: 4.2.3.1, "Maximum Safe Temperature Rise" (1986).
The electrode was then removed and the heating pattern observed by
placing a liquid crystal film over the anterior thigh area.
-17-
CA 02247268 1998-08-24
WO 97/37719 PCT/US96/12891
There was no sign of any real edge effect around the circumference
of the electrode between resistive and lossy dielectric regions. The
construction of
Example 1 clearly achieved a reduction of edge effect compared to a
commercially
available dispersive electrode.
S
Examples 2-6
Several more samples were prepared which visually had different
coverages and patterns of spray paint but otherwise in accordance with Example
1.
When electrodes prepared according to these Examples 2 - 6 were tested
according
to the same AAMI standard on the same anterior thigh of the same human
patient,
the reduction in edge effect was proportional to the completeness with which
the
flat black paint covered the outer edge of the electrode.
Example 7
To prove that CQM compatibility existed in an electrode of the
present invention, an electrode was prepared according to Example 1, and a
0.06
mm ( 1 /4") wide strip was then cut out of the aluminum foil along the midline
of the
electrode. This electrode was then applied to a forearm of the same human, and
an
alternating current (AC) impedance scan was between the two conductors of this
split return electrode from 1 MHZ to 1 Hertz. In the range from 350 KHz to 25
KHz, the AC impedance was measured from 55 to 124 ohms, which is within the
CQM limits of all major electrosurgical generator manufacturers. Fig. 3
provides
the graphical results.
Examples 8-14
Electrodes were prepared according to Example 2 above, except that
the outer border was completely and uniformly covered in the same paint , with
an
abrupt transition to the bare A1 metal center about 1 cm in from the outer
edge of
the electrode, rather than a gradual shading as occurred in the spraying of
electrodes in Examples 2-6.
-18-
CA 02247268 1998-08-24
WO 97/37719 PCT/US96/12891
Thermograms of these electrodes showed that, unexpectedly, there
was also a reduction of the temperature at the external edge of the electrode.
Moreover, with an electrode that had a substantially uniform
covering of spray paint over the whole surface of the A1 foil, the thermogram
did
not show a thermal pattern typical of a capacitive plate.
These data demonstrated that the nature of the shading of the
electrically insulating paint was not the sole reason for a reduction in edge
effect,
and further, that the paint was also not acting as a capacitive layer.
Analysis of the paint using a Schlumberger 1260 spectrum
impedance analyzer showed a phase angle of close to -90°, but not
exactly at -90°,
indicative that the current flow had a small resistive component, i.e., within
the
definition of the lossy dielectric properties of electrodes of the present
invention.
The testing voltage used was less than 3 volts but the the actual current
density was
similar to the actual current density generated by the electrosurgical
generator.
Therefore, the electrically insulative paint was acting as a lossy
dielectric layer as defined according to the present invention, which at high
electrosurgical power output was permitting passage of current in a very
limited
resistive manner, i.e., in the manner of a lossy dielectric capacitor.
Examples I S - 19 and Comparative Example 20
Additional electrodes corresponding to the electrodes of Examples
2-6 were made and tested using an Agema 470 Infra Red camera to measure
temperature quantitatively. Testing of the electrodes found that the area of
highest
temperature rise during usage was very sharply confined to the corners of the
electrode.
As a control (Comparative Example 20), a standard resistive-type
electrode was prepared without any paint as a lossy dielectric layer and
having a
I29 cmz (20 square inch) (with 2.54 cm radiused corners). For this control
electrode to have a 4° C maximum temperature rise, the portion of the
electrode
having an increase of from 3 - 4°C represented only about 2 - 4% of the
total
-19-
CA 02247268 1998-08-24
WO 97/37719 PCT/US96/12891
surface area of the electrode. Thus, a temperature reduction of 25% was
achievable, if the heat in the corners could be reduced, with no other
reduction in
edge effect.
Electrodes were produced according to Examples 2-6 with a painted
border that was wider by 1.35 cm at the corners of the electrode than at the
middle
of each edge and with different coating weights of paint being knife coated on
the
A1 foil (3, 6, 9, 13, and 17 grains / 4 in. X 6 in. (10.2 x 15.2 cm) Examples
15-19,
respectively) . Besides providing a high impedance layer at the edge of the
electrode to drive current distribution inward, the wider corners drove the
current
from the corners laterally towards the middle of each edge.
The area between the square and circle was then cut out, and the
open area in the middle of the plate was filled with a second thickness of Al
foil.
Both layers were then laminated to a layer of the same conductive adhesive as
used
in prior examples. These electrodes were then tested using the same AAMI
standard
on the anterior thighs of the same human.
Fig. 4 shows the thermogram results with the eiectrode on the right
thigh (left side of photograph} being the electrode of Example 17 and the
electrode
on the left thigh (right side of photograph) being an electrode of Comparative
Example 20 having a surface of 129 cm2 (20 square inch).
As the coating weight of the paint increases, the area of maximum
current density (and temperature in tissue of the patient beneath the
electrode) of
Examples 15-19 shifts from the outer corners of the electrode in toward the
bare Al
foil center of the electrode. More importantly, at a coating weight in about
the
middle of the range of coating weights used (Example 17, 9 grains), a much
more
diffuse heat distribution was noted, as seen in Fig. 4. Table 1 shows the
results.
-20-
CA 02247268 1998-08-24
WO 97/37719 PCT/US96/12891
Table 1
Example Coating Wt. Max. Tissue Temp.
(grains) Rise vs.
Comp. Example 20 (C)
15 3 -0.4
16 6 -0.5
17 9 -0.6
18 13 -0.1
19 17 -0.2
Comp. 20 None --
More importantly, at a coating weight in about the middle of the
range of coating weights tried, (i. e., about 9 grains), the increase in
maximum
temperature at the corners and at the edges is both reduced and more evenly
distributed.
Examples 21--22
Electrodes from Examples 15-19 were reproduced, except that the
width of the painted border at the middle of each edge was increased from 0.95
cm
(1/4") to 1.3 cm ('/2") to 1.9 cm (3/4"). Table 2 shows the results. With the
width
of the border increasing, the net temperature reduction was not as great.
Table 2
Example Width of PaintedMax. Tissue Temp. Rise
Border (cm) vs.
Comp. Example 20 (C)
17 0.95 -0.6
21 1.3 -0.2
22 I .9 0.5
Comparative Examples 23 and 24
To contrast the lossy dielectric properties of electrodes of the
present invention with materials known to act as pure dielectric materials ,
two
-21-
CA 02247268 1998-08-24
WO 97/37719 PCT/US96/12891
constructions were made up similar to those Examples 8-14, except that an
acrylate
pressure sensitive adhesive (PSA) having a formulation of isooctyl
acrylate/acrylic
acid in a ratio of 96:4 and a 0.1 mm (0.47 mil) PET film were each substituted
for
the paint. In Comparative Example 23, only the thinnest layer obtainable of
acrylate
adhesive (0.025 mm) was used in an attempt to create lossy dielectric
properties
using a truly dielectric material. In Comparative Example 24, a combination of
the
acrylate adhesive and the PET film was used. The results are shown Fig. 5. The
current is sharply concentrated at the outer edge of the bare metal in the
center of
the plate. This is further indication that the electrically insulating paint
is acting as
something less than a good dielectric, since the thermogram results seen in
Fig. 5
are inferior to the thermogram results seen in Fig. S, even with the heaviest
coating
weight (17 grains) of paint.
In other words, a very thick layer of paint still does not force the
current to the center as much as the thinnest layer of acrylate adhesive
available. As
1 S such, the paint cannot be considered to be a true dielectric material like
the acrylate
adhesive and PET film and becomes a preferred material to provide lossy
dielectric
properties for the dispersive electrode.
Examples 25-29 and Comparative Example 30
Having thus established that the current distribution on a 129 cm2
(20 square inch) electrode was made more even by the use of a lossy dielectric
material coating the outer edge of an aluminum electrode, an electrode of this
construction but smaller in area was made to demonstrate that the smaller
electrode
yielded a maximum temperature rise similar to a conventional dispersive
electrode.
An electrode was produced having a conductor area of 97 cm2 (15
square inches). Paint (No. 7715 from Rustoleum) was coated around the edges at
5
different coating weights (1, 2, 3, 5, and 7 grains / 10.2 cm x 15.2 cm (4x6),
(Examples 26-30, respectively) and electrodes were prepared and tested in the
same
manner as the above Examples. The results are shown in Table 3 below and in
Fig.
6 where left thigh-right side electrode is Comparative Example 31 and the
right
thigh-left side electrode is Example 27. Using 3 grains of paint, the maximum
-22-
CA 02247268 1998-08-24
WO 97/37719 PCT/US96/12891
temperature rise for the Example 27 electrode was no greater than the control
Comparative Example 3 i prepared in the same manner as Comparative Example 20.
Table 3
Example Coating Wt. Max. Tissue Temp. Rise
(grains) vs.
Comp. Example 30 (C)
25 1 0.8
26 2 0.2
27 3 0.0
28 5 0.4
29 7 0. S
Comp. 30 None --
S
Using a lossy dielectric material on electrodes of the present
invention permitted a substantial size reduction (25% decrease in area)
without a
significant increase in maximum tissue temperature greater than the increase
in
maximum tissue temperature of a conventional, resistive electrosurgical
dispersive
electrode having a conductor area of about 129 cm (20 sq. in.). For the
electrode
of Example 27, the 25% decrease in area did not create any increase in maximum
tissue temperature.
The present invention permits one skilled in the art to construct a
lossy dielectric electrode having the same or smaller temperature rise with a
smaller
1 S sized electrode or a smaller temperature rise with a same sized electrode.
A
dispersive electrode of smaller size can be significantly less expensive to
make.
Examples 31-58 and Comparative Examples 59-65
The electrodes of Examples 15-19 were reproduced, except that a
variety of different coating weights and paints and inks were employed. A
variety
of commercially available dispersive electrodes were gathered. Impedance
(Z)/area
(S2/ 129 cm2 ), resistance (R)/area (S2 / 129 cm2), reactance(X) /area (SZ/
129 cm2),
tan b, and S (°) were measured. Table 4 shows the results.
-23-
CA 02247268 1998-08-24
WO 97/37719 PCT/US96/12891
Table
4
ExampleLossy Coat. Z/ areaR / X/ areatan s ()
DielectricWt. s
Layer (Grains/W =29 area (S?/(S?l
4"X6") cm 129 =29
cm cm
31 7790 2.75 2.020 0.702 -1.898 0.370 20.285
32 7790 6.96 5.360 1.024 -5.265 0.195 11.010
33 7790 9.32 7.420 1.066 -7.347 0.145 8.250
34 7790 13.08 10.340 1.189 -10.2680.116 6.610
35 7790 18.1 14.080 1.563 -13.9930.112 6.370
36 7776 3.280 2.710 0.778 -2.599 0.299 16.654
37 7776 6.050 4.450 0.744 -4.383 0.170 9.636
38 7776 9.530 7.460 0.929 -7.406 0.125 7.146
39 7776 12.760 9.900 1.021 -9.849 0.104 5.919
40 7730 1.700 2.150 0.618 -2.062 0.300 16.676
41 7730 2.970 4.320 0.826 -4.237 0.195 11.034
42 7730 6.080 7.990 0.955 -7.931 0.120 6.865
43 7730 8.830 12.080 1.207 -12.0220.100 5.731
44 7730 11.830 16.240 1.573 -16.1600.097 5.558
45 7727 1.510 3.100 0.716 -3.016 0.237 13.353
46 7727 1.950 3.910 0.881 -3.807 0.231 13.026
47 7727 3.690 7.740 1.307 -7.634 0.171 9.712
48 7727 5.500 11.170 1.299 -11.0950.117 6.678
49 7727 7.550 15.160 1.658 -15.0730.110 6.277
50 7715 1.150 1.360 0.687 -1.178 0.584 30.272
51 7715 1.680 1.580 0.590 -1.462 0.404 21.976
52 7715 3.080 2.440 0.675 -2.342 0.288 16.077
53 7715 4.880 3.180 0.702 -3.099 0.226 12.755
54 7715 6.590 4.010 0.847 -3.918 0.216 12.204
55 800 1 pass 4.670 1.121 -4.535 0.247 13.887
56 800 2 pass 9.630 2.685 -9.246 0.290 16.192
57 801 1 pass 4.180 0.758 -4.108 0.185 10.461
58 801 2 pass 8.970 1.444 -8.854 0.163 9.260
C-59 Coraplate 33.205 5.346 -32.7720.163 9.264
C-60 Elmed 61.529 4.689 -61.3500.076 4.371
C-61 Mera 64.004 4.576 -63.8400.072 4.099
SAS
C-62 Diatemp 47.747 2.581 -47.6780.054 3.098
II
C-63 0.05 46.030 2.781 -45.9450.061 3.464
PET
C-64 3M 7149 0.516 0.387 0.258 1.809 61.07
C-65 3M 7146 J 1.3021.302 0.516 2.242 65.96
I
7790, 7776, 7730, 7727, and 7715 are all Rustoleum branded enamel paints.
800 and 801 are both Summit branded white inks.
Coraplate is a capacitive dispersive electrode commercially available from
Cora-ve~trieb of Mainz, Germany
Elmed is a capacitive dispersive electrode commercially available from Ebned
of Addison , Illinois
Mera SAS is a capacitive dispersive electrode commercially available from Mera
ofTokyo, Japan
Diatemp II is a capacitive dispersive electrode commercially available from
NDM of Dayton, Ohio
0.05 PET is an experimental dispersive electrode prepared according to the
disclosure of U.S. Pal. No. 4,387,714 (Geddes et
1 ~ al.)
3M 7149 and 7146 are resistive dispersive electrodes commercially available
from 3M Company, St. Paul, MN.
-24-
CA 02247268 1998-08-24
WO 97/37719 PCT/US96/12891
As seen by an analysis of the data shown in Table 4, the variety of
coating weights and paints and inks to generate lossy dielectric layers on
electrically
conductive surfaces result in biomedical electrodes that neither function like
capacitive electrodes (Comparison Examples C-59--C-63) nor function like
resistive
electrodes (Comparison Examples C-64 and C-65).
All test results shown in Table 4 are normalized to unit area or
dimensionless values. Therefore, one skilled in the art can readily determine
how it
is possible to control the extent of lossy dielectric properties on biomedical
electrodes for either electrosurgical or cardiac stimulation uses.
One should strive to obtain a value of tan b in the range approaching
a pure capacitive biomedical electrode (~ 0) (as in Comparison Examples C-59--
C-
63) while also striving to limit Z/ area as much as possible (as in Comparison
Examples C-64 and C-65). Only biomedical electrodes of the present invention
with lossy dielectric properties as described above succeed in providing both
low
tan 8 values and low Z/ area values.
Variations in the invention have also employed several types of
paints and printing inks as lossy dielectrics materials, serving to achieve
the twin
goals of reduced edge effect and size reduction in electrodes. With
appropriate
thinness of any dielectric material, that material could be made to act as a
lossy
dielectric and be used in the construction of the electrode of the present
invention.
Further shapes other than squares with inscribed circles can be made
to work using the present invention, as well as means other than knife coating
(screen printing, gravure printing, ink bubble jet technology, etc.) can be
used to
create or deposit a lossy dielectric layer on a conductive substrate.
Further, multiple areas around the outer edge of an electrode can be
coated with different thicknesses of paint or with different types of paint or
ink to
provide gradual "shading" of the lossy dielectric material. While not
necessary for
the advantages of the present invention, a gradual shading technique can
optimize
the performance of the electrode.
Other methods of creating the effects of the lossy dielectric
properties for a dispersive electrode of the present invention include (a) the
addition
-25-
CA 02247268 1998-08-24
WO 97/37719 PCT/US96/12891
of a sheet or scrim of a lossy dielectric material in the field of conductive
adhesive
32 rather than to the electrically conductive surfaces 14 and 16; (b) the
addition of a
lossy dielectric, pressure-sensitive adhesive coated sheet or film to the
field 32 of
conductive adhesive on at least a portion of the skin contacting surface; and
means
of altering (by photochemistry, electrochemistry, or otherwise) to impart
lossy
dielectric properties on a portion of electrically conductive surfaces 14 or
16 or in a
portion of field 32.
The choices available to one skilled in the art because of the breadth
of the present invention permit construction of electrodes using a simple and
cost-
effective means of production, which can yield a product that can be
commercially
produced in preference to a construction that requires multiple conductive
elements,
layers, or pieces. A smaller electrode with the same performance
characteristics
will require less raw materials to make, and be more convenient for use by the
customer at the same time.
Various modifications and alterations of the present invention will be
apparent to those skilled in the art without departing from the scope and
spirit of
this invention, and it should be understood that this invention is not limited
to the
illustrative embodiments set forth herein. The claims follow.
-26-