Note: Descriptions are shown in the official language in which they were submitted.
CA 02248212 1998-08-28
WO 97/37614 PCT/US97105268
PERFORATED SUBMUCOSAL TISSUE GRAFT CONSTRUCTS
Field of the Invention
This invention relates to tissue graft constructs
useful in promoting regrowth and healing of damaged or
diseased tissue structures. More particularly this
invention is directed to perforated submucosal tissue graft
constructs formed from submucosal tissue of a warm-blooded
vertebrate and a method for making said constructs.
Background and Summary of the Invention
It is known that compositions comprising the
tunica submucosa delaminated from both the tunica
muscularis and at least the luminal portion of the tunica
mucosa of the intestine of warm-blooded vertebrates can be
used as tissue graft materials. See, for example, U.S.
Patent Nos. 4,902,508 and 5,281,422. The compositions
described in those patents are characterized by excellent
mechanical properties, including high compliance, a high
burst pressure point, and an effective porosity index which
allows such compositions to be used beneficially for
vascular graft constructs and in connective tissue
replacement applications. When used in such applications
the submucosal graft constructs appear to serve as a matrix
for the regrowth of the tissues replaced by the graft
constructs. Significantly, too, in over 600 cross-specie s
implants, submucosa-derived graft compositions have never
been shown to elucidate a tissue graft rejection reaction.
Submucosa-derived matrices for use in accordance
with the present invention are collagen based biodegradable
matrices comprising highly conserved collagens,
glycoproteins, proteoglycans, and glycosaminoglycans in
their natural configuration and natural concentration. One
extracellular collagenous matrix for use in this invention
is submucosal tissue of a warm-blooded vertebrate.
Submucosal tissue can be obtained from various sources, for
CA 02248212 1998-08-28
WO 97137614 PCT/US97/05268
-2-
example, intestinal tissue harvested from animals raised
for meat production, including, pigs, cattle and sheep or
other warm-blooded vertebrates. Vertebrate submucosal
tissue is a plentiful by-product of commercial meat
production operations and is thus a low cost tissue graft
material.
One limitation of the submucosal graft constructs
described in the above mentioned patents is that the size
of the graft is restricted by the size of the source
l0 material from which the submucosal tissue is prepared. For
example, the size of a submucosal tissue graft prepared
from intestinal tissues is limited by the length and
circumference of the source segments intestinal tissue.
Yet several applications of submucosal tissue graft
constructs, including hernia repair, skin graft, meningeal
coverings, repair of gastroschisis (congenital stomach
defects) and organ tissue replacement, often require larger
sheets of graft material than can be prepared directly from
natural sources.
Large sheets of submucosal tissue can be prepared
from smaller segments of submucosal tissue through
conventional techniques such as weaving, knitting or the
use of adhesives. However, commercial implementation of
such techniques are often impractical and expensive.
Additionally the use of adhesives or chemical pretreatment
to promote adhesion of the tissue strips can compromise the
biotropic properties of the submucosal grafts. Thus there
is a need for an inexpensive, easily manufactured, large
area submucosal tissue graft construct that retains its
biotropic properties.
In accordance with one embodiment of the present
application large area submucosal tissue graft constructs
are formed from multiple pieces of vertebrate submucosa-
derived matrices. Unitary sheets (i.e., single piece graft
constructs) of submucosal tissue are prepared in accordance
with the present invention by fusing multiple strips of
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/05268
-3-
submucosal tissue to each other to form a sheet of tissue
having a surface area larger than any one of the component
strips of submucosal tissue. The process comprises the
steps of overlapping at least a portion of one strip of
submucosal tissue with at least a portion of another strip
of submucosal tissue and applying pressure at least to said
overlapped portions under conditions allowing dehydration
of the submucosal tissue. Under these conditions the
overlapped portions will become "fused" to form a unitary
large sheet of tissue. These large area graft constructs
consist essentially of submucosal tissue, free of
potentially compromising adhesives and chemical
pretreatments, and have a greater surface area and greater
mechanical strength than the individual strips used to form
the graft construct.
Individual strips of submucosal tissue as
prepared from the tissues of a warm-blooded vertebrate have
mechanical properties that are directionally specific
(i.e., physical properties vary along different axes of the
tissue). These directional characteristics are governed
primarily by collagen orientation within the tissue. The
collagen fibers are the load bearing constituents within
intestinal submucosal tissue and are predominantly
orientated parallel to the axis of the intestine lumen.
This longitudinal disbursement of collagen in intestinal
submucosal tissue contributes to the directional
variability in physical properties of the submucosal tissue
constructs.
Unitary pseudoisotropic multi-laminate graft
constructs can be prepared from multiple strips of
submucosal tissue. The term "pseudoisotropic" as used
herein describes a graft material having approximately
similar physical properties along each axis of the graft
material. These pseudoisotropic multi-laminate graft
constructs are prepared from individual strips of
submucosal tissue or sheets of submucosal tissue comprising
CA 02248212 2005-03-07
64005-602
-4-
strips of submucosal tissue. The method of preparing the
pseudoisotropic graft constructs comprises overlapping a
portion of a first strip (or sheet) with a second strip (or
sheet), wherein the second strip (or sheet) is orientated in
a plane parallel to the first strip (or sheet) but rotated
so that the longitudinal axis of the first strip (or sheet)
forms an angle relative to longitudinal axis of the second
strip (or sheet). Additional strips (or sheets) can be
added in a similar manner to create a multi-laminate
structure having a desired number of laminate layers. The
individual submucosal strips (or sheets) are then fused to
one another to form a unitary multi-laminate pseudoisotropic
construct by applying pressure at least to the overlapped
portions of submucosal tissue.
Summarv of the Invention
The present invention is directed to an improved
submucosal tissue graft construct. The improvement
comprises forming a plurality of perforations in the
submucosal tissue graft constructs. The perforations allow
extracellular fluids to pass through the tissue graft
material, decreasing fluid retention within the graft and
enhancing the remodeling properties of the tissue grafts.
The perforation of the submucosal tissue is especially
beneficial for multi-laminate tissue graft constructs
wherein the perforations also enhance the adhesive force
between adjacent layers.
According to another aspect of the invention,
there is provided a unitary multi-laminate tissue graft
construct comprising multiple strips of submucosa, wherein
each of said multiple strips has first and second planar
surfaces and said graft construct formed to have a planar
surface with a surface area greater than the surface area of
CA 02248212 2005-03-07
64005-602
- -4a-
any one planar surface of the individual strips used to form
said construct, said graft further provided with a plurality
of perforations that allow fluid to pass through the graft
construct.
According to a further aspect of the invention,
there is provided a unitary multi-laminate tissue graft
construct comprising multiple strips of submucosa, said
graft construct comprising first and second planar surfaces
wherein the planar surfaces are perforated with at least
2.0 perforations per cm2, said perforations providing fluid
communication between the first and second planar surfaces.
Brief Description of the Drawings
Fig. la-c is a diagrammatic representation of a
homolaminate graft construct formed from multiple strips of
submucosal tissue.
Fig. 2a-c is a diagrammatic representation of a
pseudoisotropic heterolaminate graft construct formed from
four strips of submucosal tissue.
Fig. 3a-c is a diagrammatic representation of a
CA 02248212 2005-03-07
64005-602
-5-
pseudoisotropic heterolaminate graft construct formed from
three sheets of submucosal tissue, wherein each sheet is
formed from multiple strips of submucosal tissue.
Fig, 9 is a diagrammatic representation of one
device suitable for forming perforations in submucosal
tissue grafts in accordance with the present invention.
Detailed Description of the Preferred Embodiments
There is provided in accordance with this
invention an improved submucosal tissue graft construct.
the improved construct comprising a submucosal tissue graft
having a plurality of perforations extending through the
graft. In preferred embodiments the perforations are
uniform in size and are evenly distributed over the entire
surface of the graft. Furthermore, the present invention
provides a method far preparing perforated submucosal
tissue graft constructs.
Submucosal tissue suitable for use in the
formation of the present graft constructs comprises
naturally associated extracellular matrix proteins,
glycoproteins and other factors. One source of submucosal
tissue is the intestinal tissue of a warm-blooded
vertebrate. Small intestinal tissue is a preferred source
of submucosal tissue for use in this invention.
Suitable intestinal submucosal tissue typically
comprises the tunics submucosa delaminated from both the
tunics muscularis and at least. the luminal portion of the
tunics mucosa. In one embodiment of the present invention
the intestinal submucosal tissue comprises the tunics
submucosa and basilar portions of the tunics mucosa
including the lamina muscularis mucosa and the stratum
compactum which layers are known to vary in thickness and
in definition dependent on the source vertebrate species.
The preparation of submucosal tissue for use in
accordance with this invention is described in U.S. Patent
No. 4, 902, 508.
CA 02248212 2005-03-07
64005-602
-6-
A segment of vertebrate intestine, preferably
harvested from porcine, ovine or bovine species,
but not excluding other species, is subjected to
abrasion using a longitudinal wiping motion to
remove the outer layers, comprising smooth muscle tissues,
and the innermost layer, i~e., the luminal portion of the
tunics mucosa. The submucosal tissue is rinsed with saline
and optionally sterilized.
The multi-laminate submucosal tissue graft
constructs of the present invention can be sterilized using
conventional sterilization techniques including
glutaraldehyde tanning, formaldehyde tanning at.acidic pH,
propylene oxide or ethylene oxide treatment, gas plasma
sterilization, gamma radiation, electron beam, peracetic
acid sterilization. Sterilization techniques which do not
adversely affect the mechanical strength, structure, and
biotropic properties of the submucosal tissue is preferred.
For instance, strong gamma radiation may cause loss of
strength of the sheets of submucosal tissue. Preferred
sterilization techniques include exposing the graft to
peracetic acid, 1-4 Mrads gamma irradiation (more
preferably 1-2.5 Mrads of gamma irradiation), ethylene
oxide treatment or gas plasma sterilization; peracetic acid
sterilization is the most preferred sterilization method.
Typically, the submucosal tissue is subjected to two or
more sterilization processes. After the submucosal tissue
is sterilized, for example by chemical treatment, the
tissue may be wrapped in a plastic or foil wrap and
sterilized again using electron beam or gamma irradiation
3o sterilization techniques.
Submucosal tissue can be stored in a hydrated or
dehydrated state. Lyophilized or air dried submucosa
tissue can be rehydrated and used in accordance with this
invention without significant loss of its biotropic and
mechanical properties.
Large area compliant sheets of submucosal tissue
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/05268
can be formed from multiple strips of submucosal tissue.
The dimensions of the individual strips of submucosal
tissue used is not critical and the term "strip of
submucosal tissue" is defined herein to include submucosal
tissue from one or more vertebrate sources or organs in a
wide variety of sizes and shapes. In one embodiment the
strips are formed from a delaminated segment of intestinal
tissue that is optionally, but preferably, cut and
flattened out to provide an elongated strips of submucosal
tissue having two generally parallel sides and opposite
ends. The term "sheet of submucosal tissue" is defined
herein to include tissue constructs comprising multiple
strips of submucosal tissue, wherein the strips are
overlapped to form a construct having a greater surface
area than any one of the individual sheets used to form
said construct. The term "layers of submucosal tissue"
refers to the individual laminae of a multi-laminate
submucosal tissue construct.
Unitary, large area sheets of submucosal tissue
are formed by overlapping individual strips of submucosal
tissue and applying pressure to the overlapped portions to
fuse the tissues together. In one embodiment pressure is
applied to the overlapped tissue under conditions allowing
dehydration of the submucosal tissue. The large area
sheets of submucosal tissue can be formed as either a
heterolaminar sheet or a homolaminar sheet. The term
"heterolaminar" as used herein refers to a multi-laminate
tissue having a variable number of laminae of submucosa
superimposed at (and fused) at different points on the
unitary graft construct. The term "homolaminar" as used
herein refers to a multi-laminate tissue graft construct
having a uniform number of laminae of submucosa at all
points on the unitary graft construct.
In one embodiment the method of forming large
sheets of submucosal tissue comprises the steps of
overlapping at least a portion of one strip of submucosal
CA 02248212 1998-08-28
WO 97/37614 PCT/i7S97/05268
_g_
tissue with at least a portion of a second strip of
submucosal tissue, and applying pressure at least to said
overlapped portions under conditions allowing dehydration
of the submucosal tissue. The amount of t;s~"A ~tTAYian
between the adjacent strips of submucosal tissue can be
varied based on the intended use and the desired properties
of the large area graft construct, provided that at least a
portion of each strip of submucosal tissue overlaps with a
portion of another strip of submucosal tissue. The applied
pressure fuses the strips of submucosal tissue to one
another along the overlapped portions, producing a
compliant unitary heterolaminar sheet of submucosal tissue.
In another embodiment, a unitary homolaminate
sheet of submucosal tissue can be prepared from strips of
submucosal tissue. The method for forming the homolaminar
tissue graft construct comprises the steps of forming a
first layer of submucosal tissue, wherein strips of
submucosal tissue are located side-by-side on a first
surface. The strips of submucosal tissue of the first
layer are located adjacent to one another so that the edges
of the individual strips are in contact with one another
without substantial overlap between one another. The first
layer of submucosal tissue is then overlaid with a second
layer of submucosal tissue. The strips of submucosal
tissue of the second layer are located adjacent to one
another similar to the strips of submucosal tissue of the
first layer (i.e., adjacent to one another so that the
edges of the individual strips are in contact with one
another without substantial overlap between one another).
In one embodiment the strips of submucosal tissue of the
second layer are orientated in the same direction as the
strips of submucosal tissue of the first layer, but offset
in relationship to the submucosal strips of the first
layer, so that the contacting edges of the individual
strips of submucosal tissue of the first layer are bridged
by the strips of submucosal tissue of the second layer (See
CA 02248212 2005-03-07
64005-602
_g_
Fig. la-c). The overlap portions of the strips of
submucosal tissue are then compressed between two surfaces,
at least one of the two surfaces being water permeable,
under conditions allowing at least partial dehydration of
the compressed submueosal tissue.
Advantageously both the heterolaminar and
homolaminar large area sheets of submucosal tissue consist
essentially of submucosal tissue, have enhanced mechanical
strength and have a greater surface area than any one of
the individual strips used to form the submucosal sheets.
Submucosal tissue typically has an abluminal and
a luminal surface. The luminal surface.is the submucosal
surface facing the lumen of the organ source and typically.
adjacent to wn inner mucosa layer in vivo whereas the
abluminal surface is the submucosal surfaee facing away
from the lumen of the organ source and typically in contact
with smooth muscle tissue in vivo. The multiple strips of
submucosal tissue can be overlapped with the abluminal
surface contacting the luminal surface, the luminal surface
contacting the luminal surface or with the abluminal
surface contacting the abluminal surface of an adjacent
strip of submucosal tissue. All of these combinations of
overlapping strips of submucosal tissue from the same or
different vertebrate or organ sources will produce a large
area sheet of submucosal tissue upon compression of at
least the overlapped portions under conditions allowing
dehydration of the tissue.
Strips of submucosal tissue can be conditioned,
~0 as described in U.S. Patent No. 5,275,826 to alter the
viscoelastic properties of the subrnucosal tissue.
In accordance with one embodiment submucosa delaminated
from the tunics muscularis and luminal portion of the
tunics mucosa is conditioned to have a strain of no more
tran ZO~. The submucosal tissue is conditioned by
stretching, chemically treating, enzymatically treating or
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/05268
-10-
exposing the tissue to other environmental factors. In one
embodiment the strips of intestinal submucosa tissue are
conditioned by stretching in a longitudinal or lateral
direction so that the strips of intestinal submucosa tissue
have a strain of no more than 200. The conditioned
submucosal strips can be used to form large area sheets or
multi-laminate structures in accordance with the present
invention. Alternatively, the submucosal material can be
conditioned after the formation of a large area sheets or
multi-laminate large area sheet constructs to produce
submucosal tissue material having a strain of no more than
200.
During formation of the large area sheets of
submucosal tissue, pressure is applied to the overlapped
portions by compressing the submucosal tissue between two
surfaces. The two surfaces can be formed from a variety of
materials and in any shape depending on the desired form
and specification of the unitary graft construct.
Typically the two surfaces are formed as flat plates but
they can also include other shapes such as screens, opposed
cylinders or rollers and complementary nonplanar surfaces.
Each of these surfaces can optionally be heated or
perforated. In preferred embodiments at least one of the
two surfaces is water permeable. The term water permeable
surface as used herein includes surfaces that are water
absorbent, microporous or macroporous. Macroporous
materials include perforated plates or meshes made of
plastic, metal, ceramics or wood.
The submucosal tissue is compressed in accordance
with one embodiment by placing the overlapped portions of
the strips of submucosal tissue on a first surface and
placing a second surface on top of the exposed submucosal
surface. A force is then applied to bias the two surfaces
towards one another, compressing the submucosal tissue
between the two surfaces. The biasing force can be
generated by any number of methods known to those skilled
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/05268
-11-
in the art including the passage of the apparatus through a
pair of pinch rollers (the distance between the surface of
the two rollers being less than the original distance
between the two plates), the application of a weight on the
top plate, and the use of a hydraulic press or the
application of atmospheric pressure on the two surfaces.
In one preferred embodiment the strips of
submucosal tissue are subjected to conditions allowing
dehydrating of the submucosal tissue concurrent with the
compression of the tissue. The term "conditions allowing
dehydration of the submucosal tissue" is defined to include
any mechanical or environmental condition which promotes or
induces the removal of water from the submucosal tissue at
least at the points of overlap. To promote dehydration of
the compressed submucosal tissue, at least one of the two
surfaces compressing the tissue is water permeable.
Dehydration of the tissue can optionally be further
enhanced by applying blotting material, heating the tissue
or blowing air across the exterior of the two compressing
surfaces.
The multiple strips of submucosal tissue are
typically compressed for 12-48 hours at room temperature,
although heat may also be applied. For example a warming
blanket can be applied to the exterior of the compressing
surfaces to raise the temperature of the compressed tissue
up to about 40°C to about 50°C. The overlapped portions are
usually compressed for a length of time determined by the
degree of dehydration of the tissue. The use of heat
increases the rate of dehydration and thus decreases the
amount of time the overlapped portions of tissue are
required to be compressed. Typically the tissue is
compressed for a sufficient time to produce a stiff but
flexible material. Sufficient dehydration of the tissue is
also indicated by a increase in impedance of electrical
current flowing through the tissue. When impedance has
increased by 100-200 ohms, the tissue is sufficiently
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/05268
-12-
dehydrated and the pressure can be released.
The compressed submucosal tissue can be removed
from the two surfaces as a unitary compliant large area
tissue construct. The construct can be further manipulated
(i.e., cut, folded, sutured, etc.) to suit various medical
applications where the submucosal material of the present
invention is required.
A vacuum can optionally be applied to submucosal
tissue during the compression procedure. The applied
vacuum enhances the dehydration of the tissue and may
assist the compression of the tissue. Alternatively the
application of a vacuum may provide the sole compressing
force for compressing the overlapped portions of the
multiple strips of submucosal tissue. For example the
overlapped submucosal tissue is laid out between two
surfaces, preferable one of which is water permeable. The
apparatus is covered with blotting material, to soak up
water, and a breather blanket to allow air flow. The
apparatus is then placed in a vacuum chamber and a vacuum
is applied, generally ranging from 14-70 inches (36-179 cm)
of Hg (7-35 psi). Preferably a vacuum is applied at
approximately 51 inches (131 cm) of Hg (25 psi).
Optionally a heating blanket can be placed on top of the
chamber to heat the submucosal tissue during the
compression of the tissue. Chambers suitable for use in
this-embodiment are known to those skilled in the art and
include any device that is equipped with a vacuum port.
The resulting drop in atmospheric pressure coacts with the
two surfaces to compress the submucosal tissue and
simultaneously dehydrate the submucosal tissue.
Optionally, large area tissue grafts can be
formed into various shapes for tissue graft applications.
For example, in organ reconstruction applications the large
area sheets can be formed in the shape of a hollow sphere
or pouch. Such a shaped construct would be advantageous in
the replacement of large regions of the urinary bladder or
CA 02248212 1998-08-28
WO 97!37614 PCT/US97105268
-13-
stomach. These shaped submucosal tissue constructs can be
formed by conventional techniques such as cutting and
suturing the tissue to form a desired shape.
Alternatively, strips of submucosal tissue can be
formed into a large sheet of submucosal tissue having a
nonplanar shape through a simple manufacturing procedure.
The method comprises the steps of placing multiple strips
of submucosal tissue between two complementary nonplanar
shaped surfaces and compressing overlapped strips of
submucosal tissue between the two surfaces. The
complementary shaped surfaces are formed such that the two
surfaces can be pressed together such that the surfaces fit
snug against one another without leaving any substantial
pockets of air between the two surfaces. Preferably at
least one of the two complementary surfaces is water
permeable.
One method of forming a shaped submucosal
construct comprises placing multiple strips of submucosal
tissue on a nonplanar shaped porous surface such that the
submucosal tissue conforms to the shape of the porous
surface. Preferably the submucosal tissue is placed on the
porous surface without stretching the material, however,
the submucosal tissue can be stretched to facilitate
covering the shaped porous surface. Each of the strips of
submucosal tissue is positioned on the porous surface to
overlap at least a portion of an adjacent strip of
submucosal tissue. The overlapped portions of the
submucosal tissue are then covered with a second shaped
surface that is complementary in shape with the first
3o porous surface and pressure is applied to compress the
submucosal tissue between the two surfaces under conditions
allowing dehydration of the submucosal tissue.
Alternatively the large area sheets of the
present invention can be shaped into a nonplanar shape by
stretching the large area sheet through the use of a die
press procedure, wherein the submucosal tissue is pressed
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/05268
-14-
into a nonplanar shape by a porous die under dehydrating
conditions such that the formed tissue graft holds its
shape. Preferably a multi-laminate large area sheet is
used in such a procedure.
Multi-laminar submucosal tissue constructs are
formed in accordance with the present invention by
overlapping a portion of one strip of submucosal tissue
with a portion of another strip of submucosal tissue. In a
similar fashion large area multi-laminar tissue graft
constructs can be formed in accordance with the present
invention by overlapping a sheet of submucosal tissue
(formed as described above) with at least a portion of a
second sheet of submucosal tissue. The size and physical
properties of the multi-laminate submucosal tissue
construct can be regulated by the number of overlapped
strips of submucosal tissue and the percent of the
overlapped portion of each strip.
The multi-laminar tissue graft constructs are
formed in accordance with the present invention by
overlapping at least a portion of one strip of submucosal
tissue with a portion of another strip of submucosal tissue
to form a first sheet. Additional strips of submucosal
tissue are overlaid onto the overlapped portions of the
first sheet to form a second sheet, wherein the edges of
the strips of the second sheet are optionally at an acute
angle to the edges of the strips in the first sheet, and
wherein said formed second sheet is coplanar with the first
sheet. The strips of submucosal tissue of the second sheet
can be positioned so that at least a portion of one strip
of submucosal tissue of the second sheet overlaps with at
least a portion of another strip of submucosal tissue of
the second sheet. Additional strips of submucosal tissue
can be overlaid on the overlapped portions of the first and
second sheets to provide additional layers of submucosal
tissue. The multiple layers of submucosal tissue are then
compressed under dehydrating conditions to form a multi-ply
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/05268
-15-
heterolaminar submucosal tissue construct having a surface
area greater than any one of the individual strips of
submucosal tissue used to form the multilayered construct.
In one embodiment of the present invention
submucosal tissue is cut to into strips, each strip having
generally parallel sides, and used to form the multilayered
heterolaminar construct of the present invention. In this
embodiment the strips of submucosal tissue of the second
sheet are overlaid onto the overlapped portions of the
l0 first sheet such that the edges of the first sheet
submucosal strips are at an angle relative to the edges of
the second sheet submucosal strips. The overlapped
portions of submucosal tissue are compressed under
dehydrating conditions to form the multilayered
heterolaminar construct.
The mufti-laminate tissue graft constructs can be
formed to have pseudoisotropic properties. These
pseudoisotropic tissue grafts are prepared from at least
three strips of intestinal submucosal tissue delaminated
from both the tunica muscularis and the luminal portion of
the tunica mucosa of a warm blooded vertebrate. Each of
the strips of intestinal submucosal tissue are
characterized as having a longitudinal axis corresponding
to the predominant orientation of the collagen fibers in
the submucosal tissue strips. The method of forming the
pseudoisotropic graft constructs comprises locating a first
strip of submucosal tissue on a first surface,'overlaying
said first strip with at least two additional strips of
submucosal tissue so that the longitudinal axes of each
individual strip of submucosal tissue forms an angle of
about 180°/N with the longitudinal axis of at least two
other strips of submucosal tissue forming the
heterolaminate graft, wherein N = the total number of
strips of submucosal tissue. (See Fig. 2a-c). For example
a pseudoisotropic graft construct formed from four (4)
strips of submucosal tissue will have an angle of 45°
CA 02248212 1998-08-28
WO 97/37614 PCT/LTS97/05268
-16-
(180°/4 = 45°) formed between the central longitudinal axes
of each strip in reference to two of the other three strips
forming the graft construct. (See Fig. 2a-c). The
submucosal tissue (at least the overlapped portions) is
then compressed between the first surface and a second
surface. In one embodiment the tissue is compressed under
conditions allowing at least partial dehydration of the
compressed submucosal tissue, and in a preferred embodiment
at least one of said surfaces is water permeable.
Advantageously the submucosal tissue grafts are fused
together in accordance with the present invention in the
absence of adhesives or sutures.
Large area tissue graft constructs having
pseudoisotropic properties can also be prepared from large
area sheets of submucosal tissue. These pseudoisotropic
tissue graft constructs comprise multiple layers of large
area sheets of submucosal tissue wherein the sheets of
submucosal tissue comprise overlapped strips of submucosal
tissue. As described above, large area sheets of
submucosal tissue can be formed from overlapped submucosal
tissue to form either heterolaminar or homolaminar sheets
of submucosal tissue. Both heterolaminar and homolaminar
sheets are suitable for forming large area pseudoisotropic
tissue graft constructs in accordance with the present
2,5 invention (See Fig. 3a-c).
One method of preparing a large area multi-
laminate tissue graft construct having pseudoisotropic
properties comprises forming a first sheet of submucosal
tissue from multiple strips of submucosal tissue and
overlaying the first sheet with at least two additional
sheets. The individual strips of submucosal tissue
comprising each sheet have a longitudinal axis
corresponding to the predominant orientation of the
collagen fibers in the submucosal tissue strips. The first
sheet is formed on a first surface by overlapping the
individual strips of submucosal tissue so that each strip
CA 02248212 1998-08-28
WO 97137614 PCT/US97/05268
-17-
is aligned with the adjacent strips and the longitudinal
axis of each strip of submucosal tissue are substantially
parallel to one another. Thus the collagen fibers of the
first sheet are aligned predominantly in a single
orientation, such that the sheet can be characterized as
having a longitudinal axis corresponding to the predominant
orientation of the collagen fibers. The sheet has a
greater surface are than any one of the individual strips
used to form the sheet.
to After the first sheet of submucosal tissue is
formed, additional submucosal sheets are formed on top of
the first sheet in the same manner that the first sheet was
formed (i.e., each sheet of submucosal tissue of the multi-
laminate comprises overlapped strips of submucosal tissue
wherein the longitudinal axes of the strips of submucosal
tissue comprising each sheet are substantially parallel to
one another). Each individual sheet is overlaid on another
sheet so that the longitudinal axes of the strips of
submucosal tissue of the overlaid sheet forms an angle of
about 180°/S (S = the total number of sheets of submucosal
tissue), with the longitudinal axes of the strips of
submucosal tissue of at least two of the other sheets
forming the multi-laminate construct. Once the total
number of sheets have been overlaid, the sheets of
submucosal tissue are compressed between the first surface
and a second surface under conditions allowing at least
partial dehydration of the compressed submucosal tissue.
In preferred embodiments at least one of said surfaces is
water permeable.
In one embodiment, after multiple strips of
submucosal tissue are overlapped with one another, the
overlapped portions are manipulated to remove trapped air
and bulk quantities of water before fusing the strips into
a single sheet of submucosal tissue. In general the
trapped air bubbles and bulk quantities of water are
squeezed out through the use of a compressing force which
CA 02248212 1998-08-28
WO 97/37614 PCT/US97105268
-18-
is moved across the surface of the overlapped portions.
The compressing force can take the form of a cylinder that
is rolled across the surface of the overlapped portions, or
alternatively the overlapped portions can be passed between
two or more rollers wherein the distance between the
surface of the opposing rollers is less than the thickness
of the submucosal sheet. The overlapped portions can then
be compressed if necessary for an additional length of time
under dehydrating conditions to fuse the multiple strips
into a single sheet of submucosal tissue in accordance with
the present invention.
The excess portions of the pseudoisotropic multi-
laminate grafts (i.e., those portions of the graft having a
laminate number less than N or S) can be removed after
formation of the multi-laminate. Furthermore, the
mechanical properties of multi-laminate submucosal material
can be tailored to the medical application needs by
adjusting the percentage of overlap between adjacent strips
of submucosal tissue, altering the number of submucosal
tissue layers, varying the angle of adjacent layers
relative to one another, changing the water permeability of
the compressing surfaces and/or the composition of the
compressing surfaces, selecting the shape of the
compressive surfaces, and varying the load applied to
compress the overlapped submucosal tissue.
The present invention is directed to a
modification that improves the efficacy of large area and
multi-laminate submucosal graft constructs as implantable
graft materials. Recent experiments have demonstrated that
the process of remodeling is slower with implanted multi-
laminate submucosal tissue graft constructs than for single
or two layered submucosal tissue grafts. In addition,
multi-laminate submucosal tissue graft constructs tend to
accumulate tissue fluid in cyst-like pockets between
adjacent laminae during the first 14-28 days after
implantation in soft tissue locations (such as the muscular
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/05268
-19-
body wall of rats). Fluid pockets are detrimental to wound
healing because they retard connective tissue ingrowth,
provide an environment conducive to bacterial growth, and
prevent the apposition of natural (native) body tissues
which promotes healing and tensile strength.
The present invention minimizes the
disadvantages associated with multi-laminate submucosal
tissue graft constructs by forming perforations in the
graft constructs. Perforation of the graft constructs has
l0 been found to enhance the graft's in vivo remodelling
properties and to enhance the adhesion of the tissue graft
layers to one another. The perforations are believed to
promote contact of the submucosal tissue with endogenous
fluids and cells (by increasing the surface area of the
implanted graft) and the perforations also serve as a
conduit allowing extracellular fluid to pass through the
graft.
In accordance with the present invention the term
"perforate" designates a bore that extends through the
entire graft construct. However, tissue graft constructs
having "holes", defined herein as a cavity that penetrates
into the tissue but does not extend through the entire
graft construct, are also within the scope of the present
invention. The spacing and size of the perforations, as
well as the depth to which the perforations penetrate into
the tissue, will be varied according to the desired
mechanical strength, porosity, size and thickness (number
of layers) and other factors related to the medical
application of the tissue graft. The size of the
perforations range from 0.5 to 3 mm, more preferably from
0.6 to 2 mm. The perforations are spaced from one another
at a distance ranging from 2 to 20 mm, more preferably from
3 to 7 mm, and in one embodiment the perforations are
uniformly spaced from one another.
The perforations are formed in the submucosal
tissue while the tissue remains at least partially
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/05268
-20-
hydrated. In large area sheets or mufti-laminate
submucosal tissue constructs, comprising multiple strips of
submucosal tissue fused together, the perforations are
preferably made after formation of the large area
sheet/multi-laminate construct and when the tissue is has
been dried to a water content of approximately 10-20% by
weight water (10-20$ hydrated). Sufficient drying of the
tissue can be determined by weighing the fresh tissue and
drying the tissue to 10-20~ of the fresh weight or the
l0 sufficient drying can be determined by impedance
measurement as previously described. After perforation of
the tissue the submucosal tissue is subjected to terminal
sterilization and stored as described previously.
In one embodiment holes (extending only part way
through the tissue) or perforations can be formed on both
sides of the tissue graft. In addition the tissue can be
modified to include perforations as well as holes that
extend only part way through the tissue. Furthermore the
submucosal tissue can be modified to include a plurality of
holes, wherein various subsets of holes extend to different
depths into the tissue relative to the other formed holes.
This can be accomplished, for example, by perforating the
individual layers of submucosal tissue before overlapping
the .layers to form the mufti-laminate construct. If some
of the layers are not perforated or if the perforations of
the individual layers are not aligned, the formed multi-
laminate construct will have holes extending to different
depths into the tissue. Preferably the tissue is
perforated, in a uniform distribution over the surfaces of
the tissue graft, thus forming a series of bores that allow
fluid communication from a the first planar surface to a
second opposite planar surface of the graft construct.
In one embodiment the perforations are formed
perpendicular to the surface of the tissue graft construct,
i.e., the longitudinal axis of the perforation/hole forms a
90° angle with the plane defining the surface of the graft.
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/05268
-21-
Alternatively the perforations can be formed so that the
axis of perforation is not perpendicular to the surface of
the graft (i.e., so that a longitudinal axis parallel to
the wall defining the perforation/hole forms an angle other
than 90° with the plane of the graft surface). In
accordance with one embodiment the perforations are formed
at an angle ranging from 45° to 90° in reference to the
surface of the graft.
The perforation of submucosal tissue is
anticipated to have its greatest impact on mufti-laminate
submucosal graft constructs. Mufti-laminar tissue grafts
can be cut without unraveling and do not delaminate when
soaked in water for a period of time (greater than one
hour) that corresponds to the time required for implanting
the sheet in a host. However, mufti-laminate tissue
constructs tend to accumulate tissue fluid in cyst-like
pockets between adjacent laminae during the first 19-28
days after implantation in soft tissue locations (such as
the muscular body wall of rats). Perforations of the
mufti-laminate graft construct will alleviate the
accumulation of fluids between the layers of the multi-
laminar construct by providing a conduit through which the
fluid can flow out of the tissue. In addition the
perforations will have a "stapling" effect that will
augment the adhesion of the laminae to each other.
Accordingly, the placement of full thickness or
partial thickness holes in mufti-laminate tissue grafts
provide the following advantages over non-perforated multi-
laminate sheets:
1. Increased passage of fluids (including tissue
fluids) through the material; and
2. Increased adhesive force between adjacent layers.
The submucosal tissue can be perforated using a
wide variety of devices know to those skilled in the art.
The method utilized to perforate the submucosal tissue is
not critical provided the aggregate structural integrity of
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/05268
-22-
the submucosal tissue is maintained.
In preferred embodiments the perforation of the
submucosal tissue does not result in the removal of
significant amounts of tissue. For example, the
perforations are formed by pressing a pointed solid object
through the tissue to press the tissue aside during the
insertion of a solid object as opposed to boring out the
material. Other means for perforating the tissue include
the use of ballistics, cutting instruments, laser beams or
enzymatic or chemical treatments.
In one embodiment, the submucosal tissue is
perforated by pressing a pin or solid needle, into/through
the tissue. Typically a 20-23 gauge solid needle is used
to form the perforations. In this manner, no significant
amount tissue is removed during the process of forming the
perforations, but rather a portion of each layer is torn
and pushed into an adjacent layer to provide a stapling
effect. This "stapling" effect can be further enhanced by
forming a portion of the perforations from one side of the
graft and forming the remaining perforations from the
opposite side of the graft.
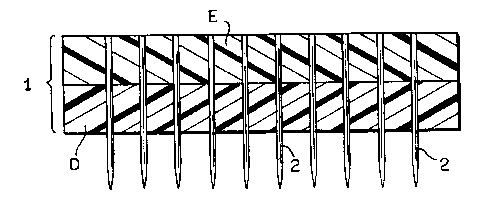
Fig. 4 depicts one embodiment of a device for
perforating the submucosal tissue graft constructs. The
device comprises a base (1) and a plurality of stainless
steel pins (2) embedded in base (1) and extending out
through the surface of the base. The base comprises Epoxy
(E) and Delrin (D) portions and has a length of 3.2 inches
(8.2 cm), a width of 1.85 inches (4.74 cm) and is 0.5
inches (1.3 cm) thick. The Epoxy and Delrin portions each
have a length of 3.2 inches (8.2 cm), a width of 1.85
inches (4.74 cm) and are 0.25 inches (0.64 cm) thick. In
accordance with this embodiment pins (2) are substantially
parallel to one another and form a 90° angle with the
surface of the base. Pins (2) are 0.040 inches (0.1 cm) in
diameter, are spaced 0.264 inches (0.67 cm) apart (center
to center of adjacent pins) within a 0.4 inches (1 cm)
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/05268
-23-
border from the edge of the device and protrude 0.25 inches
(0.69 cm). from the base. The device thus holds a total of
fifty pins.
Example 1
Submucosal tissue was prepared from vertebrate
intestinal tissue in accordance with the procedure
described in U.S. Patent No. 4,902,508. Strips of
submucosal tissue were formed from a segment of intestinal
tissue of a warm-blooded vertebrate, said segment
comprising the tunica submucosa delaminated from both the
tunica muscularis and at least the luminal portion of the
tunica mucosa of said segment of intestinal tissue. The
segment of intestinal tissue was cut along the longitudinal
axis of the segment and laid flat. The tissue was then
further sliced into a series of strips each having
generally parallel sides.
Multiple strips of submucosal tissue were
organized on a 12 by 12 inches (31 by 31 cm) perforated
stainless steel plate wherein a portion of one strip of
submucosal tissue overlaps a portion of the adjacent strip
of submucosal tissue. A second 12 by 12 inches (31 by 31
cm) perforated stainless steel plate was then placed on top
of the submucosal tissue. The perforated stainless steel
plates used,in this embodiment has 0.045 inches (0.11 cm)
perforations arranged straight center and located 0.066
inches (0.17 cm) apart. A 50-100 pound (22.7-45.4 kg)
weight was placed on top of the second stainless steel
plate and the tissue was compressed for 24 hours at room
temperature.
Example 2
Strips of submucosal tissue were prepared as
described in Example 1. Multiple strips of submucosal
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/05268
-29-
tissue were laid out between two perforated, stainless
steel plates so that a portion of one strip of submucosal
tissue overlapped a portion of the adjacent strip of
submucosal tissue. The "plate-submucosa-plate" apparatus
was placed on a flat surface and covered with blotting
material, to soak up water, and a breather blanket to allow
air flow. The apparatus was then sealed into a nylon bag
that has a vacuum port. A vacuum was applied (greater than
28 inches (71.8 cm) of Hg) to pull air out of the vacuum
bag and the resulting drop in atmospheric pressure
simultaneously compressed and dehydrated the submucosal
tissue. After 24 hours of applying a vacuum, the produced
sheet was moist and very flexible. No seams from the
layering of the submucosal tissue were visible and the
strength of a prototype 8-thickness sheet as determined by
ball burst test was 80 pounds (36.3 kg).
Example 3
Strips of submucosal tissue were prepared as
described in Example 1. The submucosal tissue strips were
organized on a mesh so that a portion of one strip of
submucosal tissue overlapped a portion of the adjacent
strip of submucosal tissue. Once the mesh was covered with
one layer of submucosal tissue a second layer of submucosal
tissue was applied on top of the first layer so that the
edges of the submucosal strips of the second layer were at
an angle relative to edges of the submucosal strips of the
first layer.
After all the strips of submucosal tissue were
placed on the mesh, another mesh was placed on top of the
submucosal tissue layers and the "mesh-submucosal tissue-
mesh" sandwich was compressed with a load and dried. This
process produced a dried large area submucosal sheet that
was pealed off the mesh as a unitary graft construct.
CA 02248212 1998-08-28
WO 97/37614 PCT/LTS97/05268
-25-
Example 4
Sterilization of Submucosal Tissue with Peracetic Acid
Submucosal tissue is soaked in a peracetic
acid/ethanol solution for 2 hours at room temperature using
a ratio of 20:1 (mls peracetic solution: grams submucosal
tissue) or greater. The peracetic acia/etnanol solution
comprises 4% ethanol, 0.1% (volume: volume) peracetic acid
and the remainder water. The 0.1% peracetic acid component
l0 is a dilution of a 35% peracetic acid stock solution
commercially available and defined as in table 1.
Preferably, the submucosal tissue is shaken on a rotator
while soaking in the peracetic acid solution. After two
hours, the peracetic acid solution is poured off and
replaced with an equivalent amount of lactated Ringer's
solution or phosphate buffered saline (PBS) and soaked
(with shaking) for 15 minutes. The submucosal tissue is
subjected to four more cycles of washing with lactated
Ringer's or PBS and then rinsed with sterile water for an
additional 15 minutes.
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/05268
-26-
Table 1: Chemical Composition of the 35~ Peracetic Acid
c~~ "+-; ~r
Composition, ~ by weight
Peracetic acid 35.5
Hydrogen peroxide 6,g
Acetic acid 39.3
Sulfuric acid 1.0
Water 17.4
Acetyl peroxide 0.0
Stabilizer 500 PPM
Typical active oxygen analysis, ~ by weight
Active Oxygen as peracid 7.47
Active Oxygen as H202 2.40
Total active oxygen 10.67
Sterilization of Submucosal Tissue with Ethylene Oxide
After preparation of the multi-laminate
constructs using sterile conditions, the material is
packaged and subjected to a second round of sterilization
(terminal sterilization). The tissue can be packaged in
plastic that is permeable to ethylene oxide and subjected
to ethylene sterilization according to procedures known to
those skilled in the art. Essentially the packaged
material is exposed to ethylene oxide for four hours at
115°F (46°C). During the sterilization the tissue is also
provided with 65~ relative humidity for at least 75 minutes
of the four-hour treatment. The high humidity enhances
uptake of the ethylene oxide by the tissue. After four
hours the ethylene oxide, ethylene chlorohydron and
ethylene glycol is flushed out with nitrogen and air.
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/05268
-27-
Example 5
Ball Burst Strength Test by Means of a Compression Cage and
a MTS Tensile Tester
The strength of multi-laminate submucosal tissue
grafts is determined through the use of a material testing
system (MTS) tensile tester. The multi-laminate tissue
construct is secured within a four sided frame clamp
(specimen clamp) to provide uniform distribution of the
stress through out the tissue construct. The initial
fixture level is set so that the top of the steel ball is
located immediately under the plane the test specimen. The
handle of the specimen clamp is lifted to its topmost
position so that the jaws of the clamp are able to accept
the test specimen. The submucosal tissue construct is cut
to fit the specimen clamp, the aperture of the clamp having
a diameter of one and three-quarter inches (1.9 cm). A
half-inches (1.3 cm) of excess material should be included
around the perimeter of the test specimen to ensure
sufficient clamping area. The submucosal tissue is placed
in jaws of the clamp and secured, the clamp force being
controlled by thumbwheel means located on the top clamp.
The clamped submucosal tissue is then pressed
down over a metal ball at a controlled rate utilizing a
tensile tester software interface to control and measure
the force placed on the test specimen. The force is
increased until failure of the specimen occurs. Failure is
defined as the maximum load which corresponds to the first
appearance of the ball through visible non-natural
discontinuities in the plane of the specimen. In the case
that the topmost position of the fixture is reached prior
to failure, the software limits will engage and discontinue
the test. The peak load value displayed on the
Microprofiler 458.01 is recorded and the specimen is
removed.
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/05268
-28-
Example 5
A multi-laminate tissue graft construct was
prepared as follows:
An ample amount of submucosal tissue is prepared
from vertebrate intestine, cut and flattened out and
disinfected with peracetic acid as described in Example 4
(approximately 70 grams of submucosal tissue is required
for a 10 cm x 15 cm device). Surgical gloves, face mask,
and cap should be worn after sterilization of the tissue
with peracetic acid to minimize the contamination from
organic matter and airborne particulate.
Strips of submucosal tissue are placed on top of
a first perforated stainless steel plate in the desired
orientation. The stainless steel plates used are
perforated stainless steel plates with 0.045 inches (0.11
cm) round perforations on straight centers and located
0.066 inches (0.17 cm) apart. After formation of a layer
of submucosal tissue the submucosal tissue is smoothed out
to remove air bubbles. Additional layers are overlaid
until the device is complete. Excess material is removed
from around the multi-laminate structure with a scissors.
The weight of the submucosal multi-laminate is recorded. A
second stainless steel plate (perforated with 0.045 inches
(0.11 cm) round perforations on straight centers located
0.066 inches (0.17 cm) apart) is placed on top of the
multi-laminate construct.
The multi-laminate construct can optionally be
"pinch rolled" to remove trapped air and water. To pinch
roll the material, the two perforated metal plates
surrounding the submucosal tissue are placed in-between two
polypropylene sheets (Kimberly Clark, class 100 "Crew
Wipe") and the entire apparatus is placed in-between two
layers of nylon bagging film (Zip Vac, Auburn WA) that are
larger than 1°x 1°. A weighted cylinder is then rolled
across the apparatus numerous times (at least three times).
CA 02248212 1998-08-28
WO 97/37614 PCTIUS97/05268
-29-
To perforate the mufti-laminate submucosal tissue
graft construct the apparatus is partially disassembled to
expose the top surface of the tissue graft and a piece of
nylon bagging film is placed directly on the top most layer
of submucosa tissue. The mufti-laminate submucosal tissue
graft construct is then inverted onto a Styrofoam work
surface, and the first stainless steel plate is carefully
removed. The exposed surface of submucosa is then covered
with a piece of nylon bagging film. The tissue graft
construct is then perforated, and then the top nylon
bagging film is removed. The mufti-laminate submucosal
tissue graft construct is then re-inverted and placed back
on the perforated stainless steel plate. The nylon bagging
film is removed from the submucosa top surface and a second
perforated stainless steel plate is placed on top of the
mufti-laminate submucosal tissue graft construct.
The mufti-laminate submucosal tissue graft
construct is then compressed under dehydrating conditions
as follows:
A layer of blotting material (NuGauze) larger
than the size of the perforated plates is placed on a table
top (or other smooth level surface). The stainless steel
plates with the mufti-laminate submucosal tissue graft
construct between them is placed on top of the blotting
material. Another layer of blotting material
(approximately the same size as the first sheet of blotting
material) is placed on top of the stainless steel plates.
A breather blanket (Zip Vac, Auburn, WA) is placed on top
of the blotting material. Preferably the breather blanket
is slightly larger than the objects it is covering.
Optionally electrodes can be placed in contact
with the submucosal tissue to allow the measurement of
impedance across the tissue. Typically the tissue is
compressed for a sufficient time to produce a stiff but
flexible material. Sufficient dehydration of the tissue is
indicated by a increase in impedance of electrical current
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/05268
-30-
flowing through the tissue. When impedance has increased
by 100-200 ohms, the tissue is sufficiently dehydrated and
the pressure can be released.
A border of chromate tape is placed on the table
top around the apparatus and the area to be vacuum pressed.
The backing is removed from the tape and a piece of the
nylon bagging film that already has the nozzle port
attached to it is placed on top of the area enclosed by die
chromate tape (see Figures 3a & 3b) and adhered to the
tape. The heating blanket, if used is turned on, and
vacuum pump is turned on. The bag should be checked for
wrinkles (smooth them out if found) and for an inadequate
seal between the chromate tape and the nylon bagging film
(correct if found). A vacuum should be drawn to a level
ranging from 25 to 30 psi"a~- After vacuuming to the
desired hydration level (approximately 24 hours), the seal
of the bag is broken at a taped region, the vacuum pump is
turned off and the unitary, perforated mufti-laminate
submucosal tissue graft construct is removed. A pair of
scissors can be used to cut off any portion of the tissue
graft that did not receive the complete amount of overlap.
Example 7
The submucosal tissue graft construct can also be
perforated after formation of the unitary mufti-laminate
construct as follows. The mufti-laminate construct is
formed in accordance with Example 3. The mesh/submucosal
tissue sandwich was removed from the drying apparatus and
the tissue was perforated. The graft was perforated by
inserting a nail between the mesh of the wire and pushing
the nail through the tissue at multiple points on the graft
surface. The perforated mufti-laminate submucosal tissue
was then cut square (4 1/2 x 4 1/2 in.) and marked for
identification purposes.
CA 02248212 1998-08-28
WO 97137614 PCT/US97/05268
-31-
Example 8
A perforated pseudoisolaminate construct was
prepared as follows: Strips of submucosal tissue were
arranged in 9 layers on a wire mesh. The first layer was
laid directly on the mesh and the remaining three layers
were overlaid on top of the first layer at an angles of 45°,
90° and 135° relative to the first layer, respectively (see
Fig. 2a-c). A second mesh was placed on top of the
submucosal tissue, and the tissue was sandwiched between
the mesh and c-clamped to the drying rack. A fan was
placed in front of the rack and turned on. Holes were
punched through the tissue in a checkerboard pattern using
the mesh as a guide. (i.e., every alternate space in the
mesh was used to perforate the tissue. Accordingly the
pattern appeared as follows:
X X X
X X
X X X
X X
The perforation of the tissue was stopped before
completion because the submucosal tissue was being
disrupted. Therefore the submucosal tissue was dried for
25 min. with the fan on high. The remaining perforations
were then made in the tissue in accordance with the
original pattern.
The sheet was allowed to dry overnight, removed,
cut square, and labelled for identification.
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/05268
-32-
Example 9
Comparison of the Strength of Perforated and Non-perforated
Submucosal Tissue
Eight layered pseudoisotropic multi-laminate
tissue graft constructs were prepared in accordance with
the present invention. The constructs were perforated
(uniformly) and the strength of these constructs was
compared to non-perforated constructs using the ball burst
test described in Example 5. Three separate experiments
were conducted and the force applied at failure was
recorded (in pounds).
a) The perforated construct comprised 1.0 mm
perforations uniformly spaced at 6.71 mm. Four non-
perforated constructs and for perforated constructs were
tested and the mean values were determined. The non-
perforated construct failed at 94.11 ~ 7 pounds (42.7
kg) whereas the perforated construct failed at 83.572 ~ 6
pounds (37.9 kg).
b) The perforated construct comprised 1.0 mm
perforations uniformly spaced at 6.71 mm. Four non-
perforated constructs and four perforated constructs were
tested and the mean values were determined. The non-
perforated construct failed at 73.71 ~ 9 pounds (33.4
kg) whereas the perforated construct failed at 62.35 ~ 2
pounds ( 2 8 . 3 kg ) .
c) Two perforated constructs were compare in this
experiment with the non-perforated control: the first
perforated construct having 1.0 mm perforations uniformly
spaced at 6.71 mm and the second having 1.0 mm perforations
uniformly spaced at 3.35 mm. Two non-perforated
constructs, two of the first perforated construct and seven
of the second perforated construct were tested and the mean
values were determined. The non-perforated construct
failed at 70.17 ~ 12 pounds (31.8 kg) whereas the first
CA 02248212 1998-08-28
WO 97/37614 PCTIUS97/05268
-33-
perforated construct failed at 79.94 ~ 8 pounds (36.3 kg)
and the second construct at 62.39 ~ 7 pounds (28.3 kg).
Comparison of Perforated and Non-perforated Submucosal
Tissue as Tissue Graft Constructs
In the following example mufti-laminate tissue
graft constructs comprising eight layers were prepared in
both perforated and non-perforated form. The perforated
constructs were perforated using the device depicted in
Fig. 4. The resulting perforated tissue constructs had
0.40" diameter bores regularly spaced intervals (6.7 mm
apart ) .
The study conducted utilized 24 rats. The rats
were divided into two groups of 12 each. In the first
group, an 8 layered, non-perforated mufti-laminate sheet of
submucosal tissue was implanted. In the second group, an 8
layered, perforated mufti-laminate sheet was implanted.
The 12 rats in each group were further subdivided into
smaller groups that differed only in the method of terminal
sterilization.
1. Subgroup #1 in each major group had no terminal
sterilization performed on the final device.
2. Subgroup #2 had 2.5 Mrad gamma irradiation applied to
the device as a terminal sterilization method.
3. Subgroup #3 had 1.5 Mrad gamma irradiation applied to
the final device as a terminal sterilization method.
4. Subgroup #4 had 1.5 Mrad e-beam irradiation terminal
sterilization method to the final device.
5. Subgroup #5 had 1.5 ethylene oxide (conducted at
Purdue University) applied to the final device as a
terminal sterilization method.
6. Subgroup #6 had 1.5 ethylene oxide (performed at
Centurion Labs) applied to the final device as a terminal
sterilization method.
Results of these studies (excluding terminal
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/05268
-34-
sterilization as a variable) showed that perforation
significantly decreased the fluid accumulation between the
multi-laminate sheets during the remodeling period. One
animal was sacrificed from each subgroup at 14 days and the
second animal was sacrificed from each subgroup at 28 days.
At 14 days, in the animals receiving the tissue grafts
lacking perforations, numerous "cysts" of serosanguinous
fluid had accumulated between the multi-laminate sheets and
around the graft. In the animals receiving the perforated
tissue grafts, the amount of fluid accumulation was
significantly less, both in terms of the number of cysts
and the size of cysts.
By 28 days, there was virtually no evidence for
fluid accumulation in the graft of the group receiving the
perforated tissue grafts whereas the group receiving the
non-perforated tissue grafts still had small pockets of
fluid present.
Accordingly, perforating the submucosal tissue
before implantation into the host has a significant effect
upon healing and remodeling. Perforations appeared to
serve as a conduit through which fluid could flow through
the entire graft rather than accumulate between sheets. In
addition, there was no visible separation of the layers of
the perforated multi-laminate tissue grafts which was at
least in part attributed to the perforations.
Example 10
Comparison of Submucosal Tissue, Dexon~, and Marlex~ as
tissue graft constructs for use in hernia repair.
The effectiveness of submucosal tissue, Dexon~
and Marlex~ as tissue graft constructs will be analyzed in
two separate animal studies. Study No. I utilizes a dog
model and Study No. 2 utilizes a rat model.
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/0526$
-35-
Study No.I - Dog Model
Thirty dogs are randomly divided into three
groups of ten dogs each. A full-thickness body wall defect
is created in the ventral lateral abdominal wall of each
dog. The defect measures 5 cm x 5 cm (W x L) and leaves
the peritoneum intact. The defect will be created lateral
to the midline, left side, and involves primarily the
abdominal aponeurosis. The lateral portion of the defect
reaches the distal portion of the abdominal skeletal muscle
layers. The defect site is repaired with one of the three
devices: small intestinal submucosa, Dexon~, or Marlex~
mesh. Ten animals are utilized in each group (i.e., ten
animals are implanted with one of the three devices). Two
animals from each group are sacrificed at each of the
following times: one week, one month, three months, six
months, and two years past implantation. The endpoint of
the study is the morphology (both macroscopic and
microscopic) of the graft materials and surrounding tissue
at the time of sacrifice.
Study No. 2 - Rat Model
The experimental design for the rat study is
identical to that described above for the dog study with
one exception: there are thirty animals in each group and
six rats are sacrificed from each group at each timepoint.
The endpoint of the study will be the same; that is, the
macroscopic and microscopic appearance of the graft
material and surrounding tissue at the time of sacrifice.
Specimen Preparation
The hernia repair devices will be prepared as
follows:
1. Intestinal submucosal tissue: the submucosal tissue
graft constructs for implantation will be prepared as
described in Example 3. Raw material will be tested
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/05268
-36-
using an axial burst test. Only lots with a mean
burst force at 3.0 lbs or greater will be used in
fabrication. The configuration parameters for the
device include the following:
a) The strips of submucosal tissue will be
overlapped (500 overlap) with adjacent strips to form a two
layered large area sheet of submucosal tissue.
b) A second sheet of submucosal tissue is
formed and layered on the first sheet at 45° angle relative
to the first sheet of submucosal tissue
c) A third sheet of submucosal tissue is formed
and layered on the first sheet at a 45° angle relative to
the second sheet
d) A forth sheet of submucosal tissue is formed
and layered on the first sheet of submucosal tissue
relative to the third sheet
Thus a homolaminate construct comprising eight
layers of intestinal submucosal tissue (delaminated from
the tunica muscularis and the luminal portion of the tunica
mucosa of a vertebrate species) is prepared. The construct
is essentially rectangular in shape having a length width
of l0cm x l5cm. The graft construct is pinch rolled to
remove air and water from between the laminate layers and
the tissue is perforated using a 20-gauge solid needles.
The perforations are evenly spaced at 6-7 mm apart. The
construct is compressed under dehydrating conditions and
sterilized with ethylene oxide.
2. Dexon~: material use for hernia repair will be
obtained through Owens & Minor of Indianapolis.
3. Marlex~: material use for hernia repair will be
obtained through Owens & Minor of Indianapolis.
The physical properties of the submucosal tissue grafts
will be characterized as follows: Five mufti-laminar
tissue graft constructs will be prepared as described in
this example. The graft constructs will first be tested
for sterility and pyrogens, respectively by NAmSA
CA 02248212 1998-08-28
WO 97137614 PCT/US97/05268
-37-
(Northwood, OH). One graft construct from the batch of
devices will be ball burst tested as described in Example
7. One construct will be stored as a reserve sample for
archival. Three of the graft constructs will be
implemented. Each graft construct will be identified by
manufacturing date and graft construct number. For Dexon~
and Marlex~, the devices will be characterized by lot
number and manufacturing date as given on the device label.
The dogs used in this study will be purchased
from LBL Kennels and the rats used in this study will be
purchased from Harlan Sprague Dawley, Inc.
Surgical Procedure
Dogs: Each animal weighs between 18 and 25
kg. Each animal will be anesthetized with intravenous
thiopental sodium, intubated, and maintained on inhalation
anesthesia with isoflurane and oxygen. Under a surgical
plane anesthesia, the surgical site will be clipped and
scrubbed. The surgical site will be located at least 3 cm
lateral (left) to the midline, and will be in a caudal
position such that the site includes only distal fibers of
the rectus abdominous muscle.
A longitudinal skin incision will be made with
dissection of the subcutaneous tissue being performed to
expose an area which measures 5 cm x 5 cm in dimensions. A
full-thickness defect will be created in the abdominal wall
removing all tissues except the skin, subcutis, and
peritoneum. The peritoneum and overlying transversalis
fascia will be left intact.
The defect site will be repaired with either the
submucosal tissue construct, the Dexon~ hernia repair
device, or the Marlex~ mesh hernia repair device. The
defect site will be filled with a section of either of
these devices that is equal in size to the defect. The
devices will be sutured to the adjacent normal body wall
tissues with 2-0 prolene suture material. The overlying
CA 02248212 1998-08-28
WO 97/37614 PCT/US97/05268
-38-
subcutaneous tissue will be closed following placement of a
penrose drain which will exit the skin adjacent to the
suture line.
Rats: Each animal will be anesthetized with an
intraperitoneal injection of pentobarbital sodium (90
mg/kg) followed by inhalation (nose cone) of methoxyflurane
and oxygen as needed to maintain a surgical plane of
anesthesia. The surgical procedure will be identical to
that described above for the dog with the following
exception: the defect site will measure 1.5 cm x 1.5 cm in
dimensions. The location of the defect will be in the same
relative location as described for the dog study; on the
ventral lateral abdominal wall. The suture used to secure
the repair devices in place will be 2-0 prolene.