Note: Descriptions are shown in the official language in which they were submitted.
CA 02249272 l998-09-l8
W O 97/35201 PCT~L97/00105
MULTl-ANTIGEN SEROLOGlCAL DL~GNOSIS
FIELD OF THE INVE:NTION
The present invention relates generally to medical diagnostic testing, and
specifically to appal dl~ls and methods for detection of antibodies in serum samples.
BACKGROUND OF THE INVENTION
Methods and apparatus for immunoassay diagnostic tests are well known in the
art, for example in diagnosing viral infections, auto-immlme disorders and otherpathologies and conditions, such as preg~ancy. These tests are based on antibody-
antigen interactions, wherein a serum sample taken from a subject is applied to an
antigen, such as a particular protein, which reacts with a specific type of antibody in the
serum that recognizes and responds to the antigen. The reacted antibody is typically
bound to a labeling substance, whose concenl~ation is then detected in order to measure
the level of the specific antibody in the serum sample to be assayed. Labeling substances
known in the art include, inter alia, radioisotopes (for radioimmunoassay), conjugated
enzyrnes (for ELISA - enzyme-linked immunoassay), and fluorescent labels.
Immunoassay tests must frequently be performed on a serum sample for more
than one type of antigen. Generally in these cases the test is performed separately for
each specific antigen-antibody reaction, for example, for a single strain of influenza virus.
The test must then be repeated for each different antigen of interest.
Double antigen testing, wherein a different label is used for each antigen, is also
known in the art. Such testing is useful for direct detection of antigens, but is not
practical for antibody detection. In this method, antibodies of two different types, each
type labeled with a different, respective fluorescent, isotopic or other marker, are added
to a sample containing antigens. The concentrations of the two types of antigens are
detected, for example by observing fluorescence in two different respective colors using
a spectrometer. This method, however, is practically impossible to implement when
more than two antigens are to be detected.
CA 02249272 1998-09-18
W O 97/3S201 PCT/IL97/00105
A number of methods have been developed for pe~ g multiple single-
antigen assays together on a given serum sample. The ~ntig~n~ in these assays are
p.ererably bound to solid-phase substrates, for more convenient h~n-lling Such assays
are p~,ro.l..ed, for example, using the EL312e reader and EL404 washer made by Bio-
Tek Instrument~, Inc., of Winsooki, Vermont, USA, or the MAGIA series of instruments
made by E. Merck Diagnostica, Darmstadt, Gel...any. Test kits using these instruments
typically include a test substrate having multiple plastic wells, wherein a dirrerenl antigen
to be tested for is bound to each of the wells. Up to 96 wells, each with a din'e.enl
antigen, may be used on a single substrate.
Alternatively, plastic or m~gnetic beads may be used to bind the antigens, as
described, for example, by D. Leahy, et al., in Transfusion 32 (1992), pages 548-553,
which is incorporated herein by reference. As described in this article, the beads are of
uniform size, and all the beads in a given test tube or test cell are bound to antigens of a
single type.
Petts, et al., in Eur. J. Clin. Microbiol. Infect. Dis. 7 (1988), pages 34-39, and
Hadfield, et al., in Journal of Tmmllnolo~ical Methods 97 (1987), pages 153-158,describe other diagnostic techniques, in which two or more di~le.ll ~ntigens are bound
to beads of lesl~ec~ e, di~rellt colors. When antibodies in a sample react with the
anti~nc~ the beads to which these antigens are bound form a dense p-ecipilale. The
color of the precipitate is thus indicative of the type of antibodies in the sample. This
type of test is useful in q~ itative assays, for example, in identifying which single type of
a group of types of antibodies is present in the sample, but it is not generally practical for
multiple-antibody diagnosis or for qu~ ;./e determinations.
Fluorescçnce-activated cell sorting (FACS) devices and methods are well known
in the art. FACS is performed using flow cytometry methods and appa-~ s, such as the
FACStar PLUSTM farnily of instruments, m~mlf~ctllred by Becton Dickinson
Immlmocytometry Systems, of San Jose, California. In flow cytometry, cells in a stream
of fluid are made to pass through a laser beam, which causes them to fluorescently emit
and/or scatter light. The intensity of this emitted or scattered light signal from each
individual cell is analyzed. In FACS, this signal is then used to cause an electrical
charge, dependent on the signal, to be applied to a droplet of fluid cont~ining the cell. A
CA 02249272 1998-09-18
W O 97/35201 PCT/IL97/00105
system of electrical deflection plates dow,l~l,e&"~ causes the droplets to be sorted and
collected according to the signals they produced.
FACS is cGnlmonly used to sort or analyze cells on the basis of their surface
anti~p-~c~ by first treating them with an antibody that has been conju~ted to a fluorescent
probe molecule. In order to test and calibrate the FACS appa.~L~Is, beads of subst~ntiPI~y
the same size as cells are inserted into the app7 al~ls for proces~ing as described by
Dulling and W~ldsçhm:~t in ISAC XVI Cytometry Suppl~mçnt 6 (1993), page 47; by
Bell and Shenton, in ISAC XVII Cytometry Supplement 7 (1994), page 38; and by
Mathai, et al., in ISAC XVII Cytometry Supplement 7 (1994), page 38, which are
incorporated herein by reference.
Additionally, the use of beads/particles for serological analysis has been described
in the European Patent Application No. 93110399.8, titled "ImmllnQassay Using
Miclopa~licles Co~ Different Detectable Substances", applicant Becton, Dickson
& Company, and inventors T. J. Mercolino, J.H. E~cl~mp and E.C. McFarland, filedJune 30,1993 and published in Bulletin 94/01, Publication No. 0577092A2 on January 5,
1994. This patent application describes a method for quantifying the amount of complex
antigen/antibody used in serological diagnosis. In this method described, particles are
used as carriers of the colored tracer and at the same time as markers for the antigen.
Furthermore, the review articles "New Developments in Particle-Based
Immunoassay: Introduction", by L.B. Bangs and published in Pure and Applied
Chemistry, Vol. 68(10) pp. 1873-9, 1996, and "Diagnostic Application of
Microspheres", by L.B. Bangs, published in Liquid- and Surface-Borne Particle
Measurement Handbook, Chapter 15, pp. 687-707, Editors J.Z. Knapp, T.A. Barber and
A. Liebe-ll.an, published by Marcell Dekker, Inc., New York, describe the use ofbeads/particles in multi-antigen diagnosis. The review articles suggest the use of multi-
antigen diagnosis for both protein diagnostics and nucleic acid diagnostics.
CA 02249272 1998-09-18
W O 97135201 PCT~L97/00105
SUMMARY OF THE INVENTION
It is an object of the present invention to provide a convenient method of
assaying multiple antibodies sim~llt~neoucly in a single sample.
In one aspect of the present invention, the multiple antibody assay is performedusing methods of flow cytometry.
It is a further object of the present invention to provide appa,al.ls for assaying
multiple antibodies sim~lt~neously in a single sample and a method for controlling
thereof.
In one aspect of the present invention, this appal ~ s operates in conjunction with
a flow cytometry system.
Yet another object of the present invention is to provide a test kit for pl epal~lion
of a sample for assaying antibodies for multiple antigens simnlt~neously.
In p,t;~lled embod;...~ .Is of the present invention, a multi-antigen test kit
c~",l"ises a multiplicity of beads, having a plurality of sizes and/or shapes and/or colors,
wherein the sizes and/or shapes and/or colors are chosen so that the beads may be sorted
according to their lespec~ e shapes, sizes and/or colors. Pre~ly the sizes of the
beads are on the order of the size of cells, and they are sorted by fluorescence-activated
cell sorting (FACS).
The term "beads" in the context of the present invention is taken to mean
min~ )re pellets or particles, which may be of any desired shape. In one pre~"edembodiment of the present invention, the beads are sul,slalllially spherical.
Additionally, throughout the specification and claims of the present patent
application, -the term "color" refers to the broader me~nin~ of wavclen~,lh, the intensity
of spectral emissions, absorbances and excitations.
P~ef~lably the test kit inchldes a plurality of di~lenl types of antigens, wherein
each antigen type is associated with a respeclive size, shape and/or color, and wherein
the specific antigen is bound to all beads in the kit having substantially the same size,
shape and/or color. It will be appreciated that the beads are many times larger than the
~nti~en.~ and the antibodies reacting therewith, so that the sizes and shapes of the beads
remain substantially l-nch~nged during use of the test kit.
CA 02249272 1998-09-lX
W O 97/35201 PCT/IL97/00105
Furthermore, in plefe-led embodiments of the present invention, the multiplicityof antigen-bound beads are mixed with a serum sample, causing specific antibodies
present in the sample to bond to corresponding specific ~nti~çn~ The multiplicity of
beads to which the sarnple has been applied are then developed, using methods known in
the art, so that a fluoresce.-l-labeled molecule is conju~Pted or bound to each of the
bonded antibodies. The developed beads are passed through flow cytometry apparatus,
which sorts the beads according to their sizes and/or shapes and/or color and analyzes
the fluo~escent light signal received from each of the beads. It will be apprecialed that in
this manner, the beads are sorted into groups, each group corresponding to a di~nt
~nti~en~ whereby the fluoresce--L light signals received from each group are indicative of
the prese.-ce and concentration level of the specific antibody in the sample associated
with the single specific Pnti~Pn
In accordance with p~ere.,ed embodiments of the present invention, the flow
cytometry appa~al-ls is adapted to provide a data readout that includes sim..lt~neous
assays of the levels of all the specific antibodies in the sample for which specific antigens
were provided. The flow cytometry appa.al~ls preferably applies calibration standards
and thresholds, as are known in the art, to the analysis of the data. ~l~rer~bly the data
output in~.ludes a list of pathologies, disorders and/or other medical conditions, wherein
each of the listed pathologies, disorders and conditions is followed by an indication of its
p,esence or absence, and/or by a measure of the concentration of antibodies in the
sample associated with the pathology, disorder or condition. The data output mayinclude a histogram or other statistical analysis.
In prefelled embodiments of the present invention, the test kit, flow cytometry
appal~ s and method are suitable for assaying viral agents, such as HIV, hepatitis,
herpes, infln~on7~ and other agents known in the art.
Additionally or alternatively, the test kit, flow cytometry appa~ s and method
are suitable for diagnosing autoimmllne disorders, such as systemic lupus erythematosus,
or my~heni~ gravis, or, in still other ~)lere..~d embodiments, for analyzing physiological
conditions, such as pregnancy, cancer, respiratory virologies, infections endangering
pregnant women, kidney disorders, liver disorders, as well as pathology, epidemiology.
CA 02249272 l998-09-l8
W O 97/35201 PCT/IL97/0010
There is therefore provided, in accordance with a plèrelled embodiment of the
present invention, a test kit for multi-antigen serological diagnosis in~ din~. a plurality of
bead groups, each group incl~din~ at least one bead; and a plurality of types of ~ntigen~,
wherein each bead group has at least one common identifying physical characteristic, and
herein each type of antigen is bound to the at least one bead of one, respective, bead
group.
Frefel~bly the at least one identifying physical characteristic includes bead size or
bead shape or bead color, and the largest dimension of each bead is between 0.5 and 10
micrometers. Preferably at least some of the beads are subst~nti~lly spherical.
Preferably the plurality of groups of beads inc3ndes at least three groups of beads,
and the plurality of types of antigens int~ des at least three dirre~ ~"l, respective, types of
~ntigen~.
Preferably at least one of the plurality of types of antigens is adapted for assaying
anti-viral antibodies. Additionally or alternatively, preferably at least one of the plurality
of types of ~ntig~nS is adapted for cletectin~ autoimm~lne disorders, cancer markers,
respiratory virologies, infections endangeriilg pregnant women, pathology, epidemiology,
kidney disorders, liver disorders.
There is further provided, in accordance with a prerelled embodiment of the
present invention, app&~alus for multi-antigen serological diagnosis of a serum sample,
inclu~ling a test kit as described above; and a sorter, adapted to sort the beads according
to their le;,~eclh/e, identifying, physical characteristics.
Plc;rel~bly the sorter sorts the beads accolding to their sizes and/or shapes and/or
color.
Preferably the sorter includes fluorescence-activated cell sorting appa,~ s, which
sorts the beads according to fluorescent light emission thereflo",. Preferably the sorter
further sorts the beads accordhlg to a physical characteristic indicative of presence or
substantial absence of predetermined antibodies on the beads.
Pl e~l ably the appa- al~ls inrludes a processor, which provides an output
responsive to the presence or substantial absence of predetermined types of antibodies in
the sample; a display, which displays inrol~l,alion relating to the sample based on said
CA 02249272 1998-09-18
W O 97135201 PCT/IL97/00105
output; and a printer, which prints inforrnation relating to the sample based on said
output.
Preferably the appa~alLIs also in~ludes a plepalalion unit, which prepares the test
kit for sorting. Preferably the prepd~lion unit includes an applicator which applies the
sample to the test kit, and a developer which develops a predetermined marker onto at
least some of the beads that have antibodies reacted with the ~ntigçn~ thereon.
There is also provided, in accordance with a pl efe. ~ td embodiment of the present
invention, a method for assaying multiple types of antibodies in a serum sample,i~cl~tlin~ the following steps:
providing a plurality of beads;
binding a plurality of types of antigens to the beads;
applying a serum sample to the beads;
developing the beads with a marker;
sorting the developed beads, so as to generate a bead count;
classifying the levels of one or more types of antibodies in the sample using the
bead count.
Preferably providing a plurality of beads includes providing a plurality of groups
of beads, all beads in each group having a common identifying physical characteristic.
Flere-ably each type of antigen is bound to subst~nti~lly all the beads in one, respective,
group of beads. ~1 ~re. ably beads from di~- e-~l groups are mixed according to the types
of antibodies to be assayed, and beads from at least three di~renl groups are mixed.
Preferably developing the beads includes conjugating a fluorescent marker to at
least some of the antibodies that have reacted with antig~n~ bound to the beads.Preferably a plurality of dirre.~nl fluo.escenl markers, respectively, are conjugated to a
plurality of d;rre~ enl types of antibodies.
P~ bly sorting the beads incl~ldes sorting the beads by size, shape and/or
color.
~ -t;re.~bly sorting the developed beads further includes:
irr~di~tin~ the beads with laser radiation;
receiving light signals from the beads;
identifying the beads according to the light signals received thel eL ~1.l; and
. . , . ~
CA 02249272 l998-09-l8
W O 97/35201 PCT/IL97/00105
counting beads from which subst~r~ti~lly similar light signals were received.
Preferably receiving light signals from the beads includes receiving scattered light
signals andlor receiving fluoresccnl emission light signals.
~ re-. bly classifying the levels of one or more types of antibodies in the sample
includes applying thresholds to the bead count.
Preferably classifying the levels of one or more types of antibodies in the sample
further includes dete~ ,l ing the plcs~,nce or substantial absence of the one or more types
of antibodies and/or determining the respecLi~re concentrations of the one or more types
of antibodies.
Plere,ably classifying the levels of one or more types of antibodies in the sample
includes classifying the levels of anti-viral antibodies and/or of antibodies indicative of
autoimm~lne disorders, cancer markers, respiratory virologies, infections en-l~n~ering
pregnant women, pathology, epidemiology, kidney disorders, liver disorders.
CA 02249272 1998-09-18
W O 97/35201 PCTtlL97/00105
BRIEF DESCRIPT~ON OF THE DRAWINGS
The present invention will be more fully understood from the following detailed
description of the prcrc,,cd embo~ s thereof, taken together with the drawings in
which:
Fig. 1 is schematic illustration of appa,a~us for analyzing antibody levels in aserum sample, in accordance with a prefe.led embodiment of the present invention;
Fig. 2 is a sçl-e.n~;c illustration of a test plepalalion unit for plepa,~lion of
samples for analysis, in accoldance with the ~cfie~lcd embodiment of the presentinvention shown in Fig. l;
Fig. 3A is a schematic illustration of beads coated with specific antigens, in
accordance with a prcrcl, cd embodiment of the present invention;
Fig. 3B is a scl~n~;c illustration ofthe beads of Fig. 3A, mixed together in a test
kit, in accordance with a prerc~ I ed embodiment of the present invention;
Fig. 3C is a sçl-.-....~l;c illustration of the test kit of Fig. 3B, after application of
the serum sample, in accordance with a plefc,-cd embodiment of the present invention;
Fig. 3D is a scl-.,~ l;c illustration of color development of the test kit and selum
sample of Fig. 3C, in accoldance with a p~cfe~led embodiment ofthe present invention;
Fig. 4 is a sc.hc...~;c illustration of a sorter for sorting beads and receivingfluorescence signals from s~l,ples for analysis, in accordance with a plefel,cd
embodiment of the present invention shown in Fig. I;
Fig. 5 is a table, represçntin~ schçmqtisqlly sample results of the operation of thê
reading unit;
Fig. 6 is a schematic illustration of an analysis and display unit for analysis and
presentation of data, in accordance ~,vith a prerc. I cd embodiment of the present invention
shown in Fig. 1; and
Fig. 7 is a schematic illustration of a Sample output of the Analysis and Display
unit
CA 02249272 1998-09-18
W O 9~/35201 PCT/IL97/00105
DETAILl :D DESCRI~TION OF PREFI :RRED EMBODIMENTS
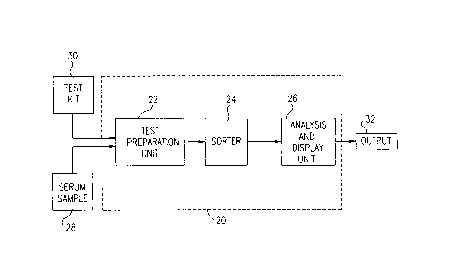
Fig. 1 illustrates sc~ ;ç~lly appal allls 20 for multi-antigen serological
diagnosis on a single sample, in accordance with a prerelled embodiment of the present
invention. Apparatus 20 preferably comprises a test plepal~lion unit 22, a sorter 24 and
an analysis and display unit 26. As will be described below in greater detail, test
plepal~lion unit 22 receives a serum sarnple 28 and a suitably pr~paled test kit 30. The
test kit and serum colll~ lion is processed as illustrated in Fig. 1 and in subsequent
figures, and outputs test results 32. These results preferably colllpl;se data relating to
the levels of specific antibodies detected in ser~m sample 28.
Fig. 2 schçn~tic~lly illustrates test plepalalion unit 22, which preferably
comprises a mixer 40, for prepal;ng test kit 30; applicator 42, for applying serum sample
28 to the prepared test kit; and developer 44, for color development of the test kit after
application of the serum sample. Mixer 40, applicator 42 and developer 44 are
preferably automated laboratory devices of types known in the art, and preferably
operate under the control of a robot 46, likewise of a type known in the art.
Alternatively, some or all ofthe operations oftest prepalalion unit 22 may be pt;lr~lllled
m~ml~lly by a laboratory technician. After these operations are completed, the test kit
with serum sample is ready for 11 ~nsrè~ to sorter 24.
Figs. 3A-3D illustrate more clearly processes executed by test prcpal~lion unit
22. As shown in Fig. 3A, test kit 30 conlplises a multiplicity of beads 50, 52, 54, having
a plurality of d;lrel elll sizes, and grouped initially according to these sizes. Preferably the
beads have dimensions in the range 0.5 to 10 micrometers and are made of plastic, for
example polypropylene, polystyrene, or latex, as described in the references cited above,
or other polymers or particles known in the art. It will be understood that although, in
accordance with one prèfelled embodiment of the present invention, beads 50, 52 and 54
shown in Figs. 3A-3D are spherical, in other prerelled embodiments of the present
invention, the beads may have di~elenl shapes and colors, and are grouped and sorted
according to their shape or size or color or combination thereof.
A first type of antigen 56 is bound, using methods known in the art, to
substantially all beads 50, which are all of the same size. Similarly, a second type of
antigen 58 is bound to subst~nti~lly all beads 52, and a third type of antigen 60 is bound
CA 02249272 1998-09-18
W O 97/35201 PCT/IL97/00105
to subst~nti~lly all beads 54. It will be understood that although for clarity of
explanation, the figures show only three diffè~ sizes and col.esponding ~ntig~nc, in
p~ .ed embodiments of the present invention, the number of di~elenl sizes and shapes
and colors can be much larger. Thus, in pl ~1 l ed embodiments of the present invention,
test kit 30 can include tens or hundreds of di~' enl types of ~ntigenS, each type bound to
beads of a dilrel e..l size and/or shape and/or color.
As shown in Fig. 3B, the beads of di~ere.ll sizes from test kit 30 are mixed
together by mixer 40 (shown in Fig. 2) in a single test cell 64, and the serum sample 28
and the set of mixed beads 50, 52, 54, are applied to the applicator 42. It will be
appreciated that each of antigenS 56, 58 and 60 of the serum sample 28 is adapted to
react with a corresponding specific antibody 66, 68 or 70, respectively, which may be
present in serum sample 28. Preferably beads 50, 52 and 54 are selected and mixed
together in p-ephl~tion for analysis, based on the specific antibodies whose levels are to
be analyzed. Alternatively, test cell 64 may be prepared by the m~nuf~ctllrer with test kit
30 in pre-mixed form.
Referring now to Fig. 3C, it will be seen that when serum sample 28iS applied byapplicator 42 (shown in Fig. 2) to the contents of test kit 30, antibodies 66 react with
ntigellc 56 on beads 50, and antibodies 68 react with ~nti~çnc 58 on beads 52. In this
t,~a---~le, however, antibodies 70 are not present in the serum sample, and therefore,
antigens 60 on beads 54 remain unreacted.
Finally, as shown in Fig. 3D, developer 44 (shown in Fig. 2) biochemically
develops the kit to which serum sample 28 has been applied, using methods and materials
known in the art, wherein reacted antibodies are conjugated to fluoresc.".L dye markers,
for example FITC (fluo~esce.n) or rhodamine. Thus, after development, beads having
antibody-reacted antigens, such as antigen 56, Will produce fluorescence of a
predett;llll;ned color when ilhlmin~te~l by suitable laser light, while beads having non-
reacted ~ntig~onc of the same type, such as antigen 56A, Will not provide fluorescence of
this color. Based on the difference in fluorescence, beads of substantially the same size,
such as beads 50 and S0A, are ~ictin~lich~hle by color as to whether antibody 66 has
reacted with an antigen 56 thereon. Distin~lishin~ these beads 50 and 50A would
otherwise be very difficult, because antigens 56,58 and 60 and antibodies 66,68 and 70
11
CA 02249272 1998-09-18
W O 97~5201 PCT/IL97/00105
are typically smaller than the beads by several orders of magnitude. Antigens 56, 58 and
60 are shown to be co,npa~le in size to the beads in Figs. 3A-3D for demonstrative
simplicity only.
.Althollgh generally all reacted antibodies are marked with the same color, in
some p,efellcd embodiments of the present invention, dil~renl types of reacted
antibodies are marked with dilre,e,-l color dyes. These embodin~nts are useful when
more than one type of antibody may react with a given ~ntig~on, for example antibodies
IgG, IgM and IgE, as are known in the art. In this case the dil~re"L colors associated
lespe~ ely with the dilrerenl antibodies are used to determine their respective
concentrations in the sample.
Fig. 4 sch~m~tic~lly illustrates sorter 24, which sorts the colored beads 50, 52and 54 following development by developer 44. In the plerelled embodiment of thepresent invention shown in Fig. 4, sorter 24 incl~ldes a fluorescence-activated cell sorting
~FACS) system, such as the FACStar PLUS system, m~n~lf~ctured by Becton Dickinson,
or any other type of FACS system known in the art. In this system, beads 50, 52 and 54
are mixed with a sheath fluid 72 and expelled through a nozzle 74, which directs them
through a laser beam 75, pl~r~l~bly in the 400-700 nm range of wavelen~h produced by
a laser 76. As each bead 50, 52, 54 passes through the laser beam, the amount and
pattern of light that the beads 50, 52, 54 scatter is measured by a scattering detector 77.
This measurement is used by processor 79 to determine the size and/or shape and/or
color of the bead, using methods known in the art, and thus to sort the beads by size
and/or shape and/or color. Since each di~l ~lll size or shape bead is bound to a di~el elll
specific antigen, dete,l.l;nil-g the beads' sizes allows sorting of the di~lenl types of
antigens.
Furthermore, when laser beam 75 strikes a bead, conventional fluorescent marker
chemicals on the bead emit a fluorescent light signal, which is detected by fluorescence
detector 78. The level of this signal is proportional to the number of antibodies that have
reacted with ~ntig~n~ on the bead. Thus, the fluo~escen~ signal received from a bead of a
given size, corresponding to a specific antigen, is responsive to the concentration level of
the specific antibody in sample 28 corresponding to the antigen. This signal is used by
processor 79 to determine the concenll ~Lion of antibodies on the bead.
12
CA 02249272 1998-09-18
W O 97/35201 PCT/IL97/OOlOS
In a conventional FACS system, the FACS system is calibrated using beads of
predetermined known sizes and shapes. Such calibration of FACS systems using beads is
known in the art for the purposes of calibrating the systems to sort biological cells of
comparable size and/or shape.
In a prerelled embodiment of the present invention, the identity of an antigen,
bound to a bead of known size, shape, and color (wavelength and brightness) is
determined in real time. The method for controlling the identifis~tion of the antigen is
pt;, Ço..,.ed in real time from the size, shape and intensity of the 5 out of 6 colors read by
the FACS system. The sixth color, FLl, of the antigen is used for deter...inillg the
antibody binding.
In the real time antigen identification method, a set of beads of known size, shape
and color, and with various FLI int.oncities~ preferably di~erent from the size, shape, and
color of the antigens to be identified, are introduced into the apparatus. Using this group
of the beads, the (0,0,0) coordinates of the matrix, which describes the parameters of the
antigen are determined. The brightness and intensity of the scattered laser signal received
are depen~ent on the ratio of ~nti~o.n~/antibodies in the sample. The pa.an.elers, namely
size, shape, and color, of the antibody are determined from the fluorescent signal emitted
by the fluor~scent chemical marker. From these measured data, the size, shape, and color
of the antigen are dt:lellllined These pal~"elers allow the identific~tion of the antigen in
real time.
By using substantially similar beads in all test kits, it is possible to standardize the
method of identifying an antigen in real time.
P,eîeiably the calibration incl~ldes detelll il~ g one or more threshold values of
size and color. During sorting of the processed test kits, the predetermined threshold
values are applied to data received by processor 79, in order to produce an output
indicative of the levels of antibodies in the sample. This output may be in the form of a
bargraph 80, as shown schematically in Fig. 5.
Referring now to the sample results shown in Fig. 5, it is noted that one bead of
"size l" emitted a signal with a color "(+)," indicating that antibody 66 has reacted with
antigen 56 on bead 50, as shown in Fig. 3D. However, since no antibodies have reacted
with antigens on other beads of "size l," such as antigen 56A on bead 50A, two beads of
13
, . . . .. . . ... .. ..
CA 02249272 1998-09-18
W O 97/35201 PCT/IL97/00105
"size l" were thus found to have emitted signals of color "(-)," inrlic~ting that the
antigen-antibody reaction has not occurred. The histogram values for beads 52 and
beads 54, listed respectively as "size 2" and "size 3" in Fig. 5, are similarly derived.
It will be appreciated that although Fig. S shows only two values of color, (+)
and (-), COl l esponding to whether the fluorescent signals received are greater or less than
a given threshold value, in other pre~.ed ~mbo~l;.ue.~l~ of the invention, multiple
thresholds may be set, corresponding to multiple dif~-e~l levels of fluorescenl emission.
The output data from sorter 24 are then analyzed and displayed by analysis and
display unit 26, shown sc~ ically in Fig. 6. Unit 26 p~eft.ably comprises output data
processor 90, which receives data from processor 79 (shown in Fig. 4). Processor 90
pools the data received regarding the sizes of the beads, the shapes of the beads, and the
color of the beads, and m~tçhes the output data with corresponding specific antigen-
antibody reactions. The fluorescent signal data are then classified in accordance with
predetermined standards for classifying antigen-antibody reactions, as are known in the
art. These standards may be preset by the m~mlf~cturer of the appa.~ s, or,
alternatively, they may be set by the user in accordance with specific test requ;le...enls.
Typically, the standards are used to classify the antibody level collea~)onding to each
specific antigen as zero, high or low, although other standards classifications may
alternatively be used. Classification results 32 are output to display 92 and printer 94,
for eAa-.-ple, in the form of tables and/or graphs.
Fig. 7 shows a typical output table lOO, in accordance with a pr~ ed
embodiment of the present invention, col..p.ising classification results 32 for samples
taken from five pati~nts and tested for five di~re.ll viruses. As illustrated by Fig. 7, in
plefe--ed embodiments of the present invention, the test kit, flow cytometry apparatus
and diagnostic methods executed thereby are adapted for assaying viral agents, such as
HIV, hepatitis, herpes, influçn7~ and other agents known in the art.
In other prefel.ed embodiments of the present invention, the test kit, flow
cytometry apparatus and diagnostic methods executed thereby are adapted for
diagnosing autoimmune disorders, such as systemic lupus erythematosus, or myasthenia
gravis, or, in still other piel~lled embodiments, for analyzing physiological conditions,
such as pregnancy such as pl egnancy, cancer, res~ lol y virologies, infections
14
CA 02249272 1998-09-18
W O 97/35201 PCT/IL97/00105
end~n~ering pregnant women, kidney disorders, liver disorders, as well as pathology,
epidemiology.
The di~nostic methods described here may be executed using any suitable
circuitry and/or software incorporated, inter alia, in processor 79 and processor 90.
It will be appreciated that the p~,ft,-ed embodiments described above are cited
by way of example, and the full scope of the invention is limited only by the following
claims: