Note: Descriptions are shown in the official language in which they were submitted.
-09-28
BOEHRINGER MANNHEIM GMBH ~.
4397/OA/
New oxazolidine derivatives, proces~es for their
production and pharmaceutical agents containing these
compounds
It is known that compounds which carry a basic and an
acidic group are able to inhibit blood platelet
aggregation when there is a very particular distance
between the basic and acidic group (Drugs of the Future
19(2):135-159 (1994). Compounds with an anti-aggregatory
effect on blood platelets are described in the patent
documents WO 93/14077, EP-A-0 537-980, EP-A-0 542 363,
WO 94/22834, WO 94/22835 and EP 0623615A1.
The present invention concerns new oxazolidine
derivatives, processes for their production as well as
pharmaceutical agents containing these substances.
It has now been found that oxazolidine derivatives
effectively inhibit the aggregation of blood platelets
and can thus be used to treat diseases which can be
attributed to thromboembolic events such as stroke,
myocardial infarction or occlusive arterial diseases as
well as inflammations, osteoporosis or tumour diseases.
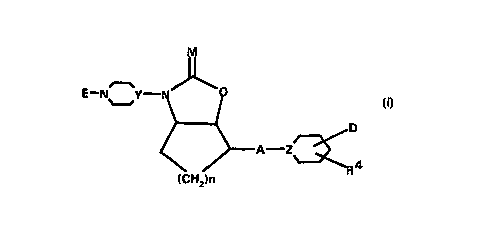
The present invention concerns compounds of the general
formula I
. , ~
CA 022~02~0 1998-09-28
E--N~_Y N ~
~ A--Z~ (I)
(CH2)n
in which
E denotes a residue of formula (a) or (b)
N~ R 10 N~
(a) (b)
M denotes oxygen, sulphur or NR00,
X denotes hydrogen or NRlR2,
W denotes nitrogen or NH or CH or CH2,
Q denotes nitrogen or CH,
Y denotes nitrogen or CH,
Z denotes nitrogen, CH or C-OH,
A denotes an alkylene chain -(CH2)p- which is
optionally substituted,
D denotes a side chain of the form -(CHR3)m-Coo-R8 or
=CR3-C~~-R3 ~
n denotes 1-3,
m denotes 0 or 1
p denotes 0-3
Rl, R2 denote independently of one another hydrogen,
lower alkyl, aryl, arylalkyl, hetaryl, acyl or an
optionally substituted carb~cyclic or heterocyclic ring
or, together with the nitrogen to which they are bound,
form an optionally substituted five-membered or six-
CA 022~02~0 1998-09-28
membered ring which can contain a further 1 to 3
heteroatoms, or denote a group (c)
RO
(c)
--C = NH
R3 denotes hydrogen or a group -oR5 or -NR6R7
R4 denotes hydrogen, lower alkyl, aryl, arylalkyl,
hetaryl or a group -oR5,
R5 denotes hydrogen, lower alkyl, aryl or arylalkyl,
R6 denotes hydrogen, lower alkyl or arylalkyl,
R7 denotes hydrogen, lower alkyl, arylalkyl, acyl,
alkylsulfonyl or arylsulfonyl,
R8 denotes hydrogen, methyl, ethyl, isopropyl, tert.-
butyl, phenyl or benzyl, in particular hydrogen,
ethyl, phenyl or isopropyl,
R10 denotes hydrogen, lower alkyl, arylalkyl, acyl,
alkylsulfonyl, arylsulfonyl or a group (c),
RO denotes hydrogen, lower alkyl, arylalkyl or a
group -NHROO~
ROO denotes hydrogen, lower alkyl, arylalkyl, acyl,
alkylsulfonyl or arylsulfonyl,
as well as their pharmacologically acceptable salts.
Lower alkyl should in all cases represent a straight-
chained or branched Cl-C6 alkyl group such as e.g.
methyl, ethyl, propyl, isopropyl, butyl, isobutyl,
pentyl or hexyl in particular methyl, ethyl, propyl,
isobutyl and pentyl.
CA 022~02~0 1998-09-28
Aryl usually denotes a phenyl residue optionally
substituted once or several-fold.
Hetaryl usually denotes an unsubstituted or once or
several-fold substituted pyridyl, pyrimidyl, piperazyl,
imidazolyl, pyrrolyl, furyl or thiophenyl residue
preferably a pyridyl, pyrimidyl, indolyl or imidazolyl
residue.
Arylalkyl usually denotes an unsubstituted or a once or
several-fold substituted benzyl, phenethyl,
phenylpropyl, phenylbutyl or phenylpentyl residue
preferably a benzyl, phenethyl or phenylpentyl residue.
C1-C6 alkyl residues, preferably methyl, ethyl or
isopropyl as well as chlorine, bromine, fluorine or
hydroxy, methoxy, benzyloxy, acetyloxy, carboxy,
ethoxycarbonyl, aminocarbonyl, methylaminocarbonyl,
dimethylamino-carbonyl, cyano, amino, methylamino,
dimethylamino, benzylamino, acetylamino, benzoylamino
and amidino groups come into consideration as
substituents.
Acyl usually denotes a formyl, acetyl, propionyl,
butyryl or benzoyl residue in particular an acetyl or
benzoyl residue.
Alkylsulfonyl usually denotes a methanesulfonyl,
ethanesulfonyl, propanesulfonyl or butanesulfonyl
residue, in particular a butanesulfonyl residue.
Arylsulfonyl usually denotes a benzenesulfonic acid or
toluenesulfonic acid residue.
CA 022~02~0 1998-09-28
A carbocyclic ring usually denotes a saturated or
unsaturated 5-6-membered ring optionally substituted
once or twice by lower alkyl such as a cyclopentyl,
cyclohexyl, cyclopentenyl or cyclohexenyl ring.
A heterocyclic ring usually denotes a saturated or
unsaturated 5-6-membered ring optionally substituted
once or twice by lower alkyl such as a pyrolidine,
piperidine, piperazine, morpholine, tetrahydro-
pyrimidine, dihydropyridine or dihydroimidazole ring
preferably a piperidine or tetrahydropyrimidine ring.
In the case that the residues Rl and R2 together with
the nitrogen to which they are bound form a five-
membered or six-membered ring this is a saturated or
unsaturated 5-6-membered ring optionally substituted
once or twice by lower alkyl such as a pyrrolidine,
piperidine, piperazine, morpholine, tetrahydro-
pyrimidine, dihydropyridine or dihydroimidazole ring
preferably a piperidine, pyrrolidine or tetrahydro-
pyrimidine ring.
The heterocyclic ring of formula (a) usually represents
a pyridine, pyridazine or pyrimidine ring in particular
a pyridine or pyrimidine ring.
A heterocyclic ring of formula (b) usually represents a
piperidine or hexahydropyrimidine ring in particular a
piperidine ring.
Compounds of the general formula I contain at least one
asymmetric carbon atom and;therefore optically active
compounds of the general formula I are also a subject
matter of the present application. Conformation isomers
CA 022~02~0 1998-09-28
of compounds of the general formula I which may occur
are an additional subject matter of the present
application.
Preferred compounds are compounds of formula I in which
n=1-2, p=O-1 and E, D, Y, Z and R4 have the given
meanings.
Compounds of formula I are particularly preferred in
which D denotes a group -CooR5, n=2, p=O, and Y=CH, Z
denotes nitrogen, M denotes oxygen and E denotes a
substituted or unsubstituted pyridine, pyrimidine or
piperidine ring.
Compounds of the general formula I in which R8 denotes
hydrogen are produced by known processes by hydrolysing
an ester of the general formula I in which R8~H.
Compounds of the general formula I in which R8~H can be
produced according to the reaction path outlined in
scheme 1.
CA 02250250 1998-09-28
Scheme 1
A- Z~_
(CH2)n (V) R4 MN3 or
TMSN3
N3 '~H ~
A--Z~ 4 (XXVI)
(CH2)n R
H 2/P d /C or
Ph3P/H20
E'N--\ H2~ OH
\_, Y ' N H2
(IV) ~ ~-- ~ 4
(CH2)n ~ R
_~y_ ~ ~ H O H ~ _ y~\N - E ( XVI )
iA Z~
cyclization
synthone
"M=C ~h (Il)
E--N~
-~ ~
(CH2)n R4 (1)
CA 022~i02~i0 1998-09-28
Scheme 1
In scheme 1 R4, A, D, E, M, Y, Z and n have the above-
mentioned meanings. As a rule cyclization synthone "M=C++"
denotes phosgene, diphosgene, triphosgene, carbonyl-
diimidazole, carbonic acid dimethyldiethyl or diphenyl
ester, chloroformic acid methyl or ethyl ester,
thiophosgene, thiocarbonyldiimidazole, carbon disulphide,
alkylisonitrile, N,N-dimethylformamide, bromocyanogen or
chlorocyanogen, (N-acyl or N-sulfonyl)-dithiocarbamic acid
dimethyl ester, dialkylcarbonyl diimide, 1-amidino-3,5-
pyrazole-nitrate in particular carbonyldiimidazole,
carbonic acid diethyl ester, chloroformic acid ethyl ester,
thiocarbonyl diimidazole or bromocyanogen. MN3 denotes a
metal azide such as lithium, sodium, potassium, tributyltin
or magnesium azide in particular lithium or sodium azide.
TMSN3 is an abbreviation for trimethylsilylazide.
Compounds of the general formula IV in which E denotes the
formula (a) can be produced according to the reaction paths
outlined in scheme 2.
Scheme 2 ~ ,, HN~N - B21
IXIIII bl Pd/C/H2
3 ~HN~eO
N~, _~ N N--H
X~= X~ (XlV
N" ~N~}OH N" ~N~=O
~XVIIII IXVII N-NO2/
11 ~' / H2NBlr/ HCI
~51 t-u~obu
~orldltlonJ x~ ~
~=w ~\ N~ N~}N~H N~ _~N N--N=O
N" _~N~N I ~ IXVIII / Bzl ~ IXVI
IXIXI O ~ d~
~ l ,,/
N =~N y--N
~w H IIVI
c) hydrolysis of the ph~h~ ; d) hydrogenation of the benzyl groups;
e) reduction of the nitroso group
CA 02250250 1998-09-28
Scheme 2
In scheme 2 X, Q, W and Y have the above-mentioned
meanings; as a rule L denotes a leaving group such as
chlorine, bromine, iodine, mesylate, triflate or
tosylate, in particular chlorine or tosylate.
Compounds of the general formula IV in which E denotes
the formula (b) can be produced by ring hydrogenation of
compounds of formula XVI or XVIII.
Compounds of the general formula V can be prepared by
the reaction paths outlined in scheme 3.
C) ~ D
~\ A~ /'='\ ~2 R4 (Vl)
( C H21 n b
(CH2)n (Vlll)
~ I X~ C
~R4 ~ A~
(CH2)n ~V) A. 4
(CH2)n (X) R
a) ~rnYi~tion; b) metallo-organic reaction; c) dehydroxylation
~.
CA 022~02~0 1998-09-28
-- 10 --
Scheme 3
In scheme 3 R4, A, D, L and n have the above-mentioned
meanings.
Some of the compounds of the general formula VI are
commercially available and can be obtained in special
cases by oxidizing an alcohol of the general formula XI
~ D
HO ~ (Xl)
~ 4
in which D and R4 have the above-mentioned meanings.
Compounds of the general formula VII can be produced by
reacting a compound of the general formula VI with a
metallo-organic compound of the general formula XX
prepared from a compound of the general formula VIII
,~CH2)n
~< (XX)
A - M
in which A and n have the above-mentioned meanings and M
denotes a metal such as lit;hium, magnesium or titanium.
CA 02250250 1998-09-28
-- 11 --
Compounds of the general formula XXV (scheme 1) in which
R8~H, p=O and Z=N can also be produced by the reaction
paths outlined in scheme 4.
CA 02250250 l998-09-28
-- 12 --
Scheme 4
NH2 L 1 ~
(CH~ ~ (CH2~ d) ~ (CH2~O
\/ tlX)
(Vlll)
( XXVI I ) J ~D
H~N O 1~~H
(CH~ ~ r ~R4
\ (CH2)n
(XXVIII) (XXXI)
d) MN3 or
~ ~ TMSN3
o~R11
'N~O N3 OH
,1\ ~ r ~R4
(CH2)n ~0 (CH2)n
~ (XXVI)
(X~X) H21PdlC or
D Ph3P/H20
11HN~~R4
o o ~ (Xll)
H-N OH H21\ OH
~ ~ ~ N~ (XXV)
(XXX)
a) potassium phthalimide; b) hydrolysis; c) acylation;
d) peroxy acid; e) deacylation
CA 022~02~0 1998-09-28
- 13 -
Scheme 4
In scheme 4 R4, D, MN3, TMSN3 and n have the above-
mentioned meanings. As a rule L1 denotes a hydroxyl or
acetyloxy group or has one of the meanings of L. As a
rule Rll denotes a methyl, ethyl, tert.-butyl, phenyl or
benzyl residue in particular a tert.-butyl or benzyl
residue.
In the case of the compounds of the general formula VIII
these are commercially available cycloalkenyl
halogenides or alcohols if p=0. If p>0 compounds of the
formula VIII are known in the literature or can be
produced according to processes described there (Brinker
U.H., Tetrahedron Lett., 1991, 4461-4464; Atkinson P.H.,
J. Chem. Soc. Perkin Trans 1, 1977, 230-238; M~ller E.,
Chem. Ber. 108, 1401-1412 (1975); Walton J.C., J. Chem.
Soc. Perkin Trans 2, 1986, 1641-1646; Balme G.,
Tetrahedron 48, 3891-3902 (1992); Fieser et al., J.
Amer. Chem. Soc., 70, 3174-3196 (1948); Lee G.M., J.
oerg. Chem., 55, 1281-1285 (1990)).
Compounds of the general formula XII are as a rule
commercially available pipecolic carboxylic acid
derivatives; in special cases compounds of the general
formula XII can be produced by reacting a commercially
available 3-piperidone or 4-piperidone of formula XXI
~ 0
HN~3 (XXI)
with a commercially available acetic acid ester of the
CA 022~02~0 1998-09-28
general formula XXII,
R800C-CH2-R3 (XXII)
in which R3 and R8 have the above-mentioned meanings or
with a Wittig reagent of the general formula XXIII
Hal~ g
R800C--(CHR3)m p--R9 (XXIII)
\R9
in which R3, R8 and m have the above-mentioned meanings,
R9 denotes butyl, phenyl or p-tolyl and Hal~ denotes
chloride, bromide or iodide.
Some of the compounds of the general formula XI are
commercially available and can in special cases be
obtained according to known methods by ring
hydrogenation of an arylcarboxylic acid of the general
formula XXIV
~ ~ D
in which R4 and D have the ~eanings stated above.
CA 022~02~0 1998-09-28
Compounds of the general formula XX can be synthesized
in situ according to general methods for the production
of metallo-organic compounds.
Some Wittig reagents of the formula XXIII are
commercially available and can be prepared from the
corresponding commercial halogen compounds and
triphosphines.
Hydrolysis of an ester of the general formula I to form
the corresponding carboxylic acid of the general formula
I is usually carried out according to standard methods
in which a carboxylic acid ester of the general formula
I in water or in a mixture of water, tetrahydrofuran,
dioxane, methanol or ethanol preferably in a
water/tetrahydrofuran mixture is treated at temperatures
between room temperature and 80~C, preferably at room
temperature with a hydroxide such as sodium, potassium
or lithium hydroxide preferably sodium or lithium
hydroxide or with an acid such as hydrochloric acid,
sulphuric acid or trifluoroacetic acid preferably
trifluoroacetic acid.
As a rule the reaction of a compound of the general
formula XIII with 1-benzylpiperazine or 4-hydroxy-
piperidine or 4-oxopiperidine (scheme 2) or the reaction
of a compound of formula IX with a compound of formula
XII (scheme 3) is carried out in an aprotic solvent such
as toluene, tetrahydrofuran, diethyl ether,
dimethylformamide or methylene chloride preferably
dimethyl formamide or tetrahydrofuran using a base such
as potassium hydride, sodium hydride, potassium
carbonate or sodium hydrogbn carbonate preferably sodium
hydride or potassium carbonate and at temperature
CA 022~02~0 1998-09-28
-- 16 --
between room temperatures and 180~C preferably at 120~C
or room temperature.
The reaction between the 3-piperidone or 4-piperidone of
formula XXI and an ester of formula XXII usually takes
place under the conditions of an aldol reaction in a
solvent such methanol, ethanol, toluene, tetrahydro-
furan, diethyl ether or dimethylformamide, preferably
tetrahydrofuran or dimethylformamide using a base such
as sodium methylate or potassium methylate or potassium
ethylate, sodium hydride, potassium hydride, lithium
diisopropylamide, potassium hexamethyldisilazide
preferably sodium hydride or lithium diisopropylamide
and at temperatures between -78~C and 90~C preferably,
however, at -78~C and room temperature.
The benzyl protecting groups are removed if necessary by
catalytic hydrogenation such as e.g. by
palladium/carbon/hydrogen.
The Mitsunobu reaction between a compound of formula
XVIII and phthalimide is carried out according to
methods known in the literature tMitsunobu O.,
Synthesis, page 1 (1981)).
The reductive amination of a ketone of formula XVI with
dibenzylamine or an amine of formula XXV is carried out
according to methods known in the literature by reacting
the ketone and amine component in a solvent such as
methanol or ethanol in the presence of a reducing agent
such as sodium cyanoborohydride or sodium triacetate
borohydride with addition of a Bronsted or Lewis acid
such as hydrochloric acid, acetic acid, titanium tetra-
chloride or titanium tetraisopropylate and at a
CA 022~02~0 1998-09-28
temperature between 0~C and 100~C preferably at room
temperature or in the presence of a hydrogenation
catalyst such as platinum dioxide and a hydrogen
atmosphere (Borch R.F., Org. Synth. Coll. Vol. 6, 499
(1988); Heinzelman R.V.Z. Chem. 8, 270 (1968); Mattson
R.J., J. Org. Chem. 55, 2552 (1990); Barney C.L. Tetr.
Letters 31, 5547 (1990); Hutchins R.O., J. Org. Chem.
46, 3571 (1981)).
The nitrosation of a compound of the general formula XIV
to form a compound of formula XV is usually carried out
with sodium nitrite or isoamyl nitrite in water or
ethanol with addition of an acid such as hydrochloric
acid or acetic acid and at a temperature between -20~C
and 80~C preferably at room temperature.
A nitroso compound of the general formula XV is reduced
according to known methods by reacting a compound of
formula XV in a solvent such as water, acetic acid,
ethanol, tetrahydrofuran or diethyl ether preferably
acetic acid or tetrahydrofuran with a reducing agent
such as elemental zinc, lithium aluminium hydride or
sodium aluminium hydride preferably elemental zinc or
lithium aluminium hydride and at a temperature between
room temperature and 120~C preferably, however, at 70~C.
A compound of the general formula XV can also be
converted into a compound of formula IV by a
hydrogenolytic process using a catalyst such as
palladium/carbon (Hatt, H.H., Org. Synth. Coll. Vol. 2,
211 (1943); Schuler F.W., J. Amer. Chem. Soc. 73, 4996
(1951).
The oxidation of an alcohol of the general formula XI to
form a ketone of the general formula VI is carried out
CA 022502~0 1998-09-28
- 18 -
according to known methods such as the Jones oxidation
(Jones E.R.H., J. Chem. Soc. 36 (1946)), the Swern
oxidation (Swern D. Tetrahedron 34, 1651 (1978), the
Dess-Martin oxidation (Dess D.B., Martin J. C., J. Org.
Chem. 48, 4155 (1983) or using a bromo-urotropin complex
as the oxidation agent (Yavari I., J. Chem. Res. (S) 274
(1994).
The Wittig reagents used are optionally produced
analogously to methods known in the literature (Buddras
J., "Angew. Chem." 80, 535 (1968); Bestmann H.J. "Angew.
Chem. 77, 620, 651 (1965); Wittig G. Ber. Deutsch.Chem.
Ges. 88, 1654 (1955)).
The Wittig reaction is carried out according to known
methods by reflux heating the reactants in an aprotic
solvent such as benzene, toluene or xylene preferably
toluene.
As a rule the phthalimide hydrolysis is carried out
according to known methods by treating the phthalimide
with hydrazine hydrate or a semi-concentrated mineral
acid such as hydrochloric acid or sulphuric acid
preferably with hydrazine hydrate or hydrochloric acid
at room temperature.
The acylation of amines with an acylating agent is
carried out as a rule in a solvent such as methylene
chloride, dimethylformamide or pyridine preferably
methylene chloride or pyridine with addition of an
auxiliary base such as triethylamine or 4-dimethylamino-
pyridine and at a temperature between -10~C and 50~C
preferably, however, at room temperature. Carboxylic
acid halogenides such as acetyl chloride, propionide
CA 022~02~0 l998-09-28
-- 19 --
bromide or benzyloxycarbonyl chloride or carboxylic acid
anhydrides such as acetic anhydride or di-tert.-butyl
dicarbonate come into consideration as an acylating
agent but acetic anhydride, benzyloxycarbonyl chloride
or di-tert.-butyl dicarbonate are preferably used.
The epoxidation of an olefin of formula VII or of
formula X or of formula VIII or of formula XXVIII is
carried out according to methods known in the literature
by reacting them with a peracid such as m-chloroper-
benzoic acid, peracetic acid or trifluoroperacetic acid
preferably m-chloroperbenzoic acid in an aprotic solvent
such as methylene chloride and at a temperature between
-30~C and 50~C preferably at room temperature; in
addition the olefins listed above can be converted into
the corresponding epoxides by means of the Sharpless
epoxidation (Sharpless K.B., Org. Syntheses, Vol. 63, 66
(1985)).
As a rule the metallo-organic reaction in scheme 3 is a
Grignard reaction which is carried out according to
methods known in the literature. However, the magnesium
reagent of formula XX can optionally be converted into a
lithium or titanium reagent before it is reacted with a
carbonyl compound of formula VI (Reetz M.T., Chem. Ber.
118, 1421 (1985)).
The conversion of an aminoalcohol of formula III into a
compound of formula I (scheme 1) is carried out
according to methods known in the literature by reacting
an aminoalcohol of formula III with diethylcarbonate
(Evans D.A., Org. Syntheses, Vol. 68, 77 (1989)) or
carbonyldiimidazole (Chadwick D.I., J. Chem. Soc. Perkin
Trans 481 (1984); Geffken D. Arch. Pharm. 313, 817
CA 022~02~0 1998-09-28
- 20 -
(1980)) or phosgene (Newman W.S., J. Am. Chem. Soc. 73,
4199 (1951)) or diphosgene or triphosgene (Hassner A.,
Synth. Commun. 23, 2839 (1993)) or chloroformic acid
methyl, ethyl or benzyl ester (Kanoshinzo, J. Org. Chem.
53, 3865 (1988)) or thiophosgene (Dubey S.K., Can. J.
Chem., 61 565, (1983)) or thiocarbonyldiimidazole
(Goering B.K., Tetrahedron Lett. 35 (38), 6997, (1994))
or carbon disulphide (Zinner H., J. Prakt. Chem., 15, 72
(1962)) or bromocyanogen (Mousseron, Bull. Soc. Chim.
Fr., 737 (1953)) or alkylisonitrile (Ito Y., J.
Organomet. Chem. 131 121 (1977)) or (N-acyl or N-
sulfonyl)-dithiocarbamic acid dimethyl ester
(Bretschneider H., Monatsh. Chem. 103, 1377 (1972);
Evers R., J. Prakt. Chem., 333, 699 (1991)) or 1-
amidino-3,5-pyrazole-nitrate (Fotsch C.H., Tetrahedron
Lett., 35, 2481 (1994) in a solvent such as methylene
chloride, dimethylformamide, toluene, xylene, ethanol,
dioxane, tetrahydrofuran, water or diethyl-ether,
preferably dimethylformamide, methylene chloride,
ethanol or tetrahydrofuran optionally with the addition
of an auxiliary base such as triethylamine or pyridine
and at a temperature between -50~C and 80~C preferably
at room temperature.
The catalytic hydrogenation of a compound of formula
XXIV is carried out in a solvent such as methanol or
ethanol with addition of a catalyst such as ruthenium
oxide, rhodium oxide or palladium/strontium carbonate
preferably rhodium oxide in a hydrogen atmosphere at a
pressure of 1-200 bar preferably at 200 bar and a
temperature between room temperature and 200~C (Rastin
R.H., I. Chem. Soc. 1855 (1949)).
The epoxide opening of an epoxide of formula V with an
amine of formula IV (scheme 1) or of an epoxide of
CA 022~02~0 1998-09-28
- 21 -
formula IX with an amine of formula XII (scheme 4) is
usually carried out in a solvent such as methanol,
ethanol, dimethylformamide or toluene preferably ethanol
or toluene and at a temperature between 0~C and 120~C
preferably 80~C.
The epoxide opening of an epoxide of formula V (scheme
1) with a metal azide is carried out according to
methods known in the literature by reacting an epoxide
of formula V with a metal azide such as lithium, sodium,
potassium, tributyltin or magnesium azide, preferably
sodium azide, in a solvent such as methanol, ethanol,
1,4-dioxane, water, dimethylformamide, tetrahydrofuran,
acetonitrile or hexamethylphosphorotriamide or in
mixtures of the said solvents but preferably in
methanol, dimethylformamide or 1,4-dioxane-water
mixtures and at a reaction temperature between -10~C and
120~C preferably 80~C (Vanderverf C.A., J. Am. Chem.Soc.
76, 1231 (1954); Saito S., Tetrahedron Lett. 30, 4153
(1989); Hudlicky T., J.Chem.Soc. Perkin Trans. I, 2907
(1991)). As a rule an epoxide of formula V is reacted
with trimethylsilyl-azide in a solvent such as methanol,
tetrahydrofuran, methylene chloride, chloroform,
dichloroethane or benzene, preferably tetrahydrofuran or
methylene chloride, without further additives or using
additives such as titanium tetraisopropylate, aluminium
triisopropylate, dichlorotitanium diisopropylate or
diethylaluminium fluoride, preferably titanium
tetraisopropylate or aluminium triisopropylate, and at a
temperature between 0~C and 100~C but preferably at room
temperature (Emziane M., Synthesis, p. 541 (1988); Saito
S., Tetrahedron Lett. 26, 5309 (1985); Blandy C.,
Tetrahedron Lett. 24, 4189 (1983); Jung M.E., J. Org.
Chem., 56, 2614 (1991)).
CA 022~02~0 l998-09-28
- 22 -
The reaction of a compound of formula XXXI with a metal
azide to form a compound of formula XXVI (scheme 4) is a
nucleophilic substitution in the case that Ll has the
meaning of L which is carried out according to standard
methods of organic synthesis.
The deacylation of a compound of formula XXX to form a
compound of formula XXV is carried out according to
standard methods in which a compound of formula XXX in
water or in a mixture of water, tetrahydrofuran,
dioxane, methanol or ethanol, preferably in a
water/tetrahydrofuran mixture, is treated with a
hydroxide such as sodium, potassium or lithium
hydroxide, preferably sodium or lithium hydroxide, or
with an acid such as hydrochloric acid, sulphuric acid
or trifluoroacetic acid, preferably trifluoroacetic
acid, or with palladium/carbon/hydrogen and at
temperatures between room temperature and 80 ~ C
preferably at room temperature.
The conversion of an azide of formula XXVI into an amine
of formula XXV is carried out according to known
methods: Suami T., Bull.Chem.Soc. Jpn., 51, 855 (1978);
(Boullanger P., Bull.Soc.Chim.Fr., p. 2149 (1973;
Ackerman K., Can.J.Chem., 50, 3886 (1972); Hanessian S.,
Chem.Ind., p. 1296 (1965); Horner L., Liebigs Ann.Chem.,
591, 117 (1955); Koziara A. Synthesis, p. 487 (1987);
Vogel E., Ang.Chem. Int.Ed.Engl., 18, 962 (1979);
Purwono B., Synlett, 3, 231 (1992).
Compounds of formula I contain one or several chiral
centres and can therefore be present in a racemic or in
an optically-active form. Racemates can be resolved
mechanically or chemically by known methods into the
CA 022~02~0 1998-09-28
-- 23 --
enantiomers. Preferably diastereomers are formed from
the racemic mixture by reaction with an optically-active
acid such as the D and L forms of tartaric acid,
diacetyltartaric acid, dibenzoyltartaric acid, mandelic
acid, malic acid, lactic acid or the various optically-
active camphorsulfonic acids such as ~-camphorsulfonic
acid.
It is of course also possible to obtain optically-active
compounds of formula I using the methods described above
by using starting materials (e.g. those of formula V or
VIII) which are already optically-active.
Within the sense of the present invention all prodrug
forms of compounds of the general formula I are also
claimed but especially carboxylic acid esters of the
general formula I in which R8 denotes a methyl, ethyl,
n-propyl, isopropyl, butyl, phenyl or benzyl residue in
particular a methyl, ethyl or benzyl residue.
Alkali salts, ammonium salts, trifluoroacetates or
hydrochlorides are used above all as pharmacologically
acceptable salts which are produced in the usual manner
e.g. by titrating the compounds with inorganic or
organic bases or acids such as e.g. sodium hydrogen
carbonate or potassium hydrogen carbonate, sodium
hydroxide solution, potassium hydroxide solution,
aqueous ammonia or amines such as e.g. trimethylamine or
triethylamine, trifluoroacetic acid or hydrochloric
acid. As a rule the salts are purified by precipitation
from water/acetone.
The new substances of formula I according to the
invention and the salts thereof can be administered
CA 022~02~0 1998-09-28
-- 24 --
enterally or parenterally in a liquid or solid form. All
the usual forms of application come into consideration
such as tablets, capsules, drageés, syrups, solutions,
suspensions etc.. Water is preferably used as the
injection medium which contains the usual additives such
as stabilizing agents, solubilizers and buffers for
injection solutions.
Such additives are e.g. tartrate and citrate buffer,
ethanol, complexing agents (such as ethylenediamine
tetraacetic acid and non-toxic salts thereof), high-
molecular polymers (such as liquid polyethylene oxide)
to regulate viscosity. Liquid carrier substances for
injection solutions have to be sterile and are
preferably filled into ampoules. Solid carrier
substances are e.g. starch, lactose, mannitol, methyl
cellulose, talcum, highly-dispersed silicic acids,
higher molecular fatty acids (such as stearic acid),
gelatine, agar-agar, calcium phosphate, magnesium
stearate, animal and vegetable fats, solid high-
molecular polymers (such as polyethylene glycols);
preparations suitable for oral application can
optionally contain flavourings and sweeteners.
The dose can depend on various factors such as manner of
application, species, age and/or individual state. The
daily doses to be administered are about 1-1000 mg/human
preferably at 100-500 mg/human and can be taken singly or
divided into several administrations.
Within the sense of the present invention the following
oxazolidininone derivatives are preferred in addition to
the compounds mentioned in;the examples and compounds
which can be derived by combining all meanings for
substituents mentioned in the claims.
CA 022~02~0 1998-09-28
-- 25 --
1) 1-t2-Oxo-3-(1-pyrimidin-4-yl-piperidin-4-yl)-
octahydro-benzooxazol-7-yl]-piperidine-4-carboxylic
acid
2) 1-{2-Oxo-3-[1-(2-pyrrolidin-1-yl-pyrimidin-4-yl)-
piperidin-4-yl]-octahydro-benzooxazol-7-yl}-
piperidine-4-carboxylic acid
3) {1-[2-Oxo-3-(1-pyrimidin-4-yl-piperidin-4-yl)-octa-
hydro-benzooxazol-7-yl]-piperidin-4-yl}-acetic acid
4) (1-{3-[1-(2-Amino-pyrimidin-4-yl)-piperidin-4-yl]-2-
oxo-octahydro-benzooxazol-7-yl}-piperidin-4-yl)-
acetic acid
5) (1-{3-[1-(2-Methylamino-pyrimidin-4-yl)-piperidin-4-
yl]-2-oxo-octahydro-benzooxazol-7-yl}-piperidin-4-
yl)-acetic acid
6) (1-{2-Oxo-3-[1-(2-phenylamino-pyrimidin-4-yl)-
piperidin-4-yl]-octahydro-benzooxazol-7-yl}-
piperidin-4-yl)-acetic acid
7) 2-Methanesulfonyl amino-3-{1-[2-oxo-3-(1-pyrimidin-
4-yl-piperidin-4-yl)-octahydro-benzooxazol-7-yl]-
piperidin-4-yl}-propionic acid
8) 1-[2-Oxo-3-(1-pyrimidin-4-yl-piperidin-4-yl)-
octahydro-benzooxazol-7-ylmethyl]-piperidine-4-
carboxylic acid
9) 1-[2-Oxo-3-(3,4,5,6-tetrahydro-2H-[1,4']bipyridinyl-
4-yl)-octahydro-benzooxazol-7-ylmethyl]-piperidine-
4-carboxylic acid
10) 1-{3-[1-(2-Amino-pyrimidin-4-yl)-piperidin-4-yl]-2-
oxo-octahydro-benzooxazol-7-ylmethyl}-piperidine-4-
carboxylic acid
11) 1-{2-Oxo-3-[1-(2-piperidin-1-yl-pyrimidin-4-yl)-
piperidin-4-yl]-octahydro-benzooxazol-7-ylmethyl}-
piperidine-4-carboxylic acid
12) 1-{3-[1-(2-Amino-pyrimidin-4-yl)-piperidin-4-yl]-2-
oxo-octahydro-benzooxaZol-7-yl}-piperidine-4-
carboxylic acid
CA 022~02~0 1998-09-28
- 26 -
13) l-{3-[l-(2-Benzylamino-pyrimidin-4-yl)-piperidin-4
yl]-2-oxo-octahydro-benzooxazol-7-ylmethyl}
piperidine-4-carboxylic acid
14) 4-{3-[1-(2-Benzylamino-pyrimidin-4-yl)-piperidin-4-
yl]-2-oxo-octahydro-benzooxazol-7-ylmethyl}-
cyclohexanecarboxylic acid
15) 4-{3-[1-(2-Benzylamino-pyrimidin-4-yl)-piperidin-4-
yl]-2-oxo-octahydro-benzooxazol-7-ylmethyl}-4-
hydroxy-cyclohexanecarboxylic acid
16) 4-[2-Oxo-3-(3,4,5,6-tetrahydro-2H-[1,4']bipyridinyl-
4-yl)-octahydro-benzooxazol-7-yl]-cyclohexane-
carboxylic acid
17) 4-{3-[1-(2-Amino-pyrimidin-4-yl)-piperidin-4-yl]-2-
oxo-octahydro-benzooxazol-7-yl}-cyclohexanecarboxylic
acid
18) 4-Hydroxy-4-[2-oxo-3-(3,4,5,6-tetrahydro-2H-
[1,4']bipyridinyl-4-yl)-octahydro-benzooxazol-7-yl]-
cyclohexanecarboxylic acid
19) 4-Hydroxy-4-{2-oxo-3-[1-(2-pyrrolidin-1-yl-
pyrimidin-4-yl)-piperidin-4-yl]-octahydro-
benzooxazol-7-yl}-cyclohexanecarboxylic acid
20) {1-[2-Oxo-3-(3,4,5,6-tetrahydro-2H-[1,4']bipyridinyl-
4-yl)-octahydro-benzooxazol-7-yl]-piperidin-4-yl}-
acetic acid
21) 2-(Butane-1-sulfonylamino)-3-{1-[2-oxo-3-(3,4,5,6-
tetrahydro-2H-[1,4']bipyridinyl-4-yl)-octahydro-
benzooxazo1-7-yl]-piperidin-4-yl}-propionic acid
22) 1-{2-[2-Oxo-3-(3,4,5,6-tetrahydro-2H-
[1,4']bipyridinyl-4-yl)-octahydro-benzooxazol-7-yl]-
ethyl}-piperidine-4-carboxylic acid
23) 1-{3-[1-(2-Methylamino-pyrimidin-4-yl)-piperidin-4-
yl]-2-oxo-octahydro-benzooxazol-7-yl}-piperidine-4-
carboxylic acid
CA 022~02~0 1998-09-28
- 27 -
24) 1-{3-~2-Oxo-3-(3,4,5,6-tetrahydro-2H-
[1,4']bipyridinyl-4-yl)-octahydro-benzooxazol-7-yl]-
propyl}-piperidine-4-carboxylic acid
25) 1-{3-[2-Oxo-3-(1-pyrimidin-4-yl-piperidin-4-yl)-
octahydro-benzooxazol-7-yl]-propyl}-piperidine-4-
carboxylic acid
26) 1-(3-{3-[1-(2-Amino-pyrimidin-4-yl)-piperidin-4-yl]-
2-oxo-octahydro-benzooxazol-7-yl}-propyl)-piperidine-
4-carboxylic acid
27) 1-[2-Oxo-3-(1-pyridazin-4-yl-piperidin-4-yl)-
octahydro-benzooxazol-7-yl]-piperidine-4-carboxylic
acid
28) {1-[2-Oxo-3-(1-pyridazin-4-yl-piperidin-4-yl)-
octahydro-benzooxazol-7-yl]-piperidin-4-yl~-acetic
acid
29) 1-[2-Oxo-3-(1-pyridazin-4-yl-piperidin-4-yl)-
octahydro-benzooxazol-7-ylmethyl]-piperidine-4-
carboxylic acid
30) 4-[2-Oxo-3-(1-pyridazin-4-yl-piperidin-4-yl)-
octahydro-benzooxazol-7-yl]-cyclohexanecarboxylic
acid
31) 1-{3-[2-Oxo-3-(1-pyridazin-4-yl-piperidin-4-yl)-
octahydro-benzooxazol-7-yl]-propyl}-piperidine-4-
carboxylic acid
32) 1-~2-Oxo-3-(4-pyrimidin-4-yl-piperazin-1-yl)-
octahydro-benzooxazol-7-yl]-piperidine-4-carboxylic
acid
33) 4-[2-Oxo-3-(4-pyrimidin-4-yl-piperazin-1-yl)-
octahydro-benzooxazol-7-yl]-cyclohexanecarboxylic
acid
34) (+)-( 3 ' a, 7 ' ~, 7a)-1-{ 3 - [ 1- ( 2-benzylamino-pyrimidin-4-
yl)-piperidin-4-yl]-2-oxo-octahydro-benzooxazol-7-
yl}-piperidine-4-carboxylic acid
CA 022~02~0 1998-09-28
- 28 -
35) 1-{2-[2-Oxo-3-(4-pyridin-4-yl-piperazin-1-yl)-
octahydro-benzooxazol-7-yl]-ethyl}-piperidine-4-
carboxylic acid
36) 1-[2-Oxo-3-(1-pyrimidin-4-yl-piperidin-4-yl)-
hexahydro-cyclopentaoxazol-6-yl]-piperidine-4-
carboxylic acid
37) 1-[2-Oxo-3-(3,4,5,6-tetrahydro-2H-tl,4']bipyridinyl-
4-yl)-hexahydro-cyclopentaoxazol-6-yl]-piperidine-4-
carboxylic acid
38) 1-{3-[1-(2-Amino-pyrimidin-4-yl)-piperidin-4-yl]-2-
oxo-hexahydro-cyclopentaoxazol-6-yl}-piperidine-4-
carboxylic acid
39) (1-{2-Oxo-3-[1-(2-phenylamino-pyrimidin-4-yl)-
piperidin-4-yl]-hexahydro-cyclopentaoxazol-6-yl}-
piperidin-4-yl)-acetic acid
40) 1-{2-[2-Oxo-3-(3,4,5,6-tetrahydro-2H-
[1,4']bipyridinyl-4-yl)-hexahydro-cyclopentaoxazol-6-
yl]-ethyl}-piperidine-4-carboxylic acid
41) 1-[2-Oxo-3-(1-pyridazin-4-yl-piperidin-4-yl)-
hexahydro-cyclopentaoxazol-6-yl]-piperidine-4-
carboxylic acid
42) 1-[2-Oxo-3-(4-pyridin-4-yl-piperazin-1-yl)-hexahydro-
cyclopentaoxazol-6-yl]-piperidine-4-carboxylic acid
43) 4-Hydroxy-4-[2-oxo-3-(3,4,5,6-tetrahydro-2H-
[1,4']bipyridinyl-4-yl)-hexahydro-cyclopentaoxazol-6-
yl]-cyclohexanecarboxylic acid
44) 1-[2-Oxo-3-(1-pyrimidin-4-yl-piperidin-4-yl)-
octahydro-cycloheptaoxazol-8-yl]-piperidine-4-
carboxylic acid
45) 1-{2-Oxo-3-[1-(2-phenylamino-pyrimidin-4-yl)-
piperidin-4-yl]-octahydro-benzooxazol-7-yl}-
piperidine-4-carboxylic acid
46) 1-[2-Oxo-3-(3,4,5,6-tetrahydro-2H-[1,4']bipyridinyl-
4-yl)-octahydro-cycloheptaoxazol-8-yl]-piperidine-4-
carboxylic acid
CA 022~02~0 1998-09-28
47) 1-{3-[1-(2-Amino-pyrimidin-4-yl)-piperidin-4-yl]-2-
oxo-octahydro-cycloheptaoxazol-8-yl}-piperidine-4-
carboxylic acid
48) (1-{3-[1-(2-Amino-pyrimidin-4-yl)-piperidin-4-yl]-2-
oxo-octahydro-cycloheptaoxazol-8-yl}-piperidin-4-yl)-
acetic acid
49) 1-{2-Oxo-3-[1-(2-piperidin-1-yl-pyrimidin-4-yl)-
piperidin-4-yl]-octahydro-cycloheptaoxazol-8-
ylmethyl}-piperidine-4-carboxylic acid
50) 1-(2-Oxo-3-{1-[2-(pyridin-4-ylamino)-pyrimidin-4-yl]-
piperidin-4-yl}-octahydro-benzooxazol-7-yl)-
piperidine-4-carboxylic acid
51) 1-(2-Oxo-3-{1-[2-(pyrimidin-2-ylamino)-pyrimidin-4-
yl]-piperidin-4-yl}-octahydro-benzooxazol-7-yl)-
piperidine-4-carboxylic acid
52) 1-(2-Oxo-3-{1-[2-(1,4,5,6-tetrahydro-pyrimidin-2-
ylamino)-pyrimidin-4-yl]-piperidin-4-yl}-octahydro-
benzooxazol-7-yl)-piperidine-4-carboxylic acid
53) 1-{3-[1-(2-Cyclohexylamino-pyrimidin-4-yl)-piperidin-
4-yl]-2-oxo-octahydro-benzooxazol-7-yl}-piperidine-4-
carboxylic acid
54) 1-{3-[1-(2-Pyrrolidin-l-yl-pyrimidin-4-yl)-piperidin-
4-yl]-2-thioxo-octahydro-benzooxazol-7-yl}-
piperidine-4-carboxylic acid
55) 1-{2-Oxo-3-[1-(2-pyrrolidin-1-yl-hexahydro-
pyrimidine-4-yl)-piperidin-4-yl]-octahydro-
benzooxazol-7-yl}-piperidine-4-carboxylic acid
56) 1-{2-Oxo-3-[1-(2-pyrrolidin-1-yl-1,4,5,6-tetrahydro-
pyrimidin-4-yl)-piperidin-4-yl]-octahydro-
benzooxazol-7-yl}-piperidine-4-carboxylic acid
57) 1-[3-(1'-Benzyl-[1,4']bipiperidinyl-4-yl)-2-thioxo-
octahydro-benzooxazol-7-yl]-piperidine-4-carboxylic
acid
CA 022~02~0 1998-09-28
- 30 -
58) 1-[2-Acetylimino-3-(3,4,5,6-tetrahydro-2N-
[1,4']bipyridinyl-4-yl)-octahydro-benzooxazol-7-yl]-
piperidine-4-carboxylic acid
59) 1-[2-Imino-3-(3,4,5,6-tetrahydro-2H-
[1,4']bipyridinyl-4-yl)-octahydro-benzooxazol-7-yl]-
piperidine-4-carboxylic acid
60) 4-(lH-Indol-3-ylmethyl)-1-[2-oxo-3-(3,4,5,6-
tetrahydro-2H-[1,4']bipyridinyl-4-yl)-octahydro-
benzooxazol-7-yl]-piperidine-4-carboxylic acid
61) 4-Butyl-1-[2-oxo-3-(3,4,5,6-tetrahydro-2H-
[1,4']bipyridinyl-4-yl)-octahydro-benzooxazol-7-yl]-
piperidine-4-carboxylic acid
62) 1-[2-Oxo-3-(3,4,5,6-tetrahydro-2H-[1,4']bipyridinyl-
4-yl)-octahydro-benzooxazol-7-yl]-4-phenethyl-
piperidine-4-carboxylic acid
63) (-)-(3'S,7'S,7R)-1-[2-Oxo-3-(3,4,5,6-tetrahydro-2H-
[1,4']bipyridinyl-4-yl)-octahydro-benzooxazol-7-yl]-
piperidine-4-carboxylic acid [a]2~D=-17.4~(c=1.18;
CHCl3)
64) (+)-(3'R,7'R,7S)-1-[2-Oxo-3-(3,4,5,6-tetrahydro-2H-
[1,4']bipyridinyl-4-yl)-octahydro-benzooxazol-7-yl]-
piperidine-4-carboxylic acid [a]2~D=+18.7~(c=1.2;
CHCl3)
The following examples show some of the variants of the
process that can be used to synthesize the compounds
according to the invention. However, they are not
intended to represent a limitation of the subject matter
of the invention. The structure of the compounds was
confirmed by lH and optionally by 13C-NMR spectroscopy
as well as by mass spectrometry. The purity of the
substances was determined by means of C, H, N as well as
thin layer chromatography.
CA 022~02~0 l998-09-28
-- 31 --
Example
(i)-(3' a, 7 ' B, 7a) -1- ~ 2-Oxo-3-(3.4.5.6-tetrahydro-2H-
rl.4~lbipYridinyl-4-yl)-octahydro-benzooxazol-7
Piperidine-4-carboxylic acid
N 2 o
N~
a) A solution of 46 g (0.4 mol) 4-chloropyridine and
123.5 g (0.86 mol) 4-piperidone-ethylene ketal is
heated in 400 ml p-xylene for 48 h under reflux.
Subsequently the reaction mixture is cooled, the
precipitated precipitate is removed by filtration,
the mother liquor is concentrated to dryness and
the residue is purified by column chromatography on
silica gel (ethyl acetate/saturated ammonia-
alkaline methanol 9/1). In this way 79.7 g (90 %)
8-pyridin-4-yl-1,4-dioxa-8-aza-spiro-[4.5]decane is
obtained as a white powder. m/e=220; Fp=65~C.
b) A solution of 79 . 7 g of the ketal produced in a) in
2 l tetrahydrofuran is admixed with 1 l 6 N
hydrochloric acid and the reaction mixture is
stirred for 2 h at room temperature. Subsequently
the tetrahydrofuran is removed in a vacuum on a
rotary evaporator, the hydrochloric acid solution
is alkalysed with semi-concentrated ammonium
hydroxide solution and;extracted four times with
100 ml methylene chloride each time. After drying
CA 022S02S0 1998-09-28
- 32 -
the combined organic extracts over sodium sulfate
and removing the solvent, the residue is purified
by column chromatography on silica gel. In this way
64.2 g (100 % yield) 2,3,5,6-tetrahydro-
[1,4']bipyridinyl-4-one is obtained as a grey
powder. m/e=176; Fp=102~C.
c) A solution of 41.6 g cis-2,3-epoxycyclohexanol
(Svante T., J. Org. Chem., 38, 1380 (1973)) and
54 g imidazole in 650 ml dimethylformamide (DMF) is
admixed at 0~C with 67.7 g tert.-butyl dimethyl-
chlorosilane. Afterwards the reaction mixture is
stirred for a further 4 hours at 0~C, it is then
admixed with 600 ml water and the aqueous solution
is extracted four times with 100 ml ethyl acetate.
After drying the combined organic phases over
sodium sulfate and removing the solvent under
reduced pressure, the residue is distilled in a
vacuum. 50 g cis-1-tert.-butyl-dimethyl-silyloxy-
2,3-epoxycyclohexane is obtained. bpo o5=74~C-
H-NMR (CDCl3):~ = 3.90 ppm (m, lH); 3.10 (broad s,
lH); 3.0 (broad s, lH); 1.65 (m, 2H); 1.40 (m, 3H);
1.15 (m, lH); 1.82 (s, 9H); 0.01 (s, 6H).
d) A mixture of 20 g epoxide lc) and 34 ml 4-
piperidine carboxylic acid ethyl ester in 160 ml
ethanol is heated for 48 hours under reflux.
Subsequently the reaction mixture is evaporated to
dryness and the residue is purified by column
chromatography on silica gel (ethyl acetate/
isohexane=1/2). 22.2 g (+)-(la,2~,3~)-1-[3-(tert-
butyl-dimethylsilanyloxy)-2-hydroxy-cyclohexyl]-
piperidine-4-carboxylic acid ethyl ester is
obtained as a white powder. m/e=385.
CA 022~02~0 1998-09-28
- 33 -
e) A solution of 18.7 g of the alcohol ld) and 12 ml
triethylamine in 200 ml methylene chloride is
admixed at 5-10~C internal temperature with 5.4 ml
methanesulfonic acid chloride. The reaction mixture
is allowed to stir for one hour at room
temperature, subsequently 50 ml saturated sodium
hydrogen carbonate solution is added, the phases
are separated, the organic phase is washed with
100 ml water and dried over sodium sulfate. After
removing the solvent on a rotary evaporator, the
residue is purified by column chromatography on
silica gel (ethyl acetate/isohexane=l/3). 21 g (+)-
(la,2B,3B)-1-[3-(tert.-butyl-dimethylsilanyloxy)-2-
methanesulfonyloxy-cyclohexyl]-piperidine-4-
carboxylic acid ethyl ester is obtained as a light-
yellow oil. lH-NMR (d6-DMSO):~=4.45 ppm (d, lH);
4.20 (broad s, lH); 3.95 (q, 2H); 3.10 (s, 3H);
2.88-263 (m, 2H); 2.50 (m, lH); 2.20-2.00 (m, 2H);
1.75-1.62 (m, lH); 1.58-1.18 (m, 7H); 1.10 (t, 3H);
0.80 (s, 9H); 0.01 (s, 6H).
f) A solution of 21 g of the silyl derivative le) in
200 ml tetrahydrofuran is admixed with 63 ml of a
1.1 molar tetrabutylammonium fluoride solution in
tetrahydrofuran. The reaction mixture is allowed to
stir for 30 hours at room temperature, then
concentrated to dryness and the residue is
chromatographed on silica gel (ethyl acetate/
isohexane=95/5). 6.3 g (+)-(la,2B,3B)-1-(3-hydroxy-
2-methanesulfonyloxy-cyclohexyl)-piperidine-4-
carboxylic acid ethyl ester is obtained as a white
powder. m/e=349.
g) A mixture of 5.48 g of the hydroxymesylate lf) and
550 mg sodium hydride is stirred for one hour at
CA 022~02~0 1998-09-28
- 34 -
5~C in 200 ml tetrahydrofuran (THF) and after
subsequent addition of 50 ml water it is stirred
for 15 hours at 50~C. Afterwards the THF is removed
on a rotary evaporator and the aqueous mixture is
extracted three times with 30 ml methylene chloride
each time. After drying the combined organic phases
over sodium sulfate and removing the solvent in a
vacuum 3.9 g (+)-trans-1-(2,3-epoxy-cyclohexyl)-
piperidine-4-carboxylic acid ethyl ester is
obtained as a yellow oil which is reacted further
without additional purification.
h) A solution of 3.4 g of the epoxide produced under
lg), 5.3 g sodium azide and 4.3 g ammonium chloride
is heated for 24 hours at 70~C in 50 ml of an
ethanol/water mixture (80/20). Subsequently the
ethanol is removed in a vacuum, the residue is
diluted with 10 ml water and the aqueous solution
is extracted three times with 15 ml methylene
chloride each time. After drying the combined
organic phases over sodium sulfate and removing the
solvent on a rotary evaporator the crude product is
chromatographed on silica gel (ethyl acetate/
isohexane: 4/1). In this way 2.74 g (+)-(la,2~,3a)-
1-(3-azido-2-hydroxy-cyclohexyl)-piperidine-4-
carboxylic acid ethyl ester is obtained as an
orange-coloured oil, which slowly solidifies. 1H-
NMR (d6-~MSO): ~=4.53 ppm (broad s, lH, OH); 4.05
(q, 2H); 3.30 (broad d, 2H); 2.80 (quasi d, lH);
2.65 (quasi d, lH); 2.50 (m, lH); 2.25 (m, 3H);
1.90-1.40 (m, 7H); 1.30-1.02 (m+t, 6H).
i) A solution of the azid~e produced in lh) in 20 ml
ethanol is admixed with 0.8 g 10 % palladium/carbon
and the mixture is hydrogenated for 6 h/40 mbar at
CA 022~02~0 1998-09-28
- 35 -
room temperature. Subsequently the catalyst is
removed by filtration and the solution is
concentrated on a rotary evaporator. In this way
2.4 g (+)-(la,2~,3a)-1-(3-amino-2-hydroxy-
cyclohexyl)-piperidine-4-carboxylic acid ester is
obtained. FAB 271.
j) A solution of 2.4 g amine li), 1.6 g ketone lb) and
3.75 g sodium triacetate borohydride in 40 ml
methylene chloride is stirred for 48 h at room
temperature. Subsequently the reaction mixture is
admixed with 10 ml water and acidified with 1 N
hydrochloric acid. After separating the phases the
aqueous acidic phase is again extracted with 10 ml
methylene chloride and then alkalized with 1 N
sodium hydroxide solution. After extracting the
alkaline mixture three times with 15 ml methylene
chloride each time and drying the combined organic
phases over sodium sulfate, the solvent is removed
on a rotary evaporator. The crude product is then
purified by means of preparative HPLC (RP 18,
methanol/buffer (pH=7.5) 70/30). In this way 2.5 g
(+)-(la~2l3l3a)-l-[2-hydroxy-3-(3~4~5~6-tetrahydr
2H-[1,4']bipyridinyl-4-ylamino)-cyclohexyl]-
piperidine-4-carboxylic acid ethyl ester is
obtained. 1H-NMR (d6-DMSO): ~=8.20 ppm (d, 2H);
6.85 (d, 2H); 4.15 (m, lH); 4.12 (q, 2H); 3.85
(broad d, 2H); 3.10 (t, lH); 3.0-2.8 (m, 4H); 2.70
(d, lH); 2.55 (m, 2H); 2.30 (broad t, 3H); 2.0 (d,
lH); 1.88 (m, 4H); 1.80-1.50 (m, 5H); 1.38-0.88 (m,
5H); 1.25 (t, 3H).
k) A solution of 2.5 g of the aminoalcohol from lj)
and 1.9 g carbonyldiimidazole in 20 ml dimethyl-
formamide is stirred for 15 h at room temperature.
CA 022~02~0 1998-09-28
- 36 -
Subsequently the reaction solution is evaporated to
dryness and the residue is purified by means of
preparative HPLC (Merck, Select B, methanol/buffer
(pH=7.5) 65/35). In this way one obtains 2.78 g
(+)-(3'a,7'~,7a)-1-[2-oxo-3-(3,4,5,6-tetrahydro-2H-
[1,4']bipyridinyl-4-yl)-octahydro-benzooxazo1-7-
yl]-piperidine-4-carboxylic acid ethyl ester as a
yellow oil. m/e=456.
1) A solution of 1.85 g of the ethyl ester lk) and
4.60 ml 1 N sodium hydroxide solution in 30 ml
methanol is stirred for 1 h at room temperature.
Subsequently the methanol is removed in a vacuum
and the product is purified by means of an ion
exchanger (Dowex 50, H form). In this way 0.95 g of
the title compound is obtained as a white powder
Fp.> 200~C. m/e (EI spectrum)=500 (measured as a
trimethylsilyl derivative).
Example la
(-)-(3'S,7'S,7R)-1-[2-Oxo-3-(3,4,5,6-tetrahydro-2H-
[1,4'lbipyridinyl-4-yl)-octahydro-benzooxazol-7-yll-
pi~eridine-4-carboxylic acid
N ~ ~0
~7~ N~
After carrying out reaction steps 2a)-2c) substitution
of the racemic cis-2,3-epoxycyclohexanol in example 2a)
CA 022~02~0 1998-09-28
by (lR,2R,3S)-l-hydroxy-2,3-epoxycyclohexane yields the
optically active compound (lS,2S,3R)-1-azido-2-hydroxy-
cyclohexyl)-piperidine-4-carboxylic acid ethyl ester
([a]D2~=-41.4~C (C1.2; CHCl3)) from which the title
compound is obtained analogously to example li)-11).
[a] D= - 17.4~ (C1.18; CHCl3).
(lR,2R,3S)-1-hydroxy-2,3-epoxycyclohexane is obtainable
according to Svante T. (J. Org. Chem. 38, 1380 (1973))
by epoxidizing (R)-cyclohex-2-enol known in the
literature (Fukazawa et al., Tetrahydron Asymmetry 4,
2323 (1993)) by means of meta-chloroperbenzoic acid.
ExamPle 2
Additional Process for the production of (+)-(la,2~,3a)-1-
(3-azido-2-hYdroxy-cyclohexYl)-piperidine-4-carboxylic
acid ethyl ester lh)
OH ~OEt
N3 ~ ~N
a) A solution of 119.65 g cis-2,3-epoxycyclohexanol
(see example lc) and 188 ml triethylamine in 400 ml
methylene chloride is admixed at 0~C with a
solution of 240 g p-toluenesulfonic acid chloride
in 500 ml methylene chloride. Subsequently the
reaction mixture is stirred for 15 hours at room
temperature, the precipitated salt is then removed
by filtration and the filtrate is washed with
100 ml saturated sodium hydrogen carbonate
solution. After drying the methylene chloride phase
CA 022~02~0 1998-09-28
- 38 -
over sodium sulfate and removing the solvent, the
crude product is purified by column chromatography
on silica gel (ethyl acetate/isohexane = 1/1). In
this way 211 g (75 %) cis-2,3-epoxycyclohexyl-
tosylate is obtained as a yellow oil, which slowly
solidifies. lH-NMR (CDCl3): ~ = 7.85 ppm (d, 2H);
7.35 (d, 2H); 4.90 (m, lH); 3.27 (broad s, lH);
3.17 (broad s, lH); 2.45 (s, 3H); 1.80 (m, 2H);
1.65 (m, 3H); 1.22 (m, lH).
b) A mixture of 112 g epoxide 2a) and 110 ml 4-
piperidine-carboxylic acid ethyl ester in 250 ml
ethanol is irradiated for 2.75 hours in a microwave
oven with an energy of 500 W so that the reaction
temperature is 65~C. Subsequently the reaction
solution is cooled to 0~C, the product is suction
filtered, washed twice with 50 ml cold ethanol each
time and washed three times with 50 ml diethyl
ether each time and dried in a vacuum at 30~C. In
this way 96 g (54 %) (la,2~,3~)-1-(3-p-toluene-
sulfonyloxy-2-hydroxy-cyclohexyl)-piperidine-4-
carboxylic acid ethyl ester is obtained. Fp = 135 -
137~C. m/e=425. 1H-NMR (CDCl3): ~ = 7.75 ppm (d,
2H); 7.22 (d, 2H); 4.85 (broad s, lH); 4.05 (q,
2H); 3.28 (d with fine resolution, lH); 2.62 (m,
2H); 2.50 (t, lH); 2.35 (s, 3H); 2.18 (m, lH); 2.05
(t, 2H); 1.75 (m, 3H); 1.55 (m, 4H); 1.32 (m, lH);
1.15 (t, 3H); 1.10 (m, lH).
c) A mixture of 37 g tosylate 2b) and 34.3 g sodium
azide in 250 ml dimethylformamide is irradiated in
a microwave oven for 20 min with an energy of 500 W
in such a way that the reaction temperature is
90~C. Subsequently the reaction solution is cooled
to room temperature, the salt is suction filtered,
CA 022~02~0 1998-09-28
- 39 -
the filtrate is evaporated to dryness in a vacuum
at 50~C, the residue is taken up in 50 ml water and
the aqueous mixture is extracted three times with
50 ml diethyl ether each time. After drying the
combined organic phases over sodium sulfate and
removing the solvent, the crude product is purified
by column chromatography on silica gel (ethyl
acetate/ isohexane=6/4). In this way 21.8 g (84 %)
of the title compound is obtained as a light grey
powder. Fp.: 61-63~C. The title compound is
obtainable according to this process in a better
yield and higher purity than by the process of lh).
Example 2a
(+)-(3'R,7'R 7S)-1-~2-Oxo-3-(3,4,5,6-tetrahYdro-2~-
[1,4']bipYridinyl-4-yl)-octahydro-benzooxazol-7-yl]-
piperidine-4-carboxylic acid
J 2 0
~ ~ OH
After carrying out reaction steps 2a)-2c) substitution
of the racemic cis-2,3-epoxycyclohexanol in example 2a)
by (lS,2S,3R)-1-hydroxy-2,3-epoxycyclohexane yields the
optically active compound (lR,2R,3S)-1-azido-2-
hydroxycyclohexyl)-piperidine-4-carboxylic acid ethyl
ester ([a]D20 = +42~ (Cl.23; CHCl3)), from which the
title compound is obtained analogously to example li)-
11). [a]D = +18.7~(Cl.2; CHCl3).
CA 022~02~0 1998-09-28
- 40 -
(lS,2S,3R)-1-hydroxy-2,3-epoxycyclohexane is obtainable
according to Svante T. (J. Org. Chem. 38, 1380 (1973))
by epoxidizing (S)-cyclohex-2-enol known in the
literature (Singh V.K., et al., Synth. Commun. 24, 375
(1994)) by means of metachloroperbenzoic acid.
Example 3
(+)-(3'a,7'~,7a)-1-~3-~1-(2-Benzylamino-pyrimidin-4-yl)-
piperidin-4-yll-2-oxo-octahydro-benzooxazol-7-Yl~-
piperidine-4-carboxylic acid
N ~ 3~
3~ 57 ~O
a) A solution of 16 ml (0.12 mol) 4-piperidone-
ethylene ketal in 100 ml ethanol is added dropwise
to a solution of 18.5 g (0.12 mol) 2,4-dichloro-
pyrimidine and 17.5 ml triethylamine in 150 ml
ethanol while cooling on ice. Subsequently the
reaction mixture is stirred for 2.5 hours at room
temperature and then it is evaporated to dryness.
The residue is taken up in 50 ml water and the
aqueous solution is extracted three times with
20 ml methylene chloride each time. After drying
the combined organic phases over sodium sulfate and
removing the solvent, the residue is recrystallized
from ethyl acetate/isohexane. In this way 21.8 g 8-
(2-chloro-pyrimidin-4-;yl)-1,4-dioxa-8-aza-spiro-
[4.5]decane is obtained as a white powder. m/e=256.
CA 022~02~0 1998-09-28
- 41 -
H-NMR (CDCl3): ~ = 7.92 ppm (d, lH; Ar-H); 6.35
(d, lH; Ar-H); 3.91 (s, 4H; ketal-CH2); 3.65 (broad
s, 4H); 1.68 (t, 4H).
b) A mixture of 8 g 2-chloropyrimidine 3a) and 7.2 ml
benzylamine is heated for 2 hours at 150~C.
Subsequently the reaction mixture is cooled to room
temperature, admixed with 30 ml water, the aqueous
solution is extracted three times with 20 ml
methylene chloride each time, the combined extracts
are dried over sodium sulfate, the solvent is
removed on a rotary evaporator and the residue is
crystallized from ethyl acetate. In this way 7 g
8-(2-benzylamino-pyrimidin-4-yl)-1,4-dioxa-8-aza-
spiro-[4.5]decane. m/e=326. lH-NMR (d6-DMS0): ~ =
7.88 ppm (d, lH; Ar-H); 7.32 (m, 5H; Ar-H); 7.25
(broad s, lH; NH); 6.12 (d, lH; Ar-H); 4.45 (d, 2H;
pH-CH2-) 3.98 (s, 4H; ketal-CH2); 3.65 (broad s,
4H); 1.72 (broad s, 4H).
c) Analogously to example lb) 6.3 g 1-(2-benzylamino-
pyrimidin-4-yl)-piperidin-4-one is obtained as a
brown oil from 7 g ketal 3b) and 80 ml 6 N
hydrochloric acid which is reacted further as a
crude product.
d) 0 77 g (+)-(la,2~,3a)-1-{2-hydroxy-3-[1-(2-
benzylamino-pyrimidin-4-yl)-piperidin-4-ylamino]-
cyclohexyl}-piperidine-4-carboxylic acid ethyl
ester is obtained analogously to example lj) from
0.42 g amine li), 0.44 g ketone 3c), 0.66 g sodium
triacetateborohydride and 0.3 ml 100 % acetic acid.
m/e=537.
CA 022~02~0 1998-09-28
- 42 -
e) 0 54 g (+)-(3~a~7lB/7a)-l-{3-[l-(2-benzylamino-
pyrimidin-4-yl)-piperidin-4-yl]-2-oxo-octahydro-
benzooxazol-7-yl}-piperidine-4-carboxylic acid
ethyl ester is obtained analogously to example lk)
from 0.77 g amino alcohol 3d) and 0.28 g carbonyl
diimidazole. pos. FAB = 562. lH-NMR (d6-DMSO): ~ =
7.75 ppm (d, lH); 7.20 (m, 5H); 7.12 (broad s, lH;
NH); 6.03 (d, lH); 4.43 (d, 2H); 4.39 (broad s,
lH); 4.05 (q, 2H); 3.80 (t, lH); 3.69 (m, lH); 3.25
(broad t, lH); 2.76 (m, 5H); 2.48 (m, 2H); 2.25 (m,
2H); 1.95-1.20 (m, 14H); 1.18 (t, 3H).
f) 0.065 g of the title compound is obtained
analogously to example 11) from 0.1 g ethyl ester
3e) and 0.4 ml 1 N sodium hydroxide solution.
m/e-534. Fp=180~C.
Example 4
(+)-(3'a~7'B~7a)-1-~3-~1-(2-Pyrrolidin-l-Yl-pyrimidin-4
yl)-piPeridin-4-yl~-2-oxo-octahydro-benzooxazol-7-yl~-
piperidine-4-carboxylic acid
~ ~ N~ 3 J~
G ~7~ N~
a) A mixture of 7.7 g chloropyrimidine derivative 3a)
and 25 ml pyrrolidine is irradiated in a microwave
oven for 15 min with an energy of 500 W in such a
way that the reaction temperature is 50~C.
CA 022~02~0 1998-09-28
Subsequently the reaction solution is evaporated to
dryness, the residue is taken up in 20 ml water and
the aqueous solution is extracted four times with
20 ml methylene chloride each time. After drying
the combined extracts over sodium sulfate and
removing the solvent, the residue is purified by
means of preparative HPLC (Merck, Select B,
methanol/buffer (pH=7.5) 75/25). In this way 7.8 g
8-(2-pyrrolidin-1-yl-pyrimidin-4-yl)-1,4-dioxa-8-
aza-spiro-[4.5]decane is obtained. m/e=290.
b) 5 9 g 1-(2-pyrrolidin-1-yl-pyrimidin-4-yl)-
piperidin-4-one is obtained analogously to example
lb) from 7.8 g ketal 4a) and 72 ml 6 N hydrochloric
acid which is reacted further as a crude product.
c) 3.4 g (+)-(la,2~,3a)-1-{2-hydroxy-3-[1-(2-
pyrrolidin-1-yl-pyrimidin-4-yl)-piperidin-4-
ylamino]-cyclohexyl}-piperidine-4-carboxylic acid
ethyl ester is obtained as a yellow oil analogously
to example lj) from 2.46 g ketone 4b), 2.7 g amine
li), 3.2 g sodium triacetate borohydride and 6 ml
100 % acetic acid. 1H-NMR (d6-DMSO + AcOH): ~ =
7.92 ppm (d, lH); 6.29 (d, lH); 4.52 (broad d, 2H);
4.15 (q, 2H); 3.79 (t, lH); 3.53 (m, 4H); 3.20 (m,
3H); 3.00 (m, 3H); 2.55 (m, 2H); 2.20-1.70 (m,
13H); 1.62-1.35 (m, 5H); 1.25 (t, 3H).
d) 2.5 g (+)-(3'a,7'~,7a)-1-{3-[1-(2-pyrrolidin-1-yl-
pyrimidin-4-yl)-piperidin-4-yl]-2-oxo-octahydro-
benzooxazol-7-yl}-piperidine-4-carboxylic acid
ethyl ester is obtained as a light-grey powder
analogously to example lk) from 3.3 g amino-alcohol
4c) and 1.6 g carbonyldiimidazole. Fp = 140~C.
CA 022~02~0 1998-09-28
- 44 -
m/e=526. lH-NMR (d6-DMSO):~ = 8.01 ppm (d, lH);
6.21 (d, lH); 4.62 (broad t, 2H); 4.20 (q, 2H);
3.99 (t, lH); 3.88 (m, lH); 3.55 (m, 5H); 2.98 (m,
5H); 2.65 (m, lH); 2.45 (m, 2H); 2.13 (m, lH);
2.08-1.40 (m, 18H); 1.33 (t, 3H).
e) 0.16 g of the title compound is obtained
analogously to example 11) from 0.4 g ethyl ester
4d) and 0.9 ml lN sodium hydroxide solution.
m/e=498. lH-NMR (d6-DMSO+AcOH): ~ = 7.60 ppm (d,
lH); 6.25 (d, lH); 4.35 (broad s, 2H); 3.78 (t,
lH); 3.59 (broad t, lH); 3.25 (m, 5H); 3.05-2.70
(m, 5H); 2.60 (broad t, lH); 2.42 (broad t, lH);
2.15 (m, lH); 1.80-1.38 (m, 14H); 1.20 (m, 4H).
Example 5
(_)-(3'a,7'~,7a)-1-~3-~1-(2-Amino-PYrimidin-4-yl)-
piperidin-4-yll-2-oxo-octahydro-benzooxazol-7-yl~-
piPeridine-4-carboxylic acid
N
~ ~N ~
a) A solution of 26 g chloropyrimidine 3a) and 120 ml
liquid ammonia in 500 ml ethanol is kept in a 1 1
autoclave for 60 hours at 5 bar and 90~C.
Subsequently the reaction mixture is evaporated to
dryness, the residue is taken up in 20 ml water and
the aqueous solution is extracted five times with
CA 022~02~0 1998-09-28
- 45 -
20 ml methylene chloride each time. After drying
the combined organic phases over sodium sulfate and
removing the solvent the solid residue is stirred
out with 40 ml ethyl acetate. In this way 5.7 g 8-
(2-amino-pyrimidin-4-yl)-1,4-dioxa-8-aza-spiro-
[4.5]decane is obtained as a yellow powder.
m/e=236.
b) 5.5 g (2-amino-pyrimidin-4-yl)-piperidin-4-one is
obtained analogously to example lb) as a yellow
solid from 6 g ketal 5a) and 65 ml 6 N hydrochloric
acid. m/e=192.
c) 1.5 g (+)-(la,2~,3a)-1-{2-hydroxy-3-[1-(2-amino-
pyrimidin-4-yl)-piperidin-4-yl)-piperidin-4-
ylamino]-cyclohexyl}-piperidine-4-carboxylic acid
ethyl ester is obtained as a yellow oil analogously
to example lj) from 1 g amine li), 0.7 g ketone
5b), 1.6 g sodium triacetate borohydride and 0.8 ml
100 % acetic acid. m/e=446. lH-NMR (d6-DMSO): ~ =
7.80 ppm (d, lH); 6.08 (d, lH); 6.00 (s, lH; NH);
4.24 (d, 2H); 4.15 (m, 4H); 3.12 (t, lH); 2.90 (m,
4H); 2.72 (broad d, lH); 2.60 (m, 3H); 2.30 (broad
t, 3H); 1.88 (m, 5H); 1.70 (m, 5H); 1.25 (t, 3H);
1.15 (m, 3H).
d) 0.3 g ((+)-(3'a,7'B,7a)-1-{3-[1-(2-amino-pyrimidin-
4-yl)-piperidin-4-yl]-2-oxo-octahydro-benzooxazol-
7-yl}-piperidine-4-carboxylic acid ethyl ester is
obtained as a light-grey powder analogously to
example lk) from 1.1 g amino-alcohol 5c) and 0.5 g
carbonyldiimidazole. m/e=472. Fp=120-122~C.
;
CA 022~02~0 1998-09-28
- 46 -
e) 0.07 g of the title compound is obtained
analogously to example 11) from 0.11 g ethyl ester
Sd) and 0.25 ml 1 N sodium hydroxide solution.
m/e=444. Fp > 200~C.
Example 6
(i)-(3'a,7'~,7a)-rl- r 2-Oxo-3-f3,4,5,6-tetrahydro-2H-
~1,4'~bipyridinYl-4-Yl-octahydro-benzooxazol-7-Yl~-
piperidin-4-yl~-acetic acid
'~'
N 2 o
3~7~ N~
OH
The title compound is obtained analogously to
examples 2b), 2c) and li)-11) by substituting the
4-piperidine-carboxylic acid ethyl ester in example
2b) by 4-piperidineacetic acid ethyl ester.
Fp=135~C (decomposition). m/e=(EI spectrum)=514
(measured as trimethylsilyl derivative).
CA 022~02~0 1998-09-28
- 47 -
Example 7
(+)-(3'a,7'~,7a)-~ 2-Oxo-3-(3,4,5,6-tetrahydro-2N-
[1 4'1bipyridinyl-4-yl-octahydro-benzooxazol-7-yll-4-(2-
phen~lethyl~-piperidine-4-carboxylic acid ethyl ester
N2O
~ ~ OEt
The title compound is obtained by substituting 4-
piperidine-carboxylic acid ethyl ester in example
2b) by 4[4-(2-phenylethyl)]piperidine-carboxylic
acid ethyl ester analogously to examples 2b), 2c)
and li)-lk) (Gilligan et al., J. Med., Chem. 37,
364 (1994)). m/e=560. lH-NMR (d6-DMSO): ~ = 8.13
ppm (d, 2H); 7.25 (m, 2H); 7.15 (m, 3H); 6.80 (d,
2H); 4.12 (q, 2H); 4.01 (m, 2H); 3.80 (t, lH); 3.70
(m, lH); 3.60 (m, 4H); 3.28 (m, lH); 2.80 (m, 5H);
2.45 (m, 2H); 2.32 (t, 3H); 2.05 (m, 2H); 1.95 (m,
2H); 1.70 (m, 9H); 1.35 (m, 5H); 1.20 (t, 3H).
CA 022~02~0 1998-09-28
- 48 -
Example 8
(+)-(3'a,7'B,7a)-~1- r 2-Thiooxo-3-(3,4,5,6-tetrahydro-2H-
~1 4'~biPyridinyl-4-Yl-octahydro-benzooxazol-7-yll-
piperidine-4-carboxylic acid
\~ N~_ N ~ O
~ 7~N ~
a) 0.2 g (+)-(3'a,7'~,7a)-1-[2-thiooxo-3-(3,4,5,6-
tetrahydro-2N-[1,4']bipyridinyl-4-yl)-octahydro-
benzooxazol-7-yl]-piperidine-4-carboxylic acid
ethyl ester is obtained analogously to example lk)
from 0.3 g amino-alcohol lj) and 0.21 g
thiocarbonyldiimidazole. Pos. FAB=472.
b) 0.1 g of the product was obtained analogously to
example 11) from 0.14 g ethyl ester 8a) and 0.35 ml
1 N sodium hydroxide solution from which 143 mg of
the title compound was isolated as a HCl salt after
addition of 0.6 ml 1 N hydrochloric acid. Pos. FAB
= 472. Fp = 180~C.
CA 022~02~0 1998-09-28
- 49 -
Example 9
(_)-(3'a,7'1~,7a)-1-~3-(l'Benzyl-~1,4'~bipiPeridinyl-4
yl)-2-oxo-octahYdro-benzooxazol-7-yll-piperidine-4
carboxYlic acid
N20
~ ?,N ~
a) A mixture of 15 g pyridine derivative la) and 4 g
ruthenium oxide is hydrogenated until hydrogen
uptake is completed (30 hours). Subsequently the
catalyst is removed by filtration, the filtrate is
concentrated in a vacuum, the residue is taken up
in 120 ml 1,4 dioxane and the solution obtained in
this way is admixed with 18 ml benzyl chloride and
15 g potassium carbonate. The reaction mixture is
then heated for 5 hours under reflux, afterwards it
is cooled, the precipitate is removed by filtration
and the filtrate is evaporated to dryness. The
crude product is purified by column chromatography
on silica gel (ethyl acetate + 10 % saturated
ammoniacal methanol). In this way one obtains 3.4 g
8-(1-benzyl-piperidin-4-yl)-1,4-dioxa-8-aza-
spiro[4.5]decane. lH-NMR (d6-DMSO): ~ = 7.30 ppm
(m, 5H); 3.85 (s, 4H); 3.45 (s, 2H); 2.84 (d, 2H);
2.52 (m, 4H); 2.25 (m, lH); 1.88 (t, 2H); 1.63 (m,
6H); 1.42 (q with fine resolution, 2H).
;
CA 022~02~0 1998-09-28
- 50 -
b) 1.2 g 1'-benzyl-[1,4']bipiperidinyl-4-one is
obtained analogously to example lb) from 1.5 g
ketal 9a) and 10 ml 6 N hydrochloric acid. m/e=272.
1H-NMR (d6-DMS0): ~ = 7.35 ppm (m, 5H); 4.01 (s,
2H); 3.22 (d, 2H); 3.01 (m, lH); 2.84 (d, 3H); 2.65
(q, 3H); 2.33 (t, 3H); 1.80 (m, 5H).
c) 1.4 g 1-[3-(1'-benzyl-[1,4']bipiperidinyl-4-
ylamino)-2-hydroxy-cyclohexyl]-piperidine-4-
carboxylic acid ethyl ester is obtained as a light-
grey solid analogously to example lj) from 1.2 g
amine li), 1.2 g ketone 9b) and 1.9 g sodium
triacetate borohydride. Fp=92~C.
d) 0.51 g (+)-(3'a,7'~,7~)-1-[3-(1'-benzyl-
[1,4']bipiperidinyl-4-yl)-2-oxo-octahydro-
benzooxazol-7-yl]-piperidin-4-carboxylic acid ethyl
ester is obtained as a white powder analogously to
example lk) from 0.99 g amino-alcohol 9c) and
0.45 g carbonyldiimidazole. Fp=148-150~C.
e) 0.12 g of the title compound is obtained
analogously to example 11) from 0.25 g ethyl ester
9d) and 0.5 ml 1 N sodium hydroxide solution.
m/e=524. 1H-NMR (D20): ~ = 7.25 ppm (m, 5H); 3.92
(t, lH); 3.60 (s, 2H); 3.45 (m, 2H); 3.01 (d, 4H);
2.82 (m, 3H); 2.48 (t, 2H); 2.38-2.00 (m, 7H);
1.95-1.60 (m, lOH); 1.35 (m, 7H).
CA 022~02~0 1998-09-28
Example 10
(+)-(3'a 7'B 7a)-1-(3-~1 4'~Bipiperidinyl-4-yl-2-oxo-
octahydro-benzooxazol-7-~1)-Piperidine-4-carboxylic acid
N~' N
~7' N ~
a) A mixture of 0.74 g N-benzyl derivative 9d) and
0.3 g 10 % palladium/carbon in 20 ml ethanol is
hydrogenated at 50~C/4 bar until the hydrogen
uptake is completed (2 hours). Afterwards the
catalyst is removed by filtration and the filtrate
is evaporated to dryness. In this way one obtains
0.355 g (+)-(3'a,7'~,7a)-1-(3-[1,4']bipiperidinyl-
4-yl-2-oxo-octahydro-benzooxazol-7-yl)-piperidine-
4-carboxylic acid ethyl ester. Pos. FAB=524.
Fp = > 200~C.
b) 0.13 g of the title compound is obtained
analogously to example 11) from 0.2 g ethyl ester
lOa) and 0.7 ml 1 N sodium hydroxide solution. Pos.
FAB (MH+) = 435. Fp > 200~C.
CA 022~02~0 1998-09-28
- 52 -
Example 11
(+)-(3'~,7'a,7a)-1- r 2-Oxo-3-(3,4,5,6-tetrahydro-2H-
~1~4'lbipyridinyl-4-yl)-octahYdro-benzooxazol-7-Y
piperidine-4-carbox~lic acid
N2~0
~ 7~N ~
- a) A mixture of 1.77 g trans-3-bromo-1,2-epoxycyclo-
hexane (Lier E. et al., Helv. Chim. Acta, 62, 932
(1979)), 1.7 ml 4-piperidinecarboxylic acid ethyl
ester and 1.8 g potassium carbonate in 30 ml
dimethylformamide is stirred for 24 hours at room
temperature. Subsequently the reaction mixture is
admixed with 400 ml water and the aqueous solution
is extracted three times with 40 ml diethyl ether
each time. After drying the combined organic phases
over sodium sulfate and removing the solvent, the
crude product is purified by column chromatography
on silica gel (ethyl acetate + 1 % saturated
ammoniacal methanol). In this way 0.6 g (+)-cis-l-
(2,3-epoxy-cyclohexyl)-piperidine-4-carboxylic acid
ester is obtained. 1H-NMR (CDCl3): ~ = 4.05 ppm (q,
2H); 3.20 (d, lH); 3.05 (t, lH); 2.98 (m, lH); 2.82
(m, 2H); 2.40 (m, 12 lines, 2H); 2.18 (m, lH); 1.85
(m, 2H); 1.70 (m, 6H); 1.50 (m, lH); 1.36 (m, lH);
1.19 (t, 3H).
CA 022S02~0 1998-09-28
- 53 -
b) 0.6 g (+)-(la,2a,3B)-1-(3-azido-2-hydroxy-
cyclohexyl)-piperidine-4-carboxylic acid ethyl
ester is obtained as a grey solid analogously to
example 2c) from 0.6 g epoxide lla) and 0.23 g
sodium azide. m/e=296. Fp = 90~C.
c) 0.2 g (+)-(la,2a,3~)-1-(3-amino-2-hydroxy-
cyclohexyl)-piperidine-4-carboxylic acid ethyl
ester is obtained analogously to example li) from
0.44 g azide llb). Pos. FAB = 270. 1H-NMR (CDCl3):
= 4.05 ppm (q, 2H); 3.60 (t, lH); 3.15 (q, lH);
3.00 (m, 2H); 2.50 (m, lH); 2.28-2.00 (m, 5H);
1.90-1.40 (m, llH); 1.25 (m, lH); 1.18 (t, 3H).
d) 0.12 g (i)-(la,2u,3~)-1-(2-hydroxy-3-(3,4,5,6-
tetrahydro-2H-[1,4']bipyridinyl-4-ylamino)-
cyclohexyl]-piperidine-4-carboxylic acid ethyl
ester is obtained analogously to example lj) from
0.2 g amine llc), 0.13 g ketone lb) and 0.31 g
sodium triacetate borohydride. Pos. FAB = 430.
e) 0.35 g (+)-(3'~,7'a,7a)-1-[2-oxo-3-(3,4,5,6-
tetrahydro-2H-[1,4']bipyridinyl-4-yl)-octahydro-
benzooxazol-7-yl]-piperidine-4-carboxylic acid
ethyl ester is obtained analogously to example lk)
from 0.42 g amino-alcohol lld) and 0.24 g carbonyl-
diimidazole. m/e = 456. lH-NMR d6-DMSO): ~ = 8.20
ppm (d, 2H); 6.85 (d, 2H); 4.05 (q+m, 5H); 3.75 (m,
lH); 3.20 (broad d, lH); 2.90 (m, 3H); 2.30 (m,
lH); 2.04 (m, 4H); 1.80 (m, 5H); 1.65 -1.35 (m,
9H); 1.20 (t, 3H).
f) 0.26 g of the title compound is obtained
analogously to example 11) from 0.3 g ethyl ester
CA 022~02~0 1998-09-28
- 54 -
lle) and 1 ml lN sodium hydroxide solution. Pos.
FAB = 428.
Example 12
(i) - ( 3 ' ~, 7 ' ~, 7a) -1- ~ 2-Oxo-3-(3,4,5,6-tetrahydro-2H-
[1,4'lbipyridinyl-4-yl)-octahYdro-benzooxazol-7-yll-
piperidine-4-carboxYlic acid
\~ N~ 3 ~ o
~57~ N~
OH
a) O.9 g (+)-(la,2~, 3~) -1- ( 3 -benzyloxycarbonyl-amino-
2-hydroxy-cyclohexyl)-piperidine-4-carboxylic acid
ethyl ester is obtained as a viscous oil
analogously to example ld) from 0.65 g cis-3-
benzyloxycarbonyl amino-1,2-epoxycyclohexane
(Brouillette W.J. et al., J. Org. Chem., 59, 4297
(1994)) and 0.6 ml 4-piperidine-carboxylic acid
ethyl ester, which is processed further as a crude
product.
b) A solution of O.9 g of the N-benzyloxycarbonylamine
produced in 12a) in 15 ml ethanol is admixed with
0.8 g 10 % palladium/carbon and the mixture is
hydrogenated at normal pressure and room
temperature until the hydrogen uptake is completed
(12 hours). Subsequently the catalyst is removed by
filtration and the solution is concentrated on a
rotary evaporator. In this way one obtains 0.5 g (+
CA 022~02~0 1998-09-28
)-(1~,2~,3~)-1-(3-amino-2-hydroxy-cyclohexyl)-
piperidine-4-carboxylic acid ethyl ester. m/e=270.
H-NMR (d6-DMSO): ~ = 4.05 ppm (q, 2H); 3.48 (dd,
lH); 3.20 (q, lH); 2.80 (dt, lH); 2.68 (dt, lH);
2.58 (t with fine resolution, lH); 2.42 (t with
fine resolution, lH); 2.25 (m, lH); 2.13 (t with
fine resolution, lH); 1.78 (m, 2H); 1.63 (m, 2H);
1.55 (m, 4H); 1.38 (m, 2H); 1.19 (t, 3H); 1.15 (m,
2H).
c) 0.33 g (+)-(la,2~,3~)-1-[2-hydroxy-3-(3,4,5,6-
tetrahydro-2N-[1,4']bipyridinyl-4-ylamino)-
cyclohexyl]-piperidine-4-carboxylic acid ethyl
ester is obtained analogously to example lj) fr~m
0.4 g amine 12b), 0.26 g ketone lb) and 0.42 g
sodium triacetate borohydride. The subsequent
reaction of the amino-alcohol produced in this way
with 0.17 g carbonyldiimidazole analogously to
example lk) yields 0.22 g ethyl ester (+)-(3'~,7'~-
7~)-1-[2-oxo-3-(3,4,5,6-tetrahydro-2H-
[1,4']bipyridinyl-4-yl)-octahydro-benzooxazol-7-
yl]-piperidine-4-carboxylic acid ethyl ester the
saponification of which with 0.6 ml 1 N sodium
hydroxide solution analogously to example 11)
yielded 0.11 g of the title compound. Fp = 150~C
(decomposition). m/e=428. 1H-NMR (d6-DMSO+AcOH):
= 4.05 ppm 8.15 (d, 2H); 7.10 (d, 2H); 4.65 (t,
lH); 4.25 (broad d, 2H); 3.98 (broad d, lH); 3.61
(m, lH); 3.05 (m, 4H); 2.70 (m, 2H); 2.35 (m, lH);
2.10 (m, lH); 2.0-1.10 (m, 16).
CA 022~02~0 l998-09-28
- 56 -
Example 13
AssaY
Microtitre plates were coated overnight with 2 ~g/ml
isolated activated GpIIb/IIIa receptor. After unbound
receptor had been removed by several washing steps, the
surface of the plate was blocked with 1 % casein and it
was washed again. The test substance was added at the
required concentrations, subsequently the plates were
incubated for 10 minutes while shaking in a linear
shaker. The natural ligand of the gpIIb/IIIa receptor,
fibrinogen, was added. After incubating for 1 hour
unbound fibrinogen was removed by several washing steps
and bound fibrinogen was determined by measuring the
change in optical density at 405 nm caused by a
peroxidase-conjugated monoclonal antibody in an ELISA
reader. Inhibition of the fibrinogen GpIIb/IIIa
interaction leads to lower optical densities. The IC50
value was determined on the basis of a concentration-
effect curve.
Literature:
The GpIIb/IIIa fibrinogen ELISA is a modification of the
assay which is described in the following references:
Nachman, R.L. & Leung, L.L.K. (1982): Complex formation
of platelet membrane glycoproteins IIb and IIIa with
fibrinogen. J. Clin. Invest. 69: 263-269.
Wright, P.S. et al. (1993): An echistatin C-terminal
peptide activated GpIIbIIIa binding to fibrinogen,
fibronectin, vitronectin and collagen type I and type
IV, Biochem. J. 293: 263-267.
CA 02250250 1998-09-28
- 57 -
Pharmacological data:
Example IC50 (nMol/l) Name
~ 25 (+)-(3'a,7'B,7a)-1-[2-oxo-3-(3,4,5,
6-tetrahydro-2H-[1,4']bipyridinyl-
4-yl)-octahydro-benzooxazol-7-yl]-
piperidine-4-carboxylic acid