Note: Descriptions are shown in the official language in which they were submitted.
CA 02250254 2006-01-30
64536-958
COMPOSITIONS AND METHODS FOR SELECTIVELY
CROSSLINKING FILMS AND IMPROVED FII.M
ARTICLES RESULTING THEREFROM
The present invention generally relates to polymeric compositions capable
of providing enhanced crosslinking efficiency to multilayer fihns having the
subject composition contained within at least one layer of said film, to a
method of
treating said film to provide enhanced crosslink within said at least one
layer of
the film and to the resultant cross-linked film product as well as articles
made
from said cross-linked film product.
The present invention is particularly useful for the manufacture of flexible
packaging films including those used to package food items.
It has long been known that the physical properties of polymers can be
altered by crosslinking. Control of crosslinking can induce a number of
desirable
changes in the physical properties of a polymer, depending on the application.
For
example, for polyolefins, the softening temperature increases, as does the
toughness, impact strength, and resismce to attack by solvents and grease.
Further, if a crosslinked polymer is stretched to induce orientation, the
material
will have a greater degree of heat-shrink characteristics than an
uncrosslinked
counterpart sample. However, these same physical properties can present
difficulties for manufacture of a product if an attempt is made to merely
substitute
the crosslinked material for an uncrosslinked raw material. This is
particularly
1
CA 02250254 1998-09-29
WO 97/36741 PCTIUS97/04796
true in manufacturing processes that rely on extrusion, coating, or spraying
to
produce a thin layer of material.
An increase in softening temperature or viscosity, for instance, may take a
polymer completely out of the useable range for a given type of equipment. A
higher softening temperature would require higher manufacturing temperatures,
which may cause other useful components of a film or coating to degrade. A
higher viscosity may mean that the material is difficult to spray or extrude,
or that
the resulting thickness of a coating is undesirably high. Some of the changes
in
physical properties obtained by crosslinking polymers are discussed in
Photoinitiated Cross-Linking of Polyethylenes and Diene Copolymers, B. Ranby,
ACS Symposium Series, 1990, Vol. 417, pp. 140-150; and Photoinitiated
Crosslinking of Low Density Polyethylene I: Reaction and Kinetics, Y. Qing, X.
Wenying, and B. Ranby, Polymer and Eng. Sci., Nov. 1991, Vol. 31, No. 22.
Various processes are known for the industrial manufacture of crosslinked
polyolefin materials. These include the use of high energy ionizing
irradiation,
such as gamma- and accelerated electron beam irradiation (e-beam), as well as
chemical crosslinking agents, such as peroxides, silanes and difunctional
compounds, monomers and oligomers which can combine with the target polymer.
One of the problems generally associated with chemically cross-linked
polymers is that the agents capable of causing the cross-link are normally
introduced into the composition prior to its being formed into a packaging
article
(e.g., film). Thus, cross-linking may occur under the elevated temperature
and/or
pressure conditions normally encountered while forming the initial film, such
as
by extrusion. By having the polymeric material cross-linked prior to or during
its
being processed into a film or the like, the processing step requires much
higher
energy, may produce a product having unacceptable properties, or, in certain
instances, is not practical at all.
Various parties have disclosed the use of high energy irradiation as a
means of cross-linking polymeric compositions. For example, German patent
Z
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
publication P 16 29 772.6 by Rosenthal discloses that a relatively thick (500
micron) polyethylene film can be irradiated with an electron beam at a dose of
5
Megarads at a penetration depth of 250 microns and then stretched to a
thickness
of 20 microns to produce a film with sides (material adjacent to a major
surface of
the film) having different properties. In that case, the treated side was said
to have
a melting point of about 160 C, while the untreated side was said to have a
melting point of 115 C. Rosenthal teaches that crosslinking can also be
accomplished using UV, gamma-rays or x-rays using a photoinitiator such as a
chlorinated aromatic or aliphatic compound. Examples cited include
tetrachloroethylene, 1,2,4,5-tetrachlorobenzene, and 1,2,4 trichlorobenzene.
Such
chlorinated aromatic materials are not desirable from a toxicity standpoint,
especially for food packaging.
European Application 0 549 372 Al discloses a method of crosslinking the
surface of a molded article made of a copolymer of an alkenylsilane and an
olefin
having at least two unsaturated double bonds, by dipping the article into a
solution
of a catalyst in hydrocarbon solvent, and then heating the article for two
hours at
80 C.
European Patent Application 0 490 854 A2 teaches a continuous process
for crosslinking polyethylene with UV light. Crosslinking occurs during
extrusion
of the polyethylene while it is in the melt state and under a nitrogen
atmosphere.
The method employs a photoinitiator such as benzophenone or a benzophenone
derivative, and triallylcyanurate (TAC) or triallylisocyanurate (TAIC). While
crosslinking aids such as TAC and TAIC are well known in the prior art, they
are
also highly toxic and unsuitable for food package applications and are taught
to
require the use of an inert atmosphere, which is costly and inconvenient.
United States Patent No. 4,737,559, issued to Kellen et al April 12, 1988
relates to pressure-sensitive adhesives for bandages. The application
discloses that
such adhesives tend to build adhesion strength over time, but that the
addition of a
crosslinking monomer with p-acryloxybenzophenone and subsequent exposure to
3
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
UV radiation yields an adhesive which has a good ability to conform to the
skin
surface, adequate initial adhesion, and limited adhesion build-up, while
providing
low residue upon removal.
In Polymer, 1993, 34(12), 2585-91, Gedde et al, describe the thermal
crosslinking of polyethylene using low molecular weight, 1,2-polybutadiene and
peroxides. Thermal crosslinking has limited applicability because of inherent
instability during the extrusion and/or molding steps. Such processes always
suffer from some degree of trade off between processability and amount of
premature crosslinking.
In Polymer Science, Ser. A, 1994, 36(5), 608-14, Zamotaev et al, describe
the UV crosslinking of polypropylene and low density polyethylene using
benzophenone and difunctional acrylates.
In Reza Kenkyu, 1993, 21(9), 974-80, Ueda et al, describe the crosslinking
of polyethylene with an excimer laser using benzophenone or 4-chlorobenzo-
phenone as photoinitiators. The excimer laser is an impractical radiation
source
because it requires focusing to a small area (10x20 mm) and is quite
expensive.
Furthermore, in this case, long irradiation times and high radiation doses
were
required.
While each of the above referenced teachings and others disclose means of
cross-linking polymeric films using irradiation, a number of problems are
associated with the resultant cross-linked product, especially when the film
is
contemplated for use as a packaging article. Packaging materials formed of one
or
more polymer layers, such as films having two major surfaces and thickness of
up
to about 50 mils, have been used to form closed packages. For example, the
packaging material may be a film which has at least one layer (normally a
surface
layer) which is suitable to provide heat sealing. The ability to be heat
sealed relies
on the ability of the material to flow when heated near its softening or
melting
temperature. Inner or core layers, on the other hand, may be present to
provide
strength, toughness, shrink characteristics and the like.
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
During manufacture, such films are sometimes exposed to radiation, such
as electron-beam radiation or other radiation to crosslink the polymeric
material
forming the film packaging material. Such techniques do not distinguish the
different layers forming a film. Thus, when the irradiation is applied, it may
adversely affect one or more layers while providing the desired beneficial
effect to
other layers of the film. For example, it is desirable to have certain surface
characteristics which provide desired sealability of the outer layer of a
polyolefin
film when subjected to highspeed packaging equipment. However, when
irradiation is applied to such films to enhance the core layers
characteristics, the
radiation indiscriminately adversely effects the sealability of the film. In
addition,
the performance of the sealant layer for its intended purpose is generally
lowered
when crosslinking is induced. This is because the higher the degree of
crosslinking of the sealant material, the less is its ability to flow at a
given
processing temperature. Thus, the resultant packaging material exhibits weaker
and sometimes defective seals.
If one lowers the dosage of irradiation to which the overall film is exposed
(if irradiation is the method of crosslinking) one may be able to lessen the
adverse
effect on the sealant material. However, when this is done, other layers which
benefit from crosslinking (e.g. to provide toughness, improved optics, or
greater
processability during manufacture) may not perform as well. Thus, the
processor
is faced with the trade-off between compromising sealing properties and other
desired properties of films, such as toughness and processability.
Furthermore, it is well known that certain resins, such as poly(vinylidene
dichloride) (PVDC) and poly(propylene) (PP), and other polymers having
tertiary
carbons within their structure, degrade upon exposure to ionizing radiation.
Thus,
improvements in physical properties associated with crosslinking by such
method
cannot always be realized in films containing such materials.
In addition to balancing the concerns associated with irradiation
crosslinking to achieve good seal properties as well as processability and
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
toughness properties, there are two technical parameters which further
complicate
this matter. The first is that materials typically used in sealant layers
(e.g.,
ethylene/vinyl acetate copolymers ("EVA") and the like) tend to crosslink to a
higher degree at a given irradiation dosage than those materials typically
used as
part of the internal layers of a film (e.g., ethylene/alpha-olefin copolymers,
such as
linear low density polyethylene (LLDPE) and the like). Stated another way, a
polymeric layer for which crosslinking is desired may be inherently less
susceptible to crosslinking than a layer of the film for which crosslinking is
not
required.
A second factor is that in irradiation processes where a tubular film is
exposed to irradiation from one side of the tube, then the other in a
"multipass"
setup, the geometry of the tubular film and the physics of irradiation are
such that
the surface layers (e.g., sealant material) will absorb more radiation than
the
internal or core layers. Thus, although the preferred approach is to minimize
crosslinking of the sealant layers, the tendency of many typical irradiation
processes, is to cause more, not less, crosslinking of the sealant material.
In order to overcome the indiscriminate crosslinking caused by high
voltage irradiation, U.S. Patent 4,863,768 (Ishio et al) teaches low voltage
irradiation of films to provide for attenuation of the radiation across the
films
cross-section (thickness). However, this method has certain defects including
the
significant financial investment in equipment needed to use this technology on
a
commercial scale, the inherent unpredictability as to the degree of
crosslinking
achieved at each layer as well as the inapplicability of this technique for
films
having a sealant/core/sealant configuration.
Still another proposed solution is the use of crosslinking enhancers
typically in the form of liquids or powders. Examples include low molecular
weight (LMW) compounds such as peroxides, and unsaturated esters such as
diallylmaleate, trimethylolpropanetrimethylacrylate, and 1,6-hexanediol
diacrylate.
These materials pose several practical problems, including difficulty of
handling
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
liquids and powders during extrusion, regulatory status (lack of food law
compliance for many of these materials), organoleptic concerns, and poor
compatibility with typical extrusion-grade polymers. Peroxides, in particular,
are
inherently thermally unstable. When used, they can initiate crosslinking of
polymers in the extruder. The reaction kinetics involved makes the extrusion
process difficult to control. Extrusion rates and overall process conditions
must be
rigorously controlled to avoid fluctuations in final film properties.
It is highly desired to have a means to selectively enhance the crosslinking
efficiency (i.e., degree of crosslinking at a unit dosage of radiation) of a
specific
layer or layers of a film in an effective and commercially acceptable manner,
preferably in a continuous process. It is further highly desired to provide a
means
to crosslink one or more layers of a film composed of a polymer (e.g.,
polypropylene, PVDC and the like) generally deemed degraded by certain
ionizing
irradiation, especially electron beam radiation. It is still further desired
to be able
to crosslink a polymer film by irradiation to provide a clear, transparent
film
product suitable for use in packaging applications.
Summary of the Invention
The present invention provides a means to selectively enhance the
crosslinking efficiency of a polymeric film and, more particularly, to achieve
enhanced crosslinking with respect to certain layer or layers of a multilayer
film.
The present invention further provides a means of achieving selective
crosslinking
of at least one layer of a polymeric film using actinic irradiation (e.g.,
ultraviolet
radiation or electron beam radiation) and, still further, to permit the
desired
crosslinking to occur on films containing polypropylene, PVDC and other
polymers, without having the resultant film exhibit the degradative effects
normally associated with such treatment.
Specifically, the present invention is directed to multi-layer film having at
least one layer for which crosslinking is desired wherein said layer contains
a PCE
CA 02250254 1998-09-29
WO 97/36741 PCTIUS97/04796
composition composed of (i) a copolymer formed from monomers comprising (a)
at least one polyene monomer, (b) at least one C2-C20 olefin monomer and,
optionally, (c) one or more additional copolymerizable monomers different from
(a) and (b) above or, alternatively, (ii) polymer mixture composed of at least
one
polymer having units derived from (a) at least one polyene monomer; and at
least
one polymer having units derived from (b) at least one C2-C20 olefin monomer
and, optionally at least one additional copolymerizable monomer different from
(a) and (b) above for this mixture. The composition, preferably, further
contains a
photoinitiator agent, especially when the radiation to be applied is ultra-
violet
radiation. The layer(s) having the PCE composition results in a crosslinked
layer
of the film having a higher degree of crosslink than other layers. The subject
PCE
composition can be used alone to form a layer of a film or can be blended with
one
or more polymers to provide a layer of a film for which crosslinking is
desired.
The present invention is further directed to a method of forming a film
having at least one selected crosslinked layer. The method comprises the
forming
of a polymeric film having at least one layer of the film's thickness which
contains
the subject crosslinking enhancer composition therein and subjecting the film
to
actinic radiation (e.g., electron beam or ultra-violet irradiation).
The present invention is still further directed to a multi-layer film product
having at least one first layer of the films thickness which comprises a
crosslinked
polymeric composition and at least one second layer of the film's thickness
comprising a polymeric composition which is crosslinked to a lesser degree
than
that of the first layer. The film product is formed according to the method
described hereinabove.
The present invention is still further directed to a package formed of a
packaging material and having a cavity capable of having or actually having an
article, wherein the package material is composed of the subject single or
multi-
layer film which has the subject crosslink enhancer composition in at least
one
CA 02250254 2006-06-12
64536-958
layer thereof and the package material has been subjected to
irradiation to cause crosslinking of the polymeric material
of said at least one layer.
In one product aspect, the invention provides a
multilayer film that is coextruded and crosslinked, said
film having at least one layer containing a polymeric
crosslink enhancer (PCE) composition, comprising: (i) a
copolymer having polymeric units derived from (a) at least
one polyene monomer, (b) at least one C2-C20 olefinic monomer
and, optionally, (c) at least one copolymerizable monomer
other than (a) or (b); or (ii) a polymer mixture composed of
at least one polymer having polymeric units derived from (a)
at least one polyene monomer, and at least one polymer
having polymeric units derived from (b) at least one C2-C20
olefinic monomer and, optionally, (c) at least one
copolymerizable monomer other than (a) or (b); wherein each
of said at least one layer formed with the PCE composition
is crosslinked to a greater degree than at least one other
layer of said film, wherein at least one layer forming a
major surface of the film is a heat sealable surface layer,
wherein the at least one of said layers containing the PCE
composition is an internal layer of the film.
In one process aspect, the invention provides a
process of forming a film having a plurality of layers,
wherein at least one of said layers is selected to have an
elevated degree of crosslink with respect to at least one
other layer, comprising (A) extruding a film having a
plurality of layers, wherein at least one of said layers
contains a polymeric crosslink enhancer (PCE) composition,
comprising: (i) a copolymer having polymeric units derived
from (a) at least one polyene monomer, (b) at least one
C2-C20 olefinic monomer and, optionally, (c) at least one
copolymerizable monomer other than (a) or (b); or (ii) a
9
CA 02250254 2006-01-30
64536-958
mixture composed of at least one polymer having polymeric
units derived from (a) at least one polyene monomer, and at
least one polymer comprising polymeric units derived from
(b) at least one C2-C20 olefinic monomer and, optionally, (c)
at least one copolymerizable monomer other than not (a) or
(b); and (B) subjecting the film to actinic radiation.
The above invention is fully described herein
below.
Brief Description of the Drawings
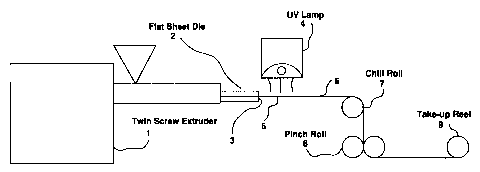
Figure 1 is a schematic diagram of a melt-state
irradiation crosslinking process. In this process, a
conical twin screw extruder (1) is attached to a flat sheet
die (2) with a die lip gap (3). A source of irradiation,
such as a Fusion Systems UV lamp (4) (equipped with a
H-bulb) is positioned at or near the die lip gap (3) in such
a way as to insure that the extruded film (5) passes through
the focal point of the lamp (4). The irradiated film (6) is
drawn over a chill roll (7) and cooled. The resulting film
is drawn through pinch rolls (8) and wound up on a take-up
reel (9);
Figure 2 is a schematic cross-section of a film
suitable for use as part of a multilayer film of the present
invention;
Figures 3 through 8 are schematic cross-sections
of alternative embodiments of films of the present
invention; and
Figures 9 through 12 are bar graphs comparing
films of the present invention to other films.
9a
CA 02250254 2006-01-30
64536-958
Description of the Invention
The present invention is directed to a new and
novel composition and method of using same to provide an
improved multi-layer film suitable for packaging foodstuffs
and other products and the like. At least one layer of the
film formed according to the present invention comprises a
polymeric crosslink enhancer (PCE) composition which
provides that said at least one layer can be crosslinked to
a higher degree than would occur in the absence of enhancer
composition without adversely affecting the desirable
characteristics of other layers present in the film.
9b
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
The present invention provides for a polymeric crosslink enhancing
composition comprising (i) a copolymer comprising units derived from (a)
polyene monomer, (b) at least one C2-C20 olefinic monomer, and optionally (c)
at
least one additional copolymerizable monomer which is different from (a) and
(b)
above or, alternatively, (ii) a mixture of a polymer formed from monomers
comprising polyene monomer (a) and a polymer formed from monomers
comprising at least one C2-C20 olefin monomer (b) and, optionally at least one
additional copolymerizable monomer different from (a) and (b) above of
composition (ii). In addition, the PCE composition of this invention
preferably
further contains a photoinitiator compound, especially when the contemplated
irradiation of the film is to be by ultra-violet radiation.
The following terms are defined herein below to aid in describing and
defining the subject invention herein and in the claims appended hereto:
"Film" shall mean a sheet, laminate, non-woven or woven web or the like
or combinations thereof, having length and breadth dimensions and having two
major surfaces with a thickness therebetween. The film can be composed of more
than one layer (laminate, plies) composed of at least two different
compositions,
extending substantially the length and breadth dimensions of the film. The
thickness of the film can be any suitable thickness of up to about 50 mils to
form a
package and is normally up to about 20 mils, preferably up to about 15 mils,
more
preferably up to about 10 mils and most preferably from 0.1 to 8 mils.
"Layer" or "ply" means herein a member forming all or a fraction of the
thickness of a film wherein the member extends the length and breadth of the
film
and is composed of a distinct composition.
"Crosslinked" or "crosslink" means herein the formation of chemical
bonds directly or indirectly (via some chemical structural entity) between two
or
more of the molecular chains of polymers within a layer of the film. The
degree of
crosslinking is typically shown by a change in the melt flow index, as
measured
according to ASTM D- 1238 with respect to uncrosslinked composition of the
/V
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
same type. Higher degrees of crosslinking are typically reported gel fraction
as
measured according to ASTM-D-2765 with values of greater than one percent
(1 %) gel indicating some degree of crosslink.
"Polyene" means herein a monomer, as defined in Hawley's Condensed
Chemical Dictionary, 12th Edition, page 932, comprising any unsaturated
aliphatic or alicyclic compound containing at least four carbon atoms in a
chain
and having at least two double bonds. The term "at least two double bonds"
refers
to carbon-carbon double bonds. One or more bonds or double bonds of carbon
and an element other than carbon can, optionally, also be present in the
polyene,
such as carbonyl.
"Substituted" means herein the result of a chemical reaction in which one
atom or group of atoms replaces another atom or group of atoms in the
structure of
a molecule. It especially refers to the substitution of a hydrogen atom, of a
hydrogen-carbon moiety, with an alkyl, aryl, hydroxy, halogen, or other
chemical
substituent.
"Polymer" means herein a molecule that has been formed by the union of a
considerable number of simple molecules with one another. The simple molecules
that will unite to provide a polymer are known as monomers and their union is
called polymerization. The polymer may comprise a union of monomers which
are all alike to provide a homopolymer, or of two or more varieties of
monomers
to provide copolymers which are sometimes specifically called copolymers,
terpolymers, tetrapolymers, etc.
"Flowability" means herein the ability of a film or layer to flow under the
influence of heat and/or pressure. This term is especially used with respect
to
films or layers capable of sealing to itself or some other material.
Flowability is
typically reported as melt flow index (MFI) conventionally measured according
to
the procedure of ASTM D-1238. Flowability is an alternative way to indicate
the
level of crosslinking as the higher the degree of crosslink a material, the
lower is
its MFI.
1/
CA 02250254 1998-09-29
WO 97/36741 PCTIUS97/04796
"Ultra-violet" or "UV" means radiation at a wavelength or a plurality of
wavelengths in the range of from 170 to 400 nm.
"Ionizing radiation" means high energy radiation capable of generating
ions and includes electron beam radiation, gamma rays and x-rays.
"E-Beam" means ionizing radiation of an electron beam generated by Van
de Graaff generator, electron-accelerator or x-ray.
"PCE" means a polymeric crosslink enhancer and refers to composition of
the subject invention and to the components thereof.
"AUPO" means herein a PCE copolymer of an advanced unsaturated
polyolefin type formed by catalytic polymerization using at least one single-
site
catalyst, preferably at least one catalyst known as metallocene catalyst, to
have
high random distribution of comonomeric units therein.
The present PCE composition can be composed of composition (i)
comprising a copolymer formed with monomeric units derived from (a) at least
one polyene monomer; (b) at least one C2-C20 olefinic monomer; and,
optionally,
(c) at least one or more copolymerizable monomers other than (a) and (b)
above.
Further, the present PCE composition may contain a compound suitable to act as
a
photoinitiator wherein said compound is blended with the PCE copolymer.
The monomer (a) of the PCE copolymer is selected from a polyene.
Examples of such polyenes are exemplified by but not limited to the following:
5-
ethylidene-2- norbomene ("ENB"), 5- methylidene-2- norbomene, 5-vinyl-2-
norbornene ("VNB"), 5-methylene-2-norbornene, 2,5-norbornadiene, butadiene,
isoprene, 1,4 - hexadiene ("HD"), 4-methyl-1,4-hexadiene, 5-methyl- 1,4-
hexadiene, 4-ethyl-1,4-hexadiene, 1,5-hexadiene, 1,6-heptadiene, 1,4-
heptadiene,
1,5-heptadiene, 5-methyl-1,4-heptadiene, 1,4-octadiene, 1,5-octadiene, 1,6-
octadiene, 5-ethyl-1,6-octadiene, 6-methyl-1,6-octadiene, 7-methyl-1,6-
octadiene,
6-ethyl-1,6-octadiene, 6-propyl-1,6-octadiene, 6-butyl-1,6-octadiene, 1,7-
octadiene, 6-methyl-1,6-nonadiene, 7-methyl-1,6-nonadiene, 6-ethyl-1,6-
nonadiene, 7-ethyl-1,6-nonadiene, 6-methyl-1,6-decadiene, 1,9-decadiene, 6-
/'Z
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
methyl-1,6-undecadiene, 1,8-nonadiene, 1,13-tetradecadiene, 1,4-dodecadiene,
1,5-cyclooctadiene, 1,4-divinylcyclohexane, 1,3-divinylcyclohexene, 1-allyl-4-
vinylcyclohexane, 1,4-divinylcyclohexane, 1,3-divinylcyclopentane, 1-allyl-3-
vinylcyclopentane, 1,5-divinylcyclooctane, 1-allyl-5-vinylcyclooctane, 1,5-
diallylcyclooctane, 1-allyl-4-isopropenylcyclooctane, 1-allyl-4-
isopropenylcyclohexane, 1-isopropenyl-3-vinylcyclopentane, 1-allyl-4-
isopropenylcyclohexane, 4-vinylcyclohexene("VCH"), dicyclopentadiene
("DCPD"), divinylbenzene and vinylisopropenylbenzene. They can be used singly
or in combination with one another as the polyene component of the polymeric
crosslink enhancer. The preferred polyenes are butadiene, ENB, VNB, HD,
DCPD and VCH and particularly preferred as part of AUPOs are ENB and VNB
and most preferred are VNB.
The monomer (a) should be capable of forming units of the PCE
copolymer wherein at least some of the units retain ethylenic unsaturation.
The monomer(s) (b) of the PCE copolymer is at least one C2-C20 olefinic
monomer such as an olefin of 2 to 20 carbon atoms. Such monomers (b) are
exemplified by, but are not limited to: ethylene, propylene, 1-butene, 1-
hexene, 3-
methyl-l-butene, 3-methyl-l-pentene, 3-ethyl-l-pentene, 4-methyl-l-pentene,
4,4-
dimethyl-l-pentene, 4-methyl-l-hexene, 4,4-dimethyl-l-hexene, 4-ethyl-l-
hexene,
3-ethyl-l-hexene, 3,5,5-trimethylhexene, 1-octene, 1-decene, 1-dodecene, 1-
tetradecene, 1-hexadecene, 1-octadecene, 1-eicosene. Typically the monomer (b)
is a C2-C8 -olefin and most typically either ethylene or propylene.
The PCE copolymer may, optionally, contain at least one third monomer
(c) selected from monomers which are other than (a) or (b) monomers described
above. Such monomer (c) are exemplified by but not limited to: vinyl
aromatics,
such as styrene or styrene derivatives and the like, cycloolefin monomers,
such as
cyclopentene, norbomene, tetracyclododecene and the like, unsaturated esters,
such as vinyl acetate, methyl acrylate, ethyl acrylate, and butyl acrylate and
the
/3
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
like, and unsaturated acids, such as acrylic acid or methacrylic acid or their
acid
salts and the like as well as polyvinyl halide such as polyvinyl chloride.
PCE copolymers of the present invention have a density at 25 C of
preferably between 0.8 and 1.0 g/cc.
The PCE copolymer, as used in PCE composition (i), will generally have a
polyene content of 0.01 to 40 mole %, preferably from 0.1 to 10 mole %. The
remainder of the PCE copolymer (the at least one C2 to C20 olefinic monomer(s)
(b) as well as any third or additional monomer(s) (c) will form 99.99 to 60
mole
%, such as 99.9 to 90 mole %, of the polymeric crosslinking enhancer. The
weight average molecular weight (Mw) of the copolymer should be at least about
20,000 daltons, preferably from at least about 10,000 to 1,000,000 daltons. A
variety of factors will determine the optimal composition for a particular end-
use
which include compatibility with any diluent polymer, degree of reactivity
with
respect to the radiation to be utilized and the like. The optimal composition
for a
particular PCE copolymer can be readily determined by minor experimentation.
PCE copolymers of the present invention are exemplified by but not
limited to ethylene-propylene-diene monomer terpolymers (EPDM's) where the
diene monomer is most commonly selected from ENB, HD, DCPD or VCH.
The PCE composition may further include a photoinitiator compound.
Such compounds are blended with the PCE copolymer to provide a substantially
uniform composition. When ultra-violet radiation is contemplated as the form
of
irradiation, the PCE composition preferably should contain the photoinitiator
in
order to increase the crosslink efficiency, i.e., degree of crosslink per unit
dose of
radiation. When E-Beam radiation is contemplated as the form of irradiation,
the
PCE composition may, optionally, include a photoinititator. Although E-Beam
radiation is not normally associated with photoinitiators as crosslinking
readily
occurs in the absence of such compounds, it has been unexpectedly found that
when the present PCE composition is employed which contains such
photoinitiator compounds, crosslink efficiency increases and, therefore, the
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
operator can use less PCE composition, attain higher degree of crosslink,
utilize
lower dosage of electron beam radiation or a combination thereof.
Suitable photoinitators include, but are not limited to, benzophenone,
ortho- and para-methoxybenzophenone, dimethylbenzophenone, dimethoxy-
benzophenone, diphenoxybenzophenone, acetophenone, o-methoxy-acetophenone,
acenaphthenequinone, methyl ethyl ketone, valerophenone, hexanophenone, a-
phenyl-butyrophenone, p-morpholinopropiophenone, dibenzosuberone, 4-
morpholinobenzophenone, benzoin, benzoin methyl ether, 3-o-
morpholinodeoxybenzoin, p-diacetylbenzene, 4-aminobenzophenone, 4'-
methoxyacetophenone, a-tetralone, 9-acetylphenanthrene, 2-acetyl-phenanthrene,
10-thioxanthenone, 3-acetyl-phenanthrene, 3-acetylindole, 9-fluorenone, 1-
indanone, 1,3,5-triacetylbenzene, thioxanthen-9-one, xanthene-9-one, 7-H-
benz[de]anthracen-7-one, benzoin tetrahydrophyranyl ether, 4,4'-
bis(dimethylamino)-benzophenone, 1'-acetonaphthone, 2'acetonaphthone,
acetonaphthone and 2,3-butanedione, benz[a]anthracene-7,12-dione, 2,2-
dimethoxy-2-phenylacetophenone, a,a-diethoxy-acetophenone, a,a-
dibutoxyacetophenone, anthraquinone, isopropylthioxanthone and the like.
Polymeric initiators include poly(ethylene%arbon monoxide), oligo[2-hydroxy-2-
methyl-l-[4-(1-methylvinyl)phenyl]propanone], polymethylvinyl ketone, and
polyvinylaryl ketones. Use of a photoinitiator is preferable in combination
with
UV irradiation because it generally provides faster and more efficient
crosslinking.
Preferred photoinitiators that are commercially available include
benzophenone, anthrone, xanthone, and others, the IrgacureTM series of
photoinitiators from Ciba-Geigy Corp., including 2,2-dimethoxy-2-
phenylacetophenone (IrgacureTM 651); 1-hydroxycyclohexylphenyl ketone
(IrgacureTM 184) and 2-methyl-l-[4-(methylthio)phenyl]-2-moropholino propan-l-
one (IrgacureTM 907). The most preferred photoinitiators will have low
migration
from the formulated resin, as well as a low vapor pressure at extrusion
temperatures and sufficient solubility in the polymer or polymer blends to
yield
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
good crosslinking efficiency. The vapor pressure and solubility, or polymer
compatibility, of many familiar photoinitiators can be easily improved if the
photoinitiator is derivatized. The derivatized photoinitiators include, for
example,
higher molecular weight derivatives of benzophenone, such as 4-
phenylbenzophenone, 4-allyloxybenzophenone, 4-dodecyloxybenzophenone and
the like. The photoinitiator can be covalently bonded to the PCE copolymer or
to
a polymer diluent, as described herein below. The most preferred
photoinitiators
will, therefore, be substantially non-migratory from the packaging structure.
The photoinitiator is added in a concentration of about 0.1 to 3 weight
percent, preferably I to 2 weight percent of the layer containing the PCE
composition. In the case where the photoinitiator is bound to a polymer, the
polymer will typically be added at such a level as to provide 0.1 to 3 percent
of
photoinitiator by weight of the layer containing the PCE composition.
In another embodiment of the present invention, the PCE composition may
be composed of a mixture of at least one polymer having units derived from a
polyene (a); and at least one polymer having units derived from C2-C20
olefinic
monomer(s) (b) alone or with monomer(s) (c), each described hereinabove. For
example, 1,2-polybutadiene, styrene/butadiene copolymers and the like having a
molecular weight (Mw) of 1,000 to 1,000,000, preferably 1,000 to 200,000 can
be
used in combination with a second polymer formed of at least one monomer (b)
and, optionally, at least one monomer (c). For purposes of this description
and the
defined invention of the claims appended hereto the term "PCE copolymer" shall
also refer to the mixture of polymers as herein described unless specifically
stated
otherwise. In view of the fact that same such polymers are substantially
compatible with polyolefins in up to about 5 weight percent, good distribution
can
be easily obtained which results in more uniform distribution of crosslinks in
the
resultant layer of the film.
When the PCE copolymer comprises a polymer mixture (ii) as described
above, the mixture will generally be of a polymer comprising a polyene in from
16
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
0.01 to 40 weight percent, preferably from about 0.1 to 10 weight percent
based on
the mixture (ii) and the polymer of monomer(s) (b) and, optionally (c) will
comprise the remainder of the polymer mixture (ii). The preferred polyene of
the
mixture is polybutadiene, styrene-butadiene copolymers and styrene-isoprene
copolymers.
The PCE copolymer may be formed by known polymerization processes
employing Ziegler-Natta transition metal catalysts as, for example, those
based on
vanadium. However, it is preferred that the resultant copolymer have the
unsaturation uniformly distributed throughout the PCE polymer molecule. That
is
to state, it is preferred that the copolymers contain unsaturated sites which
are
essentially isolated from each other. Conventional polymerization processes
tend
to incorporate multiple identical units adjacent to one another (in blocks)
resulting
in polymers with less random distribution of unsaturation within the polymer
molecule.
A preferred set of PCE copolymers are PCE copolymers (i) and, of these
the most preferred are those which are produced by at least one single-site
catalyst, preferably at least one metallocene catalyst, to provide polymeric
materials with a super-random distribution of comonomers. A single-site
catalyst
is defined as a catalyst which contains a single type of active center. The
resulting
polymer from a single-site catalyst exhibits a narrow molecular weight
distribution
frequently has a polydispersity (Mw/Mn) of less than 3, and narrow
compositional
distribution. A metallocene catalyst is defined as an organometallic compound
with at least one pi-bound cyclopentadienyl-moiety (or substituted
cyclopentadienyl moiety) and most frequently two pi-bound cyclopentadienyl-
moieties or substituted moieties. This includes other C5 pi bound moieties
such as
indenyls or fluorenyls or derivatives thereof. These materials often display
higher
regio-regularity and, in certain cases, higher stereoregularity than
conventionally
prepared copolymers, such as conventionally prepared PCE copolymers. For the
/~-
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
purposes of this disclosure such PCE copolymers are referred to as advanced
unsaturated poly(olefin)s (hereinafter "AUPO").
AUPO's are produced in more chemically homogeneous form, with a
molecular weight distribution that is narrow, with less catalyst residue than
conventionally prepared PCE copolymers. They are thus beneficial in forming
films for packaging applications according to the present invention,
especially
food packaging. They also offer physical properties superior in utility and
scope to
current copolymer technology.
AUPO's can be described as copolymers identical in composition to that
described above for PCE copolymers (i). They differ however in the process by
which they are manufactured which involves the use of at least one single-site
catatlyst and preferably a metallocene catalyst. Typical polyenes which
traditionally have been used are limited to highly substituted polyenes such
as 5-
ethylidene-2-norbomene, dicyclopentadiene or 1,4-hexadiene. More reactive
polyenes often cannot be employed using vanadium-based catalytic systems (used
in conventional EPDM technology, for example) as they tend to crosslink
prematurely leading to gelled, difficult to process materials. It is highly
desirable
to be able to prepare AUPO's which contain more reactive polyenes to
facilitate
crosslinking and other polymer modification/grafting reactions. Such materials
can be crosslinked by chemical (such as peroxide, silane or sulfur), or by
ionizing
or nonionizing radiation processes.
A first improvement obtained by the use of these AUPO materials is that
they require less energy to be crosslinked to a given level of crosslinking,
and
provide more versatility in crosslinking, than conventional PCE copolymers of
the
same type. AUPO's crosslink to a substantially higher degree than saturated
resins at a given energy level. This improvement is largely based on improved
distribution of the polyene component along the polymer backbone leading to
improved crosslink efficiency.
(sr,
CA 02250254 1998-09-29
WO 97/36741 PCTIUS97/04796
A second improvement with AUPO resins involves improved
regioselectivity over vanadium-based catalysts giving less chain scission,
which
competes with crosslinking.
A third improvement of AUPO's over conventional PCE copolymers of
the same type involves the oxidative and light stability of the resin. This
leads to
unsaturated resins with a lower yellowness index.
A fourth improvement of AUPO's over conventional PCE copolymers is
there greater ease of processing to form films and layers.
AUPO's, by virtue of their higher crosslinking efficiency, offer enhanced
orientability, toughness, puncture resistance, tear resistance, impact,
tensile
strength, and/or elongation, and thus are suitable as for aiding in the
formation of a
core and/or abuse layer in multilayer films, bags and laminates. They can be
selectively crosslinked at a lower radiation (e.g., electron-beam) dosage than
currently used materials so that ionizing radiation-sensitive resins, such as
vinylidene dichloride copolymers and polypropylene can be used without
substantial degradation, and with improved organoleptic quality.
AUPO's also offer comparably better blends with improved physical
properties compared with blends using conventional PCE copolymers (including
amorphous EPDM resins) due to improved grafting reactions and reduced chain
mobility. For example, melt crosslinking a blend of two or more polymers which
exhibit appreciable solubility at elevated temperatures but phase separate on
cooling could be improved if one of the components crosslinks and/or grafts
thus
reducing the tendency to phase segregate and, thus, improve the aging
characteristics. This can result in improved optics in the final film made
from
these materials. Improvements in blend properties can also be realized since
there
can be closer matching of resin densities and refractive indices, and also
provides
for reduced yellowness. Multicomponent blends containing AUPO's with
improved optics and physical properties can be thereby produced.
/'j
CA 02250254 2006-01-30
64536-958
AUPO's can be chosen with regard to their molecular weight and
crystallinity in order to tailor blends of these materials with other resins
in order to
optimize physical properties of a film made from the blend, e.g. optical
properties
such as gloss, haze, and clarity. This degree of tailoring is not possible
with more
conventionally produced PCE copolymers.
AUPO's also offer outstanding shrink characteristics, i.e. higher free
shrink, lower shrinking temperatures, and improved orientability compared with
conventional ethylene/alpha-olefin copolymers.
Methods of making these materials using metallocene catalysts are
disclosed in International Application WO 88/04674 by Welborn, et al.
Typical
examples of AUPO'S are those copolymers described above except that they are
prepared using a single-site catalytic process, such as metallocenes. These
include, but are not limited to, terpolymers of ethylene-propylene-polyene
monomer, ethylene-butene-polyene monomer, ethylene-hexene-polyene monomer,
ethylene-heptene-polyene monomer, ethylene-octene-polyene monomer, ethylene-
4-methyl-l-pentene-polyene monomer, ethylene-norbornene-polyene monomer,
and ethylene-styrene-polyene monomer, where the diene is selected from ENB,
VNB, HD, DCPD, VCH, 1,7-octadiene, I,9-decadiene or DVB.
Preferred AUPO resins contain highly reactive vinyl groups without
premature crosslinking or gelation such as reaction extruder or reactor or the
like.
The preferred dienes include 5-vinyl-2-norbornene. Unsaturated PCE copolymers
of olefins and 5-vinyl-2-norbornene can be prepared using simple metallocene
catalysts such as Cp2ZrC12 without premature crosslinking. In this case, the
cyclic-olefinic group is polymerized leaving the pendant vinyl group available
for
subsequent crosslinking or modification/grafting reactions. Alpha,omega-
dienes,
such as 1,7-octadiene and 1,9-decadiene, and other acyclic dienes containing
an
alkyl substituent alpha to one of the vinyl groups are also prefenrd. Examples
of
such acyclic dienes are 3-methyl-1,5-hexadiene, and 3-methyl-l,7-octadiene.
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
Vinyl-unsaturated materials exhibit outstanding crosslinking characteristics
which
lead to films, bags or laminates with improved physical properties.
Preferred AUPO resins comprise single-site catalyzed polymeric materials
with a density of between 0.8 and 1.0 g/cc, such as between 0.84 and 0.96
g/cc,
between 0.86 and 0.94 g/cc, between 0.88 and 0.92 g/cc, and between 0.89 and
0.91 g/cc. All density values falling within any of these stated ranges are
also
included herein.
The PCE composition of the present invention can be used alone to
provide at least one layer of a film or can be used in conjunction with one or
more
diluent polymers suitable for forming the at least one layer for which
enhanced
crosslinking is desired. The amount of PCE composition (either as PCE
copolymer alone or further with photoinitiator, as described above) to be
combined with diluent polymer(s) may be from about 0.1 to 99.9 weight percent
of the composition forming the target layer. The exact amount will depend on
the
degree of crosslinking desired, the compatibility of the subject PCE
compsition
and the diluent polymers used in a particular instance and, therefore, all
values of
weight percentages and ranges between 0.1 and 99.9 weight percent are made
part
of the present teaching.
The diluent polymers are exemplified by, but not limited to:
homopolymers and copolymers of olefins, such as polyethylene, including high
density polyethylene, low density polyethylene, ultra-low density
polyethylene,
linear low density polyethylene, polypropylene, as well as ethylene/propylene
copolymers, polystyrene copolymers, ethylene/acrylate or alkacrylate
copolymers,
ethylene/acrylic acid or alkacrylic acid copolymers and ionomers,
ethylene/vinyl
acetate and the like and mixtures thereof.
The crosslink may occur between and/or among molecules of PCE
copolymer. Further, the PCE copolymer may crosslink or react with molecules or
fragments of molecules of the diluent polymer. For example, a crosslink may be
formed between a first and a second PCE copolymer molecule or crosslinks may
2~
CA 02250254 1998-09-29
WO 97/36741 PCTIUS97/04796
occur among a first PCE copolymer molecule, a second PCE copolymer molecule
and a third PCE copolymer. Crosslink may also occur between at least one PCE
copolymer and diluent polymer molecule or a fragment of such molecules.
Diluent polymer molecules may have residual ethylenic unsaturation suitable as
a
site for entering into a crosslinking with another molecule. In the case of
polymers having tertiary carbon-hydrogen bonds, such as polypropylene and the
like, which may undergo scission of the polymer molecule upon ionizing
irradiation, it has been found that the presence of the subject PCE coplymer
inhibits scission to occur and/or provides a means of recombining the polymer
fragments formed with thermselves or as part of the PCE copolymer. Thus, the
degradative effect of scission commonly associated with ionizing irradiation
of
certain polymers is substantially reduced.
The PCE composition is preferably a solid at ambient temperatures which
is usually between 20 and 25 C. When the PCE copolymer is used in a
composition which will be used in conjunction with diluent polymer, the melt
flow index (MFI) is chosen to be compatible with the rheology of the PCE
copolymer with the polymer diluent or with the materials of the other layers
of the
film, if present. PCE copolymers of low weight average molecular weight (LMW)
of about 5,000 grams/mole or less, are less preferred because they present
difficulties in handling, because of the extra step which may be required to
compound such low molecular weight compounds with the diluent polymer
forming the layer's matrix, and because of the tendency of these low molecular
weight materials to bloom or migrate through the film after extrusion if
crosslinking is delayed. It is preferred to provide the PCE composition as
solid
pellets so that they are easily blended with the other polymeric raw materials
(such
as ethylenic polymers), which are also typically provided in pellet form.
Thus,
these materials can be preblended prior to being fed to an extruder or other
apparatus used to form the film structures. The polymeric crosslinking
enhancer
composition will preferably have a low yellowness index prior to, and
following,
zz
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
irradiation and should possess good organoleptic properties (i.e. not impart
off-
odors or flavors to foods).
The melt flow index (MFI) of the solid PCE copolymer should be between
0.01 and 100 dg/min under ASTM D- 1238 at 2.16 kg/190 C, although higher MFI
materials may be used. The preferred MFI is between 0.1 and 20 dg/min, and
more preferably between 0.1 and 10 dg/min, as such MFI polymers are typically
used in packaging applications (ASTM D-1238, Condition E).
The number average molecular weight (Mõ) of the PCE copolymer of the
invention is preferably at least 10,000 daltons, and more preferably at least
15,000,
20,000, 40,000, or 60,000 daltons. The Mõ is normally between 10,000 and
1,000,000, preferably between 10,000 and 200,000 daltons, such as between
20,000 and 100,000, between 30,000 and 80,000, between 40,000 and 70,000, and
between 50,000 and 60,000 daltons (grams/mole).
The weight average molecular weight (MW) of the PCE copolymer should
be at least about 10,000 daltons and preferably at least 20,000 daltons. The
preferred M,,, can be between 20,000 and 1,000,000 daltons, such as between
30,000 and 350,000, between 50,000 and 250,000, between 70,000 and 170,000,
more preferably between 90,000 and 130,000 daltons.
The viscosity average molecular weight (Mõ) of the PCE copolymer can be
between 20,000 and 1,000,000, preferably between 30,000 and 350,000, such as
between 50,000 and 250,000, between 75,000 and 150,000, more preferably
between 90,000 and 125,000 daltons. For example, when the subject film has a
food contact end-uses, a M,, of at least 120,000 daltons is preferred for
purposes of
compliance with current U.S. food law (FDA) regulations.
Although the PCE copolymer may be amorphous, it is preferred that the
copolymer be senii-crystalline. Thus, AUPO's used as PCE copolymers in the
present invention can have crystallinity ranging from 0 to about 70% or
greater,
such as ranges of from 0.001% to 45% for materials with propylene as the
predominant monomer (b), and from 0.001% to 70% for materials with ethylene
2-3
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
as the predominant monomer (b). For the purposes of this disclosure,
crystallinity
is defined as the fraction of crystals formed as determined by conventional X-
ray
diffraction methods.
Conventional polyolefins typically have a certain number of double bonds
within their molecular structure. The PCE copolymers of the present invention
contributes additional unsaturation to this "background" or baseline
unsaturation
primarily due to the polyene present. Too low a polyene content (taking into
account the percent of the polyene in the copolymer, the percentage
unsaturation
of the polyene monomer itself, the amount of antioxidant present, and the
percentage of the PCE copolymer in a blend of PCE composition and diluent
polymer, where present) can result in insufficient crosslinking enhancement.
It is
preferred that the PCE copolymer contain at least 10 carbon-carbon ethylenic
double bonds (C=C) per 100,000 carbon atoms of the copolymer molecule. The
number of ethylenic double bonds may range from 10 to 33,333 double bonds per
100,000 carbon atoms with from about 20 to about 1000 being preferred. It is
understood that all numerical values and ranges within the specific ranges
disclosed are incorporated herein by reference. Thus, the PCE copolymer will
provide more double bonds to the layer's composition because of the presence
of
the polyene, than a similar polymer without the polyene. The most desirable
unsaturation is vinyl unsaturation (also called terminal or pendent
unsaturation),
but internal double bonds can also be used. Such polymers will contain
unsaturation at a level significantly higher than that represented by the
polymer
end groups, and are also characterized by a uniform distribution of
unsaturation
within the matrix (i.e., random copolymers). The unsaturation of such polymers
is
most readily characterized by infra-red (IR) spectroscopy.
Too high a polyene content (again taking into account the percent of the
polyene in the copolymer, the percentage unsaturation of the polyene monomer
itself, the percentage of the PCE composition and diluent polymer, where
present)
can result in extrusion gels or inclusions which can, if severe enough, affect
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
orientation stability (if an oriented film is being made) and can even result
in
bubble breaks. Even if not severe enough to cause the film to break, the
optics
(gloss, clarity, haze) of the final film can be adversely affected by such
inclusions.
For packaging applications where optics are important, this can mean film
which
is not commercially acceptable. Thus, it is preferred to have the polyene
content
of the PCE copolymer within a range to provide from 10 to about 10,000 and
preferably from 20 to 1,000 ethylenic double bonds per 100,000 carbon atoms
present in the layer composition.
For example, the calculated levels of unsaturation (number of C=C bonds
per 100,000 carbon atoms) for a representative group of polyenes useful as PCE
copolymers are as shown in Table 1 herein below:
TABLE 1
Wei ng t% polyene in PCE Copolymer C=C/100 000 C
ENB 14-HD
0 0 0
1 116 171
2 233 341
3 349 512
4 465 682
5 581 852
6 697 1022
7 813 1192
The values of unsaturation given in Table 1 assume that (1) ethylene and
propylene are the two other monomers in the PCE copolymer, and (2) the ratio
by
weight of ethylene to propylene is 3:1.
The present invention provides a means of providing a desired degree of
crosslinking of polymeric material of a particular layer or layers of a film
while
not adversely effecting any other layers of the film. It is useful in
providing the
desired crosslinking of the target layer(s) of a multi-layer film having
resins,
which are normally adversely effected by ionizing irradiation, especially that
of E-
Z S
CA 02250254 2006-01-30
64536-958
Beam irradiation as, for example, polypropylene, vinylidene dichloride
copolymers and the like. Such polymers tend to undergo chain scission and
resultant degradation when exposed to E-Beam radiation at dosages of 10 to 100
kiloGrays. It has been found that the PCE compositions of the present
invention
when used as a layer or a blend component of a layer, unexpectedly impaits the
desired level of crosslink and its associated properties while minimizing or
substantially eliminating the degradation products and results normaIly
encountered.
The film of the invention can be made by any conventional means,
including coextrusion, lamination, extrusion coating, or corona bonding,
irradiated
and optionally oriented. The above steps can be carried out in various order
and/or repeated as known to those skilled in the art. The materials to be used
in
forming the subject layer of the film may be formed, for example, by initially
mixing the PCE composition with diluent polymer (if desired) during the film-
forming extrusion step by using a single or twin screw extruder in any of
various
mixing sections in manners well known in the art. In some instances, it may be
preferable to pre-compound the materials prior to the film-forming extrusion
step.
Irradiation can be done by any conventional means. In the irradiation process,
the
film is subjected to an actinic radiation treatment, such as ultra-violet,
corona
discharge, plasma, X-ray, ganuna ray, beta ray, or high energy electron
treatment,
such as electron-beam radiation, which induces cross-linking between molecules
of
the in;adiated material.
The ionizing irradiation of polymeric films is disclosed in U.S. Patent No.
4,064,296, to Bornstein, et. al.,
Ionizing radiation dosages are commonly referred to in
terms of the radiation unit "RAD", with one million RADS, also known as a
megarad, being designated as "MR", or, in terms of the radiation unit kiloGray
(kGy), with 10 kiloGray representing I MR, as is known to those skilled in the
art. A suitable radiation dosage of high energy electrons is in the range of
between
26
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
and 200 kGy, preferably between 20 and 150 kGy, and more preferably between
30 and 120 kGy such as between 40 and 90 kGy. Preferably, irradiation is
carried
out by an electron accelerator and the dosage level is determined by standard
dosimetry methods. Other accelerators such as a Van de Graaff or resonating
5 transformer may be used. The radiation is not limited to electrons from an
accelerator since any ionizing radiation may be used. As can be seen from the
descriptions of preferred films for use in the present invention, the most
preferred
amount of radiation is dependent upon the film and its end use and the exact
dosage can be readily determined by one skilled in the art.
The film may, alternately, be irradiated with ultra-violet radiation. In this
embodiment the PCE composition may, preferably, contain at least one
photoinitiator agent described herein above. The radiation should be emitted
from
a source capable of emitting radiation of the wavelength of from 170 to 400
nanometers (nm). The radiation dosage should be at least 0.1 Joule per cm2 and
preferably from 0.5 to 10 Joules per cm2 and most preferably from 0.5 to about
5
Joules per cm2. The dosage required on a particular application will depend on
the
configuration of the layer in the film, the composition of the layer, the
temperature
of the film being irradiated and the particular wavelength used. The dosage
required to cause crosslinking to occur for any particular set of conditions
can be
determined by the artisan.
Any UV source capable of being positioned at or near the die lip so that the
film passes through the focal point of a lamp while the layer to be
crosslinked is
still in the melt state can be used.
In another embodiment of the invention, the layer need not be crosslinked
upon extrusion, but may be crosslinked at some later time, at the convenience
of
the processor, and typically in conjunction with other processing steps. In
this
embodiment, the crosslinking may take place at room temperature or at an
elevated temperature which is below the melting point of the film as a whole.
For
example, a film having layers with different melting points can be heated to a
2~
CA 02250254 2006-01-30
64536-958
temperature between the two melting points and then irradiated. The
crosslinking
effect would be greatly enhanced in the layer with the lower melting point,
yielding some of the benefits of melt-phase crosslinking without tying the
crosslinking step to the time and location of the extrusion step.
The present invention as described herein relates to improved methods and
materials for making multilayer thermoplastic films, however, one of ordinary
skill in the art will readily recognize that it is applicable to thermoplastic
objects in
a variety of forms such as cups, bottles and trays. In addition, a fihn or
coating
made according to the present invention may be applied to a variety of
substrates,
including other polymeric materials, paper, glass, silica, and metal, as well
as
fabrics made from natural and synthetic fibers.
A common measure of the amount of crosslinking in an irradiated film is gel
content or percent (%) gel. The weight fraction of polymer insoluble in
a.suitable
solvent, such as boiling toluene or boiling xylenes, is referred to as the %
gel and
this is an indication of degree of crosslinking. It is determined by placing a
0.4 to 0.5
gram sample weighed to 0.1 mg into a cellulosic or Teflon extraction
thimble.
About 100 ml solvent is poured into a 400 ml Erlenmeyer flask having a block-
tin
condenser with copper cover, and borosilicate glass siphon cup. Three to six
boiling
stones (carborundum or equivalent) are added to the flask. The flask is then
set on a
hot plate, the thimble is placed in the siphon cup, and the siphon cup and
condenser are positioned into the flask. The toluene is brought to a boil, and
the
heat is adjusted to yield a reflux rate of between two and four drops per
second.
The material is refluxed for twenty one hours. The thimble is then removed
with
forceps. The sample is air dried under a hood for at least two hours. The
sample
is transferred to a vacuum oven heated at 50 C under 25 to 30 inches of
mercury
vacuum, and the sample is dried in the oven for 24 hours. The gel is weighed
on
an analytical balance. Gel % is calculated by the forrnula:
Gel weight, g X 100 = % gel
Sample weight, g
28
CA 02250254 2006-01-30
64536-958
The sample is extracted for a second 21 hours to assure complete dissolution
of all
soluble portions. If the gel of the second extraction is more than 3%
(absolute)
less than the gel of the first extraction, subsequent extractions are run.
An alternate measure of the amount of crosslinking in an in-adiated film is
"flowability". A lower flowabilility value indicates a greater degree of
crosslinking.
The following is a detailed description of the drawings:
Figure 1 is a schematic diagram of a melt-state irradiation crosslinking
process. In this process, a conical twin screw extruder (1) is attached to a
flat sheet
die (2) with a die lip gap (3). A source of irradiation, such as a Fusion
Systems
UV lamp (4) (equipped with a H-bulb) is positioned at or near the die lip gap
(3)
in such a way as to insure that the extruded film (5) passes through the focal
point
of the lamp (4). The in,adiated fihn (6) is drawn over a chill roll (7) and
cooled.
The resulting film is drawn through pinch rolls (8) and wound up on a take-up
reel
(9);
Figure 2 illustrates a layer 11 which contains a PCE composition of the
present invention to provide enhanced crosslink at a given irradiation dosage.
Such a layer can be combined with other layers to provide a multilayer film.
Figure 3 shows a multilayer film having layers 11 and 12. Layer 12 is a
heat sealable layer which can be formed of any polymeric material, such as a
polyolefin; more preferably ethylenic polymers, such as ethylene/alpha-olefin
or
ethylene/unsaturated ester copolymers, such as ethylene/vinyl acetate
copolymer,
and ethylene/alkyl acrylate copolymer, as well as polyamides, or polyesters.
Layer
11 is as described above.
Figure 4 shows a multilayer film with layers 11, 12, and 13. Layer 13 is an
abuse-resistant layer useful as an outermost layer of a film for packaging
applications. This layer can be formed by any polymeric material such as a
polyolefin, more preferably ethylenic polymers, such as ethylene/aipha-olefin
or
29
CA 02250254 1998-09-29
WO 97/36741 PCTIUS97/04796
ethylene/unsaturated ester copolymers, polypropylene, polyamide, polyester,
and
the like. The layers 11 and 12 are as described above.
Figure 5 shows a multilayer film with layers 11, 12, 13 and 14. Layer 14 is
an adhesive layer in films where such a material beneficially ensure or
enhance
interlaminar bond strength between any or all of layers 11, 12, and 13. The
specific placement of layer 14 in a film of the invention, as shown in Figure
4, is
by way of example only. Such adhesives may be polymeric, such as an acid or an
acid anhydride-grafted polyolefins. Alternatively, layer 13 can represent a
conventional adhesive or glue of any suitable kind, e.g. polyurethane adhesive
where a laminate of the multilayer film 10 with another is contemplated. The
remaining layers are as described above.
Figure 6 shows a multilayer film with layers 11, 12, 13, 14, and 15. Layer
comprises an oxygen barrier material, such as ethylene-vinyl alcohol copolymer
(EVOH), vinylidene dichloride/vinyl chloride copolymer, vinylidene
15 dichloride/methyl acrylate copolymer, polyester, or polyamide, etc. The
remaining
layers are as described above.
Figure 7 shows a multilayer film with layers 11, 12, 13, 14, 15, and 16.
Layer 16 comprises a core or internal layer which contributes bulk,
shrinkability,
toughness, or some other function or property to the overall film. Layer 16
can
comprise any of the polymers disclosed for the other layers. The remaining
layers
are as described above.
Figure 8 shows a multilayer film with layers 11, 12, 13, 14, 15, 16, and 17.
Layer 17 comprises an adhesive layer in cases where such a material can be
beneficial in ensuring or enhancing interlaminar bond strength. Such adhesives
have already been described with respect to Figure 4. The remaining layers are
as
described above.
The present invention can be used to crosslink to the polymeric
composition of different layers at different levels. For example, an abuse-
resistant
layer (13) and/or an internal layer (16) can be crosslinked to a greater
extent than a
3d
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
sealant layer (12). This may be accomplished by varying the amount of PCE
composition contained in each target layer.
Figures 9 through 12 are bar graphs comparing monolayer films of the
present invention to other, control films that do not have a crosslinking
enhancer.
These graphs are described in detail below with respect to the examples.
The present invention can be used to enhance the crosslink content of one
or more layers of a film. The film has two major surfaces and a thickness
which
extends from one major surface to the other. The film thickness is composed of
n
layers where n is a positive integer of from I to an upper value Z which can
be any
positive integer of two or greater and usually is a value of from 2 to 14,
preferably
2 to 12. The film will have x layers for which enhanced crosslink is desired
(target layers) where x is an integer of from I to an upper value of (Z-1).
The
target layers may be any layer or combination of layers of the film including
layer(s) providing one or both of the film's major surfaces or any of the
layers
spaced away from the major surfaces (core layers) or a combination thereof.
The
choice of layers will be dictated by the configuration of the film and the
layer(s)
for which crosslink is desired without causing deterioration of the other
layers and,
thereby, provide an improved film product.
The invention may be further understood by reference to the examples
shown below. These examples are given for illustrative purposes only and are
not
meant to be a limitation on the invention described herein or defined by the
claims
appended hereto. All parts and percentages are by weight unless otherwise
stated.
Tables 2, 2A, and 2B identify the materials used in the examples. The
Tables thereafter describe the films made with these materials.
3/
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
TABLE 2
MATERIAL* TRADENAME SOURCE
PEI DowlexTM 2045.03 Dow
PE2 DPF 1150.02 Dow
PE3 AffinityTM PL 1270 Dow
PE4 AffinityTM PF 1140 Dow
PE5 XU59001.00 Dow
PE6 XU59202.01 Dow
PE7 DPF 1150.01 Dow
PE8 AffinityTM XU 59220.04 Dow
PE9 DowlexTM 2045.14 Dow
PEIO DowlexTM 2037 Dow
EV 1 XV 65.93 Exxon
EV2 LD-318.92 Exxon
EV3 EscoreneTM LD-761.36 Exxon
EV4 PE 1335 Rexene
OBI XU 32034.06 Dow
OB2 E-151 Evalca
PAl GrilonTMCF6S EMS
EM 1 EMACTM SP 1305 Chevron
AB 1 10,075 ACP concentrate Teknor Color
AB2 80,274 ACP concentrate Teknor Color
AD1 AdmerTM SF 700 A Mitsui
*PE 1= LLDPE, an ethylene/ 1-octene copolymer with a density of 0.920 gm/cc
and a 1-octene content of about 6.5 wt%.
PE2 = a single site, branched, ethylene/ 1-octene copolymer with a density of
0.901 gm/cc and a 1-octene content of about 12.5 wt%.
PE3 = a single site, branched, ethylene/ 1-octene copolymer with a density of
0.898 gm/cc and a 1-octene content of about 13 wt%.
PE4 = a single site, branched, ethylene/ 1-octene copolymer with a density of
0.8965 gm/cc and a 1-octene content of about 14 wt%.
PE5 = a single site, branched, ethylene/ 1-octene copolymer with a density of
0.91
gm/cc and a 1-octene content of about 10 wt%.
PE6 = a single site, branched, ethylene/ 1-octene copolymer with a density of
0.900 gm/cc and a 1-octene content of about 13 wt%.
3Z-
CA 02250254 2006-01-30
64536-958
PE7 = a single site, branched, ethylene/ 1-octene copolymer with a density of
0.901 gm/cc and a 1-octene content of about 12.5 wt%.
PE8 = a single site, branched, ethyleneJ 1-octene copolymer with a density of
0.896 gm/cc and a 1-octene content of about 14 wt%.
PE9 = LLDPE, an ethylene/ 1-octene copolymer with a density of 0.920 gm/cc and
a 1-octene content of about 6.5 wt%.
PE 10 = LMDPE, an ethylene/ 1-octene copolymer with a density of 0.935 gm/cc.
and a 1-octene content of 2.5 wt%.
EV 1= ethylene vinyl acetate copolymer with 15 wt% vinyl acetate comonomer.
EV2 = ethylene vinyl acetate copolymer with 9 wt% vinyl acetate comonomer.
EV3= ethylene vinyl acetate copolymer with 28 wt% vinyl acetate comonomer.
EV4 = ethylene vinyl acetate copolymer with 3.3 wt% vinyl acetate monomer.
OB 1= 96 wt.% vinylidene dichloride/methyl acrylate copolymer with 8.5 wt.%
methyl acrylate comonomer, 2 wt.% epoxidized soybean oil, and 2 wt. % butyl
acrylatelmethyl methacrylate/butyl methacrylate terpolymer.
OB2 = ethylene/vinyl alcohol copolymer (44 mole % ethylene ).
PA 1= nylon 6,12 copolymer.
EM1= ethylene/methyl acrylate copolymer with 20 wt.% methyl acrylate
comonomer.
AB l= 89.8 wt% low density polyethylene (Exxon LD 203.48) + 10 wt% synthetic
amorphous silica (SyloidTM 74X6500 from Davison Chemical) + 0.2 wt% calcium
stearate.
AB2= about 82 wt9'o low density polyethylene (Exxon LD 203.48) + 10 wt%
synthetic amorphous silica (SyloidTM 74X6500 from Davison Chemical) + 0.2
wt% calcium stearate + small amount of pigments.
ADI = anhydride-grafted polyolefin blend.
33
CA 02250254 2006-01-30
64536-958
TABLE 2A
CE] KeltanTM 2308 DSM
CE2 Pol sarTM 227P Bayer
CE3 VistalonTM 8731 Exxon
CE4 DutralTM 4033 Enichem
CE5 VistalonTM 3708 Exxon
CE6 PolysarTM 847XP Bayer
CE7 NordelTm 2722-el.ec DuPont
CE8 KeltanTM 5509 DSM
CE9 Ro aleneTM IM.7200 uniro aI .
CEIO TafinerTm TP 3]$0 Mitsui.
CE 11 Ro aleneTM JM'7100 1'.Tniro ai
CEI2 EP 18ISP . JSR
CE13 KeltanTM 5808 DSM
CE14 NordelT"' 5892 (2760 DuPont
CE15 NordelTM 3681 (2744) DuPont
CE16 EP 57P JSR
CE17 DutralTM 4028 Enichem
CE 18 Dutral''m 403? Enichem
CE19 KeltanTM E80.1 k. DSM
CE 20 NordelTM 2722-P DuPont
The crosslinking enhancers copolymer (CEI through CE 20 ) were EPDM resin
type PCE copolymers having the composition and properties listed in Table 2B
below.
34
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
TABLE 2B
PCE C2 DIENE DIENE MOONEY MFI
(wt%) a (wt%)a TYPE VISCOSITY (dg/min)
ML1+8 MLI+4
@ 100 C @ 125 C
CE1 73 2.0 ENB*
CE2 75 3.0 ENB 24.2 14.3
CE3 74 3.0 ENB 30.3 21.0
CE4 76 3.0 ENB 26.9 15.3
CE5 65 3.5 ENB 79.2 47.0
CE6 74 4.0 ENB 85.0 57.0
CE7 72 6.0 HD** 31.1 23.2
CE8 73 4.5 ENB
CE9 76 4.5 ENB 65.8 46.4
CE 10 75 4.7 ENB 12.6 6.6
CE 11 74 5.0 ENB 6.0 b
CE12 75 5.9 ENB
CE13 67 6.0 ENB
CE14 71 6.0 HD 80.4 58.9
CE15 71 6.0 HD 61.9 46.1
CE 16 72 7.1 ENB
CE 17 76 3-5 ENB
CE18 73 3-5 ENB
CE19 -- --- ENB 3 0c
CE20 HD 31.9 24.1
a Monomer content determined by ASTM D-3900.
b MFI determined by ASTM D-1238 at 230 C/21.6 kg.
c MFI determined by ASTM D-1238 at 230 C/ 10.0 kg.
* ENB = 5-ethylidene-2- norbornene
* * HD = 1,4 - hexadiene
Mooney Viscosity was measured in accordance with ASTM 1646. In this
procedure a Mooney viscometer is used to measure the effects of time of
shearing
3~
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
and temperature on the comparative viscosities of rubber compounds. Rotor
torque in "Mooney units" (1 MU = 0.833 N=m) is recorded over a 4 or 8 minute
period, typically passing through a broad minimum, and the minimum is reported
as the Mooney viscosity.
Certain materials were blended together to form some of the film
structures, and these blends are identified as follows:
PEB 1= 90 parts PE 1+ 10 parts AB 1.
PEB2 = 92.5 parts EV2+ 7.5 parts PEI.
PEB3 = 90 parts PE6 + 10 parts PE1.
PEB4 = 85 parts PE7 + 15 parts PE 1.
PEB5 = 75 parts PE6 + 25 parts PEI.
PEB6 = 75 parts PE7 + 25 parts PEI.
CEB 1= 90 parts PE2 + 10 parts CE3.
CEB2 = 90 parts PE2 + 10 parts CE2.
CEB3 = 70 parts PE2 + 20 parts PE1+10 parts CE2.
CEB4 = 60 parts PE2 + 30 parts PE1+10 parts CE2.
CEB5 = 60 parts PE2 + 30 parts PE1+10 parts CE3.
Some of the PCE copolymers were further analyzed to determine
molecular weight and molecular weight distribution. The results of the
analysis
appear in Table 3.
3~
CA 02250254 2006-01-30
64536-958
TABLE 3
n w z z+1 Txl iSper3l ty tT1nSiC
x 1000 x 1000 1 1000 00 4w/Mn ~~0s'~Y
/mol) mol). o1) ol) ol) ~gl
56 121 9 .15 1.41 ...
39 162 74 1,058 . 117 .18 1.26
.45 1.31
17 115 3 302 10
JE
2 00 89 73 173 .22 1.8 17 191 76 . 1$8 2.47 10
' 1 153 505 94 110 .87 1.151 192 135 66 165 .73 1.84
18 152 35 84.9 1.86 1.11
14 53 48 64 1,096 192 3.96 1.88
E 15 59 06 526 881 163 3.47 1.70
0 1 1168 11 1,094 116 5.41 . 1.19
Some of the polymeric crosslinking enhancers were also analyzed to
5 determine melt flow index at different ASTM D-1238 conditions, and also to
determine density (at room temperature) using heptane displacement in a
TM
Toyoseiki densimeter. The results of these analysis appears in Table 4 below.
TABLE 4
PCE Melt Flow Index Density
type (d min) cc)
2.16 kg/190 C 10.0 kg/190 C 2.16 kg/230 C
CE2 1.33 11.30 2.69 0.878
CE3 0.29 5.35 0.80 0.877
CE4 1.43 10.33 2.98 0.873
CE5 0.14 1.66 0.28 0.839
CE6 0.08 0.99 0.15 0.879
CE7 0.23 5.05 0.56 0.874
CE9 0.10 2.98 0.22 0.879
CE 10 4.06 27.40 7.48 0.879
CE 14 0.01 0.41 0.03 0.870
CE15 0.02 0.72 0.05 0.868
CE20 0.16 3.78 0.46 0.873
37
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
Example 1-16
Sixteen monolayer films in accordance with the invention were extrusion
cast. Each film was then exposed to electron beam irradiation, at a given
power
setting (beam current), in an E-beam irradiation unit. By adjusting the power
settings one can directly change the dosage received. Thus, the beam current
is
substantially directly proportional to received dosage. The process was
repeated
twice with additional samples of each film, but at different power settings
(different radiation dosage). The composition of these films, and two
comparative
films (COMP. A and B) produced in the same manner, are given in Tables 5 and 6
below. Each film had a thickness of about 12 mils.
TABLE 5
Example Film Structure
COMP. A 100% PEI
1 90% PE 1+ 10% CE7
2 90% PE I+ 10% CE3
3 90% PE 1+ 10% CE4
4 90% PE 1+ 10% CE2
5 90% PE 1+ 10% CE5
6 90% PE 1+ 10% CE6
7 90% PE 1+ 10% CE9
8 90% PE 1+ 10% CE 10
3,P
------ -- ------ --
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
TABLE 6
Example Film Structure
COMP.B 100% PE2
9 90% PE2 + 10% CE7
90% PE2 + 10% CE3
11 90% PE2 + 10% CE4
12 90% PE2 + 10% CE2
13 90% PE2 + 10% CE5
14 90% PE2 + 10% CE6
90% PE2 + 10% CE9
16 90% PE2+ 10% CE 10
Figures 9 and 10 provide bar graphs depicting the received dosage, in
KGy, versus the gel % determined for each film sample. Numerals in the drawing
5 correspond to the respective samples. It can be seen that very substantial
improvements in crosslinking efficiency (values shown at the top of each bar),
as
measured by % gel, are obtained by films of the present invention when
compared
to a control film of LLDPE.
10 Example 17-28
Seven three-layer films, and one control film (COMP. BB) were cast
coextruded to produce a substrate. In each case, the substrate was exposed to
electron beam irradiation, at a dosage of about 50 kGy, in an irradiation
unit.
After irradiation of the substrate, four additional layers were added to the
substrate
15 by simultaneous extrusion coating process. The resulting seven-layer films
was
then oriented by conventional trapped bubble method to produce crosslinked
heat
shrinkable films. The composition of these films is given in Table 7 below.
The
thickness (in mils) of each layer of the film of Example 17 was measured,
before
orientation, and was determined to be:
3~
CA 02250254 1998-09-29
WO 97/36741 PCTIUS97/04796
layer I layer 2 layer 3 layer 4 layer 5 layer 6 layer 7
4.9 13.2 1.0 1.8 1.0 2.9 1.7
After orientation, the final thickness of the film of Example 17 was 2.7 mils.
In a similar manner, the thickness (in mils) of each layer of the films of
Examples 18 to 23 and COMP.BB was measured, before orientation and was
determined to be:
layer 1 layer 2 layer 3 layer 4 layer 5 layer 6 layer 7
5.4 9.8 1.0 1.9 1.0 2.6 1.8
After orientation, the final thickness of the film of each of Example 18 to
23 was 2.2 mils.
In each film, layer 1 would preferably form the food or product contact
layer, and sealant layer, for a typical packaging application. The composition
of
each layer of the films is given in Table 7 below. The double slash (//)
indicates
where a substrate is adhered to an extrusion coated layer.
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
TABLE 7
EXAMPLE STRUCTURE
COMP.BB PEB3/PE2/ EV 1//OB 1/EM 1/PE7/PEB2
17 PE5/CEB 1/EV 1//OB 1/EM 1/PE7/PEB2
18 PEB3/CEB2/ EV 1//OB 1/EM 1/PE7/PEB2
19 PEB3/CEB 1/ EV 1//OB 1/EM 1/PE7/PEB2
20 PEB3/CEB3/ EV 1//OB 1/EM 1/PE7/PEB2
21 PEB3/CEB4/ EV I//OB 1/EM 1/PE7/PEB4
22 PEB3/CEB5/ EV 1//OB 1/EM 1/PE7/PEB4
23 PEB5/CEB4/ EV 1//OB 1/EM 1/PEB6/PEB6
Some of the film formulations, and COMP.BB, were rerun at four different
dosage levels of E-Beam irradiation in an irradiation unit to evaluate the gel
% as
a function of level of irradiation. Table 7A shows the dosage received, in
KGy,
versus the gel % determined for these selected films. The % gel was in each
example determined for the substrate (layers 1 to 3).
TABLE 7A
X. Gel %
@30 kGy @44 kGy @57kGy @7lkGy
OMP. BB 10
18 8 15
19 13
20 6 14
17"1
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
Examples 24-29
Six monolayer films were extrusion cast, and then exposed to electron
beam irradiation, at a given power setting (beam current), in an E-beam
irradiation
unit. The process was repeated with additional samples of each film, but at
two
different power settings. The composition of these films, and the three
comparative films (COMP. A, B, and C) produced in the same manner, are given
in Tables 8 and 9. Figure 11, with respect to the samples of Table 8, and
Figure
12, with respect to the samples of Table 9, graphically show the received
dosage,
in kGy, versus the gel % determined for each film example.
TABLE 8
Example Film Structure
COMP. A 100% PEI
24 95% PEI + 5% CE3
25 90% PE 1+ 10% CE3
26 95% PE i+ 5% CE5
27 90% PE 1+ 10% CE5
TABLE 9
Example Film Structure
COMP.B 100% PE2
28 90% PE2 + 10% CE5
COMP. C 100% PE8
29 90% PE8 + 10% CE5
The bar graphs of Figures 11 and 12 clearly show that one achieves higher
crosslink efficiency with the films of the present invention than the
comparative
films of examples Comp. A, B and C.
'r~Z
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
Preparation and Characterization of Representative Advanced Unsaturated
Poly(olefins) (AUPO's)
Example 30
Polymerizations listed in Tables 10 and 11 herein below were each
conducted in a 2L stainless steel autoclave reactor equipped with an overhead
helical impeller at either 50 C and 50 psig ethylene or 75 C and 60 psig
ethylene
using bis(cyclopentadienyl)zirconium(IV)dichloride (Cp2ZrC12) and
methylaluminoxane (MAO) in dry, degassed toluene. The reactor was charged
with toluene (300 to 1300 g), 1-hexene (50 to 200 g) or norbornene (10-100 g),
diene (0.1 to 20 g), and MAO (2 to 20 g of MAO/toluene solution containing 10
wt% Al), and saturated with ethylene at either 50 C and 50 psig or 75 C and
60
psig. The polymerization was commenced by addition of the metallocene catalyst
(0.1 to 3 mg Cp2ZrClZ in 10 mL toluene) to the reactor and the polymerizations
were allowed to proceed for 0.5 to 2 hours. The reactor was quickly vented and
the contents discharged into methanol, filtered and dried. Tables 10 and 11
show
the amount and type of starting materials, and the amount and type of
polymers,
made by this procedure. "E" herein means ethylene, "H" herein means 1-hexene,
and "NB" herein means norbornene. A-1 through A-8 represent polymers
produced in each of eight polymerization reactions in accordance with the
above-
described procedure.
~3
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
TABLE 10
Ethylene/1-Hexene/Pol ey ne Copolymer
- la -2a -3a -45 -5a A-6a
oluene 300 300 500 506 146 154
(grams)
1-hexene 64 7 108 112 178.4 175
(grams)
iene NB NB 5-VNB 5-VNB 5-VNB NB
(type)
iene 3.3 .6 5.1 15.1 8.3 8.7
(grams)
AO 3.0 .0 .0 .0 1.5 1.5
(grams)
atalyst .25 .38 .25 1.0 .38 .5
(mg)
eaction .5 .0 1.7 1.7 1.5 1.7
ime
(hours)
olymer -H-ENB -H-ENB i-H- VNB -H-VNB B-H-VNB -H-ENB
(type)
olymer 6 52 65 50.4 104 109
(grams)
a) 50 psig ethylene and 50 C
CA 02250254 1998-09-29
WO 97/36741 PCTIUS97/04796
Table 11
Ethylene/Norbornene/Polyene Copolymer
-7 k-8 b
oluene (grams) 1285 1235
B (grams) 16 5
iene (type)
iene (grams) .3 16.5
AO (grams) 15 12
atalyst (mg) .8 1.4
eaction time 2.0 1.2
(hours)
olymer(type) -NB-VNB -NB-VNB
olymer (grams) 103 76
b) 60 psig ethylene and 75 C.
The AUPO's described above were found to be compositionally pure as
indicated by a single melting endotherm by differential scanning calorimetry
(DSC) at 10 C per minute and with narrow polydispersities by gel permeation
chromatography (GPC). Table 12 summarizes the characterization of the
terpolymers.
7'S~
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
TABLE 12
AUPO A-1 A-2 A-3 A-4 A-5 A-6
Density (g/cc) 0.913 0.919 0.906 0.915 0.908 0.909
Mol% H 5.1 3.7 5.5 4.5 5.4 5.1
Mol% diene 1.2 2.2 0.8 1.9 0.9 1.1
(ENB) (ENB) (VNB) (VNB) (VNB) (ENB)
T. ( C) 90 91 91 96 93 93
AHf (J/g) 60 40 50 60 70 70
I2 (dg/min) 2.8 6.3 0.6 4.6 4.5 10.5
MW x 10 81 68 124 70 nd nd
(grams/mole)
MW / Ml 1.9 1.9 1.9 1.9 nd nd
To further illustrate the breadth of the invention, copolymers of ethylene
and norbornene, as well as unsaturated terpolymers based on these monomers
with
VNB (at two different levels) were also prepared. An ethylene-norbomene
copolymer was prepared in a 20 gallon jacketed stainless steel autoclave
equipped
with baffles and an overhead turbine-style stirrer. The reactor was charged
with
48.1 kg of toluene containing 0.2 wt% MAO, 4.2 kg of norbornene solution
(65wt% in toluene) and heated to 75 C and pressurized to 60 psig ethylene.
After the system equilibrated, the polymerization was initiated by addition of
a
total of 38 mg of Cp2ZrC12 and ethylene was fed in on demand. The
polymerization was allowed to proceed for 4.25 hours. It was then terminated
by
addition of 100 mL of methanol. The reactor was vented, the contents
discharged
and precipitated into methanol and the polymer filtered and dried in a vacuum
oven. 7.1 kg of polymer was isolated. The polymer (A9 ) had an MFI (190 C and
2.16 kg) of 1.7 dg/min with a Tm of 75 C. The polymer was found, by carbon-13
NMR, to contain 10 mol% NB and the microstructure was consistent with an
--- -
CA 02250254 2006-01-30
= -
64536-958
addition polymerization with NB monomer inserted without ring-opening. The
reaction conditions for the unsaturated E-NB terpolymers A-7 and A-8 is listed
in
Table 11 and the characterization of these materials A-7; A-8 and A-9 are
listed in
Table 13.
Table 13
AUPO A-7 A-8 A-9
Density (g/cc) 0.958 0.952 0.958
Mol% NB 14.0 11.2 10.0
MoI% diene 0.2 0.9 0
(VNB) (VNB)
Tm ( C) 65 68 75
I2 (dg/min) 13.5 3.9 1.7
MWx 10 103 63 112
(grams/mole)
MN./Mn 2.3 1.8 2.4
The resins listed above were found to contain 0% gel prior to exposure to
electron -beam radiation. To further explore the utility of AUPO's as
crosslinking
enhancers, blends containing said materials (10 % unsaturated resin) were
prepared on a Brabender mixing chamber with a LLDPE (DowlexM2045.14 of
Dow Chemical Co.); 6.5 wt% octene, (0.920 g/cc, 1.0 dg/min). These blends
were:
PEB7 = 90% PE9 + 10% CE5
PEB8 = 90% PE9 + 10% A5
PEB9 = 90% PE9 + 10% A6
PEB 10 = 90% A 10 + 10% CE5
47
CA 02250254 1998-09-29
WO 97/36741 PCTIUS97/04796
The effect of electron -beam irradiation on the AUPO resins and their blends
is
shown in Table 14. The data clearly shows that VNB is a more efficient diene
at
promoting crosslinking than ENB, and that high levels of crosslinking could be
achieved using these AUPO's either alone or as a crosslinking enhancer to
improve the crosslinking in other resins. It also clearly shows that the AUPO
resins, as a component of a blend, enhanced the crosslinking of LLDPE giving
higher gel contents at lower doses than the base resin.
Table 14
Gel Content of AUPO Crosslink Enhancers and Their Blends
Resin Dose (kGray)
37 70
Al (E-H-ENB) 46.9 1.9 74.4 6.3
A2 (E-H-ENB) 45.0 8.3 66.9 4.3
A3 (E-H-5VNB) 66.3 3.5 83.0 4.0
A4(E-H-5VNB) 60.7 3.7 78.1 0.9
A5(E-H-5VNB) 37.2 12.1 nd
A6 (E-H-ENB) nd nd
PE9 (LLDPE) . 0 15
PEB7 (EPDM) 24.0 4.1 25.7 7.3
PEB8 (E-H-5VNB) 21.4 4.1 37.2 12.1
PEB9 (E-H-ENB) 30 45.6 3.5
Table 14 demonstrates that AUPO type PCE copolymers (A1-A5) can be
employed on their own to generate highly crosslinked films, and to enhance
crosslinking (PEB8 and PEB9) in other resins.
CA 02250254 2006-01-30
64536-958
Table 15
%Gel Content of E-NB Co-(Ter)polymers as a Function of Dose
Dose (kGray) 35 70 100
A9 0 3 35
PEB 10 5 19 37
A7 3 43 50
A8 51 73 80
Table 15 clearly shows that enhanced crosslinking of E-NB copolymers
can be achieved by blending in a PCE copolymer of the presnt invention or by
copolymerization with a diene monomer. The level of diene determines the
extent
to which the crosslinking can be enhanced.
Films formed with at least one layer containing the subject PCE
composition and subjected to irradiation, as described herein above and
illustrated
by Examples 1-30 above, are particularly useful in the production of bags for
packaging fresh red meat, smoked and processed meat, pork, cheese, poultry,
and
the like, as described in e.g. U.S. Patent Nos. 3,741,253 (Brax et al.),
3,891,008
(D'Entremont), 4,048,428 (Baird), and 4,284,458 (Schirmer),.
However, the film can also be used in other
applications. For example, the film can be used as a shrink film in packaging
applications for packaging food and non-food items. Films in which the present
invention can be beneficially used are described in e.g. U.S. Patent Nos.
4,551,380
and 4,643,943, both to Schoenberg.
In addition, the present invention can also be used with films
having oxygen, moisture, or odor barrier functionality, as described in e.g.
4,064,296 (Bornstein et al.), 4,724,185 (Shah), 4,839,235 (Shah), and
5,004,647
(Shah).
49
CA 02250254 1998-09-29
WO 97/36741 PCTIUS97/04796
Example 31-34
Four oxygen barrier films were formed with layers of PCE copolymer of
the present invention (Examples 31 and 34) as well as a comparative control
film
(COMP.D). Each had the layer structure:
A/B/C/D/CB/A
These films were made by a coextrusion of the layers, and each film was
irradiated and oriented. A small amount of anhydrous aluminum silicate (an
antiblock) and mono- and diglyceride/propylene glycol (an antifog) were
compounded into the resin blend of the two outside layers, such that, after
compounding, the additives comprised about 6% of the total compounded blend.
The film of COMP.D was compositionally and structurally as shown below:
50% PE9 90% OB2 50% PE9
25% PE10 PE10 AD1 AD1 PE10 25% PE10
25% EV4 10%PA 1 25% EV4
The films of Examples 31 and 32 had the same general formulation as
shown above for COM.D except that the second and sixth layers (the "B" layers)
comprised 90% PE 10 + 10% CE3.
The film of Examples 33 and 34 had the same general formulation as
shown above for COMP.D except that the third and fifth layers (the "C" layers)
comprised 90% AD 1+ 10% CE3.
Data comparing the power settings of the E-Beam irradiation unit and MFI
of these examples is given in Table 16 herein below.
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
TABLE 16
Example Beam Current Melt Flow Index
(mA) (dg/min.)
COMP. D 15.0 6.3
31 12.2 6.4
32 15.0 3.7
33 9.0 14.4
34 12.0 5.9
A review of the data of Table 16 shows two benefits of the present
invention.
First, the data shows that two films with identical composition and
structure ( COMP.D and Example 32), except for the presence of 10% of a
crosslinking enhancer in the B layers of Ex. 32, were irradiated at the same
dosage, yet had very different MFI values. The lower MFI value of Ex. 32
reflects
the increased crosslinking of the film of Ex. 32 as the result of the presence
of the
crosslinking enhancer. As pointed out in the description of the invention,
crosslinking improves processability in making oriented films.
Secondly, the data shows that two films with identical composition and
structure (COMP.D and Example 31), except for the presence of 10% of a
crosslinking enhancer in the B layers of Ex. 31, had nearly the same MFI (6.3
vs.
6.4) even though the film of Ex. 31 had been irradiated at a power setting of
only
12.2 mA, compared with the higher power setting of 15.0 for COMP. D. Again,
as pointed out in the description section of this specification, higher levels
of
crosslinking generally degrade the performance of sealing layers. By lowering
the
power setting of irradiation to which the extruded tape is exposed, the
flowability
of the sealant is less severely affected, and the sealant will perform better.
5~~
CA 02250254 2006-01-30
64536-958
Examule 35
Films formed according to the present invention were prepared for use as
or in connection with a patch as described in e.g. U.S. Patent No. 4,755,403
(Ferguson). An
exemplary patch structure was made having the formulation:
87% PEI 879'o PEI
10% CE2 EV3 EV3 10% CE2
3 loEV1 396EV1
This patch material was a tubular patch self-welded to itself at the //
interface. It was coextruded, and irradiated at a dosage of 98 kGy. The patch
thickness after orientation was 4.5 mils. The patch film was oriented and
rendered
heat shrinkable. This material can be used alone, or as a patch for a bag or
other
film.
For comparative purposes, a tubular patch film was formed by coextrusion
in the same manner as the above exemplary patch of the present invention. The
film was also irradiated with E-Beam at a dosage of 98 kGy and oriented by
stretching to a thickness of 4.5 mils. In the case of this control film the
layer
structure was:
90% PEI 909b PE1
10% EV2 EV3 EV3 10% EV2
The two patch materials were measured for gel % and it was detenmined
that the exemplary film had a ge19b of 55.0% while the comparative film had a
gel
% of only 46.5%.
52
CA 02250254 2006-01-30
64536-958
Example 36
Films were formed in which the outer layer was crosslinked by UV
irradiation. The films contained unsaturated block copolymers in combination
with a second polymer. The polymers were compounded in a Brabender mixing
chamber, pressed to make pressed-film samples, or plaques, and exposed to a
low
,M
intensity UV source (Amergraph lamp, primarily UVA output) for ten minutes at
room temperature in the solid state. At ten minutes the Amergraph radiometer
measurement was 1600 mJ/sq cm at 365 nm.
Unsaturated yolymer additive
The blends were: 69% by.weight of an ethylene/vinyl acetate resin having
9% by weight vinyl acetate (EVA 9) and 29% by weight of an unsaturated
polymer and 2% by weight of benzophenone. The unsaturated polymers were
Kraton D1107, a linear styrene-isoprene-styrene triblock copolymer available
from Shell (Sample A); Kraton D1102, a linear styrene-butadiene-styrene
triblock
copolymer available from Shell (Sample B); and Stereon 840, a styrene-
butadiene block polymer available from Firestone, Akron, Ohio (Sample C). Gel
content was analyzed as above.
Table 17
Gel Content Data
Sample A 30%
Sample B 6%
Sample C 3%
The only unsaturated block copolymer to yield significant gel levels was
Sample A, the styrene-isoprene-styrene block copolymer. However, the styrene-
butadiene-styrene block copolymer did not yield significant gel content under
these conditions.
53
CA 02250254 2006-01-30
64536-958
Comparative Examnle
For comparative purposes, a chemieaily crosslink polymer eomposition
was formed and exposed to UV radiation in the same manner as Sample A above.
EVA9 resin was compounded with 2 weight percent of triallylcyanurate (TAC)
and 2 weight percent of benzophenone as initiator. The material formed was
labeled "Sample G". The gel content of this Sample G was 26% and, therefore,
similar in crosslink as that of Sample A.
Samples A and G were selected for extrusion in multilayer test films.
Sample G was found to be difficult to extrude due to blooming and bleeding
(separation of one component so that it selectively appears on the surface of
the
extrudate) of the TAC. To obtain a sample, 50 weight percent of ethylenelvinyl
acetate with 9% vinyl acetate was added. In comparison, Sample A did not
exhibit blooming.
Further, fihns were formed having multiple layers as follows. EVA-9 resin
and an ethylene/vinyl acetate resin with 15% vinyl acetate (EVA15) were
coextruded as a bilayer annular tape, electronically crosslinked, and cooled.
The
tape was then coated by coextruding three more layers, a Saran/PVDC barrier
layer blend, and ethylene/vinyl acetate with 28% vinyl acetate layer, and a
layer of
the resin of Samples A or G. The Saraii/PVDC layer was in contact with the EVA
15 layer, and the Sample resins were located on the outside. The tapes were UV
irradiated using lamps from Fusion Systems, Inc., Rockville, MD. The doses
were
450 and 900 mJ/sq. cm. The tape was then biaxially oriented (stretched) at a
racking ratio of about 3x in the transverse direction and about 4x in the
longitudinal direction. Gel content of the outside layer was then determined
and is
reported below. The gel content from Sample A was higher than from Sample G.
The film from Sample A was tested and showed no tendency for film "pick-off"
during the sealing process, had excellent grease resistance and good optics.
54
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
Table 18
Irradiated Film Samples
Crosslinked Layer Dose, mJ/sq. cm. at 360 nm % Gel
Sample A 450 20
Sample A 900 66
Sample G 450 0
Sample G 900 0
These examples demonstrate a UV system that produces a crosslinked
network. The resulting film dose can eliminate applied seal delamination,
increase grease resistance, and still maintain good optics. These results also
show
that the films having the present PCE composition performed significantly
better
than films which were chemically crosslinked.
Example 37
Benzophenone was dissolved in low molecular weight 1,2-polybutadiene
(1,2-PBD) with gentle warming. The liquid was poured onto pellets of LLDPE
and was evenly distributed by tumble mixing to provide a PCE composition. The
final composition was 5% by weight 1,2-PBD and 1% by weight benzophenone.
The apparatus in Figure 1 was used to extrude and irradiate the mixture. The
lamp
was positioned directly above the die lip. Linear extrusion rate was varied by
varying a combination of extruder rpm and take-up speed. Gel content of the
resulting film was determined as described above. The results are reported in
Table 19 below.
SS'~
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
Table 19
Gel Content Data - Linear Low Density Polyethylene
Linear Extrusion Rate Gel Content Film Thickness
(ft./min.) (% by wt.) (mil)
2.66 50 11.4
2.83 45 8.1
8.50 22 6.6
These data demonstrate that useful gel contents can be achieved in
polyethylene by this process. These data also show that 1,2-polybutadiene is
as
effective as TAC as a crosslinking aid. In addition, high gel contents can be
achieved in relatively thick films. These films gave 0% gel content in the
absence
of UV.
Example 38
This example was carried out exactly like that in Example 37, except that
LLDPE polymer was substituted with an ethylene-propylene copolymer (having
3.1% ethylene). The following data were obtained:
Table 20
Gel Content for EP Copolymer
Linear Extrusion Rate Gel Content Film Thickness
(ft./min.) (% by wt.) (mil)
2.8 37 7.1
5.7 19 7.4
9.0 7 9.2
These data show that useful crosslinking can be obtained by this method in
propylene copolymers.
S(o
CA 02250254 2006-01-30
64536-958
Example 39
The same equipment and method described in Example 37 was also used
in this example except that the 1,2 PBD polymer was substituted with a
developmental LLDPE copolymer composed of ethyleneloctene/polyene having
430 C=C per 100,000 carbon atoms, in the form of terminal vinyl unsaturation
by
IR analysis. With the addition of 1% benzophenone, this PCE composition gave a
gel content of 90% when extruded at a linear rate between 2 and 3 ftJmin. and
irradiated with UV irradation. Again there was no gel content in the absence
of
UV irradiation.
Example 40
In this example, two formulations were prepared in a Brabender mixing
chamber by melt blending the components to compare chemically crosslinked
material to that of the present invention. The first formulation was
conunercially
available LLDPE (AttaneM4201) with 1% triallylcyanurate (TAC) and 1%
benzophenone. The second was the.developmental unsaturated LLDPE
copolymer described in Example 39 above, with 1% benzophenone. A 10 inch
Fusion Systems lamp (H-bulb) mounted on a conveyor belt was used to irradiate
pressed films of the above formulations (15-20 mil thick) at room temperature
(UV doses measured at 365 nm). The following results were obtained:
57
CA 02250254 2006-01-30
64536-9-58
Table 21
Gel Content at Various UV Doses in the Solid State
Formulation UV Dose Gel Content
(7/cm2 @ 365 nm) (% by wt.)
Attane 4201, l% TAC, 0.2 0.0
1% benzophenone
same as above 0.4 1.4
same as above 0.6 1.7
unsaturated LLDPE, 0.2 19.6
1% benzophenone
same as above 0.4 25
same as above 0.8 41
These data show that a high degree of crosslinking was not obtained for
chemical system with TAC in the solid state; however, the PCE copolymer
(developmental unsaturated LLDPE), a high degree of crosslinking was obtained
in the solid state at relatively low doses of UV radiation. These data when
combined with the previous examples, further demonstrate that even higher gel
contents for a given system can be obtained when in-adiation occurs in the
melt
state.
Example 41
In this example, a PCE copolymer was prepared by grafting. In a
Brabender mixing chamber, an ethylene-alkyl acrylate-maleic anhydride
terpolymer (Lotader 3200, AtoChem Inc.) was melt compounded and reacted with
5% by weight hydroxyl terminated 1,2-PBD (Nisso-PB , G-3000, Nippon Soda
Co., Ltd.). To the fonmed graft copolymer, 1% benzophenone was further
incorporated by melt blending. A pressed film was irradiated at room
temperature
as described above to a UV dose of 0.8 J/cm2 (measured at 365 nm), which
58
CA 02250254 1998-09-29
WO 97/36741 PCTIUS97/04796
resulted in a gel content of 23% by weight. In contrast, the unirradiated film
had a
gel content of 0.8% by weight, as a result of the difunctional nature of the
1,2-
PBD.
Example 42
In this example, two formulations were prepared in a Brabender mixing
chamber by melt blending the components. The first formulation was PCE
copolymer of developmental unsaturated LLDPE combined with 1% 4-
allyloxybenzophenone. The second was the same developmental unsaturated
LLDPE with 1%, 4,4'-diallyloxybenzophenone. A 10 inch Fusion Systems lamp
(H-bulb) mounted on a conveyor belt was used to irradiate pressed films of the
above formulations (15-20 mil thick) at room temperature (UV doses measured at
365 nm). The following results were obtained:
Table 22
Gel Content at Various UV Doses in the Solid State
Formulation UV Dose Gel Content
(J/cm2 @ 365 nm) (% by wt.)
Unsat. LLDPE, 1% 4- 0.2 18.5
allyloxybenzophenone
same as above 0.4 19.5
same as above 0.8 18.5
Unsat. LLDPE, 1% 4,4' - 0.2 11.0
diallyloxybenzophenone
same as above 0.4 22.0
same as above 0.8 34.7
6-7
CA 02250254 2006-01-30
64536-958
These data show that useful crosslinking can be obtained even in the solid
state for polyethylene-polyene copolymer and that useful crosslinking can be
obtained from substituted benzophenone.
Example 43
Pellets of EVA-9 were coated with a mixture of low molecular weight 1,2-
polybutadiene (1,2-PBD) and benzophenone to form a PCE composition.
Benzophenone was dissolved in the 1,2-PBD with gentle warming prior to
coating. The final composition was 5% by weight 1,2-PBD and 1% by weight
benzophenone. The twin screw extruder in Figure 1 was used to extrude and
pelletize the mixture. The resulting pellets were fed into a Randcastle micro-
extruder, which had the UV lamp in Figure 1 mounted at the lip of the 6" flat
sheet
die. The linear extrusion rate was varied by varying a combination of extruder
rpm and take-up speed. Gel content of the resulting film was determined as
described above. The following results were obtained:
Table 23
Gel Content Data for EVA-98
Linear Extrusion Rate Gel Content F'ilm Thickness
(ft./min.) (% by wt.) (mil)
8.0 28 2.1
11 42 1.5
' EVA 9(polyethylene/vinyl acetate resin with 9% vinyl acetate comonomer LD
318.92 available from Exxon Corp., Houston, TX) compounded with 5% by
weight 1,2-PBD and 1% by weight benzophenone and extruded using a Brabender
twin-screw extruder.
These data clearly show that PCE compositions containing EVA-9 can be
UV crosslinked to high gel contents.
CA 02250254 1998-09-29
WO 97/36741 PCT/US97/04796
Example 44
The Randcastle micro-extruder described above was used to make a three
layer film that was irradiated in a similar fashion. The skin layers of the
structure
were a PCE composition composed of a blend of LLDPE, with 5% by weight 1,2-
polybutadiene (1,2-PBD) and 1% by weight benzophenone. The skin layer blend
was compounded prior to coextrusion as described above. The core layer of the
structure was LLDPE (Dowlex 3010). The approximate ratio of the layer gauges
was 1:1:1 based on the extruder rpm.
Table 24
LLDPEa/LLDPEb/LLDPEa
Linear Extrusion Total Gel Content Skin Gel Content' Film Thickness
Rate (ft./min.) (% 2a) (% 26 (total, mil)
4.5 21 7 32 11 6.7
8.0 24 4 36 6 3.7
11 20 2 30 3 2.9
a Skin layer LLDPE is Dowlex 2045.03 with 5% 1,2-PBD and l% Benzophenone,
twin screw compounded prior to coextrusion.
b Middle layer LLDPE is Dowlex 3010.
Approximate gel content of skin layers calculated assuming 2/3 of structure.
These data clearly indicate that the skin layers of a multilayer film can be
substantially crosslinked by UV irradiation.
Example 45
The Randcastle micro-extruder described in Example 43 was used to make
a three layer film that was irradiated as in Example 8. The skin layers of
this
CA 02250254 1998-09-29
WO 97/36741 PCTIUS97/04796
structure were LLDPE (Dowlex 3010, Dow Chemical). The core layer of the
structure was a PCE composition comprosed of a blend of LLDPE (Dowlex
2045.03, Dow Chemical) with 5% 1,2-PBD (Nisso PB , B-1000, Nippon Soda
Co., Ltd.) and 1% acrylated benzophenone derivative (Ebecryl P-36, UCB
Radcure Inc.), that was compounded prior to coextrusion as described in
Example
7. The approximate ratio of the layer gauges was 1:1:1 based on the extruder
rpm.
Table 25
LLDPEa/LLDPEb/LLDPEa
Linear Extrusion Total Gel Content Core Gel Content' Film Thickness
Rate (ft./min.) (% 2(y) (% 2(y) (total, mil)
8.0 17 4 51 12 5.5
14 27 5 81 15 3.8
a Skin layer LLDPE is Dowlex 3010.
b Core layer LLDPE is Dowlex 2045.03 with 5% 1,2-PBD and 1% acrylated
benzophenone, twin screw compounded prior to coextrusion.
Approximate gel content of core layer calculated assuming 1/3 of structure.
These data clearly indicate that an internal layer of a multilayer film can be
substantially crosslinked by UV with these additives and apparatus.