Note: Descriptions are shown in the official language in which they were submitted.
CA 02252038 1998-10-09
WO 97/38662 PCT/US97/02793
1
TITLE OF THE INVENTION
BUCCAL POLAR SPRAY OR CAPSULE
BACKGROUND OF THE INVENTION
It is known that certain biologically active compounds are better
absorbed through the oral mucosa than through other routes of
administration, such as through the stomach or intestine. However,
formulations suitable for such administration by these latter routes present
their own problems. For example, the biologically active compound must
be compatible with the other components of the composition such as
propellants, solvents, etc. Many such tormulations nave been proposed.
For example, U.S.P. 4,689,233, Dvorsky et al., describes a soft gelatin
capsule for the administration of the anti-coronary drug nifedipine dissolved
in a mixture of polyether alcohols. U.S.P. 4,755,389, Jones et ~I.,
describes a hard gelatin chewable capsule containing nifedipine. A
chewable gelatin capsule containing a solution or dispersion of a drug is
described in U.S.P. 4,935,243, Borkan et al. U.S.P. 4,919,919, Aouda et
al, and U.S.P. 5,370,862, Klokkers-Bethke, describe a nitroglycerin spray
for administration to the oral mucosa comprising nitroglycerin, ethanol, and
other components. An orally administered pump spray is described by
Cholcha in U.S.P. 5,186,925. Aerosol compositions containing a hydro-
carbon propellant and a drug for administration to a mucosal surface are
described in U.K. 2,082,457, Su, U.S.P. 3,155,574, Silson e~ al., U.S.P.
5,011,678, Wang et al., and by Parnell in U.S.P. 5,128,132. It should be
noted that these references discuss bioavailabiiity of solutions by inhalation
rather than through the membranes to which they are administered.
SUMMARY OF THE INVENTION
A buccal aerosol spray or soft bite gelatin capsule using a polar
. solvent has now been developed which provides biologically active com-
pounds for rapid absorption through the oral mucosa, resulting in fast onset
CA 02252038 1998-10-09 -
WO 97/38662 PCT/US97/02793
2
of effect.
The buccal aerosol spray compositions of the present invention, for
transmucosal administration of a pharmacologically active compound soluble
in a pharmacologically acceptable polar solvent comprising in weight% of
total composition: polar solvent 75-99.8%, active compound 0.0025-40%,
suitably additionally comprising, by weight of total composition a flavoring
agent 0.05-5%. Preferably the composition comprises: polar solvent 75-
99%, active compound 0.025-20%, flavoring agent 0.1-2.5%; most
suitably polar solvent 75-98%, active compound 0.125-12.5%, flavoring
agent 0.1-2.5%.
The soft bite gelatin capsules of the present invention for trans-
mucosal administration of a pharmacologically active compound, at least
partially soluble in a pharmacologically acceptable polar solvent, having
charged thereto a composition comprising in weight % of total composition:
polar solvent 40-99.8%, emulsifier 0-20%, active compound 0.0003-35%,
provided that said composition contains less than 10% of water, suitably
additionally comprising, by weight of the composition: flavoring agent
0.05-60%. Preferably, the soft bite gelatin capsule comprises: polar solvent
50-99.8%, emulsifier 0-15%, active compound 0.0003-26%, flavoring
agent 0.5-55%; most suitably: polar solvent 70-99.5%, emulsifier 0-10%,
active compound 0.015-24.0%, flavoring agent 0.1-50%.
It is an object of the invention to coat the mucosal membranes either
with extremely fine droplets of spray containing the active compounds or a
solution or paste therof from bite capsules.
It is also an object of the invention to administer to a mammalian need
. of-came preferably man, a predetermined amount of a biologically active
compound by this method or from a soft gelatin bite capsule.
CA 02252038 2005-09-23
960402 RV 10 3 PHCO 3.0-002
A further object is a pump spray container containing a composition
of the spray formulation, and a metered valve suitable for releasing from
said container a predetermined amount of said composition.
The solvent i~ a low molecular weight, pharmacologically acceptable
alcohol, water, glycerine or polyethylene glycol or mixtures thereof.
A further object is a soft gelatin bite capsule containing a composition
as set forth above. The formulation may be in the form of a viscous solu-
tion or paste containing the active compounds. Although solutions are
preferred, paste fills may also be used where the active compound is not
soluble or only partially soluble in the solvent of choice. Where water is
used to form part of the solvent or paste composition, it should not exceed
10% thereof. (All percentages herein are by weight unless otherwise
indicated.)
The polar solvent is chosen such that it is compatible with the gelatin
shell and the active compound. The solvent preferably dissolves the active
compound. However, other components wherein the active compound is
not soluble or only slightly soluble may be used and will forma paste fill.
Soft gelatin capsules are well known in the art. Sea, for example,
U.S.P. 4,935,243, Borkan et al. -
The capsules of the present invention are
intended to be bitten into to release the low viscosity solution or paste
therein, which wilt then coat the buccal mucosa with the active compounds.
Typical capsules, which are swallowed whole or bitten and then swallowed,
deliver the active compounds the stomach, which results in significant lag
time before maximum blood levels can be achieved or subject the compound
-~o~a~ large first pass effect. Because of the enhanced absorption of the
compounds through the oral mucosa and no chance of a first pass effect,
CA 02252038 1998-10-09
WO 97/38662 PCT/US97/02793-
4
use of the bite capsules of the invention will eliminate much of the lag time,
resulting in hastened onset of biological effect. The shell of a soft gelatin
capsule of the invention may comprise, for example: gelatine 50-75%,
glycerine 20-30%, colorants 0.5-1.5%, water 5-10%, and sorbitol 2-10%.
The spray compositions of the invention are intended to be
administered from a pump spray.
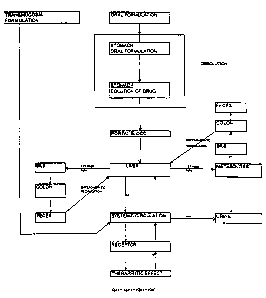
BRIEF DESCRIPTION OF THE DRAWING
The figure is a schematic diagram showing routes of absorption and
processing of pharmacologically active substances in a mammalian system.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
The preferred active compounds of the present invention are nicotine,
clemastine, testosterone, estradiol, progesterone, fluoxetine, and piroxicam
in their nonionized form or as the free base of the pharmaceutically
acceptable salts thereof (provided, for the aerosol or spray compositions,
they are soluble in the spray solventl. These compounds are soluble in the
non- polar solvents of the invention at useful concentrations or can be
prepared as pastes at useful concentrations. These concentrations may be
less than the standard accepted dose for these compounds since there is
enhanced absorption of the compounds through the oral mucosa. This
aspect of the invention is especially important when there is a large
(40-99.99%? First pass effect.
As solvents for the sprays there may be used low molecular weight
polyethyleneglycols (PEGI of 400-1000 Mw (preferably 400-600). Low
molecular weight alcohols and polyols, such as glycerin may also be present
and water may also be used.
Suitable solvents for the capsules include low molecular weight
polyethylenegiycols (PEG1 of 400-1000 Mw (preferably 400-600. Low
CA 02252038 1998-10-09
WO 97/386b2 PCT/US97/02793
molecular weight alcohols and polyols, such as glycerin may also be present
and water may also be used. However, these should only be used sparingly
in the bite capsule compo-sitions as they may migrate into the gelatin shell
and weaken it.
5
It is expected that some glycerin and water used to make the gelatin
shell will migrate from the shell to the fill during the curing of the shell.
Likewise, there may be some migration of components from the fill to the
shell during curing and even throughout the shelf-fife of the capsule.
Therefore, the values given herein are for the compositions as prepared, it
being within the scope of the invention that minor variations will occur.
The preferred flavoring agents are synthetic or natural oil of pepper-
mint, oil of spearmint, citrus oil, fruit flavors, chocolate, sweeteners
(sugars,
aspartame, saccharin, etc.), and combinations thereof.
The active substances include the active compounds selected from
the group consisting of alkaloids, anti histamines, steroid hormones, non-
steroidal anti-inflammatories, analgesics and anti-depressants, benzo-
diazepines, such as tamezepam.
Clemastine hydrogen fumarate is a known (Taoist°, Sandozl anti-
histamine. Both the spray and capsule of the invention advantageously coat
the oral mucosa with an immediately available dose of clemastine which can
be rapidly absorbed. This is highly desirable, as during an acute asthma
attack.
Nicotine is a component of tobacco products which is considered
addictive. Smokers wishing to stop smoking have a dual problem. First, is
the addictive properties of nicotine itself. Second, is that the habit is
. associated with smoking activities, i.e., puffing, inhaling, etc. Both the
spray and capsule of the invention dissociate these two problems. By
CA 02252038 2005-09-23
960402 RV10 6 PHCO 3.0-002
presenting nicotine in a form which can be readily absorbed, the spray and
capsule allow the smoker to temporarily continue nicotine use but terminate
smoking. Once the habit of smoking is stopped, the former smoker can
then be weaned off nicotine use, as by less frequent use and/or by use of
a lower concentration spray or- capsule. Advantageously, during this
regimen, the user is exposed to none of the carcinogens present in tobacco
smoke.
Testosterone is a hormone produced by gonadal cells. Testosterone,
especially the esters thereof (e.g., acetate, propionate, enanthate, and
cypionate), is used in the treatment of hypogonadism.
Estradiol is an estrogen steroid secreted from the ovaries. Estradiol,
especially the esters thereof (e.g., diacetate, and benzoatel, is used as
estrogen replacement therapy, especially in post-menopausal women.
Progesterone is a hormone produced by the corpus luteum. Fluo-
xetine is an antidepressant also known as Prozac. Piroxicam is a known
(Feldene~, Pfizer) anti-inflammatory.
The formulations of the present invention comprise an active com-
pound or a pharmaceutically acceptable salt thereof. The term
"pharmaceutically acceptable salts" refers to salts prepared from
pharmaceutically acceptable non-toxic acids or bases including organic and
inorganic acids or bases.
When an active compound of the present invention is acidic, salts
may be prepared from pharmaceutically acceptable non-toxic bases. Salts
derived from ail stable forms of inorganic bases include aluminum,
~amiir~onium, calcium, copper, iron, lithium, magnesium, manganese,
potassium, sodium, zinc, etc. Particularly preferred are the ammonium,
CA 02252038 1998-10-09
WO 97/38662 PCT/US97/02793-
7
calcium, magnesium, potassium, and sodium salts. Salts derived from
pharmaceutically acceptable organic . non-toxic bases include salts of
primary, secondary, and tertiary amines, substituted amines including
naturally occurring substituted amines, cyclic amines and basic ion-
s exchange resins such as arginine, betaine, caffeine, choline, N,N'-
dibenzylethylenediamine, diethylamine, 2-diethylaminoethanol, 2-dimethyl-
aminoethanol, ethanolamine, ethylenediamine, N-ethylmorpholine, N-ethyl-
piperidine, glucamine, glucosamine, histidine, isopropylamine, lysine,
methylglucosamine, morpholine, piperazine, piperidine, polyamine resins,
procaine, purine, theobromine, triethylamine, trimethylamine, tripropylamine,
etc.
When an active compound of the present invention is basic, salts may
be prepared from pharmaceutically acceptable non-toxic acids. Such acids
include acetic, benzenesulfonic, benzoic, camphorsulfonic, citric, ethane-
sulfonic, fumaric, giuconic, glutamic, hydrobromic, hydrochloric, isethionic,
lactic, malefic, mandelic, methanesulfonic, mucic, nitric, pamoic, panto-
thenic, phosphoric, succinic, sulfuric, tartaric, p-toluenesulfonic, etc.
Particularly preferred are citric, hydrobromic, malefic, phosphoric, sulfuric,
and tartaric acids.
In the discussion of methods of treatment herein, reference to the
active compounds is meant to also include the pharmaceutically acceptable
salts thereof. While certain formulations are set forth herein, the actual
amounts to be administered to the mammal or man in need of same are to
be determined by the treating physician.
CA 02252038 1998-10-09
WO 97/38662 PCT/US97/02793
8
The invention is further defined by reference to the following
examples, which are intended to be illustrative and not limiting.
EXAMPLE 1
Clemastine Spray
A spray of the invention comprises the following formulation:
Amount Preferred Amount Most-Preferred Amount
Polar solvent 75-99% 90-99% 97-99%
Clemastine fumarate 0.12-12.5% 0.25-7.25% 0.25-6.5%
Flavoring agent 0.05-5% 0.1-2.5% 0.2-2%
It is particularly preferred to formulate the spray delivering
1.34mg/activation:
Amount
Polar solvent:
Ethanol 66
Water 31
Clemastine fumarate 2.8%
Peppermint Oil 0.2%
EXAMPLE 2
Nicotine Spray
A spray of the invention comprises the following formulation:
Amount Preferred Amount Most-Preferred Amount
Polar solvent 75-99.8% 96-99.8% 96-99.8%
Nicotine 0.0125-10% 0.125-2.5% 0.2-1.15%
Flavoring agent 0.05-5 % 0.1-2.5 % 0.2-2.0%
It is particularly preferred to formulate the spray delivering
.Ø~5mg/activation:
CA 02252038 1998-10-09
WO 97/38662 PCT/US97/02793
9
Amount
Polar solvent:
Ethanol 49.37%
Water 49.37
Nicotine 1.06%
Peppermint Oil 0.2%
EXAMPLE 3
Nicotine Sulfate
A spray of the invention comprises the following formulation:
Amount Preferred Amount Most-Preferred Amount
Polar solvent 75-99.8% 96-99.8% 96-99.8%
Nicotine Sulfate 0.0125-10% 0.125-2.5% 0.2-2.0%
Flavoring agent 0.05-5% 0.1-2.5% 1-2%
It is particularly preferred to formulate the spray for 0.5mg of nicotine
sulfate:
Amount
Polar solvent:
Ethanol 9.84%
Water 88.9%
Nicotine Sulfate 1.06%
Peppermint Oil 0.2%
CA 02252038 1998-10-09
WO 97/38662 PCT/US97/02793
EXAMPLE 4
Fluoxetine Hydrochloride Spray
A spray of the invention comprises the following formulation:
Amount Preferred Amount Most-Preferred Amount
5 Polar solvent 75-99% 75-98% 75-95%
Fluoxetine 0.125-25 % 2.5-20% 5-12.5
Hydrochloride .
Flavoring agent 0.05-5% 0.1-2.5% 0.1-2.0%
It is particularly preferred to formulate the spray delivering
5mg/activation:
Amount
Polar solvent:
Ethanol 48.4%
Water 10.0%
Polyethyleneglycol 30.0%
Fluoxetine HCI 10.6%
Oil of Orange 1.0%
EXAMPLE 5
Testosterone Spray Delivering 3mg/Activation
A spray of the invention comprises the following formulation:
Amount Preferred Amount Most-Preferred Amount
Polar Solvent 55-99% 75-95% 85-93%
Testosterone 0.12-10% 0.25-7.5% 0.25-6.5%
Flavoring Agent 0.05-3% 0.1-2.5% 0.1-2.5%
CA 02252038 1998-10-09
WO 97/38662 PCT/US97/02793
11
It is particularly preferred to formulate the spray:
Amount
Polar Solvent:
Water 10%
Polyethyleneglycol 65%
Ethanol 16.6%
Testosterone 6.4%
Orange Aroma 1.0%
Oil of Citrus 1.0%
EXAMPLE 6
Estradiol Spray Delivering 0.1 mg/Activation
A spray of the invention comprises the following formulation:
Amount Preferrg~,1 Amount Most-Preferred Amount
Polar Solvent 75-99% 75-95% 85-99%
Estradiol 0.0025-2.5% 0.025-1.5% 0.125-1.0%
Flavoring Agent 0.05-3% 0.1-2.5% 1-2%
It is particularly preferred to formulate the spray:
Amount
Polar Solvent:
Water 10%
Polyethyleneglycol 75
Ethanol 13.79%
Estradiol 0.21
Peppermint 1.0%
CA 02252038 1998-10-09
WO 97/38662 PCT/US97/02793-
12
EXAMPLE 7
Progesterone Spray Delivering 0.32mg/Activation
A spray of the invention comprises the following formulation:
Amount Preferred Amount Most-Preferred Amoun
Propellent 55-99% 75-99.8% 85-99.5%
Progesterone 0.12-10% 0.25-6.25% 0.25-1
Flavoring Agent 0.05-3% 0.1-2.5% 1-2%
It is particularly preferred to formulate the spray:
Amount
Polar Solvent:
Water 10%
Polyethyfeneglycol 75
Ethanol 13.32
Progesterone 0.68%
Peppermint 1.0%
EXAMPLE 8
Clemastine Bite Capsule
A bite capsule of the invention comprises the following fill
formulation:
Amount Preferred Amount Most-Preferred Amount
Polar solvent 55-99% 66-97% 85-99.5%
Emulsifier 0-20% 0-15% 0-10%
Clemastine fumarate 0.1-4% 0.03-3% 0.04-1.5%
Flavoring agent 0.05-5 % 0.1-2. 5 % 0.1-2. 5
CA 02252038 1998-10-09
WO 97/38662 PCT/US97/02793-
13
It is particularly preferred to formulate the composition fill for a
1.34mg capsule:
Amount
Polar Solvent:
Polyethyleneglycol 89.6%%
Water 5.3%
Glycerine 4.4%
Clemastine fumarate 0.50%
Peppermint Oil 0.1
Saccharine 0.1
EXAMPLE 9
Testosterone Bite Capsule
A bite capsule of the invention comprises the following fill
formulation:
Amount Preferred Amount Most-Preferred Amount
Polar solvent 55-99% 66-97% 85-99.5%
Emulsifier/wetting 0-20% 0-15% 0-10%
agents
Testosterone" 0.01-3.7% 0.4-3% 0.7-2%
Flavoring agent 0.05-5 % 0.1-2. 5 % 0.1-2. 5
*'or esters thereof, preferably, the acetate, propionate, and enenthate esters
CA 02252038 1998-10-09
WO 97/38662 PCT/US97/02793
14
It is particularly preferred to formulate the fill composition for the 5mg
capsule:
Amount
Polar solvent:
Polyethyleneglycol 85.0%
Glycerine 6.15
Lecithin 6.0%
Testosterone 1.85
Peppermint Oil 1.0%
EXAMPLE 10
Estradiol Bite Capsule
A bite capsule of the invention comprises the following fill
formulation:
Amount Preferred Amount Most-Preferred
Amount
Polar solvent 75-99% 75-99.8% 85-99.5%
Emulsifier/wetting0-20% 0-15% 0-10%
agents
Estradiol ~ 0.0003-2 0.003-0.75 % 0.02-0.2
%
Flavoring agent0.05-5 % 0.1-2.5 % 0.1-2.5
~"or esters thereof,preferably,he diacetate and benzoate esters
t
It is particularly preferred to formulate the fill composition for a 0.5mg
capsule:
Amount
Polar solvent:
Polyethyleneglycol 85
Glycerine 8.82 %
Lecithin 5.0%
. FstFadiol 0.18
Oil of Peppermint 1.0%
CA 02252038 1998-10-09
WO 97/38662 PCT/US97/02793-
EXAMPLE 11
Progesterone Bite Capsule
A bite capsule of the invention comprises the following fill
formulation:
5 Amount Preferred AmountMost-Preferred Amount
Polar solvent 75-99.8% 75-98.8% 85-99.5%
Emulsifier/wetting0-20% 0-15% 0-10%
agents
Progesterone 0.0003-4% 0.0003-3% 0.75-2%
10 Flavoring agent0.05-5% 0.1-2.5% 0.1-2.5%
It is particularly preferred to formulate the fill formulation for a 3mg
capsule:
Amount
15 Polar solvent:
Polyethyleneglycol 85
Glycerine 7.89
Lecithin 5.0%
Progesterone 1.11
Oil of Peppermint 1.0%
EXAMPLE 12
Fluoxetine Bite Capsule
A bite capsule of the invention comprises the following fill
formulation:
Amount Preferred Amount Most-Preferred Amount
Polar solvent 75-99.8% 75-99.8% 85-99.5%
Emulsifier/wetting0-20% 0-15% 0-10%
agents
. FIEroxetine 0.2-9.25% 0.4-6% 0.75-4%
HCI
Flavoring agent 0.05-5% 0.1-2.5% 0.5-3%
CA 02252038 1998-10-09
WO 97/38662 PCT/US97/02793-
16
It is particularly preferred to formulate the fill formulation for a 5mg
capsule:
Amount
Polar solvent:
Polyethyleneglycol85%
Glycerine 7,15%
Lecithin 5.0%
Fluoxetine HCI 1.85%
Oil of Peppermint 1.0%
EXAMPLE 13
Piroxicam Bite Capsule
A bite capsule of the invention comprises the following fill
formulation:
Amount Preferred AmountMost-Preferr d Amount
Polar solvent 75-99.8% 75-99.8% 95-99.5%
Emulsifier/wetting0-20% 0-15% 0-10%
agents
Piroxicam 0.02-9.25%0.4-4% 0.75-4%
Flavoring agent0.05-5 0.1-4.5 % 0.5-3%
%
It is particularly preferred to formulate the fill formulation for a 5mg
capsule:
Amount
Polar solvent:
Polyethyleneglycol 85 %
Glycerine 7.5
Lecithin 5.0%
Piroxicam 1,85%
..Oi~~flt Peppermint1.0~
CA 02252038 1998-10-09
WO 97/38662 PCT/US97/02793
17
EXAMPLE 14
Clemastine Fumarate
with Phenylpropanolamine
Hydrocyloride
Bite Capsule
A bite capsule of invention comprisesthe following
the fill
formulation:
Amount Preferred Amount st-Preferred
Mo Amount
Polar solvent 40-99% 50-98% 70-98%
Emulsifier 0-20% 0-15% 0-10%
Clemastine
fumarate 0.01-1.85% 0.03-0.74% 0.05-0.185%
Phenylpropanolamine
HCI 1-30% 1.5-20% 1.8-10%
Flavoring agent 0.05-5 % 0.1-2.5 % 0.1-2. 5
It is particularly preferred to formulate the composition fill for a
clamastine 1.34mg/25mg phenylpropanolamine capsule:
Amount
Polar Solvent:
Polyethyleneglycol 78.73%
Water 5.3%
Glycerine 4.4%
Clemastine fumarate 0.5%
Phenylpropanolamine 9.2%
HCI
Saccharine 0.37%
Peppermint Oil 1.5%
CA 02252038 1998-10-09
WO 97/38662 PCT/US97/02793
18
EXAMPLE 15
Clemastine/Pseudoephedrine Bite Capsule
A bite capsule of the invention comprises the following fill
formulation:
Amount Prefe rred AmountMost-Preferred
Am
t
oun
Polar solvent 40-99% 60-95% 70-90%
Emulsifier 0-20% 0-15% 0-10%
Clemastine
fumarate 0.01-1.85% 0.03-0.74% 0.05-01.85%
Pseudoephedrine
HCI 3-30% 5-25 /a 10-23%
Flavoring agent 0.05-5% 0.1-2.5% 0.1-2.5%
It is particularly preferred to formulate the composition fill for a
1.34mg ciemastine fumerate/60mg pseudoephridrine HCI capsule:
Amount
Polar Solvent:
Polyethyleneglycol 65.71 %%
Water 5.30%
Glycerine 4.40%
Clemastine fumarate 0.50%
Pseudoephedrine HCI 22.22%
Peppermint Oil 1.5%
Saccharine 0.37%
CA 02252038 1998-10-09
WO 97/38662 PCT/US97/02793
19
EXAMPLE 16
Nicotine 0.5mg Bite Capsule ,
A bite capsule of the invention comprises the following fill
formulation:
Amount Preferred Amount Most-Preferred Amount
Polar solvent 30-99.8% 35-99.8% 40-99.5%
Emulsifier/wetting 0-20% 0-15% 0-10%
agents
Nicotine' 0.018-0.74% 0.037-0.37% 0.37-0.20%
Flavoring agent 0.05-60% 0.1-55% 0.5-50%
' or as nicotine sulfate
It is particularly preferred to formulate the fill for a 0.5mg capsule:
Amount
Polar Solvent:
Polyethylenegiycol89.6%%
Water 5.5
Glycerine 4.40%
Peppermint Oil 0.1
Na Saccharine 0.1
Nicotine 0.185%