Note: Descriptions are shown in the official language in which they were submitted.
CA 02253693 1998-11-09
Title: METHOD AND APPARATUS FOR CONCENTRATING A
GAS USING A SINGLE STAGE ADSORPTION ZONE
FIELD OF THE INVENTION
This invention relates to a method and apparatus using
a single absorption zone for producing an enriched stream of a first
gas from a stream containing the first gas and at least one second
gas. In one embodiment, the method and apparatus may be used to
obtain a concentrated stream of oxygen from air.
BACKGROUND OF THE INVENTION
Various different methods have been developed for
separating gases and producing a concentrated stream of a selected
gas. One particular method which has been used in industry is the
pressure swing adsorption process. Generally, these processes use an
adsorbent which, under elevated pressure conditions, preferentially
adsorbs a targeted gas over other gases present in a gas stream.
Accordingly, the adsorbent could be selected to preferentially adsorb
an undesirable gas from a gas stream thereby leaving a gas stream
having an increased concentration of the gasses remaining in the
gas stream. An example of such a process would be the use of a
pressure swing adsorption process to produce an oxygen enriched
air stream. The adsorbent would be selected to preferentially adsorb
nitrogen over oxygen. Thus, after the adsorption process is
conducted, the pressurized air in contact with the adsorbent
contains a higher percentage by volume of oxygen. This oxygen
enriched air may then be vented from the adsorption zone and the
adsorbent purged (at reduced pressure conditions) to remove the
adsorbed nitrogen. Alternately, such a process may be used to
preferentially adsorb a targeted gas (e.g. oxygen) thereby also
producing an enriched stream of oxygen.
Various different processes have been designed to
utilize the selective absorption ability of zeolite. Examples of these
CA 02253693 1998-11-09
-2-
include, Bansal (United States Patent Number 4,973"339), Stanford
(United States Patent Number 4,869,733) and Haruna et al (United
States Patent Number 4,661,125).
The process and apparatus of Bansal, Stanford and
Haruna et al each utilize two adsorption zones. The use of two
adsorption zones is undesirable as it unnecessarily complicates the
apparatus since it requires additional valuing and control means to
cycle each adsorption bed through a pressurization cycle and a
purging cycle. Further, this adds to the cost of the apparatus and
decreases the reliability of the apparatus.
Other disadvantages of existing designs is the
requirement to use expensive valve control means. In particular,
solenoids are frequently required to switch the adsorption zone
from a pressurization mode to a purging mode. These controls are
expensive and also prone to failure after extensive use.
Further, existing designs utilize electronics (e.g. micro-
processors) to control the cycling of the adsorption zone. This adds
to the cost of the equipment and also requires an electrical power
source to operate the process. Further, the electronic components
may be damaged in harsh environments and this limits the
applications of the some existing designs.
SUMMARY OF THE INVENTION
Despite the fact the method and apparatus of the instant
invention use only a single adsorption zone, the instant invention
may provide a continual, relatively steady, flow of an enriched gas
stream while the adsorption zone is being purged. Further, this
stream of enriched gas may have a flow rate which is generally
constant throughout the entire cycle of the adsorption zone from
the pressurization (adsorption) stage to the purging (de-adsorption)
stage.
CA 02253693 1998-11-09
-3-
To this end, in accordance with the instant invention
there is provided a concentrator for obtaining an enriched stream of
a first gas from a gas containing the first gas and at least one second
gas, the concentrator comprising:
(a) a pressurizable adsorption zone having an inlet port
and containing a member for reversibly adsorbing the
at least one second gas to produce the enriched stream;
(b) a storage container;
(c) a passageway extending between the pressurizable
adsorption zone and the storage container, the storage
container being expandable to an expanded position to
store at least a portion of the enriched stream when the
storage container is in flow communication with the
pressurizable adsorption zone;
(d) a member positioned in the passageway and
moveable between a first position in which the storage
container is in flow communication with the
pressurizable adsorption zone and a second position in
which the storage container is isolated from the
pressurizable adsorption zone;
(e) a purge valve associated with the pressurizable
adsorption zone and moveable between a closed
position and an open position in which the
pressurizable adsorption zone can be purged; and,
(f) an outlet port in flow communication with the
storage container.
In accordance with another embodiment of the instant
invention, there is provided a pressure swing adsorption apparatus
for producing an enriched stream of a first gas from a gas containing
the first gas and at least one second gas, the apparatus comprising:
(a) means for reversibly adsorbing the at least one
CA 02253693 1998-11-09
-4-
second gas to produce the enriched stream;
(b) expandable storage means for storing at least a
portion of the enriched stream;
(c) outlet means in flow communication with the
storage container for venting the enriched stream from
the apparatus;
(d) means for alternatingly connecting in flow
communication and then isolating the expandable
storage means and the means for reversibly adsorbing
the at least one second gas; and,
(e) purging means for removing at least a portion of the
at least one second gas from the means for reversibly
adsorbing the at least one second gas when the
expandable storage means is isolated from the means
for reversibly adsorbing the at least one second gas
whereby the expandable storage means expands to store at least a
portion of the enriched stream when the storage means is in flow
communication with the means for reversibly adsorbing the at least
one second gas.
In one embodiment, the storage container is drivingly
connected to the purge valve, whereby the purge valve is moved to
the open position when the storage container expands to the
expanded position.
In another embodiment, the concentrator includes an
actuator operatively mechanically connecting the storage container
to the purge valve. The purge valve may be a mechanical valve.
In one embodiment, the storage container comprises a
vessel having expandable walls and the vessel defines a reservoir
for storing the enriched stream, the size of the reservoir increasing
as the walls expand.
In an alternate embodiment the storage container
CA 02253693 1998-11-09
-5-
comprises a vessel having a moveable member mounted therein
and moveable between a first position and a second position, the
vessel defines a reservoir for storing the enriched stream, and the
size of the reservoir increases as the moveable member moves from
the first position to the second position. The concentrator may
include an actuator drivingly connecting the moveable member to
the purge valve whereby movement of the moveable member from
the first position towards the second position actuates the purge
valve to move to the open position. Alternately, the member may
be a pressure actuated valve whereby movement of the purge valve
to the open position causes the pressure actuated valve to move to
the closed position.
In another embodiment, the concentrator may also
comprise a biasing member to bias the moveable member to the first
position. The biasing member may be gravity if the moveable
member is moved upwardly during the expansion mode.
Alternately it may be an elastomeric member (eg. if the side walls
resiliently expand outwardly during the expansion mode or if an
elastomeric member is compressed during the expansion mode) or
it may be a spring which is compressed during the expansion mode.
In a preferred embodiment, the concentrator comprises
an oxygen concentrator to produce a concentrated stream of oxygen
enriched gas. In a more preferred embodiment, the gas comprises
air, the first gas is oxygen and the at least one second gas comprises
nitrogen such that the concentrator produces an oxygen enriched
stream of air.
In one embodiment, the flow of the enriched stream of
the first gas through the outlet port is less than the flow of gas
through the inlet port. The outlet port may be open at all times
when the concentrator is in use.
The member preferably comprises an adsorbent such as
CA 02253693 1998-11-09
-6-
a zeolite molecular sieve. If a stream of oxygen enriched air is to be
obtained from air, the member may comprise a nitrogen adsorbent.
In accordance with another embodiment of the instant
invention, there is provided a method for producing an enriched
gas having an increased concentration of a first gas from a gas
containing the first gas and at least one second gas comprising the
steps of:
(a) the step of introducing the gas into a vessel
containing a member for adsorbing the at least one
second gas;
(b) the step of pressurizing the vessel for a time
sufficient for the member to adsorb at least a portion of
the second gas to produce the enriched gas;
(c) the step of alternately passing the enriched gas to an
expandable container having an outlet port to expand
the container and isolating the expandable container
from the vessel; and,
(d) the step of purging the vessel when the expandable
container is isolated from the vessel.
In accordance with another embodiment of the instant
invention, there is provided a method for producing an enriched
gas having an increased concentration of a first gas from a gas
containing the first gas and at least one second gas comprising:
(a) introducing the gas into a vessel containing an
adsorbent for adsorbing the at least one second gas;
(b) pressurizing the vessel for a time sufficient for the
adsorbent to adsorb at least a portion of the second gas
to produce the enriched gas;
(c) opening a flow valve to connect the vessel in flow
communication with an expandable container;
(d) allowing the expandable container to expand to store
CA 02253693 1998-11-09
_7_
at least a portion of the enriched gas;
(e) opening a purge valve to purge the vessel and
closing the flow valve after the expandable container
has expanded to by a preset amount;
r
(f) purging at least a portion of the second gas from the
vessel; and,
(g) contracting the expandable container to release at
least a portion of the enriched gas stored in the
expandable vessel.
In one embodiment, the method further comprises the
step of venting the enriched gas from the expandable container
when the expandable container is isolated from the vessel.
In one embodiment, the method further comprises the
step of venting the enriched gas from the expandable container at all
times during the operation of the method.
In one embodiment, the method further comprises the
step of automatically passing the enriched gas to the expandable
container when the vessel reaches a preset pressure.
In one embodiment, the method further comprises the
step of automatically purging the vessel when the expandable
container expands by a preset amount.
In one embodiment, the method further comprises the
step of automatically isolating the expandable container from the
vessel upon the commencement of the step of purging the vessel.
A further advantage of the instant invention is that the
expansion of the storage container (i.e. the reservoir for storing the
enriched gas) may be used to actuate the purging cycle when the
storage container expands to a desired level. Accordingly, an
electronic controller is not required to time the process. Further, no
gas sensors are required to determine when to actuate a particular
part of the cycle of the adsorption zone.
CA 02253693 1998-11-09
_8_
A further advantage of the instant invention is that the
expandable container may be drivingly linked to the purging valve.
In this embodiment, simple actuation means may be used to move
the purging valve to the open position so as to initiate the purging
cycle. Accordingly, solenoids and other complicated switching
apparatus are not required. Further, the expandable container may
be operatively connected to the purge valve by mechanical linkages
and, in addition, the purge valve may be a simple mechanical valve
(e.g. a seat valve). Accordingly, no electrical power supply is
required to initiate the purging cycle.
It will be appreciated that, according to the instant
invention, a concentrator, and in a preferred embodiment an
oxygen concentrator, may be designed wherein a source of
pressurized gas (eg. air) which is fed to the adsorption zone is the
driving source of the entire apparatus. Accordingly, the resultant
device, which uses only an external motive force, may be
manufactured as a lightweight reliable unit.
In accordance with the instant invention, the apparatus
may be designed to trigger the end of the purging cycle and thereby
commence the pressurization (adsorption) cycle as the expandable
storage container contracts to a pre-determined position. This
position may be pre-determined based upon the volume of the
adsorption zone and the time required to complete the purging cycle
as well as the flow rate of enriched gas from the reservoir. In this
way, a continual flow of enriched gas may be produced by the
apparatus. Further, the apparatus is energy efficient since the timing
of the cycles is based upon the actual completion of a cycle (i.e. the
contraction of the expandable reservoir) as opposed to a electronic
timing means which would initiate a cycle regardless of the
concentration of the enriched gas exiting the apparatus.
CA 02253693 1998-11-09
_g_
BRIEF DESCRIPTION OF THE DRAWINGS
These and other advantages of the instant invention
will be more fully and particularly understood in connection with
the following description of a preferred embodiment of the
invention in which:
Figure 1 is a view illustrating in diagrammatic form an
apparatus according to the instant invention wherein the
adsorption zone is supplying enriched gas to the expandable
container;
Figure 2 is a further view illustrating in diagrammatic
form the apparatus of Figure 1 wherein the apparatus has
commenced the purging cycle; and,
Figure 3 is a schematic diagram of a an alternate purge
valve.
DESCRIPTION OF PREFERRED EMBODIMENT
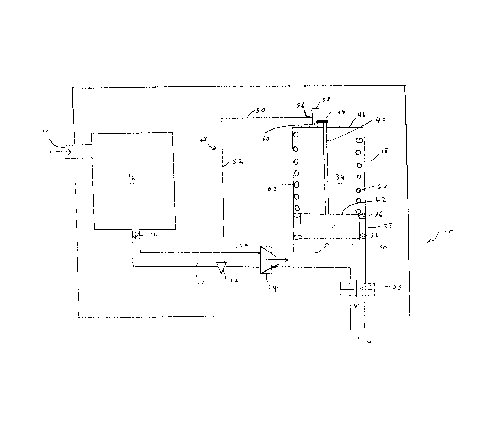
Concentrator 10 comprises inlet port 12, outlet port 14,
adsorption zone 16 and expandable container 18. Passageway 20
extends between adsorption zone 16 and expandable container I8.
Passageway 20 is provided with purge valve 22 and valve 24.
Adsorption zone 16 may be of any particular
construction which is known in the art for pressure swing
adsorption apparatus. In the preferred embodiment, the adsorption
zone 16 comprises a vessel distinct from expandable container 18
that may be subjected to an increased pressure during which a
selected fluid is adsorbed into adsorption media provided in
adsorption zone 16 leaving a fluid having an increased
concentration of the remaining (unadsorbed) fluids in adsorption
zone 16. It will be appreciated that adsorption zone 16 may comprise
a bed containing the adsorbent material through which the fluid
flows as it passes through adsorption zone 16.
CA 02253693 1998-11-09
-10-
The fluid may be a liquid or a gas. If the fluid is a liquid,
then concentrator 10 may be used, for example, to selectively
remove an impurity from a fluid stream (eg. the fluid selectively
adsorbed into carbon) such as water or a pesticide. The concentrator
may also be used for pressure swing fractional distillation.
In a preferred embodiment, the fluid is a gas and, more
preferably, the concentrator is an oxygen concentrator. The
following description is based upon the use of concentrator 10 as an
oxygen concentrator; however, the concentrator may be used for
other pressure swing operations of fluids.
If concentrator 10 is an oxygen concentrator, then the
feed gas which is introduced into adsorption zone 16 via inlet port
12 comprises an oxygen containing gas and, more preferably, air.
The adsorbent material in adsorption zone 16 accordingly comprises
a material which selectively adsorbs nitrogen (the largest
constituent of air) thereby leaving air containing an enriched Ievel
of oxygen in adsorption zone 16. Such adsorbent material are
known in the art. Examples of such material are zeolites and, in a
particularly preferred embodiment, the adsorbent is clinoptilolite.
It will be appreciated that, in an alternate embodiment,
the desired product may be the fluid adsorbed onto the adsorbent
media. In such a case, the product exiting purge valve 22 could be
fed to a container or other apparatus as may be desired.
The remaining part of this description of the preferred
embodiment is premised upon concentrator 10 including an
adsorbent to remove nitrogen from air thereby producing a stream
of oxygen enriched air. It is to be understood that the feed gas stream
fed to adsorption stream 16 may comprise at least any two gases and
the adsorbent material may be selected to adsorb the one or more of
such gases leaving a gas stream having an enhanced concentration
of the remainder of such gases.
CA 02253693 1998-11-09
-11-
Adsorption zone 16 operates under pressure.
Accordingly, means must be provided to raise adsorption zone 16 to
the desired pressure. In the preferred embodiment, the air fed to
inlet port 12 comprises a stream of pressurized air (eg at a pressure
of 5 to 30 psig). It will be appreciated that, in an alternate
embodiment, a compressor or other means may be provided as part
of apparatus 10 to feed an air stream into adsorption zone 16 and to
pressurize adsorption zone 16 to the required pressure. The exact
pressure which is required for the adsorption media to adsorb the
targeted gas, and the length of the adsorption cycle, will depend on
the thermodynamics of the adsorption media.
Valve 24 is provided in passageway 20 to alternately (i.e.
cyclicly), connect adsorption zone 16 and expandable container 18 in
flow communication and to then isolate adsorption zone 16 from
expandable container 18. Valve 24 may be any member which is
movable between a first position in which adsorption zone 16 is
isolated from expandable container 18 and an open position in
which adsorption zone 16 and expandable container 18 are in flow
communication. Further, valve 24 may be positioned at any point
between the two vessels. For example, valve 24 could be positioned
as part of outlet port 26 of adsorption zone 16. Further, if adsorption
zone 16 and expandable container 18 are a single unit separated by a
wall (not shown), valve 24 may be positioned in the wall.
While adsorption zone 16 is undergoing the
adsorption portion of the cycle, adsorption zone 16 is sealed
sufficiently such that adsorption zone 16 will be raised to the
required pressure. Preferably, no gas flow out of adsorption zone 16
is permitted during this part of the method. Accordingly purge
valve 22 is preferably in the fully closed position shown in Figure 1
and valve 24 is preferably in the fully closed position shown in
Figure 2. Adsorption zone 16 is thus isolated so as to allow pressure
CA 02253693 1998-11-09
-12-
to build up therein. Due to the inflow of air through inlet port 12,
pressure will build up in adsorption zone 16 and nitrogen will be
adsorbed in the adsorbent media.
Based upon the volume of adsorption zone 16, the
adsorption characteristics of the adsorbent in adsorption zone 16 and
the rate of air input into adsorption zone 16, the length of time
required to achieve the desired concentration of oxygen in the free
gas in adsorption zone 16 may be calculated. Further, a person
skilled in the art will be able to determine the pressure at which this
desired oxygen concentration will be achieved.
Any valve mechanism (either mechanically or
electrically operated) may be used for valve 24. Preferably, valve 24
is a pressure actuated member which will open to bring adsorption
zone 16 into flow communication with expandable container 18
when adsorption zone 16 reaches the pressure at which the required
oxygen concentration will have been achieved (eg. a check valve).
One advantage of this approach is that valve 24 may open, and the
adsorption cycle therefore terminate, when a desired preset pressure
is achieved. Thus no sensors are required to monitor the progress of
the adsorption cycle. The adsorption cycle automatically terminates
when the requisite pressure is reached. Preferably, valve 24 is a
mechanical member which is biased (eg. by a spring) to the closed
position and which will open when the pressure upstream thereof
(i.e. in adsorption 16 and in passageway 20) reaches a preset pressure
(which may be the pressure at which the desired concentration of
oxygen is achieved in the free gas in adsorption zone 16).
When valve 24 is in the open position shown in Figure
1, oxygen enriched air will pass from adsorption zone 16 through
passageway 20 and into expandable container 18.
Outlet port 14 is in flow communication which
expandable container 18. When valve 24 is in the open position
CA 02253693 1998-11-09
-13-
shown in Figure 1, outlet port 14 is also, indirectly, in flow
communication with adsorption zone 16. When valve 24 opens,
expandable container 18 expands so as to receive at least a portion of
the oxygen enriched air which exits adsorption chamber 16.
Accordingly, outlet port 14 provides a flow of oxygen enriched air
when valve 24 is open and the oxygen enriched air is passing into
expandable container 18. Further, when valve 24 closes and
adsorption zone 16 is isolated from expandable container I8,
expandable container 18 preferably will have by then stored a
sufficient supply of oxygen enriched air so that outlet port 14 may
still provide a flow of oxygen enriched air even while adsorption
chamber 16 is undergoing a purge cycle.
In a preferred embodiment, outlet port 14 has an
aperture 28 which is open at all times when concentrator 10 is in
operation. Aperture 28 is preferable of a pre-set opening size so as to
provide a generally continuous flow of oxygen enriched air through
outlet port 14. It will also be appreciated that, if desired, aperture 28
may have a variable opening size so as to vary the flow rate of
oxygen enriched air through outlet port 14. This may be desirable if,
for example the apparatus is used for medical purposes, eg.
providing a source of oxygen enriched air to a patient. Further,
aperture 28 may be operable so as to seal outlet port 14 (or alternately
a valve to close outlet port 14 may be provided). This may be
desirable if, for example there is backpressure from downstream
equipment.
Preferably, expandable container 18 and aperture 28 are
sized so that outlet 14 provides a generally constant flow rate of
oxygen enriched air during both the adsorption cycle and the purge
cycle of adsorption zone 16. To this end, outlet port 14 preferably has
a reduced flow rate of gas therethrough than the flow rate of air into
adsorption zone 16 via inlet port 12. Preferably, the flow rate of gas
CA 02253693 1998-11-09
-14-
through outlet port 14 is about half that of the flow rate into inlet
port 12. For example, if inlet 12 has a flow rate of 6 - 8 litres per
minute of air, then outlet port 14 preferably has a flow rate
therethrough of 2 - 4 litres per minute of oxygen enriched air. This
flow rate may be achieved by, for example, selecting the cross
sectional area of outlet port 14 or including a flow restriction, such
as aperture 28, in outlet port 14, to achieve this result. It will be
appreciated that two or more adsorption zones 16 may be connected
to one or more expandable containers 16. A regulator could also be
used to control the output rate from port 14.
Expandable container 18 may be any storage container
for storing a gas which has an expandable reservoir 30 for storing at
least a portion of the oxygen enriched gas produced in adsorption
zone 16. In one preferred embodiment, expandable container 18 may
be a storage vessel having expandable walls. In this embodiment,
container 18 may have at least one wall which will expand when
container 18 is subjected to an increased pressure. For example, one
or more of the walls of container 18 may be composed of an
elastomeric material. Alternately, in another preferred
embodiment, container 18 may have a flexible side wall which is
movable between a first, compacted portion and a second expanded
position when reservoir 30 is filled with oxygen enriched air. For
example, expandable container 18 may be in the shape of a bellows.
Alternately, or in addition, container 18 may comprise a
vessel having a movable member mounted therein and movable
between a first position and a second position. The size of reservoir
increases as the movable member moves from the first position
to the second position. An example of such a construction is shown
in Figures 1 and 2. In these Figures, the movable member comprises
30 piston 32.
In the embodiment of Figures 1 and 2, expandable
CA 02253693 1998-11-09
-15-
container 18 is a longitudinally extending member and, is preferably
vertically oriented. Piston 32 may be movably mounted in container
18 by any means known in the art. Further, piston 32, and the
means for movably mounting piston 32 in container 18, preferably
isolate reservoir 30 from upper portion 34 of container 18. In this
way, the oxygen enriched gas which enters container 18 will remain
in reservoir 30 instead of passing upwardly by piston 32 to upper
portion 34. Piston 32 preferably moves upwardly into upper portion
34 of container 18 due solely to a pressure of the oxygen enriched
stream passing through valve 24. Piston 32 may be so mounted by a
plurality of O-rings 36 which are positioned between piston 32 and
side wall 38 of container 18. The O-rings, in conjunction which
piston 32 seal reservoir 30 from upper portion 34. However, it will
be appreciated that other means, such as a bellows, bearings or a cam
may be used to movably mount piston 32.
When the adsorption cycle of adsorption zone I6 is
completed, valve 24 will open allowing a stream of oxygen enriched
air to pass into container 18. At this time, piston 32 may be in
approximately the position shown in Figure 1 (i.e. in a contracted
position towards the bottom of container 18). Oxygen enriched air
will enter container 18 and pass through aperture 28 through outlet
port 18. However, as the flow of oxygen enriched air through
aperture 28 is restricted, pressure will build up in container 18
which will force piston 32 into upper portion 34. As additional
oxygen enriched air enters container 18, the pressure will be
maintained in container 18 and piston 32 will continue to move
into upper portion 34, for example until the position shown in
Figure 2 is reached.
As oxygen enriched air exits adsorption zone 16, the
pressure in adsorption zone 16 will decrease. For example, the
pressure in adsorption zone 16 may reach 20 to 30 psig at the end of
CA 02253693 1998-11-09
-16-
an adsorption cycle. As the pressure is reduced, for example to about
psig, nitrogen will commence being released by the zeolite thus
decreasing the concentration of oxygen in the air exiting adsorption
zone 16. At this time, it is desirable to purge the zeolite in
5 adsorption zone 16. Advantageously, in one embodiment of the
instant invention, the purge cycle may be commenced
automatically.
In particular, apparatus 10 may include an actuator
which drivingly connects the expandable container (e.g. piston 32) to
purge valve 22 whereby movement of piston 32 from a first
contracted position (as shown in Figure 1) to a second expanded
position (as shown in Figure 2) actuates the purge valve to move it
to the open position. As the flow rate of oxygen enriched air
through aperture 28 may be predetermined and as the volume of
container 18 is predetermined, a person skilled in the art rnay
determine the distance through which piston 32 will travel as the
oxygen enriched air exits adsorption chamber 16. By designing
container 18 so as to permit piston 32 to move this distance, piston
32 may be in the upper position shown in Figure 2 when the oxygen
enriched air has been vented from adsorption zone 16 and the
pressure in adsorption zone 16 has been reduced to a point wherein
it is desirable to purge adsorption zone 16.
Preferably, piston 32 is drivingly connected to purge
valve 22 so as to actuate purge valve 32 when piston 32 is in the
upper position shown in Figure 2. At that time, purge valve 32 will
be in the open position allowing air to exit therethrough (as shown
in Figure 2). When purge valve 22 opens, the pressure in passage
way 20 will drop to a sufficient degree such that valve 24 (which is
preferably pressure operated) will close thus isolating reservoir 30
from adsorption zone 16. In this position, the pressurized air
entering inlet port 12 may pass through the adsorbent material in
CA 02253693 1998-11-09
-17-
adsorption zone 16, exit adsorption zone 16 via outlet port 26 and
exit passageway 28 via the purge valve thereby removing the
nitrogen which was releasable adsorbed by the adsorption media
from apparatus 10.
The driving connection between piston 32 and purge
valve 22 may be either mechanical or electrical but is preferably
mechanical. As shown in Figures 1 and 2, extension member 40
extends upwardly from upper surface 42 of piston 32. Movable arm
44 is fixedly mounted to extension member 40. Accordingly,
movable arm 44 moves longitudinal with respect to container 18 as
piston 32 moves longitudinally within container 18. When piston
32 is in the contracted position shown in Figure 1, moveable arm 44
is positioned adjacent surface 46 of container 18 and, when piston 32
is in the expanded position, as shown Figure 2, moveable arm 44 is
spaced a distance from surface 46 of container 18.
Movable arm 44 may be mechanically linked to purge
valve 32 such as by connector member 48. Connector member 48
comprises a mechanical linkage which extends from movable arm
44 to purge valve 32. If container 18 extends vertically, then
connector member 48 may have a first horizontal portion 50 and a
second vertical portion 52 extending downwardly from the end of
horizontal portion 50 distal to moveable arm 44. Connector member
48 has a first end 54 which is operatively connected to purge valve
22 and a second end 56 which is positioned to engage and be
actuated by movable arm 44.
If container 18 is vertically disposed, then connector
member 48 may be operatively engaged by movable arm 44 so as to
move first end 54 upwardly as piston 32 moves upwardly and to
move first end 54 downwardly as piston 32 moves downwardly.
This may be achieved by having at least a first arm 58 provided on
second end 56. As container 18 expands, piston 32 moves upwardly.
CA 02253693 1998-11-09
-18-
At some point, movable arm 44 will engage first arm 58. Further
movement of piston 32 will cause first arm 58 to move upwardly
(due to its engagement with movable arm 44). As moveable arm 58
moves upwardly, purge valve 22 is moved to the open position.
When purge valve 22 has been opened a sufficient amount of time,
valve 24 will close and adsorption zone 16 will be purged. During
this purging cycle, piston 32 will move downwardly into reservoir
30 thereby forcing oxygen enriched air through aperture 28. The
movement of piston 32 may be due to the pressure of gravity (if
container 18 is vertically disposed). In addition, or alternately, a
biasing member, such as spring 62 may urge piston 32 downwardly
to the contracted position.
As air exits aperture 28, piston 32 will move
downwardly and, accordingly, arm 44 will move downwardly. If
purge valve 22 is a vertically operable valve, then purge valve 32
may cause first arm 58 to move downwardly in conjunction with
movable arm 44 (such as by the force of gravity and/or a biasing
means urging purge valve 22 to the closed position) thus closing
purge valve 22. Alternately, or in addition, second end 56 may have
a second arm 60. In this embodiment, vertical portion 52 is a
generally non-compressible member (eg. a rod) and as piston 32
moves downwardly, movable arm 44 will engage second arm 60
thereby driving first end 54 downwardly so as to close purge valve
22.
Preferably, connector member 48 moves essentially
only due to movable arm 44 pushing up longitudinally outwardly
on first arm 58 and longitudinally inwardly on second arm 60.
Further, arms 58 and 60 are preferably spaced apart. In this way,
piston 32 will move upwardly a defined amount before causing
purge valve 22 to open thus allowing reservoir 30 to be filled a pre-
set amount before the purge cycle commences. Further, piston 32
CA 02253693 1998-11-09
-19-
may move downwardly by a preset amount until it engages second
arm 60 thereby closing purge valve 22 and completing the purging
cycle. The distance between the arms is preferably sufficient to allow
the purging cycle to be conducted while piston 32 is still pumping
air from reservoir 30 through aperture 28. In a particularly preferred
embodiment, by the time piston 32 is in the contracted position
shown in Figure 1, purge valve 22 has been closed for a sufficient
amount of time to allow adsorption zone 16 to have reached the
requisite pressure to have produced an oxygen enriched stream and
to cause valve 24 to open. Thus, a continuous supply of oxygen
enriched air through aperture 28 may be obtained.
In this embodiment, it may be seen that the actuator for
purge valve 22 is a mechanical linkage comprising member 40,
movable arm 44 and connector 48. Purge valve 22 is accordingly
actuated by vertical movement of piston 32. Purge valve 22 is
preferably a mechanical valve that is moved to the open position by
vertical motion of connector 48. An example of such a valve is a
seat valve which is lifted upwardly by upward motion of first end
54. However, other valves, such as a gate valve or a ball valve
which rnay be opened to an open position by vertical motion of first
end 54 may be utilized.
In an alternate embodiment, arms 40, 58 and 60 may
define electrical connections and contact between arms 44 and 58
may actuate a circuit to open purge valve 22 and connection
between arm 44 and arm 60 may consequentially close the circuit to
close purge valve 22. For example, vertical portion 52 may be
composed of a bimetal member or a muscle wire which contracts
when heated. When movable arm 44 contacts first arm 58, an
electrical connection may be made causing an electrical current to
flow through vertical portion 52 thereby heating the vertical portion
and causing it to contract. This contraction will cause purge valve 22
CA 02253693 1998-11-09
-20-
to open. When the electrical connection is broken (i.e. arm 44 is no
longer in contact with first arm 58 or alternately the circuit is broken
when movable arm 44 contacts second arm 60) the current flow
through vertical potion 52 will be terminated thus allowing vertical
portion 52 to cool and expand thereby sealing purge valve 22.
An alternate embodiment of purge valve 22 is shown
in Figure 3. In this embodiment, the purge valve comprises a bi-
metal strip 64 having an outer metal member 66 affixed to an inner
metal member 68. The two metals have different thermal
coefficients of expansion. Accordingly, when contact is made
between arms 44 and 58, an electrical connection may be made
causing an electric current to pass, eg. via an electrically conductive
member 48, to and through bi-metal strip 64 thereby heating the
strip. If the inner metal member 68 has a greater thermal expansion
than the outer metal member 66, the heating of bi-metal strip 64
will cause the bi-metal strip 64 to bend inwardly in the direction of
arrow A thereby uncovering opening 70 so that the purge cycle may
begin. When movable arm 44 engages arm 60, or brakes contact with
first arm 58, the circuit may be closed causing the electric heating
current to terminate and allowing bi-metal strip 64 to cool. When
bi-metal strip 64 cools, inner metal member 68 will contract more
than the outer metal member 66 thereby causing the bi-metal strip
to curve downwardly and close opening 70. In an alternate
embodiment, it will be appreciated that bi-metal strips 64 may be
positioned on the outside passageway 20.
In a further alternate embodiment, valve 22 may be
actuated by a solenoid. Once again, contact between movable arm 44
and first arm 58 may complete an electric circuit so as to actuate a
solenoid to open any desired valve which may function as a purge
valve. When movable arm 44 engages arm 60, or brakes contact
with first arm 58, the circuit may be closed causing the solenoid to
CA 02253693 1998-11-09
-21-
move to its starting position thereby closing the purge valve.
By constructing a concentrator according to the instant
invention, a concentrator may be constructed whereby the
pressurized air fed to adsorption zone 16 may be the only motive
force to open purge valve 22 and valve 24. Further, it provides the
requisite motive force to cause container 18 to expand. Thus, by
using simple mechanical linkages and movable or expandable
elements, a gas concentrator having a simple, rugged construction
may be developed.
In addition, aperture 28 may be in an open position at
all times so as to provide a continual supply of enriched gas to
outlet 14 even when adsorption chamber 16 is being purged. This is
due to reservoir 30 contracting during the purge cycle thereby
driving the enriched stored gas from reservoir 30 to aperture 28.
Another advantage of the instant invention is that the
expansion of container 18 may be used to time the purging cycle of
adsorption zone 16. Accordingly, electronic timers or concentration
sensors are not required to provide input to a controller to
determine when the purge cycle should be commenced or
terminated.