Note: Descriptions are shown in the official language in which they were submitted.
CA 022~4120 1998-ll-lO
WO 97/43273 PCT/US97/08148
SUBSTITUTF.D 1.3-BEN7,0n~0XO~,FS
This invention relates to novel substituted 1,3-benzodioxole compounds which
have ~nti~ hetic, antihyperglycemic, and antiobesity properties. The present invention
also relates to pharm~eutic~l compositions cont~ining these compounds, methods for
the preparation of these compounds, and methods for the use of these compounds in
treating diabetes and/or hyperglycemia and/or obesity in ~ ls
BACKGROUND OF THF INVE~TION
Bloom, et al., U.S. Patent 5,061,727, disclose substituted 5-(2-((2-aryl-2-
hydroxyethyl)amino)propyl)- 1 ,3-benzodioxoles of general formula (I)
R1 ~ X (I)
wherein R1 and R4 may be one or more groups which may be the same or different and
are selected from the group consisting of hydrogen, C1 to C4 alkyl, C1 to C4 alkoxy,
hydroxy, halogen, trifluoromethyl, carboxy, hydroxyalkyl, alkoxycarbonyl, C1 to C4
thioalkyl, sulfonyl and sulfinyl; X is a divalent radical consisting of
OR' R' O-y
~,N~ or ~'N
wherein R' is selected from the group consisting of hydrogen, Cl to C4 alkyl and C1
to C4 acyl and Y is selected from the group consisting of carbonyl and thiocarbonyl; R2
and R3 may be the same or different and are selected from the group consisting of
hydrogen and C1 to C4 alkyl; Rs and R6 are selected from the group consisting of- hydrogen, carboxy, alkoxycarbonyl, hydroxymethyl, -CH2OCH2COOR7 and -
CH2OCH2CH2OR7, where R7 is hydrogen or C1 to C4 alkyl; with the provision that
~ Rs and R6 may not both be hydrogen; which have antihyperglycemic and antiobesity
activity.
CA 022~4120 1998-11-10
Wo 97/43273 PCT/US97/08148
The synthesis, anti~ betic effects, and antiobesity effects of (R,R)-5-[2-[[2-(3-
chlorophenyl)-2-hydroxyethyl~amino]propyl)- 1,3-benzodioxole-2,2-dicarboxylate
(which is one of the compounds disclosed by Bloom, et al. in U.S . Patent 5,061,727)
are detailed in Bloom, et al. J. Med. Chem., 1992, 35, 3081, Largis, et al. Drug Dev
Res., 1994,32, 69, and Bloom, et al. Drugs of ~he Future, 1994, 19, 23.
The compounds of the present invention possess greatly increased potency at
human ,B3 receptors in comparison to the compounds in Bloom, et al., U.S. Patent5,061,727. They retain high selectivity for the ,B3 receptor and show much higher
10 antiobesity and antihyperglycemic activity in animal models than the compounds of the
prior art. The compounds have intrinsic activity at human ,~3 receptors and can directly
bring about antihyperglycemic and antiobesity effects, but may also be hydrolyzed in
vivo to deliver a compound of the type disclosed in Bloom, et al., U.S. Patent
5,061,727 where Rs and R6 are carboxy. Thus the compounds may act as prodrugs.
15 Therefore, the compounds of this invention are useful in treating diabetes,
hyperglycemia, and obesity, exhibiting minim~l side effects such as heart rate increase
and muscle tremor in humans and animals, when form~ t~d into phann~euti~l
compositions.
l)ESCR~PT~ON OF THE INVFNT~ON
The compounds of the present invention achieve their ~ntirli~betic,
antihyperglycemic, and antiobesity effects by acting as selective agonists at ,B3
adrenergic receptors. The stim~ tion of these receptors on white and brown
25 adipocytes promotes both lipolysis (breakdown of fat) and energy expenditure.Selective stimulation of ,B3 adrenergic ~~c~lol~ is important for chronic tre~t~n.o.nt.
Stimulation of other ,~-receptors could cause side effects such as increased heart rate
(,Bl effect) and muscle tremor (~2 effect). The compounds of the present invention
show high selectivity for ,B3 adrenergic receptors.
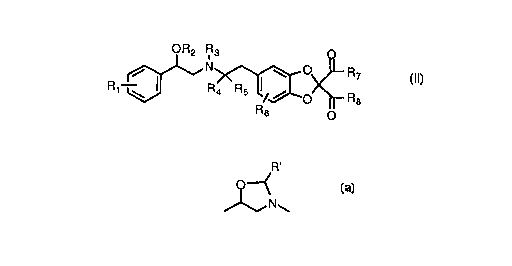
According to the present invention there are provided new compounds of the
formula (II):
CA 022~4120 1998-ll-lO
WO 97/43273 PCT/~S97/08148
OR2 R3 Q
R1 ~~ R?'~[~~<~R7 (II)
R6 o
wherein:
R1 and R6 are independently hydrogen, C1 to C6 alkyl, trifluoromethyl, cyano,
Cl to C6 alkoxy, or halogen;
R2 is hydrogen or Cl to C6 trialkylsilyl;
R3 is hydrogen or Cl to C6 alkoxycarbonyl;
or R2 and R3 are joined to form a ring:
~ N~
wherein R' is hydrogen, Cl to C6 alkyl, or aryl;
R4 and Rs are independently hydrogen or C1 to C6 alkyl;
R7 and R8 are independently ORg or NR1oR1 1;
Rg is hydrogen, C1 to C12 alkyl, Cl to C12 cycloalkyl, Cl to C12 silylalkyl,
aryl, arylallcyl, alkoxyalkyl, heteroaryl, -CHR12COOR13, -CHR12C(O)R13, -
CHR12CONRloRll, -CHR12OCOOR13, or -CHR12OC(O)R13, with the provision
that Rg is not hydrogen in both R7 and R8;
R1o and R11 are independently hydrogen, C1 to C12 alkyl, arylalkyl, aryl,
furanylalkyl, or alkoxycarbonylalkyl;
R12 and R13 are independently hydrogen, Cl to C12 alkyl, aryl, or aralkyl;
and the pharmaceutically acceptable salts thereof, the enantiomers thereof, the racemic
mixtures thereof, and the diastereomeric mixtures thereof.
In the description above, aryl may be phenyl or napthyl; arylalkyl may be
phenyl Cl to C6 alkyl or naphthyl C1 to C6 alkyl; alkoxy alkyl may be C1 to C6
alkoxy C1 to C6 alkyl; and heteroaryl may be pyridyl, thiophenyl, furanyl, imi~i~7.r)1yl,
oxazolyl, or thiazolyl. The aryl, arylalkyl and heteroaryl groups referred to above may
CA 02254120 1998-11-10
WO 97/43273 PCT/US97/08148
be optionally substituted with one or more moieties select~d from halogens, Cl-C6
alkyl, C,-C6 alkoxy, -CF3, -CN, or -OH groups. In a more preferred embo~lim~nt of
this invention, the phenyl Cl to C6 alkyl group may be optionally substituted by one or
more substituents selected from F, Cl, Br, CH3 or CF3.
s
This invention also provides processes for preparing the compounds of the
invention which comprise one of the following:
a) reacting a compound of formula:
OR2 R3
Rl ~ R~oXco2RAo
wherein R2 - R6 are as defined above and Rg~ is OAg or R8 as defined above excepting
OH with a compound of formula:
I-CHR1 2oc(o)Rl3
wherein R12 and R13 are as defined above to give a compound of formula II wherein
Rg is
-CHR12OC(O)R13
or b) reacting a compound of formula:
OR2 R3
R ~J ~N ~OxCO2H
R4 R5 ~ o COR8
R6
25 wherein Rl-R6 and R8 are as defined above with compound of formula RgOH whereRg is as defined above excepting hydrogen to give a corresponding compound of
formula II;
CA 02254120 1998-ll-lO
WO 97/43273 PCT/US97/08148
or c) hydrolysing a compound for formula:
OR2 R3
R1--~ I ~[ OXCOR,
wherein Rl, R4, Rs, R6, R7 and R8 are as defined above and R2 is trialkylsilyl and R3
is (Cl-C6 alkoxy)carbonyl to give a corresponding compound of formula 1 wherein R2
and R3 are both hydrogen,
or d) reacting a compound of formula II wherein R2 and R3 are both hydrogen with an
aldehyde of forrnula:
R'CHO
wherein R' is as defined above to give a corresponding compound of forrnula I wherein
R2 and R3 are joined to forrn a ring:
o~R'
~N
or e) reacting a compound of formula:
I R2 R3
R ~ '~Ooxco2RH
R6
CA 02254120 1998-ll-lO
WO 97/43273 PCT/US97/08148
wherein Rl, R4, Rs and R8 are as defined above with the proviso neither R2 or R3 is
hydrogen with a compound of formula:
Z-R9
s
wherein Z is Cl, Br, I, methanesulfonate or p-toluenesulfonate and Rg is as defined in
above excepting hydrogen, to give a corresponding compound of formula II;
or f) hydrolysing a compound of formula:
OR2 R3
R g3r,~ ~0XCoOR9~
wherein R1, R2, R3~ R4, Rs and R6 are as defined in Claim 1, R8 is ORg' or
NR1oRl 1 and Rg~ is Cl-C12 alkyl, C1-C12 cycloalkyl, C1-C12 silyalkyl, aryl,
15 arylalkyl, alkoxyalkyl or heteroaryl to give a compound of formula:
OR2 R3
R ~/ ~ ~ OXCOOH
wherein RI-R6 and R8 are as defined above providing that R2 and R3 are both
20 hydrogen or joined to form a ring:
or g) reacting a compound of formula:
CA 022~4120 1998-11-10
wo 97/43273 PCT/US97/08148
OR2 R3
N~O~COORg
R1 ~ R4 5 ~ ~ COR8
wherein R1-R6 and R8 are as defined above and Rg~ is as defined above, with a
5 compound of forrnula:
HNR I IR 12
wherein Rl I and R12 are as defined above to give a compound of formula:
~I R2 R3
R~ OXCONRllRl2
wherein RI-R6, Rg, Rll and R12 are as defined above.
The most ~l~fell~d compounds of this invention are the following and the
pharmaceutically acceptable salts thereof:
5-{2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl)-benzo[1 ,3~dioxole-
2,2-dicarboxylic acid bis-(2-methoxy-ethyl) ester;
5- ~ 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino] -propyl ~ -benzo[ 1 ,3]dioxole-
2,2-dicarboxylic acid diphenethyl ester;
5- { 2-~2-(3-chloro-phenyl)-2-hydroxy-ethylamino] -propyl } -benzo[ 1 ,3]dioxole-
2,2-dicarboxylic acid bis-(2-butoxy-ethyl) ester;
5- {2-~2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl~-benzo[1,3]dioxole-
- 2,2-dicarboxylic acid bis-(2-phenoxy-ethyl) ester;
5- { 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino] -propyl ) -benzo[ 1 ,3]dioxole-
2,2-dicarboxylic acid bis-(2-ethoxy-ethyl) ester;
CA 022~4120 1998-11-10
WO 97/43273 PCTIUS97/08148
5- ~ 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl ) -benzo[ 1 ,3]dioxole-2,2-dicarboxylic acid bis-(2-tert-butoxy-ethyl) ester;
5- { 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino~-propyl ) -benzo[ 1 ,3]dioxole-2,2-dicarboxylic acid (2-tert-butoxy-ethyl) ester;
55-(2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl)-benzo[1,3]dioxole-
2,2-dicarboxylic acid bis-(2-isobutoxy-ethyl) ester;
5-~2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl)-benzo[1,3]dioxole-
2,2-dicarboxylic acid bis-(benzyl) ester;
5-~2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-benzo[1,3]dioxole-
102,2-dicarboxylic acid bis-(cyclohexyl) ester;
5- { 2-12-(3-chloro-phenyl)-2-hydroxy-ethylamino] -propyl } -benzo[ 1 ,3]dioxole-
2,2-dicarboxylic acid bis-(cyclopentyl) ester;
5- ( 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl ) -benzo[ 1 ,3]dioxole-2,2-dicarboxylic acid dioctyl ester;
155- ( 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl ~ -benzo[ 1 ,3]dioxole-
2,2-dicarboxylic acid dipentyl ester;
5- { 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl ) -benzo[ 1 ,3]dioxole-2,2-dicarboxylic acid dihexyl ester;
carbonic acid 3-chloro-benzyl ester 2-(3-chloro-benzyloxycarbonyloxy)-4-(2-
20[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl3-phenyl ester;
5-~2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-benzo[1,3]dioxole-
2,2-dicarboxylic acid bis-(1-phenyl-ethyl) ester;
5- { 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino] -propyl ) -benzo[ 1 ,3]dioxole-
2,2-dicarboxylic acid diheptyl ester;
255- ~ 2- [2- (3-chloro-phenyl)-2-hydroxy-ethylamino] -propyl ) -benzo[ 1 ,3]dioxole-
2,2-dicarboxylic acid dinonyl ester;
5- ~ 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl ) -benzo[ 1 ,3]dioxole-2,2-dicarboxylic acid bis-decyl ester;
5-{2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-benzo[1,3]dioxole-
302,2-dicarboxylic acid didodecyl ester;
5- { 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl } -benzo[ 1 ,3]dioxole-2,2-dicarboxylic acid bis-(2-isopropoxy-ethyl) ester;
5- { 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl } -benzo[ 1 ,3]dioxole-2,2-dicarboxylic acid isopropyl ester;
CA 022~4120 1998-11-10
WO 97143273 PCTIUS97/08148
5- ~ 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino] -propyl) -benzo[ 1 ,3]dioxole-2,2-dicarboxylic acid ethyl ester;
5-(2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl)-benzo[1,3]dioxole-
2,2-dicarboxylic acid bis-methoxycarbonylmethyl ester;
55-{2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl)-benzo~1,3]dioxole-
2,2-dicarboxylic acid bis-propoxycarbonylmethyl ester;
5-(2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-benzo[1 ,3]dioxole-
2,2-dicarboxylic acid bis-(1-methoxycarbonyl-ethyl) ester;
5-(2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl)-benzo[1 ,3]dioxole-
102,2-dicarboxylic acid bis-isopropoxycarbonylmethyl ester;
5-(2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-benzo[1,3]dioxole-
2,2-dicarboxylic acid bis-ethoxycarbonylmethyl ester;
5- ( 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl } -benzo[ 1 ,3]dioxole-2,2-dicarboxylic acid bis-(1-ethoxycarbonyl-ethyl) ester;
155- ( 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl} -benzo[1 ,3]dioxole- 2,2-dicarboxylic acid bis-trimethylsilanylmethyl ester;
5-(2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-benzo[1 ,3]dioxole-
2,2-dicarboxylic acid bis-(2-trimethylsilanyl-ethyl) ester;
5- ( 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl ) -benzo[ 1 ,3]dioxole-202,2-dicarboxylic acid bis-(3-trimethylsilanyl-propyl) ester;
5-(2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-benzo[1,3]dioxole-
2,2-dicarboxylic acid bis-(3,3-dimethyl-butyl) ester;
5-(2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl~-benzo[1,3]dioxole-
2,2-dicarboxylic acid bis-cyclohexylmethyl ester;
255- ( 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl ) -benzo[ 1 ,3]dioxole-
2,2-dicarboxylic acid bis-(4-methyl-pentyl) ester;
5- ( 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino] -propyl ) -benzo[ 1 ,3]dioxole-
2,2-dicarboxylic acid bis-(2-cyclohexyl-ethyl ) ester;
5- ( 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino] -propyl ) -benzo[ 1 ,3~dioxole-
- 302,2-dicarboxylic acidbis-(3-cyclopentyl-propyl )ester;
5-(2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-benzo[1,3]dioxole-
2,2-dicarboxylic acid bis-cyclopropylmethyl ester;
5-(2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl)-benzo[1 ,3]dioxole-
2,2-dicarboxylic acid bis-(l-methyl-cyclopropylmethyl) ester;
CA 022~4120 1998-11-10
WO 97/43273 PCT/US97/08148
- 10 -
5-(2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl)-benzo[1,3]dioxole-
2,2-dicarboxylic acid bis-cyclobutylmethyl ester;
5- { 2-~2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl ) -benzo[ I ,3]dioxole-2,2-dicarboxylic acid bis-(2-cyclopentyl-ethyl) ester;
55- { 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylarnino]propyl 3 benzo[ 1 ,3]dioxole-2,2-dicarboxylic acid bis-acetoxymethyl ester;
5-{2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]propyl}benzo~1,3]dioxole-
2,2-dicarboxylic acid bis-propionyloxymethyl ester;
5-{2-~2-(3-chloro-phenyl)-2-hydroxy-ethylamino]propyl)benzo[1,3]dioxole-
102,2-dicarboxylic acid bis-butyryloxymethyl ester;
5- { 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]propyl l benzo[ 1 ,3]dioxole-
2,2-dicarboxylic acid bis-isobutyryloxymethyl ester;
5- { 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]propyl ) benzo[ 1 ,3]dioxole-
2,2-dicarboxylic acid bis-heptanoyloxymethyl ester;
155- { 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]propyl } benzo[ 1 ,3]dioxole- 2,2-dicarboxylic acid bis-(4-methyl-pentanoyloxymethyl) ester;
5-(2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]propyl)benzo[1,3]dioxole-
2,2-dicarboxylic acid bis-hexanoyloxymethyl ester;
5 - ~ 2- [2- (3 -chloro-phenyl)-2-hydroxy-ethylamino]propyl ) benzo[ 1 ,3]dioxole-
202,2-dicarboxylic acid bis- (2,2-dimethyl-propionyloxymethyl) ester;
5- { 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]propyl ) benzo[ 1 ,3]dioxole-
2,2-dicarboxylic acid bis cyclohexanecarbonyloxymethyl ester;
5- { 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]propyl ) benzo[ 1 ,3]dioxole-
2,2-dicarboxylic acid bis-(l-propionyloxy-ethyl) ester;
255-{2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]propyl~benzo~1,3]dioxole-
2,2-dicarboxylic acid bis-[1-(2,2-dimethyl-propionyloxy-ethyl) ester;
5-{2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]propyl)benzo[1,3]dioxole-
2,2-dicarboxylic acid bis-(3,3-dimethyl-butyryloxymethyl) ester;
5-~2-[2-(3-chloro-phenyl)-2-hydroxy-ethylarnino]propyl~benzo[1,3]dioxole-
302,2-dicarboxylic acid bis-[1-(3,3-dimethyl-butyryloxy)-ethyl)}ester;
5- { 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]propyl ) benzo[ 1 ,3]dioxole-
2,2-dicarboxylic acid bis-(3-cyclopentyl-propionyloxymethyl) ester;
5-{2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]propyl)benzo[1,3]dioxole-
2,2-dicarboxylic ~cid bis-benzoyloxymethyl ester;
CA 022~4120 1998-ll-lO
WO 97143273 PCT/US97/08148
5-~2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino~propyl}benzo[1,3]dioxole-
2,2-dicarboxylic acid bis-(benzoyloxy-ethyl) ester;
5- ~ 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]propyl ) benzo[ 1 ,3]dioxole-
2,2-dicarboxylic acid bis-(2,2-dimethyl-butyryloxymethyl) ester;
5- ~ 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl ) -benzol 1 ,3]dioxole-2,2-dicarboxylic acid bis-amide;
5- { 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino] -propyl ) -benzo[ 1 ,3]dioxole-
2,2-dicarboxylic acid bis-2-propyl amide;
5-{2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl)-benzo[l ,3]dioxole-
2,2-dicarboxylic acid bis-n-butyl amide;
5- { 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino] -propyl } -benzo[ 1 ,3]dioxole-
2,2-dicarboxylic acid bis-phenylmethyl amide;
5-{2-~2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl~-benzo[1,3]dioxole-
2,2-dicarboxylic acid bis-(2-furanylmethyl) amide;
5-{2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl)-benzo[1,3]dioxole-
2,2-dicarboxylic acid bis-(glycine ethyl ester) amide;
5- ( 2-[2-(3-chloro-phenyl)-3-oxazolidinyl] -propyl } -benzo[ 1 ,3]dioxole-2,2-
dicarboxylic acid;
Also according to the present invention there is provided a method of treating
diabetes and/or hyperglycemia and/or obesity in humans or other .~ s which
comprises administering to a human or other m:~mm~l an antiobesity effective amount
or an antihyperglycemia effective amount of a compound of the present invention.
It is understood that the effective dosage of the active compounds of this
invention may vary depending upon the particular compound ntili7sd, the mode of
a(lmini~tration, the condition, and severity thereof, of the condition being treated, as
well as the various physical factors related to the individual being treated. For treating
diabetes mellitus and/or hyperglycemia generally satisfactory results may be obtained
when the compounds of this invention are ~rlmini~tered to the individual in need at a
daily dosage of from about 0.1 mg to about 1 mg per kilogram of body weight,
~ preferably ~rlmini~tered in divided doses two to six times per day, or in a sustained
release form. For most large m~mm~ls, the total daily dosage is from about 3.5 mg to
about 140 mg. This regimen may be adjusted to provide the optimal therapeutic
response.
CA 022~4120 1998-ll-lO
WO 97/43273 PCT/US97/08148
When treating obesity, in conjunction with diabetes and/or hyperglycemia, or
alone, generally satisfactory results can be obtained when the com~oullds of this
invention are ;~-imini~tered at a daily dosage of from about 0.1 mg to about 1 mg per kg
5 of body weight, preferably given in divided doses two to six times per day or in a
sustained release form. For most large m~mm~ls, the total daily dosage is from about
3.5 to about 140 mg, preferably from about 3.5 to about 5 mg. In the case of a 70 kg
human adult, the total daily dose will generally be from about 7 mg to about 70 mg and
may be adjusted to provide the optimal therapeutic result.
The tablets, pills, capsules, and the like may also contain a binder such as gumtrag~canth, acacia, corn starch or gelatin; excipients such as dicalcium phosphate; a
rli~integrating agent such as corn starch, potato starch, alginic acid; a lubricant such as
magnesium stearate; and a sweetening agent such as sucrose, lactose or saccharin.
15 When a dosage unit form is a capsule, it may contain, in addition to materials of the
above type, a liquid carrier such as a fatty oil. Various other materials may be present
as coatings or to modify the physical form of the dosage unit. For instance, tablets may
be coated with shellac, sugar or both. A syrup or elexir may contain, in addition to the
active ingredients, sucrose as a sweetening agent, methyl and propyl parabens as20 preservatives, a dye and a flavoring such as cherry or orange flavor.
These active compounds may also be administered parenterally. Solutions or
suspensions of these active compounds can be prepared in water suitably mixed with a
surfactant such as hydroxypropylcellulose. Dispersions can also be prepared in
25 glycerol, liquid polyethylene glycols and mixtures thereof in oils. Under ordinary
conditions of storage and use, there preparations contain a preservative to prevent the
growth of micoorganisms.
The pharn~ eutic~l forms suitable for injectable use include sterile aqueous
30 solutions or dispersions and sterile powders for the extemporaneous plep~ on of
sterile injectable solutions or dispersions. In all cases, the form must be sterile and
must be fluid to the extent that easy syringability exists. It must be stable under the
conditions of manufacture and storage and must be preserved against the corl~n~ g
action of microorganisms such as bacteria and fungi. The carrier can be a solvent or
35 dispersion medium containing, for example, water, ethanol, polyol (e.g. glycerol,
CA 022~4120 1998-ll-lO
WO 97/43273 PCT/US97/08148
- 13-
propylene glycol and liquid polyethylene glycol), suitable mixtures thereof, andvegetable oils.
The compounds of the present invention also possess utility for increasing lean
meat deposition and/or improving lean meat to fat ratio in edible ~nim~lc, i.e. ungulate
~nim:3l.c and poultry.
Animal feed compositions effective for increasing lean meat deposition and for
improving lean meat to fat ratio in poultry, swine, sheep, goats, domestic pets and
cattle are generally prepared by mixing the compounds of the present invention with a
sufficient amount of animal feed to provide from about 1 to 1000 ppm of the compound
in the feed. Animal feed supplements can be prepared by admixing about 75% to 95%
by weight of a compound of the present invention with about 5% to about 25% by
weight of a suitable carrier or diluent. Carriers suitable for use to make up the feed
supplement compositions include the following: alfalfa meal, soybean meal, cottonceed
oil meal, linseed oil meal, sodium chloride, cornmeal, cane molasses, urea, bone meal,
corncob meal and the like. The carrier promotes a uniform distribution of the active
ingredients in the finished feed into which the supplement is blended. It thus performs
an illJyOl ~U1t function by ensuring proper distribution of the active ingredient
throughout the feed. The supplement is used as a top dressing for the feed, it likewise
helps to ensure uniformity of distribution of the active material across the top of the
dressed feed.
The preferred m~ ted swine, cattle, sheep and goat feed generally contain
from 0.01 to 400 grams of active ingredient per ton of feed, the c~lilllulll amount for
these animals usually being about 50 to 300 grams per ton of feed. The pn~e.lcd
poultry and domestic pet feed usually contain about 0.01 to 400 grams and preferably
10 to 400 grams of active ingredient per ton of feed.
- 30 For parenteral administration the compounds of the present invention may be
prepared in the form of a paste or a pellet and administered as an implant, usually under
~ the skin of the head or ear of the animal in which increase in lean meat deposition and
improvement in lean mean to fat ratio is sought. In general, parenteral ~imini~ration
involves injection of a sufficient amount of the compounds of the present invention to
provide the animal with 0.001 to 100 mg/kg/day of body weight of the active
CA 022~4120 1998-ll-lO
WO 97/43273 PCT/US97/08148
- 14-
ingredient. The preferred dosage for swine, cattle, sheep and goats is in the range of
from 0.001 to 50 mg~g/day of body weight of active ingredient; whereas, the preferred
dose level for poultry and domestic pets is usually in the range of from 0.001 to 35
mgJlcg/day of body weight.
Paste formulations can be prepared by dispersing the active compounds in a
ph~rtn~reutic~lly acceptable oil such as peanut oil, sesame oil, corn oil or the like.
Pellets containing an effective amount of the compounds of the present invention can be
prepared by admixing the compounds of the present invention with a diluent such as
10 carbowax, carnuba wax, and the like, and a lubricant, such as m~gnecillm or calcium
stearate, can be added to improve the pelleting process. It is, of course, recognized that
more than one pellet may be administered to an animal to achieve the desired dose level
which will provide the increase in lean meat deposition and improvement in lean meat to
fat ratio desired. Moreover, it has been found that implants may also be made
15 periodically during the animal treatment period in order to ."~inl~i" the proper drug
level in the animal's body. The method of the present invention has several advantages;
for the pet owner or veterinarian who wishes to increase leanness and trim unwanted fat
from pet ~nim~, the present invention provides the means by which this can be
accomplished. For the poultry and swine raisers, using the method of the present20 invention yields leaner animals which command higher prices from the meat industry.
Further, according to the present invention there are provided pharm~rell~ic~l
compositions of matter comprising an effective amount of the compounds of the present
invention in combination with a pharmaceutically acceptable carrier; as well as a method
25 for increasing the content of lean meat in edible ~nim~l~, which comprises
administering to edible m~mm~I~ an effective amount of the compound.
Also according to the present invention there are provided processes for
producing the compounds of the present invention.
PROCE~ OF THE INVI~NTION
The compounds of the present invention may be prepared according to one of
the general processes outlined below.
CA 02254120 1998-11-10
WO 97/43273 PCT/US97/08148
- 15-
As outlined in Scheme I, a disodium carboxylate 1 is converted to a disilver
carboxylate and treated with an iodo derivative 2 to yield the diester compounds 3
wherein R1, R4, Rs, R6, R12, and R13 are as defined above.
5 Scheme I
OH H
~ N ~ OxCO2Na 1 ) AgNO3
R~ ~ R6 1 ~OJ~R
~ >~ R~2 13
Alternatively, a dicarboxylic acid 4 (Scheme II) is treated with an alcohol RgOHand an acid catalyst to yield the diester compounds 5 wherein Rl, R4, Rs, R6, and Rg
10 are as defined above.
Scheme II
OH H
R~--~ R~/~oXCO H RgOH, H+
R6
R~ R?~;;~O>~OR9
R6 o
CA 02254l20 l998-ll-lO
WO 97/43273 PCT/US97/08148
- 16-
As outlined in Scheme III, the diester compounds 5 can also be produced by
5 protecting the hydroxy and amino groups of compound 6 with R2 and R3 groups,
respectively, basic hydrolysis of the ethyl esters, alkylation of the carboxyl groups of
compound 7 with an alkylating agent Z-Rg, and removing the protecting groups R2
and R3, wherein Rl, R2, R3, R4, R5, R6, and Rg are as defined above and Z is Cl,
Br, I, methanesulfonate, trifluoromethanesulfonate, or para-toluenesulfonate.
Scheme III
OH H O>~OEt 1) add R2 and R3
4 5 o OEt 2) OH-
6 O
OR2 ,R3
N>~.~O~,~LOH
R 1--~1 R4 R5 ~/~ O~ OH
7 6 o
1) base,Z-Rg ~N~[O~ LoR9
2) remove R2and R3 RlR4 R6 o~OR9
lS 5 4 o
As outlined in Scheme IV, a dicarboxylate 1 is treated with an aldehyde R'CHO
to yield the oxazaline compounds 8, wherein Rl, R4, Rs, R6, and R' are as defined
above.
CA 02254120 1998-11-10
WO 97/43273 PCT/US97/08148
Scheme IV
OH H o
R1 ~ R~Oo><~oNa R'CHO
R6 o
R'
OX~ OH
R6 o
As outlined in Scheme V the diester compounds 5 can be hydrolyzed under
basic conditions to a monoester 9a and/or 9b, wherein R1, R4, Rs, R6, and Rg are as
defined above. One or both of the diastereomers 9a and 9b may be produced in the hydrolysis reaction.
Scheme V
H O
R--~ RX;~ o~lL OR9 OH-
4 5 /~o ~rORg
R6 o
R,~ and/or R,~ OR
9a 9b
CA 02254120 1998-11-10
Wo 97/43273 PC~/USg7/08148
- 18-
As illustrate in Scheme VI, a diester compound 5 is reacted with an amine
HNRllR12toyieldthediamide compounds 10, wherein Rl, R4, R5, R6, Rg, Rll,
and R12 are as defined above.
Scheme VI
H O
,~1 N ~ O~ ~L ORg
R~ ~;~ R4 Rs ~ ~O~OR9 HNRl1R12
6 o
H O
R ~--R~CO~NRl1R12
R6 o
The compounds of this invention were tested for antihyperglycemic and
antiobesity activity according to the following procedures.
Hum~n Beta Adrener~ic ReceDtor Selectivity
The activity of the test compounds on human ,B-adrenergic r~ce~to.~ was
determined with Chinese ha~ er ovary (CHO) cells transfected with human ,B2, or ,B1
adrenergic receptors. Agonist activity is in~lic~te~ by increased cAMP levels in the
CHO cells. Selectivity of the test compounds for the ,B3 receptor was assessed by
comparison with results in ~2 and ~1 adrenergic receptor transfected cells.
Procedure:
1). Chinese hamster ovary (CHO) cells transfected with human ,~2, or ,B1 ad,,_.lcl~,ic
receptors were used in the assay.
25 2). Cells were grown to confluent conditions in 24 well plates.
CA 022~4120 1998-11-10
WO 97/43273 PCT/US97/08148
- 19-
3). Drugs were dissolved in DMSO at a concentration of 10 ~M.
4). Cells were incubated with drug at 10 nM conrentration for 10 rnin at 37~ C.
- Isoproterenol (Standard 1) was used as the standard compound and assayed at 10 ~lM
which gives a maximal cAMP elevation in all 3 cell types.
5 5). Cell cAMP concentrations were assayed using a scin~ill~tion proximity assay kit
from Amersham Corp., Chicago, IL.
6). Activities for the test compounds are expressed as a percentage of the isoproterenol
response.
Production of the CHO cells transfected with human ~2. or ,B1 adlen~ly,ic
receptors, and compound test procedures utili7ing the CHO cells, are described by
Emorine et al. in their article "Molecular Characterizanon of the ~llrnan Beta3-AdrenergicReceptor", Science 1989, 245(8), 1118-1121 and by Muzzin et al. in thearticle "AnAdiposeTissue-SpecificBeta3-AdrenergicReceptor", J. Biol. Chem.l991,
266, 24053-24058.
Fffects on Free Fatty Acid l,evels in Rats
Rats respond to a single oral dose of ,B3 agonists by increasing plasma free fatty
acids (FFA) in response to ,B3 receptor stim~ tion on the plasma membrane of the fat
cell. 5-~2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid diisopropyl ester (Standard 2) was used as a standard compound.
All test compounds were dosed at 0.1 mg/kg and compared to the response by Standard
2.
Procedure:
1). Drugs were dissolved in DMSO at 10 mg/ml.
2). Twenty ~1 of the DMSO-drug solution was added to 10 mL methyl cellulose:tween-
80 (0.5%:0.1 %) for a final concentration of 20 ~lg/mL.
3). Methyl cellulose:tween-80 drug suspension was given via gavage (1 mL/200g body
weight; or 0.1 mg/kg) to rats and blood was collected 50 min later.
4). Plasma was analyzed for free fatty acids using a kit supplied by Biochçntic~l
Diagnostics Inc. (Brentwood, N.Y.).
CA 022~4120 1998-11-10
WO 97143273 rCT/US97/08148
- 20-
5). Drug response was calculated from the formula below.
% FFA Response= FFA (compound) - FFA vehicle x 100
FFA (Standard 2) - FFA vehicle
s
Effects on Hyperglycemia in Mice
On the morning of Day 1 (baseline), 35 mice (male, db/db (C57BLtKsJ),
Jackson Laboratories, 2 to 7 months of age and 35 to 60 g) were fasted for 4 h,
10 weighed, and a baseline blood sample was collected from the tail-tip of each mouse
without anesthesia, placed directly into a fluoride-cont~ining tube, mixed, and
mz-int~ined on ice. Food was then returned to the mice. The plasma was seperated and
the levels of glucose in the plasma were d~e..-~ined by an Abbot VP Analyzer.
Because of the variable plasma glucose levels of the db/db mice, the 5 mice having the
15 most extreme (i.e., highest or lowest) plasma glucose levels were excluded and the
re.~ g 30 mice were randomly assigned into 7 groups of equivalent mean plasma
glucose level (vehicle control, ciglitazone (Standard 3), and 5 test compound groups).
On the afternoon of Days 1, 2, and 3 the vehicle (0,2 mL of 2% Tween 80/saline w/v)
or test compounds were ~Aminist~ed (p.o.) to the ad libitum fed mice. On the morning
20 of Day 4, the food was removed from the cages for 3 h, a blood sample was collected,
and the mice were then given the fourth ~lmini~ration of test compound or vehicle.
Additional blood samples were collected at 2 and 4 h after test coml)ound
~dministration. Plasma glucose levels were determined. To assess test compound
activity, the percent change of an animal s plasma glucose level on Day 4 (mean of 2
25 and 4 h values) from its level before test compound administration (Day 1 b~celine
sample) was determined as follows:
Mean of 2 and 4 h samples (Day 4) x 100
Baseline sample (Day 1)
A 50-60% reduction of plasma glucose levels in the hyperglycemic dbtdb mice
represents a normalization of glucose levels.
CA 02254l20 l998-ll-lO
W097/43273 PCTrUS97/08148
- 21 -
Table I
Compound a Rat Freedb/db Mice
(Example) ~la ~2 Fatty Plasma
Acidb GlucoseC
1 4% 65%
0.022 ~M0-071 mg/kg 0.128 mg/kg
3 ocj 2070
- O77 2C,~
~C 7 ll5~o
6 ~C/o 14%
7 2~,o 22%
8 1 ~,'~ 50~,7
9 16~/~ 22C,~
0% 59C,~
1 n: 113
2 1 ~77 4~C~o
5~~'~ 6~07~
' 5 18% 46~,o
:.6 n- 0.128 mg/kg
7 n- nt
60,7~ 25%
2~ 3n%
, ~ oO7~ 4 %
~ 1 2~ 0.086 mg/kg
22 0.015 ~M 45%
23 nt 57%
24 nt nt
6% 30C~o
26 7C~ 42%
27 5% 7~C,j
28 2~,'~ 2 ~ CYo
29 2C ~ 9 ~'~
0~,7J 75O7~
31 5O7~ 0.090 mg/kg
32 2% 33%
33 5.90 ~M0.100 ~M0-080 mglkg 0.201 m~g
34 207~ 74c.j
~% 69~,'~
36 'C,~ 37C,j
37 '~% 33%
38 0.274 ~M0-086 mg/kg
39 1% 71%
8.4 ~M0.420 ~M0.071 m~g
41 22% 36%
CA 02254120 1998-ll-lO
WO 97/43273 PCT/US97/08148
- 22-
Compound Rat Free db/db Mice
(Example) ~1a ~2a Fatty Plasma
Al~idb GlucoseC
42 0~,S ~9C,~
43 007J ~C j
44 OCaJ 4C~aJ
1% 6C~
46 ln% 23~,7J
47 C,~ 37~,~
48 ' ~ 29C/o
49 ~ 50%
CC,~ 19%
51 0% 2%
52 2% 23%
53 2% 20%
54 7C,j 24%
0~S 31%
56 8~,7J 31%
57 C C~ 30~,S
5 3 c,O 35c,o
5~ o _7C~o
3C j r 2~,S
~n 3% ~OC,O
5O~J 5%
0.079 ~M 42%
3.00 ~M 0.048 lM 39%
71 14Cr~ 0.076 mg~kg
72 13O~7J 43%
73 0% 3%
74 0% 6%
5.4 ~M 0.058 ~M 0.02~. mg/kg 0.110 mglkg
76 0.980 ~M 0.074 ~M 42%
77 5% 25%
78 5.0 ~M 0.325 ~M 0-027 mg~kg 0-028 mg/kg
79 0% 61%
2.4 ~M 0.055 ~M 81%
81 0.380 ~M 0.059 ~M 61%
2 n: 84c~j
3 n 3~,~J
J4 n: ~iS~,~
n: ~0%
,6 n ~0%
CA 02254120 1998-11-10
Wo 97l43273 PcT/us97losl48
Compound Rat Free db/db Mice
~ (Example) ~la ~2a Fatty Plasma
Ac db GlucoseC
87 n- r~ %
89 n- ' J%
7~ 1
~1 7~ ~707J
32 nt ~0%
~3 n: 7 5~
~4 n- 78%
~5 OC~ 73C~o
~6 1% 71C~o
~7 4% 3~C~o
~8 7% 8~-%
~9 16% 5 %
00 2% 10%
01 3% 38%
- 04 2% 83t,'~o
a Human ,B receptors expressed in Chinese hamster ovary cells, compounds tested at 10
nM, results expressed as % of isoproterenol activity (increase in cAMP) at 10 IlM.
S EC50 (~lM) values determined for selected compounds.
b Elevation of plasma free fatty acids in rats, compounds tested at 0.1 mg/kg, results
expressed as % of 5-~2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl)-
benzo~1,3]-dioxole-2,2-dicarboxylic acid diisopropyl ester response (78% increase) at
0.1 mg/kg. EDso (mg/kg) values determined for selected compounds.
10 C ED50 (mg/kg/day) values for lowering of plasma glucose.
The following non-limiting specific examples further illustrate the present
invention.
15EXAMPLE 1
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-(2-methoxy-ethyl) ester
hydrochloride salt
20To a stirred mixture of 5- l 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-
propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid (1.0 g, 2.37 mmol), and 2-methoxy-
CA 022~4120 1998-ll-lO
WO 97/43273 PCT/US97/08148
- 24-
1-ethanol (10 mL) was added excess HCl(g). The rnixture became homogeneous and
was stirred at 23 ~C. After 12 h, the solution was concentrated, and cnro~,natographed
on silica gel, eluting with CHC13/MeOH (1/0, then 40/1, 20/1 and 10/1) to give
fractions con~ining 5- ~ 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl } -benzo[1,3]dioxole-2,2-dicarboxylic acid bis-(2-methoxy-ethyl) ester (RpO.37 (10/1
CHC13/MeOH)) as a viscous oil. This oil was dissolved in Et20 (10 rnL) and HCl(g)
was bubbled through the solution for 1 min. The resulting solution was evaporated to
give 1.06 g (78 %) of 5-(2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl)-
benzo[1,3]dioxole-2,2-dicarboxylic acid bis-(2-methoxy-ethyl) ester hydrochloride salt
as a white solid; IH NMR (300 MHz, CDC13): ~ 1.33 (brm, 3H), 2.75-2.88 (brm,
lH), 3.10-3.30 (brm, 2H), 3.35 (s, 6H), 3.40-3.53 (brm 2H), 3.63-3.68 (complexm,4H), 4.40-4.48 (brm, 4H), 5.40-5.50 (brd, IH), 5.50-5.75 (brs, lH), 6.70-6.91
(complex m, 4H), 7.18-7.38 (m, 2H), 7.43 (s, lH), 8.60-8.85 (brs, lH), 9.90-10.15
(brs, lH); MS (ES) m/z (relative intensity): 538 (M+-HCI, 100).
EXAMPLE 2
~-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid diphenethyl ester hydrochloride
salt
The title compound was prepared from 5-~2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl }-benzol l ,3]dioxole-2,2-dicarboxylic acid and 2-phenylethanol
accord-ing to the procedure of Example 1 as a colorless oil; lH NMR (300 MHz,
CDC13): d 1.30-1.45 (brs, 3H), 2.80-2.90 (brm, lH), 2.89 (t, J = 6.9 Hz, 4H), 3.10-
3.30 (brm, 2H), 3.39-3.69 (brm, H), 4.40 (t, J = 6.8 Hz, 4H), 5.45-5.61 (brs, 2 H),
6.70-6.91 (brm, 3 H), 7.10-7.35 (complex m, 13H), 7.40-7.51 (brs, lH), 8.60-8.85(brs, lH), 9.91-10.20 (brs, lH); MS (ES) m/z (relative intensity): 630 (M+-HCI, 40),
144 (100).
CA 02254120 1998-ll-lO
WO 97/43273 PCT/US97/08148
- 25-
- EXAMPLE 3
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzoll,3]dioxole-2,2-dicarboxylic acid bis-(2-butoxy-ethyl) ester
hydrochloride salt
The title compound was prepared from 5-(2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino] -propyl ~ -benzo[1,3]dioxole-2,2-dicarboxylic acid and 2-butoxyethanol
accord-ing to the procedure of Example 1 as a colorless oil; lH NMR (300 MHz,
CDCl3): ~ 0.78-0.93 (m, 6H), 1.15-1.78 (m, 12H), 2.70-3.62 (brm, 5H), 3.31 -3.4710(m, 4H), 3.57-3.73 (m, 4H), 4.29-4.53 (m, 4H), 5.47-5.57 (brs, lH), 6.70-6.95 (m,
3H), 7.20-7.49 (m, 4H); MS (ES) m/z (relative intensity): 622 (M+-HCl, 20), 214
(10), 158 (1()).
EXAMPLE 4
155-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dic~rboxylic acid bis-(2-phenoxy-ethyl) ester
hydrochloride salt
The title compound was prepared from 5-~2-[2-(3-chloro-phenyl)-2-hydroxy-
20ethylamino]-propyl )-benzo[1,3]dioxole-2,2-dicarboxylic acid and 2-phenoxyethanol
according to the procedure of Example 1 as an off-white gummy solid; lH NMR (300MHz, CDC13): o 1.23-1.40 (brs, 3H), 2.70-2.90 (brm, lH), 3.05-3.30 (brrn, 2H),
3.31-3.52 (brm, 2H), 4.12 (t, J = 4.4 Hz, 4 H), 4.57 (t, J = 4.8 Hz, 4 H), 5.38-5.60
(brs, 2H), 6.70-7.0 (complexm, IOH), 7.15-7.47 (complex m, 7 H), 8.60-8.80 (brs,25lH), 9.90-10.20 (brs, 1 H).; MS (ES) m/z (relative intensity): 662 (M~-HCl, (100),
594 (20), 498 (35).
EXAMPLE 5
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
- 30benzo[1,3]dioxole-2,2-dicarboxylic acid bis-(2-ethoxy-ethyl) ester
hydrochloride salt
The title compound was prepared from 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl l -benzo~ 1,3]dioxole-2,2-dicarboxylic acid and 2-ethoxyethanol
35according to the procedure of Example I as an oily off-white gummy solid; lH NMR
CA 022~4120 1998-ll-lO
WO 97/43273 PCT/US97/08148
- 26-
(300 MHz, CDC13): ~ 1.10-1.20 (t overlaps with d, J = 6.9 Hz, 9H), 1.31-1.41 (brm,
2 H), 2.75-2.90 (brm, lH), 3.10-3.30 (brm, 2H), 3.50 (brq, J = 6.9 Hz, 4H), 3.62-
3.71 (brm, 4H), 4.37-4.47 (brm, 4H), 5.30-S.70 (brm, 2H), 6.70-6.90 (brm, 4H),
7.19-7.50 (brm, 3H), 8.50-8.85 (brs, lH), 9.90-10.30 (brs, lH); MS (ES) mlz
(relative intensity): 566 (M~-HCI, (60).
EXAMPLE 6 and EXAMPLE 7
5-{2-~2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[lj3]dioxole-2,2-dicarboxylic acid bis-(2-tert-butoxy-ethyl) ester
and
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid (2-tert-butoxy-ethyl) ester
To a stirred mixture of 5- (2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-
propyl~-benzo[1,3]dioxole-2,2-dicarboxy}ic acid (1.0 g, 2.37 mmol), and 2-t-butoxy-
l-ethanol (10 mL) was added p-toluenesulfonic acid (451 mg, 2.37 mmol). The
mixture became homogeneous and was stirred at 23 ~C. After 12 h, an additional
portion of p-toluenesulfonic acid (351 mg, 1.84 mmol) was added. After a total of 90
h, the solution was concentrated, and chromatographed on silica gel, eluting with
CHC13/MeOH (1/0, then 40/1, 20/1 and 10/1) to give 100 mg (7 %) of 5-{2-[2-(3-
chloro-phenyl)-2-hydroxy-ethylamino]-propyl ~ -benzo[1,3]dioxole-2,2-dicarboxylic
acid bis-(2-tert-butoxy-ethyl) ester (Rf~0.25 (10/1 CHC13/MeOH)) as a yellow oil and
320 mg (26 %) of 5-(2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl~-
benzo[1,3]dioxole-2,2-dicarboxylic acid (2-tert-butoxy-ethyl) ester (Rf=0.09 (10/1
CHCl3/MeOH)) as an off-white solid.
5- ~ 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl ) -benzo[1,3]dioxole-
2,2-dicarboxylic acid bis-(2-tert-butoxy-ethyl) ester; IH NMR (300 MHz, CDC13): ~
1.15 (d, 3H), 1.68 (s, 18 H), 1.85-2.40 (brm, 2H), 2.50-2.75 (m, 2H), 2.81-2.96
(m, lH), 3,41-3.51 (m, 4H), 3.56-3.64 (m, 2H), 3.65-3.76 (m, 4H), 4.30-4.41 (m,
2H), 6.61-6.87 (m, 4H), 7.15-7.33 (m, 2H), 7.44 (s, lH); MS (ES) m/z (relative
intensity): 622 (M+, 90),522 (M+-t-BuOCH2CH3, 60), 3S2 (40), 266 (100).
CA 02254120 1998-11-10
WO 97/43273 PCTIUS97/08148
- 27 -
- 5- (2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl ) -benzo[1,3]dioxole-
2,2-dicarboxylic acid (2-tert-butoxy-ethyl) ester; lH NMR (300 MHz, CDCl3): ~ 1.10
- (brm, 12H), 2.25-2.70 (brm, 2H), 3.00-3.30 (brm, SH), 3.51-3.61 (m, 2H), 4.25-
4.47 (m, 2H), 5.20-5.35 (brm, lH), 6.50-6.70 (brm, 4H), 7.15-7.44 (m, 3H), 8.70-9.70 (brs, lH). MS (ES) m/z (relative intensity): 522 (M+, 100).
EXAMPLE 8
S-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl~
ben~o[1,3]dioxole-2,2-dicarboxylic acid bis-(2-isobutoxy-ethyl) ester
The title compound was prepared from 5-~2-~2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl }-benzo[1,3]dioxole-2,2-dicarboxylic acid and 2-isobutoxyethanol
according to the procedure of Example 1 as a colorless oil; lH NME~ (300 MHz,
CDCl3): ~ 0.70-0.95 (m, 12H), 1.10-2.0 (complexm, 1 lH), 3.09-3.29 (m, 4H), 3.51-
3.58 (m, lH), 3.60-3.72 (m, 4H), 4.31-4.48 (m, 2H), 6.70-7.00 (m, 4H), 7.15-7.50(m, 4H); MS (ES) m/z (relative intensity): 622 (M+-HCl, 100).
EXAMPLE 9
5 {2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benYol1,3]dioxole-2,2-dic~rboxylic acid bis-(benzyl) ester
hydrochloride salt
The title compound was prepared from 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylarnino]-propyl ) -benzo[1,3]dioxole-2,2-dicarboxylic acid and benzyl alcohol
according to the procedure of Example 1 as a gummy white solid: lH NMR (300 MHz,CDCl3): ~ 1.26-1.32 (m, 3H), 2.76 (brt, lH), 3.01-3.21 (brrn, 2H), 3.36-3.49 (brrn,
2H), 5.24 (s, 4H), 5.41 (d, J=9.5 Hz, lH), 6.70-6.88 (m, 3H), 7.12-7.44 (complexm, 14H), 8.70-8.83 (brs, lH), 9.95-10.10 (brs, lH); MS (ES) m/z (relative intensity):
602 (M+, 100).
CA 02254120 1998-11-10
WO 97143273 PCT/US97/08148
- 28 -
EXAMPLE 10
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo~l,3]dioxole-2,2-dicarboxylic acid bis-(cyclohexyl) ester
hydrochloride salt
s
The title compound was prepared from 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl)-benzo~1,3]dioxole-2,2-dicarboxylic acid and cyclohex~nc-l
according to the procedure of Example 1 as a yellow gum: lH NMR (300 MHz,
CDC13): ~ 1.10-1.40 (complex m, 7H), 1.45-1.60 (brrn, 4H), 1.63-1.81 (brm, 8H),
101.82-1.95 (brm, 4H), 2.72-2.90 (brm, lH), 3.10-3.30 (brm, 2H), 3.37-3.55 (m,
2H), 4.90-5.03 (m, 2H), 5.37-5.52 (brs, lH), 6.71-6.90 (complex m, 4H), 7.20-7.32
(m, 2H), 7.44 (s, lH), 8.55-8.80 (brs, lH), 9.90-10.20 (brs, lH); MS (ES) m/z
(relative intensity): 585 (M+- HCl), 532 (10).
15EXAMPLE 11
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-(cyclopentyl) ester
The title compound was prepared from 5-(2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid and cyclopentanol as a
20 yellow gum according to the procedure of Example 1, leaving out the final HCl(g~/Et20
hydrochloride salt forming step: IH NMR (300 MHz, CDC13): ~ 1.07 (d, J=6.2 Hz,
3H), 1.50-2.00 (complex m, 16H), 2.52-2.70 (complex m, 3H), 2.85-2.95 (m, 2 H),
4.55-4.65 (m, lH), 5.29-5.38 (m, 2H), 6.65-6.72 (m, lH), 6.76 (s, lH), 6.84 (d,
J=8.0 Hz, lH), 7.18-7.30 (m, 3H), 7.37 (s, lH); MS (ES) mtz (relative intensity):
25558 (M+, 100), 518 (10), 490 (20).
EXAMPLE 12
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl~-
benzo[l,3]dioxole-2,2-dicarboxylic acid dioctyl ester
The title compound was prepared from 5-~2-~2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl ~ -benzo[1,3]dioxole-2,2-dicarboxylic acid and 1 -octanol as a
gummy white solid according to the procedure of Example 1, leaving out the finalHCl(g)/Et20 hydrochloride salt forming step: IH NMR (300 MHz, CDC13): ~ 0.78-
350.90 (m, 6H), 0.95-1.40 (brm, 23H), 1.52-1.87 (m, 6H), 2.55-2.75 (brs, lH), 2.89-
CA 02254l20 l998-ll-lO
WO 97/43273 PCT/US97/08148
- 29-
~ 3.30 (brm, 4H), 4.12-4.33 (m, 4H), 5.15-5.35 (brs, lH), 6.60-6.81 (m, 3H), 7.12-
7.45 (m, 4H); MS (EI) m/z (relative intensity): 645 (M+, 5), 504 (100), 348 (100), 319
~ (100), 180 (100).
EXAMPLE 13
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid dipentyl ester
The title compound was prepared from 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl } -benzo[1,3]dioxole-2,2-dicarboxylic acid and 1 -pentanol as a
brown gum according to the procedure of Example 1, leaving out the final HCl(g)/Et20
hydrochloride salt forrning step: IH NMR (300 MHz, CDC13): ~ 0.90 (t, J=6.9 Hz,
3H), 1.21-1.41 (brm, lOH), 1.67 (t, J=7.0 Hz, 4H), 1.80-2.10 (brs, lH), 2.80 (t,J=6.5 Hz, lH), 3.05-3.19 (brm, 2H), 3.45 (d, J=10.4 Hz, 2H), 4.28 (t, J = 6.7 Hz,
4H), 5.46 (d, J=9.6 Hz, lH), 5.50-5.90 (brs, lH), 6.71-6.90 (m, 3H), 7.20-7.35 (m,
3H), 7.43 (s, lH), 8.76 (brs, lH), 1.05 (brs, lH); MS (ES) m/z (relative intensity):
562 (M+, 100).
EXAMPLE 14
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl~-
benzo[l,3]dioxole-2,2-dicarboxylic acid dihexyl ester
The title compound was prepared from 5-(2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl~-benzo[1,3]dioxole-2,2-dicarboxylic acid and 1-hexanol as a white
solid according to the procedure of Example 1, leaving out the final HCl(g)/Et20hydrochloride salt forming step: lH NMR (300 MHz, CDC13): ~ 0.75-0.90 (m, 6H),
1.00-1.41 (brm, l5H), 1.47-1.85 (m, 6H), 2.60-3.50 (brm, SH), 4.15-4.30 (m, 4H),5.18-5.35 (brs, lH), 6.67-6.82 (m, 3H), 7.15-7.45 (m, 4H); MS (ES) m/z (relativeintensity):
- 30
CA 02254120 1998-ll-lO
WO 97/43273 PCT/US97/08148
- 30-
EXAMPLE 15
Carbonic acid 3~chloro-benzyl ester 2-(3-chloro-
benzyloxycarbonyloxy)-4-{2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino3-propyl}-phenyl ester
The title compound was prepared from 5-{2-~2-(3-chloro-phenyl)-2-hydroxy-
ethylarnino]-propyl}-benzo[1,3]dioxole-2,2-dicarboxylic acid and 3-chlorobenzyl
alcohol as a tan solid according to the procedure of Example 1, leaving out the final
HCl(g)/Et2O hydrochloride salt forming step: lH NMR (300 MHz, CDCl3): ~ 1.28 (d,10J=6.3 Hz, 3H), 1.80-2.40 (brs, lH), 2.79 (t, J=8.8 Hz, lH), 3.05-3.17 (brm, 2H),
3.37-3.49 (m, 2H), 5.23 (s, 4H), 5.43 (m, lH), 6.72-6.88 (m, 3H), 7.10-7.44
(complexm, 12H), 8.76 (brs, lH), 10.02 (brs, lH); MS (ES) m/z (relative intensity):
670 (M+, 100)
15EXAMPLE 16
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylaminol-propyl}-
benzo[1,3]dioxole-2,2-dicarboxylic acid bis-(1-phenyl-ethyl) ester
The title compound was prepared from 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-
20ethylamino]-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid and 1-phenylethanol as a
gummy tan solid according to the procedure of F~mple 1, leaving out the final
HCl(g)/Et20 hydrochloride salt forming step: IH NMR (300 MHz, CDCl3): ~ 0.90-
1.22 (m, 6H), 1.30-1.61 (m, 6H), 2.39-2.70 (brm, lH), 2.83-3.19 (brm, 2H), 3.20-3.50 (brm, 2H), 5.15-5.35 (brs, lH), 5.90-6.02 (brm, lH), 6.45-6.82 (m, 3H), 7.11-
257.40 (m, 4H), 8.6~ (brs, IH), 9.45 (brs, lH); MS (ES) m/z (relative intensity): 630
(M+, 100).
EXAMPLE 17
5-{2.[2-(3-Chloro~phenyl)-2-hydroxy-ethylamino]-propyl}-
30benY.o[1,3]dioxole-2,2-dicarboxylic acid diheptyl ester
The title compound was prepared from 5-(2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylarnino]-propyl ) -benzo[1,3]dioxole-2,2-dicarboxylic acid and 1 -heptanol as a
brown gum according to the procedure of Example 1, leaving out the final HCl(g)/Et20
35hydrochloride salt forming step: IH NMR (300 MHz, CDCl3): o 0.80-0.92 (m, 6H),
CA 02254l20 l998-ll-lO
WO 97/43273 PCT/US97/0814B
- 31 -
~ 1.23-1.40 (brm, l9H), 1.60-1.75 (m, 4H), 2.80 (t, J=8.8 Hz, lH), 3.05-3.30 (brm,
2H), 3.38-3.50 (m, 2H), 4.27 (q, J=6.7 Hz, 4H), 5.43 (d, J=8.4 Hz, lH), 6.72-6.90
~ (m, 4H), 7.20-7.33 (m, 2H), 7.44 (s, lH), 8.70 (brs, lH), 10.10 (brs, lH); MS (ES)
m/z (relative intensity): 618 (M+, 100).
s
EXAMPLE 18
5-{2-~2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[1,3]dioxole-2,2~dicarboxylic acid dinonyl ester
The title compound was prepared from 5-~2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylaminol-propyl ~ -benzo[1,3]dioxole-2,2-dicarboxylic acid and 1 -nonanol as a
brown gum according to the procedure of Example 1, leaving out the final HCl~,)/Et20
hydrochloride salt forming step: IH NMR (300 MHz, CDC13): S 0.88 (t, J-6.8 Hz,
9H), 1.25-1.40 (m, 22H), 1.54-1.60 (m, 2H), 1.63-1.74 (m, 4H), 2.79 (brt, J = 8.8
15Hz, lH), 3.()8-3.30 (m, 2H), 3.40-3.55 (m, 2H), 4.28 (t, J=6.8 Hz, 4H), 5.46 (d,
J=8.7 Hz, lH), 6.70-6.90 (m, 3H), 7.15-7.35 (m, 3H), 7.43 (s, lH), 8.72 (brs, lH),
9.98 (brs, lH); MS (ES) m/z (relative intensity): 674 (M+, 100), 548 (M+-CgH20, 5).
EXAMPLE 19
205-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-decyl ester
The title compound was prepared from 5-(2-~2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl}-benzo[1,3]dioxole-2,2-dicarboxylic acid and and 1-decanol as a
25brown gum according to the procedure of Example 1, leaving out the final HCl~,)/Et20
hydrochloride salt forming step: lH NMR (300 MHz, CDCl3): ~ 0.88 (t, J=6.8 Hz,
9H), 1.15-1.40 (m, 24H), 1.52-1.60 (m, 4H), 1.62-1.74 (m, 4H), 2.80 (brt, J = 8.8
Hz, lH), 3.08-3.28 (m, 2H), 3.35-3.52 (m, 2H), 4.28 (t, 1=6.7 Hz, 4H), 5.45 (d,
J=8.8 Hz, lH), 6.70-6.90 (m, 3H), 7.18-7.35 (m, 3H), 7.43 (s, lH), 8.71 (brs, lH),
3010.02 (brs, lH); MS (ES) m/z (relative intensity): 702 (M+, 100).
CA 02254120 1998-11-10
WO 97143273 PCTIUS97/08148
- 32-
EXAMPLE 20
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid didodecyl ester
S The title compound was ~ par~d from 5-~2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid and 1-clodec~nc-l as a
brown gum according to the procedure of Example 1, leaving out the final HCl(g)/Et20
hydrochloride salt forming step: lH NMR (300 MHz, CDCl3): o 0.88 (t, J=6.8 Hz,
9H), 1.20-1.40 (m, 30H), 1.51-1.60 (m, 4H), 1.62-1.73 (m, 4H), 2.75-2.85 (m,
10lH), 3.05-3.25 (m, 2H), 3.35-3.52 (m, 2H), 4.28 (t, J=6.8 Hz, 4H), 5.45 (d, J=8.8
Hz, lH), 6.60-6.90 (m, 3H), 7.17-7.40 (m, 3H), 7.43 (s, lH), 8.78 (brs, lH), 10.05
(brs, lH); MS (ES) m/z (relative intensity): 594 (M+, 100).
EXAMPLE 21
155-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-(2-isopropoxy-ethyl) ester
The title compound was prepared from 5-(2-~2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl}-benzo~1,3]dioxole-2,2-dicarboxylic acid and 2-isopropoxyethanol
20as a brown solid according to the procedure of Fy;~mrle 1, leaving out the final
HCl(g,~/Et2O hydrochloride salt forming step: lH NMR (300 MHz, Cl)Cl3): ~ 1.11 (d,
J=6.1 Hz, 12H), 1.30 (d, J=6.2 Hz, 3H), 2.79 (t, J=8.7 Hz, lH), 3.06-3.28 (m,
2H), 3.40-3.60 (m, 2H), 3.62-3.70 (complexm, 4H), 3.90-4.12 (brm, 2H), 4.35
4.45 (complexm, 4H), 5.43 (d, J = 8.7 Hz, lH), 6.61-6.90 (m, 3H), 7.11-7.32 (m,
253H), 7.44 (m, lH), 8.70 (brs, lH), 9.92 (brs, lH); MS (ES) m/z (relative intensity):
758 (M+, 30), 546 (100).
EXAMPLE 22
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
30benzo[l,3]dioxole-2,2-dicarboxylic acid isopropyl ester
To a stirred solution of 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-
propyl~-benzo[1,3]dioxole-2,2-dicarboxylic acid Ws-isopropyl ester hydrochloride salt
(3.0 g, 5.53 mmol) and 20 mL of i-PrOH was added was added lN NaOH (11.1 mL,
11.1 mmol). After stirring at 23 ~C for 3 days, the mixture was con-~e~ ed to
CA 02254120 1998-11-10
WO 97/43273 PCTIUS97/08148
- dryness, dissolved in 10 % MeOHJCH2C12, and filtered through a small pad of silica
gel. The filtrate was concentrated to a colorless gum, and ¢iturated with Et20 to give
- 1.0 g (39 ~o) of 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl)-
benzo~1,3]dioxole-2,2-dicarboxylic acid isopropyl ester as a white solid; lH NMR(300 MHz, CDC13): o 0.93 (d, J=6.1 Hz, 3H), 1.17 (d, J=6.3 Hz, 6H), 2.30-2.53
(m, 2H), 2.67-2.80 (m, 2H), 2.85-3.00 (m, lH), 4.65-4.75 (brs, lH), 4.91 (hept,
J=6.3 Hz, lH), 6.52-6.80 (complexm, 3H), 7.17-7.45 (complexm, 4H); MS (ES) m/z
(relative intensity): 464 (M+, 100).
EXAMPLE 23
5-{2-[2-(3-Chloro-phenyl)~2-hydroxy-ethylamino]-propyl}-
ben~.o[l,3]dioxole-2t2-dicarboxylic acid ethyl ester
The title compound was prepared from 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl}-benzoL1,3]dioxole-2,2-dicarboxylic acid bis-ethyl ester
hydrochloride according to the procedure of Example 22 as a brown solid: lH NMR
(300 MHz, CDC13): o 1.20 (d, J=6.7 Hz, 3H)1 1.33 (t, J=7.1 Hz, 3H), 2.70 (m, lH),
2.90-3.22 (m, 4H), 3.23-3.48 (m, lH), 4.34 (q, J=7.1 Hz, 2H), 5.30 (m, lH), 6.55-
6.90 (m, 3H), 7.10-7.45 (m, 4H); MS (ES) m/z (relative intensity): 450 (M++1, 50 ).
EXAMPLE 24
5-{2-~2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-methoxycarbonylmethyl
ester
Step 1
5-(2-{tert-Butoxycarbonyl-[2-(3-chloro-phenyl)-2-hydroxy-ethyl]-
amino}-propyl)-ben~.o[1,3]dioxole-2,2-dicarboxylic acid bis-ethyl ester
~ 30 To a stirred solution of 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-ethylarnino]-
propyl}-benzo[1,3]dioxole-2,2-dicarboxylic acid diethyl ester hydrochloride salt (5.0
g, 9.72 mmol), and THF (90 mL) was added i-Pr2NEt (4.23 mL, 3.14 g, 24.3 mmol)
and (Boc)2O (2.12 g, 9.72 mmol). After 6 h, an additional portion of (Boc)2O (200
mg, 0.92 mmol) is added. After a total of 22 h, the solution was quenched with 10 mL
of sat. aq. NaHC03, and extracted with 3 x 100 rnL Et20. The combined organics
CA 02254120 1998-11-10
WO 97/43273 PCT/US97/08148
- 34-
were washed with 1 x 150 mL of brine, dried over MgSO4, filtered and concentrat~d to
a yellow oil. Flash chromatography on silica gel, eluting with hex~ne~/EtOAc (V1)
gave 5.07 g (90 %) of product as a sticky, off-white solid; IH NMR (300 MHz,
CDC13): ~ 1.22 (d, J = 6.9 Hz, 3H), 1.30-1.47 (m, l5H), 2.47-2.54(m, lH), 2.57-
2.70 (m, lH), 3.05-3.15 (m, lH), 3.46-3.68 (m, lH), 4.08-4.18 (m, lH), 4.29-4.42(m, 4H), 4.75 (brd, J = 8.6 Hz, lH), 5.51 (brs, lH), 6.55-6.88 (complexm, 4H),
7.20-7.44 (m, 3H); MS (ES) m/z (relative intensity): 578 (M+, 1), 504 (20), 336 (60),
298 (20), 198 (100), 180 (40).
Step 2
5-(2-{tert-Butoxycarbonyl-~2-(3-chloro-phenyl)-2-hydroxy-ethyl]-
amino}-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid
To a stirred solution of 5-(2-{tert-butoxycarbonyl-[2-(3-chloro-phenyl)-2-
hydroxy-ethyl]-amino)-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid bis-ethyl ester
(4.42 g, 7.65 mmol), MeOH (100 mL) and H20 (25 mL) was added S N NaOH (7.64
mL, 38.2 mmol). After 19 h, the solution was concentrated to an aqueous mixture and
acidifîed to pH = I with I N aq. HCI which turns the solution rnilky-white. Extraction
with 3 x 100 rnL EtOAc, washing the combined organics with 1 x 200 mL brine,
drying over Na2SO4, filtration and concentration gave 3.96 g (99 %) of product as a
white solid; IH NMR (300 MHz, DMSO-d6). ~ 1.16 (d, J = 6.7 Hz, 3H), 1.31 (s,
9H), 2.55-2.70 (m, lH), 2.80-2.92 (m, lH), 3.05-3.23 (m, 2H), 3.71-3.93 (m, 2H),4.62-4.87 (m, 2H), 5.30-5.90 (brs, lH), 6.59-6.99 (complexm, 4H), 7.14-7.47
(complexm, 3H), 8.60-8.80 (brs, lH); MS (ES) m/z (relative intensity): 522. (M++25 H, 50).
Step 3
5-(2-{tert-Butoxycarbonyl-[2-(3-chloro-phenyl3-2-hydroxy-ethyl]-
amino}-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid bis-
30 methoxycarbonyl-methyl ester
To a stirred solution of 5-(2- ~ tert-butoxycarbonyl-[2-(3-chloro-phenyl)-2-
hydroxy-ethyl3-amino } -propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid (500 mg,0.96 mmol) and DMF (10 mL) was added K2C03 (132 mg, 0.96 mmol) and methyl-2-
bromo~cet~te (0.23 mL, 366 mg, 2.40 mmol). After stining at 50 ~C for 4 h, the
CA 02254120 1998-ll-lO
W O 97/43273 PCTrUS97/08148
~ reaction mixture was cooled to 23 ~C, quenched with 5 mL sat. aq. NaHCO3, and
extracted with 3 x 30 mL of EtOAc. The combined organics were washed with 2 x 50~ mL brine, dried over MgSO4, filtered and concentrated to a yellow oil. Flash
chromatography on silica gel, eluting with hexane/EtOAc (4/1 to 2/1), gave 508 mg (79
%) of product as a colorless oil; IH NMR (300 MHz, CDCl3): ~ 1.22 (d, J = 6.9 Hz, 3
H), 1.41 (brs, 9H), 2.50-2.60 (brm, lH), 2.60-2.75 (brm, lH), 3.06-3.15 (brm,
lH), 3.45-3.60 ~brm, lH), 3.76 (s, 3H), 3.77 (s, 3H), 4.05-4.20 (brm, lH), 4.70-4.79 (brm, lH), 4.79(s, 2H), 4.80(s, 2H), 5.42-5.51 (brs, lH), 6.60-6.91
(complexm, 4H), 7.20-7.43(complexm, 3H); MS (ES) m/z (relative intensity): 666
(M+, (100), 610 (25).
Step 4
5-~2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-benzo[1,3}-
dioxole-2,2-dicarboxylic acid bis-methoxycarbonylmethyl ester
To a stirred solution of 5-(2- ~tert-butoxycarbonyl-[2-(3-chloro-phenyl)-2-
hydroxy-ethyl]-amino3-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid bis-
methoxycarbonyl-methyl ester (330 mg, 0.50 mmol) and CH2C12 (10 mL) was added
trifluoroacetic acid (0.08 mL, 113 mg, 0.99 mmol). After stirring at 23 ~C for 1 h,
additional trifluoroacetic acid (0.08 mL,113 mg,0.99 mmol) was added. After a total
of 22 h, the mixture was quenched with 5 mL of sat. aq. NaHCO3, and extracted with
3 x 30 mL of EtOAc. The combined organics were washed with 1 x 50 mL of brine,
dried over MgSO4, filtered and concentrated to a yellow oil. Flash chromatography on
silica gel, eluting with CHC13/MeOH (20/1 to 10/1) gave 145 mg (52 %) of product as
a white gum; lH NMR (300 MHz, CDC13): o 1.33 (m, 3H), 1.70-2.40 (brm, 2H),
2.73-2.90 (brt, lH), 3.04-3.30 (m, 2H), 3.37-3.53 (m, 2H), 3.75 (s, 3H), 3.76 (s,
3H), 4.80 (s, 2H), 4.81 (s, 2H), 5.40 (m, lH), 6.73-6.93 (complexm, 4H), 7.15-
7.32 (m, 2H(), 7.43 (s, lH); MS (ES) m/z (relative intensity): 566 (M+, 80), 508 (M+ -
CO2CH3+H, 100), 450 (M+ - 2CO2CH3+2H, 30).
CA 02254120 1998-11-10
Wo 97143273 PCT/USg7/08148
- 36-
EXAMPLE 25
5-{2- [2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo~l,3]dioxole-2,2-dicarboxylic acid bis-propoxycarbonylmethyl
ester
s
Step 1
5-(2-{tert-Butoxycarbonyl-[2-(3-chloro-phenyl)-2-hydroxy-ethyl]-
amino}-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid bis-
propoxycarbonylmethyl ester
The title compound was prepared from 5-(2-{tert-butoxycarbonyl-[2-(3-chloro-
phenyl)-2-hydroxy-ethyl]-amino}-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid and
propyl 2-bromoacetate according to the procedure of Example 24, step 3 as a colorless
oil: lH NMR (300 MHz, CDC13): ~ 0.91 (t, J=7.4 Hz, 6H), 1.21 (d, J=6.9 Hz, 3H),
1.40 (s, 9H), 1.55-1.72 (m, 4H), 2.47-2.70 (m, lH), 3.05-3.17 (m, lH), 3.46-3.60(m, lH), 4.11 (t, J=6.6 Hz, 4H), 4.70-4.85 (m, 4H), 5.47 (s, lH), 6.60-6.92 (m,
3H), 7.20-7.35 (m, 3H), 7.41 (s, lH); MS (ES) m/z (relative intensity): 722 (M+,100).
Step 2
5-~2-[2-(3-Chloro-phenyl)-2-hydroxy~ethylamino]-propyl}-benzo[1,31-
dioxole-2,2-dicarb~xylic acid bis-propoxycarbonylmethyl ester
The title compound was plGpared from 5-(2-~tert-butoxycarbonyl-[2-(3-chloro-
phenyl)-2-hydroxy-ethyl]-amino}-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid
bis-propoxyoxycarbonylmethyl ester according to the procedure of Example 24, step 4
as a brown gum: IH NMR (300 MHz, CDCl3): ~ 0.80-0.95 (m, 9H),1.40-1.80 brm,
6H), 2.80-3.74 (brm, 7H), 4.05-4.20 (m, 4H), 4.70-4.85 (m, 2H), 5.10 (brs, lH),
6.70-6.95 (brm, 3H), 7.15-7.40 (brm, 4H); MS (ES) m/z (relative intensity): 622 (M+,
100).
CA 02254l20 l998-ll-lO
WO 97/43273 PCT/US97/08148
~ EXAMPLE 26
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
- benzo[l,3]dioxole-2,2-dic~rboxylic acid bis-(l-methoxycarbonyl-ethyl) ester
Step 1
5-(2-{tert-Butoxycarbonyl-[2-(3-chloro-phenyl)-2-hydroxy-ethyl]-
amino}-propyl)-ben~.oll,3]dioxole-2,2-dicarboxylic acid bis-(l-
methoxycarbonyl-ethyl~ester
The title compound was ~repa-~d from 5-(2- ~ tert-butoxycarbonyl-[2-(3-chloro-
phenyl)-2-hydroxy-ethyl]-amino ) -propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid and
methyl 1-bromopropionate according to the procedure of Example 24, step 3 as a
colorless oil: IH NMR (300 MHz, CDC13): o 1.22 (d, J=6.9 Hz, 3H), 1.38 (s, 9H),
2.50-2.70 (m, 2H), 2.89 (s, 3H), 2.96 (s, 3H), 3.07-3.15 (m, lH), 3.45-3.60 (m,
lH), 3.70-3.83 (m, 8H), 4.05-4.18 (m, lH), 4.72-4.80 (m, lH), 5.22-5.32 (m, lH),6.60-6.90 (m, 3H), 7.20-7.32 (m, 3H), 7.41 (s, lH); MS (ES) m/z (relative intensity):
694 (M+, 10()), 638 (M+-t-Bu, 20), 594 (M+-Boc, 20).
Step 2
5-{2-12-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,31dioxole-2,2-dicarboxylic acid bis-(l-methoxycarbonyl-
ethyl)ester
The title compound was prepared from 5-(2-ttert-butoxycarbonyl-[2-(3-chloro-
phenyl)-2-hydroxy-ethyl]-amino)-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid
bis-methoxycarbonyl- l-ethyl ester according to the procedure of Fy~mple 24, step 4 as
a colorless gum: IH NMR (300 MHz, CDCl3): o 1.28 (d, J=6.7 Hz, 3H), 1.30-2.00
(brm, 2H), 1.55-1.65 (m, 6H), 2.70-2.80 (m, lH), 3.05-3.30 (m, 2H), 3.35-3.69
(m, 2H), 3.68-3.80 (m, 6H), 5.10-5.18 (m, lH), 5.22-5.31 (m, 2H), 6.70-6.95 (m,
- 30 3H), 7.15-7.30 (m, 3H), 7.36 (s, lH); MS (ES) m/z (relative intensity): 594 (M+,
100).
CA 02254120 1998-ll-lO
WO 97143273 PCT/US97/08148
- 38-
EXAMPLE 27
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-isopropoxycarbonylmethyl
ester
Step 1
5-(2-{tert-Butoxycarbonyl-[2-(3-chloro-phenyl)-2-hydroxy-ethyl]-
amino}-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid bis-
isopropoxycarbonyl-methyl ester
10The title compound was prepared from 5-(2-{tert-butoxycarbonyl-[2-(3-chloro-
phenyl)-2-hydroxy-ethyl]-amino)-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid and
isopropyl 2-bromoacetate according to the procedure of Example 24, step 3 as a
colorless oil: lH NMR (300 MHz, CDC13): ~ 1.15-1.30 (m, 15H), 1.40 (s, 9H), 2.45-
2.70 (m, 2H), 3.06-3.15 (m, lH), 3.45-3.60 (m, lH), 4.05-4.18 (m, 2H), 4.70-4.8015(m, 4H), 5.06 (sept, J=6.3 Hz, 2H), 5.46 (brs, lH), 6.60-6.90 (m, 3H), 7.20-7.35
(m, 3H),7.41 (s, lH).; MS (ES) m/z (relative intensity): 722 (M+, 100).
Step 2
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]~propyl}-benzo
20[1,3]dioxole-2,2-dicarboxylic acid bis-isopropoxycarbonylmethyl ester
The title compound was plcpa~,d from 5-(2-~tert-butoxycarbonyl-[2-(3-chloro-
phenyl)-2-hydroxy-ethyl]-amino)-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid
bis-isopropoxycarbonylmethyl ester according to the procedure of Example 24, step 3
25as a brown gum: IH NMR (300 MHz, CDC13): ~ 1.17-1.30 (m, 15H), 2.72-2.82 9m,
lH), 3.10-3.29 (m, 3H), 3.38-3.50 (m, lH), 4.70-4.80 (m, 4H), 4.95-5.18
(complexm, 3H), 6.72-6.95 (m, 3H), 7.15-7.30 (m, 3H), 7.36 (s, lH), 8.73 (brs,
lH), 9.86 (brs, lH); MS (ES) m/z (relative intensity): 622 (M+, 100).
CA 022~4120 1998-11-10
WO 97/43273 PCT/US97/08148
- 39-
EXAMPLE 28
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzoll,3]dioxole-2,2
dicarboxylic acid bis-ethoxycarbonylmethyl ester
s
Step 1
5-(2-{tert-Butoxycarbonyl-[2-(3-chloro-phenyl)-2-hydroxy-ethyll-
amino}-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid bis-
ethoxycarbonyl methylester
The tit~e compound was prepared from 5-(2-(tert-butoxycarbonyl-[2-(3-chloro-
phenyl)-2-hydroxy-ethyl]-amino}-propyl)-benzo~1,3]dioxole-2,2-dicarboxylic acid and
ethyl bromoacetate according to the procedure of Example 24, Step 3 as a colorless oil;
1H NMR (300 MHz, CDC13): ~ 1.17-1.30 (m, 9H), 1.39 (s, 9H), 2.48-2.54 (brm,
lH), 2.55-2.61(brm, lH), 3.07-3.13 (m, 2H), 3.47-3.55 (brm, lH), 4.05-4.20 (brrn,
lH), 4.21 (q, J = 7.0 Hz, 4H), 4.20-4.33 (brm, lH), 4.78 (s, 2H), 4.79 (s, 2 H),5.45-5.50 (brm, 1 H), 6.61-6.85 (m, 4H), 7.20-7.43 (m, 3H); MS (ES) m/z (relative
intensity): 694 (M+, (100), 638 (15), 594 (15).
Step 2
5-{2-~2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl~-benzo[1,3]
dioxole-2,2-dic~rboxylic acid bis-ethoxycarbonylmethyl ester
The title compound was prepared from 5-(2-{tert-butoxycarbonyl-[2-(3-chloro-
phenyl)-2-hydroxy-ethyll-amino~-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid
bis-ethoxycarbonylmethyl ester according to the procedure of Example 24, Step 4 as a
white gum; lH NMR (300 MHz, CDC13): ~ 1.15-1.27 (m, 3H), 1.31-1.41 (t, J=6.8
Hz, 6H), 2.53-3.51 (m, 6H), 3.60-3.80 (m, 4H), 4.00-4.10 (brs, lH), 4.15-4.30 (q,
J=6.7 Hz, 4H), 4.70-4.85 (brm, lH), 6.60-6.95 (m, 3H), 7.10-7.50 (m, 4H); MS
(ES) m/z (relative intensity): 594 (M+, 10), 522 (80), 508 (80), 436 (100).
CA 022~4l20 l998-ll-lo
Wo 97/43273 PCT/USg7/08148
- 40-
EXAMPLE 29
S-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-(l-ethoxycarbonyl-ethyl)
ester
s
Step 1
5-(2-{tert-Butoxycarbonyl-[2-(3-chloro-phenyl)-2-hydroxy-ethyl]-
amino}-propyl)-ben~o[1,3]dioxole-2,2-dicarboxylic acid bis-(l-
ethoxycarbonyl-ethyl) ester
The title compound was prepared from 5-(2- ( tert-butoxycarbonyl-[2-(3-chloro-
phenyl)-2-hydroxy-ethyl]-amino)-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid and
ethyl 2-bromopropionate according to the procedure of Example 24, Step 3 as a
colorless oil; IH NMR (300 MHz, CDC13): ~ 1.15-1.30 (complex m, 9H), 1.40 (brs,
9H), 1.52-1.69 (m, 6H), 2.47-2.58 (m, lH), 2.58-2.70 (m, lH), 3.05-3.16 (m, lH),3.42-3.54 (m, lH), 4.05-4.30 (complexm, 5H), 4.77 (m, lH), 5.19-5.30 (m, 2H),
5.49 (brs, lH), 6.57-6.67 (m, lH), 6.71-6.80 (m, lH), 6.82-6.91 (m, 2H), 7.20-
7.35 (m,2H),7.41 (brs, lH); MS (ES) m/z (relative intensity): 722 (M+, 100).
Step 2
5-~2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-(l-ethoxycarbonyl-ethyl)
ester The title compound was prepared from 5-(2-(tert-butoxycarbonyl-~2-(3-chloro-
phenyl)-2-hydroxy-ethyl]-arnino)-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acidbis-ethoxycarbonyl-1-ethyl ester according to the procedure of Example 24, Step 4 as a
colorless oil; IH NMR (300 MHz, CDCl3): o very complex due to 4 dia~ Gom~
present; MS (ES) m/z (relative intensity): 622 (M+, 40), 522 (M+-CH3CHCO2Et,
100).
CA 022~4120 1998-11-10
WO 97/43273 PCT/US97108148
- 41 -
EXAMPLE 30
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-trimethylsilanylmethyl ester
S Acetyl chloride (0.59 g, 7.5 mmol) was added to trimethylsilylmeth~ncl (4.03
g, 37.5 mmol) with stirring at room temperature. After 0.5 h, 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino] -propyl ) -benzo[1,3]dioxole-2,2-dicarboxylic acid(1.05 g, 2.5 mmol) was added in portions. The resulting solution was st~rred for 3
days, and then treated with 50 ml of 50% sodium bicarbonate solution, followed by
extraction with ethyl acetate (50 ml). The organic extract was dried over anhydrous
sodium sulfate, filtered, and evaporated. The residue was passed through a pad of silica
gel, eluting with hexanes, and ether-hexanes/l:l until no more trimethylsilylmeth;~nol
could be detected. The filter pad was then eluted with ethyl acetate, and the solvent was
evaporated to give 1.14 g of the desired product as a colorless gum. Conversion to the
HCl salt yielded 1.16 g of white foam; lH NMR (CDC13) ~ 0.06 (s, 18 H), 1.33 (d, J
= 6.5 Hz, 3 H), 2.80 (m, lH), 3.15 (m, lH), 3.20 (m, 1 H), 3.48 (m, 2 H), 4.014 (s,
2 H), 4.015 (s, 2 H), 5.45 (bd, J = 9.7 Hz, 1 H), 5.60 (bs, 1 H), 6.80 (m, 3 H), 7.25
(m, 2 H), 7.45 (s, I H), 8.70 (bs, 1 H), 10.10 (bs, 1 H); IR (KBr): 1763 cm-l (C=O);
MS (ES) m/z 594 (MH+); [a]2D5 -23~ (c, 1.0, CHC13). Anal. Calcd. for
C2gH40ClNO7Si2HCI: C, 53.32; H, 6.39; N, 2.22; Cl, 11.24; Si, 8.91. Found: C,
52.65; H, 6.70; N, 2.11; Cl, 11.47; Si, 9.00.
EXAMPLE 31
5-~2- 12-(3-Chloro-phenyl)-2-hydroxy-ethylamino] -propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-(2-trimethylsilanyl-ethyl)
ester
The title compound was prepared from 5-12-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino] -propyl ) -benzo[1,3]dioxole-2,2-dicarboxylic acid and 2-
trimethylsilylethanol according to the procedure of Example 30 as a white foam (HCl
salt); IH NMR (CDC13) ~0.03 (s, 18 H), 1.06 (t, J = 8.6 Hz, 4 H), 1.33 (bs, 3 H),
1.90 (bs, lH), 2.80 (bs, IH), 3.18 (bs, 2H), 3.48 (bs, 2 H), 4.37 (t, J = 8.6 Hz, 4
H), 5.45 (bs, 1 H), 6.80 (m, 3 H), 7.26 (m, 2 H), 7.43 (s, 1 H), 8.70 (bs, 1 H),10.10 (bs, 1 H); IR (KBr): 1763 cm-l (C=O); MS (ES) m/z 622 (MH+); [a]25 -23~ (c,
CA 02254120 1998-ll-lO
WO 97143273 PCTfUS97/08148
1.0, CHCl3). Anal. Calcd. for C30H44ClNO7Si2-HCl: C, 54.70; H, 6.88; N, 2.13; Cl,
10.76; Si, 8.53. Found: C, 53.96; H, 7.05; N, 1.86; Cl, 8.84; Si, 10.33.
EXAMPLE 32
55-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[1,3]dioxole-2,2-dicarboxylic acid bis-(3-trimethylsilanyl-propyl)
ester
The title compound was prepared from 5-~2-[2-(3-chloro-phenyl)-2-hydroxy-
10ethylamino]-propyl )-benzo[1,3]dioxole-2,2-dicarboxylic acid and 3-
trimethylsilylpropanol according to the procedure of Example 30 as a white foam (HCl
salt); IH NMR (CDCl3) ~ 0.01 (s, 18 H), 0.46 (m, 4 H), 1.33 (d, J = 6.3 Hz, 3 H),
1.70 (m, 4 H), 2.80 (m, lH), 3.18 (m, 2H), 3.46(m 2 H), 4.24(t, J = 7.0 Hz, 4 H),
5.47 (bd, J = 9.7 Hz, 1 H), 5.70 (bs, 1 H), 6.80 (m, 3 H), 7.25 (m, 2 H), 7.43 (s, 1
15H), 8.70 (bs, 1 H), 10.10 (bs, 1 H); IR (KBr): 1765 cm-l (C=O); MS (ES) m/z 650
(MH+); [a]2D5 -22~ (c, 1.0, CHCl3). Anal. Calcd. for C32H4gClNO7Si2-HCl: C,
55.96; H, 7.()4; N, 2.()4 Cl, 10.32; Si, 8.18. Found: C, 55.82; H, 7.21; N, 1.87; Cl,
10.22; Si, 7.76.
20EXAMPLE 33
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylaminol-propyl}-
benzo~1,3]dioxole-2,2-dicarboxylic acid bis-(3,3-dimethyl-butyl) ester
The title compound was prepared from ~-{2-[2-(3-chloro-phenyl)-2-hydroxy-
25ethylamino]-propyl}-benzo[1,3]dioxole-2,2-dicarboxylic acid and 3,3-dimethylbutanol
according to the procedure of Example 30 as a white foam (HCl salt); lH NMR
(CDC13) ~ 0.90 (s, 18 H), 1.33 (bs, 3 H), 1.62 (t, J = 7.4 Hz, 4 H~, 2.80 (bs, lH),
3.18 (bs, 2H), 3.48 (bs, 2 H), 4.35 (t, J = 7.4 Hz, 4 H), 5.45 (bs, 1 H), 6.80 (m, 3
H), 7.26 ~m, 2 H), 7.43 (s, 1 H), 8.70 (bs, 1 H), 10.10 (bs, 1 H); IR (KBr): 1765
30cm-l (C=O); MS (CI) m/z 590 (MH+); [~12DS -24~ (c, 1.0, CHCl3). Anal. Calcd. for
C32H44ClNO7HCl: C, 61.34; H, 7.08; N, 2.24; Cl, 11.32. Found: C, 60.79; H,
7.46; N, 2.09; Cl, 11.95.
CA 02254120 1998-ll-lO
WO 97143273 PCT/US97/08148
- 43 -
~ EXAMPLE 34
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-cyclohexylmethyl ester
S The title compound was prepared from 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl}-benzo[1,3]dioxole-2,2-dicarboxylic acid and cyclohexylmeth~nc-l
according to the procedure of Example 30 as a white foam (HCl salt); IH NMR
(CDC13) ~ 0.80-1.80 (m, 22 H), 1.33 (bs, 3 H), 2.80 (m, lH), 3.18 (m, 2H), 3.48
(m, 2 H), 4.10 (m, 4 H), 5.50 (bs, 1 H), 6.80 (m, 3 H), 7.25 (m, 2 H), 7.43 (s, 1 H),
8.70 (bs, 1 H), 10.10 (bs, 1 H); IR (KBr): 1765 cm-l (C=O); MS (Cl) m/z 614
(MH+); [o~]2D5 -22~ (c, 1.0, CHC13). Anal. Calcd. for C34H44ClNO7-HCI: C, 62.77;H, 6.82; N, 2.15; Cl, 10.90. Found: C, 61.83; H, 7.27; N, 2.07; Cl, 10.25.
EXAMPLE 35
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[1,3]dioxole-2,2-dicarboxylic acid bis-(4-methyl-pentyl) ester
The title compound was prepared from 5-l2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylaminol-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid and 4-methylpentanolaccording to the procedure of Example 30 as a colorless gum (HCI salt); IH NMR
(CDC13) ~ 0.87 (d, J = 6.5 Hz, 12 H), 1.33 (bs, 3 H), 1.10-2.0 (m, 10 H), 2.80 (bs,
lH), 3.18 (bs, 2H), 3.48 (bs, 2 H), 4.28(m, 4 H), 5.60 (bs, 2 H), 6.80 (m, 3 H),7.25 (m, 2 H), 7.43 (s, 1 H), 8.70 (bs, 1 H), 10.10 (bs, 1 H); IR (KBr): 1765 crn~l
(C=O); MS (Cl) rn/z 590 (MH+); [~12D5 -24~ (c, 1.0, CHCl3). Anal. Calcd. for
C32H44CINO7-HCI: C, 61.34; H, 7.08; N, 2.24; Cl, 11.32. Found: C, 60.50; H,
7.36; N, 2.13; Cl, 11.66.
EXAMPLE 36
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo~1,3]dioxole-2,2-dicarboxylic acid bis-(2-cyclohexyl-ethyl ) ester
The title compound was prepared from 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino] -propyl } -benzo[1,3]dioxole-2,2-dicarboxylic acid and 2-cyclohexylethanol
according to the procedure of Example 30 as an off-white foam (HCI salt); IH NMR
CA 02254120 1998-11-10
WO 97/43273 PCTtUS97/08148
- 44 -
(CDCl3) ~0.80-1.80 (m, 26 H), 1.32 (d, J = 6.3 Hz, 3 H), 2.80 (m, lH), 3.18 (m,
2H)7 3.48 (m, 2 H), 4.32 (t, J = 6.9 Hz, 4 H), 5.45 (bd, J = 9.7 Hz, 1 H), 6.80 (m, 3
H), 7.25 (m, 2 H), 7.45 (s, 1 H), 8.70 (bs, 1 H), 10.0 (bs, I H); IR (KBr): 1765 cm~l
(C=O); MS (CI) m/z 642 (MH+); [~]2D5 -24~ (c, 1.0, CHC13). Anal. Calcd. for
S C36H4gClNO7-HCI: C, 63.71; H, 7.13; N, 2.06; Cl, 10.45. Found: C, 63.07; H,
7.54; N, 2.07; Cl, 12.81.
I~:XAMPLE 37
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino~-propyl}-
10benzo[1,31dioxole-2,2-dicarboxylic acid bis-(3-cyclopentyl-propyl )
ester
The ~itle compound was prepared from 5-~2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylarnino]-propyl ) -benzo[1,3]dioxole-2,2-dicarboxylic acid and 3-
15 cyclopentylpropanol according to the procedure of Example 30 as a colorless gum(HCI salt); lH NMR (CDC13) ~ 0.80-1.80 (m, 26 H), 1.33 (d, J = 6.3 Hz, 3 H), 2.80
(m, lH), 3.18 (m, 2H), 3.48 (m, 2 H), 4.28 (t, J = 6.8 Hz, 4 H), 5.45 (bd, J = 9.7
Hz, 1 H), 6.80 (m, 3 H), 7.25 (m, 2 H), 7.45 (s, I H), 8.70 (bs, 1 H), 10.10 (bs, 1
H); IR (KBr): 1765 cm-l (C=O); MS (CI) m/z 642 (MH+); [~]2D5 -21~ (c, 1.0, CHCl3).
Anal. Calcd. for C36H4~CINO7-HCI: C, 63.71; H, 7.13; N, 2.06; Cl, 10.45. Found:
C, 63.69; H, 7.64; N, 2.00; Cl, 10.13.
EXAMPLE 38
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo~l,3~dioxole-2,2-dicarboxylic acid bis-cyclopropylmethyl ester
The title compound was prepared from 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid and cyclopropylm~ h~nol
according to the procedure of Example 30 as a white solid (HCl salt); IH NMR
(CDC13) ~ 0.33 (m, 4 H), 0.61 (m, 4 H), 1.21, (m, 2 H), 1.32 (bs, 3 H), 2.80 (m,lH), 3.19 (m, 2H), 3.48 (m, 2 H), 4.15 (d, J = 7.3 Hz, 4 H), 5.50 (bd, 1 H), 5.70
(bs, 1 H), 6.80 (m, 3 H), 7.25 (m, 2 H), 7.45 (s, l H), 8.70 (bs, 1 H), 10.10 (bs, 1
H); IR (KBr): 1757, 1778 cm~l (C=O); MS (ES) m/z 530 (MH+); [OL]2D5 -26~ (c, 1.0,
CA 022~4l20 l998-ll-lO
WO 97/43273 PCT/US97/08148
- 45-
~ CHC13). Anal. Calcd. for C2gH32ClNO7-HCI: C, 59.37; H, 5.69; N, 2.47; Cl, 12.53.
Found: C, 59.19; H, 5.88; N, 2.29; Cl, 12.87.
EXAMPLE 39
55-{2-12~(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
ben~o[1,3~dioxole-2,2-dicarboxylic acid bis-(l-methyl-
cyclopropylmethyl) ester
The ~tle compound was prepared from 5-(2-[2-(3-chloro-phenyl)-2-hydroxy-
10ethylamino]-propyl ~-benzo[1,3]dioxole-2,2-dicarboxylic acid and 1-
methylcyclopropyl-methanol according to the procedure of Example 30 as a white solid
(HCl salt); 1H NMR (CDC13) ~ 0.39 (m, 4 H), 0.52 (m, 4 H), 1.10, (s, 6 H), 1.32 (d,
J = 6.5 Hz, 3 H), 2.80 (m, IH), 3.19 (m, 2H), 3.48 (m, 2 H), 4.10 (s, 4 H), 5.50(bd, 1 H), 5.70 (bs, 1 H), 6.80 (m, 3 H), 7.25 (m, 2 H), 7.45 (s, 1 H), 8.70 (bs, 1
15H), 10.10 (bs, 1 H); IR (KBr): 1763 cm-l (C-O); MS (ES) m/z 558 (MH+); [a]2DS -25~
(c, 1.0, CHC13). Anal. Calcd. for C30H36CINO7HCI: C, 60.61; H, 6.10; N, 2.36;
Cl, 11.94. Found: C, 60.02; H, 6.58; N, 2.17; Cl, 11.20.
EXAMPLE 40
205-~2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[1,3]dioxole-2,2-dicarboxylic acid bis-cyclobutylmethyl ester
The htle compound was prepared from 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl ) -benzo[1,3]dioxole-2,2-dicarboxylic acid and cyclobutyl~ ol25according to the procedure of Example 30 as an off-white solid (HCl salt); lH NMR
(CDCl3) ~ 1.32 (d, J = 6.5 Hz, 3 H), 1.85 (m, 8 H), 2.05 (m, 4 H), 2.69 (m, 2 H),
2.80 (m, lH), 3.19 (m, 2H), 3.48 (m, 2 H), 4.10 (s, 4 H), 5.50 (bd, 1 H), 5.70 (bs,
1 H), 6.80 (m, 3 H), 7.25 (m, 2 H), 7.45 (s, 1 H), 8.70 (bs, 1 H), lO.10 (bs, 1 H);
IR (KBr): 1759, 1779 cm-l (C=O); MS (ES) m/z 558 (MH+); [oL]2D5 -27~ (c, 1.0,
30CHC13). Anal. Calcd. for C30H36CINO7-HCI: C, 60.61; H, 6.10; N, 2.36; Cl, 11.94.
Found: C, 60.68; H, 6.30; N, 2.31; Cl, 11.71.
CA 02254l20 l998-ll-lO
WO 97/43273 PCT/US97/08148
- 46-
EXAMPLE 41
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-(2-cyclopentyl-ethyl) ester
The title compound was prepared from 5-(2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl~-benzo~1,3]dioxole-2,2-dicarboxylic acid and 2-cyclopentylethanol
according to the procedure of Exarnple 30 as an amber gum (HCl salt); lH NMR
(CDC13) ~ 1.32 (d, J = 6.3 Hz, 3 H), 1.60 (m, 20 H), 2.80 (m, lH), 2.90 (m, 2 H),
3.15 (m, lH), 3.20 (m, lH), 3.48 (m, 2 H), 4.30 (t, J = 6.9 Hz, 4 H), 5.50 (bd, 1
H), 6.80 (m, 3 H), 7.25 (m, 2 H), 7.43 (s, 1 H), 8.70 (bs, 1 H), 10.10 (bs, 1 H); IR
(KBr): 1765 cm-l (C=O); MS (CI) m/z 614 (MH+); [OL]2D5 -24~ (c, 1.0, CHCl3). Anal.
Calcd. for C34H44CINO7-HCl: C, 62.77; H, 6.82; N, 2.15; Cl, 10.90. Found: C,
64.04; H, 8.24; N, 1.62; Cl, 8.99.
General Method to Prepare 5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-
ethylamino]-propyl~-ben~o~1,3]dioxole-2,2-dicarboxylic acid bis-
acetoxy-alkyl esters
Step 1
Preparation of Acyloxyalkyl Iodides
These esters were prepared by reacting the appropriate acid chlorides with
forrnaldehyde or acetaldehyde in the presence of a catalytic amount of anhyrous ZnC12
according to Ulich, L. H. and Adams, R. J. Amer. Chem. Soc. 1921, 43, 660- 666.
25 The resultant acyloxyalkyl chlorides were converted in to the co~ ,onding
acyloxyalkyl iodide derivatives by reacting them with NaI in boiling benzene according
to Fujimoto, K., Ishihara, S., Yanagisawa, H., Ide, J., Nakayama, E., Nakao, H.,Sugawara, S., Iwata, M. J. Antibiotics 1987,19, 370.
CA 022~4120 1998-ll-lO
WO 97/43273 PCT/US97tO8148
- 47 -
Step 2
5-{2- [2-(3-Chlorophenyl)-2-hydroxyethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid disilver salt
To a stirred solution of 5- f 2-~2-(3-chlorophenyl)-2-hydroxyethylarnino]-
propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid disodium salt (3.0 gm, 6.0 mmol) in
distilled water, (in the dark) a solution of AgNO3 (2.3 g, 12.0 mrnol) was addeddropwise. After stirring at room temperature for 1 h the separated solid was filtered
and washed with water and acetone. The solid was dried at room temperature undervacuum to give a colorless solid; yield 100%; mp 183-185; M+H 422.1.
Step 3
5-{2- [2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-acetoxyalkyl esters
To a stirred suspension of 5-(2-[2-(3-chlorophenyl)-2-hydroxyethylarnino]-
propyl)-benzol1,3ldioxole-2,2-dicarboxylic acid disilver salt (2.0 gms, 3 mmol) in
anhydrous chloroforrn the apl,lu,."iately substituted acyloxyalkyl iodide (9 rnmol)
dissolved in chloroform (30 mL) was added. The reaction mixture was stirred at room
20 temperature, under dark for 16 h. The reaction mixture was filtered and washed with
5% Na2S2O3 solution in distilled water. The chloroform layer was dried over
anhydrous MgSO4 and filtered. It was evaporated to dryness and the residue obtained
was dissolved in 5 mL of ethyl acetate and 60 mL of hexane was added. The sep~edsolid was filtered and washed with hexane. The compounds of Exarnples 42-58 below
25 were prepared according to this general procedure.
EXAMPLE 42
5-{2-12-(3-Chloro-phenyl)-2-hydroxyethylamino]propyl}benzo[1,3]
dioxole-2,2-dicarboxylic acid bis-acetoxymethyl ester
To a stirred suspension of 5-~2-[2-(3-chlorophenyl)-2-hydroxyethylaminol-
- propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid disilver salt (2.0 gms, 3 mrnol) in
anhydrous chloroforrn, acetoxymethyl iodide (9 mmol) dissolved in chloroform (30mL) was added. The reaction rnixture was stirred at room temperature, under dark for
16 h. The reaction rnixture was filtered and washed with 5% Na2S2O3 solution in
CA 022~4120 1998-ll-lO
WO 97143273 PCT/US97/08148
- 48 -
distilled water. The chloroform layer was dried over anhydrous MgSO4 and filtered.
It was evaporated to dryness and the residue obtained was dissolved in S mL of ethyl
acetate and 60 mL of hexane was added. The separated solid was filtered and washed
with hexane to yield an ivory color solid; mp 132-134; yield 97%; M+H 566.
s
EXAMPLE 43
5-{2-[2-(3-Chloro-phenyl)-2-hydroxyethylamino]propyl}benzo[1,3]
dioxole-2,2-dicarboxylic acid bis-propionyloxymethyl ester
To a stirred suspension of 5-{2-[2-(3-chlorophenyl)-2-hydroxyethylamino]-
propyl}-benzo[1,3]dioxole-2,2-dicarboxylic acid disilver salt (2.0 gms, 3 mrnol) in
anhydrous chloroform, propionyloxymethyl iodide (9 mmol) dissolved in chloroform(30 mL) was added. The reaction mixture was stirred at room temperature, under dark
for 16 h. The reaction mixture was filtered and washed with 5% Na2S2O3 solution in
distilled water. The chloroform layer was dried over anhydrous MgSO4 and filtered.
It was evaporated to dryness and the residue obtained was dissolved in 5 mL of ethyl
acetate and 60 mL of hexane was added. The separated solid was filtered and washed
with hexane to yield a colorless solid; mp 118-20; yield 88%; M+H 594.
EXAMPLE 44
5-{2-[2-(3-Chloro-phenyl)-2-hydroxyethylamino]propyl}benzo[1,3]
dioxole-2,2-dicarboxylic acid bis-butyryloxymethyl ester
To a stirred suspension of 5-{2-~2-(3-chlorophenyl)-2-hydroxyethylamino]-
propyll-benzo[1,3]dioxole-2,2-dicarboxylic acid disilver salt (2.0 gms, 3 mmol) in
anhydrous chloroform butyryloxymethyl iodide (9 mmol) dissolved in chlc,lorolln (30
mL) was added. The reaction mixture was stirred at room temperature, under dark for
16 h. The reaction mixture was filtered and washed with 5% Na2S2O3 solution in
distilled water. The chloroform layer was dried over anhydrous MgSO4 and filtered.
It was evaporated to dryness and the residue obtained was dissolved in 5 mL of ethyl
acetate and 60 mL of hexane was added. The separated solid was filtered and washed
with hexane to yield a white solid; mp 70-72 (dec); yield 85%; M+H 622.
CA 02254120 1998-ll-lO
WO 97143273 PCT/US97/08148
- 49 -
EXAMPLE 4S
5-{2-[2-(3-Chloro-phenyl)-2-hydroxyethylamino]propyl}benzo[1,3]
~ dioxole~2,2-dic~rboxylic acid bis-isobutyryloxymethyl ester
5To a stirred suspension of 5-~2-[2-(3-chlorophenyl)-2-hydroxyethylamino]-
propyl}-benzo[1,3~dioxole-2,2-dicarboxylic acid disilver salt (2.0 gms, 3 rnrnol) in
anhydrous chloroform isobutyryloxymethyl iodide (9 mrnol) dissolved in chloroforrn
(30 rnL) was added. The reaction mixture was stirred at room temperature, under dark
for 16 h. The reaction mix~ure was filtered and washed with 5% Na2S2O3 solution in
10~lictilled water. The chloroform layer was dried over anhydrous MgSO4 and ~ltered.
It was evaporated to dryness and the residue obtained was dissolved in 5 rnL of ethyl
acetate and 60 mL of hexane was added. The separated solid was filtered and washed
with hexane to yield a white solid; mp 1 14-116 (dec); yield 78%; M+H 622.
15EXAMPLE 46
5-{2-[2-(3-Chloro-phenyl)-2-hydroxyethylamino]propyl}benzoll,3]
dioxole-2,2-dicarboxylic acid bis-heptanoyloxymethyl ester
To a stirred suspension of 5-12-[2-(3-chlorophenyl)-2-hydroxyethylamino]-
20propyl}-benzo[1,3]dioxole-2,2-dicarboxylic acid disilver salt (2.0 gms, 3 mmol) in
anhydrous chloroforrn heptanoyloxymethyl iodide (9 mmol) dissolved in chlororc,~(30 mL) was added. The reaction mixture was stirred at room temperature, under dark
for 16 h. The reaction mixture was filtered and washed with 5% Na2S2O3 solution in
distilled water. The chlu~of~.n~ layer was dried over anhydrous MgSO4 and filtered.
25It was evaporated to dryness and the residue obtained was dissolved in 5 mL of ethyl
acetate and 60 mL of hexane was added. The separated solid was filtered and washed
with hexane to yield a white solid; mp 94-96; yield 18%; M+H 707.
EXAMPLE 47
305-{2-[2-(3-Chloro-phenyl)-2-hydroxyethylamino]propyl}benzo[1,3]
dioxole-2,2-dica~rboxylic acid bis-(4-methyl-pentanoyloxymethyl) ester
To a stirred suspension of 5-~2-[2-(3-chlorophenyl)-2-hydroxyethylarnino]-
propyl}-benzo[1,3]dioxole-2,2-dicarboxylic acid disilver salt (2.0 gms, 3 mmol) in
35anhydrous chloroforrn pentanoyloxymethyl iodide (9 rnrnol) dissolved in chlc~rorol.
CA 02254120 1998-ll-lO
WO 97/43273 PCT/US97/08148
- 50 -
(30 mL) was added. The reaction mixture was stirred at room temperature, under dark
for 16 h. The reaction mixture was filtered and washed with 5% Na2S2O3 solution in
distilled water. The chloroform layer was dried over anhydrous MgSO4 and filtered.
It was evaporated to dryness and the residue obtained was d~ssolved in 5 mL of ethyl
acetate and 60 rnL of hexane was added. The separated solid was filtered and washed
with hexane to yield a white solid; mp 105-108 (dec); yield 19%; M+H 679.
EXAMPLE 48
5-{2-[2-(3-Chloro-phenyl~-2-hydroxyethylamino]propyl}benzo[1,3]
dioxole-2,2-dicarboxylic acid bis-hexanoyloxymethyl ester
To a stirred suspension of 5-{2-[2-(3-chlorophenyl)-2-hydroxyethylamino]-
propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid disilver salt (2.0 gms, 3 mrnol) in
anhydrous chloroforrn hexanoyloxymethyl iodide (9 mmol) dissolved in chlolol~
(30 mL) was added. The reaction mixture was stirred at room temperature, under dark
for 16 h. The reaction mixture was filtered and washed with 5% Na2S2O3 solution in
distilled water. The chloroform layer was dried over anhydrous MgSO4 and filtered.
It was evaporated to dryness and the residue obtained was dissolved in 5 rnL of ethyl
acetate and 60 mL of hexane was added. The separated solid was filtered and washed
with hexane to yield a white solid; mp 106-109; yield 20%; M+H 679.
EXAMPLE 49
5-{2-[2-(3-Chloro-phenyl)-2-hydroxyethylaminolpropyl}benzo[1,3]
dioxole-2,2-dicarboxylic acid bis- (2,2-dimethyl-propionyloxymethyl)
ester
To a stirred suspension of 5-(2-l2-(3-chlorophenyl)-2-hydroxyethylamino]-
propyl}-benzo[1,3]dioxole-2,2-dicarboxylic acid disilver salt (2.0 gms, 3 mmol) in
anhydrous chloroform 2,2-dimethylpropionyloxymethyl iodide (9 rnmol) dissolved in
chlolofo~ (30 mL) was added. The reaction mixture was stirred at room temperature,
under dark for 16 h. The reaction mixture was filtered and washed with 5% Na2S2O3
solution in distilled water. The chloroform layer was dried over anhydrous MgSO4and filtered. It was evaporated to dryness and the residue obtained was dissolved in 5
rnL of ethyl acetate and 60 mL of hexane was added. The separated solid was filtered
and washed with hexane to yield a ivory solid; mp 72 (dec); yield, 48%; M+H 651.
CA 022~4120 lsss-ll-lo
wo 97/43273 PCT/USg7/08148
EXAMPLE 50
5-{2-12-(3-Chloro-phenyl)-2-hydroxyethylamino]propyl}benzo[1,3]
dioxole-2,2-dicarboxylic acid bis-cyclohexanecarbonyloxymethyl ester
s
To a stirred suspension of 5-(2-[2-(3-chlorophenyl)-2-hydroxyethylamino]-
propyl}-benzol1,3]dioxole-2,2-dicarboxylic acid disilver salt (2.0 gms, 3 mmol) in
anhydrous chloroform cyclohexanecarbonyloxymethyl iodide (9 mmol) dissolved in
chloroforrn (30 mL) was added. The reaction mixture was stirred at room ~ p~ ture,
under dark for 16 h. The reaction mixture was filtered and washed with 5% Na2S2O3
solution in distilled water. The chloroform layer was dried over anhydrous MgSO4and filtered. It was evaporated to dryness and the residue obtained was dissolved in 5
mL of ethyl acetate and 60 mL of hexane was added. The separated solid was filtered
and washed with hexane to yield a white solid; mp 86 (dec); yield 52%; M+H 703.
EXAMPLE 51
5-~2-~2-(3-Chloro-phenyl)-2-hydroxyethylamino]propyl}benzo[1,3]
dioxole-2,2-dicarboxylic acid bis-(1-propionyloxy-ethyl) ester
To a stirred suspension of 5-12-[2-(3-chlorophenyl)-2-hydroxyethylamino]-
propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid disilver salt (2.0 gms, 3 mmol) in
anhydrous chloroform 1-propionyloxyethyl iodide (9 mmol) dissolved in chloroform(30 mL) was added. The reaction mixture was stirred at room temperature, under dark
for 16 h. The reaction mixture was filtered and washed with 5% Na2S2O3 solution in
distilled water. The chlorofo,lll layer was dried over anhydrous MgSO4 and filtered.
It was evaporated to dryness and the residue obtained was dissolved in 5 mL of ethyl
acetate and 6() rnL of hexane was added. The separated solid was filtered and washed
with hexane to yield a white solid; mp 130-132 (dec); yield, 68%; M+H 622
CA 022~4120 1998-11-10
WO 97/43273 rCT/US97/08148
- 52-
EXAMPLE 52
5-{2-[2-(3-Chloro-phenyl)-2-hydroxyethylamino]propyl}benzo[1,3]
dioxole- 2,2-dicarboxylic acid bis-[1-(2,2-dimethyl-propionyloxy-ethyl)
ester
s
To a stirred suspension of 5-~2-[2-(3-chlorophenyl)-2-hydroxyethylamino]-
propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid disilver salt (2.0 gms, 3 mmol) in
anhydrous chloroform 1-(2,2-dimethylpropionyloxy)ethyl iodide (9 mmol) dissolved in
chloroform (30 mL) was added. The reaction mixture was stirred at room telllp~latul~,
under dark for 16 h. The reaction mixture was filtered and washed with 5% Na2S2O3
solution in distilled water. The chloroform layer was dried over anhydrous MgSO4and filtered. It was evaporated to dryness and the residue obtained was dissolved in 5
mL of ethyl acetate and 60 mL of hexane was added. The separated solid was filtered
and washed with hexane to yield a white solid; mp 92; yield, 58%; M+H 679.
EXAMPLE 53
5-{2-[2-(3-Chloro-phenyl)-2-hydroxyethylamino]propyl}benzo~1,3]
dioxole-2,2-dicarboxylic acid bis-(3,3-dimethyl-butyryloxymethyl) ester
To a stirred suspension of 5-{2-[2-(3-chlorophenyl)-2-hydroxyethylamino]-
propyl}-benzo[1,3]dioxole-2,2-dicarboxylic acid disilver salt (2.0 gms, 3 mmol) in
anhydrous chlolofolln 3,3-dimethylbutyryloxymethyl iodide (9 mmol) dissolved in
chloroform (30 mL) was added. The reaction mixture was stirred at room l~m~e-~lure,
under dark for 16 h. The reaction mixture was filtered and washed with 5% Na2S2O3
solution in distilled water. The chloroform layer was dried over anhydrous MgSO4and filtered. It was evaporated to dryness and the residue obtained was dissolved in 5
mL of ethyl acetate and 60 mL of hexane was added. The separated solid was filtered
and washed with hexane to yield a white solid; mp 104-105 (dDec); yield 98%; M+H678.
CA 022~4l20 l998-ll-lo
Wo 97143273 PCT/USg7/08148
- 53 -
~ EXAMPLE 54
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]propyl}benzo[1,3]
dioxole-2,2-dicarboxylic acid bis-[1-(3,3-dimethyl-butyryloxy)-
ethyl)}ester
s
To a stirred suspension of 5-(2-[2-(3-chlorophenyl)-2-hydroxyethylamino]-
propyl}-benzo[1,3]dioxole-2,2-dicarboxylic acid disilver salt (2.0 gms, 3 mmol) in
anhydrous chloroform 3,3-dimethylbutyryloxyethyl iodide (9 mrnol) dissolved in
chlolofc)r-~l (30 mL) was added. The reaction mixture was stirred at room tenl~e.~u~e,
under dark for 16 h. The reaction mixture was filtered and washed with 5% Na2S2O3
solution in distilled water. The chloroform layer was dried over anhydrous MgSO4and filtered. It was evaporated to dryness and the residue obtained was dissolved in 5
mL of ethyl acetate and 60 mL of hexane was added. The separated solid was filtered
and washed with hexane to yield a white solid; mp 96-98 (dec); yield, 72%; M+H 706.
EXAMPLI: 55
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]
propyl}benzo[1,3~dioxole-2,2-dicarboxylic acid bis-(3-cyclopentyl-
propionyloxymethyl~ ester
To a stirred suspension of 5-{2-[2-(3-chlorophenyl)-2-hydroxyethylarnino]-
propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid disilver salt (2.0 gms, 3 rnmol) in
anhydrous chloroform 3-cyclopentylpropionyloxy iodide (9 nunol) dissolved in
chloroform (30 mL) was added. The reaction mixture was stirred at room tell~pel~lul~,
under dark for 16 h. The reaction mixture was filtered and washed with 5% Na2S2O3
solution in distilled water. The chloroform layer was dried over anhydrous MgSO4and filtered. It was evaporated to dryness and the residue obtained was dissolved in 5
mL of ethyl acetate and 60 mL of hexane was added. The separated solid was filtered
and washed with hexane to yield a white solid; mp 155-157 (dec); yield, 98%; M+H730.
CA 02254120 1998-11-10
WO 97/43273 PCT/US97108148
- 54 -
EXAMPLE 56
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-
ethylamino]propyl}benzo[1,3]dioxole-2,2-dicarboxylic acid bis-
benzoyloxymethyl ester
s
To a stirred suspension of 5-(2-[2-(3-chlorophenyl)-2-hydroxyethylamino]-
propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid disilver salt (2.0 gms, 3 rnrnol) in
anhydrous chloroform benzoyloxymethyl iodide (9 rnmol) dissolved in chloroform (30
rnL) was added. The reaction mixture was stirred at room temperature, under dark for
16 h. The reaction mixture was filtered and washed with 5% Na2S2O3 solution in
distilled water. The chloroform layer was dried over anhydrous MgSO4 and filtered.
It was evaporated to dryness and the residue obtained was dissolved in 5 mL of ethyl
acetate and 60 mL of hexane was added. The separated solid was filtered and washed
with hexane to yield a white solid; mp 150-153 (dec); yield 98%; M+H 690.
EXAMPLE 57
5-{2-[2-~3-Chloro-phenyl)-2-hydroxyethylaminolpropyl}benzo[1,3]
dioxole-2,2-dicarboxylic acid bis-(benzoyloxy-ethyl) ester
To a stirred suspension of 5-(2-[2-(3-chlorophenyl)-2-hydroxyethylamino]-
propyl}-benzo[1,3]dioxole-2,2-dicarboxylic acid disilver salt (2.0 gms, 3 mrnol) in
anhydrous chloroform 1-benzoyloxyethyl iodide (9 rnmol) dissolved in chlo -~rc)~ (30
mL) was added. The reaction mixture was stirred at room temperature, under dark for
16 h. The reaction mixture was filtered and washed with ~% Na2S2O3 solution in
(li.ctilled water. The chloroform layer was dried over anhydrous MgSO4 and filtered.
It was evaporated to dryness and the residue obtained was dissolved in S mL of ethyl
acetate and 60 mL of hexane was added. The separated solid was filtered and washed
with hexane to yield an ivory solid; mp 82-83 (dec); yield 96%; M+H 718.
CA 022~4120 1998-ll-lO
WO 97143273 PCT/US97/08148
- 55 -
- EXAMPLE 58
5-{2~[2-(3-Chloro-phenyl)-2-hydroxyethylamino]propyl}benzo[1,3~
dioxole-2,2-dicarboxylic acid bis-(2,2-dimethyl-butyryloxymethyl) ester
To a stirred suspension of 5-{2-[2-(3-chlorophenyl)-2-hydroxyethylarnino]-
propyl}-benzo[1,3]dioxole-2,2-dicarboxylic acid disilver salt (2.0 gms, 3 mmol) in
anhydrous chloroform 2,2-dimethylbutyryloxymethyl iodide (9 rnmol) dissolved in
chloroform (30 mL) was added. The reaction mixture was stirred at room temperature,
under dark for 16 h. The reaction mixture was filtered and washed with 5% Na2S2O3
solution in distilled water. The chloroform layer was dried over anhydrous MgSO4and filtered. It was evaporated to dlyness and the residue obtained was dissolved in 5
mL of ethyl acetate and 60 mL of hexane was added. The separated solid was filtered
and washed with hexane to yield a ivory solid; mp 121-123 (dec); yield 37%; M+H
679.
EXAMPLE 59
5-{(2R)~2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[1,3]dioxole-2,2-dicarboxylic acid bis-[l-
(cyclohexyloxycarbonyloxy)-ethyl] ester
Cyclohexyl 1-iodoethyl carbonate (5.6 g, 18 8 mmol) in CHCl3 (10 mL) was
added dropwise into a cold (0 ~C) suspension of (R, R)-5-[2-[2-(3-chlorophenyl)-2-
hydroxyethylamino]propyl]-1,3-benzodioxole-2,2-dicarboxylic acid disilver salt (4.0
g, 6.3 mmol) and CHC13 (100 mL). After the addition, the mixture was allowed to
come to room temperature and stirred for 24 h. Diethyl ether (300 mL) was added into
the reaction mixture and the precipitated solids were filtered and discarded. The filtrate
was concentrated in vaccuo at room temperature and the residue was quickly
chromatographed through silica gel, eluting anhydrous solvent (EtOAc / hexane, 1/1),
to give a light yellow solid (1.25 g, 26% yield): mp 78-81 ~C; (+)FAB m/e 762
- 30 (M+H)+. Analysisfor: C3gH4gClNO13: Calcd: C, 59.89; H, 6.35; N, 1.84; Found:
C, 60.88; H, 6.53; N, 1.99
CA 02254120 1998-ll-lO
WO 97/43273 PCT/US97/08148
- 56-
EXAMPLE 60
5-{2 [2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-
propyl}benzo[l,3~dioxole-2,2-dicarboxylic acid bis-amide
S A mixture of 5-~2-[2-(3-ehloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[1,3]dioxole-2,2-dicarboxylic aeid bis-ethyl ester (2.4 g, 5 mrnol) and ~.. ,.-;~
(excess) was stirred for 48 h. The reaction mixture was ccnce~ dted and the residue
obtained was extracted with chloroform: methanol (3:1). It was washed with waterand dried over anhydrous MgSO4. The organic layer was filtered and coneentrated.
10 The residue obtained was chromatographed over silica gel eluted with 9:1 chloroform:
methanol to yield a brown solid; mp 98; yield. 1.0 g; 45%; M+H 420.
I :XAMPLE 61
5-{2-12-(3-Chloro-phenyl)-2-hydroxy~ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-2-propyl amide
A mixture of 5-{2-[2-(3-ehloro-phenyl)-2-hydroxy-ethylamino]-propyl}-benzo-
[1,3]dioxole-2,2-diearboxylic acid bis-ethyl ester (2.4 g, 5 mmol) and isopropylamine
(10 mL, excess) was refluxed in ethanol for 48 h. The reaetion mixture was
20 eoneentrated and the residue obtained was extracted with chloroform: methanol (3:1).
It was washed with water and dried over anhydrous MgSO4. The organic layer was
filtered and concentrated. The residue obtained was chromatographed over silica gel
eluted with 9:1 chloroforrn: methanol to yield a colorless spongy solid; mp 60; yield
1.5 g; 60%; M+H 504.
EXAMPLE 62
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy~ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-n-butyl amide
A mixture of 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-benzo-
[1,3]dioxole-2,2-dicarboxylic acid bis-ethyl ester (2.4 g, 5 mmol) and n-butylamine
(10 mL, excess) was refluxed in ethanol for 48 h. The reaction mixture was
eoncentrated and the residue obtained was extracted with chloroform: methanol (3:1).
It was washed with water and dried over anhydrous MgSO4. The organic layer was
filtered and concentrated. The residue obtained was chromatographed over siliea gel
CA 02254120 1998-11-10
WO 97/43273 PCT/US97/08148
~ eluted with 9:1 chloroform: methanol to yield a brown semi solid; yield 1.6 g; 61%;
M+H 532.
EXAMPLE 63
55-{2-~2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[1,3]dioxole-2,2-dicarboxylic acid bis-phenylmethyl amide
A mixture of 5-{2-~2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl)-
benzo[ 1 ,3]dioxole-2,2-dicarboxylic acid bis-ethyl ester ~2.4 g, 5 rnmol) and
10benzylamine (10 mL, excess) was refluxed in ethanol for 48 h. The reaction mixture
was concentrated and the residue obtained was extracted with chloroform: methanol
(3:1). It was washed with water and dried over anhydrous MgSO4. The organic layer
was filtered and concentrated. The residue obtained was chromatographed over silica
gel eluted with 9:1 chloroform: methanol to yield a colorless spongy solid; mp 101;
15yield 2.0 g; 67%; M+H 600.
EXAMPLE 64
5-~2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-(2-furanylmethyl) amide
A mixture of 5- { 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl } -
benzo[1,3]dioxole-2,2-dicarboxylic acid bis-ethyl ester (2.4 g, 5 mmol) and 2-
furanylmethylamine (10 mL, excess) was refluxed in ethanol for 48 h. The reaction
mixture was concentrated and the residue obtained was extracted with chloroform:25 meth~nQl (3:1). It was washed with water and dried over anhydrous MgSO4. The
organic layer was filtered and concentrated. The residue obtained was
chromatographed over silica gel eluted with 9:1 chloroform: methanol to yield a brown
solid; mp 50; yield 400 mg; 14%; M+H 581.
30EXAMPLE 65
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-(~lycine ethyl ester) amide
A mixture of glycine ethyl ester.HCI (1.1 gm, 8 mmol) and sodium methoxide
35(424 mg, ~ mmol) was stirred at room temperature in ethanol for fifteen minutes. At
CA 022~4120 1998-11-10
WO 97/43273 PCT/US97108148
- 58 -
the end, 5- l 2-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl } -
benzo[1,3]dioxole-2,2-dicarboxylic acid bis-ethyl ester (970 mg, 2 mmol) was added
to the reaction mixture and refluxed for 48 hours. At the end ethanol was removed.
The residue obtained was added to ice cold water and seperated solid was filtered. The
solid was dried and purified by silica gel column chromatography by eluting it with 3:1
chloroform:methanol to yield a colorless solid; yield 500 mg; 42%; M+H 592.
EXAMPLE 66
5-{2-[2-(3-Chloru-phenyl)-3-oxazolidinyl~-propyl}-benzo[1,31dioxole-
2,2-dicarboxylic acid
5- (2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl } -benzo[1,3]dioxole-
2,2-dicarboxylic acid sodium salt (0.46 g, 1.0 mmol) was dissolved in 4 mL of 37%
formaldehyde, and stirred at room temperature for 0.5 h. Trifluoroacetic acid (0.23 g,
2.0 mmol) was then added dropwise, and the resulting white suspension was stirred
for 24 h. The precipitate was filtered, washed with water, and dried in vacuo to give
0.40 g of white solid; IH NMR (CDC13) ~ 1.02 (d, J = 6.4 Hz, 3 H), 2.50 (m, 1 H),
3.00 (m, 2H), 3.20 (m, 1 H), 3.80 (m, 1 ~I), 4.75 (d, J = 4.5 Hz, 1 H), 4.90 (d, J =
4.5 Hz, 1 H), 5.12 (t J = 7.3 Hz, 1 H), 6.70 (m, 1 H), 6.86 (m, 2 H), 7.40 (m, 2 H),
7.50 (s, 1 H); IR (KBr): 1740 cm-l (C=O); MS (CI) n~/z 433 (M~). Anal. Calcd. for
C21H20ClNO7-HCl: C, 58.14; H, 4.65; N, 3.23; Cl, 8.17. Found: C, 57.08; H, 4.78;N, 3.32; Cl, 7.51.
EXAMPLE 67
5-((2R)-2-{tert-Butoxycarbonyl-[(2R)-2-(3-chloro-phenyl)-2-hydroxy-
ethyl]-amino}-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid bis-
diethylcarbamoylmethyl ester
A mixture of 5-((2R)-2-~tert-butoxycarbonyl-[(2R)-2-(3-chloro-phenyl)-2-
hydroxy-ethyl]-amino)-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid (0.26 g, 0.5
mmol) and anhydrous potassium carbonate (0.28 g) in anhydrous dimethyl rc,~ .--ide
was treated with 2-bromo-N,N-diethyl~cet~mide (0.39 g, 2 mmol), and stirred at room
temperature for 3 days. It was then diluted with water and hexanes, and the resulting
suspension was filtered. The precipitate was washed with saturated sodium bicarbonate
solution, water, and hexanes, and then dried in vacuo to give 0.27 g of white solid; lH
CA 02254120 1998-11-10
Wo 97/43273 PCT/USg7/08148
- 59-
NMR (CDCl3) ~ 1.0-1.5 (m, 24 H), 2.6 (m, 2H), 3.0-3.5 (m, lOH), 4.0 (m, 1 H~,
4.7-5.0 (m, 4H), 5.5, 6.0 (m, 1 H), 6.80 (m, 3 H), 7.26 (m, 2 H); IR (KBr): 1772,
- 1668 cm-l (C=O); MS (ES) m/z 748 (MH+).
EXAMPLE 68
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy~ethylamino]-propyl~-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-diethylcarbamoylmethyl
ester
A solution of 5-((2R)-2- { tert-butoxycarbonyl-[(2R)-2-(3-chloro-phenyl)-2-
hydroxy-ethyl]-amino) -propyl)-benzoL 1,3]dioxole-2,2-dicarboxylic acid bis-diethyl-
c~ Joylmethyl ester (0.16 g, 0.2 mmol) in methylene chloride (2 ml) was treated
with 0.08 ml of trifluoroacetic acid at room temperature for 18 h. The rnixture was then
evaporated, and the residue washed with ether to give 0.12 g of white solid (TFA salt);
lH NMR (DMSO-d6) ~ 1.0-1.2 (m, 15 H), 2.6 (m, 2H), 3.0-4.0 (m, llH), 5.0 (m, 4
H), 6.1 (m, 1 H), 6.80 (m, 3 H), 7.26 (m, 2 H), 8.7 (bs, 1 H),9.2 (bs, 1 H); IR
(KBr): 1765, 1654 cm-l (C=O); MS (ES) m/z 648 (MH+).
EXAMPLE 69
5-{2-~2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[1,3]dioxole-2,2-dicarboxylic acid cyclopropylmethyl ester
The title compound was prepared from 5-(2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylarnino]-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid and cyclopropylm~th~nol
according to the procedure of Example 1 as an off-white solid; IH NMR (DMSO) ~
0.25 (q,2H), 0.49 (q,2H), 1.01 (d,3H), 1.10 (m,lH), 2.49 (m, lH), 2.85-3.20
(m,4H), 3.94 (d, 2H) 4.85 (bt, lH), 6.58 (d, lH), 6.76 (m,2H), 7.35(m,3H),
7.46(s,1H); IR (KBr): 1653 cm-l(C=O), 1747 cm-l (C=O); MS (CI) m/z 476 (MH+).
Anal. Calcd. for C24H26ClNO7: C, 60.57; H, 5.51; N, 2.94; Cl, 7.46. Found: C,
56.38; H, 5.16; N, 2.61; Cl, 7.60.
CA 02254120 1998-ll-lO
WO 97/43273 PCTIUS97/08148
- 60-
EXAMPLE 70
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid cyclobutylmethyl ester
S The title compound was prepared from 5-(2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylarnino]-propyl3-benzo[1,3]dioxole-2,2-dicarboxylic acid and cyclobutylm~.th~nol
according to the procedure of Example 1 as an off-white solid; IH NMR (I)MSO)
~1.01 (d,3H), 1.75(m,4H), 1.92(m,2H), 2.49 (m, lH), 2.65 (m,lH), 2.85-3.20
(m,4H), 4.09 (d, 2H), 4.85 (bt, lH), 6.58 (d, lH), 6.76 (m,2H), 7.35(m,3H),
7.46(s,1H); IR (KBr): 1652 cm-l(C=O), 1761 cm-l (C=O); MS (CI) rn/z 490 (MH+).
Anal. Calcd. for C2sH28clNo7: C, 61.29; H, 5.76; N, 2.86; Cl, 7.24. Found: C,
57.13; H, 5.15; N, 2.43; Cl, 6.83.
EXAMPLE 71
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[1,3]dioxole-2,2-dicarboxylic acid bis 2-(3-Thienyl)ethyl- ester
The title compound was prepared from 5-{2-[2- (3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl}-benzo[1,3]dioxole-2,2-dicarboxylic acid and 2-(3-thienyl)ethanol
according to the procedure of Example 1 as yellow crystals (HCl salt); lH NMR
(CDC13) ~1.33 (d, 3 H), 2.82 (m, lH), 2.95 (t,4H), 3.20(m, 2H), 3.48 (bd, 2 H),
4.45 (t, 4 H), 5.50 (bd, 1 H), 5.60(bs,1H), 6.75-7.00(m, 7H), 7.21-7.32(m, 5H),
7.45(s,1H), 8.70 (bs, 1 H), 10.10 (bs, 1 H); IR (KBr): 1765 cm-l (C=O); MS (CI)
rn/z 642 (MH+); [a]25 -21 ~ (c, 1.0, CHC13). Anal. Calcd. for C34H32ClS2NO7-HCl:
C, 56.63; H, 4.75; N, 2.06; S, 9.45; Cl, 10.46. Found: C, 55.89; H, 4.95; N, 1.87;
S, 9.45; Cl, 9.95.
EXAMPLE 72
~-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid 2~(3-Thienyl)ethyl ester
The title compound was prepared from 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl ~ -benzo[1,3]dioxole-2,2-dicarboxylic acid bis 2-(3-thienyl)ethyl-
ester according to the procedure of Example 22 as an off-white solid; lH NMR
CA 02254120 1998-11-10
WO 97143273 PCTIUS97/08148
- 61 -
- (DMSO) ~i0.96 (d,3H), 2.45 (m, lH), 2.72-3.10 (m,6H), 4.25(t,2H), 4.80 (bt, lH),
5.92 (bs, lH), 6.60(d,1H), 6.79(d,2H), 7.05(d,1H), 7.17(s,1H), 7.30-7.50(5H); IR(KBr): 1652 cm-l(C=O), 17Sl cm~l (C=O); MS (CI) m/z 532 (MH+). Anal. Calcd.
for C26H26CISNO7: C, 58.70; H, 4.93; N, 2.63; S, 6.01; Cl, 5.98. Found: C, 54.17;
H, 4.44; N, 2.24; S, 5.13; Cl, 5.98.
EXAMPLE 73
5-{2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis 2-(Chloro)ethyl ester
The title compound was prepared from 5-12-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl } -benzo[1,3]dioxole-2,2-dicarboxylic acid and 2-chloroethanol
according to the procedure of Example 1 as yellow crystals (HCI salt); 1H NMR
(DMSO) ~1.13 (d, 3 H), 2.65 (m, lH), 3.15-3.52(m, 4H), 3.85 (t, 4 H), 4.60 (t, 4H), 5.55 (bd, I H), 6.40(bs,1H), 6.86(d,1H), 7.08(m,2H), 7.40-7.58(m,4H), 8.85
(bs, 1 H), 9.40 (bs, 1 H); IR (KBr): 1770 cm~l (C=O); MS (Cl) m/z 548 (MH+); [al25
-28~ (c, 1.0, DMSO). Anal. Calcd. for C24H26CI3NO7-HCI: C, 49.42; H, 4.67; N,
2.40;CI, 25.9. Found: C, 49.69; H, 4.38; N, 2.32; Cl, 23.20.
EXAMPLE 74
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylaminol-propyl}-
benzoll,3]dioxole-2,2-dicarboxylic acid bis-(2-ethylbutyl) ester
The title compound was prepared from 5-(2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino] -propyl ) -benzo[1,3]dioxole-2,2-dicarboxylic acid and 2-ethylbutanol
according to the procedure of Example I as an off-white gum (HCI salt); IH NMR
(CDC13~ ~ 0.80-0.95 (m, 12 H), 1.25-1.45 (m, 10 H), 1.50-1.65 (m, 3 H), 2.75-2.85
(m, lH), 3.10-3.40 (m, lH), 3.47-3.49 (m, 2 H), 4.25 (d, 5 H), 5.50 (d, 1 H), 6.70-
6.80 (m, 1 H), 6.80-6.90 (m, 2 H), 7.20-7.45 (m, 3 H), 7.46 (s, 1 H), 8.74 (bs, 1
H), 10.06 (bs, 1 H); IR (KBr): 2964 cm~l (-OH), 1766 cm-l (C=O); MS (ES) m/z
590.4 (MH+); [a]2D5 -21~ (c, 1.0, CHCl3). Anal. Calcd. for C32H44ClNO7-HCl: C,
61.34; H, 7.08; N, 2.24; Cl, 11.32. Found: C, 61.09; H, 7.46; N, 2.12; Cl, 10.77.
CA 02254120 1998-11-10
WO 97/43273 PCT/US97/08148
EXAMPLE 75
S-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-(3-methylbutyl)ester
S The title compound was prepared from 5-l2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl 1 -benzo[1,3]dioxole-2,2-dicarboxylic acid and 3-methylbutanol
accord-ing to the procedure of Example 1 as an off-white foam (HCl salt); lH NMR(CDC13) ~ 0.90 (d, 12 H), 1.35 (d, 2 H), 1.55-1.80 (m, 6H), 2.70-2.85 (m, lH),
3.45-3.55 (m, 3 H), 4.30 (t, 4 H), 5.45 (bd, 1 H), 5.65 (bs, 1 H), 6.70-6.80 (m, 1
H), 6.80-6.9() (m, 3 H), 7.25-7.35 (m, 3H), 7.43 (s, lH), 8.74 (bs, 1 H), 10.08 (bs,
1 H); IR (KBr): 3303 cm-l (-OH), 1765 cm-l (C=O); MS (ES) m/z 562.4 (MH+); [~325
-24~ (c, 1.0, CHC13). Anal. Calcd. for C30H40ClNO7-HCI: C, 60.20; H, 6.74; N,
2.34; Cl, 11.85. Found: C, 59.98; H, 7.04; N, 2.24; Cl, 12.09.
EXAMPLE 76
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
ben~o[l,3]dioxole-2,2-dicarboxylic acid (3-methylbutyl)ester
The title compound was prepared from 5-1(2R)-2-[(2R)-2-(3-chloro-phenyl)-2-
hydroxy-ethylamino]-propyl)-benzo[},3]dioxole-2,2-dicarboxylic acid bis-(3-methyl-
butyl)ester according tO the procedure of Example 22 as an off-white solid; lH NMR
(DMSO) ~ 0.80-0.85 (m, 6H), 1.02-1.09 (m, 3H), 1.45 (q, 2H), 1.55-1.70 (m, lH),
3.01-3.05 (m, 2H), 3.15-3.25 (m, lH), 3.26-3.40 (m, 2H), 4.15 (t, 2H), 5.00-5.08(m, lH), 6.45 (bs, lH), 6.55-6.65 (m, lH), 6.75-6.90 (m, 2H), 7.45-7.49 (m, SH),7.51 (s, lH); ~R (Kbr): 3394 cm~l (-OH), 1651 cm~l (C=O); MS (ES) rn/z 492.0
(MH+); 1~]2DS -22~ (c, 1.0, CHC13). Anal. Calcd. for C2sH30ClNO7: C, 61.04; H,
6.15; N, 2.85; Cl, 7.20. Found: C, 59.80; H, 6.10; N, 2.89; Cl, 7.10.
EXAMPLE 77
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-adamantan-l-ylmethyl ester
The title compound was prepared from 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl}-benzo[1,3]dioxole-2,2-dicarboxylic acid and a~ m~nt~n-l-
CA 022~4120 1998-11-10
W O 97/43273 PCT~US97/08148
- 63 -
ylm--th~nol according to the procedure of Example 1 as a white solid (HCI salt); lH
NMR (CDCl3) ~ 1.25 (m, 2H), 1.35 (d, 3 H), 1.51 (s, 12 H), 1.55-1.61 (m, 2H),
1.61-1.67 (m, 4 H), 1.67-1.75 (m, 6 H), 1.97 (s, 4 H), 3.20 (s, 2H), 3.42-3.49 tm,
2H), 3.88 (s, 4H), 4-11-4.16 (q, lH), 5.45 (bd, lH), 5.65 (bd, lH), 6.75 (m, lH),
56.78-6.89 (m, 2H), 7.23-7.30 (m, 3H), 7.44 (s, lH), 8.74 (bs, lH), 10.115 (bs,
lH); IR (KBr) 3418 cm~l (-OH), 1767 cm~1 (C=O); MS (ES) m/z 718.4 (MH+); ~o~]25
-15~ (c, 1.0, CHCl3). Anal. Calcd. for C42Hs2ClNO7: C, 68.94; H, 9.22; N, 1.90; Cl,
9.40. Found: C, 67.48; H, 7.49; N, 1.48; Cl, 8.10.
EXA MPLE 78
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino~-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-(2,2-dimethyl-propyl) ester
15The title compound was prepared from 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl ~-benzol 1,3]dioxole-2,2-dicarboxylic acid and 2,2-
dimethylpropanol according to the procedure of Fx~mple I as an off-white foam (HCl
salt); IH NMR (CDC13) ~ .924 (s, 18 H), 1.45 ~d, 2H), 1.75 (s, 2H), 2.80 (m, lH),
3.17 (m, lH), 3.45 (m, lH), 3.98 (s, 4H), 5.45-5.55 (bd, lH), 5.63-5.67 (bs, lH),
206.75-6.80 (m, 2H), 6.80-6.89 (m, 2H), 7.25-7.35 (m, 3H), 7.440 (s, lH), 8.75 (bs,
lH), 10.10 (bs, lH); IR (KBr) 3355 cm-l (-OH), 1767 cm-l (C=O); MS (ES) m/z
562.3 (MH+); [~12DS -23~ (c, 1.0, CHC13). Anal. Calcd. for C30H40ClNO7: C, 60.20;
H, 6.90; N, 2.34; Cl, 11.85. Found: C, 59.74; H, 6.97; N, 2.23; Cl, 11.60.
25EXAMPLE 79
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[1,3]dioxole-2,2-dicarboxylic acid adamantan-1-ylmethyl ester
The title compound was prepared from 5- ( (2R)-2-[(2R)-2-(3-chloro-phenyl)-2-
30hydroxy-ethylamino]-propyl ) -benzo[1,3]dioxole-2,2-dicarboxylic acid bis-~m~nt~n-
1-ylmethyl ester according to the procedure of Example 22 as an off-white solid; lH
NMR (DMSO) ~ 1.03-1.09 (m, 3H), 1.44 (s, 6 H), 1.45-1.64 (m, 8H), 1.86 (s, 4H),
3.02-3.15 (m, lH), 3.25-3.55 (m, 2H), 3.70-3.80 (m, 2H), 4.58 (s, 2H), 5.06-5.10(m, lH), 6.52-6.65 (m, lH), 6.79-6.88 (m, 2H), 7.32-7.49 (m, 3H), 7.49 ~s, lH);
CA 02254120 1998-ll-lO
WO 97/43273 PCT/US97/08148
- 64-
IR (KBr) 3384 cm~l (-OH), 1758 cm~l (C=O), 1651 cm~l (C=O); MS (ES) m/z 570.3
(MH+); [a]2D5 -29~ (c, 1.0, CHCl3). Anal. Calcd. for C31H36ClNO7: C, 65.31; H,
6.37; N, 2.46; Cl, 6.39. Found: C, 64.19; H, 6.20; N, 2.33; Cl, 6.33.
EXAMPLE 80
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl~-
benzo[l,3]dioxole-2,2-dicarboxylic acid (2,2-dimethyl-propyl)ester
The title compound was prepared from 5- { (2R)-2-[(2R)-2-(3-chloro-phenyl)-2-
hydroxy-ethylamino]-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid bis-(2,2-
dimethyl-propyl) ester according to the procedure of Example 22 as a white solid; IH
NMR (DMSO) ~ 0.868 (s, 9 H), 1.02-1.06 (m, 3H),3.00-3.02 (m, lH), 3.24-3.30
(m, lH), 3.35 (s, 5H), 3.80 (t, 2H), 4.55 (s, lH), 5.03 (m, lH), 6.52 (m, lH), 6.61
(m, lH), 6.79 (m, lH), 7.34 (m, 3H), 7.48 (s, lH); IR (KBr) 3398 cm-1 (-OH), 1748
cm-l (C=O), 1654 cm-1 (C=O); MS (ES) rn/z 492.3 (MH~; [o~l25 - 93~ (c, 1.0,
CHCl3). Anal. Calcd. for C2sH30ClNO7: Ct 61.04; H, 6.15; N, 2.85; Cl, 7.21.
Found: C, 56.96; H,5.65; N, 2.63; Cl, 7.05.
EXAMPLE 81
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]- propyl}-
benzo[1,3]dioxole-2,2-dicarboxylic acid (3,3-dimethylbutyl) ester
The title compound was prepared from 5- ~ (2R)-2-[(2R)-2-(3-chloro-phenyl)-2-
hydroxy-ethylamino]-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid bis-(3-methyl-
butyl)ester according to the procedure of Example 22 as an off-white solid; lH NMR
(DMSO) ~ 0.87 (s, 9 H), 1.04 (m, 3H), 1.50 (t, 2 H), 3.34 (s, 8H), 4.20 (t, 2H),4.95-5.05 (m, lH), 6.35 (m, lH), 6.60 (m, lH), 6.75 (m, lH), 7.34-7.46 (m, 3H),
7.48 (s, lH); IR (KBr) 3410 cm-l (-OH), 1745 cm-l (C=O), 1651 cm-l (C=O); MS
(ES) m/z 506.4 (MH+); Anal. Calcd. for C26H32CINO7: C, 61.72; H, 6.37; N, 2.77;
Cl, 7.01. Found: C, 60.69; H, 6.20; N, 2.63; Cl,7.06.
CA 02254120 1998-11-10
W O 97143273 PCTrUS97/08148
- 65 -
EXAMPLE 82
5-{(2R)-2-[(2R)-2-(3~Chloro~phenyl)-2-hydroxy-ethylamino]-propyl}-
~ ben~o[l,3]dioxole-2,2-dicarboxylic aicd bis-(l-methyl-
cyclohexylmethyl) ester.
s
The title compound was prepared from 5-~2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino] -propyl } -benzo[1,3]dioxole-2,2-dicarboxylic acid and 1 -methylcyclohexyl-
methanol according to the procedure of Example 1 as an off-white solid; IH NMR
(DMSO-d6,400MHz) ~ 0.84 (s, 6H, CH3, CH3), 1.09 (d, J = 6.4 Hz, 3H, CH3), 1.1-
1.4 (m, 20H, cyclohexyl), 2.6 (m, lH, CH), 3-3.3 (m, 3H, CH, CH2), 3.4 (brs, lH,CH), 4.04 (s, 4H, OCH2, OCH2), 5.05 (m, lH, CH), 6.35 (d, J = 4.17 Hz, lH,
OH), 6.85 (dd, J = 7.9, 1.32 Hz, lH, Ar-H), 7.07 (s, lH, Ar-H), 7.09 (d, J = 8.35
Hz, Ar-H), 7.38-7.42 (m, 3H, Ar-H), 7.5 (s, lH, Ar-H), 8.8 (brs, lH, NH), 9.4
(brs, lH, NH); IR (KBr, cm~~) 3400 (OH), 2900 (NH), 1760 (CO); MS mle 641
(M+); Analysis for: C36H48ClNO7xHCI: Calc'd: C, 63.71; H, 7.28; N, 2.06; Found: C,
63.72; H, 7.03; N, 1.91.
EXAMPLE 83
5-{(2R)-2-1(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3~dioxole-2,2-dicarboxylic aicd bis-(2-cyclohexyl-2-methyl-
propyl) ester.
The title compound was prepared from 5-(2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl } -benzo[1,3]dioxole-2,2-dicarboxylic acid and 2-cyclohexyl-2-
methyl~r~anol according to ~he procedure of Example 1 as an off-white solid; lH
NMR (DMSO-d6,400MHz) ~ 1.1 (s, 6H, CH3, CH3), 1.45 (s, 6H, CH3, CH3), 1.5
(s, 6H, CH3, CH3), 2,6 (m, lH, CH), 3-3.3 (m, 3H, CH, CH2), 3.4 (brs, lH, CH),
4.04 (s, 4H, OCH2, OCH2), 5.05 (m, lH, CH), 6.35 (d, J = 4.17 Hz, lH, OH), 6.85
(dd, J = 7.9, 1.32 Hz, lH, Ar-H), 7.07 (m, 2H, Ar-H), 7.38-7.42 (m, 3H, Ar-H),
7.5 (s, lH, Ar-H), 8.8 (brs, lH, NH), 9.4 (brs, lH, NH); IR (KBr, cm~l) 3400
(OH), 2900 (NH), 1760 (CO); MS mle 698 (M+H)+; Analysis for: C40Hs6ClNO7xHCl:
Calc'd: C, 65.38; H, 7.82; N, 1.91; Found: C, 65.32; H, 7.96; N, 1.94.
CA 02254l20 l998-ll-lO
WO 97143273 PCT/US97/08148
- 66-
EXAMPLE 84
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo~l,3]dioxole-2,2-dicarboxylic aicd bis-(2-methyl-2-nitro-propyl)
ester.
s
The title compound was prepared from 5-{2-[2-(3-ehloro-phenyl)-2-hydroxy-
ethylamino]-propyl } -benzo[1,3]dioxole-2,2-diearboxylie aeid and 2-methyl-2-
ni~ upanol according to the procedure of Example 1 as an off-white solid; IH NMR(DMSO-d6,400MHz) ~i 0.78 (s, 12H, 4 x CH3), 0.8-1.0 (m, 4H, CH2), 1- 1.2 (m,
llH, eyelohexyl, CH3), 1.5-1.65 (m, 10 H, cyclohexyl), 2.6 (m, lH, CH), 3-3.3 (m,
3H, CH, CH2), 3.4 (brs, lH, CH), 4.04 (s, 4H, OCH2, OCH2), 5.05 (m, lH, CH),
6.35 (d, J = 4.17 Hz, lH, OH), 6.83 (dd, J = 7.9, 1.32 Hz, lH, Ar-H), 7.06 (s lH,
Ar-H), 7.09 (d, J = 8.35 Hz), 7.38-7.42 (m, 3H, Ar-H), 7.5 (s, lH, Ar-H), 8.8 (brs,
lH, NH), 9.4 (brs, lH, NH); IR (KBr, cm~~) 3400 (OH), 2900 (NH), 1760 (CO);
Analysis for: C28H34ClN3O~IxHCl: Cale'd: C, 50.92; H, 5.34; N, 6.36; Found: C,
50.72; H, 5.42; N, 5.96.
EXAMPLE 85
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic aicd bis-(2,2,4-trimethyl-pentyl)
ester.
The title compound was prepared from 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl ~ -benzo[1,3]dioxole-2,2-diearboxylie aeid and 2,2,4-
~imethylpentanol aeeording to the proeedure of Example 1 as an off-white solid; IH
NMR (DMSO-d6,400MHz) ~ 0.65-0.9 (m, 24H, 8 x CH3), 1.09 (d, J = 6.4 Hz, 3H,
CH3), 1.1- 1.4 (m, 4H, CHz, CH2), 2.62 (m, IH, CH), 3-3.3 (m, 3H, CH, CH2), 3.4
(brs, lH, CH), 4.04 (s, 4H, OCH2, OCHz), 5.05 (m, lH, CH), 6.34 (d, J = 3.95 Hz,lH, OH), 6.85 (dd, J = 7.9, 1.32 Hz, lH, Ar-H), 7.07 (m, 2H, Ar-H), 7.38-7.42 (m,
3H, Ar-H), 7.5 (s, lH, Ar-H), 8.7 (brs, lH, NH), 9.1 (brs, lH, NH); IR (KBr, em~1~ 3300 (OH), 2900 (NH), 1760 (CO); MS m/e 646 (M+H)+; Analysis for:
C36HszClNO7xHCl: Calc'd: C, 63.33; H, 7.83; N, 2.05; Found: C, 62.96; H, 7.71; N,
2.44.
CA 02254l20 l998-ll-lO
WO 97/43273 PCT/US97/08148
- 67 -
EXAMPLE 86
5-{(2R)-2-[(2R~-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]~propyl}-
- benzo[1,3]dioxole-2,2-dicarboxylic aicd bis-(2,2-dimethyl-pentyl)
ester.
s
The tide compound was prepared from 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl)-benzo[1,3]dioxole-2,2-dicarboxylic acid and 2,2-
dimethylpentanol according to the procedure of Example 1 as an off-white solid; lH
NMR(DMSO-d6,400MHz)~0.8 (m, 18H, 6xCH3), 1.1-1.2 (m, llH, CH2CH3),
2.6 (m, lH, CH), 3-3.3 (m, 3H, CH, CH2), 3.4 (brs, lH, CH), 4.0 (s, 4H, OCH2,
OCH2), 5.09 (m, lH, CH), 6.36 (d, J= 4.17 Hz, lH, OH), 6.86 (dd, J= 7.9, 1.32
Hz, lH, Ar-H), 7.07 (s lH, Ar-H), 7.09 (d, J = 8.35 Hz, Ar-H), 7.35-7.42 (m, 3H,Ar-H), 7.48 (s, lH, Ar-H), 8.84 (brs, lH, NH), 9.48 (brs, lH, NH); IR (KBr, cm~l)
3300 (OH), 2900 (NH), 1760 (CO); MS mle 617 (M+); Analysis for:
C34H48ClNO7xHCl: Calc'd: C, 62.38; H, 7.55; N, 2.14; Found: C, 62.47; H, 7.51; N,
2.31.
EXAMPLE 87
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylaminol~propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic aicd bis-(tetrahydro-furan-3-
ylmethyl) ester.
The title compound was prepared from 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl ) -benzo[1,3]dioxole-2,2-dicarboxylic acid and tetrahydrofuran-3-
ylmethanol according to the procedure of Example 1 as an off-white solid; lH NMR(DMSO-d6,400MHz) ~ 1.03 (d, J = 6.37 Hz, 3H, CH3), 1.5 (m, 2H, CH2), 1.9 (m,
2H, CH2), 2.6 (m, 3H, CH), 3-3.3 (m, 3H, CH, CH2), 3.4 (brs, lH, CH), 3.6-3.7
(m, 6H, CH2), 4.1-4.15 (m, 6H, CH2), 5.09 (m, lH, CH), 6.36 (brs, lH, OH), 6.86
(m, lH, Ar-H), 7.07 (m, 2H, Ar-H), 7.38-7.42 (m, 3H, Ar-H), 7.47 (s, lH, Ar-H),
8.84 (brs, 2H, NH, NH); IR (KBr, cm~l) 3300 (OH~, 2900 (NH), 1760 (CO); MS
mle 590 (M+H)+; Analysis for: C30H36ClNO9xHCl: Calc'd: C, 57.51; H, 5.95; N,
2.24; Found: C, 57.80; H, 6.30; N, 2.21.
. . .. ~. ~ . .
CA 02254120 1998-ll-lO
WO 97/43273 PCT/US97/08148
- 68 -
EXAMPLE 88
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
ben~o~1,3]dioxole-2,2-dicarboxylic aicd bis-(3-hydroxy-2,2,4-
trimethyl-pentyl) ester.
The ti~e compound was prepared from 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl)-benzo[1,3~dioxole-2,2-dicarboxylic acid and cyclopropylmetl,allol
according to the procedure of Example 1 as an off-white solid; IH NMR (DMSO-
d6,400MHz) ~0.7-0.95 (m, 27H, 9 x CH3), 1.8 (m, lH, CH), 2.1 (m, lH, CH), 2.6
(m, lH, CH), 3-3.4 (m, 6H, OH, CH, CH2), 3.6-3.8 (brs, 2H, CH), 4.04 (m, 2H,
OCH2, OCH2), 4.6 (m, lH, CH), 4.85 (m, lH, CH), 5.05 (m, lH, CH), 6.35 (d, J =
3.7 Hz, lH, OH), 6.85 (dd, J = 7.9, 1.32 Hz, lH, Ar-H), 7.09 (m, 2H, Ar-H), 7.38-
7.42 (m, 3H, Ar-H), 7.5 (s, lH, Ar-H), 8.78 (brs, lH, NH), 9.2 (brs, lH, NH); IR(KBr, cm~l) 3400 (OH), 2900 (NH), 1750 (CO); MS mle 678 (M+H)~; Analysis for:
C36H52ClNO9xHCl: Calc'd: C, 60.50; H, 7.47; N, 1.95; Found: C, 60.30; H, 8.09; N,
1.62.
EXA MPLE 89
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic aicd bis-(2,2-dimethyl-3-phenyl-
propyl) ester.
The title compound was prepared from 5-(2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl l -benzo[1,3]dioxole-2,2-dicarboxylic acid and 2,2-dimethyl-3-
phenylpropanol according to the procedure of Example 1 as an off-white solid; lHNMR (DMSO-d6,400MHz) ~0.82 (s, 12H, 4 x CH3~, 1.08 (d, J = 6.37 Hz, 3H,
CH3), 2.6 (m, lH, CH), 3-3.3 (m, 3H, CH, CH2), 3.4 (brs, lH, CH), 3.96 (s, 4H,
OCH2, OCH2), 5.04 (m, lH, CH), 6.35 (d, J= 4.17 Hz, lH, OH), 6.89 (dd, J =
8.13, 1.53 Hz, lH, Ar-H), 7.05 (m, 4H, Ar-H), 7.14 (m, 8H, Ar-H), 7.38-7.42 (m,
3H, Ar-H), 7.5 (s, lH, Ar-H), 8.81 (brs, lH, NH), 9.38 (brs, lH, NH); IR (KBr,
cm~~) 3400 (OH), 2900 (NH), 1760 (CO); MS mle 714 (M+H)+; Analysis for:
C42H48ClNO7xHCI: Calc'd: C, 67.19; H, 6.58; N, 1.87; Found: C, 66.69; H, 6.53; N,
1.83.
CA 02254l20 l998-ll-lO
WO 97143273 PCT/US97/08148
- 69-
EXAMPLE 90
5~{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic aicd bis-(tetrahydro-pyran-2-
ylmethyl) ester.
s
The tide compound was prepared from 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl)-benzo~1,3]dioxole-2,2-dicarboxylic acid and tetrahydropyran-2-
ylmethanol according to the procedure of Example 1 as an off-white solid; IH NMR(DMSO-d6,400MHz) ~ 1.10 (d,J = 6.37 Hz, 3H, CH3), 1.1-1.2 (m, 2H, CH2), 1.3-
1.6 (m, 8H, CH2), 1.7-1.8 (m, 2H, CH2), 2.6 (m, lH, CH), 3-3.5 (m, 9H, CH,
CH2), 3.8 (m, 2H, CH), 4.19-4.2 (s, s, 4H, OCH~, OCH2), 5.04 (m, lH, CH), 6.35
(d, J = 4.17 Hz, IH, OH), 6.85 (d, J = 8.13 Hz, lH, Ar-H), 7.07 (m, 2H, Ar-H),
7.36-7.42 (m, 3H, Ar-H), 7.48 (s, lH, Ar-H), 8.76 (brs, lH, NH), 9.18 (brs, lH,
NH); IR (KBr, cm~l) 3400 (OH), 2900 (NH), 1760 (CO); MS m/e 618 (M+H)+;
Analysis for: C32H40ClNOgxHCI: Calc'd: C, 58.72; H, 6.31; N, 2.14; Found: C,
57.97; H, 6.46; N, 2.08.
EXAMPLE 91
5-{(2R)-2~[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic aicd bis-(tetrahydro-furan-2-
ylmethyl) ester.
The title compound was prepared from 5-(2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl ) -benzo[1,3]dioxole-2,2-dicarboxylic acid and tetrahydrofuran-2-
ylmethanol according to the procedure of Example 1 as an off-white solid; IH NMR(DMSO-d6,400MHz) ~ 1.1 (d, J = 6.37 Hz, 3H, CH3), 1.43-1.6 (m, 2H, CH2) 1.65-
1.8 (m, 4H, CH2), 1.98-2.0 (m, 2H, CH2), 2.6 (m, lH, CH), 3-3.3 (m, 3H, CH,
CH2), 3.4 (brs, lH, CH), 3.57-3.7 (m, 4H, CH2), 4.05 (m, 2H, CH2), 4.2 (m, 2H,
CH2), 4.3 (m, 2H, CH2), 5.04 (m, lH, CH), 6.34 (d, J = 3.95 Hz, lH, OH), 6.84
~ 30 (dd, J = 8.13, 1.32 Hz, lH, Ar-H), 7.07 (m, 2H, Ar-H), 7.36-7.44 (m, 3H, Ar-H~,
7.48 (s, lH, Ar-H), 8.77 (brs, lH, NH), 9.2 (brs, lH, NH); IR (KBr, cm~~) 3400
(OH), 2900 (NH), 1760 (CO); MS mle 590 (M+H)+; Analysis for: C30H36ClNO9xHCl:
Calc'd: C, 57.51; H, 5.95; N, 2.24; Found: C, 56.37; H, 5.90; N, 2.20.
CA 02254120 1998-11-10
WO ~7/43273 PCT/US97/08148
- 70 -
EXAMPLE 92
5~{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[1,3]dioxole-2,2-dicarboxylic aicd bis-(5-methyl-[1,3]dioxan-5-
ylmethyl) ester.
The title compound was l~r~ .,d from 5-~2-~2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl ~ -benzo[1,3]dioxole-2,2-dicarboxylic acid and S-methyl-
[1,3]dioxan-5-ylmethanol according to the procedure of Example 1 as an off-whitesolid; ~H NMR (DMSO-d6,400MHz) ~ 0.7 (s, 3H, CH3), 0.74 (s, 3H, CH3), 1.1 (d,
J = 6.59 Hz, 3H, CH3), 2.6 (m, lH, CH), 3-3.3 ~m, 8H, CH, CH2), 3.6-3.7 (m, 4H,
CH2), 4.6-4.8 (m, 4H, CH2), 5.04 (m, lH, CH), 6.34 (brs, lH, OH), 6.84 (dd, J=
8.13, 1.32 Hz, lH, Ar-H), 7.07 (m, 2H, Ar-H), 7.36-7.44 (m, 3H, Ar-H), 7.48 (s,
lH, Ar-H), 8.77 (brs, lH, NH), 9.2 (brs, lH, NH); IR (KBr, cm~l) 3400 (OH),
2900 (NH), 1760 (CO); MS mle 650 (M+H)+; Analysis for: C32H40ClNOllxHCl:
lS Calc'd: C, 55.98; H, 6.02; N, 2.04; Found: C, 54.67; H, 6.58; N, 2.05.
EXAMPLE 93
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,33dioxole-2,2-dicarboxylic aicd bis-(1-methyl-cyclohex-3-
enylmethyl) ester.
The title compound was prepared from 5-{2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl }-benzo[1,3]dioxole-2,2-dicarboxylic acid and 1-methylcyclohex-3-
enylmethanol according to the procedure of Example 1 as an off-white solid; ~H NMR
(DMSO-d6,4()0MHz) ~0.84 (s, 6H, 2 x CH3), 1.09 (d, J = 6.4 Hz, 3H, CH3), 1.25-
1.4 (m, 4H, CH2), 1.6-1.65 (m, 2H, CH2), 1.8-2.0 (m, 6H, CH2), 2.6 (m, lH, CH),
3-3.3 (m, 3H, CH, CH2), 3.4 (brs, lH, CH), 4.04 (m, 4H, OCH2, OCH2), 5.05 (m,
lH, CH), S.SS-5.65 (m, 4H, CH), 6.35 (d, J = 4.17 Hz, lH, OH), 6.85 (dd, J = 7.9,
1.32 Hz, lH, Ar-H), 7.07 (m, 2H, Ar-H), 7.38-7.42 (m, 3H, Ar-H), 7.5 (s, lH, Ar-H), 8.8 (brs, lH, NH), 9.4 (brs, lH, NH); IR (KBr, cm~~) 3400 (OH), 2900 (NH),
1760 (CO); MS mle 638 (M+H)+; Analysis for: C36H44ClNO7xHCI: Calc'd: C, 64.09;
H, 6.72; N, 2.08; Found: C, 64.69; H, 7.19; N, 1.67.
.
CA 022~4120 1998-11-10
WO 97143273 PCT/US97/08148
- 71 -
- EXAMPLE 94
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
~benzo[l,3]dioxole-2,2-dicarboxylic aicd bis-(3-hydroxy-2,2-dimethyl-
propyl) ester.
s
The title compound was prepared from 5-(2-[2-(3-chloro-phenyl)-2-hydroxy-
ethylamino]-propyl ) -benzo[1,3]dioxole-2,2-dicarboxylic acid 3-hydroxy-2,2-dimethyl-
propanol according to the procedure of Example 1 as an off-white solid; IH NMR
(DMSO-d6,400MHz) ~0.78 (s, 12H, 4 x CH3), 1.09 (d, J = 6.4 Hz, 3H, CH3), 2.6
(m, lH, CH), 3-3.3 (m, 7H, CH, CH2), 3.4 (brs, lH, CH), 4.04 (s, 4H, OCH2,
OCH2),4.65 (m, 2H, OH), 5.05 (m, lH, CH), 6.34 (d, J = 4.17 Hz, lH, OH), 6.85
(dd, J = 7.9, 1.53 Hz, lH, Ar-H), 7.07 (m, 2H, Ar-H), 7.37-7.42 (m, 3H, Ar-H),
7.48 (s, lH, Ar-H), 8.78 (brs, lH, NH), 9.2 (brs, lH, NH); IR (KBr, cm~l) 3400
(OH), 2900 (NH), 1760 (CO); MS mle 594 (M+H)+; Analysis for: C30H40ClNO9xHCl:
Calc'd: C, 57.14; H, 6.55; N, 2.22; Found: C, 56.44; H, 6.62; N, 2.38.
EXAMPI,E 95
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[1,3]dioxole-2,2-dicarboxylic acid (2-ethoxy-ethyl) ester
Through a stirred room te.llpc,.at-lre solution of 8.0 g (0.019 mole) 5-(2-[2-(3-
chloro-phenyl)-2-hydroxy-ethylamino]-propyl ) -benzo[1,3]dioxole-2,2-dicarboxylic
acid and 30 mL of 2-ethoxyethanol was bubbled HCl(g) for 5 min. After heating at 100
~~C for 12 h, TLC (9/1 CH2Cl2/MeOH) indicated the formation of both 5-12-[2-(3-
chloro-phenyl)-2-hydroxy-ethylamino]-propyl 3 -benzo[1,3]dioxole-2,2-dicarboxylic
acid bis-(2-ethoxy-ethyl) ester (R~ = 0.8; 9/1 CH2Cl2/MeOH) and 5-1(2R)-2-[(2R)-2-
(3-chloro-phenyl)-2-hydroxy-ethylamino]-propyl } -benzo[1,3]dioxole-2,2-dicarboxylic
acid (2-ethoxy-ethyl) ester (Rf = 0.2; 9/1 CH2Cl2/MeOH). After cooling to room
t~,..pel~ture, excess 2-ethoxyethanol was removed via Kugelrohr ~ 3tion (0.05 mm,
oven teml)eldture = 95 ~C), and the brown residue was chromatographed on silica gel,
eluting with 0 to 10% MeOH in CH2Cl2, to give 1.0 g (0.002 mole, a 10% yield) of the
title compound as an off-white solid; IH NMR (300 MHz, DMSO-d6): ~ 1.12 (m, J =
6.3 Hz, 3H), 2.5 (m, 3H) 3.35 (m, 8H), 5.06 (m, lH), 6.3 (s, lH, OH) 6.5 (m, lH),
6.6 (m, lH), 6.8 (m, 2H), 7.0 (m, lH), 7.5 (m, 2H), 8.0 (m, lH, NH), d 9.2 (m,
lH, COOH); MS (ES) m/z (relative intensity): 494 (M++H, 100).
CA 02254120 1998-ll-lO
WO 97143273 PCT/US97/08148
EXAMPLE 96
5-{(2R)-2- [(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-[2-(3-bromo-phenyl)-
ethyl]ester hydrochloride salt
The title compound was plepal~d as a brown oil from 5-(2-[2-(3-chloro-
phenyl)-2-hydroxy-ethylamino] -propyl } -benzo[1,3]dioxole-2,2-dicarboxylic acid and
2-(3-bromophenyl)ethanol according to the procedure of Example 1; IH NMR (300
MHz, CDCI3): ~ 1.12 (m, J = 6.3 Hz, 3H), 2.79 (m, 8H), 3.5 (m 2H), 4.18 (s, lH,
OH), 5.56 (m, lH), 6.8 (m, 3H), 7.0 (m, 4H), 7.33 (m, 4H), 7.5 (m, 4H), 8.5 (m,
lH, NH); MS (ES) m/z (relative intensity): 788 (M'+H, 100).
EXAMPLE 97
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dic~rboxylic acid bis-(2-m-tolyl-ethyl) ester
hydrochloride salt
The title compound was prepared as a brown oil from 5-{2-[2-(3-chloro-
phenyl)-2-hydroxy-ethylamino~-propyl ) -benzo[1,3]dioxole-2,2-dicarboxylic acid and
2-(3-methylphenyl)ethanol according to the procedure of Example l; yield: 81%; IH
NMR (300 MHz, CDCI3): ~ 1.12 (m, J = 6.3 Hz, 3H),2.27 (s, 6H), 2.79 (m, 7H),
3.19 (m, lH), 3.48 (m, 2H), 4.29 (m, J = 6.6 Hz, 4H), 5.49 (m, lH,) 6.75 (m, J =8.1 Hz, lH), 6.83 (m, lH), 7.05 (m, 8H), 7.20 (m, lH), 7.27 (m, lH), 7.43 (s, lH),
7.5 (m, 2H); MS (ES) m/z (relative intensity): 658 (M++H, 100).
CA 02254120 1998-11-10
WO 97/43273 PCT/US97/08148
EXAMPLE 9~
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid diallyl ester
Step I
(R,R)-5-{2-[[2-(3-Chloro-phenyl)-2-hydroxy-ethyl]-(2,2,2-trichloro-
ethoxycarbonyl)-amino]-propyl}-benzo[1,3]dioxole-2,2-dicarboxylic
acid diethyl ester
10To a 0 ~C solution of 3.0 g (5.83 mmol) (R,R)-5-~2-[2-(3-chloro-phenyl)-2-
hydroxy-ethylamino]-propyl } -benzo[1,3]dioxole-2,2-dicarboxylic acid diethyl ester
hydrochloride salt and 60 mL CH2CI2 was added 2.54 rnL (1.88 g, 14.57 rnmol) of i-
Pr2NEt followed by 0.84 mL (1.30 g, 6.12 mmol) of 2,2,2-trichloroethyl
chloroformate. After stirring at room temperature for 6 h, the reaction mixture was
15quenched with 10 mL sat. aq. NaHCO3, and extracted with 3 x 100 mL Et2O. The
combined organics were washed with I x 100 lN HCI, 1 x 100 mL brine, dried over
MgSO4, filtered and evaporated to a yellow oil. Flash chromatography on silica gel,
eluting with hexanes/EtOAc (4/1 to 2/1), gives 3.46 g (5.30 mrnol, a 91% yield) of the
title compound (R~ = 0.32; 2/1 hexanes/EtOAc) as an oily, off-white solid; IH NMR
20300 MHz, CDCI3): ~ 1.20-1.39 (complex m, 9H), 2.60-2.80 (m, lH), 2.80-2.91 (m,lH), 3.01-3.19 (m, lH), 3.23-3.31 (m, lH), 3.40-3.53 (m, lH), 4.10-4.23 (m, lH),4.30-4.42 (complex m, 4H), 4.70-4.89 (m, 3H), 6.65-6.88 (complex m, 4H), 7.15-
7.39 (complex m, 3H); MS (ES) m/z (relative intensity): 654 (M+ + H, 100).
25Step 2
5-{(2R)-2-[[(2R)-2-(tert-Butyl-dimethyl-siloxy)-2-(3-chloro-phenyl)-
ethyl~-(2,2,2-trichloro-ethoxycarbonyl)-amino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid diethyl ester
30To a -78 ~C solution of 3.75 g (5.47 mmol) (R,R)-5-~2-[[2-(3-chloro-phenyl)-
2-hydroxy-ethyl] -(2,2,2-trichloro-ethoxycarbonyl)-amino]-propyl ~ -benzo[1,3]dioxole-
2,2-dicarboxylic acid diethyl ester and 60 mL of CH2CI2 was added 1.27 mL (1.17 g,
10.94 mmol) of 2,6-lutidene followed by 1.38 mL (1.59 g, 6.03 mmol) of TBSOTf.
After stirring at -78 ~C for 1.5 h, the reaction mixture was quenched with 50 rnL sat.
aq. NaHCO3 and warmed to room temperature. After extraction with 3 x 150 mL Et2O,
CA 022~4120 lsss-ll-lo
WO 97/43273 PcTluss7/o8l48
- 74-
the combined organics were washed with 1 x 200 mL sat. CuSO4, 1 x 200 mL brine,
dried over MgSO4, filtered and evaporated to a cloudy white oil. Flash
chromatography on silica gel, eluting with hexanes/EtOAc (8/1 to 4/1) gave 3.42 g
(4.46 mmol, an 82% yield) of the title compound (Rf = 0.31; 4/1 hexanes/EtOAc) as a
5gummy colorless solid; IH NIVIR (300 MHz, CDCl3): ~ -0.13-(-0.08) (m, 3H), 0.00-
0.06 (m, 3H), 0.85-0.91 (m, 9H), 1.05 (d, J=6.7 Hz, 3H), 1.33 (t, J=7.1 Hz, 6H),2.60-2.75 (m, lH), 2.77-2.96 (m, lH), 3.00-3.31 (m, 2H), 3.77-4.00 (m, lH), 4.35(q, J=7.1 Hz, 4H), 4.65-4.88 (m, 2H), 5.02-5.20 (m, lH), 6.60-6.85 (m, 4H), 7.15-
7.37 (m, 3H); MS (ES) m/z (relative intensity): 790 (M++Na, 100), 768 (M++H, 20).
Step 3
5-{(2R)-2-[[(2R)-2-(tert-Butyl-dimethyl-siloxy)-2-(3-chloro-phenyl)-
ethyl]-(2,2,2-trichloro-ethoxycarbonyl)-amino]-propyl}-
benzo[l,3]dioxole-2,2-dic~rboxylic acid
To a room temperature solution of 3.31 g (4.31 mmol) 5-~(2R)-2-[~(2R)-2-
(tert-butyl-dimethyl-siloxy)-2-(3-chloro-phenyl)-ethyl]-(2,2,2-trichloro-
ethoxycarbonyl)-amino]-propyl~-benzo[1,3]dioxole-2,2-dicarboxylic acid diethyl ester,
100 mL MeOH and 25 mL H20 was added 4.31 mL (21.56 mmol) of a 5N NaOH
20solution. After stirring at room temperature for 80 h, the solvent was evaporated, and
the resulting slurry was acidifled to pH 1 with 1 N HCI. The reaction mixture isextracted with 3 x 100 mL EtOAc, and the combined organics were washed with 2 x 30
mL brine, dried over Na2SO4, filtered and evaporated to give 3.01 g (4.23 mmol, a
98% yield) of the title compound (R~ = 0.0; 10/1 CHCI3/MeOH) as an off-white solid;
25IH NMR (300 MHz, CDCl3): ~ -0.19-(-0.11) (m, 3H), 0.01 -0.09 (m, 3H), 0.81 -0.91
(m, 9H), 0.93-0.97 (m, 3H), 2.55-2.97 (complex m, 3H), 3.07-3.35 (m, 2H), 3.80-
4.40 (br m, 2H), 4.60-4.83 (m, 2H), 5.03-5.18 (m, lH), 6.60-6.92 (m, 4H), 7.15-
7.39 (m, 3H); MS (ES) m/z (relative intensity): 734 (Mt+Na, 30), 712 (M++H, 100).
CA 022~4120 1998-11-10
wo 97/43273 PCT/US97/08148
Step 4
5-{(2R)-2-[[(2R)-2-(tert-Butyl~dimethyl-siloxy)-2-(3-chloro-phenyl)-
- ethyll-(2,2,2-trichloro-ethoxycarbonyl)-amino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid diallyl ester
s
To a room temperature solution of 950 mg (1.34 mmol) 5-{(2R)-2-[[(2R)-2-
(tert-butyl-dimethyl-siloxy)-2-(3-chloro-phenyl)-ethyl]-(2,2,2-trichloro-
ethoxycarbonyl)-amino]-propyl } -benzo[1,3]dioxole-2,2-dicarboxylic acid and 15 rr~
DMF was added 267 mg (2.67 mmol) of KHCO3 and 0.26 mL (471 mg, 2.80 mrnol)
of allyl iodide. After stirring at room temperature for 16 h, the reaction was quenched
with 10 mL sat. aq. NaHCO3 and extracted with 3 x 100 mL Et2O. The combined
organics were washed with I x 150 mL brine, dried over MgSO4, filtered and
evaporated to a colorless oil. Flash chromatography on silica gel, eluting with
hex~n~c/EtOAc (8/1 to 4tl), gave 670 mg (0.85 mmol, a 63% yield) of the title
compound (R~ = 0.62; 2/1 hexaneslEtOAc) as a colorless oil; IH NMR (300 MHz,
CDCI3): ~-0.19-(-0.11) (m, 3H), 0.00-0.08 (m, 3H), 0.87 (s, 9~1), 1.04 (d, J=6.7Hz, 3H), 2.60-2.74 (m, lH), 2.77-2.98 (m, lH), 3.03-3.17 (m, lH), 3.18-3.30 (m,
lH), 3.75-3.98 (m, lH), 4.65-4.87 (m, 6H), 5.03-5.18 (m, lH), 5.29 (d, J=17.3 Hz,
2H), 5.34 (d, ~=23.7 Hz, 2H), 5.82-5.97 (complex m, 2H), 6.60-6.88 (m, 4H),
7.15-7.37 (m, 3H); MS (ES) m/z (relative intensity): 814 (M++Na, 100).
Step 5
5-{(2R)-2-1[(2R)~2-(3-Chloro-phenyl)-2-hydroxy-ethyl]-(2,2,2-
trichloro-ethoxycarbonyl)-amino]-propyl}-benzo[1,3]dioxole-2,2-
dicarboxylic acid diallyl ester
To a 0 ~C solution of 650 mg (0.82 mmol) 5-~(2R)-2-[[(2R)-2-(tert-butyl-
dimethyl-siloxy)-2-(3-chloro-phenyl)-ethyl]-(2,2,2-trichloro-ethoxycarbonyl)-amino]-
propyl~-benzo[1,31dioxole-2,2-dicarboxylic acid diallyl ester and 20 rnL THF was30 added 1.0 mL of HF-pyridine, and the reaction mixture was warmed to room
temperature. After 22 h, TLC indicated that some starting material remained (Rf = 0.62
(Vl hexanes/EtOAc), and an additional 1.0 mL of HF-pyridine was added. After a
total of 26 h, TLC indicated the disappearance of starting material and the formation of
the title compound (Rf = 0.28 (2/1 hexanes/EtOAc). The reaction mixture was slowly
quenched with 30 mL of sat. aq. NaHCO3, extracted with 2 x 75 mL Et2O and then
CA 022~4120 1998-11-10
WO 97/43273 PCT/US97/08148
- 76-
with 1 x 75 mL EtOAc. The combined organics were washed with 1 x 100 mL brine,
dried over MgSO4, filtered and evaporated to a colorless oil. Flash chromatography on
silica gel, eluting with hexanes/EtOAc (2/1), gave 460 mg (0.68 mmol, an 83% yield)
of the title compound (Rf = 0.28; 2/1 hexanes/EtOAc) as a colorless oil; lH NMR (300
S MHz, CDCl3): ~ 1.20-1.37 (m, 3H), 2.60-2.78 (m, 2H), 2.80-2.91 (m, lH), 2.98-
3.18 (m, lH), 3.23-3.40 (m, lH), 3.41-3.54 (m, 2H), 4.10-4.26 (br m, lH), 4.73-
4.92 (SH), 5.22-5.40 (complex m, 4H), 5.81-5.98 (complex m, 2H), 6.62-6.87 (m,
4H),7.17-7.41 (m, 3H); MS (ES) m/z (relative intensity): 700 (M++ Na, 100).
Step 6
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylaminol-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid diallyl ester
A mixture of 370 mg (0.55 mmol) 5-~(2R)-2-~[(2R)-2-(3-chloro-phenyl)-2-
hydroxy-ethyl]-(2,2,2-trichloro-ethoxycarbonyl)-amino] -propyl } -benzo[1,3]dioxole-
2,2-dicarboxylic acid diallyl ester,10 mL glacial acetic acid and 357 mg (5.46 mmol) of
Zn dust were stirred at room temperature for 40 h. The reaction mixture was filtered
through celite, poured into 50 rnL brine, and extracted with 3 x 50 mL EtOAc. The
combined organics were washed with 3 x 75 mL sat. NaHCO3,1 x 75 mL brine, dried
over Na2SO4, filtered and evaporated to a colorless oil. Flash chromatography on silica
gel, eluting with CHCl3/MeOH (20/1 to 10/1), gave 180 mg (0.36 mmol, a 66% yield)
of the title compound (Rf = 0.35; 10/1 CHCI3/MeOH) as a colorless gum; lH NMR
(300 MHz, CDCI3): o 1.20-1.33 (m, 3H), 2.70-2.81 (m, lH), 3.00-3.19 (m, 2H),
3.36-3.45 (m, 2H), 4.50-5.50 (br s, 2H), 4.70-4.80 (m, 4H), 5.10-5.20 (m, lH),
5.25-5.40 (m, 4H), 5.82-6.00 (complex m, 2H), 6.70-6.91 (m, 3H), 7.17-7.30 (m,
3H),7.41 (s, lH); MS (ES) m/z (relative intensity): 502 (M+,100).
CA 022~4120 1998-11-10
WO 97/43273 PCT/US97108148
~ EXAMPLE 99
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
- benzo[l ,3]dioxole-2,2-dicarboxylic acid bis-(3-phenyl-allyl)ester
Step 1
5-{(2R)-2-[[(2R)-2-(tert-Butyl-dimethyl-siloxy)-2-(3-chloro-phenyl)-
ethyll-(2,2,2-trichloro-ethoxycarbonyl)-amino]-propyl}-
benzo[l,3]dioxole-2,2-dicarboxylic acid bis-(3-phenyl-allyl) ester
The title compound was prepared as a colorless oil according to the procedure
of Example 4, Step 4 except that cinnamyl bromide was used in place of allyl iodide;
yield 66%; Rf = 0.68 (2/1 hexanes/EtOAc); IH NMR (300 MHz, CDCl3): ~ -0.19-(-
0.11) (m, 3H), -0.01-0.05 (m, 3H), 0.83-0.90 (m, 9H), 1.02 (d, J=6.7 Hz, 3H),
2.60-2.75 (m, IH), 2.77-2.96 (m, IH), 3.04-3.33 (m, 2H), 3.76-4.00 (m, lH), 4.65-
4.85 (m, 2H), 4.90-4.98 (m, 4H), 5.02-5.20 (m, lH), 6.20-6.33 (m, 2H), 6.60-6.85(m, 5H), 7.15-7.45 (m, 14H); MS (ES) m/z(relative intensity) 944 (M+, 100).
Step 2
5-{(2R3-2-[[(2R)2-(3-Chloro-phenyl)-2-hydroxy-ethyl]-(2,2,2-
trichloro-ethoxycarbonyl)-amino]-propyl}-benzoll,3]dioxole-2,2-
dicarboxylic acid bis-(3-phenyl-allyl) ester
The title compound was prepared as a colorless oil according to the procedure
of Example 4, Step 5 except that 5- { (2R)-2-[[(2R)-2-(tert-butyl-dimethyl-siloxy)-2-(3-
chloro-phenyl)-ethyl]-(2,2,2-trichloro-ethoxycarbonyl)-amino]-propyl } -
benzo[1,3]dioxole-2,2-dicarboxylic acid bis-(3-phenyl-allyl) ester was used in place of
5- ( (2R)-2-[~(2R)-2-(tert-butyl-dimethyl-siloxy)-2-(3-chloro-phenyl)-ethyl]-(2,2,2-
trichloro-ethoxycarbonyl)-amino]-propyl ) -benzo[1,3]dioxole-2,2-dicarboxylic acid
diallyl ester; yield 78%; R~ = 0.31 (2/1 hexanes/EtOAc); IH NMR (300 MHz, CDC13):
~ 1.20-1.35 (m, 3H), 2.58-2.80 (m, lH), 2.81-3.20 (m, 2H), 3.22-3.56 (m, 2H),
4.10-4.31 (m, 3H), 4.70-4.95 (m, SH), 6.17-6.43 (m, 2H), 6.60-6.90 (m, SH), 7.15-
7.45 (m, 14H).; MS (ES) m/z (relative intensity): 830 (M++H, 100).
CA 02254120 1998-11-10
WO 97143273 PCT/US97/08148
- 78 -
Step 3
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl}-
benzo[1,3]dioxole-2,2-dicarboxylic acid bis-(3-phenyl-allyl)ester
The title compound was prepared as a colorless oil according to the procedure
5of Example 4, Step 6 except that 5-{(2R)-2-[[(2R)2-(3-chloro-phenyl)-2-hydroxy-ethyl]-(2,2,2-trichloro-ethoxycarbonyl)-amino]-propyl ) -benzo[1,3]dioxole-2,2-
dicarboxylic acid bis-(3-phenyl-allyl) ester was used in place of 5- ~ (2R)-2-[~(2R)-2-(3-
chloro-phenyl)-2-hydroxy-ethyl]-(2,2,2-trichloro-ethoxycarbonyl)-amino]-propyl } -
benzo[1,3]dioxole-2,2-dicarboxylic acid diallyl ester; yield 59%; Rf = 0.35 (10/1
10CHCl3/MeOH); IH NMR (300 MHz, CDCl3): ~ 1.15 (d, J=6.3 Hz, 3H), 2.50-3.70
(brs, 2H), 2.60-2.71 (m, IH), 2.80-2.92 (m, 2H), 2.95-3.15 (m, 2H), 4.83-4.95 (m,
5H), 5.25-5.40 (m, 4H), 6.08-6.30 (complex m, 2H), 6.66-6.91 (m, 5H), 7.15-7.40
(m, 14H); MS (ES) m/z (relative intensity): 654 (M+, 100).
15EXAMPLE 100
5-{(2R) 2-~(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino~-propyl}-
benzo[1,3]dioxole-2,2-dicarboxylic acid dicyclooctyl ester
The title compound was prepared as a white gum from 5-(2-[2-(3-chloro-
20phenyl)-2-hydroxy-ethylamino]-propyl}-benzo[1,3]dioxole-2,2-dicarboxylic acid and
cyclooctanol according to the procedure of Example l; yield 84 %; Rf = 0.30 (10/1
CHCI3/MeOH); lH NMR (300 MHz, CDCI3): ~ 1.30-1.39 (brd, 3H), 1.40-1.92 (m,
28H), 2.72-2.~7 (m, lH), 3.06-3.30 (m, 2H), 3.39-3.52 (m, 2H), 3.80-3.91 (m,
lH), 5.02-5.13 (m, lH), 5.40-5.80 (m, lH), 6.71-6.89 (m, 4H), 7.21-7.37 (m, 2H),257.44 (s, lH), 8.73 (brs, lH), 10.11 (brs, lH); MS (ES) m/z (relative intensity). 679
(M+, 100).
EXAMPLE 101
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-propyl~-
30benzo[1,3]dioxole-2,2-dicarboxylic acid bis-(4-benzyloxy-but-2-
enyl)ester
To a room temperature solution of 110 mg (0.25 mmol) of 5-~2-[2-(3-chloro-
phenyl)-3-oxazolidinyl]-propyl }-benzo[1,3ldioxole-2,2-dicarboxylic acid and 5 rnL of
35DMF was added 69 mg (0.69 mmol) of K2CO3 followed by 195 mg (0.76 mmol) of
CA 02254120 1998-11-10
Wo 97/43273 PCT/US97/08148
- 79 -
~ cis-4-benzyloxy-2-buten-1-methane sulfonate. After heating at 50 ~C for 20 h, the
reaction mixture was cooled to room temperature, poured into 50 mL of brine and
- extracted with 2 x 50 mL EtOAc. The combined organics were washed with 1 x 50 n~
sat. aq. NaHCO3, 1 x 50 mL brine, dried over Na2SO4, filtered and evaporated to a
S yellow oil. Flash chromatography on silica gel, eluting with CHCI3/MeOH (40/1 to
10/1), gave 89 mg (0.12 mmol, a 47% yield) of the title compound (R~ = 0.30 (10/1
CHCI3/MeOH) as a yellow oil. IH NMR (300 MHz, CDCl3): ~ 0.98-1.10 (m, 3H),
2.44-2.78 (m, 3H), 2.82-3.00 (m, 2H), 4.11-4.20 (m, 4H), 4.40-4.70 (m, 2H), 4.85-
4.95 (m" 4H), 5.70-5.79 (m, 2H), 5.81-5.92 (m, 2H), 6.72-6.90 (m, 3H), 7.17-7.47(m, 14H); MS (ES) m/z (relative intensity): 742 (M+, 100).
EXAMPLE 102
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-
propyl}ben~o[l,3]dioxole-2,2-dicarboxylic acid phenethyl ester
To a 0 ~C solution of 0.630 g (1.0 mmol) of 5-1(2R)-2-[(2R)-2-(3-chloro-
phenyl)-2-hydroxy-ethylamino]-propyl ) -benzo~ 1,3]dioxole-2,2-dicarboxylic acid bis-
(2-phenethyl) ester in 12 mL CH3CN was added 5.6 mL 1 N NaOH (5.6 mmol), and
the resulting solution was stirred at room temperature for 12 h. The solution was
concentrated, 10 mL of water and 10 mL of ether were added, the pH of the rnixture
was adjusted to pH 8 with sat. aq. NH4Cl, and the resulting white precipitate was
collected by filtration. After washing with sat. aq. NaHCO3, water and ether, the
resulting solid was dried under vacuum to give 0.4 g (0.76 mmol, a 76% yield) of the
title compound (Rf = 0.39; 9/1 CHCI3/MeOH) as a tan solid; mp 75-82 ~C; ~H NMR
(300 MHz, DMSO-d6): â 1.00 (d, 3H), ~ 2.5 (br m, 4H), ~ 2.84-3.10 (m, SH),
3.35 (br, water), ~ 4.28 (t, 2H), ~ 4.90 (m, lH), ~ 6.6 (d, lH), ~ 6.76 (d, 2H), ~
7.21 (br s, 6H), ~ 7.36 (m, 3H), o 7.46 (s, lH); MS (ES) m/z (relative intensity): 526
(M+, 100).
CA 02254120 1998-11-10
WO 97/43273 PCT/US97/08148
- 80-
I~:XAMPLE 103
5 {(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-
propyl}benzo[l,3]dioxole-2,2-dicarboxylic acid (l-phenyl-ethyl) ester
S The title compound was prepared as a tan solid according to the procedure ofExample 102 from 5-~(2R)-2-[(2R)-2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-
propyl}benzo[1,3]dioxole-2,2-dicarboxylic acid bis-(l-phenylethyl) ester; yield: 22 %;
Rf = 0.39 (9/1 CHCI3/MeOH; mp 71-79 ~C; lH NMR (300 MHz, DMSO-d6): ~ 1.00
(d, 3H), ~ l.S (br m, 3H), ~ 2.4-2.75 (m, lH), o 3.15 -3.45(br m, SH), â 4.90 (br d,
10lH), ~ 5.20 (br d, lH), ~ 5.90 (q, lH), o 6.6 (d, lH), â 6.76 (d, 2H), o 7.21 (br s,
6H), ~ 7.36 (m, 3H), ~ 7.46 (s, lH); MS (ES) m/z (relative intensity): 526 (M+, 100).
EXAMPLE 104
5-{(2R)-2-[(2R)-2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-
15propyl}benzo~l,3]dioxole-2,2-dicarboxylic acid bis-(3-benzyloxy-
propyl)ester
The title compound was ~lepal~d as a yellow gum from 5-~2-[2-(3-chloro-phenyl)-2-hydroxy-ethylaminol-propyl ) -benzo[1,3]dioxole-2,2-dicarboxylic acid and
203-benzyloxy-propanol according to the procedure of Example 1; yield: 38 %; Rf = 0.52
(9/1 CHCI3/MeOH); 1H NMR (300 MHz, CDCI3): ~ 1.32 (d, 3H), o 2.00 (m, 4H),
2.75 (m, lH), ~ 3.30 (s, 2H), ~ 3.50 (m, 4H), o 4.40 (br m, 12H), ~ 5.49 (s, lH), ~
6.68-6.85 (m, 3H), ~ 7.24-7.40 (m, 13H), â 7.43 (s, lH); MS (ES) m/z (relative
intensity): 5l8 (M+-HCI, 100).