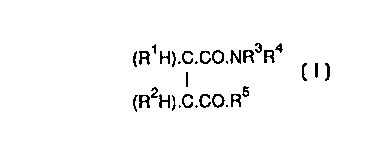Note: Descriptions are shown in the official language in which they were submitted.
CA 022~600 1998-11-18
WO 97/47199 PCT/GB97tO1484
- 1 -
Herbicidal Compositions
This invention relates to the use of certain surfactants as adjuvants in agrochemical formulations,
particularly herbicidal compositions using gylphosate and similar herbicides.
Our PCT Application PCT/GB 95/02785 (published as WO96/16930) relates to a class of
5 surfactants of the general formula [formula (I) in PCT/GB 95/02785j:
(R1 H).C.Co.NR3R
(R2H).C.Co.R5
where R1, R2 R3, R4 and R5 have defined meanings (generally one of R and R2 is an alkyl or
10 alkenyl group and the other is hydrogen, -NR R is a polyhydroxylhydrocarbylamino group,
particularly a glucamino group, and R is an -NR R group, a different amino group or a
polyoxyalkylene group which may include a substituent at the end of the chain). The application
describes various uses for these compounds, including as laundry d~tergenl~i, pigment dispersant
surfactants and as adjuvant surfactants in agrochemical formulations including as adjuvants in
15 herbicidal compositions based on glyphosate type herbicides.
In current practice, using glyphosate type herbicides, the application rate to ensure effectiveness
against difficult to kill weeds, particularly broad leaved weeds, is typically about 30 to 50% higher
than would be needed to kill general weeds. It would be very desirable to have an adjuvant that
would make such herbicides as effective against the difficult to kill weeds as they now are against
20 the relatively easier to kill weeds.
The present invention is based on our discovery that the su, r~ nts of our earlier PCT case can
perform as adjuvants to give enhanced effectiveness of glyphosate type herbicides against
weeds, particularly broad leaved and perennial weeds, that are generally relatively more difficult
to kill. In particular, this discovery applies to compounds of the where R is a (gluc)amino group
25 or a polyoxyalkylene group, corresponding to compounds of the sub-formulae (1 a) and (1 b) of
Application No PCT/GB 95/02785.
The present invention accordingly provides a method of killing weeds which are difficult to kill,
which comprises applying to the weeds a herbicidal composition including as active herbicide a
glyphosate type herbicide compound and as adjuvant a compound of the formula (I):
(R H).C.Co.NR3R4 (I)
(R H).C.CO.R where
one of R1 and R2 in the succinic acid moiety is C6 to C22 alkenyl or alkyl and the other is
hydrogen;
SUBSTITUTE SHEET (RULE 26)
CA 022~600 1998-ll-18
WO 97/47199 PCT/GB97/01484
- 2 -
R4 is hydrogen, C1 to C22 hydrocarbyl, particularly C1 to C20 alkyl;
R5 is a group: -NR3R4 where R3 and R are independently as defined above; or
R5 is a group: -O.(AO)n.R where: AO is an alkylene oxide, particularly an ethylene oxide,
residue;
5 n is 1 to 200; and
R6 is hydrogen, or C1 to C22 hydrocarbyl, particularly C1 to C6 alkyl.
The invention is specifically applicable to the use of compounds of the formula (la) or (Ib):
(R1 H).C.Co.NR3R4 (la)
1 0 (R2H).C.Co.NR3R4
or
(R1H).C.Co.NR3R4 (Ib)
(R2H).C.C O.O.(A O)n.R6
15 where R1, R2, R3, R4 and R5 are as defined above. A particularly advantageous sub-class of the
compounds of the formula (1 a) above, which have good compatibility with glyphosate type
herbicides in aqueous formulation, are compounds of the formula (la'):
(R11H).C.Co.NR13R14 (la')
(Rt2H).c.co NR13R14 where:
one of R11 and R12 in the succinic acid moiety is.C6 to C12 alkenyl or alkyl and the other is
hydrogen;
each group R13 is a glycosyl group, particularly glucosyl group; and
each group R is hydrogen, or a C1 to C4 alkyl group.
25 The surfactant adjuvant compounds used in this invention can be made by the methods described
in PCT/GB 95/02785. When made by such methods, compounds used in this invention are, as
described in that Ap~ ' on, typically mixtures of isomers corresponding to the two senses of
anhydride ring opening during synthesis.
The invention particularly includes a method of killing weeds which are difficult to kill, which
30 comprises applyi"g to the weeds a herbicidal composition including as active herbicide a
glyphosate type he~b;o ~e compound and as adjuvant a compound of the formula (la), particularly
formula (la'), and/or (Ib) as defined above. Specifically the invention includes a method of killing
weeds which are difficult to kill, which comprises applying to the weeds a herbicidal co~,,posillun
including as active herbicide a glyphosate amine salt, particularly glyphosate isopropylamino salt
35 ~N-phosphonomethylglycine isopropylamine salt), and as adjuvant a compound of the formula (la),
particularly formula (la'), and/or (Ib) as defined above
CA 022~600 1998-ll-18
WO 97/47199 PCT/GB97/01484
- 3 -
In referring to weeds as being difficult to kill we mean that on I-, ' ~~tion of glyphosate type
herbicides, the weeds either require a significantly higher ~rplicAtion of herbicide to kill them than
the generality of weeds or that they are not killed rapidly. In particular this applies to broad leaved,
dicotyledon weeds (annuals and perennials) and to grass (generally monocotyledon) perennial
5 weeds. Examples include the broad leaved (dicotyledon) weeds of the species: Chenopodivm
album, Solanum nigrum, Lactuca sallgna, A~,a,d~,tl~us retroflexus and Erigeron canadensis
(annuals) and Cirsum arvense (perennial) and similar related weeds from these genera and
perennial monocotyledon weeds of the species: Lolium perenne, Convolvulus arl~ensis and
Agropyron repens and similar related weeds from these genera. Among the grass weeds,
10 Agropyron repens is particularly recognised as being difficult to kill.
The term glyphosate type herbicide refers to N-phosphonylmethylglycine herbicides particularly
herbicide compounds of the formula:
MO . C(O) . CH2 . N(Z) . CH2 . P(O) (OM)2
where M, and Z (as z11) are as defined in PCT Application No PCT/GB 95/02785. In particular,
15 such compounds where Z is hydrogen are very desirable for use as herL. ~ :~'es and thus the
compound HO.C(O).CH2.NH.CH2.P(O).(OH)2 and its salts with agriculturally accepl ~le
salt-forming species are especially useful in the invention. Suitable salt-forming species include
alkaii metal, particularly sodium, potassium, or rubidium; alkaline earth metal particularly
magnesium or calcium; ammonium; primary, secondary, tertiary or quaternary aliphatic
20 ammonium, preferably where the total number of carbon atoms is not more than about twelve;
trialkylsulphonium, preferably where the total number of carbon atoms is not more than about six,
such as trimethylsulphonium, ethyl dimethylsulphonium and propyl dimethylsulphonium.
In eSpeci~lly useful compounds, each M is hydrogen, alkali metal, ammonium, monoalkyl
a~"~,onium or trialkylsulphonium moiety. In the most preferred compounds, one M is an alkali
25 metal, ammonium, monoalkyl a",l"on.um, or trialkylsulphonium, and two are hydrogen. Examples
of especially preferred compounds include isopropylamine N-phosphonomethylglycine,
trimethylsulphonium N-phosphonomethylglycine and sodium sesqui-N-phosphonomethyl-glycine
Combinations of two or more such compounds can be used in the present invention.The mechanism by which these surfactants are es~eci~lly effective against difficult to kill weeds is
30 not certain, but is seems that either the surfactant forms lamellar and/or liquid crystalline phases or
structures in the coatings on the leaves of the weeds enhancing rel~ntion of the active component
on the leaf surface, or the adjuvant enhances absorption of the active component into the leaves of
the weed (the structures referred to above may assist in this), or the surfactant enhances
translocation of the active herbicide within the weed after absorption. Whatever the mechanism,
35 the effect seems to be substantial and is seen, at equal rates of application of active herbicide in an
increase in the speed of killing weeds, especially difficult to kill weeds and/or that weeds, especi~lly
difficult to kill weeds, are killed with lower application rates of the active herbicide than would
CA 022~600 1998-11-18
WO 97/47199 PCT/GB97/01484
- 4 -
otherwise be needed, in particular reducing effective application rates to those typical for effective
control of weeds that are not difficult to kill. In addition to enhanced effectiveness and/or speed of
action, particularly in killing difficult to kill weeds, the adjuvants used in the present invention may
also reduce the effect of seed spread from treated weeds, and/or provide improved rain fastness of
5 the applied herbicides.
The invention is particularly ~plic~hle to the use of glyphosate itself (N-phospl1onomethylglycine
isopropylamine salt) as the surfactants used in the invention are especially effective with
glyphosate. Indeed, using glyphosate as the active herbicide with adjuvants used in this invention
can give formulations which are more effective, particularly in their effectiveness at lower
10 concentrations and/or in their speed of action, than otherwise similar formulations made using the
closely related herbicide sulphosate, which has the same core acid active entity but as the
dimethylsulphate salt. As is noted above this use of glyphosate (N-phosphonomethylglycine
isopropylamine salt) forms a specific aspect of the invention.
The enhanced herbicidal effect arising from the use of compounds of the formula (I) is particularly
15 noticeable against broad leaved (dicotyledon) weeds especially of the species: Chenopodium
album, Solanum nigrum, Lact~ca saligna, Amaran~hus retroflexus and Erigeron canadensis
(annuals) and Cirsum arvense (perennial) and similar related weeds from these genera and
perennial (generally monocotyledon) weeds of the species: Lolium perenne, Convolvulus arvensis
and Agropyron repens and similar related weeds from these genera. Good and enhanced effects
20 have also been observed against grass (monocotyledon) annual weeds for example of the species:
Sorghum halepense and similar related weeds indicating the broad spectrum activity of
formulations including the adjuvants used in this invention
Accordi, Igly, the invention particularly includes a method of killing a weed selected from
Chenopodium album, Solanum nigrum, Lactuca saligna, Amaranthus re~roflexus, Erigeron
25 canadensis and Cirsum arvense and/or a weed selected from Lolium perenne, Convolvulus
arvensis and Agropyron repens which comprises applying to the weeds a herbicidal composition
including as active herbicide a glyphosate type herbicide compound, particularlyN-phosphonomethylglyCine isopropylamine salt, and as adjuvant a compound of the formula (la) or
(Ib) above, specifically and in particular an adjuvant of the formula (la') above.
30 The amounts of the materials used will generally be within the broad ranges typically used in the art
with similar materiais. I loJ~vor, as the compositions used in this invention can be more effective,
it may be possible to reduce the amount or concentration of the active herbicide as compared with
conventional formulations. Considered as propo~lions of spray compositions (so-called 'tank
mixes'~ at current spray volume ~pplic~tion rates of about 300 I.ha the concentration of active
35 herbicide will typically be from about 0.05 to about 3%, more usually from about 0.1 to about 0.5
-
CA 022~600 lsss-ll-ls
WO 97/47199 PCT/GB97/01484
- 5 -
and particularly about 0.2%, and the concentration of adjuvant will typically be from about 0.02 to
about 2% more usually from about 0.2 to about 1% and particularly about 0.1%. The weight ratio
of active herb.~:do to adjuvant surfactant will typically be from about 1 :5 to about 10:1, more usually
about 1:2 to about 4:1 and particulariy about 2:1. Where low volume spraying methods are used,
5 the concent~dtions will typically be co"eslJon l; )gly higher, but the ratio of active herbicide to
adjuvant will generally remain within the stated ratios. Thus for low volume spraying at formulation
application rates of 20 to 50 I.ha , the concentration of active herbicide will typically be from
about 0.3 to about 30%, more usually from about 0.5 to about 15 and particularly about 5%, and
the concentration of adjuvant will typically be from about 0.1 to about 15% more usually from
10 about 0.5 to about 10% and particularly about 7%, with ratios typically corresponding to the above
ranges. Expressed as amounts of the components applied to unit area of field, the active herbicide
will typically be applied at a rate of about 300 to about 4000 g.ha 1, more usually about 750 to
about 2000 g.ha , and particularly about 1000 to about 1500 g.ha , and correspondingly, the
amount of adjuvant surfactant applied will typically be from about 150 to about 4000 g.ha~1, more
15 usually about 500 to about 2000 g.ha~1.
The adiuvant surfactants, especially those of the formula (1 a'), used in this invention have good
compatibility with glyphosate type herbicides, they can be formulated into herbicidal concentrates
for dilution to tank mixes. In such formulations, the active herbicide will typically be at a
concentration of from about 5 to about 60%, more. usually from about 10 to about 40%, and the
~n adjuvant surfactant, particularly an adjuvant surfactant of the formula (1 a'), at a concentration of
from aboùt 3 to about 50%, more usually from about 5 to about 30%, by weight of the concentrate.
The adjuvants used in this invention can be incorporated into aqueous herbicide formulations as
such. However, we have found that the use of accessories or co-solvents can usefully improve the
solubility of the adjuvants as used with the glyphosate herbicides. Suitable accessories and
25 co-solvents are generally water soluble and/or miscible organic solvents for the adjuvant
surfactants and include glycols, such as ethylene glycol and particularly propylene glycol (so-called
'monopropylene glycol'), low molecular weight polyglycol ethers and their mono alkyl ethers such
as di- and tri-ethylene glycol, di- and tri-propylene glycol and their C1 to C4 mono alkyl ethers and
dimethyl isosorbide. When used the proportion of accessory or co-solvent will typically be from
30 about 10 to about 150%, more usually from about 25 to about 75% by weight of the adjuvant used.
CA 0225~i600 1998-11-18
WO 97/47199 PCT/GB97/01484
~ 6 ~
The adjuvant surfactants used in this invention have relatively low toxicity and are readily
biodegradable. In addition, in combination with the glyphosate type herbicide in aqueous
formulation, the adjuvant surfactants are much less susceptible to foaming than the alkyi
polyglycoside surfactants that have been suggested for use, particulariy with sulfosate
5 (N-phosphonomethyl glycine trimethylsulphonium salt).
The following Examples illustrate the invention. All parts and percentages are by weight unless
otherwise stated.
Materials
MPG mono-propylene glycol
10 GAS technical grade N-phosphonomethyl glycine as the isopropylamine salt in aqueous
solution (nominally 62% active salt)
GSS technical grade N-phosphonomethyl glycine as the trimethylsulphonium salt in
aqueous solution (nominally 52% active salt)
ETA conventional ethoxylated tallow amine adjuvant (95+% active)
15 APS alkyl polysaccharide adjuvant composition from ICI (about 75% APS)
water the water used to make up the spray formulations used in the Examples had a
standard hardness of 342 ppm.
glucamide adjuvants:
Active Material
Form.No
AF1 70% tetradecenyl succinic acid glucamide PEG 600 ester + 30% MPG
AF2 70% octadecenyl succinic acid glucamide PEG 200 ester + 30% MPG
AF3 70% tetradecenyl succinic acid glucamide PEG 600 ester + 30% MPG
AF4 70% octenyl succinic acid glucamide PEG 600 ester t 30% MPG
AF5 50% decenyl succinic acid diglucamide + 50% MPG
AF6 decenyl succinic acid diglucamide (100%)
AF7 50% octenyl succinic acid diglucamide + 50O/o MPG
AF8 octenyl succinic acid diglucamide (100%)
AF9 50% dodecenyl succinic acid diglucamide + 50% MPG
notes: in the glucamide adjuvants listed above, glucamide groups are -N(methyl)(glucosyl)
groups and PEG groups are polyethylene glycolyl groups of the stated approximatemolecular weight.
Example 1
Aqueous herbicide formulations were made up using GAS and GSS as the active agents and using
various adjuvants. The formulations were made up as aqueous solutions containing:
for giyphosate formu!ations: for sulphosate formulations:
material amount material amount
CA 022~600 l998-ll-l8
Wo 97/47199 PCTtGB97/01484
- 7 -
GAS 7.3 ml.l~1 GSS 6.7 ml.l~
adjuvant 1.8 ml.l adjuvant 2.0 ml.1~
composition composition
water remainder water remainder
The formulations were used in a test spraying programme in which the formulation was sprayed at
an application rate of 3001.ha (thus providing 2.2 I.ha GAS, equivalent to 1276 g.ha 1 active salt
and 2.0 I.ha GSS. equivalent to 1137 g.ha active salt) onto field test plots containing growing
plants of the weeds Erigeron canadensis (ERICA) and Lactuca saligna (LACSA) using 4
5 replications. The percentage of weed plants killed by the herbicide was ~sessed after 7, 14 and
28 days and was reported as the % efficacy. The formulations used and the weed control results
obtained are summarised in Table 1 below.
Table 1
Ex Formulation Efficacy (%)
No Active Adjuvant ERICA LACSA
7 14 28 7 14 28
C1 a GAS none 57 93 100 42 86 98
C1 b ETA 59 95 100 42 92 99
1 a AF1 87 99 100 69 97 100
1 b AF2 64 98 100 42 92 99
1 c AF3 81 98 100 67 92 99
C1 d GSS none 50 92 99 41 92 98
C1 e AL 2042 80 98 1 00 62 93 99
C1 f ETA 77 97 100 69 94 99
1 d AF1 52 98 1 00 39 93 99
1 e AF3 56 98 100 38 94 99
Exam~le 2
10 Aqueous herbicide formulations were made up using GAS and GSS as the active agents and using
various adjuvants at the concentrations used in Example 1. The formulations were used in a test
spraying programme in which the formulation was sprayed, at the rate described in Example 1,
onto greenhouse test plots containing growing plants of the weeds Chenopodium al~vm (CHEAL),
Sorghum halepense (SORHA) and Amaranthvs retroflexus (AMARE) using 4 replications. The
15 percentage of weed plants killed by the herbicide was assessed after 7. 14 and 28 days and was
reported as the % efficacy. The formulations used and the weed control results obtained are
summarised in Table 2 below.
Table 2
CA 022~600 1998-11-18
WO 97147199 PCTIGB97/01484
- 8 -
Ex Formulation Efficacy (%)
No Active Adjuvant CHEAL SORHA AMARE
7 14 28 7 14 28 7 14 28
C2a GAS none 34 50 85 60 80 99 50 80 100
C2b ETA 65 70 99 90 90 100 82 100 100
2a AF4 98 99 100 99 100 100 99 100 100
2b AF1 98 99 100 99 100 100 99 1 00 100
2c AF2 92 97 100 94 99 100 98 100 100
2d AF3 93 98 100 95 99 1 00 99 100 100
C2c GSS none 41 90 98 58 90 100 60 93 1 00
C2d AL 2042 93 99 100 95 100 100 97 100 100
2e AF1 93 99 100 96 100 100 98 100 100
Example 3
Example 2 was repeated using different adjuvants (and controls) and the formulations and results
are summarised in Table 3 below.
Table 3
Ex Formulation Efficacy (%)
No Active Adjuvant CHEALSORHA AMARE
7 14 28 7 14 28 7 14 28
C3a GAS none 34 50 85 60 80 99 50 80 100
C3b ETA 65 70 98 90 90 100 82 100 100
3a AF5 91 99 100 93 100 1 00 94 100 1 00
3b AF6 94 98 100 98 1 00 100 97 100 100
3c AF7 90 96 100 94 99 100 92 100 100
3d AF8 93 99 100 95 100 100 96 100 100
3e AF9 89 99 100 92 100 100 95 100 100
C3c GSS none 41 90 98 58 90 100 60 93 100
3f AL 2042 93 99 100 95 1 00 100 97 100 100
3g AF9 90 98 100 95 99 1 00 97 100 100
3h AF8 90 98 100 95 99 100 97 100 100
Examole 4
Example 1 was repeated using adjuvant AF5 (and controls) and the formulations and results are
summarised in Table 4 below.
Table 4
CA 02255600 1998-ll-18
WO 97/47199 PCT/GB97/01484
g
Ex Formulation Efficacy (%)
No Active Adjuvant ERICA MATIN CIRAR
7 14 28 7 14 28 7 14 2B
C4a GAS none 67 81 94 76 100 100 63 100 100
C4b ETA 71 82 95 90 100 100 67 100 100
4a AF5 80 92 99 94 100 100 77 100 100
C4c GSS none 66 77 90 72 100 100 49 100 100
C4d AL 2042 80 91 99 88 100 100 75 100 100
4b AF5 71 77 96 82 100 100 51 100 100