Note: Descriptions are shown in the official language in which they were submitted.
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97/00936
I
Cytostatic sterols
The present invention relates to the use of cholesterol derivatives with an oxo group
- and a conjugated double bond in the steroid nucleus and a hydroxyl group in the
s side-chain as inhibitors of cell growth and/or as inducers of death in abnormal cells.
International patent application no. WO 96/28175 describes the use of the
compound 27 hydroxycholesterol:
rOH
HO~J
0 as an anticancer agent, especially against metastatic liver cancer, colon cancer and
breast cancer. As with native cholesterol this compound has an unsaturated bond at
~5, that is between the 5 and 6 carbons in the steroid B-ring. However, Zander et al
in J Chem Research (S)1977 219 suggests tha~ the corresponding 25-hydroxylated
~5 analogue does not possess cytotoxic activity in an hepatocyte assay.
s WO 96/28175 broadly suggests certain analogues of this compound such as 25, 26
or 27 aminocholesterol, but does not disclose any ~4 derivatives, that is compounds
with an unsaturated bond between the 4 and 5 carbons in the steroid A-ring.
Other ~5 cholesterols which have shown some cytostatic activity include the
compound 7~-hydroxycholesterol with the formula:
,. ~
HO~OH
which was first described as a component of the Chinese drug Bombyx cum botryte,a fungal infestation of silk worm, as described by Kwok-Ping Cheng et al, J ChemResearch ~S) 1977, 217 and Hietter et al, Cancer Biochem Biophys 1986 vol 9,
CA 022~677 1998-11-19
wo 97/45440 PCT/SE97J00936
pp75-83. International patent application no. WO 91 11452 describes glycosylation
of such compounds at the 3~.-hydroxy and Rong et al C. R. Acad. Sc. Paris 1985,
300 Serie III, 89-94 describes the corresponding bis-hemisuccinate.
Moog et al in Biochimie 1991 73 (10) pp 1321-6 investigate the immunosuppressiveeffects of the ~5 compound 7,25-dihydroxycholesterol:
~ OH
HO~OH
which strongly inhibits Iymphocyte response to different stimuli. Activity appeared
0 to be related to protein kinase C and the incorporation of the dihydroxycholesterol
into the cell membrane. Apparently the free ~5 oxysterols do not depress tumour
growth in living ~nim~ls but Moog et al in Anticancer Res 13 (4) 953-8 (1993) and
Ji, Moog et al, Canc. Biochem. Biophys 11 (1) 45-47 (1990) report that the
phosphate diester of 7,B,25-dihydroxycho]esterol with a pyrimidine nucleoside iss usable in a cytotoxic role. However Ji's observation that similar nuc}eoside-
cholesterol esters act at the level of nucleic acid synthesis make the contribution to
cytotoxicity played by the steroid viz a viz the nucleoside difficult to determine.
Analogues of the ~5 compound 7~-hydroxycholesterol with branched, unsaturated
side chains are implicated in rat hepatocyte toxicity at high (33 llg/ml)
concentrations by Nagano et al, J Chem Res (S) 1977, 218, as are certain
hydroxylated, unsaturated side chains in ~5 analogues lacking the 7-hydroxy group.
This work also describes the ~4 compound:
CA 02255677 1998-11-19
W O 97/45440 PCTISE97/00936
HO~J
where the double bonds are in different rings of the sterol possessed modest
cytostatic activity at the relatively high concentration of 33 ~g/ml.
s We have now discovered that compounds with a 3-keto group and conjugated
double bond at ~4 in conjunction with an hydroxylated side chain have cytostaticactivities which are many times more potent than the compounds of the prior art.
In accordance with a first aspect of the invention there is provided compounds of the
0 forrnula I:
X
~~
O~ OH
wherein X is a straight or branched, hydroxy substituted Cl-Cl~ hydrocarbon chain,
for use in medicine.
1S A preferred group of compounds within the scope of the invention has the formula
IA:
~'~,~ R"
:~/
O~OH
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97/00936
wherein one of R' and R"is OH and the other is H. Particularly preferred
compounds of Formula I include 7~,25-dihydroxy-4-cholesten-3-one, 7a,25-
dihydroxy-4-cholesten-3-one and 7~,27-dihydroxy-4-cholesten-3-one.
s The enantiomer 7~B,25-dihydroxy-4-cholesten-3-one does not appear to have beenpreviously described in the literature and thus a further aspect of the invention
provides this compound, preferably in substantially pure form, for instance > 75%,
preferably > 90% and most preferably > 95% enantiomerically pure.
0 The invention further provides pharmaceutical compositions comprising the
compounds of the invention, preferably those within Formula IA, in admixture with
a physiologically acceptable diluent or pharmaceutical carrier.
Advantageous aspects of the invention include the use of the above group of
5 compounds in the preparation of a medicament for the treatment of conditions
associated with rapidly growing cells, such as various cancers and virus transformed
mammalian cells. Representative cancers (which may or may not be virally
transformed) include sarcomas, such as soft tissue sarcoma~ myeloproliferative
tumours such as leukaemia, glioblastoma, pancreatic, ovarian and adenocystic
20 cancers. A further area of application is psoriasis, a disorder in which there is a loss
of control of normal epidermal turnover. Increased mitosis of epidermal cells results
in thickening of the epidermis and the production of imperfect keratin scales.
Thus the invention also provides a method for the treatment of conditions associated
25 with rapidly growing cells comprising the ~lmini.ctration of an effective amount of a
compound of formula I, or more preferably Formula IA to a human or animal in
need thereof.
A preferred aspect of the invention provides the above compounds for use in the
30 preparation of a medicament or in methods for the treatment of breast carcinoma or
colonic carcinoma in a human or animal. A particularly preferred aspect of the
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97/00936
invention provides use of the above groups of compounds for use in the preparation
of a medicament or in methods for the treatment of malignant melanoma cells.
The compounds of the invention include the corresponding pharmaceutically
s accceptable derivatives as are known in the steroid art and which release the
respective compound of Formula I in vivo. Suitable derivatives include ethers and
esters of the hydroxy groups and/or derivatives of the oxo group. Representativeesters and ethers include glycosides, such as the hexoses described in the
abovementioned WO 91 11452, and bis-hemisuccinates, phosphates, glycoside
o phosphates, silyl ethers, acetate, formate and other fatty esters, such as the oleate,
glucuronides, phosphodiesters, cyclodextrins, such as 2-hydroxy-,~-cyclodextrin and
the like as are known in the steroid art. Other derivatives include the corresponding
oximes and pharmaceutically acceptable salts.
As shown in greater detail below, the compounds of the invention are subject to
intracellular metabolism and such active metabolites are within the scope of theinvention. For instance, 7,25-dihydroxycholesl-4-en-3-ones may be metabolized tothe corresponding cholestenoic acids. The administration to a mamal, including
humans, of such active metabolites for the indications specified above is thus to be
regarded as an aspect of the invention.
The nature of the pharmaceutical preparation of the invention will depend on thedisease being treated and the examples below are not intended to be limiting.
For oral administration, the compounds can be formulated into solid or liquid
preparations such as capsules, pills, tablets, troches, powders, solution, suspensions,
or emulsions.
For parenteral administration, the compounds may be administered as injectable
- 30 dosages of a solution, suspension or emulsion of the compound in a physiologically
acceptable diluent with a pharmaceutical carrier which can be a sterile liquid such as
, . . . . .
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97/00936
water, alcohols, oils and other acceptable organic solvents, with or without theaddition of a surfactant and other ph~ ceutically acceptable adjuvants.
The compounds can also be a~lmini.ctered in the form of a depot injection or implant
5 preparation which may be formulated in such manner as to permit a sustained
release of the active ingredient.
For topical application the compounds can be a-~ministered in the form of an
unguent, cream, ointment, or a lotion.
The invention will be further described below by way of example only with
reference to the enclosed Figures, in which:
Fig. I outlines a simplified scheme of the metabolism of LDL (low density
5 lipoprotein) cholesterol and major autooxidation products of fibroblasts and the
formation of potent HMG-CoA reductase suppressors. The names of the steroids arelisted in Table I. In addition to hydrolysis and esterification (not shown) major
reactions of sterols are I, 27-hydroxylation, II, 7a-hydroxylation; III, 3-oxidation
with isomerization of the 5-double bond; and IV, oxidation to a 27-carboxy group.
20 Hydrolyzed LDL cholesterol is metabolized via these reactions (filled arrows).
Oxidation of a 7~-hydroxy group (V) to a ketone is also observed. Minor reactions
noted are shown by broken arrows. The formation of 7cl,25-dihydroxy-4-cholesten-3-one by 25-hydroxylation of sterols (not shown) was observed under specific
conditions. Reactions I, II and III were obstructed in virus-transformed fibroblasts
25 displaying a defective suppression of HMG-CoA reductase by LDL cholesterol and
autooxidation products of cholesterol. Sterol metabolites with an apparently normal
suppressive effect also in transformed cells are indicated in frame.
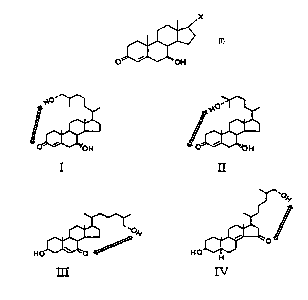
Fig. 2 shows structures of the three naturally occurring cholesterol derivatives that
30 were potent suppressors of HMG-CoA reductase also in transformed fibroblasts.The sterols 7cc,27-dihydroxy-4-cholesten-3-one (I), 70~,25-dihydroxy-4-cholesten-3-
one (II), and 27-hydroxy-7-oxo-cholesterol (III) did not seem to require further
CA 022~677 1998-11-19
WO 97/45440 PCTISE97/00936
metabolism in order to suppress HMG-CoA reductase in human fibroblasts.
Common to these sterols is the presence of an oxo group with a conjugated doublebond in the steroid nucleus and a distal hydroxyl group in the side chain. The sterols
are drawn in such a way that their ap~al~nl structural similarities are illustrated. For
comparison, 27-hydroxylated 3~-hydroxy-5a-cholest-8(14)-en-15-one (IV) is also
shown.
Fig. 3 discloses gas chromatographic-mass spectrometic GC/MSanalysis of neutral
oxysterols isolated from the medium after incubating normal fibroblasts with
o lipoproteins. Normal human fibroblasts (protein contents 1.1 mg, dish size: 143
cm2) were incubated with 10 ml of medium containing 10 % FCS (fetal calf serum);(cholesterol concentration: 1,2 mM) for 24 h, and the medium was then taken for
analysis by GG/MS. Fragment ion current chromatograms characteristic of the
trimethylsilyl ethers of oxysterols were constructed by the computer from mass
spectra taken every 2h during the analysis and for the pupose of illustration the
intensities of the ions (m/z) were multiplied by a~lopliate factors. The principal
sterols indicated by the numbers are listed in Table I. The equivalent of about 0.2 ml
of medium was injected onto a Finnigan SSQ 710 instrument housing a 25-m fused-
silica column coated with methyl silicone, and the oven temperature was
programmed from ~ 85 till 280"C at a rate of 5~C x min ~' .
Fig. 4 relates to HPLC analy.~cs of 'H-labeled 7a,27-dihydroxy-4-cholesten-3-oneand 7a-hydroxy-3-oxo-4-cholcslcnoic acid isolated from the medium of normal
human fibroblasts. The cells (().7 mg of protein) were incubated for 68 h with LDL
25 (4% FCS) labeled with [3Hlcholes~eryl oleate, and the medium was then taken for
analysis by HPLC. For experimental details, see "General Methods" hereafter and
Table IV. After the injections, fractions of the HPLC column emuent were collected
in scintillation vials with 15-60 s intervals, and the radioactivity was then
determined. For purpose of illustration, unlabeled 7a,27-dihydroxy-4-cholesten-3-
30 one (results shown in top chromatograms) or 70c-hydroxy-3-oxo-4-cholestenoic acid
(as methyl ester derivative, bottom chromatograms) were injected together with the
products, and the peaks of these compounds are seen in the UV chromatograms. A
. .
CA 022~677 1998-11-19
wo 97/45440 PCT/SE97/00936
column of silica (LiChrosper) connected to a UV detector was used with
hexane/isopropyl alcohol (90: 10) as mobile phase, and the flow rate was 1.0 ml x
mln
Fig 5. shows effects of LDL on HMG-CoA reductase in normal (O) and
virustransformed O) human fibroblasts. Activities of HMG-CoA reductase in the
fibroblasts were determined after incubation for 24 h with media containing
diffferent concentrations of FCS. In the absence of FCS, media contained 10% LDS(lipoprotein deficient serum). All cells were preincubated for 24 h in media
0 containing 10% LDS. The concentrations of cholesterol in FCS and LDS were 1.2
and 0.1 mM, respectively. The control activities of HMG-CoA reductase in normal
and transformed cells were 72% and 101 pmol/min/mg of protein, respectively.
Fig. 6 discloses time-response curves for the LDL-induced production of 7a,27-
dihydroxy-4-cholesten-3-one (--), 27-hydroxy-7-oxocholesterol (--), and 7(x,25-
dihydroxy~-cholestn-3-one (8), and suppression of HMG-CoA reductase (O) in
normal human fibroblasts. The cells (protein content: 1.7 mg, dish size: 143 cm2)
were incubated with medium (15 ml) containing 10% FCS and were harvested at the
indicated times. The concentration of cholesterol in FCS was 1.2 mM. All cells were
preincubated for 24 h in media containing 10% LDS (see also Table III). For
comparison, the production of 27-hydroxycholesterol (~) is also shown.
The invention will now be illustrated by way of example only with reference to the
following non-limiting examples.
2s
General methods
The steroids used were obtained in the following way: Diosgenin ((25R)-5-spirosten
-3~-ol) was from Sigma (USA) and was used as the starting material for the
synthesis of 27-oxygenated steroids (Arunachalam et al, (1981) J. Org. Chem. vol.
46, 2966-2968; Fieser et al., (1967) Reagents for Organic Synthesis, p. 1059, John
Wiley and Sons Inc., New York; Shoda et al., (1993) Steroids, vol. 58, 119-125). In
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97/00936
addition, 5-cholestene-3,B,7cc,25-triol was prepared from the 3-acetate,25-
trimethylsilyl ether derivative of 25-hydroxycholesterol, and after hydrolysis, this
steroid was further oxidized to 7~c,25-dihydroxy-4-cholesten-3-one as described for
the corresponding 27-hydroxysteroids (Shoda et al., supra). 25-Hydroxycholesterol
was oxidized in the same way to 25-hydroxy-4-cholesten-3-one. The other steroidswere those used in a previous study (Axelson et al (1995) J. Lipid Res vol. 36, 290-
298) and we refer to this article as a disclosure on how to obtain these compounds.
Normal human fibroblasts (line GM 08333) were obtained from NiGMS, Corriell
o Institute for Medical Research (Camden, NJ) and SV40 virus-transformed human
fibroblasts 90-VA IV) were a kind gift from Dr. Stein (University of Colorado,
Boulder, CO). Human colonic carcinoma (WiDr), breast carcinoma (MDA 231),
and malignant melanoma (SK-MEL-2) cell lines were from American Type Culture
Collection.
Cell lines were grown in monolayers in tissue culture flasks maintained in a 95%air, 5% CO2 atmosphere at 37~C in humidified incubator and were cultured in either
Dulbecco's Modified Eagle's Medium (MDA 213) or Minimal Eagle's Medium (the
other cells) supplemented with essential and non-essential amino acids and 10%
20 FCS (v/v). For experimental purposes, cells were cultured in dishes. Cells were
seeded at a density of 5,000 cells per cm2. The experiments were started 48-72h
later, at which time a cell density of approximately 20,000 per cm2 had been
reached. When the metabolism of cholesterol or oxysterols was studied, normal ortransformed fibroblasts (cell number 3-6 x lo6 in 57- 143 cm2 dishes) were first25 preincubated for 24 h in medium containing 10% LDS and were then incubated for
3-68 h with 7-10 ml of medium containing 4-10% FCS (with our without 3H-labeled
cholesterol or cholsteryl oleate) or were incubated with the oxysterol in 10% LDS
for 24-48 h. Control cells were incubated in the same way, but only for 15 min.
Effects of cyclosporin A (CsA), ketoconazole, and oxysterols were tested on normal
30 and transformed fibroblasts at concentrations of 10-30 ~lM, 30 ,~M, and 0.12 ~M,
respectively, in cell media containing 0- 10% FCS and 10-0% LDS. The substances
were added to the incubation media in freshly prepared ethanol solutions, and the
, . ,
CA 022=,=,677 1998-11-19
wo 97/45440 PCT/SE97/00936
ethanol concentrations of media became 0.1 -0.5%. Control cells were incubated in
the same way, but without CsA, ketoconazole, or oxysterols. The dish size and
volume of media when HMG-CoA reductase activity was to be determined were 20
cm2 and 5 ml, respectively, and the incubations were carried out in duplicate for 3-
s 24 h. Each oxysterol was tested in 2-5 separate experiments. Determination of
HMG-CoA reductase activity was then carried out as described previously (Axelsonet al (1995) J. Biol. Chem. 270, 15102, Cavenee et al. (1981) J. Biol. Chem. 256,
2675 and Edwards, P.A. et al. (1979) J. Lipids. Res. 20, 40.
o The procedure for extraction and purification of oxysterols present in incubation
media and cells was essentially the same as described previously (Axelson 1995
supra). Following the collection of a neutral oxysterol fraction from the lipophilic
anion exchanger a fraction containing steroids with a free carboxyl group was eluted
with 0.15 M acetic acid in 95% aqueous methano~ prior to elution of a fraction
5 containing stronger acids (including steroid sulfates) with 0.5 M potassium
acetate/potassium hydroxidc, apparent pH 10.0 in 72% aqueous methanol (Axelson
et al. J. Biol. Chem. (1991) 266, 17770).
Trimethylsilyl ethers of oxysterols and methyl ester trimethylsilyl ether derivatives
20 of steroid acids were prepared (Axelson 1991 supra) and were analyzed by gas
chromatography mass spcc~romctry (GC/MS) as described in Axelson 1995 supra.
3H-Labeled cholesterol, cholcslcryi oleate, and 25-hydroxycholesterol and/or their
radioactive metabolites werc analysed by HPLC prior to or after group fractionation
25 and purification as described above.3H-labeled cholesterol and cholesteryl esters
were extracted from small aliquots of the incubation media with mixtures of
isopropyl alcohol and hexane and from cells with mixtures of ethanol and water
prior to separation by straight-phase HPLC using hexane/isopropyl alcohol, 98:2
(v/v), as the mobile phase (Axelson 1995 supra). ~ppropriate fractions from the
30 HPLC effluent were collected in vials, and the radioactivity was then deterrnined by
scintillation counting. Radioactive neutral and acidic metabolites of [3H]cholesterol
or [3H]cholesteryl oleate were isolated from media and cells as described above and
CA 022~677 1998-ll-lg
Wo 97/45440 PCT/SEg7/00936
ll
were then characterized by HPLC: For this purpose, three HPLC systems were used
in the following order. Reversed phase HPLC was carried out on a column of
LiChrospher (250 x 4 mm, Hibar, lOORP- 18, 5 ~m, Merck, Darmstadt, Germany)
using a pump (Constametric III) and a variable wavelength detector (Spectra
Monitor D from LDC/Miton Roy, Riviera Beach, ~;L) set at 220 or 240 nm and a
Rheodyne Model 7125 injector with a 100,ul loop. The mobile phase used for
neutral metabolites was a mixture of methanol/ethanol/water, 80:20: 10 (by volume,
flow rate I ml x min~l), and fractions were collected between 0 and 11 min (fraction
1; containing polar metabolites, e.g. 7a,27-dihydroxy-4-cholesten-3-one, retention
o time about 4.5 min) and between 11 and 14 min (fraction 2; containing 7a-hydroxy-
4-cholesten-3-one and 27-hydroxycholesterol having retention times 11.5 and 12.5min, respectively). The mobile phase was then changed to 85% a~ueous methanol
(flow rate I ml x min-~) for separation of sterols in fraction 1 or for separation of
steroid acids (as methyl ester derivatives). In the former case, a fraction of the
effluent containing 7a,27-dihydroxy-4-cholesten-3-one (retention time about 8.5
min) was collected between 8.0 and 9.0 min, and in the latter case a fraction of the
effluent containing 7a-hydroxy-3-oxo-4-cholestenoic acid (retention time about 12
min) was collected between 11.0 and 13.0 min. These fractions and fraction 2
(containing 7a-hydroxy-4-cholesten-3-one and 27-hydroxy-cholesterol) were then
reanalyzed by straight-phase HPLC. The latter was carried out with an instrumentsimilar to that above, but with a column (250 x 4.5 mm) of LiChrosper (Hibar, Si100, 5 ~M, Merck). The mobile phase was hexane/isopropyl alcohol, 94:6 (v/v),
when fraction 2 was analyzed (retention times of 7a-hydroxy-4-cholesten-3-one and
27-hydroxycholesterol were about 8 and 9 min, respectively), and 90:10 (v/v), when
2s the fractions containing 7~,27-dihydroxy-4-cholesten-3-one or 7a-hydroxy-3-oxo-4-
cholestenoic acid methyl ester were analyzed. The flow rate was 1.0 ml x min~l in all
cases. The HPLC emuent during the latter analyses was collected in scintillationvials with 15-60-s intervals, and after addition of scintillation fluid, the radioactivity
was determined.
25-[3H]Hydroxycholesterol and its metabolites in media and cells were analyzed by
HPLC following extraction. Medium was extracted with ethanol, and, after
. .. ...
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97/00936
centrifugation and removal of the supernatant, the pellet was re-extracted with
ethanol/isopropyl alcohol, 1:1 (v/v). The extracts were combined and the solventwas then evaporated. Nonpolar compounds were dissolved in hexane, and, after
removal, the solid residue was dissolved in 60% aqueous methanol, which was
s passed through a column (1.5 x 0.8 cm) of octadecylsilane-bonded silica
(Preparative Cl8; Walters Associates Inc., Milford, MA) and collected. The
methanol in the eluate was then removed in vacuo, and the aqueous solution was re-
extracted on the same column. After washing the column with water, adsorbed
steroids (polar metabolites) were eluted with methanol/chloroform, l: l (v/v), and
o were combined with the nonpolar metabolites present in the hexane fraction. This
combined extract was evaporated to dryness and dissolved in methanol or
hexane/isopropyl alcohol, 90:10 (v/v), prior to analysis by reversed-phase HPLC
(mobile phase; methanol/ethanol/water, 80:20:10 (v/v) or straight-phase HPLC
(mobile phase: hexane/isopropyl alcohol, 97:3 (v/v).
1s
Example 1
Formation of oxy~enated cholesterol derivatives in human fibroblasts
Table I shows the oxygenated cholesterol derivatives identified in the neutral and
20 acidic fractions from media (containing 10% FCS) after incubation with normalhuman fibroblasts and their gas chromatographic/mass spectrometric characteristics
as trimethylsilyl ethers and methyl ester trimethylsilyl ether derivatives,
respectively.
CA 022~677 1998-ll-lg
Wo 97/4s440 PCT/SE97/00936
13
TABLEI Structurea Fig RI Mol~ lsr &
Steroidname 3e signiffcant ionsC
Neutral C27 Steroids m/z
7O~-Hydroxycholesterol C5-3,B,7a-ol 1 3115 546, 456
7a-Hydroxy-4-cholesten-3- C4-7a-ol-3One 2 3210 472,457,382,269
one
7~-Hydroxycholesterol Cs-3,~,7~-ol 3 3235 546,456
7-Oxocholesterol C5-3~-ol-7-one 4 3375 472,382,367,129
24-Hydroxycholesterol C53~,24 (R/S)ol 5 3385 S46,413,145,129
25-Hydroxycholesterol C5-3~25 ol 6 3405 546,456,131
7cc,25-Dihydroxycholesterol C5-3~,7a-25-ol 7 3390 634,544,131
7c~,25-Dihydroxy-4- C4-7a,25-ol-3- 8 3490 560,545,412,131
cholesten-3-one one
27-Hydroxycholesterol C5-3~,27-ol 9 3455 546,456,417,129
7a,27-Dihydroxycholesterol C5-3,B,7a,27OI 10 3445 634,544
7a,27-Dihydroxy-4- C4-70c,27-ol-3- 11 3545 560,545,470,269
cholesten-3-one one
7~,27-Dihydroxycholesterol C5-3,B,7~,27OI 12 3555 634,544
27-Hydroxy-7-oxo- C5-3,B,27-ol-7- 13 3710 560,545,470,129
cholesterol one
C27-steroid acids
3,~-hydroxy-5-cholestenoate CA5-3~-ol 14 3425 502,412,373,129
3,B,70c-Dihydroxy-5- CA5-3,B,7a-ol 15 3415 590,500
cholestenoate
7cc-Hydroxy-3-oxo-4- CA4-7a-ol-3- 16 3515 516,501,426,269
cholestenoate one
- 3~,7~-Dihydroxy-5- CA5-3,B 7~ old 17 3530 590,500
cholestenoate
3~-Hydroxy-7-oxo-5- CA5-3,~-ol-7- 18 3680 516,426,411,129
cholestenoate oned
CA 022~677 1998-ll-l9
WO 97/45440 14 PCT/SE97/00936
a C, cholestane;CA, cholestanoate; superscript indicates position of
double bond; Greek letters denote configuration of hydroxyl groups.
b RI, Retention Index, Kovats, on a fused silica capillary column coated
s with cross-linked methyl silicone
c Intensities of charged ions with m/z values above 200-300 were
enhanced relative to those of lighter fragments; base peak is shown in
italics; m/z, mass/charge.
d Tentative identification, reference compound not available.
e enumerated peaks on Fig 3.
Table II shows the production of oxysterols in normal and virus transformed human
fibroblasts when incubated with lipoproteins. The amounts of neutral and acidic
oxygenated cholesterol derivatives were determined in the media (10 ml) Cont~ining
5 10% FCS (cholesterol concentration 1.2 mM) after incubation with fibroblasts for
48 hours. Cells incubated for 0.25 h served as controls.
CA 022~677 1998-11-19
WO 97/45440 15 PCT/SE97/00936
Table II Amountb of steroid found in media - pmol
Structur~a Normal fibroblastsCVirus-transformedC
filbroblasts
0.25 h; n=2 48 h; n=4 0.25 h; n=2 48 h; n=4
C5-3~,7a-ole 171: 117-225 54: 39 - 81 156: 81 - 228 60: 54 - 123
C4-7a-ol-3-one <6: <3 - <6 <12: <6 - <18 <9: <6 - <9 69: 51 - 159
C -3,~,7,~-ol 138: 108 - 165 114: 99 - 156 144: 81 - 207 129: 75 - 216
Cs-3~-ol-7-onee 879: 840 - 915 816: 609-1140 993: 633-1350 867: 657-1881
Cs-3,~,24-ol 6: <6 - 6 6: <6 - 9 3: <3 - 6 9: <6 - <18
C -3~,25-ol 6: <3 - 12 45: 25 - IS0 9: <3 - 18 27: 15 - 30
Cs-3,~,7a-25-ol <3: <3 - 3 <6: <3 - <9 <3: <3 - 3 <3: <3 - <3
C4-7Oc,25-ol-3- <6: <3 - <3 21: <9 - 27 <3: <3 - <3 <3: <3 - <3
one
Cs-3~,27-ol 12: 9 - 18 219: 153 - 267 12: 9 - 12 21: 15 - 33
Cs-3~,7a,27OI <3: <3 - <3 <3: <3 - <3 <3: <3 - <3 <3: <3 - <3
C4-7a,27-ol-3- <12: <12 -<12 270: 144 - 321 <9: <3 - <12 <12: <9 - <15
one
Cs-3,B,7,~,2701 <3: c3 - <3 42: 33 - 48 <3: <3 - <3 <3: <3 - <3
Cs-3~,27-ol-7- <9: <6 - <12 81: 33 - 108 <12: <3 - <18 <9: <6 - 15
one
CAs-3,~-ol 21: 12-27 21: 18-27 15: 12- 15 15: 12- 18
CAs-3~,7a-ol 9: 6 - 9 6: 3 - 6 6: 6 - 6 6: 3 - 6
CA4-7a-ol-3- 21: 18 - 21 75: 63 - 117 18: 15 - 18 9: 6 - 15
one
CAs-3,~,7~ ol 12: 9 - 12 51: 45 - 72 3: <3 - 3 <3: <3 - <3
CAs-3~ ol 7 <3 <3 <3 27: 18 - 30 <3: <3 - <3 <3: <3 - <3
one
a For abbreviations and steroid names see Table I
b Expressed as median: range, < = cm amount at or below detection limit
~ Protein contents of normal and virus-transformed fibroblasts were 0.6
s mg and 1.4 mg respectively. The size of the incubation dishes was 57
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97/00936
16
cm2. All cells had been preincubated for 24 h in media containing 10%
LDS.
d n = number of incubations.
e Can also be formed by autooxidation of cholesterol during incubations
or during purification of samples.
Fig. 3 shows a GC/MS analysis of neutral C27-steroids isolated from the medium
after incubating normal fibroblasts with lipoproteins.
o As can be seen from Table I, when normal fibroblasts are incubated for 28-48 hours
with lipoproteins (media containing 10% FCS), 13 neutral and 5 acidic C27 steroids
were found in the media.
The identification was based on the GC retention indices of the derivatives and mass
spectra, which were compared with those of the authentic steroids. Most of the
steroids had additional oxygen groups both at C-7 and in the side chain. No
additional steroids were identified in the cell extracts, and, with the exception of the
autooxidation products of cholesterol (see above), the amounts of oxysteroids in the
cell extracts were low and barely detectable (<10-20% of those in media). Because
of this, oxysterols in the cells were usually not analyzed, unless otherwise stated.
The quantitative results after incubating normal and transformed fibroblasts for 48
hours with lipoproteins can be seen from Table II. In addition to 27-
hydroxycholesterol which is formed from LDL cholesterol (Axelson 1995 supra),
2s the amounts of three other 27-hydroxylated sterols also increased 10-20-fold when
incubating normal fibroblasts. These sterols were 70c,27-dihydroxy-4-cholesten-3-
one, 27-hydroxy-7-oxocholesterol and 7~,27-dihydroxycholesterol. In fact, the
former of these was the major oxysterol formed. The amounts of corresponding C27 -
acids also increased (about 5-10-fold). Also, 25-hydroxycholesterol and 70~,25-
dihydroxy-4-cholesten-3-one increased, but the amounts of the former varied
considerably, possibly indicating that a part of this sterol had been produced by
autooxidation of cholesterol. A decrease of the amount of 70c-hydroxycholesterol
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97/00936
17
was also noted indicating a consumption of this sterol during the incubations. The
amounts of the other steroids were similar to those of the controls, suggesting that
they were present in FCS when added to the media.
5 Much smaller amounts of oxysterols were formed by the transformed fibroblasts
(Table II). For example, the amounts of 27-hydroxycholesterol only increased about
2-fold, and the other 27-hydroxylated sterols were barely detectable in the media.
Only the amounts of 7a-hydroxy-4-cholesten-3-one increased significantly (this
was not the case in normal fibroblasts) which may be related to a decrease of the
o amount of its potential precursor 7cc-hydroxycholesterol. These results indicated that
27-hydroxylation of sterols may be obstructed in transformed fibroblasts (see
below).
The oxysterols were also studied with regard to the time course of their cellular
production, as shown in Table III which shows time-response for the production of
oxysterols in normal fibroblasts when incubated with lipoproteins. The amounts of
neutral oxygenated cholesterol derivatives were determined in the media (15 ml)
containing 10% FCS (cholesterol concentration 1.2 mM) after incubation for 0.25 h
with norrnal human fibroblasts (protein content 1.7 mg, size of dishes 143 cm2) All
20 cells had been preincubated for 24 h in media containing 10% LDS.
CA 022~677 1998-11-19
WO 97/4~440 18 PCT/SE97/00936
TABLE III
structurea Amount (~mol )of oxysterol found in media after
incnh~tion for:
0.25h 3h 8h 24h
C5-3~,70~-ol 92 92 31 15
C4-7a-ol-3-one 55 65 58 45
C -3,B,7,B-ol 92 92 58 66
Cs-3~-ol-7-oneb 508 500 369 412
Cs-3,~,24-ol 15 15 10 10
C -3~,25-ol 28 28 10 15
Cs-3,B,7~c,25-ol 25 20 5 5
C4-7a,25-ol-3-one 33 28 25 30
C5-3,~,27-ol 23 30 70 240
C5-3~,7a,27-ol 2 2
C4-7a,27-ol-3-one 65 58 138 308
Cs-3~,7,~,27-ol 3 3 15 13
Cs-3,B,27-ol-7-one 8 5 43 108
For abbreviations and steroid names, see Table I
b Can also be formed by autooxidation of cholesterol during the
incubations or during purification of samples.
s
As shown in Table III incubation of normal human fibroblasts for different lengths
of time showed that the formation of 27-hydroxylated sterols had started 3-8 h after
exposure to lipoproteins. No production of 25-hydroxylated sterols was observed
during the first 24 h. A decrease of the amounts of 7a-hydroxycholesterol was
o noted after 8 h of incubation.
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97100936
19
Example 2
Metabolism of LDL cholesterol and 7-oxv~enated sterols in normal fibroblasts
The cell lines, cell culture conditions, analysis of oxysterols and steroid acids and
s the HPLC procedures were as in Example 1.
The metabolism of 7a-hydroxycholesterol, 7~-hydroxycholesterol, and 7-
oxocholesterol was studied by incubating these sterols (5 nmol) with normal human
fibroblasts (protein content about 0.5 mg, dish size 57 cm2) for 24 or 48 h in media
10 (10 ml) containing 10% LDS. The values given in percent represent the distribution
of observed metabolites.
When 7a-hydroxycholesterol was incubated (24 h), the major metabolites found
were 7a-hydroxy-4-cholesten-3-one (57%) and 7a,27-hydroxy-4-cholesten-3-one
s (43%) (acids were not analyzed). Only a small amount of 7a,27-hydroxy-
cholesterol (0.5%) was noted suggesting that oxidation/isomerization of 7a-
hydroxycholesterol to 70c-hydroxy-4-cholesten-3-one precedes 27-hydroxylation.
The corresponding enzymc activities have been found previously in fibroblasts
(Skrede et al (1986) J Clin Invest 78, 729). When 7a-hydroxy-4-cholesten-3-one
20 was incubated with the fibroblasts for 48 h, the steroid was extensively converted to
7a,27-dihydroxy-4-chole.sten-3-onc (37%) and 7a-hydroxy-3-oxo-4-cholestenoic
acid (63%). A small amount of '~-hydroxylated 7a-hydroxy-4-cholesten-3-one
(0.5%) was also found. Thesc rcsult.s show that 7a-hydroxycholesterol can be
converted to 7a-hydroxy-4-chole.sten-5-one, 7a,27-dihydroxy-4-cholesten-3-one,
25 and 7a-hydroxy-3-oxo-4-cholestenoic acid by normal fibroblasts and this couldexplain the appearance of these metabolites and the disappearance of 7a-
hydroxycholesterol in media during the incubations with FCS (Table II).
The metabolism of 7~-hydroxycholesterol differed from that of 7a-hydroxy-
30 cholesterol. When this sterol was incubated for 48 h with normal fibroblasts, the
major metabolites were 7~,27-dihydroxycholesterol (1 %) and 3,B,7~,-dihydroxy-5-
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97/00936
cholestenoic acid (62%). In addition, a large portion (about one-third) of 7~-
hydroxycholesterol was converted to 7-oxocholesterol (20%), 27-hydroxy-7-
oxocholesterol (1%), and 3,~-hydroxy-7-oxo-5-cholestenoic acid (14%). Oxidation
of 7~-hydroxy group also occurred when 7~,27-dihydroxycholesterol was
s incubated.
Surprisingly, no conversion of 7,~-hydroxycholesterol to the corresponding 3-oxo-~4
derivative was observed, which was consistent with the absence of 7,B-hydroxylated
3-oxo-~4 steroids in media after incubating fibroblasts with FCS (Table I). This0 implies that prior art 7~-hydroxycholesterol derivatives are unable in vivo to mimic
the effects of the compounds of the invention.
Incubation of 7-oxocholesterol with normal fibroblasts resulted in the formation of
27-hydroxy-7-oxocholesterol (55~o) and the corresponding C27-acid (35%). A smallamount was converted to 7~-hydroxycholesterol (10%), but a conversion to 7a-
hydroxylated products was not observed. Thus, the formation of C2~-steroids having
oxygen groups in both the 7- and 27-positions by normal fibroblasts could be due to
the presence of autooxidation products of cholesterol in the medium. However, this
did not exclude the possibility that 70c,27-dihydroxy-4-cholesten-3-one and 7a-
hydroxy-3-oxo-4-cholestenoic acid could also be derived from 27-
hydroxycholesterol. 7a-Hydroxylation of 27-hydroxycholesterol in human
fibroblasts was first noted by the present applicants (Axelson et al. (1995) J. Lipid
Res. 36, 290) and was later found to be occurring generally in these cells (Zhang et
al. (1995) Biochem. Biophys. Acta. 1256, 353). The product 7a,27-
dihydroxycholesterol is extensively converted to 7a,27-dihydroxy-4-cholesten-3-
one and the corresponding acid in the cells.
In order to determine whether LDL cholesterol (via 27-hydroxycholesterol) could be
converted to 7c~,27-dihydroxy-4-cholesten-3-one and the acid, the contribution from
7a-hydroxycholesterol (which is always present when the medium contains
lipoproteins) had to be accounted for. This was made possible by the following
CA 022~677 1998-11-19
WO 97/45440 21 PCT/SE97/00936
experiment. LDL and other lipoproteins in FCS were first labeled with
[3H]cholesteryl oleate and were then incubated with normal fibroblasts in the
presence and absence of cyclosporin A (CsA), a selective inhibitor of the sterol 27-
hydroxylase (Axelson 1995 supra, Princen et al. (1991) Biochem J. 275, 501.
s Dalback-SJoberg et al. ( 1993) Biochem. J. 293, 203) When lipoproteins are labeled
in this way, the cellular uptake of [3H]cholesteryl oleate is due solely to an LLD
receptor-dependent process (i.e. a physiological uptake of LDL (Axelson 1995
supra). After the incubations, radioactive 27-hydroxycholesterol, 7a,27-dihydroxy-
4-cholesten-3-one, and 7a-hydroxy-3-oxo-4-cholestenoic acid were analyzed by
0 HPLC. 3H-Labeled 7a-hydroxy-4-cholesten-3-one, the direct metabolite of 7a-
hydroxycholesterol, was also determined. If 3H-labeled 70c-hydroxycholesterol (free
or esterified) was present in the medium, its 3-oxidized metabolite was expected to
accumulate in the presence of CsA, since the drug prevented further metabolism
(see above). The amount of metabolite would then reflect the contribution to 7a,27-
dihydroxy-4-cholesten-3-one and the acid from 7a-hydroxycholesterol in the
absence of CsA. Obviously, essentially no formation of 27-hydroxylated compoundswas expected in the presence of CsA (see also Fig. 1). For comparison, fibroblasts
were also incubated with lipoproteins labeled with [3H]cho]esterol, whose cellular
uptake is not entirely dependent on LDL receptors.
The results of these incubations are ~.umnl~ized in Table IV, which shows the
formation of radioactive metabolites from LDL [3H ]cholesteryl oleate in normal
fibroblasts. The amounts of 3H-labeled 27-hydroxycholesterol, 7a,27-dihydroxy-4-cholesten-3-one and 7a-hydroxy-3-oxo-4-cholestenoic acid were determined in
2s media and cells after incubating normal human fibroblasts (protein content, 0.7 mg;
size of dishes, 57 cm2) for 68 h with media (10 ml) containing LDL (4% FCS;
cholesterol concentration, 1.2 mM) labeled with [3H]cholesteryl oleate or
[3Hlcholesterol. When 27-hydroxylation of sterols was obstructed by cyclosporin A
(CsA, 10 ,uM), the accumulation of 3H-labeled 7a-hydroxy-4-cholesten-3-one
indicated the presence of autooxidized [3H]cholesterol or [3H]cholesteryl oleate (i.e.
free or esterified 7a-hydroxycholesterol) in the media during the incubation.
CA 022~677 1998-11-19
WO 97/4S440 22 PCT/SE97/00936
TABLE IV
Structurea ~mo~lnt of 3H -labeled oxysterol foundb - dpm
FCS + ~3H]rh~ t~ryl FCS + r3H]cholesterolC
oleatec
Control +CsA Control +CsA
Cs-3~,27-ol 12 000 <1 970 7 090 <120
C4-7a,27-ol-3-one 5 140 <1 140 88 800 12 800
CA4-7a-ol-3-one lO 800 <120 26 300 l 690
C4-7a-ol-3-one <410 <410 l 450 25 300
For abbreviations and steroid names, see Table I,
b Values represent the sum of the amounts found in medium and cells;
< = an amount at or below detection level
c FCS was preincubated for 16 h at 20~C with [3H]cholesteryl oleate
(48 x 106 dpm) or [3H]cholesterol (49 x lo6 dpm). All cells had been
preincubated for 24 h in media containing 10% LDS.
0 In addition to 27-hydroxycholesterol, both 70~,27-dihydroxy-4-cholesten-3-one and
7a-3-oxo-4-cholestenoic acid werc found a~s 3H-labeled compounds after incubation
with [3H]cholesteryl ocate. The HPLC analyses of these metabolites are shown (Fig.
4). Since no acccumulation of radio;lctivc 70~-hydroxy-4-cholesten-3-one was
observed in the corresponding incubation with CsA (Table IV), autooxidation (70c-
hydroxylation) of ~3H]cholesleryl oleate had not occurred during the incubations. In
the incubations with [3H]choles(erol. much larger amounts of radiactive 7a,27-
dihydroxy-4-cholesten-3-one and the corresponding acid were found, although the
amount of 27-hydroxycholesterol was less than with [3H]cholesteryl oleate.
However, the incubation with l3H]cholesterol and CSA resulted in a significant
accumulation of 3H-labeled 7a-hydroxy-4-cholesten-3-one suggesting that most of
the 27-oxygenated metabolites had been produced from autooxidized
[3H]cholesterol (70c-hydroxycholesterol). The difference in chemical stability toward
oxygen between [3H]cholesteryl oleate and [3H]cholesterol was surprising, but was
CA 022~677 1998-11-19
WO 97/45440 23 PCT/SE97/00936
confirmed by exposing them to air and heat in an a~ueous/methanolic environment
for 24 h. No autooxidation products (<0.1 %) of [3H]cholesteryl oleate could be
detected, whereas about 2% of [3H]cholesterol were autooxidized. Thus, the results
demonstrate that LDL cholesteryl esters are hydrolyzed and are converted to 27-
s hydroxycholesterol, which is then 7a-hydroxylated and oxidized to 7a,27-
dihydroxy-4-cholesten-3-one and 7a-hydroxy-3-oxo-4-cholestenoic acid in normal
human fibroblasts. The latter seems to be the major metabolic end product of this
extended LDL pathway in fibroblasts.
o Example 3:
Metabolism of LDL cholesterol and side-chain hydroxylated sterols in transformedfibroblasts
The cell lines, cell culture conditions, analysis of oxysterols and steroid acids and
5 the HPLC procedures were as in Example l.
In contrast to normal fibroblasts, only small amounts of 27-oxygenated sterols were
detected in media after incubating transformed human fibroblasts with lipoproteins
(Table II). Although the increased amounts of 70~-hydroxy-4-cholesten-3-one could
20 indicate that 27-hydroxylation of sterols was obstructed in these cells (see above),
the lack of 27-hydroxylated sterols in the media could also be due to a decreased
cellular uptake of LDL and oxysterols, or to an increased forrnation of conjugates
(e.g. fatty acid esters or sulfate esters).
2s Table V shows the distribution of 3H-labeled cholesterol and cholesteryl esters after
incubating normal and transformed fibroblasts with lipoproteins labeled with
radioactive cholesterol or cholesteryl oleate for 48 h. Distribution of radioactivity
after incubating normal and virus-transformed human fibroblasts for 48 hours in
media containing lipoproteins (10% FCS) labeled with [3Hlcholesteryl or
30 13H]cholesteryl oleate. The total concentration of unlabelled cholesterol in FCS was
1.2 mM. Incubations for 0.25 h served as controls.
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97/00936
24
TABLE V
Incubation conditions;l Distribution of free & esterified [3H] cholesterolC
Time Addnb In the Medium In the Cells
h Free¦ Esters ¦ Total Free ¦ Esters ¦ Total
normal cells
0.25 C/FCSd 95 4 99 1 <1
48 C/FCSd 82 3 85 11 4 15
48 C/FCS~ 80 4 84 11 4 16
0.25 CO/FCSd 2 98 100 <l <1 <1
48 CO/FCS 10 81 91 6 4 9
48 CO/FCS 11 77 88 9 3 12
transformed cells
0.25 CtFCSd 95 4 99 1 <1
48 C/FCSd 66 12 79 17 4 21
48 C/FCS 73 5 78 18 4 22
0.25 CO/FCSd 1 99 100 <I <l <1
48 COlFCSd 38 49 86 11 3 14
48 CO/FCSd 34 51 84 12 3 16
a Protein content of normal and virus transformed fibroblasts were 0.6
mg and 1.4 mg respectively. The size of the incubation dishes was 57
cm2. All cells had been preincubated for 24 h in media containing 10%
LDS. Results from these incubations are also shown in Table II.
b Additions to the incubation medium: The amounts of [3H] cholesterol
and ~3H] cholesteryl oleate added to the medium were 22.8 x lo6 dpm
and 18.9 x 106 dpm respectively. The sterols were preincubated with
FCS for 16 h at 20~C.
c Percentage of recovered radioactivity. The total recovery was >90% of
the radioactivity added.
d C/FCS: [3H]Cholesterol in FCS, CO/FCS: [3H]Cholesterol oleate in
FCS
CA 022~677 1998-11-19
WO 97/45440 25 PCTISE97/00936
Results on the oxysterol production from the same incubations are those shown inTable II. As seen in Table V, the cellular uptake and retention (cell content) of
[3H]cholesterol in normal and transformed cells were about 16 and 22%,
respectively. About 4% had been esterified by both cell types. After the incubations
5 with [3H]cholesteryl oleate, the retention of the compound was about 1 l ~o in the
normal cells and 15% in the transformed cells~ although a major portion had beenhydrolyzed in the cells. About 9% and 35% of hydrolyzed ~3H]cholesterol were also
present in media of the normal and transformed fibroblasts, respectively, due to an
efflux of hydrolyzed LDL cholesterol from the cells (Axelson 1995 supra, Fielding
lo et al 1995 J Lipid Res 36, 211-228). When the cellular content and the eMux of
cholesterol in the incubations with ~3H]cholesteryl oleate in the normal and thetransformed cells was 19% (32%/mg of protein) and 50% (36%/mg of protein),
respectively. These results show that the uptake of LDL (reflected by that of
[3H]cholesteryl oleate) was not decreased but possibly increased in the transformed
5 cells also when the protein content of the cells was taken into account. Thus, a
possible reduced formation of 27-hydroxylated sterols by transformed fibroblasts(Table II) was not due to a decreased uptake of LDL.
In order to determine whether the shortage of 27-hydroxy-cholesterol and other 27-
20 hydroxylated sterols in media of transformed fibroblasts could be due to an
increased metabolism (other than formation of C27-acids) or conjugation, the
metabolism of 25-hydroxycholesterol was studied. The major reason for selecting
this sterol instead of 27-hydroxycholesterol was that 25 hydroxycholesterol was
available in a 3H-labeled form, so that major metabolites or conjugates could be2s traced and would not escape detection. Because of their similarities in structure
(both having a 3~-hydroxy-~5 structure and one hydroxyl group in the side chain)~
the cellular handling of the two sterols was expected to be similar (except that the
25-hydroxy group could not be oxidized to a carboxyl group). Table VI shows the
metabolism of 25-[3H]hydroxycholesterol in normal and transformed fibroblasts. In
30 particular it shows the distribution of recovered radioactivity after incubating normal
and transformed fibroblasts for 48 h with media containing 3H-labeled and
CA 022~5677 1998-11-19
WO 97/45440 PCT/SE97/00936
26
unlabeled 25-hydroxycholesterol and 10% LDS (cholesterol concentration 0.1 mM)
lncubations for 0.25 h served as controls.
TABLE VI
~adioactive comp. Relative amount founda b
Normal fibroblasts ¦ Transforrned fibroblasts
0.25h ¦48h ¦48h 0.25h¦48h¦48h
In the medium
25-[3H] 47 5 4 73 21 21
hydroxycholesterol
Non-polar metabolitesC <1 <1 <I <1 2 2
Polar metabolitesd 2 43 37 2 2 2
Total radioactivitye 57 57 51 79 30 29
In cells
25-[3H] 35 30 34 18 49 50
hydroxycholesterol
Non-polar metabolitesC <1 <1 <I <I 5 5
Polar metabolitesd <I 4 5 <I 2 3
Total radioactivitye 43 43 49 22 70 71
a The amounts of 3H-labeled and unlabeled 25-hydroxycholesterol added
to the medium (10 ml containing 10%LDS) were 1.4 x lo6 dpm and 1.2
nmol respectively. Protein contents of normal and virus transforrned
fibroblasts were 0.6 mg and 1.4 mg respectively. The size of the
incubation dishes was 57 cm2. All cells had been preincubated for 24
o hours in media containing 10%LDS.
b Figures represent % recovered radioactivity. The total recovery was
about 90% of the radioactivity added.
c Retention time on straight phase HPLC was 3.5 - 4.0 min using
hexane/isopropyl alcohol (97:3) as the mobile phase and a flow rate of 1
ml x min~~. It was tentatively identified as 25-hydroxycholesterol
esterified with a fatty acid.
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97/00936
27
d retention time on reversed phase HPLC was 3.0 - 4.5 min using
methanol/ethanol/water (80:20: 10) as the mobile phase and a flow rate
of I ml x min ~~. In this fraction, 7(xt25-dihydroxy-4-cholesten-3-one
was identified by gas chromatography-mass spectrometry, and the
amounts were similar to those calculated from the radioactivity.
e Also includes other radioactive compounds, each accounting for less
than I % of the total recovery.
In addition to 25-[3H]hydroxycholesterol, two major radioactive metabolites, one10 polar and one nonpolar, were found by HPLC. Other metabolites constituted less
than I % each of the recovered radioactivity. No radioactivity (<0.1 %) corresponding
to oxidized 25-[3H]hydroxycholesterol without a 7~c-hydroxy group (i.e. 25-
hydroxy-4-cholesten-3-one, see below) was found. Neither did we find any
radioactivity (<I %) in fractions containing weak acids (e.g. having a free carboxyl
group) or stronger acids (e.g. glucuronides or mono- or disulfates) which were
isolated from the extracts by anion exchange chromatography. After collecting a
fraction of the HPLC effluent containing the polar metabolite and derivatization, it
was identified by GC/MS as 70~,25-dihydroxy-4-cholesten-3-one (Zhang supra). Thenonpolar metabolite(s) had a retention time (3.7 min), which was similar to that of
20 the 3-acetate derivative of 25-hydroxycholesterol (retention time 4.9 min) bystraight-phase HPLC (retention time of free 25-hydroxycholesterol was 11.8 min). It
was therefore tentatively characterized as being fatty acid esters of 25-
3H]hydroxycholesterol. This was supported further by recovery of free 25-
[3H]hydroxycholesterol after treating the nonpolar metabolite(s) with mild alkali in
25 a methanolic solution. Table VI clearly reveals large differences in the handling of
25-[3H]hydroxycholesterol between the two cell lines. Intact 25-
~3H]hydroxycholesterol was found mainly in the cells (32% in the normal and 50%
in the transformed cells). A large portion (about 43%; 71 %/mg of protein) of 25-
[3H]hydroxycholesterol had been converted to 7a,25-dihydroxy-4-cholesten-3-one
30 by normal cells (62) but much less so (about 3~; 2%/mg of protein) by the
transformed cells. This sterol was recovered mainly in the media. On the other hand,
esterification of 25-[3H]hydroxycholesterol was noted only in the transformed cells,
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97/00936
28
although the amount of esters was relatively small (about 7%). These results show
that 25-hydroxycholesterol is readily taken up by both norrnal and transformed cells,
but whereas the sterol is extensively 7a-hydroxylated in normal cells, this reaction
is obstructed in transformed cells. The results also suggested that the apparentshortage of 27-hydroxycholesterol in transformed cells was due to a decreased
formation rather than an increased conjugation, since only a minor amount of theanalogous sterol 25-hydroxycholesterol was esterified and since no other conjugates
were found.
After these observations, the rates of oxidation/isomerization of 3~,7a-dihydroxy-~5
steroids to 3-oxo-~4 steroids in normal and transformed cells were also investigated.
7a,27-Dihydroxycholesterol (1.2 nmol) was therefore incubated with normal and
transformed fibroblasts (protein contents 0.4 and 1.1 mg, respectively, size of dishes
57 cm 2~ for 48 h in media ( 10 ml) containing l O~o LDS, and the metabolites were
then analyzed by GC/MS. Incubations for 15 min served as controls. The analyses
showed that this sterol was readily taken up by the cells, since only about 1%
remained in the media. In media from normal cells, about 26% and 39% were
recovered as 7a,27-dihydroxy-4-cholesten-3-one and 7a-hydroxy-3-oxo-4-
cholestenoic acid, respectively. The corresponding values for the transformed cells
were 28% and 19%. Trace amounts (about 1%) were converted to 3~,7a-dihydroxy-
5-cholestenoic acid in the transformed cells. No 7-oxo-, 7~-hydroxy-, or other
metabolites were found. Thus, almost the same amount of 7a,27-
dihydroxycholesterol were oxidized by the normal and transformed fibroblasts.
However, when the number of incubated cells (cell protein content) were taken into
account, the oxidation rate in transformed cells was calculated to be about 25% of
that of normal cells. These studies show that the ap~ar~llt activities of 27- and 7a-
hydroxylating enzymes are much lower in transformed than in normal fibroblasts
(estimated to be <2% of the normal activity when corrected for the cellular protein
content or total uptake of LDL), whereas the enzyme catalyzing oxidation of 3~-
hydroxy-~5 sterols is affected to a lesser extent.
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97/00936
29
Example 4:
Effects of LDL and oxysterols on HMG-CoA reductase in normal and tumor-
transformed human fibroblasts
Norrnal human fibroblasts (line GM 08333) from NiGMS, Corriell Institute for
Medical Research (Camden NJ) and SV-40 virus transformed human fibroblasts
(90-VA VI), a kind gift from Dr. Stein (University of Colorado, Boulder, CO), were
grown in monolayers in tissue culture flasks maintained in a 95% airtS% CO2
atmosphere at 37~C in a humidified incubator. Cells were cultured in Dulbecco's
o Minimal Eagle's Medium supplemented with essential and non-essential amino
acids and 10% fetal calf serum (FCS, from Life Technologies, Inc., Stockholm,
Sweden). Cells were then seeded in dishes (20 cm2) at a density of 5,000 cells per
cm2 and the media (5 ml) contained lO~o FCS. The experiments were started 48-72
h later, at which time a cell density of approximately 20,000 per cm2 had been
reached (the cells were subconfluent also at the end of the incubations). All cells
were then incubated for 24 h in medium (5 ml) containing 10% lipoprotein deficient
serum, prepared by treating FCS with Cab-O-Sil (Weinstein (1979) Circulation 54,Suppl. ~ 59), before LDL (0.5-8% FCS; cholesterol concentration, 1.2 mM) and
oxysterols (tested at a concentration of 0.12 ~M) were added to the incubation
media, the latter in freshly prepared ethanol solutions. The ethanol concentrations of
the media then became 0.2%. Control cells were incubated in the same way but
without sterols. After an incubation period of 24 h, the cells were rinsed twice with
phosphate buffered saline and harvested for assay of cellular HMG-CoA reductase
activity as described in Cavance et al 1981 J Biol Chem 256, 2675. In brief, cell
Iysate were incubated in 200 mM potassium phosphate, 20 mM dithiotheritol, 40
mM glucose-6-phosphate, 5 mM NADPH, and 5 units/ml of glucose-6-phosphate
dehydrogenase. After a 15-min preincubation at 37~C, 0.9 nmoltL [14C]HMG-CoA
(57mCi/mmol) and unlabelled HMG-CoA (the final concentration of HMG-CoA
was 100 ~M) were added for a 60-min incubation at 37~C. The final reaction
volume for each sample was 60 ,~LL. The reaction was stopped by addition of 5 ~L S
M HCI which also allowed lactonization of the produced [14C]mevalonate. After
addition of known amounts of [3H]mevalonolactone (internal standard) one ali~uot
CA 022~677 1998-11-19
WO 97/45440 PCTlSEg7/00936
was used to separate [14C]mevalonolactone from [14C]HMG-CoA by ion-exchange
chromatography (Edwards et al 1977 J. Lipid Res. 20, 40) and one aliquot was used
for spectrophotometric determination of protein content. The [3H]- and [14C]-
radioactivity was analyzed by a scintillation counter which was equipped with a
s program for automatic correction for quenching and spill-over (Beckman LS
5000TA).
Table VII shows the effects of LDL and oxysterols on HMG-CoA reductase in
normal and transformed fibroblasts. In particular it shows the effects of LDL (0.5-
0 8% FCS; cholesterol concentration 1.2 mM) and of oxysterols (0.12 ~LM in media
containing 10% LDS) on HMG-CoA reductase in normal and virus transformed
human fibroblasts.. The activities of HMG-CoA reductase were determined after
incubating the fibroblasts for 24 h. All cells were preincubated for 24 h in media
containing 10% LDS.
CA 022~677 l998-ll-l9
WO 97/45440 31 PCT/SEg7/00936
TABLE VII
Sl~ a Obs~ HMGCoA re-luc~ce ~Supp-
metabolismb a~livilyC ressiond
Norrnal Trans- %
cells formed
LDL cholesterol 27-hydroxylation15-31 40-88 32-57
C4-7a-ol-30ne 27-hydroxylation 26 50 24
C5-3,~,7,B-ol 7-oxidation or 39 60 21
27-hydroxylation
C5-3~-ol-7-one 27-hydroxylation 38 100 62
5a-C 8(14)-3l3-ol-15- 27-hydroxylation 22 41 19
one
C5-3,B,25-ol 7a-hydroxylation 37 77 40
C5-3~B,27-ol 7a-hydroxylation 33 79 46
Cs-3~,7a,25-ol 3-oxidation/ isomerizn 23 49 26
Cs-3,~,7a,27-ol 3-oxidation/ isomerizn 30 60 30
C4-27-ol-3-one 27-oxidation to acid 31 38 7
C4-7a,27-ol-3-one 27-oxidation toacid 33 34
Cs-3,B,27-ol-7-one 27-oxidation to acid 7 33 26
C4-25-ol-3-one none 33 37 4
C4-7a,25-ol-3-one none 26 31 5
CA4-7a-ol-3-one none 30 47 17
~ For abbreviations and steroid names see Table I
b Metabolism observed in normal human fibroblasts. The ~ctivities of
the enzymes catalysing 27-hydroxylation, 7a-hydroxylation and 3-
s oxidation with isomerisation of the 5-double bond of sterols were shown
to be reduced in tumour-transformed fibroblasts.
c % of control. The activities of HMG-CoA reductase in normal and
transformed fibroblasts were 58 and 96 pmol/min/mg protein
respectively.
CA 022~677 1998-11-19
WO 97/45440 32 PCT/SE97/00936
d Difference in degree of suppression of HMG-CoA reductase in normla
and transformed fibroblasts induced by LDL or the oxysterol
5 By relating the intracellular production of different oxysterols to the observed
~up~l~ssion of HMG-CoA reductase in normal and transformed fibroblasts and
using inhibitors of sterol-metabolizing enzymes, it was a~)pal~nl that:
1. LDL cholesterol and a number of oxysterols (including) 27-
o hydroxycholesterols, 3,B-hydroxy-Sa-cholest-8(14)-en-lS-one, and the
autooxidation products of cholesterol 25-hydroxycholesterol, 7-oxo-cholesterol, 70~-
hydroxycholesterol and 7,B-hydroxycholesterol), which have been considered to bepotent suppressors of HMG-CoA reductase, all appear to have to be metabolized
prior to being biologically active.
2. In contrast, their metabolites 7c~,27-dihydroxy-4-cholesten-3-one, 3,~,27-
dihydroxy-S-cholesten-7-one and 7a,25-dihydroxy-4-cholesten-3-one (and most
likely 3,~,27-dihydroxy-So~-cholest-8(14)-en-lS-one) do not seem to require further
metabolism in order to be active, i.e. are true suppressors of HMG-CoA reductase.
3. Of the above compounds, one was remarkably more potent in the
inhibition of HMG-CoA reductase and thus an additional aspect of the invention
provides the compound 3,B,27-dihydroxy-5-cholesten-7-one, preferably in
substantially pure form, for instance >75%, preferably > 90% pure and most
25 preferably >95% enantiomerically pure. This aspect of the invention further
provides the use in medicine of this compound and/or its active metabolites such as
the cholestenoate. A preferred use is in the manufacture of medicament for
suppressing HMG-CoA reductase activity in a cell or m~rnm~l, including humans.
Representative for this aspect of the invention include its a~lministration to a human
30 or mammal to reduce serum cholesterol and/or the biosynthesis of cholesterol.
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97/00936
33
4. Certain sterols with these structures also showed suppression of HMG-
CoA reductase in other human neoplastic cells, including breast carcinoma, colonic
carcinoma and m~lign~nt melanoma cells, which all displayed a defective regulatory
response to LDL. This is shown in Example 5.
Example 5:
Effects of LDL and oxysterols on HMG-CoA reductase in human mali~nant cells
Human breast carcinoma (MDA 231), colonic carcinoma (WiDr) and malignant
0 melanoma (SK-MEL-2) cell lines from American Type Culture Collection, U.S.A.,
were grown in monolayers in tissue culture flasks maintained in a 95% air/5% CO2atmosphere at 37~C in a humidified incubator and were cultured in either
Dulbecco's Modified Eagle's Medium (MDA 231 ) or Minimal Eagle's Medium (the
other cells) supplemented with essential and non-essential amino acids and 10%
fetal calf serum (FCS, from Life Technologies, Inc., Stockholm, Sweden). Cells
were then seeded in dishes (20 cm2) at a density of 5,000 cells per cm2 and the
media (5 ml) contained 10% FCS. The experiments were started 48-72 h later, at
which time a cell density of approximately 20,000 per cm~ had been reached (the
cells were subconfluent also ~t the end of the incubations). All cells were thenincubated for 24 h in medi~ (5 ml ) containing 10% lipoprotein deficient serum,
prepared by treating FCS with C;lh-O-Sil (Weinstein supra), before LDL (2% FCS;
cholesterol concentration, l.~ m~1) and oxysterols (tested at a concentration of 0.12
IlM) were added to the incubation media, the latter in freshly prepared etha,nolsolutions. The ethanol concentr~lions of the media then became 0.2%. Control cells
were incubated in the same way but without sterols. After an incubation period of 24
h, the cells were rinsed twice with phosphate buffered saline and harvested for assay
of cellular HMG-CoA reductase activity as described (Cavenel supra). In brief, cell
Iysates were incubated in 200 mM potassium phosphate, 20 mM dithiotheritol, 40
mM glucose-6-phosphate, 5 mM NADPH, and 5 units/mL of glucose-6-phosphate
dehydrogenase. After a 1 5-min preincubation at 37~C, 0.9 nmol/L l l 4C]HMG-CoA
(57 mCi/mmol) and unlabelled HMG-CoA (the final concentration of HMG-CoA
was 100 ~LM) were added for a 60-min incubation at 37~C. The final reaction
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97/00936
34
volume for each sample was 60 ~LL. The reaction was stopped by addition of 5 ,uL 5
M HCI which also allowed lactonization of the produced [14C]mevalonate. After
addition of known amounts of [3H]mevalonolactone (internal standard) one aliquotwas used to separate [14C]mevalonolactone from [14C]HMG-CoA by ion-exchange
s chromatography (Edwards supra) and one aliquot was used for spectrophotometricdetermination of protein content. The [3H]- and [ 14C]-radioactivity was analyzed
by a scintillation counter which was equipped with a program for automatic
correction for quenching and spill-over (Beckman LS 5000TA).
lo Table VIII shows the effects of LDL and oxysterols on HMG-CoA reductase in
human malignant cells. ~n particular it shows the effects of LDL (2% FCS;
cholesterol concentration 1.2 mM and of selected oxysterols (0.12 ~lM in media
containing 10% LDS) on HMG-CoA reductase in breast and colonic carcinoma cells
and malignant melanoma cells after incubation for 24 hours. All cells had been pre-
s incubated for 24 h in media containing 10% LDS. For comparison the
corresponding values on transformed human fibroblasts are also shown.
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97/00936
3s
TABLE vm Activity of HMG-CoA reductase
Structurea Breast Colonic Malignant Trans- Median
carcinoma carcinoma melanoma formedb
cells cells cells fibroblasts
3~-hvdroxv-~S sterols '~o of contro~
Cs-3~-ol 67 79 121 68 74
(LDL cholesterol)
Cs-3,B-ol-7-one 96 91 123 100 98
SaC~('4)3-,B-ol-lS- 66 80 101 41 73
one
C -3~,25-ol - 94 d 77 86
C5-3~,27-ol 75 86 75 79 77
C -3~,7~c,27-ol - 74 73 60 73
3-oxo-~4 sterols
C4-25-ol-3-one 43 55 _d 37 43
C4-25-ol-3-one 48 54 S9 38 S l
;l C, cholestane; ~Up~l~Clipt indicates postion of double bond; Greek
letters denote configuration of hydroxyl groups.
b For comparison, the values of tumor transformed human fibroblasts are
S also shown (see Example 4). The corresponding values for normal
fibroblasts were 18-38%.
c The activities of HMG-CoA reductase in breast carcinoma cells,
colonic carcinoma cells, malignant melanoma cells and transformed
fibroblasts were S9, 120, 137 and 86 pmol/min per mg protein
o respectively.
d not studied.
The results show that the compounds of this type are potent suppressors of
cholesterol production in many different cells, including those having low activities
of sterol-metabolizing enzymes. This is in contrast to most other oxysterols that
have been used as HMG-CoA reductase suppressors. Like tumor cells, normal cells
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97/00936
36
such as macrophages, a~pare~ y lack the 70~-hydroxylating enzyme, which is
required for the forrnation of HMG-CoA reductase suppressors from cholesterol orside-chain hydroxylated 3,B-hydroxy-5 sterols. These cells are believed to play an
important role in the development of atherosclerotic plaques when located in thes blood vessel wall Although the mech~ni.cms behind the development of
atherosclerosis are not known in detail, excessive accumulation of esterified
cholesterol in blood vessel wall cells is believed to be of major importance. Inrelation to this it should be pointed out that we have not found any evidence that this
group of sterols stimulates esterification of cholesterol in cells, a reaction which
0 seems to be triggered by side-chain hydroxylated 3,~-hydroxy-~5 sterols (e.g. 25-
hydroxycholesterol). Furthermore, in contrast to the conventional pharmacological
treatment of hypercholesterolemia and atherosclerosis using competitive inhibitors
of HMG-CoA reductase (compactin or lovastatin (mevinolin)), the described group
of sterols also reduces the cellular uptake of LDL-cholesterol by suppressing the
5 number of LDL receptors on the cell surface. This is shown in Example 6.
Example 6:
Effects of oxysterols on LDL-receptors of normal human fibroblasts
Normal human fibroblasts (line GM 08333) from NiGMS, Corriell Institute for
Medical Research (Camden, NJ), were grown in monolayers in tissue culture flasksmaintained in 95% air/5% Co2 atmosphere at 37~C in an humidified incubator.
Cells were cultured in Dulbecco's Minimal Eagle's Medium supplemented with
essential and non-essential amino acids and 10% fetal calf serum (FCS, from LifeTechnologies, Inc., Stockholm, Sweden). Cells were then seeded at a density of
6,000 cells per cm~ in 50 cm2 dishes in the same medium (5 ml). The experiment
was started 96 h later, at which time a cell density of approximately 20,000 per cm2
had been reached (the cells were subconfluent also at the end of the incubations).
The effect of the sterols on LDL-receptor activity (rate of high-affinity = receptor
mediated degradation of LDL) of cells was then tested. The cells were washed twice
in phosphate buffered saline and were incubated with 5 ml of medium containing
lO~o lipoprotein deficient serum (LDS) prepared by treating ~;CS with Cab-O-Sil
CA 022~677 1998-11-19
wO 97/45440 PCT/SE97/00936
37
(Weinstein supra). (Cell growth was not negatively affected when this serum was
used). The sterols 24-hydroxy-4-cholesten-3-one, 25-hydroxy-4-cholesten-3-one and
27-hydroxy-4-cholesten-3-one were then added to the incubation media
(concentration I .1 llM) in freshly prepared ethanol solutions and the ethanol
s concentrations of the media were then 0.2%. To the control cells were added the
same volume of ethanol but without the sterols. After incubation for 19 h, 50 ~g of
125I-labeled LDL (specific activity 150-380 cpm/ng of protein, prepared from
Nal25I (from Amersham, specific activity >350 Ci/mol) as described in Langer et al
1972 J Clin Invest 51, 1528 and containing less than 1% of radioactivity as freeo iodide) was added to the medium and the incubations continued for additional 4 h.
Cellular degradation of ~25I-labeled LDL was then determined from the formation of
acid-soluble radioactivity in the incubation medium (Vitols et al 1984 Blood 63
1186). All incubations were carried out in duplicate. The degradation of 125I-
labeled LDL in the control cells were found to be 105-109 ng/h x mg cell protein.
The corresponding values for cells treated with 24-hydroxy-4-cholesten-3-one, 25-
hydroxy-4-cholesten-3-one and 27-hydroxy-4-cholesten-3-one were 40-41, 58-59
and 62-69 ng/h x mg cell protein, respectively. This shows that the sterols reduced
the LDL-receptor activity of the cells by approximately 50% under the conditionsused.
Since it is known that cell growth is dependent on the production of mevalonate, the
effects of various oxysterols, including the "new" group of potent HMG-CoA
reductase suppressors, on proliferation and viability of normal and tumor-
transformed human fibroblasts were also tested. In addition to the sterols described
2s in previous examples, several sterols with a 7,B-hydroxy group were included in the
tests. Although these were potential metabolites of cholesterol, many of them were
not produced in detectable amounts in fibroblasts (e.g. sterols with 7~-hydroxy-3-
oxo-~4 structure). However, we have previously shown that 7~B-hydroxysterols canbe formed from the corresponding 7a-hydroxy sterols when incubated with isolatedhuman liver mitochondria (Shoda (1993) Hepatology 17, 395).
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97/00936
38
The effects of oxysterols on growth and viability of human fibroblasts are shown in
Example 7. The concentrations of oxysterols used were intentionally much lower
(1/5 - 1/10) than used in most other studies on cell growth inhibition or toxicity of
oxysterols (Smith et al (1989) Free Radical Biology & Medicine, 7 285). This was5 because we did not want to cause non-specific damages to cell membranes
(Sevanian (1986) Fd. Chem. Toxic. 24, 1103) (the oxysterols can "replace"
cholesterol in the membranes) and thereby induce cell death. In fact, the
concentration of 1.25 ~LM used was similar to that required for suppression of LDL-
receptors on the cells (see Example 3).
Example 7:
Effects of ox~/sterols on proliferation and viabilitv of normal and tumor transformed
human fibroblasts
Normal human fibroblasts (line GM 08333) and SV-40 virus transformed human
fibroblasts (90-VA VI) were grown in monolayers in tissue cultered flasks
rn~int~ined in a 95% air/5% CO2 atmosphere at 37~C in a humidified incubator andwere cultured in Dulbecco's Minimal Eagle's Medium supplemented with essential
and non-essential amino acids and 10% fetal calf serum (FCS). The experiments
were started 24-48 h after seeding, at which time a cell density of approximately
10,000 per cm2 had been reached (the cells were subconfluent also at the end of the
incubations). All cells were then incubated for 24 h in the media (2 ml) lackingserum, before oxysterols (tested at concentrations 1.25 ~M and 2.5 ,uM) were added
to the incubation media in freshly prepared ethanol solutions. The ethanol
concentrations of the media then became 0.5-1.0%. Control cells vere incubated
with the same volumes of ethanol but without sterols. The proliferation of cells was
registered by counting in a light microscope the cell number in marked areas in the
dishes after incubations for 48 h (sterol concentration 2.5 ~M) or 72 h (sterol
concentration 1.25 ~M) and cell death was recognized microscopically as
detachment or Iysis of cells in the monolayer cultures.
CA 02255677 1998-11-19
WO 97/45440 PCT/SE97/00936
39
TABLE IX Cell viability (%d)
StructureD Normalcells Transformedcells
1.25 ~M 2.5 ,uM 1.25 ~M 2.5 ~I
72hC 48hC 72hC 48hC
Control 127 123 160 150
3,B-hydroxy-~5 sterols
Cs-3,~,25-ol 91 117 45 179
Cs-3,~,27-ol 97 117 83 144
3,B-7a-dihydroxy-~s sterols
Cs-3,B,7a-ol 60 140 40 65
Cs-3~,7a,25-ol 119 117 43 62
C5-3~,7a,27-ole 94 105 43 27
3~,7~-dihydroxy-~5 sterols
C -3,B,7,B-ol 156 77 144 131
C -3,B,7~,25-ol 140 62 34 36
Cs-3~,7,~,27-ol 121 138 130 137
3,B-hydroxy-7-oxo-~ssterols
Cs-3,B-ol-7-one 163 128 118 209
C5-3~,27-ol-7-one 112 117 64 102
3-oxo-~4 sterols
C4-25-ol-3-one 85 127 46 162
C4-27-ol-3-one 91 117 58 135
7a-hydroxy-3-oxo-~4sterols
C4-7a-ol-3-one 127 92 78 45
C4-7a,25-ol-3-one 119 76 6 0
C4 7a,27-ol-3-onee 108 87 83 93
7~-hydroxy-3-oxo-~4sterols
C4-7,B-ol-3-one 164 89 90 74
C4-7,B,25-ol-3-one 121 g7 10 0
C4-7,B,27-ol-3-one 122 62 11
CA 022SS677 1998-11-19
WO 97/45440 PCT/SE97/00936
a C, cholestane; superscript indicates position of double bond; Greek
letters denote configuration of hydroxyl groups.
b Concentration of sterol in medium
c Incubation period
S d Number of viable cells in relation to the number of cells at the
beginning of the experiment (in %)
e Inconsistent effects of sterol on transforrned fibroblasts (noted in other
experiments)
o It is ~pa~ t from the above that:
1. Tumor-transforrned fibroblasts were selectively affected by the
oxysterols when compared with normal cells, resulting in not only ceased growth
but also in cell death of the forrner cells. The effect on the normal cells was
relatively small.
2. 7-hydroxylated sterols are more potent than sterols lacking a hydroxyl
group or having an oxo group in this position. Surprisingly, the latter group included
the potent HMG-CoA reductase suppressors 25-hydroxy-4-cholesten-3-one, 27-
20 hydroxy-4-cholesten-3-one and 3,~,27-dihydroxy-5-cholesten-7-one, suggesting that
the induction of cell death was nol mainly due to a suppression of HMG-CoA
reductase.
3. Sterols with an hydroxy group in the side chain were more potent than
2s the corresponding sterols without such a group.
4. Highly potent inducers of death in the transforrned cells were 7-
hydroxy-~4-sterols with a hydroxyl group in the side chain (i.e. 7a,25-dihydroxy-4-
cholesten-3-one, 7~,25-dihydroxy-4-cholesten-3-one and 7~,27-dihydroxy-4-
30 cholesten-3-one). One of the sterols belonging to this group, 7cc,27-dihydroxy-4-
cholesten-3-one, was less potent in this assay than the others. Possibly, this was due
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97/00936
to a more rapid metabolism (it was converted into the corresponding C27-acid) bythe transformed fibroblasts.
5. The strong lethal effect of 7-hydroxy-~4-sterols with a hydroxyl group in
s the side chain was not limited to tumor-transformed fibroblasts, but was also seen in
other human malignant cells, as shown in Example 8 (tested with 7~,27-dihydroxy-4-cholesten-3-one) .
Example 8:
o Effects of 7~27-dihydroxv-4-cholesten-3-one on proliferation and viability of human mali~nant cells
Human colonic carcinoma (WiDr) and malignant melanoma (SKMEL-2) cell lines
from American Type Culture Collection, U.S.A., and SV-40 virus transformed
human fibroblasts (90-VA VI), a kind gift from dr. Stein (University of Colorado,
Boulder, CO, U.S.A.), were grown in monolayers in tissue cultured flasks
maintained in a 95% air/5% CO2 atmosphere at 37~C in a humidified incubator and
were cultured in Dulbecco's Minimal Eagle's Medium supplemented with essential
and non-essential amino acids and 10% fetal calf serum (FCS, from Life
20 Technologies, Inc., Stockholm, Sweden). Cells were seeded at a density of 5,000
cells per cm2 in 9 cm2 dishes in the media (2 ml ) containing 10% FCS. The
experiments were started 24-48 h later, at which time a cell density of approximately
10,000 per cm2 had been reached (the cells were subconfluent also at the end of the
incubations). All cells were then incubated for 24 h in the media (2 ml) lacking2s serum, before 7~,27-dihydroxy-4-cholesten-3-one (and 5-cholestene-3,~,7~,27-triol
for comparison) (tested at the concentration 2.5 ~lM) were added to the incubation
media in freshly prepared ethanol solutions. The ethanol concentration of the media
then became 1.0%. Control cells were incubated with the same volumes of ethanol
but without sterols. The proliferation of cells was registered by counting in a light
microscope the cell number in marked areas in the dishes after incubations for 24 h
and 48 h, and cell death was recognized microscopically as detachment or lysis of
cells in the monolayer cultures.
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97/00936
42
TABLL X
Sterol structurell Cell viability (%)c
Coloniccarcinoma Malignant Transformed
cells melanoma cells fibroblasts
24 h 48 h 24 h 48 h 24 h 48 hb
None = Control 126 87 122 113 123 95
C5-3~,7~,27-ol 94 124 128 157 88 86
C4-7~,27-ol-3-one 35 24 86 26 42 0
;' C, cholestane; superscript indicates position of double bond; Greek
letters denote configuration of hydroxyl groups.
b Incubation period
c Number of viable cells in relation to the number of cells at the
beginning of the experiment (in %)
The results clearly show that the compounds of the invention are potent inducers of
o death in both human colonic and malignant melanoma cells. It should be pointed out
that malignant melanoma is a form of cancer that is extremely resistant to
chemotherapy.
Because of the high potency of these sterols and their selectivity in toxicity
(affecting tumor-transformed human cells), they can be expected to be highly useful
for treatment of various cancer diseases, in addition to those tested above.
Furthermore, because of their effects on cell growth one may expect that these
sterols should also be useful for treatment of diseases caused by abnormally fast-
growing non-tumor cells and psoriasis, particularly when the sterols can be applied
locally.
CA 022C7C7677 1998-11-19
WO 97/45440 PCTISE97/00936
Example 9
Synthesis of 7~B.25-dihydroxy-4-cholesten-3-one and 70c,25-dihydroxy-4-cholesten-
3-one
a) Preparation of 25-hydroxycholestrol acetate
The acetate is prepared from 2 mg dried commercially available 25-
hydroxycholesterol (Sigma) to which 0.5 ml pylidine is added prior to ultrasound.
0.5 ml acetic anhydride is added and the mixture resubjected to ultrasound. The
reaction mixture is allowed to stand at room temperature for 2.5 hours and the
reaction is quenched with 5 ml H20 and allowed to stand for l 5 minutes. 3 ml ethyl
acetate is added and the mixture agitated and subjected to ultrasound and then
centrifuged for several minutes. The aqueous phase (the bottom phase) is transferred
to a fresh vessel and saved. 3 ml of H2O is added to the ethyl acetate phase and the
5 mixture is shaken/ultrasounded/centrifuged and decanted to the vessel. This
procedure is repeated to result in an aqueous phase of 3 x 3 ml.
b) Trimethylsilylation
The resulting ethyl acetate phase is rinsed with a small amount of ethyl acetate into a
20 fresh vessel and blown dry. The trimethylsilyl ether (TMS) of the 25 hydroxy group
is prepared by subjecting the product to 0.5 ml pyridine/hexamethyldisilane/
trimethylchlorosilazane1 3:2:1 which is allowed to react at 60~ for 30 minutes. The
product is blown dry and dissolved in hexane, ultrasounded and transferred to a 25
ml Florence flask with a little hexane.
c) TBB-oxidation
The resulting product is subject to TBB-oxidation as follows:
The hexane phase is blown dry, dissolved in l.5 ml concentrated acetic acid and
30 ultrasounded. 1.5 mg copper II bromide, lO ,ul TBB (tert-butylperbenzoate) and an
agitating bead is added and the reaction proceeds under N2 at 100~C for 5 minutes.
The product is allowed to cool and is transferred to a separating funnel. The flask is
CA 022=,=,677 1998-11-19
wo 97/45440 PCT/SE97/00936
44
rinsed out into the funnel with 5 ml H2O, then 40 ml hexane. The mixture is agitated
and the aqueous phase discarded. 5 ml of 5% NaHCO3 is added and the product
agitated, while allowing for gas evolution. The bicarbonate phase is discarded. The
latter step is repeated. The product is washed twice with 5 ml H2O. Th pH should be
5 neutral. The hexane phase is transferred to a Florence flask and blown dry.
d) Hydrolysis
The resulting product is hydrolysed as follows:
0 5 ml of 5% KOH in methanol is added, ultrsounded and the mixture is incubated for
1 hour at 50~C in a water bath with gentle agitation. The reaction is 4uenched with 5
ml H2O which is mixed and the product is neutralised to ca. pH 7 with concentrated
acetic acid. The product is subject to a C18 column (ODS silica) (1.5 x 0.8 cm)
rinsed with 10 ml H2O. The product is eluted with 8 ml methanol, evaporated and
dissolved in I ml methanol. 10~1 is retained for GLC.
e) Purification
The resulting product is purified on a Unisil column (Clarkson Chem Co,
Williamsport PA, USA) (mesh 200-325, 3 x 0.8 cm packed in hexane, washed with
20 5 ml hexane/ethyl acetate 1.1) after being evaporated and dissolved in 3 ml
hexane/ethyl acetate 1:1. 11 fractions are collected as follows:
product (3 ml hexane/ethyl acetate 1:1) & additional 3 ml
hexane/ethyl acetate 1:1
2,3 2 x 3 ml hexane/ethyl acetate 40:60
4,5 2 x 3 ml hexane/ethyl acetate 30:70
6,7 2 x 3 ml hexane/ethyl acetate 20:80
8,9 2 x 3 ml hexane/ethyl acetate 10:90
6 ml ehtyl acetate
11 6 ml methanol
30 The flask is rinsed with all fractions, the fractions are evaporated and dissolved in
0.5 ml methanol. 20 ~1 is retained for GLC. The remainder is stored at -70~C
CA 022~677 1998-11-19
WO 97/45440 PCT/SE97/00936
pending GLC analysis. Fractions 4 and 5 contain 5-cholestene-3~,7,~,25 triol.
Fractions 6, 7 & 8 contain 5-cholestene-3,B,7cc,25-triol.
f~ Oxidation with cholesterol oxidase
The 3,B-hydroxy group is oxidised and the 5-double bond isomerised) via cholesterol
oxidase (C-1512, Sigma) as follows. Fractions 4 and 5 are dissolved in 0.5 ml
isopropanol. 6 ml of 0.5 M phosphate buffer, pE~ 7.0 and 5 U cholesterol oxidaseadded. The mixture is incubated at 37~C for 3 hours with gentle agitation. The
reaction is then quenched with 9 ml methanol and subjected to the C18 column,
0 (1.5 x 0.8 cm). The eluent is pooled and evaporated until H2O remains (ca 6 ml). I
ml methanol is added and after mixing the product is resubjected to the same C18column. When the product has gone through, the column is rinsed with 10 ml H2O
(2+2+6) and eluted with 8 ml methanol direct into a Florence flask. The product is
evaporated, transferred to test tube with methanol, evaportated and dissolved in 0.5
ml ethanol. 20~1 is retained for GLC and GS/MS.
g) HPLC Purification
The resulting 7a,25-diydroxy-4-cholesten-3-one and 7~,25-diydroxy-4-cholesten-3-one are then purified with revcrsed phase HPLC (column: LiChrosper, 250 x 4 mm,
20 Hibar, 100 RP-18, 5 ,u, Merck) in 85% methanol, UV detector at 240 nm, retention
time 7.3 minutes for the 7,B anomcr and 7.8 minutes for the 7a anomer. The effluent
is collected over a Florence fl~k. cv~porated, transferred to a test tube with
methanol, evaporated and dissol~ed in 0.5 ml ethanol. 20 ,ul is retained forGLC and
GC/MS.
CA 022~677 1998-11-19
wO 97/45440 PCT/SEg7/00936
46
Example 10
Preparation of 7,~,27-dihydroxy-4-cholesten-3-one
A Preparation of 27-hydroxycholesterol
a) A Clemmensen reduction of diosgenin is carried out with fresh zink
amalgam prepared from 60 g zink filings, 4.5 g mercury II chloride, 3.0 ml conc.HCI and 75 ml H20. The mixture is agitated in a multinecked round flask for 5
minutes and decanted. 200 ml ethanol and 1.2 g diosgenin (Sigma) is added and
0 refluxed. Over 45 minutes 60 ml conc. HCI is added dropwise, followed by 15 min
continued reflux. The mixture is allowed to cool to room temperature. 1.5 1 ice-cold
water is added dropwise and the reaction allowed to stand under refrigeration for
around an hour. The water is then filtered away and the dry mass transferred to a
Florence flask. 30 ml diethyl ether is added and the mixture stirred at room
temperature for lo minutes. The ether is filtered off and mass transferred to a small
evaporating flask an dissolved in a minimum of ethyl acetate using a little warmwater. Crystallisation is commenced firstly at room tenlpe,dtuie and later overnight
in the coolroom to afford tetrahydrodiosgenin (16~,27-dihydroxycholesterol).
b) Chromo-oxidation
The product is oxidised in 4 batches as follows. 20 mg chrome trioxide in 0.1 mlH2O and 0.2 ml acetic acid is added dropwise to a stirred mixture of 125 mg
tetrahydrodiosgenin and 0.62 g NaAc in 22.5 ml glacial acetate. The reaction
continues for 18 hours at 25 ~C. A few drops of methanol are then added to destroy
excess reagent. The mixture is diluted with 25 ml cold water and extracted with 40
ml MeCl2. The methylene chloride phase is rinsed with around 10 ml H2O and then
with 5% sodium bicarbonate and H2O until neutral by pH paper. The MeCI2 is driedwith a spoon of waterfree Na2SO4 and the MeCI2 phase filtered down into a
Florence flask and evaporated. A GLC sample retained. The remaining product is
subjected to a silicon column 8 x 0.8 cm in 30% ethyl acetate/TMP
(trimethylpentane) in one fif~h aliquots. The fractionation comprises:
5 x 10 ml 30% ethyl acetate/TMP
CA 0225~677 1998-11-19
WO 97/45440 PCTISE97/00936
47
10 x 5 ml 405'o ethyl acetate/TMP.
Around 0.1% of each fraction is assayed by GLC and the fractions containing 16-
keto-27-hydroxycholesterol are pooled (generally fractions 3-6 of the 40% blend).
s c) Reduction of 16-keto-27 hydroxycholesterol
The resulting product from step b) (170 mg) is transferred to a small Plorence flask
and 0.1 1 g KOH in 3.5 ml triethylene glycol, 0.1 ml hydrazine and a couple of
agitation beads are added. The mixture is refluxed for 15 minutes and then
refrigerated for I hour. The mixture is dropwise added to 15 ml 0.5 M HCI, filtered
0 and rinsed with ice cold water. The dry material is transferred to a small evaporating
flask and recrystallised with ethyl acetate. Purity is assayed by GLC & GC/MS.
Yield: 75 mg 27-hydroxycholesterol, 45 mg as clystals.
B Preparation of 7~,27-dihydroxy-4-cholesten-3-one
a) The acetate of the product of step A is prepared in the same manner as
Example 9 step a), with the exception that the reaction was quenched with 5 ml
H2O. The TMS step b) of Example 9 is omitted.
20 b) The resulting ethyl acetate phase is blown dry and subject to Na2CrO4oxidation by the addition of 500 ~I concentrated acetic acid and 200 ~11 Na2CrO4.
The reaction proceeds overnight at room temperature with a magnetic stirrer. Theoxidation is stopped with 3ml of H2O added cautiously to the reaction vessel sitting
in an ice bath in a ventilation hood. The mixture is allowed to stand for 15 minutes
25 and is then neutralised to pH 7 with lM NaOH.
c) The resulting product is blown dry and reduced with 3 mg NaBH4
following after being dissolved in I ml dry ethanol (from a freshly opened bottle).
The reaction proceeds in an ultrasound bath for 30 minutes and then around 1.5
30 hours at room temperature. The reaction is quenched with 100 ml acetone and 3 ml
H2O. The product is subjected to a rinsed C18 column with 5 ml H2O. The product
CA 022~677 1998-11-19
WO 97/45440 PCTISE97/00936
48
is eluted with 8 ml methanol (2+2+4 ml)into a Florence flask. 50 ,ul is assayed via
TMS and GLC.
d) The resulting product is evaporated dry and hydrolysed via the addition
of 0.3 ml isoplopallol and ultrasound, followed by the addition of 3 ml methanol.
259 ml of 12.5 M NaOH is added mixed and ultrasounded. The product is incubated
at 50~C for 2 hours in a water bath. The reaction is stopped by blending in 6 mlH2O. The product is neutralised to pH 7 with conc. acetic acid and then sub3ected to
a rinsed C18 column. The 4 fractions comprise:
0 1 product
2 10 ml H2O via round flask & ultrasound (2+2+6 ml)
3 5 ml 30% methanol (do.)
4 product is eluted with 10 ml methanol (do.)
50 ~1 is assayed via TMS and GLC.
e) The remainder of the product is purified on a Unisil column as
described in example 9 part d, wherein the 50 ,ul check samples are assayed byGLC
after TMS treatment. Fraction 6 contains 5-cholestene-3,~,7~,27 triol and fraction 8
contains 5-cholestene-3~,7a,27 triol.
e) Fraction 6 from step d) is oxidised by cholesterol oxidase as described in
example 9, step e) and the resultant 7,B,27-dihydroxy-4-cholesten-3-one purified by
HPLC as described in example 9. Retention time 8.7 minutes.