Note: Descriptions are shown in the official language in which they were submitted.
FIL~ J~ T'II~AM~E~
-'I L, ,~ N -LA
S016-974PCT.200
Case 1/1024-Dr. Wy/ks
New triazolopurines, processes for preparing them and
their use as ph~r~-ceutical compositions
The invention relates to new triazolopurine derivatives
and fundamental triazolopurine substances, processes for
preparing them and their use as pharmaceutical
compositions.
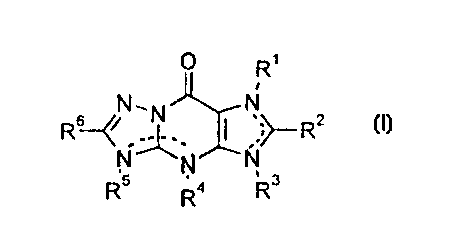
-,' The new triazolopurine derivatives have the structure of
general formula (I):
NX~,~R2
R R4 R
(I)
wherein
Rl or R3 denotes hydrogen, an optionally substituted
~- Cll0-alkyl, C2l0-alkenyl or C2l0-alkynyl, an
optionally substituted C38-cycloalkyl, an
optionally substituted C48-cycloalkenyl or an
optionally substituted C68-cycloalkynyl;
Rl or R3 denotes an optionally substituted C6l0-aryl-Cl6-
alkyl~ C6l0-aryl-c2-6-alkenyll C6l0-aryl-
C26-alkynyl, an optionally substituted C6l0-aryl
or an optionally substituted heteroaryl, an
optionally substituted 5-, 6- or 7-membered
heterocyclic group which contains as heteroatoms
one or more atoms from the group comprising
CA 022~6736 1998-12-02
nitrogen, oxygen or sulphur, the heterocycle
being linked via a carbon atom of the ring;
R1 or R3 denotes an optionally substituted
Cl10-alkyloxycarbonyl, an optionally substituted
C2l0-alkenyloxycarbonyl, an optionally
substituted C210-alkynyloxycarbonyl, an
optionally substituted C3a-cycloalkyloxy-
carbonyl, an optionally substituted
C610-aryloxycarbonyl, an optionally substituted
C6l0-aryl-Cl6-alkyloxycarbonyl, A-O-CO- wherein A
denotes an optionally substituted 5-, 6- or 7-
} membered heterocyclic group which contains as
heteroatoms one or more atoms selected from the
group comprising nitrogen, oxygen or sulphur,
wherein A is linked via a carbon atom of the
ring,
Rl or R3 is an optionally substituted C110-alkyl-
sulphonyl, C210-alkenylsulphonyl,
C210-alkynylsulphonyl, an optionally substituted
C3a-cycloalkylsulphonyl, an optionally
substituted C6l0-arylsulphonyl, an optionally
substituted C610-aryl-C16-alkylsulphonyl, a group
A-SO2- wherein A is as hereinbefore defined and
is linked via a carbon atom of the ring;
R1 or R3 denotes an optionally substituted
C110-alkylcarbonyl, an optionally substituted
C210-alkenylcarbonyl, an optionally substituted
C210-alkynylcarbonyl, an optionally substituted
C38-cycloalkylcarbonyl, an optionally
substituted C610-arylcarbonyl, an optionally
substituted C610-aryl-C16-alkylcarbonyl, a group
A-CO- wherein A is as hereinbefore defined;
R1 or R3 denotes a group of general formula
CA 022~6736 1998-12-02
H~
wherein
R denotes hydrogen, phenyl, substituted phenyl, an
optionally substituted benzyl, an optionally
substituted C36-cycloalkyl group, a branched or
unbranched Cl10-alkyl, preferably Cl4-alkyl,
C2l0-alkenyl or C2l0-alkynyl group which may
optionally be substituted by hydroxy, phenyl,
substituted phenyl, amino or substituted amino;
1, .
R2 denotes hydrogen, hydroxy, amino, halogen,
nitro, CF3, COOH, mercapto, Cl6-alkylmercapto,
an optionally substituted Cll0-alkyl, an
optionally substituted C2l0-alkenyl, an
optionally substituted C210-alkynyl, an
optionally substituted C38-cycloalkyl, an
optionally substituted C58-cycloalkanone, an
optionally substituted C48-cycloalkenyl, an
optionally substituted C68-cycloalkynyl, an
optionally substituted C6l0-aryl, an optionally
substituted C6l0-aryl-Cl6-alkyl, C6l0-aryl-
'~ C26-alkenyl, C6-lo-aryl-c2-6-alkynyl~
C38-cycloalkyl-Cl6-alkyl, C38-cycloalkyl-
C26-alkenyl, or C38-cycloalkyl-C26-alkynyl;
R2 denotes an optionally substituted 5-, 6- or 7-
membered heterocyclic group which contains as
heteroatom one or more atoms selected from the
group comprising nitrogen, oxygen or sulphur,
wherein the nitrogen may optionally be
substituted and the heterocyclic group may be
linked via a carbon or nitrogen atom directly or
via a Cl6-alkyl, C26-alkenyl or C26-alkynyl
~ 35 bridge;
CA 022~6736 1998-12-02
R2 denotes an optionally substituted
C110-alkylsulphonyl, C210-alkenylsulphonyl or
C210-alkynylsulphonyl, an optionally substituted
C38-cycloalkylsulphonyl, an optionally
substituted C610-arylsulphonyl, an optionally
substituted heteroarylsulphonyl or an optionally
substituted C610-aryl-C16-alkylsulphonyl;
R2 denotes an optionally substituted
Cl1O-alkylsulphonyloxy, C2l0-alkenylsulphonyloxy
or C210-alkynylsulphonyloxy, an optionally
- substituted C38-cycloalkylsulphonyloxy, an
optionally substituted C610-arylsulphonyloxy, an
optionally substituted C610-aryl-C16-
alkylsulphonyloxy or an optionally substituted
heteroarylsulphonyloxy;
R2 denotes an optionally substituted
Cl1O-alkylsulphonylamino, C2l0-alkenylsulphonyl-
amino or C210-alkynylsulphonylamino, an
optionally substituted C3B-cycloalkyl-
sulphonylamino, an optionally substituted
. C610-arylsulphonylamino, an optionally
,j substituted C610-aryl-C16-alkylsulphonylamino, or
an optionally substituted heteroarylsulphonyl-
amino;
R2 denotes an optionally substituted
Cl1O-alkylaminocarbonyl, Cl1O-dialkylamino-
carbonyl, C110-alkyl- C2-1o- alkenylaminocarbonyl,
C2l0-alkenylaminocarbonyl, C2l0-dialkenylamino-
carbonyl, C210-alkynylaminocarbonyl or
C11O-alkyl-C210-alkynylaminocarbonyl, an
optionally substituted C38-cycloalkyl-
aminocarbonyl, an optionally substituted
C610-arylaminocarbonyl, an optionally
substituted C610-aryl-C16-alkylaminocarbonyl, or
CA 022~6736 1998-12-02
an optionally substituted heteroarylamino-
carbonyl;
R2 denotes an optionally substituted
C110-alkyloxycarbonylamino, C210-alkenyloxy-
carbonylamino or C210-alkynyloxycarbonylamino,
an optionally substituted C38-cycloalkyloxy-
carbonylamino, an optionally substituted
C610-aryloxycarbonylamino, an optionally
substituted C6-10 - aryl- Cl-6 - alkyloxycarbonylamino
or an optionally substituted heteroaryloxy-
. carbonylamino;
i
R2 denotes an optionally substituted
C110-alkylaminocarbonyloxy, C210-alkenylamino-
carbonyloxy or C210-alkynylaminocarbonyloxy, an
optionally substituted C38-cycloalkylamino-
carbonyloxy, an optionally substituted
C610-arylaminocarbonyloxy, an optionally
substituted C610-aryl-C16-alkylaminocarbonyloxy
or an optionally substituted heteroarylamino-
carbonyloxy;
R2 denotes an optionally substituted C110-alkyl-N-
i 25 amidino, C210-alkenyl-N-amidino or C210-alkynyl-
N-amidino, an optionally substituted
C38-cycloalkyl-N-amidino, an optionally
substituted C610-aryl-N-amidino, an optionally
substituted C610-aryl-C16-alkyl-N-amidino or an
optionally substituted heteroaryl-N-amidino;
R2 denotes an optionally substituted C110-alkyloxy,
C210-alkenyloxy or C2-10-alkynyloxy, an optionally
substituted C38-cycloalkyloxy, an optionally
substituted C610-aryloxy, an optionally
sub~tituted C610-aryl-C16-alkyloxy or an
optionally substituted heteroaryloxy;
CA 022~6736 1998-12-02
.. . . . ..
R2 denotes an optionally substituted
C110-alkyloxycarbonyl, C210-alkenyloxycarbonyl or
C210-alkynyloxycarbonyl, an optionally
substituted C38-cycloalkyloxycarbonyl, an
optionally substituted C610-aryloxycarbonyl, an
optionally substituted C6-10-aryl-C16-alkyloxy-
carbonyl or an optionally substituted
heteroaryloxycarbonyl;
R2 denotes an optionally substituted
- C110-alkylcarbonyloxy, C210-alkenylcarbonyloxy or
C210-alkynylcarbonyloxy, an optionally
substituted C38-cycloalkylcarbonyloxy, an
optionally substituted C610-arylcarbonyloxy, an
optionally substituted C610-aryl-C16-alkyl-
carbonyloxy or an optionally substituted
heteroarylcarbonyloxy;
20 R2 denotes an optionally substituted
C1l0-alkylthio, C2l0-alkenylthio or
C210-alkynylthio, an optionally substituted
C38-cycloalkylthio, an optionally substituted
C610-arylthio, an optionally substituted
C610-aryl-C16-alkylthio or an optionally
~~ substituted heteroarylthio;
R2 denotes an optionally substituted amine,
preferably NR8R9;
R2 denotes an optionally substituted group of the
formula
~ , (CH2)1,2 ~ (~1
CA 022~6736 1998-12-02
R2 denotes an optionally substituted group of the
formula
(CH2)1.2 ('-H2)-,2 (CH2),,2
-Y~ _y~ -Y~G~
wherein Y is a single bond or an alkylene, an
alkenylene or an alkynylene having up to 6,
- preferably up to 4 carbon atoms in the chain;
..
10 R2 denotes an optionally substituted group of the
formula
(CH2),,2 (~H2)-,2
-Y~ -Y~
C,-C6-AIkY C,-C6-AIkYI
wherein Y is a single bond or an alkylene, an
~ alkenylene or an alkynylene ha~ing up to 6,
'~ preferably up to 4 carbon atoms in the chain;
R2 denotes an optionally substituted group of the
formula
CA 022~6736 1998-12-02
_yZ~ CH2)0,1,2
~ 12 ~ Y ~
wherein Y is a single bond or an alkylene, an
~ alkenylene or an alkynylene having up to 6,
preferably up to 4 carbon atoms in the chain;
R4 or R5 denotes a hydrogen atom, an optionally
substituted Cl10-alkyl, C2l0-alkenyl or
C2l0-alkynyl, an optionally substituted
C38-cycloalkyl, an optionally substituted
C48-cycloalkenyl or an optionally substituted
C68-cycloalkynyl, an optionally substituted
C6-lo~arYl~Cl6~alkYl~ C6-lo-aryl-c2-6-alkenyll
C6l0-aryl-C26-alkynyl, an optionally substituted
C6l0-aryl or an optionally substituted
heteroaryl;
: 20 R4 or Rs denotes an optionally substituted 5-, 6- or 7-
~ membered heterocyclic group which contains as
heteroatoms one of more atoms selected from the
group comprising nitrogen, oxygen or sulphur,
the heterocyclic group being bound via a carbon
atom of the ring;
R4 or Rs denotes an optionally substituted
Cl10-alkyloxycarbonyl, an optionally substituted
C2l0-alkenyloxycarbonyl, an optionally
substituted C2l0-alkynyloxycarbonyl, an
optionally substituted C38-cycloalkyloxy-
carbonyl, an optionally substituted C6l0-aryl-
CA 022~6736 l998-l2-02
C16-alkyloxycarbonyl, an optionally substituted
C6_10 - aryloxycarbonyl;
R4 or R5 denotes A-0-C0- wherein A denotes an optionally
substituted 5-, 6- or 7-membered heterocyclic
group which contains as heteroatoms one or more
atoms selected from the group comprising
nitrogen, oxygen or sulphur;
R4 or Rs denotes an optionally substituted
Cl1O-alkylsulphonyl, C2l0-alkenylsulphonyl,
. C2l0-alkynylsulphonyl, an optionally substituted
( C38-cycloalkylsulphonyl, an optionally
substituted C6l0-arylsulphonyl, a group A-S02-
wherein A is as hereinbefore defined and is
linked via a carbon atom of the ring;
R4 or R5 denotes an optionally substituted
C110-alkylcarbonyl, an optionally substituted
C210-alkenylcarbonyl, an optionally substituted
C210-alkynylcarbonyl, an optionally substituted
C38-cycloalkylcarbonyl, an optionally
substituted C610-arylcarbonyl, an optionally
substituted C6l0-aryl-Cl6-alkylcarbonyl, a group
- 25 A-C0- wherein A is as hereinbefore defined;
R4 or R5 denotes a group of general formula
H~
,N ~
R O
wherein
R denotes hydrogen, phenyl, substituted phenyl, an
optionally substituted benzyl, an optionally
substituted C36-cycloalkyl group, a branched or
unbranched C110-alkyl, preferably C14-alkyl,
C210-alkenyl or C210-alkynyl group, which may
CA 022~6736 1998-12-02
optionally be substituted by hydroxy, phenyl,
substituted phenyl, amino or substituted amino;
R6 denotes hydrogen, hydroxy, -CHO, amino, halogen,
nitro, CF3, COOH, mercapto, Cl6-alkylmercapto,
an optionally substituted Cll0-alkyl, an
optionally substituted C2l0-alkenyl, an
optionally substituted C2l0-alkynyl, an
optionally substituted C38-cycloalkyl, an
optionally substituted Cs8-cycloalkanone, an
optionally substituted C48-cycloalkenyl, an
optionally substituted C68-cycloalkynyl, an
optionally substituted C6l0-aryl, an optionally
substituted C6l0-aryl-Cl6-alkyl, C6l0-aryl-
C26-alkenyl, C6l0-aryl-C26-alkynyl, an optionally
substituted C38-cycloalkyl-Cl6-alkyl,
C38-cycloalkyl-C26-alkenyl or C38-cycloalkyl-
C2 6-alkynyl;
20 R6 denotes an optionally substituted 5-, 6- or 7-
membered heterocyclic group which contains as
heteroatom one or more atoms selected from the
group comprising nitrogen, oxygen or sulphur,
wherein the nitrogen may optionally be
substituted and the heterocyclic group may be
linked via a carbon atom or N-atom directly or
via a Cl6-alkyl, C26-alkenyl or C26-alkynyl
bridge;
30 R6 denotes an optionally substituted
C,l0-alkylsulphonyl, C2l0-alkenylsulphonyl or
C2,0-alkynylsulphonyl, an optionally substituted
C38-cycloalkylsulphonyl, an optionally
substituted C6,0-arylsulphonyl, an optionally
substituted C6,0-aryl-Cl6-alkylsulphonyl or an
optionally sub~tituted heteroarylsulphonyl;
CA 022~6736 1998-12-02
R6 denotes an optionally substituted
Cl1O-alkylsulphonyloxy, C2l0-alkenylsulphonyloxy
or C2l0-alkynylsulphonyloxy, an optionally
substituted C38-cycloalkylsulphonyloxy, an
optionally substituted C6l0-arylsulphonyloxy, an
optionally substituted C6l0-aryl-Cl6-alkyl-
sulphonyloxy or an optionally substituted
heteroarylsulphonyloxy;
10 R6 denotes an optionally substituted
Cl-10-alkylsulphonylamino, C2-10-alkenylsulphonyl-
amino or C2l0-alkynylsulphonylamino, an
optionally substituted C38-cycloalkylsulphonyl-
amino, an optionally substituted
C6l0-arylsulphonylamino, an optionally
substituted C610-aryl-C16-alkylsulphonylamino or
an optionally substituted heteroarylsulphonyl-
amino;
20 R6 denotes an optionally substituted Cl10-alkyl-
aminocarbonyl, Cl10-dialkylaminocarbonyl,
Cl-lo-alkyl-c2-lo-alkenylaminocarbonyl~
C2l0-alkenylaminocarbonyl, C2l0-dialkenylamino-
carbonyl, C210-alkynylaminocarbonyl or
Cl10-alkyl-C2l0-alkynylaminocarbonyl, an
optionally substituted C38-cycloalkylamino-
carbonyl, an optionally substituted
C6l0-arylaminocarbonyl, an optionally
substituted C6l0-aryl-Cl6-alkylaminocarbonyl or
an optionally substituted heteroarylamino-
carbonyl;
R6 denotes an optionally substituted
Cl10-alkylaminocarbonylamino,
C2l0-alkenylaminocarbonylamino or
C2l0-alkynylaminocarbonylamino, an optionally
substituted C38-cycloalkylaminocarbonylamino, an
CA 022~6736 1998-12-02
........... ... .
optionally substituted C610-arylaminocarbonyl-
amino, an optionally substituted C6l0-aryl-
Cl6-alkylaminocarbonylamino or an optionally
substituted heteroarylaminocarbonylamino;
R6 denotes an optionally substituted
C110-alkyloxycarbonylamino,
C2l0-alkenyloxycarbonylamino or
C2l0-alkynyloxycarbonylamino, an optionally
substituted C38-cycloalkylcarbonylamino, an
optionally substituted C610-aryloxycarbonyl-
amino, an optionally substituted C610-aryl-
C16-alkyloxycarbonylamino or an optionally
substituted heteroaryloxycarbonylamino;
R6 denotes an optionally substituted
C110-alkylaminocarbonyloxy,
C2l0-alkenylaminocarbonyloxy or
C210-alkynylaminocarbonyloxy, an optionally
substituted C38-cycloalkylaminocarbonyloxy, an
optionally substituted C610-arylamino-
carbonyloxy, an optionally substituted
C610-aryl-Cl6-alkylaminocarbonyloxy or an
optionally substituted heteroarylamino-
carbonyloxy;
. ~ .,
R6 denotes an optionally substituted C110-alkyl-N-
amidino, C210-alkenyl-N-amidino or C210-alkynyl-
N-amidino, an optionally substituted
C38-cycloalkyl-N-amidino, an optionally
substituted C610-aryl-N-amidino, an optionally
substituted C6l0-aryl-Cl-6- alkyl-N-amidino or an
optionally substituted heteroaryl-N-amidino;
35 R6 denotes an optionally substituted C110-alkyloxy,
C2l0-alkenyloxy or C2l0-alkynyloxy, an optionally
substitutea C38-cycloalkyloxy, an optionally
CA 022~6736 1998-12-02
substituted C610-aryloxy, an optionally
substituted C610-aryl-C16-alkyloxy or an
optionally substituted heteroaryloxy;
5 R6 denotes an optionally substituted
Cl10-alkylcarbonyl, an optionally substituted
C2l0-alkenylcarbonyl, an optionally substituted
C2l0-alkynylcarbonyl, an optionally substituted
C38-cycloalkylcarbonyl, an optionally
substituted C6l0-arylcarbonyl, an optionally
substituted C6l0-aryl-Cl6-alkylcarbonyl;
i R6 denotes a group A-C0- wherein A is as
hereinbefore defined;
R6 denotes an optionally substituted
Cll0-alkyloxycarbonyl, C2l0-alkenyloxycarbonyl or
C2l0-alkynyloxycarbonyl, an optionally
substituted C38-cycloalkyloxycarbonyl, an
optionally substituted C6l0-aryloxycarbonyl, an
optionally substituted C6l0-aryl-Cl6-
alkyloxycarbonyl or an optionally substituted
heteroaryloxycarbonyl;
~ 25 R6 denotes an optionally substituted
~ Cll0-alkylcarbonyloxy, C2l0-alkenylcarbonyloxy or
C2l0-alkynylcarbonyloxy, an optionally
substituted C38-cycloalkylcarbonyloxy, an
optionally substituted C6l0-arylcarbonyloxy, an
optionally substituted C610-aryl-Cl6alkyl-
carbonyloxy or an optionally substituted
heteroarylcarbonyloxy;
R6 denotes an optionally substituted
Cll0-alkylthio, C2l0-alkenylthio or
C2l0-alkynylthio, an optionally substituted
C38-cycloalkylthio, an optionally substituted
CA 022~6736 1998-12-02
14
C610-arylthio, an optionally substituted
C610-aryl-C16-alkylthio or an optionally
substituted heteroarylthio;
5 R6 denotes an optionally substituted
Cl1O-alkylcarbonyloxy-Cl6-alkyl,
C210-alkenylcarbonyloxy-C16-alkyl or
C210-alkynylcarbonyloxy-C16-alkyl, an optionally
substituted C38-cycloalkylcarbonyloxy-C16-alkyl,
an optionally substituted C610-arylcarbonyloxy-
Cl6-alkyl, C6l0-aryl-Cl6-alkylcarbonyloxy-
- C16-alkyl or heteroarylcarbonyloxy-C16-alkyl;
R6 denotes an optionally substituted group of the
formula
R10O-B-(CH) -
wherein n=1, 2, 3 or 4, where B denotes a
C610-aryl or a single bond;
R6 denotes an optionally substituted amine,
preferably NR9R9;
! 1 . 25 R6 denotes an optionally substituted group of the
formula
~ ~(CH2)1,2
~
R6 denotes an optionally substituted group of the
formula
CA 022~6736 1998-12-02
.. . . ...
(CH2)1.2 (~H2)1,2 (~CH2)-,2
-Y~ -Y~ -Y~G~
(~H2)-,2 (CH2)1,2
-y~ -y~
C~-C6-Alkyl C,-C6-AIkY
wherein Y is a single bond or an alkylene, an
~ alkenylene or an alkynylene having up to 6,
preferably 4 carbon atoms in the chain;
R6 is an optionally substituted group of the
formula
_y~\ ~CH2)0,1,2
~7 ~CH2)0,1,2
~ )0,12 ~ Y ~
wherein Y is a single bond or an alkylene, an
alkenylene or an alkynylene having up to 6,
preferably 4 carbon atoms in the chain;
R8 denotes hydrogen, an optionally substituted
C36-cycloalkyl group, a branched or unbranched
alkyl, alkenyl or alkynyl group having up to 10
carbon atom~, preferably an alkyl group having 1
to 4 carbon atoms which may optionally be
CA 022~6736 l998-l2-02
16
substituted by hydroxy, phenyl, substituted
phenyl, benzyl, substituted benzyl, amino,
substituted amino, Cl8-, preferably
Cl4-alkyloxy, or -(CH2)m-NHCOORl~ wherein m = 1,
2, 3 or 4;
R8 denotes a 5-, 6- or 7-membered heterocyclic
group which is carbon linked directly or via a
Cl4-alkyl chain and which contains one or more
heteroatoms selected from the group comprising
nitrogen, oxygen or sulphur and may optionally
~ be mono- or polysubstituted, preferably
( monosubstituted, by benzyl, optionally
substituted benzyl, Cl4-alkyl, halogen, -ORl~,
- CN, -NO2, NH2, -OH, =O, -COOH, -SO3H or -COORl~;
R8 denotes a bicyclic heterocyclic group which is
C-linked directly or via a Cl4-alkyl chain and
which contains one or more heteroatoms selected
from the group comprising nitrogen, oxygen or
sulphur and is optionally mono- or
polysubstituted, preferably monosubstituted, by
benzyl, optionally substituted benzyl,
Cl_4- alkyl, halogen, -ORl~, - CN, -NO2, -NH2, -OH,
=0, - COOH, -SO3H, - COORl~;
R9 denotes hydrogen, an optionally substituted
C36-cycloalkyl group, a branched or unbranched
alkyl, alkenyl or alkynyl group having up to 10,
preferably 1 to 4 carbon atoms, which may
optionally be substituted by hydroxy, phenyl,
substituted phenyl, benzyl, substituted benzyl,
amino, substituted amino, Cl8-, preferably
Cl4-alkoxy, or -(CH2)m-NHCOORl~ wherein m equals
1, 2, 3 or 4;
CA 022~6736 1998-12-02
R9 denotes a 5-, 6- or 7-membered heterocyclic
group which is carbon linked directly or via a
Cl4-alkyl chain and which contains one or more
heteroatoms selected from the group comprising
nitrogen, oxygen or sulphur and may optionally
be mono- or polysubstituted, preferably
monosubstituted, by benzyl, optionally
substituted benzyl, Cl4-alkyl, halogen, -ORl~,
-CN~ -NO2, NH2, -OH, =0, -COOH, -SO3H or -COOR10;
R9 denotes a bicyclic heterocyclic group which is
carbon linked directly or via a Cl4-alkyl chain
~:J and which contains one or more heteroatoms
selected from the group comprising nitrogen,
oxygen or sulphur and may optionally be mono- or
polysubstituted, preferably monosubstituted, by
benzyl, optionally substituted benzyl,
Cl4-alkyl, halogen, -ORl~, -CN~ -NO2~ -NH2, -OH~
=0, ~ COOH, ~ SO3H, ~ COORl~;
or
RB and R9 together with the nitrogen atom form a
saturated or unsaturated 5- or 6-membered ring
which may contain as further heteroatoms
nitrogen, oxygen or sulphur, whilst the
-, 25 heterocyclic group may be substituted by a
'~~ branched or unbranched alkyl group having 1 to 4
carbon atoms, preferably methyl, or may carry
one of the following groups
3 0 - ( CH2 ) n ~ phenyl,
-(CH2)n-NH2, =O, a ketal - preferably
-O-CH2-CH2-~-~
- (CH2)nNH-Cl 4-alkyl,
- (CH2)n~N(Cl a~alkYl)2~
- (CH2)n-NHCOORl~, (n = 2, 3 ~ 4) ~ halogen,
- ORl~ ~ - CN ~ - NO2 ~ -NH2 ~ - CH2NRaR9 ~
-OH -COOH, -S03H, -COORl~, -CONRBR9~ -SO2-Rl~;
CA 022~6736 1998-12-02
, ., . , , . ~ , ,, .. ,, , . , . ~
R10 denotes hydrogen Cl4-alkyl, C24-alkenyl,
C24-alkynyl, a benzyl or phenyl group which is
optionally mono- or polysubstituted by OCH3,
optionally in the form of the racemates, the
enantiomers and diastereomers thereof and their
mixtures, as well as optionally the
pharmacologically acceptable acid addition salts
thereof.
It will be appreciated that the dotted lines linking pairs
of nitrogen atoms in formula (I) indicate the presence of
i a double bond in one of two alternative positions, such
that both of R1 and R3, or of R4 and R5 are not
simultaneously present.
CA 022~6736 1998-12-02
The compounds of general formula (I) form the following
isomers:
R6~/ ~ R2 R6~/ 1~R\~R2
(Ia) (Ib)
( ) R'~ /~R R6~1 1 \~R
(Ic) (Id)
the isomers of general formulae (Ia) and (Ib) being
preferred, particularly those wherein Rl or R3 denotes
hydrogen or C14-alkyl. Compounds of general formula (Ia)
and (Ib) wherein Rl and R3 denote hydrogen are
particularly preferred; in this case isomers (Ia) and (Ib)
are tautomers.
~ Preferred compounds are the compounds of general formulae
'~~ (Ia) to (Id) wherein
Rl or R3 denotes hydrogen, Cl4-alkyl, benzyl, preferably
hydrogen;
R2 denotes hydrogen, a Cl8-alkyl, C28-alkenyl or
C28-alkynyl group which may optionally be
substituted, by -CN, -CH2NR8R9, -OH
(polysubstitution also possible), -ORl~, -NR8R9,
-NHCORl~, -NHCONR8R9, -NHCOORl~, halogen, -OCORl~,
-OCO-pyridyl, -OCH2COOH, -OCH2COORl~, -So2R7,
-S-R7, -NHCONH-phenyl, -OCH2-CONR8R9, -OCH2CH2OH,
CA 022~6736 1998-12-02
- S~2 - CH2 - CH2 - O - CORl~, - OCH2 - CH2 - NR8R9,
-SO2-CH2-CH2-OH, -CONHSO2Rl~, -CH2CONHSO2Rl~,
-OCH CH ORl~ -COOH, -COORl~, -CONR8R9, -CHO, -SR ,
-SORl~, -SO2Rl~, -SO3H, -SO2NR8R9, -OCH2-CH2OCORl~,
-CH=NOH -CH=NORl~, =O, -CORll, -CH(OH)R ,
-CH(ORl~) 2~ -CH=CH-R12, OCONR8R9, optionally mono-
or poly-substituted, preferably mono-methyl-
substituted, 1,3-dioxolane or 1,3-dioxane;
10 R2 denotes phenyl-C16-alkyl, preferably phenyl-
C14-alkyl, phenyl-C26-alkenyl or phenyl-
C26-alkynyl, wherein the phenyl ring is
' optionally substituted either directly or via a
C14-alkylene bridge by one or more, preferably
one, of the groups -C13-alkyl, -CN, -NR8R9, -NO2,
-OH, -OR , -CH2-NH-SO2-Rl~, -NHCORl~, -NHCONR8R9
halogen, -OCOR10, -OCO-pyridyl, -OCH2 COOH,
- OCH2COORl~, - CH20CORl~, - So2R7, - OCH2 - CONR8R9,
-OCH2CH20H, -OCH2-CH2-NR8R9, -CONHSO2Rl~,
-OCH2CH2ORl~, -COOH, -COORl~, -CF3, cycl opropyl,
-CONR8R9, -CH2OH, -CH2ORl~, -CHO, -SRl~, -SORl~,
-SO2Rl~, -SO3H, -SO2NR8R9, -OCH2-CH2OCORl~, -CH=NOH,
CH NORl~ -CORll -CH(OH)Rll, -CH(OR ) 2~
-NHCOORl~, -CH2CONHSO2Rl~, -CH=CH-Rl2, OCONR8R9,
-CH2-O-CONR8R9, -CH2-CH2-O-CONR8R9, optionally
~- mono- or poly- preferably mono-methyl-
substituted 1,3-dioxolane or 1,3-dioxane;
R2 denotes C37- cycloalkyl-C16-alkyl,
C37-cycloalkyl-C26-alkenyl, C37-cycloalkyl-
C26-alkynyl, wherein the cycloalkyl group is
optionally substituted either directly or via a
C1~-alkylene bridge by one or more, preferably
one, of the groups -CN, NR8R9, =O, -OH, -ORl~,
-NR8R9, -NHCOR10, -NHCONR8R9, halogen, -OCOR10,
-OCO-pyridyl, -OCH2COOH, -OCH2COOR1~, -CH20COR1~,
- So2R7, - OCH2 - CONR8R9, - OCH2 CH20H, - OCH2 - CH2 - NR8R9,
CA 022~6736 1998-12-02
~ . .... ..
-OCH2CH20Rl~, -COOH, -COORl~, -CONR8R9, -CH20H,
-CH20R~, -CHO, -SRl~, -SORl~, -SO Rl~ -SO H
-SO2NR8R9, -OCH2-CH20CORl~, -CH=NOH, -CH=NORl~,
-COR , -CH(OH)Rll, -CONHSO2Rl~, -CH(ORl~)
-NHCOORl~, -CH=CH-Rl2, -OCONR8R9, -CH2-O-CONR8R9,
-CH2-CH2-O-CONR8R9, methyl-substituted 1,3-
dioxolane or 1,3-dioxane;
R2 denotes a group of the formula
A-Cl6-alkyl, A-CONH-Cl6-alkyl,
A-CONH-C26-alkenyl, A-CONH-C26-alkynyl,
A-NH-CO-Cl6-alkyl, A-NH-CO-C26-alkenyl,
~ A-NH-CO - C2 - 6 - alkynyl, A - C2 - 6 - alkenylene,
A-C2 6-alkynylene or A-, wherein A is a C- or N-
linked 5-, 6- or 7-membered heterocyclic group
which contains one or more heteroatoms from the
group comprising nitrogen, oxygen or sulphur and
may optionally be mono- or poly-, preferably
monosubstituted by benzyl, optionally
substituted benzyl, C14-alkyl, halogen, -OR10,
-CN, -NO2, -NH2, -CH2NR8R9, -OH, =O, a ketal,
ethyleneketal, - COOH, - SO3H, - COORl~, - CON8R9,
- CORll SO Rl~ or CONR8R9;
. 25
denotes C37-cycloalkyl, preferably cyclopentyl
or cyclohexyl, which may optionally be
substituted by =O, -OH, -OR10, OCOR10 or
~ -OCO-pyridyl;
R2 denotes phenyl which is optionally substituted
by -OH, halogen, -OR10, C14-alkyl, preferably
-CH3, -NH2, -COOH, -SO3H, -COORl~, -OCH2COORl~, - CN
or - oCH2CONR8R9;
R2 denotes a norbornane, norbornene,
- C36-dicycloalkylmethyl, preferably
CA 022~6736 1998-12-02
dicyclopropylmethyl, adamantane or noradamatane
group optionally substituted by Cl4-alkyl,
preferably methyl;
5 R2 denotes -CH=CH-phenyl in which the phenyl ring
is mono- or polysubstituted by methoxy, hydroxy
or halogen;
R2 is a [3.3.0]-bicyclooctane, preferably a
[3.3.0]-bicyclooctan-2-yli
R2 is a C-linked piperidine or furan;
R2 is an amine of general formula NRaR9;
R4 or Rs denotes hydrogen, an optionally branched
Cl8-alkyl, C28-alkenyl or C28-alkynyl group
which is substituted by -CN, -CH2NR8R9, -OH
(polysubstitution also possible), -ORl~, -NR8R9,
-NHCORl~, -NHCONR8R9, halogen, -OCORl~,
-OCO-pyridyl, -OCH2COOH, -OCH2COORl~, -So2R7,
-S-R7, -NHCONH-phenyl, -OCH2-CONR8R9, -OCH2CH20H,
- S~2 - CH2 - CH2 - O - CORl ~, - OCH2 - CH2 - NR8R9,
-SO2-CH2-CH2-OH, -CONHSO2Rl~, -CH2CONHSO2Rl~,
i .25 -OCH2CH20Rl~, -COOH, -COORl~, -CONR8R9, -CHO, -SRl~,
~ -SORl~, -SO2Rl~, -SO3H, -SO2NR8R9, -OCH2-CH20CORl~,
=O -CH=NOH -CH=NORl~, -CORll, -CH(OH)R ,
-CH(ORl~) 2~ -CH=CH-Rl2, -OCONR8R9, optionally mono-
or poly-, preferably mono-methyl-substituted
1,3-dioxolane or 1,3-dioxane;
R4 or Rs denotes phenyl-Cl6-alkyl, preferably phenyl-
Cl4-alkyl, phenyl-C26-alkenyl or phenyl-
C26-alkynyl, wherein the phenyl ring is
optionally substituted either directly or via a
Cl4-alkylene bridge by one or more, preferably
one of the groups, Cl3-alkyl, -CN, -NR8R9, -NO2,
CA 022~6736 1998-12-02
, OR , -CH2-NH-SO2-Rl~, -NHCORl~ NHCO 8 9
halogen, -OCORl~, -OCO-pyridyl, -OCH2COOH,
-OCH2COORl~, -CH20CORl~, -So2R7, -oCH2-CoNR8R9,
-OCH2CH20H, -OCH2-CH2-NRBR9, -CONHSO2Rl~,
-OCH2CH2ORl~, -COOH, -COOR10, -CF3, cyclopropyl,
-CONR8R9, -CH2OH, -CH2ORl~, -CHO, -SR10, -SOR10,
-SO2R1~, -SO3H, -So2NR5R9, -OCH2-CH2OCOR1~, -CH=NOH,
CH NoR10 -COR11 -CH(OH)R11, -CH(OR ) 2 '
-NHCOOR10, -CH2CONHSO2R1~, -CH=CH-R12, -OCONR9R9,
-CH2-O-CONR8R9, -CH2-CH2-o-CoNR5R9, optionally
mono- or poly-, preferably mono-methyl-
- substituted 1,3-dioxolane or 1,3-dioxane;
R4 or Rs denotes an optionally substituted C37-cycloalkyl
group;
R4 or R5 denotes C37-cycloalkyl-C16-alkyl,
C37-cycloalkyl-C26-alkenyl, C37-cycloalkyl-
C26-alkynyl, wherein the cycloalkyl group is
optionally substituted either directly or via a
Cl4-alkylene bridge by -CN, -NR9R9, =O, -OH,
-ORl~, -NR5R9, -NHCORl~, -NHCoNR5R9, halogen,
-OCORl~, -OCO-pyridyl, -OCH2COOH, -OCH2COORl~,
- CH20CORl~, - So2R7, - OCH2 - CONR9R9, - OCH2CH20H,
-OCH2-CH2-NR8R9, -OCH2CH2OR1~, -COOH, -COOR10,
-CONR8R9, -CH2OH, -CH2OR1~, -CHO, -SR10, -SORl~,
-SO2Rl~, -SO3H, -SO2NR8R9, -OCH2-CH2OCORl~, -CH=NOH,
-CH=NOR10 -CORll, -CH(OH)Rll, -CONHSO2Rl~,
CH(OR ) 2, -NHCOORl~~ -CH=CH-Rl2 OCONR8 9
-CH2-O-CONR8R9, -CH2-CH2-O-CONR8R9, optionally
mono- or poly-, preferably mono-methyl-
substituted 1,3-dioxolane or 1,3-dioxane;
R4 or R5 denotes a group of the formula
A-Cl6-alkyl, A-CONH-Cl6-alkyl,
A-CONH-C26-alkenyl, A-CONH-C26-alkynyl,
.
CA 022~6736 1998-12-02
24
A-NH-CO-Cl6-alkyl, A-NH-CO-C26-alkenyl,
A-NH-CO-C26-alkynyl, A-C26-alkenyl or
A-C26-alkynyl, wherein A is a C- or N-linked
heterocyclic group which contains one or more
heteroatoms selected from the group comprising
nitrogen, oxygen or sulphur and may optionally
be mono- or polysubstituted, preferably
monosubstituted, by C14-alkyl, halogen, -ORl~,
-CN, -NO2, -NH2, -CH2NR8R9, -OH, =O, a ketal,
ethyleneketal, -COOH, -SO3H, -COORl~, -CONR8R9,
-COR11 SO R10 or CONR8R9;
: R denotes hydrogen, a C18-alkyl, C28-alkenyl or
C28-alkynyl group which is substituted by -CN,
-CH2NRaR9, -OH (polysubstitution also possible),
-ORl~, -NR8R9, -NHCORl~, -NHCONR8R9, -NHCOORl~,
halogen, -OCOR10, -OCO-pyridyl, -OCH2COOH,
-OCH2COOR1~, -So2R7, -S-R7, -NHCONH-phenyl,
-OCH2-CONR8R9, -OCH2CH20H, -SO2-CH2-CH2-O-CORl~,
-OCH2-CH2-NR8R9, -SO2-CH2-CH2-OH, -CONHSO2R1~,
-CH2CONHSO2R1~, -OCH2CH20R1~, -COOH, -COORl~,
-CONR8R9, -CHO, -SR10, -SORl~, -S02Rl~, -SO H
-SO2NR8R9, -OCH2-CH20COR1~, -CH=NOH, -CH=NOR10,
-COR11 -CH(OH)R11, -CH(OR10) 2/ -CH=CH-R12~
-OCONR8R9, optionally mono- or poly-, preferably
mono-methyl-substituted 1,3-dioxolane or 1,3-
dioxane;
R6 denotes phenyl-C16-alkyl, preferably phenyl-
C14-alkyl, phenyl-C26-alkenyl or phenyl-
C26-alkynyl, wherein the phenyl ring is
optionally substituted either directly or via a
C14-alkylene bridge by one or more of the
groups, -C13-alkyl, -CN, -NR8R9, -NO2, -OH, -OR10,
-CH2-NH-SO2-R1~, -NHCOR10, -NHCONR8R9, halogen,
-OCOR10, -OCO-pyridyl, -OCH2COOH, -OCH2COOR1~,
-CH20CORl~, -So2R7, -OCH2-CONR8R9, -OCH2CH20H,
CA 022~6736 1998-12-02
-OCH2-CH2-NR8R9, -CONHSO2R1~, -OCH2CH20R1~, -COOH,
-COOR10, -CF3, cyclopropyl, -CONRaR9, -CH20H,
-CH20R1~, -CHO, -SR10, -SOR10, -S02R1~, -S03H
-SO2NR8R9, -OCH2-CH20COR1~, -CH=NOH, -CH=NOR10,
-COR11, -CH(OH)R11, -CH(OR10) 2~ -NHCOORl~~
-CH2CONHSO2Rl~, -CH=CH-Rl2, -OCONR8R9
- CH2 - O - CONR8R9, - CH2 - CH2 - O - CONR8R9, - CO - Rl~,
-CO-C14-alkyl-NR8R9, optionally mono- or poly-,
preferably mono-methyl-substituted 1,3-dioxolane
or 1,3-dioxane;
R6 denotes C37-cycloalkyl-C16-alkyl,
C37-cycloalkyl-C26-alkenyl, C37-cycloalkyl-
C26-alkynyl, wherein the cycloalkyl group is
optionally substituted either directly or via a
C14-alkylene bridge by one or more, preferably
one, of the groups -CN, NR8R9, =0, -OH, -OR10,
~ -NR8R9, -NHCOR10, -NHCONR8R9, halogen, -OCOR10,
-OCO-pyridyl, -OCH2COOH, -OCH2COOR1~, -CH20COR1~,
-So2R7, -OCH2-CONR8R9, -OCH2CH20H, -OCH2-CH2-NR8R9,
-OCH2CH20R1~, -COOH, -COOR10, -CONR8R9, -CH20H,
-CH20R1~, -CHO, -SR10, -SOR10, -SO R10 -SO H
-SO2NR8R9, -OCH2-CH20COR1~, -CH=NOH, -CH=NOR10,
-COR , -CH(OH)R11, -CONHSO2R1~, -CH(OR10)
, 25 -NHCOOR~, -CH=CH-R12, -OCONR8R9, -CH -O-CONR8R9
-CH2-CH2-O-CONR8R9, methyl-substituted 1,3-
dioxolane or 1,3-dioxane;
R6 denotes a group of the formula
A-C16-alkyl, A-coNH-cl-6-alkyl~
A-CONH-C26-alkenyl, A-CONH-C26-alkynyl,
A-NH-CO-Cl6-alkyl, A-NH-CO-C26-alkenyl,
A-NH-CO-C26-alkynyl, A-C26-alkenyl or
A-C26-alkynyl or A-,
wherein A is a C- or N-linked 5-, 6- or 7-
membered heterocyclic group which contains one
CA 022~6736 1998-12-02
or more heteroatoms from the group comprising
nitrogen, oxygen or sulphur and may optionally
be mono- or polysubstituted, preferably
monosubstituted, by benzyl optionally
substituted benzyl, Cl4-alkyl, halogen, -OR10,
-CN, -NO2, -NH2, -CH2NR8R9, -OH, =O, a ketal,
ethyleneketal, -COOH, -SO3H, -COORl~, -CONR8R9,
~ -CORll, -SO2-Rl~ or -CONR8R9;
10 R6 denotes -CHO, -COORl~, -CONR8R9;
R6 denotes C37-cycloalkyl, preferably cyclopentyl
or cyclohexyl, optionally substituted by =O,
-OH, -ORl~, OCOR10 or -OCO-pyridyl;
R6 denotes phenyl optionally substituted by -OH,
halogen, -ORl~, Cl4-alkyl, preferably -CH3, -NH2,
-COOH, -SO3H, -COORl~, -OCH2COORl~, -CN or
-OCH2CONR8R9;
R6 denotes a norbonane, norbornene, C36-dicyclo-
alkylmethyl, preferably dicyclopropylmethyl,
~ adamantane or noradamantane group optionally
substituted by Cl4-alkyl, preferably methyl;
' R6 denotes -CH=CH-phenyl, wherein the phenyl ring
may be mono- or polysubstituted by methoxy,
hydroxy or halogen;
30 R6 denotes a [3.3.0]-bicyclooctane, preferably a
[3.3.0]-bicyclooctan-2-yl;
R6 denotes a C-linked piperidine or furan;
35 R7 denotes Cl4-alkyl which is optionally
substituted by -OH, -OCORl~, -OCO-pyridyl, -NH2,
CA 022~6736 1998-12-02
-NR8R9 or -NHCOR10, preferably -CH2-CH2-OH,
- CH2- CH2OCORl~, - CH2- CH2- CH2- OH or - CH2- CH2CH2OCORl~;
R8 denotes hydrogen, an optionally substituted
C36-cycloalkyl group, a branched or unbranched
alkyl, alkenyl or alkynyl group having up to 10
carbon atoms, preferably a C14-alkyl group which
may optionally be substituted by hydroxy,
phenyl, substituted phenyl, benzyl, substituted
benzyl, amino, substituted amino or C18-alkoxy,
preferably C14-alkoxy, or -(CH2)~-NHCOOR1~ where m
= 1, 2, 3 or 4;
(
R3 denotes a 5-, 6- or 7-membered heterocyclic
group which is C-linked directly or via a
C14-alkyl chain, and which contains one or more
heteroatoms selected from the group comprising
nitrogen, oxygen or sulphur and may optionally
be mono- or polysubstituted, preferably
monosubstituted by benzyl, optionally
substituted benzyl, C14-alkyl, halogen, -OR10,
-CN, -NO2, -NH2, -OH, =O, -COOH, -SO3H or -COOR10;
R8 denotes a bicyclic heterocyclic group C-linked
~- 25 directly or via a C14-alkyl chain, which
' contains one or more heteroatoms from the group
comprising nitrogen, oxygen or sulphur and may
optionally be mono- or polysubstituted,
preferably monosubstituted by benzyl, optionally
substituted benzyl, C14-alkyl, halogen, -ORl~,
- CN, - NO2, - NH2, - OH, = O, - COOH, - SO3H or -COORl~;
R9 denotes hydrogen, an optionally substituted
C36-cycloalkyl group, a branched or unbranched
alkyl, alkenyl or alkynyl group having up to 10
carbon atoms, preferably a C14-alkyl group,
which may optionally be substituted by hydroxy,
CA 022~6736 1998-12-02
phenyl, substituted phenyl, benzyl, substituted
benzyl, amino, substituted amino or Cla-alkoxy,
preferably Cl4-alkoxy, or -(CH2)m-NHCOORl~ wherein
m is 1, 2, 3 or 4, preferably hydrogen;
R9 denotes a 5-, 6- or 7-membered heterocyclic
group which is C-linked directly or via a
Cl4-alkyl chain, and which contains one or more
heteroatoms selected from the group comprising
nitrogen, oxygen or sulphur and may optionally
be mono- or polysubstituted, preferably
-~ monosubstituted by benzyl, optionally
substituted benzyl, Cl4-alkyl, halogen, -ORl~,
-CN, -NO2, -NH2, -OH, =O, -COOH, -SO3H or -COORl~;
R9 denotes a bicyclic heterocyclic group C-linked
directly or via a Cl4-alkyl chain, which
contains one or more heteroatoms from the group
comprising nitrogen, oxygen or sulphur and may
optionally be mono- or polysubstituted,
preferably monosubstituted by benzyl, optionally
substituted benzyl, Cl4-alkyl, halogen, -ORl~,
-CN, -NO2, -NH2, -OH, =O, -COOH, -SO3H or -COORl~,
or
,- 25 R8 and R9 together with the nitrogen atom form a
.~ ,
saturated or unsaturated 5- or 6-membered ring
which may contain nitrogen, oxygen or sulphur as
additional heteroatoms, whilst the heterocyclic
group may be substituted by a branched or
unbranched Cl4-alkyl group, preferably methyl,
or may carry one of the following groups:
- ( CH2 ) n-phenyl,
-(CH2)n-NH2, =O, a ketal - preferably
-O-CH2-CH2-O-~
-(CH2)nNH-C14-alkyl,
- (CH2) n~N (Cl-8-alkyl ) 2 ~
CA 022~6736 1998-12-02
29
-(CH2)n-NHCOOR1~, (n = 1, 2, 3, 4), halogen,
-OR10, -CN, -NO2, -NH2, -CH2NR3R9,
-OH, -COOH, -SO3H, -COORl~, -CONR9R9, -SO2-Rl~;
R10 denotes hydrogen, C14-alkyl, C24-alkenyl,
C24-alkynyl, a benzyl or phenyl group which is
optionally mono- or polysubstituted by OCH3;
Rll denotes Cl4-alkyl, C24-alkenyl, C24-alkynyl,
optionally substituted phenyl, optionally
substituted benzyl, C36-cycloalkyl;
R12 denotes -COOR10, -CH20R1~, -CoNR3R9, hydrogen,
C13-alkyl, optionally substituted phenyl or
- CH2NR3R9,
optionally in the form of the racemates,
enantiomers and diastereomers thereof and
mixtures thereof, and optionally the
pharmacologically acceptable acid addition salts
thereof.
Also preferred are compounds of general formula (Ia) to
(Id)
O R1 0
N~N1 - N N~NJ~ N
N ~r~N~ R~/ ~ \~R2
R4 R4 R3
(Ia) (Ib)
CA 022~6736 1998-12-02
R6~,N ' N ~ N~ R2 R6~N ~ N ~ N'~ R2
Nl N N N ~' N
R5 R5 R3
(Ic) (Id)
wherein
Rl or R3 denotes hydrogen, C14-alkyl or benzyl;
. R2 denotes hydrogen, C18-alkyl, preferably
Cl6-alkyl;
R2 denotes phenyl which is optionally substituted
by halogen, preferably fluorine or chlorine,
Cl4-alkyl, Cl4-alkyloxy, hydroxy or NR9R9;
15 R2 denotes phenyl-Cl6-alkyl, preferably benzyl,
phenyl-C26-alkenyl or phenyl-C26-alkynyl,
wherein the phenyl ring is optionally
substituted by halogen, preferably fluorine or
chlorine, Cl4-alkyl, C14-alkyloxy, hydroxy or
NR8R9;
i: i
'~~~ R2 denotes an optionally substituted amine,
preferably NR8R9;
.
25 R2 denotes a 5- or 6-membered heterocyclic group
which is optionally C- or N-linked either
directly or via a C14-alkylene bridge,
containing one or more heteroatoms selected from
the group comprising nitrogen or oxygen and
optionally substituted by benzyl or Cl4-alkyl;
R2 denotes a C36-cycloalkyl which may optionally be
substituted by =0, hydroxy, Cl4-alkyl or
Cl4-alkyloxy;
CA 022~6736 1998-12-02
R2 denotes norbornane, norbornene, adamantane or
noradamantane optionally substituted by
Cl4-alkyl, preferably methyl;
R4 or R5 denotes hydrogen, C18-alkyl, phenyl-Cl4-alkyl,
preferably benzyl, phenyl-C26-alkenyl or phenyl-
C26-alkynyl, wherein the phenyl ring is
optionally substituted by halogen, preferably
chlorine or fluorine, hydroxy, C14-alkyl,
Cl4-alkyloxy or NR8R9;
R6 denotes hydrogen, Cl8-alkyl, wherein the alkyl
chain may be substituted by halogen, hydroxy,
=O, C14-alkyloxy, NRaR9, phenyloxy, -O-phenyl-
C14-alkyloxy, benzyloxy, -O-benzyl-O-
C14-alkyloxy, -OCO-C14-alkyl, -OCO-phenyl,
-OCO-benzyl, -OCO-pyridyl, -O-C24-alkylene, -CO-
C14-alkyl, -CHO, =NOH, -COOH, -COO-Cl4-alkyl,
-COO-phenyl, -COO-benzyl, -CONR8R9,
-NHCO-Cl4-alkyl, -NHCO-phenyl,
-CO-C14-alkyl-NR8R9, -SO20H, -SO2-C14-alkyl or
-SO2-phenyl;
25 R6 denotes phenyl which may optionally be
substituted by halogen, preferably chlorine or
fluorine, hydroxy, C14-alkyl, C14-alkyloxy,
benzyloxy, phenyloxy, NR8R9, -OCO-Cl 4- alkyl,
-OCO-phenyl, -OCO-benzyl, -OCO-pyridyl,
-O-C24-alkylene, -CO-Cl4-alkyl, -Cl4-alkyl-NH2,
-C14-alkyl-OH, -C14-alkyl=NOH, -COOH,
-COO-C14-alkyl, -COO-phenyl, -COO-benzyl,
-CONR8R9, -CO-Cl4-alkyl-NH2, -SO20H,
-SO2-Cl~-alkyl or -SO2-phenyl;
R6 denotes phenyl-Cl4-alkyl, preferably benzyl,
phenyl-C26-alkenyl or phenyl-C26-alkynyl,
CA 022~6736 1998-12-02
32
wherein the phenyl ring may be optionally
substituted by halogen, preferably chlorine or
fluorine, hydroxy, C14-alkyl, C14-alkoxy,
benzyloxy, phenyloxy, -NR8R9, -OCO-C14-alkyl,
-OCO-phenyl, -OCO-benzyl, -OCO-pyridyl,
-O-C24-alkylene, -CO-C14-alkyl, -C14-alkyl-NR8R9,
-C14-alkyl-OH, -C14-alkyl=NOH, -COOH,
-COO-C14-alkyl, -COO-phenyl, -COO-benzyl,
- CONR8R9, - CO - Cl _4 - alkyl-NH2, -SO2OH,
-SO2-C14-alkyl or -SO2-phenyl;
. R6 denotes a 5- or 6-membered heterocyclic group
c optionally C- or N-linked either directly or via
a C14-alkylene bridge, which contains one, two
or three heteroatoms selected from nitrogen or
oxygen and is optionally mono- or
polysubstituted by benzyl or Cl4-alkyl;
R6 denotes a C36-cycloalkyl or C36-cycloalkyl-
C14-alkyl group which may optionally be
substituted by =O, hydroxy, Cl4-alkyl or
Cl4-alkyloxy;
R6 denotes norbornyl, norbornenyl, adamantyl or
noradamantyl optionally substituted by
Cl4-alkyl, preferably methyl;
R6 denotes -CHO, -COOH, -COO-Cl4-alkyl,
-COO-phenyl, -COO-benzyl or -CONR8R9;
R6 denotes an amine of general formula NR8R9;
R8 denotes hydrogen, a branched or unbranched
Cl4-alkyl group;
R8 denotes a C-linked 5- or 6-membered heterocyclic
~ group which contains one, two or three
CA 022~6736 1998-12-02
heteroatoms selected from the group comprising
nitrogen, oxygen and sulphur and is optionally
substituted by benzyl, C14-alkyl, Cl4-alkyloxy,
halogen, -CN, -NO2, -NH2, -OH or =O;
R9 denotes hydrogen, a branched or unbranched
Cl4-alkyl group;
R9 denotes a C-linked 5- or 6-membered heterocyclic
group which contains one, two or three
heteroatoms selected from the group comprising
- nitrogen, oxygen or sulphur and is optionally
~: substituted by benzyl, C14-alkyl, C14-alkyloxy,
halogen, -CN, -NO2, -NH2, -OH or =O;
or
R8 and R9 together with the nitrogen atom form a
saturated or unsaturated 5- or 6-membered ring
which may contain nitrogen or oxygen as
additional heteroatoms, whilst the heterocyclic
group may be substituted by a branched or
unbranched C14-alkyl group, preferably methyl,
or by a -(CH2)14-phenyl group, preferably
benzyl,
optionally in the form of their racemates,
enantiomers, diastereomers and mixtures thereof,
and optionally the pharmacologically acceptable
acid addition salts thereof.
Also preferred are compounds of general formulae (Ia) and
(Ib)
O R1 0
N_N~ N N_N~ N
R6~ ~ R2 r r
R4 R4 R3
(Ia) (Ib)
CA 022~6736 1998-12-02
~ , _, ,.. .~
34
wherein
~1 or R3 denotes hydrogen, C14-alkyl, preferably
C13-alkyl and benzyl;
R2 denotes hydrogen, C16-alkyl, preferably
C14-alkyl;
R2 denotes cyclopentyl, cyclohexyl, cyclopentanone,
cyclohexanone, hydroxycyclopentane or
hydrocyclohexane;
. .
' R2 denotes a morpholine group optionally
substituted by C14-alkyl, preferably methyl, a
piperidinyl group, a piperazinyl group
optionally substituted by benzyl or C14-alkyl,
preferably methyl, pyridyl, tetrahydrofuranyl,
tetrahydropyranyl or furyl;
20 R2 denotes a phenyl group optionally substituted by
C14-alkyl, halogen or hydroxy;
R2 denotes a phenyl-C14-alkyl, preferably benzyl,
wherein the phenyl ring is optionally
substituted by halogen, preferably fluorine or
chlorine, Cl4-alkyl, C14-alkyloxy, hydroxy or
NR8R9;
R2 denotes an amine of general formula NR3R9;
R2 denotes a norbornenyl, norbornyl, adamantyl or
noradamantyl optionally substituted by
Cl4-alkyl, preferably methyl;
35 R4 denotes hydrogen, Cl7-alkyl, preferably
Cl5-alkyl, phenyl-Cl3-alkyl, preferably benzyl,
wherein the phenyl ring may be substituted by
CA 022~6736 1998-12-02
halogen, preferably chlorine or fluorine,
hydroxy, C14-alkyl, Cl4-alkyloxy or NR8R9;
R6 denotes hydrogen, C16-alkyl, preferably
C14-alkyl, wherein the alkyl chain may be
substituted by halogen, hydroxy, =O,
C14-alkyloxy, NR8R9, phenyloxy, -O-phenyl-O-
C14-alkyloxy, benzyloxy,
-O-benzyl-O-C14-alkyloxy, -OCO-C14-alkyl,
-OCO-phenyl, -OCO-pyridyl, -OCO-benzyl, -O-
C2 _4 - alkylene, -CO-C1 4 - alkyl, - CHO, =NOH, -COOH,
-COO-C14-alkyl; -COO-phenyl, -COO-benzyl,
-CONR8R9, -NHCO- Cl_4- alkyl, - NHCO -phenyl,
-CO-Cl 4- alkyl-NR8R9, -SO20H, -SO2-C1 4- alkyl or
-SO2-phenyl;
R6 denotes phenyl, wherein the phenyl ring may be
substituted by halogen, preferably fluorine or
chlorine, hydroxy, C14-alkyl, C14-alkyloxy or
NR8R9;
R6 denotes phenyl-C13-alkyl, preferably benzyl,
wherein the phenyl ring may be substituted by
halogen, preferably fluorine or chlorine,
hydroxy, C14-alkyl, C14-alkyloxy, benzyloxy,
phenyloxy, NR8R9, -OCO-C14-alkyl, -OCO-phenyl,
-OCO-benzyl, -OCO-pyridyl, -O-C24-alkylene,
-CO-C14-alkyl, -C14-alkyl-NR8R9, -C14-alkyl -OH,
-C14-alkyl =NOH, -COOH, -COO - Cl_4 - alkyl,
-COO-phenyl, -COO-benzyl, -CONR8R9,
-CO-Cl 4- alkyl-NR8R9, -SO20H, -SO2-C1 4- alkyl or
-SO2-phenyl;
R6 denotes a cyclopentyl, cyclohexyl, cyclohexyl-
C13-alkyl, preferably cyclohexylmethyl,
cyclopentanone, cyclohexanone,
CA 022~6736 1998-12-02
hydroxycyclopentane or hydroxycyclohexane linked
via a single bond or via a C14-alkylene chain;
R6 denotes a furan, tetrahydrofuran, a-pyran,
~-pyran, dioxolane, tetrahydropyran, dioxane,
thiophene, thiolane, dithiolane, pyrrole,
pyrroline, pyrrolidine, pyrazole, pyrazoline,
imidazole, imidazoline, imidazolidine, triazole,
pyridine, piperidine, pyridazine, pyrimidine,
pyrazine, piperazine, triazine, morpholine,
thiomorpholine, oxazole, isoxazole, oxazine,
thiazole, isothiazole, thiadiazole, oxadiazole
~ or pyrazolidine linked via a single bond or via
a Cl4-alkylene chain;
R6 denotes -CHO, -COOH, -COO-C14-alkyl;
-COO-phenyl, -COO-benzyl, -CO-NH-C14-alkyl,
-CO-N(C14-alkyl) 2 or -CO-NH-phenyl;
20 R6 denotes an amine of general formula NR8R9;
R8 denotes hydrogen, a branched or unbranched
C14-alkyl group;
i 25 R8 denotes a C-linked pyrrole, pyrroline,
pyrrolidine, pyrazole, pyrazoline, imidazole,
~ imidazoline, imidazolidine, triazole, pyridine,
piperidine, pyridazine, pyrimidine, pyrazine,
piperazine, morpholine, oxazole, isoxazole,
thiazole, isothiazole or thiadiazole optionally
substituted by chlorine, bromine, C14-alkyl,
C14-alkyloxy, NO2, NH2 or OH;
R9 denotes hydrogen, a branched or unbranched
C14-alkyl group;
CA 022~6736 1998-12-02
..... .. . . ..
R9 denotes a C-linked pyrrole, pyrroline,
pyrrolidine, pyrazole, pyrazoline, imidazole,
imidazoline, imidazolidine, triazole, pyridine,
piperidine, pyridazine, pyrimidine, pyrazine,
piperazine, morpholine, oxazole, isoxazole,
thiazole, isothiazole or thiadiazole optionally
substituted by chlorine, bromine, C14-alkyl,
C14-alkyloxy, NO2, NH2, OH,
or
R8 and R9 together with the nitrogen atom form a
saturated or unsaturated 5- or 6-membered ring
-~ which may contain nitrogen or oxygen as
additional heteroatoms, wherein the heterocyclic
group may be substituted by a branched or
unbranched C14-alkyl group, preferably methyl,
or by a -(CH2)14-phenyl group, preferably
benzyl, optionally in the form of the racemates,
the enantiomers and the diastereomers thereof
and mixtures thereof, and optionally the
pharmacologically acceptable acid addition salts
thereof.
Other preferred compounds are those of general formula
(Ib)
o
R ~ --1~ ~ R
N ~ N
R4 R3
(Ib)
~ wherein
30 R2 denote~ hydrogen, C14-alkyl, phenyl, benzyl,
wherein the phenyl ring is optionally
substituted by fluorine, pyridyl, piperidinyl,
morpholinyl, piperazinyl, 4-benzylpiperazinyl,
CA 022~6736 1998-12-02
furyl, tetrahydrofuranyl, tetrahydropyranyl,
NR8R9, cyclopentyl, cyclohexyl, adamantyl,
noradamantyl, norbornyl or norbornenyl;
R3 denotes hydrogen, C13-alkyl or benzyl;
R4 denotes hydrogen, C15-alkyl or benzyl;
R6 denotes hydrogen, C14-alkyl, preferably methyl,
which may optionally be substituted by OH,
chlorine, bromine, C14-alkyloxy or NR8R9, -CHO,
-COOH, -coo-cl-4-alkyl~ preferably -COOCH3,
phenyl, phenyl-C13-alkyl optionally substituted
by fluorine or benzyloxy, preferably benzyl,
phenyloxy-cl-3-alkyl optionally substituted by
methoxy, preferably phenyloxymethyl,
benzyloxy-Cl3-alkyl optionally substituted by
methoxy, preferably benzyloxymethyl,
benzyloxybenzyl, benzoyloxymethyl,
pyridylcarbonyloxymethyl, cyclopentyl, furyl,
cyclohexylmethyl, pyridylmethyl, N-pyrrolyl-
methyl or N-morpholinomethyl;
R8 denotes hydrogen, C14-alkyl or pyridyl;
'~ J 25
R9 denotes hydrogen, C14-alkyl or pyridyl,
optionally in the form of the racemates, the
enantiomers and the diastereomers thereof and
mixtures thereof, and optionally the
pharmacologically acceptable acid addition salts
thereof.
Other preferred compounds are those of general formula
(Ia)
CA 022~6736 l998-l2-02
~ . . .. . . ..
,~ R1
N N N
R4
(Ia)
wherein
R1 denotes hydrogen, C13-alkyl or benzyl;
- R2 denotes hydrogen, C14-alkyl, phenyl, benzyl,
'- wherein the phenyl ring is optionally fluorine-
substituted, pyridyl, piperidinyl, morpholinyl,
piperazinyl, 4-benzylpiperazinyl, furyl,
tetrahydrofuranyl, tetrahydropyranyl, NR8R9,
cyclopentyl, cyclohexyl, adamantyl,
noradamantyl, norbornyl or norbornenyl;
R4 denotes hydrogen, Cl_5 - alkyl or benzyl;
R6 denotes hydrogen, C14-alkyl, preferably methyl,
which may optionally be substituted by OH,
chlorine, bromine, C14-alkyloxy or NR8R9, -CHO,
-COOH, -COO-C14-alkyl, preferably -COOCH3,
phenyl, phenyl-C13-alkyl optionally substituted
- by fluorine or benzyloxy, preferably benzyl,
phenyloxy-Cl3-alkyl optionally substituted by
methoxy, preferably phenyloxymethyl, benzyloxy-
C13-alkyl, optionally substituted by methoxy,
preferably benzyloxymethyl, benzyloxybenzyl,
benzoyloxymethyl, pyridylcarbonyloxymethyl,
cyclopentyl, furyl, cyclohexylmethyl,
pyridylmethyl, N-pyrrolylmethyl or N-
morpholinomethyl;
R8 denotes hydrogen, C14-alkyl or pyridyl;'
CA 022~6736 1998-12-02
R9 may denote hydrogen, Cl4-alkyl or pyridyl,
optionally in the form of the racemates, the
enantiomers, the diastereomers thereof and
mixtures thereof and optionally the
pharmacologically acceptable acid addition salts
thereof.
Also of interest are compounds of general formula (Ic)
O R1
Nl N N
R5
(Ic)
wherein
15 Rl denotes hydrogen or C13-alkyl;
R2 denotes hydrogen, C14-alkyl, cyclopentyl,
cyclopentanone, hydroxycyclopentane, furan or
benzyl;
Rs denotes C16-alkyl, preferably C14-alkyl, most
preferably methyl, ethyl or tert.-butyl;
R6 denotes hydrogen, benzyl or cyclopentyl,
optionally in the form of the racemates, the
enantiomers, the diastereomers and mixtures
thereof, and optionally the pharmacologically
~ acceptable acid addition salts thereof.
Also of interest are compounds of general formula (Id)
CA 022~6736 1998-12-02
41
R6~/N 1~ N'~ R2
Nl N ,N3
R5 R
(Id)
~ wherein
5 R1 denotes hydrogen or C13-alkyl;
R2 denotes hydrogen, C14-alkyl, cyclopentyl,
- cyclopentanone, hydroxycyclopentane, furan or
benzyl;
R5 denotes C16-alkyl, preferably C14-alkyl, most
preferably methyl, ethyl or tert.-butyl;
R6 denotes hydrogen, benzyl or cyclopentyl,
optionally in the form of the racemates, the
enantiomers, the diastereomers and mixtures
thereof, and optionally the pharmacologically
~ acceptable acid addition salts thereof.
Also preferred are compounds of general formula (Ib)
: O
N r r
R4 R3
(Ib)
wherein
R2 denotes hydrogen, methyl, ethyl, n-propyl, i-
propyl, n-butyl, tert.-butyl, phenyl, benzyl, 4-
fluorobenzyl, pyridyl, N-piperidinyl, N-
~ morpholinyl, N-piperazinyl, 4-benzylpiperazinyl,
CA 022~6736 1998-12-02
42
2-furyl, 3-tetrahydrofuranyl, 4-
tetrahydropyranyl, -NMe2, cyclopentyl,
cyclohexyl, adamantan-1-yl, noradamantan-3-yl,
norbornan-2-yl or 5-norbornen-2-yl;
R3 denotes hydrogen, methyl, ethyl, n-propyl or
benzyl;
R4 denotes hydrogen, methyl, ethyl, n-propyl,
i-propyl, n-butyl, tert.-butyl, n-pentyl or
benzyl;
., ~.. .
R6 denotes hydrogen, methyl, ethyl, n-propyl,
i-propyl, n-butyl, tert.-butyl, phenyl, benzyl,
cyclopentyl, 2-furyl, 2-pyridylmethyl, 3-
pyridylmethyl, 4-pyridylmethyl,
cyclohexylmethyl, phenylethyl, N-morpholino-
methyl, N-pyrrolylmethyl, (3-pyridyl)-NH-CH2-,
PhCO-O-CH2-, pyridyl-CO-O-CH2-, Ph-O-CH2-,
(4-MeO-Ph)-O-CH2-, (4-MeO-Ph)-CH2-O-CH2-,
(4-Ph-CH2-O-Ph)-CH2-, 4-F-Ph-CH2-, 3,4-F-Ph-CH2-,
-COOH, -COOMe, -CH2-OH, -CH2-OMe, -CH2OEt or
-CH2-NMe2~
optionally in the form of the racemates, the
enantiomers, the diastereomers and mixtures
thereof and optionally the pharmacologically
acceptable acid addition salts thereof.
Of particular interest are compounds of general formula
(Ia)
R~
N N N
R4
(Ia)
CA 022~6736 1998-12-02
wherein
R1 denotes hydrogen, methyl, ethyl, n-propyl or
benzyl;
R2 denotes hydrogen, methyl, ethyl, n-propyl, i-
propyl, n-butyl, tert.-butyl, phenyl, benzyl, 4-
fluorobenzyl, pyridyl, N-piperidinyl, N-
morpholinyl, N-piperazinyl, 4-benzylpiperazinyl,
2-furyl, 3-tetrahydrofuranyl, 4-
tetrahydropyranyl, -NMe2, cyclopentyl,
~~ cyclohexyl, adamantan-l-yl, noradamantan-3-yl,
norbornan-2-yl or 5-norbornen-2-yl;
15 R4 denotes hydrogen, methyl, ethyl, n-propyl,
i-propyl, n-butyl, tert.-butyl, n-pentyl or
benzyl;
R6 denotes hydrogen, methyl, ethyl, n-propyl,
i-propyl, n-butyl, tert.-butyl, phenyl, benzyl,
cyclopentyl, 2-furyl, 2-pyridylmethyl, 3-
pyridylmethyl, 4-pyridylmethyl,
cyclohexylmethyl, phenylethyl, N-morpholino-
methyl, N-pyrrolylmethyl, (3-pyridyl)-NH-CH2-,
: . 25 PhCO-O-CH2-, pyridyl-CO-O-CH2-, Ph-O-CH2-,
(4-MeO-Ph)-O-CH2-, (4-MeO-Ph)-CH2-O-CH2-,
(4-Ph-CH2-O-Ph)-CH2-, 4-F-Ph-CH2-, 3,4-F-Ph-CH2-,
-COOH, -COOMe, -CH2-OH, -CH2-OMe, -CH20Et or
- CH2 - NMe2 ~
optionally in the form of the racemates, the
enantiomers, the diastereomers and mixtures
thereof and optionally the pharmacologically
acceptable acid addition salts thereof.
Also preferred are compounds of general formula (Ib)
CA 022~6736 1998-12-02
44
N r r
R4 R (Ib)
wherein
R2 denotes hydrogen, ethyl, n-propyl, i-propyl,
n-butyl, tert.-butyl, phenyl, benzyl, 2-pyridyl,
3-pyridyl, 4-pyridyl, N-piperidinyl,
N-morpholinyl, N-piperazinyl, 4-benzyl-
f - piperazinyl, 3-tetrahydrofuranyl, 4-tetrahydro-
pyranyl, -NMe2, cyclopentyl, cyclohexyl,
adamantan-1-yl, noradamatan-3-yl, norbornan-2-yl
or 5-norbornen-2-yl;
R3 denotes hydrogen;
15 R4 denotes hydrogen, methyl, ethyl, n-propyl,
i-propyl, n-butyl, tert.-butyl, n-pentyl or
benzyl;
R6 denotes hydrogen, methyl, ethyl, n-propyl,
i-propyl, n-butyl, tert.-butyl, phenyl, benzyl,
t- cyclopentyl, 2-furyl, 2-pyridylmethyl,
3-pyridylmethyl, 4-pyridylmethyl, cyclohexyl-
methyl, 2-phenylethyl, N-morpholinomethyl,
N-pyrrolylmethyl, (3-pyridyl)-NH-CH2-,
Ph-COO-CH2-, 3-pyridyl-COO-CH2-, Ph-O-CH2-, (4-
MeO-Ph)-O-CH2-, (4-MeO-Ph)-CH2-O-CH2-,
4-F-Ph-CH2-, 3,4F-Ph-CH2-, -CH2-OH, -CH2-OMe,
-CH2-OEt, -CH2-NMe2, -COOMe or -COOH,
optionally in the form of the racemates, the
enantiomers, the diastereomers and mixtures
thereof, and optionally the pharmacologically
acceptable acid addition salts thereof.
CA 022~6736 1998-12-02
~ ~ . . . .....
Also of particular interest are compounds of general
formula (Ia)
~ R1
R6~/ ~ /~ R2
(Ia)
wherein
R1 denotes hydrogen;
R2 denotes hydrogen, ethyl, n-propyl, i-propyl,
-- n-butyl, tert.-butyl, phenyl, benzyl, 2-pyridyl,
3-pyridyl, 4-pyridyl, N-piperidinyl,
N-morpholinyl, N-piperazinyl, 4-benzyl-
piperazinyl, 3-tetrahydrofuranyl, 4-tetrahydro-
pyranyl, -NMe2, cyclopentyl, cyclohexyl,
adamantan-1-yl, noradamatan-3-yl, norbornan-2-yl
or 5-norbornen-2-yl;
R4 denotes hydrogen, methyl, ethyl, n-propyl,
i-propyl, n-butyl, tert.-butyl, n-pentyl or
benzyl;
J R6 denotes hydrogen, methyl, ethyl, n-propyl,
~ i-propyl, n-butyl, tert.-butyl, phenyl, benzyl,
cyclopentyl, 2-furyl, 2-pyridylmethyl,
3-pyridylmethyl, 4-pyridylmethyl, cyclohexyl-
methyl, 2-phenylethyl, N-morpholinomethyl,
N-pyrrolylmethyl, (3-pyridyl)-NH-CH2-,
Ph-COO-CH2-, 3-pyridyl-COO-CH2-, Ph-O-CH2-, (4-
MeO-Ph)-O-CH2-, (4-MeO-Ph)-CH2-O-CH2-,
4-F-Ph-CH2-, 3,4-F-Ph-CH2-, -CH2-OH, -CH2-OMe,
-CH2-OEt, -CH2-NMe2, -COOMe or -COOH,
optionally in the form of the racemates, the
enantiomers, the diastereomers and mixtures
CA 022~6736 1998-12-02
.
46
thereof, and optionally the pharmacologically
acceptable acid addition salts thereof.
In addition, the invention relates to a new category of
compounds which contain basic structures of general
formulae (Ia) to (Id).
~<,N - N ~~<,N ' N J~ N,~
~, ,
(Ia) (Ib)
O ~ O
~<,N 1~ N~ ~ ~<N - N ~ N
N N N N N IN
~ ~ ~
(Ic) (Id)
Preferred compounds are derivatives of general formula
N N N,
~ H
which have a substituent in positions 2, 4 and 6.
If required, the compounds of general formulae (Ia) to
(Id) may be converted into the salts thereof,
particularly, for pharmaceutical use, into the
physiologically acceptable salts thereof with an inorganic
or organic acid. Suitable acids for this purpose include
hydrochloric acid, hydrobromic acid, sulphuric acid,
phosphoric acid, methanesulphonic acid, acetic acid,
fumaric acid, succinic acid, lactic acid, citric acid,
CA 022~6736 1998-12-02
47
tartaric acid or maleic acid. Moreover, mixtures of these
acids may be used.
The alkyl group~ meant here (including those which are
components of other groups) are branched and unbranched
alkyl groups having 1 to 10 carbon atoms, preferably 1 to
4 carbon atoms, such as: methyl, ethyl, n-propyl,
iso-propyl, butyl, iso-butyl, sec.-butyl, tert.-butyl,
pentyl, iso-pentyl, hexyl, heptyl and octyl.
Unless otherwise specified, substituted alkyl groups
,~ (including those which are components of other groups)
may, for example, carry one or more of the following
substituents: halogen, hydroxy, mercapto, Cl6-alkyloxy,
amino, alkylamino, dialkylamino, cyano, nitro, =O, -CHO,
-COOH, -COO-Cl6-alkyl, -S-Cl6-alkyl.
Alkenyl groups (including those which are components of
other groups) are the branched and unbranched alkenyl
groups with 2 to 10 carbon atoms, preferably 2 to 3 carbon
atoms, provided that they have at least one double bond,
e.g. the alkyl groups mentioned above provided that they
have at least one double bond, such as for example vinyl
(provided that no unstable enamines or enolethers are
'J 25 formed), propenyl, iso-propenyl, butenyl, pentenyl and
hexenyl.
Unless otherwise specified, substituted alkenyl groups,
(including those which are components of other groups),
may for example carry one or more of the following
substituents: halogen, hydroxy, mercapto, Cl6-alkyloxy,
amino, alkylamino, dialkylamino, cyano, nitro, =O, -CHO,
-COOH, -coo-Cl6-alkyl, -s-cl6-alkyl-
The term alkynyl groups (including those which arecomponents of other group~) refers to alkynyl groups
having 2 to 10 carbon atoms provided that they have at
CA 022~6736 1998-12-02
,, . . , ,, , , , _, .
48
least one triple bond, e.g. ethynyl, propargyl, butynyl,
pentynyl and hexynyl.
Unless otherwise specified, substituted alkynyl groups,
(including those which are components of other groups),
may for example carry one or more of the following
substituents: halogen, hydroxy, mercapto, C16-alkyloxy,
amino, alkylamino, dialkylamino, cyano, nitro, =O, -CHO,
- COOH, - COO -Cl-6- alkyl, -S-C16-alkyl.
Examples of cycloalkyl groups having 3 to 6 carbon atoms
include cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl, which may also be substituted by branched or
unbranched C14-alkyl, hydroxy and/or halogen or as
hereinbefore defined. The term halogen generally refers
to fluorine, chlorine, bromine or iodine.
The word aryl denotes an aromatic ring system having 6 to
10 carbon atoms which, unless otherwise specified, may
carry one or more of the following substituents, for
example: C16-alkyl, C16-alkyloxy, halogen, hydroxy,
mercapto, amino, alkylamino, dialkylamino, CF3, cyano,
nitro, -CHO, -COOH, -COO-C16-alkyl, -S-C16-alkyl. The
preferred aryl group is phenyl.
~ Examples of N-linked cyclic groups of general formula
NR3R9 are as follows: pyrrole, pyrroline, pyrrolidine, 2-
methylpyrrolidine, 3-methylpyrrolidine, piperidine,
piperazine, N-methylpiperazine, N-ethylpiperazine, N-(n-
propyl)-piperazine, N-benzylpiperazine, morpholine,
thiomorpholine, imidazole, imidazoline, imidazolidine,
pyrazole, pyrazoline, pyrazolidine, preferably morpholine,
N-benzylpiperazine, piperazine and piperidine, wherein the
above-mentioned heterocycles may also be substituted by
C14-alkyl, preferably methyl, or may be substituted as in
the definitions.
CA 022~6736 1998-12-02
.
.. . .,, , , ~ ._, . , .. . . _
49
Examples of C-linked 5- or 6-membered heterocyclic rings
which may contain nitrogen, oxygen or sulphur as
heteroatoms include, for example, furan, tetrahydrofuran,
2-methyltetrahydrofuran, 2-hydroxymethylfuran,
tetrahydrofuranone, r-butyrolactone, a-pyran, ~-pyran,
dioxolane, tetrahydropyran, dioxane, thiophene,
dihydrothiophene, thiolane, dithiolane, pyrrole,
pyrroline, pyrrolidine, pyrazole, pyrazoline, imidazole,
imidazoline, imidazolidine, triazole, tetrazole, pyridine,
piperidine, pyridazine, pyrimidine, pyrazine, piperazine,
triazine, tetrazine, morpholine, thiomorpholine, oxazole,
; isoxazole, oxazine, thiazole, isothiazole, thiadiazole,
- oxadiazole and pyrazolidine, whilst the heterocycle may be
substituted as in the definitions.
"=O" means an oxygen atom linked by a double bond.
The compounds according to the invention have an affinity
for adenosine receptors and thus constitute a new category
of adenosine antagonists. In general, adenosine
antagonists may exhibit a therapeutically useful activity
in cases where diseases or pathological situations are
connected with the activation of adenosine receptors.
Adenosine is an endogenous neuromodulator with
predominantly inhibitory effects on the CNS, heart,
kidneys and other organs. The effects of adenosine are
mediated through at least three receptor subtypes:
adenosine Al, ~ and A3 receptors.
In the CNS, adenosine develops inhibitory effects
predominantly by activating Al receptors: presynaptically
by inhibiting synaptic transmission (inhibiting the
release of neurotransmitters such as acetylcholine,
dopamine, noradrenalin, serotonin, glutamate, etc.), and
postsynaptically by inhibiting neuronal activity.
CA 022~6736 1998-12-02
Al antagonists cancel out the inhibitory effects of
adenosine and promote neuronal transmission and neuronal
activity.
Al antagonists are therefore of great interest in the
treatment of degenerative diseases of the central nervous
system such as senile dementia of the Alzheimer's type and
age-associated disorders of memory and learning
performance.
The disease includes, in addition to forgetfulness in its
mild form and total helplessness and absolute dependence
'; on care in the most severe form, a range of other
accompanying systems such as sleep disorders, motor-
coordination disorders up to the clinical picture of
Parkinson's disease as well as increased lability affect
and depressive symptoms. The disease is progressive and
can result in death. Therapy up till now has been
unsatisfactory. Hitherto, there has been a complete
absence of specific therapeutic agents. Attempts at
therapy with acetylcholinesterase inhibitors exhibit some
effect in a small proportion of patients but are connected
with a high level of side effects.
,
The pathophysiology of Alzheimer's disease and SDAT is
characterised by a severe impairment of the cholinergic
system, but other transmitter systems are also affected.
As a result of the loss of presynaptic cholinergic and
other neurons and the resulting lack of provision of
neurotransmitters, neuronal transmission and neuronal
activity is significantly reduced in the areas of the
brain essential for learning and memory.
Selective adenosine Al receptor antagonist~ promote
neuronal transmission by increased provision of
neurotransmitters, they increa~e the excitability of
CA 022~6736 1998-12-02
postsynaptic neurons and can thus counteract the symptoms
of the disease.
The high receptor affinity and selectivity of some of the
compounds claimed should make it possible to treat
Alzheimer's disease and SDAT with low doses, so that
hardly any side effects can be expected which cannot be
attributed to the blockade of A1 receptors.
Another indication for centrally acting adenosine Al
~ antagonists is depression. The therapeutic success of
antidepressant substances appears to be connected to the
regulation of A1 receptors. A1 antagonists may lead to
the regulation of adenosine A1 receptors and thus present
a new therapeutic approach to the treatment of depressive
patients.
Other fields of use particularly for A2-selective
adenosine antagonists are neurodegenerative diseases such
as Parkinson's disease and also migraine. Adenosine
inhibits the release of dopamine from central synaptic
nerve endings by interaction with dopamine-D2 receptors.
A2 antagonists increase the release and availability of
dopamine and thus offer a new therapeutic principle for
treating Parkinson's disease.
In migraine, vasodilation of cerebral blood vessels
mediated by A2 receptors appears to be involved.
Selective A2 antagonists inhibit vasodilation and may thus
be useful in treating migraine.
Adenosine antagonists may also be used in the treatment of
peripheral indications.
For example, the activation of Al receptors in the lungs
may lead to bronchoconstriction. Selective adenosine Al
antagoniqts relax the smooth muscle of the trachea, cause
CA 022~6736 1998-12-02
bronchodilation and may thus be useful as antiasthmatic
agents.
By activating A2 receptors, adenosine may also lead, under
certain circumstances, to respiratory depression and
stoppage of breathing. A2 antagonists cause respiratory
stimulation. For example, adenosine antagonists
(theophyllin) are used for treating respiratory distress
and for preventing "sudden infant death" in premature
babies.
Important fields of therapy for adenosine antagonists are
also cardiovascular diseases and kidney diseases.
In the heart, adenosine causes inhibition of electrical
and contractile activity by activating Al receptors. In
conjunction with coronary vasodilation mediated by A2
receptors, adenosine has a negative chronotropic,
ionotropic, dromotropic, bathmotropic and bradycardiac
effect and lowers the minute output.
Adenosine Al receptor antagonists are able to prevent
damage to the heart and lungs caused by ischaemia and
subsequent reperfusion. Consequently, adenosine
, 25 antagonists may be used for the prevention and early
treatment of damage to the heart caused by ischaemic
reperfusion, e.g. after coronary bypass surgery, heart
transplants, angioplasty or thrombolytic treatment of the
heart and similar interventions. The same is true of the
lungs.
In the kidneys, the activation of A1 receptors causes
vasoconstriction of afferent arterioles and, consequently,
a fall in renal blood flow and glomerular filtration. Al
antagonists act as powerful potassium-saving diuretics on
the kidneys and can thus be used for kidney protection and
CA 022~6736 1998-12-02
for the treatment of oedema, renal insufficiency and acute
renal failure.
Because of the adenosine antagonism on the heart and the
- 5 diuretic activity, Al antagonists may be used to
therapeutic effect for various cardiovascular diseases,
such as cardiac insufficiency, arrhythmias
(bradyarrhythmias) associated with hypoxia or ischaemia,
conduction disorders, hypertension, ascites in liver
failure (hepato-renal syndrome) and as an analgesic in
circulatory disorders.
.~.
! ~
Surprisingly, some of the compounds according to the
invention display an affinity for the A3-adenosine
receptor. A3 antagonists inhibit the degranulation of
mast cells caused by activation of the A3 receptor and are
therefore therapeutically useful in all diseases and
pathological situations connected with mast cell
degranulation; e.g. as anti-inflammatory substances in
hypersensitivity reactions such as asthma, allergic
rhinitis, urticaria, in myocardial reperfusion injury,
scleroderma, arthritis, autoimmune diseases, inflammatory
bowel diseases, and the like. Cystic fibrosis - also
known as mucoviscidosis - is a congenital metabolic
,~ ) 25 disorder caused by a genetic defect on a certain
chromosome. As a result of increased production and
increased viscosity of the secretions of the mucous glands
in the bronchi, there may be severe complications in the
airways. Early investigations have shown that A1
antagonists increase the efflux of chloride ions, e.g. in
CF PAC cells. On the basis of these findings it is to be
expected that the compounds according to the invention
will regulate the disrupted electrolyte bands of the cells
and alleviate the symptoms of the disease in patients
- 35 suffering from cystic fibrosis (mucovicidosis).
CA 022~6736 1998-12-02
~ .. . ,.. . , , .. . _.
The A1 receptor binding values obtained have been
determined analogously with Ensinger et al. in "Cloning
and functional characterisation of human A1 adenosine
receptor - Biochemical and Biophysical Communications,
Vo.187, No. 2, 919-926, 1992" and are assembled in Table
20.
The A3 receptor binding values assembled in Table 21 were
determined analogously to Salvatore et al. "Molecular
10 cloning and characterization of the human A3-adenosine
receptor" (Proc. Natl. Acad. Sci. USA 90, 10365-10369,
1993).
The new compounds of general formula (Ia) to (Id) may be
15 administered orally, transdermally, by inhalation or
parenterally. The compounds according to the invention
are present as active ingredients in conventional
preparations, e.g. in compositions consisting essentially
of an inert pharmaceutical carrier and an effective dose
20 of the active substance, such as for example plain or
coated tablets, capsules, lozenges, powders, solutions,
suspensions, emulsions, syrups, suppositories, transdermal
systems, etc. An effective dose of the compounds
according to the invention is between 1 and 100,
J 25 preferably between 1 and 50, most preferably between 5 and
30 mg/dose for oral use, between 0.001 and 50, preferably
between 0.1 and 10 mg/dose for intravenous or
intramuscular use. According to the invention, solutions
containing 0.01 to 1.0, preferably 0.1 to 0.5~ active
30 substance are suitable for inhalation. It is preferable
to use powders for administration by inhalation. It is
also possible to use the compounds according to the
invention as a solution for infusion, preferably in
physiological saline or nutrient saline solution.
CA 022~6736 1998-12-02
The compounds according to the invention may be prepared
by the following methods. In order to synthesise the two
isomers
N N N R6~/ ~C \~R~
(Ia) (Ib)
. .
of general formula (Ia) and (Ib), wherein the substituents
are as hereinbefore defined, the following procedure is
used.
In a first step, aminoguanidine (1) is reacted with a
carboxylic acid derivative of general formula (2) to
obtain a triazole of general formula (3) (diagram 1).
This reaction may be carried out in accordance with the
procedures published in J. Chem. Soc. 1929, 816; J. Org.
Chem 1926, 1729 or Org. Synthesis 26, 11.
, H2N~NH ~ NH + ~r Step1 R6~/ -~H
,
(1) (2) (3)
Diaqram 1:
Then, in a second step, the triazole of general formula
(3) is reacted with alkylcyanoacetate in a cyclising
reaction under alkaline conditions to obtain a
triazolopyrimidine of general formula (4) (Diagram 2).
CA 022~6736 1998-12-02
.. ... . , , , . ., . , . _. . ~ .
56
R ~/ 1~ Step2 R6~/
(3) (4)
Diaqram 2:
The base may be an alkali or alkaline earth metal
alkoxide, e.g. of methanol, ethanol, isopropanol, n-, sec-
or tert.-butyl alcohol. Suitable alkali and alkaline
earth metals include for example lithium, sodium,
potassium, magnesium and calcium. Sodium methoxide,
sodium ethoxide, sodium isopropoxide and potassium
tert.-butoxide are particularly preferred as the base.
Furthermore, alkali or alkaline earth metal hydrides may
be used as bases. The hydrides of sodium, lithium,
potassium, magnesium and calcium are preferred. Suitable
inert sol~ents are dimethylformamide, dimethylacetamide,
methylene chloride and tetrahydrofuran. Additionally,
alkali or alkaline earth metal hydroxides of lithium,
sodium, potassium, magnesium and calcium may also be used,
but preferably sodium hydroxide, potassium hydroxide,
lithium hydroxide and calcium hydroxide in alcoholic or
aqueous solution.
In addition to the alkylcyanoacetates, cyanoacetic acid
may also be used. The mixture thus obtained is stirred at
ambient temperature for 0.5 to 4 hours, preferably 1 to 2
hours and then mixed with a compound of general formula
(3) and stirred for 2 to 12, preferably 4 to 6 hours,
preferably at reflux temperature. The reaction mixture is
then mixed with water at ambient temperature and
acidified, after which the solid is filtered off, washed
and dried. Examples of suitable acids are formic acid,
CA 022~6736 1998-12-02
acetic acid or inorganic acids such as hydrochloric or
sulphuric acid.
In a third step, the groups R4 and R5 are introduced into
the triazolopyrimidine of general formula (4) and the
compounds of general formulae (5) and (12) are obtained
(Diagram 3).
O O O
R6~/ ~3~ Step 3 R6~/ ~'13~ N--N~
N N NH2 N N NH2 R5N N NH2
H R
(5) (1 2)
Diaqram 3:
For this, a compound of general formula (4) is dissolved
in 5 to 40 times, preferably 10 to 20 times the amount of
a polar solvent, such as dimethylformamide,
dimethylacetamide, methylene chloride or tetrahydrofuran,
preferably dimethylformamide, and most preferably
anhydrous, possibly absolute dimethylformamide. The
resulting solution is combined with a base and a
corresponding alkylating agent. Suitable bases include
the alkali or alkaline earth metal carbonates of lithium,
sodium, potassium and calcium such as sodium carbonate,
lithium carbonate, potassium carbonate, calcium carbonate
and preferably potassium carbonate. Moreover, the
hydrogen carbonates of lithium, sodium and potassium may
be used. It is also possible to use the alkali or
alkaline earth metal hydroxides of lithium, sodium,
potassium, magnesium or calcium, preferably sodium
hydroxide, potassium hydroxide, lithium hydroxide and
calcium hydroxide in alcohols or water. Other bases which
may be consid~red are the alkoxides of the alkali and
alkaline earth metals already mentioned in step (2).
CA 022~6736 1998-12-02
. . .
58
Furthermore, the above-mentioned alkali and alkaline earth
metal hydrides may be used, preferably in inert solvents
such as dimethylformamide, dimethylacetamide, methylene
chloride, ethers, tetrahydrofuran and toluene. Suitable
alkylating agents include alkyl halides, such as alkyl
chloride, alkyl bromide, especially alkyl iodide and alkyl
tosylates, mesylates, triflates and dialkylsulphates. The
alkyl groups of the alkylating agents correspond to the
definitions for R4 and R5 given hereinbefore. The
reaction mixture is stirred at ambient temperature for 0.5
to 4 days, preferably 1 to 2 days and evaporated to
dryness. The product can be worked up, on the one hand,
~--' by stirring the residue with water and a solvent, e.g. a
~ halogenated solvent such as carbon tetrachloride,
methylene chloride, preferably methylene chloride,
whereupon the compound of general formula (5) can be
isolated as a solid by filtering. The compound of general
formula (12) can be obtained from the filtrate by removing
the solvent. Moreover, in the event that no solid is
obtained, in order to isolate the compound of general
formula (12) from the filtrate, the phases of the filtrate
may be separated, the aqueous solution may be extracted
with an halogenated solvent, preferably methylene
chloride, and the combined organic phases may be dried and
worked up. The compounds of general formulae (5) and (12)
are obtained by chromatographic purification of the
residue (Diagram 3).
~ By nitrosing a compound of general formula (5), a compound
of general formula (6) is obtained in the fourth step
(Diagram 4).
CA 022~6736 1998-12-02
.,, . ~
59
o o
--N~ R~ --1~
N Nl NH2 N N NH2
R4 R4
(5) (6)
Dia~ram 4:
According to the invention, there are two alternative
processes which may be used.
Step 4, alternative A:
A compound of general formula (5) is dissolved or
suspended in a polar solvent, the polar solvent possibly
consistlng of any of the above-mentioned solvents and
dimethylacetamide or an alcohol, e.g. methanol, ethanol,
~ propanol, isopropanol and butanol. Dimethylformamide is
particularly preferred. It is advisable to use the chosen
solvent as an anhydrous, optionally absolute solvent. The
mixture thus obtained is cooled to below 0~C, preferably
-5~C. A nitrosing agent, e.g. an alkyl nitrite such as
methyl, ethyl, n-propyl, isopropyl, n-butyl, sec.-butyl,
tert.-butyl and pentyl nitrite, but preferably isoamyl
nitrite, is added to this mixture, whilst the temperature
should not rise above 0~C. The mixture is stirred at this
temperature for 2 to 48, preferably 4 to 24, most
preferably 8 to 12 hours. Then stirring is continued at
ambient temperature if required to finish off the
reaction. The solvent is eliminated in vacuo, the residue
is filtered with a solvent, e.g. diethylether, washed with
water and the residue thus obtained is dried ( in vacuo) .
Ste~ 4 alternative B:
A compound of general formula (5) is mixed with an aqueous
acid, the acid being an organic acid, formic acid, acetic
acid, propionic acid, preferably acetic acid or an
CA 022~6736 1998-12-02
inorganic acid, e.g. hydrochloric acid or dilute sulphuric
acid, preferably hydrochloric acid and mixtures thereof.
The mixture is heated to 30 to 100, preferably 50 to 80,
most preferably 70~C and mixed with a nitrosing agent,
preferably sodium nitrite, most preferably sodium nitrite
in water and kept at the above temperature for 0.5 to 2
hours, preferably 1 hour, and then kept for 0.5 to 2
hours, preferably 1 hour, at 0 to 20, preferably 5 to 15,
most preferably 10~C. The product is filtered, the
residue is washed and dried in vacuo.
In the fifth step the nitroso compound (6) is reduced to
form a diamine derivative of general formula (7) (Diagram
5)
O O
N_ ~ NO N~N ~ NH2
R6 ~/ ~ ~ Step 5 R6
R4 R4
(6) (7)
Diaqram 5:
The following alternative methods may be used for this
purpose.
Step 5, alternative A:
The nitroso compound (6) is suspended in water with
stirring and combined with a suitable reducing agent.
Suitable reducing agents are dithionite in acid or
alkaline solution. Also ammonium sulphite, lithium,
sodium or potassium hydrogen sulphite, triethylphosphite,
triphenylphosphine or lithium aluminium hydride can be
used. Moreover, the nitroso group may be catalytically
hydrogenated using transition metal catalysts based on
palladium, platinum, nickel or rhodium or by transfer
CA 022~6736 1998-12-02
61
hydrogenation, e.g. with cyclohexane or ammonium formate
as the hydrogen source. However, it is preferable to use
sodium dithionite. Apart from the aqueous ammonia
solution which is preferably used, alkali or alkaline
earth metal hydroxide solutions, e.g. lithium, sodium,
potassium hydroxide or magnesium, calcium and barium
hydroxide solutions, but preferably the dilute solutions,
may be used as bases. Furthermore, the methyl, ethyl,
isopropyl, n-propyl, isobutyl, tert.-butyl and n-butyl
alkoxides of the above-mentioned alkali and alkaline earth
metals may be used. This suspension is heated to 20 to
100~C, preferably 50 to 90~C, most preferably 60 to 80~C
~ and mixed with an acid, preferably at the beginning, but
most preferably within the first 30 minutes. Possible
acids are inorganic acids, hydrochloric and sulphuric acid
and organic acids such as formic acid and acetic acid and
mixtures thereof, sulphuric acid being preferred whilst
50~ sulphuric acid is particularly preferred. The
resulting mixture is refluxed until the reaction is
complete. On cooling, the product is precipitated as a
hemisulphate. Moreover, the product can be precipitated
by neutralising with an aqueous base, e.g. lithium
hydroxide, sodium hydroxide, potassium hydroxide,
preferably sodium hydroxide solution, most preferably 30
by weight sodium hydroxide solution by neutralising and
then filtered off as a free base.
Step 5, alternative B:
The nitroso compound (6) is added to a mixture of aqueous
base and an alcohol, with stirring. Then, after the
nitroso compound has been dissolved or suspended, the
reducing agent is added, preferably in solution. Possible
reducing agents are those mentioned in Method A, an
aqueous solution of sodium dithionite being particularly
preferred. Then the mixture is stirred at ambient
temperature for 0.5 to 6, preferably 1 to 4, most
CA 022~6736 1998-12-02
.. , . . , .. . . . _ ..
62
preferably 1.5 to 3 hours. The product precipitated is
isolated by filtering.
In the sixth step, the isomeric compounds (8) are prepared
from the diamines of general formula (7) (Diagram 6).
O O O
N_NJ~NH2 N_ J~,NHCOR2 N_ NJ~NH2
Step 6 R6~/ 1 ~ + R~
N N NH2 N N NH2 N N NHCOR2
R4 R4 R4
(7) (8)
Diaqram 6:
For this, the amino compound of general formula (7) is
suspended in an organic solvent and mixed with an organic
base. After the mixture has been kept at ambient
temperature for 40 to 60 minutes, preferably 20 to 50
minutes, most preferably 25 to 45 minutes, the mixture is
cooled to 0 to 15~C, preferably 5 to 10~C and combined
with a carboxylic acid derivative wherein the group R2 is
as hereinbefore defined. Suitable organic solvents are
-- dimethylformamide, dimethylacetamide, methylene chloride,
toluene and tetrahydrofuran, of which dimethylformamide is
preferred and anhydrous, optionally absolute
dimethylformamide is particularly preferred. Suitable
organic bases are dimethylaminopyridine, pyridine, tert.-
amines, such as trimethylamine, triethylamine,
diisopropylethylamine, DBU (diazabicycloundecene) or one
of the inorganic bases mentioned earlier, particularly
alkali or alkaline earth metal carbonates or hydrogen
carbonates. However, dimethylaminopyridine is
particularly suitable as the base. Carboxylic acid
derivatives which may be used according to the invention
are the carboxylic acid halides, preferably carboxylic
acid chlorides or carboxylic acids if they are suitably
CA 022~6736 1998-12-02
activated. This may be done, for example, by reacting
with chloroformic acid esters, carbonyldiimidazole,
carbodiimides, such as dicyclohexylcarbodiimide, EDAC, or
benzotriazoles, such as HOBt (1-hydroxy-1-H-benzotriazole)
5 and TBTU. Similarly, the corresponding aldehyde may also
be used instead of the carboxylic acid derivative and the
~ intermediate product may be oxidised with
iron(II)chloride, azodicarboxylic acid esters and other
oxidising agents. The mixture is then stirred at ambient
temperature for 0.5 to 4 hours, preferably 1 to 3 hours,
most preferably 2 hours, at 5 to 20~C, preferably 7 to
f 15~C, most preferably 10~C and overnight at ambient
temperature and the solvent is then eliminated in vacuo.
The residue is taken up in water, the solid is filtered
15 off, washed and optionally purified by recrystallisation
or chromatography, preferably column chromatography.
In the seventh step, a compound of general formula (6),
(7) or (8) is cyclised to form the compound of general
20 formula (9) in accordance with methods A to C (Diagram 7).
~ ~.
CA 022~6736 1998-12-02
64
N_ N ~NHCOR2 S
--1 Method A
N Nl NH2
R (8)
O O
N-N ~ NH2 Step7,~ N-N ~ N
N 1 N NH2 Method BN--1 N N~ R
-~ R4 (7) R4 H
(9)
o
~ N-N ~ NO Step 7l
R _l I Method C
N N ~ NH2
R (6)
Diagram 7:
5 Step 7, Method A:
A compound of general formula (8) is suspended in an
alcohol and refluxed with a base for 24 hours. After
-- cooling, the mixture is made alkaline, the solid is
filtered off and dried. If desired, the product is
purified by recrystallisation or chromatography,
preferably column chromatography. Bases which may be used
here include the alkali metal and alkaline earth metal
hydroxides, e.g. the hydroxides of lithium, sodium,
potassium, calcium and barium and mixtures thereof in the
form of aqueous solutions or optionally in admixture with
an alcohol and/or with a water-miscible ether. Suitable
alcohols are methanol, ethanol, n-propanol, isopropanol,
n-butanol and tert.-butanol. Suitable ethers include, in
particular, cyclic ethers, e.g. tetrahydrofuran and
dioxane. By analogous methods, cyclisation may also be
CA 022~6736 1998-12-02
~ carried out with inorganic acid chlorides such as thionyl
chloride, phosphorusoxychloride or phosphorus
pentachloride and with polyphosphoric acid.
5 Another analogous method consists of reacting the
carboxylic acid amide under acid conditions with inorganic
acid/methanol, preferably hydrochloric acid/methanol or
under basic conditions with alkali metal/methanol,
preferably sodium/methanol and then carrying out thermal
10 cyclisation.
Step 7, Method B:
In another process it is possible to prepare the compound
(9) wherein R2 is hydrogen starting from the diamine (7).
15 To do this, (7) is stirred with an equimolar amount,
~ preferably with an excess of formamide for 0.5 to 3 hours,
preferably 1 to 2 hours, most preferably 1.5 hours at 50
to 250~C, preferably 100 to 225~C, most preferably 180 -
200~C. The formamide remaining is distilled off in vacuo
and the residue is taken up in water. The solid thus
obtained is filtered off, washed with water and the
resulting product is purified optionally by
recrystallisation or chromatography, preferably column
chromatography.
Step 7, Method C:
Moreover, the compound of general formula (9) may be
obtained directly from the nitroso compound of general
formula (6) if R2 denotes a group NR8R9, wherein R8 and R9
are as hereinbefore defined. To a solution of the desired
~ N-formylamine in diglyme, phosphorusoxychloride is added
at -10 to +20~C, preferably -5 to +5~C, most preferably
0~C. Then the mixture is stirred in an ice bath and the
nitroso compound (6) is added. The mixture is stirred for
15 to 120 minutes, preferably 30 to 80 minutes, most
preferably 40 to 60 minutes, at 30 to 120~C, preferably 50
to 100~C, most preferably 70 to 80~C and then added to ice
CA 022~6736 1998-12-02
, . . , ., _ . .. , . ., ,. , . __
66
water. The solid product is filtered off, washed and
dried and optionally purified by recrystallisation or
purified by chromatography, preferably column
chromatography.
The compounds of general formula (Ia) and (Ib) may be
obtained in an eighth step from the compound of general
formula (9) (Diagram 8).
R'
R6~' 1~ /~ R2
O N N N
--N~ \~R2 Step 8 R4
R4 H R6~ R
(9) N N N~ 3
R4 R
o (Ib)
Diaqram 8:
For this, the starting compound is taken up in an organic
' solvent and reacted with a base and a suitable alkylating
'~- agent. The reaction mixture is stirred overnight at
ambient temperature. If required, the reaction may also
be carried out at elevated temperature. The duration of
the reaction may be up to one week under certain
circumstances. When the reaction has ended the mixture is
evaporated to dryness. The residue is taken up in an
aqueous alkaline solution, whilst the base may be lithium
hydroxide, sodium hydroxide, potassium hydroxide,
preferably sodium hydroxide and most preferably 2N sodium
hydroxide solution. The solution is then washed with a
non-polar organic solvent such as benzene, toluene or
xylene, preferably toluene and adjusted to a pH of 6. The
solid precipitated i~ filtered off and optionally purified
CA 022~6736 1998-12-02
67
by chromatography, preferably column chromatography. In
this way, both the dialkylation products and the
monoalkylation products of the compounds of general
formula (Ia) and (Ib) may be prepared and isolated.
Suitable organic solvents for the alkylation include inert
solvents such as dimethylformamide, dimethylacetamide,
methylene chloride, alkylether, preferably diethylether,
tetrahydrofuran, benzene, toluene, xylene and particularly
dimethylformamide. Suitable bases include carbonates such
~ as lithium carbonate, sodium carbonate, potassium
carbonate, calcium carbonate and hydrogen carbonates of
the above-mentioned alkali metals and alkaline earth
metals as well as the hydroxides and alkoxides thereof.
The alkoxides of the above-mentioned alkali and alkaline
earth metals with methanol, isopropanol and tert.-butanol
are preferred, sodium ethoxide being particularly
preferred. In the case of the carbonates, hydrogen
carbonates, alkoxides and hydroxides, it may be
advantageous to use alcohol or water as solvent. Other
bases which may be used for alkylation are sodium hydride,
potassium hydride, lithium hydride and calcium hydride in
the above-mentioned inert solvents. Moreover, suitable
alkylating agents are hydrocarbon halides such as alkyl
chlorides, alkyl bromides and alkyl iodides as well as
hydrocarbon or alkyl tosylates, -mesylates and -triflates
and the alkyl sulphates, alkyl iodides being preferred.
The invention further relates to a process for preparing
compounds of general formula (Id) and (Ic)
O
R6~N ~CN\~R2 R6~, _~N~R2
r N N~ ~N N N
R5 R3 R5
(Id) (Ic)
CA 022~6736 1998-12-02
68
~ Starting from a compound of general formula (12) a
compound of general formula (13) may be obtained (Diagram
9) in accordance with the reaction of (5) to obtain (6)
described hereinbefore (cf. Diagram 4, Step 4).
O O
R6 ~/ l ~ Step 4 R6
(12) (13)
Diaqram 9:
This may be converted in accordance with the method of
Step 5 (cf. Diagram 5) into a compound of general formula
~(14) (Diagram 10).
O O
N_NJ~,NO N_NJ' NH2
R~ 1~ ~ R~ ~
(13) (14)
Diaqram 10:
In accordance with the alternative methods described
hereinbefore for reacting compound (7) to obtain compound
(8) (cf. Diagram 6, Step 6) the compounds of general
formula (14) may be converted into the compounds of
general formula (15) (Diagram 11).
CA 022~6736 1998-12-02
69
O O O
N_ N ~NH2 N_ NJ~,NHCOR2 N_NJ' NH2
,NlN~NH2 Nl~N~NH Nl~N~NHCOR2
R5 R5 R5
(14) (15)
Diaqram 11:
From this, the compounds of general formula (16) may be
obtained (Diagram 12) in Step 7 in accordance with the
( alternative reactions of cyclisation described above (cf.
Diagram 7).
N-N ~ NHCOR2 Ste
rl~ Me~od A
R5 (1 5)
O O
Rs~/ l ~ Step 7, ' RC~/ l ~ \~ R2
R5 (14) R5 H
(16)
o
N-N ~ NO Step
R5 (13)
Diaqram 12:
From these, the compounds of general formulae (Ic) and
(Id) may be obtained by the alkylation reactions described
CA 02256736 1998-12-02
,,, _.,
in Steps 3 and 8 and may then be isolated if required by
suitable methods of purification or separation, e.g.
crystallisation or column chromatography (Diagram 13).
,R
R6~/N ~ N ~ N~ 2
O N N N
--N~ \~R2 SteP 8 R +
jN N N~ O
._ 5 H N_N~ N
(16) ~Nl~ ~N~
R R3 (~)
Diaqram 13:
By introducing suitably substituted groups Rl, R2, R3, R4,
R5 or R6, the triazolopurines (Ia), (Ib), (Ic) and (Id)
which may be prepared by the methods described above can
be further functionalised using generally known methods.
If desired, suitable protecting groups may be used.
Diagram 14 which follows illustrates, by way of example,
possible modifications to the group R6 of the compounds
(Ib) which may arise according to the invention.
The reactions outlined by way of example in Diagram 14
lead to reactive triazolopurines which may be used as
starting compounds for further structural variations
(esterifications, alkylations, substitutions etc.).
According to the invention, all the groups R1, R2, R3, R4,
R5 or R6 may be functionalised. These modifications are
not restricted to compounds of general formula (Ib) but
may also be transferred to the triazolopurines of general
formulae (Ia), (Ic) and (Id). Diagram 14 serves as an
explanation by way of example of possible structural
variations of the triazolopurines (Ia), (Ib), (Ic) and
CA 022~6736 1998-12-02
, . . ,, ~ ~ . . ...
(Id), without restricting the invention to the reaction
sequences shown.
R~ ~N\~ 2 R6 MeO~CH2--O-CH2Alcyl--
N N N~ 3(b)
R4 R
[Oxidation ]
O O
N ~ N ~Halogena~on~ N ~ N
R4 R 14 R3
R': -CH2AIkYI-, [ Oxidation ] R': -CH2Alky~,
[Oxidation ] ~ X: Halogen,
O O
H N J~ N HO N~ N
,~ R~ R ,~ R~ R
O N N N~ ~ N N N
R4 R3 R":-Alkyl-, R4 R3
Diagram 14:
The present invention is explained more fully by means of
descriptions of synthesis of triazolopurines given by way
of example. These Examples serve as an illustration
without restricting the invention to their scope.
CA 02256736 1998-12-02
. . _, .
Step 1: SYnthesis of 5-substituted 3-amino-1,2,4-triazoles
(3):
N ' N' H
N NH2
(3)
The unsubstituted 3-amino-1,2,4-triazole is commercially
obtainable, the 5-substituted 3-amino-1,2,4-triazoles
required may be prepared in accordance with methods known
from the literature as published, for example, in J. Chem.
Soc. 1929, 816, J. Org. Chem. 1926, 1729 or Org. Synthesis
26, 11. The carboxylic acids R6-COOH and nitriles R6-CN
are commercially obtainable or may be prepared by methods
known from the literature.
Table 1:
No. R6 Yield M (~C)
(%)
1.1. -H commercially
obtainable
1.2.-Methyl 76 145-148
- 1.3. -Ethyl 75 152
~-- 1.4.-n-Propyl 62 140-143
1.5.-i-Propyl 67 103-104
1.6.-n-Butyl 62 118
1.7.-t-Butyl 39 130-131
1.8.Benzyl- 70 167-169
1.9.Cyclopentyl- 64 168-172
1.10.2-Furyl- (Hemisulfate) 76 207-208
1.11.Phenyl- 68 185-186
1.12.Cyclohexylmethyl- 51 186-188
1.13.2-Phenylethyl- 53 139-140
1.14.(4-MeO-Ph)-O-cH2- 41
1.15.(4-MeO-Ph)-CH2-O-CH2 54 153
CA 022~6736 1998-12-02
., _ . .
1.16. (4-Ph-CH2O-Ph)-CH2- 57 196-200
1.17. 4-Fluorobenzyl- 100 163-164
1.18. 3,4-Difluorobenzyl- 81 135-137
1.19. 3-Pyridylmethyl- 58 192-196
SteP 2: SYnthesis of 2-substituted 4H-5-amino-1,2,4-
triazolo[l,5-a]pyrimidin-7-one derivatives (4)
R ~ N~
H (4)
General method:
2.3g (0.1 mol) of sodium are dissolved in 10 ml of abs.
ethanol, then 14. 7 g (0.13 mol) of ethyl cyanoacetate are
added. The mixture is stirred for 1 - 2 hours at ambient
temperature, then mixed with 0.1 mol of the desired 5-
substituted 3-aminotriazole and refluxed for 4 - 6 hours.
The cooled reaction mixture is mixed with water and made
acidic with stirring, then the solid is filtered off,
washed and dried.
- Using thls process the following compounds were prepared:
Table 2:
No R6 Yield Mp (~C)
(%)
2.1. -H 73 ~370
2.2. -Methyl 76 ~300
2.3. -Ethyl 81 ~300
2.4. -n-Propyl 80 290 (Decomp.)
~ 2.5. -i-Propyl 81 294 (Decomp.)
2.6. -n-Butyl 77 256
2.7. -t-Butyl 78 ~300
2.8. Benzyl- 82 300 (Decomp.)
CA 022~6736 l998-l2-02
, .. . . .. .
74
2.9. Cyclopentyl- 83 310 (Decomp.)
2.10. 2-Furyl- 61
2.11. Phenyl- 75
2.12. Cyclohexylmethyl- 81 > 300
2.13. 2-Phenylethyl- 83 295-296
2.14. (4-MeO-Ph)-O-cH2- 68 302
2.15. (4-MeO-Ph)-CH2-O-CH2 89 245
~ 2.16. (4-PhCH2O-Ph)-CH2- 96 296-298
No R6 Yield Mp (~C)
(%)
....~
2.17. 4-Fluorbenzyl- 79 300-302
2.18. 3,4-Difluorbenzyl- 62 290-292
2.19. 3-Pyridylmethyl- 82 ~300
Step 3: SYnthesis of the alkylated triazoloPYrimidine
derivatives (5) and (12):
O O
R~N~ 3~ N-N~
(5) (12)
General method:
66 mMol of the relevant 5-amino-1,2,4-triazolo[1,5-a]-
pyrimidin-7-one (4) are dissolved in 10 - 20 times the
quantity of anhydrous dimethylformamide and mixed with
76 mMol of potassium carbonate and 76 mMol of the desired
alkyl iodide. The reaction mixture is stirred at ambient
temperature for one 1 - 2 days and evaporated to dryness.
The residue is stirred with water and methylene chloride.
In many cases, after this treatment, compound (5) can be
filtered off as a solid and isolated in this way. The
isomer (12) can then be obtained from the filtrate as
described below. If no solid is obtained during stirring
CA 022~6736 1998-12-02
, . .. ... _ _--. .. .. . .. .
or in order to isolate compound (12) from the filtrate,
the phases of the filtrate are separated, the aqueous
phase is extracted with methylene chloride and the
combined organic phases are dried over magnesium sulphate
and evaporated to dryness. Compound (5) and optionally
(12) are isolated by chromatographic purification of the
residue. The alkyl halides required R'-X (wherein R' =
R4, R5) are commercially obtainable or may be prepared by
methods known from the literature.
Using this method the following substances were prepared:
~,
CA 022~6736 1998-12-02
~ , . . .
76
Table 3:
No. R6 R4 R5 Yield Mp (~C)
(%)
3.1 -H -Methyl - 80310(Decomp.)
3.2 -H -n-Propyl - 58 184- 186
3.3 -H - -n-Propyl 18 183
3.4-Methyl -n-Propyl - 61 228
3.5 -Ethyl -n-Propyl - 65 194
3.6-n-Propyl -n-Propyl - 69 170
3.7-i-Propyl -n-Propyl - 50 138
3.8 -Butyl -n-Propyl - 62 167
3.9-t-Butyl -n-Propyl - 64 164- 165
3.102-Furyl- -n-Propyl - 45 260 - 262
3.11Cyclopentyl- -n-Propyl - 64 148- 150
3.12Cyclopentyl- - -n-Propyl 22 173
3.13Benzyl- -n-Propyl - 60 208
3.14Benzyl- -Methyl - 52 296
3.15Benzyl- -Ethyl - 58 213
3.16Benzyl -i-Propyl - 29 232
3.17Benzyl- -n-Butyl - 55 175
3.18Benzyl- -Pentyl - 63 188
3.19Benzyl- Benzyl- - 51 180- 181
t 3.20Phenyl- -n-Propyl - 30 270 - 2713.21Cyclohexylmethyl- -Ethyl - 67 210-212
3.222-Phenylethyl- -Ethyl - 29 173-174
3.23(4-MeO-Ph)-O-CH2- -Ethyl - 58 177-180
3.24(4-MeO-Ph)-CH2-O-CH2- -Ethyl - 100 *
8.25(4-PhCH2-O-Ph)-CH2- -Ethyl - 63 240-242
3.264-Fluorobenzyl- -Ethyl - 53
3.273,4-Difluorobenzyl- -Ethyl - 61 228
~ 3.283-Pyridylmethyl- -Ethyl - 94 186-188
*Isomer sepd,dlion in r~ /,;.,9 step
CA 022~6736 1998-12-02
77
Ste~ 4: Synthesis of the nitroso compounds (6) and (13)
RB~,N - N ~ N ~ N ~NO
(6) (13)
1~ General methods (alternative A):
10 mMol of 5-amino-1,2,4-triazolo[1,5-a]pyrimidin-7-one
(4), (5) or (12) are dissolved or suspended in 10-30 ml of
anhydrous dimethylformamide and the mixture is cooled to
-5~C. 20 mMol of isoamyl nitrite are added in such a way
that the temperature does not rise above 0~C and the
mixture is stirred at this temperature for 4 - 24 hours,
then as necessary at ambient temperature until the
reaction is complete. The solvent is eliminated in vacuo,
the residue is stirred with ether, filtered off, washed
with water and dried in vacuo.
- 20 The following substances were prepared by this method:
Table 4a:
No. R6 R4 R5 Yield (%) Mp (~C)
4.1 -H -H - 85 >350
4.2 -H -Methyl - 79 248
4.3 -H -n-Propyl - 81 211
(Decomp.)
4.4 -Methyl -n-Propyl - 88 226
(Decomp.)
4.5 -Ethyl -n-Propyl - 94 190
CA 022~6736 1998-12-02
4.6 -n-Propyl -n-Propyl - 96 206
(Decomp.)
4.7 i Plopyl -n-Propyl - 72 210
(Decomp.)
4.8 -n-Butyl -n-Propyl - 95 196
(Decomp.)
4.9 -t-Butyl -n-Propyl - 93 200
(Decomp.)
4.10 2-Furyl- -n-Propyl - 85 261
4.11Cyclopentyl- -n-Propyl - 97 224
(Decomp.)
..
4.12Cyclopentyl- -H - 89 225
(Decomp.)
4.13 Benzyl- -H - 97 238
(Decomp.)
4.14 Benzyl- -n-Propyl - 95 208
(Decomp.)
No. R6 R4 R5 Yield (%) Mp (~C)
4.15 Benzyl- -Methyl - 96 233
(Decomp.)
4.16 Benzyl- -Ethyl - 97 226
(Decomp.)
4.17 Benzyl -i-Propyl - 88 190
(Decomp.)
4.18 Benzyl- -n-Butyl - 93 196
4.19 Benzyl- -n-Pentyl - 74 190
(Decomp.)
4.20 Benzyl- Benzyl- - 96 236
4.21 Phenyl- -n-Propyl - 92 257 - 258
(Decomp.)
4.22 -H - -n-Propyl 63 206
(Decomp.)
4.23Cyclopentyl- - -n-Propyl 74 146
4.24Cyclohexylmethyl- -Ethyl - 83 208-209
4.252-Phenylethyl- -Ethyl - 90 200-201
CA 022~6736 1998-12-02
, . ... .. , .. , . . _ . , .
79
4.26(4-MeO-Ph)-O-CH2- -Ethyl - 93 214-216
4 27(4-Meo-ph)-cH2-o-cH2- -Ethyl - 56 156-158
4.28(4-phcH2-o-ph)-cH2- -Ethyl - 97 201
(Decomp.)
4.294-Fluorobenzyl- -Ethyl - 89 241-242
4.303,4-Difluorobenzyl- -Ethyl - 97 234-236
4.313-Pyridylmethyl- -Ethyl - 44 229
General method (alternative B):
~ 20 mMol of 5-amino-1,2,4-triazolo[1,5-a]pyrimidin-7-one
(4), (5) or (12) are suspended in 80 to 100 ml of glacial
acetic acid. The mixture is heated to 70~C, mixed with a
solution of 20 mMol of sodium nitrite in 2-4 ml of H2O and
stirred for 0.5-2 hours at 70~C, then for a further 0.5-2
hours at 10~C. The product is filtered off, washed and
dried in vacuo. Using this process the following
10 compounds were prepared:
Table 4b:
No. R6 R4 R5 Yield (%) Mp (~C)
4.32 Benzyl- -H 65 236 (Decomp.)
SteP 5: SYnthesis of the diamines (7) and (14):
~,
~NH ~Nl'~NH
(7) (14)
General method (alternative A):
12.7 mMol of the nitroso compound (6) or (13) are
suspended in 86 ml of H2O with stirring and 4.9 g
(2.9 mMol) of Na2S2O4 are added. The suspension is heated
to 80~C and within 30 minutes 15 ml of 50~ H2SO4 are added.
CA 022~6736 1998-12-02
The mixture is refluxed until the reaction is complete.
After cooling, the product is usually obtained as a
hemisulphate. If no solid is precipitated, the mixture is
neutralised with 30~ sodium hydroxide solution and the
S free base precipitated is filtered off.
General method (alternative B):
11.5 mMol of nitroso compound (6) or (13) are added to a
mixture of 175 ml of 25~ aqueous ammonia and 36 ml of
ethanol, with stirring. Then the mixture is stirred at
30~C until the nitroso compound has substantially
dissolved, a solution of 6.1 g (34.7 mMol) of Na2S2O4 in
57 ml of H2O is added dropwise and stirring is continued
for a further 1.5 to 3 hours at ambient temperature. If
some starting compound is still present, 10~ of the above
quantity of Na2S2O4 are added and stirring is continued
until the reaction is complete. The product is isolated
by filtering.
The compounds described in Table 5, inter alia, were
prepared by one of these methods (A or B, see Table 5):
CA 022~6736 1998-12-02
., .. . ~ . . . . . ....
D 8 1
Table 5:
~,
No. R6 R4 R5 Alter- YieldSalt formMelting point in
native in % ~C
5.1 -H -H - A 73 Hemisulfate ~300
5.2 -H -Methyl - A 65 - 267 (Deco",p.)
5.3 -H -n-Propyl - A 65 - 185-188
5.4 -Methyl -n-Propyl - A 70 - 174
5.5 -Ethyl -n-Propyl - A 78 - 170-172
5.6 -n-Propyl -n-Propyl - A 82 - 148-150
5.7 -i-Propyl -n-Propyl - A 52 - 193
5.8 -n-Butyl -n-Propyl - A 71 - 140-142
5.9 -t-Butyl -n-Propyl - B 90 - 168
5.10 Cyclopentyl- -H - A 74 Hemisulfate 224
5.11 Cyclopentyl- -n-Propyl - A 48 Hemisulfate 195-197
5.12 2-Furyl- -n-Propyl - B 75 - 204-205
5.13 Benzyl -i-Propyl - A 8 Hemisulfate 223
5.14 Benzyl- -n-Propyl - A 62 Hemisulfate 217
5.15 Benzyl- -Methyl - B 91 - 248
D 82
o
Ul
No. R6 R4 R5 Alter- Yield Salt form Melting point
~, native in % in ~C
5.16Benzyl- -Ethyl - B 95 - 175
5.17Benzyl- -n-Butyl - A 48Hemisulfate 203-205
5.18Benzyl- -n-Pentyl - B 92 - 146
5.19Benzyl- -H - A 47Hemisulfate 218-220
5.20Benzyl- Benzyl- - B 80 - 273
5.21Phenyl- -n-Propyl - A 79Hemisulfate 260
5.22 -H - -n-Propyl A 81 - 222-224
5.23Cyclopentyl- - -n-Propyl A 86 - 175
5.24Cyclohexylmethyl- -Ethyl - B 94 - 158-160
5.252-Phenylethyl- -Ethyl - B 94 - 204-205
5.26(4-MeO-Ph)-O-CH2- -Ethyl - B 86 - 154-156
5.27(4-MeO-Ph)-CH2-O-cH2- -Ethyl - B 88 - 133-139
5.28(4-phcH2-o-ph)-cH2- -Ethyl - B 84 - 155
5.294-Fluorobenzyl- -Ethyl - B 88 - 202-205
5.303,4-Difluorobenzyl- -Ethyl - B 95 - 209-212
5.303-Pyridylmethyl- -Ethyl - B 82 - 179-180
SteD 6: Synthesis of amides (8) and (15):
~ R ~ N ~'
+ +
o
R~ J~ N-N~NH2
N N NHCOR N 1 N NHCOR2
R4 R5
(8) (15)
General method (alternative A):
30 mMol of diamino compound (7) or (14) are suspended in
165 ml of anhydrous dimethylformamide and mixed with
45 mMol (or in the case of hemisulphates 78 mMol) of 4-
dimethylaminopyridine. The mixture is stirred for 30
. minutes at ambient temperature, cooled to 5 - 10~C and a
'~. solution of 39 mMol of the desired carboxylic acid chloride
in 16 ml of anhydrous dimethylformamide is added. Then the
mixture is stirred for 2 hours at 10~C and overnight at
ambient temperature, after which the solvent is eliminated
in vacuo. The residue is added to water, the solid is
filtered off and washed and optionally purified by
recrystallisation or chromatography.
The carboxylic acid chlorides required are commercially
available or may be prepared by methods known from the
literature.
CA 022~6736 1998-12-02
84
Using this method the compounds of formulae (8) and (15)
described in Table 6a were prepared, inter alia:
:
CA 02256736 1998-12-02
D 8 5
ulTable 6a:
No. R6 R4 R5 R2 Yield inMeltingpointin
~, % ~C
6.1 -H -H Cyclopentyl- 86284 (Decomp.)
O 6.2 -H -Methyl - Benzyl- 35 306
6.3 -H -n-Propyl - Benzyl- 65 178
6.4 -H -n-Propyl -Cyclopentyl- 95 192
6.5 -Methyl -n-Propyl -Cyclopentyl- 71 208
6.6 -Ethyl -n-Propyl -Cyclopentyl- 72 209
6.7 -Ethyl -n-Propyl - -Ethyl 77
6.8 -n-Propyl -n-Propyl -Cyclopentyl- 90 210
6.9 -n-Propyl -n-Propyl - Phenyl- 68 205
6.10 -n-Propyl -n-Propyl -4-Fluorobenzyl- 84 200
6.11 -i-Propyl -n-Propyl -Cyclopentyl- 71 148
6.12 -n-Butyl -n-Propyl -Cyclopentyl- 66 173
6.13 -t-Butyl -n-Propyl -Cyclopentyl- 60 100
6.14 Cyclopentyl- -n-Propyl - -Ethyl 76 205
6.15 Cyclopenlyl- -n-Propyl - -Methyl 70 243
6.16 Cyclopentyl- -n-Propyl - Benzyl- 76 160
6.17 2-Furyl- -n-Propyl -Cyclopentyl- 74
6.18 2-Furyl- -n-Propyl - -Ethyl 73
6.19 Benzyl- -H -Cyclopentyl- 51 254-257
6.20 Benzyl- Benzyl- -Cyclopentyl- 90
6.21 Benzyl- -Ethyl -3-Tetrahydrofuranyl- 60 227
6.22 Benzyl- -Ethyl -4-Tetrahydropyranyl- 65 237
D 8 6
No. R6 R4 R5 R2 Yield inMelting pointin
% ~C
6.23Benzyl- -Ethyl - 2-Pyridyl- 63 223
6.24Benzyl- -Ethyl - 4-Pyridyl- 55
6.25Benzyl- -n-Propyl - -Ethyl 61 183
6.26Benzyl- -n-Propyl - -n-Propyl 62 166
6.27Benzyl- -Methyl - Cyclopentyl- 88 205-208
6.28Benzyl- -Ethyl - Cyclopentyl- 90 188-189
6.29Benzyl- -n-Propyl - Cyclopentyl- 77 170
6.30Benzyl- -n-Butyl - Cyclopentyl- 60 163
6.31Benzyl- -n-Pentyl - Cyclopentyl- 67 162
6.32Phenyl- -n-Propyl - -Ethyl 47 165-166
6.33Phenyl- -n-Propyl - Cyclopentyl- 68 >300
6.34 -H - -n-PropylCyclopentyl- 69 248
6.35Benzyl- -Ethyl - 2-Pyridyl- 40 223
6.36Benzyl- -Ethyl - 4-Pyridyl- 55
6.37Benzyl- -Ethyl - 3-Pyridyl- 88 206
6.38Benzyl- -Ethyl - Cyclohexyl- 86 186-187
6.39Benzyl- n-Butyl- - -H 84 231-232
6.40Benzyl- -H - Phenyl- 72 276-279
6.41Benzyl- -H - 2-Furyl- 69 305-307
6.423-Pyridylmethyl--Ethyl - Cyclopentyl- 73 163-165
6.433-Pyridylmethyl--Ethyl - -Ethyl 71 186-187
6.442-Furyl- -n-Propyl - 2-Furyl- 81 228
D 8 7
No.R6 R4 R5 R2 Yield inMelting pointin
% ~C
6.45 Cyclohexylmethyl--Ethyl - -Ethyl 81 204-207
6.46 Cyclohexylmethyl--Ethyl - Cyclopentyl-70 196-198
6.47 2-Phenylethyl- -Ethyl - -Ethyl 95 182-183
6.48 2-Phenylethyl- -Ethyl - Cyclopentyl-81 155-156
6.49 Cyclopentyl- -n-Propyl - 3-Tetrahydrofuranyl- 65 179-180
6.50 Cyclopentyl- -n-Propyl - Cyclopentyl-41 306-308
6.51 (4-MeO-Ph)- OCH2- -Ethyl - -Ethyl 93 160-164
6.52 (4-MeO-Ph)- OCH2- -Ethyl - Cyclopel,lyl-76 176-178
6.53 (4-MeO-Ph)-CH2-O-cH2--Ethyl - Cyclopentyl-91 133-136
6.54 (4-PhCH2O-Ph)-cH2- -Ethyl - Cyclopentyl-86 190
6.55 Cyclohexylmethyl--Ethyl - ", ~ 98
6.56 4-Fluorobenzyl- -Ethyl - Cyclopentyl-62 206-208
6.57 4-Fluorobenzyl- -Ethyl - tert.-Butyl- 53
6.58 4-Fluorobenzyl- -Ethyl - ~ 75
6.59 4-Fluorobenzyl- -Ethyl - ,~ 91
6.60 3,4-Difluorobenzyl--Ethyl - Cyclopentyl-100 222-224
6.61 3,4-Difluorobenzyl--Ethyl - ,~ 100
6.62 Benzyl- -Ethyl - ,~ 87
6.63 3-Pyridylmethyl- -Ethyl - ,~ 79 134-137
General procedure (alternative B):
6.6 mMol of a diamine (7) or (14) are suspended with
7.3 mol of the correqponding carboxylic acid in 15-25 ml
of acetonitrile. 7.3 mMol of N-methylmorpholine are
slowly added with stirring. Then a solution of 7.3 mMol
of isobutyl chloroformate in 6-7 ml of acetonitrile is
added dropwise at 20-25~C within 10-20 minutes. The
suspension obtained is stirred for 4-6 hours at ambient
temperature. For processing, the suspension is filtered
and washed with acetonitrile. The resulting filtrate is
concentrated and chromatographed over silica gel.
_,
Using this process, the compounds of formula (8) and (15)
described in Table 6b were prepared:
Table 6b:
No. R6 R4 R5 R2 Yield in
%
6.64 4-fluorobenzyl- Ethyl- - ,~ 26
SteD 7: Reactions of cyclisation to obtain the
triazoloPurines (9) and (16)
R6~ l ~ \~ R2 R~ \~ R2
(9) (16)
General method (Method A. alternative A):
14.4 mMol of (8) or (15) are suspended in 75.5 ml of H2O
and 37.8 ml of ethanol and refluxed with 17.4 ml of 50
NaOH and 4.82 g (65 mMol) of Ca(OH) 2 for 24 hours. The
mixture is allowed to cool, made acidic, the solid is
CA 022~6736 1998-12-02
89
filtered off and dried. The product is optionally
purified by recrystallisation or chromatography.
Using this process the compounds of general formulae (9)
and (16) described in Table 7a were prepared, inter alia.
CA 022~6736 1998-12-02
D 90
o
ul Table 7a-
No. R6 R4 R5 R2 Yield inMellin~ point
% in ~C
7.1 -H -H -Cyclopentyl- 80 >360
O 7.2 -H -Methyl - Benzyl- 35 306
7.3 -H -n-Propyl - Benzyl- 68 222-223
7.4 -H -n-Propyl -Cyclopentyl- 59 250
7.5 -Methyl -n-Propyl -Cyclopentyl- 62 262-264
7.6 -Ethyl -n-Propyl -Cyclopentyl- 58 253
7.7 -Ethyl -n-Propyl - -Ethyl 68 229
7.8 -n-Propyl -n-Propyl -Cyclopentyl- 75 256
7.9 -n-Propyl -n-Propyl - Phenyl- 49300 (Decomp.)
7.10-n-Propyl -n-Propyl -4-Fluorbenzyl- 65 221
7.11i Plopyl -n-Propyl -Cyclopentyl- 62 260-261
7.12-n-Butyl -n-Propyl -Cyclopentyl- 72 246
7.13-t-Butyl -n-Propyl -Cyclopentyl- 64 310
7.14Cyclopentyl- -n-Propyl - -Ethyl 85 245-248
7.15Cyclopentyl- -n-Propyl - -Methyl 56 248-250
7.16Cyclopentyl- -n-Propyl - Benzyl- 70 233
7.172-Furyl- -n-Propyl -Cyclopentyl- 61>300(Decomp.)
7.182-Furyl- -n-Propyl - -Ethyl 44>300(Decomp.)
7.19Benzyl- -H Cyclopentyl- 40270 (Decomp.)
7.20Benzyl- Benzyl- -Cyclopentyl- 13 270
7.21Benzyl- -Ethyl -3-Tetrahydrofuranyl-60 227
t~ - ' }
D 91
o
Ul
No. R6 R4 R5 R2 Yield in Melting point
~, % in~C
7.22 Benzyl- -Ethyl - 4-Tetrahydropyranyl- 64 237
7.23 Benzyl- -Ethyl - 2-Pyridyl- 21 287
7.24 Benzyl- -Ethyl - 4-Pyridyl- 8 346
7.25 Benzyl- -n-Propyl - -Ethyl 57 223
7.26 Benzyl- -n-Propyl - -n-Propyl 53 202
7.27 Benzyl- -Methyl - Cyclopentyl- 13 265-267
7.28 Benzyl- -Ethyl - Cyclopentyl- 56 242
7.29 Benzyl- -n-Propyl - Cyclopentyl- 67 234-235
7.30 Benzyl- -n-Butyl - Cyclopentyl- 53 238-240
7.31 Benzyl- -n-Pentyl - Cyclopentyl- 56 231-236
7.32 Phenyl- -n-Propyl - -Ethyl 97 ~300 (Decomp.)
7.33 Phenyl- -n-Propyl - Cyclopentyl- 78 ~300 (Decomp.)
7.34 -H - -n-Propyl Cyclopentyl- 36 217-220
7.35 -H - -Ethyl Cyclopentyl- 2 4 260
7.36 Benzyl- -Ethyl - 3-Pyridyl- 11 331-332
7.37 Benzyl- -Ethyl - Cyclohexyl- 74 260-262
7.383-Pyridylmethyl- -Ethyl - Cyclopentyl- 60 261-263
7.393-Pyridylmethyl- -Ethyl - -Ethyl 62 225-227
7.40 2-Furyl- -n-Propyl - 2-Furyl- 9 385-387
7.41 Benzyl- -H - 2-Furyl- 14 352-354
7.42Cyclohexylmethyl- -Ethyl - -Ethyl 73 230-232
7.43Cyclohexylmethyl- -Ethyl - Cyclope"lyl- 62 273-274
D 92
o
Ul
No.R6 R4 R5 R2 Yield inMelting point
~, % in ~C
7.44 2-Phenylethyl--Ethyl - -Ethyl 31 282-283
7.45 2-Phenylethyl--Ethyl - Cyclopentyl-53 292-294
7.46 Cyclopentyl-n-Propyl -3-Tetrahydrofuranyl- 56 289-290
7.47 Cyclopentyl-n-Propyl - Cyclopentyl- 41 306-308
7.48 (4-MeO-Ph)-OCH2--Ethyl - -Ethyl 47 225
7 49 (4-MeO-Ph)-OCH2--Ethyl - Cyclopentyl-20 234-236
7 50 (4-MeO-Ph)-CH2-O-CH2- -Ethyl - Cyclopentyl- 70 1827.51 (4-PhCH2O-Ph)-CH2--Ethyl - Cyclopentyl-70 251
7.52 4-Fluorobenzyl--Ethyl - Cyclopentyl-71 252-254
7.53 4-Fluorobenzyl--Ethyl - -tert.-Butyl 73 262-264
7.54 4-Fluorobenzyl--Ethyl - ~ 53 337-339
7.55 4-Fluorobenzyl--Ethyl - ,~ 45 325-327
7.56 3,4-Difluorobenzyl- -Ethyl - Cyclopentyl- 60 264-2667.57 3,4-Difluorobenzyl- -Ethyl - ~ 58 315-317
7.58 Benzyl- -Ethyl - ,~ 67 320-322
7.59 3-Pyridylmethyl--Ethyl - ,~ 57 330-332
General method (Method A. alternative B):
4 mMol of an amide (8) or (15) are stirred in 10-15 ml of
tetrahydrofuran and 25-35 ml of H20 with 46 mMol of
lithium hydroxide monohydrate at 45-65~ for 14 days. Then
5 the mixture is acidified with 6 N hydrochloric acid and
suction filtered. The crude product is recrystallised
from methanol. The resulting product is separated by
chromatography on a silica gel column.
10 The compounds of general formula (9) described in Table 7b
were prepared by this method.
Table 7b:
No. R6 R4 R2 Yield (%)Mp (~C)
7.62 Cyclohexylmethyl- Ethyl- ""OE~ 10 246-248
7.63 Cyclohexylmethyl- Ethyl- ~) 3258-260(Decomp.)
General method (Method B):
10.1 mMol of (7) or (14) are stirred into 34 ml of
formamide for 1.5 hours at 200~C. The remaining formamide
20 is distilled off in vacuo and the residue is stirred with
water. The solid is filtered off, washed with water and
the product is purified by recrystallisation or
chromatography.
25 Using this process, the compounds (9) and (16) listed in
Table 7c were prepared:
CA 022~6736 1998-12-02
94
~ No. R6 R4 R5 R2 Yield Mp (~C)
(%)
7.64-Ethyl -n-Propyl - -H 84 >300
7.65Cyclopentyl- -H - -H 53 215
7.66Cyclopentyl- -Methyl - -H 7 ~300
7.673-Pyridylmethyl- -Ethyl - -H 58 328-330
7.68Cyclopentyl- -n-Propyl - -H 58287 (Decomp.)
7.692-Furyl- -n-Propyl - -H 74~300 (Decomp.)
7.70Benzyl- -Methyl - -H 9 ~300
.~ 7.71Benzyl- -n-Propyl - -H 75 ~300
7.72Benzyl- -i-Propyl - -H 90 ~300
7.73Benzyl- -n-Butyl - -H 75 ~300
7.74Benzyl- -H - -H 63 ~300
7.75Phenyl- -n-Propyl - -H 75 295-296
(Decomp.)
7.76Cyclopentyl- - -n- -H 35 202-203
Propyl
7.773-Pyridylmethyl- -Ethyl - -H 58 328-330
7.78Cyclohexylmethyl- -Ethyl - -H 87 328-330
7.792-Phenylethyl- -Ethyl - -H 84 273
General method (Method C):
At 0~C, 0.66 ml (7.2 mMol) of phosphorusoxychloride are
added to a solution of 6.8 mMol of the desired N-
formylamine in 6 ml of diglyme. The mixture is stirred
for 30 minutes in an ice bath and then 4.8 mMol of nitroso
compound (6) or (13) are added. The mixture is stirred at
80~C for 30 - 60 minutes and added to ice water, the solid
product is filtered off, washed and dried. The product is
~ purified by crystallisation or by chromatography.
Using this process, compounds (9) described in Table 7d
were prepared:
CA 022~6736 1998-12-02
. . ., ~, . .
Table 7d:
No. R6 R4 R2 Yield (%) Mp (~C)
7.80Benzyl- -n-Propyl N-Morpholinyl- 21 298
7.81Benzyl- -n-Propyl4-Benzyl-1-piperazinyl- 21 266-268
7.82Benzyl- -n-Propyl Dimethylamino- 9 272-274
~ 7.83Benzyl- -n-Propyl N-Piperidinyl- 22 294-297
The formylamines required are prepared by methods known
from the literature by reacting the amines with 88% formic
acid and acetic anhydride.
Step 8: Reactions of alkYlation
General method (alternative A):
4 mMol of 1,2,4-triazolo[1,5-a]purin-9-one unsubstituted
in the 4- and 5-position [(9) with R4 = H; (16) with Rs =
H] are taken up in 26 ml of anhydrous dimethylformamide
and mixed first with a solution of 0.1 g (4.2 mMol) of
sodium in 4 ml of anhydrous ethanol, then with 4.2 mMol of
the desired alkyliodide. The mixture is stirred overnight
~ at ambient temperature and then evaporated to dryness.
The residue is dissolved in 2 N of NaOH, the solution is
~~- washed with toluene and then adjusted to pH 6. The solid~-- 20 precipitated is filtered off and purified by
chromatography. If required, the dialkylation products
(Ia) and (Ib) may be isolated.
The following compounds (9) were prepared using this
method:
CA 022~6736 1998-12-02
Table 8a:
No. R6 R4 R5 R2 Yield (%) Mp (~C)
8.1Benzyl- -Methyl - -H 9 ~300
8.2Cyclopentyl- -Methyl - -H 7 >300
8.3 -H -Methyl - Cyclopentyl- 44 285
8.4 -H -Ethyl - Cyclopentyl- 57 249-253
8.5 -H -n-Propyl - Cyclopentyl- 52 255
8.6 -H -n-Butyl - Cyclopentyl- 45 243
8.7 -H -t-Butyl - Cyclopentyl- 10 190-191
8.8 Benzyl- -Ethyl - -t-Butyl 24 243-244
8.9 Benzyl- -Ethyl - Phenyl- 24 343-345
8.10 -H -n-Butyl - Cyclopentyl- 45 243
8.11 -H - -t-Butyl Cyclopentyl- 4 149-150
Moreover, the following compounds (Ia) and (Ib) could be
obtained by this method:
~ R' O
N_N~N N_NJ~ N
~N lN~N~ N N N,
14 14 R
~...
: (Ia) (Ib)
10 Table 8b:
No R6 R4 R1 orR3 R2 Yield Mp (~C)
(%)
8.12 Benzyl- -Ethyl -Ethyl -H 4 ~300
8.13 -H -Methyl -Methyl Cyclopentyl- 4 206
8.14Cyclopentyl- -n-Propyl -n-Propyl -H 10 146
8.15Cyclopentyl- -n-Propyl -n-Propyl -H 5 151
8.16 Benzyl- -Ethyl -Ethyl 2-Furyl- 16 187-189
CA 022~6736 1998-12-02
General method (alternative B):
20.4 mMol of 1,2,4-triazolo[1,5-a]purin-9-one
unsubstituted in the 4- and 5-position [(9) with R4 = H;
(16) with Rs = H] are stirred together with 40.8 mMol of
potassium carbonate and 40.8 mMol of tert.-butylbromide in
100 ml of anhydrous dimethylformamide at 70~C for 7 days.
The mixture is evaporated to dryness, the residue is
combined with 100 ml of 2N NaOH, the aqueous phase is
washed thoroughly with toluene and neutralised. The
precipitate is filtered off, extracted with CH2Cl2/CH3OH
9:1 and the extract is evaporated down. The isomeric
alkylation products (9) and (16) are isolated by
chromatography on silica gel with CH2C12/CH3OH 95:5 (Table
8c). The following compounds were prepared using this
method.
T a ble 8 c:
N ~ R 6 R 4 R 5 R 2 Yield M p (~ C)
(%)
8.1 7 -H -t-B utyl - C yclo p e ntyl- 1 0 1 9 0-1 91
8.1 8 -H - -t-B utylC yclo p e ntyl- 5 1 4 9-1 5 0
- 20 General method (alternative C):
0.05 Mol of starting compound (9) are dissolved in 500 ml
of absolute dimethylformamide and stirred with 0.055 Mol
of potassium carbonate and 0.055 Mol of benzyl bromide
overnight at ambient temperature. For processing, the
solvent is distilled off in vacuo and the residue is taken
up in methylene chloride/water. The organic phase is
separated off, washed once with water, dried over
magnesium sulphate and evaporated down. The residue is
crystallised with ether. The compounds (Ia/Ib) thus
obtained are listed in Table 8d.
CA 022~6736 1998-12-02
, . . , . . ... , ~
98
Tabl~ 8d:
No. R6 R4 R1 or R2 Yield Mp (~C)
R (%)
8.19 (4-MeO-Ph)-CH2-o-cH2- Ethyl-Benzyl- Cyclopentyl- 85 127-128
8.20 (4-PhcH2o-ph)-cH2- Ethyl-Benzyl- Cyclopentyl- 66 138-140
IX. Functionali~ation of the triazolopurine~ of general
formula (I):
IX.a. Deblocking of triazoloPurines (Ia/Ib) with Protected
hydroxyl functions:
~...
General diagram of synthesis taking as example the
compounds of general (formula Ib):
O O
Y~ R2 ~ ~ R2
DDQ: 2,3-Dichloro-5,6-dicyano-benzoquinone
The above reaction was carried out using a method known
from the literature (Tetrahedron 1986, 42, 3021). The
following compounds were obtained, inter alia:
Tabl~ 9:
No. R6 R4 R1 or R2 Yield Mp (~C)
R3 (%)
9.1 HO-CH2- Ethyl- Benzyl- Cyclopentyl- 97 204-207
IX.b. Deblockinq of triazolopurines (Ia)/(Ib) with
protected amine functions in the side chain:
General diagram of synthesis taking as example the
~ compounds of general formula (Ib):
CA 022~6736 1998-12-02
.. ~ ... .. ..
99
o o
6~/N--N ~ N~ A H2 R6~/ N ~ \~ N NH
R4 R ~ Cat. R4 R3
0.83 mMol of a 2-(4-N-benzyl-1-piperazinyl)-1,2,4-
triazolo[1,5-a]purin-9-one are taken up in 30 ml of
glacial acetic acid and hydrogenated at 60~C and at 5 bar
for 6 hours in the presence of 0.1 g of 10% Pd on
~ charcoal. The catalyst is filtered off, the residue is
evaporated to dryness and stirred with CH30H, the solid
product is filtered off, washed and dried. Using this
method the following was obtained:
Tabl~ 10:
No. R6 R4 R1 or R2 Yield SaltformMp (~C)
R3 (%)
10.1 Benzyl- n-Propyl- H- 1-Piperazinyl- 53 Acetate 260
IX.c. Cleavinq of Protective amine groups from the
triazolopurine core structure:
General diagram of synthesis taking as example the
! compounds of general formula (Ib):
O O
R6~ R2 H2, R6~ R2
N N N Cat. N N NH
R4 \~ R4
3-N-benzylated triazolopurines of general formula (I) are
dissolved in methanol or absolute glacial acetic acid and
hydrogenated under a hydrogen atmosphere (1-5 bar) at
25-80~C with the help of a palladium catalyst (e.g. Pd/C)
over a period of 2.5-40 hours. The catalyst is filtered
CA 022~6736 1998-12-02
. . .
100
off, the filtrate is evaporated down and the residue is
purified over silica gel. The purified product is
crystallised from the eluate, preferably with ether and
then, if possible, crystallised as a salt (preferably
methanesulphonate). In this way the following compounds
were obtained.
Tabl~
No. R6 R4 R2 Salt form Yield Mp (~C)
%
11.1Methoxymethyl- Ethyl- Cyclopentyl- - 27 246-248
11.2Ethoxymethyl- Ethyl- Cyclopentyl- - 45 224-225
11.3Hydroxymethyl- Ethyl- Cyclopentyl- - 37 305-307
11.4Benzoyloxymethyl- Ethyl- Cyclopentyl- - 54 238-239
~ .
CA 022~6736 1998-12-02
101
No. R6 R4 R2 Salt form Yield Mp
% (~C)
11.5 Dimethylaminomethyl- Ethyl- Cyclopentyl- Methanesulfonate 29 224-
225
11.6 N-Morpholinyl-methyl- Ethyl- Cyclopentyl- Methanesulfonate 47 184
11.7 Phenoxymethyl-Ethyl- Cyclopentyl- - 40 229
IX.d. Alkylation of hydroxy-substituted triazolopurine:
General diagram of synthesis taking as example the
compounds of general formula (Ib):
, i .
O O
--<'N1~ \~R2 -- R6~ R2
IN4 ~R3 N N N~
3 mMol of hydroxyalkyl- or hydroxyaryl-substituted
triazolopurines of general formula (Ia), (Ib), (Ic) or
(Id) are dissolved in 30 ml of anhydrous
dimethylformamide, mixed with 3.6 mMol of sodium hydride
(60% suspension in mineral oil), stirred for 1 hour at
~~ ambient temperature, mixed with 3.6 mMol of alkyliodide
and stirred for 1-2 days at 25-60~. To complete the
reaction, 50% sodium hydride and alkylhalide are added.
For processing, the reaction mixture is evaporated down to
leave the residue which is taken up in ethylene chloride
and water, acidified with 2N hydrochloric acid, the
aqueous phase is extracted once more with methylene
chloride, the combined organic phases are washed with
saline solution and dried over magnesium sulphate and then
evaporated down. The residue is purified over silica gel
and the eluate is crystallised from ether.
The following compounds were prepared using this method:
CA 022~6736 1998-12-02
, . . , . , ., . ~ , ,, . ,, , , . _ , .. .. .. .
102
TablP 12:
No. R6 R4 R1 or R2 Yield Mp (~C)
R3 (%)
12.1 Methoxymethyl- Ethyl-Benzyl- Cyclopentyl- 37 116-118
12.2 Ethoxymethyl- Ethyl-Benzyl- Cyclopentyl- 36 120-123
IX.e. Acylation of hydroxy-substituted triazolo~urines:
General diagram of synthesis taking as example the
compounds of general formula (Ib):
O O
HO N ~ N ~ N pyridine N _ ~U~ N
N N N carboxylic acid N N N
R4 ~R3 chloride R4 ~R3
The hydroxyl compound is taken up in pyridine and, whilst
it is being cooled with ice, stoichiometric quantities of
acid chloride are added. When conversion is complete the
mixture is added to ice water and acidified with 2N
hydrochloric acid. The aqueous phase is extracted several
times with methylene chloride. The combined organic
phases are washed with saline solution, dried over
magnesium sulphate and evaporated down. The residue is
purified over silica gel and the eluate is crystallised
from ether.
The following compounds were prepared by this method:
Table 13:
No. R6 R4 R1 or R2 Yield
R3 (%)
12.1 ~ Ethyl- Benzyl- Cyclopentyl- 73
O - CH~
12.2 ~ Ethyl- Benzyl- Cyclopentyl- 93
.N O-CH~-
CA 022~6736 1998-12-02
.... . .... . ..
103
IX.f. Halogenation of hydroxy-substituted triazolopurines:
General diagram of synthesis taking as example the
compounds of general formula (Ib):
O O
N~N~ NEt3 ~Nl~N'R~R2
The hydroxylated triazolopurines are converted into the
corresponding halides in the usual way with halogenating
reagents such as SOCl2, SOBr2, POCl3 in inert solvents in
the presence of bases (e.g. NEt3).
The following compounds were prepared analogously:
Tabl~ 14:
No. R6 R4 R1 or R2 Yield Mp (~C)
R3 (%)
14.1 Br-CH2- Ethyl-Benzyl- Cyclopentyl- 66 188-189
IX.q. Nucleo~hilic substitution in haloqen-substituted
~ . .
trlazolopurlnes:
General diagram of synthesis taking as example the
compounds of general formula (Ib):
o Alcoholor ~
y ~ R2 ~ R6~ RZ
X:C~orine,bro~e,io~ne
CA 02256736 1998-12-02
104
Using generally known standard procedures, halogen-
substituted triazolopurine derivatives may be converted
into the corresponding amines and alkyloxy/aryloxy
compounds by reacting with nucleophiles (e.g. alcohols,
amines) in the presence of a base. For preparation of the
amines, please see the methods of synthesis described for
Step 8 hereinbefore. The alkyloxy/aryloxy compound may be
synthesised in accordance with the general method under
point IX.d. The following compounds were prepared
10 analogously:
Tabl~ 15:
No. R6 R4 R1 or R2 Yield Mp (~C)
R3 (%)
15.1 (CH3)2N-CH2- Ethyl- Benzyl-Cyclopentyl- 100
15.2 N-Morpholinomethyl-Ethyl- Benzyl- Cyclopentyl- 96
15.3 Phenyloxymethyl- Ethyl-Benzyl- Cyclopentyl- 86 155
IX.h. Oxidation of hydroxy-substituted triazolopurines:
General diagram of synthesis taking as example the
compounds of general formula (Ib):
O O
- HO N _ N ~ N Oxidation N _ J~ N
</ 1 J~ \~ R2 R6~ R2
R4 R R R
Using generally known standard procedures (e.g.
Tetrahedron Letters 1979, 5, 399-402) hydroxy-substituted
triazolopurine derivatives may be converted into
triazolopurines with functionalities of a higher oxidation
state (e.g. aldehydes, carboxylic acids). The following
compounds were prepared analogously:
CA 022~6736 1998-12-02
. . ~ _ ~ .,
105
Tabl~ 16:
No. R6 R4 R1 or R2 Yield Mp (~C)
R3 (%)
16.1 -CHO Ethyl- Benzyl- Cyclopentyl- 48 153-154
16.2 -COOH Ethyl- Benzyl- Cyclopentyl- 38 157-158
IX.i. Derivatisation of triazolopurine carboxylic acids:
General diagram of synthesis taking as example the
compounds of general formula (Ib):
O O
o N_ J~ N N_ J~ N
~<' I~ ~ '~ R2 R6~/ ~ R2
HO R4 R3 N N N
Using methods known from the literature, triazolopurine
carboxylic acids may be converted into a plurality of
carboxylic acid derivatives (e.g. halides, anhydrides,
amides, ester, etc.). The following general method may be
used to prepare the corresponding triazolopurine-methyl
carboxylates:
0.003 mol of triazolopurine carboxylic acid are suspended
in a 1 molar solution of SOCl2 (1 . 5 eq) in MeOH. The
resulting clear solution is stirred at ambient
temperature. After the reaction is complete the solvent
is distilled off in vacuo and the residue remaining is
purified by chromatography on silica gel. Using this
process the following were prepared:
Tabl~ 17:
No. R6 R4 R1 or R2 Yield Mp (~C)R3 (%)
17.1 -COOCH3 Ethyl- Benzyl- Cyclopentyl- 71 155-157
CA 022~6736 1998-12-02
.. ... . . .
106
IX.k. Reductive amination of triazolopurine aldehydes:
General diagram of synthesis taking as example the
compounds of general formula (Ib):
O , O
~ R2 ) R6~ R2
O N N N~ 2)Red. N N N~
Using generally known standard procedures, triazolopurine
aldehydes may be converted into the corresponding amines
by reaction with primary or secondary amines and
subsequent reduction or hydrogenation of the Schiff's base
formed as an intermediate. The following compounds were
prepared accordingly:
Tabl~ 18:
No. R6 R4 R1 or R2 Yield Mp (~C)
R3 (%)
18.1 ~NH CH2- Ethyl- Benzyl- Cyclopentyl-
CA 022~6736 1998-12-02
2 o ~ u~
s E G N 3 N G
~ _ sO 2 s 2
r N N a~ N a7 N a~ N O~
.C ~ .C ~ ,C ~ ,C
o m m, m, m, m,
a)
~ C .C O O O O O
O ~ C ~') ~ ~ ~ ~ O
5 Q A A A A A
1~
CJ~ N
O ~
~ ~'Z, ~'Z'~
~ O~z 'k ~C
C/ In
r-Z~Z U~c~ ~ I l ' '
~D~
~0
~~; o ~ 2
-
I I I I I
~ ,
_C
U~
~"
E Q~ c~ I I I I I
~n
0 ~
a~ tD ~ O ~ N C') ~t U7
U~
CA 02256736 1998-12-02
. .
~ N ~ N e ~ I ~
$ 2 c a~ c 0 c a~ p N ~ ~
t~S ~' O ~;~ O Q c~Q ~ Q 3 Q ~ ~ Q ~ Q ~
Z ~ C t~I a) C O ~' r c O
~, c ~ ,_ c~ N ~C ~ ~ N c~ ~
E N _ N _ N O N O ~ O N O n Q N ~ N , N
~ m ~- m ~ m ~~ m, ~~ m 1~a m ~~ ~~ ~ m ~ m OQ m ~
C ~ l N N C~l ~ ~~
o o C~
o
~ ' ' ' ' ' ' ' ' ' '
~ ~ ~' o ';? ~3 o ~ G
~ ~ ~ ~ ~ z~ ~Z) [z~
- o~ I I I I I I I I I I
Q
E z ~ ~ ~ C~
~L~
CA 02256736 1998-12-02
E ~ 3 ' ~ t ~ ~ ~
Z ~ p ~ y ~~ ~ a) 2. w ~ ~
3 q N C~ ,~
E ~ ~ a ~ 'C ~ ON ~ m ~ m, ,~J
~) C Q Q O 0
, ~ C ~ 1~ o ~ IS) o1~ 0 N ~1 C~ C'~l
5 r~ ~J) A ~C) ~ ~ N
~ ~ N
~ ~
o
. . I I . . . . .
ll ll
~ s s ~ ~ C 2 ' 2 2
~, . . . . .
o ~"
~y I I I I I I I I I I
$: o ~D ~ co ~ O ~ ~ ~ ~ U~
CA 02256736 1998-12-02
..... . ... . .... _ . ... .. . .
~ ~ o ~c ~ ~
E~ q ~ q ~. $ ~ ~ 5 ~ o
ZE a) ~ 'n ~ ~ a) ~ a~ ~ a~ a) o ~ ~ Q a~
~ t . t c a a) c a c ~ c ~ ~ a~ In- ', a) ~'
~ .C ,,~ r) N~ ~ ~ g ~
- 5 ~ ~ A C~ ~ A N
C~ I I I I I I o S .
o
C! ' ~ I
lY
I 2 ~ ~ ~ ~ ~' 2 2 ~
c 5 ' c ' c c C
C! ~ ~ ~ O ~ ~ O ~ I
'- C~ I I I I I I I I I I
~ O C~ O ~ C~l C~
CA 02256736 1998-12-02
.... . . . .. . . ..
E ~ C ~ c ~ c ~ c ~ ~ 2 ~ ' a,
~ ~ 2 ' ~ ~ t a~ c cn ~
g ~ - ~D U') N U~ Q ~C Q 1~ ~ C Q ~
E ~ o ~ ~ m o ~ ~ ~ ~ c~ ~ c~ ~ ' w c ~ o ~
~ .,.~ ._ ._ ._ , , ,
g co c~ o o E ~ c~ 8 ~ 8 E
-- 0 ~ ~ O ~ C~ ~ C~ o ~ o ~ o
~ C, C~
~1
Q Q Q Q Q Q Q Q Q Q
~ ,0 ,0 , , ,0 0 0 0 0 0
' c c c ~ c ~ ~ ~ c c
O ~ s I ~lC~ ~
~ C~
I I I I I I I I I I
o ~ ~ o~ a~ o ~ ~ c~ ~ u~
~ z ~ ~ ~ ~ ~ ~ ~ ~ ~ ~
CA 02256736 1998-12-02
E ~ c ~ ~C ~ C ~a ~ C a
t~ C ,0 2'Q ~ ~ ,o ~ U) ,0 U) o C~) lo ~~ Io ~ lo
Z q ~~ ~ a) O ~ a) ~ o~
~C ~ ~ a~ D C OC C C C C C - C C C
o g o g ~ c O Qg ~ g ~ N Q g Q
C ~ ~, ~ E ~ E ~ E C~l o ~ '~ '~ ~ ~
~A a) A OA a) 1~ A ~ A O
o ~o ~o O I I I I
~' ~
"" , ~
-- ~>.O o
0. Qo ~ ~ ~) s
~ I s
o ~" Q
~~ I I I ~ s~
~ o ~D ~ co a~ o ~ c~
CA 02256736 1998-12-02
~ .
D
Example-R1 or Melting
No. -R3 -R2 -R4 -R5 R6 pointin Chemical Name
~ ~C
56 -n-Propyl -H -n-Propyl - O 146 6-Cyclopentyl-1,4-di-n-propyl-1,2,4-
O l,i ~1~ [1,5-a]purin-9-one
57 -n-Propyl -H -n-Propyl - ~ 151 6-Cyclopentyl-3,4-di-n-propyl-1,2,4-
[1 ,5-a]purin-9-one
58 -H ~ -n-Butyl - -H 243 2-Cyclopentyl-4-n-butyl-1,2,4-~i -'c [1,5-
a]purin-9-one
59 -H ~3 -Ethyl - \~ 287 6-Benzyl-4-ethyl-2-(2-pyridyl)-1,2,4-
l, - I [1 ,5-a]purin-9-one
-H ~N -Ethyl \_ ~3 346 6-Benzyl-4-ethyl-2-(4-pyridyl)-1,2,4-
l,i '- [1,5-a]purin-9-one
61 -H G ~ -H 149-150 2-Cyclope"tyl 5-tert.-butyl-1,2,4-triazolo-
[1 ,5-a]purin-9-one
62 -H ~ ~ - -H 190-191 2-Cyclopentyl4-tert.-butyl-1 ,2,4-triazolo-
[1 ,5-a]purin-9-one
63 -H ~ -Ethyl - \~ 331-332 6-Benzyl-4-ethyl-2-(3-pyridyl)-1,2,4-
ll Nc [1,5-a]purin-9-one
64 -H ~ -n-Propyl - ~ 385-387 2,6-Di-(2-furyl)-4-n-propyl-1,2,4-triazolo-
[1 ,5-a]purin-9-one
-H -N(CH3)2 -n-Propyl - \~ 272-274 6-Benzyl-2-dimethylamino-4-n-propyl-
1,2,4-lli ~'~ [1,5-a]purin-9-one
114
D
o
Example -R1 or Melting
No. -R3 R2 -R4 -R5 R6 pointin Chemical Name
~ ~C
66 -H ~ -Ethyl - \~) 260-262 6-Benzyl-2-cyclohexyl ~ cthyl-1,2,4-
O t~ [1,5-a]purin-9-one
67 -H - N~ -n-Propyl - \~ 294-297 6-Benzyl-2-(N-piperidinyl)4-n-propyl-1,2,4-
t~ 1,5-a]purin-9-one
68 -H ~ -Ethyl - \~3 243-244 6-Benzyl-2-tert.-butyl4-ethyl-1,2,4-triazolo-
[1 ,5-alpurin-9-one
69 -H ~ -Ethyl - \~3 343-345 6-Benzyl4-ethyl-2-phenyl-1,2,4-tri~olo-
11 ,5-alpurin-9-one
-H ~ -Ethyl - \~ 261-263 2-Cyclopent~rl q cthyl4-(3-pyridylmethyl)-
1,2,4-l~ [1,5-a]purin-9-one
71 -H -Ethyl -Ethyl - \~) 225-227 2,4-Diethyl-6-(3-pyridylmethyl)-1,2,4-
N
lli :'- [1,5-alpurin-9-one
72 -Ethyl ~ -Ethyl - \~ 187-189 6-Benzyl-1 ,4-diethyl-2-(2-furyl)-1,2,4-
l,' :'~ [1,5-alpurin-9-one
73 -H -H -Ethyl - \~ 328-330 4-Ethyl-6-(3-pyridylmethyl)-1,2,4-triazolo-
[1 ,5-alpurin-9-one
74 -H -H -Ethyl - \ 0 328-330 6-Cyclohexylmethyl4-ethyl-1,2,4-triazolo-
[1 ,5-a]purin-9~ne
-H -Ethyl -Ethyl - \ 0 230-232 6-Cyclohexy'~"ell,yl-2,4~iethyl-1,2,4-
[1 ,5-a]purin-9-one
~ c g 5 f~ O ~ e ~ g ~ 5
E C ~ a~ $ ~ o ~ ~~ a) E ~, o ~, ~ 3~ ~
Q _ C C o Q~ N ~ ., C ~ ~ ~ 3 _ ~ ~
N llJ Q ~ U~ ~ 0 ~ C ~ ~ C N o Q
C) ~ 1- ~ N --- ~D 2 C~ l E ~ Q 1~ ~ E
c ~ ~) ~ o a~
5 ~ ~ c~ o~ a) o c~
I
$ ~ ~
~o ~o o
J
U~
a~: S S S S o o s s s
.
I s ~ s ~ O
I I I I I I I I (~ 3
~ O CD ~ a~ a~ o ~ c~
Il~
CA 02256736 1998-12-02
,, .. , , , . . , . . .,, . _
c c , ~,a E c, ~P
E ~ c c ~ C C,~ C ~ ~ C s u~
E a) ~ o ~ c u~ o
~ q c ~ c q S 9~ q ~, c S
Z ~ ~ C ~ ~ ac~ 2' ~ q ~ ~ ~ ~ ~ ~Q ~
~ c ' ~ ~ ~ ~' a) ~ ~Q 8 ~ 8 ~ c ~Q 8 c ~-
~ N~ N~ o ~ ~~ S, N ~ ~ S
~ a~ Q m ~ , m g~ O~
~ n~ ~ n ~ ~ ~ c a~ n
~' ~ In c~ o o
._ O , a) ~ ~ O
o ~ [~ ~ IL 1
O O
J
Lt')
a~ ' ' ' ~ ~ S S S
o ~
a
o U~ cc~ 1~ oo 0 o ~ ~
~ z ~ a~ 0 0 0
Il~
CA 02256736 1998-12-02
.. ~, . . . . . .. . . .
c
~ ~ s ~ E ~ I ~ o ~
U~ C o ~ o ~ _ o -- C
~ ~ O o N Q c Q S O S ~
s O s ~ t ~ , ~ s, lo 9' ~ E ~
a~ -- I S ~ O ~ ~ ~ aS~ NO ~ NO ~ ~~ a~ U~
q ~ q '_ ~ S q
S ~ ~ ~ ~ C N C~l C ~ ~D N
c ~ c 7 c o ~ ~ L) ~ ~ 7 ~ ~ ~ N
~) ~) C O~D C O ~ ~ N ~ C~l C ~ ~C ~) C t~ ~1
ao ~
C ~--
._ ~C ~ l ~ O
~D ~-- ~ ~ 0~ ~.) O ~ 00 c
~ '~ ~ N C~
~Y g ~ O 0 0~ 0~ 0 0
o o ~ ~
r~
~ s s s s s s s s
0 [~
Q ~ un ~ ~ oo cn ~
X a) a~ 0 a) ~ a) ~
llJ
CA 02256736 1998~12~02
.. . .. .
u~
c ~ e
E ~ S
Z ~ c a~ ~ C ~ ~ ~ ~ ~ ~ ~ a y C a~ O
s ~1~ E ~ ~ E c ~ s ~ ~ s ~ ' N ~ c ~ ~ ~ C
o o u~
~~ ~~~
u~
s s s s s s ~ s
'~ l l l l l l l l
C! I ~ I I I ~ I I
a)
~ ~ O O O O O O O o
CA 02256736 1998-12-02
. . .. . . .
c ~ E ~~ ~ W
_ Q ~ c ~ a) o c ~ Q ~
:~ ~ c , O ~ C ~ n a~ q N
~ ~ E C~l Q E; ~~ Q C ~- ~ --O ~~
E ~ ~O ~ a~ 0~ ~ ~L ~ E ~ o .;. o N
g , ~,1 ~ ~C-- ~ ON tl;~ ~ N NO
~, ~ N ~ Q N _. N ~- N C N ~- N C N E
u~ ~, m ~ m ~ m C m ~ m C m C m E a)
C~ ~ E ~ Q' ~ O
c ~ ~ U~
_ O O r-- U) U~ ~n
~'~J ~~ O G I ~ z ~J
$ ' r
u)
s s s s s s s s
~ ~
53 ~3 5 3 5 3 5 3 5~ 3 ~
C O a~ O
Z
111
CA 02256736 1998-12-02
120
The structures of the examples of compounds (Ia) to (Id)
synthesised hereinbefore were confirmed by NMR-
spectroscopy.
NMR-spectroscopic data of selected compounds:
Example (3)
H-NMR (DMSO-d6): ~ = 14.10 (lH, s, broad, NH); 8.24
(lH, s, H2); 7.35-7.14 (5H, m, Aryl-H); 4.12 (2H, t, J =
7.5 Hz; N-CH2CH2CH3)i 4.09 (2H, s, -CH2-Phenyl); 1-73 (2H~
m, N-CH2CH2CH3); 0.89 (3H, t, J = 7.5 Hz, N-CH2CH2CH3).
- Example (4)
lH-NMR (DMSO-d6): ~ = 13.28 (lH, s, broad, NH); 8.24
(lH, s, H2); 7.35-7.14 (5H, m, Aryl-H); 4.15 (2H, t, J =
7.5 Hz, N-CH2CH2CH2CH3); 1.68 (2H, m, N-CH2CH2CH2CH3);
1.32 (2H, m, N-CH2CH2CH2CH3); 0.90 (3H, t, J = 7.5 Hz, N-
CH2cH2cH2cH3 ) -
Example (5)
H-NMR (DMSO-d6): ~ = 13.22 (lH, s, broad, NH); 8.25
(lH, s, H2); 7.35-7.14 (5H, m, Aryl-H);5.33 (lH, m, CH-
Isopropyl); 4.09 (2H, s, -CH2-Phenyl);
1.55 (6H, d, J = 7.0 Hz, (CH3)2-Isopropyl).
Example (6)
H-NMR (DMSO-d6): ~ = 13.66 (lH, s, broad, NH); 7.34-
7.14 (5H, m, Aryl-H);
4.11 (2H, t, J = 7.5 Hz, N-CH2CH2CH3); 4.07 (2H, s, -CH2-
Phenyl);
2.79 (2H, qu, J = 7.5 Hz, -CH2CH3); 1.71 (2H, m, N-
CH2cH2cH3)i
1.29 (3H, t, J = 7.5 Hz, -CH2-CH3); 0.90 (3H, t, J = 7.5
Hz, N-CH2CH2CH3).
Example (7)
CA 022~6736 1998-12-02
121
H-NMR (DMSO-d6): ~ = 13.70 (lH, s, broad, NH); 7.43-
7.14 (5H, m, Aryl-H);
4.09 (2H, t, J = 7.5 Hz, N-CH2CH2CH3); 4.07 (2H, s, -CH2-
Phenyl);
2.75 (2H, t, J = 7.5 Hz, -CH2CH2CH3); 1.74 (4H, m, N-
CH2CH2CH3i -CH2CH2CH3)i 0.92 (6H, m, N-CH2CH2CH3; -
CH2cH2cH3)-
Example (8)
1H-NMR (DMSO-d6): ~ = 7.34-7.12 (5H, m, Aryl-H); 4.07
(2H, s, CH2-Phenyl);
- 3.55 (3H, s, N-CH3); 3.23 (lH, m, CH-Cyclopentyl); 2.14-
1.52 (8H, m, CH2-Cyclopentyl).
Example (9)
1H-NMR (DMSO-d6): ~ = 13.68 (lH, s, broad, NH); 7.40-
7.14 (5H, m, Aryl-H);
4.19 (2H, qu, J = 7.5 Hz, N-CH2CH3); 4.09 (2H, s, CH2-
Aryl); 3.24 (lH, m, CH-Cyclopentyl); 2.26-1.50 (8H, m,
CH2-Cyclopentyl); 1.26 (3H, t, J = 7.5 Hz, N-CH2CH3).
Example (10)
H-NMR (DMSO-d6): ~ = 13.65 (lH, s, broad, NH); 7.38-
7.14 (5H, m, Aryl-H);
4.11 (2H, t, J = 7.5 Hz, N-CH2CH2CH3); 4.08 (2H, s,
Phenyl-CH2-); 3.23 (lH, m, CH-Cyclopentyl); 2.14-1.49 (10
H, m, CH2-Cyclopentyl; N-CH2CH2CH3); 0.89 (3H, t, J = 7.5
Hz, N-CH2CH2CH3).
Example (11)
H-NMR (DMSO-d6): ~ = 13.85 (lH, s, broad, NH); 7.62-
7.33 (5H, m, Aryl-H);
4.34 (2H, t, J = 7.5 Hz, N-CH2); 4.28 (2H, s, CH2-Phenyl);
3.43 (lH, m, CH-Cyclopentyl); 2.33-1.38 (12H, m, CH2-
Cyclopentyl; N-CH2-_2CH2-CH3);
1.10 (3H, t, J = 7.5 Hz, N-(CH2)3-CH3).
Example (12)
CA 022~6736 1998-12-02
.. , . , . .. . .. . ., . . ~
122
H-NMR (DMSO-d6): ~ = 12.58 (lH, s, broad, NH); 7.52-
7.14 (10 H, m, Aryl-H);
5.52 (2H, s, N-CH2-Aryl); 4.18 (2H, s, -CH2-Aryl); 3.31
(lH, m, CH-Cyclopentyl);
2.21-1.50 ~8H, m, CH2-Cyclopentyl).
Example (13)
lH-NMR (DMSO-d6): ~ = 12.38 (lH, s, broad, NH); 7.34-
7.14 (5H, m, Aryl-H);
4.08 (2H, t, J = 7.5 Hz, N-CH2CH2CH3); 4.6 (2H, s, -CH2-
Phenyl); 3.69; 3.59 (8H, m, morpholin-H); 1.70 (2H, m, N-
CH2CH2CH3); 0.88 (3H, t, J = 7.5 Hz, N-CH2CH2CH3).
Example (14)
lH-NMR (DMSO-d6): ~ = 12.26 (lH, s, broad, NH); 7.38-
7.14 (10 H, m, Aryl-H);
4.08 (2H, t, J = 7.5 Hz, N-CH2CH2CH3); 4.06 (2H, s, CH2-
Phenyl); 3.57; 2.46 (8H, m, -CH2-Piperaz.); 3.52 (2H, s,
N-CH2-Phenyl); 1.70 (2H, m, N-CH2CH2CH3);
0.88 (3H, t, J = 7.5 Hz, N-CH2CH2CH3).
Example (15)
H-NMR (DMSO-d6): ~ = 7.40-7.14 (5H, m, Aryl-H); 4.76
(3H, s, broad, NH, NH2);
4.08 (2H, t, J = 7.5 Hz; N-CH2CH2-CH3); 4.06 (2H, s, -CH2-
Phenyl); 3.48; 2.79 (8H, 2m, CH2-Piper.); 1.90 (3H, s,
CH3COOH); 1.70 (2H, m, N-CH2CH2-CH3) ; 0.88 (3H, t, J = 7.5
Hz, N-CH2CH2-CH3).
Example (16)
lH-NMR (DMSO-d6): ~ = 13.82 (lH, s, broad, NH); 7.34-
7.14 (5H, m, Aryl-H);
4.19 (2H, qu, J = 7.0 Hz, N-CH2CH3); 4.08 (2H, s, -CH2-
Phenyl); 3.93; 3.43 (4H, 2m, 2 CH2-0); 3.09 (lH, m, -CH-
THP); 2.00-1.68 (4H, m, 2-CH2-THP); 1.25 (3H, t, J = 7.0
Hz, N-CH2CH3).
Example (17)
CA 022~6736 1998-12-02
. _ , . .. .... ..
123
lH-NMR (DMSO-d6): ~ = 13.84 (lH, s, broad, NH); 7.34-
7.14 (5H, m, Aryl-H);
4.18 (2H, qu, J = 7.0 Hz, N-CH2CH3); 4.08 (2H, s, -CH2-
Phenyl); 4.08-3.74 (4H, m, 2 CH2-0-3THF); 3.62 (lH, m, CH-
3 THF); 2.29 (2H, m, CH2- 3 THF); 1.25 (3H, t, J = 7.0 Hz,
N CH2CH3).
Example (18)
lH-NMR (DMSO-d6): ~ = 13.64; 12.76 (2H, 2s, broad,
2NH); 7.35-7.14 (5H, m, Aryl-H); 4.02 (2H, s, -CH2-
Phenyl); 3.23 (lH, m, CH-Cyclopentyl); 2.14-1.50 (8H, m,
CH2-Cyclopentyl).
Example (19)
lH-NMR (DMSO-d6): ~ = 13.42; 13.20 (2H, 2s, broad, 2
NH); 8.24 (lH, s, H2);
3.20 (lH, m, CH-Cyclopentyl); 2.17-1.50 (lH, m, CH2-
Cyclopentyl).
Example (20)
H-NMR (DMSO-d6): ~ = 13.86 (lH, s, broad, NH); 8.22
(lH, s, H2); 3.58 (3H, s, N-CH3); 3.23 (lH, m, CH-
Cyclopentyl); 2.14-1.52 (8H, m, CH2-cylcopentyl).
Example (21)
H-NMR (DMSO-d6): ~ = 14.04 (lH, s, broad, NH); 8.24
(lH, s, H2); 4.13 (2H, t, J = 7.5 Hz, N-CH2CH2CH3); 3.21
(lH, m, CH-Cyclopentyl); 2.14-1.52 (10 H, m, CH2-
Cyclopentyl; N-CH2CH2CH3); 0.90 (3H, t, J = 7.5 Hz, N-
CH2cH2cH3)-
Example (22)
H-NMR (DMSO-d6): ~ = 13.68 (lH, s, broad, NH); 4.12
(2H, t, J = 7.5 Hz, N-CH2-CH2CH3)j3.20 (lH, m, CH-
Cyclopropyl); 2.45 ~3H, s, -CH3); 2.12-1.52 (10 H, m, CH2-
Cyclopentyl); 0.89 (3H, t, J = 7.5 Hz, N-CH2-CH2CH3).
Example (23)
CA 022~6736 1998-12-02
124
H-NMR (DMSO-d6): ~ = 13.64 (lH, s, broad, NH); 4.12
(2H, t, J = 7.5 Hz, N-CH2-CH2CH3)j3.20 (lH, m, CH-
Cyclopentyl); 2.80 (2H, qu, J = 7.5 Hz, -CH2-CH3);
2.13-1.52 (10 H, m, CH2-Cyclopentyl; N-CH2-CH2CH3); 1.29
(3H, t, J = 7.5 Hz, -CH2-CH3)i 0.90 (3H, t, J = 7.5 Hz, -
N-cH2-cH2cH3)-
Example (24)
lH-NMR (DMSO-d6): ~ = 13.88 (lH, s, broad, NH); 7.35-
7.14 (5H, m, Aryl-H);
4.13 (2H, s, -CH2-Phenyl); 4.11 (2H, t, J = 7.5 Hz, N-
- CH2CH2CH3); 3.19 (lH, m, CH-Cyclopentyl); 2.09-1.52 (l=H,
m, cH2-cyclopentyl; N-CH2CH2CH3);
0.89 (3H, t, J = 7.5 Hz, N-CH2CH2CH3).
Example (25)
lH-NMR (DMSO-d6): ~ = 13.60 (lH, s, broad, NH); 7.43-
7.14 (5H, m, Aryl-H);
4.36 (2H, t, J = 7.5 Hz, N-CH2(CH2)3-CH3); 4.19 (2H, s, -
CH2-Phenyl); 3.40 (lH, m, CH-Cyclopentyl); 2.31-1.24 (14H,
m, CH2-Cyclopentyl; N-CH2(CH2)3-CH3);
0.89 (3H, m, N-(CH2)4-CH3).
Example (26)
lH-NMR (DMSO-d6): ~ = 13.85 (lH, s, broad, NH); 8.16
(lH, s, H6); 7.35-7.14 (5H, m, Aryl-H); 4.15 (2H, s, -CH2-
Phenyl); 3.59 (3H, s, N-CH3).
Example (27)
lH-NMR (DMSO-d6): ~ = 14.02 (lH, s, broad, NH); 8.19
(lH, s, H6); 7.35-7.14 (5H, m, Aryl-H); 4.18 (2H, s, -CH2-
Phenyl); 4.15 (2H, t, J = 7.5 Hz, N-CH2CH2CH3); 1.75 (2H,
m, N-CH2CH2CH3); 0.93 (3H, t, J = 7.5 Hz, N-CH2CH2CH3).
Example (28)
lH-NMR (DMS0-d6): ~ = 13.50, 12.92 (2H, 2s, broad,
2NH); 8.10 (lH, s, H6);
CA 022~6736 1998-12-02
125
3.24 (lH, m, CH-Cyclopentyl); 2.17-1.52 (8H, m, CH2-
Cyclopentyl).
Example (29)
lH-NMR (DMSO-d6): ~ = 13.70 (lH, s, broad, NH); 8.18
(lH, s, H6); 3.59 (3H, s, C-CH3); 3.25 (lH, m, CH-
Cyclopentyl); 2.15-1.52 (8H, m, CH2-Cyclopentyl).
Example (30)
lH-NMR (DMSO-d6): ~ = 13.70 (lH, s, broad, NH); 8.20
(lH, s, H6); 4.25 (2H, qu, J = 7.0 Hz, N-CH2-CH3); 3.27
- (lH, m, CH-Cyclopentyl); 2.21-1.52 (8H, m, CH2-
Cyclopentyl); 1.29 (3H, t, J = 7.0 Hz, N-CH2-CH3).
Example (31)
H-NMR (DMSO-d6): ~ = 13.74 (lH, s, broad, NH); 8.18
(lH, s, H6); 4.14 (2H, t, J = 7.5 Hz, N-CH2-CH2CH3); 3.25
(lH, m, CH-Cyclopentyl); 2.15-1.54 (10 H, m, CH2-
Cyclopentyl; N-CH2-CH2CH3); 0.90 (3H, t, J = 7.5 Hz, N-
CH2-CH2CH3).
Example (33)
H-NMR (DMSO-d6): ~ = 13.62 (lH, s, broad, NH); 4.11
(2H, t, J = 7.5 Hz, N-CH2CH2CH3); 3.23 (lH, m, CH-
Cyclopentyl); 2.37 (3H, s, -CH3); 2.14-1.50 (10 H, m, CH2-
Cyclopentyl, N-CH2CH2CH3); 0.90 (3H, t, J = 7.5 Hz, N-
CH2cH2cH3)-
Example (34)
lH-NMR (DMSO-d6): ~ = 8.22 (lH, s, Hz); 4.13 (2H, t, J
= 7.5 Hz, N-CH2CH2CH3)i
2.75 (2H, qu, J = 7.5 Hz, -CH2CH3); 1.74 (2H, m, N-
CH2CH2CH3); 1.29 (3H, t, J = 7.5 Hz, CH2-CH3); 0.92 (3H,
t, J = 7.5 Hz, N-CH2CH2CH3).
Example (35)
CA 022~6736 1998-12-02
126
H-NMR (DMSO-d6): ~ = 13.62 (lH, s, broad, NH); 4.12
(2H, t, J = 7.5 Hz, N-CH2CH2CH3); 3.25 (lH, m, CH-
Cyclopentyl); 2.74 (2H, qu, J = 7.5 Hz, -CH2CH3);
2.17-1.51 (10 H, m, CH2-Cyclopentyli N-CH2CH2CH3); 1.29
(3H, t, J = 7.5 Hz, -CH2CH3); 0.90 (3H, t, J = 7.5 Hz, N-
CH2CH2CH3).
Example (36)
lH-NMR (DMSO-d6): ~ = 13.58 (lH, s, broad, NH); 4.12
(2H, t, J = 7.5 Hz, N-CH2CH2CH3); 3.24 (lH, m, CH-
Cyclopentyl)j2.69 (2H, t, J = 7.5 Hz, -CH2CH2CH3);
2.15-1.50 (12H, m, CH2-Cyclopentyl; (CH2CH2CH3)2); 0.95;
~- o.90 (6H, 2t, J = 7.5 Hz, (CH2CH2CH3)2)-
Example (37)
H-NMR (DMSO-d6): ~ = 13.62 (lH, s, broad, NH); 4.13
(2H, t, J = 7.5 Hz, N-CH2CH2CH3); 3.24 (lH, m, CH-
Cyclopentyl); 3.05 (lH, m, CH-Isoprop.);
2.14-1.50 (10 H, m, CH2-Cyclopentyl; N-CH2CH2CH3); 1.32
(6H, d, J = 7.5 Hz, CH3-Isoprop.); 0.90 (3H, t, J = 7.5
Hz, CH2CH2CH3).
Example (38)
H-NMR (DMSO-d6): ~ = 13.62 (lH, s, broad, NH); 4.12
(2H, t, J = 7.5 Hz, N-CH2CH2CH3); 3.24 (lH, m,
Cyclopentyl-H); 2.70 (2H, t, J = 7.5 Hz, CH2-CH2CH2CH3);
2.15-1.26 (14H, m, CH2-Cyclopentyl; N-CH2CH2-CH3, CH2-
CH2CH2CH3); 0.90 (6H, m, N-CH2CH2CH3); (-CH2)3-CH3).
Example (39)
lH-NMR (CDC13): ~ = 4.36 (2H, t, J = 7.5 Hz, N-CH2CH2CH3);
3.40 (lH, m, CH -Cyclopentyl); 2.32-1.63 (10 H, m, CH2-
Cyclopentyl; N-CH2CH2CH3); 1.46 (9H, s, C(CH3)3; 1.02 (3H,
t, J = 7.5 Hz, N-CH2CH2CH3).
Example (40)
H-NMR (DMSO-d6): ~ = 14.28 (lH, s, broad, NH); 8.26-
7.45 (5H, m, Aryl-H);
CA 022~6736 1998-12-02
127
4.14 (2H, t, J = 7.5 Hz, N-CH2CH2CH3); 2.71 (2H, t, J =
7.5 Hz, -CH2CH2CH3);
1.76 (4H, m, (-CH2CH2CH3)2); 0.98; 0.93 (6H, 2t, (-
CH2CH2CH3)2)-
Example (41)
H-NMR (DMSO-d6): ~ = 7.44-7.05 (4H, m, Aryl-H); 4.13
(2H, s, CH2-Phenyl);
4.11 (2H, t, J = 7.5 Hz, N-CH2CH2-CH3); 2.68 (2H, t, J =
7.5 Hz, -CH2CH2-CH3)i
1.74 (4H, m, (-CH2CH2-CH3)2); 0.95; 0.89 (6H, 2t, J = 7.5
Hz, (cH2cH2-cH3)2)-
Example (42)
lH-NMR (DMSO-d6): ~ = 13.64 (lH, s, broad, NH); 4.12
(2H, t, J = 7.5 Hz; N-CH2CH2CH3); 2.76 (4H, m, (CH2CH3)2);
1.73 (2H, m, N-CH2CH2CH3));
2.29 (6H, m, (CH2-CH3)2); 0.90 (3H, t, J = 7.5 Hz, N-
CH2cH2cH3)-
Example (43)
H-NMR (DMS0-d6): ~ = 14.19 (lH, s, broad, NH); 8.31
(lH, s, H2);
8.16-7.38 (5H, m, Aryl-H); 4.21 (2H, t, J = 7.5 Hz, N-
CH2CH2CH3)i
1.83 (2H, m, N-CH2CH2CH3); 0.98 (3H, t, J = 7.5 Hz, N-
CH2cH2cH3)-
Example (44)
lH-NMR (DMS0-d6): ~ = 13.78 (lH, s, broad, NH); 8.19-
7.43 (5H, m, Aryl-H);
4.21 (2H, t, J = 7.5 Hz, N-CH2CH2CH3); 2.83 (2H, qu, J =
7.5 Hz, -CH2CH3)i
1.81 (2H, m, N-CH2CH2CH3); 1.33 (3H, t, j = 7.5 Hz, -
CH2CH3)i
0.95 (3H, t, J = 7.5 Hz, -N-CH2CH2CH3).
Example (45)
CA 022~6736 1998-12-02
128
H-NMR (DMSO-d6): ~ = 13.70 (lH, s, broad, NH); 8.19-
7.38 (5H, m, Aryl-H);
4.21 (2H, t, J = 7.5 Hz, N-CH2CH2CH3); 3.28 (lH, m, CH-
Cyclopentyl);
2.19-1.50 (10 H, m, CH2-Cyclopentyl; N-CH2CH2CH3); 0.94
(3H, t, J = 7.5 Hz, N-CH2CH2CH3).
Example (46)
lH-NMR (DMSO-d6): ~ = 8.26 (lH, s, Hz); 7.84; 7.10;
6.69 (3H, 3m, Furyl-H);
4.10 (2H, t, J = 7.5 Hz, N-CH2CH2CH3); 1.81 (2H, m, N-
CH2CH2CH3) i
0.98 (3H, t, J = 7.5 Hz, N-CH2CH2CH3).
Example (47)
lH-NMR (DMSO-d6): ~ = 13.56 (lH, s, broad, NH); 7.70;
6.91; 6.50 (3H, 3m, Furyl-H); 4.00 (2H, t, J = 7.5 Hz, N-
CH2CH2CH3); 2.66 (2H, qu, J = 7.5 Hz, -CH2CH3);
1.61 (2H, m, N-CH2-CH2-CH3); 1.14 (3H, t, J = 7.5 Hz, N-
CH2cH2cH3)i
0.77 (3H, t, J = 7.5 Hz, -CH2CH3).
Example (48)
lH-NMR (DMSO-d6): ~ = 13.84 (lH, s, broad, NH); 8.00;
. ~
7.18; 6.78 (3H, 3m, Furan-H); 4.26 (2H, t, J = 7.5 Hz, N-
CH2CH2CH3); 3.37 (lH, m, CH-Cyclopentyl);
2.28-1.62 (10 H, m, CH2-Cyclopentyl; N-CH2CH2CH3);
1.04 (3H, t, J = 7.5 Hz; N-CH2CH2CH3).
Example (49)
H-NMR (DMSO-d6): ~ = 12.84 (lH, s, broad, NH); 8.26
(lH, s, H2);
4.32 (2H, t, J = 7.5 Hz, N-CH2CH2CH3); 3.18 (lH, m, CH-
Cyclopentyl);
2.09-1.52 (10 H,m, CH2-Cyclopentyli N-CH2CH2CH3);
0.83 (3H, t, J = 7.5 Hz, N-CH2CH2CH3).
CA 022~6736 1998-12-02
129
Example (50)
lH-NMR (DMSO-d6): ~ = 8.28 (lH, s, H6); 8.15 (lH, s,
H2);
13.35 (2H, s, broad).
Example (51)
H-NMR (DMSO-d6): ~ = 13.74 (lH, s, broad, NH); 8.34
(lH, s, H6);
4.63 (2H, qu, J = 7.5 Hz, N-CH2CH3); 3.33 (lH, m, CH-
Cyclopentyl);
2.24-1.57 (8H, m, CH2-Cyclopentyl); 1.47 (3H, t, J = 7.5
Hz, N-cH2cH3)-
Example (52)
lH-NMR (DMSO-d6): ~ = 13.72 (lH, s, broad, NH); 8.34
(lH, s, H6);
4.53 (2H, t, J = 7.5 Hz, N-CH2CH2CH3); 3.34 (lH, m, CH-
Cyclopentyl);
2.20-1.57 (8H, m, CH2-Cyclopentyl); 1.87 (2H, m, N-
CH2CH2CH3)i
1.06 (3H, t, J = 7.5 Hz, N-CH2CH2CH3).
Example (53)
lH-NMR (DMSO-d6): ~ = 8.19 (lH, s, H6); 4.00 (3H, s, N-
CH3)i
3.58 (3H, s, N-CH3); 3.42 (lH, m, CH-Cyclopentyl); 2.18-
1.57 (8H, m, CH2-Cyclopentyl).
Example (55)
H-NMR (DMS0-d6): ~ = 14.12 (lH, s, broad, NH); 8.44
(lH, s, Hz);
4.53 (2H, t, J = 7.5 Hz, N-CH2CH2CH3); 3.28 (lH, m, CH-
Cyclopentyl);
2.17-1.55 (8H, m, CH2-Cyclopentyl)i 1.87 (2H, m, N-
CH2CH2CH3)i
1.05 (3H, t, J = 7.5 Hz, N-CH2CH2CH3).
CA 022~6736 1998-12-02
130
Example (56)
lH-NMR (DMSO-d6): ~ = 8.28 (lH, s, H2); 4.36 (2H, t, J
= 7.5 Hz, N-CH2CH2CH3)i
4.12 (2H, t, J = 7.5 Hz, N-CH2CH2CH3); 3.21 (lH, m, CH-
Cyclopentyl).
2.12-1.57 (12H, m, CH2-Cyclopentyl; (N-CH2CH2CH3)2);
0.92; 0.87 (6H, 2t, J = 7.5 Hz, (N-CH2CH2CH3)2).
Example (57)
lH-NMR (DMSO-d6): ~ = 8.02 (lH, s, H2); 4.45 (2H, t, J
= 7.5 Hz, N-CH2CH2CH3)i
4.14 (2H, t, J = 7.5 Hz, N-CH2CH2CH3); 3.24 (lH, m, CH-
Cyclopentyl);
2.14-1.57 (12H, m, CH2-Cyclopentyl; (N-CH2CH2CH3)2;
0.93; 0.89 (6H, 2t, J = 7.5 Hz, (N-CH2CH2CH3)2;
CA 022~6736 1998-12-02
... ~ .. . .. . . .... .
131
The following Table contains KiA1 (human) and KiA2 (rat)
receptor binding values.
Table 20:
Example KjA1 KjA2
No.: [rM] [nV]
7 ,1 '5
~ g v~
c ,4
'0 ',7 ,vO
3~5
'3 5" 2
''5 1(,3 1''31
~,0 8,~ 3292
36 5,8 731
37 6,~ .~07
38 6,~ ~2
39 6,(~ ~9
48 11,4 4455
The following Table contains KiA1 (human) receptor binding
values.
Table 21:
Example KjA3
No. [r M]
v ~,7
",3
~2 20
~9 3,8
~3 2
''5 26
v8 22
~8 30
;8 5,3
82 25
CA 02256736 1998-12-02
132
The compounds of general formula (I) may be used on their
own or combined with other active substances according to
the invention, possibly also together with other
pharmacologically active substances. Suitable
preparations include, for example, tablets, capsules,
suppositories, solutions, syrups, emulsions or dispersible
powders. Corresponding tablets may be obtained, for
example, by mixing the active substance or substances with
known excipients such as inert diluents, e.g. calcium
carbonate, calcium phosphate or lactose, disintegrants
such as corn starch or alginic acid, binders such as
starch or gelatine, lubricants such as magnesium stearate
' or talc, and/or agents for achieving delayed release such
as carboxymethyl cellulose, cellulose acetate phthalate or
polyvinyl acetate. The tablets may also be made up of
several layers.
Coated tablets may be prepared analogously by coating
cores produced in the same way as the tablets with agents
conventionally used in tablet coatings, e.g. collidone or
shellack, gum arabic, talc, titanium dioxide or sugar. To
achieve delayed release or prevent incompatibilities, the
core may also be made up of several layers. Similarly,
the tablet coating may be made up of several layers to
- 25 achieve delayed release, in which case the excipients used
for the tablets may be used.
Syrups of the active substances according to the invention
or combinations of active substances may additionally
contain a sweetener such as saccharin, cyclamate, glycerol
or sugar and a flavouring improving agent, e.g. a
flavouring such as vanillin or orange extract. They may
also contain suspension adjuvants or thickeners such as
sodium carboxymethylcellulose, wetting agents, e.g.
condensation products of fatty alcohols with ethylene
oxide, or preservatives such as p-hydroxybenzoates.
CA 022~6736 1998-12-02
133
Injectable solutions are produced in the usual way, e.g.
by adding preservatives such as p-hydroxybenzoates or
stabilisers such as alkali metal salts of ethylenediamine
tetraacetic acid and are transferred into injection vials
or ampoules.
The capsules containing one or more active substances or
combinations of active substances may be produced by
mixing the active substances with inert carriers such as
lactose or sorbitol and packing them into gelatine
capsules.
! Suitable suppositories may be prepared, for example, by
mixing with carriers intended for this purpose such as
neutral fats or polyethyleneglycol or derivatives thereof.
A therapeutically active daily dose is between 1 and
800 mg, preferably 10 to 300 mg per adult.
The Examples which follow illustrate the invention without
restricting its scope:
Examples of pharmaceutical formulations
25 A) Tablets per Tablet
Active substance 100 mg
Lactose 140 mg
Corn starch 240 mg
Polyvinylpyrrolidone 15 mg
Magnesium stearate 5 mq
500 mg
The finely ground active substance, lactose and some of
the corn starch are mixed together. The mixture is
screened, and then moistened with a solution of
polyvinylpyrrolidone in water, kneaded, moist-granulated
and dried. The granules, the remaining corn starch and
CA 022~6736 1998-12-02
134
the magnesium stearate are screened and mixed together.
The mixture is compressed into tablets of suitable shape
and size.
5 B) Tablets per Tablet
Active substance 80 mg
Corn starch 190 mg
Lactose 55 mg
Microcrystalline cellulose35 mg
Polyvinylpyrrolidone 15 mg
~~ Sodium carboxymethyl starch 23 mg
Magnesium stearate 2 mq
400 mg
The finely ground active substance, some of the corn
starch, lactose, microcrystalline cellulose and
polyvinylpyrrolidone are mixed together, the mixture is
screened and processed with the remaining corn starch and
water to form a granulated material which is dried and
screened. The sodium carboxymethyl starch and the
magnesium stearate are added to this, then mixed together
and the mixture is compressed to form tablets of suitable
slze .
C) Coated tabletsper coated tablet
Active substance 5 mg
Corn starch 41.5 mg
Lactose 30 mg
Polyvinylpyrrolidone3 mg
Magnesium stearate0.5 mq
80 mg
The active substance, corn starch, lactose and
polyvinylpyrrolidone are thoroughly mixed and moistened
with water. The moist mass is pressed through a 1 mm mesh
screen, dried at about 45~C and the granules are then
CA 022~6736 1998-12-02
135
passed through the same screen again. After the addition
of magnesium stearate, curved tablet cores measuring 6 mm
in diameter are pressed out in a tablet making machine.
The tablet cores thus produced are coated in known manner
with a covering consisting essentially of sugar and talc.
The finished coated tablets are polished with wax.
D) Ca~sules per caPsule
Active substance 50 mg
Corn starch 268.5 mg
Magnesium stearate 1.5 mg
320 mg
The substance and corn starch are mixed together and
moistened with water. The moist mass is screened and
dried. The dry granules are screened and mixed with
magnesium stearate. The finished mixture is packed into
size 1 hard gelatine capsules.
CA 022~6736 1998-12-02
136
E) Ampoule solution
Active substance 50 mg
Sodium chloride 50 mg
Water for injections 5 ml
The active substance is dissolved at its own pH or
optionally at pH 5.5 to 6.5 in water and sodium chloride
is added to render the solution isotonic. The resulting
solution is filtered free from pyrogens and the filtrate
is transferred under aseptic conditions into ampoules
~~ which are subsequently sterilised and sealed by fusion.
The ampoules contain 5 mg, 25 mg and 50 mg of active
substance.
F) Suppositories
Active substance 50 mg
Solid fat 1650 mq
1700 mg
The hard fat is melted. At 40~C the ground active
substance is homogeneously dispersed therein. It is
cooled to 38~C and poured into slightly chilled
suppository moulds.
G) Oral Sus~ension
Active substance 50 mg
Hydroxyethylcellulose 50 mg
Sorbic acid 5 mg
(70~) Sorbitol 600 mg
Glycerol 200 mg
Flavouring 15 mg
3 5 Water ad 5 ml
Distilled water is heated to 70~C. Hydroxyethylcellulose
is dissolved therein with stirring. After the addition of
CA 022~6736 1998-12-02
137
sorbitol solution and glycerol the mixture is cooled to
ambient temperature. At ambient temperature the sorbic
acid, flavouring and substance are added. To eliminate
air from the suspension it is evacuated with stirring.
;'':
CA 022~6736 1998-12-02