Note: Descriptions are shown in the official language in which they were submitted.
CA 02257362 1998-12-O1
WO 97/48690 - PCT/US97/10337
PROCESS FOR THE SELECTIVE ALKYLAT10N OF EPOXY-ALCOHOLS
1 . f=ield of the Inyention
The present invention relates to a novel process for the preparation of 1-{4-
[2-
(Cyclopropylmethoxy)ethyl]phenoxy}-3-isopropylamino-propane-2-of hydrochloride
by
selective afkylation of an intermediate alkoxide with an alkylating agent and
a base.
2. Backq_rround of the Invention
1 o The processes typically employed in producing 1-{4-[2-
(Cyclopropylmethoxy)ethyl]phenoxy}-3-isopropylamino-propane-2-of (Betaxolol)
involved protecting the phenol functional group so that the alcohol could be
alkylated. This
involves extra protection and subsequent deprotection steps which make the
reaction
cotsiplicated as well as gives low yields which may further require
chromatography to purify
product.
US 4,252,984 to Manoury et al., describes benzylation of the phenolic alcohol
of 4-hydroxyphenethanoic acid. The ethanoic acid group is then reduced to an
alcohol and
subsequently alkylated with (bromomethyl)cyclopropane. Reduction with H2 in
the
presence of a catalyst deprotects the compound back to a phenolic compound. In
a final step,
2 o addition of isopropylamine produces the end product, Betaxolol. A silica
gel column is used to
purify the compound.
US 4,760,182 to Ippolito et al., describes a process for producing Betaxolol
by
converting 4-hydroxyphenethanol to a phenoxide anion with a base and then
reacting.the
phenoxide anion with epichlorohydrin to produce 1-[4-(2-hydroxyethyl)phenoxy]-
2,3-
epoxypropane. 1-[4-{2-hydroxyethyl)phenoxy]-2,3-epoxypropane is reacted with a
primary amine to produce an intermediate of Betaxolol. Protection and
deprotection steps
are necessary to obtain the final product.
US 5,034,535 to Keding et al., describes reacting 4-[2-methoxyethyl]phenol
with
(S)-5-hydroxymethyl-3-isopropyloxazolidin-2-one sulfonic acid ester to prepare
an
intermediate in the preparation of S-metoprolol.
The processes typically employed in producing Betaxolol involve extra
protection and
deprotection steps which make the reaction complicated. Specifically, the
epoxide of 1-[4-
(2-hydroxyethyl)phenoxy]-2,3-epoxypropane is not stable toward alkylating
reagent since
the usual alkylation condition will cause polymerization of the epoxy-alcohol.
This is due to
CA 02257362 2004-11-12
-2-
the molecule possessing both a nucleophilic and an electrophilic center. The
alkoxide
of 1-[4-)2-hydroxyethyl)phenoxy]-2,3-epoxypropane can react with the
alkylating
reagent as well as the epoxide moiety of 1-[4-(2-hydroxyethyl)phenoxy]-2,3-
epoxypropane, leading to the formation of multiple products/polymers.
It is therefore advantageous and preferable to have a process wherein the
protection and deprotection steps can be eliminated. In addition, it would be
a further
advantage to have a process which produces highly pure Betaxolol.
SUMMARY OF THE INVENTION
The present invention relates to a process for the selective alkylation of an
alcohol in an epoxy-alcohol compound comprising the steps of reacting the
epoxy-
alcohol compound in the presence of an alkylating reagent, a solvent and a
strong base.
More especially, the invention relates to a process for the selective
alkylation
of the ethanol hydroxy moiety of 1-[4-(hydroxyethyl)phenoxy]-2,3-epoxypropane
comprising the steps of reacting the 1-[4-(hydroxyethyl)phenoxy]-2,3-
epoxypropane in
the presence of an alkylating reagent, a solvent and a strong base.
The present invention further relates to an improved process for the
production
of Betaxolol.
DETAILED DESCRIPTION OF THE INVENTION
The present invention relates to a process for producing selective alkylations
of alcohol hydroxy groups in compounds which also contain an epoxide
functional
group. The alcohol hydroxy group of a compound can react with an alkylating
reagent
in the presence of a strong base without polymerization of the compound due to
the
reactivity of the epoxide with the alkylating reagent.
The present invention also relates to a novel process for producing
intermediates of Betaxolol. The synthetic process for producing intermediates
of
Betaxolol with the novel alkylation process of the present invention is shown
in
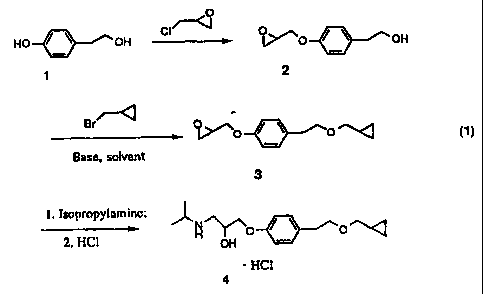
Scheme 1. The phenolic alcohol of 4-hydroxyphenethanol (1) is usually more
readily
CA 02257362 2004-11-12
-3-
alkylated than the alcohol hydroxy moiety in the same compound. Typically, for
the
alcohol hydroxy moiety to be alkylated, the phenolic alcohol had to be
protected, thus
adding extra protection and deprotection steps. Such protection and
deprotection steps
included benzylation of the phenol prior to alkylation of the ethanol moiety
and then
subsequent hydrogenolysis back to the phenol. After the hydrogenolysis, the
epoxide
could then be added to the phenol and synthesis of Betaxolol continued.
However, with
the process of the present invention, such protection and deprotection steps
are
eliminated. The process of the present invention thus eliminates extra steps
and also
results in higher yields of product.
The selective alkylation of the present invention utilizes a reactive
alkylating
reagent and a strong base. The present invention relates to a process for
producing
selective alkylations of alcohol hydroxy groups in compounds which also
contain an
epoxide functional group. As an example, in the production of the Betaxolol,
Betaxolol
may be produced in three steps with only one isolation necessary. In Scheme l,
the
competition between the reaction of the ethanol hydroxy moiety of 1-[4-(2-
hydroxyethyl)phenoxy]-2,3-epoxypropane (2) with an incoming alkylating reagent
and
with the epoxide of 1-[4-(2-hydroxyethyl)phenoxy]-2,3-epoxypropane (2) was
optimized such that self polymerization with the epoxide was eliminated.
CA 02257362 2004-11-12
-4-
Scheme 1.
O
HO ~ \ OH CI -; O~,]~O / \ OH
70 1
Bra / \
q.~'~o o '''~
Base, solvent
3
I. Isopropyl;~minc;
2. HCl ~ off 0 / \ O'
t 5 ~ HCl
4
Suitable alkylating agents used in the selective alkylation of the present
invention
20 include subsiiiuted haloalkyls, halo-substituted cycloaikyls, halo-
substituted
cycloalkylalkyls, halo-substituted arylalkyls, halo-substituted aryl, halo-
substituted
alkoxies, halo-substituted arylalkoxies, halo-substituted cycloalkoxies, halo-
substituted
cycloalkylalkoxies, halo-substituted heterocyclics or halo-substituted
(heterocyclic)alkyls.
In addition, sulfonated substituted alkylating agents may be used instead of
halo-substituted
2 5 alkylating agents. Alkyl groups include straight or branched chain alkyl
radicals containing
from 1 to 6 carbon atoms including, but not limited to, methyl, ethyl, n-
propyl, iso-propyl,
n-butyl, iso-butyl, sec-butyl, t-butyl, n-pentyl, 1-methylbutyl, 2,2-
dimethylbutyl, 2-
methylpentyl, 2,2-dimethylpropyl, n-hexyl and the like.
Suitable strong bases used in the selective alkylation of the present
invention include,
30 but are not intended to be limited to, potassium tert-butoxide, 1,8-
Diazabicyclo [5.4.0]
undec-ene (DBU), alkyllithiums including, but not limited to butyllithium
(BuLi), and
CA 02257362 2004-11-12
-5-
lithium diisopropytamide (LDA), and phenyllithium. The most preferred base is
potassium
tart-butoxide.
Solvents which may be used with the present invention include, but are not
intended
to be limited to, Dimethyl Sulfoxide (DMSO), N,N-dimethylformamide (DMF), N,N-
Dimethylacetamide (DMA), acetonitrile, dichloromethane, ethyl acetate, and
tetrahydrofuran (THF).
The temperature of the reaction is typically run at from about -10°C to
about 25°C.
A more preferred temperature range is from about -10°C to about
10°C. A most preferred
temperature range is from about -5°C to about 5°C.
t o In accordance with the teaching of the present invention, the selective
alkylation of
1-[4-(2-hydroxyethyl)phenoxy]-2,3-epoxypropane (2) in the presence of a strong
base
produces intermediate, 1-(4-j2-(Cyclopropylmethoxy)ethyl]phenoxy}-2,3-
epoxypropane
(3). 1-[4-[2-(Cyclopropylmethoxy)ethyl]phenoxy]-2,3-epoxypropane may then be
reacted with isopropylamine followed by an acid including, but not intended to
be limited to.
hydrochloric acid to yield Betaxolol (4).
The following example is merely an example of the process of the present
invention
utilizing the selective alkylation procedure in the presence of a base.
Example 1
A reaction flask was charged with 1-[4-(2-Hydroxyethyl)phenoxy]-2,3-
epoxypropane (140 grams, 0.72 moles), bromomethylcyclopropane (90 milliliters
(mL),
125 grams, 0.92 miilimoles) and N,N'-dimethylacetamide (700 mL). The mixture
was
blanketed with nitogen and stirred at room temperature for 70 minutes and then
cooled to 0°
C. Potassium tert-butoxide (120 grams, 1.1 moles) was slowly added. After the
addition
was completed, the reaction mixture was maintained at 0° C far 3 hours.
The reaction mixture was diluted with aqueous hydrochloric acid (7 Normal, 500
mL). The aqueous mixture was extracted three times with 500 mL portions of
heptane. The
combined organic extracts were washed twice with 500 mL portions of water and
3o concentrated to an oil by vacuum distallation to give 179 grams (i00%
yield) of 1-(4-[2-
(Cyclopropylmethoxy)-ethyl]-phenoxy}-2,3-epoxypropane which was used for the
preparation of Betaxolol without additional purification.
Example 2
The oil obtained in Example 1 (179 grams) was dissolved in 400 mL of
isopropyiamine. After the reaction solution was refluxed for 2 days, the
isopropylamine was
distilled off and the residue was dissolved in 200 mL of toluene. The toluene
was removed by
CA 02257362 2004-11-12
-6-
vacuum distillation to give 222 grams of 1-(4-[2-
(Cyclopropylmethoxy)ethyl]phenoxy)-
3-isopropylamino-propane-2-of (Betaxolol free base) which was used for the
preparation
of the hydrochloride salt without additional purification. The Betaxoiol free
base was
dissolved in 300 mL of toluene containg 20 mL of isopropanol. A stream of
hydrogen chloride
gas was passed through the above solution at 0° C until the reaction
mixture pH was less than
3Ø The solvent was removed by vacuum distillation, and the residue was
crystallized from
400 mL of acetone to give 102 grams (99% pure) of Betaxolol hydrochloride.