Note: Descriptions are shown in the official language in which they were submitted.
CA 022~7948 1998-12-10
W O 98/~M~5 rCT~US97/1113
TITLE
PHARMACEUTICAL PREPARATION
BACKGROUND OF THE INVENTION
The present invention relates to the co-~-lmini~tration,
either simlllt~neously, separately or sequentially of a selective IKS
antagonist and a beta-adrenergic receptor blocking agent for use in
preventing, treating and termin~ting cardiac arrhythmias, such as
atrial, supraventricular and ventricular ectopy, tachycardia, flutter
or fibrillation, including atrial, supraventricular and ventricular
arrhythmias resulting from myocardial ischemic injury in a patient
in need thereof. This invention also relates to a pharmaceutical
formulation which comprises a selective IKS antagonist and a beta-
adrenergic receptor blocking agent along with a pharmaceutically
acceptable carrier.
Arrhythmias often occur a.s complications to cardiac
diseases such as myocardial infarction and heart failure. In a serious
case, arrhythmias give rise to ventricular fibrillation and can cause
sudden death.
Though various antiarrhythmic agents are now available on
the market, agents exhibiting both satisfactory effects and high safety
profiles, are not yet available for patients. For example, antiarrhythmic
agents of Class I, according to the classification of Vaughan-Willi~ms,
which cause a selective inhibition of the maximum velocity of the
upstroke of the action potential (Vmax) are inadequate for preventing
ventricular fibrillation. In addition, they have problems regarding
safety, namely, they cause a depression of the myocardial contractility
and have a tendency to induce arrhythmias due to an inhibition of the
impulse conduction. Beta-adrenergic receptor blocking agent which
belong to Class II are of limited value since their effects are either
limited to a certain type of arrhythmia or are contraindicated because
of their cardiac depressant properties in certain patients with cardio-
vascular disease. Their safety, however, is higher than that of the
antiarrhythmic agents of Class I.
CA 022~7948 1998-12-10
WO 98/0040~ PCT/US97/11131
Antiarrhythmic agents of Class III are drugs which cause a
selective prolongation of the duration of the action potential without a
significant depression of the Vmax. Until recently, drugs in this class
were limited to sotalol and amiodarone, both of which have been shown
to possess Class III properties. However, Sotalol also possesses Class II
effects which may cause cardiac depression and be contraindicated in
certain susceptible patients. Amiodarone is severely limited by side
effects. Drugs of this class are expected to be effective in preventing
ventricular fibrillations. Pure Class III agents, by definition, are not
considered to cause myocardial depression or an induction of
arrhythmias due to the in~ibition of the action potential conduction as
seen with Class I antiarrhythmic agents.
Recently, a novel group of Class III agents have been
disclosed which antagonize the IKs channel found in heart muscle.
These compounds IKs channel antagonists are effective in treating and
preventing all types of arrhythmias including ventricular and atrial
(supraventricular) arrhythmias. These novel compounds are disclosed
and claimed in U.S. Patent Application, Serial Nos. 08/411,240;
08/516,467; and 08/516,226 which are hereby incorporated by
reference. These novel compounds are especially useful for controlling
reentrant arrhythmias and preventing sudden death due to ventricular
fibrillation. These compounds are also effective in treating and
preventing impaired cardiac pump functions.
In the treatment of arrhythmia, IKS antagonists have
demonstrated effectiveness when delivered orally in amounts r~nging
from about 0.01 to about 1 mg per kg of body weight per day, in a
single dose or in 2 to 4 divided doses.
The activity of the compounds described herein as anti-
arrhythmic agents is measured by their ability to block the IKS and
IKr currents as determined by the following test protocol.
Outward potassium currents are measured in single guinea
pig ventricular myocytes using a whole-cell voltage clamp technique
described in detail elsewhere (Sanguinetti and Jurkiewicz, 1990, Two
components of cardiac delayed rectifier K+ current: differential
CA 022~7948 1998-12-10
WO 98/00405 PCT/US97/11131
sensitivity to block by Class III antiarrhythmic agents. J. Gen Physiol.
96: 195-215). Myocytes are isolated by enzymatic (collagenase and
protease) digestion of Langandorf perfused hearts. Single cells are then
voltage clamped using l mm square-bore pipettes filled with 0.5 M
Kgluconate, 25 mM KCl, 5 mM K(2)ATP. Cells are bathed in a
solution cont~ining, in mN: 132 NaCl, 4KCl, 1.2 MgCl2~ 10 HEPES, 10
glucose: pH 7.2, temp. 35~C.
Each cell is maintained at a holding potential of -50 mV.
Test depolarizations are applied as voltage ramps from -85 to -50 mV,
and as steps to -10 mV (0.5 s) and +50 mV (1.0 s). IKI is measured as
peak outward current during the voltage ramp. IKr i.c measured as tail
currents upon repolarization from -lO mV to -50 mV. IKS is measured
as time-dependent current during the pulse to +50 mV. Currents are
measured during control, then after exposure to drug at two different
concentrations .
Employing this test the compounds described herein as
selective IKS channel antagonists, have an IC50 of less than 100 nM as
IKS antagonists. The compounds of this invention are at least 10 times
more potent in the blockade of IKs than of blockade of IKr-
Beta-adrenergic receptor blocking agents, or "beta-
blockers", are a class of ph~rm~ceutically active compounds which
decrease the positive chronotropic, positive inotropic, bronchodilator
and vasodilator responses caused by beta-adrenergic receptor agonists.
The m~gnitll~le of this decreased response is proportional to the existing
sympathetic tone and the concentration of beta-blocker at the receptor
sites. Beta-adrenergic receptor blockage is said to reduce cardiac output
in both healthy subjects and patients with heart disease. While the
mech~ni~m of antihypertension effects of beta-adrenergic receptor
blocking agents has not been established, possible mech~ni.cm~ of action
include reduction in cardiac output, reduction in plasma renin activity,
and central nervous system sympatholytic action. The ~lmini~tration of
beta-adrenergic receptor antagonists has been shown effective in
- reducing the incidence of mortality and sudden death in postinfarction
CA 022~7948 1998-12-lO
W O 98/00405 PCT~US97/11131
patients (Yusaf et al., Prog Cardiovasc Dis 17: 335-371, 198~; Lau et
al., N Eng J Med 327: 248-254, 1992).
While both selective IKS channel blockers and beta-
adrenergic receptor blocking agents have been proven effective when
~lmini~tered separately, it is considered to be in the best interest of the
patient to reduce the amount of these compounds provided to the patient.
Any reduction of one or the other compound would be considered
helpful, but this is particularly true of beta-adrenergic receptor blocking
agents which are known to have significant side effects in some humans.
SUMMARY OF THE INVENTION
A method is presented for use in preventing, treating and
termin~ting cardiac arrhythmias, such as atrial, supraventricular and
ventricular ectopy, tachycardia, flutter or fibrillation, including atrial,
supraventricular and ventricular arrhythmias resulting from myocardial
ischemic injury in a patient in need thereof which comprises the co-
a~mini.~tration, either simultaneously, separately or sequentially of a
selective IKS antagonist and a beta-adrenergic receptor blocking agent.
This invention also relates to a pharmaceutical formulation which
comprises a selective IKS antagonist and a beta-adrenergic receptor
blocking agent along with a pharmaceutically acceptable carrier.
DETAILED DESCRIPTION OF THE INVENTION
A method is presented for use in preventing, treating and
termin~ting cardiac arrhythmias, such as atrial, supraventricular and
ventricular ectopy, tachycardia, flutter or fibrillation, including atrial,
supraventricular and ventricular arrhythmias resulting from myocardial
ischemic injury in a patient in need thereof which comprises the co-
~lministration, either simultaneously, separately or sequentially of a
selective IKS antagonist and a beta-adrenergic receptor blocking agent.
This invention also relates to a pharmaceutical formulation which
comprises a selective IKS antagonist and a beta-adrenergic receptor
blocking agent along with a pharmaceutically acceptable carrier.
CA 022~7948 1998-12-10
WO 98/00405 PCT/US97/11131
By a "selective IKs antagonist" is meant those compounds
which when studied in the test disclosed above have an IC50 of less than
100 nM as IKS blockers. The compounds of this invention are at least
10 times more potent in the blockade of IKs than of blockade of IKr.
Beta-adrenergic receptor blocking agents are compounds
which decrease the positive chronotropic, positive inotropic, bronch-
odilator and vasodilator responses caused by beta-adrenergic receptor
agonists. The magnitude of this decreased response is proportional to
the existing sympathetic tone and the concentration of beta-adrenergic
receptor blocking agent which reaches the receptor sites.
Examples of compounds which fit the definition of beta-
adrenergic receptor blocking agent include but are not limited to
timolol, sotalol, esmolol, cateolol, propranolol, betaxolol, penbutolol,
metoprolol, acebutolol, atenolol, metoprolol, pindolol, and bisoprolol,
and their salts, hydrates, solvates and any crystal forrns in which they
may occur.
Examples of compounds which fit the definition of selective
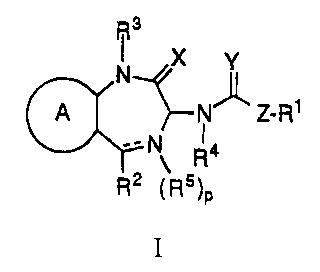
IKS antagonists include, but are not limited to, the following:
N~X y
(~ ~N z R
\
R2 ( R5)p
I
or a pharmaceutically acceptable salt thereof, wherein
A is 1 ) thieno,
2) pyrido, or
3) benzo either unsubstituted or substituted with -NH2
- -NHS02 (C1-3 alkyl), Cl 3 alkyl or Cl 3 alkoxy;
CA 022~7948 1998-12-10
WO 98/00405 PCT/US97/11131
X is 1 ) =0,
2) =S,
3) =N-NH2,
4) =N-OH or
5) =H2,
Y is 1 ) =0,
2) =N-CN or
3) --H2;
Z i 1) C1 6 alkylene, either straight or branch chain and either
unsubstituted or substituted with phenyl or spiro-piperidine,
2) C2 4 alkenylene, either straight or branch chain,
3) -(CH2)m-W-(CH2)n- wherein m and n are independently 0,
1, 2, 3 or 4 and W is -O-, -S- or -NH,
4) 4-(5-methylisoxazole-3-yl),
5) C3-6 cycloalkylene, or
6) single bond,
p is 0 or 1;
R1 is 1) phenyl, either unsubstituted or substituted with one or two
substituents selected from
a) -NO2,
b) -Cl, Br, F, or I,
c) -CF3,
d) -Cl 3 alkyl,
e) -Cl 3 alkoxy,
f) -CN,
g) -methylenedioxy,
2) C5 7 cycloalkyl,
3)
~N- CO2t-Bu,
CA 022~7948 1998-12-10
WO 98/00405 PCT/US97/11131
4) mono- or bicyclic heterocyclyl of 5 to 10 members one or
two of which are sulfur, nitrogen or oxygen, the rem~ining
being carbon, such as 2-thienyl, 2-furanyl, 2-indolyl, 2-
quinoxolinyl, or 2-(2,3-dihydro benzofuranyl)
S) Cl 3 alkyl, or
6) indan-S-yl;
R2 is 1) phenyl, either unsubstituted or substituted with Cl 3 alkoxy
or 4,4-dimethyloxazolin-2-yl,
2) C1 6 alkyl, either straight or branched chain, and either
unsubstituted or substituted with C1 3 alkoxy or C1 3
alkoxy-Cl 3 alkoxy,
3) C5 7 cycloalkyl,
4) 2- or 3-furyl,
5) 1-methylpiperidin-2-yl, or
6) if R2 is phenyl, the 2-position of the phenyl can be joined to
the 4-position nitrogen of the diazepine ring through a
carbonyl group and the double bond between the 4-nitrogen
and the 5-carbon becomes a single bond;
R3 is 1) hydrogen or
2) Cl-3 alkyl either unsubstituted or substituted with
-N(CH3)2, -OH, -CF3, or
3) -CF3;
R4 is 1 ) hydrogen,
2) Cl 6 alkyl, the chain of carbon atoms of which can be
interrupted by one or two non-adjacent oxygen atoms and
which is either unsubstituted or substituted with C1-3
alkoxycarbonyl, -OH or
--O--~3No2, or
3) tetrazol-S-yl;
CA 022~7948 1998-12-lo
W 098100405 PCTrUS97/11131
R5 is hydrogen or oxygen or is joined to R2 to form the partial
structure:
~N
~0; and
~J ,
the bond represented by is:
1 ) a double bond when p is zero or when p is 1 and RS is
oxygen, or
2) a single bond when R5 is hydrogen or R5 is joined to R2 to
form the partial structure:
\~N~
[~~
This invention is meant to include the individual
diastereomers where such exist and mixtures thereof and enantiomers
and mixtures of the enantiomers.
The ph~ ceutically acceptable salts of the compounds of
Formulas I include the conventional non-toxic salts or the quarternary
ammonium salts of the compounds of Formula I formed, e.g., from
non-toxic inorganic or organic acids. For example, such conventional
non-toxic salts include those derived from inorganic acids such as
hydrochloric, hydrobromic, sulfuric, sulfamic, phosphoric, nitric and
the like; and the salts prepared from organic acids such as acetic,
propionic, succinic, glycolic, stearic, lactic, malic, tartaric, citric,
ascorbic, pamoic, maleic, hydroxymaleic, phenylacetic, glutamic,
benzoic, salicylic, sulfanilic, 2-acetoxybenzoic, fumaric, toluenesulfonic,
methanesulfonic, ethane disulfonic, oxalic, isethionic, and the like.
The pharmaceutically acceptable salts of the present
invention can be synthesized from the compounds of Formula I which
,,._
CA 022~7948 1998-12-10
WO 98/00405 PCTrUS97/11131
contain a basic or acidic moiety by conventional chemical methods.
Generally, the salts are prepared by reacting the free base or acid with
stoichiometric amounts or with an excess of the desired salt-forming
inorganic or organic acid or base in a suitable solvent or various
combinations of solvents.
One embodiment of this invention are novel compounds
useful in the novel method of treatment of this invention wherein:
A is benzo;
X and Y are oxygen;
R3 is methyl;
R4 is hydrogen; and
R2 is C1 6 alkyl.
Specific novel compounds representative of this
embodiment are those of the following structure and specified in
Table I:
TABLE I
CH3
~NJ--R
R2
CA 02257948 1998-12-lo
W O 98/00405 PCTnUS97/11131
- 10-
Rl R2
2?4-diClPh -CH3
2,4-diClPh -C2H~S
2,4-diClPh -t-Bu
4-CF3Ph i-C3H7
cyclohexyl i-C3H7
2,4-diClPh i-C3H7
Another embodiment of the compounds useful in the novel
method of treatment of this invention is that wherein:
A lS
~ ;
X and Y are oxygen;
R3 is methyl;
R4 is hydrogen; and
R2 is phenyl.
A class of novel compounds within this embodiment is that
with structural formula:
CA 02257948 1998-12-10
W 098/00405
1 1
CH3
~,~N Z- R
~3
wherein
Z is C1 6 alkylene or a bond and
R 1 is phenyl, phenyl substituted with -Cl, -Br, -I, -F, or -CF3, or R 1
is cyclohexyl.
Specific novel compounds representative of this class are
those depicted in the following Table II:
TABLE II
Z Rl
-(CH2)2- 2,4-diClPh
-(CH2)2- 4-ClPh
-(CH2)2- 2,4-diFPh
-(CH2)2- 2-ClPh
-(CH2)2- 4-CF3Ph
-CH2- 4-CF3Ph
-(CH2)2- 3-CF3Ph
-(CH2)2- 2-CF3Ph
-(CH2)2- cyclohexyl
- cyclohexyl
CA 02257948 1998-12-10
W 098/00405 PCTrUSg7/1113
- 12 -
TABLE II (Cont'd)
Z Rl
-(CH2)3- cyclohexyl
-CH2- cyclohexyl
-(CH2)2- Ph
-CH2- Ph
-(CH2)2- 4-CNPh
-(CH2)2- 3-ClPh
-(CH2)3- Ph
-(CH2)2- 3-CNPh
-(CH2)3 2-thienyl
Another class of novel compounds within this embodiment
is that with structural formula:
CH~
~N Z- R1
wherein Z is C2 4 alkenylene and R1 is phenyl or phenyl substituted
with -Cl, -Br, -F, -I, -CF3, Cl 3 alkyl, C1 3 alkoxy or methylenedioxy.
Specific novel compounds representative of this class are
those depicted in the following Table III:
CA 02257948 1998-12-lO
W O 98/00405 PCT~US97/11131
TABLE III
Z R1
-CH=CH- 4-NO2Ph
-CH=CH- 2,4-diClPh
-CH=CH- 3-ClPh
-CH=CH- 2-ClPh
-CH=CH- 2,4-di FPh
-CH=CH- 2,6-diClPh
-CH=CH- 4-CF3Ph
-CH=CH- 2-BrPh
CA 02257948 1998-12-lo
W 098/0040S PCTrUS97/11131
- 14 -
TABLE III (Cont'd)
-CH=CH- 4-lPh
-CH=CH- 4-BrPh
-Cl CH- Ph
CH3
-CH=CH- Ph*
-CH=CH- 8,4-diClPh
-CH=CH- 4-CH3Ph
-CH=CH- 4-CH30Ph
-CH=CH-3,4-methylenedioxyPh
-CH=CH- 3-BrPh
*This compound is disclosed in U.S. Patent 4,820,834
A third embodiment of the compounds useful in the novel
method of treatment of this invention is that wherein:
Z is -NH-.
Compounds representative of this embodiment are those
disclosed in the following Tab~e IV.
CA 02257948 1998-12-10
WO 98t00405 PCT/US97/11131
- 15 -
TABLE IV
(~ H
A R 1 R2R3 Y
benzo 3-CH3Ph Ph ~ O
OH
benzo 2,4-diClPh Ph-CH3 O
benzo 3-CH3Ph ~Ç n-C3H7 o
benzo -CH2 Cyclohexyl Ph-CH3 =N-CN
benzO 3-CH3Ph Ph-CH3 O
benzo 5-indanyl Ph ~ O
GH
3-CH3Ph Ph-CH3 O
Other specific compounds included within the broadest
genus but not included in one of the embodiments previously described
are as shown in Table V.
CA 02257948 1998-12-lo
W O 98/00405 PCTtUS97tlll31
- 16 -
TABLE V
(~N Z-R
N l4
R2
Representative of compounds wherein p is 1 is the
compound of structural formula:
CH3
0~$N ~--
~0
Representative of compounds wherein the bond between the
4 and 5 positions is a single bond is the compound of structural formula:
CH3
N H H~--
CH3 CH3
_ . , . .,, ~ .
CA 02257948 1998-12-10
W O 98/00405 PCTAUS97/11131
Representative of compounds wherein the bond -------
represents a single bond and R5 is joined to R2 is the compound of
structural formula:
CH3
~H ~--~'CI
~0
Another embodiment of this invention is a group of
compounds, active in the novel method of treatment of this invention,
which are novel compounds ~ se. These novel compounds are
depicted in the following Table VI.
H3
NJ~ Z R
~N ¦4
~3
Another embodiment of this invention is a group of
compounds which are active in the novel method of treatment of this
invention. These compounds are depicted as follows:
.
CA 02257948 1998-12-10
W O 98/00405 PCTAUS97/11131
- 18 -
CF3
~H (CH2)~\\y
,~
R
where
X and Y are independently hydrogen, chloro, fluoro, bromo, iodo,
or trifluoromethyl and
n is 0, 1 or 2;
R is hydrogen, fluoro, chloro, bromo, iodo, or trifluoromethyl,
methyl, or methoxy; and
the racemates, mixtures of enantiomers, individual diastereomers or
individual enantiomers with all isomeric forms and ph~ ceutically
acceptable salts, hydrates or crystal forms thereof, which are
antiarrhythmic agents.
Yet another embodiment of this invention is a group of
compounds which are active in the novel method of treatment of this
invention. These compounds are depicted as follows:
CH3
(~CH3
N~ J~ 2
R1
CA 02257948 1998-12-10
WO 98/00405 PCT/US97/11131
- 19-
R1and R2 are independently
1 ) phenyl, either unsubstituted or substit~lte~l with one or two
substituents selected from
a) -NO2, OH,
b) -Cl, Br, F, or I,
c) -CF3,
d) -C1 3 alkyl,
e) -Cl 3 alkoxy,
f) -CN,
g) -methylenedioxy, and
Z is 1) Cl 6 alkyl, either straight or branched chain,
2) substituted C1 6 alkyl, either straight or branched
chain, wherein the substituents are selected from F,
OH, NO2,
3) C2 4 alkenylene, either straight or branched chain,
4) -(CH2)m-W-(CH2)n- wherein m and n are
independently 0, 1, 2, 3 or 4 and W is -O-, -S-
or -NH,
5) C3-6 cycloalkane,
6) C3-6 cycloalkylene, or
7) single bond;
The selective IKS blockers of the present invention have the
pharmacological properties required for antiarrhythmic agents of Class
III, namely they demonstrate prolongation of QTc-interval, and dose
dependent increases in ventricular refractoriness. This is accomplished
- without effecting heart rate, mean arterial pressure and PR and QRS
intervals. Modest increases in LV+dP/dt (left ventricular change in
pressure with time) is observed. Further, these compounds suppress
the induction of PVS (Programmed Ventricular Stimulation) induced
ventricular tachyarrhythmi~.
Individually, these compounds are effective in treating
CA 022~7948 1998-12-lO
W O 98100405 PCTAUS97/11131
- 20 -
and preventing all types of arrhythmias including ventricular, atrial and
supraventricular arrhythmias. The compounds of th~ present invention
are especially useful for controlling reentrant arrhythmias and prevent
sudden death due to ventricular fibrillation. These compounds are also
effective in treating and preventing impaired cardiac pump functions.
In the novel method of this invention of treating
arrhythmia, a selective IKs antagonist is a~lministered in an amount
ranging from about .0001 to about 10 mg per kg of body weight per
day, preferably from about .0001 to about 2 mg per kg of body weight
per day, and more preferably, when intravenous delivery of the
compounds is employed, from about 0.0003 to about 0.3 mg per kg of
body weight per day, or when given orally from about 0.01 to about 1
mg per kg of body weight per day, in a single dose or in 2 to 4 divided
doses of each compound. The beta-adrenergic receptor blocking agent
is a~lministered in an amount ranging from about 1 mg per day to about
300 mg poer day and more preferably from about 2 mg/day to about
250 mg per day.
The activity of the compounds described herein as
antiarrhythmic agents is measured by their ability to block the IKS
and IKr currents as determined by the following test protocol.
Outward potassium currents are measured in single guinea
pig ventricular myocytes using a whole-cell voltage clamp technique
described in detail elsewhere (Sanguinetti and Jurkiewicz, 1990, Two
components of cardiac delayed rectifier K+ current: differential
sensitivity to block by Class III antiarrhythmic agents. J. Gen Physiol.
96: 195-215). Myocytes are isolated by enzymatic (collagenase and
protease) digestion of Langandorf perfused hearts. Single cells are then
voltage clamped using 1 mm square-bore pipettes filled with 0.5 M
Kgluconate, 25 mM KCl, S mM K(2)ATP. Cells are bathed in a
solution cont~ining, in mN: 132 NaCl, 4KCl, 1.2 Mgcl2~ 10 HEPES, 10
glucose: pH7.2, temp. 35~C.
Each cell is m~int~ined at a holding poter.tial of -50 mV.
Test depolarizations are applied as voltage ramps from -85 to -50 mV,
and as steps to -10 mV (0.5 s) and +50 mV (1.0 s). IKI is measured as
CA 02257948 1998-12-lo
W O 98/00405 PCTAUS97/11131
- 21 -
peak outward current during the voltage ramp. IKr is measured as tail
currents upon repolarization from -10 mV to -50 mV. IKs is measured
as time-dependent current during the pulse to +50 mV. Currents are
measured during control, then after exposure to drug at two different
concentrations.
Employing this test the compounds described herein as
selective IKS blockers have an Ic50 of less than 100 nM as IKS blockers.
The compounds of this invention are at least 10 times more potent in the
blockade of IKS than of blockade of IKr.
Typical synthetic schemes employed in making the
compounds herein are illustrated below.
SCHEME 1
HO Z R1 \N~~
~ ~f N ~NH2
1. (COCI)2, \ 2. Ph
2. (2), Et3N
or
EDC b~ ~ ~N Z-
HOBT \~N H
3 Ph
CA 02257948 1998-12-lo
W 098/00405 PCT~US97/11131
- 22 -
SC~nE~nE 2
N~Br
5 Ph
H2NR1 \N ~~
~N H
6. Ph
_ .
CA 02257948 1998-12-lO
W O 98/0040S
PCTAUS97/11131
- 23 -
SCHEME 3
H2SO4 02N J~ N 02N J~N
~3 7. 8. [~
1 R1-Z Cl
2. TiCI3
3. Chromatography
~ N Z-R1
H2N ~N H CH3SO2CI
9.
~ ~ ~N~z
CH3SO2HN ~N H
10. W
CA 02257948 1998-12-lO
W 098/00405 PCTAJS97/11131
- 24 -
SCHEME 4
OCNJ~ N N~
2. Cl \~N H H Cl
13. Ph
SCHEME 5
HJ~
(~ 2 n-BuLi (~O EtOH-H20 (~O
3. PhcoNMe2
Ph Ph
o
1 BOC-Gly, DCC ~5 Pyridine
Ph Ph
= ~ =
N~
. . .
CA 02257948 1998-12-10
W O 98/00405 PCT~US97/11131
- 25 -
SCHEME 6
- S OH
J~,CN HO'~S~' ~H~
Et3N Ph
Cl ~ N~
2. H2NNH2 Ph
3. AcOH
CA 02257948 1998-12-lo
W O 98/00405 PCTnUS97/11131
- 26 -
SCHEME 7
(~5 N (~= N
Ph Ph
SnCI2 (~NH2
Ph Ph
= ~ N~
N~ or
CA 02257948 1998-12-10
WO 98/00405 PCT/US97/11131
SCHEME 8
R 1 NCO
or \ ~ O
Ph R1-Z-CO2H Ph
EDC, HOBT
CA 02257948 1998-12-10
WO 98/00405 PCT/US97/11131
- 2~S -
SCHEME 9
R3 1. Lawesson's R3
~N _4 Reagent ~N~
HNCBZ l~ HNCBZ
N ~f N
Ph Ph
R3= H, Me R3
1 HBr ~ N~--
~CI Ph
R3= H /
R3 = Me
H2NN~ Ra-Ni
Nl H2
Ph ¢~N H~)
.
CA 02257948 1998-12-10
WO 98/00405 PCT/US97/11131
- 29 -
SCHEME 10
H N
c~H2 GLYEthyl ~~ <J
N
1) KOtBu/lsoamylnitrite ~ ~ J
2) ethyl isocyanate Nl ~ n
3) hydrog~nation
CA 02257948 1998-12-10
WO 98/00405 PCTNS97111131
- 30 -
SCHEME 1 1
N~O xs NaHCO3 N~O
NH2 + R X CH3CN ~N
X= Br, Cl, OTs, OMs.
CIJ~--~\, ~NsN R4
CH2CI2 0~C
CA 02257948 1998-12-10
W O 98/00405 PCTrUSg7/1113
- 31 -
SCHEME I 2
O
O O=<
~N~ (cH2)Qxs NaHCO3
NH2 + Br CH3CN
~ CH2CL2, R.T.
O O
1~1'
~N (C~
W ~
CA 02257948 1998-12-10
WO 98/00405 PCI/US97/11131
- 32 -
SCHEME 13
(Boc)20, THF \~N~O R-MgX, THF
NaH ~ -78~C
~NH \~ NBoc
// // 80-30%
O O
X = Br, Cl
NJ~' Boc 1) HCI EtOAc '~N~O
2)1N NaOH ~S5
R2 R2
K-OtBu, Toluene \N ~1 ) ethyl isocyanate, THF
r /~ ~ ~~ 2)10% Pd/C MeOH
isoamylnitrite ~ )= NOH
-78~C ~ N or
R2 Na2S204/THF/H20
CH
\N ~ EDC, HOBT, TEA \ O O
~$ R1 z coo H ~ N~ZR1
R2
CA 02257948 1998-12-lo
W O 98l0040S PCTtUS97tlll31
- 33 -
SCHEME 14
H_~ DMF-DMA C\H3 O
~N H Toluene ~ N-Cbz
CH3
~NH2
CH3
/ EDC, HOBT, TEA
R1-Z-COOH
~NH ZR
CH3
SCHEME 15
~NH~ MCPBA
CA 02257948 1998-12-lo
W 098100405 PCTAUS97111131
- 34 -
SCHEME 16
CF3
H~ ~O I-cH2cF3 ~~
N Cs2CO3 \~\~ N
r~\ 1~
Y~ ~s
N3 KOtBu
CF3 CF3
O ~ O
N H2 H2, Pd/C~ N3
EtOH
\ D-Cbz-Phe-OH, EDC, HOBT
CF3
NH N H Cbz
H2, Pd/C, EtOH
CA 02257948 1998-12-lo
W O 98/00405 PCT~US97/11131
- 35 -
CF3
NH ~H.
g~ Separate Diastereomers
CF3 ~CF3
H- D-Phe-H ~ " H- D-Phe-H
1 ) PhNCS
2) TFA
CF3 CF3
O ~ O
~NH2 [~ NH2
~S ~
R-(CH2)nCOCI or
R-(CH2)nCO2H, EDC, HOBT
CF3 ~CF3
~NH~L(CH2)n-R ~ NJL(CH2)n-R
n=0,1, or 2 ~
R = O or ~--~ X X, Y= H, Cl, CF3, Br
CA 022~7948 1998-12-lo
W O98/0040S PCT~US97/11131
- 36 -
EXAMPLES
EXAMPLE 1
CH3
\ ~0 o
(3 R)
W
(E)-(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1,4-benzo-
diazepin-3 -yll -3 -phenyl -2-propenamide
A solution of (E)-3-phenyl-2-propenoyl chloride (367 mg,
2.2 mmol) in methylene chloride (1 mL) was added to a solution of
3(R)-amino- 1,3-dihydro- 1 -me~yl-5-phenyl-2H- 1,4-benzodiazepin-2-
one (J. Org. Chem. 1987, 52, 3232-3239) (531 mg, 2.0 mmol) and
trie~ylamine (307 mL, 225 mg, 2.2 mmol) in methylene chloride (10
mL). The mixture was stirred at room temperature for 25 min. and
the solvent was evaporated under reduced pressure. The residue was
purified by flash column chromatography on silica gel, eluting with
CH2C12/Et20 (95:5) and the residue was triturated with Et2O. The
solid was collected and dried in vacuo at 70~C to give (E)-(+)-N-[(3R)-
2,3-dihydro- 1 -methyl-2-oxo-5 -phenyl- 1 H- 1,4-benzodiazepin-3-yl] -3-
phenyl-2-propenamide as a colorless solid (170 mg, 21%), m.p. 140-
142~C, [oC]D +86.7~ (c=0.173, CH2C12).
dH (CDC13) 7.70-7.26 (16H, m), 6.63 (lH, d, J 15.6 Hz), 5.68 (lH, d, J
8.3 Hz), and 3.50 (3H, s).
Anal. Calcd. for C25H21N3O2Ø15 (C2H5)2O:
C, 75.63; H, 5.58; N, 10.33.
Found: C, 75.29; H, 5.57; N, 10.33%.
CA 02257948 1998-12-lO
W O 98/00405 PCT~US97/11131
- 37 -
Employing the procedure substantially as described above,
but substituting an appropriate acid chloride for the !E)-3-phenyl-2-
propenoyl chloride, the following compounds were prepared:
EXAMPLE 2
CH3
~0 o
(+)-N-[(3R)-2,3-Dihydro-1 -methyl-2-oxo-5-phenyl- 1 H- 1,4-benzo-
diazepin-3-yllben7.~mide
m.p. 224-225~C, [a]D +89.2~ (c = 0.141, CH2C12).
dH (CDC13) 8.04 (lH, d, J 8.1 Hz), 7.96 (2H, d, J 6.8 Hz), 7.64-7.36
(lOH,m),7.27(2H,t,J7.6Hz),5.74(1H,d,J7.8Hz),and3.51 (3H,
s).
Anal. Calcd. for C23Hl gN302Ø20H20:
C, 74.06; H, 5.24; N, 11.26.
Found: C, 74.13; H, 5.12; N, 11.16%.
EXAMPLE 3
N
CA 02257948 1998-12-10
WO 98/00405 PCT/US97/11131
- 3~ -
First diastereoisomer to elute:
(-)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1,4-
benzo-diazepin-3-yll(trans-2-phenyl- 1 -cyclopropane)carboxamide
m.p. 180-181~C, [oc]D -155.8~ ( c = 0.434, CH2C12).
dH (CDCl3) 7.62-7.09 (15H, m), 5.59 (lH, d, J 8.1 Hz), 3.47 (3H, s),
2.52-2.45 (lH, m), 1.90-1.84 (lH, m),1.69-1.56 (lH, m), and 1.38-1.32
(lH, m).
Anal. Calcd. for C26H23N3o2.o.25H2o:
C, 75.43; H, 5.72; N, 10.15.
Found: C, 75.38; H, 5.64; N, 9.94%.
Second diastereoisomer to elute:
(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1,4-
benzo-diazepin-3-yll(trans-2-phenyl- 1 -cyclopropane)carboxamide
m.p. 104-107~C, [oc~D +328.2~ (c = 0.098, CH2C12).
dH (CDC13) 7.62-7.13 (lSH, m), 5.60 (lH, d, J 8.3 Hz), 3.48 (3H, s),
2.59-2.54 (lH, m), 1.93-1.87 (lH, m),l.62-1.56 (lH, m, overlaps with
water), and 1.33-1.25 (lH, m).
Anal. Calcd. for C26H23N302Ø50H20Ø45PhCH3:
C, 76.13; H, 5.95; N, 9.14.
Found: C, 76.10; H, 5.94; N, 9.17%.
EXAMPLE 4
N ~3
CA 02257948 1998-12-10
WO 98/00405 PCT/US97/11131
- 39 -
(+)-N- [(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5 -phenyl- 1 H- 1,4-
benzodiazepin-3-yll - 1 H-indole-2-carboxamide
m.p. 167-177~C, [a]D +113~ (c = 1.103, CH2C12).
dH (CDC13) 9.15 (lH, br s), 8.10 (lH, d, J 9.0 Hz), 7.75-7.10 (14H, m),
5.75(1H,d,J9.OHz),and3.50(3H,s).
Anal. Calcd. for C25H2oN4o2:
C, 73.51; H, 4.94; N, 13.72.
Found: C, 73.31; H, 4.80; N, 13.62%.
EXAMPLE 5
~~
(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5 -phenyl- 1 H- 1,4-benzo-
diazepin-3-yllhept~n~mide
m.p. 49-54~C, [a]D +69.5~ (c=1.000, MeOH).
Anal. Calcd. for C23H27N3O2Ø40H20:
C, 71.81; H, 7.28; N, 10.92.
Found: C, 71.90; H, 7.09; N, 10.85%.
EXAMPLE 6
N'l--
CA 02257948 1998-12-10
WO 98/00405 PCI/US97/11131
- 40 -
(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- lH- 1,4-benzo-
diazepin-3-yllhexanamide
[a]D +72.6~ (c=0.920, MeOH).
Anal. Calcd. for C22H25N302:
C, 72.70; H, 6.93; N, 11.56.
Found: C, 72.44; H, 6.75; N, 11.25%.
EXAMPLE 7
I ~o O
~N
~3
(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1,4-benzo-
diazepin-3-yllpent~n~mide
[~C]D +68.2~ (c=1.310, MeOH).
Anal. Calcd. for C21H23N3O2Ø25CHC13:
C, 68.21; H, 6.26; N, 11.26.
Found: C, 68.2; H, 6.29; N, 11.17%.
CA 022~7948 1998-12-10
WO 98/00405 PCT/US97/11131
- 41 -
EXAMPLE 8
CH3
1 ~0 o
(+)-N-[(3R)-2,3-Dihydro-1 -methyl-2-oxo-5-phenyl-lH-1,4-benzo-
diazepin-3-yll -3-phenylpropanamide
Oxalyl chloride (158 mL, 230 mg, 1.81 mmol) was added
to a mixture of 3-phenylpropanoic acid (249 mg, 1.66 mmol) and DMF
(1 drop) in THF (10 mL) and the mixture was stirred at room tempera-
ture for 40 min. 3(R)-Amino-1,3-dihydro-1-methyl-S-phenyl-2H-1,4-
benzodiazepin-2-one (J. Or~. Chem. 1987, 52, 3232-3239) (400 mg,
1.51 mmol) and triethylamine (252 mL, 183 mg, 1.81 mmol) were
added and the mixture was stirred at room temperature for 18 h. The
mixture was poured into saturated aqueous sodium hydrogen carbonate
(20 mL) and extracted with ethyl acetate (3 x 20 rnL). The combined
organic fractions were dried (Na2so4) and the solvent was evaporated
under reduced pressure. The residue was purified by flash column
chromatography on silica gel, eluting with CH2cl2lEt2o (95:5) and the
residue was recrystallized from toluene/hexane to give (+)-N-[(3R)-2,3-
dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H-1,4-benzodiazepin-3-yl]-3-
phenylpropanamide as a colorless solid (380 mg, 63%), m.p. 179~C,
[a]D +100.4~ (c = 0.225, CH2c12).
dH (CDC13) 7.62-7.57 (2H, m), 7.47-7.21 (13H, m), 5.54 (lH, d, J 8.1
Hz), 3.47 (3H, s), 3.03 (2H, t, J 7.8 Hz), and 2.73-2.67 (2H, m).
Anal. Calcd. for C25H23N302Ø15H20:
C, 75.04; H, 5.87; N, 10.50.
Found: C, 75.06; H, 5.78; N, 10.55%.
CA 02257948 1998-12-10
WO g8/00405 PCT/US97/11131
- 42 -
Employing the procedure subst~nti~lly as described above,
but substituting an appropriate carboxylic acid for the 3-phenyl-
propanoic acid, the following compounds were prepared:
EXAMPLE 9
CH3
1~0 o C
[~ (3R)
E-(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-S-phenyl- 1 H- 1,4-benzo-
diazepin-3-yll -3-(3.4-dichlorophenyl)-2-propenamide
m.p. 145-147~C, [a]D +77.8~ (c=0.126, CH2C12).
dH (CDC13) 7.64-7.25 (14H, m), 6.61 (lH, d, J 15.6 Hz), 5.65 (lH, d, J
8.0 Hz), and 3.50 (3H, s).
Anal. Calcd. for C25Hl9N3o2cl2:
C, 64.67; H, 4.12; N, 9.05.
Found: C, 64.57; H, 4.25; N, 9.01%.
EXAMPLE 10
CH3
~N H i N~2
~3 (3R)
,
CA 02257948 1998-12-10
W 098/00405 PCTAUS97/11131
- 43 -
E-(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-S -phenyl- 1 H- 1 ,4-benzo-
diazepin-3-yll3 -(4-nitrophenyl)-2-propenamide
m.p. 165-166 ~C, [a]D +80.5~ (c=0.126, CH2Cl2).
dH (CDC13) 8.26 (lH, d, J 8.8 Hz), 7.74-7.28 (13H, m), 6.76 (lH, d, J
15.6 Hz), 5.66 (1H, d, J 8.0 Hz), and 3.51 (3H, s).
Anal. Calcd. for C25HlgN404:
C, 68.17; H, 4.58; N, 12.72.
Found: C, 68.25; H, 4.65; N, 12.57%.
EXAMPLE 1 1
CH3
¦ ~0 o C
E-(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1 ,4-benzo-
diazepin-3-yll -3 -(2~4-dichlorophenyl)-2-propenamide
m.p. 137-139~C, [oc]D +66.0~ (c=0.144, CH2C12).
dH (CDCl3) 8.02 (lH, d, J 15.6 Hz), 7.73-7.26 (13H, m), 6.66 (lH, d, J
15.6 Hz), 5.81 (lH, d, J 8.8 Hz), and 3.53 (3H, s).
Anal. Calcd. for C2sHl9cl2N3o2:
C, 64.67; H, 4.12; N, 9.05.
Found: C, 64.28; H, 4.24; N, 8.83%.
CA 02257948 1998-12-lo
W O 98/00405 PCTrUS97/11131
- 44 -
EXAMPLE 12
~3~ H iCH3
E-(+)-N-[(3R)-2,3-Dihydro-1 -methyl-2-oxo-5-phenyl-lH-1,4-
benzodiazepin-3 -yll-3-(4-methylphenyl)-2-propenamide
m.p. 133-135~C, [a]D +90.4~ (c=0.125, CH2C12).
dH(CDC13)7.68-7.19(15H,m),6.59(1H,d,J 15.6Hz),5.70(1H,d,J
8.0 Hz), 3.50 (3H, s), and 2.38 (3H, s).
Anal. Calcd. for C26H23N3o2:
C, 76.26; H, 5.66; N, 10.26.
Found: C, 75.93; H, 5.82; N, 10.10%.
EXAMPLE 13
CH3
~ ~~ ~ OCH3
E-(+)-N-[(3R)-2,3-Dihydro-l-methyl-2-oxo-5-phenyl-lH-1,4-benzo-
diazepin-3-yll -3 -(4-methoxyphenyl)-2-propenamide
m.p. 129-133~C, [a]D +89.9~ (c 0.188, CH2C12).
CA 02257948 1998-12-lO
W 0 98/00405 PCTAUS97/11131
- 45 -
dH (CDC13) 7.65-7.24 (14H, m), 6.92 (lH, d, J 8.8 Hz), 6.50 (lH, d, J
15.6 Hz), 5.69 (lH, d, J 8.0 Hz), 3.84 (3H, s), and 3.50 (3H, s).
Anal. Calcd. for C26H23N303Ø30H20:
C, 72.48; H, 5.52; N, 9.75.
Found: C, 72.75; H, 5.60; N, 9.36%.
EXAMPLE 14
H~
(+)-N-[(3R)-2,3 -Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1 ,4-benzo-
diazepin-3-yll-3-(2.4-dichlorophenyl)propanamide
m.p. 92-95~C, [a]D 90.5~ (c = 0.196, CH2C12).
dH (CDCl3) 7.62-7.15 (13H, m), 5.52 (lH, d, J 8.1 Hz), 3.47 (3H, s),
3.10(2H,t,J7.6Hz),and2.68(2H,dd,J7.6,2.8Hz).
Anal. Calcd. for C2sH2lcl2N3o2.o.2oH2o:
C, 63.89; H, 4.59; N, 8.94.
Found: C, 63.86; H, 4.62; N, 8.87%.
CA 02257948 1998-12-10
WO 98/00405 PCT/US97/11131
- 46 -
EXAMPLE lS
N H ~CI
~3
E-(~)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-S-phenyl- 1 H- 1 ,4-benzo-
diazepin-3 -yll -3 -(3-chlorophenyl)-2-propenamide
m.p. 229-231~C, [a]D +86.2~ (c = 0.225, CH2C12).
dH (CDC13) 7.64-7.26 (lSH, m), 6.62 (lH, d, J 15.6 Hz), 5.66 (lH, d, J
8.1 Hz), and 3.50 (3H, s).
Anal. Calcd. for C2~H20clN3O2:
C, 69.85; H, 4.69; N, 9.77.
Found: C, 70.20; H, 4.83; N, 9.41%.
EXAMPLE 16
CH3
1~0 o C
E-~)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5 -phenyl- 1 H- 1 ,4-benzo-
diazepin-3-yll-3-(2-chlorophenyl)-2-propenamide
m.p. 128-131~C, [a]D = +61.7~ (c = 0.196, CH2C12).
CA 02257948 1998-12-lo
W O 98/00405 PCTnUS97/11131
- 47 -
dH (CDC13) 8.06 (lH, d, J 15.6 Hz), 7.65-7.2~ (14H, m), 6.62, (lH, d, J
15.6Hz),5.68(1H,d,J8.3Hz),and3.50(3H,s).
Anal. Calcd. for C25H20ClN302Ø20H20:
C, 69.27; H, 4.74; N, 9.69.
Found: C, 69.21; H, 4.68; N, 9.45%.
EXAMPLE 17
CH3
N'J~
E-(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1 ,4-benzo-
diazepin-3 -yll -3 -(2.4-difluorophenyl)-2-propenamide
m.p. 121-123~C, [a]D +76.8~ (c = 0.111, CH2Cl2).
dH (CDCl3) 7.71 (lH, d, J 15.9 Hz), 7.64-7.24 (1 lH, m), 6.92-6.84 (2H,
m), 6.69 (lH, d, J 15.9 Hz), 5.67 (lH, d, J 8.1 Hz), and 3.50 (3H, s).
Anal. Calcd. for C2sHl9F2N3o2.o. l~H2~:
C, 69.31; H, 4.47; N, 9.70.
Found: C, 69.28; H, 4.57; N, 9.31%.
CA 02257948 1998-12-10
WO 98/0040S PCT/US97/11131
- 48 -
EXAMPLE 1
~ ~H~
(+)-N- [(3R)-2,3 -Dihydro- 1 -methyl -2-oxo-5 -phenyl- 1 H- 1 ,4-benzo-
diazepin-3-yll-3-(4-chlorophenyl)propanamide
m.p. 203-205~C, [a]D +99.2~ (c = 0.300, CH2C12).
dH (CDC13) 7.62-7.16 (14H, m), 5.52 (lH, d, J 8.1 Hz), 3.47 (3H, s),
2.99 (2H, t, J 7.7 Hz), and 2.67 (2H, t, J 7.7 Hz).
Anal. Calcd. for C25H22ClN3O2:
C, 69.52; H, 5.13; N, 9.73.
Found: C, 69.50; H, 5.15; N, 9.72%.
EXAMPLE 19
CH3
\_~~ o C
~3
E-(+)-N-[(3R)-2,3-Dihydro-l-methyl-2-oxo-5-phenyl-lH-1 ,4-benzo-
diazepin-3-yll -3-(2~6-dichlorophenyl)-2-propenamide
m.p. 121-124~C, [a]D +69.0~ (c = 0.342, CH2C12).
,
CA 02257948 1998-12-10
WO 98/00405 PCTtUS97/11131
- 49 -
dH (CDCl3) 7.79 (lH, d, J 16.1 Hz), 7.64-7.15 (13H, m), 6.78 (lH, d, J
15.8Hz),5.69(1H,d,J8.1 Hz),and3.50(3H,s).
Anal. Calcd. for C25HlgC12N3O2Ø15PhCH3:
C, 65.44; H, 4.23; N, 8.79.
Found: C, 65.40; H, 4.38; N, 8.85%.
EXAMPLE 20
~s N H ~CF
~3
E-(+)-N-[(3R)-2,3-Dihydro- I -methyl-2-oxo-S-phenyl-lH-1 ,4-benzo-
diazepin-3 -yll -3- l 4-(trifluoromethyl)phenyll -2-propenamide
m.p. 133-137~C, [a]D +68.7~ (c = O.l lS, CH2Cl2).
dH (CDC13) 7.72-7.25 (lSH, m), 6.71 (lH, d, J 15.6 Hz), 5.67 (lH, d, J
8.1 Hz), and 3.51 (3H, s).
Anal. Calcd. for C26H2oF3N3o2:
C, 67.38; H, 4.35; N, 9.07.
Found: C, 67.38; H, 4.45; N, 8.95%.
CA 02257948 1998-12-10
WO 98/00405 PCT/US97/11131
- 50 -
EXAMPLE 21
CH3
~N ~"' H ~
Cl
(+)-5 -Chloro-N- [(3R)-2,3 -dihydro - 1 -methyl -2-oxo-5-phenyl- 1 H- 1,4-
benzodiazepin-3-yllindole-2-carboxamide
m.p. 160-164~C, [a]D +103.8~ (c = 0.160, CH2C12).
dH (CDC13) 9.71 (lH, br s), 8.13 (lH, d, J 7.8 Hz), 7 68-7.09 (13H, m),
5.75 (lH, d, J 7.8 Hz), and 3.53 (3H, s).
Anal. Calcd. for C25H1gCIN402Ø25H20Ø15PhCH3:
C, 67.84; H, 4.49; N, 12.15.
Found: C, 67.80; H, 4.41; N, 12.07%.
EXAMPLE 22
~: N H ~3
(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1,4-benzo-
diazepin-3-yll-2.2-diphenyletll~n:~mide
m.p. 200-201~C, [a]D +97.0~ (c = 0.168, CH2C12).
CA 02257948 1998-12-10
WO 98/00405 PCT/IIS97/11131
dH (CDC13) 7.60-7.22 (20H, m), 5.58 (lH, d, J 8.1 Hz), 5.08 (lH, s),
and 3.44 (3H, s).
Anal. Calcd. for C30H25N3O2Ø1SPhCH3:
C, 78.79; H, S.SS; N, 8.88.
Found: C, 78.81; H, 5.63; N, 9.07%.
EXAMPLE 23
~ H ~ F
(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-S-phenyl- lH- 1,4-benzo-
diazepin-3 -yll -3 -(2~4-difluorophenyl)propanamide
m.p. 79-81~C, [oclD +92.9~ (c = 0.105, CH2C12).
dH (CDC13) 7.62-7.56 (3H, m), 7.50-7.19 (8H, m), 6.82-6.76 (2H, m),
5.52 (lH, d, J 8.1 Hz), 3.47 (3H, s), 3.01 (2H, t, ~ 7.6 Hz), and 2.69 (2H,
m).
Anal. Calcd. for C25H21F2N302:
C, 69.27; H, 4.88; N, 9.69.
Found: C, 68.96; H, 4.99; N, 9.47%.
CA 02257948 1998-12-10
W O 98/00405 PCTrUS97/11131
- 52 -
EXAMPLE 24
~N H
,~,
(+)-N-[(3R)-2,3-Dihydro-1-methyl-2-oxo-5-phenyl-lH-1,4-benzo-
diazepin-3-yll -2-phenyletll~n~mide
m.p. 241-242~C (dec.), [a]D +85.5~ (c = 0.159, CH2Cl2).
dH (CDCl3) 7.59-7.55 (3H, m), 7.46-7.22 (12H, m), 5.51 (lH, d, J
8.1Hz), 3.72 (2H, s), and 3.44 (3H, s).
Anal. Calcd. for C24H21 N3o2.o.ssH2o:
C, 73.28; H, 5.66; N, 10.68.
Found: C, 73.25; H, 5.38; N, 10.47%.
EXAMPLE 25
N H
~ Y
(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- l H- 1,4-benzo-
diazepin-3-yll -3-(2-chlorophenyl)propanamide
m.p. 158.5-159.5~C, [a]D +95.8~ (c = 0.224, CH2C12).
CA 02257948 1998-12-10
WO 98/0040S PCT/US97/11131
- 53 -
dH (CDC13) 7.62-7.57 (3H, m), 7.47-7.16 (1 IH, m), 5.55 (lH, d, J 8.1
Hz), 3.47 (3H, s), 3.14 (2H, t, J 7.9 Hz), and 2.75-2.69 (2H, m).
Anal. Calcd. for C25H22CIN302Ø15H20:
C, 69.09; H, 5.17; N, 9.67.
Found: C, 69.05; H, 5.12; N, 9.63%.
EXAMPLE 26
~N H ~--~CF3
(+)-N-[(3R)-2,3-Dihydro-l-methyl-2-oxo-S-phenyl-lH-1,4-benzo-
diazepin-3-yll-3-14-(trifluoromethyl)phenyllpropanamide
m.p. 175-176~C, [a]D +86.5~ (c = 0.141, CH2C12).
dH (CDC13) 7.62-7.54 (5H, m), 7.47-7.22 (9H, m), 5.52 (lH, d, J 8.1
Hz), 3.47 (3H, m), 3.08 (2H, t, J 7.6Hz), and 2.72 (2H, m).
Anal. Calcd. for C26H22F3N302Ø80H20:
C, 65.08; H, 4.93; N, 8.76.
Found: C, 65.03; H, 4.63; N, 8.72%.
CA 02257948 1998-12-10
WO 98/00405 PCTtUS97/11131
- 54 -
EXAMPLE 27
~N H CF3
,~
(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1,4-benzo-
diazepin-3-yll -2-r4-(trifluoromethyl)phenyllethanamide
m.p. 224-226~C, ~a]D +68.0~ (c = 0.153, CH2C12).
dH (CDC13) 7.63-7.55 (4H, m), 7.51-7.33 (8H, m), 7.26-7.23 (2H, m),
5.51 (lH, d, J 8.1 Hz), 3.77 (2H, s), and 3.46 (3H, s).
Anal. Calcd. for C25H20F3N3O2:
C, 66.51; H, 4.47; N, 9.31.
Found: C, 66.46; H, 4.36; N, 9.10%.
EXAMPLE 28
~N H ~CF3
~ Y
(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1,4-benzo-
diazepin-3-yll-3-~3-(trifluoromethyl)phenvllpropanamide
m.p. 135-136~C, ~a]D +78.8~ (c = 0.134, CH2C12).
CA 02257948 1998-12-10
WO 98/00405 PCT/US97/11131
dH (CDC13) 7.62-7.56 (3H, m), 7.49-7.22 (llH, m), 5.53 (lH, d, J 8.1
Hz), 3.47 (3H, s), 3.08 (2H, t, J 7.3 Hz), and 2.72 (2H, m).
Anal. Calcd. for C26H22F3N302:
C, 67.09; H, 4.76; N, 9.03.
Found: C, 67.03; H, 4.73; N, 9.13%.
EXAMPLE 29
¢~ N H ~~~
~ y
(+)-3-Cyclohexyl-N-[(3R)-2,3-dihydro- 1 -methyl-2-oxo-S-phenyl- 1 H-
1 4-benzodiazepin-3-yllpropanamide
m.p. 144.5-145.5~C, [oc~D +83.1~ (c = 0.116, CH2C12).
dH (CDC13) 7.62-7.56 (3H, m), 7.46-7.21 (7H, m), 5.55 (lH, d, J 8.3
Hz), 3.48 (3H, s), 2.41-2.36 (2H, m), 1.77-1.58 (7Hs m), 1.31-1.16 (4H,
m), and 0.98-0.90 (2H, m).
Anal. Calcd. for C25H29N302:
C, 74.41; H, 7.24; N, 10.41.
Found: C, 74.46; H, 7.27; N, 10.58%.
CA 02257948 1998-12-lo
W O 98/00405 PCTrUS97/11131
- 56 -
EXAMPLE 30
CH3
~
(+)-N-[(3R)-2,3 -Dihydro- 1 -methyl-2-oxo-5 -phenyl- 1 H- 1 ,4-benzo-
diazepin-3-yll-3-1 2-(trifluoromethyl)phenyllpropanamide
m.p. 1 10-11 3~C, [oc]D +79.2~ (c = 0.376, CH2C12).
dH (CDC13) 7.65-7.57 (4H, m), 7.50-7.22 (lOH, m), 5.55 (lH, d, J 8.0
Hz), 3.47 (3H, s), 3.20 (2H, t, J 7.9 Hz), and 2.70 (2H, dt, J 7.9, 3.3 Hz).
Anal. Calcd. for C26H22F3N3o2:
C, 67.09; H, 4.76; N, 9.03.
Found: C, 66.97; H, 4.76; N, 8.93%.
EXAMPLE 3 1
CH3
H iCN
(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- lH- 1 ,4-benzo-
diazepin-3 -yll -3 -(4-cyanophenyl)propanamide
m.p. 81-85~C, [a]D +91.0~ (c = 0.11 1, CH2C12).
CA 02257948 1998-12-10
WO 98/00405 PCTtUS97/11131
- 57 -
dH (CDC13) 7.64-7.55 (4H, m), 7.48-7.16 (lOH, m), 5.50 (lH, d, J 8.3
Hz), 3.47 (3H, s), 3.08 (2H, t, J 7.6 Hz), and 2.74-2.69 (2H, m).
Anal. Calcd. for C26H22N402Ø60H20Ø50PhCH3:
C, 73.93; H, 5.62; N, 11.69.
Found: C, 73.98; H, 5.61; N, 11.71%.
EXAMPLE 32
CH3
N ~CI
,~
(+)-N- [(3R)-2,3 -Dihydro- 1 -methyl-2-oxo-5 -phenyl- 1 H- I ,4-benzo-
diazepin-3-yll-3-(3-chlorophenyl)propanamide
m.p. 157-159~C, [a]D +90.7~ (c = 0.134, CH2C12).
dH (CDC13) 7.62-7.57 (3H, m), 7.47-7.12 (1 lH, m), 5.53 (lH, d, J 8.1
Hz), 3.47 (3H, s), 3.00 (2H, t, J 7.3 Hz), and 2.71-2.66 (2H, m).
Anal. Calcd. for C2sH22clN3o2.o.s5H2o:
C, 67.96; H, 5.27; N, 9.51.
Found: C, 67.99; H, 5.18; N, 9.26%.
CA 02257948 1998-12-lo
W O 98/00405 PCTrUS97/11131
- 5~s -
EXAMPLE 33
¢~ N H ~3
~Y
E-(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1 ,4-benzo-
diazepin-3-yll-3-(2-bromophenyl)-2-propenamide
m.p. 1 13-1 1 6~C, [oC]D +44.2~ (c = 0.1 13, CH2Cl2).
dH (CDCl3) 8.03 (lH, d, J 15.6 Hz), 7.64-7.16 (14H, m), 6.57 (lH, d, J
15.6 Hz), 5.68 (lH, d, J 8.1 Hz), and 3.50 (3H, s).
Anal. Calcd. for C25H20BrN302Ø60H20Ø30PhCH3:
C, 63.48; H, 4.58; N, 8$.19.
Found: C, 63.49; H, 4.38; N, 8.19%.
EXAMPLE 34
CH3
~ ~O o Br
E-(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1 ,4-benzo-
diazepin-3-yll-3 -(3-bromophenyl)-2-propenamide
m.p. 221-223 d~C, [a]D +65.5~ (c = 0.206, CH2C12)-
CA 02257948 1998-12-10
W O 98/00405 PCTrUS97/11131
_ 59 _
dH (CDC13) 7.69 (lH, br s), 7.64-7.57 (4H, m), 7.51-7.37 (6H, m),
7.29-7.19 (4H, m), 6.62 (lH, d, J 15.6 Hz), 5.66 (lH, d, J 8.1 Hz), and
3.50 (3H, s).
Anal. Calcd. for C25H20BrN302Ø35H20Ø20PhCH3:
C, 63.54; H, 4.46; N, 8.42.
Found: C, 63.50; H, 4.39; N, 8.42%.
EXAMPLE 35
CH3
N~
~Y
E-(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-S-phenyl- 1 H- 1 ,4-benzo-
diazepin-3-yll -3-(4-iodophenyl)-2-propenamide
m.p. 137-140~C, [a~D +67.9~ (c = 0.268, CH2Cl2).
dH (CDCl3) 7.75-7.72 (2H, m), 7.64-7.36 (gH, m), 7.29-7.16 (SH, m),
6.63 (lH, d, J 15.6 Hz), 5.66 (lH, d, J 8.1 Hz), and 3.50 (3H, m).
Anal. Calcd. for C25H20IN3o2.o.3ophcH3:
C, 59.29; H, 4.06; N, 7.65.
Found: C, 59.29; H, 3.90; N, 7.40%.
EXAMPLE 36
CA 02257948 1998-12-10
WO 98/00405 PCT/US97/11131
- 60 -
CH3
~;~~ ~ Br
E-(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-S-phenyl- 1 H- 1,4-benzo-
diazepin-3-yll-3-(4-bromophenyl)-2-propenamide
m.p. 121-124~C, [oc]D +75.6~ (c = 0.201, CH2C12).
dH (CDC13) 7.64-7.57 (3H, m), 7.55-7.35 (1 lH, m), 7.28-7.24 (lH, m),
6.62 (lH, d, J 15.6 Hz), 5.66 (lH, d, J 8.1 Hz), and 3.50 (3H, s).
Anal. Calcd. for C25H2oBrN3o2:
C, 63.30; H, 4.25; N, 8.86.
Found: C, 63.50; H, 4.20; N, 8.78%.
EXAMPLE 37
~N H
,~
Y
(+)-N-[(3R)-2,3 -Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1,4-benzo-
diazepin-3-yll -4-phenylbut~n~mide
m.p. 65-74~C, [a]D +77.4~ (c = 0.155, CH2C12).
dH (CDC13) 7.62-7.56 (3H, m), 7.46-7.19 (12H, m), 5.55 (lH, d, J 8.1
Hz), 3.47 (3H, s), 2.71 (2H, t, J 7.6 Hz), 2.42-2.37 (2H, m), and 2.09-
2.01 (2H, m).
. .
CA 02257948 1998-12-10
WO 98/00405 PCT/US97/11131
- 61 -
Anal. Calcd. for C26H25N3o2~o~3oH2o:
C, 74.91; H, 6.19; N, 10.08.
Found: C, 74.93; H, 6.05; N, 10.07%.
EXAMPLE 38
N~
g~
(+)-N- [(3R)-2,3 -Dihydro- 1 -methyl-2-oxo-5 -phenyl- 1 H- 1,4-benzo-
diazepin-3 -yll -5 -methyl -3 -phenylisoxazole-4-carboxamide
m.p. 123-126~C, [a]D +122.0~ (c = 0.199, CH2Cl2).
dH (CDCl3) 7.79-7.76 (2H, m), 7.62-7.32 (l lH, m)5 7.26-7.21 (2H, m),
5.61 (lH, d, J 7.9 Hz), 3.42 (3H, s), and 2.76 (3H, s).
Anal. Calcd. for C27H22N403Ø40H20:
C, 70.85; H, 5.02- N, 12.24.
Found: C, 70.84; H, 4.91; N, 11.92%.
EXAMPLE 39
CH3
1~ ,N~--~CN
- I 11
CA 02257948 1998-12-lo
W O 98/00405 PCTnUS97/11131
- 62 -
(+)-N- [(3R)-2 ,3 -Dihydro- 1 -methyl-2-oxo-S -phenyl- 1 H- 1 ,4-benzo-
diazepin-3-yll -3-(3 -cyanophenyl)propanamide
m.p. 110-112~C, [a]D +~4.2~ (c = 0.202, CH2Cl2).
dH (CDC13) 7.63-7.22 (14H, m), 5.51 (lH, d, J 8.1 Hz), 3.47 (3H, s),
3.06 (2H, t, J 7.8 Hz), and 2.74-2.6~s (2H, m).
Anal. Calcd. for C26H22N4o2.o.5oH2o:
C, 72.37; H, 5.37; N, 12.98.
Found: C, 72.52; H, 5.12; N, 12.59%.
EXAMPLE 40
~~
H ~
(+)-N-[(3R)-2,3 -Dihydro- 1 -methyl-2-oxo-5 -phenyl- lH- 1 ,4-benzo-
diazepin-3-yllcyclohexaneth~n~mide
m.p. 144-146~C, [a]D +72.1~ (c=1.000, MeOH).
Anal. Calcd. for C24H27N302Ø20H20:
C, 73.33; H, 7.03; N, 10.69.
Found: C, 73.27; H, 7.02; N, 10.76%.
CA 02257948 1998-12-10
WO 98/00405 PCT/US97/11131
- 63 -
EXAMPLE 41
", 1 ~0
~3
(+)-4-Cyclohexyl-N-[(3R)-2,3-dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H-
1 .4-benzodiazepin-3-yllbllt~n~mide
[a]D +57.7~ (c=0.440, MeOH).
Anal. Calcd. for C26H31 N3O2:
C, 74.79; H, 7.48; N, 10.06.
Found: C, 74.8;0 H, 7.78; N, 10.05%.
EXAMPLE 42
~5~N~
(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-S-phenyl- lH- 1,4-benzo-
diazepin-3-yll -4-methylpent~n~mide
m.p. 123-125~C, [a]D +66.8~ (c=0.500, MeOH).
Anal. Calcd. for C22H25N302Ø45H20:
C, 71.12; H, 7.03; N, 11.31.
~ound: C, 71.08; H, 6.81; N, 11.42%.
CA 022~7948 1998-12-10
WO 98/00405 PCTIUS97/11131
- 64 -
EXAMPLE 43
H3C ~ /~
,NH
~N H
~d (R)
(+)-N-[(3R)-2,3 -Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1,4-benzo-
diazepin-3 -yll -2.3 -dihydrobenzofuran-2-carboxamide
Diisopropylethylamine (0.3 mL, 223 mg, 1.72 mmol) was
added to a stirred, cooled (0 ~C) solution of 3(R)-amino-1,3-dihydro-l-
methyl-5-phenyl-2H-1,4-benzodiazepin-2-one (J. Or~. Chem. 1987, 52,
3232-3239) (400 mg, 1.5 mmol), 2,3-dihydrobenzofuran-2-carboxylic
acid (274 mg, 1.7 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodi-
imide hydrochloride (583 mg, 3.0 mmol), and 1-hydroxybenzotriazole
(479 mg, 3.1 mmol) in DMF (4.5 mL). The mixture was stirred at
room temperature for 18 h., poured into aqueous hydrochloric acid
(3M, 12 mL) and extracted with ethyl acetate (3 x 20 mL). The
combined organic fractions were washed with saturated aqueous sodium
hydrogen carbonate (20 mL) and brine (20 mL), dried (MgSO4) and
evaporated under reduced pressure. The residue was crystallized from
2-chloro-2-methylpropane~exane to give (+)-N-[(3R)-2,3-dihydro-l-
methyl-2-oxo-5-phenyl- 1 H- 1,4-benzodiazepin-3 -yl] -2,3-dihydrobenzo-
furan-2-carboxamide as a colorless solid (156 mg, 25%), m.p. 141-
180~C, [a]D ~ 127.1 ~ (c=0.425, CHCl3).
dH (CDCl3) (3:1 Mixture of diastereoisomers) 8.44 (lH, m), 7.65-6.91
(13H, m), 5.52 (lH, m), 5.28 (lH, m), and 3.70-3.40 (5H, m).
Anal. Calcd. for C25H2lN3o3.o.25 Hexane
C, 73.50; H, 5.70; N, 9.71.
Found: C, 74.12; H, 5.57; N, 9.71%.
CA 022~7948 1998-12-10
WO 98/00405 PCT/US97tlll31
- 65 -
EXAMPLE 44
(+)-N-[(3R)-2,3-dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- l ,4-benzo-
diazepin-3 -yl] - 1 '-(1,1 -dimethylethoxycarbonyl)spiro(cyclohexan-4,4'-
piperidine)- 1 -carboxamide
Step A:
EtO2C CO2Et
N
Diethyl l-benzylpiperidine-4.4-diacetate
Ethanol (120 mL) was cooled in ice and ammonia bubbled
through to give a saturated solution. 1-Benzyl-4-piperidone (40.0g,
21 lmmol) and ethyl cyanoacetate (47.8g, 423 mmol) were added, the
reaction vessel stoppered and stored at O C overnight. The solid was
collected, washed with ethanol and ether and dried in vacuo to give a
yellow solid (68.86g). The solid (58.86g) was dissolved in a mixture of
sulfuric acid (70 mL, 98%) and water (60 mL) and heated under reflux
for three days the mixture cooled and most of the ~vater evaporated. The
residue was azeotroped with ethanol (4x750 mL), further ethanol (SOO
mL) added and the mixture heated under reflux for 20h, cooled in ice
and sodium carbonate (lOOg) added slowly with vigorous stirring. The
ethanol was evaporated under reduced pressure, water (800 mL) added
and the mixture extracted with methylene chloride (3x400 mL). The
combined organic extracts were dried (Na2so4) and the solvent
evaporated to give diethyl l-benzylpiperidine-4,4-diacetate (37.51g).
A small portion of this was purified by flash column chromatography.
CA 022~7948 1998-12-10
W 098/00405 PCT~US97tlll31
- 66 -
NMR (300 MHz, CDC13) d: 7.2-7.4 (m, SH), 4.11 (q, J=7.3Hz,4H), 3.50
(s, 2H), 2.56 (s, 4H), 2.4 (m, 4H), 1.7 (m, 4H), 1.24 (t, J=7.3Hz, 6H).
Step B:
HO ,~ OH
N~
Ph
1 -Benzylpiperidine-4~4-diethanol
A solution of the diester (12.2 g, 35 mrnol) in ether (25
mL) was added to a cooled (-30~C) and stirred suspension of LiAlH4
(2.1 g, 55 mmol) in ether (400 mL), under argon. THF (60 mL) was
added and the reaction mixture allowed to warm to room temperature.
After recooling to 0~C, water (2.2 mL), lM NaOH (4.4 mL) and water
(S mL) were added, the reaction mixture stirred vigorously for 30 min
and the solid filtered off, washing well with ether. The combined
filtrates were evaporated to afford a white solid which was tritutrated
with ether to give 8 g of l-benzylpiperidine-4,4-diethanol.
m.p. 75-78~C
NMR (300 MHz, CDCl3) d: 7.2-7.4 (m, 5H), 3.7 (t, J = 6.8 Hz, 4H),
3.52 (s, 2H), 2.7 (brs, 2H), 2.43 (m, 4H), 1.66 (t, J = 6.8 Hz, 4H), 1.5
(m, 4H).
Step C:
HO~S OH
N
BOC
CA 022~7948 1998-12-10
WO 98/0040S PCT/US97/11131
- 67 -
l -t-Butoxycarbonylpiperidine-4.4-diethanol
The benzylamine (2.07 g, 7.9 rnmol) was dissolved in
methanol (60 mL), BOC2O (1.72 g, 7.9 mmol) added and the mixture
hydrogenated at 50 psi over 10% palladium hydroxide on charcoal (200
mg) for 1~ hours. The reaction mixture was filtered through celite,
washed with methanol and the filtrate evaporated to give 1-t-butoxy-
carbonylpiperidine-4,4-diethanol (2.0 g).
NMR (300 MHz, CDC13) d: 3.7 (m, 4H), d 3.3 (m, 6H), 1.65 (t, J = 6.8
Hz, 4H), 1.41 (s, 9H).
Step D:
MeO2SO x~OSO2Me
BOC
l-t-Butoxycarbonylpiperidine-4~4-diethanoL bis(methanesulfonate)
The diol (2.41 g, 8.9 mrnol) was dissolved in dichloro-
methene (50 mL), the solution cooled to -20~C under argon before
addition of triethylamine (3.7 mL, 26 mmol) and methanesulfonyl
chloride (1.6 mL, 20 mmol). After 30 min., the reaction mixture
was poured into ice cold 10% citric acid and extracted with ether (X3).
The combined extracts were washed with water, saturated NaHCO3 and
brine, dried (MgSO4) and the solvent evaporated to afford 1-t-butoxy-
carbonylpiperidine-4,4-diethanol, bis(methanesulfonate) (3.2g).
NMR (300 MHz, CDCl3) d: 4.32 (t, J = 7.1 Hz, 4H), 3.4 (m, 4H), 3.04
(s, 6H), 1.89 (t, J = 7.1 Hz, 4H).
CA 02257948 1998-12-lo
W O 98/00405 PCTrUS97/11131
- 68 -
Step E:
EtOOC~OOEt
BOC
Diethyl 3-t-butyloxycarbonyl-3-azaspiror5.51undecane-9.9-dicarboxylate
To a slurry of 60% NaH (2.04 g, 0.51 mole) in toluene
(160 mL), under argon, was slowly added diethyl malonate (3.72 mL,
24.3 mmol). The mixture was cooled to 0~C and the bis-mesylate 1 (7.0
g, 16.3 mmol) added as a solid and the mixture heated to reflux for 18
hours. The reaction was quenched into 10% citric acid (100 mL) and
the product extracted with CH2C12 (2x150 mL). The extracts were
dried (Na2SO4), concentrated to an oil, and chromatographed on silica
to give 3.83 g (60% ) of diethyl 3-t-butyloxycarbonyl-3-azaspiro-
[S .S]undecane-9,9-dicarboxylate .
lH NMR (CDC13) d: 1.22 (t, 6H), 1.4 (s, 9H), 2.0 (m, 4H), 3.35 (m,
4H), 4.2 (q, 4H).
Step F:
~OH
~N
BOC
CA 022~7948 1998-12-10
WO 98/0040S PCT/US97/11131
- 69 -
3-t-Butyloxycarbonyl-3-azaspiror5.5lundecane-9-carboxylic acid
To a solution of the diester 2 (3.69 g, 0.0093 m) in TH~
(50 mL) was added lN LiOH (47 mL). The reaction was stirred for 3
days at 25~C, diluted with water (50 mL) and pH adjusted to 2.2 with
KHSO4. The product was extracted into ethyl acetate (2x75 mL), dried
(Na2SO4), and concentrated to a foam (3.5 g). The solid was melted in
a flask at 140~C for 2 hours, cooled and the oil dissolved in THF (15
mL), lN LiOH (10 mL) added and mixture stirred overnight at 30~C.
The reaction was concentrated to remove THF, diluted with water (20
mL) and washed with diethyl ether (10 mL). The pH was adjusted to
2.5 with KHSO4 and product extracted (3xS0 mL) with ethyl acetate.
The extracts were dried (Na2so4)~ filtered and concentrated to yield 3-
t-butyloxycarbonyl-3-azaspiro[5.5]undecane-9-carboxylic acid as a foam
(2.48 g, 90%).
1 H NMR (CDCl3, partial) d: l .45 (s, 9H), 3.4 (m, 4H).
Employing the procedure substantially as described in
Example 43 but substituting an appropriate acid for the 2,3-dihydro-
benzofuran-2-carboxylic acid, the following compounds were prepared:
Step G:
CH3
H ~ CH3
(+)-N-[(3R)-2,3-Dihydro-1 -methyl-2-oxo-5-phenyl-lH-1 ,4-benzo-
diazepin-3-yl]- l '-( l ,1 -dimethylethoxycarbonyl)spiro(cyclohexan-4,4'-
piperidine)- l -carboxamide
m.p. 135-138~C, fa]D +58.8~ (C=0.925, CHCl3).
CA 02257948 1998-12-10
W 098/00405 PCT~US97/11131
- 70 -
dH (CDCl3) 7.61-7.23 (lOH, m), 5.54 (lH, d, J 9.0 Hz), 3.47 (3H, s),
3.37 (4H, m), 2.28 (lH, m), and 1.81-1.18 (21H, s).
Anal. Calcd. for C32H40N404:
C, 70.56; H, 7.40; N, 10.29.
Found: C, 70.21; H, 7.40; N, 10.16%.
EXAMPLE 45
O~ ~
1~ (R)
. ~
(+)-N- [(3R)-2,3 -Dihydro- 1 -methyl-2-oxo-5 -phenyl -1 H -1 ,4-benzo-
diazepin-3-yll-3-(furan-2-yl)propanamide
m.p. 115-118~C, [a~D +65.8~ (c=0.800, CHCl3).
dH (CDCl3) 7.62-7.26 (1 lH, m), 6.28 (lH, dd, J 3.2, 2.0 Hz), 6.08 (lH,
dd, J 3.2, 0.7 Hz), 5.58 (lH, d, J 8.1 Hz), 3.48 (3H, s), 3.04 (2H, t, J 7.6
Hz), and 2.75 (2H, m).
Anal. Calcd. for C23H21N303Ø3Hexane:
C, 72.07; H, 6.15; N, 10.17.
Found: C, 71.78; H, 6.30; N, 9.77%.
CA 02257948 1998-12-10
WO 98/00405 PCT/US97/11131
EXAMPLE 46
N~~
H
~ Y
(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1,4-benzo-
diazepin-3-yll-4-(2-thienyl)b~lt~n~mide
m.p. 170-180~C, [a]D +63.5~ (c=l .OOO, MeOH).
Anal. Calcd. for C24H23N302SØ95H20:
C, 66.32; H, 5.77; N, 9.67.
Found: C, 66.32; H, 5.34; N, 9.40%.
EXAMPLE 47
,~ ~ ,N
~0
(+)-N-I (3R)-2,3-Dihydro- l -methyl-2-oxo-S-phenyl- 1 H- 1,4-benzo-
diazepin-3-yllcyclohexylcarboxamide
m.p. 213-214~C, [a]D +62.4~ (c=l.OOO, MeOH).
Anal. Calcd. for C23H24N302:
C, 73.77; H, 6.46; N, 11.22.
Found: C, 73.86; H, 6.81; N, 11.15%.
CA 02257948 1998-12-10
WO 98100405 PCT/US97/11131
- 72 -
EXAMPLE 48
~N H ~~
(E)-(+)-N-[(3R)-2,3 -Dihydro- 1 -methyl-2-oxo-5 -phenyl- 1 H- 1,4-benzo-
diazepin-3-yll-3-(3,4-methylenedioxyphenyl)-2-propenamide
m.p. 143-145~C, [a]D +62.3~ (c=0.960, MeOH).
Anal. Calcd. for C25H2lN3o4.o.loH2o.o.2oEt2o:
C, 69.78; H, 5.27; N, 9.46.
Found: C, 69.78; H, 4.98; N, 9.28%.
EXAMPLE 49
~N H~¢N~
(+)-N- [(3R)-2,3 -dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1,4-benzo-
diazepin-3-yll -2-quinoxalinecarboxamide
[a]D +85.8~ (c=0.360, MeOH).
Anal. Calcd. for C25HlgN5O2:
C, 69.96; H, 4.90; N, 15.33.
CA 022~7948 1998-12-lo
W O 98t00405 PCTrUS97/11131
- 73 -
Found: C, 69.95; H, 4.72; N, 15.25%.
EXAMPLE 50
(+)-N-[(3R)-2,3 -Dihydro-2-methyl-2-oxo-5-phenyl- 1 H- 1,4-benzo-
diazepin-3-yll -2-(phenylamino)acetamide
Step A:
CH3
~"" N'~' Br
~ H
N-[(3R)-2,3-Dihydro-1 -methyl-2-oxo-5-phenyl-lH-1,4-benzodiazepin-
3-yll -2-bromoacetamide
Bromoacetyl bromide (165 mL, 383 mg, 1.9 mmol) was
added to an ice cooled solution of 3(R)-amino-1,3-dihydro-1-methyl-5-
phenyl-2H-1,4-benzodiazepin-2-one (J. Org. Chem. 1987, 52, 3232-
3239) (500 mg, 1.88 mmol) and triethylamine (264 mL, 192 mg, 1.9
mmol) in methylene chloride (10 mL) and the mixture was stirred at
room temperature for 1 h. The mixture was washed with water (3 x 10
mL), dried (MgSO4) and the solvent was evaporated -under reduced
pressure to give N-[(3R)-2,3-dihydro-1-methyl-2-oxo-5-phenyl-lH-1,4-
benzodiazepin-3-yl]-2-bromoacetamide as a colorless foam (760 mg,
100%).
dH (CDC13) 8.24 (lH, d, J 7.8 Hz), 7.64-7.24 (9H, m), 5.48 (lH, d, J
~ 7.8 Hz), 4.00 (2H, m), and 3.50 (3H, s).
CA 022~7948 1998-12-lo
W O 98/00405 PCTAUS97/11131
- 74 -
.
Step B:
CH3 o
N-I--CH2--NH~
(+)-N-~(3R)-2,3 -Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1,4-benzo-
diazepin-3-yll -2-(phenylamino)acetamide
Aniline (297 mL, 304 mg, 3.26 mmol) was added to a
solution of N- [(3R)-2,3 -dihydro- l -methyl -2-oxo-5 -phenyl- 1 H- 1,4-
benzodiazepin-3-yl]-2-bromoacetamide (600 mg, 1.55 mmol) in ethanol
(25 mL) and the mixture was heated under reflux for 24 h. The
mixture was cooled and the solid was collected and recrystallized from
ethanol (20 mL) to give (+)-N-[(3R)-2,3-dihydro-1-methyl-2-oxo-5-
phenyl- 1 H- 1,4-benzodiazepin-3-yl} -2-(phenylamino)acetamide as a
colorless solid (500 mg, 81%), m.p. 245-246~C, [a]D +119~ (C=0.850,
CHCl3).
dH (CDCl3) 8.26 (lH, d, J 8.3 Hz), 7.63-7.20 (12H, m), 6.81 (lH, t, J
7.3 Hz), 6.72 (2H, d, J 7.6 Hz), 5.56 (lH, d, J 8.3 Hz), 3.95 (2H, d, J 1.5
Hz), and 3.45 (3H, s).
Anal. Calcd. for C24H22N4o2:
C, 72.34; H, 5.57; N, 14.06.
Found: C, 72.37; H, 5.59; N, 14.32%.
Employing the procedure subst~nti~lly as described above,
but substituting 2-chloroaniline or 4-(trifluoromethyl)aniline for the
aniline, the following compounds were prepared:
CA 02257948 1998-12-10
W O 98/00405 PCT~US97tlll31
EXAMPLE 51
CH3
~" NJ~ CH' N~
/~ Cl
(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1,4-benzo-
diazepin-3-yll -2-(2-chlorophenylamino)acetamide
m.p. 222-224~C, [a]D +111~ (c=0.973, CHC13).
dH (CDC13) 8.15 (lH, d, J 8.3 Hz), 7.60-7.16 (12H, m), 6.71 (2H, m),
5.57 (lH, d, J 8.3 Hz), 4.01 (2H, d, J 2.7 Hz), and 3.45 (3H, s).
Anal. Calcd. for C24H21 ClN402:
C, 66.59; H, 4.89; N, 12.94.
Found: C, 66.40; H, 4.94; N, 12.92~o.
EXAMPLE 52
CH3
~$ N ~ N ~C~
(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1,4-benzo-
diazepin-3-yll-2-~4-(trifluoromethyl)phenylaminolacetamide
m.p. 218-219~C, [oc]D +91.9~ (c = 0.419, CHC13).
CA 022~7948 1998-12-lo
W 098/00405 PCTrUS97/11131
- 76 -
dH (CDC13) 8.13 (lH, d, J 9.0 Hz), 7.70-7.25 (12H, m), 6.72 (2H, d, J
8.7 Hz), 5.60 (lH, d, J 9.0 Hz), 4.05 (2H, m), and 3.50 (3H, s).
Anal. Calcd. for C25H2lF3N4o2.o.7H2o:
C, 62.68; H, 4.71; N, 11.69.
Found: C, 62.47; H, 4.32; N, 11.44%.
EXAMPLE 53
CH3
" N~~~
H
~ Y
(+)-N-[(3R)-2,3 -Dihydro- l -methyl-2-oxo-5 -phenyl- 1 H- 1,4-benzo-
diazepin-3-yll -2-(phenoxy)acetamide
Phenol (104 mg, 1.1 mmol) was added to a suspension of
sodium hydride (60% dispersion in mineral oil, 44 mg, 1.1 mmol) in
toluene (10 mL). When hydrogen evolution had stopped, N-[(3R)-2,3-
dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1,4-benzodiazepin-3-yl] -2-bromo-
acetamide (400 mg, 1.04 mmol) was added and the mixture was stirred
at room temperature for 18 h. The mixture was washed with water (3 x
15 mL), dried (MgSO4) and the solvent was evaporated under reduced
pressure. The residue was triturated with 2-propanol and the solid was
collected and recrystallized from 2-propanol (5 mL) to give (+)-N-
[(3R)-2,3-dihydro- l -methyl-2-oxo-5-phenyl- lH- 1,4-benzodiazepin-3-
yl]-2-(phenoxy)acetamide as a colorless solid (112 mg, 27%), m.p. 126-
128~C, [a]D +81.6 (C=0.692, CHCl3).
dH (CDCl3) 8.49 (lH, d, J 8.2 Hz), 7.64-7.01 (14H, m), 5.61 (lH, d, J
8.2 Hz), 4.65 (lH, d, J 14.6 Hz), 4.58 (lH, d, J 14.6 Hz), and 3.50
(3H, s).
~ . ,, ~,
CA 02257948 1998-12-10
WO 98/00405 PCTtUS97/11131
Anal. Calcd. for C24H21N303:
C, 72.17; H, 5.30; N, 10.52.
Found: C, 71.84; H, 5.25; N, 10.41 %.
Employing the procedure substantially as described above,
but substituting 2,4-dichlorophenol, thiophenol or 2,4-dichloro-
thiophenol for the phenol, the following compounds were prepared:
EXAMPLE 54
CH3
N~,O~(',I
~ H
(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- lH- 1,4-benzo-
diazepin-3-yll-2-(2 4-dichlorophenoxy)acetamide
m.p. 206~C, [oC]D +31.1~ (c=0.289, CHC13).
dH (CDC13) 8.75 (lH, d, J 9.0 Hz), 7.65-7.20 (1 lH, m), 6.90 (lH, d, J
8.7 Hz), 5.60 (lH, d, J 9.0 Hz), 4.65 (2H, m), and 3.50 (3H, s).
Anal. Calcd. for C24Hl9cl2N3o3.o.3H2o:
C, 60.85; H, 4.17; N, 8.87.
Found: C, 60.80; H, 4.04; N, 8.87%.
CA 02257948 1998-12-10
WO 98/00405 PCTtUS97/11131
- 78 -
EXAMPLE 55
~~
H
~ '~
(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- lH- 1 ,4-benzo-
diazepin-3 -yll -2-(phenylthio)acetamide
[a]D +104.9~ (c=0.316, CHC13).
dH (CDCl3) 8.50 (lH, d, J 9.0 Hz), 7.60-7.20 (14H, m), 5.50 (lH, d, J
9.0 Hz), 3.75 (2H, m), and 3.45 (3H, s).
Anal. Calcd. for C24H21N3O2S:
C, 69.37; H, 5.10; N, lO.l l.
Found: C, 68.98; H, 5.06; N, 9.76%.
EXAMPLE 56
~S~CI
~o~ H Cl
~ Y
(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl-] H- l ,4-benzo-
diazepin-3 -yll -2-(2 ~4-dichlorophenylthio)acetamide
[a]D +97.4~ (c=0.286, CHC13).
CA 022~7948 1998-12-10
WO 98/00405 PCT/US97/11131
- 7~ -
dH (CDC13) 8.35 (lH, d, J 9.0 Hz), 7.70-7.20 (12H, m), 5.50 (lH, d, J
9.0 ~z), 3.70 (2H, m), and 3.50 (3H, s).
Anal. Calcd. for C24H19Cl2N3O2S:
C, 59.51; H, 3.95; N, 8.67.
Found: C, 59.32; H, 3.95; N, 8.65%.
EXAMPLE 57
H 1
,~
(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5 -phenyl- 1 H- 1,4-benzo-
diazepin-3-yll -3-(phenylamino)propanamide
3-Bromopropionyl chloride (2.01 mL, 3.428 g, 20 mmol)
was added to an ice cooled solution of 3(R)-amino-1,3-dihydro-1-
methyl-5-phenyl-2H-1,4-benzodiazepin-2-one (J. Or~,. Chem. 1987, 52,
3232-3239) (5.0 g, 18.8 mmol) and triethylamine (2.79 mL, 2.02 mg,
20 mmol) in methylene chloride (85mL) and the mixture was stirred at
room temperature for 18 h. The mixture was washed with saturated
aqueous sodium hydrogen carbonate (85 mL), water (2 x 85 mL), and
brine (85 mL), dried (MgSO4) and the solvent was evaporated under
reduced pressure. A sample (0.5 g, 1.25 mmol) was dissolved in
ethanol (25 mL), aniline (230 mL, 233 mg, 2.5 mmol) was added and
the mixture was heated under reflux for 70 h. The mixture was cooled
and the solid was collected and recrystallized from ethanol to give (+)-
N-[(3R)-2,3-dihydro-1-methyl-2-oxo-5-phenyl-lH-1,4-benzodiazepin-3-
yl]-3-(phenylamino)propanamide as a colorless solid, m.p. 218-221~C,
[~C]D +58.2~ (c=0.585, CHCI3).
CA 022~7948 1998-12-lo
W 098/00405 PCTAUS97/11131
- 80 -
dH (CDC13) 7.60-6.71 (16H, m), 5.54 (lH, d, J 8.1 Hz), 3.54 (2H, t, J
6.1 Hz), 3.52 (3H, s), and 2.70 (2H, m).
Anal. Calcd. for C25H24N4O2Ø5Et0H:
C, 71.70; H, 6.25; N, 12.87.
Found: C, 71.42; H, 5.98; N, 12.84%.
EXAMPLE 58
CH3
~=~N H N J~
~ Y
(+)- 1 -[(3R)-2,3 -Dihydro- 1 -methyl-2-oxo-S-phenyl- 1 H- 1,4-benzo-
diazepin-3-yll-3-(2.4-dichlorophenyl)urea
2,4-Dichlorophenylisocyanate (188 mg, 1.0 mmol) was
added to a solution of 3(R)-amino-1,3-dihydro-1-methyl-S-phenyl-2H-
1,4-benzodiazepin-2-one (J. Org. Chem. 1987, 52, 3232-3239) (265 mg,
1.0 mmol) in tetrahydrofuran (20 mL). The mixture was stirred at
room temperature for 18 h. and the solvent was evaporated under
reduced pressure. The residue was purified by flash column
chromatography on silica gel, eluting with CH2cl2lMeoH (99.5:0.5)
and the residue was crystallized from CH2cl2lhexane to give (+)-1-
[(3R)-2,3-dihydro-1 -methyl-2-oxo-5-phenyl-lH-1,4-benzodiazepin-3-
yl]-3-(2,4-dichlorophenyl)urea as a colorless solid, m.p. 215-216.5~C,
[a]D +76.2~ (c=0.261, CHC13).
dH (CDC13) 8.10 (lH, d, J 9.0 Hz), 7.65-6.95 (13H, m), 5.50 (lH, d, J
9.0 Hz), and 3.50 (3H, s).
Anal. Calcd. for C23Hl8cl2N4o2.o.3H2o:
C, 60.22; H, 4.09; N, 12.21.
CA 022~7948 1998-12-10
WO 98/00405 PCT/US97/11131
- 81 -
Found: C, 60.28; H, 3.89; N, 12.10%.
EXAMPLE 59
CH3
H~\O
g~ O
(-)-3-Cyclohexyl-N-[(3R)-2,3-dihydro- 1 -methyl-2-oxo-4-oxido-5-
phenyl- 1 H- 1.4-benzodiazepin-3 -yllpropanamide
3-Chloroperoxybenzoic acid (80%, 0.32 g, 1.5 mmol) was
added to a solution of (+)-3-cyclohexyl-N-[(3R)-2,3-dihydro-1-methyl-
2-oxo-5-phenyl-lH-1,4-benzodiazepin-3-yl]propanarnide (0.60 g, 1.5
mmol) in dichloromethane (25 mL) and the mixture was stirred at room
temperature for 18 h. Further 3-chloroperoxybenzoic acid (80%, 0.1 g,
0.5 mmol) was added and the mixture was stirred for 24 h. The
mixture was washed with saturated aqueous sodium hydrogen carbonate
(4 x 25 mL), water (2 x 25 mL) and brine (25 mL,), dried (MgS04) and
the solvent was evaporated under reduced pressure. The residue was
recrystallized from toluene~exane (65:35) to give (-)-3-cyclohexyl-N-
[(3R)-2,3-dihydro- 1 -methyl-2-oxo-4-oxido-5-phenyl- 1 H- 1,4-
benzodiazepin-3-yl]propanamide as colorless prisms, m.p. 222-224~C,
[~C]D -80.7~ (c=1.15, CHC13).
dH (CDC13) 7.71-7.23 (lOH, m), 6.01 (lH, d, J 9.3 Hz), 3.54 (3H, s),
2.48 (2H, m), and 1.76-0.89 (13H, m).
Anal. Calcd. for C2sH29N3o3.o.sH2o:
C, 70.06; H, 7.06; N, 9.81.
Found: C, 70.10; H, 6.80; N, 9.79%.
CA 022~7948 1998-12-lo
W 098/00405 PCTrUS97/11131
- 82 -
EXAMPLE 60
N-[2,3-Dihydro- 1 -(2-dimethylaminoethyl)-2-oxo-5-phenyl- 1 H- 1,4-
benzodiazepin-3 -yll -3 -(2.4-dichlorophenyl)propanamide
Step A:
CH3~ ,CH3
N
~N
,~
2,3-Dihydro- l -(2-dimethylaminoethyl)-5 -phenyl- 1 H- 1 ,4-benzodiazepin-
2-one
2,3-Dihydro-5-phenyl- l H- 1 ,4-benzodiaz~pin-2-one
(1.00 g, 4.23 mmol) was added to hexane washed sodium hydride
(60% dispersion in mineral oil, 186 mg, 4.65 mmol) in DMF (5 mL).
Further DMF (10 mL) was added and the mixture was stirred at room
temperature. 2-(Dimethylamino)ethyl chloride hydrochloride (0.73 g, 5
mmol) was added to hexane washed sodium hydride (60% dispersion in
mineral oil, 200 mg, 5.0 mmol) in DMF (5 mL) and the mixtures were
combined. Potassium iodide (1 crystal) was added and the mixture was
stirred at 110~C for 30 min. The solvent was evaporated under reduced
pressure, water was added and the mixture was extracted with ethyl
acetate. The combined organic fractions were washed with water (2 x),
dried (MgSO4) and the solvent was evaporated under reduced pressure
to give 2,3 -dihydro- l -(2-dimethylaminoethyl)-5-phenyl- 1 H- 1,4-
benzodiazepin-2-one (1.21 g, 93%).
CA 022~7948 1998-12-10
W O 98/00405 PCT~US97/11131
- 83 -
dH (CDCl3) 7.63-7.16 (9H, m), 4.77 (lH, d, J 10.6 Hz), 4.41 (lH, m),
3.80 (lH, m), 3.78 (lH, d, J 10.6 Hz), 2.49 (2H, m), and 2.13 (6H, s).
Step B:
CH3'N'CH3
~$
2,3-Dihydro- 1 -(2-dimethylaminoethyl)-3-hydroxyimino-5 -phenyl- 1 H-
1.4-benzodiazepin-2-one
2,3-Dihydro- 1 -(2-dimethylaminoethyl)-5-phenyl- 1 H- 1,4-
benzodiazepin-2-one (1.21 g, 3.9 mmol) was dissolved in toluene (20
mL). The mixture was cooled to -78 ~C and potassium t-butoxide
(l.OM solution in t-butanol, 4.72 mL, 4.72 mmol) was added. The
mixture was stirred at -78 ~C for 20 min., then isoamyl nitrite (0.63
mL, 0.55 g, 4.72 mmol) was added. The mixture was stirred at -78~~
for 90 min. then allowed to warm to room temperature and poured into
aqueous citric acid (lM, 10 mL). The pH was adjusted to S.O with
aqueous sodium hydroxide then to 7.0 with saturated a(lueous sodium
hydrogen carbonate. The mixture was extracted with ethyl acetate (50
mL) and the organic layer was aged at room temperature. The solid
which formed was collected and dried in vacuo to give 2,3-dihydro-l -
(2-dimethylaminoethyl)-3-hydroxyimino-5-phenyl- 1 H- 1,4-
benzodiazepin-2-one (0.876 g, 66%) as a solid, m.p. 232-234~C.
dH (d6-DMSO) 10.90 (lH, s), 7.72-7.25 (9H, m), 4.40 (lH, m), 3.80
(lH, m), 2.50 (2H, m), and 1.85 (6H, s).
CA 022~7948 1998-12-lo
WO 98100405 PCTrUS97/11131
- 84 -
Step C:
CH3\ ,CH3
N
~ NH2
,~
Y
3-Amino-2,3-dihydro- 1 -(2-dimethylaminoethyl)-5-phenyl- 1 H- 1,4-
benzodiazepin-2-one
Ethyl isocyanate (320 mL, 287 mg, 4.0 rnmol) was added to
a mixture of 2,3-dihydro-1-(2-dimethylaminoethyl)-3-hydroxyimino-5-
phenyl-lH-1,4-benzodiazepin-2-one (0.91 g, 2.7 mmol) and triethyl-
amine (0.56 mL, 0.41 g, 4.0 mmol) in THF (30 mL). The mixture was
heated under reflux for 7 h., further ethyl isocyanate (167 mL, 150 mg,
2.1 mmol) was added and the mixture was heated under reflux for 12 h.
The mixture was cooled, the solvent was evaporated under reduced
pressure and ethyl acetate (75 mL) and water (25 mL) were added. The
organic phase was washed with water (4 x 25 mL), dried (MgSO4) and
evaporated under reduced pressure. The residue was dissolved in
ethanol (100 mL), palladium on carbon (10%, 100 mg) was added and
the mixture was shaken under hydrogen (50 p.s.i.) for 4.5 h. Further
palladium on carbon (10%, 100 mg) was added and the mixture was
shaken under hydrogen (50 p.s.i.) for 1.5 h. The mixture was filtered
and the solvent was evaporated under reduced pressure. The residue
was purified by flash column chromatography on silica gel, eluting with
CH2C12/MeOH to give 3-amino-2,3-dihydro-1-(2-dimethylaminoethyl)-
5-phenyl-lH-1,4-benzodiazepin-2-one (180 mg, 17%).
dH (CDCl3) 7.75-7.17 (9H, m), 4.45 (lH, s), 4.40 (lH, m), 3.82 (lH,
m), 2.47 (4H, m), and 2.08 (6H, s).
CA 02257948 1998-12-10
WO 98/00405 PCT/US97/11131
- g5 -
Step E:
N~
(l
~N I J~ ~CI
~0~ Cl
~ D
N-~2,3-Dihydro- 1 -(2-dimethylaminoethyl)-2-oxo-5-phenyl- 1 H- 1,4-
benzodiazepin-3 -yll -3 -(2~4-dichlorophenyl)propanamide
Triethylamine was added to a mixture o~ 3-amino-2,3-
dihydro- 1 -(2-dimethylaminoethyl)-5 -phenyl - 1 H- 1,4-benzodiazepin-2-
one (180 mg, 0.6 mmol), 3-(2,4-dichlorophenyl)propanoic acid (131
mg, 0.6 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide
hydrochloride (115 mg, 0.6 mmol) and l-hydroxyberzotriazole (81 mg,
0.6 mmol) in DMF (15 rnL) until the pH was 9Ø The mixture was
stirred at room temperature for 72 h. The solvent was evaporated
under reduced pressure and ethyl acetate was added. The mixture was
was washed with water, saturated aqueous sodium hydrogen carbonate
and water, dried (MgSO4) and evaporated under reduced pressure. The
residue was triturated with acetone and recrystallized from i-
PrOH/MeOH to give N-[2,3-dihydro-1-(2-dimethylaminoethyl)-2-oxo-5-
phenyl- 1 H- 1,4-benzodiazepin-3-yl]-3-(2,4-dichlorophenyl)-propanamide
as a solid, m.p. 199-201~C.
dH (CDC13) 7.60-7.15 (13H, m), 5.50 (lH, d, J 8.0 Hz), 4.40 (lH, m),
3.80 (lH, m), 3.10 (2H, t, J 7.5 Hz), 2.70 (2H, t, J 7.5 Hz), 2.40 (2H,
m), and 2.05 (6H, s).
Anal. Calcd. for C28H28cl2N4o2:
C, 64.25; H, 5.39; N, 10.70.
Found: C, 64.23; H, 5.40; N, 10.61%.
CA 022~7948 1998-12-10
WO 98/00405 PCT/US97/11131
- 86 -
EXAMPLE 61
CH3
~"" N~3,
~ HCI
(+)-3(R)- ~ N-[3-(4-chlorophenyl)prop- l -en-3-yl]amino } - 1,3-dihydro-
1-methyl-S-phenyl-2H-1~4-benzodiazepin-2-one hydrochloride
A mixture of 3(R)-amino-1,3-dihydro-l-methyl-5-phenyl-
2H-1,4-benzodiazepin-2-one (J. Org. Chem. 1987, 52, 3232-3239) (265
mg, 1 mmol), E-l-chloro-4-(3-chloro-1-propenyl)benzene (281 mg, 1.5
mmol), potassium carbonate (276 mg, 2 mmol) and potassium iodide
(25 mg, 0.15 mmol) in acetonitrile (2 mL) was heated under reflux for
4 h. The mixture was cooled and poured into ethyl acetate (10 mL) and
water (5 mL). The layers were separated and the aqueous layer was
extracted with ethyl acetate (S mL). The combined organic fractions
were washed with brine, dried (Na2so4) and the solvent was evaporated
under reduced pressure. The residue was purified by flash column
chroma-tography on silica gel, eluting with EtOAc/Hexane (65:35
increasing to 100:0). The first compound to elute was suspended in
ethanol (1 mL) and ethanolic HCl (6 M, 0.11 mL) was added. The
mixture was stirred, then the solvent was evaporated under reduced
pressure. The residue was triturated with ether and the solid was
collected and dried in vacuo to give (+)-3(R)-{N,N-bis[1-(4-chloro-
phenyl)propen-3 -yl]amino ) - 1,3-dihydro- 1 -methyl-5 -phenyl-2H- 1,4-
benzodiazepin-2-one hydrochloride (235 mg, 39%) as a tan solid, m.p.
138-145~C, [a]D +9.2~ (c=0.500, MeOH).
CA 022~7948 1998-12-10
W 098/00405 rCT~US97/11131
- 87 -
dH (d6-DMSO) 11.2 (lH, br s), 7.77-7.31 (17H, m), 6.85 (2H, br m),
6.54 (2H, m), 5.20 (lH, br s), 4.60-4.00 (4H, m), and 3.46 (3H, s).
Anal. Calcd. for C34H29C12N3O.HClØ10EtOH:
C, 67.60; H, 5.08; N, 6.92.
Found: C, 67.60; H, 5.03; N, 7.03%.
The second compound to elute was suspended in ethanol
(0.5 mL) and ethanolic HCl (6 M, 0.035 mL) was added. The mixture
was stirred, then the solvent was evaporated under reduced pressure.
The residue was triturated with ether and the solid was collected and
dried in vacuo to give (+)-3(R)-~N-[3-(4-chlorophenyl)propen-3-
yl]arnino ~ - 1,3-dihydro- 1 -methyl-5-phenyl-2H- 1,4-benzodiazepin-2-one
hydro-chloride (56 mg, 12%) as a yellow solid, m.p. 156-162~C, [oc]D
+35~ (c=0.100, MeOH).
dH (d6-DMSO) 10.3 (lH, br s), 10.0 (lH, br s), 7.79-7.34 (13H, m),
6.78 (lH, d, J lS.9 Hz), 6.40 (lH, dt, Jd 15.9, Jt 9.0 Hz), 5.13 (lH, s),
4.00 (2H, m), and 3.46 (3H, s).
Anal. Calcd. for C25H22ClN3O.HClØ10EtOHØ40H20:
C, 65.20; H, 5.30; N, 9.05.
Found: C, 65.14; H, 5.09; N, 9.33%.
Employing the procedure substantially as described above,
but substituting 1-(2-bromoethoxy)-4-nitrobenzene or 4-chlorobenzene-
propanol methanesulfonate for the E-l-chloro-4-(3-chloro-1-propenyl)-
benzene, the following compounds were prepared:
CA 02257948 1998-12-10
WO 98/00405 PCT/US97tlll31
_ ~g _
EXAMPLE 62
I~ N/
HCI
NO2
(+)-3(S)-{N,N-Bis[2-(4-nitrophenoxy)ethyl]amino)-1,3-dihydro-
1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one hydrochloride
m.p. 126-145~C. ralD +5.0~(0.100~ CHCI~).
dH (d6-DMSO) 8.20 (4H, d, J 9.2 Hz), 7.75-7.36 (9H, m), 7.08 (4H, d, J
9.2 Hz), 4.90 (lH, br s), 4.50 (4H, br s), 4.30-3.60 (5H, br m), and 3.34
(3H, s).
Anal. Calcd. for C32H29N507.HCIØ15EtOH:
C, 60.71; H, 4.87; N, 10.96.
Found: C, 60.70; H, 4.~7; N, 10.70%.
EXAMPLE 63
~" N~--~~
~ HCI
CA 02257948 1998-12-lo
W O 98t00405 PCTAUS97/11131
- 89 -
(+)-3(R)-{N-[3-(4-Nitrophenoxy)ethyl]amino}-1,3-dihydro-1 -methyl-
5-phenyl-2H-1.4-benzodiazepin-2-one hydrochloride
m.p. 154-160~C, [a]D +84.6~(0.500, MeOH).
dH (d6-DMSO) 10.2 (lH, br s), 8.25 (2H, d, J 9.0 Hz), 7.83-7.41 (9H,
m), 7.09 (2H, d, J 9.0 Hz), 5.21 (lH, s), 4.57 (2H, m), 3.70 (2H, m),
3.47 (3H, s), and 3.40 (lH, m).
Anal. Calcd. for C24H22N404.HClØ15EtOHØ20H20:
C, 61.13; H, 5.13; N, 11.74.
Found: C, 61.12; H, 4.92; N, 11.64%.
EXAMPLE 64
CH3
~? 3~
~ HCI
(+)-3(R)- { N-[3-(4-Chlorophenyl)prop- l -yl]amino ) - 1,3 -dihydro- 1 -
methyl-5- phenyl-2H-1.4-benzodiazepin-2-one hydrochloride
m.p. 167-168~C, [a]D +20.8~ (c=0.500, MeOH).
dH (d6-DMSO) 9.9 (2H, br m), 7.78-7.26 (13H, m), 5.08 (lH, s), 3.45
(3H, s), 3.20 (lH, m), 3.00 (lH, m), 2.70 (2H, t, J 7.4 Hz), and 2.05
(2H, m).
Anal. Calcd. for C25H24CIN30.HCI:
C, 66.08; H, 5.55; N, 9.25.
Found: C, 65.81; H, 5.49; N, 9.30%.
CA 022~7948 1998-12-lo
W 098/00405 PCT~US97/11131
- 90 -
EXAMPLE 65
C\H3
~,~"" N
,~
Y
(+)-Phenylmethyl N-[(3R)-2,3-dihydro-1-methyl-5-phenyl-2-thioxo-
1 H - 1 4-benzodiazepin-3-yll carbamate
A mixture of (+)-phenylmethyl N-[(3R)-2,3-dihydro-l-
methyl-5 -phenyl -2-oxo- 1 H - 1 ?4-benzodiazepin-3 -yl] carbamate (4.0 g, 10
mmol) and 2,4-bis(4-methoxyphenyl)-1,3-dithia-2,4-diphosphetane-2,4-
disulfide (4.5 g, 11 mmol) in toluene (100 mL) was heated under reflux
for 75 min. The mixture was cooled and the volume was reduced to 30
mL by evaporation under reduced pressure. The residue was purified
by flash column chromatography on silica gel, eluting with
EtOAc/Hexane (75:25) to give (+)-phenylmethyl N-[(3R)-2,3-dihydro-
l-methyl-5-phenyl-2-thioxo-lH-1,4-benzodiazepin-3-yl]carbamate as a
solid, m.p. 128-131~C, ~a]D +22.5~ (c=0.656, CHC13).
dH (CDC13) 7.65-7.26 (lSH, m), 5.50 (lH, d, ~ 8.8 Hz), 5.14 (2H, s),
and 3.86 (3H, s).
Anal. Calcd. for C24H2lN3o2s.o.25H2o:
C, 68.63; H, 5.16; N, 10.01.
Found: C, 68.28; H, 5.21; N, 10.06%.
Employing the procedure subst~nti~lly as described
above, but substituting phenylmethyl N-[2,3-dihydro-S-phenyl-2-
oxo-lH-1,4-benzodiazepin-3-yl]carbamate for the (+)-phenylmethyl
N-~(3R)-2,3-dihydro-1 -methyl-S-phenyl-2-oxo-lH-1,4-benzodiazepin-
3-yl]carbamate, the following compound was prepared:
CA 02257948 1998-12-10
WO 98/00405 PCT/US97111131
- 91 -
EXAMPLE 66
i~ N ~O--
,~
Y
Phenylmethyl N-~2,3-dihydro-5-phenyl-2-thioxo-1 H- 1 ,4-benzodiazepin-
3-yllcarbamate
dH (d6-DMSO) 10.85 (lH, s), 8.42 (lH, d, J 8.6 Hz), 7.65-7.10 (14H,
m), 5.10 (2H, s), and 5.05 (lH, d, J 8.6 Hz).
EXAMPLE 67
CH3 S
~NH ~
3-Cyclohexyl-N-(2,3-dihydro- 1 -methyl-5-phenyl-2-thioxo- 1 H- 1,4-
benzodiazepin-3-yl)propanamide
- Hydrogen bromide was bubbled at room temperature
through a solution of (+)-phenylmethyl N-[(3R)-2,3-dihydro-1-methyl-
5-phenyl-2-thioxo-lH-1,4-benzodiazepin-3-yl]carbamate (0.9 g, 2.1
n~nol), acetic acid (5 mL) and dichloromethane (5 mL). After 2 h., the
solvent was evaporated under reduced pressure, ether was added and the
CA 022~7948 1998-12-lO
W O 98/00405 P~ ',7/11131
- 92 -
solid was collected and dried in vacuo. A sample (0.58 g, 1.8 mmol)
was suspended in THP (10 mL), triethylamine (0.24 mL, 0.18 g, 1.8
mmol) was added and the mixture was stirred at room temperature for
3 h. In a separate flask, oxalyl chloride (0.20 mL, 0.29 g, 2.3 mmol)
was added to a solution of cyclohexanepropionic acid (0.33 mL, 0.30 g,
1.9 mmol) and DMF (1 drop) in THF (10 mL) and the mixture was
stirred at room temperature for 3 h. The two mixtures were combined,
triethylamine (0.32 mL, 0.23 g, 2.3 mmol) was added and the mixture
was stirred at room temperature for 2.5 h. The solvent was evaporated
under reduced pressure, water was added and the mixture was extracted
with ethyl acetate. The combined organic fractions were washed with
water, saturated aqueous sodium hydrogen carbonate, water (2 x) and
brine, dried (Na2SO4) and the solvent was evaporated under reduced
pressure. The residue was purified by flash column chromatography on
silica gel, eluting with CH2C12/MeOH (99.5:0.5) and the residue was
recrystallized from EtOAc/Hexane to give 3-cyclohexyl-N-(2,3-
dihydro- 1 -methyl-5-phenyl-2-thioxo- 1 H- 1,4-benzodiazepin-3-
yl)propanamide as a solid, m.p. 219-221~C.
dH (CDC13) 7.95 (lH, br d, J 8.6 Hz), 7.65-7.30 (9H, m), 5.72 (lH, d, J
8.6 Hz), 3.87 (3H, s), 2.41 (2H, t, J 7.6 Hz), and 1.80-0.85 (13H, m).
Anal. Calcd. for C25H29N30SØ25H20:
C,70.81;H,7.01;N,9.91.
Found: C, 70.80; H, 6.91; N, 9.95%.
Employing the procedure subst~nti~lly as described above,
but substituting phenylmethyl N-[2,3-dihydro-5-phenyl-2-thioxo-lH-
1,4-benzodiazepin-3-yl]carbamate for the (+)-phenylmethyl N-[(3R)-
2,3 -dihydro- 1 -methyl-S -phenyl-2-thioxo- 1 H- 1,4-benzodiazepin-3-
yl]carbamate and an appropriate acid for the cyclohexanepropionic acid,
the following compounds were prepared:
CA 02257948 1998-12-10
WO 98/00405 PCT/US97/11131
- 93 -
EXAMPLE 68
NH'~--
N
~ Y
3-Cyclohexyl-N-(2,3-dihydro-5-phenyl-2-thioxo- 1 H- 1,4-benzodiazepin-
3-yl)propanamide
m.p. 113-119~C.
dH (CDC13) 9.8 (lH, br s), 7.75-7.25 (lOH, m), 5.75 (lH, d, J 8.1 Hz),
2.41 (2H, m), and 1.80-0.85 (13H, m).
Anal. Calcd. for C24H27N30SØ8CH2C12:
C, 62.91; H, 6.09; N, 8.87.
Found: C, 62.88; H, 5.70; N, 9.12%.
EXAMPLE 69
~ ~NH ~
3-Cyclohexyl-N-(2,3-dihydro-2-hydrazono-5-phenyl- 1 H- 1,4-
benzodiazepin-3yl)propanamide
Hydrazine (53 mL, 56 mg, 1.8 mmol) was added to a
solution of 3-cyclohexyl-N-(2,3-dihydro-1-methyl-5-phenyl-2-thioxo-
CA 022~7948 1998-12-lo
W O 98t00405 PCT~US97/11131
- 94 -
lH-1,4-benzodiazepin-3-yl)propanamide (120 mg, 0.25 mmol) in
methanol (3 mL). The mixture was stirred at room temperature for 3
h. and the solvent was evaporated under reduced pressure. Ethyl acetate
was added and the mixture was washed with water and brine, dried
(Na2SO4) and the solvent was evaporated under reduced pressure. The
residue was purified by flash column chromatography on silica gel,
eluting with CH2Cl2/MeOH (99.5:0.5 increasing to 98:2) to give 3-
cyclohexyl-N-(2,3-dihydro-2-hydrazono-5-phenyl- 1 H- 1,4-benzodiaze-
pin-3yl)propanamide as a foam.
dH (CDCl3) 7.55-7.00 (1 lH, m), 5.75 (lH, d, J 7.6 Hz), 3.50 (2H, br s),
2.37 (2H, t, J 8.0 Hz), and 1.80-0.85 (13H, m).
Anal. Calcd. for C24H29NsOØ8CH30HØ15CH2C12:
C, 67.82; H, 7.41; N, 15.85.
Found: C, 67.79; H, 7.46; N, 16.05%.
EXAMPLE 70
NOH o
~N~/'\
,~
(E)- and (Z)-3-Cyclohexyl-N-(2,3-dihydro-2-hydroxyimino-5-phenyl-
1 H- 1 ~4-benzodiazepin-3-yl)propanamide
A mixture of 3-cyclohexyl-N-(2,3-dihydro-1-methyl-S-
phenyl-2-thioxo-lH-1,4-benzodiazepin-3-yl)propanamide (740 mg, 1.83
mmol), hydroxylamine hydrochloride (140 mg, 2 mmol) and triethyl-
amine (280 mL, 203 mg, 2 mmol) in methanol (lS mL)/THF (15 mL)
was stirred at room temperature for 3 h. The solvent was evaporated
under reduced pressure and the residue was purified by flash column
CA 022~7948 1998-12-lO
W O 98/00405 PCTrUS97/1113
_ 95 _
chromatography on silica gel, eluting with CH2cl2lMeoH (98:2). The
residue recrystallized from ethyl acetate. The first isomer to crystallize
was recrystallized from ethyl acetate to give(E)-3-cyclohexyl-N-(2,3-
dihydro-2-hydroxyimino-5-phenyl- lH- 1,4-benzodiaz~pin-3-
yl)prop~n~mide as a solid, m.p. 196~C.
dH (d6-DMSO) 12.20 (lH, s), 9.00 (lH, d, J 8.0 Hz), 7.70-7.30 (lOH,
m), 5.45 (lH, d, J 8.0 Hz), 2.30 (2H, m), and 1.80-0.75 (13H, m).
The second isomer to c~ystallize was recrystallized from
methanol to give (Z)-3-cyclohexyl-N-(2,3-dihydro-2-hydroxyimino-5-
phenyl-lH-1,4-benzodiazepin-3-yl)propanamide as a solid, m.p. 219~C.
dH (d6-DMSO) 9.95 (lH, s), 8.95 (lH, s), 8.75 (lH, d, J 8.0 Hz), 7.50-
7.00 (9H, m), 5.70 (lH, d, J 8.0 Hz), 2.25 (2H, m), and 1.75-0.75
(13H, m).
Anal. Calcd. for C24H28N402:
C, 71.26; H, 6.98; N, 13.85.
Found: C, 70.89; H, 6.99; N, 13.55%.
EXAMPLE 71
H3
NH--~O
~ Y
- 3-Cyclohexyl-N-(2,3-dihydro- 1 -methyl-5-phenyl- 1 H- 1,4-benzodiazepin-
3yl) prop~n~mide
Freshly prepared Raney nickel (400 mg) was added to a
solution of 3-cyclohexyl-N-(2,3-dihydro-1-methyl-S-phenyl-2-thioxo-
lH-1,4-benzodiazepin-3-yl)propanamide (200 mg, 0.5 mmol) in ethanol
(20 mL) and the mixture was stirred at room temperalure for 2 h. The
CA 02257948 1998-12-lo
W 098/0040S PCT~US97/11131
- 96 -
mixture was filtered and the solvent was evaporated under reduced
pressure. The residue was purified by flash column chromatography
on silica gel, eluting with CH2cl2lMeoH (99.75:0.25) to give 3-cyclo-
hexyl-N-(2,3-dihydro-1-methyl-5-phenyl-lH-1,4-benzodiazepin-3yl)
propanamide as a foam.
dH (CDCl3) 7.60-6.80 (9H, m), 6.37 (lH, br d, J 6.6 Hz), 5.53 (lH, m),
3.60 (2H, m), 2.77 (3H, s), 2.21 (2H, t, J 8.0 Hz), and 1.85-0.80
(13H, m).
Anal. Calcd. forC25H31N30Ø2CH2Cl2:
C, 74.45; H, 7.79; N, 10.34.
Found: C, 74.68; H, 7.87; N, 10.23%.
EXAMPLE 72
1 -(2,3-Dihydro- 1 -methyl-2-oxo-S-phenyl- 1 H-thieno-[2,3-e] - 1.4-
diazepin-3-yl)-3-(3 -methyl-phenyl)urea
Step A:
S NH2
~ O
(2-Arnino-3-thienyl)phenylmethanone
Triethylamine (6.8 mL, 4.94 g, 49 mrnol) was added
to a heated (33~C) mixture of b-oxobenzenepropanenitrile (18.6 g, 128
mmol) and 1,2-dit~ ne-2,5-diol (9.8 g, 64 mmol) in ethanol (120 mL)
and the mixture was stirred at SOC~ for 18 h. The mixture was cooled
and the solvent was evaporated under reduced pressure.
Dichloromethane was added, the mixture was washed with aqueous
hydrochloric acid (O.SM), aqueous sodium hydroxide (lM) and brine,
CA 022~7948 1998-12-10
WO 98/00405 PCT/US97/11131
- 97 -
dried (Na2SO4) and the solvent was evaporated under reduced pressure.
The residue was recrystallized from acetonitrile (150 mL) to give (2-
amino-3-thienyl)-phenylmethanone as an orange solid (5.7 g, 44%).
dH (CDC13) 7.70-7.35 (SH, m), 6.95 (2H, br s), 6.90 (lH, d, J 6.3 Hz),
and 6.15 (lH, d, J 6.3 ~Iz).
Step B:
H O
~N_~
~3N
2.3 -Dihydro-5-phenyl- 1 H-thienor2~3 -el - 1.4-diazepin-2-one
A solution of 1,3-dihydro-1,3-dioxo-2H-isoindole-2-acetyl
chloride (8.6 g, 38 mmol) in dichloromethane (20 mL) was added
slowly to a cooled (0~C) mixture of (2-amino-3-thienyl)phenyl-
methanone (6.8 g, 33 mmol), pyridine (6.34 mL, 6.20 g, 78 mmol) and
4-dimethylamino-pyridine (0.79 g, 6.5 mmol) in dichloromethane (130
mL). The mixture was stirred at 0~C for 30 min., diluted with
dichloromethane (80 mL) and washed with aqueous hydrochloric acid
(lM), saturated aqueous sodium hydrogen carbonate and brine. The
mixture was dried ~a2SO4) and the solvent was evaporated under
reduced pressure. The residue was triturated with ethanol and the solid
was collected and dried in vacuo to give N-(3-benzoylthien-2-yl)-1,3-
dihydro-1,3-dioxo-2H-isoindole-2-acetamide as a solid (9.8 g, 76%).
A mixture of N-(3-benzoylthien-2-yl)-1,3-dihydro-1,3-
- dioxo-2H-isoindole-2-acetamide (10.9 g, 28 rnrnol) and hydrazine (1.9
mL, 1.94 g, 60 mmol) in THF (500 mL) was heated under reflux for 4
h. The mixture was cooled, filtered and the solvent was evaporated
under reduced pressure. Saturated aqueous sodium hydrogen carbonate
was added and the mixture was extracted with ethyl acetate. The
.
CA 022~7948 1998-12-10
WO 98/00405 PCTIUS97tlll31
- 98 -
combined organic fractions were washed with brine, dried (Na2SO4)
and the solvent was evaporated under reduced pressure. Acetic acid
(300 mL) was added and the mixture was heated under reflux for 15
min. The mixture was cooled and the solvent was evaporated under
reduced pressure. Saturated aqueous sodium hydrogen carbonate was
added and the mixture was extracted with ethyl acetate. The combined
organic fractions were washed with brine, dried (Na2so4) and the
solvent was evaporated under reduced pressure to give 2,3-dihydro-5-
phenyl-lH-thieno[2,3-e]-1,4-diazepin-2-one as a foam (3.5 g, 52%).
dH (CDCl3) 9.75 (lH, br s), 7.90-7.30 (SH, m), 6.87 (lH, d, J 6.0 Hz),
6.82 (lH, d, J 6.0 Hz), and 4.45 (2H, s).
Step C:
,0
~Sy ~
~ )
~3N
2,3-Dihydro-1-methyl-5-phenyl-lH-thieno~2~3-el-1.4-diazepin-2-one
Sodium hydride (60% dispersion in mineral oil, 757 mg,
11.3 mmol) was added to a cooled (0~C) solution of 2,3-dihydro-5-
phenyl-lH-thieno[2,3-e]-1,4-diazepin-2-one (2.61 g, 10.8 mmol) in
DMF (7 mL). Further DMF (10 mL) was added and the mixture was
stirred for 30 min. A solution of iodomethane (0.67 mL, 1.53 g, 10.8
mmol) in ether (20 mL) was added and the mixture was stirred for 1 h.
The mixture was poured into water and the mixture was extracted with
ethyl acetate. The combined organic fractions were washed with brine,
dried (Na2SO4) and evaporated under reduced pressure. The residue
was purified by flash column chromatography on silica gel, eluting with
CA 022~7948 l998-l2-lO
W 098/00405 PCTrUS97/11131
_ 99 _
CH2C12/MeOH (95:5) to give 2,3-dihydro-1-methyl-5-phenyl-lH-
thieno[2,3-e]-1,4-diazepin-2-one (l.S g, 54%).
dH (CDC13) 7.67-7.35 (SH, m), 7.00 (lH, d, J 6.0 Hz), 6.85 (lH, d, J
6.0 Hz), 4.45 (2H, br s), and 3.50 (3H, s).
Step D:
,0
~S~ ~
~NH2
~3N
3-Amino-2,3-dihydro- 1 -methyl-S -phenyl- 1 H-thieno[2,3-e] -1 ,4-diazepin-
2-one
2,3-Dihydro- 1 -methyl-S-phenyl- 1 H-thieno[2,3-e] -1 ,4-
diazepin-2-one (1.5 g, 5.8 mmol) was dissolved in toluene (30 mL).
The mixture was cooled to -10~C and potas,sium t-butoxide (1.7 g, iS.l
mmol) was added. The mixture was stirred at -10"C for 15 min., then
isoamyl nitrite (1.0 mL, 0.87 g, 7.4 mmol) was added. The mixture
was stirred at -10~C for 1 h. then allowed to warm to room temperature
and poured into water (50 mL) and acetic acid (3 mL). The mixture
was extracted with ethyl acetate and the combined organic fractions
were washed with brine, dried (Na2so4) and the solvent was evaporated
under reduced pressure. The residue was purified by flash column
chromatography on silica gel, eluting with EtOAc/Hexane to give 2,3-
dihydro- 1 -methyl-3-hydroxyimino-5-phenyl- 1 H-thieno[2,3-e]- 1,4-
diazepin-2-one (0.80 g, 48%).
2,3-Dihydro- 1 -methyl-3-hydroxyimino-S-phenyl- 1 H-thieno
[2,3-e]-1,4-diazepin-2-one (0.80 g, 2.8 mmol) was dissolved in ethanol
(40 mL) and Raney nickel (2 g) was added. The mixture was shaken
under hydrogen (50 p.s.i.) for 5 days, ~ ling further Raney nickel (10
g) in portions. The mixture was filtered and the solvent was evaporated
CA 022~7948 1998-12-lo
W O 98/00405 PCTrUS97/11131
- 100-
under reduced pressure. The residue was purified by flash column
chromatography on silica gel, eluting with CH2Cl2/~eOH to give 3-
amino-2,3 -dihydro- 1 -methyl-S-phenyl- 1 H-thieno[2,3-e] - 1,4-diazepin-2-
one (248 mg, 33%).dH (CDC13) 7.50-7.30 (SH, m), 7.05 (lH, d, J 6.0 Hz), 6.85 (lH, d, J
6.0 Hz), 4.57 (lH, s), 3.55 (3H, s), and 1.70 (2H, br s).
Step E:
CH3
~NHJ~ NH~
,~
1 -(2,3 -Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H-thieno [2,3-e] - 1,4-diazepin-
3-yl)-3-(3-methylphenyl)urea
3-Methylphenylisocyanate (60 mL, 62 mg, 0.46 mmol) was
added to a solution of 3-amino-2,3-dihydro-1-methyl-S-phenyl-lH-
thieno[2,3-e]-1,4-diazepin-2-one (124 mg, 0.46 mmol) in tetrahydro-
furan (S mL). The mixture was stirred at room temperature for 2 h.
and the solvent was evaporated under reduced pressure. The residue
was crystallized from EtOAc (4 mL) to give 1-(2,3-dihydro-1-methyl-
2-oxo-S-phenyl-lH-thieno[2,3-e]-1,4-diazepin-3-yl)-3-(3-methyl-
phenyl)urea as a solid (94 mg, 50%).
m.p. 128- 130~C.
dH (CDCl3) 8.70 (lH, s), 7.65-6.75 (12H, m), 5.55 (lH, d, J 9.0 Hz),
3.55 (3H, s), and 2.30 (3H, s).
Anal. Calcd. for C22H20N402SØ25H20:
C, 64.62; H, 4.99; N, 13.70.
Found: C, 64.68; H, 4.96; N, 13.70%.
CA 022~7948 1998-12-10
WO 98/00405 PCT/US97/11131
- 101 -
EXAMPLE 73
CH3
N~O O
3-Cyclohexyl-N-(2,3-dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H-thieno[2,3-
el - 1.4-diazepin-3 -yl)propanamide
Triethylamine (75 mL, 54 mg, 0.54 mrnol) was added to
a mixture of 3-amino-2,3-dihydro-1-methyl-5-phenyl lH-thieno[2,3-e]-
1,4-diazepin-2-one (82 mg, 0.3 mmol), cyclohexanepropanoic acid (52
m~, 47 mg, 0.3 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodi-
imide hydrochloride (58 mg, 0.3 mmol) and l-hydroxybenzotriazole
(42 mg, 0.3 mmol) in DMF (1.5 mL). The mixture was stirred at room
temperature for 18 h. and ethyl acetate (60 mL) was added. The
mixture was washed with aqueous citric acid (10%), saturated aqueous
sodium hydrogen carbonate and brine, dried (Na2SO4) and the solvent
was evaporated under reduced pressure. The residue was purified by
flash column chromatography on silica gel, eluting with EtOAc/Hexane
to give 3 -cyclohexyl-N-(2,3 -dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H-
thieno[2,3-e]-1,4-diazepin-3-yl)propanamide as a solid (56 mg, 46%).
m.p. 189-190~C.
dH (CDCl3) 7.65-6.85 (8H, m), 5.65 (lH, d, J 8.0 Hz), 3.55 (3H, s),
2.40 (2H, t, J 7.0 Hz), and 1.80-0.85 (13H, m).
Anal. Calcd. for C23H27N302SØ5H20:
. C, 66.00, H, 6.74; N, 10.04.
Found: C, 66.25; H, 6.76; N, 9.83%.
CA 022~7948 1998-12-lo
W 098/00405 PCTAUS97/11131
- 102-
EXAMPLE 74
~=~NH--~ O
3-Cyclohexyl-N-(5-cyclohexyl-2,3 -dihydro-2-oxo- 1 H- 1,4-
benzodiazepin-3-yl) propanamide
Phenylmethyl N-[5-cyclohexyl-2,3-dihydro-2-oxo-lH-1,4-
benzodiazepin-3-yl]carbamate (150 mg, 0.38 mmol) was dissolved in
hydrogen bromide in acetic acid (30%, 0.5 mL). After 2 h., ether was
added and the solid was collected and dried in vacuo. THF (3 mL) and
triethylAmine (0.45 mL, 33 mg, 0.32 mmol) were added and the
mixture was stirred at room temperature for 3 h. In a separate flask,
oxalyl chloride (38 mL, 56 mg, 0.44 mmol) was added to a solution of
cyclohexanepropionic acid (61 mL, 56 mg, 0.36 mmol) and DMF (1
drop) in THF (2 mL) and the mixture was stirred at room temperature
for 3 h. The two mixtures were combined, triethylamine (61 mL, 44
mg, 0.44 mmol) was added and the mixture was stirred at room
temperature for 3 h. The solvent was evaporated under reduced
pressure and ethyl acetate was added. The mixture was washed with
water (2 x), saturated aqueous sodium hydrogen carbonate, water and
brine, dried (Na2so4) and the solvent was evaporated under reduced
pressure. The residue was recrystallized from i-PrOH to give 3-
cyclohexyl-N-(5-cyclohexyl-2,3-dihydro-2-oxo- 1 H- 1,4-benzodiazepin-
3-yl)propanamide as a solid, m.p. 133-138~C.
dH (CDCl3) 7.85 (lH, br s), 7.62-6.95 (SH, m), 5.40 (lH, d, J 8.7 Hz),
2.77 (lH, m), 2.34 (2H, m), and 2.05-0.75 (23H, m~.
Anal. Calcd. for C24H33N302Ø7C3H70H:
C, 71.64; H, 8.89; N, 9.60.
CA 022~7948 1998-12-10
WO 98/0040~ PCT/US97/11131
- 103-
Found: C, 71.28; H, 8.70; N, 9.82%.
EXAMPLE 75
H2N ~N I ~ ~CI
(+)-N-[(3R)-7-Amino-2,3-dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1,4-
benzodiazepin-3 -yll -3 -(2~4-dichlorophenyl)propanamide
Step A:
To a mixture of 3(R)-amino-1,3-dihydro-1-methyl-5-
phenyl-2H-1,4-benzodiazepin-2-one (J. Or~. Chem. 1987, 52, 3232-
3239) (3.9~ g, 15.0 mmol) in concentrated sulfuric acid (15 mL) cooled
in an ice-bath was added dropwise a solution of potassium nitrate (2.12
g, 21.0 mmol) in concentrated sulfuric acid (6 mL). The mixture was
stirred with cooling for 2 h., then stirred at ambient temperature for 1.5
h. Ice (80 g) was added and the mixture was basified with concentrated
ammonium hydroxide to pH 9. The resulting mixture was extracted
with ethyl acetate (3 x 220 mL). The combined organic fractions were
washed with brine, dried (Na2SO4) and the solvent was evaporated
under reduced pressure. The residue was purified by flash column
chromatography on silica gel, eluting with chloroform/methanol (97:3).
The material which eluted was further purified by flash column
chromatography on silica gel, eluting with ethyl acetete/methanol (95:5).
The material which eluted was stirred under n-butyl chloride (30 mL)
and the solvent was evaporated under reduced pressure to give an
inseparable mixture of 3(R)-amino-1,3-dihydro-1-methyl-7-nitro-5-
phenyl-2H-1,4-benzodiazepin-2-one and 3(R)-amino-1,3-dihydro-1-
CA 022~7948 1998-12-10
WO 9810040~; PCTIUS97/11131
- 104-
methyl-7-nitro-5-(2-nitrophenyl)-2H- 1,4-benzodiazepin-2-one (3.81 g)
in a 3:1 ratio as a yellow solid.
dH (CDC13) (mononitro compound) 8.43 (lH, dd, J 9, 3 Hz), 8.23 (lH,
d, J 3 Hz), 7.59 (2H, m), 7.52 (2H,m), 7.44 (2H,m), 4.47 (lH,s), 3.53
(3H,s), and 2.42 (2H, br s); (dinitro compound) 8.49 (lH, dd, J 9, 3),
8.42 (lH, m), 8.18 (lH, d, J 3 Hz), 8.01 (lH, m), 7.67 (lH, t, J 6 Hz),
7.6-7.4 (2H, m), 4.52 (lH,s), 3.56 (3H,s), and 2.42 (2H, br s).
Step B:
A solution of 3-(2,4-dichlorophenyl)propionic acid (482
mg, 2.2 mmol), DMF (0.017 mL, 0.22 mmol), and thionyl chloride
(0.24 mL, 3.3 mmol) in chloroform (2.5 mL) was heated at reflux for 1
h. The solvent was evaporated under reduced pressure to give 3-(2,4-
dichlorophenyl)propionyl chloride (520 mg, 100%). To a solution of
mixed 3(R)-amino- 1,3-dihydro- 1 -methyl-7-nitro-5-phenyl-2H-1,4-
benzodiazepin-2-one and 3(R)-amino-1,3-dihydro-1-methyl-7-nitro-5-
(2-nitrophenyl)-2H-1,4-benzodiazepin-2-one (3:1) (621 mg, 2 mmol)
and triethylamine (0.305 mL, 2.2 mmol) in methylene chloride (10
mL), was added a solution of 3-(2,4-dichlorophenyl)propionyl chloride
(520 mg, 2.2 mmol) in methylene chloride (1.5 mL). The mixture was
stirred for 30 min., the solvent was partially evaporated under reduced
pressure, and the reaction mixture was purified by flash column
chromatography on silica gel, eluting with methylene chloride/ether
(90:10) to give a mixture of (~)-N-~(3R)-2,3-dihydro-1-methyl-7-nitro-
2-oxo-5 -phenyl- 1 H- 1,4-benzodiazepin-3 -yl] -3 -(2,4-dichlorophenyl)-
propanamide and (+)-N-[(3R)-2,3-dihydro-1-methyl-7-nitro-2-oxo-5-
(2-nitrophenyl)- 1 H- l ,4-benzodiazepin-3-yl]-3-(2,4-dichlorophenyl)-
propanamide (850 mg, 84%) in a 3:1 ratio as a solid ~vhite foam.
dH (CDC13) (mononitro compound) 8.45 (lH, dd, J 9, 3 Hz), 8.25 (IH,
d J 3 Hz), 7.54 (3H, m), 7.45 (2H, m), 7.38 (lH, d, J 2 Hz), 7.26-7.18
(4H, m), 5.50 (lH, d, J 8 Hz), 3.52 (3H, s), 3.10 (2H, m), and 2.70 (2H,
m); (dinitro compound) 8.51 (lH, dd, J 9, 3 Hz), 8.40 (lH, m), 8.21
(lH, d J 3 Hz), 7.98 (lH, m), 7.68 (lH, t, J 6 Hz), 7.60 (lH, m), 7.44
,
CA 022~7948 1998-12-10
WO 98/00405 PCr/US97/11131
- 105-
(lH, m), 7.26-7.15 (4H, m), 5.52 (lH, d, J 8 Hz), 3.55 (3H, s), 3.10
(2H, m), and 2.70 (2H, m).
Step C:
To a solution of mixed N-[(3R)-2,3-dihydro-1-methyl-
7-nitro-2-oxo-S-phenyl- 1 H- 1,4-benzodiazepin-3-yl] -3-(2,4-dichloro-
phenyl)propanamide and (+)-N-[(3R)-2,3-dihydro-1-methyl-7-nitro-2-
oxo-5-(2-nitrophenyl)-lH- 1,4-benzodiazepin-3-yl]-3-(2,4-dichloro-
phenyl)propanamide (3:1) (770 mg, 1.5 mmol) in acetic acid (6 mL)
was added dropwise in portions over 1.5 h. a solution of 15% titanium
(III) chloride in 20-30% hydrochloric acid (7.8 mL, 9.0 mmol). The
resulting solution was stirred 30 min., basified with 20% sodium
hydroxide solution (pH 9), diluted with water (80 mL) and extracted
with ethyl acetate (3 x 100 mL). The combined organic fractions were
washed with brine, dried (Na2SO4) and the solvent was evaporated
under reduced pressure. The residue was purified by flash column
chromatography on silica gel, eluting with ethyl acetate/hexane (75:25
increasing to 100:0). The first compound to elute was crystallized from
ethyl acetate to give (+)-N-[(3R)-7-amino-2,3-dihydro-1-methyl-2-oxo-
5-phenyl- 1 H- 1,4-benzodiazepin-3 -yl] -3-(2,4-dichlorophenyl)-
propanarnide (413 mg, 57%) as a pale yellow solid, m.p. 179-180~C,
[a]D +60.2~ (c= 0.500, CHC13).
dH (CDC13) 7.60 (2H, d, J 7 Hz), 7.49-7.36 (5H, m) 7.24 (lH, d, J 9
Hz),7.17(2H,m),6.99(1H,dd,J9,3Hz),6.64(1H,d,J3Hz),5.54
(lH, d, J 8 Hz), 4.80-3.50 (2H, br s), 3.39 (3H, s), 3.09 (2H, t, J 8 Hz),
and 2.68 (2H, dt, Jd 3, Jt 8 Hz).
Anal. Calcd. for C25H22C12N402:
C, 62.38; H,4.61; N, 11.64.
- Found: C, 62.58; H, 4.68; N, 11.65%.
~ The second compound to elute was crystallized from ethyl
acetate to give (+)-N-[(3R)-7-amino-2,3-dihydro-1-methyl-2-oxo-S-
(2-aminophenyl)- 1 H- 1,4-benzodiazepin-3 -yl] -3-(2,4-dichlorophenyl)-
propanamide (114 mg, 15%) as a pale yellow solid, m.p. 188-189~C,
CA 022~7948 1998-12-lo
W 098/00405 PCTrUS97/11131
- 106-
[~~]D +50.0~ (c=0.100, MeOH).
dH (CDC13) 7.36 (2H, m), 7.25 (lH, d, J 9 Hz), 7.15 (3H, m), 7.00 (lH,
m), 6.88 (2H, m), 6.79 (lH, m), 6.60 (lH, bs), 5.52 (lH, d, J 8 Hz),
4.10-2.80 (4H br s), 3.40 (3H, m), 3.09 (2H, t, J 8 Hz), and 2.69 (2H,
m).
Anal. Calcd. for C25H23Cl2N5O2Ø05EtOAc:
C, 60.43; H, 4.71; N, 13.99.
Found: C, 60.79; H, 4.74; N, 13.83%.
EXAMPLE 76
CH3So2NH~$N N~--~CI
(+)-N-[(3R)-2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl-7-methane-
sulfonamido- 1 H- 1,4-benzodiazepin-3 -yl]-3-(2~4-dichlorophenyl)-
propanamide
Methanesulfonyl chloride (0.040 mL, 0.52 mmol) was
added to a solution of (+)-N-[(3R)-7-amino-2,3-dihydro-1-methyl-2-
oxo-5-phenyl- 1 H- 1,4-benzodiazepin-3 -yl] -3-(2,4-dichlorophenyl)-
propanamide (193 mg, 0 40 mmol) and pyridine (0.065 mL, 0.80
mmol) in methylene chloride (1.6 mL). The resulting solution was
stirred 2 h. The solution was diluted with ethyl acetate (12 mL), washed
with lN HCI, water, saturated sodium bicarbonate solution, water, and
brine (3 mL each), dried (Na2SO4) and the solvent was evaporated
under reduced pressure. The re~sidue was dissolved in warm toluene,
treated with charcoal, and filtered. The filtrate was diluted with hexane,
the mixture was cooled, and the resulting precipitate was collected and
CA 022~7948 1998-12-10
W 098/00405 rCTAUS97/11131
- 107-
dried in vacuo to give (+)-N-[(3R)-2,3-dihydro-1-methyl-2-oxo-5-
phenyl-7-methanesulfonamido- 1 H- 1,4-benzodiazepin-3-yl] -3-(2,4-
dichlorophenyl)propanamide (152 mg, 68%) as a white solid, m.p. 130-
148~C, [a]D +111.6~ (c=0.500, CHC13).
dH (CDC13) 7.55-7.32 (9H, m), 7.24 (2H, dd, J 10, 2 Hz), 7.17 (lH, dd,
J 9, 2 Hz), 7.05 (lH, d, J 3 Hz), 5.49 (lH, d, J 8 Hz), 3.41 (3H, s), 3.08
(2H, t, J 8 Hz), 2.97 (3H, s), and 2.71 (2H, dt, Jd 3, Jt 8 Hz).
Anal. Calcd. for C26H24C12N4O4S:
C, 55.82; H, 4.32; N, 10.01.
Found: C, 56.12; H, 4.47; N, 9.89%.
EXAMPLE 77
CH,
N~ N ~CI
~HCI
N-(2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H-pyrido[4,3-e] - 1,4-
diazepin-3-yl)-3-(2.4-dichlorophenyl)propanamide hydrochloride
Step A:
To a solution of 2,3-dihydro-1-methyl-5-phenyl-1H-
pyrido[4,3-e]-1,4-diazepine-2-one (J. Med. Chem.. 1965, 8, 722-724)
(1.63 g, 6.5 mmol) in toluene (32 mL) under argon cooled to -20~C
(ice/methanol bath) was added potassium t-butoxide (1.83 g, 16.3
mmol). The resulting purple suspension was stirred 15 min. at -20~C
and isoamyl nitrite (1.05 mL, 7.8 mmol) was added. The mixture was
stirred at -20~C for 30 min., then poured into a mixture of water (50
mL), acetic acid (3 mL), and ethyl acetate (65 mL). The mixture was
CA 022~7948 1998-12-lo
W O 98/00405 PCT~US97/11131
- 108-
stirred to dissolve all solids and the layers were separated. The aqueous
layer was extracted with ethyl acetate (65 mL). The combined organic
fractions were washed with saturated sodium bicarbonate solution and
brine (20 mL each), dried (Na2so4)~ and the solvent was evaporated
under reduced pressure. The residue was triturated with cold toluene
and the solid was collected and dried in vacuo to give 2,3-dihydro-3-
hydroxyimino- 1 -methyl-5-phenyl- 1 H-pyrido[4,3-e] - 1,4-diazepine-2-one
(1.22 g, 67%) as a yellow solid, m.p. 223-224~C.
dH (CDCl3) 8.92 (lH, bs), 8.73 (lH, d, J 7 Hz), 8.62 (lH, s), 7.80 (2H,
dd,J7, 1 Hz),7.59(1H,m),7.48(2H,m),7.26(1H,d,J7Hz),and
3.50 (3H,s).
Step B:
A mixture of 2,3-dihydro-3-hydroxyimino-l-methyl-5-
phenyl-lH-pyrido[4,3-e]-1,4-diazepine-2-one (1.77 g, 6.3 mmol) and
freshly prepared Raney nickel (3.2 g) in 1: 1 ethanol/methanol (l90 mL)
was shaken on a Parr hydrogenation apparatus under hydrogen (50 psi)
for 4 h. The mixture was filtered through filter aid and the filtrate was
evaporated under reduced pressure. The residue was purified by flash
column chromatography on silica gel, eluting with methanol/
chloroform/acetic acid (S:9S:l increasing to 10:90:1). The material
which eluted was stirred under chloroform (30 mL) with potassium
carbonate (0.3 g) and water (0.2 mL) for 5 min. The mixture was d~ied
(Na2S04) and the solvent was evaporated under reduced pressure to
give 3 -amino-2,3-dihydro- 1 -methyl-S-phenyl- 1 H-pyrido[4,3-e] - 1,4-
diazepine-2-one (276 mg, 16%), as a yellow solid, m.p. 109-123~C.
dH (CDC13) 8.72 (lH, d, J 6 Hz), 8.58 (lH, s), 7.61 (2H, m), 7.51 (lH,
m), 7.43 (2H, m), 7.26 (lH, m), 4.47 (lH ,s), 3.50 (3H, s), and 2.1
(2H, bs).
High res. mass spectrum: Theoretical mass for C15Hl4N4O (M+l):
267.124586. Measured mass (M+ 1): 267.123654.
, ,
CA 022~7948 1998-12-10
WO 98100405 PCT/US97/11131
- 109-
Step C:
A solution of dicyclohexylcarbodiimide (87 mg, 0.42
mmol) in methylene chloride (0.17 mL) was added to a solution of 3-
amino-2,3-dihydro-1 -methyl-5-phenyl-lH-pyrido[4,: -el-l ,4-diazepine-
2-one (93 mg, 0.35 mmol) and 3-(2,4-dichlorophenyl)propionic acid
(83 mg, 0.38 mmol) in tetrahydrofuran (0.5 mL) under argon. The
resulting mixture was stirred for 5 h., filtered, and the filtrate was
evaporated under reduced pressure. The residue was purified by
preparative plate chromatography on silica gel eluting with methanol/
chloroform/acetic acid (5:95:1). The purified material was stirred
under chloroform (S mL) with potassium carbonate (0.1 g) and water (2
drops) for S min. The mixture was dried (Na2SO4) and the solvent was
evaporated under reduced pressure. The residue was suspended in
ethanol (2 mL) and ethanolic HCI (6.8 M, 0.147 mL) was added. The
mixture was stirred, the resulting precipitate was collected and dried in
vacuo to give N-(2,3-dihydro-1-methyl-2-oxo-5-phenyl-lH-pyrido[4,3-
e]-1,4-diazepin-3-yl)-3-(2,4-dichlorophenyl)propanamide hydrochloride
(32 mg, 18%) as a white solid, m.p. 218-219~C.
dH (d6-DMSO) 9.38 (lH, d, J % Hz), 8.86 (lH, bs), 8.~9 (lH bs), 7.79
(lH, d, J 6 Hz), 7.56 (3H, m), 7.51 (2H, m), 7.39 (2H, m), 7.25 (lH,
m), 7.16 (lH, m), 5.37 (lH, d, J 8 Hz), 3.44 (3H, s) 2.94 (2H, t, J 7 Hz),
and 2.64 (2H, t, J 7 Hz).
Anal. Calcd. for C24H20cl2N4o2~Hcl:
C, 57.22; H, 4.20; N, 11.12.
Found: C, 56.87; H, 4.18; N, 11.09%.
CA 022~7948 1998-12-lo
W O 98/00405 PCT~US97/11131
- 110-
EXAMPLE 78
C H~
/~ H
~ Y
N-(2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H-pyrido[4,3-e] - 1,4-
diazepin-3-yl)-3-(cyclohexvl)propanamide
A solution of dicyclohexylcarbodiimide (87 mg, 0.42
mmol) in methylene chloride (0.17 mL) was added to a solution of 3-
amino-2,3-dihydro- 1 -methyl-5-phenyl- 1 H-pyrido[4,3-e] - 1,4-diazepine-
2-one (93 mg, 0.35 mmol) and cyclohexanepropionic acid (0.065 mL,
0.38 mmol) in tetrahydrofuran (0.5 mL) under argon. The resulting
mixture was stirred for 5 h., filtered, and the filtrate was evaporated
under reduced pressure. The residue was purified by preparative plate
chromatography on silica gel eluting with methanol/chloroform/acetic
acid (5:95:1). The purified material was stirred under chloroform (5
mL) with potassium carbonate (0.1 g) and water (2 drops) for 5 min.
The mixture was dried (Na2SO4) and the solvent was evaporated under
reduced pressure. The residue was crystallized from toluene to give N-
(2,3-dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H-pyrido[4,3-e]- 1,4-diazepin-3-
yl)-3-(cyclohexyl)-propanamide (47 mg, 33%) as a white crystalline
solid, m.p. 170-173~C.
dH (CDC13) 8.75 (lH, d, J 6 Hz), 8.61 (lH, s), 7.58 (2H, m), 7.52 (lH,
m), 7.45 (2H, m), 7.31 (lH, d, J 6 Hz), 7.21 (lH, d, J 8 Hz), 5.54 (lH,
d, J 8 Hz), 3.51 (3H, s), 2.39 (2H, m), 1.73 (4H, m), 1.63 (3H, m), 1.85-
1.12 (4H, m), and 0.94 (2H, m).
Anal. Calcd. for C24H2gN402Ø10PhCH3:
C, 71.70; H, 7.02; N, 13.54.
CA 02257948 1998-12-lo
W O 98/00405 PCTrUS97/11131
- 111 -
Found: C, 71.78; H, 7.01; N, 13.57%.
Employing the procedure subst~nti~lly as described above,
but substituting 3-(4-trifluoromethylphenyl)-propionic acid for the
cyclohexanepropionic acid, the following compound was prepared:
EXAMPLE 79
N~N~ ~CF3
N-(2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H-pyrido[4,3 -e] - 1,4-
diazepin-3-yl)-3-(4-trifluoromethylphenyl)propanamide
m.p. 191 -192~C.
dH (CDC13) 8.76 (lH, d, J 6 Hz), 8.61 (lH, s), 7.56 (4H, m), 7.52 (lH,
m), 7.42 (2H, d, J 7 Hz), 7.38 (2H, m), 7.30 (lH, d, J 6 Hz), 7.22 (lH,
d,J8Hz),5.51 (lH,d,J8Hz),3.50(3H,s),3.09(2H,t,J8Hz),and
2.73 (2H, t, J 8 Hz).
Anal. Calcd. for C25H21F3N402Ø20PhCH3:
C, 65.39; H, 4.70; N, 11.56.
Found: C, 65.69; H, 4.64; N, 11.95%.
CA 022~7948 1998-12-lo
W O 98/0040~ PCT~US97/11131
- 112-
EXAMPLE 80
CH3
~N~
N-(2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H-pyrido[3,4-e] - 1,4-
diazepin-3 -yl) -3 -(2~4-dichlorophenyl)propanamide
Step A:
To a solution of 2,3-dihydro- 1 -methyl-5-phenyl- 1 H-
pyrido[3,4-e]-1,4-diazepine-2-one (Can. J. Chem. 1987, 65, 1158-1161)
(1.43 g, 5.7mmol) in toluene (28 mL) under argon cooled to -20~C
(ice/methanol bath) was added potassium t-butoxide (1.59 g, 14.2
mmol). The resulting purple suspension was stirred 15 min. at -20 ~C
and isoamyl nitrite (0.92 mL, 6.8 mmol) was added. The mixture was
stirred at -20~C for 30 min., then poured into a mixture of water (25
mL), acetic acid (2.5 mL), and ethyl acetate (55 mL). The mixture was
stirred to dissolve all solids and the layers were separated. The aqueous
layer was extracted with ethyl acetate (2 x 55 mL). The combined
organic fractions were washed with saturated sodium bicarbonate
solution and brine (20 mL each), dried (Na2S04), and the solvent was
evaporated under reduced pressure. The residue was triturated with
hexane and the solid was collected and dried in vacuo to give 2,3-
dihydro-3-hydroxyimino- 1 -methyl-5-phenyl- 1 H-pyrido[3,4-e]-1,4-
diazepine-2-one (1.60 g, 100%) as a tan foam.
dH (CDC13) 8.77 (lH, s), 8.50 (lH, d, J 4 Hz), 7.81 (2H, dd, J 8, 1 Hz),
7.60 (lH, m), 7.49 (3H, m), 7.32 (lH, d, J 5 Hz), and 3.55 (3H,s).
CA 022~7948 1998-12-10
W O 98/00405 PCTrUS97/11131
- 113 -
Step B:
A solution of stannous chloride dihydrate (3.72 g, 16.5
mmol) in concentrated hydrochloric acid (11 mL) was added dropwise
to 2,3-dihydro-3-hydroxyimino- l -methyl-5-phenyl- 1 H-pyrido[3,4-e]-
1,4-diazepine-2-one (1.54 g, 5.5 mmol) cooled in an ice bath. The
resulting solution was stirred at ambient temperature for 3 h. The
solution was diluted with water (20mL), basified with concentrated
ammonium hydroxide (l~ mL), and extracted with ether (4 x 75 mL).
The combined organic fractions were washed with brine (30 mL), dried
(Na2SO4), and the solvent was evaporated under reduced pressure. The
residue was purified by flash column chromatography on silica gel,
e}uting with methanoVchloroform/acetic acid (5:95:1 increasing to
10:90:1). The material which eluted was stirred under chloroform (20
mL) with potassium carbonate (0.3 g) and water (2 drops) for 5 min.
The mixture was dried (Na2SO4) and the solvent was evaporated under
reduced pressure. The residue was stirred under hexane, and the
resulting solid was collected to give 3-amino-2,3-dihydro-1-methyl-5-
phenyl-lH-pyrido[3,4-e~-1,4-diazepine-2-one (241 mg, 16%) as a yellow
solid, m.p. 94- 118~C.
dH (CDC13) 8.79 (lH, s), 8.48 (lH, d, J 5 Hz), 7.62 (2H, dd,3 8, 1 Hz),
7.51 (lH, m), 7.45 (2H, m), 7.24 (lH, dd, J 5, 1 Hz), 4.47 (lH ,s), 3.55
(3H, s), and 2.2 (2H, bs).
Anal. Calcd. for C15H 14N4o-o-25(c2H5)2o:
C, 67.46; H, 5.84; N, 19.67.
Found: C, 67.28; H, 5.66; N, 19.53%.
High res. mass spectrum: Theoretical mass for C15H14N4O (M+l):
267.124586. Measured mass (M+l): 267.123093.
Step C:
A solution of oxalyl chloride (0.023 mL, 0.26 mmol) in
methylene chloride (0.2 mL) was added dropwise to a solution of 3-
(2,4-dichlorophenyl)propionic acid (4~ mg, 0.22 mmol) and DMF (l
drop) in methylene chloride (0.5 mL) cooled in an ice-bath. The
resulting solution was stirred I h. with cooling. The solvent was
CA 022~7948 1998-12-lo
W O 98/0040S PCTrUS97/11131
- 114-
evaporated under reduced pressure to give 3-(2,4-dichlorophenyl)-
propionyl chloride (52 mg, 100%). To a solution of 3-amino-2,3-
dihydro-1-methyl-5-phenyl-lH-pyrido[3,4-e]-1,4-diazepine-2-one (53
mg, 0.20 mmol) and pyridine (0.021 mL, 0.22 mmol) in methylene
chloride (3 mL), was added a solution of 3-(2,4-dichlorophenyl)-
propionyl chloride (52 mg, 0.22 mmol) in methylene chloride (0.5 mL).
The mixture was stirred for 1 h., the solvent was partially evaporated
under reduced pressure, and the reaction mixture was purified by flash
column chromatography on silica gel, eluting with methanol/ether (5:95
increasing to 7.5:92.5). The material which eluted w-dS crystallized
from toluene/hexane to give N-(2,3-dihydro-1-methyl-2-oxo-S-phenyl-
1 H-pyrido[3,4-e] - 1,4-diazepin-3-yl)-3-(2,4-dichlorophenyl)propanamide
(38 mg, 38%) as a white crystalline solid, m.p. 220-221~C.
dH(CDC13)8.81 (lH,s),8.52(1H,d,JSHz),7.56(2H,dd,J7,2Hz),
7.51 (lH,m),7.44(2H,d,J6Hz),7.40(1H,m),7.27(2H,m),7.18
(2H,dd,J8,2Hz),5.48(1H,d,J8Hz),3.55(3H,s),3.10(2H,t,J7
Hz), and 2.71 (2H, dt, Jd 2 Jt 8 Hz).
Anal. Calcd. for C24H20C12N402Ø25PhCH3:
C, 63.06; H, 4.52; N, 11.43.
Found: C, 63.03; H, 4.48; N, 11.25%.
EXAMPLE 81
N-[2,3-Dihydro-1 -methyl-2-oxo-S-isopropyl-lH-1,4-benzodiazepin-3-
yll -3-(2~4-dichlorophenyl)propanamide
Step A:
~N THF [~N
~ O BOC
CA 02257948 1998-12-10
WO 98/0040S PCT/US97/11131
- 115-
To a solution of the benzodiazepine (1.0 g, 5.3 mmol) in
THF (20 mL) at -78~C under argon was added 60% (NaH, 2.52 g, 6.3
mmol) Boc anhydride (1.27 g, 5.8 mmol) and the mixture stirred at
-78~C for 1/2 hour. The reaction was then allowed to warm to 25~C
and stirred for 2 hours before quenching into cold aq. NH4CI (10%)
and extracting the product into ethyl acetate (3xS0 mL). Concentration
of the dried (Na2S04) extracts gave an oil which was passed through
silica (EtOAc/hexane) to give 1.35 g product (89%).
1H NMR (CDCl3) d: 1.60 (s, 9H), 3.40 (s, 3H), 3.95 (brd, lH), 4.80
(brd, lH), 7.20 (d, lH), 7.30 (q, lH), 7.60 (t, lH), 7.92 (d, lH).
Step B:
CH3
MgCI ~N~
CH3 CH3
To a solution of the BOC-benzodiazepine (4.0 g, 13.8
mmol) in THF (80 mL) under argon was rapidly added a solution of
isopropylmagnesium chloride (2.0 M) in THF (7.66 mL, 15.3 mmol).
The reaction was stirred for 1/2 hour, quenched into aq NH4Cl (50
mL), and extracted with ethyl acetate (2x200 mL). The organic extracts
were concentrated and chromatographed on silica (1:1, EtOAC/hexane)
to give 1.55 g (34%) of product.
lH NMR (CDC13) d: 1.14 (d, 3H), 1.19 (d, 3H), 1.40 (s, 9H), 3.13 (s,
3H), 3.2-3.8 (m, 3H), 5.45 (brs, lH), 7.28 (dt, lH), ~7.48 (dt, lH), 7.56
- (dt, lH), 7.72 (dd, lH).
CA 022~7948 1998-12-lo
W O 98/00405 PCTnUS97/11131
- 116 -
Step C:
H~ ,BOC 1) HCI, EtOAc NH3o
o H 2) LiOH, H20 [~
To a 0~C solution of the isopropylphenone (1.55 g) in ethyl
acetate was added anhydrous HCI gas over 90 min. The reaction was
then concentrated in vacuo to give a solid which was dissolved in H2O
(40 rnL) and the pH adjusted to 11.0 with lN LiOH. After 30 min. at
pH = 11.0 the pH was adjusted to 7.0 with lN HCl and product extracted
into ethyl acetate. The organic extracts were dried (Na2SO4), filtered
and concentrated to give a solid 1.22 g, 100%.
1H NMR (CDC13) d: 0.95 (d, 3H), 1.30 (d, 3H), 3.16 (septet, lH), 3.36
(s, 3H), 3.60 (d, lH), 4.60 (d, lH), 7.2-7.3 (m, 2H), 7.45-7.55 (m, 2H).
Step D:
The benzodiazepine obtained in Step C was converted to the
oxime as described in Example 80 Step A.
Step E:
The oxime (2 gms) was dissolved in acetic acid (150 mL)
and 10% Pd/C (1 gm) added. The mixture was stirred rapidly under an
atmosphere of hydrogen for 90 min or until complete by HPLC. The
reaction was filtered, the catalyst washed with methylene chloride (200
mL) and the filtrates concentrated in vacuo to an oil. The oil was
dissolved in saturated aqueous sodium bicarbonate (100 mL) and
product extracted with ethyl acetate (3 x 150 mLs). Concentration of
the dried (Na2SO4) extracts gave 2.60 gms (97%).
Step F:
-
CA 022~7948 1998-12-10
WO 98/00405 PCTIUS97/11131
- 117-
The anine was coupled with 3-(2,4-dichlorophenyl)-
propionic acid as described in Example 43 to yield N-(2,3-dihydro-1-
methyl-2-oxo-5-isopropyl- 1 H- 1,4-benzodiazepin-3-yl)-3-(2,4-
dichlorophenyl)propanamide .
1H NMR (CDCl3) d: 0.92 (d, 3H), 1.25 (d, 3H), 2.65 (dt, 2H), 3.05 (t,
2H), 3.15 (SepT, lH), 3.40 (s, 3H), 5.38 (d, l~I), 7.0-7.6 (m, 8H).
The following compounds were prepared in a similar
manner as described in Example 81, using the appropriate Grignard
reagent in place of isopropyl magnesium chloride.
EXAMPLE 82
N-[2,3-dihydro-1 -methyl-2-oxo-5-isopropyl- 1 H- 1,4-benzodiazepin-3-
yll -3-cyclohexylpropanamide
m.p. 164-165~C
CHN: Anal. Calcd. for C22H31N3O2:
C, 71.51; H, 8.46; N, 11.37
Observed: C, 71.72; H, 8.39; N, 11.32
EXAMPLE 83
N-~2,3 -dihydro- 1 -methyl-2-oxo-5 -isopropyl- 1 H- 1,4-benzodiazepin-3-
yll -3 -f4-trifluoromethylphenyl)propanamide
m.p. 187-188~C
1H NMR (CDC13) d: 0.92 (d, 3H), 1.25 (d, 3H), 2.66 (dt, 2H), 3.04 (t,
2H), 3,15 (SepT, lH), 3.40 (S, 3H), 5.38 (d, lH), 7.14 (brd, lH), 7.25-
7.6 (m, 8H).
Employing subst~n~i~lly the same methods described in
Example 80, but replacing Step E with the reduction method described
below, the following compound,s were prepared:
CA 02257948 1998-12-10
W O 98100405 PCTrUS97/11131
- 118-
~N ~0
To a solution of the oxime 1 (1.28 g, 0.()048 mole) in H20
(130 ml) and THF (65 ml) was added sodium dithionite (Na2S2O4)
(13.0 g, 0.075 mole). The mixture was stirred for 2 hours then diluted
with saturated a~ueous sodium bicarbonate (50 ml) and product
extracted into ethyl acetate (2 x 150 ml). The organic extracts were
combined, dried over Na2SO4, filtered, and concentrated to give an oil
( 1.0 g). The oil was chromatographed on silica using ethyl acetate
followed by 10% methanol/methylene chloride to give pure amine
0.778g (64%).
1H NMR (DMSO) d 3.32 (s, 3H), 4.30 (s, lH), 6.64 (d, d, lH), 6.76
(d, lH), 7.35 (dt, lH), 7.58-7.74 (m, 3H), 7.88 (m, lH).
EXAMPLE 84
N-[2,3-dihydro- 1 -methyl-2-oxo-5-(2-furanyl)- 1 H- 1,4-benzodiazepin-3-
yll-3-cyclohexylpropanamide
m.p. 168-169~C
CHN: Anal. Calcd. for C23H27N303:
C, 70.21; H, 6.92; N, 10.68
Observed: C, 70.15; H, 6.67; N, 10.64
CA 022~7948 1998-12-10
WO 98100405 PCT/US97/11131
- 119-
EXAMPLE ~5
N-[2,3-dihydro- 1 -methyl-2-oxo-5-(2-furanyl)- 1 H- 1,4-benxodiazepin-3-
yll-3-(4-trifluoromethylphenyl)propanamide
m.p. 155-157~C
CHN: Anal. Calcd. for C24H20N3O3F3:
C, 63.29; H, 4.432; N, 9.23
Observed: C, 63.22; H, 4.44; N, 9.07
EXAMPLE 86
N-[2,3-dihydro- l -methyl-2-oxo-5-(2-furanyl)- 1 H- 1,4-benzodiazepin-3-
yll -3-(2~4-dichlorophenyl)propanamide
m.p. 132-133~C
CHN: Anal. Calcd. for C23H l gN303CI2
C, 60.54; H, 4.20; N, 9.21
Found: C, 60.62; H, 4.07; N, 9.07
EXAMPLE 87
N-[2,3-dihydro- l -methyl-2-oxo-5-(3-furanyl)- 1 H- l ,4-benzodiazepin-3 -
yll -3-cyclohexylpropanamide
m.p. 199-200~C
1H NMR (CDCI3) d: 0.9-1.~ (brm, 3H), 2.38 (t, 2H), 3.42 (S, 3H), 5.55
(brd, lH), 6.90 (S, lH), 7.2-7.77 (m, 7H)
EXAMPLE ~
N-[2,3-Dihydro- 1 -methyl-2-oxo-5-(3 -furanyl)- 1 H- 1,4-benzodiazepin-3-
yll -3 -(4-trifluoromethylphenyl)propanamide
m.p. 213-214~C
1H NMR (CDC13) d: 2.71 (dt, 2H), 3.05 (t, 2H), 3.42 (~S, 3H), 5.72 (d,
lH), 6.~2 (brS, lH), 7.2-7.7 (m, l lH)
CA 02257948 1998-12-lo
W O 98100405 PCTrUS97/11131
- 120-
EXAMPLE 89
N-[2,3-Dihydro- 1 -methyl-2-oxo-5-[2'-(4,4-dimethyl-2-oxazolinyl)-
phenyl]-1 H- 1 ~4-benzodiazepin-3-yll-3-(2~4-dichlorophenyl)-
propanamide
The subject compound was prepared su~st~nti~lly as
described in Example 81.
m.p. 194-195~C
CHN: Anal. Calcd. for C3oH28N4o3cl2
C, 63.95; H, 5.01; N, 9.94
Found: C, 63.70; H, 5.01; N, 9.96
EXAMPLE 90
N-[2,3,4,5-Tetrahydro- 1 -methyl-2-oxo-5-isopropyl- 1 H- 1 ,4-benzo-
diazepin-3yll -3-cyclohexylpropanamide
~H3 o
~N~ ~ H 10% Pd/C
~N)'NH J--~ MeOH
CH3 CH3
CIH3 o
~ N
CH3 CH3
A solution of N-[2,3-dihydro-1-methyl-2-oxo-5-isopropyl-
CA 02257948 1998-12-10
WO 98/00405 PCT/US97/11131
- 121 -
lH-1,4-benzodiazepin-3-yl]-3-cyclohexylpropanamide (50 mg) in
methanol (10 mL), cont~ining 10% Pd/C (50 mg) was stirred under 1
atmosphere of hydrogen for l ~ hours. Filtration of the reaction,
concentration and cryst~lli7~tion ffrom diethyl ether gave 21 mg N-
[2,3,4,5-tetrahydro- 1 -methyl-2-oxo-5-isopropyl- 1 H- 1,4-benzodiazepin-
3-yl] -3 -cyclohexylpropanamide.
CHN: Anal. Calcd. for C22H33N3o2
C, 71.12; H, 8.95; N, 11.31
Observed: C, 70.9~; H, 8.97; N, 11.15
m.p. 114-115~C
EXAMPLE 91
N-[2,3-dihydro- 1 -methyl-2-oxo-5-methyl- 1 H- l ,4-benzodiazepin-3-yl] -
3 -(2~4-dichlorophenyl)propanamide
Step A:
CH3\ ~ / CH3
H_~ / \O C~
CH3 CH3
To CBZ-benzodiazepine (250 mg, 0.776 mmol) in toluene
(25 mL) at reflux was added dropwise a solution of DMF dimethylacetal
~ (1.09 mL) in toluene (10 mL). The reaction was refluxed for 5 hours,
cooled and concentrated to an oil. The oil was triturated with ether to
give a white solid (124 mg).
lH NMR (CDCl3) d: 2.50 (s, 3H), 3.42 (s, 3H), 5.12-5.20 (m, 3H), 6.62
(d, lH), 7.25-6.4 (m, 7H), 7.5-7.6 (m, 2H).
CA 02257948 1998-12-lo
W O 98/00405PCT~US97/11131
- 122-
Step B:
~N.CBZ ~ ~NH~
CH3 CH3
The CBZ-amine-N-me~yl amide (190 mg) was treated
with 30% HBr/AcOH (0.8 mL) for 1 hour at room temperature. The
reaction mixture was poured into ether (10 mL) at 0~C and the solid
filtered. Solid dissolved in 10% Aq. NaOH (5 mL) and CH2C12 (10
mL) and organic layer separated, dried (Na2SO4), filtered and
concentrated to an oil (172 mg, 110%).
lH NMR (CDC13) d: 2.42 (s, 3H), 3.05 (brs, 2H), 3.40 (s, 3H), 4.40 (s,
lH), 7.2-7.6 (m, 4H).
Step C:
o
o HO ~,CI
EDC, HOBT,TEA,
DMF
CH3
CH3
~5~ NH~--~CI
CH3
CA 02257948 1998-12-10
WO 98/0040~ PCT/US97/11131
- 123-
N- [2,3 -dihydro - 1 -methyl-2-oxo-5 -methyl- 1 H - 1,4-benzodiazepin-3 -yl] -3 -
(2,4-dichlorophenyl)propanamide was prepared in a similar manner as
described previously in Example 43.
m.p. 194-195~C
CHN: Anal. Calcd. for C2oHl 9N3o2cl2
C, 59.42; H, 4.74; N, 10.39
Obselved: C, 59.50; H, 4.74; N, 10.44
1H NMR (CDC13) d: 2.49 (brs, 3H), 2.65 (dt, 2H), 3.05 (t, 2H), 3.42 (s,
3H), 5.35 (d, lH), 71-7.6 (m, 8H).
EXAMPLE 92
N-[2,3-Dihydro- 1 -methyl-2-oxo-[4,5-a] -(1 -oxo- 1,3-dihydro-2H-
isoindole)- 1 H- 1,4-benzodiazepin-3-yl] -3 -(2,4-dichlorophenyl)-
propanamide
~$N~--~CI
~<~
~ ~ J~CI
To a solution of N-[2,3-dihydro-1-methyl-2-oxo-5-~2'-(4,4-
dimethyl-2-oxazolinyl)phenyl] - 1 H- 1,4-benzodiazepin-3-yl~ -3 -(2,4-
CA 022~7948 1998-12-lo
W O 98/00405 PCT~US97/11131
- 124-
dichlorophenyl)propanamide (100 mg, 0.178 mmol) in methylene
chloride was slowly added methyl trifluoromethanesulfonate (22 mL,
0.198 mmol). After stirring 5 minutes, sodium borohydride (7.6 mg,
0.20 mmol) in asolute ethanol (0.5 mL) was added and reaction stirred
30 min. the product was extracted into ethyl acetate and purified by
column chromatography on silica (60% ethyl acetate/hexane) to give 30
mg N- ~2,3 -dihydro- 1 -methyl-2-oxo- [4,5 -a] -(1 -oxo- 1,3 -dihydro -2H-
isoindole)- 1 H- 1,4-benzodiazepin-3-yl] -3 -(2,4-dichlorophenyl)-
propanamide.
1H NMR (CDC13) d: 2.70 (m, 2H), 3.12 (t, 2H), 3.55 (s, 3H), 5.68 (s,
lH), 5.90 (d, lH), 6.85 (dd, lH), 7.05 (brd, lH), 7.1-7.5 (m, 9H), 7.85
(d, lH).
MS M+1-494.
EXAMPLE 93
~(1oN H
,~,
Y
3R-(+)-3-(Phenylthio)-N-[2,3-dihydro- 1 -methyl-2-oxo-5-phenyl- lH-
1 ~4-benzodiazepin-3-yllpropanamide
To a stirred solution of 3-bromopropionic acid (l.Og,
6.5mmol) in DMF (20 mL) was added K2CO3 (1.8 g, 13 mmol) and
thiophenol (0.72 g, 6.5 mmol). This was heated to 50~C for lh. The
mixture was then diluted with 200 mL H2O and extracted with 2 x 100
mL EtOAc. The combined organics were washed with 100 mL H2O
and dried with Na2SO4. This was evaporated to give 1.52g of a
colorless oil, 1.18g corrected for residual DMF by NMR.
CA 02257948 1998-12-lO
W 098/0040S PCTrUS97/1113
- 125-
The above oil was taken up in 30 mL DMF and 1-(3-
dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (2.45g,
12.8mmol) and 1-hydroxybenztriazole hydrate ( 1.73g, 12.8mmol) were
added. This was stirred for 5 min at rt. 3-(R)-Amino-1,3-dihydro-l-
methyl-5-phenyl-2H-1,4-benzodiazepin-2-one (0.66g, 2.6mmol) was
then added and the reaction was stirred at rt overnight. The reaction
was diluted wi~ 200 mL H2O and extracted with 2xl50mL EtOAc.
The combined organics were washed with lxlOOmL H20, dried with
Na2SO4 and evaporated. The residue wa.s chromatographed over silica
eluting with 2% MeOH:CHCl3. Collected pure fractions, evaporated.
Evaporated from diethyl ether to give 770mg of a white foam.
Anal. Calcd for C25H23N3o2s-o.o5Hexane:
C, 70.04; H, 5.51; N, 9.69.
Found:C 69.91, H 5.40, N 9.78.
EXAMPLE 94
~N H s,CH3
,~
3R-(+)-5-(Methylthio)-N-[2,3-dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H-
1.4-benzodiazepin-3 -yllpropanamide
- To an aqueous solution of K2co3 (0.76g,5.5mmol) was
added 5-bromopentanoic acid and sodium ~iomethoxide. This was
stirred at rt overnight. The reaction was diluted with 50 mL H2O and
acidified to pH=0 with 6N HCl. Extracted with 2 x 50 mL EtOAc.
Dried with Na2SO4, evaporated to give 0.55g of a yellow oil.
CA 022~7948 1998-12-lo
W O 98/00405 PCTAUS97/11131
- 126-
The above oil was taken up in 10 mL DMF and 1-(3-
dimethyl-aminopropyl)-3-ethylcarbodiimide hydrochloride (1.30g,
6.8mmol) and l-hydroxybenztriazole hydrate (0.92g, 6.8mmol) were
added. 3 -(R)-Amino- 1,3 -dihydro- 1 -methyl-S-phenyl-2H- 1,4-
benzodaizepin-2-one (0.85g, 3.4 mmol) was then added and the reaction
was stirred overnight at rt. The reaction was diluted with 100 mL
H2O and extracted with 2 x 50 mL EtOAc. Combined organics were
dried with brine and Na2S04, and evaporated to give yellow oil. The
residue was chromatographed over silica eluting with 50:50 EtOAc:Hex
to 100% EtOAc. Pure fractions were collected to give 1.33g of a
colorless oil, 0.4g of which was chroma-tographed over silica eluting
with 2% MeOH:CH2C12. Pure fractions were collected, and evaporated
from ethyl ether:hexane to give a white powder mp. 61-65~C.
Anal. Calcd for C22H25N3o2s-o.3sH2o:
C, 65.76; H, 6.45; N, 10.46.
Found: C, 65.81; H, 6.21; N, 10.57.
EXAMPLE 95
Me
1~ " N ~ N
N-cyano-N'-cyclohexylmethyl-N"-(1,3-dihydro- 1 -methyl-2-oxo-5-
phenyl-2H- 1.4-benzodiazepin-3-yl)guanidine
A solution of 3-(R)-amino-1,3-dihydro-1-methyl-5-phenyl-
2H-1,4-benzodiazepin-2-one (lg, 3.7 mmole) in acetonitrile (20 mL)
was treated with diphenylcyanocarbonimidate (0.9 g, 3.7 mmole) and
stirred at room temperature for thirty minutes. Cyclohexylmethyl-
CA 02257948 1998-12-10
WO 98/0û405 PCT/US97/11131
- 127-
amine (0.84 g, 7.4 mmole) was then added and the reaction stirred at
room temperature for two hours. The reaction was poured into 100 mL
of 0.1 N HCI and extracted with 3 x 100 mL portions of ethyl acetate.
The organic layers were combined and washed once with saturated
sodium bicarbonate (50 mL), dried over anhydrous m~nesium sulfate,
filtered, and concentrated at reduced pressure. The residue was
chromatographed on silica gel eluting with 50% ethyl acetate/hexane to
give 0.875 g of the product. The analytical sample was crystallized
from ethyl acetate.
m.p. 158-161~C.
Anal. Calcd. for C25H28N6O:
C, 70.07; H, 6.59; N, 19.61.
Found: C, 70.05; H, 6.59; N, 19.64%.
EXAMPLE 96
Me
N ~CI
N-( 1 ,3-Dihydro- 1 -methyl-2-oxo-5 -phenyl-2H- 1 ,4-benzodiazepin-3-yl)-
4-(4-chlorobenzyl)-4-piperidinecarboxamide dihydrochloride
Step A: Preparation of N-tert-butyloxycarbonyl-4-(4-chloro-
benzyl)-4-piperidinecarboxylic acid
-
A solution of N-Boc-ethylisonipecotate (51.4 g, 200
mmole) in THF (lL) at -60~ C was treated with a solution of lithium
bistrimethylsilyl amide (220 mL of a 1 N solution in THF, 220 mmole).
CA 022~7948 1998-12-10
WO 98/00405 PCT/US97/11131
- 128-
After stirring at -60~C for S minutes, a solution of 4-chlorobenzyl
chloride (33.8 g, 210 mmole) in THF (200 mL) was added and the
reaction allowed to warm to room temperature. Most of the THF
(about 800 mL) was removed by evaporation at reduced pressure. The
rem~in~ler was poured into 1 L of 1 N HCI and extracted with two 800
mL portions of ethyl acetate. The organic layers were combined and
washed once with saturated sodium bicarbonate (500 mL), dried over
anhydrous magnesium sulfate, filtered, and concentrated at reduced
pressure. The residue was chromatographed on silica gel eluting with
10%-20% ethyl acetate/hexane to give the product ester which was used
directly. The material thus obtained was dissolved in THF (100 mL)
and IPA (100 mL) and treated with 350 mL of 10 N NaOH. The
mixture was heated to reflux for 30 hours. The reaction was cooled to
room temperature and poured over a mixture of crushed ice (2 L), 6 N
HCI (500 mL) and saturated potassium hydrogen sulfate (1 L). The
mixture was extracted with two 1 L portions of ethyl acetate. The
organic layers were combined and dried over anhydrous magnesium
sulfate, filtered, and concentrated at reduced pressure to give 52 g of the
product.
m.p. 179-180~C,
lH NMR CDC13 d 7.26 (d, J = 8 Hz, 2 H), 7.03 (d, J = 8 Hz, 2 H),
3.98 (m, 2H), 3.0-2.8 (m, 2H), 2.84 (s, 2H), 2.10-2.00 ( m, 2H), 1.55-
1.40 (m, 2H), 1.45 (s, 9H)
Step B: Preparation of N-(1,3-dihydro- 1 -methyl-2-oxo-5-phenyl-
2H- 1,4-benzodiazepin-3 -yl)-4-(4-chlorobenzyl)-4-piper-
idinecarboxamide dihydrochloride
A mixture consisting of N-tert-butyloxycarbonyl-4-(4-
chlorobenzyl)-4-piperidinecarboxylic acid (1.48 g, 4.18 mmole), 3-(R)-
amino- 1,3-dihydro- 1 -methyl-5-phenyl-2H- 1,4-benzodiazepin-2-one (1 g,
3.7 mmole), hydroxybenzotriazole (1.17 g, 8.66 mmole), 1-(3-dim-
ethylaminopropyl)-3-ethylcarbodiimide hydrochloride (1.49 g, 7.70
mmole), diisopropylethyl amine (0.53 g, 4.13 mmole), and DMF (10
mL) was stirred at room temperature for 18 hours. The reaction was
CA 022~7948 1998-12-1o
W O 98/00405 PCTnUS97/11131
- 129-
poured into 1 N HCI and extracted with ethyl acetate (4 X 50 mL). The
organic layers were combined and washed once with saturated sodium
bicarbonate (50 mL), once with saturated sodium chloride (50 mL),
dried over anhydrous magnesium sulfate, filtered, and concentrated at
reduced pressure. The residue was chromatographed on silica gel
eluting with 25%-50% ethyl acetate~exane to give 2.34 g of the product
amide which was used directly. The material thus obtained was
dissolved in ethyl acetate (50 mL) and HCI (g) was bubbled into the
reaction for 5 minutes. The reaction was concentrated at reduced
pressure and the residue recrystallized from ethyl acetate to give 1.13 g
of the product as a pale yellow .solid.
m.p. 190- 195~C.
Anal. Calcd. for C29H29ClN4O2-2 HCI:
C, 60.68; H, 5.44; N, 9.76.
Found: C, 60.47; H, 5.5; N, 9.42~b.
Utili7ing the procedures subst~nti~lly as desribed above
except substituting N-Boc-ethylnipecotate for N-Boc-ethyl isonipecotate
there were obtained the following compounds
EXAMPLE 97
Me
~"N~
N-( 1 ,3-dihydro- 1 -methyl-2-oxo-5-phenyl-2H- 1 ,4-benzodiazepin-3-yl)-
3-(4-chlorobenzyl)-3-piperidinecarboxamide hydrochloride A + B
somers
CA 02257948 1998-12-10
WO 98/00405 PCT/US97/11131
- 130-
Iso m er A
m .p.205 - 210~ C.
A nal. C alcd. for C 29 H 28 CIN 402-H Cl-O.S C H 3 C H 2 ~ H ~0.8 H 2 ~:
C,62.67; H,6.07; N,9.75.
Fou~nd: C,62.69; H,5.94; N,9.42 % .
Iso m er B
m .p.200 - 205~ C.
A nal. C alcd. for C 29 H 28 ClN 4 O 2-H Cl.-0.1 C H 3 C H 2 O C O C H 3-1.6 H 2 ~:
C,61.39; H,5.96; N,9.74.
Fouuld: C,61.39; H ,5.66; N,9.56 % .
E X A M P L E 98
~ Br ~ O ~_"~ ~ O
~3
Cl~ N~--O
~ O
CA 022~7948 1998-12-1o
W O 98/00405 PCT~US97tlll31
- 131 -
(+)-3-Cyclohexyl-N-[2,3-dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1,4-
benzodiazepin-3-yll-~-(ethoxycarbonylmethyl)propanamide
3-(R)-Amino- 1,3-dihydro- l -methyl-5-phenyl-2H- 1,4-
benzodiazepin-2-one (5.0 g, 18.8 mmol) in acetonitrile (100 mL) was
mixed with ethyl bromoacetate (2.1 mL, 18.8 mmol) and sodium
hydrogen carbonate (4.0 g) was suspended in the mixture. The mixture
was stirred and heated at reflux for 2 h. After that time, the reaction
was cooled to room temperature, diluted with 150 mL water, and
extracted with ethyl acetate (3 x 100 mL). The organic layers were
combined, dried with magnesium sulfate, gravity filtered, and the
solvent was removed in vacuo. The resulting oil was chromatographed
over silica in 3: l ethyl acetate:hexane, yielding the mono-alkylated
product (2.58 g, 39%) as well as the starting 1,4-benzodiazepin-2-one
and bis-alkylated material. To a solution of 3-cyclohexylpropionic acid
(1.0 g, 6.40 mmol) in methylene chloride (30 mL) was added oxalyl
chloride (0.56 mL, 6.40 mmol) and catalytic (N,N)-dimethyl formamide
( 2 drops). After 0.5 h, a solution of the acetate (2.25 g, 6.40 mmol) in
methylene chloride (10 mL) was added and the reaction was stirred for
0.25 h. The reaction was then diluted with methylene chloride (150
mL) and saturated aqueous sodium hydrogen carbonate (150 mL ) was
added. The aqueous portion was extracted again with methylene
chloride (2 x 100 mL) and the organic.s were combined, dried with
magnesium sulfate, gravity filtered, and the solvent was removed in
vacuo. The resulting oil was chromatographed over silica with 1 :1 ethyl
acetate:hexane, yielding a foam that was crystallized with ether, giving
2.0 g (64%) of the product.
m.p. 120-122~C, [a]D + 0.63~ (c=0.79; MeOH).
Anal. Calcd. for C29H35N304:
C, 71.14; H, 7.21; N, 8.58.
Found: C, 71.13; H, 7.13; N, 8.75%.
The following compound was prepared in a m~nner
subst~nti~lly as desribed above except substituting ethyl bromobutyrate
for ethyl bromoacetate.
CA 02257948 1998-12-10
WO 98/00405 PCT/US97/11131
- 132-
EXAMPLE 99
~ ~0
o
3-Cyclohexyl-N-[2,3 -dihydro- l -methyl-2-oxo-5-phenyl- 1 H- 1,4-
benzodiazepin-3 -yll -N-(ethoxycarbonylpropyl)propanamide
m.p. 103-105~C, [oc]D 0.00~; c=0.85; MeOH.
Anal. Calcd. for C31H39N304.-0.40 mol H2O:
C, 70.94; H, 7.64; N, 8.01.
Found: C, 70.91; H, 7.44; N, 8.12%.
CA 02257948 1998-12-10
WO 98/00405 PCT/US97/11131
- 133-
EXAMPLE 100
N~ ~,0
~ ~l NH2
O
~ N/\,O ~o~ Cl
~3
0~
~3 ~~\
N-[2,3-Dihydro- 1 -methyl-2-oxo-5 -phenyl- 1 H- 1 ,4-benzodiazepin-3-yl] -
N- ~2-(2-methoxyethoxy)ethyllhex~n~mide
3-(R)-Amino- l ,3-dihydro- 1 -methyl-5-phenyl-2H- 1,4-
benzodiazepin-2-one (1.33 g, 5.0 mmol) in N,N-dimethyl formamide
(30 mL ) was mixed with l-bromo-2-(2-methoxyetho~.y)ethane (1.35
mL, 5.0 mmol) and triethylamine (l.O mL ). The mixture was stirred
and heated at reflux for 4 h. After that time, the reaction was cooled to
room temperature, diluted with 150 mL water, and extracted with ethyl
acetate (3 x 100 mL ). The organic layers were combined, dried with
magnesium sulfate, gravity filtered, and the solvent was removed in
CA 022~7948 1998-12-lo
W 098/00405 PCT~US97/11131
- 134-
vacuo. The resulting oil was chromatographed over silica in 1:1 ethyl
acetate:hexane, yielding the mono-alkylated product (1.2 g, 65%) as
well as the starting 1,4-benzodiazepin-2-one and bis-alkylated material.
To a solution of the mono-alkylated material (1.2 g, 3.27 mmol) in
methylene chloride (20 mL) was added hexanoyl chloride (0.96 mL,
3.27 mmol) and the reaction was stirred for 0.25 h. The reaction was
then diluted with methylene chloride (150 mL) and saturated aqueous
sodium hydrogen carbonate (150 mL) was added. The aqueous portion
was extracted again with methylene chloride (2 x 100 mL) and the
organics were combined, dried with magnesium sulfate, gravity filtered,
and the solvent was removed in vacuo. The resulting oil was
chromatographed over silica with 1 :1 ethyl acetate:
hexane, yielding an oil, giving 580 mg (38%) of the product.
[a~D 0.00~; c=0.27; MeOH.
Anal. Calcd. for C27H35N3O4~-0~80 mol H2O:
C, 67.56; H, 7.69; N, 8.75.
Found: C, 67.56; H, 7.39; N, 8.85%.
CA 02257948 1998-12-10
W O 98/00405 PCTAUS97/11131
- 135-
EXAMPLE 101
O
C~ OH
NH2
[~ O
~5~.",N~----OH Cl
~3
$ N
OH
(+)-N- [2,3-Dihydro- 1 -methyl-2-oxo-5 -phenyl- 1 H- 1 ,4-benzodiazepin-3 -
yll-N-(5-hydroxypentyl)hex~n~mide
3-(R)-Amino- 1 ,3-dihydro- 1 -methyl-5-phenyl-2H-1,4-
benzodiazepin-2-one (1.33 g, 5.0 mmol) in acetonitrile (40 mL ) was
mixed with 5-chloropentan-l-ol (0.61 g, 5.0 mmol) and sodium
hydrogen carbonate (2.0 g) was suspended in the mixture. The mixture
was stirred and heated at reflux for 12 h. After that time, the reaction
was cooled to room temperature, diluted with 100 mL water, and
extracted with ethyl acetate (3 x 75 mL ). The organic layers were
combined, dried with magnesium sulfate, gravity filtered, and the
CA 022~7948 1998-12-10
WO 98100405 PCT/US97/11131
- 136-
solvent was removed in vacuo. The resulting oil was chromatographed
over silica in 1:49 methanol:chloroform yielding the mono-alkylated
product (1.1 g, 62%) as well as the starting 1,4-benzodiazepin-2-one and
bis-alkylated material. To a solution of the monoalkylated material
(0.50 g, 1.42 mmol) in methylene chloride (30 mL ) was added
hexanoyl chloride (0.20 mL, 1.42 mmol) and the reaction was stirred
for 0.25 h. The reaction was then diluted with methylene chloride (100
mL) and saturated aqueous sodium hydrogen carbonate (100 mL ) was
added. The aqueous portion was extracted with methylene chloride
(2x75 mL ) and the organics were combined, dried with magnesium
sulfate, gravity filtered, and the ~solvent was removed in vacuo. The
resulting oil was chromatographed over silica with 1:1 ethyl
acetate:hexane, yielding a foam, giving 360 mg (64%) of the product.
foam, [oc]d + 8.36~ (c=0.61, MeOH).
Anal. Calcd. for C27H35N302.-0.25 mol H20:
C, 71.42; H, 7.~s~; N, 9.25.
Found: C, 71.47; H, 7.89; N, 9.12%.
CA 02257948 1998-12-lO
W O 98/00405 PCT~US97/11131
- 137-
EXAMPLE 102
I O
N~ Br CO2Et
NH2
~N
O
~~ CO2Et ,.
0~,
CO2Et .
(+)-N-[2,3-Dihydro-1 -methyl-2-oxo-S-phenyl- 1 H- 1 ,4-benzodiazepin-3-yl~ -N-(ethoxycarbonylpentyl)hex~n~mide
3 -(R)-Amino- 1 ,3-dihydro- l -methyl-5-phenyl-2H- 1 ,4-
benzodiazepin-2-one (1.33 g, S.0 mmol) in acetonitrile (40 mL ) was
mixed with ethyl-6-bromohexanoate (0.~9 mL, 5.0 mmol) and sodium
hydrogen carbonate (2.0 g) was suspended in the mixture. The mixture
was stirred and heated at reflux for 10 h. After that time, the reaction
was cooled to room temperature, diluted with 100 ml water, and
extracted with ethyl acetate (3x75 mL ). The organic layers were
combined, dried with magnesium sulfate, gravity filtered, and the
CA 022~7948 1998-12-10
W 098/00405 PCT~US97111131
- 13P~-
solvent was removed in vacuo. The resulting oil was chromatographed
in 1:49 methanol:chloroform, yielding the mono-alkylated product
(0.56 g, 28%) as well as the starting 1,4-benzodiazepin-2-one and bis-
alkylated material. To a solution of the mono-alkylated material (0.56
g, 1.37 mmol) in methylene chloride (20 mL) was added hexanoyl
chloride (0.19 mL, 1.37 mmol) and the reaction was stirred for 0.25 h.
The reaction was then diluted with methylene chloride (100 mL) and
saturated aqueous sodium hydrogen carbonate (100 mL) was added.
The aqueous portion was extracted again with methylene chloride (2x75
mL) and the organics were combined, dried with magnesium sulfate,
gravity filtered, and the solvent was removed in vacuo. The resulting
oil was chromatographed over silica with 1:1 ethyl acetate:hexane,
yielding a foam, giving 0.40 g (58%) of the product.
m.p. 59-65~C, [a]d (+)52.7~ (c=0.48,MeOH).
Anal. Calcd. for C30H39N3o4.-o.2o mol CH2cl2:
C, 69.4; H, 7.6; N, ~S.04.
Found: C, 69.44; H, 7.68; N, 7.71%.
The following compound was prepared in a manner
subst~nti~lly as described above except substituting ethyl bromoacetate
for ethyl 6-bromohexanoate.
CA 02257948 1998-12-10
WO 98/00405 PCT/US97/11131
- 139-
EXAMPLE 103
l O O
N
o
(+)-N-[2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1,4-benzodiazepin-3-yll -N-(ethoxycarbonylmethyl)hex~n~mide
foam, [Oc]d + 2.04~ (c=0.98; MeOH).
Anal. Calcd. for C26E~31N304:
C, 69.47; H, 6.95; N, 9.35.
Found: C, 69.41; H, 7.03; N, 9.26%.
EXAMPLE 104
~0 o
(+)-3-Cyclohexyl-N-~2,3-dihydro- 1 -methyl-2-oxo-S-phenyl- 1 H- 1,4-
benzodiazepin-3-yll -N-(hydroxymethyl)propanamide
(+)-3-Cyclohexyl-N-[2,3-dihydro- 1 -methyl-2-oxo-5-
phenyl-lH-1,4-benzodiazepin-3-yl]propanamide (2.0 g, 5.0 mmol) was
dissolved in tetrahydrofuran (30 mL), cooled to 0~C and methyl
CA 022~7948 1998-12-lo
W 098/00405 PCT~US97/11131
- 140 -
magnesium chloride (3M, 2.0 mL) was added. After 0.25 h,
paraformadehyde (0.15 g,l0 mmol) was added, and the mixture was
allowed to warm to room temperature. The reaction was then diluted
with ethyl acetate (150 mL) and saturated aqueous sodium hydrogen
carbonate (150 mL) was added. The aqueous portion was extracted
again with ethyl acetate (2 x 100 mL) and the organics were combined,
dried with magnesium sulfate, gravity filtered, and the solvent was
removed in vacuo. The resulting oil was chromatographed over silica
with 1:1 ethyl acetate:hexane, yielding a foam (0.80 g, 37%).
foam, [a]d + 124~ (c=0.69, MeOH).
Anal. Calcd. for C26H31N303:
C, 72.03; H, 7.21; N, 9.69.
Found: C, 71.66; H, 7.08; N, 9.78%.
The following compound was prepared in a manner
substantially as described above starting from (+)-N-~2,3-dihydro-l-
methyl-2-oxo-5-phenyl-lH-1 ,4-benzodiazepin-3-yl]hex~n~mide.
EXAMPLE 105
l O O
) N OH
[~
(+)-N-[2,3-Dihydro- 1 -methyl-2-oxo-5-phenyl- 1 H- 1 ,4-henzodiazepin-3-
yll-N-(hydroxymethyl)hex~n~mide
m.p. 154-156~C, ~~~]d + 190.~¢~ (c=0.24, MeOH).
Anal. Calcd. for C23H27N3O3-0.30 mol H20:
C, 69.26; H, 6.97; N, 10.53.
CA 02257948 1998-12-10
WO 98/0040S PC'r/US97/11131
- 141 -
Found: C, 69.29; H, 6.81; N, 10.6%.
EXAMPLE 106
(+)-3-Cyclohexyl-N-[2,3-dihydro- 1 -methyl-2-oxo-S-phenyl- 1 H- 1,4-
benzodiazepin-3-yll -N-(tetrazolylmethyl)propanamide
(+)-3-Cyclohexyl-N-[2,3 -dihydro- 1 -methyl-2-oxo-S-
phenyl- 1 H- 1,4-benzodiazepin-3-yl]-N-(hydroxymethyl)propanamide
(0.67 g, 1.56 mmol) was dissolved in methylene chloride(100 mL),
along with tetrazole (0.33 g, 4.7 mmol), and then N,N-diisopropyl-
dibenzyl-phosphoramidite (1.07 g, 3.1 mmol). After 2 h, the mixture
was diluted with methylene choride (150 mL), and extracted with
saturated aqueous sodium hydrogen carbonate (3 x lQ0 mL). The
organic layers were combined, dried with magnesium sulfate, gravity
filtered, and the solvent was removed in vacuo. The resulting oil was
chromatographed twice over silica with 1:1 ethyl acetate:hexane,
yielding two constitutional isomers, a (65 mg, 9%) and b (56 mg,
7.5%).
- Isomer A:
m.p. 96-98~C, ~a1d +188.9~ (c=0.19, MeOH).
Anal. Calcd. for C27H31N7O2-0.30 mol TFA:
C, 63.78; H, 6.07; N, 18.86.
Found: C, 63.7; H, 6.12; N, 1~.76%.
CA 022~7948 1998-12-10
WO 98/00405 PCT/US97111131
- 142-
Isomer B:
m.p. 92-95~C, [oc]d +81.3~ (c=0.31, MeOH).
Anal. Calcd. for C27H31N7O20.35 mol TFA:
C, 63.31; H, 6.01; N, 18.66.
Found: C, 63.35; H, 6.02; N, 18.74%.
3~XAMPLE 107
NJ~O~
~3
3R-(+)-3-(Benzyloxycarbonylamino)-2,3-dihydro- 1 -methyl-2-oxo-5-
phenyl- 1 H- 1 ~4-benzodiazepine
To a stirring solution of 3-(R)-amino-1,3-dihydro-1-
methyl-5-phenyl-2H-1,4-benzodiazepin-2-one (2.0 g, 7.5 mmol) in
methylene chloride (45 mL ) at 0~C was added benzyl chloroformate
(1.2 mL, 8.3 mmol) and the reaction was allowed to warm to room
temperature. The reaction mixture was diluted with methylene chloride
(150 mL ), and extracted with saturated aqueous sodium hydrogen
carbonate (150 mL ). The a~ueous portion was extracted with
methylene chloride (2 x 100 mL ) and the organics were combined,
dried with magnesium sulfate, gravity filtered, and the solvent was
removed in vacuo. The resulting oil was chromatographed over silica
with 1: 1 ethyl acetate:hexane, yielding a white foam (3.0 g, 99.7%)
[~C]d +57.5~ (c=1.17; MeOH).
Anal. Calcd. for C24H20N303.- 0.70 mol H20 ~0.15 mol CHC13:
C, 67.62; H, 5.06; N, 9.8.
Found: C, 67.6; H, 5.02; N, 9.75%.
CA 022~7948 1998-12-10
WO 98/0040~ PCT/US97/11131
- 143 -
The following compounds were prepared subst~nti~lly as
described in Example 81.
EXAMPLE 108
N- [2,3 -Dihydro- 1 -methyl-2-oxo-5-ethyl- 1 H- 1,4-benzodiazepin-3-yl] -3 -
(2.4-dichlorophenyl)propanamide
m.p. 156-15~S~C.
CHN: Anal. Calcd. for C2lH2lcl2~3o2-o.s H2O:
C, 59.02; H, S.l9; N, 9.83.
Found: C, 58.99; H, 4.89; N, 9.88.
EXAMPLE 109
N-[2,3-Dihydro- 1 -methyl-2-oxo-S-t-butyl- 1 H- 1,4-benzodiazepin-3-yl]-
3-(2.4-dichlorophenyl)propanamide
m.p. 170-171~C.
CHN: Anal. Calcd. for C23H2scl2N3o2-o.7 H2O:
C, 60.18; H, 5.80; N, 9.16.
Found: C, 60.17; H, 5.30; N, 9.30.
EXAMPLE 110
N-[2,3-Dihydro- 1 -methyl-2-oxo[4'-(4,4-dimethyl-2-oxazolinyl)phenyl]-
1 H- 1.4-benzodiazepin-3-yll -3-(2.4-dichlorophenyl)propanamide
m.p. 188-190~C.
CHN: Anal. Calcd. for C30H28N403C12:
C, 63.95; H, 5.01; N, 9.94.
Found: C, 63.96; H, 5.02; N, 10.08.
EXAMPLE 111
N-[2,3-Dihydro- 1 -methyl-2-oxo-5-(4-methoxyphenyl)- 1 H- 1,4-
benzodiazepin-3 -yll -3-(2.4-dichlorophenyl)propanamide
CA 02257948 1998-12-lO
W 098/00405 PCT~US97/11131
- 144-
m.p. 188- 189~C.
CHN: Anal. Calcd. for C26H23C12N303-0.45 H20:
C, 62.91; H, 4.67; N, 8.47.
Found: C, 61.89; H, 4.78; N, 8.33.
EXAMPLE 112
(+)-3,5-Dichloro-N-[3R-2,3-dihydro-2-oxo-5-phenyl- 1 -(2,2,2-
trifluoroethyl)-lH-benzolel~1 ~41diazepin-3-yllbenzamide.
~N HJ~
Cl
Step A: Preparation of 2,3-dihydro-2-oxo-5-phenyl-1-(2,2,2-
trifluoroethyl)- lH-benzo[e] [1,4]diazepine.
A solution of 5-phenyl-1,4-benzodiazepine-2-one
(J. Org. Chem., 1962, 27, 3788)(50 g, 0.211 mole) in DMF (100 mL)
was treated with cesium carbonate (103.5 g, 0.317 mole) and
trifluoroethyl iodide (109.7 g, 0.525 mole). The mixture was stirred at
50~C overnight. The reaction mixture was then poured into water (2 L)
and extracted with ethyl acetate (3 X 1 L). The combined ethyl acetate
fractions were dried over anhydrous m~nesium sulfate, filtered and
concentrated at reduced pressure. The residue was crystallized from
ethyl ether to give 45 g (68 %) of the product. MP = 130 - 131~C;
lH NMR (CDC13, 300 MHz) d 7.65-7.60 (m, 2H), 7.60-7.45 (m, SH),
7.40-7.20(m, 2H), 5.25 (dq, J = 14, 8.6 Hz, lH), 4.82(d, J = 10.5 Hz,
lH), 4.15 (app sextet,3 = P~.6 Hz, lH), 3.81 (d, J = 10.5 Hz, lH)
CA 022~7948 1998-12-lo
W O 98/00405 PCT~US97/11131
- 145-
Step B: Preparation of 3-Azido-S-phenyl-1-(2,2,2-trifluoroethyl)-
1 H-benzo[e] [ 1 ,4]diazepine.
To a stirring solution of S-phenyl-1-(2,2,2-trifluoroethyl)-
lH-benzo[e][1,4]diazepine (70 g,0.22 mol) in THF (lS00 mL) cooled to
-70~C was added potassium tert-butoxide(1.1 eq, 0.24 mol, 240 mL of a
1 N solution in THF) dropwise over lS min. A solution of 2,4,6-
triisopropylbenzenesulfonylazide (74.8 g, 0.24 mol) in THF (250 ml)
was added over S min. This was stirred for 10 minutes and acetic acid
(40 mL, 0.63 mol) was added and the reaction allowed to warm to
ambient temperature. The reaction was poured into satd. NaHCO3
(lS00 mL) and ethyl acetate (lL). The phases were separated and the
aqueous phase was extracted with ethyl acetate(S00 mL). The combined
organic layers were washed with water (500 mL) then brine (300mL).
The organic layers were dried with Na2SO4 and evaporated to a brown
foam. This was triturated with ethyl ether to give 65 g of a white
powder. The filtrate was concentrated and chromatographed over silica
gel eluting with 30% ethyl acetate/hexane to give another 8.9 g. The
combined yield was 74 g( 93%). MP = 159 - 160~C;
1H NMR (CDC13, 300 MHz) d 7.70-7.26 (m,9H), 5.28-5.12 (m,lH),
4.63 (s,lH), 4.35-4.10 (m,lH).
Step C: Preparation of racemic 3-Amino-5-phenyl- 1-(2,2,2-
trifluoroethyl)- 1 H-benzo[e] [ 1 ,4]diazepine.
To a stirring solution of 3-Azido-2-oxo-5-phenyl-1-(2,272-
trifluoroethyl)-2,3-dihydro- 1 H-benzo[e~ ~1 ,4]diazepine (83 .4mmoL30g)
in 300mL ethanol and lSOmL THF was added 10%Pd/C (10 wt%, 3.0g)
Hydrogen gas was bubbled through the solution for 8h. The reaction
was filtered and evaporated under reduced pressure. The residue was
crystallized from ethyl ether to give 20.0g of white crystals. Another
4g was recovered from evaporation and recryst~lli7~tion of the filtrates.
Combined yeild, 86.7%.
CA 022~7948 1998-12-1o
W 098/00405 PCTrUS97111131
- 146-
MP = 141 - 143~C;
1 H NMR (CDC13,300 MHz) d 7.70-7.26 (m,9H), 5.28-5.12 (m, l H),
4.57 (s,1 H), 4.35-4.10 (m, l H).
Step D: Preparation of 2-Amino-N-[2-oxo-5-phenyl- 1 -(2,2,2-
trifluoroethyl)-2,3 -dihydro- 1 H-benzo[e] [1,4]diazepin-
3-yl] -3 -phenylpropionamide
To a stirring solution of 3-Amino-2-oxo-5-phenyl-1-(2,2,2-
trifluoroethyl)-2,3-dihydro-1 H-benzo[e] [1,4]diazepine (92.2 mmol,
30.74g) in DMF (300mL) was added N-Benzyloxy-D-Phenylalanine
(92.2 mmol, 27.6g), 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide
hydrochloride (0.12mol,22.95g) and 1-hydroxybenztriazole hydrate
(46.1mmol,6.23g). This was stirred at room temperature for 2h. The
reaction was then diluted with lL of 10~7 KHSO4 and extracted with
ethyl acetate (2x600 mL). The organic layers were combined and
washed with saturated sodium hydrogen carbonate (600mL). They were
dried with brine and sodium sulfate and evaporated under reduced
pressure. 66.58g of an orange foam, which contained ethyl acetate by
NMR. NMR 1H (CDC13) d 7.75-7.18 (m, 20H), 5.62-5.55 (m,lH),
5.48-5.00 (m, 4H), 4.72-4.60 (m, lH), 4.25-4.05 (m,lH) 3.32-3.05 (m,
2H). This material was carried on without further purification.
To a stirring solution of 2-(N-Benzyloxyamino)-N-[2-oxo-
5-phenyl- 1 -(2,2,2-trifluoroethyl)-2,3-dihydro- 1 H-benzo[e] [1,4]diazepin-
3-yl]-3-phenyl propionamide in lL ethanol was added 10% Pd/C (15
wt%) and hydrogen was bubbled through the reaction for 2h and then
left stirring under 1 atm. hydrogen overnight. Hydrogen was bubbled
through the reaction for an additional three hours the following
morning. The reaction was then filtered, the catalyst was rinsed with
lL methylene chloride and evaporated under reduced pressure. The
resulting solid was dried under vacuum overnight to give 44.46g of a
white solid. This was chomatographed over silica, eluting with 1 %
MeOH:EtOAc. The pure upper Rf fractions were collected and
CA 022~7948 1998-12-lo
W O 98/00405 PCTAUS97/11131
- 147-
evaporated under reduced pressure. The mixed fraclions were
collected, evaporated and rechromatographed. The pure fractions were
collected and combined with the above pure fractions to get a combined
yield of 18.1 lg, 83.5% of the upper Rf diastereomer. 1H NMR
(CDCl3,300 MHz) d 8.94 (d, J-8.6Hz, lH), 7.65-7.10 (m, 9H), 5.64 (d,
J=8.6 Hz, lH), 5.28-5.12 (m, lH), 4.57 (s, lH), 4.35-4.10 (m, lH)
3.71 (dd, J=9.8 and 3.9 Hz, lH), 3.34 ( dd, J=13.9 and 3.9 Hz,lH), 2.79
(dd, J=13.9 and 10.0 Hz, lH). The Absolute stereochemistry at C-3 of
the benzodiazepine ring was determined to be (R) by X-Ray analysis.
The lower Rf material corresponding to C-3(S) was isolated
as well.
Step E: Preparation of 3(R)-(+)-3-Amino-5-phenyl- 1 -(2,2,2-
trifluoroethyl)- 1 H-benzo [e] [1,4] diazepine .
To a stirring solution of 2-Amino-N-[2-oxo-5-phenyl-1-
(2,2,2-trifluoroethyl)-2,3-dihydro- l H-benzo[e] [1,4]diazepin-3-yl]-3-
phenylpropionamide (13.6 g, 28.3 mmol) in methylene chloride (136
mL) was added phenyl isothiocynate (3.87 mL, 34.0 mmol). This was
stirred overnight at ambient temperature. The reaction was then cooled
in ice, trifluoroacetic acid (2.73 mL, 0.283 mol) added and the reaction
allowed to warm to ambient temperature. After stirring at ambient
temperature for 2.5 hours the reaction was evaporated under reduced
pressure, chromatographed with 90:10:1:1 methylene chloride:
methanol:acetic acid:water. The low Rf spot was collected and
evaporated under reduced pressure with no heat. The residue was taken
up in 600 mL methylene chloride and washed with 300 mL saturated
NaHCO3 and 300 mL water. The solution was dried over Na2so4 and
evaporated under reduced pressure. The residue wa~ crystallized from
ethyl acetate:hexanes to give 6.65 g of a white powder .
MP = 162 - 164~C;
lH NMR (CDC13,300 MHz) d 7.70-7.26 (m,9H), 5.28-5.12 (m,lH),
4.57 (s,lH), 4.35-4.10 (m,lH).
CA 022~7948 1998-12-10
WO 98/00405 PCT/US97111131
- 14~-
[a]D = +72.9~ (c=0.7, MeOH)
The (-)-3S enantiomer was prepared in the same fashion
from the Lower Rf product of Step D.
MP = 156 - 158~C;
lH NMR (CDC13,300 MHz) d 7.70-7.26 (m,9H), 5.28-5.12 (m,lH),
4.57 (s,lH), 4.35-4.10 (m,lH).
[a]D = -71.2~ (c=0.66, MeOH)
Step F: Preparation of (+)-3,5-Dichloro-N-[3R-2,3-dihydro-2-oxo-
5- phenyl-1-(2,2,2-trifluoroethyl)-lH-benzo[e][1,4]diazepin-3-
yl]benzamide:
To a stirring solution of (+)-3R-3-amino-5-phenyl-1-
(2,2,2-trifluoroethyl)-lH-benzo[e][1,4] diazepine (5.6 g, 16.8 mmol) in
DMF (50 mL) was added 1-(3-Dimethylaminopropyl-3-ethylcarbodi-
imide hydrochloride(4.44 g, 23.0 mmol), and l-hydroxybenztriazole
hydrate (3.11 g, 23.0 mmol) and 3,5-Dichlorobenzoic acid (3.21 g, 16.8
mmol). This was stirred at ambient temperature for 2 hours. The
reaction was diluted with 500 mL satd. NaHCO3 and extracted with 2 x
300 mL ethyl acetate. The combined organics were washed with 10%
KHSO4 (200 mL), brine (200 mL), dried over Na2SO4, and
evaporated to a white foam. This was chromatographed over a 75 x 200
mm silica column eluting with 20% ethyl acetate:hexane. The pure
fractions were collected and evaporated under reduced pressure to give
8.5 g of a white foam which was crystallized from 15% ethyl
acetate:hexane to give 5.3 g o~ a white powder . mp=140-143~C,
[a]D=+47~9~; lH NMR (CDC13, 300 MHz) d 7.85-7.75 (m, 2 H), 7.70-
7.20 (m, 9 H), 5.78 (d, J=8.1 Hz,l H), 5.30-5.15 (m, lH), 4.30-4.15 (m,
lH)
Analysis Calcd. for C24Hl 6cl2F3N3o2:
C, 56.93; H, 3.19; N, 8.30;
Found: C, 56.81; H, 3.17; N, 8.17.
CA 02257948 1998-12-10
WO 98/00405 PCT/US97111131
- 149-
The following examples were prepared by a procedure substantially as
described for Example 1, Step F.
EXAMPLE 113
(-)-2-(3,4-Dichlorophenyl)-N-[3R-2,3-dihydro-2-oxo-5-phenyl-
1-(2~2~2-trifluoroethyl)-lH-benzo~ell l.41diazepin-3-yllacetamide.
~CF3 Cl
" H ~
,~
Y
mp=219-221~C; ~a]D=-10.8~;
1H NMR (CDC13,300 MHz) d 7.65-7.15 (m, 12H), 5.78 (d, J=8.1 Hz,
lH), 5.25-5.10 (m, lH), 4.25-4.05 (m, lH), 3.56 (s, 2H);
Analysis Calcd. for C25H 1 ~C12F3N302-0.85 H2O:
C, 56.06; H, 3.71; N, 7.84.
Found: C, 56.03; H, 3.53; N, 7.82.
EXAMPLE 114
(-)-2-(3,5-Dichlorophenyl)-N-~3R-2,3-dihydro-2-oxo-5 -phenyl-
1-(2~2.2-trifluoroethyl)-lH-benzo~el~1.41diazepin-3-yllacetamide
CA 02257948 1998-12-lo
W O 98/00405 PCTrUS97/11131
- 150-
"" NH
mp=93-100~C, [a]D = -5-7~;
lH NMR (CDC13,300 MHz) d 7.65-7.15 (m, 12H), 5.78 (d, J=8.1 Hz,
lH), 5.25-5.10 (m, lH), 4.25-4.05 (m, lH), 3.65 (s, 2H);
Analysis Calcd. for C25Hl 8cl2F3N3o2:
C, 57.71; H, 3.49; N, 8.08;
Found: C, 57.41; H, 3.48; N, 8.12.
EXAMPLE 115
(-)-2-[3 ,5-Bis(trifluoromethyl)phenyl] -N-[3R-2,3 -dihydro-2-oxo-
S-phenyl- 1 -(2,2,2-trifluoroethyl)- 1 H-benzo[e] [ 1 ,4]diazepin-3 -
yllacetamide
~ ~'
m.p. foam ~C, [a]D = -9.7~ (c=0.59,MeOH).
Anal. Calcd. for C27HlgFgN302-0.75 H20:
C, 53.96; H, 3.27; N, 6.99.
CA 02257948 1998-12-lo
W O 98/00405 PCTAUS97/11131
- 151 -
Found: C, 53.96; H, 3.1; N, 6.98%.
EXAMPLE 116
(-)-2-(4-Trifluoromethylphenyl)-N-[3R-2,3-dihydro-2-oxo-5-
phenyl- 1 -(2.2~2-trifluoroethyl)- 1 H-benzol el r 1 .41diazepin-3-yllacetamide
CF3
N H C F3
,~
~ Y
m.p. 253-255 ~C, [a]D =-9.2~ (c=0.25, MeOH).
Anal. Calcd. for C26H1 gF6N3O2-0.05 ethyl etherO.55 H20:
C, 59.03; H, 3.9; N, 7.88.
Found: C, 59.05; H, 3.82; N, 7.78%.
EXAMPLE 1 17
2-(3 -Trifluoromethylphenyl)-N-[3R-2,3-dihydro-2-oxo-5 -phenyl-
1 -(2.2,2-trifluoroethyl)-lH-benzo~el~1 ~41diazepin-3-yllacetamide
~ "N~CF3
m.p. 172-173 ~C, [a]D = +5.9~ (c=0.56, CHC13).
CA 02257948 1998-12-lo
W O 98/0040~ PCT~US97/11131
- 152-
Anal. Calcd. for C26H1gF6N302-0.60 H20:
C, 58.89; H, 3.84; N, 7.92.
Found: C, 58.92; H, 3.71; N, 7.98%.
EXAMPLE 118
(+)-2-(2-Trifluoromethylphenyl)-N-[3R-2,3-dihydro -2-oxo-5-
phenyl-1-(2.2.2-trifluoroethyl)-lH-benzolel~1 .41diazepin-3-yllacetamide
NJ~3
m.p. 170-171 ~C, [a]D = ~9.0~ (c=0.48, CHC13).
Anal. Calcd. for C26HlgF6N3O2-0.25 H20:
C, 59.6; H, 3.75; N, 8.02.
Found: C, 59.64; H, 3.68; N, 7.97%.
EXAMPLE1 1 9
(-)-2-(2,4-Dichlorophenyl)-N-[3R-2,3-dihydro-2-oxo-5-phenyl-
1-(2.2.2-trifluoroethyl)-lH-benzo~el~ 1 .41diazepin-3-yllacetamide
' h
~ Y
CA 02257948 1998-12-10
W 098/00405 rCTAUS97/11131
- 153-
m.p. 143-145 ~C, [a]D = -22.6~ (c=0.73; MeOH).
Anal. Calcd. for C2sHl 8N3o2cl2~3:
C, 57.71; H, 3.49; N, 8.08.
Found: C, 57.75; H, 3.52; N, 8.09%.
EXAMPLE 120
(-)-2-(3 -Chlorophenyl)-N-[3R-2,3-dihydro-2-oxo-S-phenyl- 1 -
(2.2.2-trifluoroethyl)-1 H-benzo~el ~ 1.41diazepin-3-yllacetamide
¢~N H Cl
,~
m.p. 188-189 ~C, [a]D = -5.4~ (c=1.03,MeOH).
Anal. Calcd. for C25H1gClF3N3O2-0.10 ethyl ether:
C, 61.84; H, 4.09; N, 8.52.
Found: C, 61.84; H, 4.05; N, 8.5%.
EXAMPLE 121
(-)-2-(4-Chlorophenyl)-N-[3R-2,3 -dihydro-2-oxo-S-phenyl-
1 -(2.2~2-trifluoroethyl)- 1 H-benzol el l 1.41diazepin-3-yllacetamide
CA 02257948 1998-12-10
W O 98/00405
- 154-
CF3
N H C I
,~
W
m.p. 246-247 ~C, [a]D =-10.1~ (c=0.45,MeOH).
Anal. Calcd. for C25H1gClF3N302-0.20 H20 0.15 ethyl ether:
C, 61.42; H, 4.21; N, 8.39.
Found: C, 61.46; H, 4.15; N, 8.39%.
Example 122
(-)-2-[2,4-Bis(trifluoromethyl)phenyl]-N-[3R-2,3 -dihydro-2-oxo-5-
phenyl- 1 -(2 ~2~2-trifluoroethyl )- I H-benzol el ~ I .4l diazepin-3 -yll acetamide
CF3
N~O ~J~C F3
~N H CF3
,~,
~ Y
Step A. 2,4-Bis(trifluoromethyl)benzonitrile
To a stirring biphasic mixture of 100mL ethanol and 250
mL of phosphate buffer (lg of NaH2PO4-H2O per S mL H2O adjusted
to pH=7.0 with 50% NaOH) and NaCN (81.3mmol,4.0g) heated to 60~C
was added 2,4-bis(trifluoromethyl) benzyl bromide (32.5mmol,10g) in
SOmL EtOH dropwise over 30min. The reaction was heated at 60~C
CA 022~7948 1998-12-lO
W 09810040S PCTrUS97/11131
- 155-
for 24h. The reaction was then evaporated under reduced pressure.
The rem~ining aqueous was extracted with 2xl50mL EtOAc. The
organic layers were combined, dried with brine and Na2SO4. The
organic phase was evaporated under reduced pressure and the residue
chromatographed over silica eluting with 10% EtOAc:Hexanes. The
pure fractions were collected and evaporated to give 7.0g of a pale
yellow oil, 85.1% NMR lH (CDC13) d 8.0-7.85 (m,3H), 4.03 (s,2H)
Step B. 2,4-Bis(trifluoromethyl)phenyl acetic acid
2,4-Bis(trifluoromethyl)benzonitrile (41.5mmol,10.51 g)
was taken up in 100mL acetic acid, 50mL conc. H2SO4, and 20mL
water. This was heated to 120~C for 3h. The reaction was then diluted
with lL ice water, and extracted with 2x300mL ethyl acetate. The
combined organics were washed with 2x200mL water, dried with brine
and Na2SO4, and evaporated under reduced pressure. The residue was
taken up in a minimum of diethyl ether and crystallized by adding
sufficient hexane to precipatate the product. The solid was collected to
give 7.74g of 2,4-bis(trifluoromethyl) phenyl acetic acid as white
crystals, 68.5 %.NMR 1 H (CDC13) d 7.93 (s,1 H), 7.80 (d, J=7.9Hz, l H),
7.55 (d, J=7.9Hz, l H), 3.94 (s,2H).
Step C. Preparation of (-)-2-[2,4-Bis(trifluoromethyl)phenyl]-N-
[3R-2,3-dihydro-2-oxo-5-phenyl- 1 -(2,2,2-trifluoroethyl)-
1 H-benzo[e] [1,4]diazepin-3-yl]acetamide
To a stirring solution the 3R-3-Amino-2-oxo-5-phenyl-1-
(2,2,2-trifluoroethyl)-2,3-dihydro- 1 H-benzo[e] [1,4~diazepine (28.4
mmol, 9.47g) in DMF (lOOmL) was added 2,4-Bis(trifluoromethyl)
phenyl acetic acid (2~.4mmol,7.74g), 1-(3-Dimethylaminopropyl)-3-
ethylcarbodiimide hydrochloride (42.6mmol,8.16g) and 1-Hydroxy-
benztriazole hydrate (14.2mmol,1.92g). This was stirred for lh at
room temperature. The reaction was then diluted with 750mL of 10%
KHSO4 and extracted with ethyl acetate (2x300mL). The organic layers
CA 02257948 1998-12-lO
W O 98/00405 PCT~US97/11131
- 156-
were combined and washed with saturated sodium hydrogen carbonate
(lx600mL). The organics were then dried with brine, and sodium
sulfate and evaporated under reduced pressure. The residue was
chromatographed over silica eluting with 20% EtOAc:Hexane. Pure
fractions were collected and evaporated. The residue was taken up in
100 mL of warm 75% isopropanol:water. This was allowed to cool
slowly and stirred overnight (16 hr) at room temperature. The
suspension was cooled briefly to @5~C and filtered. The white solid
was dried overnight at 60~C to give 10.5 g of material that melted at
132-134~C. X-Ray diffraction confirrns crystallinit~.
NMR 1H (CDC13) d 7.95-7.25 (m,13H), 5.60 (d,J=8.1Hz,lH), 5.30-
5.10 (m,lH), 4.25-4.06 (m,lH), 3.96 (s,2H)
Anal. Calcd. for C27H 18F9N3O2:
C, 55.20; H, 3.09; N, 7.15.
Found: C, 55.03; H, 3.14; N, 7.10%.
EXAMPLE 123
( i)-2-(3,5-Dichlorophenyl)-N-[2,3-dihydro-2-oxo-5-phenyl-
1 -(2.2~2-trifluoroethyl)- 1 H-benzolel ~ 1 ~41diazepin-3-yll acetamide
F3C Cl
N ~CI
,~
W
m.p. 219-220 ~C. racemic
Anal. Calcd. for C25Hl gN3O2C12F3:
C, 57.71; H, 3.49; N, 8.08.
Found: C, 57.94; H, 3.48; N, 8.02%.
CA 02257948 1998-12-10
WO 98/00405 PCT/US97tlll31
- 157-
EXAMPLE 124
2-(3,5-dichloro-4-methoxyphenyl)-N-[3R-2,3-dihydro-2-oxo-5-
phenyl-1-(2.2~2-trifluoroethyl)-lH-benzo~ell 1~41diazepin-3-yllacetamide
¢~~ ~OCH3
m.p. 100-104 ~C, ~a]D = -8.9~ (c=0.55,MeOH).
Anal. Calcd. for C26H20cl2F3N3o3:
C, 56.74; H, 3.66; N, 7.63.
Found: C, 55.67; H, 3.47; N, 7.41%.
The following examples were prepared by procedures
substantially as described in example 1 except substituting the
appropriate fluoro substituted aminobenzophenone in step A.
EXAMPLE 125
(+)-2-(3,5-Dichlorophenyl)-N-[2,3-dihydro-5-(4-fluorophenyl)-2-
oxo-1-~2.2.2-trifluoroethyl)-1H-benzorell 1~41diazepin-3-yllacetamide
,
CA 02257948 l998-l2-lO
W O 98/00405 PCTrUS97/11131
- 158-
Cl
F3C
¢~ N ~J~C I
F
m.p. foam ~C, [a]D = +3.4~ (c=0.55; MeOH).
Anal. Calcd. for C25H17N302C12F4:
C, 55.78; H, 3.18; N, 7.81.
Found: C, 55.73; H, 3.25; N, 7.72%.
EXAMPLE 126
(-)-2-(2,4-Dichlorophenyl)-N-[2,3 -dihydro-S -(4-fluorophenyl)-2-
oxo-l -(2.2~2-trifluoroethyl~-lH-benzolelrl ~41diazepin-3-yllacetamide
~$ H C
F
m.p. foarn ~C, [a]D = - 11 ~ (c=0.68; MeOH).
Anal. Calcd. for C25H17N302F4:
C, 55.78; H, 3.18; N, 7.81.
Found: C, 55.82; H, 3.41; N, 7.42%.
CA 02257948 1998-12-10
WO 98/0040S rCT~US97/11131
- 159-
EXAMPLE 127
(+)-2-(3 ,5-Bis(trifluoromethyl)phenyl)-N-[2,3-dihydro-5-(4-
fluorophenyl)-2-oxo- 1 -(2,2,2-trifluoroethyl)- l H-benzo[e]
r 1 ~41diazepin-3-yll-acetamide
CF3
¢~S~~ CF3
F
m.p. foam ~C, [a]D = +2.8~ (c=0.67; MeOH).
Anal. Calcd. for C27Hl7N302FlO:
C, 53.56; H, 2.83; N, 6.94.
Found: C, 53.56; H, 2.93; N, 6.91%.
EXAMPLI~ 128
(-)-2-[2,4-Bis(trifluoromethyl)phenyl] -N-[2,3-dihydro--5-(4-
fluorophenyl)-2-oxol-(2,2,2-trifluoroethyl)-lH-benzo[e][1 ,4
diazepin-3-yllacetamide
CA 02257948 1998-12-10
WO 98/00405 PCT~US97/11131
- 160-
F~C ,~CF3
F
[a]D = -14~ (c=0.63; MeOH).
Anal. Calcd. for C27H17N302F10:
C, 53.56; H, 2.83; N, 6.94.
Found: C, 53.3; H, 2.89; N, 7.05%.
EXAMPLE 129
3-Cyclohexyl-N-[2,3-dihydro-5-(2-fluorophenyl)-2-oxo- l -
(2.2~2-trifluoroethyl- l H-benzo~el l l ~41diazepin-3-yllpropionamide
CF3
H ~/'~
g~_F
m.p. 202-204 ~C.
lH NMR d (CDC13) 7.72 (m,8H), 5.65 (d,J=8.3Hz,lH), 5.35-5.08
(m,lH), 4.32-4.15 (m,lH), 2.37 (t,J=7.8Hz,2H), 1.80-1.55 (m,7H),
1.45-Anal. Calcd. for C26H27F4N3o2:
C, 63.8; H, 5.56; N, 8.58.
CA 02257948 1998-12-10
WO 98/00405 PCT/US97/11131
- 161 -
Found: C, 63.82; H, 5.54; N, 8.56%.
EXAMPLE 130
3,4-Dichloro-N-[2,3-dihydro-5-(2-fluorophenyl)-2-oxo- 1 -
(2.2.2-trifluoroethyl)- 1 H-benzol el I 1 .41diazepin-3-yllbenzamide
~_ H J~C I
F
m.p. 168-170 ~C.
1H NMR d (CDC13) 8.03 (d,J=2.0,1H), 7.86 (d,J=7.8Hz,lH), 7.7~-7.05
(m,9H), 5.80 (d,J=7.8Hz,lH), 5.27-5.15 (m,lH), 4.35-4.20 (m,lH)
Anal. Calcd. for C24Hl 5C12F4N302:
C, 54.98; H, 2.88; N, 8.01.
Found: C, 54.96; H, 2.89; N, 8.12%.
EXAMPLE 131
Antiarrhythmic efficacy of the combined ~lministration of
low dose IKS blocker (-)-2-[2,4-bis(trifluoromethyl)phenyl]-n-[3r-2,3-
dihydro-2-oxo-5-phenyl- 1 -(2,2,2-trifluoroethyl)- 1 h-benzo[e] [ 1 ,4~
diazepin-3-yl]acetamide and the beta-adrenergic receptor antagonist
timolol in anesthetized dogs with previous anterior myocardial
infarction was studied. This study was conducted to t~st the hypothesis
the concomitant ~lminis-tration of low dose IKS blocher (-)-2-[2,4-
Bis(trifluoromethyl)phenyl] -N- [3R-2,3 -dihydro-2-oxo-5-phenyl- 1-
(2,2,2-trifluoroethyl)-1 H-benzo[e] [ 1 ,4]diazepin-3-yl]acetamide and
CA 022~7948 1998-12-10
WO 98/00405 PCT/US97/11131
- 162-
low dose beta-adrenergic receptor antagonist timolol, which when
~lmini~tered individually at low dose afford no significant anti-
arrhythmic protection, would in combination provide significant
protection against the development of malignant ventricular arrhythmias
in a canine model of previous myocardial infarction. In the absence of
protective intervention, this postinfarction canine preparation displays a
very high incidence ( 80%) of malignant ventricular tachycardia
degenerating into ventricular fibrillation and death in response to the
development of ischemia at a site remote from previous myocardial
infarction (Patterson et al., Am. J. Cardiol. 50: 1414-1423, 1982;
Wilber et al., Am. Heart J. 109: 8-18, 1985; Lynch
et al., J Pharmacol Exp Therap 277: 671-678, 1996).
METHODS
Surgical Preparation.
Male or female purpose-bred mongrel dogs (7-12 kg)
were preanesthetized with sodium thiamylal (5.0 mg/kg i.v.) and general
anesthesia was induced with isoflurane. A left thoracotomy was
performed in the 4th intercostal space, the pericardillm incised, and the
heart suspended in a pericardial cradle. Anterior myocardial infarction
was produced by a two hour occlu~sion of the left anterior descending
coronary artery followed by reperfusion. Surgical incisions were
closed, and the ~nim~ls were allowed to recover.
Electrophysiologic Testing, Programmed Ventricular Stimulation (PVS)
and Acute Posterolateral Myocardial Ischemia
Purpose-bred male or female mongrel dogs at 5-20 days
after anterior myocardial infarction were anesthetized with alpha
chloralose (80.0-100.0 mg/kg i.v.). The ~nim~ls were ventilated by
means of a cuffed endotracheal tube and a volume-cycled respirator
(Model 613, Harvard Apparatus, S. Natick, MA). Systemic arterial
pressure was monitored via the cannulated left common carotid artery
(Model P23XL pressure transducer, Gould Inc, Cleveland, OH), and the
CA 022~7948 1998-12-10
WO 98/00405 PCT/US97/11131
- - 163 -
right femoral vein was isolated and c~nn~ ted for drug ~lmini~tration.
The heart was re-exposed via a left thoracotomy, and was suspended in a
pericardial cradle. A platinum epicardial bipolar electrode (3 mm
electrode post separation) was sutured to the surface of the left atrial
appendage for atrial pacing, and a stainless steel bipolar plunge
electrode (5 mm length, 3 mm electrode post separation) was inserted
into the interventricular septum near the right ventricular outflow tract
(RVOT) adjacent to the site of left anterior descending coronary artery
occlusion for the introduction of ventricular extrastimuli during
programmed ventricular stimlll~tion (PVS). One acrylic button per zone
containing fixed stainless steel bipolar plunge electrodes (see below for
specifications) was sutured into the infarcted anterior region of the left
ventricle distal to the site of coronary artery occlusion and within the
area of myocardial scarring as ascertained visually and by palpation (IZ,
infarct zone) and into the non-infarcted posterolateral region of the left
ventricle (NZ, non-infarct zone) for the measurement of ventricular
electrophysiologic parameters (excitation thresholds and refractory
periods). Lead II electrocardiogram was monitored continuously.
After stabilization of the preparation and the measurement
of baseline electrocardiographic and cardiac electrophysiologic
parameters, PVS consisting of the introduction of 1-3 ventricular
extrastimuli during sinus rhythm and atrial pacing was performed at the
RVOT site. If ventricular extrastimuli could not be inserted at the
RVOT site, programmed pacing was attempted at the IZ site.
Ventricular extrastimuli were introduced at 2x diastolic threshold
voltage or, if extrastimuli were not introduced adequately, at 4x
diastolic threshold voltage. Responses to baseline programmed
ventricular stimulation were categorized as: 1) non-inducible (NI): less
than 5 nonstim~ ted ventricular complexes; 2) nonsustained
ventricular tachycardia (NSVT): 5 or more nonstimulated ventricular
complexes terminating spontaneously with a duration less than
15 seconds; 3) unimorphic sustained ventricular tachycardia (UVT):
unimorphic ventricular tachycardia with a duration exceeding 15
seconds; 4) polymorphic sustained ventricular tachycardia (PVT):
CA 022~7948 1998-12-10
W 098/00405 PCT~US97/11131
- 164-
polymorphic ventricular tachycardia with a duration exceeding 15
seconds, and 5) ventricular tachycardia degenerating into ventricular
fibrillation (VT/VF): an unstable ventricular tachycardia degenerating
spontaneously into ventricular fibrillation. In this study, progra~ led
pacing was continued until the induction of either sustained VT or
VT/VF, or until the end of the pacing protocol with either NSVT or no
response (NI) occurring. In many preparations ultimately responding to
programmed stim~ tion with either SVT or VT/VF, reproducible
NSVTs were initiated early in the pacing protocol. Only postinfarction
preparations responding to baseline PVS with inducible sustained
ventricular tachyarrhythmias (UVT, PVT or VT/VF) were entered into
the present study. Baseline response to PVS with inducible ventricular
tachyarrhythmias has been found to correlate strongly with the
development malignant ventricular tachycardia, fibrill~tion and
arrhythmic death in response to a subsequent episode of myocardial
ischemia at a site remote from previous infarction in this ~nim~l model
(Wilber et al., Am. Heart J. 109: 8-18, 1985).
Following the completion of baseline and post treatment
electrophysiologic and PVS testing, the tip of a silver wire electrode
was inserted through the wall and into the lumen of the proximal left
circumflex (LCX) coronary artery. An anodal current of 200 ,uA was
applied to the intim~l surface of the coronary artery via this electrode,
producing intim~l injury, thrombus formation and ultimately acute
myocardial ischemia in the left circumflex coronary artery distribution.
The onset of acute posterolateral myocardial ischemia was noted electro-
cardiographically. Upon the development of lethal ischemic arrhythmias
or at 3 hours after the onset of acute posterolateral myocardial ischemia,
the hearts were excised and wet thrombus mass in th~ left circumflex
coronary artery determined. Anterior myocardial infarct size was
determined by cutting the heart into 1 cm thick transverse sections
which then were incubated in 0.4% triphenyltetrazolium chloride
solution. Reaction with triphenyltetrazolium forms a red precipitate in
viable tissue, whereas infarcted tissue remains pale. Infarct size was
CA 022~7948 1998-12-10
W O 98/00405
- 165-
quantitated gravimetrically and was expressed as a percentage of total
left ventricle.
The responses (i.e. occurrence of vs protection from the
development of lethal ventricular arrhythmia) of five experimental
groups to the development of acute posterolateral ischemia at a site
remote from previous myocardial infarction were compared in this
study: 1) the laboratory cohort of aqueous vehicle (saline or PEG/
saline)-treated control ~nim~ (n=40); 2) a dedicated soy bean oil-based
microemulsion vehicle (20.0% soy bean oil, 2.0% glycerin, 1.2%
lecithin and 76.8% water)-treated control group (n=10); 3) low dose
0.0003 mg/kg i.v. IKS blocker (-)-2-~2,4-Bis(trifluoromethyl)phenyl]-N-
[3R-2,3-dihydro-2-oxo-5-phenyl- 1 -(2,2,2-trifluoroethyl)- 1 H-
benzo[e][1,4]diazepin-3-yl]acetamide in soy bean oil-based
microemulsion (n=10); 4) low dose 0.001 mg/kg i.v. beta-adrenergic
receptor blocking agent timolol in 5% dextrose in saline vehicle (n=6);
and 5) the combination of low dose 0.0003 mg/kg i.v. IKS blocker
(-)-2-[2,4-Bis(trifluoromethyl)phenyl] -N-[3R-2,3 -dihvdro-2-oxo-5-
phenyl- 1 -(2,2,2-trifluoroethyl)- 1 H-benzo[e] [ 1 ,4]diazepin-3-yl]acetamide
with low dose 0.001 mg/kg i.v. beta-adrenergic receptor blocking agent
timolol (n=10). In the individual and combination IKS blocker (-)-2-
[2,4-Bis(trifluoromethyl)phenyl] -N-[3R-2,3-dihydro-2-oxo-5-phenyl- 1-
(2,2,2-trifluoroethyl)-lH-benzo[e][1,4]diazepin-3-yl]acetamide and beta-
adrenergic receptor blocking agent timolol treatment groups,
treatments were ~lmini.~tered i.v. 15 min prior to the conduct of post
treatment PVS and production of ischemia at a site remote from
previous infarction.
Statistics.
- Data are expressed as mean + S.E.M. Comparisons among
treatment groups were made using a single factor ANOVA or a Fisher's
exact test, when appropriate. P 0.05 was the criterion for statistical
significance.
CA 02257948 1998-12-lo
W 098/00405 PCTAJS97/11131
- 166 -
RESULTS
All ~nim~l~ in all treatment groups responded to baseline
PVS with inducible ventricular tachyarrhythmias as an entty criterion.
The incidences of ischemic le~al ventricular arrhythmias (ventricular
fibrillation; VF) occur~ng in the laboratory control cohort, dedicated
microemulsion vehicle control group, low dose IKS blocker (-)-2-[2,4-
Bis(trifluoromethyl)phenyl] -N-[3R-2,3-dihydro-2-oxo-5-phenyl- 1-
(2,2,2-trifluoroethyl)- 1 H-benzo[e] [ 1 ,4] diazepin-3-yl]acetamide, low dose
beta-adrenergic receptor blocking agent timolol, and combined low do.se
IKS blocker (-)-2-[2,4-Bis(trifluoromethyl)phenyl]-N-[3R-2,3-dihydro-
2-oxo-5-phenyl- 1 -(2,2,2-trifluoroethyl)- 1 H-benzo [e] ~1 ,4]diazepin-3 -
yl]acetamide with beta-adrenergic receptor blocking agent timolol
groups are s-lmm~rized below.
P Value
Incidents of Lethal P Value Compared Co-l-pa-~d to
Ischemic toLaboratory Microemulsion
Ventricular Vehicle Control Vehicle Control
Tlt;aLIllellt GroupArrhythmic (VF) Cohort Group
Laboratory Vehicle34t40 (85%) -- NS
Control Cohort
Microemulsion 8/10 (80%) N S --
Vehicle Control
Group
Low Dose IKs 6/10 (60%) NS NS
Blocker 0.0003
mg/kg i.v.
Low Dose ~ Blocker4/6 (67%) NS NS
0.001 mg/kg i.v.
Timolol
Combined Low Dose3/10 (30%) 0.001 0.03
IKs Blocker 0.0003
mg/kg i.v. + Low
Dose 13 Blocker
0.001 mg/kg i.v.
Timolol
NS = nonsignificant (P>0.05).
CA 022~7948 1998-12-10
W 098/00405 rcTrusg7/ll131
- 167-
Neither low dose IKS blocker (-)-2-[2,4-
Bis(trifluoromethyl)phenyl~ -N-[3R-2,3-dihydro-2-oxo-5-phenyl- 1-
(2,2,2-trifluoroethyl)-lH-benzo[e][1,4]diazepin-3-yl]acetamide nor low
dose beta-adrenergic receptor antagonist timolol ~clmini.~tered
individually afforded significant protection against the development of
malignant ischemic ventricular arrhythmias and death compared to
either the laboratory vehicle control cohort or the dedicated
microemulsion vehicle control group. In contrast, the combined
~lmini.~tration of both low dose IKS blocker (-)-2-[2,4-
Bis(trifluoromethyl)phenyl] -N-[3R-2,3-dihydro-2-oxo-5-phenyl- 1-
(2,2,2-trifluoroethyl)-lH-benzo[e][1,4]diazepin-3-yl]acetamide and low
dose beta-adrenergic receptor antagonist timolol provided significant
protection against development of malignant ischemic ventricular
tachyarrhythmia and arrhythmic death, which signifies a synergistic
~ salutary antiarrhythmic interaction between block of IKS and beta-
adrenergic receptor blockade.