Note: Descriptions are shown in the official language in which they were submitted.
CA 02259109 1999-01-18
-1-
Title: KRAFT PULPING PROCESS
FIELD OF THE INVENTION
This invention relates generally to the Kraft process for the
production of bleached cellulosic fibrous pulp. The invention is
concerned more particularly with an improvement in the Kraft process by
which impurities such as chloride, as well as minor non-process elements
such as calcium, manganese, iron and zinc can be removed from pulping
chemicals used in the process.
BACKGROUND OF THE INVENTION
In the Kraft process, cellulosic fibrous feed material (wood) is
heated in a digestion stage with a "white liquor" which contains sodium
sulfide and sodium hydroxide to dissolve hemicelluloses, lignin and other
extractable or organic materials contained in the fibrous material. The
digested fibrous pulp so obtained is separated from the resultant "black
liquor", the latter being sent to a recovery stage and the former being sent
to a bleaching stage. The above process is of particular application to the
pulping of wood chips.
In the recovery stage, the black liquor is concentrated by
evaporation of water therefrom and the concentrated liquor is then
burned in a boiler (sometimes referred to as a"furnace") to yield a smelt
containing sodium carbonate and sodium sulfide. The smelt is quenched
with water to form a raw "green liquor" which is then clarified. The
clarified green liquor is causticized with lime to convert the sodium
carbonate present in the liquor to sodium hydroxide. Calcium carbonate is
precipitated during the causticization of the liquor and is separated
therefrom as a mud and calcined to regenerate lime for further
causticization. The filtered causticized green liquor is the white liquor
which is used in the digestion stage and is recycled to treat further fibrous
material.
An appreciable portion of the inorganic chemicals contained
in the black liquor are lost to the recovery boiler flue gas, either by
CA 02259109 1999-01-18
-2-
entrainment or volatilization. To conserve chemicals it is common
practice to collect dust by electrostatic precipitation (ESP dust) from the
boiler exhaust, and recycle the dust by redissolution of same in the black
liquor. Additional sodium sulfate is usually added to the process to make
up any net loss of chemicals in the cycle.
In the Kraft process described above, sodium chloride and
other impurities entering with the wood and input chemicals tend to
build up to a steady state concentration in the pulping liquors since they
have no deliberate outlet. This is a particular problem for coastal mills
such as those located on the coast of British Columbia where logs are
transported in seawater and become saturated in chloride. Other than in
exceptional cases such as these coastal mills, chloride contamination has
not been a serious problem in the past, as impurity outlets occur due to
normal spills, leakages and other losses. As mills improve their operating
practices however, there is a reduced outlet for contamination and an
increasing tendency for build-up of impurities. Means of removal of
contaminants is becoming increasingly necessary.
The pulp produced in the Kraft mill is subjected to bleaching
and purification in a bleach plant operation. Treatment chemicals,
commonly in aqueous solutions, and wash water are used in the bleaching
and purification of the pulp, and result in one or more aqueous bleach
plant effluents containing spent chemicals and spent wash water. Such
bleach plant effluents usually are discharged, after treatment, to water
bodies. The treatment of bleach plant effluents represents considerable
expense. The possibility of recycling bleach plant effluents to eliminate
their discharge and associated treatment is very attractive and has been an
industry goal for some time. Recycling of bleach plant effluent would go a
long way towards elimination of pollution from pulp mill operations.
In 1972, Rapson patented (U.S. Pat. No. 3,698,995) a process for
reducing the discharge of the bleach plant effluent by recycling the filtrates
to the pulping liquor regeneration process. This process was later
improved by Reeve et al. in U.S. Pat. No. 4,039,372. The Rapson/Reeve
CA 02259109 2007-04-13 -3-
process was installed at Great Lakes Paper in Thunder Bay, Ontario and
began operation in 1977 but was abandoned in 1985. One of the main reasons
for the apparent failure of this process was related to concerns regarding
corrosion and pulp quality because of inadequate removal of chloride
contamination. Chloride originating from chlorine based chemicals used in
pulp bleaching, was recycled with the bleach filtrate to the kraft recovery
process from whence it had no adequate outlet.
The Rapson/Reeve process included some provision for
chloride removal by evaporation and crystallization of sodium chloride from
white liquor. This chloride removal process did not prove sufficient,
however.
In the Kraft pulping chemical recovery cycle, it is well-known
that chloride and potassium become enriched relative to sodium sulfate in the
flue gas dust retained by the electrostatic precipitator in the recovery
boiler.
These elements decrease the melting point of the dust, leading to plugging of
the boiler tubes, which leads to decreased boiler efficiency. This impurity
enrichment has beneficial aspect, however and several prior art processes
have taken advantage of this enrichment to facilitate removal of chloride and
potassium impurities from the Kraft pulping process.
An improved process for recycling bleach plant filtrate (called
BFR) is disclosed in U.S. Patent No. 5,352,332 (Maples et al.) and was
recently
installed at the Canton N.C. mill of Champion International. A key feature of
the BFR process is its ability to remove chlorine and potassium contamination
from the Kraft pulping process by treatment of the electrostatic precipitator
(ESP) dust catch. According to the patent this is done by selectively leaching
the chloride and potassium from the dust with a minimal amount of water or
by recrystallizing sodium sulfate after dissolution of the dust in water.
The leaching technique is described in more detail by Moy et
al. ("Removal of Sodium Chloride from Kraft Recovery System": Pulp
Paper Mag. Can. 75(4): T150 (April 1974)). The basic principle of this
CA 02259109 1999-01-18
-4-
leaching process is that sodium chloride is much more soluble that
sodium sulfate. In fact, the solubility of sodium sulfate in a saturated
solution of sodium chloride is significantly reduced, according to the
well-known common ion effect. The idea is to add just enough water to
dissolve the sodium chloride, leaving most of the sodium sulfate behind.
In practice this idea is unworkable as it produces a thick non-pumpable
paste containing 45-60% undissolved solids. One reason that this material
is difficult to filter is the presence of fine particles of oxides of non-
process
elements such as calcium, manganese, iron, zinc etc which are mixed in
with the sodium salts.
Moy found that by decreasing the solids concentration to
20-25% the slurry became workable. However the dilution water dissolved
much more sodium sulfate, which would then be lost to waste with the
sodium chloride. This problem was partially overcome by using a
saturated solution of sodium chloride/sodium sulfate instead of water to
dilute the thick slurry. In practice, the slurry is dewatered using, for
example, a rotary drum vacuum filter. A portion of the filtrate, which is a
saturated solution of sodium chloride/sodium sulfate is then recycled for
use in diluting the slurry.
The other problem with the basic leaching process is that
sodium carbonate, a valuable component of the dust, is leached out of the
liquor along with the sodium chloride. Moy overcame this problem by
converting the sodium carbonate to sodium sulfate by neutralizing the salt
slurry to a pH of 3.5 to 5.5 using sulfuric acid. This additional sodium
sulfate was largely retained with the solids removed by the filter.
When Champion International installed the BFR system
discussed above, they determined that the dust leaching process described
above was not satisfactory. Several shortcomings were cited. According to
Earl ("Removal of Chloride and Potassium from the Kraft Recovery
Cycle": Paper by Paul F. Earl, P. David Kick and Jean-Claude Patel), the
leaching process does not effectively remove potassium from the system.
In addition, the amount of sodium sulfate lost with the sodium chloride
CA 02259109 1999-01-18
-5-
in the filtrate was still considered excessive.
A new process, developed by Sterling Pulp Chemicals, was
installed at Champion wherein all of the dust is dissolved in water.
Sulfuric acid is added to convert sodium carbonate to sodium sulfate. The
liquor is then concentrated by evaporation. Sodium sulfate is then
recrystallized from the concentrated liquor and separated from the sodium
chloride liquor by filtration. A portion of the filtrate, containing sodium
chloride as well as potassium chloride is purged from the system.
According to Earl, the amount of sodium sulfate lost is much less with this
system than with the leaching process of the prior art. It is claimed that
this process recovers 90% of the sodium sulfate values in the dust while
removing 90% of the chloride and greater than 60% of the potassium. In
addition, it is claimed that the relatively coarse sodium sulfate crystals are
much easier to filter than the fine dust particles of the leaching process.
While apparently quite effective in its operation, the major
disadvantage of the recrystallization process is the high capital and
operating cost associated with the evaporator employed to concentrate the
dissolved dust liquor.
A common problem with all of the above described prior art
processes is that non-process elements such as calcium , manganese, iron
and zinc which are present in the ESP dust are largely insoluble in water.
As a result they are filtered out along with the recovered sodium sulfate
and recycled back to the kraft pulping process. It is well known that these
non-process elements are undesirable and a process which also removes
these non-process elements would be preferred.
SUMMARY OF THE INVENTION
Broadly speaking, the invention is based on the recognition
that it is possible to purify pulping chemicals used in a Kraft pulping
process by treating the chemicals with an amphoteric ion exchange resin to
remove sodium chloride, if the pulping chemicals are collected from the
process at a point at which the sulfide content of the chemicals is low or if
the chemicals are pre-treated to remove sulfide. The purified pulping
CA 02259109 1999-01-18
-6-
chemicals can then be returned to the Kraft process. In the context of the
present invention, references to "removal" of sulfide include conversion
of the sulfur to another species.
More specifically, the invention provides an improvement in
a Kraft pulping process in which pulping chemicals are used to treat
cellulosic fibrous feed material to form pulp in a process loop in which the
fibrous feed material is heated with a white liquor in a digestion stage, to
produce fibrous pulp and a black liquor, and in which the black liquor is
treated in the recovery cycle which includes the steps of concentrating the
black liquor, burning the concentrated black liquor in a recovery boiler to
yield a smelt, treating the smelt to form a said white liquor, and returning
the white liquor to the digestion stage. The improvement comprises the
further steps of: collecting pulping chemicals from the process loop for
treatment, at a point in the loop which is selected so that (a) the sulfide
content of the chemicals is low, or (b) the chemicals can be pre-treated to
remove sulfide, and in case (b), effecting such pre-treatment; subsequently
treating the collected pulping chemicals to remove sodium chloride in an
ion exchange unit containing an amphoteric ion exchange resin; and
returning the treated pulping chemicals to the process.
A corresponding apparatus for performing the process is also
provided.
The ion exchange unit used in the process of the invention
contains an amphoteric ion exchange resin. This type of ion exchange
resin is also called a'snake-cage polyelectrolyte', and was first reported by
Hatch ("Preparation and Use of Snake-Cage Polyelectrolytes": Industrial
and Engineering Chemistry Vol. 49 No. 11 November 1957). Although
several different types of amphoteric resins can be employed, the preferred
type is one in which acrylic acid is polymerized inside a quaternary amine
strong base anion exchange resin, as described by Hatch in U.S. Patent No.
3,041,292. The Hatch ion exchange process is sometimes referred to as "ion
retardation".
Resins of the type described by Hatch may be defined as a
CA 02259109 1999-01-18
-7-
granular amphoteric ion exchange agent, individual granules of which are
each an insoluble composite body of at least two intimately associated solid
resin ingredients, including an insoluble cross-linked resin, one of which
resin ingredients contains cation exchanging groups and another of which
resin ingredients contains anion exchanging groups. A more specific
definition is a bead form of polystyrene cross-linked with divinylbenzene
and having nuclear substituted quaternary trialkyl ammonium groups,
wherein said quaternary ammonium groups are neutralized by carboxylic
acid groups which are pendant on chains of polyacrylic acid entrapped
within the resin beads (see U.S. Patents Nos. 3,076,140 and 4,235,717, as well
as Dorfner "Ion Exchangers" -- Walter de Gruyter Berlin, New York, 1991).
Although amphoteric ion exchange resins traditionally are
used in particulate form, an amphoteric ion exchange resin may be made
in the form of a membrane for use in a diffusion dialysis process very
similar to that used to separate acids from salts. Water is used to strip
sodium chloride from the membrane. The diffusion dialysis process using
an amphoteric ion exchange resin in membrane form is essentially the
same process as ion retardation in a fixed bed.
It has been recognized in the literature that 'ion retardation'
which employs amphoteric snake-cage polyelectrolyte ion exchange resins,
can be used to separate sodium chloride from sodium hydroxide or
sodium sulfate. The present inventors have unexpectedly found that
these resins also have a high selectivity for sodium sulfide and that, in a
Kraft process, the resins will remove sodium sulfide, in addition to
sodium chloride, from white or green liquors. The sulfide contained in
the liquor undergoing treatment would then be lost. Normally sodium
sulfide is a valued, desired component in kraft pulping liquors and it
would not be acceptable to lose sodium sulfide along with sodium
chloride, unless there is a surplus of sulfur in the kraft cycle which needs
to be removed. It would therefore be disadvantageous to employ the ion
retardation process to remove chloride from kraft liquors under normal
circumstances.
CA 02259109 1999-01-18
-8-
In the Kraft process, the strong black liquor is burned in a
recovery boiler. Because of the relatively high volatility of the sodium
chloride contained in the strong black liquor, a considerable quantity of
sodium chloride is found in dust collected by the electrostatic precipitator
(ESP dust). Most of the sulfur contained in the black liquor is initially
present as sulfate, but is reduced to sulfide in the bottom of the boiler in
the char bed. This material passes into the smelt. Any sulfur that escapes
into the combustion gas, is oxidized to sulfate, producing sodium sulfate
according to the following reaction:
Na20 + H2S + 2 02 ----> Na2SO4 + H20
The sodium sulfate is collected as dust in the electrostatic
precipitator. Consequently, the ESP dust contains a high concentration of
sodium chloride, along with sodium sulfate, but virtually no sodium
sulfide. In accordance with a first aspect of the invention, this ESP dust is
dissolved in water and fed to the ion exchange unit. The purified sodium
sulfate solution produced by the ion exchange unit is next mixed with
black liquor and fed back to the recovery boiler, where most of the sulfate
is converted back to sulfide. The sodium chloride eluted from the ion
exchanger is wasted. Thus, chloride contamination is removed from the
kraft recovery cycle with very little loss of sulfur.
More specifically, the improvement provided by this aspect of
the invention involves collecting dust from exhaust gases produced in the
recovery boiler; dissolving the dust collected from the exhaust gases in
water to produce a solution containing sodium chloride and sodium
sulfate; filtering the solution to produce a filtrate solution and a solid
product; feeding the filtrate solution to an ion exchange unit selected to
remove sodium chloride and produce a purified sodium sulfate product;
removing said sodium chloride from the ion exchange unit by water
elution; and recycling at least one of said products to said recovery cycle.
This aspect of the invention is based on the recognition that
CA 02259109 1999-01-18
-9-
dust in the exhaust gases from the Kraft recovery boiler is enriched in
chloride and potassium and that at least the chloride can be removed by
the process steps of the inventive improvement, leaving a valuable
product that can be returned to the recovery cycle of the Kraft process.
In one embodiment of this aspect of the present invention,
the dust from the kraft recovery boiler is dissolved in water to the greatest
extent possible. The resulting solution still contains a significant amount
of suspended material, for example oxides of non-process elements such
as calcium, manganese, iron and zinc. These insolubles are first filtered
out using a conventional solid liquid separation device such as leaf or
drum filter. The filtered liquor, which now contains only trace levels of
these non-process elements, is then passed through a bed of an ion
exchange resin which has the ability to sorb sodium chloride while
excluding other salts such as sodium sulfate and sodium carbonate. This
chloride-depleted sodium sulfate/carbonate solution is then recycled back
to the Kraft pulping process.
Unlike the Earl process, an additional evaporator is not
required specifically to operate this process. The water that is used to
dissolve the dust does of course represent an additional load on the
evaporators that typically are used to concentrate the black liquor, so in
principle there is no reduction in energy consumption. In practice,
however there may be a significant saving. The black liquor evaporators
often have extra capacity, in which case there will be no additional capital
cost. In any case, the black liquor evaporators are typically multiple effect
units, which reduces the energy required to accomplish a given amount of
evaporation by typically 70% compared to single effect evaporation.
An additional benefit of this aspect of the present invention
is that, unlike both the recrystallization and leaching prior art processes,
it
is not necessary to neutralize the carbonate salt with sulfuric acid to
convert it to sodium sulfate.
The chloride that has been taken up by the ion exchange resin
is desorbed with water to yield a sodium chloride solution containing only
CA 02259109 1999-01-18
-10-
very low levels of sodium sulfate. This stream can then be purged from
the system.
In this embodiment of the invention, a portion of the
potassium ions will follow the sodium sulfate rich stream and a portion
will follow the sodium chloride rich stream. Unlike the selectivity that
the ion exchange resin shows for chloride, it shows very little, if any
selectivity for potassium. The mole fraction of potassium in each stream
remains the same as that in the original dust itself. Thus, while this
embodiment is highly efficient in chloride removal, it has very limited
capacity for potassium removal.
This invention may also be used in conjunction with the
recrystallization process described above. In the recrystallization process,
the mother liquor which has been separated from the sodium sulfate
crystals is normally purged from the system. This stream does however
contain a significant quantity of valuable sodium sulfate. This stream can
be treated with an ion exchange unit employing amphoteric ion exchange
resin to remove the chloride from this stream. The chloride
depleted/sulfate rich stream can then be recycled back to the kraft pulping
process while the chloride eluate is purged.
In another embodiment of the invention, the precipitator
dust is leached with a reduced amount of water. Chloride is preferentially
dissolved from the dust, producing a solution which is saturated in
chloride salt. The amount of sodium sulfate dissolved from the dust is
limited, because its solubility is reduced by the high concentration of
sodium chloride, owing to the common ion effect. The slurry is then fed
to a solid/liquid separation device such as a rotary drum vacuum filter.
The solids, which are depleted in chloride are recycled to the Kraft pulping
process, while the filtrate is then passed through a bed of amphoteric ion
exchange resin which has the ability to sorb sodium chloride while
excluding other salts such as sodium sulfate. The chloride-depleted
sodium sulfate solution is then recycled to the Kraft pulping process. The
chloride that has been taken up by the ion exchange resin is desorbed with
CA 02259109 1999-01-18
-11-
water to yield a sodium chloride solution containing only very low levels
of sodium sulfate which can be purged from the system.
A major advantage of this embodiment of the invention
over the prior art leaching process such as that of Moy is that it is not
necessary to minimize the concentration of sodium sulfate in the leachate.
In the prior art leaching process this leachate, together with its contained
sodium sulfate values, is purged to waste along with the chloride
impurities. In the present invention the sulfate contained in the leachate
is separated from the chloride impurities and recycled back to the Kraft
pulping process. In fact, it is possible to improve the efficiency of the
leach
in removing chloride and potassium by allowing a higher concentration of
sulfate in the leachate without increasing the loss of sulfate.
A key feature of this particular embodiment of the invention
is its improved potassium removal efficiency over the previously
described embodiment. The potassium present in the precipitator dust
exists principally as potassium chloride and potassium sulfate. Like
sodium chloride the solubility of potassium chloride in the leach liquor is
quite high. Unlike sodium sulfate, whose solubility has been depressed by
the presence of the high sodium chloride concentration, the solubility of
potassium sulfate has not been depressed, since it does not have a
common ion with the sodium chloride. As a result, the leachate will be
significantly enriched in potassium compared to the dust itself. When the
filtrate is fed to the ion exchange unit, the potassium will distribute itself
between the chloride rich and sulfate rich streams in the same mole
fraction in which it is fed. A significant portion of the potassium will thus
find its way out of the system along with the chloride rich stream and be
purged from the system.
Unlike the Moy process, with the present invention it is not
necessary to neutralize the carbonate content of the dust with sulfuric acid.
Any sodium carbonate that is dissolved from the dust is rejected by the
amphoteric ion exchange resin and is recycled back to the Kraft pulping
process along with the sulfate.
CA 02259109 1999-01-18
-12-
If the chloride depleted/sulfate rich stream from the ion
exchange unit is recycled directly back to the Kraft pulping process, a
significant amount of the potassium dissolved from the dust will also be
recycled. In a preferred embodiment, the purified sulfate rich salt stream
from the ion exchange unit is recycled back to the leach step instead of
being returned directly to the Kraft pulping process. As a result, the
leachate will be further enriched in potassium. In fact, in principle, the
only way that potassium can leave the system is with the chloride eluate
stream from the ion exchange unit. Providing that the solubility of
potassium salt is not exceeded in the leach liquor, potassium will build up
in the leach liquor until the removal rate of potassium by the ion
exchange unit equals the feed rate of potassium contained in the
precipitator dust fed to the system.
In practice, some chloride and potassium will remain in the
solids collected from the filter. However this can be further minimized by
utilizing a small portion of the sulfate rich stream from the ion exchange
unit to wash the dewatered solids before they are harvested from the filter.
As with the embodiments described above, with this
preferred embodiment it is not necessary to neutralize the carbonate salts
with sulfuric acid to convert them to sodium sulfate. Because the
amphoteric ion exchange resin takes up very little carbonate, it will build
up in the leach liquor until the solubility of sodium carbonate, or possibly
burkeite, a double salt of Na2SO4-Na2CO3, is exceeded. The presence of
high concentrations of sodium chloride and sodium sulfate will tend to
reduce the solubility of sodium carbonate, owing to the common ion
effect. As a result, carbonate will tend to follow the sodium sulfate and be
retained in the filter cake and ultimately recycled back to the Kraft pulping
process.
The volume of liquid requiring ion exchange treatment with
this embodiment is appreciably reduced compared to the embodiment in
which the dust is completely dissolved. This reduces the size and cost of
the associated ion exchange equipment and represents another significant
CA 02259109 1999-01-18
-13-
advantage for this embodiment.
In Kraft process plants in which chloride contamination is
high, such as at coastal mills or when mill closure is practised to a high
degree, the extent of chloride removal afforded by treatment of ESP dust
may not suffice. In such instances, chloride removal must be augmented
in some way, to avoid build-up to unacceptable levels in the kraft liquor.
In accordance with a second aspect of the invention, chloride
is removed directly from the Kraft liquors. The best choice is the white
liquor since this is the cleanest kraft liquor available, although green
liquor could also be treated. It is first necessary to remove the sulfide from
the white liquor. This can be accomplished by oxidizing the white liquor
by oxygen in a boiler as is done with black liquor in a recovery boiler. The
oxidation can be also accomplished by air or oxygen under less strenuous
conditions according to various techniques already known to those skilled
in the art. One such process is the OXYPROTM process from Air Products &
Chemicals. Although the oxidation of white liquor occurs in stages
according to a number of chemical reactions, it can be expressed in
simplified form by the following reaction, which is similar to that
occurring in the recovery boiler described above:
Na2S + 202 -----> Na2SO4
After the sodium sulfide is oxidized to sodium sulfate, the
oxidized white liquor, which contains sodium hydroxide, sodium
carbonate, sodium sulfate and sodium chloride, can be processed by the
ion exchanger, which removes the sodium chloride. To avoid fouling the
ion exchange unit with suspended material, it is advisable to filter the
oxidized white liquor prior to ion exchange treatment. The purified,
oxidized white liquor can be mixed with the black liquor and fed to the
recovery boiler where most of the sulfate is converted back to sulfide. The
sodium chloride recovered from the ion exchanger is discharged as waste.
In a conventional Kraft process, residual lignin still
CA 02259109 1999-01-18
-14-
contained in the pulp after digestion of the wood with white liquor, is
removed in a subsequent delignification stage prior to bleaching. In order
to reduce the quantity of chloride impurities entering the recovery cycle,
many mills are converting to oxygen delignification from older chlorine
delignification technology as part of their mill closure program.
Considerable quantities of sodium hydroxide are consumed in the oxygen
delignification process to maintain optimum pH and assist in
solubilization. If virgin sodium hydroxide is employed and the effluent
from the oxygen delignification stage is recycled to kraft recovery, a
sodium excess will result. To avoid this, it is common to employ oxidized
white liquor as a source of sodium hydroxide.
According to a preferred embodiment of this invention,
white liquor is first oxidized to convert the sulfide to sulfate. It is then
purified of chloride impurities by the ion exchange unit. After removal of
chloride impurities, the purified, oxidized white liquor is then utilized in
the oxygen delignification process. The effluent from the oxygen
delignification process is then recycled back to the recovery cycle. In this
way the sodium hydroxide and sulfur values contained in the white liquor
are retained while significant quantities of chloride contamination can be
removed. There are no incremental costs associated with the white liquor
oxidation since it is required anyway to produce oxidized white liquor for
the oxygen delignification process.
Sodium hydroxide is used in a number of other mill
processes such as oxidative extraction, peroxide bleaching and gas
scrubbing. In many of these applications, oxidized white liquor could be
advantageously substituted for virgin sodium hydroxide and the resulting
effluents from these processes may be recycled to recovery as part of a mill
closure program. In such cases, chloride contamination could be removed
by treating the oxidized white liquor with the ion exchange system as
described above with oxygen delignification, prior to utilization of the
oxidized white liquor and subsequent recycle to recovery.
CA 02259109 1999-01-18
-15-
BRIEF DESCRIPTION OF DRAWINGS
In order that the invention may be more clearly understood,
reference will now be made to the accompanying drawings which
illustrate a number of preferred embodiments of the invention, and in
which:
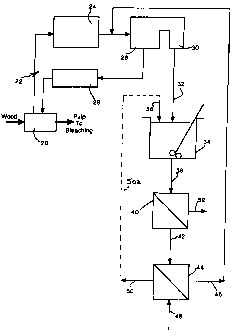
Figs. 1 to 6 are each block diagrams illustrating three
embodiments of the process and apparatus provided by the invention.
DESCRIPTION OF PREFERRED EMBODIMENTS
In each of the drawings, the principal steps in a conventional
Kraft pulping process are shown in block diagram form, in conjunction
with symbols representing further steps in the improvement provided by
the invention. Like reference numerals have been used to denote like
parts in all figures.
Reference will first be made to Fig. 1 by way of example, in
briefly describing the overall Kraft process. The process is well known and
has been described in some detail earlier; the detailed description therefore
will not be repeated here. For present purposes, it is sufficient to note that
pulping chemicals are used to treat cellulosic fibrous feed material (wood)
to form pulp, in a process loop in which the feed material is heated in a
digestion stage represented at 20 with a "white liquor" to produce a
digested fibrous pulp that is then sent to a bleaching stage. The black
liquor is treated in a recovery cycle generally indicated at 22 including an
evaporation stage 24 in which the black liquor is concentrated, and a stage
indicated at 26 in which the concentrated black liquor is burnt in a boiler to
yield a smelt. As discussed previously, the smelt is quenched with water
to form a raw "green liquor" which is then clarified. The clarified green
liquor is causticized with lime to convert the sodium carbonate present in
the liquor to sodium hydroxide. The causticized green liquor is filtered to
produce the white liquor which is used in the digestion stage. These steps
are represented generally at 28 in Figs. 1, 2 and 3. Reference numeral 30
indicates an electrostatic precipitator on the boiler exhaust, by which dust
is collected from the flue gases (ESP dust). In the drawings, the dust
CA 02259109 1999-01-18
-16-
delivered from the electrostatic precipitator 30 is indicated by the arrow 32.
The dust contains sodium sulfate and has been enriched in chloride and
potassium contamination relative to the Kraft pulping liquors.
In the most basic embodiment of the first aspect of the
invention shown in Fig. 1, the dust is fed to a dissolving tank 34. Water 36
is added to dissolve the dust. The resulting salt solution is fed via line 38
to a filter 40 (e.g. a leaf or drum filter). The filtrate 42 is then fed to an
ion
exchange unit 44 equipped with an amphoteric ion exchange resin. The
ion exchange resin takes up the chloride contamination, leaving a purified
sodium sulfate solution 46 which is recycled back to the Kraft pulping
process. Water 48 is employed to elute the chloride from the ion exchange
resin, thereby producing a sodium chloride eluate solution 50 which is
normally purged as waste.
In this embodiment, solid material 52 removed by filter 40
comprises primarily metal oxide impurities and is also discarded as a waste
product.
A disadvantage of the embodiment of Fig. 1 is that the rather
low concentration of chloride in the dust will result in a low concentration
of chloride in the solution fed to the ion exchange unit. Consequently, a
rather large volume of dilute eluate waste solution is produced as shown
in Table II (see examples). In addition, a rather large quantity of water is
required to effect elution of the chloride from the resin. It would be
desirable to reduce water consumption. Furthermore if reuse of the
sodium chloride were contemplated, for example as feedstock for sodium
chlorate production, a more concentrated solution would be preferred.
In a preferred embodiment, a portion of the eluate 50
containing chloride is substituted for fresh water 36 to dissolve the ESP
dust 32 in tank 34 instead of being discharged to waste -- as indicated by
line 50a in Fig. 1. This will increase the concentration of chloride in the
solution fed to the ion exchange unit and subsequently increase the
concentration of chloride in the portion of the eluate that is discharged to
waste. For example, if 50% of the total eluate produced by the ion
CA 02259109 1999-01-18
-17-
exchange unit is discharged to waste and the remainder is recycled to the
dissolution tank, the concentration of chloride in the feed solution will
increased by a two-fold factor. The operating conditions of the ion
exchange would be adjusted to deal with the higher concentration of
chloride in the feed. The volume of eluate discharged to waste could be
further reduced by recycling a larger proportion of the eluate to the
dissolution tank and reducing the amount of fresh water used for dust
dissolution by a corresponding amount.
One negative consequence of increasing the concentration of
sodium chloride in the solution is that the solubility of the sodium sulfate
will be reduced owing to the common ion effect. Nevertheless, because
the amount of chloride in the dust is low relative to the amount of
sodium sulfate, the volume of waste chloride can be reduced by substantial
amount while the solubility of sodium sulfate would only be reduced by a
relatively small amount. For example, if 75% of the eluate shown in
example 1 was employed for dust dissolution in place of fresh water, the
level of chloride would be increased from about 8 g/L to about 32 g/L, a
400% increase. The solubility of sodium sulfate would be reduced by
somewhat less than this amount. Since the solubility of pure sodium
sulfate is in excess of 400 g/L, the reduction in solubility would less than
32/400x 100% = 8%.
As discussed above, the ion exchange resin shows no
preference for potassium ions over sodium. As a result this most basic
embodiment (Fig. 1) is not very effective in removing potassium
contamination.
In a modification of the Fig. 1 embodiment illustrated in Fig.
2, only a portion of the dust is dissolved in dissolving tank 34. The
amount of fresh water employed is reduced, so that sodium chloride is
dissolved, but very little additional sodium sulfate is dissolved. The
remaining dust which is primarily sodium sulfate is separated from the
liquid by a suitable filter 40. In this embodiment, a rotary drum vacuum
filter is preferred. The solid sodium sulfate product 52 collected from the
CA 02259109 1999-01-18
-18-
filter 40 contains a reduced concentration of chloride is recycled back to the
Kraft process. The filtrate 42 contains a high concentration of sodium
chloride as well as some sodium sulfate and is then fed to the ion
exchange unit 44 (also containing an amphoteric ion exchange resin). The
ion exchange resin removes the chloride leaving a purified sodium sulfate
solution 46 which is recycled to the Kraft recovery cycle 22.
If the dust contains relatively low levels of chloride, the
amount of liquid processed by the ion exchange unit in relation to the
amount of dust processed will be rather small. In this case the slurry solids
concentration would tend to become rather high and therefore difficult to
handle. To deal with this situation, a portion of the filtrate can be recycled
directly back to the dissolving tank via line 56 to reduce the solids
concentration of the slurry.
In a preferred embodiment shown in Fig. 3, the purified (i.e.
chloride depleted) sodium sulfate solution 46 produced by the ion
exchange unit is recycled back to the dissolving tank 34 for leaching the
dust to replace all or a portion of the fresh water. By this means, the
potassium concentration will build up in the leach solution and therefore
the concentration of potassium in the chloride eluate solution will also be
increased. As a result, the effectiveness of the system for potassium
removal will be enhanced. As with the above embodiment, it may be
advantageous to recycle a portion of the filtrate via line 56 to the
dissolving tank, to reduce the solids concentration of the slurry. To lower
the chloride content of the leached dust, a small portion of purified
sodium sulfate solution from the ion exchange unit can be recycled to the
filter via line 58 to wash the solids before they are harvested.
Figs. 4 and 5 illustrate a second aspect of the invention, in
which chloride contamination is removed directly from the Kraft pulping
liquors.
Referring to Fig. 4, Kraft liquor is collected following process
stage 28 and in advance of the digestion stage 20 and is delivered to a white
liquor oxidation stage indicated at 60. As discussed previously, sodium
CA 02259109 1999-01-18
-19-
sulfide in the liquor is oxidized. The oxidized white liquor is then fed to
tank 34, followed by filter 40 and ion exchanger 44 as discussed in
connection with previous embodiments. The ion exchange resin in unit
44 removes chloride, leaving a purified white liquor which is recycled back
to the Kraft process. Preferably, the solution is returned upstream of
evaporation stage 24, although other locations are possible, as indicated by
the broken line in Fig. 4. In the other embodiments, alternative locations
for returning the recycled solutions also are possible. Reverting to Fig. 4,
the recycled solution is mixed with the black liquor prior to being fed to
the recovery boiler 26. In the boiler, most of the sulfate in the incoming
stream 46 is converted back to sulfide.
It should also be noted that, in the embodiments of Figs. 4
and 5, tank 34 is optional. In some cases, the solution may be fed directly
to filter 40. It should also be noted that treatment of white liquor may be
used to supplement removal of chloride from ESP dust. In this case the
tank 34 would still be necessary to dissolve the ESP dust. The oxidized
white liquor could entirely or partially replace the water that is employed
to dissolve the ESP dust.
Fig. 5 shows a further embodiment, in which white liquor is
again subjected to white liquor oxidation in stage 60 before being fed to the
ion exchange unit. In this embodiment, however, the process also
includes an oxygen delignification stage indicated at 62. In this
embodiment, the oxidized white liquor, after removal of chloride
impurities by the ion exchange unit 44, is fed to the oxygen delignification
stage 62, providing sodium hydroxide for the oxygen delignification
process. The effluent from the oxygen delignification process is then
recycled back to the Kraft process loop for reconversion of the sulfate
values to sulfide as discussed previously in connection with Fig. 4. In this
way, the sodium hydroxide and sulfur values contained in the oxidized
white liquor are retained, while significant quantities of chloride
contamination are removed by the ion exchange unit 44.
It is also possible to employ this invention to remove
CA 02259109 2007-04-13
-20-
chloride from bleach filtrates prior to recycle to the kraft recovery cycle.
Bleach filtrates contain a high concentration of dissolved organic material
which if discharged to the environment causes pollution. In addition to the
organic pollutants, bleach filtrates contain a significant quantity of
valuable
inorganic chemicals such as sodium and sulfur compounds. These bleach
filtrates also contain appreciable quantities of chloride, some of which
originates from the kraft cycle and some of which originates from bleaching
chemicals. These inorganic compounds are withdrawn from the kraft
recovery cycle and enter the bleaching operation along with the pulp fibres.
The bleach filtrates do not contain any sulfide; however, oxidants such as
chlorine dioxide employed in the bleaching process would oxidize any sulfide
to sulfate. They can therefore be purified of chloride contamination utilizing
amphoteric ion exchange resin according to this invention prior to recycle to
the kraft recovery cycle, without losing significant sulfur values. In order
to
reduce the load on the black liquor evaporators, it may be desirable to pre-
concentrate the bleach filtrates by suitable means such as an evaporator. This
pre-concentration step should be done prior to ion exchange treatment to
reduce the flow to be treated by the ion exchanger. Furthermore, it has been
found that the ion exchange unit of this invention performs more efficiently
on a pre-concentrated solution.
In this embodiment shown in Fig. 6 a conventional bleach plant
is indicated at 63 downstream of the oxygen delignification stage 62 (Fig. 5).
Bleach filtrates 64, which are collected from some point in the bleach plant
63,
such as after what is known as the "DlOO" bleach stage, are first filtered
with
a suitable filter such as the filter 40 referred to in previous embodiments to
remove suspended material such as fibres to produce a clarified solution 42,
which is fed to a concentration device such as an evaporator unit 67. The
condensed distillate 69 from the evaporator can be discharged to waste or
reused in the mill. The concentrated bleach filtrate 66 is then fed to an ion
exchange unit 44 containing an amphoteric ion exchange resin which removes
the alkali chloride salt as described previously. Purified, concentrated
bleach
CA 02259109 2007-04-13 -21-
filtrate 68 can then be recycled to the kraft recovery cycle 22 as with
previous
embodiments. By doing this the organic pollutants are returned to the kraft
recovery cycle where they are burned in the recovery boiler, the valuable
inorganic chemicals such as sodium and sulfur are recovered, but the chloride
is not recycled.
The embodiment of Fig. 6 can be used in conjunction with or
separately from other embodiments of the invention.
EXAMPLES
Example 1
A sample of ESP dust was obtained from an inland mill. A
chemical analysis obtained by x-ray fluorescence is shown in Table I. The
dust was dissolved in hot water. It was noted that the liquid contained an
appreciable quantity of suspended material. The solution was then filtered
using WhatmanTM 44 filter paper under vacuum in a Buchner funnel. The
filtered solution was fed to the bottom of an ion exchange column containing
a bed of amphoteric ion exchange resin measuring 2 inches in diameter by 24
inches in height. The amphoteric ion exchange resin was prepared by
polymerizing acrylic acid inside a quaternary amine strong base anion
exchange resin according to the method outlined by Hatch in U.S. Patent No.
3,041,292. After the void of water was displaced from the resin bed, the
chloride depleted purified salt solution was collected. Next, water was fed to
the top of the ion exchange resin bed. After displacing the entrained void of
feed solution from the bed, the eluate was collected from the bed. The
average flow rate of solution passed through the bed was approximately 0.4
L/min. The cycle was repeated several times and composite samples of the
purified salt and eluate streams were collected and analyzed from one cycle.
These results are shown in Table II.
Table I shows that the total concentration of the major non-
process elements (Ca, Mn, Fe, Zn) in the dust is 0.184%. When the
concentration of these elements in the filtrate which was fed to the ion
CA 02259109 1999-01-18
-22-
exchange unit is adjusted for dilution it can be calculated that more than
99% of these elements have been removed by filtration.
Approximately 97% of the chloride contamination was
removed from the sulfate solution by the ion exchange unit, while only
7.8% of the sulfate values were lost to the chloride eluate stream. The
system was very effective in removing chloride contamination from the
sulfate. On the other hand, very little of the potassium contamination was
removed from the sulfate, the ratio of potassium to sulfate in the purified
salt being the same as that in the feed (0.1).
Although the concentration of the non-process elements in
the eluate is near to the detection limits of the analysis (approximately 0.1
mg/L), it can be seen that only a small portion of these elements are taken
up by the ion exchange resin and recovered in the eluate. The majority of
the small quantity of non-process elements remaining after filtration are
found in the purified salt solution.
Table I: Typical Inland Mill ESP Dust Analysis
Na 26.3%
K 3.18%
Ca 0.05%
Mn 0.0273%
Fe 0.1%
Zn 0.0068%
CA 02259109 1999-01-18
-23-
Table II
volume [Na] [K] [S04] [Cl]
(L) (g/L) (g/L) (g/L) (g/L)
feed 1.105 92 9.66 156 8.14
eluate 1.10 12.1 1.24 12.2 8.33
purified 1.11 77.7 8.55 146 0.23
salt
[Ca] [Mn] [Fe] [Zn]
(mg/L) (mg/L) (mg/L) (mg/L)
feed 3.5 0.2 1.7 0.18
eluate 0.3 0.1 0.4 <0.1
purified salt 3.4 0.1 1.4 0.2
Example 2
A solution of sodium sulfate and sodium chloride was
prepared to simulate a solution obtained from leaching precipitator dust
with a sodium sulfate according to the process described by Moy. In this
solution the sodium chloride concentration approaches saturation while
the concentration of sodium sulfate approaches saturation at that level of
sodium chloride. The concentration ratio of sodium chloride to sodium
sulfate is approximately 3:1.
The simulated leach solution, which was heated to
approximately 60 C, was fed to the bottom of the ion exchange column
described in example 1. After the void of water was displaced from the
resin bed, the chloride depleted purified salt solution was collected. Next,
water at about 60 C was fed to the top of the ion exchange resin bed. After
displacing the entrained void of feed solution from the bed, the eluate was
collected from the bed. The average flow rate of solution passed through
CA 02259109 1999-01-18
-24-
the bed was approximately 0.34 L/min. The cycle was repeated several
times and composite samples of the purified salt and eluate streams were
collected and analyzed from one cycle. These results are shown in Table
III.
It can be seen from these results that approximately 83% of
the chloride contamination was removed from the sulfate solution. This
stream would be recycled back to the Kraft pulping process or reused for
leaching more dust. Only 6.9% of the sulfate values were lost to the
chloride eluate stream which would be discharged to waste.
Table III
volume [NaC1] [Na2SO4]
(L) (g/L) (g/L)
feed 0.31 270 72
eluate 0.31 223 4.9
purified salt 0.31 51.2 66
Example 3
Synthetic electrostatic precipitator dust typical of a British
Columbia coastal mill was prepared by mixing solid sodium sulfate and
sodium chloride salts together to contain 25% NaC1. Approximately 3 kg
of synthetic dust was mixed with 3 litres of purified salt solution produced
by the ion exchange unit from synthetic leach liquor as in example 1. The
leaching was conducted over a period of about 2 hours at 60 C in two equal
sized batches. The slurry was filtered under vacuum and the resulting
cake was washed with a small amount of water so that the total volume of
liquid collected from the filter was 3 litres. The filtrate was held at about
45 C for two days and a very small amount of solids (est. <10 g) was noted
CA 02259109 1999-01-18
-25-
on the bottom of the container, indicating that it was close to saturation as
it left the filter. The composition of the initial and final liquids and
solids
are shown in Table IV. The approximate composition of the final solids
was calculated from the material balance.
Table IV
amount [NaCI] [Na2SO41 g NaCl g Na2SO4
initial 3 L 62 g/L 67.3 g/L 186 202
liquid
final 3 L 272 g/L 83.7 g/L 816 251
liquid
initial 3 kg 25% 75% 750 2250
solid
final solid ca 5% ca. 95% ca. 120 ca. 2201
Approximately 84% of the sodium chloride was leached from
the synthetic dust while only 2% of the sodium sulfate was leached out,
neglecting the amount that precipitated in the container after filtration. In
the practice of the invention the final liquid obtained from the leach
would be fed once again to the ion exchange unit while the final solid
would be recycle to the Kraft pulping process.
Example 4
Approximately 2.2 kg of synthetic dust containing 75%
sodium sulfate ([SO4] = 50.7%), 20% sodium chloride ([C1]= 14.5%) and 5%
potassium chloride ([K]= 2.6%) was leached with approximately 2.2 litres of
purified sodium sulfate solution produced by the ion exchange unit in
example 2 in the manner described in example 3. The remaining solids
were filtered and then the filtrate was treated once again with the ion
exchange unit as above. This leach/filtration/ion exchange process was
CA 02259109 1999-01-18
-26-
repeated ten times employing fresh dust each time, at which point the
composition of the leach no longer change appreciably. The purified dust
produced from the final leach was analyzed along with the composition of
the filtrate solution which was fed to the ion exchange unit as well as the
chloride containing eluate and purified salt solutions produced by the ion
exchange unit.
Analysis of the final solids collected from the filter indicated
that they contained 95.5% of the initial sulfate values, however the
potassium content was only 17.9% of initial and the chloride content was
only 8.8% of the initial. The composition of the liquids entering and
leaving the ion exchange unit during one cycle of the final run are shown
in Table V.
Table V
volume (L) [K] (g/L) [SO41 (g/L) [Cl] (g/L)
feed 0.299 40.2 87.0 156
eluate 0.300 16.9 4.55 125
purified 0.313 21.5 82.6 39.8
salt
Example 5
White liquor was treated by the ion exchange unit described
in Example 1. The results are summarized in the following table:
CA 02259109 1999-01-18
-27-
Table VI
volume (L) [NaOH] [Cl-] [SO4=] [S=]
(g/L) (g/L) (g/L) (g/L)
feed (white liquor) 0.872 84.4 3 2.77 8.47
eluate 0.867 0 3.2 0.23 6.67
purified liquor 0.872 94 0.34 2.53 1.72
It can be seen from the data in this table that 78% of the
sulfide values in the white liquor fed to the ion exchange unit report to
the eluate stream along with the chloride impurities. Little of the sodium
hydroxide and sulfate values are taken up by the resin. This illustrates the
fact that sulfide values are removed from the pulping chemicals, which
would be detrimental if the chemicals were not pre-treated in accordance
with the embodiment of Fig. 4 or 5.
Example 6
A synthetic, low sulfidity, oxidized white liquor was prepared by adding
equal amounts of Na2S, Na2S2O3, NaSO4 and NaCl to NaOH. Sodium
sulfide would be converted to sodium thiosulfate (Na2S2O3) if the white
liquor were only partially oxidized. If the sodium sulfide is entirely
converted to sodium sulfate, the white liquor is said to be fully oxidized.
This composition was chosen to demonstrate resin selectivity towards
various components of the oxidized white liquor. The ion exchange unit
used was similar to the one used in Example 1. The oxidized white liquor
was filtered and fed to the bottom of the ion exchange bed. After the
displacement of the void of water, a purified (ie. chloride-depleted) caustic
solution was collected. In the next step, water was fed to the top of the ion
exchange resin bed. After displacing the entrained void of feed solution
CA 02259109 1999-01-18
-28-
from the bed, the chloride-rich eluate was collected from the bed. The
average flow rate of the solution passed through the bed was
approximately 0.10 L/min. The composition of oxidized white liquor feed
and the experimental results are shown in Table VII. The hydroxide
recovery was 92% whereas the chloride and sulfide removal were 99% and
96% respectively. With respect to the other components of oxidized white
liquor, the distribution is shown in Table VII. An interesting discovery
was that more that 50% of the sodium thiosulphate was sorbed onto the
resin and was subsequently eluted with water, ending up in the
chloride-rich eluate stream. A further experiment was carried out with a
more representative partially oxidized white liquor containing 24 g/L
NaCI. The composition of oxidized white liquor feed and the
experimental results are shown in Table VIII. The hydroxide recovery was
90% whereas the chloride removal was about 98%. About 41% of Na2S2O3
was also recovered in eluate stream along with the chloride. These results
indicate that if the white liquor is only partially oxidized, some of the
sulfur is lost as NaZS2O3, with the chloride, to the eluate stream. In order
to minimize loss of sulfur, the sulfide in the white liquor should be fully
oxidized to sulphate. In cases where there is an excess of sulfidity in the
recovery cycle, it may however be preferable to lose a certain amount of
sulfur in this manner.
Table VII: Removal of Chloride from Oxidized White Liquor
volume NaOH Na2S Na2CO3 Na2S2O3 Na2SO4 NaC1
(L) (M) (M) (M) (M) (M) (M)
Feed 0.63 2.01 0.13 0.1 0.07 0.07 0.15
Eluate 0.63 0.15 0.13 0.0 0.04 0.01 0.15
Purified 0.64 1.73 0.00 0.1 0.03 0.06 0.0
Caustic
CA 02259109 1999-01-18
-29-
Table VIII: Removal of Chloride from Partially Oxidized White Liquor
volume NaOH Na2S2O3 NaCI
(L) (M) (M) (M)
Feed 0.64 2.72 0.34 24.05
Eluate 0.64 0.31 0.14 24.0
Purified Caustic 0.64 2.44 0.21 0.64
Example 7
This example illustrates that alkali metal chloride can be
removed from a chlorine dioxide bleach filtrate. This application is
important in the context of system closure, when the bleach plant effluents
are recycled back to the recovery cycle and as a result, the level of chloride
is increased. A 10 mL volume of chlorine dioxide bleach filtrate (100%
C1O2 substitution) at a pH of 2.26 and at room temperature, with a sodium
chloride content of 0.21 g/L and C102 content of 4.3 ppm was fed to the top
of an ion exchange resin bed 1 cm in diameter and 20 cm in height,
containing amphoteric ion exchange resin similar to that of example 1.
After the displacement of the void of water, a purified (i.e.
chloride-depleted) bleach filtrate solution was collected. In the next step,
lOmL of water was fed to the top of the ion exchange resin bed. After
displacing the entrained void of bleach filtrate feed solution from the bed,
the chloride-rich eluate was collected from the bed. The composition of
the bleach filtrate feed and the experimental results are shown in Table IX,
trial 1. Because of the low pH, a small amount of chloride (0.047 g/L) is in
the form of HCI which is not retained by the resin. Since D-stage filtrate is
usually mixed with E-stage filtrate at a pulp mill, the filtrate was next
neutralized with sodium hydroxide to a pH of 7.2 and then concentrated
(by evaporation) to a sodium chloride concentration of 2.78 g/L. A small
amount of sodium sulphite was added to destroy the C1O2 residue prior to
CA 02259109 1999-01-18
-30-
concentration. Residual fibre and particulates were separated after
concentration and the resulting solution was fed to the ion exchange unit.
The composition of the effluent and the experimental results are shown in
Table IX, trial 2. The chloride removal efficiency is improved as a result of
concentrating the effluent and raising the pH. Although concentrating the
effluent will be considered an extra cost, the lower volume processed
means that a much smaller ion exchange unit will be required and the
capital cost is reduced. The discrepancy in mass balance is probably due to
the small size and configuration of the column used and the uptake of
other salts by the resin. Better separation efficiency and mass balances can
be expected if the ion exchange unit of Example 1 is used.
Table IX: Removal of Chloride from Dloo Bleach Filtrate
Trial 1 Trial 2
NaC1(g/L) NaCI (%) NaCI(g/L) NaC1 (%)
Feed (Dloo) 0.203 100 2.78 100
Eluate 0.128 63 2.31 83
Purified Filtrate 0.036 18 0 .41 14