Note: Descriptions are shown in the official language in which they were submitted.
CA 02260080 1999-01-11
DEVICES, SYSTEMS AND METHODS FOR DETERMINING
PROPER PLACEMENT OF EPIDURAL CATHETERS
FIELD OF THE INVENTION
This invention relates to the field of catheters and, more
particularly, relates to a method and to a system for inserting catheters for
10 administering epidural anesthesia and for confirming the placement of a
catheter in an epidural space of a spine of a subject.
BACKGROUND
Catheters of various types and sizes have been used by physicians
extensively. One use of the catheter is in providing regional anesthesia with
minimal physiologic alterations. When used at the start of an operation,
regional anesthesia minimizes the total dosage of inhalation or intravenous
anesthetic drugs required, hastens awakening, and permits early ambulation.
20 When administered at the conclusion of surgery, regional anesthesia produces
post-operative analgesia with reduced risk of respiratory depression.
When prolonged analgesia is required, a catheter is inserted into the
caudal or lumbar epidural space to provide intermittent or continuous injections25 of local anesthetics. Continuous lumbar epidural anesthesia is a well-established
and accepted technique in adult patients. It differs from caudal epidural
anesthesia by the location where the needle is inserted. Caudal epidural
anesthesia, however, is notable for its simplicity, safety, and effectiveness and is
one of the most frequently used regional anesthetic techniques for operations
30 below the diaphragm in children.
Epidural and spinal anesthesia require the administration of an
anesthetic agent into the epidural or subarachnoid spaces respectively of the
spine. Epidural anesthesia requires substantially more anesthetic agent than
35 spinal anesthesia and, if the anesthetist inadvertently penetrates the
duraarachnoid membrane while endeavoring to administer an anesthetic agent
to the epidural space, a dangerous quantity of anesthetic agent can be placed inthe subarachnoid space, possibly causing paralysis or even death.
- 1 -
CA 02260080 1999-01-11
The majority of physicians prefer the use of the midline approach
for spinal puncture. Generally, with the midline approach, the spinal injection
is made at the center of the patient's back with the needle oriented in a plane
5 parallel to the centerline of the spine. The needle tip is inserted into the back in
a straight line toward the midline of the spine (2) between the second (4) and-
third (6) lumbar vertebrae, a direction generally represented by the arrow (A)
shown in Figure 1. In this technique the epidural needle passes through the
supraspinous, interspinous and ligamentum flavum structures before entering
10 the epidural space. Insertion of the needle into the epidural space is complicated
by the lack of feedback as to the position of the needle tip, coupled with the
imperative need to avoid puncturing the dura mater which surrounds the spinal
cord, since there is potential for catastrophic trauma to the spinal cord with the
epidural needle. Extreme caution must therefore be exercised in the positioning
15 of the needle tip, which must pierce through the tough, resilient, leather-like
ligamentum flavum. and then stop immediately within the narrow epidural
space, short of puncturing the dura mater.
The needle must be moved through the ligamentum flavum very
20 slowly and in a carefully controlled fashion. At the same time, pressure is
applied to the plunger of the attached syringe which is filled either with air or
saline solution. The object is to continuously test for loss of resistance to
injection, experienced when the needle lumen enters the epidural space after
clearing the ligamentum flavum. This loss of resistance is experienced by little if
25 any resistance to injected air or fluid, and a negative aspiration test then indicates
that the needle lumen is properly positioned in the epidural space. Special
syringes, known as loss of resistance syringes and characterized by very low
friction between the plunger and the barrel of the syringe, are used for
positioning the needle lumen in the epidural space. Once correct positioning of
30 the needle is achieved, the resistance syringe is separated from the epiduralneedle and another syringe, loaded with the anesthetic is attached, after which
the anesthetic is injected.
It is important to understand the demands placed upon the
35 anesthesiologist's dexterity by this procedure. It is of critical importance that the
needle traverse the ligamentum flavum in a carefully measured and controlled
manner. Typically, this is achieved by applying resistance to the advancing
needle with the anesthesiologist's non-dominant hand (the left-hand if the
- 2 -
CA 02260080 1999-01-11
anesthesiologist is right-handed) while the dominant hand applies pressure to
the plunger of the resistance syringe to test for resistance to injection while at the
same time slowly advances the needle. Variations of this technique may be
adopted according to personal preference, for example the needle may be
5 advanced continuously while pressure on the syringe barrel is also maintained
continuously to test for resistance. In the alternative, the needle is advanced in
very small increments, e.g., 1 millimeter, testing for resistance to injection after
each advance.
The difficulty of correctly positioning the needle lumen in the
epidural space has spurred many attempts to develop methods and devices for
detecting and indicating correct needle placement. These approaches have
generally exploited the low resistance to injection and subatmospheric pressure
characteristic of the epidural space. One such technique involves placement of a15 drop of saline solution on the open hub of an epidural needle. The drop will be
"sucked-in" as the needle lumen enters the epidural space where, for reasons notwell understood, prevails sub-atmospheric pressure. Other means used for this
purpose include capillary attachments with fluid indicators developed by Odom,
or inflated balloons by Macintosh, which deflate upon entering the epidural
20 space. It is also known to use spring loading devices to facilitate the loss of
resistance phenomena which occur as the epidural needle passes from the dense
ligamentum flavum into the lesser resistance of the epidural space.
U.S. Pat. No. 5,024,662, describes an attachment for a resistance
25 syringe for aiding the anesthesiologist in correct placement of the epidural
needle. The attachment has an elastomeric band retained to the syringe barrel bya ring which slides onto the syringe barrel against the finger flange of the syringe
to anchor the ends of the elastic band to the barrel while a midportion of the
band is pulled by the plunger of the syringe. Consequently, the plunger is urged30 by elastic force into the syringe barrel, but is held back by fluid, air or liquid in the
barrel, until the needle lumen enters the epidural space. At that point the
contents of the syringe are injected into the epidural space under the force of the
stretched band, providing the anesthesiologist with immediate kinesthetic
indication of correct needle placement. While this arrangement works well,
35 disposable elastomeric drivers have been developed by this applicant which are
of still greater simplicity and very low cost.
CA 02260080 1999-01-11
U.S. Pat. No. 4,518,383 teaches an instrument for epidural and spinal
anesthesia in which an outer hollow TuohyTM needle has a bent pointed tip to
locate the epidural space and an inner hollow needle with a pointed tip
projecting forwardly of the outer TuohyTM needle in alignment therewith to
5 penetrate the dura with a minimum of cutting of tissue. Likewise, U.S. Pat. No.
4,737,146 discloses another version of epidural catheter in which a rigid epidural
needle is inserted into an epidural space and an epidural catheter is introducedthrough the needle into the epidural space through a lateral opening in the tip of
the needle.
U.S. Patent No. 5,081,990 describes catheters for epidural injection of
drugs with electrodes at the distal end for measuring effect of the drugs. This
device, however, is not used for determining the placement of the catheter priorto medicant injection and, by placing electrodes into the epidural space and
15 directly contacting the dural tissue, risks electrochemical reaction between the
electrode and the dural tissue in the presence of medicament, saline or body
fluids.
U.S. Patent No. 5,423,877 describes a catheter for simultaneous
20 application of electrical stimulation and infusion of analgesic medication tonerve fibers in the spinal cord. The correct placement of the catheter requires
constant communicating with the patient to determine the paresthesia in
corresponding dermatome and myotome regions. Therefore, the determination
of proper catheter location is only possible and limited to a certain group of
25 patients with conscious, communicable, cooperative, calm and oriented state. In
addition, such determination is depended on only subjective but not objective
evaluation and the results tend to be unreliable and time consuming to obtain.
Additionally, as the metal electrodes are set distally at the end of
30 the catheter tubing, correct placement of the electrode may be detected, but proper
delivery of the injected anesthetic may not be reliably established. For instance,
the injected anesthetic may leak proximal to the epidural space if there is any
damage to the catheter during the insertion procedure.
Therefore, presently the confirmation of catheter placement can be
made only after observing the clinical effects from subsequent local anesthesia
drug injection. This clinical effect may take up to twenty minutes, depending onthe type of local anesthesia injected. What is needed is a simple, rapid and
- 4 -
CA 02260080 1999-01-11
effective method of determining proper placement of the catheter prior to the
introduction of anesthesia.
5 SUMMARY OF THE INVENTION
The present invention provides systems and methods for
determining the proper insertion and for confirming the proper placement of a
spinal or epidural catheter. In one embodiment, a method comprises: a)
10 providing: i) a subject in need of having a catheter inserted in the spine; ii) an
epidural catheter having first and second ends; iii) an aqueous solution capableof conducting electricity and suitable for administration in the epidural space of
said spine of said subject; and iv) an electrical conduction means; b) contacting
the epidural space of the spine of said subject with said first end of said catheter;
15 c) attaching said electrical conduction means to said second end of said catheter;
d) administering said aqueous solution through said catheter into said epidural
space; and e) conducting electricity with said electrical conduction means underconditions such that said aqueous solution conducts electricity in said epiduralspace. In a preferred embodiment, the methods further comprise the step f)
20 measuring the motor response of said subject caused by said conducting of
electricity. While the present invention is not limited by the type of electrical
conduction means, in one embodiment, the electrical conduction means
comprises a connector configured for attachment to said second end of said
catheter and having a conductive element in communication with a source of
25 electricity.
In another embodiment, the methods comprise: a) providing: i) a
subject in need of having a catheter inserted in the spine; ii) a
non-metal-containing epidural catheter having first and second ends; iii) an
30 aqueous solution capable of conducting electricity and suitable for administration
in the epidural space of said spine of said subject; and iv) a connector configured
for attachment to said second end of said catheter, said connector having a
conductive element in communication with a source of electricity; b) contacting
the epidural space of said spine of said subject with said first end of said catheter;
35 c) attaching said connector to said second end of said catheter; d) administering
said aqueous solution through said catheter into said epidural space; e)
conducting electricity with said conductive element of said connector under
conditions such that said aqueous solution conducts electricity in said epidural- 5 -
CA 02260080 1999-01-11
space; and f) measuring the motor response of said subject caused by said
conducting of electricity. In a preferred embodiment, the methods further
comprise the step g) administering an anesthetic to said subject.
In another embodiment, the methods comprise: a) providing: i) a
subject in need of having a catheter inserted into the spine; ii) an epidural
catheter having first and second ends, said catheter comprising a metal element
disposed within the length of the catheter and housed completely within said
catheter such that no metal is exposed outside said first end of said catheter; iii)
an aqueous solution capable of conducting electricity and suitable for
administration in the epidural space of said spine of said subject; and iv) a
connector configured for attachment to said second end of said catheter, said
connector having a conductive element in communication with said metal
element and a source of electricity; b) contacting the epidural space of said spine
of said subject with said first end of said catheter; c) attaching said connector to
said second end of said catheter; d) administering said aqueous solution throughsaid catheter into said epidural space; e) conducting electricity with said
conductive element of said connector under conditions such that said aqueous
solution conducts electricity in said epidural space; and f) measuring the motorresponse of said subject caused by said conducting of electricity. In a preferred
embodiment, the methods further comprise the step g) administering an
anesthetic to said subject. The present invention is not limited by the nature of
the metal element. In one embodiment, the metal element comprises a wire
disposed within said catheter; in a preferred embodiment, said wire contacts said
connector directly.
In another embodiment, a method is provided for confirming the
placement of a catheter in an epidural space of a spine of a subject, the catheter
having a proximal end and a distal end. The method comprises the steps of: (a)
inserting the distal end of the catheter into the spine of the subject for contact
with the epidural space; (b) conducting electricity to the distal end of the catheter;
and (c) observing a motor response of the subject caused by conducting the
electricity to the distal end of the catheter in order to confirm the placement of
the catheter in the epidural space. The method may further comprise the step of
adjusting the position of the catheter such that the distal end of the catheter
contacts the epidural space of the spine of the subject. As well, this method may
further comprise the step of administering an anesthetic to the subject.
- 6-
CA 02260080 1999-01-11
In addition, the electricity may be conducted to the distal end of the
catheter, at least in part, by electrical conduction means. The electrical
conduction means may be comprised of a conductive element in
communication with a source of electricity. Further, the conductive element
5 may be comprised of a metal element disposed within the catheter. As indicatedabove, the metal element may be comprised of a wire. However, alternately, the
metal element may be comprised of a removable needle having a distal end. The
removable needle may be disposed within the catheter such that no portion of
the needle is exposed outside the distal end of the catheter. However, when the
10 metal element is comprised of a removable needle, the needle is preferably
disposed within the catheter such that the distal end of the needle is exposed
outside the distal end of the catheter for contacting the epidural space of the
spine of the subject. Either a hollow or a non-hollow needle may be used.
However, the needle is preferably hollow such that fluids may be conducted
15 therethrough.
Preferably, the exposed distal end of the needle is not left in the
epidural space for extended periods of time. Therefore, once the location or
placement of the catheter is confirmed by electrical stimulation, the removable
20 needle is preferably removed or withdrawn, at least in part, such that the distal
end of the needle is no longer exposed outside the distal end of the catheter sothat the metal of the needle is no longer in direct contact with the epidural space.
More preferably, the needle is withdrawn completely from the catheter so that norigid objects or elements remain in the epidural space of the subject.
Alternately, the catheter itself may be comprised of a hollow needle
such that fluids may be conducted therethrough to a distal end of the needle.
The fluids may be conducted directly through the bore of the needle or through asuitable second catheter, having an outside diameter smaller than the diameter
30 of the bore of the needle, which may be inserted in the bore. The distal end of
the needle defines the distal end of the catheter for contacting the epidural space
of the spine of the subject. In this case, the conducting step of this embodiment
of the method is comprised of conducting electricity to the distal end of the
needle. Further, the needle may include an insulating coating about at least a
35 portion of the needle, wherein the distal end of the needle is exposed outside the
insulating coating.
CA 02260080 1999-01-11
Preferably, the hollow needle is not left in the epidural space for
extended periods of time. Therefore, once the location or placement of the
catheter is confirmed by electrical stimulation, an injection of an anesthetic or
other medication may be introduced into the epidural space and the hollow
5 needle is preferably subsequently withdrawn. Further, the second smaller
diameter catheter described above may be inserted into the epidural space
through the bore of the needle. The second smaller diameter catheter may be leftin the epidural space and used for the administration of medications following
the withdrawal or removal of the needle.
In another preferred embodiment, the methods further comprise
the introduction of a test dose of anesthetic into the epidural space and
monitoring of the minimum current required to induce a motor response in the
subject. In this manner, it is possible to ascertain whether a catheter placed in an
15 epidural space is improperly placed intravascularly. In one embodiment, the test
dose is lidocaine, and in a preferred embodiment the test dose comprises 3 to 6
ml of 1.5% lidocaine.
The present invention is not limited by the condition of the subject,
20 in one embodiment, the subject is unable to communicate; in such an
embodiment, the subject may be unconscious. Likewise, the present invention is
not limited by the nature of the aqueous solution. In one embodiment, the
aqueous solution comprises a saline solution, while in another embodiment, the
aqueous solution comprises epimorphine solution.
In one embodiment, the systems of the present invention comprise:
a) an epidural catheter having first and second ends, said first end suitable for
administration of fluids in the epidural space of the spine of a subject; and b) a
connector attached to said second end of said catheter, said connector having a
30 conductive element suitable for communication with a source of electricity.
Another embodiment comprises a system, comprising: a) a metal-containing
catheter having first and second ends, said first end suitable for administration of
fluids in the epidural space of the spine of a subject, said catheter comprising a
metal element disposed the length of said catheter and housed completely
35 within said catheter such that no metal is exposed outside said first end of said
catheter; and b) a connector attached to said second end of said catheter, said
connector having a conductive element suitable for communication with a
source of electricity.
- 8 -
CA 02260080 1999-01-11
In another embodiment, the present invention comprises a device,
comprising a connector having first and second ends and a conductive element,
said connector being dimensioned such that it is suitable for attachment to a
5 catheter that is suitable for insertion into an epidural space of the spine of a
subject. In a preferred embodiment, the device further comprises a catheter,
having a distal end, suitable for insertion into an epidural space of the spine of a
subject, wherein said first end of said connector is attached to said catheter. In a
particularly preferred embodiment, the device further comprises a metal
10 element disposed within said catheter. If desired, the metal element is disposed
within the length of said catheter and housed completely within said catheter
such that no metal is exposed outside said distal end of said catheter.
In still a further embodiment, the invention comprises a device for
15 confirming the placement of a catheter in an epidural space of a spine of a
subject. The device is comprised of the catheter having a distal end for
contacting the epidural space of the spine of the subject and electrical conduction
means in communication with the catheter for conducting electricity, at least inpart, to the distal end of the catheter to cause a motor response of the subject in
20 order to confirm the placement of the catheter in the epidural space.
In this embodiment, the electrical conduction means may be
comprised of a conductive element in communication with a source of
electricity. Further, the conductive element may be comprised of a metal
25 element disposed within the catheter. The metal element may be comprised of aremovable needle having a distal end. The removable needle may be disposed
within the catheter such that no portion of the needle is exposed outside the
distal end of the catheter. However, the removable needle is preferably disposedwithin the catheter such that the distal end of the needle is exposed outside the
30 distal end of the catheter for contacting the epidural space of the spine of the
subject. Either a hollow or a non-hollow needle may be used. However, the
needle is preferably hollow such that fluids may be conducted therethrough.
Preferably, the exposed distal end of the needle is not left in the
35 epidural space for extended periods of time. Therefore, once the location or
placement of the catheter is confirmed by electrical stimulation, the removable
needle is preferably removed or withdrawn, at least in part, such that the distal
end of the needle is no longer exposed outside the distal end of the catheter so
CA 02260080 1999-01-11
that the metal of the needle is no longer in direct contact with the epidural space.
More preferably, the needle is withdrawn completely from the catheter so that norigid objects or elements remain in the epidural space of the subject.
Alternately the device may be comprised of: (a) the catheter having
a distal end for contacting the epidural space of the spine of a subject, wherein
the catheter is comprised of a hollow needle such that fluids may be conducted
therethrough to a distal end of the needle and wherein the distal end of the
needle defines the distal end of the catheter for contacting the epidural space of
the spine of the subject; and (b) a source of electricity in communication with the
needle such that electricity is conducted to the distal end of the needle to cause a
motor response of the subject in order to confirm the placement of the catheter
in the epidural space. The fluids may be conducted directly through the bore of
the needle or through a suitable second catheter, having an outside diameter
smaller than the diameter of the bore of the needle, which may be inserted in the
bore. Further, the needle may include an insulating coating about at least a
portion of the needle, wherein the distal end of the needle is exposed outside the
insulating coating.
Preferably, the hollow needle is not left in the epidural space for
extended periods of time. Therefore, once the location or placement of the
catheter is confirmed by electrical stimulation, an injection of an anesthetic or
other medication may be introduced into the epidural space and the hollow
needle is preferably subsequently withdrawn. Further, the second smaller
diameter catheter described above may be inserted into the epidural space
through the bore of the needle. The second smaller diameter catheter may be leftin the epidural space and used for the administration of medications following
the withdrawal or removal of the needle.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is an illustration of the spine of a human showing the
location of one method of inserting an epidural catheter tube;
Figure 2 is an illustration of a modified epidural catheter connector
useful in one embodiment of the present invention;
- 10 -
CA 02260080 1999-01-11
Figure 3 is an illustration of an epidural catheter with a wire
disposed therein, useful in one embodiment of the present invention;
Figure 4 is a schematic illustration of the clinical use of one
5 embodiment of the present invention;
Figure 5 is an illustration of an epidural catheter comprised of a
hollow needle having an insulating coating; and
Figure 6 is a schematic illustration of the clinical use of the epidural
catheter shown in Figure 5.
DEFINITIONS
"Subject" as used herein refers to a vertebrate. Preferably, the
vertebrate is a human.
"Catheter" as used herein refers to a tubular medical device for
20 insertion into canals, vessels, passageway or body cavities to permit injection or
withdrawal of fluids or to keep a passage open wherein the fluid travels in the
interior of the tubular structure. A preferred catheter is capable of insertion into
an epidural space of a subject having a spine for the introduction of fluid. In such
a catheter, there may be a metal element disposed within the catheter for the
25 conduction of electricity. The metal element may extend to the end of the
catheter such that the metal element is exposed outside of the catheter for
conducting electricity directly to the epidural space of the subject. In this
instance, the metal element is preferably removable from the catheter such that
the metal element is not exposed to the epidural space of the subject for
30 prolonged periods of time. However, in a preferred embodiment, the metal
element does not extend to the end of the catheter such that, when it is inserted
into an epidural space of a subject, there is no directly exposed metal component
in the epidural space of the subject. In this instance, an aqueous solution capable
of conducting electricity is used, as described below, such that the metal element
35 conducts electricity indirectly to the epidural space of the subject as a result of the
aqueous solution.
CA 02260080 1999-01-11
Alternately, the catheter may be comprised of a hollow needle such
that fluids may be conducted therethrough to a distal end of the needle. The
fluids may be conducted directly through the bore of the needle or through a
suitable second catheter, having an outside diameter smaller than the diameter
5 of the bore of the needle, which may be inserted in the bore. In this alternate
embodiment, the needle is preferably suitable for insertion into the epidural
space of the spine of the subject and preferably has sufficient rigidity to permit
epidural placement percutaneously. The distal end of the needle is provided for
contacting the epidural space of the spine and directing conducting electricity
10 thereto. Further, the needle preferably includes an insulating coating about at
least a portion of the needle for inhibiting the conduction of electricity
therethrough. However, the distal end of the needle is exposed outside of the
insulating coating for contacting the epidural space.
"Aqueous solution" as used herein refers to a liquid capable of
conducting electricity. In one embodiment, the aqueous solution is water based
and may be a "saline solution" (e.g., a solution of water with potassium, sodiumand/or magnesium salt). In a preferred embodiment, the aqueous solution is
isotonic. Another preferred aqueous solution is a solution comprising an
20 anesthetic such as an "epimorphine solution" (e.g., a morphine solution
designed for epidural injection, e.g., 1 to 5 mg dosages at 0.5 mg/ml or 2 to 10 mg
doses at 1 mg/ml).
"Epidural space" as used herein is the space defined between the
25 ligamentum flavum on the posterior or back side of the spinal cord and the
anterior longitudinal ligament on the anterior or frontal side of the spinal cord.
"Motor response" as used herein refers to a reaction of the body of a
subject to an electrical stimulus to the epidural space. Commonly, the motor
30 response-is muscle spasm or twitch.
"Connector" as used herein refers to an element designed to attach
to the end of a catheter and adapted to connect with a fluid control means (e.g., a
syringe) such that fluid may be introduced to or withdrawn from the catheter.
"Conductive element" as used herein refers to an element capable
of conducting electricity to the inside of a catheter. In the preferred embodiment,
such introduction may be due to direct communication with the inside of the
- 12-
CA 02260080 1999-01-11
catheter or with a electrically conductive material (solid or liquid) that is incommunication with the inside of a catheter. Commonly, the conductive
element is metal, and in a preferred embodiment, the conductive element is a
screw. However, the conductive element may be comprised of either a hollow or
a non-hollow needle.
"Electrical conduction means" as used herein refers to an element
capable of introducing electricity to the interior of a catheter that is attached to a
source of electricity. In a preferred embodiment, the electrical conduction means
10 is a connector having a conductive element that is connected to a source of
electricity.
"Unable to communicate" as used herein refers to a subject that is
unable to effectively communicate paresthesia or anesthetic effect on the
15 subject's pain or perception of pain to a treating physician. This condition may
be the result of the nature of the subject (e.g., speaks a language foreign to the
physician or is not human) or may be the result of the subject's condition (e.g.,
age of the subject or due to a condition preventing communication, such as beingmute). Commonly, the subject will be unable to communicate due to being
20 "unconscious" (unable to perceive any extracorpeal stimulus).
"Anesthetic" as used herein refers to a chemical composition, the
application of which can reduce a subject's perception of pain.
DETAILED DESCRIPTION OF THE INVENTION
In the present invention, a catheter assembly suitable for
introduction into an epidural space is provided. In a preferred embodiment, the
30 catheter assembly comprises a catheter tube of a size suitable for insertion into an
epidural space, a connector attached to the proximal end of the catheter tube, and
a connector having a conductive element. When desired, an appropriate
connector may be constructed with modification of existing epidural catheter
connectors. For example, the connector by Smiths Industries (sold by
35 Concord/Portex and described in U.S. Patent No. 5,464,400, herein incorporated
by reference) can be modified with an electrode. Figure 2 shows such a
modification. The connector shown in side view has an electrode inserted 20.
Likewise, the same electrode 20 is shown in the connector in top view 30,
- 13-
CA 02260080 1999-01-11
demonstrating the that the electrode extends from the outside of the connector
40 to the interior lumen of the connector 50. Thus, in this embodiment an
electric source may be attached to the electrode 20 on its outer exposed portionand the electric current will reach the interior lumen 50 of the connector
For this preferred embodiment, the catheter is placed in an epidural
space of a subject and the conductive element is attached to an electric source.An aqueous solution capable of conducting electricity is introduced into the
catheter and an electric current is applied to the conductive element of the
10 connector. The electric current provides a stimulus to the region of placement of
the catheter and motor responses by the subject indicate the successful placement
of the catheter. For example, if the subject exhibits motor responses at low
current (ie., 1-10 mA), the catheter has been inserted into the epidural space. On
the other hand, if the subject only exhibits a motor response at a higher current
15 (ie., > 'lOmA), then the catheter is outside of the epidural space. If the subject
exhibits a motor response at a very low current (ie., < lmA), the catheter may be
placed in subarachnoid space, subdural space or directly against a spinal root. Of
course, these amperage settings are intended as guidelines and can be readily
adjusted for the individual subject and/or species.
In addition to determining the proper placement in an epidural
space, the present invention can be utilized to avoid an improper vascular
placement in the epidural space. For example, by observance of the current
required for motor response before and after a test dose of local anesthetic
25 injected (e.g., 3 to 6 ml test dose of 1.5% lidocaine) into the epidural space, one
may also detect the intravascular placement of catheter within an epidural space.
In proper epidural catheter placement, the minimum current required to exhibit
a subject's motor response will gradually increase over time. On the other hand,this minimum current will remain unchanged if the placement of the catheter is
30 intravascular as the injected local anesthetic is inadvertently injected
systemically rather than locally. Thus, the present invention is useful in
determining proper placement of a catheter into an epidural space as well as
determining if the catheter is improperly inserted intravascularly in the epidural
space.
It should be understood that the terms "distal" and "proximal" as
used herein mean the following. The distal end of the catheter is the tip of the
- 14-
CA 02260080 1999-01-11
catheter which is first inserted into the epidural space. The proximal end is the
opposite end of the catheter.
While the present invention is not limited by the nature of the
catheter tube, in one embodiment, it will be of a size suitable for placement in an
epidural space. The catheter size is preferably that of a number 4 French body
(1.67 mm). The usable length of the catheter is not critical and may be
approximately 45-180 cm. The particular material of which the catheter is made
is not critical and may be any of the types of material used in known catheters,10 such as known bipolar cardiac pacing catheters.
While the catheter will normally be sufficiently rigid to permit
epidural placement, if the materials used do not permit sufficient rigidity for this
purpose, a stylet may be used to control the placement of the catheter. In this
15 case a stylet passage is present which permits insertion of the stylet into the
catheter. Likewise, a stylet may be inserted into the catheter via passage for
purposes of manipulating the catheter during placement in the epidural region.
Other catheters and systems and methods for inserting spinal catheters are set
forth in U.S. Patents No. 4,973,312; 4,985,022; 5,069,674; and 5,135,525 all herein
20 incorporated by reference.
The catheter, when in use, is inserted into the spinal epidural space.
This may be accomplished by means of a 15, 16 or 17 gauge epidural needle or it
may be applied directly during spinal surgery. Special needles for epidural
25 injection are described in U.S. Patent No. 5,628,734, herein incorporated by
reference.
The method of using an epidural injection needle in administering
an epidural anesthetic preferably employs the midline approach. The midline
30 approach is known to the ordinary skilled artisan and, therefore, will not bedescribed here in detail. A description of this method can be found in the
Illustrated Handbook of Local Anesthesia, Year Book Medical Publishers, Inc.,
1969. It should be understood, however, that an injection needle may also be
employed in a spinal injection made using the lateral approach.
Air lock within the catheter or high resistivity aqueous solutions
may hinder the flow of current down the length of the catheter tube. Therefore,
in one embodiment of the present invention a metal element is disposed within
- 15-
CA 02260080 1999-01-11
the lumen of the catheter tube to ensure proper conduction of electricity through
the length of the catheter. In one embodiment, the metal element is a wire. In
such an embodiment, the wire may be connected to the conductive element of
the connector and traverse the length of the catheter tube. To avoid
5 electrochemical reaction of the metal element to the subject's dural tissue in the
presence of the fluid contents of the catheter tube or the subject's body fluids, in a
preferred embodiment, the metal element does not reach the end of the catheter
tubing such that no metal element is directly contacting the subject's dural tissue.
One embodiment of a wire within a catheter is illustrated in Figure 3. A catheter
tube (60) has a wire (70) extending from the catheter's proximal end (80) towardthe distal end (90), but the wire (70) does not extend entirely to the distal end.
As indicated, the metal element is preferably comprised of a wire as
described above. However, the metal element may be comprised of any metal
15 device or structure suitable for this purpose, such as a hollow or non-hollow needle.
Further, in the preferred embodiment described above, the metal
element does not extend to the distal end of the catheter and an aqueous solution
20 is used for conducting electricity from the metal element to the epidural space of
the subject. However, alternately, the metal element may extend to the end of
the catheter such that the metal element is exposed outside of the catheter. In
this instance, the metal element conducts the electricity directly to the epidural
space of the subject.
However, where the metal element conducts electricity directly to
the epidural space, the metal element preferably does not contact, and is not
exposed to, the epidural space of the subject for extended periods of time. As aresult, the metal element is preferably removable in this embodiment. In order
30 to facilitate the removal of the metal element, the metal element is also
preferably comprised of a needle in this embodiment. Thus, once the location or
placement of the catheter is confirmed by electrical stimulation, the removable
needle is removed or withdrawn, at least in part, such that its distal end is nolonger exposed outside the distal end of the catheter. Accordingly, the metal of35 the needle is no longer in direct contact with the epidural space. More preferably,
the needle is withdrawn completely from the catheter so that no rigid objects orelements remain in the epidural space of the subject.
- 16-
CA 02260080 1999-01-11
Further, the catheter itself may be comprised of a hollow needle
such that fluids may be conducted therethrough to a distal end of the needle.
The fluids may be conducted directly through the bore of the needle. Alternately,
the fluids may be conducted through a suitable second catheter, having an
5 outside diameter smaller than the diameter of the bore of the needle, which isinserted in the bore. The distal end of the needle defines the distal end of thecatheter and is provided for contacting the epidural space of the spine of the
subject. Thus, electricity is conducted to the epidural space directly by the distal
end of the needle. Further, the needle is suitable for insertion into the epidural
10 space of the spine of the subject and preferably has sufficient rigidity to permit
epidural placement percutaneously. As well, the hollow needle of this
embodiment preferably includes an insulating coating for inhibiting the
conduction of electricity therethrough. The insulating coating may cover all or a
portion of the outer surface of the needle. However, in any event, the distal end
15 of the needle is exposed outside of the insulating coating for contacting theepidural space. As a result, the electricity is conducted to the distal end of the
catheter.
Preferably, in this alternate embodiment, the hollow needle is not
20 left in the epidural space for extended periods of time. Therefore, once the
location or placement of the catheter is confirmed by electrical stimulation, aninjection of an anesthetic or other medication may be introduced into the
epidural space and the hollow needle is preferably subsequently withdrawn.
Further, the second smaller diameter catheter described above may be inserted
25 into the epidural space through the bore of the needle. The second smaller
diameter catheter may then be left in the epidural space and used for the
administration of medications following the withdrawal or removal of the
needle.
This alternate embodiment of the invention is further illustrated in
Figure 5. Referring to Figure 5, the catheter (190) is comprised of the hollow
needle (200) for conducting fluids, such as an anesthetic, therethrough from a
proximal end (220) to the distal end (210) of the needle (200). The distal end (210)
of the needle (200) defines the distal end of the catheter for contacting the
epidural space of the spine of the subject. Further, the insulating coating (230)
extends substantially between the proximal end (220) and the distal end (210) ofthe needle (200). Although the insulating coating (230) may be comprised of a
separate tubular element formed from an insulating material for receiving the
- 17-
CA 02260080 1999-01-11
needle (200) therein, the insulating coating (230) is preferably comprised of a layer
of an insulative material integral with or affixed to the outer surface of the
needle (200). The distal end (210) of the needle (200) is exposed outside the
insulating coating (230) such that the distal end (210) may contact the epiduralspace and conduct electricity thereto. The proximal end (220) of the needle (200)
is attached to or is connected or communicates with a source of electricity.' Thus,
electricity is conducted through the needle (200) inside the insulated coating (230)
from the proximal end (220) to the distal end (210) such that the electricity isdirectly conducted to the epidural space.
The present invention is not limited by the electrical source. In a
preferred embodiment, the electrical source comprises a nerve stimulator, such
at the Dakmed Model 750 with digital display. Likewise, while the present
invention is not limited to a specific method of introducing electricity to a
15 conductive element, in one embodiment the negative lead from a nerve
stimulator is connected to the conductive element of the connector and the
positive lead is connected directly to the subject.
Drugs (medicaments) are inserted into the epidural region by the
20 catheter by injection from a drug administering device, such as a hypodermic
syringe, and directed to the interior of the catheter. The drug exits the catheter
through the outlet opening on the distal end of the catheter. While the present
invention is not limited by the nature of the medicant, one medicant suitable isdescribed in U.S. Patent No. 5,545,648, herein incorporated by reference. Where
25 the catheter comprises a hollow needle as described above, the drugs may be
directly inserted into the epidural region by injection through the distal end of
the needle.
The catheter of the present invention may be used not only on
30 human subjects but also on other animals, preferably vertebrates, and most
preferably mammals. As animals other than humans cannot communicate to
express anesthetic effect, an objective means of determining the proper
placement the catheter becomes very valuable.
The following example serves to illustrate a certain preferred
embodiment and aspects of the present invention and is not to be construed as
limiting the scope thereof.
CA 02260080 1999-01-11
EXAMPLE 1
In this example, a method of using low current stimulus through
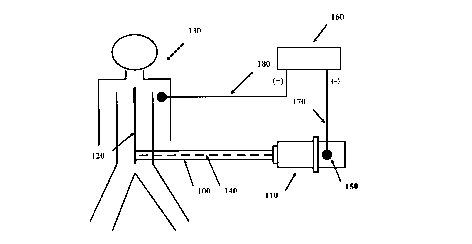
an epidural catheter to determine proper placement of the catheter is described.Figure 4 is a schematic diagram of the method described in this example.
A catheter (100) with a connector (110) on its proximal end is
inserted into an epidural space (not shown) of the spine (120) of a subject (130). A
wire (140) is disposed within the- catheter (100) and extends from a conductive
10 element (150) on the connector (110) down through the catheter (100), but does
not reach the distal end (not shown) of the catheter (100).
The conductive element (150) of the connector (110) is set such that
it communicates with the inner cavity (not shown) of the connector (110) near,
15 preferably touching, the wire (140), as well as extending outside the connector
(110) such that it can be attached to an electric source (160). The electric source
(160) is a nerve stimulator (Dakmed Model 750 digital) set at 1 Hz.
In operation, the distal end of the catheter (100) is placed in the
20 region of an epidural space. Saline solution is introduced into the catheter (100)
and the negative lead (170) from the electric source (160) is attached to the
conductive element (150). The positive lead (180) from the electric source (160) is
attached to the subject (130). Low current electrical stimulation is provided bythe electric source (160) and motor response from the subject (130) is assessed.25 The output current is gradually increased from zero mA at 1 Hz (one pulse persecond) until a motor response of the subject (130) is visible. If the subject
responds to low amperage current (ie., 1 to 10 mA), the placement of the catheter
is correct. If the subject (130) only responds to higher amperage current, the
catheter is not in an epidural space.
EXAMPLE 2
Using sterile technique, a nerve stimulator (Dakmed model 750
digital) is connected to an existing epidural catheter via an ECG adaptor (Arrow35 Corporation as described in U.S. Patent No. 4,644,960, herein incorporated byreference). 0.2-1 ccs of sterile normal saline solution is injected via the catheter to
prime the catheter and the ECG adaptor. The negative lead of the nerve
stimulator is attached to the metal hub of the ECG adaptor. The nerve
- 19-
CA 02260080 1999-01-11
stimulator frequency is set at a rate of I Hz. The output current is gradually
increased from zero until motor activity/twitch response is visible. Depending
on the observed positive or negative response to low current stimulation (1 to 10
mA), the catheter's placement is considered to be correct or incorrect. A standard
5 test dose (3ml of 1.5% lidocaine with 1:200,000 epinephrine) is then injected.
The subject is then assessed clinically for any change in heart rate,
blood pressure and sensory functions in 3 minutes. By observing the minimum
current required for motor responses before and after this test dose, intravascular
10 placement can be detected. The minimum current required to induce a motor
response increases after the standard test dose is administered if the catheter is
placed properly in the epidural space (ie., not intravascularly). If the catheter is
improperly placed intravascularly in the epidural space, the minimum current
required to induce a motor response is unchanged.
EXAMPLE 3
This example teaches a method of producing a connector
dimensioned such that it is suitable for connection to an epidural catheter, such
20 connector having a conductive element suitable for connection to a source of
electricity. Figure 2 illustrates the product. A metal sheet metal screw 20 is
inserted into the side of an epidural catheter connector 10 (commercially
available from Smith Industries, Keene, NH). The metal screw 20 is suitable as aconductive element in the connector 10.
EXAMPLE 4
This example provides an alternative method of producing a
connector dimensioned such that it is suitable for connection to an epidural
30 catheter, such connector having a conductive element suitable for connection to
a source of electricity. An epidural catheter connector (Flextip PlusTM, Arrow
International Inc., Reading, PA) is connected to an Arrow-Johans ECG Adapter
for right atrial electrocardiography (RAECG, Arrow International Inc., Reading,
PA), having a conductive element.
From the above, it is clear that the present invention provides a
simple and effective method for determining the proper placement of a catheter
into an epidural space prior to the introduction of anesthesia.
- 20 -
... , ., ~ , .. ~
CA 02260080 1999-01-11
EXAMPLE 5
Referring to Figures 5 and 6, in this example, a method and device
are provided for confirming the placement of a catheter (190) in the epidural
space of the spine of the subject using low current stimulus conducted through ahollow needle (200) directly to the epidural space. In this embodiment, the
catheter (190) comprises the hollow needle (200) for conducting fluids
therethrough. In particular, the fluids are conducted directly through the bore of
10 the needle (200). However, alternately, the fluids may be conducted through asuitable second catheter (not shown), having an outside diameter smaller than
the diameter of the bore of the needle, which is inserted in the bore.
Further, the electricity is conducted to a distal end (210) of the
15 needle (200) for contact with the epidural space (240) of the subject. The needle
(200) has sufficient rigidity to permit epidural placement percutaneously. As
well, the needle (200) has an insulating coating (230) extending substantially
between a proximal end (220) and the distal end (210) of the needle (200). The
distal end (210) extends outside of the insulating coating (230) and is suitable for
20 insertion into the epidural space (240). The proximal end (220) may also extend
outside the insulating coating (230) and is suitable for connection to or
communication with an electrical source.
In operation, the negative lead of a nerve stimulator is attached to
25 the proximal end (220) of the needle (200). The nerve stimulator frequency is set
at a rate of 1 Hz. The output current is gradually increased from zero until a
motor response (twitch response) is visible. Depending upon the observed
positive or negative response to low current stimulation (1 to 10 mA), the
placement of the catheter is considered to be correct or incorrect. When
30 incorrect, the placement may be adjusted and the method performed again.
- 21 -