Note: Descriptions are shown in the official language in which they were submitted.
CA 02260315 1999-01-25
11-ACETYL-12,13-DIOXABICYCLO[8.2.1]-TRIDECENONE
DERIVATIVES, PROCESSES FOR THEIR PREPARATION AND
PHARMACEUTICAL COMPOSITIONS COMPRISING THEM
BACKGROUND OF THE INVENTION
The present invention relates to novel N-substituted
(2R,3S,4S,5R,6R,10R,11R)-3-[(2,6-dideoxy-3-C-methyl-3-O-
methyl-a-L-ribohexopyranosyl)-oxy]-5-[(3,4,6-trideoxy-3-
amino-(3-D-xylohexopyranosyl)-oxy]-2,4,6,8,10-pentamethyl-il-
acetyl-12,13-dioxabicyclo[8.2.1]-tridec-8-en-l-one compounds
with motilin-agonistic properties and to the acid addition
salts thereof and also to pharmaceutical formulations
containing these compounds and to processes for the
preparation of these compounds. The compounds according to
the invention are ring-contracted N-demethyl-N-isopropyl
derivatives of erythromycin A with a modified side chain.
The antibiotic erythromycin A is known to have, in
addition to its antibiotic effects, also gastrointestinal
side effects which are undesirable for antibiotics, inter
alia a great increase in the contraction activity in the
gastrointestinal region with gastric and intestinal cramps,
nausea, vomiting and diarrhoea.
There have been several attempts to modify erythro-
mycin A so as to obtain derivatives in which the antibiotic
effect is virtually no longer present, but an effect
influencing the motility of the gastrointestinal tract is
retained. U.S. Patent No. 5,418,224 (= EP 550,895)
discloses ring-contracted N-demethyl-N-isopropyl-
erythromycin A derivatives having gastrointestinally
effective motilin-agonistic properties.
Furthermore, similar ring-contracted erythromycin
derivatives are known from U.S. Patent No. 5,106,961 (= EP
382,472), but these have antibiotic effects.
CA 02260315 1999-01-25
SUMMARY OF THE INVENTION
It is an aspect of the present invention to provide
novel, orally-effective ring-contracted derivatives of
erythromycin A without an antibiotic effect and with
properties having a beneficial effect on the motility of the
gastrointestinal tract with an improved activity profile.
It has now been found that the novel ring-contracted
N-demethyl-N-isopropyl derivatives of erythromycin A, the
side chain of which in the 11 position of the cyclic parent
structure has been modified by oxidation, are not
antibiotically effective, but have selective
motilin-agonistic properties and stimulate the motility of
the gastrointestinal tract in a beneficial way and show
effects enhancing the tone of the lower esophagus sphincter
and the tone of the stomach.
Because of their activity profile, the substances
according to the invention are suitable for the treatment of
motility disturbances in the gastrointestinal tract and
moreover are distinguished by good compatibility and good
oral effectiveness.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
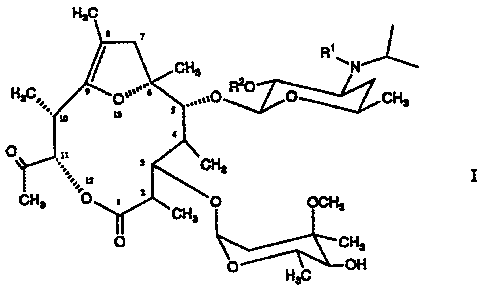
The present invention therefore relates to novel
(2R,3S,4S,5R,6R,10R,11R)-2,4,6,8,10-pentamethyl-ll-acetyl-
12,13-dioxabicyclo[8.2.1]-tridec-8-en-l-one compounds of
formula I
,
CHS
H~=,,' O''~~ ...,,0 ~0 O CHa
30 '
O ,.;=,n, 3 CHO
GFia =O , ' CH (~~Z S
Hs
H3 C OH
35 wherein
-2-
CA 02260315 1999-01-25
R' denotes hydrogen or methyl, and
R2 denotes hydrogen or lower alkanoyl,
and the stable and physiologically compatible acid addition
salts thereof.
If in compounds of Formula I one substituent is or
contains lower alkyl, this may be branched or unbranched,
and may have 1 to 4 carbon atoms. The compounds of Formula
I in which R1 is methyl have proved particularly beneficial.
R2 preferably represents hydrogen. If R2 is lower
alkanoyl, acetyl is preferred.
The compounds of Formula I can be obtained in that, in
known manner, in a compound of the general formula II,
HOC
7 ~
CHs R
~..% 0~~~~' ..; ~,.0 ~ O CH3
w ~
OH '
CH'
H3C ; II
-,v
~~O 1CH$ O OCH'
CHa 0 CH'
OH
HsC
in which R1 and R 2 have the above meanings, the
2',3'-dihydroxypent-2'-y1 side chain in the 11 position of
the cyclic parent structure is converted by oxidative glycol
cleavage into an acetyl side chain and, if desired, a methyl
group R1 is introduced into the resulting compound of
Formula I in which R' denotes hydrogen, or the methyl group
R1 is cleaved off in the resulting compound of Formula I in
which R' denotes methyl, and, if desired, free compounds of
Formula I are converted into the stable acid addition salts
thereof, or the acid addition salts are converted into the
free compounds of Formula I.
-3-
CA 02260315 1999-01-25
The oxidative glycol cleavage of the 21,3'-dihydroxy-
pent-2'-yl side chain in the 11 position of the cyclic
parent structure of compounds of Formula II can be performed
using suitable oxidation agents such as lead tetraacetate in
solvents suitable for this purpose. Suitable solvents
include non-polar or weakly polar solvents such as benzene,
toluene or xylene. The reaction may be performed at
temperatures between 0 C and 40 C, preferably at room
temperature.
The resulting compounds of Formula I in which R1
denotes hydrogen can, if desired, subsequently be alkylated
in known manner to give the corresponding N-methyl
compounds. The alkylation can take place in known manner by
reaction with a methyl halide or as reductive alkylation by
reaction with formaldehyde under reducing conditions, and
can be carried out, for example, in the presence of a
reducing agent, for example of a complex borohydride
compound such as sodium cyanoborohydride, sodium
triacetoxyborohydride or sodium borohydride. If desired,
the alkylation can also be carried out by reaction with a
methyl halide, especially methyl iodide, or with a
methylsulfonic acid ester. The alkylation is advantageously
carried out in an organic solvent which is inert under the
reaction conditions. Suitable solvents for the reductive
alkylation include cyclic ethers such as tetrahydrofuran
(= THF) or dioxane, aromatic hydrocarbons such as toluene,
or alternatively lower alcohols. The alkylation can be
carried out at temperatures between room temperature and the
boiling point of the solvent. The alkylation with a methyl
derivative, for example a methyl halide such as methyl
iodide, is advantageously carried out in the presence of a
base such as, for example, an alkali metal carbonate or a
tertiary organic amine.
The methyl group R1 can, if desired, subsequently be
cleaved off from the compounds of Formula I in which R1
denotes methyl. The demethylation can be effected in known
-4-
CA 02260315 2007-06-05
manner by treating the compound with a halogen, in particular
iodine and/or bromine, in an inert solvent in the presence of
a suitable base. Suitable bases include, for example, alkali
metal alcoholates, alkali metal hydroxides and alkali metal
salts of weak organic acids.
The compounds of Formula I can be isolated from the
reaction mixture and purified in known manner. Acid addition
salts can be converted in conventional manner into the free
bases, and the free bases can, if desired, be converted in
known manner into pharmacologically compatible acid addition
salts. To avoid secondary hydrolysis reactions, it is
advantageous to use only equivalent amounts of acids for the
salt formation.
Examples of suitable pharmacologically acceptable acid
addition salts of the compounds of Formula I include the
salts thereof with inorganic acids, for example carbonic
acid, hydrohalic acids, especially hydrochloric acid, or with
organic acids, for example lower aliphatic mono- or
dicarboxylic acids such as maleic acid, fumaric acid, lactic
acid, tartaric acid or acetic acid.
The starting compounds of Formula II in which R2 is
hydrogen are known from U.S. Patent No. 5,418,224, and can be
prepared according to the processes described therein.
The starting compounds of Formula II in which R2 is lower
alkanoyl can be prepared by reacting compounds of Formula II
in which R2 is hydrogen with carboxylic acids of the general
formula III,
R3 - COOH I I I
in which R3 is lower alkyl, or reactive derivatives of these
acids, in known manner.
Suitable reactive derivatives of acids of Formula III
include, in particular, optionally mixed acid anhydrides and
-5-
CA 02260315 1999-01-25
acid halides. For example, acid chlorides or acid bromides
of the acids of Formula III or mixed esters of the acids of
Formula III with organic sulfonic acids, for example with
lower alkane sulfonic acids optionally substituted by
halogen, such as methanesulfonic acid or trifluoromethane-
sulfonic acid, or with aromatic sulfonic acids such as
benzenesulfonic acids or with benzenesulfonic acids
substituted by lower alkyl or halogen, e.g. toluenesulfonic
acids or bromobenzene-sulfonic acids, can be used. The
reaction may be performed as an acylation in an organic
solvent which is inert under the reaction conditions at
temperatures between -20 C and room temperature. Suitable
solvents include di-lower alkyl ketones, for example
acetone, halogenated hydrocarbons such as dichloromethane or
aromatic hydrocarbons such as benzene or toluene or cyclic
ethers such as THF or dioxane, or mixtures of these
solvents.
The acylation can advantageously be carried out in the
presence of an acid-binding reagent, particularly if an
anhydride or a mixed anhydride of the acids of Formula III
with a sulfonic acid is used as acylation agent. Suitable
acid-binding reagents include inorganic bases such as alkali
metal carbonates, for example potassium carbonate, or
organic bases soluble in the reaction mixture, such as
tertiary nitrogen bases, for example tert. lower alkylamines
and pyridines, such as triethylamine, tripropylamine,
N-methylmorpholine, pyridine, 4-dimethylaminopyridine,
4-diethylaminopyridine or 4-pyrrolidinopyridine.
If desired, a methyl group R' may be introduced into a
resulting compound of Formula II in which Rl is hydrogen, or
the methyl group R' in a resulting compound of Formula II in
which R1 is methyl can be cleaved off. Such methylation or
demethylation operations may be performed in known manner,
for example under the conditions described for the
introduction or cleavage of a methyl group in the compounds
of Formula I.
-6-
CA 02260315 1999-01-25
The novel compounds of Formula I and the physio-
logically compatible acid addition salts thereof have
interesting pharmacological properties, especially
motilin-agonistic properties stimulating the motility of the
gastrointestinal tract. In this case, they are
distinguished by a beneficial activity profile with
surprisingly good oral effectiveness. They are free of
antibiotic effects and have a high selective affinity for
motilin receptors, whereas in dose ranges with
motilin-agonistic effectiveness they show no practically
relevant affinity for other receptors in the
gastrointestinal tract such as adrenaline, acetylcholine,
histamine, dopamine or serotonin receptors. The compounds
have surprisingly good liver compatibility, which makes them
suitable for applications over relatively long periods.
In the healthy state, the autonomic nervous system and
hormones in the gastrointestinal tract cooperate to ensure
controlled digestion of food consumed and in order to
generate a controlled contraction activity of the
gastrointestinal tract not only immediately after intake of
food but also when the gastrointestinal tract is empty.
Motilin is a known gastrointestinal peptide hormone which
stimulates the motility of the gastrointestinal tract and
induces a coordinated motility throughout the
gastrointestinal tract in the fasting state and after intake
of food.
The compounds of Formula I show motilin-like
physiological effects in that they act as agonists for
motilin receptors. Thus, the compounds of Formula I show
pronounced stimulating effects in the gastrointestinal
region and at the lower esophagus sphincter. In particular,
they bring about an increased rate of gastric emptying, an
increase in the stomach tone and a long-lasting increase in
the resting tone of the esophagus sphincter. Because of
their motilin-like activity profile, the substances are
suitable for the treatment of conditions which are
-7-
CA 02260315 1999-01-25
associated with motility disturbances in the
gastrointestinal tract and/or reflux of chyme from the
stomach into the esophagus. Thus, the compounds of
Formula I are indicated, for example, for gastroparesis with
a wide variety of causes, disturbances of the stomach tone,
disturbances of gastric emptying and gastro-esophageal
reflux, dyspepsia and postoperative disturbances.
The gastrointestinally effective properties of the
compounds of Formula I can be demonstrated in standard
pharmacological test methods in vitro and in vivo.
Description of the test methods
1. Determination of the binding capacity of the test
substances to motilin receptors.
The affinity of the compounds of Formula I for motilin
receptors is measured in vitro on a fraction of a tissue
homogenate from rabbit antrum. The displacement of
radioactively labelled iodinated motilin from motilin
receptor binding by the test substances is determined.
The receptor binding studies are carried out by a
modification of the process of Borman et al. (Regulatory
Peptides 15:143-153 (1986). To prepare the 125iodine-
labelled motilin, motilin is iodinated enzymatically using
lactoperoxidase in known manner, for example in analogy to
the method described by Bloom et al. (Scand. J.
Gastroenterol. 11:47-52 (1976).
To obtain the fraction of tissue homogenate used in the
test from rabbit antrum, the antrum from which the mucosa
have been removed is comminuted and homogenised in 10 times
the volume of a cold homogenisation buffer solution (50 mM
tris-HC1 buffer, 250 mM sucrose, 25 mM KC1, 10 mM MgC121
pH 7.4) with the addition of inhibitors (1 mM iodoacetamide,
1 M pepstatin, 0.1 mM methylsulfonyl fluoride, 0.1 g/l
trypsin inhibitor, 0.25 g/1 bacitracin) with a homogenizer
at 1500 revolutions per minute for 15 sec. The homogenizate
is then centrifuged at 1000 g for 15 minutes, the resulting
-8-
CA 02260315 1999-01-25
residue is washed four times with homogenization buffer
solution and finally re-suspended in 0.9% strength sodium
chloride solution (in a volume corresponding to 5 times the
amount by weight of the antrum). The tissue fraction
obtained in this way, which is referred to as "crude
membrane preparation", is used for the test.
For the binding test, 200 jil of the crude membrane
fraction (0.5 - 1 mg of protein) in 400 l of a buffer
solution A (50 mM tris-HC1 buffer, 1.5% bovine serum albumen
(BSA), 10 mM MgC1Z1 pH 8.0) are incubated with 100 l of
iodinated motilin diluted in buffer solution B (10 mM tris-
HC1 buffer, 1% BSA, pH 8) (final concentration 50 pM) at
30 C for 60 min. The reaction is stopped by adding 3.2 ml
of cold buffer solution B, and bound and non-bound motilin
are separated from one another by centrifugation (1000 g,
15 minutes) . The residue obtained as pellets after the
centrifugation is washed with buffer solution B and counted
in a gamma counter. The displacement studies are carried
out by adding increasing amounts of the substance to be
tested to the incubation medium. The test substance
solutions employed are aqueous solutions which are prepared
by suitable dilution of 60 x 10-4 molar aqueous stock
solutions. Test substances which are sparingly soluble in
water are initially dissolved in 60% strength ethanol, and
this solution is diluted with sufficient water for the
ethanol concentration in the solution to be tested not to
exceed 1.6% by volume. The IC50 of the particular test
substance is determined from the resulting measured data as
that concentration which brings about 50% inhibition of the
specific binding of the iodinated motilin to the motilin
receptors. From this the corresponding pIC50 value is
calculated. The pIC50 values given in Table 1 below were
determined for the substances of Examples 1 and 2 using the
above method. The Example numbers quoted relate to the
preparation examples described below.
-9-
CA 02260315 1999-01-25
Table 1
Example No. PIC50
1 8.01
2 7.75
2. In vivo determination of the effect of the substances
on the stomach tone.
The stomach tone plays an important role in gastric
emptying. An increased stomach tone contributes to an
increased rate of gastric emptying.
The influence of substances on the stomach tone is
determined on beagles with the aid of a barostat which is
connected to a plastic pouch in the stomach of the dog and
permits measurement of volume or pressure in the stomach of
the dog. With the barostat, the stomach volume is
determined at a constant pressure in the stomach or the
stomach pressure is determined at a constant volume in the
stomach. When the stomach tone increases, a reduced stomach
volume is detected at a given pressure, and an increased
pressure at a given volume. In the test model used to
investigate the increase in stomach tone effected by the
substances, the change in stomach volume caused by the
substances is measured at constant pressure. The stomach of
the test animals is relaxed by intake of lipids, i.e. the
stomach tone decreases, which causes the stomach volume to
increase correspondingly. The reduction in % of the stomach
volume which has been increased by administration of lipids
which occurs after intake of the substance due to a re-
increase in stomach tone is measured as a measurement of the
stomach tone-increasing effect of the substances.
The substance of Example 1 in this test model,
administered i.d. in the readily compatible dose of
2.15 mole/kg, showed a reduction in the stomach volume
increased after lipid administration by 59.5%. Oral
administration of the above test substance in the same dose
-10-
CA 02260315 1999-01-25
of 2.15 mole/kg caused an unusually marked reduction in the
stomach volume, the lipid-induced relaxation of the stomach
volume being practically completely hindered. These
findings can be used as clear indications of a particularly
high, in particular high oral, bioavailability of the
substances according to the invention.
3. In vivo determination of the effect of the substances
on the resting tone of the lower esophagus sphincter.
This determination is carried out on male, conscious,
fasting beagles which, before the start of the test, have
each been provided with an esophagus fistula and a duodenal
cannula. The pressure of the lower esophagus sphincter is
measured by means of a perfused catheter system which has a
lateral opening and which is connected to a pressure
transducer and a recorder. The catheter is passed through
the esophagus fistula into the stomach and then slowly
withdrawn manually (= pull-through manometry). A peak is
recorded when the catheter part with the lateral opening
passes through the high-pressure zone of the lower esophagus
sphincter. The pressure in mm Hg is determined from this
peak.
In this way, initially the basal pressure of the
esophagus sphincter is determined as control value.
Subsequently, the test substance is administered orally and,
after 15 min., the pressure at the lower esophagus sphincter
is measured at 2-minute intervals for a period of 60 min.
The increase in the pressure after administration of test
substance compared with the previously determined basal
pressure is calculated.
In this test, the basal tone of the esophagus sphincter
was increased by 143% by a dose of 2.15 mole/kg of the
substance of Example 1. This effect persisted throughout
the entire 60 min. duration of the test.
Because of their effects in the gastrointestinal tract,
the compounds of Formula I are suitable in gastroenterology
-11-
CA 02260315 1999-01-25
as medicaments for larger mammals, especially humans, for
the prophylaxis and treatment of motility disturbances in
the gastrointestinal tract.
The doses to be used may differ between individuals and
will naturally vary depending on the nature of the condition
to be treated and the form of administration. For example,
parenteral formulations will generally contain less active
substance than oral preparations. However, in general
medicament forms with an active substance content of 1 to
100 mg per single dose are suitable for administration to
larger mammals, especially humans.
As medicinal agents, the compounds of Formula I may be
contained with conventional pharmaceutical auxiliary
substances in pharmaceutical formulations such as, for
example, tablets, capsules, suppositories or solutions.
These pharmaceutical formulations may be prepared by known
methods using conventional solid vehicles such as, for
example, lactose, starch or talcum, or liquid diluents such
as, for example, water, fatty oils or liquid paraffins, and
using customary pharmaceutical auxiliary substances, for
example tablet disintegrants, solubilizing agents or
preservatives.
The following examples are intended to illustrate the
invention in greater detail without restricting its scope in
any way.
Example 1: (2R,3S,4S,5R,6R,10R,11R]-3-[(2,6-dideoxy-3-C-
methyl-3-O-methyl-a-L-ribohexopyranosyl)-oxy]-5-[(3,4,6-
trideoxy-3-(N-methyl-N-isopropylamino)-(3-D-xylo-
hexopyranosyl)-oxy]-2,4,6,8,10-pentamethyl-ll-acetyl-12,13-
dioxabicyclo[8.2.1]-tridec-8-en-1-one (compound of Formula
I, Rl = methyl, R2 = hydrogen)
A) 100 g [2R(2'R,3'R),3S,4S,5R,6R,10R,11R]-11-(2',3'-
dihydroxypent-2'-yl)-3-[(2,6-dideoxy-3-C-methyl-3-O-
methyl-a-L-ribohexopyranosyl)-oxy]-5-[(3,4,6-trideoxy-
-12-
CA 02260315 1999-01-25
3-(N-methyl-N-isopropylamino)-(3-D-xylohexopyranosyl)-
oxy]-2,4,6,8,10-pentamethyl-12,13-dioxabicyclo[8.2.1]-
tridec-8-en-l-one (= compound of Formula II, R' =
methyl, R2 = hydrogen) was dissolved in 2500 ml toluene
under a nitrogen atmosphere. 100.0 g lead tetraacetate
was added to this receiving mixture, and the resulting
suspension was stirred for 5 hours at room temperature.
Then the reaction mixture was washed successively with
saturated sodium hydrogen carbonate solution and then
with water until the washing water reacted neutrally.
The organic phase was dried over sodium sulfate and
evaporated at reduced pressure. Chromatography of the
remaining residue on silica gel (mobile solvent: methyl
tert. butyl ether = MTBE) yielded 81.9 g of the title
compound as a white powder, melting point = 198 -
200 C, optical rotation [a]D =-24.6 (c = 1.0 in
CH2C1z ) .
B) 1.1 g of the compound obtained above was dissolved in
1 ml acetonitrile. 0.17 g of malonic acid was added to
this receiving mixture, and the mixture was heated to
60 - 70 C. Once the solid constituents had dissolved,
10 ml MTBE was added and the mixture was heated to
boiling for 5 minutes with reflux cooling. Then
another 10 ml MTBE was added, and the mixture was
cooled to room temperature with stirring. The
resulting crystals were filtered out from the solution,
washed twice with 10 ml MTBE each time, and were dried
at 60 C in a vacuum. 1.2 g of the monomalonate of the
title compound were obtained, melting range: 115.6 -
174.6 C (indefinite).
Example 2: (2R,3S,4S,5R,6R,10R,11R)-3-[(2,6-dideoxy-3-C-
methyl-3-O-methyl-a-L-ribohexopyranosyl)-oxy]-5-[(3,4,6-
trideoxy-2-O-acetyl-3-(N-methyl-N-isopropylamino)-(3-D-xylo-
hexopyranosyl)-oxy]-2,4,6,8,10-pentamethyl-11-acetyl-12,13-
-13-
CA 02260315 1999-01-25
dioxabicyclo[8.2.1]-tridec-8-en-1-one (compound of Formula
I, R' = methyl, R2 = acetyl)
A) 210.0 g of the starting compound of Example 1
(= compound of Formula II, R1 = methyl, R 2 = hydrogen)
was dissolved in 2.4 liters acetone under a nitrogen
atmosphere, and 85.8 g potassium carbonate was added
thereto. 63.4 g of acetic anhydride was added to this
receiving mixture, and the resulting suspension was
stirred for 20 hours at room temperature. Then the
reaction mixture was poured onto a mixture of 2,400 g
ice and 1,000 ml water and was stirred for 30 minutes.
The aqueous phase was extracted three times with ethyl
acetate, the organic phases were combined, and the
excess solvent was evaporated in a vacuum.
Recrystallisation of the resulting crude product from
n- p e n t a n e y i e 1 d e d 2 0 0 g
[2R (2' R, 3' R) , 3S, 4S, 5R, 6R, 10R, 11R] -11- (2' , 3' -dihydroxy-
pent-2'-yl)-3-[(2,6-dideoxy-3-C-methyl-3-O-methyl-a-L-
ribohexopyranosyl)-oxy]-5-[(3,4,6-trideoxy-2-O-acetyl-
3-(N-methyl-N-isopropylamino)-R-D-xylohexopyranosyl)-
oxy]-2,4,6,8,10-pentamethyl-12,13-dioxabicyclo[8.2.1]-
tridec-8-en-l-one (= compound of Formula II, R1 =
methyl, R2 = acetyl), melting point = 128 - 130 C.
B) 10.1 g of the product obtained above were reacted with
9.1 g of lead tetraacetate in the manner described in
Example 1. 6.0 g of the title compound was obtained as
a white solid, melting point = 164 C, optical rotation
[a]D = -23.2 (c = 1.0 in CHzClZ)
Example I: Capsules containing (2R,3S,4S,5R,6R,lOR,11R)-3-
[(2,6-dideoxy-3-C-methyl-3-O-methyl-a-L-ribohexopyranosyl)-
oxy]-5-[(3,4,6-trideoxy-3-(N-methyl-N-isopropylamino)-(3-D-
xylohexopyranosyl)-oxy]-2,4,6,8,10-pentamethyl-ll-acetyl-
12,13-dioxabicyclo[8.2.1]-tridec-8-en-l-one:
-14-
CA 02260315 1999-01-25
Capsules containing the active substance were produced
using the following auxiliary substances and ingredients per
capsule:
(2R,3S,4S,5R,6R,10R,11R)-3-[(2,6-dideoxy-3-C-
methyl-3-O-methyl-a-L-ribohexopyranosyl)-oxy]-
5-[(3,4,6-trideoxy-3-(N-methyl-N-isopropylamino)-
(3-D-xylohexopyranosyl)-oxy]-2,4,6,8,10-pentamethyl-
11-acetyl-12,13-dioxabicyclo[8.2.1]-tridec-8-en-
1-one 20 mg
Corn starch 60 mg
Lactose 301 mg
Ethyl acetate (= EA) q.s.
The active substance, the corn starch and the lactose were
processed to form a homogenous, pasty mixture using ethyl
acetate. The paste was comminuted, and the resulting
granules were placed on a suitable metal sheet and dried at
45 C to remove the solvent. The dried granules were passed
through a comminuting machine and mixed with the following
additional auxiliary substances in a mixer:
Talc 5 mg
Magnesium stearate 5 mg
Corn starch 9 mg
and then poured into 400 mg-capacity capsules (= capsule
size 0).
The foregoing description and examples have been set
forth merely to illustrate the invention and are not
intended to be limiting. Since modifications of the
disclosed embodiments incorporating the spirit and substance
of the invention may occur to persons skilled in the art,
the invention should be construed broadly to include all
variations falling within the scope of the appended claims
and equivalents thereof.
-15-