Note: Descriptions are shown in the official language in which they were submitted.
CA 02261004 2005-06-O1
DOCKET NO. 1862PCT
- 1 -
BIOCOMPATIBLE, IMPLANTABLE
HEARING AID MICROACTUATOR
Technical Field
The present invention relates to the field of implantable
biocompatible transducers, particularly those useful for a
microactuator included in a fully implantable hearing aid system.
Background Art
A Patent Cooperation Treaty ("PCT") patent application
published 27 March 1997, International Publication no.
WO 97/11575, entitled "Implantable Hearing Aid" ("the 11575
Published PCT Patent Application") describes an implantable
hearing aid system which uses a tiny implantable microactuator.
A PCT patent application published 21 August 1997, International
Publication no. WO 97/30565 entitled "Improved Biocompatible
Transducers" ("the 30565 Published PCT Patent Application")
discloses improved implantable microactuators that are useful in
the fully implantable hearing aid system disclosed in the 11575
Published PCT Patent Application. The fully implantable hearing
aid system disclosed in the 11575 and 30565 Published PCT Patent
Applications can operate for a period of five years on a set of
batteries, and produce sound levels of 110 dB. The fully
implantable hearing aid system described in these Published PCT
Patent Applications is extremely compact, sturdy, rugged, and
provides significant progress towards addressing problems with
presently available hearing aids.
As described in the 11575 Published PCT Patent Application,
the microactuator is preferably implanted into a fenestration
that pierces the promontory of the cochlea, and uses
stress-biased lead lanthanum zirconia titana.te ("PLZT") transduc-
er material. Stress-biased PLZT materials exhibit very high
deflections and generate very high forces in comparison with
other existing piezoelectric materials and/or structures. Such
materials provide in a monolithic structure both a layer of
conventional PLZT and a compositionally reduced layer from which
the PLZT oxide has been converted to an electrically conductive
cermet material. During operation of the transducer, the PLZT
CA 02261004 2005-06-O1
- 2 -
layer expands and contracts laterally upon application of an
alternating current ("AC") voltage across the disk. Expansion and
contraction of the PLZT layer flexes the disk back-and-forth due to
differential expansion between the PLZT layer and the unexpanding
cermet layer. However, the cermet layer in that transducer material
includes a metallic form of lead ("Pb) as one of its constituent
elements.
Microactuators disclosed in the 11575 Published PCT Patent
Application include a membrane diaphragm that provides good biological
isolation for the transducer. Moreover, use of the membrane diaphragm
fully preserves, or may, through the use of hydraulic amplification,
actually enhance transducer performance by magnifying the transducer's
deflection or displacement. Although the transducers disclosed in the
11575 Published PCT Patent Application usually attach the cermet layer
to the membrane diaphragm and fully enclose that layer, a possibility
still exists that lead may leach from the transducer.
Disclosure of Invention
It is a feature of one embodiment of th.e present invention to
provide a fully biocompatible transducer.
Another feature of the present invention is to provide a fully
biocompatible transducer that, in preferred embodiments, is suitable
for use in a microactuator included in an implantable hearing aid
system.
Another feature of the present invention is to provide a
transducer for a microactuator that, in preferred embodiments, is
fabricated from only biocompatible materials.
Yet another feature of preferred embodiments, of the present
invention is to provide a transducer for a microactuator that is non
toxic.
sriefly, the present invention is a biocompatible, implantable
microactuator adapted for inclusion in a fully implantable hearing aid
system. The microactuator includes a hollow body having an open first
end and, in a preferred embodiment, open first and second faces that
are separated from the first end. The microactuator includes a first
flexible diaphragm that seals the body's first end, and that is
adapted for deflecting outward from and inward toward the body. A
second flexible diaphragm seals across the body's first face, and a
CA 02261004 2005-06-O1
DOCKET N0. 1862PCT
- 3 -
third flexible diaphragm seals across the body's second face
thereby hermetically sealing the body. An incompressible liquid
fills the hermetically sealed body.
In a preferred embodiment first and second biocompatible,
unimorphs are mechanically coupled to and an integral part of
respectively the second and third flexible diaphragms. The first
and second unimorphs are adapted for receiving an electrical
driving signal upon the application of Wr11C~1 the first and second
unimorphs directly deflect respectively t:he second and third
flexible diaphragms. Deflections of the second and third
flexible diaphragms are coupled by the liquid within the body to
the first flexible diaphragm.
Both the first and second unimorph include a plate of
biocompatible piezoelectric material, preferably a lead zirconia
titanate ("PZT") or PLZT material, to which.is bonded a layer of
biocompatible metal, preferably titanium, nickel, platinum,
rhodium, palladium, gold, or a shape memory alloy such as nickel-
titanium Naval Ordinance Laboratory ("Nitinol"). The layer of
biocompatible metal may be processed to apply a stress-bias to
the plate of biocompatible piezoelectric material. The first and
second unimorphs also include a thin, biocompatible electrode
applied to each of the plates of biocompatible piezoelectric
material opposite the respective layers of biocompatible metal.
Application of the electrical driving signal across the layer of
biocompatible metal and the biocompatible electrode causes both
of the unimorphs to deflect. To effect a stress-bias of the
unimorphs, the unimorphs are annealed at a high temperature such
as 500 °C to relieve all uncontrolled stress, and, in the
instance of the shape memory alloy Nitinol, to establish a hard
austenitic phase of the material. Cooling the unimorphs from the
elevated temperature applies the requisite stress-bias to the
unimorph.
These and other features, objects and advantages will be
understood or apparent to those of ordinary skill in the art from
the following detailed description of the preferred embodiment
as illustrated in the various drawing figures.
CA 02261004 2005-06-O1
DOCKET NO. 1862PCT
- 4 -
Brief Description of Drawings
FIG. 1 is a cross-sectional view illustrating a
stress-biased PLZT transducer in accordance with the 11575
Published PCT Patent Application;
FIG. 2 is a cross-sectional view illustrating a stress-
biased unimorph transducer in accordance with the present
invention that has improved biocompatibility;
FIG. 3 is a phase diagram for a shape memory Nitinol alloy;
FIG. 4 is a cross-sectional view illustrating a stress
biased unimorph transducer in accordance with the present
invention that has improved biocompatibility;
FIG. 5 is a graph depicting deflection sensitivity of a
stress-biased unimorph transducer, such as those depicted in
FIGS. 2 and 4, for various thicknesses of a metal layer that is
bonded to a piezoelectric plate;
FIG. 6A is a partially sectioned elevational view of a
microactuator for a fully implantable hearing aid system; and
FIG. 6B is a cross-sectional elevational view of the
microactuator taken along the line 6B-6B in FIG. 6A.
Best Mode for Carrying Out the Invention
FIG. 1 is a cross-sectional elevational view depicting a
transducer 22 as disclosed in the 11575 Published PCT Patent
Application. The 11575 Published PCT Patent Application
discloses that the transducer 22 is preferably fabricated from
a thin circular disk of stress-biased PLZT material. This
material may be one of the materials manufactured by Aura
Ceramics and sold under the "Rainbow" product designation. These
PLZT unimorphs provide a monolithic structure one side of which
is a layer 22a of conventional PLZT material. The other side of
the PLZT unimorph is a compositionally reduced layer formed by
chemically reducing the oxides in the native PLZT material to
produce a conductive cermet layer 22b. The conductive cermet
layer 22b typically comprises about 30 ~ of the total disk
thickness, and typically may have a composition that contains up
to 60 % lead. Removing of the oxide from one side of the
unimorph shrinks the conductive cermet layer 22b which bends the
whole disk and puts the PLZT layer 22a under compression. The
CA 02261004 2005-06-O1
DOCKET NO. 1862PCT
- 5 -
PLZT layer 22a is therefore convex while the conductive cermet
layer 22b is concave.
For use in the fully implantable hearing aid system, as
depicted in FIG. 1 the PLZT layer 22a and the conductive cermet
layer 22b are respectively overcoated with thin metal layers to
provide a PLZT electrode 24 and a cermet electrode 26. The
electrodes 24 and 26 may be applied to t:he transducer 22 in
various different ways such as plating, evaporation, metal
spraying etc. Application of a voltage across the electrodes 24
and 26 causes the disk to become either more or less bowed,
depending upon the polarity of the applied potential.
Although in the fully implantable hearing aid system's
microactuator disclosed in the 11575 Published PCT Patent
Applications the transducer 22 does not contact a subject's body,
and may be hermetically sealed within the microactuator, some of
the properties of the metallic form of lead in the conductive
cermet layer 22b may be undesirable.
FIG. 2 illustrates an alternative structure for the
transducer first proposed in the 11575 1?ublished PCT Patent
Application that eliminates the metallic form of lead containing
cermet conductive cermet layer 22b. In FIG. 2, a metal laminated
unimorph 32 consists of a plate 34 of biocompatible piezoelectric
material, such as a biocompatible PLZT or biocompatible PZT, onto
which is deposited a conductive metallic layer 36. For
biocompatible PLZT and PZT materials, lead occurs in an oxide
form which appears to be non-toxic.
The 11575 Published PCT Patent Application discloses that
in fabricating the laminated unimorph 32 for the fully
implantable hearing aid system the piezoelectric plate 34 is
lapped down to a thickness from 1.0 to 6.0 mils, and then coated
with a thin chromium layer 38 onto which is plated a thin nickel
layer 36. The thin nickel layer 36 stres.~es the piezoelectric
plate 34 thereby mimicking the stress-bias of the conductive
cermet layer 22b in the PLZT unimorph transducer 22. A thin,
biocompatible metal electrode 44 is applied to the plate 34
opposite the layer 36. In practice it has been found that the
stress applied with a plated nickel layer 36 is usually low, and
CA 02261004 2005-06-O1
DOCKET N0. 1862PCT
- 6 -
that it is difficult to control the stress-bias because of the
changing parameters in the plating bath.
The 11575 Published PCT Patent Application also proposes
that a metal laminated unimorph 32 may be fabricated by applying
a thin layer 36 of a shape memory alloy, such as 5 to 20 microns
of Nitinol, nickel-titanium-copper, or copper-zinc-aluminum, to
the piezoelectric plate 34. After a layer 36 of such material
has been applied to the piezoelectric plate 34, heating or
cooling the shape memory alloy establishes a phase in which the
alloy layer 36 applies compressive or tensi7_e stress to the plate
34. As is apparent to those skilled in the art of shape memory
alloys, hysteresis in a phase transition of the alloy maintains
that stress upon removal of the heating or cooling. However, it
has been determined experimentally that applying a shape memory
alloy layer 36 to the plate 34 as described without a careful
thermal procedure does not necessarily yield a satisfactory
structure.
The stress-bias required for the fully implantable hearing
aid system's transducer can be simply obtained through differen
tial thermal expansion of materials deliberately created during
the fabrication of the unimorph. Since in general for the same
temperature change metals expand more than ceramic dielectric
materials, a ceramic layer may be easily subj ected to compressive
stresses similar to those in Aura Ceramic's' Rainbow products.
However, the bond between the metal and the ceramic dielectric
should be as thin and strong as possible.
The transducers required for the fully implantable hearing
aid system are fairly thin. The desired overall transducer
thickness ranges from 1.0 to 6.0 mils, providing a compromise
between good deflection (for thin transducers) and ease of
fabrication and ruggedness with thick transducers. Consequently,
it is possible to produce suitable stress-biased structures by
vacuum deposition such as sputtering, evaporating or spraying
metal onto a hot piezoelectric substrate, thereby producing a
suitable metal phase. Upon cooling, greater contraction of the
metal layer in comparison with the piezoelectric layer creates
the required stress-bias. In principal, such a process yields
CA 02261004 2005-06-O1
DOCKET N0. 1862PCT
a type of stress-bias similar to that produced by reduction and
fabrication of Aura Ceramics' Rainbow products.
It is believed that metals with physical properties similar
to those of the Rainbow material's conductive cermet layer 22b
will yield near optimum performance for t:he fully implantable
hearing aid system's transducer. The thermal expansion of most
piezoelectric materials is about 2-4 ppm/°C, while the cermet
expansion coefficient is about 10 ppm/°C. fence it is desirable
to find biocompatible metals which have an expansion coefficient
similar to that of the conductive cermet layer 22b, and that have
suitable mechanical strength. Materials having such characteris-
tics include Nitinol, titanium, platinum, rhodium, palladium,
gold, and nickel, if suitably prepared. The first two materials,
i.e. Nitinol and titanium, have excellent biocompatible proper-
ties. Both the thermal expansion coefficient (11 ppm/°C) and the
elastic modulus (8 x 1011 Pa) of the austenitic phase of Nitinol
match closely the corresponding properties of the conductive
cermet layer 22b of Aura Ceramics' Rainbow products (respectively
10 ppm/°C and 7 x 1011 Pa) . Therefore, for i~he same thickness of
the conductive layers 22b and 36, and for t;he dielectric layers
22a and 34, it is reasonable to expect that the same stress-bias
will develop upon cooling since the difference between the
transducers' processing temperature and operating temperature are
approximately the same.
FIG. 3 depicts a thermal expansion diagram for Nitinol.
Upon cooling from high temperature along a line 52 in FIG. 3 to
a temperature T1, Nitinol exhibits a contraction coefficient of
11 ppm/°C. Along the line 52 of the phase diagram, Nitinol
exists as a harder austenitic phase. Upon reaching the tempera-
ture T1 (around 80 °C), a phase change commences that converts
Nitinol's hard austenitic phase material to a softer martensitic
phase. Conversion to the martensitic phase during cooling of
Nitinol from the temperature T1 to a temperature T2 along a line
54 causes Nitinol to expand significantly, i.e. up to 0.5 0 of
its length. Linear expansion during Nitinol's phase transforma-
tion from the austenitic phase to the martensitic form may be as
much as 2000 ppm, equivalent to expansion of the material over
almost 200 °C if the phase change did not occur. At T2 (around
CA 02261004 2005-06-O1
DOCKET N0. 1862PCT
_ g _
75 °C) , Nitinol completes its conversion to the martensite phase.
Further cooling of Nitinol below T2 along a line 56 in FIG. 3,
Nitinol exhibits the martensitic contraction (expansion)
coefficient of 6 ppm/°C. Nitinol's martensitic phase has an
elastic modulus which is 2 to 3 times lower than the austenitic
elastic modulus, and the martensitic phase's yield strength is
from 3 to 5 times less than the austenitic phase.
Heating Nitinol along the line 56 from below the martensitic
transition temperature T2 produces an expansion of the material
up to a temperature T3 (about 90 °C) . Further heating of the
material above the temperature T3 along a line 58 commences
conversion of Nitinol's softer martensitic phase material to the
harder austenitic phase. Conversion to the austenitic phase
during heating of Nitinol from the temperature T3 to a tempera-
ture T4 along a line 58 causes, Nitinol to contract signifi-
cantly, i.e. up to 0.5 % of its length. Ate T4 (around 95 °C),
Nitinol completes its conversion to the austenitic phase. The
temperature hysteresis in Nitinol's phase change cycle is
approximately of 10-20 °C, but can be larger.
Because Nitinol's martensitic phase is much softer than the
material's austenitic phase, use of the material in the
martensitic phase to apply a pre-established stress-bias to the
plate 34 depicted in FIG. 2 requires a substantially thicker
layer 36 of Nitinol than if the material i.s in the austenitic
phase. Use of a thick Nitinol layer 36 is more expensive, and
is undesirable for various other reasons. ,Also, if stress-bias
is applied to the plate 34 by thermal expansion, a significant
portion of the desired stresses will be released if Nitinol
undergoes the phase transition to the martensitic form.
Consequently, using a shape memory allow which has a phase
change temperature well below the operating temperature of the
transducer, i.e. 36 °C, or, in general, room temperature, is
preferable for a transducer to be included in the fully
implantable hearing aid system. In the bulk material, lowering
the martensitic phase transition temperature T1 of Nitinol may
be accomplished by adding small percentages of copper, iron,
cobalt or chromium to the nickel-titanium alloy. However, for
the fully implant able hearing aid system' s transducer, the layer
CA 02261004 2005-06-O1
DOCKET N0. 1862PCT
- 9 -
36 is often applied from the vapor phase, as it is difficult to
find nickel-titanium alloy foils having the required thickness.
(However, it appears that foils having the requisite thickness
may be obtained on special order from Shape Memory Applications,
Inc. of Santa Clara, California.)
The martensitic phase transition temperature T1 of sputtered
nickel-titanium films is typically approximately 60-80 °C, which
is too high for the fully implantable hearing aid system's
transducer. It has been observed that adding impurities inadver-
tently (as for example contaminants present on the surface of a
poorly cleaned sample) may lower the martensitic phase transition
temperature Tl significantly, without greatly affecting Nitinol's
mechanical properties. Alternatively, it has been observed that
co-sputtering nickel-titanium together with metallic impurities,
such as copper, iron, cobalt or chromium, typically with all
constituent elements mixed in the sputtering target, may produce
a martensitic phase transition temperature T1 well below room
temperature. Furthermore, in many sputtering systems it has also
been observed that depositing the nickel-titanium layer 36 with
a large anode-cathode separation produce a lower martensitic
phase transition temperature Tl.
In practice then, Nitinol material suitable for the
transducer used in the fully implantable hearing aid system may
be prepared as follows. First, the nickel-titanium alloy
including the copper, iron, cobalt or chromium impurity is
sputtered from the target unto the plate :34, which may be at
moderate temperature (e. g. 100-200 °C). Typically the sputtered
Nitinol material will be amorphous. The laminated unimorph 32
is then heated in an inert atmosphere or vacuum to 500 °C for
approximately one-half (0.5) hour to form the desired high
strength austenitic phase. Heating the laminated unimorph 32 to
500 °C also removes all stresses, and sets the temperature range
over which the material will be stressed by differential thermal
contraction. Alternatively, the nickel-titanium alloy including
the copper, iron, cobalt or chromium impurity may be deposited
directly on the plate 34 at high temperature. A typical
sputtering rate may be 6 minutes per micron of the Nitinol layer
36, with 2 kW of power, at a pressure of 2 x 10-3 micron of
CA 02261004 2005-06-O1
' DOCKET NO. 1862PCT
- 10 -
argon. The austenitic phase is preserved during cooling to room
temperature. For Nitinol in the austenitic: phase, the optimum
thickness for the layer 36 is about 35 microns for a 75 microns
thick plate 34 , i . a . about 0 . 5 . For a 50 micron thick PLZT plate
34, the optimum thickness for the Nitinol layer 36 is about 23
microns.
As an alternative to Nitinol, sputtered films of either pure
nickel or pure titanium that are annealed at 500 °C and cooled
can also be used very advantageously for the fully implantable
hearing aid system's transducer, although nickel is not quite as
biologically inert as titanium. Nickel's 7_arge coefficient of
expansion (13 ppm/°C) permits a thinner layer 36 to produce the
desired compression of the ceramic laminated unimorph 32.
Titanium's coefficient of expansion (9 ppmf°C) closely matches
that of the conductive cermet layer 22b. The coefficients of
expansion of other metals such as platinum, rhodium, palladium,
or gold are also similar to that of the conductive cermet layer
22b.
For nickel, the optimum thickness of the layer 36 is
approximately 0.26 of the thickness of the plate 34, and the
bowing of the laminated unimorph 32 is about 20 % larger at the
same voltage than with Nitinol. For titanium, the optimum
thickness of the layer 36 is about 0.40 of the thickness of the
plate 34, and the bowing of the laminated unimorph 32 is about
10 % greater at the same voltage than for N:itinol. As compared
to the PLZT transducer 22, the optimum nickel structure provides
up to a 30 % improvement for the same thickness of the ceramic
plate 34 and the same applied voltage.
The temperature difference, from the annealing temperature
to room temperature, over which the differential contraction
occurs together with the difference in t:he coefficients of
thermal expansion between the plate 34 and the layer 36 applies
a stress-bias to the laminated unimorph 32. Moreover, because
the layer 36 may be applied incrementally, it is therefore
possible to establish a particular stress-bias for the laminated
unimorph 32 using the method described above, and to then
subsequently increase the thickness of the layer 36 at a lower
temperature, without significantly changing the existing stress-
CA 02261004 2005-06-O1
DOCKET NO. 1862PCT
- 11 -
bias. The ability to establish a particular stress-bias in the
piezoelectric plate 34 independently of the total thickness of
the layer 36 permits establishing a thickness for the layer 36
which produces optimum deflection of the laminated unimorph 32
in response to a voltage applied across the layer 36 and the
electrode 44. Such tailoring of the conductive cermet layer 22b
is impossible for the Aura Ceramics' Rainbow products.
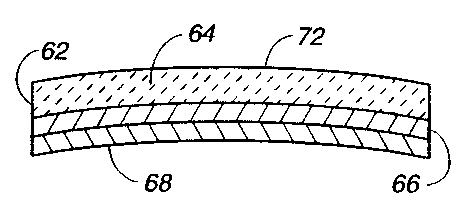
FIG. 4 depicts a stress-biased laminated unimorph 62 in
accordance with the present invention. The laminated unimorph
62 typically includes a 75 micron (3 mils) thick piezoelectric
plate 64 preferably of a biocompatible. ceramic PZT or PLZT
material. Using titanium for the stress-biasing material, the
optimum thickness for the metal layer 66-68 is 30 microns,
although a 20 micron thickness produces 95 o the deflection of
1S a 30 micron thick layer 66-68. Thus, for example, a 10 micron
thick metallic layer 66 of titanium (or any suitable thickness
to provide the desired stress-bias) is first sputtered onto the
laminated unimorph 62, annealed at 500 °C as described above, and
then cooled. Subsequently, another metallic layer 68, e.g. 10
microns or any other appropriate thickness of titanium or any
other suitable metal, can then be sputtered. or bonded onto the
layer 66 without annealing to increase the total thickness of the
metal layers 66 and 68 to that which provides optimum deformation
characteristics for the laminated unimorph 62. The deposition
or bonding of the layer 68 does not significantly change the
stress-bias applied by the layer 66, although insignificant
stresses may sometimes result from the deposition of the layer
68. A thin, biocompatible metal electrode '72 is applied to the
plate 64 opposite the layers 66 and 68.
The layers 66 and 68 of FIG. 4 may be of different materi-
als, if so desired. For example the first layer 66 may be
titanium, providing excellent adhesion to the plate 64, while the
second layer 68 may be nickel which sticks well to titanium.
Vice versa, the first layer 66 may be nickel to create a
particular stress-bias with a relatively thin layer of metal.
The nickel layer 66 may then be subsequently coated with titanium
to improve the biocompatibility of the laminated unimorph 62.
Typically, the laminated unimorph 62 has a i~otal thickness~from
CA 02261004 2005-06-O1
DOCKET NO. 1862PCT
- 12 -
1.0 to 6.0 mils, with the combined thickness of the layers 66 and
68 equaling from 0.5 to 0.15 the thickness of the plate 64.
Since it is difficult to cut a bowed material, often it is
desirable to shape the plate 64 to its desired size as a flat
sheet, and then deposit the metal layers 66 and 68. Note that
nickel can be readily plated in various forms (e. g. electrolytic
or electroless), to the required thickness. Very thin layers of
chromium (a few hundred Angstroms) can be applied to the plate
64 if necessary to improve adhesion before depositing the layer
66.
Other materials which may be used for the transducer of the
fully implant able hearing aid consist of a PLZT or PZT plate that
is physically bonded to a metal sheet at high temperature. Here
both a ceramic plate and a metal sheet are heated to the same
high temperature, with a solder or other suitable bonding
material disposed between the ceramic plate and the metal sheet.
Upon cooling, the unimorph thus created exhibits the desired
stress-bias. The biocompatible material used for the metal sheet
include titanium, nickel, platinum, rhodium, palladium, gold, or
Nitinol foils, about 0.5 to 3.5 mils thicl~:, in the same met-
al/ceramic thickness ratio as described above. To avoid possible
leaching of the metallic form of lead, suitable bonding materials
include various types of lead free solder, for example indium
alloys particularly in paste form. Such solder pastes may be
screen printed unto the metal sheet or the piezoelectric ceramic
plate in very thin layers e.g. 5 microns thick. The metal sheet
thickness is about 0.5 to 0.15 of the thickness of the piezoelec-
tric ceramic plate. Suitable bonding temperatures are 150-400
°C. Typically a weight is put on the stacked ceramic plate and
metal sheet to press them together during bonding. The weight
may be removed during cooling. Usually the piezoelectric ceramic
plate will first be coated with a very thin layer of silver paste
that is then fired onto the piezoelectric ceramic at high
temperature. An electrically conductive metal electrode on the
ceramic side, opposite the bonded metal sheet, should be as thin
as possible, to avoid creating stresses which oppose the stresses
generated by the bonded metal sheet. These stress-biased struc-
tures, 1.25 inch in diameter, 15 mils thick produce axial
CA 02261004 2005-06-O1
DOCKET NO. 1862PCT
- 13 -
displacements of 100 micron in response to a.n applied signal of
150 volts. Hence for a 4 mil thick structure, 2.5 mm in
diameter, with 10 V drive, the expected axial transducer
displacement is about .6 micron, more than adequate for the
transducer included in the microactuator of the fully implant able
hearing aid system.
FIG. 5 depicts generally deflection sensitivity of a
laminated unimorph, such as the unimorph:~ 32 and 62, as a
function of the thickness of the metal layer 36 or 66-68. A
graph 76, indicated by "+" symbols, in FIG. 5 depicts deflection
sensitivity versus thickness of the metal layer 36 or 66-68 for
a 2.0 mm square or disk-shaped plate 34 or 64 of 3203 PZT ceramic
piezoelectric material that is 75.0 microns thick computed using
a formula by Timoshenko for bimetallic springs, Journal Optical
Society of America, vol. 11, no. 233, 1925. A line graph 78 in
FIG. 5 depicts deflection sensitivity versus thickness of the
metal layer 36 or 66-68 for the same piezoelectric material
computed using a formula by Chu et al., J. Micromech. Microeng.
3 ( 1993 ) , also for bimetallic springs . As depicted by the graphs
76 and 78, increasing the thickness of the metal layer 36 or
66-68 initially increases the deflection sensitivity of the
unimorph 32 or 62. Moreover, the deflection sensitivity of the
unimorph 32 or 62 continues to increase with increasing thickness
of the metal layer 36 or 66-68 until reaching a maximum value
that extends across a broad range of thicknesses.
Heating the laminated unimorph 32 or E2 to 500 °C exceeds
the Curie point of the piezoelectric ceramic material. Therefore
the piezoelectric ceramic material needs to be repoled preferably
as the laminated unimorph 32 or 62 cools. Alternatively the
laminated unimorph 32 or 62 can be repoled after cooling. PZT
materials suitable for the laminated unimorph 32 or 62 are
identified by various commercial names such as PZT-4, PZT-5A,
PZT-5H, PZT-8, C3100, and C3200, and in particular 3203, 3199 or
3211, the latter materials being manufactured by Motorola, Inc.
Desirable properties for the laminated unimorph 32 or 62 include
a very high value for the piezoelectric constant d31, which
determines transverse contraction, and for good mechanical
DOCKET NO. 1862PCT
CA 02261004 2005-06-O1
- 14 -
machinability. The 3203 material appears best in these respects,
and is the presently preferred material.
FIGS. 6A and 6B depict a microactuator 82 adapted for
inclusion in a fully implantable hearing aid system. The
microactuator 82 includes a hollow body 84 , depicted only in FIG .
6B, from one end of which projects an L-shaped, flanged nozzle
86. The flanged nozzle 86 has-an open first end 88 that is
sealed by a flexible diaphragm 92 that may be deflected outward
from and inward toward the flanged nozzle 86. As described in
l0 greater detail in the 30565 Published PCT Patent Application, the
first end 88 and the diaphragm 92 are adapted for implantation
into a fenestration formed through a promontory that is located
between a subject's middle and inner ear. The body 84 has two
open faces 94a and 94b that axe separated from the first end 88.
Each of 'the faces 94a and 94b are respectively sealed by flexible
diaphragms 96a and 96b which, in combination with the diaphragm
92, hermetically seal the body 84. As depicted in FIG. 6B, the
diaphragms 96a and 96b respectively have cross-sectional areas
that are larger than a cross-sectional area of the diaphragm 92.
While the preceding description of the body 84 identifies various
individual parts thereof, the body 84 may, in fact, be provided
by a one-piece can formed from a material suitable for the
diaphragms 96a and 96b.
The hermetically sealed hollow body 84 is filled with the
incompressible liquid 98. Respectively secured to each of the
diaphragms 96a and 96b are transducers 102 which face each other.
In accordance with the present invention, the transducers 102 of
the microactuator 82 are provided by the laminated unimorphs 32
or 62. Each of the transducers 102 are electrically connected
to a miniature cable 104 to expand or contract in opposite
direction toward or away from each other in response to the same
voltage applied across each of the transducers 102. This driving
motion of the transducers 102 applied to the diaphragms 96a and
96b forces the liquid 98 toward or away from the diaphragm 92
that is located in a subject's inner ear thereby deflecting the
diaphragm 92. While the microactuator 82 :preferably employs a
pair of transducers 102, a microactuator 82 in accordance with
the present invention may have only a single transducer 102, or
each transducer 102 of the pair may have a different shape and/or
SlZe:
CA 02261004 1999-O1-18
WO 98/03134 PCT/US97/12678
- 15 -
As described in greater detail above, the transducer 102
exhibits maximum deflection sensitivity when the combined thick-
nesses of the metal layer 36 or 66-68 together with that of the
diaphragm 96a or 96b is within the broad range of optimum
thickness computed using the theories of Timoshenko and/or Chu.
Thus, the laminated unimorph 62 may be built by first depositing
the layer 66, and by then bonding the layer 66 to the diaphragm
96a or 96b which therefore provides the second layer 68. One
method for assembling the laminated unimorph 62 is by first
bonding the layer 66 to the diaphragm 96a or 96b, which is then
secured to the remainder of the body 84 by. electron-beam or laser
welding. Alternatively, the diaphragm 96a or 96b may first be
welded onto the remainder of the body 84, after which the layer
66 is bonded to the diaphragm 96a or 96b.
An alternative stress-biased unimorph which may be used for
the transducer 102 is a NASA Langley Research Center's THin-layer
composite UNimorph ferroelectric DrivER and sensor ("THUNDER")
high-displacement actuator. As described in NASA publications,
the THUNDER transducer consists of a piezoelectric plate
intimately attached to a pre-stressing layer with LARC-SI, a NASA
Langley Research Center developed polyimide adhesive. Reported-
ly, the pre-stressing layer, provided by adhesive backed foils,
is thermally bonded together and to the piezoelectric plate in
vacuum. This initial vacuum and thermal processing yields a
partially pre-stressed THUNDER unimorph. Fabrication of the
THUNDER unimorph is then completed by pressing the partially pre-
stressed unimorph onto a curved fixture while heating it to
increase the unimorph's pre-stress.
Industrial Applicability
To isolate the transducers 102 from a subject's body, the
body 84 and the transducers 102 of the microactuator 82 are
preferably enclosed within a hermetically sealed titanium housing
112. As illustrated in FIG. 6B the housing 112 is joined to the
flanged nozzle 86 around a perimeter of a flange 114 such as by
electron-beam or laser welding. Alternatively, the body 84 and
the transducers 102 may be enclosed with a with parylene coating
thereby isolating them from a subject's body.
CA 02261004 1999-O1-18
WO 98/03134 PCT/US97/12678
- 16 -
Anatomical considerations permit the transducers 102 to
extend a considerable distance into a subject's middle ear
cavity, and also permit shapes for the body 84 and the transduc-
ers 102 that differ from those depicted in FIGs. 6A and 6B. The
base of the body 84 adjacent to the flanged nozzle 86 can be very
narrow and the length of the body 84 and transducers 102
extending outward from the flanged nozzle 86 enlarged so that the
volume of the liquid 98 displaced by the transducers 102 becomes
quite large. In this way, the faces 94a and 94b and the
transducers 102 can be shaped, twisted and tilted to fit a
subject's middle ear cavity, and are not restricted to the space
available locally at the implantation site.
While the illustrations of FIGS. 6A and 6B depict the
diaphragms 96a and 96b as being oriented parallel to the
diaphragm 92 with the diaphragms 96a and 96b parallel to each
other, other orientations of the diaphragms 96a and 96b with the
respect to the diaphragm 92 are within the scope of the present
invention. Accordingly, the diaphragms 96a and 96b can be
oriented at a skewed angle with respect to the flanged nozzle 86
and diaphragm 92 to prevent the transducers 102 from interfering
with an ossicular chain 21 or other structures located within a
subject's middle ear. The flanged nozzle 86 provides good
anchoring to the promontory without requiring extra room which
would otherwise reduce space available for the transducers 102.
Although the present invention has been described in terms
of the presently preferred embodiment, it is to be understood
that such disclosure is purely illustrative and is not to be
interpreted as limiting. While it appears that stress-biased
transducers 102 offer superior performance for the microactuator
82, it also appears that transducers 102 that are not stress-
biased offer performance adequate for the fully implantable
hearing aid system. Consequently, without departing from the
spirit and scope of the invention, various alterations, modifica-
tions, and/or alternative applications of the invention will, no
doubt, be suggested to those skilled in the art after having read
the preceding disclosure. Accordingly, it is intended that the
following claims be interpreted as encompassing all alterations,
i
CA 02261004 1999-O1-18
WO 98103134 PCT/US97/12678
- 17 -
modifications, or alternative applications as fall within the
true spirit and scope of the invention.