Note: Descriptions are shown in the official language in which they were submitted.
CA 0226112~ 1999-01-1~
~ .
~ WO 98/03506 ; ~ Tli!S AM~l~7r3~ PCT/EP97/03570
Description ~ ~ ! ?' L !~.T i 0 - I
Substituted pyrazolylpyrazole derivatives, process for their preparation and
their use as herbicides
The invention relates to substihlted pyrazolylpyrazole derivatives, to their
preparation and to intermediates for their preparation, and to their use as
herbicides.
US 5,405,829 discloses pyrazolylpyrazoles having an unsubstituted aminogroup as herbicidally active compounds.
WO 94/08999 describes herbicidally active pyrazolylpyrazoles, inter alia
15 having a substituted amino group.
WO 96/09303 likewise discloses substituted pyrazolylpyrazoles having
herbicidal properties.
20 However, the herbicidal activity of the known compounds is frequently
insu~ricia"t, or else there are problems with the selectivity in major
agricultural crops.
It is an object of the present invention to provide novel substituted
25 pyrazolylpyrazoles which do not have these disadvantages and which are
superior to the prior art compounds in terms of biological properties.
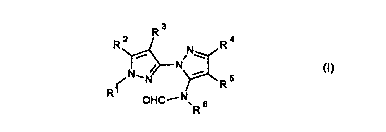
It has now been found that substituted pyrazolylpyrazoles of the formula I
CA 0226112~ 1999-01-1
R j~ ,N~ R 4
--N ~R 5 (1)~
OHC--N~ 6
in which
Rl is C,-C4-alkyl,
R2 is C1-C4-alkyl, C,-C4-alkylthio, C,-C4-alkylsulfinyl, C,-C4-alkylsulfonyl,
C,-C4-alkoxy, or is a C,-C4-alkyl, C,-C4-alkylthio, C,-C4-alkylsulfinyl,
C,-C4-alkylsulfonyl or C,-C4-alkoxy, which is mono- or
polysubstituted by halogen,
R' and R2 together form the group -(CH2)n-,
R3 is hydrogen or halogen,
10 R4 is hydrogen orC,-C4-alkyl,
R5 is hydrogen, nitro, cyano, -COOR7, the group
-C-NR8R9 -C-R1 0
Il 11
x or X
R6 is hydrogen, C,-C6-alkyl, C3-C7-cycloalkyl, C3-C7-cycloalkyl-C1-C4-
alkyl, C2-C6-alkenyl, C3-C6-alkynyl, or is a C,-C6-alkyl, C2-C6-alkenyl
or C3-C6-alkynyl which is mono- or polysubstituted by halogen,
hydroxyl, cyano, C,-C4-alkoxy, C,-C4-alkylthio, C,-C4-alkoxycarbonyl,
hydroxycarbonyl, C,-C4-alkylcarbonyl, or is a C1-CB-alkyl, C3-C7-
cycloalkyl or C3-C7-cycloalkyl-C1-C4-alkyl which is interrupted once or
more than once by oxygen or sulfur, the cycloalkyl optionally being
mono- or polysubstituted by methyl,
or is phenyl or benzyl, or is a phenyl or benzyl which is mono- or
polysubstituted by halogen, nitro, cyano, C1-C4-alkoxy or halo-C1-C4-
alkyl,
R7, R8 and R9 independently of one another are each hydrogen or C1-C4-
25 alkyl,
CA 0226112~ 1999-01-1~
R3 and R9 together with the adjacent nitrogen atom form a morpholino
group, a piperidino group or a pyrrolidino group,
R'~ is hydrogen, C,-C4-alkyl, or is a C,-C4-alkyl which is mono- or
polysubstituted by halogen,
5 X is oxygen or sulfur,
m is3,40r5,
have superior herbicidal activity to the prior art compounds.
Preference is given to those compounds of the formula I in which
10 R' is methyl,
R2 is difluoromethoxy
R' and R2 together form the group -(CH2)4-,
R3 is chlorine or bromine,
R4 is hydrogen,
Rs is nitro or cyano,
R6 is hydrogen, C1-C6-alkyl, C2-C6-alkenyl or C3-C6-alkynyl, or is a C,-
C6-alkyl which is substituted by cyano, C1-C4-alkoxy or C1-C4-
alkoxycarbonyl .
20 The term "alkyl" stands for a straight or branched chain of carbon atoms.
The term "alkenyl" stands for a straight or branched chain of carbon atoms
which is interrupted once or more than once by double bonds.
25 The term "alkynyl" stands for a straight or branched chain of carbon atoms
which is interrupted once or more than once by triple bonds.
The compounds of the formula I according to the invention can be
prepared by
A) reacting a compound of the formula ll
CA 0226112~ 1999-01-1~
-
~ N~ (I l),
in which R1, R2, R3, R4 and Rs are each as defined in the formula I
with a suitable formylating reagent to give compounds of the formula
la
~ N~N~ (la),
OHC--NH
in which R', R2, R3, R4 and R5 are each as defined in the formula I
and Rs is hydrogen, or
10 B) reacting a compound of the formula la in which R1-R5 are each as
defined in the fommula I with a suitable alkylating agent of the
formula llb
R6-Z (llb),
in which R6 is as defined in the formula I and Z is a leaving group in
the presence of a base, or
C) reacting a compound of the formula lll
- CA 0226112~ 1999-01-1
R ~ N R
R~N--N )~( 5 (Ill),
R_NH R
in which R'- Rs are each as defined in the formula I with a suitable
forrnylating reagent, or
5 D) reacting a compound of the formula IV
R j~ N,N l(IV),
,~N--N
NH2
in which Rl-R3 are each as defined in the formula I with a suitable
formylating reagent, followed by nitration, or
E) if R2 is C,-C4-alkylsulfinyl or C,-C4-alkylsulfonyl, or is a C,-C4-
alkylsulfinyl or C1-C4-alkylsulfonyl which is mono- or polysubstituted
by halogen, reacting a compound of the formula lla
1 5 R3 R4
N =~
R 1 1 S ~ ~--R 5
N--N
R1 NH2 (lla),
in which R', R3, R4 and Rs are each as defined in the formula I and R" is
C,-C4-alkyl or a C,-C4-alkyl which is mono- or polysubstituted by halogen
with an oxidizing agent.
.. . ...
CA 0226112~ 1999-01-1
Suitable oxidizing agents are, for example, hydrogen peroxide, perbenzoic
acids, sodium periodate or potassium permanganate.
Suitable formylating agents are, for example, the mixed anhydride of acetic
5 acid and fommic acid, or formic acid.
Suitable nitrating agents are, for example, concentrated nitric acid or
mixtures of fuming nitric acid with concentrated sulfuric acid.
10 The reactions are advantageously carried out by reacting the compounds
at a temperature of -30 to 150~C.
Suitable bases are, for example, alkali metal hydroxides and alkaline earth
metal hydroxides, alkali metal carbonates and bicarbonates and alkaline
15 earth metal carbonates and bicarbonates, alkoxides, alkali metal hydrides,
tertiary aliphatic and aromatic amines, such as triethylamine and pyridine,
and heterocyclic bases.
Leaving groups are, for example, bromine, chlorine or iodine.
Tha reaction according to process variants A) and B) can be carried out in
the absence or presence of a solvent, using, if required, those solvents or
diluents which are inert toward the respective reactants. Examples of such
solvents or diluents are aliphatic, alicyclic and aromatic hydrocarbons, such
25 as hexane, cyclohexane, petroleum ether, benzene, toluene and xylene,
ethers, such as diethyl ether, 1,4-dioxane and tetrahydrofuran, nitriles,
such as acetonitrile, amides, such as dimethylformamide,
dimethylacetamide or N-methylpyrrolidone, or sulfoxides, such as dimethyl
sulfoxide.
The compounds of the formulae ll and lll used as starting material are
known. Their preparation is described in EP 542388 and WO 9408999.
The compounds of the formula lla used as starting material can be
CA 0226112~ 1999-01-1~
prepared by preparing compounds of the formulae V or Vl
R3 R3
R2s(0~NHNH2 R2S J NHNH2
N--N (V) N--N (Vl)
R R1
in which R', R2 and R3 are each as defined in formula I and m is 1 or 2 by
processes known per se, such as described in JP 621 58 260, from
5 compounds of the formulae Vll or Vlll
R2S(O) ~ NH2 R2S ~ _ NH2
N--N (Vll) N--N (Vlll)
R R1
The compounds of the formulae Vll and Vlll where R' and R2 are each as
defined in the formula I and R3 is halogen and m is 1 or 2 can be prepared
15 - by reacting compounds of the formulae Vll or Vlll in which R3 is hydrogen
with a halogenating agent.
The compounds of the formula Vll in which R', R2 and R3 are each as
defined in the formula I and m is 1 or 2 can be prepared by reacting
compounds of the formula Vlll with an oxidizing agent.
The compounds of the formula Vlll in which R' and R2 are each as defined
in the formula I and R3 is hydrogen can be prepared either by reacting a
compound of the formula IX
/s CN
< ~ (IX)
\5/ \ CN
CA 0226112~ 1999-01-1~
with a hydrazine of the formula X
R1NHNH2 (X),
5 where R' is as defined in the formula 1, if appropriate in the presence of a
solvent, to give initially a compound of the formula Xl
CN
H5~ Xl)
N--N
R1
in which R1 is as defined in the formula 1, which is then allowed to react
with a compound of the formula Xll
R2Y (Xll)
in which R2 is as defined in the formula I and Y is a leaving group, such as
chlorine or bromine, and saponifying and decarboxylating the thus-
20 obtained compound of the formula Xlll
CN
R2S _~ . NH2 (Xlll)
N--N
R1
by methods known from the literature (cf. for example Zeitschrift fur
Chemie 420, (1968),
or by reacting a compound of the formula XIV
R2S CN
\~/ (XIV)
R2S CN
~ ~ , . . .
CA 0226112~ 1999-01-1~
in which R2 is as defined in the formula I with a hydrazine of the formula X,
if appropriate in the presence of a solvent, such as water, to give a
compound of the formula Xlll.
5 The starting materials required for preparing these intermediates are
known or can be prepared similar to processes known per se.
The preparation can be carried out in the absence or presence of a
solvent, using, if required, the abovementioned solvents.
Suitable oxidizing agents are, for example, hydrogen peroxide, perbenzoic
acids, sodium periodate or potassium permanganate.
Suitable halogenating agents are, for example, sulfuryl chloride, sodium
15 hypochloride, N-chlorosuccinimide, N-bromosuccinimide, bromine or
chlorine.
Suitable bases are, for example, alkali metal hydroxides and alkaline earth
metal hydroxides, sodium methoxide, alkali metal hydrides, alkali metal
20 carbonates and alkaline earth metal carbonates, tertiary aliphatic and
aromatic amines, such as triethylamine and pyridine, and heterocyclic
bases. The reactions are carried at a temperature of -30 to 1 50~C.
The compounds according to the invention are worked up in the customary
25 manner. They are purified by crystallization or column chromatography.
As a rule, the compounds according to the invention are colorless or pale
yellow crystalline or viscous substances, some of which are readily soluble
in chlorinated hydrocarbons such as, for example, methylene chloride or
30 chloroform, ethers such as, for example, diethyl ether or tetrahydrofuran,
alcohols such as, for example, methanol or ethanol, ketones such as, for
example, acetone or butanone, amides such as, for example,
dimethylformamide, or else sulfoxides such as, for example, dimethyl
sulfoxide.
.. . . . . . . .. ... . ..
CA 0226112~ 1999-01-1~
The compounds according to the invention show a good herbicidal activity
on broad-leaved weeds and grasses. Selective use is possible in a variety
of crops, for example in oilseed rape, beet, soybeans, cotton, rice, maize,
barley, wheat and other cereal species. Individual compounds are also
5 suitable as selective herbicides in beet, cotton, soybeans, maize and
cereals. Equally, the compounds can be employed for controlling weeds in
perennial crops such as, for example, forests, plantations of woody
ornamentals, orchards, vineyards, citrus stands, nut orchards, banana
plantations, coffee plantations, tea plantations, rubber plantations, oil palm
10 plantations, cocoa plantations, and in soft fruit and hop fields.
The compounds according to the invention can be used for example in the
following plant genera:
dicotyledonous weeds of genera such as Sinapsis, Lepidium, Galium,
15 Stellaria, Matricaria, Anthemis, Galinsoga, Chenopodium, Brassica, Urtica,
Senecio, Amaranthus, Portulaca, Xanthium, Convolvulus, Ipomea,
Polygonum, Sesbania, Ambrosia, Cirsium, Sochus, Solanum, Lamium,
Veronica, Abutilon, Datura, Viola, Galeopsis, Papaver, Centaurea and
Chrysanthemum;
20 monocotyledonous weeds of the genera such as Avena, Alopecurus,
Echinochloa, Setaria, Panicum, Digitaria, Poa, Eleusine, Brachiaria,
Lolium, Bromus, Cyperus, Elymus, Sagittaria, Monochoria, Fimbristylis,
Eleocharis, Ischaemum and Apera.
25 When applied pre- and post-emergence, the rates of application vary
between 0.001 and 5 kg/ha, depending on the type of application.
The intensity of action and speed of action can be promoted for example
by activity-enhancing additives such as organic solvents, wetting agents
30 and oils. Such additives may therefore allow a reduced dosage of active
substance.
The active substances according to the invention or mixtures of these are
advantageously used in the form of preparations such as powders,
..... . . . . ... ..
- CA 0226112~ 1999-01-1~
materials for spreading, granules, solutions, emulsions or suspensions,
with an addition of liquid and/or solid carriers or diluents and, if appropriate,
tackifiers, wetting agents, emulsifiers and/or dispersants.
5 Examples of suitable liquid carriers are aliphatic and aromatic
hydrocarbons such as benzene, toluene, xylene, cyclohexanone,
isophorone, dimethyl sulfoxide, dimethylformamide, and furthermore
mineral oil fractions and vegetable oils.
10 Suitable solid carriers are minerals such as, for example, bentonite, silica
gel, talc, kaolin, attapulgite, limestone and products of vegetable origin
such as, for example, meals.
Surfactants which may be mentioned are, for example, calcium
15 lignosulfonate, polyethylene alkylphenyl ethers, naphthalenesulfonic acids
and their salts, phenolsulfonic acids and their salts, formaldehyde
condensates, fatty alcohol sulfates, and also substituted benzenesulfonic
acids and their salts.
20 The amount of active substance(s) in the various products can vary within
wide limits. For example, the compositions comprise approximataly 10 to
90% by weight of active ingredient, approximately 90 to 10% by weight of
liquid or solid carriers and, if appropriate, up to 20% by weight of
surfactants.
The compositions can be applied in the customary manner, for example
using water as the carrier in amounts of spray mixture of approximately
100 to 1000 liters/ha. Application of the compositions by the low-volume
and ultra-low-volume method is also possible, as is their application in the
30 form of microgranules.
These products can be prepared in a manner known per se, for example
by grinding or mixing processes. If desired, products comprising the
individual components may also be mixed only shortly prior to use as is the
. . . , ~
CA 0226112~ 1999-01-1~
12
case, for example, under practice conditions when using the tank mix
method.
The examples below illustrate the preparation of the compounds according
to the invention.
Example 1.2
1 -(3-Chloro-4,5,6,7-tetrahydropyrazolo-[1,5-a]-pyridin-2-yl)-5-formylamino-
10 4-pyrazolecarbonitrile
At 5-8~C,147 g (1.44 mol) of acetic anhydride were added dropwise with
stirring over a period of 15 minutes to 76.32 g (1.77 mol) of formic acid.
The mixture was then heated to 60~C for 2 hours. After cooling, this
15 solution was added dropwise to a suspension of 116.5 g (0.44 mol) of 5-
amino-(3-chloro-4,5,6,7-tetrahydropyrazolo-[1,5-a]-pyridin-2-yl)-4-
pyrazolecarbonitrile in 850 ml of acetonitrile. The mixture was stirred for 20
hours at room temperature and for 4 hours at 60~C. The reaction mixture
was concentrated and the residue was poured with stirring into an ice-cold
20 solution of 290 g (2.1 mol) of potassium carbonate in 3 1 of water and
stirred for one hour, and the solid was filtered off with suction and washed
twice with 2 l of water each time. The solid was dried at 50~C (200 mbar)
and then recrystallized from methanol.
25 Yield: 80.6 g (62.5% of theory)
Mp.: 186~C
Example 1.17
30 1-(3-Chloro-4,5,6,7-tetrahydropyrazolo-[1,5-a]-pyridin-2-yl)-5-(N-formyl-N-
propargylamino)-4-pyrazolecarbonitrile
A suspension of 66.8 g (0.23 mol) of 1-(3-chloro-4,5,6,7-
tetrahydropyrazolo-[1,5-a]-pyridin-2-yl)-5-formylamino-4-
~ . , ~
CA 0226112~ 1999-01-1~
13
pyrazolecarbonitrile, 37.3 g (0.27 mol) of potassium carbonate and a
spatula tip of potassium iodide in 500 ml of dimethylfommamide were stirred
for 30 minutes at a bath temperature of 80~C. 32.12 9 (0.27 mol, 30.07 ml)
of 3-bromo-1-propyne (80% strength solution in toluene) were then added
5 dropwise and stirring was continued for 3 hours. The reaction mixture was
poured into water, and the product was extracted with ethyl acetate, dried
over sodium sulfate and concenl~ated. The residue was recrystallized first
from diisopropyl ether and then from methyl tert-butyl ether.
Yield: 47.8 9 (63.2% of theory)
Mp.: 116-117~C
The following compounds are prepared in a similar manner, the
preparation of the compounds marked *) having already been described
15 above:
The abbreviations used here denote:
C = cyclo
Et = ethyl
20 Me = methyl
.. Ph = phenyl
CA 02261125 1999-01-15
.
14
Table 1:
R 3
R ~ N
CHO
Formula of Table 1
Example No. R3 R5 Mp.[ C] ornD
1.1 H H
1.2~ Cl H 179-181
1.3 Cl Me 87-88
1.4 Cl Et 1.5668
1.5 Cl C3H7
1.6 Cl C4Hg
1.7 Cl C5H1,
1.8 Cl CH(CH3)2 1.5575
1.9 ClCH(CH3)CH(CH3)2
1.10 ClCH(CH3)CH2CH3
1.11 Cl CH2CH(CH3)2
1.12 Cl c-C3H5
CA 0226112~ 1999-01-1~
Example No. R3 R5 Mp. [ C] orn"
1.13 Cl c-C5Hg
1.14 Cl CH2CH=CH2 1.5699
1.15 Cl CH2CCICH2
1.16 ClCH2CH=C(CH3)2
1.17~ Cl CH2C-CH 116-117
1.18 Cl ~
~o
1.19 Cl CH2CH2OMe
1.20 ClCH2CH2CH2OMe
1.21 Cl(CH2CH2O)2Me
1.22 ClCH(CH l)CH20Me
1.23 Cl CH2CH(OMe)?
1.24 Cl CH2CH(OEt)2
1.25 Cl
1.26 ~~~
Cl ~~J
1.27 ~o~
Cl o~CH3
CH3
1.28
Cl
1.29 Cl ~,~
~ CA 0226112~ 1999-01-1~
Example No. R3 R6 Mp. [ C] ornn20
1.30 CH2CH2SEt
Cl
1.31 Cl CH2CN
1.32 Cl CH2COOMe
1.33 CH2COOEt
Cl
1.34 CH(CH3)COOMe
Cl
1.35 CH(CH3)COOEt
Cl
1.36 - ClCH2CH2COOMe
1.37 ClCH2CH2COOEt
1.38 ClCH2Ph
1.39 Br H
1.40 Br Me
1.41 Br Et
1.42 BrC3H7
1.43 BrCH(CH3)2
1.44 CH2CH=CH2
1.45 CH2C-CH
1.47 CH2CH2OMe
1.48 CH2CH(OMe)2
.. ... .. .
CA 02261125 1999-01-15
Example No. R3 R6 Mp. [~C] orn~20
1.49 CH2CN
1.50 CH2COOMe
CA 0226112~ 1999-01-1~
18
Table 2
~ NO2
RN~
CHO
Formula of Table 2
Example No. R3 R6 Mp.[~C] ornD20
2.1 H H
2.2 Cl H 150-154
2.3 Cl Me 87-90
2.4 Cl Et 100-105
2.5 Cl C3H7
2.6 Cl C4Hg
2.7 Cl C5H"
2.8 Cl CH(CH3)2 11 9-1 21
2.9 ClCH(CH3)CH(CH3)2
2.10 ClCH(CH3)CH2CH3
2. 11 Cl CH2CH(CH3)2
2.12 Cl c-C3H5
CA 0226112~ 1999-01-1~
19
Example No. R3 R5 Mp. [ C] orn,,20
2.13 Cl c-C5Hg
2.14 Cl CH2CH=CH2
2.15 Cl CH2CCICH2
2.16 ClCH2CH=C(CH3)2
2.17 Cl CH2C--CH 1.5655
2.18 Cl
~o
2.19 Cl CH2CH2OMe
2.20 ClCH2CH2CH2OMe
2.21 Cl(CH2CH2O)2Me
2.22 ClCH(CH3)CH2OMe
2.23 Cl CH2CH(OMe)2
2.24 Cl CH2CH(OEt)2
2.25
Cl o
2.26 /\~o~
Cl ~~J
2.27 ~~
Cl o~CH3
CH3
2.28 Cl ~
CA 0226112~ 1999-01-1~
Example No. R3 R6 Mp. [ C] ornD20
2.29 ~~~
Cl o
2.30 Cl CH2CH2SEt
2.31 Cl CH2CN
2.32 Cl CH2COOMe
2.33 Cl CH2COOEt
2.34 ClCH(CH3)COOMe
2.35 ClCH(CH3)COOEt
2.36 Cl CH2CH2COOMe
2.37 Cl CH2CH2COOEt
2.38 Cl CH2Ph
2.39 Br H
2.40 Br Me
2.41 Br Et
2.42 Br C3H7
2.43 Br CH(CH3)2
2.44 Br CH2CH=CH2
2.45 Br CH2C-CH
-- . .~,
CA 02261125 1999-01-15
Example No. R3 R6 Mp. [ C] orn,,2
2.46 Br CH2CH2OMe
2.47 Br CH2CH(OMe)2
2.48 Br CH2CN
2.49 Br CH2COOMe
Table 3
F2HCO~ N~N~
N--N ~' C N
RN~
CHO
Formula of Table 3
Example No. R3 R5 Mp. [~C] or nD20
3.1 H H 175-177
3.2 Cl H 114-117
3.3 Cl Me 1.5321
3.4 Cl Et 1.5219
3.5 Cl C3H7
3.6 Cl C4Hg
3.7 Cl C5H,
CA 0226112~ 1999-01-1~
Example No. R3 R5 Mp. [ C] or nD
3.8 Cl CH(CH3)2
3.9 ClCH(CH3)CH(CH3)2
3.10 ClCH(CH3)CH2CH3
3.11 ClCH2CH(CH3)2
3.12 Cl c-C3H5
3.13 Cl c-C5Hg
3.14 Cl CH2CH=CH2 1.5216
3.15 Cl CH2CCICH2
3.16 ClCH2CH=C(CH3)2
3.17 H CH2C-CH 1.5267
3.18 Cl CH2C-CH 68-71
3.19 Cl ~O
3.20 Cl CH2CH2OMe
3.21 ClCH2CH2CH2OMe
3.22 Cl(CH?CH?O)2Me
3.23 ClCH(CH3)CH2OMe
3.24 ClCH2CH(OMe)2
3.25 ClCH2CH(OEt)2
.,
CA 02261125 1999-01-15
23
Example No. R3 R6 Mp. [ C] ornD
3.26 Cl ~
o
3.27 Cl /\~
o
3.28 Cl ~o~
o~CH3
3.29 Cl
5 3 30 Cl
3.31 Cl CH2CH2SEt
3.32 Cl CH2CN
3.33 Cl CH2COOMe 1.5222
3.34 Cl CH2COOEt
3.35 ClCH(CH3)COOMe
3.36 ClCH(CH3)COOEt
3.37 Cl CH2CH2COOMe
3.38 Cl CH2CH2COOEt
3.39 Cl CH2Ph
3.40 Br H
3.41 Br Me
CA 0226112~ 1999-01-1~
24
Example No. R3 R6 Mp. [ C] ornD
3.42 Br Et
3.43 Br C3H7
3.44 Br CH(CH3)2
3.45 Br CH2CH=CH2
3.46 Br CH2C--CH
3.47 Br CH2CH2OMe
3.48 Br CH2CH(OMe)2
3.49 Br CH2CN
3.50 Br CH2COOMe
~ CA 02261125 1999-01-15
Table 4
F2HCO ~ N~N~
--N )~'NO2
R6 N~
CHO
Formula of Table 4
Example No. R3 R5 Mp. [ C] ornD
4.1 H H
4.2 Cl H 191-193
4.3 Cl Me 111 -112
4.4 Cl Et 84-85
4.5 Cl C3H7
4.6 Cl C4Hg
4.7 Cl C5Ht,
4.8 Cl CH(CH3)2 1.5306
4.9 ClCH(CH3)CH(CH3)2
4.10 ClCH(CH3)CH2CH3
4.11 ClCH2CH(CH3)2
4.12 Cl c-C3H5
,,
CA 0226112~ 1999-01-1~
26
Example No. R3 R5 Mp. [~C] ornD20
4.13 Cl c-C5Hg
4.14 Cl CH2CH=CH2
4.15 Cl CH2CCICH2
4.16 ClCH2CH=C(CH3)2
4.17 Cl CH2C-CH 1.5467
4.18 Cl ~
~o
4.19 Cl CH2CH~OMe
4.20 ClCH2CH2CH2OMe
4.21 Cl-(CH2CH2O)2Me
4.22 ClCH(CH3)CH2OMe
4.23 Cl CH2CH(OMe)2
4.24 Cl CH2CH(OEt)2
4.25 Cl
4.26 ~\~o~
Cl ~~J
4.27 ~1'~~
Cl o~ ~CH3
CH3
4.28 /\~
Cl
CA 0226112~ 1999-01-1~
27
Example No. R3 R6 Mp. [ C] or nD
4.29 Cl
4.30 Cl CH2CH2SEt
4.31 Cl CH2CN
4.32 Cl CH2COOMe
4.33 Cl CH2COOEt
4.34 ClCH(CH3)COOMe
4.35 ClCH(CH3)COOEt
4.36 Cl CH2CH2COOMe
4.37 Cl CH2CH2COOEt
4.38 Cl CH2Ph
4.39 Br H
4.40 Br Me
4.41 Br Et
4.42 Br C3H7
4.43 Br CH(CH3)2
4.44 Br CH2CH=CH2
4.45 Br CH2C-CH
CA 02261125 1999-01-15
28
Example No. R3 R6 Mp. [~C] ornD20
4.46 Br CH2CH2OMe
4.47 Br CH2CH(OMe)2
4.48 Br CH2CN
4.49 Br CH2COOMe
CA 0226112~ 1999-01-1~
Table 5
F3C ~ N
H C ~ N - N ~ CN
R~N~
C H O
Formula of Table 5
Example No. R3 R6 Mp. [ C]ornD
5.1 H H
5.2 Cl H
5.3 Cl Me
5.4 Cl Et
1 5 5.5 ClC3H7
5.6 ClC4Hg
5.7 ClC5H"
5.8 ClCH(CH3)2
5.9 ClCH(CH3)CH(CH3)2
5.10 ClCH(CH3)CH2CH3
5.11 ClCH2CH(CH3)2
5.12 Clc-C3H5
- CA0226112~ 1999-01-1~
Example No. R3 R5 Mp. [~C~ ornD20
5.13 Cl c-C5Hg
5.14 ClCH2CH=CH2
5.15 CH?CCICH2
5.16 ClCH2CH=C(CH3)2
5.17 ClCH2C-CH
5.18 Cl ~
~o
5.19 ClCH2CH2OMe
5.20 ClCH2CH2CH2OMe
5.21 Cl(CH2CH2O)2Me
5.22 ClCH(CH3)CH2OMe
5.23 ClCH2CH(OMe)2
5.24 ClCH2CH(OEt)2
5.25 Cl
5.26 Cl
5.27 Cl ~~~
o ,~1~ CH3
CH3
5.28 Cl ~
.. ... ..
. CA 02261125 1999-01-15
. 31
Example No. R3 R6 Mp. [ C] ornD20
5.29 Cl
5.30 Cl CH2CH2SEt
5.31 Cl CH2CN
5.32 Cl CH2COOMe
5.33 Cl CH2COOEt
5.34 ClCH(CH3)COOMe
5.35 ClCH(CH3)COOEt
5.36 Cl CH2CH2COOMe
5.37 Cl CH2CH2COOEt
5.38 Cl CH2Ph
5.39 Br H
5.40 Br Me
5.41 Br Et
5.42 Br C3H7
5.43 Br CH(CH3)2
5.44 Br CH2CH=CH2
5.45 Br CH2C-CH
CA 02261125 1999-01-15
32
Example No. R3 R6 Mp. [~C] ornD20
5.46 Br CH2CH2OMe
5.47 Br CH2CH(OMe)2
5.48 Br CH2CN
5.49 Br CH2COOMe
Table 6
F3C ~ N,N l
N--N )~'~ NO2
R ~ N~
CHO
Formula of Table 6
Example No. R3 R5 Mp. [ C] ornD
6.1 H H
6.2 Cl H
6.3 Cl Me
6.4 Cl Et
6.5 Cl C3H7
6.6 Cl C4Hg
6.7 Cl C5H1t
CA 0226112~ 1999-01-1~
33
Example No. R3 R5 Mp. [ C] ornD
6.8 Cl CH(CH3)2
6.9 ClCH(CH3)CH(CH3)2
6.10 ClCH(CH3)CH2CH3
6.11 Cl CH2CH(CH3)2
6.12 Cl c-C3H5
6.13 Cl c-C5Hg
6.14 Cl CH2CH=CH2
6.15 Cl CH2CCICH2
6.16 ClCH2CH=C(cH3)2
6.17 Cl CH2C-CH
6.18 Cl /~
~o
6.19 Cl CH2CH2OMe
6.20 ClCH2CH2CH2OMe
6.21 Cl(CH2CH2O)2Me
6.22 ClCH(CH3)CH2OMe
6.23 Cl CH2CH(OMe)2
6.24 Cl CH2CH(OEt)2
6.25 Cl ~
CA 0226112~ 1999-01-1~
34
Example No. R3 R6 Mp. [ C] ornD
6.26 Cl ~
o
6.27 Cl ~o~
o ~J~ CH3
CH3
6.28 Cl
6.29 Cl
6.30 Cl CH2CH2SEt
6.31 Cl CH2CN
6.32 Cl CH2COOMe
6.33 Cl CH2COOEt
6.34 Cl CH(CH3)COOMe
6.35 Cl CH(CH3)COOEt
6.36 Cl CH2CH2COOMe
6.37 Cl CH2CH2COOEt
6.38 Cl CH2Ph
6.39 Br H
6.40 Br Me
6.41 Br Et
CA 0226112~ 1999-01-1~
Example No. R3 R6 Mp. [ C] or nD
6.42 Br C3H7
6.43 Br CH(CH3)2
6.44 Br CH2CH=CH2
6.45 Br CH2C-CH
5 6.46 Br CH2CH2OMe
6.47 Br CH2CH(OMe)2
6.48 Br CH2CN
6.49 Br CH2COOMe
10 The Use Example which follows illustrates the invention:
Use Example
In the greenhouse, the compounds listed in the table were applied by
15 pipetting to a water surface of about 170 cm2, using test plants both pre-
emergence and in the 1-3 leaf stage.
After 2 weeks, it became evident that the compounds according to the
invention have a strong activity against weeds in rice and do not cause any
20 damage to the rice crops.
Compound of Waterapplication O E S C S M
Example kg of a.s./ha R C A Y C O
Y H G P P O
S C P D J V
A G Y I U A
25 1.4 0.05 0 4 3 4 4 4
..... ~ ...... .
CA 0226112~ 1999-01-1
36
Compound of Water appl ~~' ,,n O E S C S M
Examplekg of a.s./ha R C A Y C O
Y H G P P O
S C P D J V
A G Y I U A
1.14 0.05 0 4 4 4 4 4
1.17 0.005 0 4 4 4 4 4
Untreated O O O O O O O
a.s. = active substance
ORYSA = Oryza sativa 0 = no damage
ECHCG = Echinochloa crus-galli 1 = weak damage
SAGPY = Sagittaria pygmea 2 = medium damage
CYPDI = Cyperus difformis 3 = strong damage
SCPJU = Scirpus juncoides 4 = total destruction
MOOVA = Monochoriavaginalis