Note: Descriptions are shown in the official language in which they were submitted.
CA 0226278~ 1999-02-08
W O 98/06364 PCTrUS97/12854
- ABSORBENT MACROSTRUCTURE MADE FROM MIXTURES OF
DIFFERENT HYDROGEL-FORMING ABSORBENT POLYMERS FOR
- s IMPROVED FLUID HANDLING CAPABILITY
TECHNICAL FIELD
This application relates to porous, absorbent macrostructures that
comprise flexibie interparticle bonded aggregates. This application
particularly relates to porous absorbent macrostructures where the
interparticle bonded aggregates are made from mixtures of particulate
absorbent polymers having different fluid handling properties, or shapes,
or both, that impart improved fluid handling capability to the
macrostructure .
BACKGROUND
The development of highly absorbent members for use as
~o disposable diapers, adult incontinence pads and briefs, and catamenial
products such as sanitary napkins, are the subject of substantial
commercial interest. A highly desired characteristic for such products is
thinness. For example, thinner diapers are less bulky to wear, fit better
under clothing, and are less noticeable. They are also more compact in
the package, making the diapers easier for the consumer to carry and
store. Compactness in packaging also results in reduced distribution
costs for the manufacturer and distributor, including less shelf space
required in the store per diaper unit.
The ability to provide thinner absorbent articles such as diapers
has been contingent on the ability to develop relatively thin absorbent
cores or structures that can acquire and store large quantities of
discharged body fluids, in particular urine. In this regard, the use of
certain absorbent polymers often referred to as "hydrogels,"
"superabsorbents" or "hydrocolloid" material has been particularly
important. See, for example, U.S. Patent 3,699,103 (Harper et al), issued
June 13, 1972, and U.S. Patent 3,770,731 (Harmon), issued June 20,
CA 0226278~ 1999-02-08
W O 98/06364 PCTrUS97112854
1972, that disclose the use of such absorbent polymers (hereafter
"hydrogel-forming absorbent polymers") in absorbent articles. Indeed, the
development of thinner diapers has been the direct consequence of
thinner absorbent cores that take advantage of the ability of these
hydrogel-forming absorbent polymers to absorb large quantities of
discharged body fluids, typically when used in combination with a fibrous
matrix. See, for example, U.S. Patent 4,673,402 (Weisman et al), issued
June 16, 1987 and U.S. Patent 4,935,022 (Lash et al), issued June 19,
1990, that disclose dual-layer core structures comprising a fibrous matrix
o and hydrogel-forming absorbent polymers useful in fashioning thin,
compact, nonbulky diapers.
Prior to the use of these hydrogel-forming absorbent polymers, it
was general practice to form absorbent structures, such as those suitable
for use in infant diapers, entirely from wood pulp fluff. Given the relatively
15 low amount of fluid absorbed by wood pulp fluff on a gram of fluid
absorbed per gram of wood pulp fluff, it was necessary to employ
relatively large quantities of wood pulp fluff, thus necessit~ting the use of
relatively bulky, thick absorbent structures. The introduction of these
hydrogel-forming absorbent polymers into such structures has allowed the
use of less wood pulp fluff. These hydrogel-forming absorbent polymers
are superior to fluff in their ability to absorb large volumes of aqueous
body fluids, such as urine (i.e., at least about 15 9/9), thus making
smaller, thinner absorbent structures feasible.
These hydrogel-forming absorbent polymers are often made by
initially polymerizing unsaturated carboxylic acids or derivatives thereof,
such as acrylic acid, alkali metal (e.g., sodium and/or potassium) or
ammonium salts of acrylic acid, alkyl acrylates, and the like. These
polymers are rendered water-insoluble, yet water-swellable, by slightly
cross-linking the carboxyl group-containing polymer chains with
conventional di- or poly-functional monomer materials, such as N, N'-
methylenebisacrylamide, trimethylol propane triacrylate or triallyl amine.
These slightly crosslinked absorbent polymers still comprise a multiplicity
of anionic (charged) carboxyl groups attached to the polymer backbone.
It is these charged carboxy groups that enable the polymer to absorb
body fluids as the result of osmotic forces, thus forming hydrogels.
.,, I
CA 0226278~ 1999-02-08
W O 98/06364 PCT~US97/12854
The degree of cross-linking determines not only the water-
insolubility of these hydrogel-forming absorbent polymers, but is also an
important factor in establishing two other characteristics of these
polymers: their absorbent capacity and gel strength. Absorbent capacity
or "gel volume" is a measure of the amount of water or body fluid that a
given amount of hydrogel-forming polymer will absorb. Gel strength
relates to the tendency of the hydrogel formed from these polymers to
deform or "flow" under an applied stress. Hydrogel-forming polymers
useful as absorbents in absorbent structures and articles such as
o disposable diapers need to have adequately high gel volume, as well as
adequately high gel strength. Gel volume needs to be sufficiently high to
enable the hydrogel-forming polymer to absorb significant amounts of the
aqueous body fluids encountered during use of the absorbent article. Gel
strength needs to be such that the hydrogel formed does not deform and
S fill to an unacceptable degree the capillary void spaces in the absorbent
structure or article, thereby inhibiting the absorbent capacity of the
structure/article, as well as the fluid distribution throughout the
structure/article. See, for example, U.S. Patent 4,654,039 (Brandt et al),
issued March 31, 1987 (reissued April 19, 1988 as U.S. Reissue Patent
32,649) and U.S. Patent 4,834,735 (Alemany et al), issued May 30, 1989.
Prior absorbent structures have generally comprised relatively low
amounts (e.g., less than about 50 % by weight) of these hydrogel-forming
absorbent polymers. See, for example, U.S. Patent 4,834,735 (Alemany
et al), issued May 30, 1989 (preferably from about 9 to about 50%
hydrogel-forming absorbent polymer in the fibrous matrix). There are
several reasons for this. The hydrogel-forming absorbent polymers
employed in prior absorbent structures have generally not had an
absorption rate that would allow them to quickly absorb body fluids,
especially in "gush" situations. This has necessitated the inclusion of
fibers, typically wood pulp fibers, to serve as temporary reservoirs to hold
the discharged fluids until absorbed by the hydrogel-forming absorbent
polymer.
More importantly, many of the known hydrogel-forming absorbent
polymers exhibited gel blocking, especially when included in the
absorbent structure at higher levels. "Gel blocking" occurs when particles
CA 0226278~ 1999-02-08
WO 98106364 PCT/US97/128S4
4 -- .
of the hydrogel-forming absorbent polymer are wetted and the particles
swell so as to inhibit fluid transmission to other regions of the absorbent
structure. Wetting of these other regions of the absorbent member
therefore takes place via a very slow diffusion process. In practical terms,
s this means acquisition of fluids by the absorbent structure is much slower
than the rate at which fluids are discharged, especially in gush situations.
Leakage from the absorbent article can take place well before the
particles of hydrogel-forming absorbent polymer in the absorbent member
are fully saturated or before the fluid can diffuse or wick past the
10 "blocking" particles into the rest of the absorbent member.
Gel blocking can be a particularly acute problem if the particles of
hydrogel-forming absorbent polymer do not have adequate gel strength
and deform or spread under stress once the particles swell with absorbed
fluid. See U.S. Patent 4,834,735 (Alemany et al), issued May 30, 1989.
Low gel strength hydrogel-forming absorbent polymers also tend to be
those having higher fluid capacities. Gel strength can be increased by
surface crosslinking of these higher fluid capacity hydrogel-forming
absorbent polymers. Unfortunately, while surface crosslinking increases
gel strength, it also tends to lower the fluid capacity of the hydrogel-
forming absorbent polymer.
Gel blocking can also occur when the hydrogel-forming absorbent
polymer is in the form of regular shaped particles, such as spherically
shaped particles. Spherical shaped particles typically result when the
hydrogel-forming absorbent polymer is formed by multi-phase
polymerization processing techniques such as inverse emulsion
polymerization or inverse suspension polymerization procedures. See
U.S. Patent 4,340,706 (Obaysashi et al), issued July 20, 1982, U.S.
Patent 4,506,052 (Flesher et al), issued March 19, 1985, and U.S. Patent
4,735,987 (Morita et al), issued April 5, 1988. Because these spherical
shaped particles are prone to gel blocking, multi-phase polymerization
processing techniques have been considered less desirable in
synthesizing hydrogel-forming absorbent polymers. See U.S. Patent
5,124,188 (Roe et al), issued June 23, 1992.
To improve capillary capability and thus minimize gel blocking,
particles of these hydrogel-forming absorbent polymers have been formed
CA 0226278~ 1999-02-08
W 0~8~0f~ PCT~US97/12854
S -- .
into interparticle crosslinked aggregate macrostructures, typically in the
form of sheets or strips. These aggregate macrostructures have been
prepared by initially mixing the particles of hydrogel-forming absorbent
polymer with a solution of a nonionic crosslinking agent such as glycerol,
s water and a hydrophilic organic solvent such as isopropanol. See U.S
Patent 5,102,597 (Roe et al), issued April 7, 1992; U.S. Patent 5,124,188
(Roe et al), issued June 23, 1992; and U.S. Patent 5,149, 344 (Lahrman
et al), issued September 22, 1992. See also U.S. Patent 5,324,561
(Rezai et al), issued June 23, 1994, which discloses an improved porous
o aggregate macrostructure where the particles of hydrogel-forming
absorbent polymer are crosslinked with cationic amino-epichlorohydrin
adducts, such as KYMENE.
Because the particulate nature of the absorbent polymer is
retained, these macrostructures provide pores between adjacent particles
that are interconnected such that the macrostructure is fluid permeable
(i.e., has capillary transport channels). Due to the interparticle crosslink
bonds formed between the particles, the resultant macrostructures also
have improved structural integrity, increased fluid acquisition and
distribution rates, and minimal gel blocking charac~erislics. Even so, the
fluid handling capability of these macrostructures is still somewhat
dependent on the fluid handling capability of the particles of hydrogel-
forming absorbent polymer from which they are made. For example,
macrostructures made from low gel strength hydrogel-forming absorbent
polymers or spherical shaped particles of hydrogel-forming absorbent
polymer are still potentially subject to gel blocking. Also, macrostructures
made from surface crosslinked hydrogel-forming absorbent polymers still
have less than optimum permeability characteristics.
Accordingly, it would be desirable to be able to make absorbent
aggregate macrostructures of bonded absorbent particles that: (1) can
use hydrogel-forming absorbent polymers made by a variety of methods;
(2) are less prone to gel blocking; (3) have optimum permeability
characteristics; and/or (4) provide an improved combination of fluid
handling capabilities.
SUMMARY
CA 0226278~ l999-02-08
W 098~ PCTrUS97/12854
The present invention relates to porous, absorbent, macrostructures
having improved fluid handling capabilities that comprise interparticle
bonded aggregates. These aggregates comprise a multiplicity of
interconnected crosslinked particles comprising substantially water-
insoluble, absorbent, hydrogel-forming polymer material. The hydrogel-
forming polymer material comprises a mixture selected of (a) mixtures of
from about 50 to about 95% of a first hydrogel-forming polymer having a
Saline Flow Conductivity (SFC) value of at least about ~ x 10-7 cm3sec/g
and a Performance under Pressure (PUP) capacity value of at least about
o 23 g/g under a confining pressure of 0.7 psi (5 kPa) and from about 5 to
about 50% of a second hydrogel-forming polymer having an Absorptive
Capacity value of at least about 25 9/9; (b) mixtures of from about 2~ to
about 40% of a first hydrogel-forming polymer in the form of spherical
shaped particles and from about 60 to about 80% of a second hydrogel-
forming polymer in the form of nonspherical shaped particles; and (c)combinations of (a) and (b). The interparticle bonded aggregate
comprises pores between adjacent particles. The pores are
interconnected by intercommunicating channels so as to form a liquid
permeable macrostructure. The circùmscribed dry volume of the
macrostructure is greater than about 0.008 mm3.
The porous, absorbent macrostructures according to the present
invention are useful, alone, or in combination with other absorbent
materials, in absorbent structures for various absorbent articles, including
diapers, adult incontinence pads, sanitary napkins, and the like. These
porous absorbent macrostructures provide a particularly desirable
combination of fluid handling properties including relatively high fluid
permeability and relatively high fluid capacitv. The macrostructures of the
present invention can also be made from a wider variety of hydrogel-
forming absorbent polymers without sacrificing these desired fluid
handling properties and without being prone to gel blocking. Indeed, the
ability to use greater quantities of spherical shaped particles of hydrogel-
forming absorbent polymers provides processing advantages such as a
more uniform flow rate during the making of these macrostructures.
BRIEF DESCRIPTION OF THE DRAWINGS
CA 0226278~ 1999-02-08
W O 98/06364 PCT~US97/12854
-
Figure 1 is a scanning electron microscope photograph of a
typical macrostructure of the present invention. The macrostructure
shwon is fabricated with the use of irregular shaped and spherical
shaped superabsorbent polymers.
Figure 2 is a scanning electron microscope photograph of a
macrostructure of the present invention. The structure is fabricated by
attaching one irregular shaped superabsorbent polymer on one side of
a fiber web, and a spherical shaped polymer on the other side.
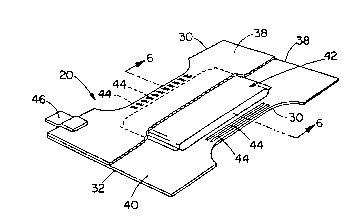
Figure 3 is a perspective view of a disposable diaper embodiment
according to the present invention wherein portions of the topsheet have
been cut-away to more clearly show the underlying absorbent core (an
embodiment of an absorbent member according to the present invention)
of the diaper wherein the absorbent member comprises a porous,
absorbent macrostructure according to the present invention.
Figure 4 is a cross-sectional view of the absorbent core of the diaper
shown in Figure 3 taken along sectional line 6-6 of Figure 8.
Figure ~ is a perspective view of a disposable diaper embodiment
according to the present invention wherein portions of the topsheet have
been cut away to more clearly show an alternative dual-layer absorbent
core embodiment.
Figure 6 is a blown-apart view of the components of a diaper
structure, one of the components being an alternative dual-layer
absorbent core where the absorbent macrostructure is in the form of a
plurality of strips.
Figure 7 is a profile of the Demand Absorbency Capacity of
various macrostructures vs. weight percent of high PUP, L-76
superabsorbent polymer in different macrostructures.
DETAILED DESCRIPTION
Definitions
"Body fluids" includes urine, menses and vaginal discharges.
"Comprising" means various components, members, steps and the
like can be conjointly employed according to the present invention.
Accordingly, the term "comprising" encompasses the more restrictive
terms "consisting essentially of" and "consisting of," these latter, more
resL,i.;tive terms having their standard meaning as understood in the art.
,
CA 0226278~ 1999-02-08
W O 98/06364 PCTAUS97/12854
All percentages, ratios and proportions used herein are by weight
unless otherwise specified.
I. Porous Absorbent Macrostructures
A. General Characteristics
Porous, absorbent macrostructures according to the present
invention are structures capable of absorbing large quantities of liquids
such as water and/or body exudates (e.g., urine or menses) and then
retaining such liquids under moderate pressures. Because of the
particulate nature of the precursor particles, the macrostructure has pores
o between adjacent precursor particles. These pores are interconnected by
intercommunicating channels such that the macrostructure is liquid
permeable (i.e., has capillary transport channels).
Due to the bonds formed between the precursor particles, the
resultant aggregate macrostructure has improved structural integrity,
15 increased liquid acquisition and distribution rates, and minimal gel-
blocking characteristics. It has been found that when the macrostructure
is contacted with liquids, the macrostructure swells generally isotropically
even under moderate confining pressures, absorbs such liquids into the
pores between the precursor particles, and then imbibes such liquids into
20 the particles. The isotropic swelling of the macrostructure allows the
precursor particles and the pores to maintain their relative geometry and
spatial relationships even when swollen. Thus, the macrostructures are
relatively "fluid stable" in that the precursor particles do not dissociate
from each other, thereby minimizing the incidence of gel blocking and
25 allowing the capillary channels to be maintained and enlarged when
swollen so that the macrostructure can acquire and transport subsequent
loadings of liquid, even excess liquid.
"Macrostructure" means a structure having a circumscribed volume
when substantially dry (i.e., circumscribed dry volume) of at least about
30 0.008 mm3, preferabiy at least about 10.0 mm3, more preferably at least
about 100 mm3, more preferably at least about 500 mm3. Typically, the
macrostructures of the present invention will have a circumscribed dry
volume much greater than about 500 mm3. In preferred embodiments of
the present invention, the macrostructures have a circumscribed dry
35 volume of between about 1000 mm3 and about 100,000 mm3.
CA 0226278~ 1999-02-08
W O 98,'C~ PCTrUS97/12854
9 - .
While the macrostructures of the present invention can have a
number of shapes and sizes, they are typically in the form of sheets, films,
cylinders, blocks, spheres, fibers, filaments, or other shaped elements.
The macrostructures will generally have a thickness or diameter between
about 0.2 mm and about 10.0 mm. Preferably for use in absorbent
products, the macrostructures are in the form of a sheet. The term
"sheet" describes macrostructures having a thickness of at least about 0.2
mm. The sheets will preferably have a thickness between about 0.5 mm
and about 10 mm, typically from about 1 mm to about 3 mm.
o The porous, absorbent macrostructures of the present invention
comprise interparticle bonded aggregates. These interparticle bonded
aggregates usually comprise about 8 or more previously independent
precursor particles. For preferred circumscribed dry volumes and sizes of
the individual precursor particles used herein, these interparticle bonded
aggregates typically are formed from about 100,000 or more individual
precursor particles. These individual precursor particles can comprise
granules, pulverulents, spheres, flakes, fibers, aggregates or
agglomerates. The individual precursor particles can have a variety of
shapes, such as cubic, rod-like, polyhedral, spherical, rounded, angular,
20 irregular, randomly-sized irregular shapes, e.g., pulverulent products of
grinding or pulverizing steps, or shapes having a large greatest
dimension/smallest dimension ratio so as to be needle-like, flake-like, or
fiber-like.
The interparticle bonded aggregate comprising the macrostructures
25 of the present invention are formed, in essence, by the joining or adhering
together of adjacent particles. The adhesive agent is essentially the
polymeric material that is present in the surface of these particles. When
these precursor particles are l,aated with a crosslinking agent and
physically associated, the polymer material present in the surface of these
30 part~cles is sufficiently plastic and cohesive (e.g., sticky) such that
adjacent particles are adhered together, typically as discrete linking
portions between the particles. The crosslinking reaction between the
particles then sets this adhered structure such that the particles in the
aggregate remain cohesively bonded together.
CA 0226278~ 1999-02-08
WO 98/06364 PCT/US97/12854
B. Precursor Absorbent Particles
The macrostructures of the present invention are formed from
precursor particles that comprise substantially water-insoluble polymer
materials capable of absorbing large quantities of liquids. Such polymer
s materials are referred to hereafter as "hydrogel-forming absorbent
polymers." Since the macrostructures of the present invention comprise
interparticle bonded aggregates, these hydrogel-forming absorbent
polymer materials will be discussed herein with respect to those forming
the precursor particles.
o Although the precursor particles can have a size varying over a
wide range, specific particle size distributions and sizes are preferred. For
purposes of the present invention, particle size is defined for precursor
particles that do not have a large greatest dimensionlsmallest dimension
ratio such as fibers (e.g., granules, flakes, or pulverulents) as the
I5 dimension of a precursor particle which is determined by sieve size
analysis. For purposes of this invention, the mass average particle size of
the precursor particles is important in determining the characteristics and
properties of the resultant macrostructures. The mass average particle
size of a given sample of precursor particles is defined as the particle size
which is the average particle size of the sample on a mass basis. A
method for determining the mass average particle size of a sample is
described in the Test Methods section of U.S. Patent 5,324,561 (Rezai et
al), issued June 23, 1994. The mass average particle size of the
precursor particies will generally be from about 20 microns to about 1500
2s microns, more preferably from about 50 microns to about 1000 microns.
In preferred embodiments of the present invention, the precursor particles
have a mass average particle size less than about 1000 microns, more
preferably less than about 600 microns, more preferably less than about
500 microns.
The particle size of materials having a large greatest
dimension/smallest dimension, such as fibers, is typically defined by their
largest dimension. For example, if absorbent, polymeric fibers (i.e.
superabsorbent fibers) are used in the macrostructures of the present
invention, the length of the fibers is used to define the "particle size." (The
denier and/or the diameter of the fibers can also be specified.) In
_ . _
CA 0226278~ 1999-02-08
W O ~81~6~ PCTAUS97/12854
exemplary embodiments of the present invention, the fibers have a length
greater than about 5 mm, preferably between about 10 mm and about 100
mm, more preferably between about 10 mm and about 50 mm.
The hydrogel-forming absorbent polymer material that comprise
these precursor particles have a multiplicity of anionic, functional groups,
such as sulfonic acid, and more typically carboxy, groups. Examples of
polymer materials suitable for use as the precursor particles herein
include those which are prepared from polymerizable, unsaturated, acid-
containing monomers. Thus, such monomers include the olefinically
o unsaturated acids and anhydrides which contain at least one carbon to
carbon olefinic double bond. More specifically, these monomers can be
selected from olefinically unsaturated carboxylic acids and acid
anhydrides, olefinically unsaturated sulfonic acids, and mixtures thereof.
See U.S. Patent 5,324,561 (Rezai et al), issued June 23, 1994, which
generally describes suitable monomers for the preparation of hydrogel-
forming absorbent polymers.
Preferred polymer materials for use in the present invention contain
carboxy groups. These polymers include hydrolyzed starch-acrylonitrile
graft copolymers, partially neutralized starch-acrylonitrile graft copolymers,
starch-acrylic acid graft copolymers, partially neutralized starch-acrylic
acid graft copolymers, saponified vinyl acetate-acrylic ester copolymers,
hydrolyzed acrylonitrile or acrylamide copolymers, slightly network
crosslinked polymers of any of the foregoing copolymers, partially
neutralized polyacrylic acid, and slightly network crosslinked polymers of
partially neutralized polyacrylic acid. These polymers can be used either
solely or in the form of a mixture of two or more different polymers. See,
for example, U.S. Patent 4,093,776 (Aoki et al), issued June 6, 1978, U.S.
Patent 4,666,983 (Tsubakimoto et al), issued May 19, 1987, and U.S.
Patent 4,734,478 (Tsubakimoto et al), issued March 29, 1988 where
representative examples of these types of hydrogel-forming absorbent
polymer are disclosed.
More preferred polymer materials for use in making the precursor
particles are slightly network crosslinked polymers of partially neutralized
polyacrylic acids and starch derivatives thereof. Preferably, the precursor
particles comprise from about 50 to about 95%, preferably about 75%,
. _ . . .
CA 0226278~ l999-02-08
W 098/06364 PCTAUS97/128S4 12
neutralized, slightly network crosslinked, polyacrylic acid (i.e. poly (sodium
acrylate/acrylic acid)). Processes for network crosslinking the polymers
and typical network crosslinking agents are described in greater detail in
U.S. Patent 4,076,663, suora.
s The individual precursor particles can be formed in any
conventional manner. For example, precursor particles can be prepared
by methods that involve aqueous solution or other solution polymerization
methods. See, for example, U.S. Reissue Patent 32,649 (Brandt et al),
reissued April 19, 1988. Precursor particles useful in the present
invention can also be manufactured using multi-phase polymerization
processing techniques such as inverse emulsion polymerization or inverse
suspension polymerization procedures. See, for example, U.S. Patent
4,340,706 (Obaysashi et al), issued July 20, 1g82, U.S. Patent 4,506,052
(Flesher et al), issued March 19, 1985, and U.S. Patent 4,735,987 (Morita
et al), issued April 5, 1988, for processes involving inverse suspension
polymerization. As will be discussed hereafter, the particular process
used in making these precursor particles can be important in determining
their fluid permeability and capacity properties, as well as the shape of the
resultant particles.
While all of the precursor particles are preferably formed from the
same polymer material with the same properties, this need not be the
case. For example, some precursor particles can comprise a starch-
acrylic acid graft copolymer while other precursor particles can comprise a
slightly network crosslinked polymer of partially neutralized polyacrylic
acid. Further, the precursor particles can vary in size, shape, absorptive
capacity, or any other property or characteristic. In a preferred
embodiment of the present invention, the precursor particles consist
essentially of slightly network crosslinked polymers of partially neutralized
polyacrylic acid, each precursor particle having similar properties.
In preferred embodiments of the present invention, the precursor
particles used to form the bonded particle aggregates are substantially
dry. "Substantially dry" means that the precursor particles have a liquid
content, typically water or other solution content, less than about 50%,
preferably less than about 20%, more preferably less than about 10%, by
weight of the precursor particles. In general, the liquid content of the
CA 0226278~ 1999-02-08
W O 98/06364 PCTAUS97/128S4
13
precursor particles is in the range of from about 0.01% to about 5% by
weight of the precursor particles. The individual precursor particles can
be dried by any conventional method such as by heating. Alternatively,
when the precursor particles are formed using an aqueous reaction
mixture, water can be removed from the reaction mixture by azeotropic
distillation. The polymer-containing aqueous reaction mixture can also be
treated with a dewatering solvent such as methanol. Combinations of
these drying procedures can also be used. The dewatered mass of
polymer material can then be chopped or pulverized to form substantially
o dry precursor particles of substantially water-insoluble, absorbent,
hydrogel-forming, polymer material.
C. Mixtures of Precursor Particles Providinq Improved Fluid
Handlinq ProPerties
The key aspect of the present invention is using mixtures of
15 precursor particles that have: (1) different fluid handling properties; (2)
different shapes; or (3) both. It has been found that mixtures of precursor
particles having different fluid handling properties, shapes or both can
impart improved overall fluid handling capability to the resultant
macrostructure. This is typically manifested as a combination higher fluid
20 permeability/performance and higher fluid capacity in the resultant
macrostructures made from these mixtures. Indeed, macrostructures
made from mixtures of precursor particles according to present invention
minimize the potential problem of "gel blocking" without sacrificing desired
fluid capacity.
Mixtures according to variant (1) of the present invention comprise
precursor particles made from: (a) a first hydrogel-forming polymer having
a relatively high Saline Flow Conductivity (SFC) value and relatively high
Performance Under Pressure (PUP) capacity (for higher gel
permeability/performance); and (b) a second hydrogel-forming polymer
30 having a relatively high Absorptive Capacity. Generally, these mixtures
~ comprise from about 50 to about 95% of the higher gel
permeability/performance hydrogel-forming polymer and from about 5 to
about 50% the higher fluid capacity hydrogel-forming polymer. Preferably,
these mixtures comprise from about 60 to about 95% of the higher gel
35 permeabilitylperformance hydrogel-forming polymer and from about 5 to
CA 0226278~ 1999-02-08
W 098/06364 PCT~US97/12854 14
about 40% of the higher fluid capacity hydrogel-forming polymer, and
more preferably from about 60 to about 80% of the higher gel
permeability/performance hydrogel-forming polymer and from about 20 to
about 40% of the higher fluid capacity hydrogel-forming polymer.
s Precursor particles useful in the present invention that have
relatively high Saline Flow Conductivity (SFC) values and relatively high
Performance Under Pressure (PUP) capacity are disclosed in copending
U.S. application Serial No. 219,574 (Goldman et al), filed March 29, 1994.
These high gel layer permeability/performance precursor particles have
o SFC values of at least about 5 x 10-7 cm3seclg, preferably at least about
10 x 10-7 cm3sec/g, and more preferably at least about 100 x 10-7
cm3sec/g. Typically, these SFC values are in the range of from about 30
to about 1000 x 10-7 cm3sec/g, more typically from about 50 to about 500
x 10-7 cm3sec/g, and more typically from about 100 to about 350 x 10-7
s cm3sec/g. These high gel layer permeability/performance precursor
particles also generally have a PUP capacity at least about 23 9/9,
preferably at least about 25 9/9, and more preferably at least about 29
9/9. Typically, these PUP capacity values are in the range of from about
23 to about 35 9/9, more typically from about 25 to about 33 9/9, more
20 typically from about 29 to about 33 9l9.
The preferred processes for obtaining precursor particles having
relatively high SFC and PUP capacity values involve surface crosslinking
of the initially formed polymers. A number of processes for introducing
surface crosslinks are disclosed in the art. These include those where: (i)
25 a di- or poly-functional reagent(s) (e.g., glycerol, 1,3-dioxolan-2-one,
polyvalent metal ions, polyquaternary amines) capable of reacting with
existing functional groups within the hydrogel-forming absorbent polymer
is applied to the surface of the hydrogel-forming absorbent polymer; (ii) a
di- or poly-functional reagent that is capable of reacting with other added
30 reagents and possibly existing functional groups within the hydrogel-
forming absorbent polymer such as to increase the level of crosslinking at
the surface is applied to the surface (e.g., the addition of monomer plus
crosslinker and the initiation of a second polymerization reaction); (iii) no
additional polyfunctional reagents are added, but additional reaction(s) is
35 induced amongst existing components within the hydrogel-forming
I
CA 0226278~ 1999-02-08
W098/06364 PCTAJS97/12854
absorbent polymer either during or after the primary polymerization
process such as to generate a higher level of crosslinking at or near the
surface (e.g., heating to induce the fo~ alio" of anhydride and/or ester
crosslinks between existing polymer carboxylic acid and/or hydroxyl
groups and suspension polymerization processes wherein the crosslinker
is inherently present at higher levels near the surface); and (iv) other
materials are added to the surface such as to induce a higher level of
crosslinking or othe~ise reduce the surface deformability of the resultant
hydrogel. Combinations of these surface crosslinking processes either
10 concurrently or in se~uence can also be employed. In addition to
crosslinking reagents, other components can be added to the surface to
aid/control the distribution of crosslinking (e.g., the spreading and
penetration of the surface crosslinking reagents.) See copending U.S.
application Serial No. 219,574 (Goldman et al), filed March 29, 1994.
I5 Suitable general "~etl,ods for carrying out surface crosslinking of
hydrogel-forming absorbent polymers according to the present invention
are disclosed in U.S. Patent 4,541,871 (Obayashi), issued September 17,
1985; published PCT application WO92/16565 (Stanley), published
October 1, 1992, published PCT application WO90/08789 (Tai), published
August 9, 1990; published PCT application W093/05080 (Stanley),
published March 18, 1993; U.S. Patent 4,824,901 (Alexander), issued
April 25, 1989; U.S. Patent 4,789,861 (Johnson), issued January 17,
1989; U.S. Patent 4,587,308 (Makita), issued May 6, 1986; U.S. Patent
4,734,478 (Tsubakimoto), issued March 29, 1988; U.S. Patent 5,164,459
(Kimura et. al.), issued November 17, 1992; published German patent
application 4,020,780 (Dahmen), published August 29, 1991; and
published European patent application 509,708 (Gartner), published
October 21, 1992. See also copending U.S. application Serial No.
219,574 (Goldman et al), filed March 29, 1994, especially Examples 1 to
4. Suitable hydrogel-forming absorbent polymers having relatively high
SFC and PUP capacity values include L761f made by Nippon Shokubai,
SXP made by Chemische Fabrik Stockhausen, XZ made by Dow
Chemical and XP-30 made by Nalco Chemical.
Precursor particles useful in the present invention that have a
relatively high Absorptive Capacity and a relatively high Absorption (MP)
CA 0226278~ 1999-02-08
W O 98/06364 PCTrUS97/128~4
16
value are disclosed in the U.S. Patent 4,076,663 (Matsuda et al), issued
February 28, 178, U.S. Reissue Patent 32,649 (Brandt et al), reissued
April 19, 1988, U.S. Patent 4,625,001 (Tsubakimoto et al), issued
November 25, 1986, U.S. Patent 4,666,983 (Tsubakimoto et al), issued
s May 19, 1987, U.S. Patent 4,734,478 (Tsubakimoto et al), issued March
29, 1988, U.S. Patent 4,735,987 (Morita et al), issued April 5, 1988, U.S.
Patent 4,973,632 (Nagasuna et al), issued November 27, 1990, U.S.
Patent 5,264,471 (Chmelir), issued November 23, 1993 and European
Patent Application 530,438 (Chambers et al), published March 10, 1993,
o all of which are. "Absorptive Capacity" refers to the capacity of a given
polymer material to absorb fluids with which it comes into contact and can
vary significantly with the nature of the fluid being absorbed and with the
manner in which the liquid contacts the polymer material. For purposes of
this invention, Absorptive Capacity is defined in terms of the amount of
S Synthetic Urine absorbed by any given polymer material in terms of grams
of Synthetic Urine per gram of polymer material. See Test Methods
section hereafter. The MP value is reflective of the demand absorbency
of the hydrogel-forming polymer, i.e. the absorptive capacity of the
polymer when subjected to an external pressure of 0.3 psi. See Test
20 Methods section hereafter.
These higher fluid capacity precursor particles have Absorptive
Capacity values of at least about 25 grams, more preferably at least about
35 grams, more preferably at least about 45 grams, of Synthetic Urine per
gram of polymer. Typically, these higher fluid capacity precursor particles
25 have an Absorptive Capacity value of from about 25 to about 70 grams,
more typically from about 40 to about 60 grams of Synthetic Urine per
gram of polymer material.
The preferred processes for obtaining these precursor particles
having relatively high Absorptive Capacity value involve aqueous solution
30 or other solution polymerization methods. As described in the above-
referenced U.S. Patent Reissue 32,649, aqueous solution polymerization
involves the use of an aqueous reaction mixture to carry out
polymerization to form the precursor particles. The aqueous reaction
mixture is then subjected to polymerization conditions which are sufficient
35 to produce in the mixture, substantially water-insoluble, slightly network
T ~
CA 0226278~ 1999-02-08
W O 98/06364 PCTrUS97112854
17 - .
crosslinked polymer material. Suitable hydrogel-forming absorbent
polymers having relatively high Absorptive Capacity and MP values
include, but are not limited to, IM 1000 made by Hoechst Celanese, L74
made by Nippon Shokubai and F201 made by Nippon Gohsei.
Mixtures according to variant (2) of the present invention comprise
precursor particles made from: (a) a first hydrogel-forming polymer in the
form of spherical shaped particles which can include spherical shaped
agglomerates of the first hydrogel-forming polymer (typically produced by
a suspension polymerization process); and (b) a second hydrogel-forming
o polymer in the form of nonspherical or irregular shaped particles which are
typically produced by a bulk polymerization process. It is believed the
reason that macrostructures made exclusively from spherical shaped
particles of hydrogel-forming absorbent polymer are prone to gel block is
due to the formation of a close, compacted structure that would have
poorer fluid permeability. It has been found that the inclusion of
nonspherical (irregular) shaped particles seems to perturb the self-
assembling nature of the spherical shaped particles during the making of
the macrostructures, especially macrostructures in sheet form. As a
result, the macrostructure is no longer prone to blocking fluid.
Generally, these mixtures according to variant (2) comprise from
about 5 to about 50% of the spherical particles and from about 50 to
about 95% the nonspherical particles. Preferably, these mixtures
comprise from about 10 to about 50% of the spherical pa~ticles and from
about 50 to about 90% the nonspherical particles, more preferably from
25 about 20 to about 40% of the spherical particles and from about 60 to
about 80% of the nonspherical particles.
Spherical shaped particles and spherical shaped agglomerates can
be obtained by multi-phase polymerization processing techniques such as
inverse emulsion polymerization or inverse suspension polymerization
30 procedures. In the inverse emulsion polymerization or inverse suspension
~ polymerization procedures, the aqueous reaction mixture is suspended in
the form of tiny droplets in a matrix of a water-immiscible, inert organic
solvent such as cyclohexane. The resultant precursor particles are
generally spherical in shape. Inverse suspension polymerization
35 procedures are disclosed in U.S. Patent 4,093,776 (Aoki et al), issued
CA 0226278~ 1999-02-08
WO ~810~ PCTrUS97/12854
18 --
June 6, 1978, U.S. Patent 4,340,706 (Obaysashi et al), issued July 20,
1982, U.S. Patent 4,446,261 (Yamasaki et al), issued, U.S. Patent
4,506,052 (Flesher et al), issued March 19, 1985, U.S. Patent 4,541,871
(Obayashi et al), issued September 17, 1985, U.S. Patent 4,698,414
(Cramm et al), issued October 6, 1987, U.S. Patent 4,735,987 (Morita et
al), issued April 5, 1988, U.S. Patent 4,833,179 (Young et al), issued May
23, 1989, and European Patent Application 522,570, published January
13, 1993. Suitable spherical shaped particles of hydrogel-forming
absorbent polymer include F201 made by Nippon Gohsei and Base 60
made by Mitsubishi Chemical.
Nonspherical or irregular shaped particles can be obtained by bulk
polymerization procedures including aqueous solution or other solution
polymerization methods. As described in the above-referenced U.S.
Reissue Patent 32,649, aqueous solution polymerization involves the use
15 of an aqueous reaction mixture to carry out polymerization to form the
precursor particles. The aqueous reaction mixture is then subjected to
polymerization conditions which are sufficient to produce in the mixture,
substantially water-insoluble, slightly network crosslinked polymer
material. The mass of polymer material thereby formed is then pulverized
20 or chopped to form the individual precursor particles. Suitable
nonspherical shaped particles of hydrogel-forming absorbent polymer
include L761f made by Nippon Shokubai, SXP or SXM made by
Chemische Fabrik Stockhausen, and 1180 or XP 30 made by Nalco
Chemical.
D. Crosslinking ~gents
In preparing macrostructures according to the present invention, a
crosslinking agent is used to provide crosslinking at the surface of the
mixture of absorbent precursor particles. This typically occurs as a result
of reacting the crosslinking agent with the polymer material in these
30 particles. Typically, the polymer material of the absorbent precursor
particles has anionic, and preferably carboxy, functional groups that form
a covalent, ester-type bond with the crosslinking agent. These portions of
the absorbent particle that have been effectively crosslinked will swell less
in the presence of aqueous (body) fluids relative to the other
35 uncrosslinked portions of the particle.
CA 0226278~ 1999-02-08
W 098~6~?C~ PCTrUS97/12854
19
Suitable crosslinking agents for this purpose can be nonionic and
possess at least two functional groups per molecule capable of reacting
with the carboxy group. See, for example, U.S. Patent 5,102,597 (Roe et
al), issued April 7, 1992, which discloses a variety of nonionic crosslinking
s agents that include polyhydric alcohols such as ethylene glycol, diethylene
glycol, triethylene glycol, tetraethylene glycol, polyethylene glycol, glycerol
(1, 2, 3-propanetriol), polyglycerol, propylene glycol, 1, 2-propanediol, 1,
3-propanediol, trimethylol propane, diethanolamine, triethanolamine,
polyoxypropylene oxyethylene-oxypropylene block copolymer, sorbitan
o fatty acid esters, polyoxyethylene sorbitan fatty acid esters,
pentaerythritol, and sorbitol; polyglycidyl ether compounds such as
ethylene glycol diglycidyl ether, polyethylene glycol diglycidyl ether,
glycerol polyglycidyl ether, diglycerol polyglycidyl ether, polyglycerol
polyglycidyl ether, sorbitol polyglycidyl ether, pentaerythritol polyglycidyl
s ether, propylene glycol diglycidyl ether, and propylene glycol diglycidyl
ether; polyaziridine compounds such as 2, 2-bishydroxymethyl butanol-
tris[3-(i-aziridine) propionate], 1, 6-hexamethyl toluene diethylene urea,
and diphenyl methane-bis-4, 4'-N,N'-diethylene urea; haloepoxy
compounds such as epichlorohydrin and a-methylfluorohydrin;
polyaldehyde compounds such as glutaraldehyde and glyoxazole;
polyamine compounds such as ethylene diamine, diethylene triamine,
triethylene tetramine, tetraethylene pentamine, pentaethylene hexamine,
and polyethylene imine; and polyisocyanate compounds such as 2, 4-
toluene diisocyanate and hexamethylene diisocyanate. The particularly
preferred nonionic crosslinking agent is glycerol
A preferred crosslinking agent for use in the present invention is an
adduct of epichlorohydrin with certain types of monomeric or polymeric
amines. See U.S. Patent 5,324,561 (Rezai et al), issued June 23, 1994,
which discloses suitable cationic amino-epichlorohydrin adduct
crosslinking agents. These amino-epichlorohydrin adducts, and
especially the polymeric resin versions of these adducts, are preferred
crosslinking agents because they react only with the polymer material at
the surface precursor particles. In addition, the cationic functional groups
(e.g., azetedinium groups) of these adducts, particularly polymeric resin
versions, are believed to react very rapidly with the anionic, typically
CA 0226278~ 1999-02-08
W O ~8/O~?C1 PCT~US97/12854
carboxy, functional groups of the polymer material of the absorbent
particles, even at room temperature (e.g., at from about 18~ to about
25~C). As a result, fairly modest levels (e.g., as low as about 1% by
weight of the particles) of these amino-epichlorohydrin adducts are
required to provide effective surface crosslinking of the polymer material
present in the absorbent precursor particles.
Suitable cationic amino-epichlorohydrin adducts useful as
crosslinking agents include those where epichlorohydrin are reacted with
monomeric di-, tri- and higher amines having primary or secondary amino
o groups in their structure such as bis-2-aminoethyl ether, N, N-
dimethylethylenediamine, piperazine, ethylenediamine, N-aminoethyl
piperazine, and dialkylene triamines such as diethylenetriamine, and
dipropylenetriamine; polymeric amines such as polyethyleneimines, and
certain polyamide-polyamines derived from polyalkylene polyamines and
saturated C3-C10 dibasic carboxylic acids. These
epichlorohydrin/polyamide-polyamine adducts are well known in the art as
wet strength resins for paper products. More preferred
epichlorohydrin/polyamide-polyamine adducts are those derived from the
polyethylene polyamines containing from two to four ethylene groups, two
primary amine groups, and from one to three secondary amine groups,
and saturated aliphatic C3-C10 dicarboxylic acids, more preferably those
containing from 3 to 8 carbon atoms, such as malonic, succinic, glutaric,
adipic, together with diglycolic acid. Cationic polyamide-polyamine-
epichlorohydrin resins particularly preferred for use herein as crosslinking
agents are commercially marketed by Hercules Inc. under the trade name
KYMENE. Especially useful are KYMENE 557H, KYMENE 557LX and
KYMENE 557 PLUS, which are the epichlorohydrin adducts of polyamide-
polyamines which are the reaction products of diethylenetriamine and
adipic acid. They are typically marketed in the form of aqueous solutions
of the cationic resin material containing from about 10% to about 33% by
weight of the resin active.
E. PreParation of Interparticle Bonded Agqregates and
Macrostructures
In preparing the interparticle bonded aggregates that comprise the
porous, absorbent macrostructures, the mixture of absorbent precursor
r -~-~~~
CA 02262785 1999-02-08
W 098/06364 PCTrUS97/12854
21
particles are treated with a sufficient amount of the crosslinking agent to
react with the polymer material at the surface of the particles so as to
cause effective crosslinking, i.e., the crosslinked surface of the particle
swells less in the presence of aqueous body fluids relative to the
5 uncrosslinked portions. What constitutes "a sufficient amount" of the
crosslinking depends upon a number of factors, including the particular
absorbent precursor particles treated, the crosslinking agent used, the
particular effects desired in forming the interparticle bonded aggregate,
and like factors. See U.S. Patent 5,102,597 (Roe et al), issued April 7,
o 1992 (nonionic crosslinking agents such as glycerol), and U.S. Patent
5,324,561 (Rezai et al), issued June 23, 1994 (cationic amino-
epichlorohydrin adduct crosslinking agents).
Besides the absorbent precursor particles and the crosslinking
agent, other components or agents can be used as aids in preparing the
15 interparticle bonded aggregates. For example, water is typicaîly used with
the crosslinking agent to form an aqueous treatment solution thereof.
Water promotes the uniform dispersion of the crosslinking agent on the
surface of the precursor particles and causes permeation of the
crosslinking agent into the surface regions of these particles. Water also
20 promotes a stronger physical association between the treated precursor
particles, providing greater integrity of the resultant interparticle bonded
crosslinked aggregates. The actual amount of water used can vary
depending upon the type of crosslinking agent used, the type of polymer
material used in forming the precursor particles, the particle size of these
25 precursor particles, the inclusion of other optional components (e.g.,
glycerol) and like factors. See U.S. Patent 5,102,597 (Roe et al), issued
April 7, 1992 (nonionic crosslinking agents such as glycerol), and U.S.
Patent 5,324,561 (Rezai et al), issued June 23, 1994 (cationic amino-
epichlorohydrin adduct crosslinking agents).
Although not absolutely necessary, organic solvents can be used,
usually to promote uniform dispersion of the crosslinking agent onto the
surface of the precursor particles. These organic solvents are typically
hydrophilic, and can include lower alcohols such as methanol and ethanol;
amides such as N,N-dimethylformamide and N,N-diethylformamide; and
35 sulfoxides such as dimethylsulfoxide. The actual amount of hydrophilic
.
CA 0226278~ 1999-02-08
W O 98/06364 PCTrUS97/12854
2~
solvent used can vary depending upon the adduct used, the polymer
material used forming the precursor particles, the particie size of these
precursor particles and like factors. See U.S. Patent 5,102,597 (Roe et
al), issued April 7, 1992 (nonionic crosslinking agents such as glycerol),
s and U.S. Patent 5,324,561 (Rezai et al), issued June 23, 1994 (cationic
amino-epichlorohydrin adduct crosslinking agents).
Other optional components can also be used with the crosslinking
agent, and especially aqueous treatment solutions thereof. It is
particularly preferred that the treatment solution include a plasticizer,
o especially when cationic amino-epichlorohydrin adducts are used as the
crosslinking agent. See U.S. Patent 5,324,561 (Rezai et al), issued June
23, 1994. Suitable plasticizers include water, alone or in combination with
other components such as glycerol, propylene glycol (i.e. 1,2-
propanediol), 1,3-propanediol, ethylene glycol, sorbitol, sucrose,
s polymeric solutions such as those involving polyvinyl alcohol, ester
precursors of polyvinyl alcohol, or polyethylene glycol, or mixtures thereof.
These other components in the plaslici~er, such as glycerol, are believed
to act as humectants, coplasticizers or both, with water being the primary
plasticizer. The preferred plasticizer for use in the present invention is a
20 mixture of glycerol and water, particularly when included as part of an
aqueous treatment solution of the cationic amino-epichlorohydrin adduct,
in a weight ratio of glycerol to water of from about 0.5:1 to about 2:1,
preferably from about 0.8:1 to about 1.7:1.
The actual amount of plasticizer used can vary depending upon the
25 particular plasticizer used, the type of polymer material used in forming
the precursor particles, and the particular flexibility effects desired from
the plasticizer. Typically, the plasticizer is used in an amount of from
about 5 to about 100 parts by weight, preferably from about 5 to about 60
parts by weight, more preferably from about 10 to about 30 parts by
30 weight, more preferably from about 15 to about 20 parts by weight, per
100 parts by weight of the precursor particles. See U.S. Patent 5,324,561
(Rezai et al), issùed June 23, 1994.
In the method of the present invention, the absorbent precursor
particles can be treated with the cationic amino-epichlorohydrin adduct,
35 typically an aqueous solution thereof, by any of a variety of techniques.
,
CA 0226278~ 1999-02-08
W O 98/06364 PCTrUS97/12854
23
These include any method for applying solutions to materials, including
coating, dumping, pouring, dropping, spraying, atomizing, condensing, or
immersing the absorbent precursor particles with the cationic amino-
epichlorohydrin adduct, or solution thereof. "Applied" means that at least
5 a portion of the surface area of at least some of the precursor particles to
be bonded together has an effective amount of the adduct on it to cause
surface crosslinking. In other words, the cationic adduct can be applied
onto some of the precursor particles, all of the precursor particles, a
portion of the surface of some or all of the precursor particles, or the entire
o surface of some or all of the precursor particles. Preferably, the adduct is
coated onto the entire surface of most, preferably all, of the absorbent
precursor particles so as to enhance the efficiency, strength, and density
of the interparticle bonds between the precursor particles, as well as the
desired surface crosslinking of the polymer material in the surface of
15 these precursor particles.
After the treatment solution has been applied onto the precursor
particles, the treated precursor particles can be mixed or layered together
by any of a number of mixing or layering techniques to insure that the
precursor particles are thoroughly coated with the treatment solution. See
20 U.S. Patent 5,102,597 (Roe et al), issued April 7, 1992 (nonionic
crosslinking agents such as glycerol), and U.S. Patent 5,324,561 (Rezai
et al), issued June 23, 1994 (cationic amino-epichlorohydrin adduct
crosslinking agents). Before, during, or after applying the treatment
solution, the precursor particles are physically associated together to form
25 an aggregate macrostructure. The precursor particles are preferably
physically associated together by applying an associating agent onto the
precursor particles and physically contacting the precursor particles at
least to the portion of the surface of the precursor particles having the
associating agent applied thereto. Associating agents useful in the
30 present invention include hydrophilic organic solvents, typically low
molecular weight alcohols such as methanol or ethanol; water; a mixture
of hydrophilic organic solvents and water; the crosslinking agents, or
mixtures thereof. Preferred associating agents are water, methanol,
ethanol, cationic polymeric amino-epichlorohydrin resins such as
35 KYMENE 557H, or 557LX or PLUS, or mixtures thereof. Typically the
.. , .. , ~
CA 0226278~ 1999-02-08
W O 98t06364 PCTAUS97/12854
24
associating agent comprises a mixture including the crosslinking agent
such that the step of applying the crosslinking is carried out
simultaneously with the step of applying the associating agent.
The associating agents can be applied to the precursor particles by
s any of various techniques and apparatus used for applying solutions to
materials including coating, dumping, pouring, spraying, atomi ing,
condensing, or immersing the associating agent on the precursor
particles. See U.S. Patent 5,102,597 (Roe et al), issued April 7, 1992
(nonionic crosslinking agents such as glycerol), and U.S. Patent
o 5,324,561 (Rezai et al), issued June 23, 1994 (cationic amino-
epichlorohydrin adduct crosslinking agents). When an associating agent
has been applied to the precursor particles, the precursor particles can be
physically contacted together in a number of different ways. For example,
the associating agent alone can hold the particles together in contact.
15 Alternatively, gravitational forces can be used to insure contact between
the precursor particles, e.g., by layering precursor particles. Further, the
particles can be placed in a container having a fixed volume so as to
insure contact between the precursor particles.
The precursor particles can alternatively be physically associated
20 together by physically constraining the precursor particles such that they
are in contact with each other. For example, the precursor particles can
be packed tightly into a container having a fixed volume such that the
precursor particles physically contact each other. Alternatively or in
combination with the above procedure, gravitational forces (e.g., layering)
~s can be used to physically associate the precursor particles. The
precursor particles can also be physically associated together by
ele~t,ostalic attraction or by the introduction of an adhering agent (e.g., an
adhesive material such as a water-soluble adhesive) to adhere them
together. The precursor particles can also be attached to a third member
30 (a substrate) such that the precursor particles are brought into contact
with each other by the substrate.
In an alternative method of forming the macrostructures of the
present invention, the aggregate of the precursor particles is shaped into
various geometries, spatial relationships, and densities to form an
35 aggregate having a defined shape, size, and/or density. The aggregate
CA 0226278~ 1999-02-08
W O 98/06364 PCTrUS97/12854
can be shaped by any conventional shaping techniques as are known in
the art. Preferred methods for shaping the aggregate include casting,
molding, or forming operations. Casting and molding techniques
generally involve introducing the precursor particles into a prepared mold
cavity and applying pressure to (compressing) the aggregate to cause the
aggregate to conform to the shape of the mold cavity. Examples of
specific molding techniques for use herein include compression molding,
injection molding, extrusion or laminating. For example, a multiplicity of
precursor particles can be added to a container having a fixed volume
o mold cavity and the aggregate compressed to conform to the shape of the
mold cavity so that the resultant macrostructure has the same shape.
Forming techniques involve performing various operations on the
aggregate to modify its shape, and/or size, and/or density. Examples of
specific forming techniques for use herein include rolling, forging,
s extruding, spinning, coating or drawing operations. For example, an
aggregate mixture of the precursor particles and at least the cationic
amino-epichlorohydrin adduct can be passed between a pair of
compaction rolls to form an aggregate sheet. Alternatively, the aggregate
mixture can be extruded through an orifice to form an aggregate having a
shape corresponding to that of the orifice. Further, the aggregate mixture
can be cast on a surface to form an aggregate having a desired shape or
surface morphology. Any or all of these techniques can also be used in
combination to form the shaped aggregate. Any suitable apparatus as
are known in the art can be used to carry out such operations, which can
be performed with the material or portions of the apparatus either hot
and/or cold. A preferred method and apparatus for continuously forming
the aggregate macrostructures of the present invention into sheets is
described in U.S. Patent 5,324,561 (Rezai et al), issued June 23, 1994
(cationic amino-epichlorohydrin adduct crosslinking agents). See
especially Figure 9 from U.S. Patent 5,324,561 and its associated
description.
Simultaneously or after the treatment solution has been applied,
the precursor particles have been physically associated together to form
an aggregate, and the aggregate has been shaped, the crosslinking agent
is reacted with the polymer material of the precursor particles, while
CA 0226278~ 1999-02-08
WO ~8~'~53~ PCT/US97/12854
26
maintaining the physical association of the precursor particles, to provide
effective surface crosslinking in the precursor particles in the aggregate
macrostructure. See U.S. Patent 5,102,597 (Roe et al), issued April 7,
1992 (nonionic crosslinking agents such as glycerol), and U.S. Patent
s 5,324,561 (Rezai et al), issued June 23, 1994 (cationlc amino-
epichlorohydrin adduct crosslinking agents). Because of the relatively
reactive cationic functional groups of the amino-epichlorohydrin adducts
that can be used as crosslinking agents in the present invention, this
crosslinking reaction can occur at relatively low temperatures, including
o ambient room temperatures. Such ambient temperature curing is
particularly desirable when the treatment solution additionally contains a
plasticizer, such as a mixture of water and glycerol. Curing at significantly
above ambient temperatures can cause the plasticizer to be driven off due
to its volatility, thus necessitating an additional step to plasticize the
resulting interparticle bonded aggregate. Such ambient curing is typically
carried out at a temperature of from about 18~ to about 35~C for from
about 12 to about 48 hours. Preferably, such ambient curing is carried out
at a temperature of from about 18~ to about 25~C for from about 24 to
about 48 hours.
Although the crosslinking reaction can occur at ambient
temperatures, such curing can also be carried out at higher temperatures
to speed up the reaction. Higher temperature curing typically involves
heating the treated and associated precursor particles to cause the
crosslinking reaction to occur in a shorter period of time, typically minutes.
This heating step can be carried out using a number of conventional
heating devices, including various ovens or dryers well known in the art.
Generally, heat curing can be carried out at a temperature above
about 50~C for a period of time sufficient to complete the crosslinking
reaction. The particular temperatures and times used in heat curing will
depend upon the particular crosslinking agents used and the polymer
material present in the precursor particles. See U.S. Patent 5,102,597
(Roe et al), issued April 7, 1992 (nonionic crosslinking agents such as
glycerol), and U.S. Patent 5,324,561 (.Rezai et al), issued June 23, 1994
(cationic amino-epichlorohydrin adduct crosslinking agents). In the case
of the preferred cationic amino-epichlorohydrin adducts, heat curing is
CA 0226278~ 1999-02-08
W 098/06364 PCT~US97/12854 27
generally carried out at a temperature in the range of from about 50~ to
about 20~~C for from about 1 to about 20 minutes. Preferably, heat
curing is carried out at a temperature of from about 180~ to about 200~C
for from about 5 to about 15 minutes.
The physical association of the treated precursor particles needs to
be maintained during the curing step so that, as crosslinking occurs,
adjacent precursor particles become cohesively bonded together. If
forces or stresses are sufficient to disassociate the precursor particles that
are present during the crosslinking reaction, insufficient bonding of the
o precursor particles can occur. This can result in aggregates having poor
structural integrity. The physical association of the precursor particles is
typically maintained by insuring minimal dissociation forces or stresses
are introduced during the curing step.
The steps for producing the macrostructures need not be carried
15 out in any specific order, and can be carried out simultaneously. For
example, the treatment solution can be applied simultaneously with the
physical association of the precursor particles, shaped into a preferred
shape and typically a desired density, and then the crosslinking agent
reacted with the polymer material of the precursor particles, either
20 immediately after the above steps are completed or after the aggregate
has been left standing for a period of time, to simultaneously surface
crosslink the precursor particles and form the aggregate macrostructure.
Typically, the precursor particles are mixed or sprayed with a solution of
the crosslinking agent, water, a humectant and/or coplasticizer (e.g.,
25 glycerol), and a hydrophilic organic solvent (e.g., methanol) to form an
adhered together aggregate. The adhered aggregate (i.e. the associated
precursor particles and the aqueous mixture) is subsequently shaped into
a densified sheet by a combination of extruding and rolling techniques as
described above. The crosslinking agent is subsequently reacted with the
30 polymer material by ambient or heat curing to simultaneously cause
crosslinking at the surface of the precursor particles and to form a
cohesive interparticle bonded aggregate macrostructure.
The macrostructures can also be treated with a plasticizer after
curing to effect surface crosslinking. Suitable plasticizers include water,
35 alone or in combination with the humectants/coplasticizers previously
CA 0226278~ 1999-02-OX
W O 98/0~'6~ PCT~US97112854
28
described, preferably glycerol. The plasticizer can be applied to the
macrostructures in a number of different ways, including spraying,
coating, atomizing, immersing, or dumping the plasticizer onto the
macrostructure. Alternatively, in the case of water alone, the
s macrostructure can be placed in a high humidity environment (e.g.,
greater than 70% relative humidity). The amount of plasticizer applied to
the macrostructure can be selected depending upon the specific
plasticizer used, and the effects desired. Typically, the amount of
plasticizer applied is from about 5 to about 100 parts by weight, preferably
from about 5 to about 60 parts by weight, per 100 parts by weight of the
macrostructure. A particularly preferred plasticizer comprises a mixture of
glycerol and water in a weight ratio of from about 0.5:1 to about 2:1,
preferably from about 0.8:1 to about 1.7:1.
Various types of fiber material can be used as the reinforcing
members in the macrostructures of the present invention. Any type of
fiber material which is suitable for use in conventional absorbent products
is also suitable for use in the macrostructures herein. Specific examples
of such fiber material include cellulose fibers, modified cellulose fibers,
rayon, polypropylene, and polyester fibers such as polyethylene
terephthalate (DACRON), hydrophilic nylon (HYDROFIL), and the like.
Examples of other fiber materials for use in the present invention in
addition to some already discussed are hydrophilized hydrophobic fibers,
such as surfactant-treated or silica-treated thermoplastic fibers derived,
for example, from polyolefins such as polyethylene or polypropylene,
polyacrylics, polyamides, polystyrenes, polyurethanes and the like. In
fact, hydrophilized hydrophobic fibers which are in and of themselves not
very absorbent and which, therefore, do not provide webs of sufficient
absorbent capacity to be useful in conventional absorbent structures, are
suitable for use in the macrostructures of the present invention by virtue of
their good wicking properties. This is because, in the macrostructures
herein, the wicking propensity of the fibers is as important, if not more
important, than the absorbent capacity of the fiber material itself due to
the high rate of fluid uptake and lack of gel blocking properties of the
macrostructures of the present invention. Synthetic fibers are generally
- r
CA 0226278~ 1999-02-08
W O 981'a~1 PCTrUS97/12854
preferred for use herein as the fiber component of the macrostructure.
More preferred are polyolefin fibers, preferably polyethylene fibers.
Other cellulosic fiber materials which can be useful in certain
macrostructures herein are chemically stiffened cellulosic fibers.
P~efer~ed chemically stiffened cellulosic hbers are the stiffened, twisted,
curled cellulosic fibers which can be produced by internally crosslinking
cellulose fibers with a crosslinking agent. Suitable stiffened, twisted,
curled cellulose fibers useful as the hydrophilic fiber material herein are
described in greater detail in U.S. Patent 4,888,093 (Dean et al), issued
o December 19, 1989; U.S. Patent 4,889,595 (Herron et al), issued
December 26, 1989; U.S. Patent 4,889,596 (Schoggen et al), issued
December 26, 1989; U.S. Patent 4,889,597 (Bourbon et al), issued
December 26, 1989; and U.S. Patent 4,898,647 (Moore et al), issued
February 6, 1990.
s "Hydrophilic" describes fibers or the surfaces of fibers which are
wetted by the liquids deposited onto the fibers (i.e., if water or aqueous
body fluid readily spreads on or over the surface of the fiber without
regard to whether or not the fiber actually imbibes fluid or forms a gel).
The state of the art respecting wetting of materials allows definition of
hydrophobicity (and wetting) in terms of contact angles and the surface
tension of the liquids and solids involved. This is discussed in detail in the
American Chemical Society Publication entitled "CONTACT ANGLE,
WErrABILITY, ANDADHESION", edited by Robert F. Gould and copyrighted in
1964. A fiber or surface of a fiber is said to be wetted by a liquid either
when the contact angle between the liquid and the fiber or surface is less
than 90~ or when the liquid will tend to spread spontaneously across the
surface of the fiber; both conditions normally coexisting.
The fiber material can be added to the macrostructures by
introducing the fibers into the l,~all"ent solution with the crosslinking, by
mixing with the precursor particles prior to applying the treatment solution,
or by adding the fiber material to the treatment solution/precursor particle
mixture. For example, the fiber material can be kneaded into the
treatment solution/precursor particle mixture. The fiber material is
preferably thoroughly mixed with the solution so that the fiber material is
uniformly dispersed throughout the macrostructure. The fibers are also
CA 0226278~ 1999-02-08
W O g8/OL,~61 PCTrUS97tl2854
preferably added before reacting the adduct with the polymer material of
the precursor particles.
F. Optional Substrate Layer
If desired, the porous absorbent macrostructure can be attached to
an optional substrate. See copending U.S. application Serial No. 142,2~3
(Hsueh et al), filed October 22, 1993. The substrate can provide a variety
of functions, including: (1 ) improving the distribution of fluids to be
absorbed by the macrostructure; and (2) supporting the macrostructure by
providing additional integrity, especially in the situation where the
o absorbent particles begin to swell after absorbing fluid. The subsl,dte can
be made from various materials known in the art such as cellulose fibers,
nonwoven webs, tissue webs, foams, polyacrylate fibers, apertured
polymeric webs, synthetic fibers, metallic foils, elastomers, and the like.
Most such substrate materials can distribute fluids to, as well as support
s the macrostructure. Preferably, the substrate is comprised of cellulosic
material or a material having cellulosic functionality. Preferred substrates
for distributing fluids are cellulosic materials, fibrous webs, cellulosic
fibrous webs, solid foams, cellulosic foams, and polyvinyl alcohol foams.
Preferred substrates for supporting the macrostructure are cellulosic
20 materials, fibrous webs, nonwoven webs, fabrics, cellulosic fibrous webs,
solid foams, cellulosic foams, and polyvinyl alcohol foams.
The substrate is preferably flexible and pliable to encourage such
properties in the resulting absorbent composite with the macrostructure.
The substrate can be substantially resilient and non-stretchable, or it can
25 be stretchable or deformable to a varying extent in response to forces
exerted normal to and in the plane of the surface of the substrate. The
thickness and basis weight (weight per unit area of substrate) of the
substrate material can vary depending on the type of substrate and
properties desired. The substrate can comprise a plurality of individual
30 sheets, or plies, of a particular substrate material, or a combination of oneor more substrate layers in a laminate. One such suitable subslrale is a
BOUNTY~9 sheet having a thickness of from about 0.02 mm to about 1.2
mm, more preferably from about 0.3 mm to about 0.8 mm, and a basis
weight of from about 5 gm/m2 to about 100 gm/m2, more preferably from
35 about 10 gm/m2 to about 60 gm/m2, and more preferably from about 15
CA 0226278~ 1999-02-08
W O 98/06364 PCTrUS97/12854
31
gmlm2 to about 40 gm/m2. Another suitable substrate is a cellulose foam
having a dry compressed thickness of from about 0.5 mm to about 3.0
mm, more preferably from about 0.8 mm to about 2.0 mm, a wet
expanded thickness of from about 0.8 mm to about 6.0 mm, more
preferably from about 1.0 mm to about 5.0 mm, and a basis weight of from
about 50 gm/m2 to about 2,000 gm/m2, more preferably from about 100
gm/m2 to about 1,000 gm/m2.
Substrates suitable for supporting the macrosl~ ucture typically have
a dry tensile strength of from about 500 gm/in to about 8,000 gm/in, more
o prererably from about 1,000 gm/in to about 3,000 gm/in, a wet tensile
sl,er,yll, of from about 200 gm/in to about 5,000 gm/in, though more
preferably from about 400 gm/in to about 1,000 gm/in, and a wet burst
nylll of from about 100 gm to about 2,000 gm, though more preferably
from about 200 gm to about 1,000 gm. Preferred suL,sl,dles of this type
include cellulosic fibrous webs such as paper towels and tissues such
those disclosed in U.S. Patent 3,953,638, issued April 27, 1976, U.S.
Patent 4,469,735, issued Sept. 4, 1984, U.S. Patent 4,468,428, issued
Aug. 28, 1984, and U.S. Patent 4,986,882, issued Jan. 22, 1991. Another
preferred substrate layer of this type is a cellulosic foam since it provides
a higher fluid wicking rate over a longer wicking distance than a cellulosic
fibrous web. P,~fer~bly, the cellulosic foam is in a compressed state so
as to further improve its fluid wicking and distribution properties. Suitable
cellulose foams can be made of regenerated rayon fibers by well-known
methods, such as those disclose~l in European patent application 293,208
(Uchida et al), published November 30,1988.
The porous absorbent macrostructure can be alldched to the
su~sb.,te by a variety of chemical, physical, and adhesive agents.
Adhesive agents for allachi"g the subsl,dte to the ",acrosl,ucture include
glues and hot melt adhesives. r,ere,dbly, the bonding between the
subslldte and macrostructure is achieved by depositing the precursor
absorbent particles on the subsl,dle, treating the deposited particles with
the solution comprising a crosslinking agent and then curing the treated
particles/subsl,dle as previously discussed. In a preferred embodiment of
this ",etl,od, a cellulosic substrate (e.g., paper towel) is used. The
precursor absorbent particles are then deposited on this cellulosic
CA 0226278~ l999-02-08
W O 98/06364 PCTrUS97/12854
32
substrate. A treatment solution comprising an amino-epichlorohydrin
adduct, preferably polymeric epichlorohydrin-polyamide/polyamine wet
strength resin such KYMENE, is then applied (e.g., sprayed) on the
cellulosic substrate and the absorbent. The treated sul,~ te/particles are
then cured at ambient temperatures such that a porous macrostructure is
formed that is bonded to the cellulosic substrate.
G. Treating Macrostructure With Latex to Im~rove Flexibility
Optionally, the above absorbent macrostructures (with or without
the optional subsl,dte) can be treated with certain l~texes. "Latex" refers
to an aqueous dispersion or emulsion of polymer particles in an aqueous
phase, and can also be referred to as an emulsion polymer. "Sinter"
refers to the fusion mechanism which occurs upon the drying of a
suspended liquid emulsion or dispersion such as a latex; the use of
"sinter" is synonymous with the phrase "film rur"~ing." Treatment of these
s ",acrûs~, uctures with these latexes has been found to dramatically
increase the flexibility of the macrosl,ucture, especially when in the form
of a sheet and even when attached to a substrate such as a paper towel.
In additiol) to improved flexibility, latex treatment according to the present
invention improves the bonding between particles of the aggregates that
comprise these macrostructures. This leads to improve",ents in the dry
and wet integrity of the macrostructure. The presence of the latex also
allows these ~"acrusll-lctures to be thermally bonded to nonwovens, such
as the backsheet of an absorbent article (e.g., a diaper).
It has been found that latexes suitable for use in the present
invention need to have certain properties. One key property of these
latexes is that they be "rubbery" at ambient temperatures or below after
they have been sintered. In other words, latexes useful in the present
invention have a glass transition temperature (Tg) of about 25~ or less.
Preferably, these latexes have a Tg of about 10~C or lower and, more
prefefably of about -10~C or lower. Another important property of these
latexes is the temperature at which they are capable of being sintered.
I ~texes useful in the present invention need to be sinterable at a,-,bier)l
temperatures or below. In other words, it is preferable for these latexes to
be sinterable at a temperature of about 25~C or lower. The ability of the
latex to be sinterable at ambient temperatures is important in avoiding
CA 0226278~ 1999-02-08
WO 98/06364 PCT/US97/12854
33
drying out the macrostructure. Another important property of these latexes
is their hydrophilicity. To be useful in the present invention, the latex,
when sintered needs to be at least somewhat hydrophilic. "Hydrophilic"
describes a material, or surface of a material, that is wettable by aqueous
s fluids (e.g., aqueous body fluids) deposited on these materials.
Hydrophilicity and wettability are typically defined in terms of contact angle
and the surface tension of the fluids and solids involved. This is
discusserl in detail in the American Chemical Society public~tion entitled
CONTACT ANGLE, WETTABILITY AND ADHESION, edited by Robert F. Gould
o (Copyright 1964).
Latexes useful in the present invention are typically prepared by
emulsion polymerization of certain olefinic (ethylenically unsaturated)
monomers. This emulsion polymerization can be carried out by
customary methods using any of a variety anionic, nonionic, caliGnic,
s zwitterionic and/or amphoteric emulsifiers to stabilize the resultant latex,
including alkyl sulfates, alkylarylalkoxy sulfates, alkylarylsulfonates and
alkali metal and/or a""nonium salts of alkyl- and alkylaryl-polyglycol ether-
sulfates; oxyethylated fatty alcohols or oxyethylated alkylphenols, as well
as block copolymers of ethylene oxide and propylene oxide; cationic
adducts of primary, secondary or tertiary fatty amines or fatty amine
oxyethylates with organic or inorganic acids, and quaternary
alkylammonium su,racla~ ; and alkylamidopropylbetaines. The olefinic
monomer can be a single type of monomer or can be mixture of different
olefinic monon,er~, i. e. to form copolymer particles dispersed or
emulsified in the aqueous phase. The latex suitable for use herein is
preferably neutral or has no ionic charge, vis-a-vis, the latex is not cationic
or anionic in nature.
Suitable l~teYes can be prepared via emulsion polymerization from
olefinic monomers that include the C2 to C4 alkyl and hydroxy alkyl
acrylates, such as those selected from the group of propyl acrylate, n-
butyl acrylate, isobutyl acrylate, 2-hydroxyethyl acrylate, 2-hydroxypropyl
acrylate, ethyl acrylate and mixtures thereof. Also suitable are C1 to C4
alkyl or hydroxy alkyl methacrylates selected from the group of propyl
methacrylate, n-butyl methacrylate, isobutyl methacrylate, 2-hydroxyethyl
methacrylate, 2-hydroxypropyl methacrylate, ethyl methacrylate, methyl
CA 0226278~ 1999-02-08
WO 98/06364 PCTAUS97112854 34 - -
methacrylate, vinyl acetate and mixtures thereof. Also suitable are
mixtures of the aforementioned C2 to C4 alkyl and hydroxy alkyl acrylates
and C1 to C4 alkyl or hydroxy alkyl methacrylates. Especially suitable for
use in the invention is an emulsion of polymethyl methacrylate.
s Particularly preferred latexes include those sold under the tradename
MONWINYL 963 by Hoechst Celanese and RHOPLEX E1845 by Rohm &
Haas.
In preparing the porous absorbent macrostructures of the present
invention having improved flexibility, the macrostructure is treated with an
o effective amount of these latexes to coat at least some of the absorbent
particles. What constitutes a "effective amount" will depend on a variety
of factors, including the particular porous absorbent mac, o~l, ucture
involved, the particular latex used, the flexibility benefits desired, and like
factors. More prererably, treating the macrostructure with about 2% by
weight latex will be sufficient to impart noticeable improvements in the
flexibility of the macrostructure. However, the macrostructure can be
effectively treated with from about 1% to about 10% by weight, and more
preferably from about 2% to about 5% by weight of latex.
The porous absorbent ",acros~,ucture can be treated with the latex
by any of the variety of methods suitable for applying additives to
conventional subsl,;dles. Suitable methods includes spraying, printing
(e.g., flexog,dphic printing), coating, e.g., gravure coating, dipping,
brushing, foaming or combinations of such application techniques.
Typically, the latex is sprayed onto the already forrned porous absorbent
,nacrosl,.lcture and then sintered at ambient temperature, e.g., at about
25~C or lower. Additionally, latex treatment can assist in the forming of a
more stable macrostructure by providing improved particle immobilization.
~ esidçs spraying on the latex on the already formed,
macrostructure, other methods can also be used to treat the porous
absorbent macrostructure with the latex. One such method involves
blending the latex with the untreated precursor absorbent particles and
then treating this latex/particle blend with the solution containing the
crosslinking agent plus any other optional components such as glycerol.
This treated latexJparticle blend can then be cured at ambient
r
CA 02262785 1999-02-08
W O 98/06364 PCTrUS97/128S4
temperature, e.g., at about 25~C or lower, to provide porous absorbent
macrostructure having improved flexibility.
Another method involves casting the latex as a thin film. The
precursor absorbent particle can then be deposited onto this cast film.
The cast film with the deposited particles is then lrealed (such as by
spraying) with a solution containing crosslinking agent and any other
optional components. This treated particle/latex film can then be cured at
ambient temperature, e.g., at about 25~C or lower, to provide porous
absorbent macrostructure having improved flexibility. In addition, the
o sintered latex film can function as a supporting subsl,~te for the
macrostructure to provide dry and especially wet integrity.
Yet another method involves pressurizing latex in a container such
that it can be blown or sprayed onto the precursor particles in the form of
a foam, after which a compression roll or the like is used to spread the
latex evenly. The blown or sprayed foam latex is, to some extent, in the
form of porous fibers which are extremely porous and further enhance the
absorbency of the macrostructure. A latex l,~ated ,-,acrosl,ucture of this
type, in which the precursor pa,li.,les swell into the porous latex fibers,
has improved structural integrity.
20 111. Uses of Macrostructures
The porous, absorbent macrostructures of the present invention
can be used for many purposes in many fields of use. For ex~n,ple, the
macrostructures can be used for packing containers; drug delivery
devices; wound cleaning devices; burn treatment devices; ion exchange
25 column r~ater;~ls; construction materials; agricultural or horticultural
materials such as seed sheets or water-retentive materials; and industrial
uses such as sludge or oil dcwatering agents, materials for the prevention
of dew formation, desiccants, and humidity control materials.
Because of the unique absorbent properties of the porous,
absorbent macrostructures of the present invention, they are especia"y
suitable for use as absorbent cores in absorbent articles, especially
disposable absorbent articles. "Absorbent article" refers to articles which
absorb and contain body exudates and more specifically refers to articles
which are placed against or in proximity to the body of the wearer to
absorb and contain the various exudates discharged from the body.
CA 0226278~ 1999-02-08
W O 98/06364 PCT~US97/12854
36
Additionally, "disposablei' absorbent articles are those which are intended
to be discarded after a single use (i.e., the original absorbent article in its
whole is not intended to be laundered or otherwise restored or reused as
an absorbent article, although certain materials or all of the absorbent
article may be recycled, reused, or composted). A preferred embodiment
of a disposable absorbent article, diaper 20, is shown in Figure 3.
"Diaper" refers to a garment generally worn by infants and incontinent
persons that is worn about the lower torso of the wearer. It should be
understood, however, that the present invention is also a~,plicable to other
o absorbent allicles such as incontinent briefs, incontinent pads, training
pants, diaper inserts, sanitar,v napkins, facial tissues, paper towels, and
the like.
Figure 3 is a perspective view of the diaper 20 of the present
invention in its uncontracted state (i.e., with all the elastic induced
15 contraction removed) with portions of the structure being cut-away to
more clearly show the construction of the diaper 20 and with the portion of
the diaper 20 which contacts the wearer facing the viewer. The diaper 20
is shown in Figure 3 to preferably comprise a liquid pervious topsheet 38;
a liquid impervious backsheet 40 joined with the topsheet 38; an
20 absorbent core 42 positioned between the topsheet 38 and the backsheet
40; elastic members 44; and tape tab fasteners 46. While the topsheet
38, the backsheet 40, the absorbent core 42, and the elastic members 44
can be assembled in a variety of well known configurations, a preferred
diaper configuration is described generally in U.S. Patent 3,860,003
25 (Buell), issued January 14, 1975. Alternatively preferred configurations
for disposable diapers herein are also disclosed in U.S. Patent 4,808,178
(Aziz et al), issued February 28, 1989; U.S. Patent 4,695,278 (Lawson),
issued September 22, 1987; and U.S. Patent 4,816,025 (Foreman),
issued March 28, 1989.
Figure 3 shows a preferred embodiment of the diaper 20 in which
the topsheet 38 and the backsheet 40 are co-extensive and have length
and width dimensions generally larger than those of the absorbent core
42. The topsheet 38 is joined with and superimposed on the backsheet
40 thereby forming the periphery of the diaper 20. The periphery derines
CA 0226278~ 1999-02-08
W O 98/06364 PCTAUS97/12854
37
the outer perimeter or the edges of the diaper 20. The periphery
comprises the end edges 32 and the longitudinal edges 30.
The topsheet 38 is compliant, soft feeling, and non-ir,itdling to the
wearer's skin. Further, the topsheet 38 is liquid pervious pe""illi"g liquids
s to readily pe"et~le through its thickness. A suitable topsheet 38 can be
manufactured from a wide range of materials such as porous foams,
retic~ ted foams, apertured plastic films, natural fibers (e.g., wood or
cotton fibers), sy"ll,etic fibers (e.g., polyester or polypropylene fibers) or
from a combination of natural and synthetic fibers. Preferably, the
o topsheet 38 is made of a hydrophobic material to isolate the wearer's skin
from liquids in the absorbent core 42.
A particularly preferred topsheet 38 comprises staple length
polypropylene fibers having a denier of about 1.5, such as ~lercules type
151 polypropylene marketed by Hercules. Inc. of ~llmington, Delaware.
"Staple length fibers" refers to those fibers having a length of at least
about 15.9 mm (0.62 inches).
There are a number of manufacturing techniques which can be
used to manufacture the topsheet 38. For exan,pl2, the topsheet 38 can
be woven, nonwoven, spunbonded, carded, or the like. A preferred
topsheet is carded, and thermally bonded by means well known to those
skilled in the fabrics art. Preferably, the topsheet 38 has a weight from
about 18 to about 25 grams per square meter, a minimum dry tensile
strength of at least about 400 grams per cer,li",eler in the machine
direction, and a wet tensile sl,e"yll~ of at least about 55 grams per
cenli"~eter in the cross-",achi"e dire~;tiG,~.
The backsheet 40 is impervious to liquids and is preferably
manufactured from a thin plastic film, although other flexible liquid
impervious materials may also be used. The backsheet 40 prevents the
exudates absorbed and contained in the absorbent core 42 from wetting
articles which contact the diaper 20 such as bedsheets and
undergarments. Preferably, the backsheet 40 is polyethylene film having
a thickness from about 0.012 mm (0.5 mil) to about 0.051 cenli",eters (2.0
mils), although other flexible, liquid impervious materials can be used.
"Flexible" refers to materials which are compliant and which will readily
conform to the general shape and contours of the wearer's body.
CA 0226278~ 1999-02-08
W O 98/06364 rCTAUS97/12854
38
A suitable polyethylene film is manufactured by Monsanto
Chemical Corporation and marketed in the trade as Film No. 8020. The
backsheet 40 is preferably embossed and/or matte finished to provide a
more clothlike appearance. Further the backsheet 40 may permit vapors
s to escape from the absorbent core 42 while still preventing exudates from
passing through the backsheet 40.
The size of the backsheet 40 is dictated by the size of the
absorbent core 42 and the exact diaper design selected. In a preferred
embodiment the backsheet 40 has a modified hourglass-shape extending
o beyond the absorbent core 42 a minimum distance of at least about 1.3
cenli",eters to about 2.5 centimeters (about 0.5 to about 1.0 inch) around
the entire diaper periphery.
The topsheet 38 and the backsheet 40 are joined together in any
suitable manner. "Joined" enco".p~sses configurations whereby the
15 topsheet 38 is directly joined to the backsheet 40 by arfi~iny the topsheet
38 directly to the backsheet 40 and config~"aLiol,s whereby the topsheet
38 is indirectly joined to the backsheet 40 by arfi~in5~ the topsheet 38 to
intermediate members which in turn are affixed to the backsheet 40. In a
preferred embodiment the topsheet 38 and the backsheet 40 are affixed
20 directly to each other in the diaper periphery by attachment means (not
shown) such as an adhesive or any other attachment means as known in
the art. For example a uniform continuous layer of adhesive a patterned
layer of adhesive or an array of separate lines or spots of adhesive can
be used to affix the topsheet 38 to the backsheet 40.
Tape tab fasteners 46 are typically applied to the back waistband
region of the diaper 20 to provide a fastening means for holding the diaper
on the wearer. The tape tab fasteners 46 can be any of those well known
in the art such as the fastening tape disclosed in U.S. Patent 3 848 ~94
(Buell) issued November 19 1974. These tape tab fasteners 46 or other
30 diaper fastening means are typically applied near the corners of the diaper
20.
The elastic ",eri,bers 44 are disposed adjacent the periphery of the
diaper 20 preferably along each longitudinal edge 30 so that the elastic
members 44 tend to draw and hold the diaper 20 against the legs of the
3s wearer. Alternatively the elastic members 44 can be disposed adjacent
CA 0226278~ 1999-02-08
W O ~8~ 6~ PCT~US97/12854
39
either or both of the end edges 32 of the diaper 20 to provide a waistband
as well as or rather than leg cuffs. For example, a suitable waistband is
disctosed in U.S. Patent 4,515,595 (Kievit et al), issued May 7, 1985. In
addition, a method and apparatus suitable for manufacturing a disposable
diaper having elastically contractible elastic members is described in U.S.
Patent 4,081,301 (Buell), issued March 28, 1978.
The elastic ",e"~bers 44 are secured to the diaper 20 in an
el~stic-~ly conlld~;ti61e condition so that in a normally unrestrained
configuration, the elastic members 44 effectively contract or gather the
o diaper 20. The elastic members 44 can be secured in an elastically
co"llactil.le condilion in at least two ways. For example, the elastic
members 44 can be sl,etched and secured while the diaper 20 is in an
uncontracted condition. Alternatively, the diaper 20 can be contracted, for
example, by ple~ling, and the elastic members 44 secured and connected
S to the diaper 20 while the elastic members 44 are in their unrelaxed or
unsl,etched condition.
In the embodiment illusl,ated in Figure 3, the elastic members 44
extend along a portion of the length of the diaper 20. Alternatively, the
elastic members 44 can extend the entire length of the diaper 20, or any
other length suitable to provide an el~stic~lly contl~ctible line. The length
of the elastic members 44 is dictated by the diaper design.
The elastic members 44 can be in a multitude of configurations.
For example, the width of the elastic members 44 can be varied from
about 0.25 millimeters (0.01 inches) to about 25 millimeters (1.0 inch) or
more; the elastic members 44 can comprise a single strand of elastic
"~aterial or can comprise several parallel or non-parallel strands of elastic
"~at~rial; or the elastic members 44 can be rectangular or curvilinear. Still
further, the elastic members 44 can be affixed to the diaper in any of
several ways which are known in the art. For example, the elastic
members 44 can be ultrasonically bonded, heat and pressure sealed into
the diaper 20 using a variety of bonding patterns or the elastic members
44 can simply be glued to the diaper 20.
The absorbent core 42 of the diaper 20 is positioned between the
topsheet 38 and the backsheet 40. The absorbent core 42 can be
manufactured in a wide variety of sizes and shapes (e.g., rectangular,
. . .
CA 0226278~ 1999-02-08
W O 98/06364 PCTrUS97/12854
hourglass, asy",mel,ical, etc.) and from a wide variety of materials. The
total absorbent capacity of the absorbent core 42 should, however, be
compatible with the design liquid loading for the intended use of the
absorbent article or diaper. Further, the size and absorbent capacity of
the absorbent core 42 can vary to acco",modate wearers ranging from
infants through adults. The absorbent core 42 comprises the porous,
absorbent macrostructures of the present invention.
A preferled embodiment of the diaper 20 has a rectangular-shaped
absorbent core 42. As shown in Figure 4, the absorbent core 42
o preferably comprises an absorbent member 48 comprising an envelope
web 50 and a porous, absorbent rnacrostructure 52 disposed in the
envelope web 50. The rrlaclosllucture 52 is encased in the envelope web
50 to minimize the potential for the precursor particles to migrate through
the topsheet and to provide an additional liquid transport layer between
s the topsheet 38 and the macrostructure 52 to enhance liquid acquisition
and minimize rewet. As shown in Figure 4, a single envelope web 50 is
wrapped about the macrostructure 52 by folding to form a first layer 54
and a second layer 56. The edges 58 of the envelope web 50 are sealed
about its periphery by any conventional means such as an adhesive 59
(as shown), ultrasonic bonds, or heaVpressure bonds, to forrn a pouch.
The envelope web 50 can comprise a number of "~al~rials including
nonwoven webs, paper webs, or webs of absorbent materials such as
tissue paper. The envelope web 50 preferably comprises a nonwoven
web similar to the webs used to form the topsheet 38. The nonwoven
web is preferably hydrophilic to allow liquids to rapidly pass through the
envelope web 50. Similar layered absorbent members (laminates) are
more fully described in U.S. Patent 4,578,068 (Kramer et al), issued
March 25, 1986.
Alternatively, the absorbent cores 42 of the present invention can
consist solely of one or more (a plurality of the) porous, absorbent
",acrosl~uctures of the present invention; can comprise a combination of
layers including the macrostructures of the present invention; or any other
absorbent core configurations including one or more of the
macrostructures of the present invention.
CA 0226278~ 1999-02-08
W 098/06364 PCT~US97112854 41
Figure 5 shows an alternative embodiment of the diaper 120
comprising a dual-layer absorbent core 142 comprising a modified
hourglass-shaped absorbent member 60 and a sheet 62 of the porous,
absorbent macrostructure positioned subjacent the absorbent member 60
s (i.e., between the absorbent member 60 and the backsheet 40).
The absorbent member 60 serves to quickly collect and temporarily
hold discharged liquids and to transport such liquids by wicking from the
point of initial contact to other parts of the absorbent member 60 and to
the macrost,.lcture sheet 62. The absorbent member 60 preferably
o comprises a web or batt of fiber n)aterials. Various types of fiber material
can be used in the absorbent member 60 such as the fiber materials
previously discussed herein. Cellulosic fibers are generally preferred for
use herein, wood pulp fibers being especially preferred. The absorbent
member 60 can also contain specific amounts of a particulate, absorbent,
polymeric composition. The absorbent member 60, for example, can
cGn~din up to about 50% by its weight of the polymeric composition. In the
more preferred embodiments, the absorbent member 60 contains from
0% to about 8% by its weight of a particulate, absorbent, polymeric
composition. In alternatively preferred embodiments, the absorbent
member 60 co"~prises chemically stiffened cellulosic fibers as previously
discussed herein. Exemplary embodiments of the absorbent member 60
useful in the present invention are described in U.S. Patent 4,673,402
(Wei~,nan et al), issued June 16, 1987; and U.S. Patent 4,834,735
(Alemany et al), issued May 30, 1989. Absorbent members having a
storage zone and an acquisition zone having a lower average density and
a lower average basis weight per unit area than the storage zone so that
the acquisition zone can effectively and erriciantly rapidly acquire
discharged liquid are especially preferred for use herein.
The absorbent member 60 can be of any desired shape, for
example, rectangular, oval, oblong, asy"~mal, ic or hourglass-shaped.
The shape of the absorbent member 60 can define the general shape of
the resulting diaper 120. In the preferred embodiments as shown in
Figure 5, the absorbent member 60 is hourglass-shaped.
The macrostructure sheet 62 of the present invention need not be
the same size as the absorbent member 60 and can, in fact, have a top
, . . ... . .
CA 0226278~ 1999-02-08
W O 98/06364 PCT~US97/12854
42
surface wh,ch is substantially smaller or larger than the top surface area
of the absorbent member 60. As shown in Figure 5 the ",aclo~t,ucture
sheet 62 is smaller than the absorbent member 60 and has a top surface
area from about 0.10 to about 1.0 times that of the absorbent member 60.
More preferably the top surface area of the macrostructure sheet 62 wili
be only from about 0.10 to about 0.75, and more p~efer~bly from about
0.10 to about 0.5 times that of the absorbent ",e"lL,er 60. In an
alternative embodiment the absorbent member 60 is smaller than the
macrostructure sheet 62 and has a top surface area from about 0.25 to
o about 1.0 times more preferably from about 0.3 to about 0.95 times that
of the macrostructure sheet 62. In this alternative embodiment the
absorbent member 60 preferably comprises chemically stiffened cellulosic
fibers as previously described.
The macrostructure sheet 62 is preferably placed in a specific
positional reldliG"ship with respect to the backsheet 40 andlor the
absorbent member 60 in the diaper. More particularly the ",acrosl,ucture
sheet 62 is positioned generally toward the front of the diaper so that the
",acrosl,ucture sheet 62 is more effectively located to acquire and hold
discharged liquids.
In alternatively preferred embodi",e"ls a plurality of
macrostructures preferably from two to six macrostructure strips or
sheets can be substituted for the single macrostructure sheet 62 shown
in Figure 5. Further additional absorbent layers members or structures
can be placed into the absorbent core 142. For example an additional
absorbent member can be positioned between the macrostructure sheet
62 and the backsheet 40 to provide reserve capacity for the absorbent
core 142 and/or a layer to distribute liquids passing through the
macrost,ucture sheet 62 to other portions of the absG,L,e"t core 142 or to
the macrostructure sheet 62. The macrostructure sheet 62 can also
alternatively be positioned over the absorbent member 60 so as to be
positioned between the topsheet 38 and the absorbent member 60.
Figure 6 shows an alternative embodiment of a diaper 220
comprising an alternative dual-layer absorbent core 242 comprising a
rectangular shaped absorbent member 260 and three elongated parallel
CA 0226278~ 1999-02-08
W098/06364 PCT~S97/12854
43
spaced macrostructure strips 262 positioned between absorbent member
260 and backsheet 40.
The absorbent member 260 serves to quickly collect and
temporarily hold discharged liquids and to transport such liquids by
5 wicking from the point of initial contact to other parts of the absorbent
member 260 and to macrostructure strips 262. This absorbent member
260 preferably comprises a web or bat of fiber ~ateriàls, more preferably
chemically stiffened cellulosic fibers as previously discussed herein.
Macrostructure strips 262 together act to acquire and hold the discharged
o liquids. By spacing macrostructure strips 262 from one another, a more
effective surface area is presented for acquiring and holding the discharge
liquids. This is particularly true since the spaced macrostructure strips
262 can swell and expand in the direction of their width, without interfering
with the ability of adjacent strips to acquire discharged liquids.
In use, the diaper 20 iS applied to a wearer by positioning the back
waistband region under the wearer's back, and drawing the reminder of
the diaper 20 between the wearer's legs so that the front waistband region
is positioned across the front of the wearer. The tape-tab fasteners 46 are
then secured pr~rably to outwardly facing areas of the diaper 20. In
20 use, disposable diapers or other absorbent articles incorporating the
porous, absorbent macrostructures of the present invention tend to more
quickly and efficiently distribute and store liquids and to remain dry due to
the high absorbent car~city of the ")acrosl,uctures. Disposable diapers
incorporating the ,,,aorusl,uctures of the present invention can also be
thinner and more flexible.
The absorbent macrostructure of the present invention may also be
used in an absorbent article such as that set forth in U.S. Patent
Application Ser. No. 08/621,030, filed March 22, 1996. Specifically, an
absorbent article coi"prising a fluid pervious topsheet, a bac~sheet and
an absorbent core positioned between the topsheet and the backsheet.
The absorbent core comprises at least one upper fluid storage component
capable of expanding in the z-direction when contacted with aqueous
body fluids to form a fluid acquisition zone, the upper fluid storage
component being in direct fluid communication with the topsheet; the
upper fluid storage component further comprising the absorbent
.. . . .
CA 0226278~ 1999-02-08
WO 98/06364 PCT/US97/12854
44
macrostructure of the present invention. The absorbent core further
comprises a fluid acquisition zone capable of receiving aqueous body
fluids, the fluid acquisition zone being at least partially surrounded by the
upper fluid storage component and positioned at least partially beneath
the fluid discharge region of the absorbent core. The absorbent core
further Co"",lisii)g a fluid acquisition/diallibution component capable of
acquiring and transporting aqueous body fluids, at least a portion of this
fluid acquisition/distribution component being positioned underneath and
in fluid communication with the upper fluid storage component, and at
o least a portion of the fluid acquisition/distribution component being
positioned underneath the fluid acquisition zone.
IV. Absorbent Article Performance
With respect to absorbent articles comprising the absorbent
macrostructures of the present invention, Applicants have discovered the
ability to overcome the inverse relationship between fluid loading level (9
or ml of fluid) and fluid acquisition rate (ml/sec) which has been generally
accepted as the norm for absorbent articles. Thus, in one aspect, the
present invention relates to an absorbent article that, in addition to being
relatively thin, exhibits the ability to maintain the rate of fluid acquisition for
each of two successive fluid loads. In another aspect, the invention
relates to an absorbent article that exhibits the ability to increase the rate
of fluid acquisition for each of two successive fluid loads. Preferably, the
articles will exhibit maintained or increased fluid acquisition rates for each
of three successive fluid loads, more pr~ferably for each of four
successive fluid loads.
The ability of an absorbent article to meet this criteria is measured
as described in detail in the Test Method section, below, using an
Acquisition Test. Briefly, fluid acquisition rates for a given absorbent
article are deterrnined for each of four successive loads of 50 ml per load,
each load being delivered at a constant rate of about 10 ml/sec) of
synthetic urine, with a 5 minute equilibration period between each load.
The articles of the present invention exhibit maintained or increased fluid
acquisition rates as the number of loads increases.
With respect to those articles that exhibit maintained fluid
acquisition rates for at least two successive loads, it is required that these
.
CA 0226278~ 1999-02-08
W O 98/06364 PCTrUS97/12854
articles (including topsheet, backsheet and absorbent core; but excluding
tape, leg cuffs, or other optional components) have a thickness, in the dry
state, of no more than about 0.5 inches, preferably no more than about
- 0.25 inches, more preferably no more than about 0.2 inches. Thickness
of the articles is measured with no pressure applied. While not a
requirement, with regard to those articles of the present invention that
exhibit increased fluid acquisition rates for at least two, three or four
successive loads, it is preferred that these absorbent articles also satisfy
the above thickness requirements.
o In addition to exhibiting maintained/increased acquisition rates for
successive fluid loads, preferred absorbent articles acquire fluid at a rate
of at least about 2 ml/sec for the first 50 ml load, more preferably at least
about 5 ml/sec. Though this is not a- criteria that must be satisfied by
these articles, meeting this criteria will more likely result in an article that is
15 useful for the intended purpose.
V. Test Methods
A. Absorptive Capacity
The Absorptive C~p~city of the absorbent particles is dete""ined
according to the Absorptive Capacity test described at Columns 27-28 of
20 U.S. Patent 5,124,188 (Roe et al), issued June 23, 1992. In this test, the
absorbent pa,~i~'es are placed within a "tea bag", immersed in an excess
of Synthetic Urine for a specified period of time, and then centrifuged for a
specific period of time. The ratio of absorbent particles final weight after
centrifuging minus initial weight (net fluid gain) to initial weight determines
25 the Absorptive Capacity.
B. Saline Flow Conductivity (SFC)
The Saline Flow Conductivity (SFC) of the precursor absorbent
pa,licles is determined according to the test procedure described in
copending U.S. application Serial No. 219,574 (Goldman et al), filed
30 March 29, 1994. In this test, a gel layer is formed from absorbent
particles swollen in Jayco synthetic urine under a confining pressure. This
test ~ssesses the ability of the hydrogel layer formed from these
absorbent particles to acquire and distribute body fluids when the pa, licles
are present at high conce"l~aliol)s and exposed to usage mechanical
35 pressures. Darcy's law and steady-state flow methods are used for
CA 0226278~ 1999-02-08
WO 98/06364 PCT/US97112854
46
determining saline flow conductivity. (See, for example, ''ABsoRsENcY,
ed. by P. K. Chatterjee, Elsevier, 1985, Pages 4243 and "CHEMICAL
ENGINEERING VOL. Il, Third Edition, J. M. Coulson and J. F. Richardson,
Pergamon Press,1978, Pages 125-127.) The test fluid for the SFC test is
Jayco synthetic urine.
C. Performance Under Pressure (PUP) Capacity
The Performance Under Pressure (PUP) Capacit~ of the precursor
absorbent particles is determined according to t~e test procedure
described in copending U.S. application Serial No. 219,574 (Goldman et
o al), filed March 29, 1994. This test determines the 60 minute gram/gram
absorption of synthetic urine for absorbent p~l licl~s that is iaterally
confined in a piston/cylinder assembly under a confining pressure of 0.7
psi (about 5 kPa). This test ~ssesses the ability of absorbent particle
layer to absorb body fluids, over a practical period of time, when the
particles are present at high basis weight and high concei,l,dlio"s and
exposed to usage pressures. These usage pressures include mechanical
pressures resulting from the weight and/or motions of the wearer,
mechanical pressures resulting from elastics and fd~le"i.~g systems, and
the hyd~ ldLic suction resulting from adjacent capillary (e.g., fibrous)
layers and/or structures as they are drained of fluid. The test fluid for the
PUP capacity test is Jayco synthetic urine. This fluid is absorbed by the
absorbent particles under demand absorption conditions at near-zero
hydrostatic pressure.
D. Demand Absorbencv CaPacitv
The Demand Absorbency Capacity of the absorbent
macrostructure is designed to measure the 60 minute gram/gram
absorption of synthetic urine for absorbent structures that are under a
pressure of 1.4 psi (about 10 kPa) at the beginning of the test (dry
state) and 0.7 psi (about 5 kPa) at the end of the test (wet, swollen
state). This tsst assesses the ability of an absorbent structure to
absorb body fluids, over a practical period of time, when the absorbent
structures are present at high basis weight and high concentrations
and exposed to usage pressures. These usage pressures include
mechanical pressures resulting from the weight and/or motions of the
wearer, mechanical pressures resulting from elastics and fastening
CA 0226278~ 1999-02-08
W O 98/06364 PCT~US97/12854
47
systems, and the hydrostatic suction resulting from adjacent capillary
(e.g., fibrous) layers and/or structures as they are drained of fluid. The
test fluid for the Demand Absorbency Capacity test is Jayco synthetic
urine. This fluid is absorbed by the absorbent structure under demand
absorption conditions.
The following describes the Demand Absorbency Capacity
measurement. The sample cell is a square shaped piston/cylinder
assembly, and has a mesh bottom of 1 Ocm x 1 Ocm in dimension.
Absorbent structures ~with and without fibers~ are sampled into a
o dimension of 3.7 cm by 3.7 cm, and placed under 1.4 psi piston. As
the sample is brought in contact with Jayco synthetic urine, it starts
to imbibe Jayco synthetic urine. The change of weight is monitored
and recorded continuously for the next 60 minutes. As a result, the
dimension of the sample is increased by about 100%, as placed under
15 a constant 1.4 psi external pressure.
E. Aquisition Test
This test simulates the introduction of urine into a diaper under
the following conditions:
1) A pressure of 0.4 psi ~about 28 g/cm2) is applied to a
20 diaper sample.
2) A total of 2 or more loadings of synthetic urine at a rate
of 10 ml/sec are applied to the diaper sample, with a 5 minute time
period (equilibration time) between each loading.
The following apparatus is employed:
25 Conditioned room: Temperature and humidity controlled within the
following limits:
Temperature: 73i2~F
Relative Humidity: 50+2%
AquisitionTester: Obtain from Concord-Renn Co., 6315 Warrick St., Cincinnati, Ohio, 45227, U.S.A.
Part
Test Bed (PLEXIGLAS)
Foam Base- 6" x 20" x 1" foam covered
withpolyethylene backsheet material -
foam type: Density 1.0 Ib/ft3
CA 0226278~ 1999-02-08
W O 98/06364 PCT~US97/12854
48
IDL 24 psi
Nozzle
Cover plate
Graduated cylinders: VWR Scientific, (100ml) Catalog number:
s (100 ml) (1,000 ml) 24711-310 (1,000 ml) Catalog number:
24711-364 or equivalent
Erlenmeyer flask: VWR Scientific Catalog number: 29135-307
or (6,000 ml) equivalent
Digital Pump: Cole-Parmer Instrument Co.; Tel. No. (800)
323-4340, U.S.A., Catalog number: G-
075323-20
Easy Load Pump Head: Cole-Parmer INsturment Co. Catalog number:
G-07518-02
Distilled water: convenient source
Dry Synthetic Urine: Jayco SynUrine
V. SPECIFIC ILLUSTRATIONS OF PREPARATION OF
MACROSTRUCTURES ACCORDING TO PRESENT INVENTION
The following examples further describe and delllGI~slldle the
preferred embodiments within the scope of the present invention. The
examples are given solely for the purpose of illusll~liG", and are not to be
construed as limitations of the present invention since many variations
thereof are possible without departing from its spirit and scope.
Example 1
This example shows how to manufacture an absorbent
macrostructure of the present invention.
The absorbent macrostructure is fabricated on a shuttle table by
first laying down superabsorbent polymer particulate on a cellulose
web, and subsequently spraying with an aqueous solution containing
KYMENE. The shuttle table is designed to move back and forth for
continuous laydown of superabsorbent polymer particulate, and has a
speed range of 3 meters/min to 30 meters/min. The shuttle table is
equipped with a fixed automatic powder feeder system for
superabsorbent polymer particulates with the range of flow rate
between 100 to 1000 grams per minute, and fixed automatic solution
spray systems of spray rates ranging between 40 and 200 grams per
T -
CA 0226278~ 1999-02-08
WO 98/06364 PCT/US97/12854
49 - .
minute. A typical operation condition includes a shuttle speed at 10
meter per minute, a feeding rate of the superabsorbent polymer
particulates at 212 grams per minute, and a solution spray rate at 76
grams per minute.
Two different superabsorbent polymer particulates are used in
this example. Both are crosslinked sodium polyacrylate. The first
superabsorbent polymer is supplied by Nippon Shokubai under the
trade name of AQUALIC CA-L76, and has a high PUP and medium gel
volume. The second crosslinked sodium polyacrylate is IM-1000
supplied by Sanyo Chemical Industry, and has a low PUP and high gel
volume. The cellulose web is a tissue towel available under the trade
name of BOUNTY, which has 20 gm per meter square of basis weight.
KYMENE is supplied by Hercules company under the trade name of
KYMENE-PLUS, which contains 30% of polymer resin. Blending of at
least two types of superabsorbent polymer particulate is carried out in
a food mixer. The spray solution contains 10% by weight of KYMENE
polymer resin, 45% of glycerol, and 45% of water. An additional
polyethylacrylate-based latex polymer, supplied by Hoechst Gosei Co.
under the trade name of MOWINYL-963, is included into the above
solution at a level of 10%. The presence of a polyethylacrylate-based
latex polymer is added to increase the softness and flexibility of the
macrostructure .
The resulting macrostructure contains 77% superabsorbent
polymer, 19% of solution and 4% cellulosic tissue towel. The total
2s basis weight of the macrostructure is 480 grams per square meter,
which is including 370 grams of superabsorbent polymer, 90 grams of
sprayed solution, and 20 grams of BOUNTY towel. The weight ratio
of the two types of superabsorbent polymers ranges from 0% to
100%.
ExamPie 2
This example shows how to manufacture an absorbent
macrostructure of the present invention.
The absorbent macrostructure is produced by following the
procedure set forth in Example 1, except the structure is made by
3s attaching the AQUALIC CA-L76 superabsorbent polymer on one side
CA 0226278~ 1999-02-08
WO 98/06364 PCTIUS97/12854
of the tissue web, and the IM-1000 superabsorbent polymer on the
other side of the web.
The resulting macrostructure contains 77% superabsorbent
polymer, 1g% of solution and 4% cellulosic tissue towel. The total
s basis weight of the macrostructure is 480 grams per square meter,
which is including 370 grams of superabsorbent polymer, 90 grams of
sprayed solution, and 20 grams of BOUNTY towel. The weight ratio
of the two types of superabsorbent polymers ranges from 0% to
100%.
o ExamRle 3
This example shows how to manufacture a baby diaper containing
an absorbent macrostructure of the present invention.
The absorbent macrostructure of Exdll,?la 1 is sampled into a
diaper core dimension, and applied with a fluid impermeable backsheet
film, wood fiber acquisition layer and a nonwoven topsheet, thereby
forming a baby diaper.
The resulting diaper has a very low caliper due to the high
concentration of superabsorbent polymer: 70% in weight as compared to
a typical baby diaper cores containing about 40% superabsorbent
polymers.
ExamPIe 5
This example shows the manufacture of a diaper comprising a
superabsorbent ",acrusl,ucture of the present invention.
The superabsorbent macrostructure is prepared following the
process of Example 1, except the concentration of superabsorbent
polymer is increased to 90% by increasing the basis weight of the
superabsorbent polymer to equal or greater than 1550 gram per square
meter. The macrost,ucture is then slitted and stretched to form a net
shaped core. The core is then applied with a fluid impermeable
backsheet film a wood fiber acquisition layer and a nonwoven topsheet to
make a baby diaper. The diaper has a very low caliper, as compared to
conventional baby diapers, due to the high concer,lldlio" of
superabsorbent polymer in the core.
CA 0226278~ 1999-02-08
WO 98/06364 PCT/US97/12854
51 .
EXamDIe 6
This example shows that employing a cationic amino-
epichlorohydrin adduct, such as KYMENE, in the absorbent
macrostructure of the present invention surprisingly enhances the
s Demand Absorbancy Capacity of the macrostructure.
Figure 7 illustrates a comparison of Demand Absorbency
Capacity of the absorbent macrostructure of the present invention, as
compared to three different samples. Specifically, curve "---o---"
illustrates the surprising effect of KYMENE to dramatically increase the
o under pressure demand absorbency capacity at the whole range of
mixing ratios of two different superabsorbent polymers. This is
compared to a similar structure prepared without using KYMENE
(Curve "---~---"), of which the 0.7 psi demand absorbency capacities
increase stepwise only at the increase of the weight percent of high
PUP superabsorbent particulates (L-76~. Curve "---~---" is also
identical to curve "~ ---", which is the test result of a layer of mixed
superabsorbent particulates without KYMENE or glue additives.
Another example of forming an absorbent structure without using
KYMENE is by first sandwiching a layer of superabsorbent polymer
particulates in between two ply of tissue, and gluing the tissue to form
a laminate composite. This laminate structure ~curve "---~---") does
not show an extraordinary relationship (as seen in curve "---o---")
between the under pressure demand absorbency capacity and the
mixing ratio of superabsorbent polymers.
All publications, patent applications, and issued patents mentioned
here,il)above are hereby incorporated in their entirety by reference.
It is understood that the examples and embodiments described
herein are for illustrative purposes only and that various modifications or
changes in light thereof will be suggested to one skilled in the art and are
to be included in the spirit and purview of this application and scope of the
appended claims.
.