Note: Descriptions are shown in the official language in which they were submitted.
CA 02263043 1999-OS-31
METHOD FOR REDUCING CARBON MONOIODE IN THE
CRACKING OF HYDROCARBON GAS STREAMS
The present invention generally relates to processes for the thermal
cracking of hydrocarbons and, specifically, to a method for providing a tube
of a
thermal cracking furnace having coke formation and carbon monoxide production
inhibiting properties when used for the thermal cracking of hydrocarbons.
In a process for producing an olefin compound, a fluid stream
containing a saturated hydrocarbon such as ethane, propane, butane, pentane,
naphtha,
or mixtures of two or more thereof is fed into a thermal (or pyrolytic)
cracking
furnace. A diluent fluid such as steam is usually combined with the
hydrocarbon feed
material being introduced into the cracking furnace.
Within the furnace, the saturated hydrocarbon is converted into an
olefinic compound. For example, an ethane stream introduced into the cracking
furnace is converted into ethylene and appreciable amounts of other
hydrocarbons. A
propane stream introduced into the furnace is converted to ethylene and
propylene,
and appreciable amounts of other hydrocarbons. Similarly, a mixture of
saturated
hydrocarbons containing ethane, propane, butane, pentane and naphtha is
converted to
CA 02263043 1999-03-08
33326CA
2
a mixture of olefinic compounds containing ethylene, propylene, butenes,
pentenes,
and naphthalene. Olefinic compounds are an important class of industrial
chemicals.
For example, ethylene is a monomer or comonomer for making polyethylene. Other
uses of olefinic compounds are well known to those skilled in the art.
As a result of the thermal cracking of a hydrocarbon, the cracked
product stream can also contain appreciable quantities of pyrolytic products
other
than the olefinic compounds including, for example, carbon monoxide. It is
undesirable to have an excessively high concentration of carbon monoxide in a
cracked product stream; because, it can cause the olefinic product to be "off
spec" due
to such concentration. Thus, it is desirable and important to maintain the
concentration of carbon monoxide in a cracked product stream as low as
possible.
Another problem encountered in thermal cracking operations is in the
formation and laydown of carbon or coke upon the tube and equipment surfaces
of a
thermal cracking furnace. This buildup of coke on the surfaces of the cracking
furnace tubes can result in an excessive pressure drop across such tubes
thereby
necessitating costly furnace shutdown in order to decoke or to remove the coke
buildup. Therefore, any reduction in the rate of coke formation and coke
buildup is
desirable in that it increases the run length of a cracking furnace between
decokings.
It is thus an object of this invention to provide an improved process for
cracking saturated hydrocarbons to produce olefinic end-products.
Another object of this invention is to provide a process for reducing
the formation of carbon monoxide and coke in a process for cracking saturated
hydrocarbons.
CA 02263043 1999-03-08
33326CA
3
A still further object of this invention is to improve the economic
efficiency of operating a cracking process for cracking saturated hydrocarbons
by
providing a method for treating the tubes of a cracking fizrnace so as to
provide
treated tubes having coke formation and carbon monoxide production inhibiting
S properties.
In accordance with one embodiment of the invention, a tube of a
thermal cracking furnace is treated with an antifoulant composition so as to
provide a
treated tube having properties which inhibit the formation of coke when
utilized in a
thermal cracking operation. The method for treating the thermal cracking tube
includes contacting under an atmosphere of a reducing gas, the tube with the
antifoulant composition which comprises a compound selected from the group
consisting of a tin compound, silicon compound, and combinations thereof.
Another embodiment of the invention includes a method for reducing a
concentration of carbon monoxide present in a cracked gas stream produced by
passing a hydrocarbon stream through a tube of a thermal cracking furnace.
This
method includes treating the tubes of the thermal cracking furnace by
contacting it
with a hydrogen gas containing a sulfur compound thereby providing a treated
tube
having properties which inhibit the production of carbon monoxide during the
thermal
cracking of hydrocarbons. The hydrocarbon stream is passed through the treated
tubes while maintaining the treated tubes under suitable cracking conditions
to
thereby produce a cracked gas stream having a reduced concentration of carbon
monoxide below the concentration of carbon monoxide that would be present in a
cracked gas stream produced by an untreated tube.
CA 02263043 1999-03-08
33326CA
4
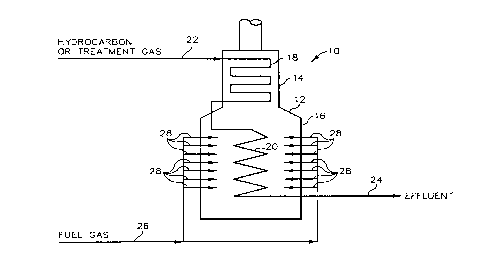
In the accompanying drawing:
FIG. 1 provides a schematic representation of the cracking furnace
section of a pyrolytic cracking process system in which the tubes of such
system are
treated by the novel methods described herein.
FIG. 2 is a plot of the weight percent of carbon monoxide in a cracked
gas stream versus the time of on-line cracker operation for tubes treated in
accordance
with an inventive method described herein and for conventionally treated
tubes.
Other objects and advantages of the invention will be apparent from
the following detailed description of the invention and the appended claims
thereof.
The process of this invention involves the pyrolytic cracking of
hydrocarbons to produce desirable hydrocarbon end-products. A hydrocarbon
stream
is fed or charged to pyrolytic cracking furnace means wherein the hydrocarbon
stream
is subjected to a severe, high-temperature environment to produce cracked
gases. The
hydrocarbon stream can comprise any type of hydrocarbon that is suitable for
pyrolytic cracking to olefin compounds. Preferably, however, the hydrocarbon
stream
can comprise paraffin hydrocarbons selected from the group consisting of
ethane,
propane, butane, pentane, naphtha, and mixtures of any two or more thereof.
Naphtha
can generally be described as a complex hydrocarbon mixture having a boiling
range
of from about 180°F to about 400°F as determined by the standard
testing methods of
the American Society of Testing Materials (ASTM).
The cracking furnace means of the inventive method can be any
suitable thermal cracking furnace known in the art. The various cracking
furnaces are
well known to those skilled in the art of cracking technology and the choice
of a
CA 02263043 1999-03-08
33326CA
suitable cracking furnace for use in a cracking process is generally a matter
of
preference. Such cracking furnaces, however, are equipped with at least one
cracking
tube to which the hydrocarbon feedstock is charged or fed. The cracking tube
provides for and defines a cracking zone contained within the cracking
furnace. The
5 cracking furnace is utilized to release the heat energy required to provide
for the
necessary cracking temperature within the cracking zone in order to induce the
cracking reactions therein. Each cracking tube can have any geometry which
suitably
defines a volume in which cracking reactions can take place and, thus, will
have an
inside surface. The term "cracking temperature" as used herein is defined as
being the
temperature within the cracking zone defined by a cracking tube. The outside
wall
temperature of the cracking tube can, thus, be higher than the cracking
temperature
and possibly substantially higher due to heat transfer considerations. Typical
pressures within the cracking zone will generally be in the range of from
about 5 psig
to about 25 psig and, preferably from 10 psig to 20 psig.
As an optional feature of the invention, the hydrocarbon feed being
charged to pyrolytic cracking furnace means can be intimately mixed with a
diluent
prior to entering pyrolytic cracking furnace means. This diluent can serve
several
positive filnctions, one of which includes providing desirable reaction
conditions
within pyrolytic cracking furnace means for producing the desired reactant end-
products. The diluent does this by providing for a lower partial pressure of
hydrocarbon feed fluid thereby enhancing the cracking reactions necessary for
obtaining the desired olefin products while reducing the amount of undesirable
reaction products such as hydrogen and methane. Also, the lower partial
pressure
CA 02263043 1999-03-08
33326CA
6
resulting from the mixture of the diluent fluid helps in minimizing the amount
of coke
deposits that form on the furnace tubes. While any suitable diluent fluid that
provides
these benefits can be used, the preferred diluent fluid is stream.
The cracking reactions induced by pyrolytic cracking furnace means
can take place at any suitable temperature that will provide the necessary
cracking to
the desirable end-products or the desired feed conversion. The actual cracking
temperature utilized will depend upon the composition of the hydrocarbon feed
stream and the desired feed conversion. Generally, the cracking temperature
can
range upwardly to about 2000°F or greater depending upon the amount of
cracking or
conversion desired and the molecular weight of the feedstock being cracked.
Preferably, however, the cracking temperature will be in the range of from
about
1200°F to about 1900°F. Most preferably, the cracking
temperature can be in the
range from 1500°F to 1800°F.
A cracked gas stream or cracked hydrocarbons or cracked hydrocarbon
stream from pyrolytic cracking furnace means will generally be a mixture of
hydrocarbons in the gaseous phase. This mixture of gaseous hydrocarbons can
comprise not only the desirable olefin compounds, such as ethylene, propylene,
butylene, aad amylene; but, also, the cracked hydrocarbon stream can contain
undesirable contaminating components, which include carbon monoxide.
It is generally observed that at the beginning or start of the charging of
a feedstock to either a virgin cracking tube or a cracking tube that has
freshly been
regenerated by decoking, the concentration of undesirable carbon monoxide in
the
cracked hydrocarbon stream will be higher or reach a maximum concentration
peak,
CA 02263043 1999-03-08
33326CA
7
which will herein be referred to as peak concentration. Once the carbon
monoxide
concentration in the cracked hydrocarbon stream reaches its peak or maximum
concentration, over time it will gadually decrease in an almost asymptotic
fashion to
some reasonably uniform concentration. While the asymptotic concentration of
S carbon monoxide will often be sufficiently low to be within product
specifications;
often, the peak concentration will exceed specifications when there are no
special
efforts taken to prevent an excessive peak concentration of carbon monoxide.
In
untreated tubes, the peak concentration of carbon monoxide can exceed 9.0
weight
percent of the cracked hydrocarbon stream. Conventionally treated tubes
provide for
a peak concentration in the range from about 6 weight percent to about 8.5
weight
percent and an asymptotic concentration in the range of from 1 weight percent
to 2
weight percent.
The novel cracker tube treatment methods described herein provide for
a reduced cumulative production of carbon monoxide in the cracked hydrocarbon
stream during the use of such treated cracker tubes, and they provide for a
lower peak
concentration and asymptotic concentration of carbon monoxide. It has been
found
that the use of cracker tubes treated in accordance with the novel methods
described
herein can result in a reduced peak concentration of carbon monoxide in a
cracked
hydrocarbon stream below that of conventionally treated tubes with the peak
concentration being in the range of from about 3 weight percent to about 5
weight
percent. The asymptotic concentration of carbon monoxide in a cracked
hydrocarbon
stream from cracker tubes treated in accordance with the novel methods
described
herein also can be lower than that of conventionally treated tubes with such
CA 02263043 1999-03-08
33326CA
8
asymptotic concentration being less than 1 weight percent. In addition to
preventing
an off spec olefin product, another advantage from having a lower carbon
monoxide
production in the cracking of hydrocarbons is that the hydrocarbons are not
converted
to carbon monoxide, but they are converted to the more desirable olefin end-
products.
A critical aspect of the inventive method includes the treatment or
treating of the tubes of a cracking furnace by contacting the surfaces of such
tubes
with an antifoulant composition while under an atmosphere of a reducing gas
and
under suitable treatment conditions. It has been discovered that the coke
formation
inhibiting properties of a cracking tube are improved by treating such
cracking tube
with the antifoulant composition in a reducing gas atmosphere as opposed to
treatment without the presence of a reducing gas. Thus, the use of the
reducing gas is
an important aspect of the inventive method.
The reducing gas used in the inventive method can be any gas which
can suitably be used in combination with the antifoulant composition during
treatment
so as to provide an enhancement in the ability of the treated tube to inhibit
the
formation of coke and the production of carbon monoxide during cracking
operation.
The preferred reducing gas, however, is hydrogen.
The antifoulant composition used to treat the tubes of the cracking
furnace in the presence of a reducing gas such as hydrogen can be any suitable
compound that provides for a treated tube having the desirable ability to
inhibit the
rate of coke formation and carbon monoxide production as compared with an
untreated tube or a tube treated in accordance with other known methods. Such
CA 02263043 1999-03-08
33326CA
9
suitable antifoulant compositions can comprise compounds selected from the
group
consisting of tin compounds, silicon compounds and mixtures thereof.
Any suitable form of silicon can be utilized as a silicon compound of
the antifoulant composition. Elemental silicon, inorganic silicon compounds
and
organic silicon (organosilicon) compounds as well as mixtures of any two or
more
thereof are suitable sources of silicon. The term "silicon compound" generally
refers
to any one of these silicon sources.
Examples of some inorganic silicon compounds that can be used
include the halides, nitrides, hydrides, oxides and sulfides of silicon,
silicic acids and
alkali metal salts thereof. Of the inorganic silicon compounds, those which do
not
contain halogen are preferred.
Examples of organic silicon compounds that may be used include
compounds of the formula
I
R1- i i-R3
wherein R,, R2, R3, and R4 are selected independently from the group
consisting of
hydrogen, halogen, hydrocarbyl, and oxyhydrocarbyl and wherein the compound's
bonding may be either ionic or covalent. The hydrocarbyl and oxyhydrocarbyl
radicals can have from 1 to 20 carbon atoms which may be substituted with
halogen,
nitrogen, phosphorus, or sulfur. Exemplary hydrocarbyl radicals are alkyl,
alkenyl,
cycloalkyl, aryl, and combinations thereof, such as alkylaryl or
alkylcycloalkyl.
Exemplary oxyhydrocarbyl radicals are alkoxide, phenoxide, carboxylate,
CA 02263043 1999-03-08
33326CA
ketocarboxylate and diketone (dione). Suitable organic silicon compounds
include
trimethylsilane, tetramethylsilane, tetraethylsilane, triethylchlorosilane,
phenyltrimethylsilane, tetraphenylsilane, ethyltrimethoxysilane,
propyltriethoxysilane, dodecyltrihexoxysilane, vinyltriethyoxysilane,
5 tetramethoxyorthosilicate, tetraethoxyorthosilicate, polydimethylsiloxane,
polydiethylsiloxane, polydihexylsiloxane, polycyclohexylsiloxane,
polydiphenylsiloxane, polyphenylmethylsiloxane, 3-
chloropropyltrimethoxysilane,
and 3-aminopropyltriethoxysilane. At present hexamethyldisiloxane is
preferred.
Organic silicon compounds are particularly preferred because such
10 compounds are soluble in the feed material and in the diluents which are
preferred for
preparing pretreatment solutions as will be more fully described hereinafter.
Also,
organic silicon compounds appear to have less of a tendency towards adverse
effects
on the cracking process than do inorganic silicon compounds.
Any suitable form of tin can be utilized as the tin compound of the
antifoulant composition. Elemental tin, inorganic tin compounds and organic
tin
(organotin) compounds as well as mixtures of any two or more thereof are
suitable
sources of tin. The term "tin compound" generally refers to any one of these
tin
sources.
Examples of some inorganic tin compounds which can be used include
tin oxides such as stannous oxide and stannic oxide; tin sulfides such as
stannous
sulfide and stannic sulfide; tin sulfates such as stannous sulfate and stannic
sulfate;
stannic acids such as metastannic acid and thiostannic acid; tin halides such
as
stannous fluoride, stannous chloride, stannous bromide, stannous iodide,
stannic
CA 02263043 1999-03-08
33326CA
11
fluoride, stannic chloride, stannic bromide and stannic iodide; tin phosphates
such as
stannic phosphate; tin oxyhalides such as stannous oxychloride and stannic
oxychloride; and the like. Of the inorganic tin compounds those which do not
contain
halogen are preferred as the source of tin.
Examples of some organic tin compounds which can be used include
tin carboxylates such as stannous formate, stannous acetate, stannous
butyrate,
stannous octoate, stannous decanoate, stannous oxalate, stannous benzoate, and
stannous cyclohexanecarboxylate; tin thiocarboxylates such as stannous
thioacetate
and stannous dithioacetate; dihydrocarbyltin bis(hydrocarbyl
mercaptoalkanoates)
such as dibutyltin bis(isoocylmercaptoacetate) and dipropyltin bis(butyl
mercaptoacetate); tin thiocarbonates such as stannous O-ethyl dithiocarbonate;
tin
carbonates such as stannous propyl carbonate; tetrahydrocarbyltin compounds
such as
tetramethyltin, tetrabutyltin, tetraoctyltin, tetradodecyltin, and
tetraphenyltin;
dihydrocarbyltin oxides such as dipropyltin oxide; dibutyltin oxide,
dioctyltin oxide,
and diphenyltin oxide; dihydrocarbyltin bis(hydrocarbyl mercaptide)s such as
dibutyltin bis(dodecyl mercaptide); tin salts of phenolic compounds such as
stannous
thiophenoxide; tin sulfonates such as stannous benzenesulfonate and stannous-p-
toluenesulfonate; tin carbamates such as stannous diethylcarbamate; tin
thiocarbamates such as stannous propylthiocarbamate and stannous
diethyldithiocarbamate; tin phosphites such as stannous diphenyl phosphite;
tin
phosphates such as stannous dipropyl phosphate; tin thiophosphates such as
stannous,
O,O-dipropyl thiophosphate, stannous O,O-dipropyl dithiophosphate and stannic
O,O-dipropyl dithiophosphate, dihydrocarbyltin bis(O,O-dihydrocarbyl
CA 02263043 1999-03-08
33326CA
12
thiophosphate)s such as dibutyltin bis(O,O-dipropyldithiophosphate); and the
like. At
present tetrabutyltin is preferred. Again, as with silicon, organic tin
compounds are
preferred over inorganic compounds.
The tubes treated with the antifoulant composition in the presence of a
reducing gas will have properties providing for a significantly greater
suppression of
either the rate of coke formation or the amount of carbon monoxide production,
or
both, when used under cracking conditions than tubes treated exclusively with
the
antifoulant composition but without the presence of a reducing gas. A
preferred
procedure for pretreating the tubes of the cracking furnace includes charging
to the
inlet of the cracking furnace tubes a reducing gas such as hydrogen containing
therein
a concentration of the antifoulant composition. The concentration of
antifoulant
composition in the reducing gas can be in the range of from about 1 ppmw to
about
10,000 ppmw, preferably from about 10 ppmw to about 1000 ppmw and, most
preferably, from 20 to 200 ppmw.
Another embodiment of the invention includes treating the tubes of a
cracking furnace by contacting such tubes with a reducing gas, such as
hydrogen,
containing a sulfur compound to thereby provide a treated tube. The sulfur
compound
used in combination with the reducing gas to treat the cracking furnace tubes
can be
any suitable sulfiu compound that provides for a treated tube having the
desirable
ability to inhibit the production of carbon monoxide when used in cracking
operations.
Suitable sulfur compounds utilized include, for example, compounds
selected from the group consisting of sulfide compounds and disulfide
compounds.
CA 02263043 1999-03-08
33326CA
13
Preferably, the sulfide compounds are alkylsulfides with the alkyl
substitution groups
having from 1 to 6 carbon atoms, and the disulfide compounds are
dialkylsulfides
with the alkyl substitution groups having from 1 to 6 carbon atoms. The most
preferred alkylsulfide and dialkylsulfide compounds are respectively
dimethylsulfide
and dimethyl disulfide.
The tubes treated with a reducing gas having a concentration of a
sulfur compound will have the ability to inhibit the amount of carbon monoxide
produced when used under cracking conditions. Also, both the peak
concentration
and the asymptotic concentration of carbon monoxide in the cracker e$luent
stream
are reduced below those of a cracked effluent stream from untreated or
conventionally
treated cracker furnace tubes. Specifically, for the tubes treated with the
reducing gas
having a concentration of a sulfur compound, the peak concentration of carbon
monoxide in the cracker effluent stream from such tube can be in the range of
from
about 3 weight percent to about 5 weight percent of the total e$luent stream.
The
asymptotic concentration approaches less than 1 weight percent of the total
effluent
stream.
The tubes treated with the reducing gas containing a sulfur compound
will have properties providing for a reduction in the production of carbon
monoxide
when used under cracking conditions below that of tubes treated with sulfur
compounds but not in the presence of a reducing gas. It is preferred to
contact the
tubes under suitable treatment conditions with the reducing gas having a
concentration of a sulfur compound. The reducing gas, which contains the
sulfur
compound, used to treat the cracker tubes is preferably hydrogen gas. The
CA 02263043 1999-03-08
33326CA
14
concentration of the sulfur compound in the hydrogen gas used for treating the
cracker tubes can be in the range of from about 1 ppmw to about 10,000 ppmw,
preferably, from 10 ppmw to about 1000 ppmw and, most preferably, from 20 to
200
PPmw.
The temperature conditions under which the reducing gas, having the
concentration of the antifoulant composition or the sulfur compound, is
contacted
with the cracking tubes can include a contacting temperature in the range
upwardly to
about 2000°F. In any event, the contacting temperature must be such
that the
surfaces of the cracker tubes are properly passivated and include a contacting
temperature in the range of from about 300°F to about 2000°F,
preferably, from
about 400°F to about 1800°F and, most preferably, from
500°F to 1600°F.
The contacting pressure is not believed to be a critical process
condition, but it can be in the range of from about atmospheric to about 500
psig.
Preferably, the contacting pressure can be in the range of from about 10 psig
to about
300 psig and, most preferably, 20 psig to 150 psig.
The reducing gas stream having a concentration of antifoulant
composition or sulfur compound is contacted with or charged to the cracker
tubes for
a period of time sufficient to provide treated tubes, which when placed in
cracking
service, will provide for the reduced rate of coke formation or carbon
monoxide
production, or both, relative to untreated tubes or tubes treated with the
antifoulant
without the presence of a reducing gas. Such time period for pretreating the
cracker
tubes is influenced by the specific geometry of the cracking furnace including
its
tubes; but, generally, the pretreating time period can range upwardly to about
12
CA 02263043 1999-03-08
33326CA
hours, and longer if required. But, preferably, the period of time for the
pretreating
can be in the range of from about 0.1 hours to about 12 hours and, most
preferably,
from 0.5 hours to 10 hours.
Once the tubes of a cracking furnace are treated in accordance with the
5 procedures described herein, a hydrocarbon feedstock is charged to the inlet
of such
treated tubes. The tubes are maintained under cracking conditions so as to
provide for
a cracked product stream exiting the outlet of the treated tubes. The cracked
product
stream exiting the tubes which have been treated in accordance with the
inventive
methods has a reduced concentration of carbon monoxide that is lower than the
10 concentration of carbon monoxide in a cracked product stream exiting
cracker tubes
that have not been treated with an antifoulant composition or a sulfur
compound or
that have been treated with an antifoulant composition or a sulfur compound
but not
with the critical utilization of a reducing gas. As earlier described herein,
the
concentration of carbon monoxide in the cracked product stream from tubes
treated in
15 accordance with the novel methods can be less than about 5.0 weight
percent.
Preferably, the carbon monoxide concentration is less than about 3.0 weight
percent
and, most preferably, the carbon monoxide concentration is less than 2.0
weight
percent.
Another important benefit that results from the treatment of cracker
tubes by the inventive method utilizing an antifoulant composition is a
reduction in
the rate of coke formation in comparison with the coke formation rate with
untreated
tubes or tubes treated with an antifoulant composition but without the
presence of a
reducing gas during such treatment. This reduction in the rate of coke
formation
CA 02263043 1999-03-08
33326CA
16
permits the treated cracker tubes to be used for longer run lengths before
decoking is
required.
Now referring to FIG. 1, there is illustrated by schematic
representation a cracking furnace section 10 of a pyrolytic cracking process
system.
Cracking furnace section 10 includes pyrolytic cracking means or cracking
furnace 12
for providing heat energy required for inducing the cracking of hydrocarbons.
Cracking furnace 12 defines both convection zone 14 and radiant zone 16.
Respectively within such zones are convection coils as tubes 18 and radiant
coils as
tubes 20.
A hydrocarbon feedstock is conducted to the inlet of convection tubes
18 by way of conduit 22, which is in fluid flow communication with convection
tubes
18. Also, during the treatment of the tubes of cracking furnace 12, the
mixture of
hydrogen gas and antifoulant composition or sulfur compound can also be
conducted
to the inlet of convection tubes 18 though conduit 22. The feed passes through
the
tubes of cracking furnace 12 wherein it is heated to a cracking temperature in
order to
induce cracking or, in the situation where the tubes are undergoing treatment,
to the
required treatment temperature. The effluent from cracking furnace 12 passes
downstream through conduit 24 where it is further processed. To provide for
the heat
energy necessary to operate cracking furnace 12, fuel gas is conveyed through
conduit
26 to burners 28 of cracking furnace 12 whereby the fuel gas is burned and
heat
energy is released.
The following examples are provided to further illustrate the present
invention.
CA 02263043 1999-03-08
33326CA
17
EXAMPLE 1
This example describes the experimental procedures used to treat a
cracking tube and provides the results from such procedures. A comparative run
and
an inventive run were performed with the results being presented in FIG. 2.
A 12 foot, 1.75 inch LD. HP-Modified tube was pretreated with sulfur
in the form of 500 ppmw dimethylsulfide for a period of three hours.
Dimethylsulfide
(DMS) was introduced with 26.4 lb/hr steam and 18.3 lb/hr nitrogen at
400°F and 12
psig several feet upstream of the electric furnace which enclosed the reactor
tube.
The average temperature in the reactor tube was 1450°F during
pretreatment. Ethane
was then charged to the experimental unit at a rate of 25.3 lb/hr, and steam
was
charged at a rate of 7.6 lb/hr while continuing to inject DMS at a
concentration of 500
ppmw. Ethane conversion to ethylene was held constant at 67%. DMS injection
was
continued at 500 ppm for 9 hours into cracking, then was reduced to 125 ppm
for the
remainder of the run. Carbon monoxide production in the cracked gas, which is
an
indirect measure of the degree of coking, was monitored throughout the run.
In a subsequent run, the same tube was pretreated with a
DMS/hydrogen mixture at a 1:1 (mole) ratio. The DMS concentration during
pretreatment was 500 ppmw and all other conditions were the same during the
pretreatment and during the cracking run. The carbon monoxide production in
the
cracked gas was monitored.
The carbon monoxide concentrations in the cracked gas for both of the
runs are shown in FIG. 2. Carbon monoxide concentration showed a peak of 8.3
wt.
for the DMS only run while a peak of only 4.5 wt. % was obtained for the
CA 02263043 1999-03-08
33326CA
18
DMS/hydrogen run. The carbon monoxide concentration in the cracked gas
remained
higher in the DMS baseline run for several hours until the coke formed on the
tube
surface minimized reactions to carbon monoxide. These results clearly
demonstrate
the advantage of utilizing DMS in a reducing environment.
EXAMPLE 2
This example describes the experimental procedure used to obtain data
pertaining to the addition of hydrogen (reducing atmosphere) with an
antifoulant
during pretreatment injection onto a cracking coil.
The experimental apparatus included a 14' long, 8 pass coil made of
1/4" O.D. Incoloy 800 tubing which was heated to the desired temperature in an
electric tube furnace. In one run, 50 ppmm tetrabutyl tin (TBT) was injected
with
steam (37.5 mol/hr) and nitrogen for a period of thirty minutes at an
isothermal
temperature of 1300°F in the furnace. The injection was then
discontinued and
ethane was charged to the reactor at a rate of 745.5 g/hr. Steam was charged
with the
ethane to the reactor at a rate of 223.5 g/hr. Carbon monoxide in the cracked
gas and
pressure drop across the reactor coil were monitored continuously throughout
the run
of eighteen minutes. Coke production in the cracking coils was then measured
by
analyzing the carbon dioxide and carbon monoxide produced when burning out the
coil with a steam/air mixture. In a subsequent run, SO ppmm tetrabutyl tin was
injected with 1.7 standard liters per minute hydrogen at identical conditions
as the
previous run. This injection was then stopped and ethane was charged to the
reactor
at identical conditions as the previous run. Again, carbon monoxide production
in the
cracked gas was monitored and coking rate in the furnace determined for this
run
CA 02263043 1999-03-08
33326CA
19
which also lasted eighteen minutes. The coking rate as measured by the carbon
dioxide produced on burning out of the reactor coil was 585 g/hr, which was
substantially less than the 1403 g/hr measured for the run that injected TBT
only. The
carbon monoxide produced in the cracked gas during the runs was also
significantly
S less for the run that injected the TBT/hydrogen mixture as compared to the
TBT only
run. The results are shown in Table I for both runs.
These data show that adding the tetrabutyl tin compound in a reducing
environment will significantly enhance the reduction of the coking rate and
the
production of carbon monoxide in the cracked gas.
Table
I
CO in
Cracked
Gas (Wt
%)
Time (min.)TBT OnlyTBT/Hydrogen
6 0.024 0
9 0.09 0.076
12 1.232 0.514
15 2.35 2.4
While this invention has been described in terms of the presently
preferred embodiment, reasonable variations and modifications are possible by
those
skilled in the art. Such variations and modifications are within the scope of
the
described invention and the appended claims.