Note: Descriptions are shown in the official language in which they were submitted.
CA 02263417 1999-02-11
w ~,
'~:~: ~ .
Arylalkanoylpyridazines
The invention relates to arylalkanoylpyridazine
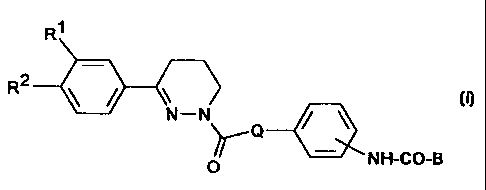
derivatives of the formula I
R~ 3 R4
R
2
R ~ ~ ~ I
N-N
~Q
O NH-CO-B
in which
B is A, OA, NH2, NHA, NAA' or an unsaturated
heterocycle which has 1 to 4 N, O and/or S
atoms and which can be unsubstituted or
mono-, di- or trisubstituted by Hal, A and/or
OA,
Q is absent or is alkylene having 1-6 C atoms,
R1, R2 in each case independently of one another are
-OH, ORS, -S-R5, -SO-R5, -SOZ-R5, Hal, -NOz,
-NH2, -NHRS or -NRSR6,
Rl and Rz together are also -O-CHZ-O-,
R3, R4 in each case independently of one another are
H or A,
R5, R6 in each case independently of one another are
A, cycloalkyl having 3-7 C atoms,
methylenecycloalkyl having 4-8 C atoms or
alkenyl having 2-8 C atoms,
A, A' in each case independently of one another are
alkyl which has 1 to 10 C atoms and which can
be substituted by 1 to 5 F and/or Cl atoms
and
Hal is F, C1, Br or I,
and the physiologically acceptable salts thereof.
1-Benzoyltetrahydropyridazines have been
described as progesterone receptor ligands, for example
in J. Med. Chem. ~$, 4878 (1995).
CA 02263417 1999-02-11
- 2 -
The invention was based on the object of
finding novel compounds which have valuable properties,
in particular those which can be used for the
preparation of pharmaceuticals.
It has been found that the compounds of the
formula I and salts thereof have highly valuable
pharmacological properties and are well tolerated.
In particular, they inhibit selectively
phosphodiesterase IV, which causes an increase of the
intracellular CAMP level (N.Sommer et al., Nature
Medicine, l, 244-248 (1995)).
The PDE IV inhibition can be determined analogously to
e.g. C.W. Davis in Biochim. biophys. Acta 797, 354-362
(1984) .
The compounds of the formula I can be employed
for the treatment of asthmatic diseases. The
antiasthmatic activity of PDE IV inhibitors is known
from T.J. Torphy et al. in Thorax, 46, 512-523 (1991)
and can be determined, for example, by the method of
T. Olsson, Acta allergologica 26, 438-447 (1971).
As cAMP inhibits bone decreasing cells and
stimulates bone increasing cells (S. Kasugai et al.,
M681 and K. Miyamoto, M 682, in Abstract of the
American Society for Bone and Mineral Research 18th
annual meeting 1996), the compounds of formula I can be
employed for the treatment of osteoporosis.
Moreover, the compounds have an inhibitory
effect on the formation of TNF (tumor necrosis factor)
and are therefore suitable for the treatment of
allergies and inflammatory diseases, autoimmune
diseases and transplant rejection reactions. They can
furthermore be used for the treatment of dysmnesia,
tumors, atherosclerosis, rheumatoid arthritis, multiple
sklerosis, Morbus Crohn, atopic dermatitis, diabetes
mellitus, ulcerative colitis and AIDS.
PDE IV inhibitors are potent compounds for the
treatment of asthmatic and inflammatory diseases,
diabetes mellitus, atopic dermatitis, psoriasis, AIDS,
CA 02263417 2005-O1-31
'30375-17
- 3 _ w
tumor growth or tumor metastasis (see e.g. EP 77 92
91) .
The antiinflammatory effect of the compounds of
the formula I and their potency for the treatment of
e.g. autoimmune diseases, multiple sclerosis or
rheumatoid arthritis can be determined analogously to
the methods of N. Sommer et al., Nature Medicine, l,
z44-248 (1995) or L. Sekut et al., Clin. Exp. Immunol.,
100, 126-132 (1995).
PDE IV inhibitors ar.e effective in the
treatment of tumors (see e.g. WO 95 35 281; WO 95 17
399 or WO 96 00 215)
The compounds of the formula I can be employed. as
pharmaceutically active ingredients in human and
veterinary medicine. Furthermore, they can be employed
as intermediates for the preparation of other
pharmaceutically active ingredients.
Accordingly,' the invention relates to the
compounds of the formula I and to a process for the
preparation of compounds of the formula I
and salts thereof, characterized in that a
compound of the formula II
R~ R4
11
N-NH
in which Rl, Ra, R3 and R4 have the abovementioned
meanings
is reacted with a compound of the formula III
L
--- Q ~ ~ ! I!,
3 o O NH-CO-E~
in which
B and Q have the abovementioned meanings and
L is C1, Br, OH or a reactive ester.ified OH
group,
CA 02263417 1999-02-11
or
- 4 -
in that a compound of the formula IV
R~ 3 R4
R
2
R ~ / ~ IV
N-N
Q
O NH2
in which
R1, R2, R3, R4 and Q have the abovementioned meanings
is reacted with a compound of the formula V
B-CO-L
in which
B has the abovementioned meaning and
L is C1, Br, OH or a reactive esterified OH group,
and/or in that a basic compound of the formula I is
converted into a salt thereof by treatment with an
acid.
The radicals, R1, R2, R3, R4, B, Q and L
hereinabove and hereinbelow have the meanings given for
the formulae I, II, III, IV and V, unless expressly
stated otherwise.
Compounds of the formula I can be chiral and
can accordingly occur in different isomeric forms. All
these forms (e. g. R- and S-forms) and their mixtures
(e.g. the R,S-forms) are embraced by the formula I.
A and A' are by preference alkyl, further
preferably alkyl which is substituted by 1 to 5
fluorine and/or chlorine atoms.
In the above formulae, alkyl is by preference
unbranched and has l, 2, 3, 4, 5, 6, 7, 8, 9 or 10 C
atoms, by preference 1, 2, 3, 4 or 5 C atoms, and is by
preference methyl, ethyl, trifluoromethyl, pentafluoro
ethyl or propyl, furthermore preferably isopropyl,
CA 02263417 2005-O1-31 .
30375-17
_ S ..
butyl, isobutyl, sec-butyl or tart-butyl, but also n-
pentyl, neo-pentyl or isopentyl.
Cycloalkyl has by preference 3-7 C atoms~and is
preferably cyclopropyl and cyclobutyl,. furthermore
preferably cyclopentyl or cyclohexyl, furthermore also
cycloheptyl. w
Methylenecycloalkyl has by preference 4-8
atoms and is preferably methylenecyclopropyl and
methylenecyclobutyl, furthermore preferably methylene-
to cyclopentyl and methylenecyclohexyl, furthermore also
methylenecycloheptyl.
Alkenyl is ~by preference v~:nyl, 1- or
2-propenyl, 1-butenyl, isobutenyl, sec-butenyl, and is
furthermore preferably 1-pentenyl, isopentenyl or
1-hexenyl.
Alkylene- is by preference unbranched and is
. preferably methylene or ethylene, furthermore
preferably propylene or butylene.
Of the radicals R~ , and . R4 , one is preferably H,
2o while the other' is preferably propyl or butyl; but
particularly preferably ethyl or methyl. Furthermore,
Rl and R2 together are also preferably each hydrogen.
Hal is by preference F, CI or Br, but also I.
The radicals Rl and R2 can be identical or
different and are in the 3- or 4-position of the phenyl
ring. For example, they are independently of one
another hydroxyl, -S-CH3, -S0-CH3, -S02CH3, F, Cl, Br or
I or together methylenedioxy. However, especially
preferably they are in each case methoxy, ethoxy,
3.0 propoxy, cyclopentoxy, or else fluoro-, difluora- or
trifluoromethoxy, 1-fluoro-, 2-fluoro-, 1,2-difluoro-,
2,2-difluoro-, 1,2,2-trifluoro- or 2,2,2-trifluoro-
ethoxy.
CA 02263417 2005-06-13
26474-945
- 5a -
The radical B is by preference 2- or 3-furyl, 2-
or 3-thienyl, 1-, 2- or 3-pyrrolyl, 1-, 2-, 4- or 5-
imidazolyl, 1-, 3-, 4- or 5-pyrazolyl, 2-, 4- or 5-oxazolyl,
3-, 4- or 5-isoxazolyl, 2-, 4- or 5-thiazolyl, 3-, 4-
or 5-isothiazolyl, 2-, 3- or 4-pyridyl, 2-, 4-, 5-
or 6-pyrimidinyl, furthermore
CA 02263417 1999-02-11
- 6 -
preferably 1,2,3-triazol-1-, -4- or -5-yl, 1,2,4-tri-
azol-1-, -3- or -5-yl, 1- or 5-tetrazolyl, 1,2,3-oxa-
diazol-4- or -5-yl, 1,2,4-oxadiazol-3- or -5-yl, 1,3,4-
thiadiazol-2- or -5-yl, 1,2,4-thiadiazol-3- or -5-yl,
1,2,3-thiadiazol-4- or -5-yl, 3- or 4-pyridazinyl,
pyrazinyl, 2-, 3-, 4-, 5-, 6- or 7-benzofuryl, 2-, 3-,
4-, 5-, 6- or 7-benzothienyl, 1-, 2-, 3-, 4-, 5-, 6- or
7-indolyl, 1-, 2-, 4- or 5-benzimidazolyl, 1-, 3-, 4-,
5-, 6- or 7-benzopyrazolyl, 2-, 4-, 5-, 6- or
7-benzoxazolyl, 3-, 4-, 5-, 6- or 7-benzoisoxazolyl,
2-, 4-, 5-, 6- or 7-benzothiazolyl, 2-, 4-, 5-, 6- or
7-benzoisothiazolyl, 4-, 5-, 6- or 7-benzo-2,1,3-
oxadiazolyl, 2-, 3-, 4-, 5-, 6-, 7- or 8-quinolyl, 1-,
3-, 4-, 5-, 6-, 7- or 8-isoquinolyl, 3-, 4-, 5-, 6-, 7-
or 8-cinnolinyl, 2-, 4-, 5-, 6-, 7- or 8-quinazolinyl.
The radical B is furthermore by preference
methyl, ethyl, propyl, n-butyl, methoxy, ethoxy,
propoxy, N-methylamino, N,N-dimethylamino, N-ethylamino
or N,N-diethylamino.
The rule that all radicals which occur more
than once can be identical or different, that is to say
are independent of one another, applies to the entire
invention.
Accordingly, the invention relates in
particular to those compounds of the formula I in which
at least one of the abovementioned radicals has one of
the preferred meanings given above. Some preferred
groups of compounds can be expressed by the following
subformulae Ia to Ie which correspond to the formula I
and in which radicals which are not defined in greater
detail have the meanings given for the formula I, but
where
in Ia, R1 and Rz are in each case independently of
one another OA,
Q is absent and
B is pyridinyl, pyrazinyl, pyrimi-
dinyl, thiazolyl, imidazolyl or
isoxazolyl;
CA 02263417 1999-02-11
-
in Ib, R1 and Rz in each case independently of one
another are OA,
Q is methylene and
B is pyridinyl, pyrazinyl, pyri mi-
dinyl, thiazolyl, imidazolyl or
isoxazolyl;
in Ic, R1 and RZ together are -O-CHZ-O-,
Q is absent or alkylene with 1-6 C
atoms and
B is pyridinyl, pyrazinyl, pyrimi-
dinyl, thiazolyl, imidazolyl or
isoxazolyl;
in Id, R1 and Rz in each case independently of one
another are OA,
Q is absent or alkylene with 1-6 C
atoms and
B is A or OA;
in Ie, R1 and RZ are in each case independently of
one another OA,
Q is absent or alkylen with 1-6 C
atoms,
B is pyridinyl, pyrazinyl, pyrimi-
dinyl, thiazolyl, imidazolyl,
isoxazolyl, A, OA or NH2.
Besides, the compounds of the formula I and
also the starting materials for their preparation are
prepared by methods known per se as they are described
in the literature (for example in the standard
publications such as Houben-Weyl, Methoden der
organischen Chemie [Methods in organic chemistry],
Georg-Thieme-Verlag, Stuttgart), under reaction
conditions which are known and suitable for the
abovementioned reactions. It is also possible to
utilize variants which are known per se but are not
mentioned in greater detail in the present text.
In the compounds of the formulae II and IV, R1,
R2, R3, R4 and Q have the abovementioned meanings, in
particular the abovementioned preferred meanings.
CA 02263417 1999-02-11
g _
In the compounds of the formulae III and IV, Q
is by preference methylene or ethylene, furthermore
preferably propylene or butylene.
B in the compounds of the formulae III and V
has the abovementioned preferred meanings, while L is
Cl, Br, OH or a reactive esterified OH group.
If L is a reactive esterified OH group, it is
by preference alkylsulfonyloxy having 1-6 C atoms
(preferably methylsulfonyloxy) or arylsulfonyloxy
having 6-10 C atoms (preferably phenyl- or p-tolyl-
sulfonyloxy, furthermore also 2-naphthalene-
sulfonyloxy).
If desired, the starting materials can also be
formed in situ, so that they are not isolated from the
reaction mixture but immediately reacted further to
give the compounds of the formula I.
On the other hand, it is possible to carry out the
reaction stepwise.
By preference, the compounds of the formula I
can be obtained by reacting compounds of the formula II
with compounds of the formula III.
Some of the starting materials of the formulae
II and III are known. If they are not known, they can
be prepared by methods known per se.
In detail, the reaction of the compounds of the
formula II with the compounds of the formula III is
carried out in the presence or absence of an inert
solvent at temperatures between approximately -20 and
approximately 150°, preferably between 20 and 100°.
Examples of suitable inert solvents are
hydrocarbons such as hexane, petroleum ether, benzene,
toluene or xylene; chlorinated hydrocarbons such as
trichloroethylene, 1,2-dichloroethane, carbon tetra-
chloride, chloroform or dichloromethane; alcohols such
as methanol, ethanol, isopropanol, n-propanol,
n-butanol or tert-butanol; ethers such as diethyl
ether, diisopropyl ether, tetrahydrofuran (THF) or
dioxane; glycol ethers such as ethylene glycol
monomethyl ether, ethylene glycol monoethyl ether
CA 02263417 1999-02-11
- 9 _
(methyl glycol or ethyl glycol), ethylene glycol
dimethyl ether (diglyme); ketones such as acetone or
butanone; amides such as acetamide, dimethylacetamide
or dimethylformamide (DMF); nitriles such as
acetonitrile; sulfoxides such as dimethyl sulfoxide
(DMSO); carbon disulfide; carboxylic acids such as
formic acid or acetic acid; nitro compounds such as
nitromethane or nitrobenzene; esters such as ethyl
acetate, or mixtures of the abovementioned solvents.
Moreover, compounds of the formula I can be
obtained by reacting compounds of the formula IV with
compounds of the formula V. As a rule, the starting
compounds of the formulae IV and V are known. If they
are not known, they can be prepared by methods known
per se.
Thus, for example, the preparation of 1-benzoyl-
tetrahydropyridazine is described in J. Med. Chem.
4878 (1995).
In the compounds of the formula V, the radical
-CO-L is a pre-activated carboxylic acid, preferably a
carboxylic acid halide.
The compounds of the formula IV are reacted
with compounds of the formula V under the same
conditions regarding reaction time, temperature and
solvent as has been described for the reaction of the
compounds of the formula II with compounds of the
formula III.
A base of the formula I can be converted into
the corresponding acid addition salt with an acid, for
example by reacting equivalent amounts of the base and
the acid in an inert solvent such as ethanol, followed
by evaporation. Acids which are suitable for this
reaction are, in particular, those which give
physiologically acceptable salts. Thus, inorganic acids
can be used, for example sulfuric acid, nitric acid,
hydrohalic acids such as hydrochloric acid or
hydrobromic acid, phosphorus acids such as
orthophosphoric acid, sulfamic acid, furthermore
organic acids, in particular aliphatic, alicyclic,
CA 02263417 1999-02-11
- 10 -
araliphatic, aromatic or heterocyclic mono- or
polybasic carboxylic, sulfonic or sulfuric acids, for
example formic acid, acetic acid, propionic acid,
pivalic acid, diethylacetic acid, malonic acid,
succinic acid, pimelic acid, fumaric acid, malefic acid,
lactic acid, tartaric acid, malic acid, citric acid,
gluconic acid, ascorbic acid, nicotinic acid,
isonicotinic acid, methane- or ethanesulfonic acid,
ethanedisulfonic acid, 2-hydroxyethanesulfonic acid,
benzenesulfonic acid, p-toluenesulfonic acid,
naphthalenemono- and -disulfonic acids, laurylsulfonic
acid. Salts with acids which are physiologically not
acceptable, for example picrates, can be used for
isolating and/or purifying the compounds of the formula
I.
On the other hand, the free bases of the
formula I can be liberated from their salts using bases
(for example sodium hydroxide, sodium carbonate,
potassium hydroxide or potassium carbonate), if so
desired.
The invention furthermore relates to the use of
the compounds of the formula I and/or of the
physiologically acceptable salts thereof for the
preparation of pharmaceutical products, in particular
via the non-chemical route . They can be brought into a
suitable pharmaceutical form together with at least one
solid, liquid and/or semi-liquid excipient or auxiliary
and, if appropriate, in combination with one or more
further active ingredients.
The invention also relates to pharmaceuticals
of the formula I and to the physiologically acceptable
salts thereof as phosphodiesterase IV inhibitors.
The invention furthermore relates to
pharmaceutical products comprising at least one
compound of the formula I and/or a physiologically
acceptable salt thereof.
These products can be used as pharmaceuticals
in human or veterinary medicine. Suitable excipients
are organic or inorganic substances which are suitable
CA 02263417 1999-02-11
- 11 -
for enteral (for example oral), parenteral or topical
administration and which do not react with the novel
compounds, for example water, vegetable oils, benzyl
alcohols, alkylene glycols, polyethylene glycols,
glycerol triacetate, gelatin, carbohydrates such as
lactose or starch, magnesium stearate, talc, petroleum
jelly. Pharmaceutical forms which are used for oral
administration are, in particular, tablets, pills,
sugar-coated tablets, capsules, powders, granules,
syrups, liquids or drops; pharmaceutical forms which
can be used, in particular, for rectal administration
are suppositories; pharmaceutical forms which can be
used for parenteral administration are, in particular,
solutions, preferably oily or aqueous solutions,
furthermore suspensions, emulsions or implants, and
pharmaceutical forms which can be used for topical
administration are, in particular, ointments, creams or
powders. The novel compounds can also be lyophilized
and the resulting lyophilizates used for example for
the preparation of injectable products. The
abovementioned products can be sterilized and/or
comprise auxiliaries such as glidants, preservatives,
stabilizers and/or wetting agents, emulsifiers, salts
for modifying the osmotic pressure, buffer substances,
colours, flavourings and/or a plurality of other active
ingredients, for example one or more vitamins.
The compounds of the formula I and their
physiologically acceptable salts can be employed for
combating diseases where a raised cAMP (cyclo-adenosine
monophosphate) level leads to the inhibition or
prevention of inflammations and to muscular relaxation.
The compounds according to the invention can be used
especially in the treatment of allergies, asthma,
chronic bronchitis, atopic dermatitis, psoriasis and
other skin diseases and autoimmune diseases.
In this connection, the substances according to
the invention are generally preferably administered in
doses of between approximately 1 and 500 mg, in
particular of between 5 and 100 mg per dose unit. The
CA 02263417 2005-06-13
26474-945
- 12 -
daily dose is preferably between approximately 0.02
and 10 mg/kg of body weight. The specific dose for each
patient, however, depends on all sorts of factors, for
example on the efficacy of the specific compound employed,
on the age, body weight, general state of health, sex, on
the diet, on the time and route of administration, and on
the excretion rate, pharmaceutical substance combination and
severity of the particular disorder to which the therapy
applies. Oral administration is preferred.
All temperatures hereinabove and hereinbelow are
given in °C. In the examples which follow, "customary work-
up" means: if required, water is added; if required, the pH
is brought to between 2 and 10, depending on the
constitution of the end product; the mixture is extracted
with ethyl acetate or dichloromethane and separated; the
organic phase is dried over sodium sulfate and evaporated;
and the residue is purified by chromatography on silica gel
and/or by crystallization.
Mass spectrometry (MS): EI (electron impact ionization)
M+ FAB (fast atom bombardment) (M+H)+
Example 1
A suspension of 4.70 g of 3-(3,4-dimethoxyphenyl)-
1,4,5,6-tetrahydropyridazine ("A") in 150 ml of THF is
treated with 2.24 g of potassium tert-butoxide and the
mixture is stirred for 30 minutes. 7.3 g of
4-nicotinoylaminobenzoyl chloride are added, and stirring is
continued for 10 hours at room temperature. The solvent is
removed and the mixture is worked up as customary. This
gives 1-(4-nicotinoylaminobenzoyl)-3-(3,4-dimethoxyphenyl)-
CA 02263417 2005-06-13
26474-945
- 12a -
1,4,5,6-tetrahydropyridazine hydrochloride, m.p. 239°
(decomposition).
The following is obtained analogously by reaction
of "A" with 4-isonicotinoylaminobenzoyl chloride:
CA 02263417 1999-02-11
- 13 -
1-(4-isonicotinoylaminobenzoyl)-3-(3,4-dimethoxy-
phenyl)-1,4,5,6-tetrahydropyridazine
hydrochloride, m.p. 247° (decomposition).
Exam~l_e 2
A solution of 2.0 g of 1-(4-aminobenzoyl)-3-
(3,4-dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
m.p. 197° [obtainable by catalytic hydrogenation of
1-(4-nitrobenzoyl)-3-(3,4-dimethoxyphenyl)-1,4,5,6-
tetrahydropyridazine, m.p. 203° in 150 ml of tetra-
hydrofuran in the presence of 3.5 g of Raney nickel at
room temperature] and 1.6 ml of pyridine in 150 ml of
acetonitrile is treated with 1.2 g of nicotinoyl
chloride hydrochloride and stirring is continued for
two hours. The solvent is removed and the residue is
worked up as customary. After recrystallization, 1-(4-
nicotinoylaminobenzoyl)-3-(3,4-dimethoxyphenyl)-
1,4,5,6-tetrahydropyridazine hydrochloride, m.p. 239°
(decomposition), is obtained.
The compounds given further below are obtained
analogously by reacting the "amine derivatives" which
follow
1-(3-aminobenzoyl)-3-(3,4-dimethoxyphenyl)-
1,4,5,6-tetrahydropyridazine, m.p. 168°;
1-(2-aminobenzoyl)-3-(3,4-dimethoxyphenyl)-
1,4,5,6-tetrahydropyridazine,
1-(4-aminobenzoyl)-3-(3-ethoxy-4-methoxyphenyl)-
1,4,5,6-tetrahydropyridazine, m.p. 154°;
1-(3-aminobenzoyl)-3-(3-ethoxy-4-methoxyphenyl)-
1,4,5,6-tetrahydropyridazine,
1-(4-aminobenzoyl)-3-(3-cyclopentyloxy-4-methoxy-
phenyl)-1,4,5,6-tetrahydropyridazine, m.p. 168°;
1-(3-aminobenzoyl)-3-(3-cyclopentyl-4-
methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-aminobenzoyl)-3-(3,4-methylenedioxyphenyl)-
1,4,5,6-tetrahydropyridazine,
1-(4-aminobenzoyl)-3-(3-methoxy-4-methylsulfonyl-
phenyl)-1,4,5,6-tetrahydropyridazine,
CA 02263417 1999-02-11
- 14 -
1-(4-aminobenzoyl)-3-(3-trifluoromethoxy-4-meth-
oxyphenyl)-1,4,5,6-tetrahydropyridazine,
with nicotinoyl chloride:
1-(3-nicotinoylaminobenzoyl)-3-(3,4-dimethoxy-
phenyl)-1,4,5,6-tetrahydropyridazine
hydrochloride, 159° (decomposition);
1-(2-nicotinoylaminobenzoyl)-3-(3,4-dimethoxy-
phenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-nicotinoylaminobenzoyl)-3-(3-ethoxy-4-
methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(3-nicotinoylaminobenzoyl)-3-(3-ethoxy-4-
methoxyphenyl)-1,4,5,6-tetrahydropyridazine
hydrochloride, 235°,
1-(4-nicotinoylaminobenzoyl)-3-(3-cyclopentyloxy-
4-methoxyphenyl)-1,4,5,6-tetrahydropyridazine
hydrochloride, m.p. 224° (decomposition);
1-(3-nicotinoylaminobenzoyl)-3-(3-cyclopentyloxy-
4-methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-nicotinoylaminobenzoyl)-3-(3,4-methylene-
dioxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-nicotinoylaminobenzoyl)-3-(3-methoxy-4-
methylsulfonylphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-nicotinoylaminobenzoyl)-3-(3-trifluoro-
methoxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine.
The compounds which follow are obtained
analogously by reacting the "amine derivatives" listed
above with isonicotinoyl chloride:
1-(4-isonicotinoylaminobenzoyl)-3-(3,4-dimethoxy-
phenyl)-1,4,5,6-tetrahydropyridazine, m.p. 247°
(decomposition);
1-(3-isonicotinoylaminobenzoyl)-3-(3,4-dimethoxy-
phenyl)-1,4,5,6-tetrahydropyridazine hydro-
chloride, 175° (decomposition);
1-(2-isonicotinoylaminobenzoyl)-3-(3,4-dimethoxy-
phenyl)-1,4,5,6-tetrahydropyridazine,
CA 02263417 1999-02-11
1-(4-isonicotinoylaminobenzoyl)-3-(3-ethoxy-4-
methoxyphenyl)-1,4,5,6-tetrahydropyridazine
hydrochloride, m.p. 266°;
1-(3-isonicotinoylaminobenzoyl)-3-(3-ethoxy-4-
5 methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-isonicotinoylaminobenzoyl)-3-(3-cyclopentyl-
oxy-4-methoxyphenyl)-1,4,5,6-tetra-hydropyridazine
hydrochloride, m.p. 244° (decomposition);
1-(3-isonicotinoylaminobenzoyl)-3-(3-cyclo-
10 pentyloxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-isonicotinoylaminobenzoyl)-3-(3,4-methylene-
dioxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-isonicotinoylaminobenzoyl)-3-(3-methoxy-4-
15 methylsulfonylphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-isonicotinoylaminobenzoyl)-3-(3-trifluoro-
methoxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine.
The compounds which follow are obtained
analogously by reacting the "amine derivatives" listed
above with picolinoyl chloride:
1-(4-picolinoylaminobenzoyl)-3-(3,4-dimethoxy-
phenyl)-1,4,5,6-tetrahydropyridazine,
1-(3-picolinoylaminobenzoyl)-3-(3,4-dimethoxy-
phenyl)-1,4,5,6-tetrahydropyridazine,
1-(2-(picolinoylaminobenzoyl)-3-(3,4-dimethoxy-
phenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-picolinoylaminobenzoyl)-3-(3-ethoxy-4-
methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(3-picolinoylaminobenzoyl)-3-(3-ethoxy-4-
methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-picolinoylaminobenzoyl)-3-(3-cyclopentyloxy-
4-methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(3-picolinoylaminobenzoyl)-3-(3-cyclopentyloxy-
4-methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-picolinoylaminobenzoyl)-3-(3,4,-methylene-
dioxyphenyl)-1,4,5,6-tetrahydropyridazine,
CA 02263417 1999-02-11
- 16 -
1-(4-picolinoylaminobenzoyl)-3-(3-methoxy-4-
methylsulfonylphenyl)-1,4,5,6-tetrahydropyrida-
zine,
1-(4-picolinoylaminobenzoyl)-3-(3-trifluoro
methoxy-4-methoxyphenyl)-1,4,5,6-tetrahydro
pyridazine.
The compounds which follow are obtained
analogously by reacting the "amine derivatives" listed
above with furan-2-carbonyl chloride:
1-(4-(furan-2-carbonylamino)benzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(3-(furan-2-carbonylamino)benzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(2-(furan-2-carbonylamino)benzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-(furan-2-carbonylamino)benzoyl)-3-(3-ethoxy-
4-methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(3-(furan-2-carbonylamino)benzoyl)-3-(3-ethoxy-
4-methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-(furan-2-carbonylamino)benzoyl)-3-(3-cyclo-
pentyloxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(3-(furan-2-carbonylamino)benzoyl)-3-(3-cyclo-
pentyloxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-(furan-2-carbonylamino)benzoyl)-3-(3,4-
methylenedioxyphenyl)-1,4,5,6-tetrahydropyrid-
azine,
1-(4-(furan-2-carbonylamino)benzoyl)-3-(3-methoxy-
4-methylsulfonylphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-(furan-2-carbonylamino)benzoyl)-3-(3-
trifluoromethoxy-4-methoxyphenyl)-1,4,5,6-
tetrahydropyridazine.
The compounds which follow are obtained
analogously by reacting the "amine derivatives" listed
above with thiophene-2-carbonyl chloride:
1-(4-thiophene-2-carbonylamino)benzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
CA 02263417 1999-02-11
- 17 -
1-(3-(thiophene-2-carbonylamino)benzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(2-(thiophene-2-carbonylamino)benzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-(thiophene-2-carbonylamino)benzoyl)-3-(3-
ethoxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(3-(thiophene-2-carbonylamino)benzoyl)-3-(3-
ethoxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-(thiophene-2-carbonylamino)benzoyl)-3-(3-
cyclopentyloxy-4-methoxyphenyl)-1,4,5,6-tetra-
hydropyridazine,
1-(3-(thiophene-2-carbonylamino)benzoyl)-3-(3
cyclopentyloxy-4-methoxyphenyl)-1,4,5,6-tetra
hydropyridazine,
1-(4-(thiophene-2-carbonylamino)benzoyl)-3-(3,4-
methylenedioxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-(thiophene-2-carbonylamino)benzoyl)-3-(3-
methoxy-4-methylsulfonylphenyl)-1,4,5,6-tetra-
hydropyridazine,
1-(4-(thiophene-2-carbonylamino)benzoyl)-3-(3-
trifluoromethoxy-4-methoxyphenyl)-1,4,5,6-
tetrahydropyridazine.
The compounds which follow are obtained
analogously by reacting the "amine derivatives" listed
above with pyrazine-2-carbonyl chloride:
1-(4-pyrazine-2-carbonylamino)benzoyl)-3-(3,4
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
m.p. 213°;
1-(3-(pyrazine-2-carbonylamino)benzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
m.p. 204°;
1-(2-(pyrazine-2-carbonylamino)benzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-(pyrazine-2-carbonylamino)benzoyl)-3-(3-
ethoxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine, m.p. 186°;
CA 02263417 1999-02-11
- 18 -
1-(3-(pyrazine-2-carbonylamino)benzoyl-3-(3-
ethoxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-(pyrazine-2-carbonylamino)benzoyl)-3-(3-
cyclopentyloxy-4-methoxyphenyl)-1,4,5,6-tetra-
hydropyridazine, m.p. 225°;
1-(3-(pyrazine-2-carbonylamino)benzoyl)-3-(3-
cyclopentyloxy-4-methoxyphenyl)-1,4,5,6-tetra-
hydropyridazine,
1-(4-(pyrazine-2-carbonylamino)benzoyl)-3-(3,4-
methylenedioxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-(pyrazine-2-carbonylamino)benzoyl)-3-(3-
methoxy-4-methylsulfonylphenyl)-1,4,5,6-tetra-
hydropyridazine,
1-(4-(pyrazine-2-carbonylamino)benzoyl)-3-(3-
trifluoromethoxy-4-methoxyphenyl)-1,4,5,6-tetra-
hydropyridazine.
The compounds which follow are obtained
analogously by reacting the "amine derivatives" listed
above with imidazole-4-carbonyl chloride:
1-(4-imidazole-4-carbonylamino)benzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(3-imidazole-4-carbonylamino)benzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-2-(imidazole-4-carbonylamino)benzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-(imidazole-4-carbonylamino)benzoyl)-3-(3-
ethoxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(3-(imidazole-4-carbonylamino)benzoyl)-3-(3-
ethoxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-(imidazole-4-carbonylamino)benzoyl)-3-(3-
cyclopentyloxy-4-methoxyphenyl)-1,4,5,6-tetra-
hydropyridazine,
1-(3-(imidazole-4-carbonylamino)benzoyl)-3-(3-
cyclopentyloxy-4-methoxyphenyl)-1,4,5,6-tetra-
hydropyridazine,
CA 02263417 1999-02-11
- 19 -
1-(4-(imidazole-4-carbonylamino)benzoyl)-3-(3,4-
methylenedioxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-imidazole-4-carbonylamino)benzoyl)-3-(3-
methoxy-4-methylsulfonylphenyl)-1,4,5,6-tetra-
hydropyridazine,
1-(4-(imidazole-4-carbonylamino)benzoyl)-3-(3-
trifluoromethoxy-4-methoxyphenyl.
The compounds which follow are obtained
analogously by reacting the "amine derivatives" listed
above with 2,4-dimethylthiazole-5-carbonyl chloride:
1-(4-(2,4-dimethylthiazole-5-carbonylamino)-
benzoyl)-3-(3,4-dimethoxyphenyl)-1,4,5,6-tetra-
hydropyridazine,
1-(3-(2,4-dimethylthiazole-5-carbonylamino)-
benzoyl)-3-(3,4-dimethoxyphenyl)-1,4,5,6-tetra-
hydropyridazine,
1-(2-(2,4-dimethylthiazole-5-carbonylamino)-
benzoyl)-3-(3,4-dimethoxyphenyl)-1,4,5,6-
tetrahydropyridazine,
1-(4-(2,4-dimethylthiazole-5-carbonylamino)-
benzoyl)-3-(3-ethoxy-4-methoxyphenyl)-1,4,5,6-
tetrahydropyridazine,
1-(3-(2,4-dimethylthiazole-5-carbonylamino)-
benzoyl)-3-(3-ethoxy-4-methoxyphenyl)-1,4,5,6-
tetrahydropyridazine,
1-(4-(2,4-dimethylthiazole-5-carbonylamino)-
benzoyl)-3-(3-cyclopentyloxy-4-methoxyphenyl)-
1,4,5,6-tetrahydropyridazine,
1-(3-(2,4-dimethylthiazole-5-carbonylamino)-
benzoyl)-3-(3-cyclopentyloxy-4-methoxyphenyl)-
1,4,5,6-tetrahydropyridazine,
1-(4-(2,4-dimethylthiazole-5-carbonylamino)-
benzoyl)-3-(3,4-methylenedioxyphenyl)-1,4,5,6-
tetrahydropyridazine,
1-(4-(2,4-dimethylthiazole-5-carbonylamino)-
benzoyl)-3-(3-methoxy-4-methylsulfonylphenyl)-
1,4,5,6-tetrahydropyridazine,
CA 02263417 1999-02-11
- 20 -
1-(4-(2,4-dimethylthiazole-5-carbonylamino)-
benzoyl)-3-(3-trifluoromethoxy-4-methoxyphenyl)-
1,4,5,6-tetrahydropyridazine.
The compounds which follow are obtained
analogously by reacting the "amine derivatives" listed
above with isoxazole-5-carbonyl chloride:
1-(4-(isoxazole-5-carbonylamino)benzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(3-(isoxazole-5-carbonylamino)benzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(2-(isoxazole-5-carbonylamino)benzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-(isoxazole-5-carbonylamino)-3-(3-ethoxy-4-
methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(3-(isoxazole-5-carbonylamino)benzoyl)-3-(3-
ethoxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-(isoxazole-5-carbonylamino)benzoyl)-3-(3
cyclopentyloxy-4-methoxyphenyl)-1,4,5,6-tetra
hydropyridazine,
1-(3-(isoxazole-5-carbonylamino)benzoyl)-3-(3-
cyclopentyloxy-4-methoxyphenyl)-1,4,5,6-tetra-
hydropyridazine,
1-(4-(isoxazole-5-carbonylamino)benzoyl)-3-(3,4-
methylenedioxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-(isoxazole-5-carbonylamino)benzoyl)-3-(3-
methoxy-4-methylsulfonylphenyl)-1,4,5,6-tetra-
hydropyridazine,
1-(4-(isoxazole-5-carbonylamino)benzoyl)-3-(3-
trifluoromethoxy-4-methoxyphenyl)-1,4,5,6-
tetrahydropyridazine.
The compounds which follow are obtained
analogously by reacting the "amine derivatives" listed
above with pyrimidine-2-carbonyl chloride:
1-(4-(pyrimidine-2-carbonylamino)benzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(3-(pyrimidine-2-carbonylamino)benzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
CA 02263417 1999-02-11
- 21 -
1-(2-(pyrimidine-2-carbonylamino)benzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-(pyrimidine-2-carbonylamino)benzoyl)-3-(3-
ethoxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(3-(pyrimidine-2-carbonylamino)benzoyl)-3-(3-
ethoxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-(pyrimidine-2-carbonylamino)benzoyl)-3-(3-
cyclopentyloxy-4-methoxyphenyl)-1,4,5,6-tetra-
hydropyridazine,
1-(3-(pyrimidine-2-carbonylamino)benzoyl)-3-(3-
cyclopentyloxy-4-methoxyphenyl)-1,4,5,6-tetra-
hydropyridazine,
1-(4-(pyrimidine-2-carbonylamino)benzoyl)-3-(3,4-
methylenedioxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-(pyrimidine-2-carbonylamino)benzoyl)-3-(3-
methoxy-4-methylsulfonylphenyl)-1,4,5,6-tetra-
hydropyridazine,
1-(4-(pyrimidine-2-carbonylamino)benzoyl)-3-(3-
trifluoromethoxy-4-methoxyphenyl)-1,4,5,6-
tetrahydropyridazine.
The compounds which follow are obtained
analogously by reacting the "amine derivatives" listed
above with pyrimidine-4-carbonyl chloride:
1-(4-(pyrimidine-4-carbonylamino)benzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
m.p. 196°;
1-(3-(pyrimidine-4-carbonylamino)benzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(2-(pyrimidine-4-carbonylamino)benzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-(pyrimidine-4-carbonylamino)benzoyl)-3-(3-
ethoxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(3-(pyrimidine-4-carbonylamino)benzoyl)-3-(3-
ethoxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
CA 02263417 1999-02-11
- 22 -
1-(4-(pyrimidine-4-carbonylamino)benzoyl)-3-(3-
cyclopentyloxy-4-methoxyphenyl)-1,4,5,6-tetra-
hydropyridazine,
1-(3-(pyrimidine-4-carbonylamino)benzoyl)-3-(3-
cyclopentyloxy-4-methoxyphenyl)-1,4,5,6-
tetrahydropyridazine,
1-(4-(pyrimidine-4-carbonylamino)benzoyl)-3-(3,4-
methylenedioxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-(pyrimidine-4-carbonylamino)benzoyl)-3-(3-
methoxy-4-methylsulfonylphenyl)-1,4,5,6-tetra-
hydropyridazine,
1-(4-(pyrimidine-4-carbonylamino)benzoyl)-3-(3-
trifluoromethoxy-4-methoxyphenyl.
The compounds which follow further below are
obtained analogously by reacting
1-(4-aminobenzylcarbonyl)-3-(3,4-dimethoxyphenyl)-
1,4,5,6-tetrahydropyridazine,
1-(3-aminobenzylcarbonyl)-3-(3,4-dimethoxyphenyl)-
1,4,5,6-tetrahydropyridazine,
1-(2-aminobenzylcarbonyl)-3-(3,4-dimethoxyphenyl)-
1,4,5,6-tetrahydropyridazine,
1-(4-aminobenzylcarbonyl)-3-(3-ethoxy-4-methoxy-
phenyl)-1,4,5,6-tetrahydropyridazine,
1-(3-aminobenzylcarbonyl)-3-(3-ethoxy-4-methoxy-
phenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-aminobenzylcarbonyl)-3-(3-cyclopentyloxy-4-
methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(3-aminobenzylcarbonyl)-3-(3-cyclopentyloxy-4-
methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-aminobenzylcarbonyl)-3-(3,4-methylenedioxy-
phenyl)-1,4,5;6-tetrahydropyridazine,
1-(4-aminobenzylcarbonyl)-3-(3-methoxy-4-
methylsulfonylphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-aminobenzylcarbonyl)-3-(3-trifluoromethoxy-4-
methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
with nicotinoyl chloride:
CA 02263417 1999-02-11
- 23 -
1-(4-nicotinoylaminobenzylcarbonyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine
hydrochloride, m.p. 225°;
1-(3-nicotinoylaminobenzylcarbonyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(2-nicotinoylaminobenzylcarbonyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-nicotinoylaminobenzylcarbonyl)-3-(3-ethoxy-4-
methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(3-nicotinoylaminobenzylcarbonyl)-3-(3-ethoxy-4-
methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-nicotinoylaminobenzylcarbonyl)-3-(3-cyclo-
pentyloxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(3-nicotinoylaminobenzylcarbonyl)-3-(3-cyclo-
pentyloxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-nicotinoylaminobenzylcarbonyl)-3-(3,4-
methylenedioxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-nicotinoylaminobenzylcarbonyl)-3-(3-methoxy-
4-methylsulfonylphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-nicotinoylaminobenzylcarbonyl)-3-(3-tri
fluoromethoxy-4-methoxyphenyl)-1,4,5,6-tetra
hydropyridazine.
The following are obtained analogously by
reacting 1-(4-aminobenzylcarbonyl)-3-(3,4-dimethoxy-
phenyl)-1,4,5,6-tetrahydropyridazine
with isonicotinoyl chloride:
1-(4-isonicotinoylaminobenzylcarbonyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine
hydrochloride, m.p. 209°C
and with ethyl chloroformate
1-(4-ethoxycarbonylaminobenzylcarbonyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
m.p. 143°.
CA 02263417 1999-02-11
- 24 -
A solution of 2.0 g of 1-(4-aminobenzoyl)-3-
(3,4-dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
m.p. 197°, and 0.8 ml of pyridine in 160 ml of
dichloromethane is treated with 0.6 ml of ethyl
chloroformate ("B") and stirring is continued for 2
hours. The solvent is removed and the residue is worked
up as customary. After recrystallization from
isopropanol/petroleum ether, 2.2 g of 1-(4-ethoxy-
carbonylaminobenzoyl)-3-(3,4-dimethoxyphenyl)-1,4,5,6-
tetrahydropyridazine, m.p. 165°, are obtained.
The compounds which follow further below are
obtained analogously by reacting the "amine
derivatives" which follow
1-(3-aminobenzoyl)-3-(3,4-dimethoxyphenyl)-
1,4,5,6-tetrahydropyridazine,
1-(2-aminobenzoyl)-3-(3,4-dimethoxyphenyl)-
1,4,5,6-tetrahydropyridazine,
1-(4-aminobenzoyl)-3-(3-ethoxy-4-methoxyphenyl)-
1,4,5,6-tetrahydropyridazine,
1-(3-aminobenzoyl)-3-(3-ethoxy-4-methoxyphenyl)-
1,4,5,6-tetrahydropyridazine,
1-(4-aminobenzoyl)-3-(3-cyclopentyloxy-4-methoxy-
phenyl)-1,4,5,6-tetrahydropyridazine,
1-(3-aminobenzoyl)-3-(3-cyclopentyloxy-4-methoxy-
phenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-aminobenzoyl)-3-(3,4-methylenedioxyphenyl)-
1,4,5,6-tetrahydropyridazine,
1-(4-aminobenzoyl)-3-(3-methoxy-4-methylsulfonyl-
phenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-aminobenzoyl)-3-(3-trifluoromethoxy-4-
methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
with "B":
1-(3-ethoxycarbonylaminobenzoyl)-3-(3,4-dimethoxy-
phenyl)-1,4,5,6-tetrahydropyridazine, m.p. 181°;
1-(2-ethoxycarbonylaminobenzoyl)-3-(3,4-dimethoxy-
phenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-ethoxycarbonylaminobenzoyl)-3-(3-ethoxy-4-
methoxyphenyl)-1,4,5,6-tetrahydropyridazine, m.p.
147°;
CA 02263417 1999-02-11
- 25 -
1-(3-ethoxycarbonylaminobenzoyl)-3-(3-ethoxy-4-
methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-ethoxycarbonylaminobenzoyl)-3-(3-cyclo-
pentyloxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine, m.p. 166°;
1-(3-ethoxycarbonylaminobenzoyl)-3-(3-cyclo-
pentyloxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-ethoxycarbonylaminobenzoyl)-3-(3,4-methylene-
dioxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-ethoxycarbonylaminobenzoyl)-3-(3-methoxy-4-
methylsulfonylphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-ethoxycarbonylaminobenzoyl)-3-(3-trifluoro-
methoxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine.
The compounds which follow are obtained
analogously with the "amine derivatives" listed above
and with methyl chloroformate:
1-(4-methoxycarbonylaminobenzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
m.p. 226°;
1-(3-methoxycarbonylaminobenzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(2-methoxycarbonylaminobenzoyl)-3-(3,4-
dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-methoxycarbonylaminobenzoyl)-3-(3-ethoxy-4-
methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(3-methoxycarbonylaminobenzoyl)-3-(3-ethoxy-4-
methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-methoxycarbonylaminobenzoyl)-3-(3-cyclo-
pentyloxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(3-methoxycarbonylaminobenzoyl)-3-(3-cyclo-
pentyloxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
1-(4-methoxycarbonylaminobenzoyl)-3-(3,4-
methylenedioxyphenyl)-1,4,5,6-tetrahydro-
pyridazine,
CA 02263417 1999-02-11
- 26 -
1-(4-methoxycarbonylaminobenzoyl)-3-(3-methoxy-4-
methylsulfonylphenyl)-1,4,5,6-tetrahydro-
pyridazine.
1-(4-methoxycarbonylaminobenzoyl)-3-(3-trifluoro-
methoxy-4-methoxyphenyl)-1,4,5,6-tetrahydro-
pyridazine.
The compounds which follow are obtained
analogously with the "amine derivatives" listed above
and with acetyl chloride:
1-(4-acetamidobenzoyl)-3-(3,4-dimethoxyphenyl)-
1,4,5,6-tetrahydropyridazine, m.p. 230°;
1-(3-acetamidobenzoyl)-3-(3,4-dimethoxyphenyl)-
1,4,5,6-tetrahydropyridazine,
1-(2-acetamidobenzoyl)-3-(3,4-dimethoxyphenyl)-
1,4,5,6-tetrahydropyridazine,
1-(4-acetamidobenzoyl)-3-(3-ethoxy-4-methoxy-
phenyl)-1,4,5,6-tetrahydropyridazine,
1-(3-acetamidobenzoyl)-3-(3-ethoxy-4-methoxy-
phenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-acetamidobenzoyl)-3-(3-cyclopentyloxy-4-
methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(3-acetamidobenzoyl)-3-(3-cyclopentyloxy-4-
methoxyphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-acetamidobenzoyl)-3-(3,4-methylenedioxy-
phenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-acetamidobenzoyl)-3-(3-methoxy-4-methyl-
sulfonylphenyl)-1,4,5,6-tetrahydropyridazine,
1-(4-acetamidobenzoyl)-3-(3-trifluoromethoxy-4-
methoxyphenyl)-1,4,5,6-tetrahydropyridazine.
CA 02263417 1999-02-11
- 27 -
Exam lp a 4
A solution of 2.0 g of 1-(4-aminobenzoyl)-3-
(3,4-dimethoxyphenyl)-1,4,5,6-tetrahydropyridazine and
0.8 ml of N-ethyl isocyanate in 160 ml of
dichloromethane is stirred for two hours at room
temperature. The solvent is removed and the residue is
worked up as customary. After recrystallization from
isopropanol/petroleum ether, 2.1 g of 1-(4-ethyl-
ureidobenzoyl)-3-(3,4-dimethoxyphenyl)-1,4,5,6-tetra-
hydropyridazine are obtained.
Analogously, by reacting with potassium cyanate the
following compound is obtained
1-(4-ureidobenzoyl)-3-(3-ethoxy-4-methoxyphenyl)-
1,4,5,6-tetra-hydropyridazine, m.p. 251°.
Example 5
Analogously to the Examples 2 and 3 the
following compounds are obtained
1-(4-nicotinoylaminobenzoyl)-3-(3-propoxy-4-
methoxy-phenyl)-1,4,5,6-tetrahydropyridazine, m.p.
239°;
1-(4-trifluoroacetamidobenzoyl)-3-(3-ethoxy-4-
methoxy-phenyl)-1,4,5,6-tetrahydropyridazine, m.p.
211°;
1-(4-ethoxycarbonylaminobenzoyl)-3-(3-propoxy-4-
methoxy-phenyl)-1,4,5,6-tetrahydropyridazine, m.p.
154°;
1-(4-isopropoxycarbonylaminobenzoyl)-3-(3-ethoxy-
4-methoxy-phenyl)-1,4,5,6-tetrahydropyridazine, m.p.
147°;
1-(4-propoxycarbonylaminobenzoyl)-3-(3-ethoxy-4-
methoxy-phenyl)-1,4,5,6-tetrahydropyridazine, m,p.
113°.
Example 6
Analogously to the Examples 2 and 3 the
following compounds are obtained by reaction of 1-(4-
CA 02263417 1999-02-11
- 28 -
aminobenzoyl)-3-(3,4-dimethoxyphenyl)-4-methyl-1,4,5,6-
tetrahydropyridazine
with nicotinoylchloride
1-(4-nicotinoylaminobenzoyl)-3-(3,4-dimethoxy-
phenyl)-4-methyl-1,4,5,6-tetrahydropyridazine, m.p.
190°;
with "B"
1-(4-ethoxycarbonylaminobenzoyl)-3-(3,4-dimethoxy
phenyl)-4-methyl-1,4,5,6-tetrahydropyridazine, m.p.
141°;
with acetyl cloride
1-(4-acetamidobenzoyl)-3-(3,4-dimethoxy-phenyl)-4-
methyl-1,4,5,6-tetrahydropyridazine, m.p. 223°.
The examples which follow relate to
pharmaceutical products:
Example A: Vials
A solution of 100 g of an active ingredient of
the formula I and 5 g of disodium hydrogen phosphate in
3 1 of twice-distilled water is brought to pH 6.5 with
2N hydrochloric acid, filter-sterilized, filled into
vials, lyophilized under sterile conditions and sealed
in sterile form. Each vial comprises 5 mg of active
ingredient.
Example B: Suppositories
A mixture of 20 g of an active ingredient of
the formula I is melted with 100 g of soya lecithin and
1400 g of cocoa butter, and the mixture is poured into
moulds and left to cool. Each suppository comprises
20 mg of active ingredient.
Example C: Solution
A solution is prepared from 1 g of an active
ingredient of the formula I, 9.38 g of NaH2P04~2H20,
28 . 48 g of Na2HP04 ~ 12Hz0 and 0 . 1 g of benzalkonium
chloride in 940 ml of twice-distilled water. The pH is
CA 02263417 1999-02-11
- 29 -
brought to 6.8, and the solution is made up to 1 1 and
sterilized by irradiation. This solution can be used in
the form of eyedrops.
Example D: Ointment
500 mg of an active ingredient of the formula I
are mixed with 99.5 g of petroleum jelly under aseptic
conditions.
Example E: Tablets
A mixture of 1 kg of active ingredient of the
formula I, 4 kg of lactose, 1.2 kg of potato starch,
0.2 kg of talc and 0.1 kg of magnesium stearate is
tableted in the customary manner in such a way that
each tablet comprises 10 mg of active ingredient.
Example F: Sugar-coated tablets
A mixture is tableted analogously to Example E,
and the tablets are subsequently coated in the
customary manner with a coating of sucrose, potato
starch, talc, tragacanth and colouring.
Example G: capsules
2 kg of active ingredient of the formula I are
filled into hard gelatin capsules in the customary
manner so that each capsule comprises 20 mg of the
active ingredient.
Example H: Ampoules
A solution of 1 kg of active ingredient of the
formula I in 60 1 of twice-distilled water is filter-
sterilized, filled into ampoules, lyophilized under
sterile conditions and sealed in sterile form. Each
ampoule comprises 10 mg of active ingredient.
Example I: Spray for inhalation
14 g of active ingredient of the formula I are
dissolved in 10 1 of isotonic NaCl solution, and the
solution is filled into commercially available pump-
CA 02263417 1999-02-11
- 30 -
operated spray containers. The solution can be sprayed
into mouth or nose. One actuation (approximately
0.1 ml) corresponds to a dose of approximately 0.14 mg.