Note: Descriptions are shown in the official language in which they were submitted.
CA 02263672 1999-02-18
W098/0~56 PCT~S97/14801
- 1 - . ,,
5METHODS AND APPARATUS FOR TRANSMYOCARDIAL
DIRECT CORONARY REVASCUL~RIZATION
~ield of the Invention
The present invention pertains generally to medical
treatment methods and devices, and more particularly to
methods and devices for transluminal direct coronary
revascularization.
Backaround of the Invention
Coronary artery disease continues to be one of the
leading causes of morbidity and mortality, throughout the
world. The typical etiology of coronary artery disease is
characterized by the build-up of atherosclerotic plaque
within the coronary arteries. Such deposits of
atherosclerotic plaque tend to fully or partially block the
flow of blood through the affected coronary arteries, and
if untreated can result in myocardial ischemica, infarction
and death.
For many years, the traditional surgical treatment of
coronary artery disease has been coronary artery bypass
surgery. In traditional coronary artery bypass surgery,
the patient is generally anesthetized and placed on
cardiopulmo~Ary bypass. A thoracotomy is performed and the
obstructed coronary blood vessels are exposed by surgical
dissection. One or more segments of the patient's saphenous
vein or internal mAmm~ry artery is/are harvested for use as
bypass graft(s). The harvested segment(s) of vein or
artery is/are then anastomosed to the obstructed coronary
artery(ies) to form bypass conduit(s) around the arterial
obstruction(s). Such traditional coronary artery bypass
surgery is expensive, extremely invasive, and is associated
. .
CA 02263672 1999-02-18
W O 98/08456 PCTrUS97/14801
2- _
with significant operative and perioperative complications.
One alternative to traditional coronary artery bypass
surgery is balloon angioplasty. In balloon angioplasty, a
flexible guide catheter is percutaneously inserted into a
peripheral artery (e.g., the femoral artery) and is
transluminally advanced through the vasculature-until the
distal tip of the catheter is within an obstructed coronary
artery. Thereafter, a balloon catheter is passed through
the guide catheter and into the obstructive lesion. The
balloon of the balloon catheter is inflated one or more
times to dilate coronary artery in the region of the
obstructive lesion. These balloon angioplasty procedures
tend to be less expensive and less traumatic than
traditional coronary artery bypass surgery. However,
balloon angioplasty procedures of this type have been
associated with a significant incidence of restenosis at
the angioplasty site. The cause and mechanism of such
restenosis continues to be the subject of ongoing study.
However, such restenosis has generally been attributed to
either a) an increase in the mass of the artery wall (e.g.,
neointima formation), b) a thickening of the artery wall
without substantial change in it's mass (e.g., vascular
remodeling) and/or c) radial contraction of the balloon-
dilated artery wall upon healing of cracks and fissures
that have been created by the balloon dilation process.
Another alternative to traditional coronary artery
bypass surgery is transluminal atheroectomy or ablation of
the obstructive matter within the coronary artery. These
t~ransluminal atheroectomy or ablation procedures are
performed by passing a catheter-mounted ablation apparatus
through the vasculature to the site of the coronary
obstruction. the catheter-mounted ablative apparatus is
then utilized to cut, shave, sonicate, pulverize or
otherwise ablate the obstructive matter from the lumen of
the coronary artery. These atheroectomy or ablative
CA 02263672 1999-02-18
W098/08456 PCT~S97/14801
procedures must be performed with caution to avoid abrasion
or damage to the artery wall, as such abrasion or damage
can result in excessive scaring and subsequent reocclusion
of the artery lumen. Furthermore, these atheroectomy or
ablative procedures may, in some cases at least, be
con~ounded by the need to meticulously contain ~nd remove
the severed fragments o~ obstructive matter in order to
prevent such fragments of obstructive matter from escaping
into the patient's circulatory system. ~xamples of such
atheroectomy catheters and other catheter-mounted ablative
apparatus are described in United States Patent Nos.
3,433,226 (Boyd), 3,823,717 (Pohlman, et al.), 4,808,153
(Parisi), 4,936,281 (Stasz), 3,565,062 (Kuris), 4,924,863
(Sterzer), 4B70,953 (Don Michael, et al.), 5,069,664
(Suess, et al.), 4,920,954 (Alliger, et al.) and 5,100,423
(Fearnot), as well as foreign patents/patent publications
EP0347098A2 (Shiber), W087-05739 (Cooper), WO89-06515
(Bernstein, et al.), WO90-0130 (Sonic Needle Corp.),
EP316789 (Don Michael, et al.), DE 3,821,836 (Schubert),
DE2438648 (Pohlman), and EP 04432~6Al (Baruch).
Other alternatives to traditional coronary artery
bypass surgery have included min;mAlly invasive endoscopic
procedures which, ostensibly at least, can be performed
through small (e.g., 1-3cm) incisions formed in the
patient's chest wall, by insertion of a thoracoscope and
associated operative instruments through such incisions.
One such thoracoscopic coronary bypass procedure is
described in United States Patent No. 5,452,733 (Sterman et
al.). If perfected, these minimally invasive coronary
artery bypass procedures may lessen the discomfort and
length of recovery time experienced by patients who undergo
such minimally invasive procedures vis a vis those who
undergo traditional coronary artery bypass surgery.
However, the performance of endoscopic surgical procedures
of this type typically requires a great deal of operator
.... - ~
CA 02263672 1999-02-18
W098/08456 PCT~S9711~01
skill and training. Furthermore, as with traditional
coronary artery bypass surgery, the patients on whom these
thoracoscopic procedures are performed are likely to
undergo general anesthesia (with or without cardiopulmonary
bypass~ and the creation of a pneumothorax due to the
formation of full-thickness incision(s) in the chest wall.
Thus, many of the drawbacks associated with traditional
coronary artery bypass surgery, are also associated with
these minimally invasive thoracoscopic procedures.
Another previously described procedure for bypassing
coronary artery obstructions utilizes a transmyocardial
passageway (e.g., an interstitial tunnel formed in the
muscular wall of the heart) to carry blood from the left
ventricle of the heart to an obstructed coronary artery.
Such procedure, hereinafter generally referred to as
"Transmyocardial Direct Coronary Revascularization" (TMDCR)
is described in United States Patent Nos. 5,287,861 (Wilk),
5,409,019 (Wilk), and 5,429,114 (Wilk). The TMDCR methods
described in these prior patents require that a catheter be
introduced into the obstructed coronary artery and advanced
through the obstructive lesion. After the catheter has
been advanced through the obstructive lesion, the distal
tip of the catheter is directed toward the artery wall and
an opening (i.e., a transmycardial passageway) is formed
through the artery wall, through the adjacent myocardium,
and into the chamber of the left ventricle. Also, in this
previously described TMDCR method, a stent is required to
be positioned within the transmyocardial passageway. Such
i,ntramyocardial stent is constructed to perform a one-way
valving function (i.e., to open and close the
transmyocardial passageway in accordance with changes in
the systolic-diastolic cardiac cycle). These TMDCR
methods, previously described in United States Patent Nos.
5,287,861 (Wilk), 5,409,019 (Wilk) and 5,429,114 (Wilk),
may be difficult or impossible to perform in patients who
CA 02263672 1999-02-18
W O 98/08456 PCT~US97/14801
5- _
suffer from total or near total obstructions of a coronary
artery, because of the necessary for advancing the catheter
through the coronary artery obstruction to accomplish
creation of the transmyocardial passageway at a location
which is downstream of the coronary obstruction.
Furthermore, because these previously described TMDCR
methods require placement of a stent within the
transmyocardial passageway, such procedures are necessarily
associated with procedural complexities associated with
measuring and precutting the stent to a precise length so
that it fits within the transmyocardial passageway without
protruding into the chamber of the left ventricle and/or
the lumen of the coronary artery. Also, any stent which is
positioned solely within the transmyocardial passageway may
be subject to repetitive flexing and/or stressing as the
myocardium undergoes its normal contraction and relaxation.
Such repeated flexing and/or stressing of the
intramyocardial stent may lead to unwanted migration,
dislodgement or damage of the stent.
In view of the above-summarized shortcomings and
complexities of the previously described TMDCR methods,
there exists a need in the art for the development o~
improved TMDCR methods and associated apparatus which may
be utilized without the need for cumbersome stenting of the
transmyocardial passageway and/or implantation of one-way
valving apparatus within the transmyocardial passageway.
Also, there exists a need for the development of a new
TMDCR methods which can be performed in patients who suffer
from total or near total coronary artery occlusion(s),
without the need for advancing a catheter through such
coronary artery occlusion(s).
Summarv of the Invention
The present invention provides new TMDCR methods, as
well as certain valving devices which are usable in
con~unction with these TMDCR methods.
.
CA 02263672 l999-02-l8
W O 98108456 PCT~US97/14801
i. T~DCR Procedures Usinq coronar~Y Vein
In accordance with the invention, there is provided a
specific TMDCR method wherein a transmyocardial passageway
is formed between a chamber of the heart (e.g., left
ventricle) and a coronary vein. In this embodiment of the
invention, blood may pass from the cardiac chambe~, through
the transmyocardial passageway, and into the coronary vein
for the purpose of improving blood flow to the myocardium
and/or to equalize or normalize pressures within the
coronary venous vasculature by draining blood from the vein
into the cardiac chamber. The coronary vein of this
embodiment may be situated next to an obstructed coronary
artery, and one or more secondary blood flow passageways
may be created between the coronary vein and the adjacent
artery, at site(s) which is/are downstream of the coronary
artery obstruction. Also, the lumen(s) of the coronary
vein and/or ad~acent coronary artery may be blocked or
embolized at appropriate positions to facilitate the flow
of blood in the desired direction(s) through the man-made
blood flow passageway(s), the coronary vein and/or the
coronary artery. Additionally, one or more valving
apparatus may be positioned within the coronary vein and/or
within the cardiac chamber, to control or intermittently
block the flow of blood through the transmyocardial~5 passageway.
ii. TMDCR Procedures Usina Unstented
TransmYocardial PassaaewaY
In accordance with the present invention, there is
p~rovided a method for coronary re-vascularization wherein
an unstented transmyocardial passageway (e.g., a puncture
tract, bore, tunnel, or other passageway) is formed between
a chamber of the heart (e.g., the left ventricle) and a
coronary vessel (e.g., a) an endogenous coronary artery; b)
an endogenous coronary vein; c) a man-made passageway which
CA 02263672 1999-02-18
W O 98/08456 rcT~usg71l4801
has been formed in the heart, and which leads to an
endogenous coronary vein; d) a man-made passageway which
has been formed in the heart, and which leads to an
endogenous coronary artery; and/or e) a man-made passageway
which has been formed in the heart between an endogenous
coronary artery and an endogenous coronary vein). The
unstented transmyocardial passageway(s) created in
accordance with this embodiment of the invention may be
utilized to improve perfusion of the myocardium by shunting
blood from the chamber of the heart (e.g., left ventricle)
into the coronary vessel (e.g., vein artery or man-made
passageway), or may alternatively be utilized to equalize
or normalize flow or pressure within the cardiac
vasculature by draining blood from one or more cardiac
vessels (e.g., vein, artery or man-made passageway), into
the chamber of the heart.
iii. Valvina Devices Positionable in Coronarv Vessels
Still further in accordance with the present
invention, there are provided several types of intraluminal
valving apparatus which may be positioned within the
lumen(s) of the coronary blood vessel(s) (i.e., artery,
vein or man-made passageway) which intersect with the
transmyocardial passageway, to intermittently block
bloodflow, in at least one direction, through the
transmyocardial passageway. These intraluminal valving
devices generally comprise tubular bodies having at least
one occluder member positioned therein, said occluder
member(s) being alternately moveable between i) open
position(s) whereby bloodflow is permitted to pass through
the transmyocardial bloodflow passageway in a desired
direction, and ii) closed position(s) whereby blood is
prevented from flowing through the transmyocardial
bloodflow passageway, in an undesired direction.
iv. Tissue Valves For TMDCR Passaaewav
, . .
CA 02263672 1999-02-18
PCT~S97/14801
W098/08456
Alternatively, the present invention also includes
endogenous tissue valve(s) which are formed in the
transmyocardial passageway to perform a desired one-way
valving function whereby blood is permitted to flow through
the transmyocardial bloodflow passageway in a first
direction, but is prevented from backflowing or
regurgitating in a second direction.
v. Intracardiac Valvinq Devices For TMDCR PassaqewaY
Still further in accordance with the present
invention, there are provided intracardiac valving devices
which are mountable within a chamber of the heart (e.g.,
left ventricle) immediately adjacent to an opening into a
transmyocardial passageway which extends from the cardiac
chamber to a coronary vessel (e.g., artery, vein or man-
made passageway). Such intracardiac valving device may beconstructed such that it will open in response to
hemodynamic pressure generated during systole and/or in
response to mechanical contraction (i.e., shortening and
thickening) of the myocardium during systole. When open,
the intracardiac valving device permits blood to flow
through the transmyocardial bloodflow passageway.
Thereafter, the valving device may be constructed to close
when diastolic pressures are present in the cardiac chamber
or when the myocardium undergoes mechanical relaxation
(i.e., lengthening and thinning during diastole. When
closed, the valving device will prevent blood from
backflowing or regurgitating from the transmyocardial
bloodflow passageway, into the cardiac chamber.
~ iv. Protrusive Stents and Stented Grafts
For ~MDCR PassaaewaYs
Still further in accordance with the present
invention, there are provided stents and stented grafts
which are positionable within the transmyocardial
passageway, and which protrude into the adjacent coronary
CA 02263672 1999-02-18
W O 98/08456 PCT~US97/14801
g
vessel (e.g., vein, artery or man-made passageway). These
protrusive stents and/or protrusive stented grafts may be
self-expanding or pressure-expandable. Optionally, one or
more valves or occluder members may be positioned within
such protrusive stents and/or stented grafts to facilitate
valving or directed movement of bloodflow in accordance
with the diastolic/systolic cardiac cycle.
Further objects and advantages of the present
invention will become apparent to those skilled in the art
upon reading and understanding of the following detailed
descriptions of preferred embodiments.
Brief Descri~tion of the Drawin~s
Figure 1 is a perspective view of a human heart
showing the typical anatomical positioning of the coronary
arteries and coronary veins of the left heart.
Figure la is a partial cut-away sectional view of a
human heart wherein a transmyocardial passageway has been
created between the left ventricle and a coronary vein, in
accordance with the present invention.
Figure lb is a partial longitudinal sectional view
through an obstructed coronary artery and adjacent coronary
vein, showing a transmyocardial passageway of the present
invention, extending between the chamber of the left
ventricle and the coronary vein.
Figure lc is a partial longitudinal sectional view
through an obstructed coronary artery and adjacent coronary
vein, showing a transmyocardial passageway of the present
invention extending between the chamber of the left
v~entricle to the coronary vein, and a secondary bloodflow
passageway extending from the coronary vein to the adjacent
coronary artery, downstream of the obstruction.
Figure ld is a partial longitudinal sectional view
through a portion of the myocardium of a human heart,
adjacent the left ventricle, showing an alternative
embodiment of the present invention wherein a
CA 02263672 l999-02-l8
W O 98/08456 PCTrUS97/14801
- 10 -
transmyocardial bloodflow passageway extends from the
chamber of the left ventricle to a secondary passageway
which has been created between the obstructed coronary
artery and the adjacent coronary vein. Figure 2 is a
longitudinal sectional view showing a first embodiment of
an intravascular valving apparatus of the present invention
operatively positioned within a coronary blood vessel
(artery, vein or man-made passageway).
Figure 2a is a perspective view of the intravascular
valving apparatus of Figure 2.
Figure 2b is an elevational view of a variant of the
intravascular valving apparatus shown in Figures 2 and 2a,
wherein a bloodflow blocking bulkhead is formed on the
upstream end of the apparatus.
Figure 3 is a longitudinal sectional view of a second
embodiment of an intravascular valving apparatus of the
present invention operatively positioned in a coronary
blood vessel (artery, vein or man-made passageway).
Figure 3a is longitudinal sectional view showing
variant of the second intravascular valving apparatus
embodiment shown in Figure 3, wherein two (2) separate
valving apparatus are respectively positioned upstream and
downstream of the junction between the transmyocardial
bloodflow passageway and the coronary blood vessel (artery,
vein or man-made passageway).
Figure 3b is a longitudinal sectional view of another
variant of the second intravascular valving apparatus
embodiment shown in Figure 3, wherein three (3) valves are
i~ncorporated within a single tubular body to accomplish
valving of bloodflow through a transmyocardial bloodflow
passageway and coronary blood vessel tartery, vein or man-
made passageway).
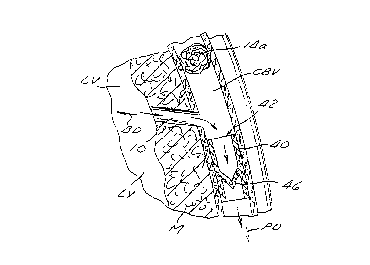
Figure 4 is a longitudinal sectional view showing a
third embodiment of an intravascular valving apparatus of
the present invention operatively positioned within a
CA 02263672 1999-02-18
W O 98/08456 PCTrUS97/14801
coronary blood vessel (artery, vein or man-made
passageway)
Figure 5 is a longitudinal sectional view showing an
intracardiac valving apparatus of the present invention
along with an optional retainer assembly (dotted lines)
useable to mount such intracardiac valving apparatus on the
inner wall of the heart.
Figure 5a is a perspective view of the intracardiac
valving apparatus o~ Figure 4 having the op~ional retainer
assembly affixed thereto.
Figures 6a and 6b are longitudinal sectional views of
a human heart wherein a bloodflow passageway has been
created between the left ventricle and a coronary blood
vessel (artery, vein or man-made passageway), and a valving
tissue valve has been created in the wall of the blood
vessel, in accordance with the present invention.
Figures 7a-7b are longitudinal sectional views of a
human heart wherein a blood vessel passageway has been
created between the left ventricle and a coronary blood
vessel (artery, vein or man-made passageway), and wherein
an elastic suture has been positioned, in accordance with
the present invention.
Figure 8a is a longitudinal sectional view showing a
protrusive stent apparatus of the present invention
implanted within a transmyocardial passageway and extending
into a coronary blood vessel (e.g., artery, vein or man-
made passageway).
Figure 8b is a longitudinal sectional view showing an
~lternative embodiment of the protrusive stent apparatus
shown in Figure 5a.
Figure 8c is a longitudinal sectional view showing
another alternative embodiment of the protrusive stent
apparatus shown in Figure 5a, having an optional tubular
covering and/or optional valve(s) incorporated therein.
Detailed Description of the Preferred Embodiments
... ....
CA 02263672 1999-02-18
W098/08456 ~CT~S97/14801
-12-
The following detailed description and the
accompanying drawings are provided for purposes of
describing and illustrating presently preferred embodiments
of the invention only, and are not intended to limit the
scope-of the invention in any way.
Upon making reference to the accompanying figures, it
will be noted that many of the figures include showings of
human cardiovascular anatomy. The various anatomical
structures shown in the figures are labeled in accordance
with the following legend:
AO . . . . . . . . . . . . . . . . . . Aorta
CBV . . . . . . . . Coronary Blood Vessel
(artery, vein or
man-made passageway)
l5CA . . . . . . . . . . . . . Coronary Artery
CAL . . . . . . . . . Coronary Artery Lumen
CV . . . . . . . . . . . . . . Coronary Vein
CVL . . . . . . . . . . Coronary Vein Lumen
IVC . . . . . . . . . . . Inferior Vena Cava
20SVC . . . . . . . . . . . Superior Vena Cava
LV . . . . . . . . . . . . . . Left Ventricle
RV . . . . . . . . . . . . . Right Ventricle
IVS . . . . . . . . Intraventricular Septum
M .. ...............................Myocardium
i. T~CRMethodUtilizinaCoronarvVein
With reference to Figures 1-4, the present invention
includes methods for improving perfusion of regions of the
myocardium M which are ischemic or otherwise affected by
t~e existence of an obstruction OB within a coronary artery
CA, by forming a transmyocardial passageway l0 which
extends from a chamber of the heart, (e.g., left ventricle
LV), to a coronary vein CV.
In some embodiments of this method, the
transmyocardial passageway l0 will simply provide a flow of
blood from the chamber of the heart and into the coronary
CA 02263672 1999-02-18
W O 98/08456 PCT~US97tl4801
-13-
vein CV, such that the blood will pass in retrograde
fashion through the coronary vein CV to perfuse the
ischemic portion of the myocardium through the coronary
vein, as sheen in Figure lb.
In other embodiments of the invention, a secondary
bloodflow passageway 12 may be created between the coronary
vein CV into which the transmyocardial passageway 10
extends and the obstructed coronary artery CA, at a
location which is downstream of the obstruction OB, as
shown in Figure lc. The formation of this secondary
bloodflow passageway 12 allows blood from the chamber of
the heart (e.g., the left ventricle LV) to initially flow
through the transmyocardial passageway 10, through a
segment of the coronary vein lumen CVL, through the
secondary bloodflow passageway 12, and into the coronary
artery lumen CAL, at a location downstream of the coronary
artery obstruction OB, as shown in Figure 2b. The
secondary bloodflow passageway 12 which extends between the
coronary vein CV and the coronary artery ~A may optionally
be stented or internally supported by a stent, sleeve or
coating (e.g., a polymer coating) to maintain patency of
the secondary passageway 12,
In at least some applications, the coronary vein lumen
CVL may be purposely blocked (e.g., ligated, embolized,
fused, welded, clamped, etc.) at site(s) upstream and/or
downstream of the transmyocardial passageway 10. As shown
in Figure lb, when the transmyocardial passageway 10 formed
for the purpose of shunting oxygenated blood into the
aoronary vein lumen CVL, a proximal embolization member 14a
may be positioned within the coronary vein lumen CVL,
immediately upstream of transmyocardial passageway 10, to
ensure that the shunted blood will flow, in the desired
retrograde direction through the coronary vein CV.
Similarly, as shown in Figure lc, when a secondary
bloodflow passageway 12 is formed to carry the oxygenated
~ ., . " . .. . . . ...
CA 02263672 1999-02-18
W O 98/08456 PCTrUS97/14801
-14- .
blood from the coronary vein lumen CVL into the coronary
artery lumen CAL, downstream of the obstruction OB, a
distal embolization member l~b may be positioned within the
coronary vein lumen CVL immediately downstream of the
secondary bloodflow passageway 12, to divert the flow of
blood through the secondary bloodflow passageway 12.
Examples of methods for forming the optional secondary
bloodflow passageway(s) 12 between the coronary vein CV and
coronary artery CA are described in United States
Provisional Specification Nos. 60/005,164, filed October
13, 1995 and 60/010,614 filed February 2, 1996, the entire
disclosures of which are expressly incorporated herein by
reference.
The proximal embolization member 14a and/or distal 14b
embolization member may comprise any suitable type of lumen
blocking matter or apparatus, examples of which are the
embolization coils described in United States Patent Nos.
5,382,260 (Dormandy, Jr. et al.), 5,108,407 (Geremia et
al.), and 5,256,146 (Ensminger, et al.). Alternatively,
the coronary vein lumen CVL may be closed off at the sites
of the proximal 14a and/or distal 14b embolization members
by any suitable alternative means, such as clamping,
clipping, ligating, fusing, welding or adhesively
con~oining the inner walls of the coronary vein lumen CVL
so as to provide the desired bloc~ing of bloodflow
therethrough.
Figure ld shows an alternative embodiment of the
method of the present invention wherein a secondary
bloodflow passageway 12 of the above-described type has
been created between the coronary vein CV and coronary
artery CA, and wherein the transmyocardial bloodflow
passageway 10a extends from the chamber of the heart (e.g.,
left ventricle) such secondary bloodflow passageway 12.
ii. TMDCR Methods Em~loYina Non-Stented
TransmYocardial PassaaewaY
CA 02263672 l999-02-l8
WO 98/08456 PCTrUS97/14801
-15-
The present invention also includes alternative TMDCR
methods wherein a transmyocardial passageway 10 is formed
between a chamber of the heart and a coronary vessel (i.e.,
a) an endogenous coronary vein, b) an endogenous coronary
artery, c) a man-made passageway in the heart which
connects to an endogenous coronary vein; d) a man-made
passageway in the heart which connects to an endogenous
coronary or e) a man-made passageway which extends between
an endogenous coronary artery and an endogenous coronary
vein), and such transmyocardial passageway 10 is allowed to
remain non-stented (e.g., devoid of any stent or internal
support member positioned therewith).
The utilization of a non-stented transmyocardial
passageway 10 in accordance with this embodiment of the
present invention eliminates the need for precise
measurement, precutting to length and insertion of a stent
apparatus within the transmyocardial passageway 10, as is
required of the previous TMDCR method described in United
States Patent Nos. 5,287,861 (Wilk), 5,409,019 (Wilk) and
5,429,114 (Wilk). When the non-stented transmyocardial
passageway 10 of the present invention is intended to
provide bloodflow from the chamber of the heart (e.g., left
ventricle) into the coronary vessel (e.g., vein, artery or
man-made passageway), the non-stented transmyocardial
passageway 10 must remain open during systolic contraction
of the myocardium. If the non-stented passageway 10 is
permitted to substantially occlude or close-off during
systolic contraction of the myocardium, such could prevent
or deter the desired ~lood flow from passing through the
transmyocardial passageway 10. In this regard, in
embodiments of the invention which utilize the non-stented
transmyocardial passageway 10, it may be desirable to
debulk, core or otherwise enlarge the diameter of the
passageway 10 during it's formation so as ensure that the
passageway 10 will remain patent and open, even during
CA 02263672 1999-02-18
W O 98108456 PCTrUS97/14801
-16- -~
systolic contraction of the myocardium. Such coring,
debulking or other enlargement of the passageway 10 may be
accomplished by any suitable means, including the use of a
hollow coring needle, laser, electrosurgical probe, or
other tissue removing/ablating device capable of debulking
and removing tissue so as to create a transmyocardial
passageway 10 of the desired diameter.
Also, it will be appreciated that the non-stented
transmyocardial passageway preferably should not fill-in
with granulation tissue or otherwise close-off as a result
of any scarring or healing process of the myocardium. In
this regard, the coring, de-bulking or other enlargement of
the non-stented passageway 10 and/or the continuing passage
of blood, therethrough, may be sufficient to prevent or
deter such scarring or natural closing of the non-stented
passageway 10. However, in applications wherein scarring
or natural closing of the non-stented passageway 10 is a
potential problem, it may be desirable to cauterize, heat,
chemically treat or coat the walls of the non-stented
passageway to prevent or deter blocking of such passageway
by scarring or ingrowth of the myocardial tissue.
iii. Valvinq A~paratus Positionable in the CoronarY
Vessel(s) to Prevent Backflow Into the
TransmYocardial Bloodflow PassaaewaY
25In many embodiments of the invention, the
transmyocardial passageway 10, lOa may function in itls
intended manner without the inclusion of any valving
apparatus, for intermittently blocking the flow of blood
therethrough. However, in at least some applications, it
may be desired to prevent the backflow of blood through the
transmyocardial passageway 10, lOa during certain phase(s)
of the cardiac cycle when the relative hemodynamic
pressures would tend to cause such backflow. In this
regard, the present invention includes intravascular
valving apparatus 20, 30, 31, 33, 40, examples of which are
CA 02263672 1999-02-18
W O 98/08456 PCT~US97/14801
-17-
shown in Figures 2-4. These intravascular valving apparatus
20, 20, 31, 33, 40 are positionable within the lumen of the
coronary blood vessel CBV (e.g., vein, artery or man-made
passageway), and operate to prevent backflow of blood into
the transmyocardial bloodflow passageway 10, 10a.
In general, each of the intravascular valving
apparatus 20, 30, 31, 33, 40 of the present invention
comprise a radially expandable cylindrical or tubular body
which is transluminally advanceable into the lumen of the
coronary blood vessel CBV (e.g., artery, vein or man-made
passageway), and which is then radially expandable so as to
become implanted at a location which is adjacent or near to
the intersection of that coronary vessel CBV with a
transmyocardial bloodflow passageway 10, 10a. The valving
apparatus 20, 30, 31, 33, 40 has an axial bore 24, 34, 42
through which blood may pass as it flows through the lumen
of the coronary blood vessel CBV or secondary passageway 12
in which the apparatus 20, 30, 31, 33, 40 is positioned.
One or more occluder members 26, 36, 46 are formed within
the apparatus 20, 30, 31, 33, 40. Such occluder member(s)
26, 36, 46 are alternately moveable between a first (e.g.,
open) position whereby blood is permitted to flow from the
transmyocardial bloodflow passageway into the coronary
blood vessel CBV or secondary passageway 12, and a second
(e.g., closed) position whereby blood is prevented or
deterred from backflowing or regurgitating from the
coronary blood vessel CBV or secondary passageway 12, into
the transmyocardial bloodflow passageway.
Individual embodiments of the intravascular valving
apparatus 20, 30, 31, 33, 40 are described in more detail
herebelow. It will be appreciated, however, that each of
the intravascular valving apparatus 20, 30, 31, 33, 40 of
the present invention offer advantages over the
intramyocardial stenting/valving apparatus described in
United States Patent Nos. 5,248,861 (Wilk), 5,409,019
CA 02263672 1999-02-18
W O 98/08456 PCTrUS97/14801
-18- _.
(Wilk) and 5,429,144 (Wilk) in that they are operatively
situated entirely within the lumen of the coronary blood
vessel CBV of secondary passageway 12 and do not extend
into the transmyocardial passage way (e.g., the first
passageway 10, lOa) which emanates from the chamber (e.g.,
left ventricle) of the heart. In this regard, the valving
apparatus 20, 30, 31, 33, 40 of the present invention do
not require precise measurement or precise cutting-to-
length, as is purportedly required of the intramyocardial
stenting/valving apparatus described in U.S. Patent Nos.
5,248,861 (Wilk), 5,409,019 (Wilk) and 5,429,144 (Wilk).
It is desirable that the valving apparatus 20, 30, 31,
33, 40 of the present invention be initially disposable in
a first radially compact diameter which is small enough to
be mounted upon or inserted into an intravascular delivery
catheter. Such intravascular delivery catheter, having the
valving apparatus 20, 30, 31, 33, 40 mounted thereon or
therewithin, is transluminally passable through the
vasculature and into the lumen of the coronary blood vessel
CBV wherein the apparatus 20, 30, 31, 33, 40 is to be
implanted. Thereafter, the apparatus 20, 30, 31, 33, 40 is
radially expanded (by self-expansion or pressure-expansion)
to a second radially expanded diameter, wherein the outer
surface of the apparatus 20, 30, 31, 33, 40 frictionally
engages the surrounding wall of the coronary blood vessel
CBV such that the apparatus 20, 30, 31, 33, 40 is thereby
implanted and retained in a stationary position. When the
valving apparatus 20, 30, 31, 33, 40 is so implanted within
the coronary blood vessel CBV, blood may flow through the
axial bore 24, 34, 42 of the apparatus 20, 30, 31, 33, 40,
as described in more detail herebelow.
It is to be appreciated that the valving apparatus 20,
30, 31, 33, 40 may be either self-expanding or pressure-
expandable. In this regard, if the valving apparatus 20,
30, 31, 33, 40 is "self-expanding", the cylindrical body of
CA 02263672 1999-02-18
W098/08456 PCT~S97/14801
- 19 - _,.
the apparatus 20, 30, 3l, 33, 40 may be formed of a shape
memory alloy or resilient material (e.g., spring metal)
which is inherently biased to it's second radially expanded
diameter. Alternately, in embodiments wherein the valving
apparatus 20, 30, 31, 33, 40 is "pressure-expandable", the
cylindrical body of the apparatus 20, 30, 31, 33, 40 may be
formed of plastically deformable material which is
initially formed it's first radially compact diameter, and
which may be pressure deformed to it's second radially
expanded diameter by the exertion of outward force from an
internally positioned balloon or other radial expansion
device.
It is to be further appreciated that the potential
useability and applicability of the intravascular valving
apparatus 20, 30, 31, 33, 40, 50 described herebelow is not
limited only to uses in connection with the improved TMDCR
methods of the present invention, but may also be useable
as a modification of the previously described TMDCR
methods, such as those of United States Patent Nos.
5,287,816(Wil~), 5,409,0l9(Wilk), and 5,429,144 (Wilk).
a. Intravascular Valvina A~aratus-First Embodiment:
Figures 2, 2a and 2b show a first embodiment of an
intravascular valving apparatus 20 which is positioned
within the lumen of a coronary blood vessel CBV (artery,
vein or man-made passageway), at a location which is
adjacent it's intersection with the transmyocardial
passageway lO. This embodiment of the valving apparatus 20
has a cylindrical body having an axial bore 24 which
e~tends longitudinally therethrough, and a side aperture 22
formed in the sidewall thereof. The side aperture 22 is
preferably the same size or larger than the diameter of the
adjacent end of the transmyocardial passageway lO, such
that blood flowing from the cardiac chamber (e.g., left
ventricle LV) through the transmyocardial passageway lO
will pass directly through the side aperture 22 and into
.... , ~,
CA 02263672 1999-02-18
W 098l084S6 PCTrUS97/14801
-20-
the bore 24 of the valving apparatus 20. An occluder
member 26, such as a hinged obturator or pliable
elastomeric leaflet is affixed to the cylindrical body of
the valving apparatus 20, and extends over and
substantially blocks the side aperture 22 so as to prevent
the flow of blood out of the side aperture 22. The
occluder member 26 is alternately moveable between a first
position wherein it blocks blood from flowing out of the
side aperture 22, and a second position wherein it permits
blood to flow into the bore 24 through the side aperture
22.
This first embodiment of the valving apparatus 20 may
be implanted in the lumen of the coronary blood vessel CBV
such that the side aperture 22 is in alignment with the
adjacent end of the bloodflow passageway 10. During
systolic contraction of the heart the relatively high
pressure within the left ventricle will force the occluder
member 26 to its second (open) position, allowing blood to
flow from the left ventricle, through the transmyocardial
passageway 10, through the side aperture 22, through the
bore 24 and into the lumen of the coronary blood vessel CBV
in the perfusive direction PD, as shown. Thereafter,
during systolic relaxation of the heart, the relatively low
filling pressure within the left ventricle LV will draw the
occluder member 26 to its first (closed) position whereby
the occluder member 26 will prevent blood from
regurgitating or moving in the backflow direction BD from
the lumen of the coronary blood vessel CBV , out of the
side aperture 22, and into the bloodflow passageway 10. In
this manner the first embodiment of the valving apparatus
20 serves to facilitate efficient pumping of oxygenated
blood from the left ventricle and into the lumen of the
coronary blood vessel CBV, to improve the flow of
oxygenated blood to an ischemic or blood-flow-deprived
region of the myocardium M.
CA 02263672 1999-02-18
W O ~8/08~r~ PCTrUS97/14801
-21-
As shown in Figure 2a, a closure member 21, in the
nature of an end cap, may be formed on the upstream end of
the apparatus 20 so as to completely or substantially block
the flow of blood through the coronary blood vessel CBV and
into the upstream end of the bore 24 of the apparatus 20.
The optional inclusion of the end closure member 21 in the
apparatus 20 may serve to obviate any need for the
placement of a proximal embolization member 14a within the
lumen of the coronary blood vessel CBV, upstream of the
valving apparatus 20.
b. Intravascular Valvinq ADparatus-Second Embodiment
Figure 3 shows a second embodiment of the
intravascular valving apparatus 30 which comprises a
generally cylindrical body having an axial bore 34
extending longitudinally therethrough and a pair of
occluder members 46 positioned therewithin, and a side
aperture 32 formed in the cylindrical sidewall of the
apparatus 30, behind the occluder members 36. Each
occluder member 36 is affixed at least one point to the
cylindrical body of the apparatus 30, and may comprise any
suitable structure or openable and closeable passage, such
as a self-sealing slit or hole, or a hinged leaflet or
pliable elastomeric member. The occluder members 46 are
alternately moveable between first positions wherein the
occluder members 36 directly contact one another so as to
prevent blood from backflowing in the backflow direction BD
through the axial bore 34 of the apparatus 30, and second
positions wherein the occluder members 36 move out of
contact with one another such that blood may flow through
the axial bore 34 of the apparatus 30 in the perfusion
direction PD. The side aperture 32 is preferably as large
as or larger than the diameter of the bloodflow passageway
10 which extends through the myocardium M from the left
ventricle LV to the lumen of the coronary blood vessel CBV.
This embodiment of the apparatus 30 is implanted in the
.. . . . . ~. .. .
CA 02263672 1999-02-18
W 098/08456 PCTAUS97/14801
-22- -~
lumen of the coronary blood vessel CBV such that its side
aperture 32 iS directly aligned with the bloodflow
passageway 10 so that blood may flow through the bloodflow
passageway 10, into the axial bore 34 of the apparatus 30.
During systolic contraction of the heart the
relatively high pressures created in the left ve~tricle LV
will force blood to flow through the passageway 10 into
the axial bore 34 of the valving apparatus 30. Such
systolic bloodflow will move the occluder members 36 to
their second (i.e., open) positions, thereby allowing the
blood to flow through the lumen of the coronary blood
vessel in the perfusion direction PD. Thereafter, when the
heart undergoes diastolic relaxation, the relatively low
filling pressures created within the left ventricle LV will
draw the occluder members 36 to their first (ie. closed)
positions, thereby preventing blood from regurgitating or
backflowing out of the side aperture 32, in the backflow
direction BD. In this manner, this second embodiment of
the intravascular valving apparatus 30 serves to facilitate
efficient pumping of oxygenated blood from the left
ventricle LV and through the lumen of the coronary blood
vessel CBV, in order to provide improved bloodflow to an
ischemic or blood-flow-deprived region of the myocardium M.
Optionally, secondary occluder members 3 8 may be
formed or mounted within the bore 34 of the apparatus 30,
upstream of the side opening 32. These optional secondary
occluder members 38 may be of the same type and
construction as the above-described downstream occluder
m~mbers 36. If present, such additional occluder members
38 will assume their first (e.g., closed) position when the
pressure of blood within the bore 34 of the apparatus 30
downstream of such secondary occluder members 38 iS greater
than the pressure of blood within the coronary blood vessel
CBV upstream of the such secondary occluder member 3 8. In
this regard, the provision of such secondary occluder
CA 02263672 1999-02-18
W098/08456 PCT~S97/14801
-23-
members 38 within the apparatus 30 will obviate the need
for placement of a proximal occlusion apparatus 14a within
the lumen of the coronary blood vessel CBV upstream of the
transmyocardial bloodflow passageway 10. The inclusion of
such secondary occluder members 38, or the alternative use
of a proximal occlusion member 14a, will be of particular
importance when the coronary blood vessel CBV is a coronary
vein CV, due to the substantial difference between
endogenous coronary venous blood pressures and those
pressures which will be created by systolic arterial
bloodflow through the coronary vein, downstream of the
transmyocardial bloodflow passageway 10.
Figure 3a shows one variant of the second embodiment
wherein two (2) separate intravascular valving apparatus
31a, 31b are respectively positioned upstream and
downstream of the transmyocardial bloodflow passageway.
The above-described occluder members 36 are formed in the
apparatus 3lb which is positioned downstream of the
transmyocardial bloodflow passageway 10 and the above-
described secondary occluder members 38 are formed withinthe apparatus 31a which is positioned upstream of the
transmyocardial bloodflow passageway 10. In this manner,
these separate intravascular valving apparatus 31a, 31b,
will function in the same manner as the apparatus 30 shown
in Figure 3, when it is equipped with the optional
secondary occluder members 38. However, it will be
appreciated that these separate intravascular valving
apparatus 31a, 31b do not have any side aperture 32, as
does the device shown in Figure 3, and accordingly, will
obviate any need for correctly sizing an aligning such side
aperture 32 with the transmyocardial bloodflow passageway
10 .
Figure 3b shows another variant of the second
embodiment wherein a single intravascular valving apparatus
33, in the nature of a tubular stent or tubular body, is
CA 02263672 1999-02-18
W O 98/08456 PCTAUS97/14801
-24- _.
provided with three (3) separate valves 26, 36, 38 at
locations which are a) at the ~unction of the
transmyocardial passageway 10 and the coronary blood vessel
CBV, b) upstream of the transmyocardial passageway 10 and
c) downstream of the transmyocardial passageway 10,
respectively. These valves 26, 36, 38 may comprise self-
sealing pliable slit openings, elastomeric leaflets, hinged
occluder members or any other suitable type of structure or
apparatus which will intermittently open and close, to
permit bloodflow in the desired direction therethrough.
For example, in applications wherein it is desired for the
transmyocardial passageway 10 to provide a flow of blood
from the cardiac chamber into the coronary blood vessel
CBV, the first valve 26 will operate to open during systole
to permit blood to flow from the transmyocardial passageway
into the coronary blood vessel CBV, but will close
during diastole to prevent backflow or regurgitation into
the cardiac chamber. Similarly, the second (upstream valve
38 will close during systole to prevent backflow of blood
through the proximal end opening of the valving apparatus
33. The third (downstream) valve 36 will open during
systole to permit the desired flow of blood entering
through the transmyocardial passageway 10, to continue on
downstream through the coronary blood vessel CBV in the
desired perfusion direction.
c. Intravascular Valvina A~aratus-Third Embodiment
Figure 4 shows a third embodiment of the intravascular
valving apparatus 40 which comprises a generally
cylindrical body having an axial bore 42 extending
longitudinally therethrough and a plurality of occluder
members 46 formed therewithin. The cylindrical body and
occluder members 46 of this third embodiment of the
apparatus 40 are the same as those of the above described
second embodiment, except that the cylindrical body of this
third embodiment is devoid of any side aperture(s) or
CA 02263672 1999-02-18
W O 98/08456 PCTrUS97/14801
-25-
openings in the cylindrical sidewall. In contrast to the
above described second embodiment 30, this third embodiment
of the apparatus 40 is implanted in the lumen of the
coronary blood vessel CBV at a location which is downstream
of the junction between the coronary blood vessel CBV and
the first bloodflow passageway 10.
It will be appreciated that the individua; features
and attributes of each of the above-described embodiments
of valving apparatus 20, 30, 31, 33, 40 may be incorE~orated
into any or all of the other above-described valving
apparatus 20, 30, 31, 33, 40 as feasible, to accomplish the
desired hemodynamic bloodflow within the coronary
vasculature.
iv. Intracardiac Valvina A~aratus For Controllin~
Bloodflow Throuqh the TransmYocardial PassaaewaY
Figures 5 and 5a show examples of intracardiac valving
apparatus 80 which may be utilized to prevent backflow of
blood through the transmyocardial passageway 10, or to
otherwise control the flow of blood through the
transmyocardial passageway 10 in accordance with the
systolic/diastolic cardiac cycle.
As shown, the intracardiac valving apparatus 80 is
positionable within the cardiac chamber (e.g., left
ventricle) immediately adjacent the opening of the
transmyocardial passageway 10 thereinto. The intracardiac
valving apparatus 80 may comprise any suitable type of
hinged, pliable or moveable occlusion member or self-
sealing slit which will operate to intermittently block or
unblock the flow of blood in at least one direction through
the transmyocardial passageway 10. In the embodiment shown
in Figures 5, 5a, the intracardiac valving apparatus 80
comprises a generally annular body having a central
aperture formed therein and an occluder member 81, such as
a pliable elastomeric flap, mounted within the aperture.
The occluder member 81 will move, in relation to
CA 02263672 1999-02-18
W O 98/08456 PCTrUS97tl4801
-26-
hemodynamic bloodflow and/or contraction of the myocardium
M, between an open position whereby blood is permitted to
pass in at least one direction through the transmyocardial
passageway 10, and a closed position whereby blood is
prevented from flowing in at least one direction through
the transmyocardial passageway 10.
The intracardiac valving apparatus 80 may be implanted
within the cardiac chamber by any suitable surgical or non-
- surgical techni~ue. Preferably, the intracardiac v'alving
apparatus 80 is initially positioned within or upon a
delivery catheter, and the delivery catheter is advanced
through the coronary blood vessel CBV, and through the
transmyocardial passageway 10. Thereafter, the
intracardiac valving apparatus 80 is released or ejected
from the delivery catheter, and is caused to radially
expand to it's operative configuration. the expanded
valving apparatus 80 is then retracted into abutting
contact with the myocardial wall, as shown.
The intracardiac valving apparatus 80 may be attached
to the myocardial wall by any suitable attachment such as
hooks, sutures, adhesives or a retaining assembly which is
operative to hold the intracardiac valving apparatus 80 in
its desired fixed position upon the myocardial wall. One
such retaining apparatus, shown in Figures 5 and 5a,
comprises an annular retaining ring 82 which is
positionable within the coronary blood vessel CBV and a
plurality of elastomeric tether members 84 which extend
between the retainer ring 82 and the intracardiac valving
apparatus 80. In this manner, the elastomeric tethers 84
will resiliently draw the retaining ring 82 and
intracardiac valving apparatus 80 toward one another, so as
to hold the intracardiac valving apparatus 80 in fixed
abutment with the myocardium M as shown.
In some embodiments of the intracardiac valving
apparatus 80, the occluder member 81 will be designed to
CA 02263672 1999-02-18
W O 98/08456 PCTAUS97tl4801
-27-
move in response to changes in hemodynamic pressure, such
that when the hemodynamic pressure within the cardiac
chamber (e.g., left ventricle) exceeds that within the
transmyocardial passageway 10, the occluder member 81 will
move to it's open position, and when the pressure within
the transmyocardial passageway 10 exceeds that within the
cardiac chamber (e.g., left ventricle) the occluder member
81 will move to itls closed position.
Alternatively, in other embodiments of~ the
intracardiac valving apparatus 80, the occluder member 81
may be designed to move in relation to contractile changes
in the myocardial muscle. In these embodiments, the
occluder member 81 will be mechanically linked or coupled
to the body of the intracardiac valving apparatus 80 such
that, when the myocardium undergoes contraction (e.g.,
shortening and thickening), the occluder member 81 will be
propelled to it~s open position, and when the myocardium
undergoes relaxation (e.g., lengthening and narrowing) the
occluder member 81 will move to it's closed position.
In this manner, the intracardiac valving apparatus 80
of the present invention serves to control the desired
bloodflow through the transmyocardial passageway 10,
without the need for customizing or precise cutting-to-size
of any intramyocardial stent, as has been described in the
prior art.
v. Tissue Valve for Prev~ntina Backflow into
the TransmYocardial ~lood~low PassagewaY
An alternative to the use of the above-described
intravascular valving apparatus 20, 30, 31, 33, 40 and/or
the intracardiac valving apparatus 80, is an endogenous
tissue valve which may be formed within the transmyocardial
passageway 10 or at either end thereof. For example,
Figures 6a-6b show an endogenous tissue valve 50 which is
formed at the junction of the transmyocardial bloodflow
~ .. .. ..
CA 02263672 1999-02-18
W098/08456 PCT~S97/14801
-28-
passageway 10 and a coronary blood vessel CBV (e.g., artery
vein or man-made passageway).
With reference to Figure 6a-6b, the endogenous tissue
valve 50 may comprise one or more segment~s) 54 of the wall
of the coronary blood vessel CBV, along with one or more
tapered segment(s) of underlying myocardial tissue 52.
This endogenous tissue valve 50 is formed such that
the segment(s) of blood vessel wall 54 and underlying
portion(s) of myocardial tissue 52 will receive suff~icient
blood supply so as not to become necrotic or infarcted.
The thickness and mass of the tissue valve 50 is preferably
defined so that, when the heart undergoes systolic
contraction the elevated pressure created within the left
ventricle LV and transmyocardial bloodflow passageway 10
will force the tissue valve 50 to an open position, as
illustrated in Figure 5a, thereby creating an opening 56
through which blood may flow into the lumen of the coronary
blood vessel CBV, in the profusion direction PD.
Thereafter, when the heart undergoes diastolic relaxation
the relatively low filling pressures within the left
ventricle LV and transmyocardial bloodflow passageway 10
will allow the tissue valve 50 to return to a second or
closed position, as illustrated in Figure 5b. When in such
second or closed position, the tissue valve 50 will
substantially or completely close off the transmyocardial
bloodflow passageway 10, so as to prevent blood from
backflowing or regurgitating in the backflow direction BD,
from the lumen of the coronary blood vessel CBV into the
transmyocardial bloodflow passageway 10.
The tissue valve 50 may be created by any suitable
means, including a procedure whereby the tissue
penetrating, cutting or boring device used to create the
transmyocardial bloodflow passageway is provided with a
tapered distal end having a configuration analogous to that
of the inner edge(s) 55 of the wall segment(s) 54 so as to
CA 02263672 1999-02-18
W O 98/08456 PCTAUS97/14801
-29- '-
form the desired tissue valve(s) or segment(s) when form
the endogenous tissue valve 50, or by another catheter-
based device which is equipped to form such tissue valve(s)
or segment(s).
It will be appreciated that the tissue valve 50 may be
formed in various configuration. For example, although the
tissue valve 50 shown in Figures 6a and 6b hereof consists
of a single flap, various alternative configurations may be
utilized wherein multiple tissue protrusions, m~ltiple
tissue flaps, or annularly tapered or funnel shapped tissue
flaps are formed to perform the desired valving function.
Any and all such configurations of endogenous tissue are
intended to be included within the scope of the term
"tissue valve~ 50 as used herein.
vi. Elastic Closure for Preventina Back~low Into the
Transmvocardial Bloodflow PassaaewaY
An alternative to the mechanical valving apparatus 20,
30, 31, 33, 40 or endogenous tissue valve 50 is the elastic
closure member 60, shown in Figure 7a and 7b.
The elastic closure member 6d may comprise one or more
sutures formed of stretchable or elastic material such as
latex or other elastomeric polymer materials. Such elastic
closure member(s) 60 are preferably passed through adjacent
portions of myocardial tissue next to the opening 66
between the transmyocardial bloodflow passageway 10 and the
lumen of the coronary blood vessel CBV (or secondary
bloodflow passageway 12).
The elastic closure member(s) 60 is the elastically
biased to a retracted state whereby the closure member(s)
60 will draw the adjacent portions of myocardium M together
so as to close off the opening 66 between the
transmyocardial bloodflow passageway 10 and the lumen of
the coronary bloodflow CBV, as shown in Figure 7b. Upon
systolic contraction of the heart the relatively high
pressures created within the left ventricle LV and
.. ..
CA 02263672 l999-02-l8
W O 98/08456 PCTrUS97/14801
-30
transmyocardial bloodflow passageway 10 will cause the
elastic closure member(s) 60 to stretch or expand, thereby
forming opening 66 through which blood may flow from the
transmyocardial bloodflow passageway 10 into the lumen of
the coronary blood vessel CBV (or secondary bloodflow
passageway 12) in the perfusion direction PD, as shown in
Figure 7a.
Thereafter, when the heart undergoes diastolic
relaxation the relatively low filling pressures wit~in the
left ventricle Lv and transmyocardial bloodflow passageway
10 will allow the elastic closure member 60 to retract,
thereby closing off the opening 66 and preventing blood
from backflowing or regurgitating from the lumen of the
coronary blood vessel CBV (or secondary bloodflow
passageway 12) into the transmyocardial bloodflow
passageway 10, in the backflow direction BD, as shown in
Figure 7b.
It will be appreciated that the elastic closure member
60 may be installed in any suitable method, such as by way
of an appropriate suturing or stapling device which
operates to attach the elastic closure member 60 at its
desired location. Such installation of the elastic closure
member 60 may be accomplished by open surgical technique or
by way of catheter-based, transluminal methodology. For
example, a catheter having a suturing or stapling device
positioned therewithin may be advanced to a position
adjacent the opening 66. Thereafter, negative pressure or
other suitable drawings means may be utilized to draw
adjacent segments of the myocardial tissue, from either
side of the transmyocardial passageway 10, into the
catheter. Thereafter, the desired elastic closure member
60 may be penetrated and threaded through the adjacent
sides of the myocardial tissue so as to form the desired
elastic closure member 60, as shown.
vii. Protrusive Stents and Stented Grafts for Stentin~ of
CA 02263672 1999-02-18
W O 98/084S6 PCT~US97114801
-3 1
the TransmYocardial Passaaewa~
In accordance with another aspect of the invention
shown in Figures 8a-8c, protrusive stents or stented grafts
may be positioned within the transmyocardial passageway 10,
and may extend into one or more adjacent coronary vessels
including a) an endogenous coronary vein, b) an endogenous
coronary artery, c) a man-made passageway in the heart
which connects to an endogenous coronary vein, d) a man-
made passageway in the heart which connects ~to an
endogenous coronary artery and/or e) a man-made passageway
which extends between an endogenous coronary vein and an
endogenous coronary artery. As described more fully
herebelow, the protrusive stent apparatus 90, 90a, 90b of
the present invention may incorporate one or more valving
apparatus to intermittently block or direct bloodflow in
accordance with various stages of the systolic/diastolic
cardiac cycle. Furthermore, such protrusive stent
apparatus may optionally be covered or juxtapositioned to
a tubular graft or sheath so as to form a discrete tubular
passageway.
Figure 8a shows a non-valved, non-covered protrusive
stent apparatus 90 of the present invention positioned
partially within a transmyocardial passageway 10, and
extending into the coronary vessel CV (e.g., artery, vein
or man-made passageway) to which such transmyocardial
passageway 10 extends. As shown, the protrusive stent
apparatus 90 is curved or bent at the junction of the
transmyocardial passageway 10 and the coronary vessel CV,
and preferably extends into the coronary vessel CV in the
desired bloodflow direction.
The protrusive stent apparatus 90 may be formed of any
suitable material, such as wire mesh or other metal or
polymeric material, and may be self-expanding or pressure-
expandable.
Figure 8b shows a variant of the protrusive stent
~ . . . .
CA 02263672 1999-02-18
W O 98/08456 PCT~US97/14801
-32-
apparatus 90a positioned partially within a transmyocardial
passageway 10, extending through a coronary vein CV,
through a secondary passageway 12, and into a coronary
artery CA. As shown the protrusive stent apparatus 9Oa is
curved or bent at the junction of the secondary passageway
12 and the coronary artery CA and preferably extends into
the coronary artery CA in the desired bloodflow direction.
Figure 8c shows alternative variations of the
protrusive stent apparatus 90b wherein an optional t~bular
covering 92 is formed on the protrusive stent 90b. Such
optional covering 92 may be any suitable tubular covering
such as woven polyester or expanded, sintered
polytetrafluoroethylene (PTFE). Additionally, or
alternatively, one or more valves such as hinged occluder
members or pliable elastomeric leaflets may be located
within the protrusive stent apparatus 90b with or without
covering 92, at locations L1 and/or L2 and/or L3 to
facilitate control and valving of bloodflow through the
transmyocardial passageway 10, coronary vein CV, secondary
passageway 12 and/or coronary artery CA. It will be
appreciated that embodiments of the protrusive valving
apparatus 9Ob which incorporates such valves at locations
L1 and/or L2 and/or L3 may be provided with appropriate
openings or apertures in any covering 92 formed thereon to
facilitate the desired inflow or outflow of blood at
specific locations thereon.
These protrusive stent apparatus 90, 9Oa, 9Ob with or
without the optional covering 92 and/or without the
optional valves at locations Ll and/or L2 and/or L3 offer
advantages over previously known intramyocardial stents in
that they do not require precise cutting to length or
precise positioning within the myocardial passageway 10.
Indeed, the protrusive stent apparatus 90, 9Oa, 9Ob of the
present invention are intended to protrude into a coronary
blood vessel CBV (e.g., artery, vein and/or man-made
CA 02263672 1999-02-18
W098/08456 PCT~S97/14801
-33-
passageway) and the length of the portion of the stent
apparatus 90, 9Oa, 9Ob which extends into such coronary
blood vessel CBV is typically not critical. In this
regard, there will exist no need for custom-fitting or
precise precutting of the stent apparatus 90, 90a, 90b
prior to implantation within the patient.
In embodiments where the stent apparatus 90, 9Oa, 9Ob
is covered by a partial or complete tubular covering, such
covering may be formed of any suitable material inc~uding
but not necessarily limited to polyester, woven polyester,
polytetrafluroethylene, expanded polytetraflouroethylene,
polyurethane; silicone, polycarbonate, autologous tissue
and, xenograft tissue.
The foregoing invention has been described hereabove
~5 with reference to certain presently preferred embodiments
and examples only. No effort has been made to exhaustively
describe all possible embodiments and examples in which the
invention may be practiced. Indeed, various additions,
deletions, modifications and alterations may be made to the
above-described embodiments and examples without departing
from the intended spirit and scope of the invention.
Accordingly, it is intended that all such additions,
deletions and modifications and alterations be included
within the scope of the following claims.