Note: Descriptions are shown in the official language in which they were submitted.
CA 02263849 1999-02-24
WO 98/18883 PCT/US97/19688
-1-
PROCESS FOR HIGHLY SHAPE SELECTIVE DEWAXING
WHICH RETARDS CATALYST AGING
This invention relates to the highly shape selective catalytic dewaxing of
petroleum charge stocks, particularly streams of high wax content which have
been hydroprocessed. In this dewaxing process, catalyst aging is retarded,
thereby extending cycle length, and catalyst tolerance to sulfur and nitrogen-
containing compounds is significantly improved. Minimization of catalyst aging
also preserves yield, since high end-of-cycle temperatures often result in non-
selective cracking.
Dewaxing processes employing constrained intermediate pore molecular
sieves as catalysts possess greater selectivity than conventional catalytic
dewaxing processes. To improve catalytic activity and to mitigate catalyst
aging, these high selectivity catalysts often contain a
hydrogenation/dehydrogenation component, frequently a noble metal. Such
selectivity benefit is derived from the isomerization capability of the
catalyst
from its metallic substituent and its highly shape-selective pore structure.
ZSM-23, and some other highly selective catalysts used for tube dewaxing,
have a unidimensional pore structure. This type of pore structure is
particularly
susceptible to blockage by coke formation inside the pores and by adsorption
of
polar species at the pore mouth. Therefore, such catalysts have been used
commercially only for dewaxing "clean" feedstocks such as hydrocrackates and
severely hydrotreated solvent extracted raffinates. In the development of
shape selective dewaxing processes, key issues to be addressed are
retardation of aging, preservation of high selectivity over the duration of
the
catalyst cycle, and maintenance of robustness for dewaxing a variety of
feedstocks.
U.S. Patent No. 4,222,543 (Pelrine) and 4,814, 543 (Chen et al.) were
the earliest patents to disclose and claim the use of constrained intermediate
pore molecular sieves for lube dewaxing. U.S. Patent No. 4,283,271 (Garwood
_~.___ _.~._ . _.._...~..__..._...._~~...~.~__._.v.___..~._..__. . . ._.
CA 02263849 1999-02-24
WO 98/18883 - PCT/US97/19688
-2-
et al.) and U.S. Patent No. 4,283,272 (Garwood et al.) later claimed the use
of
these catalysts for dewaxing hydrocrackates in energy efficient
configurations.
Also directed to dewaxing with constrained intermediate pore molecular sieves
are 5,135,638 (Miller), 5,246,566 (Miller) and 5,282,958 (Santilli). None of
these patents was, however, directed to catalyst durability. Pelrine's
examples
were directed to start-of-cycle performance with furfural raffinates as feeds.
The catalysts used in Pelrine's examples typically age rapidly when exposed to
these feeds.
Previous inventions have addressed the problem of catalyst aging and
extension of cycle length in dewaxing processes involving intermediate pore
zeolites, such as ZSM-5. The techniques disclosed in these inventions are not
generally applicable to the catalysts of this invention. U.S. Patent No.
5,456,820 (Forbus et al.) discloses a process in which a tube boiling range
feedstock is catalytically dewaxed in the presence of hydrogen over a catalyst
comprising an intermediate pore zeolite in the decationized form. Catalyst
cycle length was found to be improved by optimizing the sequencing of various
solvent extracted feedstocks.
U.S. Patent No. 4,892,646 (Venkat et al.) discloses a process for
increasing the original cycle length, subsequent cycle lengths and the useful
life
of a dewaxing catalyst comprising an intermediate pore zeolite (i.e., ZSM-5)
and
preferably, a noble metal such as Pt. The catalyst is pretreated with a low
molecular weight aromatic hydrocarbon at a temperature greater than
800°F, for
a time sufficient to deposit between 2 and 30% of coke, by weight, on the
catalyst. The pretreatment may be conducted in the presence of hydrogen gas.
U.S. Patent No. 4,347,121 (Mayer et al., hereinafter Mayer) claimed
catalytic dewaxing of hydrocrackates containing less than 10 ppm nitrogen with
a hydrofinishing step upstream of the dewaxing catalyst. Mayer is, however,
directed to ZSM-5 and ZSM-11. The hydrofinishing step is employed for the
purpose of base oil stabilization not to improve the aging characteristics of
ZSM-5 or ZSM-11. Commercial experience dewaxing hydrocrackates with
ZSM-5 shows negligible aging.
_ _.. r , i rt
CA 02263849 1999-02-24
WO 98/18883 PCT/US97/19688
-3-
Chen, et al (U.S. Patent 4,749,467), discloses a method for extending
dewaxing catalyst cycle length by employing the combination of low space
velocity and a high acidity intermediate pore zeolite. The high acid activity
and
low space velocity reduce the start-of-cycle temperature. Because catalyst
deactivation reactions are more temperature sensitive than are dewaxing
reactions, low operating temperatures reduce the catalyst aging rate. The same
principle has been found to apply to unidimensional constrained intermediate
pore molecular sieves.
Dewaxing catalysts comprising intermediate pore molecular sieves
containing noble metals have been found to have relatively high aging rates
when dewaxing heavy hydrocrackate feeds at a space velocity of 1 LHSV or
greater. The catalyst eventually lines out at high temperature, resulting in
non-
selective cracking and significant yield loss. The aging rate and yield loss
with
time can be reduced somewhat by operation at a relatively low space velocity.
Additionally, noble metal-containing constrained intermediate pore catalysts
age very rapidly when exposed to feedstocks having even modest levels of
nitrogen and sulfur, such as mildiy hydrotreated solvent refined feeds or
hydrocrackates produced at low hydrocracker severity.
It has been discovered, however, that the use of a high activity
hydrotreating catalyst (a catalyst which can operate effectively at high space
velocities and relatively low temperatures is considered a high activity
catalyst)
upstream of the dewaxing catalyst (preferably in one vessel, creating a
synergistic catalyst system) is extremely effective for reducing the dewaxing
catalyst aging rate and eventual line out temperature. The synergistic
catalyst
system also permits operation at significantly higher space velocities than
would be possible with the dewaxing catalyst operating alone. The synergistic
combination of hydrotreating and dewaxing catalysts offers the potential for
longer cycle length while processing difficult feeds with moderate amounts of
nitrogen, sulfur and aromatics, such as low conversion hydrocrackates. This
invention is also effective with hydrotreated raffinates and some neat
raffinates.
CA 02263849 1999-02-24
WO 98/18883 PCT/US97/19688
This is an unexpected improvement, since nitrogen and sulfur are generally
known to be effective poisons for catalysts loaded with noble metals.
There are also economic advantages from the invention. It is
significantly less expensive to load a dewaxing reactor with a combination of
hydrotreating catalyst and noble metal containing dewaxing catalyst than it is
to
load a reactor with the dewaxing catalyst alone. This also avoids gas
separation and clean-up typical of prior art.
The prior art discussed in the background above demonstrates that
previous attempts to retard aging and yield loss have been focused on
restricting conditions of the dewaxing process to specific parameters, such as
temperature or space velocity. Alternately, the dewaxing catalyst itself has
been altered by additional steps such as precoking or is formulated to high
alpha requirements, both of which can reduce catalyst selectivity. The instant
invention retards aging much more effectively than methods previously
disclosed. It is also much less expensive and time consuming to implement.
The dewaxing catalysts of this invention are very effective hydrogenation
catalysts when acting alone, nearly completely saturating the aromatics in the
feed. It is, therefore, unexpected that adding a high activity hydrotreating
catalyst ahead of, and preferably in, the same reactor with the dewaxing
catalyst results in dramatic minimization of aging. Catalyst line-out time and
eventual equilibration temperature are reduced. Furthermore, the upper space
velocity limit for stable operation of the dewaxing catalyst is substantially
extended. The catalyst combination of the instant invention appears to have a
different aging mechanism than the dewaxing catalyst operating alone,
permitting higher space velocity operation simultaneously with a lower aging
rate.
The synergistic catalyst combination of the instant invention performs
well for hydrocracked feeds in addition to permitting the processing of feeds
with moderately high levels of nitrogen and sulfur. Such feeds would
ordinarily
poison either of these catalysts alone causing rapid and uncontrollable aging.
The invention may be summarized as follows:
........__M. _r~..___...._._~..~.~~._~..._....
CA 02263849 2003-10-10
-5-
A process for catalytically dewaxing a lubricant feedstock
whereby the aging of the dewaxing catalyst and eventual line-out
temperature are minimized. Applicable feedstocks are
preferentially hydrocrackates or hydrotreated raffinates but
include raffinate products of conventional solvent extraction
processes. The feedstock is contacted in the presence of hydrogen
with the catalyst system at a space velocity (based on the dewaxing
catalyst volume) between 0.2 and 10 and in a temperature range
between 450°F and 800°F. The catalyst system comprises a high
activity hydrotreating catalyst operating upstream of a dewaxing
catalyst, preferably (although not restricted to operating) in the
same reactor vessel. The hydrotreating and dewaxing catalysts each
preferably contain one or more noble metals with the dewaxing
catalyst also containing a constrained intermediate pore molecular
sieve.
In accordance with one aspect of the present invention there
is provided a process for catalytically dewaxing a hydrocarbon feed
having less than 300 ppm N in the presence of hydrogen through the
use of a synergistic catalyst system comprising: a) contacting
said feed in the presence of a high activity hydrotreating catalyst
which comprises at least one metal supported on an inorganic base
which is effective for reducing, when operating at the same
conditions as a subsequent dewaxing catalyst, the aromatics content
of the feed, as measured by UV absorbtivity, by at least 60 wt.%;
b) conducting the product from step a) above directly to dewaxing
step c) below without light products separation; and c) contacting
said product from step a) above with a dewaxing catalyst
comprising: i) a constrained intermediate pore molecular sieve
having at most one pore channel of 10-membered oxygen rings wherein
any intersecting channels having 8-membered oxygen rings; and
ii) a noble metal.
DESCRIPTION OF THE DRAWINGS
Figure 1 provides the aging profile for 0.2% Pt/ZSM-23
catalyst when used alone to dewax a hydrocracked heavy vacuum gas
oil (HVGO).
Figure 2 shows an aging profile at start-of-cycle for a 0.2%
Pt/ZSM-23 catalyst used to dewax a hydrocracked HVGO contaminated
with 0.25% of raw HVGO.
CA 02263849 2002-12-05
-5 a-
Figure 3 illustrates the aging profile for a 0.5°: Pt/ZSM-23
dewaxing catalyst using several different heavy hydrocrackate
feeds.
Figure 4 shows the aging profile for a 0.2% Pt/ZSM-23
dewaxing catalyst when used in synergistic combination with a high
activity hydrotreating catalyst. Results using two different feeds
are illustrated.
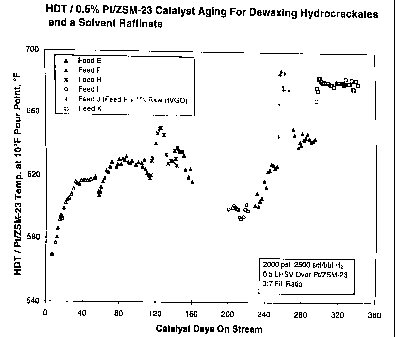
Figure 5 illustrates the aging profile for a 0.5°. Pt/ZSM-23
dewaxing catalyst when employed in a catalyst system with a noble
metal hydrotreating catalyst, using several different hydrocrackate
feeds and a solvent refined raffinate.
Figure 6 illustrates the aging profile for the catalyst
system employing a noble metal hydrotreating catalyst and 0.5%
Pt/ZSM-23 operating at several space velocities, using a heavy
hydrocrackate feed.
CA 02263849 1999-02-24
WO 98/18883 PCT/US97/19688
Figure 7 is an aging profile for the synergistic combination of noble metal
hydrotreating catalyst and 0.5% PtIZSM-23 when a hydrotreated raffinate is
used.
Detailed Description of the Invention
Feed
The present process is capable of operating with a wide range of feeds
of mineral oil origin to produce a range of lubricant base oils with good
performance characteristics. Such characteristics include low pour point, low
cloud point, and high Viscosity Index. The quality of the lube base stock and
is
dewaxing yield are dependent on the quality of the feedstock and its
amenability to processing by the catalysts of the instant invention.
Feedstocks
for this process are derived from the atmospheric residuum fraction of crude
oil
including vacuum gas oils and vacuum residues, as well as those produced by
Fisher Tropsch processing of synthesis gas.
Prior to dewaxing, crude fractions used to make lubricant stocks are
generally subjected to one or more refining steps which remove low Viscosity
Index components such as heteroatoms, aromatics, and polycyclic naphthenes.
This upgrading step can be accomplished by solvent extraction,
hydroprocessing, or a combination of the two steps. If the Viscosity Index
improvement occurs by a single hydroprocessing step, the upgrading process is
typically accompanied by a significant amount of conversion of the feed to
products boiling below the initial boiling point of the feed and is termed
hydrocracking. Hydroprocessing used in conjunction with solvent extraction
will
generally not result in significant conversion of feed to light products. Low
boiling range conversion hydroprocessing is termed hydrotreating.
Hydroprocesses used for Viscosity Index improvement typically operate at
hydrogen partial pressures above 1000 psig and remove most of the sulfur and
nitrogen-containing species in the material being treated. Because nitrogen
and sulfur act as poisons for noble metal-containing catalysts, preferred
r r r. ~.._.____.~..~...
CA 02263849 1999-02-24
WO 98/18883 PCT/US97/19688
-7-
feedstocks for this invention are those which have been hydroprocessed.
However, some solvent refined raffinates are also suitable for dewaxing by the
catalysts of the instant invention.
The Viscosity Index of the dewaxed lubricant base oil is directly related
to the Viscosity Index of the entrained oil in the waxy feedstock, as
determined
by solvent dewaxing, and to the wax content of the feedstock. Because the
catalytic system of this invention has paraffin isomerization ability, tube
base
stocks having very high Vi can be produced by dewaxing high wax content
feedstocks such as slack waxes, foots oils, derivatives of waxy crude vacuum
gas oils, and waxes produced by Fischer-Tropsch processing of synthesis gas.
Pretreating of Feed
If hydrocracking is employed as a pre-treatment step, an amorphous
bifunctional catalyst is preferably used to promote the saturation and
subsequent ring opening of the low quality aromatic components in the feed to
produce hydrocracked products which are relatively more paraffinic.
Hydrocracking is typically carried out at high pressure primarily to minimize
catalyst aging and to favor the removal of sulfur and nitrogen-containing
species. Consistent with these process objectives, the hydrogen pressure in
the hydrocracking stage is at least 800 psig (about 5500 kPa abs.) and usually
is in the range of 1000 to 3000 psig (about 6900 to 20700 kPa abs). Normally,
hydrogen partial pressures of at least 1500 psig (about 10500 kPa abs.) are
preferred. Hydrogen circulation rates of at least about 1000 SCFIBbI (about
180 n.l.l.-'), preferably in the range of 2000 to 8000 SCFIBbI (about 900 to
1800
n.l.l.-' ) are suitable.
Lube hydrocracker severity is generally set by the Viscosity Index target
of the base oil being produced with higher severity (higher feed conversion to
light byproducts) being required for higher VI. In some instances,
particularly
those in which a high shape selective noble metal-containing dewaxing catalyst
is used downstream of the hydrocracker, denitrogenation and desulfurization
considerations may necessitate hydrocracker operation at higher severity than
CA 02263849 1999-02-24
WO 98/18883 PCT/US97/19688
_8_
required to meet the target base oil Viscosity Index. This results in lower
base
oil yields and can offset the benefits of using a highly shape selective
dewaxing
catalyst. It is a primary motivation behind the instant invention to develop a
catalyst system which is both highly selective for dewaxing but which has high
tolerance for feedstock impurities such as nitrogen and sulfur. This enables
operation of the hydrocracker to meet only the required base stock VI and
maximizes overall base oii yield. A dewaxing catalyst system which is capable
of processing feeds with moderate levels of sulfur and nitrogen can also be
used to leverage the pressure of the upstream hydroprocessing unit, thus
saving capital expense.
Hydrocrackers used primarily to produce high quality fuels in which the
high boiling by-product is used for tubes manufacture will often operate at
higher severity than tubes-dedicated hydrocrackers. In these cases, conversion
is dictated primarily by fuels considerations.
For hydrocrackers dedicated to tube manufacture, the conversion of the
feed to products boiling below the tube boiling range, typically to
650°F- (about
343°C-) products is generally not more than 50 wt.% of the feed.
Conversion to
650°F- products will exceed 30 wt% only for the poorest quality feeds
and for
instances where base oil VI targets exceed those of conventional base stocks
(95-100 VI).
The conversion may be maintained at the desired level by control of the
temperature in the hydrocracking stage which will normally be in the range of
600° to 800°F (about 315° to 430°C) and more
usually in the range of about
650° to 750°F (about 345° to 400°C). Space
velocity variations may also be
used to control severity although this will be less common in practice in view
of
mechanical constraints on the system. Generally, the space velocity will be in
the range of 0.25 to 2 LHSV hr.-' and usually in the range of 0.5 to 1.5 LHSV.
Significant aromatics saturation occurs in the hydrocracking process
although the degree of saturation is limited thermodynamically by the
hydrocracking catalyst temperature. High temperatures shift the equilibrium of
exothermic reactions such as aromatics saturation in the reverse direction of
n _...r..~ _ .....__~ ____._..
CA 02263849 1999-02-24
WO 98/18883 PCT/US97/19688
_g_
the desired reaction path. Therefore, hydrocrackates will typically have
aromatics contents of 10-20 wt%, generally no lower than 5%, and higher than
30% only for iow conversion, low pressure operation.
Hydrocracking catalysts are bifunctional in nature including a metal
component for promoting the desired aromatics saturation, denitrogenation, and
desulfurization reactions and an acidic component for catalyzing cracking and
ring opening reactions. Usually a combination of base metals is used, with one
metal from the iron group (Group VIII) in combination with a metal of Group
VIB.
Thus, the base metal such as nickel or cobalt is used in combination with
molybdenum or tungsten. A particularly effective combination for high pressure
operation is nickel/tungsten. Noble metal containing catalysts are not
typically
used for single stage tube hydrocrackers since they have relatively low
tolerance to the sulfur and nitrogen levels found in typical hydrocracker
feeds,
such as vacuum gas oils. The amounts of the metals present on the catalyst
are conventional for a base metal tube hydrocracking catalysts of this type
and
generally will range from 1 to 10 wt.% of the Group VIII metals and 10 to 30
wt.% of the Group Vl metal, based on the total weight of the catalyst. The
metals may be incorporated by any suitable method including impregnation onto
the porous support after it is formed into particles of the desired size or by
addition to a gel of the support materials prior to calcination. Addition to
the gel
is a preferred technique when relatively high amounts of the metal components
are to be added, e.g., above 10 wt.% of the Group VI metal. These techniques
are conventional in character and are employed for the production of tube
hydrocracking catalysts.
The metal component of the catalyst is generally supported on a porous,
amorphous metal oxide support, and alumina or silica-alumina are preferred for
this purpose. Other metal oxide components may also be present in the
support although their presence is less desirable. Consistent with the
requirements of a tube hydrocracking catalyst, the support should have a pore
size and distribution which is adequate to permit the relatively bulky
components of the high boiling feeds to enter the interior pore structure of
the
i i
CA 02263849 1999-02-24
WO 98/18883 PCT/US97/19688
-10-
catalyst where the desired hydrocracking reactions occur. To this extent, the
catalyst wilt normally have a minimum pore size of about 50 A, i.e., with no
less
than about 5% of the pores having a pore size less than 50 A pore size, with
the
majority of the pores having a pore size in the range of 50-400 A (no more
than
5% having a pore size above 400 A), preferably with no more than about 30%
having pore sizes in the range of 200-400 A. Preferred catalysts for the first
stage have at least 60% of the pores in the 50-200 A range. The properties of
some typical tube hydrocracking (LHDC) catalysts suitable for use in the
hydrocracking are shown in Table 1.
Table 1
LHDC Catalyst Properties
Form 1.5mm. cyl. 1.5mm. tri. 1.5mm. cyl.
Pore Volume, cc/gm 0.331 0.453 - 0.426
Surface Area, m2/gm 131 170 116
Nickel, wt. pct. 4.8 4.6 5.6
Tungsten, wt. pct. 22.3 23.8 17.25
Fluorine, wt. pct. - - 3.35
SiOz/A1z03 Binder - - 62.3
Real Density, gm/cc 4.229 4.238 4.023
Particle Density, gm/cc 1.744 1.451 1.483
Packing Density, gm/cc 1.2 0.85 0.94
If necessary to obtain the desired conversion, the catalyst may be
promoted with fluorine, either by incorporating fluorine into the catalyst
during
its preparation or by operating the hydrocracking in the presence of a
fluorine
compound which is added to the feed. Alumina-based catalysts are typical of
those which require fluorine promotion. Silica-alumina or zeolitic based
catalysts have requisite intrinsic acidity and do not generally require
fluorine
addition. Fluorine containing compounds may be incorporated into the catalyst
by impregnation during its preparation with a suitable fluorine compound such
__ _..w.~...~.w___ ~.___ _~.._._.
CA 02263849 1999-02-24
WO 98/18883 PCT/US97/19688
-11-
as ammonium fluoride (NH4F) or ammonium bifluoride (NH4F HF) of which the
latter is preferred. The amount of fluorine used in catalysts which contain
this
element is preferably from about 1 to 10 wt.%, based on the total weight of
the
catalyst, usually from about 2 to 6 wt.%. The fluorine may be incorporated by
adding the fluorine compound to a gel of the metal oxide support during the
preparation of the catalyst or by impregnation after the particles of the
catalyst
have been formed by drying or calcining the gel. If the catalyst contains a
relatively high amount of fluorine, as well as high amounts of the metals as
noted above, it is preferred to incorporate the metals and the fluorine
compound
into the metal oxide gel prior to drying and calcining the gel to form the
finished
catalyst particles.
The catalyst activity may also be maintained at the desired level by in
situ fluoriding in which a fluorine compound is added to the stream which
passes over the catalyst in this stage of the operation. The fluorine compound
may be added continuously or intermittently to the feed or, alternatively, an
initial activation step may be carried out in which the fluorine compound is
passed over the catalyst in the absence of the feed, e.g., in a stream of
hydrogen in order to increase the fluorine content of the catalyst prior to
initiation of the actual hydrocracking. In situ fluoriding of the catalyst in
this way
is preferably carried out to induce a fluorine content of about 1 to 10%
fluorine
prior to operation, after which the fluorine can be reduced to maintenance
levels
sufficient to maintain the desired activity. Suitable compounds for in situ
fluoriding are orthofluorotoluene and difluoroethane.
The metals present on the catalyst are preferably used in their sulfide
form and to this purpose pre-sulfiding of the catalyst should be carried out
prior
to initiation of the hydrocracking. Sulfiding is an established technique and
it is
typically carried out by contacting the catalyst with a sulfur-containing gas,
usually in the presence of hydrogen. The mixture of hydrogen and hydrogen
sulfide, carbon disulfide or a mercaptan such as butyl mercaptan is
conventional for this purpose. Presulfiding may also be carried out by
. .. ~.. ._..._.~ -r""-_ ... _~~._._ __ _ ._ . .._.. ~..~,..~..~ .,..-_._ .. _
r
CA 02263849 1999-02-24
WO 98/18883 PCT/US97/19688
-12-
contacting the catalyst with hydrogen and a sulfur-containing hydrocarbon oil
such as a sour kerosene or gas oil.
Hydrocracking is the preferred process route for upgrading base oil
Viscosity Index prior to dewaxing for this invention. However, other processes
are practiced commercially for this purpose and are suitable for application
of
the technology described herein. Such processes include solvent extraction by
either furtural, n-methyl-2-pyrrolidone (NMP), or phenol, and hydrotreating.
The
raffinate product of solvent extraction is typically dewaxed by dilution with
solvent with subsequent filtration or by catalytic dewaxing. Unidimensional
molecular sieves discussed in prior art are not suitable for dewaxing
raffinates
since the high nitrogen and sulfur levels of these materials results in
unacceptably low catalyst life. The instant invention is more robust for
dewaxing feeds with moderate levels of nitrogen and sulfur and is suitable for
dewaxing raffinates although raffinates having less than 5000 ppmw sulfur and
50 ppmw nitrogen are preferred.
The primary difference between hydrotreating and hydrocracking is in the
degree of boiling range conversion which occurs with conversion to
650°F-
products typically being less than 10% of the feed characteristic for
hydrotreating. Hydrocracking can act alone as a VI improvement step for
treating vacuum gas oils to produce conventional quality tube stocks.
Hydrotreating, as defined here, does not provide as significant a boost in
Viscosity Index and must be used in conjunction with another VI improvement
step, such as solvent extraction, to produce conventional quality base stocks.
Hydrotreating occurs typically over a base metal catalyst similar in
composition to lube hydrocracking catalysts although hydrotreating catalysts
do
not require an acidic support. Operating pressures and temperatures are
similar to those suitable for hydrocracking although while in practice
hydrocrackers operate at H2 partial pressures above 1500 psig, hydrotreaters
may operate at significantly lower pressures, less than 1000 psig for example.
The degree of denitrogenation and desulfurization for hydrotreating may be as
high as for hydrocracking but may be much lower because of lower operating
..~_.r.~ ._w.___ __._._
CA 02263849 1999-02-24
WO 98118883 - PCT/US97/19688
-13-
pressures. Materials which have been hydrotreated are suitable feedstocks for
the instant invention giving acceptable catalyst aging. However, highly shape
selective catalysts of prior art do not provide acceptable catalyst life for
hydrotreated feedstocks having moderate levels of nitrogen and sulfur.
Dewaxina Step Employina Synerpistic Catalyst System
The dewaxing feedstocks following the VI improvement processing step
contain quantities of waxy straight chain, n-paraffins, together with higher
isoparaffins, naphthenes and aromatics. Because these contribute to
unfavorable pour points, it is necessary to remove these waxy components.
Dilution with solvents, usually methylethyl ketone, toluene, and methyisobutyl
ketone, followed by filtration at low temperatures is the traditional method
for
dewaxing solvent refined and hydroprocessed lube stocks. To catalytically
remove the undesirable waxy components without removing the desirable
isoparaffinic components which contribute to high Viscosity Index in the
product, dewaxing with a shape-selective dewaxing catalyst is necessary. This
catalyst removes the n-paraffins together with the waxy, slightly branched
chain
paraffins, while leaving the more branched chain iso-paraffins in the process
stream. Shape selective dewaxing is more fully explained in U.S. Patent No.
4,919,788, to which reference is made for a description of this process.
Unidimensional constrained intermediate pore molecular sieves have been
found to be particularly shape selective and have been found useful for
dewaxing very clean feedstocks. These catalysts typically contain a metal
component to enhance activity and retard aging and therefore also have the
ability to convert wax into tube by isomerization.
The catalytic dewaxing step in this invention is carried out with a catalyst
system comprising two catalysts acting in synergy. The initial catalyst is a
high
activity hydrotreating catalyst. Such a catalyst is capable of operating at
relatively high space velocities and low temperatures. Since it is preferred
to
practice this invention in a single reactor vessel, the hydrotreating catalyst
must
have sufficient activity at the temperature at which the dewaxing catalyst
CA 02263849 1999-02-24
WO 98/18883 PCT/US97/19688
-14-
operates. Therefore hydrotreating catalysts containing noble metals such as
platinum or palladium are preferred in this invention since they have good
hydrogenation activity if poisoning with heteroatoms can be avoided. Catalysts
containing Group VII and Group VIII metals can be used but are less desired
generally because they have lower activity than noble metal catalysts. The
amount of noble metals present on the catalyst can range from 0.1 % to 5 wt.%,
preferably between 0.2 wt.% and 2 wt.%. Noble metals may be used in
combination such as platinum and palladium in preferred ratios between 2:1
and 1:5 platinum-to-palladium.
The metals may be incorporated by any suitable convention method.
The metal component of the catalyst is generally supported on a porous,
amorphous metal oxide support. A silica-alumina combination with low acid
activity is acceptable. Other metal oxide components may also be present in
the support although their presence is less desirable. The hydrotreating step
employed in this invention differs significantly from hydrotreating used in
combination with solvent extraction to improve base stock Viscosity Index.
Firstly, the hydrotreating catalyst upstream of the dewaxing catalyst provides
no
VI boost to the finished lube. Base oil VI is nearly identical for the case
where
the dewaxing catalyst operates alone or in tandem with the hydrotreating
catalyst. Secondly, the effluent from the hydrotreating catalyst passes
directly
over the dewaxing catalyst without any pressure reduction or light product
separation steps. As typically practiced, both hydrocrackers and hydrotreaters
do not operate in cascade with a catalytic dewaxer.
The second catalyst is a selective dewaxing catalyst based on a
constrained intermediate pore crystalline material, such as a zeolite or a
silica
alumino-phosphate. A constrained intermediate crystalline material is defined
as having no more than one channel of 10-membered oxygen rings with
possible intersecting channel having 8-membered rings. ZSM-23 is the
preferred molecular sieve for this purpose although other highly shape-
selective
zeolites such as ZSM-22, ZSM-48, ZSM-50 or the synthetic ferrierite ZSM-35
~ .~.
CA 02263849 2002-12-05
-I S-
may also be used. Silicoaluminophosphates such. as SAPO-11, SAPO-31
and SAPO-41 are also suitable for use as the selective dewaxing
catalyst.
The synthetic zeolite ZSM-23 is described. in U.S,. Patent Nos.
4,076,842 and 4,104,151 to which reference is rriade for a description
of this zeolite, its preparation and properties. The synthetic
zeolite designated ZSM-48 is more particularly described by U.S.
Patent Nos. 4,375,573 and 4,397,827. The synthetic zeolite
designated ZSM-50 is more particularly described by U.S. Patent No.
4,640,829.
The intermediate pore-size synthetic crystalline material
designated ZSM-35 ("zeolite ZSM-35" or simply "ZSM-35"), is
described in U.S. Patent No. 4,106,245 to which. reference is made
for a description of this zeolite and its preparation. The
synthesis of SAPO-11 is described in U.S. Patent Nos. 4,943,424 and
4,440,871. The synthesis of SAPO-41 is described in U..S. Patent No.
4,440,871.
Ferrierite is a naturally-occurring mineral, described in the
literature, see, e.g., D.W. Breck, ZEOLITE MOLECULAR SIEVES, John
Wiley and Sons (1974), pages 125-127, 146, 219 and 625, to which
reference is made for a description of this zeolite.
The dewaxing catalysts used in this invention include a metal
hydrogenation-dehydrogenation component which is preferably a noble
metal although not restricted to a noble metal or a combination of
noble metals. Although it may not be strictly necessary to promote
the selective cracking reactions, the presence of this component has
been found to be desirable to promote certain isomerization
reactions and to enhance catalytic activity. The presence of the
noble metal component leads to product improvement, especially VI,
and stability. Aging of the shape-selective dewaxing catalyst is
significantly retarded in the present invention by syne>rgistic
combination with the upstream hydrotreating catalyst. The shape-
selective, catalytic dewaxing is normally carried out i.n the
presence of hydrogen under pressure. The metal is preferably
platinum or palladium or a combination of platinum and palladium.
CA 02263849 1999-02-24
WO 98/18883 PCT/US97119688
-16-
The amount of the metal component is typically 0.1 to 10 percent by weight.
Matrix materials and binders may be employed as necessary.
Shape-selective dewaxing using the highly constrained, highly shape-
selective catalyst with hydrotreating catalysts upstream in a synergistic
system
may be carried out in the same general manner as other catalytic dewaxing
processes. Both catalysts may be in the same fixed bed reactor or the
hydrotreating catalyst may be upstream in a separate bed. A single reactor
vessel is preferred. Conditions will therefore be of elevated temperature and
pressure with hydrogen, typically at temperatures from 250° to
500°C (about
580° to 930°F), more usually 300° to 450°C (about
570° to 840°F) and in most
cases not higher than about 370°C (about 700°F). Pressures
extend up to
3000 psi, and more usually up to 2500 psi. Space velocities extend from 0.1 to
10 hr' (LHSV), over the synergistic catalyst system more usually 0.2 to 3 hr'.
Operation at a higher space velocity than can be achieved with the dewaxing
catalyst operating alone with acceptable aging, yet with a relatively low
aging
rate at equilibrium, is a critical feature of the instant invention. Hydrogen
circulation rates range from 100 to 1000 n.l.l.-', and more usually 250 to 600
n.l.l.-' .
Reference is made to U.S. Patent No. 4,919,788 for a more extended
discussion of shape-selective catalytic dewaxing. As indicated previously,
hydrogen may be used as an interbed quench in order to provide optimal
temperature control in the reactor.
The degree of conversion to lower boiling species in the dewaxing stage
will vary according to the extent of dewaxing desired at this point, i.e., on
the
difference between the target pour point and the pour point of the feed. It
must
be noted that the catalyst system of the instant invention is employed
primarily
to enhance the cycle length of the shape-selective catalyst. Product
characteristics will be similar to those found in other shape-selective
dewaxing
processes. The degree of conversion also depends upon the selectivity of the
shape-selective catalyst which is used. At lower product pour points, and with
relatively less selective dewaxing catalysts, higher conversions and
n ..?..~....d.._...._v...~......_.__._.
CA 02263849 1999-02-24
WO 98/18883 PCT/US97/19688
-17-
correspondingly higher hydrogen consumption will be encountered. In general
terms conversion to products boiling outside the tube range, e.g.,
315°C-, more
typically 343°C-, will be at least 5 wt.%, and in most cases at least
10 wt.%, with
conversions of up to about 40 wt.% being necessary only to achieve the lowest
pour points or to process high wax content feeds with catalysts of the
required
selectivity. Boiling range conversion on a 650°F+ (343°C+) basis
will usually be
in the range of 10-25 wt.%.
After the pour point of the oil has been reduced to the desired value by
selective dewaxing, the dewaxed oil may be subjected to treatments such as
mild hydrotreating or hydrofinishing, in order to remove color bodies and
produce a tube product of the desired characteristics. Fractionation may be
employed to remove light ends and to meet volatility specifications.
EXAMPLES
Aging experiments were conducted with hydrocrackates {primarily those
derived from heavy vacuum gas oils), a light neutral raffinate, a hydrotreated
raffinate, and hydrocracked stocks contaminated with vacuum gas oil. The
experiments show benefits for a pre-hydrotreating step on dewaxing catalyst
and eventual lineout temperature, and ability to operate stably at high space
velocities. Properties of the aging feedstocks used in these experiments are
given by Table 2.
Feedstocks A, C, and E through M were derived by hydrocracking a
heavy vacuum gas oil (HVGO) from a mix of Persian Gulf crudes. These
materials differ from each other by the hydrocracking severity used to produce
them. High conversion hydrocracking increases tube VI and reduces sulfur and
nitrogen levels. Feedstock D was produced in a similar manner by
hydrocracking an Arab Light heavy vacuum gas oil and Feed I represents a
hydrocracked light vacuum gas oil.
To test the robustness of the synergistic catalyst system, Feeds B and J
were produced by contaminating hydrocracked Feeds A and F with 0.25 and
1 % raw HVGO respectively. Feedstock J contained the highest level of
_..__..~.y,._...~.~__~____e._ _._~.. ..~.._-..-._.,.._..~-__ T
~ i
CA 02263849 1999-02-24
WO 98/18883 PCT/US97/19688
-18-
nitrogen of the feeds processed here at 39 ppm. Feed K represents a light
vacuum gas oil commercially extracted with furfural to produce a nominal 100
VI
solvent dewaxed base oil. It contained the highest sulfur content (2300 ppm)
of
any of the feeds tested.
Feed L represents an NMP-extracted light neutral which was
subsequently hydrotreated at mild conditions (<5% 650°F+ conversion,
1000
psig HZ). It has sulfur and nitrogen contents lower than the furfural
raffinate
(Feed K) but substantially higher than the hydrocrackates.
r ~.. _.. __ . _.
CA 02263849 1999-02-24
WO 98/18883 PCT/US97/19688
-19-
N d
N C N ~ M ~ GO~ O~ N '
_ !C M V nn ~ of ~
N
N ~ w N ~ ~ ~ NrnM n
~
YI N v tm oton n aoao
M N
r- d'
O
O O ~~ N
_ O r
~
~ r~ ~o nn ofo~o
m U u~ ~ ~ ~W
~ a~
> V n
Z J
M ' M ~N N N
= J N ~ c <on aoo~o~
S
~oto w n N~ cryn v~ n to
~ r'~ o
C71 = j ~io ~ o ~ Nn aoof ~
m
2
Q N ~ ' N ~~ n N nM ~
~
~1 = N N u~ ~on ~ o~o~
pi
=
~ u? ~ Fv~ ~ ~M ~ ao
tul = > ~ ~ o ~n c o~rn
ao
Z
~ ~ O ~ n
I = ~ ~ N O ~ ~ ~ O
f
~
Q
U O ~on ~r' n ~y n ~ N ~ o>n
. , '
UI = j ~ n ~ nm ~ o~m
n
'.
Z
+ O
> v .- o ' v ~ ~ ~~'~'c~'n
N
, m c '
07 1 of ~ nn aoi a~ .
N = v rn
O
n O l'~O .p N~ ~ N ~~ ~ 00
~
QI o > ~ ei N nn o~rn
z z
a~
D
w
L
D
H
0 o
07 c a
o m Oo 2R?Ro?Rm N o
(L V ~ ~ n u'm 10
7
io N c
a J
O tL
5 o '
a
.c
a
o ~ g a m o
N
d a A~'o ~ c c
n.~.~
C7E ? g' 3 U
E
c E
o ' o
z
'
a
o p2~ v~ v
n ~
a
___.. "~.".Y.r_ . . .__~..~....,~......_.~...~.-._.... .._ 1.
CA 02263849 1999-02-24
WO 98/18883 PCT/US97/19688
-20-
Example 1
The first two experiments were conducted with a 0.2% Pt/ZSM-23 which
was prepared by platinum addition by ion exchange to an alumina-bound ZSM
23. In both experiments, the liquid flow rate was held primarily at 1 LHSV
over
the PtIZSM-23, hydrogen partial pressure was primarily 2000 psi, and HZ flow
rate was held at 2500 scf/bbl.
The ZSM-23 catalyst in the first experiment was run for 112 days without
a pre-hydrotreating step. Feed A (Table 2) was used throughout the run.
Because Feed A had a low level of sulfur and nitrogen relative to many of the
other feeds evaluated, catalyst aging on this feedstock should be optimistic
when compared to other feedstocks. Despite the relatively low level of
impurities in the feed during the first 30 days on stream, the catalyst aged
at
2.6°F/day before reaching a period of slower aging (0.28°F/day)
at 1 LHSV
lasting until the end of the run (see Figure 1 ). From 60 to 110 days on
stream,
the liquid flow rate was held primarily at 0.5 LHSV with periodic activity
checks
at 1 LHSV. Therefore, the 0.28°F/day aging rate observed for this
period is
likely optimistic when compared to continuous operation at 1 LHSV. When
operating at 0.5 LHSV, catalyst aging was reduced to an acceptable level of
0.03°F/day but the operating temperature required to meet a product
pour point
of 10°F was fairly high at approximately 670°F (vs. start-of-
cycle at less than
600°F) While the catalyst showed a 3% yield benefit over solvent
dewaxing at
start-of-cycle, it gave a 4-5% debit versus solvent dewaxing during the period
of
slow aging reflecting non-selective cracking at the high catalyst temperatures
(Table 3).
The same fresh PtIZSM-23 catalyst was used to dewax the same heavy
hydrocrackate contaminated with approximately 0.25% raw HVGO (Feed B) to
test catalyst robustness for treating feeds with moderate levels of nitrogen
and
sulfur. Catalyst aging at 1 LHSV was initially very high at 4.5°Flday
with start-
of-cycle temperature requirement to reach a 10°F pour product higher
than
670°F. Reducing space velocity to 0.6 hr' after 7 days on stream only
slightly
~~
CA 02263849 1999-02-24
WO 98/18883 PCT/US97/19688
-21-
reduced the temperature requirement to reach target pour point and throughout
the early part of the catalyst cycle, tube yield was 4% lower than for solvent
dewaxing (Table 3). Clearly the PtIZSM-23 had limited ability to process a
feedstock having even a moderately lower nitrogen content (4 ppm).
Example 2
A 200 day aging run was conducted with a 0.5% PtIZSM-23 with several
hydrocrackated HVGOs (Figure 3). Platinum was added by ion exchange. The
additional platinum improves the hydrotreating ability of the catalyst of
Example
2 versus the 0.2% Pt/ZSM-23 of Example 1. The aging run was conducted at a
space velocity of 0.5 hr' over PtIZSM-23, a hydrogen partial pressure of 2000
psig, and with a hydrogen circulation rate of 2500 scf/bbl.
The catalyst aged at approximately 0.64°F/day for the first 140
days on
stream before reaching a period of lower aging (0.08°Flday). The lower
initial
aging rate and longer period to reach a "lined-out" state is consistent with
Chen's observation (U.S. Patent 4,749,467) and the catalyst formulation is
clearly more selective than that used in Example 1 (see Table 3). However, the
lineout temperature still exceeded 660°F and, in that respect, showed
no
improvement over the catalyst of Example 1. From the data in Figures 1 and 3,
it can be determined that both catalysts would have approxirnately the same
life
when operating at the same space velocity.
PUZSM-23 has significant activity for saturating aromatics as shown by
Table 4. A good relative indicator of the aromatics content of a base oil,
widely
used within the industry, is ultraviolet absorbtivity at 226 nm. Table 2 shows
that 226 nm absorbtivity is reduced by at least 85% and in some cases over
95% by dewaxing over Pt/ZSM-23.
CA 02263849 1999-02-24
WO 98/18883 PCT/IJS97/19688
-22-
Example 3
The same fresh ZSM-23 catalyst used in the first experiment was used to
dewax hydrocrackate Feeds D and F with an upstream hydrotreating bed. The
fill ratio of the hydrotreating catalyst to dewaxing catalyst was 1. The
hydrotreating catalyst, a Pt-Pd/Si02A1z03, having a Pt-Pd ratio of 1:3.3 was
maintained at 600°F for the 58 day duration of the study. The aging run
conducted at a hydrogen partial pressure of 2000 psi and feed rate of 2500
scf/bbl. Liquid was charged at a liquid hourly space velocity of 1 h~' over
each
catalyst (0.5 hr' LHSV overall). Figure 4 shows that the dewaxing catalyst
reached a near equilibrated state in only 10 days and for the two feedstocks
evaluated, aged at less than 0.1 °F per day. Catalyst lineout occurred
at a
temperature significantly lower than for the Pt/ZSM-23 operating alone (Figure
1 ) when the systems are compared at constant space velocity over the
dewaxing catalyst. But even more unexpected is that the lineout temperature of
640°F to 665°F compares favorably with Pt/ZSM-23 operating alone
at the
same space velocity over the entire reaction system. In other words, for a
fixed
reactor volume, replacing half of the catalyst volume with a high activity
hydrotreating catalyst results in the same eventual lineout temperature as if
the
reactor was completely loaded with dewaxing catalyst but with the advantage of
a far shorter lineout period. An additional advantage is that the
prehydrotreating step appears to benefit dewaxing selectivity for equilibrated
systems {1 % yield advantage vs. solvent dewaxing compared to 4-5% yield
debit vs. solvent dewaxing for Pt/ZSM-23 operating alone).
Analysis of the feedstock and liquid product UV absorbtivities showed a
greater than 90% reduction in the 226 nm absorbtivity over the high activity
noble metal hydrotreating catalyst (Table 5). By comparing the data of Tables
4
and 5, it can be concluded that the hydrotreating catalyst had a slightly
better
capacity for aromatics reduction than did the Pt/ZSM-23 dewaxing catalyst.
Feedstock sulfur was reduced by 80% over the hydrotreating catalyst while the
nitrogen species were not measurably converted.
I n _ T..~.~_____._......_._.._..__...
CA 02263849 1999-02-24
WO 98/18883 PCT/US97/19688
-23-
V> >I N N 1~ 00
N of
1n
a0
M
d
O
l0
rr
C 'O
t0
t0 ~ M fr
et
d'
O
41
C
J , d..
C10
of d
C w
R w
d M M
~ t
0
Q Iw Z tC t~0 O
O t0 to
H N
t0
t0
S'
'C O
O h 1n In NM?
In
O r o 0 0 0
J Gi
M
N
N
N
!~' t O
' >
w
d Z O 1n 1L~ O IfJ
> tn -
d J r 0 0 ~ o
O o
N
r
w Y ~ O
e a t
Q m N ~ Z~'7Y
H
d
w d d d d d d N
d d
~3
Q
W
O O O w w
_ _ _ ~ '
Q Q
Q S
y M M !~ th eh
N N N N N
~ ~ ~ ~ d
(~ r f/7 N N N
~ ~
N M ~, ~ E,~,
M = v ~ ~ 1(7
~ ~
~
v
d v ~ v _~ =7
~ ~
y ~ ~ ~ ~ ~
CI CI CI
1( N N ~! N ~ d
~ d 47
' '
l 0W o o o o a u.
1 - ~ ~ u- ~ o
a
CA 02263849 1999-02-24
WO 98/18883 PCT/L1S97/19688
-24-
Examale 4
A 330 day aging experiment was conducted with the 0.5% Pt/ZSM-23
catalyst of Example 2 and the hydrotreating catalyst of Example 3 loaded
upstream of the dewaxing catalyst in a 3:7 fill ratio. The hydrotreating
catalyst
was maintained at the same temperature as the PtIZSM-23 catalyst, consistent
with preferred operation of a single reactor vessel. Neither catalyst was
presulfided. Both catalysts were reduced in H2 at 500°F prior to
introducing
liquid feed. Liquid flow rate wax maintained at 0.5 LHSV over the dewaxing
catalyst. Several feedstocks were dewaxed by this catalyst system including
hydrocrackates, hydrotreated raffinates, and a raw raffinate. For the bulk of
the
experiments, hydrogen partial pressure was maintained at 2000 psig and
hydrogen flow was 2500 scf/bbl. An aging profile for the entire run is given
by
Figure 5.
For the first 120 days on stream, the catalyst system processed
feedstocks which were also used in the 0.5% Pt/ZSM-23 aging run of Example
2. While the dewaxing catalyst operating alone required 140 days to reach a
pseudo-equilibrated state of operation at 660°F, the HDTIPt/ZSM-23
catalyst
system lined out in only 40 days at temperatures of 620-630°F for the
two
feedstocks evaluated. In addition to the reduced line out period and lower
equilibrated temperature, the HDT/Pt/ZSM-23 catalyst system showed a 1 VI
and a 1 % yield benefit over the Pt/ZSM-23 operating alone (Table 3). If the
results of Examples 2 and 4 are compared at equivalent space velocity over the
entire reaction system by adjusting the results of Example 2 to a 0.35 hr'
LHSV,
the HDT/PtIZSM-23 system still offers a 10-20°F advantage over Pt/ZSM-
23
operating alone in the eventual line out temperature. Assuming an equilibrated
aging rate of 0.1 °F/day, this activity benefit translates into an
additional half
year of catalyst life.
At approximately 120 days on stream, a low conversion heavy
hydrocrackate having a nitrogen content of 6.3 ppm nitrogen (Feed H) was
dewaxed and after an initial equilibration period, the catalyst system lined
out at
i ~ T r _
CA 02263849 1999-02-24
WO 98/18883 PCT/US97119688
-25-
635°F. Lube yield and Viscosity Index showed significant advantages for
this
catalytic dewaxing process against solvent dewaxing (Tabled 3). Later in the
aging run, a hydrocrackate contaminated with 1 % raw HVGO (Feed J) and
containing 470 ppm sulfur and 39 ppm N was dewaxed for approximately 20
days. After an equilibration period, the catalyst system lined out at
675°F and
provided tube yield equivalent to solvent dewaxing and Viscosity Index
significantly higher. These results demonstrate the robustness of the
synergistic catalyst in comparison to Example 2 in which Pt/ZSM-23 operating
alone showed poor activity and selectivity when dewaxing a feedstock
containing much lower levels of impurities.
At approximately 200 days on stream, a light hydrocrackate (Feed 1 ) was
dewaxed with negligible aging and high selectivity relative to solvent
dewaxing
showing that the aging and selectivity advantages of the synergistic catalyst
system are not restricted to heavy feedstocks. Also a light neutral furfural
raffinate (Feed K) having 2300 ppm sulfur and 16 ppm nitrogen was dewaxed
for over one month without measurable aging again demonstrating the
robustness of the catalyst system for processing feedstocks containing even
moderately high levels of impurities.
The experiment illustrated by Figure 5 demonstrated that the
hydrotreating catalyst need only to fill a fairly small fraction of the
dewaxing
reactor for the invention to have advantages over loading the reactor with
dewaxing catalyst alone. The catalyst system employing hydrotreating catalyst
followed by Pt/ZSM-23 (1:2 fill ratio) lined out after only 30 days and showed
negligible aging thereafter. This catalyst system lined out at 635°F
while
running Feed F; PtIZSM-23 operating alone lined out at 660°F (Figure
3).
Assuming an apparent activation energy of 45 kcallmol for dewaxing consistent
with ZSM-23 dewaxing data from variable flow rate experiments, it is expected
that Pt/ZSM-23 operating alone processing Feed F would line out at
650°F at
0.33 LHSV. Thus, at equivalent overall space velocity, the HDT/ZSM-23
approach offers a 15°F activity advantage over ZSM-23 operating alone.
Figure 5 also demonstrates the robustness of the HDTIZSM-23 catalyst system
___~~.~.-.._........-~ . _ ~._.....~...._. _._~_.~..~....~.....~.~....~..~.~_
. .. _ r.. . ._._....__.
I II I
CA 02263849 1999-02-24
WO 98/18883 PCT/US97/19688
-26-
for processing higher nitrogen containing feedstocks. Little activity debit,
rapid
equilibration, and insignificant aging were observed when the combination
catalyst system was used to dewax a feed containing over 6 ppm nitrogen
(Feed G, Table 1 ). This improvement is doubly unexpected because the noble
metal hydrotreating catalyst gives only a modest conversion of nitrogen and
sulfur in the feed, both of which are well known to be effective poisons for
noble
metal-containing dual functional catalysts.
Example 5
A subsequent experiment was conducted (see Figure 6) using the same
fresh hydrotreating catalyst as in Example 3 and 4 and another 0.5% PtIZSM-
23 loaded in a 2:3 fill ratio by volume. A hydrocrackate having similar
properties to Feed F in Table 2 was dewaxed at various space velocities for a
period of 140 days. The overall system was operated at rates up to 2 LHSV
over the ZSM-23, well in excess of previous data. Even at these high feed
rates, there were no appreciable signs of aging after a 20 day fine out period
at
catalyst start up. Throughout the run, a substantial advantage over solvent
dewaxing for both lobe yield and VI was obtained independent of space
velocity.
Example 6
Fresh hydrotreating catalyst and Pt/ZSM-23 catalyst, both as in Example
5, were loaded in a 3:7 fill ratio and used to dewax a hydrocracked heavy
vacuum gas oil (Feed F of Table 2). To determine the performance of the
invention for lower activity pre-hydrotreating, the hydrotreating catalyst was
presulfided in a mixture of 98% H2/2% HZS up to a temperature of 700°F
before
the introduction of liquid feed. As shown by Table 5, the effectiveness of the
hydrotreating catalyst was significantly diminished as the 226 nm reduction
over
the HDT catalyst was only 61 %. However, the catalyst system showed a similar
period of equilibriation to the unpoisoned system of Example 4 of
approximately
days. The catalyst system equilibrated at a temperature of 638°F which
represents a 22°F advantage, at constant space velocity over the
dewaxing
r__ r. ~ r.r
CA 02263849 1999-02-24
WO 98/18883 PCT/US97/19688
-27-
catalyst, over the case where the dewaxing catalyst was operated without the
benefit of the upstream hydrotreating catalyst (Example 2}. After processing
the
hydrocracked HVGO for 55 days, the catalyst system was used to dewax a
mildly hydrotreated NMP-extracted raffinate (Feed L) over a 90 day period at
various space velocities.
Feed L had sulfur and nitrogen levels comparable to the furfural raffinate
dewaxed in Example 5 (Feed K}. As Figure 7 shows, the catalyst system
performed with stability at space velocities up to 1.9 hr' over the Pt/ZSM-23
thus demonstrating that the advantage of the synergistic catalyst system for
high space velocity operation extends from hydrocrackates to feeds with even
moderately high levels of sulfur and nitrogen impurities.
Example 7
ZSM-48 was prepared according to U.S. Patent 5,075,269 and was ion
exchanged to contain a platinum loading of 0.5 wt%. The aging behavior of the
PtIZSM-48 was evaluated for dewaxing a heavy hydrocrackate (Feed M} in two
separate experiments. In the first experiment, the PtIZSM-48 was used alone to
dewax the feed while in the second experiment, the hydrotreating catalyst of
Example 3 was loaded upstream of the Pt/ZSM-48 in a 3:7 fill ratio. In both
experimental runs, the catalysts were reduced in HZ at 500°F before
liquid feed
introduction. The hydrotreating catalyst was maintained at the same
temperature as the dewaxing catalyst. Consistent with the data of Table 5, the
hydrotreating catalyst of the second experiemntal run was found to reduce the
226 nm absorbtivity of the liquid by 90%.
In both experimental runs, the dewaxing catalyst lined out in a period of
to 40 days. However, the synergistic hyudrotreating/dewaxing catalyst
system exhibited an activity advantage over the dewaxing catalyst operating
alone of 15°F at constant LHSV over the dewaxing catalyst and
6°F, by
interpolation, when the comparison is made at constant overall space velocity
30 (see Table 6).
i n 1 I
CA 02263849 1999-02-24
WO 98/18883 PCT/US97/19688
-28-
Example 8
The hydrotreating catalyst of Example 3 was tested for benzene
hydrogenation activity (BHA). Tests were performed at 100°C,
atmospheric
pressure (1 atm). Partial pressure benzene = 43 torr. Partial pressure
hydrogen = 717 torr. There is a HZlbenzene molar ratio of 17:1. Space velocity
is WHSV = 5 hr -'. The BHA rate constant is 0.024 moles benzene per gram
catalyst per hour at 100°C.
r . J n T. T