Note: Descriptions are shown in the official language in which they were submitted.
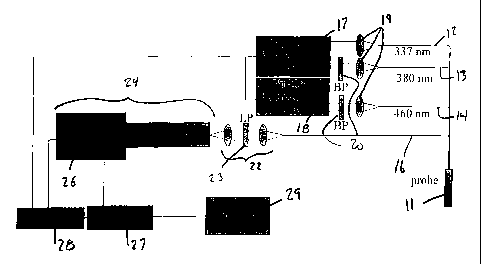
1015202530CA 02264870 2004-12-15llES_CM.l_âIl.QlSAPPARATUS FOR THE CHARACTERIZATION OF TISSUE OF EPITHELIAL LINEDVISCUSBACKGROUND OF THE INVENTIONThe present invention relates to apparatus for investigating epithelial lined viscus, and forcharacterizing normal and dysplastic tissue of the endocervical canal.The most prevalent of preinvasive conditions of the female lower genital tract iscervical intraepithelial neoplasia (CIN). The traditional definition calls it a spectrum ofintraepithelial changes that begins as a generally well differentiated intraepithelialneoplasm, which has traditionally been classified as a very mild dysplasia, and ends withinvasive carcinoma. Neoplastic changes are conï¬ned to the squamous epithelium andinclude nuclear pleomorphism, loss of polarity, and presence of abnormal mitoses. CIN isgraded l to 3, based on the amount of undifferentiated cells present from the basementmembrane to the surface epithelium. When one third of that distance is involved, the gradeis 1; when more than one third and up to two thirds is involved, the grade is 2; when morethan two thirds is involved. the grade is 3. Full-thickness involvement from the surfaceepithelium to the basement membrane is referred to as carcinoma in situ (CIS). Themedian transit time from CIN to CIS depends on the grade of CIN: for grade I CIN, thetime is approximately 6 years; for grade 2 CIN, approximately 2 years; and for grade 3,approximately 1 year. Despite some debate in the past about CIN and CIS representingtwo distinct entities, it is currently believed that CIN and CIS are part of a spectrum ofdisease that leads to invasive cancer of the cervix. The diagnosis and treatment of CIN arethus part of the prevention of invasive cervical cancer. An accepted method to classifycervical tissues is the new Bethesda system as presented in Wright et al. , âPathology ofthe Female Genital Tract,â 156-177, Springer-Verlag, (1994). In accordance with thatsystem, lesions with HPV and CIN are classiï¬ed as squamous intraepithelial lesions1015202530WO 98/05253CA 02264870 2004-12-15PCTIUS97/13300-2-(SILS) where they may be further separated as high grade SIL (CIN Il, CIN III. CIS) andlow grade SIL (CIN 1, HPV). Normal, metaplastic and non-speciï¬c inï¬ammation tissuesare classiï¬ed as nonâSILs.Cervical intraepithelial neoplasia is usually detected by screening Pap smears fromasymptomatic women. Patients with abnormal Pap smears are referred for colposcopy andpossibly biopsy. Acetic acid is applied to the cervix, and areas with abnormal DNAcontent, such as those with CIN, tum white. The colposcope, a mounted magnifying lens,is used to direct biopsies of the abnormal white areas. Abnormal conï¬gurations of bloodvessels, called vascular atypia, signal disordered growth and help the clinician know whichother areas require biopsy. An appropriate evaluation of the abnormal Pap smear involvesreview of the referral and repeat Pap smears, endocervical curettage, and multiple biopsiesof the aceto white areas; the results of such analysis will indicate whether the patient hasCIN.While the predictive accuracy of colposcopy is a matter of debate in the ï¬eld withsome researchers ï¬nding excellent overall accuracy with others ï¬nding accuracy to bepoor for CIN but good for condyloma.Recently, there has been intensive research to explore the use of opticalspectroscopy for the diagnosis of disease in human tissue. Several studies havesuccessfully demonstrated the use of ï¬uorescence. infrared absorption and Ramanspectroscopies for disease diagnosis in various organ systems. Auto and dye inducedï¬uorescence have shown promise in recognizing atherosclerosis and various types ofcancers. Many groups have utilized autoï¬uorescence for differentiation of normal andabnormal tissues from the human breast and lung, urinary bladder and gastrointestinaltract.U.S. Patent No. 6,258,576 discloses a system that uses ï¬uorescence spectroscopy todiscriminate diseased (pre-cancerous and cancerous) from non diseased1015202530W0 98/052513CA 02264870 2004-12-15PCT /U S97! 13300_ 3 -(nonnal tissues and inï¬ammation) tissue as well as differentiate cancer and high grade pre-cancers from low grade precancerous lesions of the human cervix in vivo. This systemprovides more effective patient management. as 1) ï¬uorescence measurements, and hencediagnostic information, can be obtained in real time and 2) the technique is non-invasive.In vitro studies in which ï¬uorescence was measured from cervical biopsies over the UVand visible regions of the spectrum have shown that the ï¬uorescence intensity ofhistologically abnormal cervix is signiï¬cantly lower than thathof the normal cervix fromthe same patient. In accordance with the above-referenced patent, the system includes a fiberoptic probe, illumination source and optical multi channel analyzer. The probe is insertedthrough the vaginal canal until its tip is ï¬ush with the surface of the cervix. The probe deliverslight at speciï¬c excitation wavelengths and collects ï¬uorescence from the entire emissionwavelength range from a predetermined area of the cervix. Duringâ colposcopy, spectra arecollected from each colposcopically abnormal area of the cervix prior to biopsy and from 1 to 4colposcopically normal areas. Using this system, laser induced ï¬uorescence acquired fromhuman cervical tissues in vivo at 337, 380 and 460 nm excitation is analyzed to identify cervicalintraepithelial neoplasia (CIN).A limitation of previous colposcopic and ï¬uorescence spectroscopic systems is thatthey are not capable of sampling the endocervix. It is known that atypical colposcopictissue patterns occur with some frequency at the transformation zone between thesquamous and columnar epithelium in the endocervical canal. See, Burke L, Antonioli DAand Ducatman BS. , pp. 47, 48, 61 and 62, Appleton andLarge, Norwalk CT (1991) This transformation zone (also known as the squamocolumnarjunction) is often located well within the endocervical canal and is not easily subjected tocolposcopy or ï¬uorescence spectroscopy using existing systems which are intendedprimarily to assess the ectocervix. In addition, cervical lesions that exist on the ectocervixoften extend into the endocervical canal, and characterization of the lesion within theendocervical canal is often an important matter.10152030WO 98105253CA 02264870 2004-12-15PCT/US97/13300. â 4 -It would therefore be desirable to provide a means to subject the endocervicalcanal, including the transformation zone, to ï¬uorescence spectroscopy.SUMMARY OF THE INVENTIONAccording to the invention there is provided a ring probe for applying a plurality ofelectromagnetic radiation wavelengths to an interior surface of endocervical canal tissue undertest and for gathering ï¬uorescence emitted from the tissue under test. The probe includes aplurality of optical ï¬bers coaxially arranged in a ring shape, a reï¬ector in operative relationshipâV with the plurality of optical fibers, the reï¬ector adapted to direct light from the probe in asubstantially elliptical pattern, and a ï¬ushing channel extending through said probe, said channeladapted to ï¬ush the tissue under test.Endocervical canal tissue may be characterized in vivo, by illuminating endocervicalcanal tissue in vivo with electromagnetic radiation wavelengths to produce a plurality ofï¬uorescence intensity spectra, detecting a plurality of emission wavelengths from theï¬uorescence intensity spectra, and characterizing the endocervical canal tissue as a function ofthe emission wavelengths. The characterizing step may distinguish squamous epithelium andcolumnar epitheliumâ tissue, normal squamous and abnormal tissue, normal columnar epitheliumand abnormal tissue, inï¬amed and abnormal tissue, low grade SIL and high grade SIL tissue, ornormal and high âgrade SIL tissue.ln addition, the illuminating and detecting steps may comprise, illuminating asubstantially cylindrical area of the endocervical canal tissue, and detecting the plurality ofemission wavelengths from selected portions of the cylindrical area. The illuminating anddetecting steps may further comprise illuminating an area of the endocervical canal in avicinity of a single pixel, and detecting the plurality of emission wavelengths from thesingle pixel, and repeating the illuminating and detecting steps to substantially cover thecylindrical surface. In another embodiment, the illuminating and detecting steps mayfurther comprise illuminating a substantially ring-shaped area of the endocervical canal,' detecting the plurality of emission wavelengths from the substantially ring-shaped area,and repeating the illuminating and detecting steps to substantially cover the cylindricalsurface. In yet another embodiment, the illuminating and detecting steps may furthercomprise, illuminating a substantially line~shaped area of the endocervical canal,detecting the plurality of emission wavelengths from the substantially line-shaped area,10152025WO 98/05253CA 02264870 2004-12-15PCT/U S97/ 13300- 5 -and repeating the illuminating and detecting steps to substantially cover the cylindricalsurface.In addition, the electromagnetic radiation wavelengths used to practice the methodof the present invention may be in the ranges of 317-357 nm, 360-400 nm and 440-480nm.An apparatus for characterizing endocervical tissue may comprise a light source foremitting a plurality of electromagnetic radiation wavelengths; a probe connected to the lightsource, the probe adapted to apply the plurality of electromagnetic radiation wavelengths to aninterior surface of endocervical canal tissue under test and to gather ï¬uorescence emitted fromthe tissue under test; a detector, connected to the probe, for detecting at least one ï¬uorescencespectrum emitted from the tissue under test, and a programmed computer connected to the dectormeans, for processing the at least one ï¬uorescence spectrum according to a predeterminedalgorithm to characterize the tissue under test.The light source may be a laser light source or a filtered white light source and theplurality of electromagnetic radiation wavelengths may be about 337 nm, about 380 nmand about 460 nm. The probe may include excitation optical ï¬bers for applying theplurality of electromagnetic wavelengths to an interior surface of the endocervical tissueunder test, and collection optical ï¬bers for gathering the ï¬uorescence emitted from theendocervical tissue under test.These and other features and advantages of the present invention will becomeapparent to those of ordinary skill in this art with reference to the following detaileddescription.1015202530CA 02264870 1999-03-02WO 98/05253 PCT/US97l13300-5-BRIEF DESCRIPTION OF THE DRAWINGSFIG. 1 is an exemplary apparatus in accordance with the present invention usableto perform the method of the present invention.FIG. 2 is another exemplary apparatus in accordance with the present inventionusable to perform the method of the present invention.FIGS. 3A-3F illustrate various states of the endocervical canal.FIGS. 4A and 4B are an exemplary single pixel probe usable in the presentinvention.FIG. 5 is another exemplary embodiment of a single pixel probe usable with thepresent invention.FIGS. 6-11 are various exemplary embodiments of a ring probe useable in thepresent invention.FIGS 12A and 12B are an exemplary embodiment of a line probe useable in thepresent invention.FIG. 13 is a graphical representation of a study of endocervical canal size.FIGS. 14 and 15 are graphs showing the optical transmission and excitationemission of cervical mucus.FIGS. 16 and 17 are graphs showing the optical transmission and excitationemission of ï¬uorinated ethylene-propylene (FEP).1015202530CA 02264870 1999-03-02WO 98/05253 PCT/US97/13300-7-FIGS. 18, 19 and 20 are exmplary ï¬uoresence spectra obtained from endocervicalcanal tissue.DETAILED DESCRIPTIONMEASEJREMELJI AEEAA ] USFigures 1 and 2 present exemplary embodiments of the apparatus of the presentinvention which are useable to practice the method of the present invention.Referring ï¬rst to Figure 1, an apparatus is disclosed using a single pixel opticalprobe. Exemplary embodiments of the single pixel probe are presented in more detailbelow with reference to Figures 4 and 5. The apparatus includes endocervical probe 11which, as described below in more detail, incorporates a number of optical ï¬bers includingexcitation ï¬bers 12, 13 and 14 and collection ï¬ber 16. The excitation ï¬bers are connectedto an illumination source which may be, for example, two nitrogen lasers 17,18 (LN300C,Laser Photonics) with a dye module. Other illumination sources, for example a Xenonlamp and ï¬lter wheel (disclosed in more detail with reference to Figure 2), may also beused. Other illumination sources may also be acceptable, including, for example, varioustypes of lasers (for example, HeCd or Ag lasers) used with or without dye modules, andvarious types of so-called white light sources (for example, Xe, Hg, or XeHg lamps) usedwith ï¬lter wheels. This illumination source produces light at frequencies that have beenselected for their ability to produce ï¬uorescence in tissue that permits characterization ofthe tissue, For example light at approximately 337, 380 and 460 nanometers has provenuseful. This light is coupled into excitation ï¬bers 12, 13, 14. For coupling, standardMicrobench components (Spindler Hoyer) and planoconvex lenses 19 were used. The lightcoming out of the two dye modules is bandpass ï¬ltered by bandpass ï¬lters 21 tominimize fluorescence from the dye being coupled into the excitation ï¬bers l2, l3 and 14.Collection ï¬ber 16 collects the ï¬uorescence which is projected through a coupling optics22 ( for example, Microbench, magniï¬cation 50/30) into a detector 24, for example anF/3.8 spectrograph (Monospec 18, Thermo Jarrel Ash, Scientiï¬c Measurement Systems,Inc.). In the coupling optics 22, longpass ï¬lter 23 (for example, color glass ï¬lters, Schott)l0I5202530WO 98/05253CA 02264870 2004-12-15PCT/US97/13300- 3 -block the reï¬ected excitation light from entering the detector. The spectrograph dispersesthe light onto an intensiï¬ed diode array 26. Exemplary diode array 26, electronics andcontroller 27 are manufactured by Princeton Instruments. The system also includes gatepulser 28 which is used to control the operation of lasers l7 and 18. Lasers l7 and 18 maybe controlled, for example at a 30 Hz repetition rate with a 5 nanosecond pulse duration,but other repetition rates and pulse durations may also be acceptable.The apparatus also includes programmed computer 29 which operates to energizelasers l7 and 18 and to analyze the ï¬uorescence spectra collected by collection ï¬ber 16 inorder to characterize the tissue sample under study. Details of this control and analysismay be found in U.S. Patent No. 6,258,576.Referring now to Figure 2, an apparatus embodying the present invention isdisclosed using a multiple pixel optical probe. Exemplary embodiments of multiple pixeloptical probes are presented in more detail below with reference to Figures 6-12. Theapparatus includes a multiple pixel optical probe 21 which incorporates excitation opticalï¬bersâ 22 and collection optical ï¬bers 23. Excitation optical fibers 22 are connected toreceive light from illumination source 24 which may be, for example, a Xenon lamp 26 incombination with a ï¬lter wheel 27. Once again, other illumination sources, including forexample, the laser source disclosed with reference to Figure I. would also be acceptable.As with the apparatus of Figure 1, illumination source 24 produces light at frequencies thathave been selected for their ability to produce fluorescence in tissue that permitscharacterization of the tissue.Collection ï¬bers 23 from probe 21 are connected to detector 28 which includes, forexample, an imaging spectrograph 29 (for example, a Chromex 250 IS), and a CCD array31 (for example, a thermoelectric cooled CCD Princeton Instruments EV 578x384). Theoutput of detector 28 is applied to computer 32 which is programmed to controlillumination source 24 and to analyze the ï¬uorescence spectra collected by collection1015202530WO 98/05253CA 02264870 2004-12-15PCT/US97/13300. - 9 -ï¬bers 23 and detected by detector 28 using, for example, the analysis methods disclosed inthe aforementioned patent.ERV C OLO âReferring now to Figures 3A-F, shown are simpliï¬ed representations of the crosssection of the os of the endocervical canal and surrounding tissue illustrating the locationsof the squamous epithelium (SE), columnar epithelium (CE) and transformation zone (TZ)of the uterus at various stages of maturity and for various medical conditions.Speciï¬cally. Figure 3A shows the neonate uterus, Figure 3B shows the premenarchaluterus, Figure 3C shows the menarchal uterus, Figure 3D shows the menstruating uterus,Figure 3B shows the menopausal uterus and Figure 3F shows the postmenopausal uterus.As can be seen, the transformation zone TZ can appear on the ectocervix (for example,menstruating, Figure 3D), or well within the edocervical canal (for example,postmenopausal, Figure 3F), or anywhere in between. Since the most common locationfor CIN and metaplasia is at or near the transformation zone, it is critical that thetransformation zone be imaged when conducting ï¬uorescence spectroscopy. This is ofparticular importance in menopause and postmenopause because most cervical carcinomasoccur at this age, and this is when the transformation zone is most deeply within theendocervical canal.Other general observations of the morphology of the endocervical canal are worthyof note. After the external os, which follows a funnel type opening, the endocervical canalenlarges and gets smaller again at the inner os. The uterus opens to its full size after theinternal os by a small angle. The canal can be filled inside with non-neoplastic additionaltissue like polyps and synechia. Polyps may fill the canal. Atrophy may be present, whichresults in an abnormal fonn of the wall (missing folds). In addition, It is known thatstenosis may occur after LEEP treatments.The folds of the columnar epithelium may typically be several centimeters deepwith varying shapes.. For example, in one uterus that was studied after removal byhysterectomy, the folds were a maximum of 7.83 mm with a mean depth of 3.38 mm. The1015202530CA 02264870 1999-03-02WO 98/05253 PCT/U S97/ 13300-10-folds were observed to have two main directions: axial and with an angle of approximately30 degrees to the axis of the canal. The top of this pine tree-like form points outwards thecanal. The folds are ï¬lled with mucus that sticks strongly to the tissue. Flushing withsaline solution will not remove the mucus. A study of the ï¬uorescence characteristics ofcervical mucus are presented below with reference to Figures 14 and 15.QP I JCAL PRQ QBESFigures 4A and 4B are a single pixel probe 11 that may be used in the apparatus ofFigure 1 in accordance with the present invention. Referring to Figure 4A, optical probe11 includes a bundle of optical ï¬bers 41 which are packed in a ï¬uorinated ethylene-propylene (FEP) tubing 42 that is substantially transparent to visible light and that alsotransmits in the ultraviolet. The FEP tubing 42 containing the ï¬bers 41 is ï¬exibly mountedwithin a second tubing 43 which may also be made of FEP. The outer diameter of tubing43 is preferably less than 2 mm, however other dimensions may be used. The outerdiameter of tube 43 is determined primarily by anatomical constraints of the endocervicalcanal, and is discussed in more detail below with reference to Figure 13. This dimensionallows the passage of the probe through an endocervical canal with a stenosis at the outer0s. The ï¬ber 41 within tubing 42 may be rotated and axially displaced within tubing 43 inorder to permit the testing of several tissue sites without moving tubing 43.Although FEP has proven useful for use as the material for the tubings used in theoptical probes of the present invention, other materials may also be acceptable, including,for example, other plastics such as polytetraï¬uorethylen (PTF E), glass and quartz.In the embodiment of Figure 4A and B, a short piece of a large diameter ï¬ber 45 isused. as a reï¬ector and the end surface 47 of ï¬ber 45 is polished with an oblique angle (forexample, 40°) relative to the axis of probe 11. Reflection of light emitted by ï¬bers 41toward the tissue sample under study (downward in Figure 4A) and reï¬ection of lightemitted by the tissue sample back toward ï¬bers 41 occurs because of total internalreï¬ection. An alternative reï¬ector may be made using an angled mirrored surface ofpolished metal, glass, sapphire, or the like.1015202530CA 02264870 1999-03-02WO 98/05253 PCT/US97Il3300-1]-Figure 4B is a cross section through section 4B-4B of Figure 4A, and shows theconï¬guration of ï¬bers 41. In the exemplary embodiment there are seven ï¬bers 41, sixillumination ï¬bers 48-53, and one collection ï¬ber 54, however any number of ï¬bers maybe used. In the exemplary embodiment, illumination ï¬bers 48 and 51 are used for 337 nm,ï¬bers 49 and 52 are used for 380 nm and ï¬bers 50 and 53 are used for 460 nm. andcollection ï¬ber 54 provides a single pixel collection for ï¬uorescence spectroscopy. Itshould be noted that any combination of illumination and collection ï¬bers may be usedwithout departing from the scope of the invention. For example, three illumination ï¬bersand one collection ï¬ber may be used, three illumination ï¬bers and three collection ï¬bersmay be used., one illumination ï¬ber and one collection ï¬ber may be used, one ï¬ber usedfor the combined purpose of illumination and collection may be used, and so forth. Fibers48-54 may be, for example, type SF S320/385T optical ï¬bers, and ï¬ber 46 may be a typeSFSISOO/1650N optical ï¬ber, both available from Fiberguide Industries, however othertypes may also be used. The single pixel embodiment results in a single substantiallyelliptical measurement and illumination spot, or pixel.The part of probe 11 that extends outside the vagina preferably has a rigid tubewith markings which may be used as an aid in positioning the probe both axially androtationally. Saline solution may be ï¬ushed though the openings 61 in tip 62 of probe llbefore or during a testing procedure.Figure 5 is an alternative embodiment of the single-pixel probe of the presentinvention. Referring to Figure 5, probe 11 includes ï¬ber bundle 41 like that of theembodiment of Figure 4A. light emitted from the end of ï¬bers 41 is focused by lens 66and reï¬ected by reï¬ecting surface 67 toward a tissue sample 68 under study. Similarly,light emitted by a tissue sample 68 is focused by lens 66, and reï¬ected by reï¬ectivesurface 67 back toward ï¬bers 41. Other structural details remain substantially as in Figure4A.1015202530CA 02264870 1999-03-02W0 98l05253 PCT/U S97! 13300-12-Referring now to Figures 6 and 7, a ring optical probe 21 is disclosed that may beused in the apparatus of Figure 2 Probe 21 includes a number of optical ï¬bers 72coaxially arranged in a ring shape. In one embodiment every other one of ï¬bers 72 areused for illumination, with the remaining ï¬bers being used for collection. Alternatively,each ï¬ber 72 may be used for both illumination and collection. In the embodiment ofFigures 10 and 11, reï¬ection of both illuminating light and collected light is done by ametal plug 73 with a polished reï¬ecting surface 74 Altemately, a sapphire tip 81 may beused as shown in Figure 8.. In yet another embodiment, the ends of ï¬bers 72 may becleaved and polished as shown in Figure 9. In the embodiment of Figure 9, every otherï¬ber may be used as an illumination ï¬ber with all remaining ï¬bers being collection ï¬bers.This would result in adjacent ï¬bers (for example, ï¬bers 72â and 72â) acting together toilluminate and detect from a single tissue area 77. Altemately, each of ï¬bers 72 may havethe combined function of illumination and collection. It should be noted that for the sakeof clarity, the surrounding tube is not shown in the embodiment of Figure 9.In all embodiments of the ring probe 21, light is reï¬ected from ï¬bers 72 toward atissue sample located adjacent the exterior wall of probe 21 and light emitted by the tissuesample is reï¬ected back toward ï¬bers 72. This results in a plurality of substantiallyelliptical measurement and illumination spots, or pixels distributed in a ring shape.In the ring probe 21 embodiment of Figures 6, 7 and 8, channel 76 may be includedto permit the ï¬ushing of the tissue under test with saline either before or during a test...The diameter of probe 21 may be, for example, approximately 2.8 mm, however otherdiameters may also work.Referring now to Figures 12A and 12B disclosed is yet another embodiment ofoptical probe 21 usable in the apparatus of Figure 2. The optical probe 21 of Figures 12Aand 12B is a line probe. The probe 21 includes of an illuminator that serves to illuminate atissue sample under study, and a collector that serves to collect light emitted by the tissuesample under study. The collector in the exemplary embodiment is made of 19 100micrometer optical ï¬bers 122 (type SFS100/110T available from Fiberguide Industries),1015202530CA 02264870 1999-03-02WO 98/05253 PCT/US97/13300-13-however, any number of optical ï¬bers may be used. In the disclosed embodiment, Thecollection of the ï¬uorescence occurs every 1.5 mm with one of the collection ï¬bers 122.The collection ï¬bers 122 are polished at an oblique angle relative to the longitudinal axisof the ï¬ber 122 (for example, 40°). The ends 123 of the collection ï¬bers 122 arepositioned at different axial locations along the probe as shown in Figure 12A. In theexemplary embodiment this results in a simultaneous collection every 1.5 mm along a lineapproximately 2.5 cm. long. The diameter of the line probe 21 of Figure 12A isapproximately 3 mm, however other diameters would also be acceptable.The illuminator of probe 21 in Figure 12A includes a diffuser 124. Diffuser 124 ismounted on the top of a bundle of ï¬bers 127. Fibers 127 may be for example typeSFS200 200 micrometer optical ï¬bers available from Fiberguide Industries, however othertypes of ï¬bers may be acceptable. A reï¬ective coating 128 over 270 degrees of diffuser124 allows a directed illumination over approximately 90 degrees of the circumference. ofprobe 21. The diffuser 124 is available, for example, from Rare Earth Medical.The diffuser 124 is packed in a FEP tubing 131 that is substantially transparent inthe visible and also in the ultraviolet. Included within tubing 131 is the collection bundle122, the diffuser 124 and ï¬ushing channels 132 used to carry saline to ports 133 in probe21 thus permitting ï¬ushing of the tissue either before or during testing. The outer diameterof tubing 131 is preferably less than 3 mm, however, other diameters may also beacceptable. This allows the passage of the probe through most endocervical canals.The probe is manually placed into the endocervical canal. Because of its stiffnessthe whole probe can be pressed against the walls of the endocervical canal while stillkeeping a minimal bendability.Because 100 micron ï¬bers 122 are used for collection the size of each measuredspot or pixel in the exemplary embodiment of Figure 12 A will be smaller thanapproximately 0.5 mm. The diameter depends on the distance of the collection ï¬ber 122 to1015202530CA 02264870 1999-03-02WO 98105253 PCT/US97/13300-14-the tissue. This distance may not be constant for all ï¬bers and typically varies fromapproximately 0.3 to 1 mm.The illumination light passes perpendicular through the collection ï¬bers 122.Therefore the jacket of these ï¬bers 122 should be removed. Collection ï¬bers 122 will thenact as cylindrical lenses.Referring now to Figure l3, presented in graphical form are the results of a studyof cervical size. Because the design of the probe used in the present invention depends onthe canal properties the geometrical aspects of the endocervical canal were studied. Adatabase of 362 patients at the MD Anderson Cancer Center contained measurements ofthe diameter of the external 0s. The obtained diameter is based on the size of a dilator usedat MD Anderson to measure the endocervical canal prior a LEEP or LEEP cone treatment.In a following checkup visit the canal is checked again to assure no stenosis occurred.This parameter was measured in another series of 22 patients with similar results.From Figure 13 it can be seen that the endocervical canal has a mean diameter atthe outer os of 5 mm. In most patients the outer os is larger than 3 mm and smaller than 7mm. The length of the canal was estimated from a uterus removed by hysterectomy. Theendocervical canal was measured to be approximately 4 cm long. These mechanicaldimensions may then be considered in determining a size of the optical probes used in thepresent invention. For example, the study reï¬ected in the graph of Figure 13 indicates thatthe optical probe should preferably be less than 3 mm in diameter if a single sized probe isto be used for all patients. Of course, probes of different sizes may also be used.In addition, in order to determine the possible effects of mucus in the endocervicalcanal, the transmission and ï¬uorescence of several samples of mucus was measured, andthe results are presented in graphical form in Figures 14 and 15. To produce these graphs,small amounts of mucus were diluted in 10 ml of normal buffered saline solution andplaced in a 1 cm pathlength.1015202530WO 98105253CA 02264870 1999-03-02PCT/US97/13300- 15 -As can be seen with reference to Figures 14 and 15, the strongest emission ofmucus is at 340 nm emission with an excitation at 280 nm. This will not interfere with themeasurements performed by the disclosed exemplary embodiments of the presentinvention.In addition, the transmission and ï¬uorescence of FEP tubing (the presentlypreferred material for use as the housing for the probes of the present invention) wasmeasured. and the results are presented in Figures 16 and 17. As can be seen withreference to Figures 16 and 17, the ï¬uorescence of the FEP tubing is low. However theautoï¬uorescence of the FEP tubing is about 1/10 of the tissue ï¬uorescence at 337 nmexcitation. There is a main emission peak at 400 nm with 320 nm excitation. It wasdetermined that this contribution could be accommodated during a probe calibrationprocedure, discussed in more detail below.C IN A P 0 ED REIn a clinical application, the present invention has as its purpose thecharacterization of epithelial viscus tissue, such as , for example, tissue of the endocervicalcanal. In general, when applied to the characterization of endocervical tissue, the presentinvention has as its purposes to: a) identify lesions extending from the ectocervix into theendocervical canal; b) detect the position of the transformation zone if present inside theendocervical canal; and c) identify squamous lesions with columnar involvement insidethe endocervical canal. In general, these purposes are accomplished by measuringï¬uorescence spectra at spatially resolved locations inside the endocervical canal over asubstantially cylindrical area of the interior surface of the tissue of the canal, and usingmathematical models to characterize that tissue as a function of the measured spectra.Before beginning a clinical procedure, the measuring apparatus should becalibrated. To calibrate the present invention (as shown, for example in Figure 1 and 2),the background signals are obtained without any excitation which reï¬ects the dark currentof the device. This background is stored and is automatically subtracted from anyï¬uorescence measurement. Next, the autoï¬uorescence of the probe is determined, for1015202530CA 02264870 1999-03-02WO 98/05253 PCT/US97/13300-16-example, by placing the probe in a brown bottle containing sterile H20 and measuringï¬uorescence spectra with the excitation light on. This signal is not subtracted from thetissue ï¬uorescence, however it may be subtracted if desired. In order to conï¬rmcalibration, a standard rhodamine solution (OD 0.446725, ( = 550 nm, 1 cm pathlength)may be measured. Based on previous clinical work, Rhodamine has been shown to haveapproximately twice the intensity of squamous cervical tissue ï¬uorescence.During spectral measurement of tissue, if improvement in the signal to noise ratiois desired, the spectra may be accumulated 100 and 200 times, respectively at 380 and 460nm At 337 nm 50 accumulations have proven sufficient. However, other methods toimprove the signal to noise ratio may also be used. For all three wavelengths a differentbackground subtraction ï¬le may be used with the corresponding accumulations.During a clinical procedure, it is desired to obtain ï¬uorescence spectra at 3excitation wavelengths along the substantially cylindrical surface of the entireendocervical canal with a spatial resolution of approximately 1.5 mm. This may beaccomplished by use of either of the apparatus of Figures 1 or 2, using any of the opticalprobes of Figures 4-12. During a procedure, the outer housing of the probe is placed andadvanced to the internal os of the endocervical canal. Fluorescence measurement are thenstarted. In the case of the single pixel probe (Figures 4 and 5), the single measuring pixelis advance both axially and angularly within the housing in order to image a sufficientnumber of pixels over the substantially cylindrical tissue surface. When using the ringprobe (Figures 6-11), the measuring ring of pixels is advance axially in order to image asufï¬cient number of pixels over the substantially cylindrical tissue surface. Finally, whenusing the line probe (Figure 12), the measuring line of pixels is incremented angularly inorder to image a sufï¬cient number of pixels over the substantially cylindrical tissuesurface For example, when using the line probe, four individual measurement may be' taken, one each at 12, 3, 6, and 9 oâclock (i.e., every 90°). This procedure takesapproximately 3 minutes to complete.10152025CA 02264870 1999-03-02W0 98/052523 PCT/U S97/ 13300-17-Either before or during a procedure, saline solution may be ï¬ushed over the tissuein order possibly to improve measurement accuracy by removing mucus or blood or loosetissue form the measurement site.In general, if the margin of the ï¬rst specimen at the endocervical side is free ofdysplasia or cancer and the second specimen shows no changes it may be assumed that thecanal is in a normal condition. If this margin is involved with changes it may be assumedthat the first 5 mm of the canal are in an abnormal state. If the margin of the endocervicalspecimen contains no changes it may be assumed that the margins extend no deeper than 2cm. If this specimen shows abnormal cells it may be assumed that the measurements in thecanal were abnormal even after 5 m. If the second specimen is marked as metaplasia itmay be assumed that the transformation zone is inside the endocervical canal. If the firstspecimen shows metaplasia the transformation zone is located around the os or on theectocervix.Figures 18, 19 and 20 present groups of normalized ï¬uorescence intensity spectraobtained in vivo from endocervical canals of several different patients using the methodand apparatus of the present invention In particular, Figure 18 is a group of normalizedï¬uorescence intensity spectra obtained with 337 nm excitation, Figure 19 is a group ofï¬uorescence intensity spectra obtained using 380 nm excitation, and Figure 20 is a groupof normalized ï¬uorescence intensity spectra obtained using 460 nm excitation.Based upon the foregoing disclosure, these and other features and advantages ofthe present invention will become apparent to those of ordinary skill in this art and it willbe appreciated that additions, deletions and changes nay be made to the disclosedembodiments without departing from the scope of the invention.1015202530CA 02264870 2004-12-15WO 98/05253 PCIâ/US97Il3300- 13 _BEE 1. Wright TC, Kurman RJ, and Ferenczy A in Pathglggv 91 the Eemalggenital lract (eds. A. Blaustein), 156-177, Springer-Verlag, New York (1994).2. Barron BA, Rjchart RM, "Statistical model of the natural history of cervicalcarcinoma: 11. Estimates of the transition time from dysplasia to carcinoma in situ,â JNC145: 1025-1030 (1970).3. Burke L, Antonioli DA and Ducatman BS., ,Appleton and Large, Norwalk CT (1991)._ 4. Mitchell MF, "Diagnosis and Treatment of Preinvasive Disease of theFemale Lower Genital Tract" The Cancer Bulletin. 42: 71-76 (1990).5. Reid R, Stanhope CR, Herschman BR, Crum CP, Agronow SJ, "Genitalwarts and Cervical cancer,â Am J Obstet Gynecol, IV: 815-823 (1984).6. Reid R, Scalzi P, "Genital Warts and Cervical Cancer,â Am J ObstetGynecol, 153(6): 611-618 (1985).7. Barrasso R, Coupez F, Ionesco M, DeBrux J, "Human Papilloma Virusesand Cervical Intraepithelial Neoplasia: The Role of Colposcopy,â Gynecologic Oncology,27: 197-207 (1987).8. Alfano RR, Pradhan A and Tang CG, "Optical spectroscopic diagnosis ofcancer in normal and breast tissues,âJ Optic Soc Am B, 6: 1015-1023 (1989).1015202530CA 02264870 1999-03-02WO 98/05253 PCT/US97/13300-19-9. Andersson ES, Johansson J, Svanberg K and Svanberg S, "Fluorescenceimaging and point measurements of tissue: applications to the demarcation of malignanttumors and atherosclerotic lesions from normal tissue,â Photochem Photobiol, 53: 807-14(1991).10. RichardsâKortum RR, Rava RP, Petras RE, Fitzmaurice. M, Sivak MV andFeld MS, "Spectroscopic diagnosis of colonic dysplasia,â Photochem Photobiol, 53: 777-786 (1991).11. Rava RP, RichardsâKortum RR, Fitzmaurice M, Cothren RM, Petras RE,Sivak M and Feld MS, "Early detection of dysplasia in colon and urinary bladder tissueusing laser-induced ï¬uorescence", Optical methods for tumor treatment and earlydiagnosis: mechanisms and technique, SPIE 1426: 68-78 (1991).12. Wong PTT, Wong RK, Caputo TA, Godwin TA and Rigas B, "Infraredspectroscopy of human cervical cells: Evidence of extensive structural changes duringcarcinogenesis,â Proc Natl Acad Sci USA, 88: 10988-10992 (1991).13. Alfano RR, Lui CH, Sha WL, Zhu HR, Akins DL, Cleary J, Prudente R andCellmer E, "Human breast tissues studied by IR fourier transform Raman spectroscopy,"Lasers in Life Sc, 4: 23-28 (1991).14. Baraga JJ, Feld MS and Rava RP, "Rapid near-infrared Ramanspectroscopy of human tissue with a spectrograph and CCD detector.â Appl. Spectr, 46:187-190 (1992).15. Schomacker KT, Frisoli JK, Compton CC, Flotte TJ, Richter JM, NishiokaNS and Deutsch TF, "Ultraviolet laser-induced ï¬uorescence of colonic tissue: Basicbiology and diagnostic potential,â Lasers in Surg Med, 12: 63-78 (1992).1015CA 02264870 1999-03-02WO 98/05253 PCT/US97/13300-20..16. Mahadevan A, Mitchell MF, Thomsen S, Silva E and Richards-KortumRR, "A study of the ï¬uorescence properties of normal and neoplastic human cervicaltissue,â Lasers Surg Med 13:647-655, (1993).17. Ramanujam N, Mitchell MF, Mahadevan A, Thomsen S, Malpica A,Wright TC, Atkinson, N and Richards-Kortum; In Vivo Diagnosis of CervicalIntraepithelial Neoplasia Using 337 Excitation, PNAS 91 : 1 0193, (1994).18. Ramanujam N, Mitchell MF, Mahadevan A, Thomsen S, Richards-KortumRR, "Spectroscopic Diagnosis of Cervical Intraepithelial Neoplasia (CIN) in vivo UsingLaser Induced Fluorescence Spectra at Multiple Excitation Wavelengths,â Lasers SurgMed, (in press) ( I 996).19. Brookner CK, Agrawal A, Trujillo EV, Mitchell MF and Richards-KortumRR, "Relative Risk of UV-Fluorescence Spectroscopy and Endoscopy are comparable,â24th. Annual Meeting of the American Society for Photobiology, Photochem PhotobiolSupp. (in press) (1996).