Note: Descriptions are shown in the official language in which they were submitted.
CA 02266353 1999-03-18
IMPRO ~ ~ NTS REL~TING TO CLE~iNING COMPOSITIONS
s
Technical Field
The prese~. nve~r_on re-ates to a clean g cor~csi~ a~
'J -O a merhcd OL. trea~i-g surfaces wi~h the SG-~- mPCS _ C .
sackground to the Invention
~ard-suvface clean r.g compositions generally comprise ore or
more sur.actants, and, optiorally, one or more hvg e-e
agents .
Typ-cally, the surfacrants used in such cleanirg
compositions are seiected from anionic, nonionic, amphoteric
and cationic surfactants. Nonionics are very commor_y sed
due to their effectiveness on fatty soils and the ease wi~h
which their foaming can be controlled. .~. known manner in
which foaming can be controlled involves incluc-ing in -he
composition relatively low levels of a solubilised
hydrophobic oil such as an iso-paraffin and a relat ve-y low
level on product of a suvfactant such as a fatty acid soap
which forms a calcium salt insoluble in dilute aqueous
solution. Both the oil and the soap components are
soluble/miscible in the composition forming a stable product
until (hard) water is added whereupon the combination of oil
and the particles of calcium salt formed in situ acts as a
~foam breaker~ for a second (main) surfactant. This greatly
aids in the rinsing of the compositions as excessive -oaming
is prevented.
AM~i~D~~ Stt~E~
CA 02266353 1999-03-18
As well as the ef'ect or. the salt content o~~ the compos'~_on
on dilucion with hard ~hracer it ia knO'hr2l to e.~ploit t:-e
changes in acidity,~al~al n ty ~hich occur or. dilut or T~us
acidic gene~al pur?ose cleaning compositions based on t'-.e
. gh oaming anior. c sur actant magnesium ?.'~a, or on sodium
PAS, ard an organic ac-d such as citric are knor,~. w'~ ch ar-
reutralised to pH ~ 0 w'-h a base such as ammon~a to give
inpror~ed cleanins e-~ecc-~eness especially as regarcs ''m.--
soaps ard lime sca'es Wl _st mairtaining c'ean_ng e~~i - er ~
'8 aga:ns~ fa~_y,'greasy aO a. I t is l~-ow. from Wo 94/09108
i-c:Lude in such o~mulations Clû-18 fatty acids wh c'n ~ave a
foarn boost_..g ef.ec. or. the surfactants in acid solut-on,
and which, on add ~-on or several volumes of a'kalire water
form a p~ecipi_ate o the fatty acid with calcium present o
~, give a oam-suppr.essing effect which aids rins ng ard
removal of the foam ~vroduced oy use of the product
In addition to surL-ac~ants i~ is known ~rom WO 96/17918 to
incorporate polymers in hard surLrace cleaning compos-t_ons
2û These polymers provide benefits both in initial and repea_ec
cleaning In order to obtain benefits from the presence o
polymers i~ is often useful to formulate compositions in an
acidic pH range Typically the pH of the compositions is in
the range 3-4 5, with pre.erred va ues around 4 5 ~ol,-mer
containing compositions are difficult to antifoam: the
presence of iso-paraffins and fatty acids reduce the
performance in neat usage and, in particular, the recleaning
properties of the product Overall the isoparaffin/fatty
acid antifoaming system does not provide a sufficier~tly low
3û foam profile a-,d ease of rinsability for some consumers
nr~
CA 022663~3 1999-03-18
srief Description of the Invention
We have determined that an effective antifoaming system can
be hased or the synergism between four different components:
a b~-anched fatty chain nonionic including a small amount o-
free monobranched alcohol; a low le~el of ethoxy'ated,~
propoxylated nonionic surfactant and a fatty acid.
~ ccordingly a first aspect o~ the present invention ~e a~es
to a cleaning composition of pH 2-6 comp~ising 0.l-30%wt on
product of an ethoxylated nonionic surfactant, less ~han
50%wt of total surfactant of anionic surfactant and 0.005-
5%wt on product of a water soluble, anionic polymer having
an average molecular weight of less than l,000,000, wherein,
i5 the ratio of polymer : nonionic is 0.l:1 or less, said
product being CHAR~CTERISED I~ THAT:
a) the ethoxylated nonionic surfactant is a material of
the general formula: R- (EO)W-OH wherein EO is a residue
of ethylene glycol, w is l-l0 and R is a C~-C22 alkyl or
~lkyl phenyl group;
b) the composition further comprises a monocarboxylic
.
fatty acid of general formula: R~-COOH wherein R is a
C~-Cl8 alkyl group;
c) the composition further comprises a mixed
propoxylated/ethoxylated nonionic surfactant of general
formula: R2- [ (EO) X ( PO) y] -OH wherein EO and PO are
residues of ethylene and propylene glycol, x is l-l0, y
is l-l0, and R2 is a C8-Cl8 alkyl group, and,
d) the composition further comprises a monobranched
alcohol of the general formula R~-OH wherein R, is a
branched C8-Cl8 alkyl group.
MJlENDED SfflET
CA 022663~3 1999-03-18
., i
.
A second aspect of the present invention provides a process
for cleaning surfaces which comprises the step of treating
the surface with a composition of pH 2-6 comprising: 0.1-
30%wt on product of an ethoxylated nonionic surfactant, lessthan 50%wt of total surfactant of anionic sur_aclant and
0.0()5-5%wt on product of a water soluble, anionic ~olymer
having an average molecular weight of less than 1,000,000,
whereir, the ratio o' polymer : nonionic is 0.1:1 o~ less,
said product being C'-~RACmERISE~ IN THAT:
a) the ethoxylated nonionic surfactant is a material O r
the general formula: R- (EO)W-OH wherein EO is a residue
of ethylene glycol, w is 1-10 and R is a C~-C22 alkyl or
alkyl phenyl group;
b) the composition ur~her comprises a monocarboxylic
ratty acid of general formula: Ri-COOH wherein Rl is a
C~-C.a alkyl group;
c) the composition further comprises a mixed
propoxylated/ethoxylated nonionic surfac_ant of gene~al
formula: R2-[(EO)x(PO)y]-OH wherein EO and PO are
residues of ethylene and propylene glycol, x is 1-10, v
is 1-10, and R~ is a C8-Cl8 alkyl group,and,
d) the composition further comprises a monobranched
alcohol of the general formula R3-OH wherein R~ is a
branched C8-Cl8 alkyl group.
It is believed that the combination of the these components
gives a superior antifoaming effect when the composition is
diluted with sufficient water of pH >6 to raise the pH of
the dilute composition to above 6.
AA~E~OED SltEET
. .
CA 022663~3 1999-03-18
WO98/122g4 PCT~7/05175
Detalled DescrlDtlon of the Inventlon
In order that the invention may be further understood it
will be described hereafter with reference to preferred
features and materials. Where percentages of components
are used they are weight percents unless stated otherwise
and where ratios are used they are weight ratios unless
stated otherwise.
Ethoxvlated Nonlonlcs
Nonionic, ethoxylated surfactants are present in the
compositions of the invention. These surfactants are
believed to engage in a synergistic interaction with the
polymer, to improve cleaning and aid the removal of soil
subsequently deposited.
Suitable nonionic detergent active compounds can be
broadly described as compounds produced by the
condensation of ethylene oxide groups, which are
hydrophillic in nature, with an organic hydrophobic
compound which may be aliphatic or alkyl aromatic in
nature. The length of the hydrophillic or polyoxyethylene
radical which is condensed with any particular hydrophobic
group can be readily adjusted to yield a water-soluble
compound having the desired degree of balance between
hydrophillic and hydrophobic elements.
Particular examples include the condensation product of
aliphatic alcohols having from 8 to 22 carbon atoms in
either straight or branched chain configuration with
ethylene oxide, such as a coconut oil ethylene oxide
condensate having from 3 to 10 moles of ethylene oxide per
mole of coconut alcohol; condensates of alkylphenols whose
CA 022663~3 1999-03-18
W 098/12294 PCTAEP97/0517S
alkyl group contains from 6 to 12 carbon atoms with 3 to
10 moles of ethylene oxide per mole of alkylphenol.
The preferred alkoxylated alcohol nonionic surfactants are
ethoxylated alcohols having a chain length of C9-C11 and
an EO value of at least 3 but less than 10. Particularly
preferred nonionic surfactants include the condensation
products of C1O synthetic alcohols with 3-8 moles of
ethylene oxide. The preferred ethoxylated alcohols have a
calculated HLB of 10-16.
The material available in the marketplace as 'DOBANOL
91.5EO~ (TM) comprises a high proportion of a C9_ll alcohol
with five moles of ethoxylation and has been found to be a
suitable source of nonionic surfactant in compositions
according to the invention.
The amount of nonionic detergent active to be employed in
the composition of the invention will generally be from
0.1 to 30%wt, preferably from 1 to 20%wt, and most
preferably from 3 to 10%wt for non-concentrated products.
Concentrated products will have 10-20%wt nonionic
surfactant present, whereas dilute products suitable for
spraying will have 0.1-5%wt nonionic surfactant present.
ln particularly preferred embodiments of the invention,
typical levels of ethoxylated nonionic surfactant fall in
the range 5-8%wt for non-concentrates and 13-16% for
concentrates.
Polvmers
In the context of the present invention, anionic polymers
are those which carry a negative charge or similar
polymers in protonated form. Mixtures of polymers can be
employed. It should be noted that the beneficial effect
CA 022663~3 1999-03-18
WO98/12294 PCT~P97/0~175
of anionic polymers is significantly reduced by the
presence of anionic surfactants. For this reason ,and for
reasons elaborated below, the level of anionic surfactants
in the compositions of the invention should be minimised.
The preferred polymers in embodiments of the present
invention are polymers of acrylic or methacrylic acid or
maleic anhydride, or a co-polymer of one or more of the
same either together or with other monomers. Particularly
suitable polymers include polyacrylic acid, polymaleic
anhydride and copolymers of either of the aformentioned
with ethylene, styrene and methyl vinyl ether.
The most preferred polymers are maleic anhydride co-
polymers, preferably those formed with styrene, acrylic
acid, methyl vinyl ether and ethylene. Preferably, the
molecular weight of the polymer is at least, 5000, more
preferably at least 50,000 and most preferably in excess
of l00,000. As the molecular weight increases the
cleaning benefit of the polymer is reduced. ~VERSICOL
Ell' (RTM) ex Allied Colloids has been found to be a
suitable polymer.
~ypically, the compositions comprise at least 0.0lwt%
polymer, on product. Preferably the level of polymer is
0.05-5.0wt% at which level the anti-resoiling benefits
become particularly significant. More preferably 0.l-
3.0wt~, most preferably 0.2-l.5wt%, of polymer is present.
We have determined that higher levels of polymer do not
give significant further advantage with common dilution
factors, while increasing the cost of compositions.
However, for very concentrated products which are diluted
prior to use, the initial polymer level can be as high as
5%w.
CA 022663~3 1999-03-18
W 0 98/12294 PCTAEP97/05175
FattY Acids
As mentioned above, it is believed essential that the
monocarboxylic fatty acid is capable of forming a calcium
salt which is no more than sparingly soluble in aqueous
solutions of foaming surfactants.
The monocarboxylic fatty acid is typically present at a
level of 0.2-4.5%wt on product: the upper levels of this
range being used for more highly concentrated
compositions.
Preferably the monocarboxylic fatty acid content is in the
range 0.2-3.0%wt, most preferably in the range 0.3-2.0%wt.
In embodiments of the invention the level of fatty acid is
preferably such that the ratio of ethoxylated nonionic to
monocarboxylic fatty acid falls in the range 4:1-1:1, more
preferably 3:1-2:1 as nonionic: moncarboxylic fatty acid.
More preferably, the monocarboxylic fatty acid is a
mixture of saturated fatty acids having an average carbon
chain length in the range 10-16, more preferably 12-14.
Coconut fatty acids or a synthetic equivalent thereof are
particularly preferred.
Pro~oxYlated/EthoxYlated Nonionics
The mixed propoxylated/ethoxylated nonionic surfactant
preferably has x is 4-8, y is l-S.
Preferably R2 is a C9-Cl7 alkyl group, i.e. while a range
of materials may be present the average chain length is of
9-17 carbons.
CA 022663~3 1999-03-18
W O 98/12294 PCTAEP97/05175
BIODAC L6S50 (TM), a commercially available (C12-
15)6EO/2PO ethoxylated alcohol, is a suitable
propoxylated/ethoxylated nonionic for use in embodiments
of the present invention.
This C12-C15 alcohol condensate has a cloud point in
distilled water of 36 - 41~C, a mean molecular weight: 534
- 623 and a carbon chain composition of:
C12 = 41%
C13 = 10%
C14 = 37%
C15 = 12%
It is preferable that the weight ratio of the fatty acid
to the propoxylated/ethoxylated nonionic falls in the
range 1:3-1:10 as fatty acid: nonionic. Ratios of 1:5-1:8
are particularly preferred, with a ratio of 1:6 being used
in typical embodiments of the invention.
Preferred levels of the propoxylated/ethoxylated nonionic
range from 2-10%wt with levels of 3-6%wt on product being
preferred.
Monobranched Alcohol
The monobranched alcohol may be added as a separate
component or, more conveniently, use may be made of the
residual monobranched alcohol which is present in certain
ethoxylated nonionic surfactants. In preferred
embodiments of the invention no additional monobranched
alcohol is added to the composition.
It is believed that during the ethoxylation process which
is commonly used to prepare ethoxylated nonionic
surfactants from alcohol feedstocks, the ethoxylation of
CA 022663~3 l999-03-l8
W O 98/12294 P~ 97/05175
- 10 -
linear alcohols proceeds more guickly than that of the
branched alcohols. Thus, where a mixed alcohol feedstock
is used, i.e. a synthetic mixture of linear and branched
alcohols, the reaction will initially proceed with the
ethoxylation of the linear alcohols while the branched
alcohols will react to a lesser extent. The product
obtained from this process can comprise a residue of
unreacted monobranched alcohol.
The material available in the marketplace as 'DOBANOL
91.5EO' (TM) comprises a high proportion of a Cgll alcohol
with five moles of ethoxylation and sufficient free
monobranched alcohol to be a suitable source of both
monobranched alcohol and ethoxylated nonionic surfactant
in compositions according to the invention.
While the pH of the composition can fall in the range 2.0-
6.0 it is preferable that the pH of the composition is
3.0-4.5. It is believed that above pH 4.5 the benefit of
the polymer falls off and below pH 3.0 surface damage may
occur. The preferred pH range is 3.2-4Ø The most
preferred pH is around 3.75. Compositions having a pH of
less than 3.0 will damage enamel surfaces.
Citric acid is particularly preferred to regulate the
acidity balance. Typical levels of citrate range from
0.5-5%, with higher levels of 5-10% being used in
concentrates and lower levels of 0.1-1% being used in
sprayable products. Citric can be replaced by other
suitable buffering agents to maintain the pH in this
range. These are preferably other polycarboxylic fatty
acids. Citric is also preferred for environmental reasons,
CA 022663~3 1999-03-18
W O 98/12294 PCT~EP97/05175
a lack of residues and it is believed to be the most
cost/weight-effective acld.
Sodium hydroxide is generally used to regulate the pH to
the required level.
Antimicrobialfi
Optional antimicrobial agents used in the compositions of
the present invention are benzoic acid derivatives,
dicarboxylic acids, Cl-C6 alkanols and mixtures thereof
Typical levels of the antimicrobial agent in formulations
range from 0.01 to 8%, with levels of 0.05-4wt%,
particularly around 2% being preferred for normal
compositions and up to two or four times that
concentration being present in so called, 'concentrated~
products. Although both the normal and concentrated
products can be used neat it will be commonplace for these
to be diluted by the user before use. For sprayable
products, which are seldom diluted prior to use, the
concentration of the antimicrobial agent will be in the
range 0.05-0.5%wt.
In general, whatever the strength of the product the ratio
of the nonionic surfactant to the antimicrobial agent will
preferably be in the range 50:1 to >1:1, more preferably
30:1 to >1:1 i.e. an excess of nonionic will be present
relative to the antimicrobial.
Amongst the benzoic acid derivatives a preferred
antimicrobial agent is salicylic acid, which gives better
hygiene results than benzoic and shows a very marked
improvement as compared with sorbic acid.
CA 022663~3 1999-03-18
W O 98112294 PCT~EP97/05175
- 12 -
.
Alternative benzoic acid derivatives are the polyhydroxyl
carboxylic acids in which at least two hydroxyl groups are
present and at least one of the hydroxyl groups is ortho-
to the carboxylic acid group. The remaining hydroxyl
group or groups can be in the remaining ortho-, para- or
meta- configurations. The polyhydroxyl carboxylic acids
exhibit the same synergy as the mono hydroxylic acid
derivative (salicylic acid) but are believed to be less
irritant.
We have determined that, in the presence of nonionic
surfactant, salicylic acid derivatives methylated at
positions 3-6 exhibit an additional antimicrobial action
over that obtained with salicylic acid. This is
particularly true for gram positive bacteria and yeasts.
The preferred alkyl substituted ortho-hydroxy aromatic
carboxylic acid of the general formula:
Rl-C6H~(OH)(COOH)
wherein Rlis Cll2 alkyl, and the hydroxyl group is ortho to
the carboxyl group on the benzene ring structure.
Preferably the alkyl substituted ortho-hydroxy aromatic
carboxylic acids are substituted at the 3, 4 or 5-
position, relative to the carboxyl group. Preferred chain
lengths for the alkyl group are Cl6, with methyl
substituted acids being particularly preferred.
Particularly preferred acids are 2-hydroxy 5-methyl
benzoic acid, 2-hydroxy 4-methyl benzoic acid and 2-
hydroxy 3-methyl benzoic acid.
Amongst the dicarboxylic acids, succinic acid is
preferred.
CA 022663~3 1999-03-18
W O 98/12294 PCT~EP97/05175
- 13 -
Amongst the alkanols, the C2-Cs alcohols are preferred.
These include ethanol and isopropanol. Isopropanol has
been found to be particularly effective and is
particularly preferred among the alkanols.
Minors and ODtional ComDonents
The composition according to the invention can contain
other minor, inessential ingredients which aid in their
cleaning performance and maintain the physical and
chemical stability of the product.
For example, the composition can contain detergent
builders. In general, the builder, when employed,
preferably will form from 0.1 to 25% by weight of the
composition.
optionally, the composition can include one or more
amphoteric surfactants, preferably betaines, or other
surfactants such as amine-oxide and alkyl-amino-
glycinates. Betaines are preferred for reasons of cost,
low toxicity (especially as compared to amine-oxide) and
wide availability. It is believed that amphoteric
surfactants show a slight synergy with some organic acids
(when present) as regards antimicrobial effects.
Typical betaines in compositions according to the
invention are the amido-alkyl betaines, particularly the
amido-propyl betaines, preferably having an aliphatic
alkyl radical of from 8 to 18 carbon atoms and preferably
having a straight chain. These betaines are preferred as
they are believed to comprise relatively low levels of
nitrosamine precursors although other betaines, such as
alkyl betaines, can be used in the compositions of the
invention.
CA 022663~3 1999-03-18
WO98/12294 PCT~P97/05175
- 14 -
Typical levels of amphoteric range from 0.01 to 8%, with
levels of l-5wt%, particularly around 2% being preferred
for normal compositions and up to four times the
concentration being present in so called, concentrated
products. As with the nonionic surfactant, lower levels
or around 0.05-1% will be employed in sprayable products
and higher levels of, typically, around 4%wt in
concentrates. In general, the ratio of the betaine to
the aromatic carboxylic acid will be in the range 1:3 to
3:1, with approximately equal levels on a weight basis
being preferred.
Metal ion sequestrants, including
ethylenediaminetetraacetates, aminopolyphosphonates (such
as those in the DEQUESTk range) and phosphates and a wide
variety of other poly-functional organic acids and salts,
can also optionally be employed. It is believed that the
hygiene performance of the composition is improved by the
presence of a metal ion se~uesterant.
Hydrotropes, are useful optional components. It is
believed that the use of hydrotropes enables the cloud
point of the compositions to be raised without requiring
the addition of anionic surfactants. The presence of both
anionic surfactants and betaine is believed to be
detrimental to the formulations as these surfactants
interact with the amphoterics, when present, and to form a
complex which inhibits the synergistic hygiene activity of
the amphoterics with the organic acid. Preferably the
formations according to the invention are free of anionic
surfactants when betaine is present, or contain low levels
of anionic surfactants, i.e. less than 50% of the total
level of surfactant present and preferably less than 50%
of the level of the betaine in the product. Anionics are
compatible with the solely alcohol ethoxylate based
compositions of the present invention when the level is
CA 022663~3 1999-03-18
W O 98/12294 PCT~EP97/05175
below 50%wt of the total surfactant present, but their
level should be minimised in view of their interactions
with the polymers. Preferably the level of anionic is
below 30% of the total surfactant content of the
composition and more preferably below 10% of the
surfactant content. It is possible to make compositions
which contain little or no anionic surfactant.
Suitable hydrotropes include, alkali metal toluene
sulphonates, urea, alkali metal xylene and cumene
sulphonates, polyglycols, >20EO ethoxylated alcohols,
short chain, preferably C2-Cs alcohols and glycols.
Preferred amongst these hydrotropes are the sulphonates,
particularly the cumene, xylene and toluene sulphonates.
Typical levels of hydrotrope range from 0-5% for the
sulphonates. Correspondingly higher levels of urea and
alcohols are required. Hydrotropes are not always
required for dilute, sprayable products, but may be
required if lower EO or longer alkyl ethoxylates are used
or the cloud point needs to be raised considerably.
Typically, the cloud point of the final composition should
preferably be in the range 45-50 Celcius. The cumene
sulphonate is the most preferred hydrotrope. For
ethoxylated nonionic levels of around 7%wt on product
levels of SCS will generally be in the range 0.6-0.8wt%
for ethoxylated nonionic levels of around 14%wt levels of
SCS will generally be in the range 1.0-1.2wt%.
Compositions according to the invention can also contain,
in addition to the ingredients already mentioned, various
other optional ingredients such as, solvents, colourants,
optical brighteners, soil suspending agents, detersive
enzymes, compatible bleaching agents, gel-control agents,
freeze-thaw stabilisers, further bactericides, perfumes
and opacifiers.
CA 022663~3 1999-03-18
W O 98/12294 PCT~EP97/05175
- 16 -
.
A suitable preservative is PROXEL LV ~TM).
It is preferred that compositions of the invention are
essentially free of solvents, i.e. that they comprise less
than 0.5% of glycol ethers and Cl-C6 linear alcohols.
The most preferred formulations according to the present
invention, excluding minors such as perfumes, preservative
and colour, comprise:
a) 5-16%wt of an ethoxylated mixed linear and branched
alcohol nonionic surfactant having an ethoxylation
value of 5-10 and an average carbon chain length of
8-14;
b) 0.2-1.5%wt of a polymer of acrylic or methacrylic
acid or maleic anhydride, or a co-polymer of one or
more of the same either together or with other
monomers having an average molecular weight of 50000-
1000000;
c) 2-10% of a 4-8EO/1-5PO ethoxylated alcohol with an
average carbon chain length of 12-15;
25 d) 0.3-2.0%wt of a saturated fatty acid having an
average carbon chain length of 12-14, and;
e) 0-5%wt of an alkali metal sulphonate hydrotrope;
In order that the invention may be further understood it
will be described further by way of the following non-
limiting examples and with reference to the sole
accompanying figure.
CA 022663~3 1999-03-18
WO 98/12294 PCTAEP97/05175
Exam~les
The following compositions (A and B) were prepared by
mixing the followlng components.
A% B%
Nonionic (Dobanol 91.5) 7.0 14.0
Nonionic EO/PO (BIODAC L6S50) 3.0 6.0
Polyacrylate Versicol E11) 0.5 1.0
Coco Fatty Acid 0.5 1.0
Citric acid 0.6 1.2
Sodium Hydroxide -- to pH 3.75 --
Sodium Cumene Sulphonate -- to cp 45-50 --
Preservative tProxel LV~ 0.016 0.016
Perfume 0.38-0.5 0.76-1.0
Water qv qv
Viscosity (mPas) 20 80
Both were stable compositions.
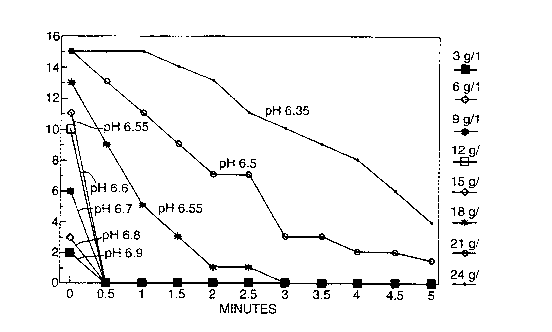
The foam profile of Example A was determined during
dilution with Italian tap water of 24 French hardness,
measuring the foam height as the product was diluted from
a concentration of 24 g/l to 3 g/1 to model the rinsing
process. Results are shown in the accompanying figure.
From the figure it can be seen that as the composition is
diluted the pH of the diluted composition rises and the
both the foam height and the foam persistence are reduced
markedly.
,