Note: Descriptions are shown in the official language in which they were submitted.
CA 02267165 1999-03-26
WO 98h 3009 1 PCT/EP97/05070
Use of 1-hydroxy-2-pyridones for the treatment of seborrheic dermatitis
Seborrheic dermatitis is understood as meaning a disorder of the scalp
which differs from simple dandruff by the presence of erythema as a sign
of inflammation, by the greater degree of scaling with occasional itching
and burning, and by the occurrence of eczematous changes to other body
sites. It can occur in the form of patches, but also more frequently affects
the whole scalp and often includes, beyond the hairline, the forehead,
around the neck and the ears. In severe cases, the scalp can have a
secondary infection, and the changes can then exhibit a spongy
consistency, vesicle and crust formation and can weep.
Seborrheic dermatitis frequently occurs even in infancy and usually remits
spontaneously at an age of 8-12 months. The scalp changes consisting of
erythema) scaling and occasionally vesicles and crusts in infants can
regress spontaneously within a few weeks, intermittently reoccur or persist
during the entire childhood. They are frequently combined with a similar
process around the eyelids, nose and ears. Later, the condition usually
occurs after puberty and can last for the whole life or even increase in
strength. Approximately 1-3% of the population are affected by this illness.
It is known that 1-hydroxy-2-pyridones and their salts exhibit activity
against normal dandruff which is characterized by a clinically noninflam-
matory scaling of the scalp occurring in nearly all people (DE 22 34 009).
The most promising type of treatment of seborrheic dermatitis until now
was the topical application of corticosteroid preparations, but more recently
topical therapy with antimycotic substances has gained importance.
While corticosteroid preparations display their activity exclusively via an
effect on the inflammatory process, the antimycotic substances such as
ketoconazole are active exclusively against the yeast fungi of the strain
Pityrosporum which is assumed to be the cause of seborrheic dermatitis.
The 1-hydroxy-2-pyridones according to the invention, however, combine
the properties of both classes of substance in one substance and exhibit
both antiinflammatory action and antimycotic activity against Pityrosporum
strains.
CA 02267165 1999-03-26
2
In comparison to ketoconazole, the substances according to the invention -
even after only a short topical contact time - concentrate rapidly in the skin
layers which are relevant for fungal growth and 'thus contribute to a rapid
cure.
While ketoconazole is inactive in vitro against gram-positive bacteria
(Kinsman et al., J. Med. Microbiol. (1983) 16) No.2, IV), the hydroxy-
pyridones according to the invention exhibit activity against gram-positive
and gram-negative aerobic and anaerobic bacteria (Dittmar et al.,
Arzneim.-Forschung, (1981 ) 31 (II), No. 8a, pp. 1317-1322). With respect
to the treatment of secondarily infected cases, this is an extremely impor-
tant finding.
Compared with ketoconazole, the compounds used according to the
invention furthermore have very crucial advantages with respect to their
processing possibilities in pharmaceutical preparations. On account of their
solubility in water, alcohols and aqueous-alcoholic solutions, the pre-
paration of hair lotions and transparent gel preparations is possible without
problems.
The preparations according to the invention can also be employed for the
treatment of Pityriasis versicolor, a superficial, noninflammatory skin
fungus disorder on the trunk.
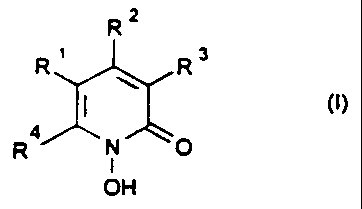
The invention therefore relates to the use of 1-hydroxy-2-pyridones of the
formula I
RZ
R' R3
R4 N O
OH
in which R1, R2 and R3, which are identical or different, are a hydrogen
atom or alkyl having 1-4 carbon atoms, and
R4 is a saturated hydrocarbon radical having 6 to 9 carbon atoms or a
radical of the formula II
CA 02267165 1999-03-26
3
Ar.Z
i
x- cx~-
Y
where
X is S or O,
Y is a hydrogen atom or up to 2 halogen atoms such as chlorine
and/or bromine)
Z is a single bond or the bivalent radicals O, S, -CR2- (R = H or
(C1-C4)-alkyl) or other bivalent radicals having 2-10 carbon and, if
appropriate, oxygen and/or sulfur atoms linked in the form of a
chain, where - if the radicals contain 2 or more oxygen and/or sulfur
atoms - the latter must be separated from one another by at least 2
carbon atoms and where 2 adjacent carbon atoms can also be
linked to one another by a double bond and the free valences of the
carbon atoms are saturated by H and/or (C1-C4)-alkyl groups,
Ar is an aromatic ring system having up to two rings which can be
substituted by up to three radicals from the group consisting of
fluorine, chlorine, bromine) methoxy, (C~-C4)-alkyl, trifluoromethyl
and trifluoromethoxy, in free or in salt form,
for the production of a pharmaceutical for the treatment of seborrheic
dermatitis.
In the radicals "Z", the carbon chain members are preferably CH2 groups. If
the CH2 groups are substituted by C~-C4-alkyl groups, CH3 and C2H5 are
preferred substituents. Exemplary radicals "Z" are:
-O-, -S-, -CH2-, -(CH2)m- (m = 2-10), -C(CH3)2-, -CH20-, -OCH2-, -CH2S-,
-SCH2-, -SCH(C2H5)-, -CH=CH-CH20-, -O-CH2-CH=CH-CH20-,
-OCH2-CH20-, -OCH2-CH2CH20-, -SCH2CH2CH2S-,
-SCH2CH2CH2CH20-) -SCH2CH20CH2CH20-,
-SCH2CH20CH2CH20-CH2CH2S- or -S-CH2-C(CHg)2-CH2-S-.
The radical "S" is a sulfur atom, the radical "O" is an oxygen atom. The
term "Ar" denotes phenyl or fused systems such as naphthyl, tetrahydro-
naphthyl and indenyl, and also isolated systems as such, which are derived
from biphenyl, diphenylalkanes, Biphenyl ethers and Biphenyl thioethers.
CA 02267165 1999-03-26
4
In the formula I, the hydrocarbon radial R4 is an alkyl or cyclohexyl radical
which can also be bonded to the pyridone ring via a methylene or ethylene
group or can contain an endomethyl group. R4 can also be an aromatic
radical which, however, is preferably bonded to the pyridone radical via at
least one aliphatic carbon atom.
Important representatives of the class of compounds characterized by the
formula I are:
6-[4-(4-chlorophenoxy)phenoxymethyl]-1-hydroxy-4-methyl-2-pyridone,
6-[4-(2,4-dichlorophenoxy)phenoxymethyl]-1-hydroxy-4-methyl-2-pyridone,
6-(biphenyl-4-oxymethyl)-1-hydroxy-4-methyl-2-pyridone, 6-(4-benzyl-
phenoxymethyl)-1-hydroxy-4-methyl-2-pyridone, 6-[4-(2,4-dichlorobenzyl-
oxy)phenoxymethyl]-1-hydroxy-4-methyl-2-pyridone, 6-(4-(4-chloro-
phenoxy)phenoxymethyl]-1-hydroxy-3,4-dimethyl-2-pyridone, 6-[4-(2,4-di-
chlorobenzyl)phenoxymethyl]-1-hydroxy-3,4-dimethyl-2-pyridone,
6-[4-cinnamyloxy)phenoxymethyl]-1-hydroxy-4-methyl-2-pyridone,
1-hydroxy-4-methyl-6-[4-(4-trifluoromethylphenoxy)phenoxymethyl]-2-pyrid-
one, 1-hydroxy-4-methyl-6-cyclohexyl-2-pyridone, 1-hydroxy-4-methyl-
6-(2,4,4-trimethylpentyl)-2-pyridone, 1-hydroxy-4-methyl-6-n-hexyl-) -6-iso-
hexyl-, -6-n-heptyl- or -6-isoheptyl-2-pyridone) 1-hydroxy-4-methyl-6-octyl-
or -6-isooctyi-2-pyridone, in particular 1-hydroxy-4-methyl-6-cyclohexyl-
methyl- or -6-cyclohexylethyl-2-pyridone, where the cyclohexyl radical can
in each case also carry a methyl radical, 1-hydroxy-4-methyl-6-(2-bicyclo-
[2,2,1 ]heptyl)-2-pyridone) 1-hydroxy-3,4-dimethyl-6-benzyl- or -6-dimethyl
benzyl-2-pyridone or 1-hydroxy-4-methyl-6-([i-phenylethyl)-2-pyridone.
The term "saturated" in this case designates those radicals which contain
no aliphatic multiple bonds, i.e. no ethylenic or acetylenic bonds.
The abovementioned compounds of the formula I can be employed either
in free form or as salts, use in free form is preferred.
If organic bases are used, poorly volatile bases are preferably employed,
for example low molecular weight alkanolamines such as ethanolamine,
diethanolamine, N-ethylethanolamine, N-methyldiethanolamine, triethanol-
amine, diethylaminoethanol, 2-amino-2-methyl-n-propanol, dimethylamino-
propanol, 2-amino-2-methylpropanediol, triisopropanolamine. Further
CA 02267165 1999-03-26
poorly volatile bases which may be mentioned are, for example,
ethylenediamine, hexamethylenediamine, morpholine, piperidine,
piperazine, cyclohexylamine, tributylamine, dodecylamine,
N,N-dimethyldodecylamine, stearylamine, oleylamine, benzylamine,
5 dibenzylamine, N-ethylbenzylamine, dimethylstearylamine,
N-methylmorpholine, N-methylpiperazine, 4-methylcyclohexylamirie,
N-hydroxyethylmorpholine. The salts of quaternary ammonium hydroxides
such as trimethylbenzylammonium hydroxide, tetramethylammonium
hydroxide or tetraethylammonium hydroxide can also be used, furthermore
guanidine and its derivatives, in particular its alkylation products. However,
it is also possible to employ as salt-forming agents, for example, low
molecular weight alkylamines such as methylamine, ethylamine or
triethylamine. Suitable salts for the compounds to be employed according
to the invention are also those with inorganic cations, for example alkali
metal salts, in particular sodium) potassium or ammonium salts, alkaline
earth metal salts such as, in particular, the magnesium or calcium salts, as
well as salts with bi- or tetravalent cations, for example the zinc, aluminum
or zirconium salt.
The active compounds to be employed in the preparations of the
compound of the formula I can be prepared, for example, according to
processes given in US 2 540 218.
For the use according to the invention of the compounds mentioned, liquid
to semisolid pharmaceutical preparations, in particular hair lotions,
shampoos, liquid soaps, as well as cream, ointment and gel preparations,
are suitable.
In this case, these are always preparations which, depending on their
actual intended use, are applied to the skin and/or to the scalp for a shorter
or longer time. Due to the addition of the compounds according to the
invention, an effective treatment of the seborrheic dermatitis is brought
about.
If the preparations according to the invention are present as shampoo, they
can be in clear liquid or opaque liquid form, in cream form or even
gelatinous. The surfactants on which these shampoos are based can be of
CA 02267165 1999-03-26
6
anionic, cationic, nonionic or amphoteric nature and can also be present as
a combination of these substances.
Preferably, however, anionic surfactants are employed on their own or as a
mixture with other anionic surfactants as base surfactants - if appropriate
with addition of amphoteric surfactants as cosurfactant.
As the sole detergent substances, amphoteric surfactants are virtually
insignificant, since their foaming behavior, thickenability and partly also
skin and eye mucous membrane tolerability are only moderate. In
combination with various anionic surfactants, however, precisely these
properties are synergistically improved. This explains the relatively great
importance of the amphoteric surfactants for the optimization of anionic
shampoo bases.
Nonionic surfactants can also be employed as cosurfactants.
Examples of anionic detergent substances of this type which may be
mentioned are: (C~ p-C2p)-alkyl- and -alkylenecarboxylates, alkyl ether
carboxylates, fatty alcohol sulfates, fatty alcohol ether sulfates, alkylol-
amide sulfates and sulfonates, fatty acid alkyolamide polyglycol ether
sulfates, alkanesulfonates and hydroxyafkanesulfonates, olefinsulfonates,
acyl esters of isothionates, a-sulfofatty acid esters, alkylbenzosulfonates,
alkylphenol glycol ether sulfonates, sulfosuccinates, sulfosuccinic acid
hemiesters and diesters, fatty alcohol ether phosphates, protein-fatty acid
condensation products, alkylmonoglyceride sulfates and sulfonates,
alkylglyceride ether sulfonates, fatty acid methyltaurides, fatty acid
sarcosinates or sulforicinoleates. These compounds and their mixtures are
used in the form of their water-soluble or water-dispersible salts, for
example the sodium, potassium, magnesium, ammonium, mono-, di- and
triethanolammonium as well as analogous alkylolammonium salts.
Examples of amphoteric surfactants which can be added to the shampoos
are: N-((C~ 2-C1 g)-alkyl)-~i-aminopropionates and N-((C12-C1 g)-alkyl)-
~3-iminodipropionates as alkali metal and mono-) di- and trialkylol-
ammonium salts; N-acylamidoalkyl-N,N-dimethylacetobetaine, preferably
N-((Cg-C~ g)-acyl)amidopropyl-N,N-dimethylacetobetaine; (C12-C1 g)-alkyl-
dimethylsulfopropylbetaine; amphoteric surfactants based on imidazoline
CA 02267165 1999-03-26
7
(trade name: Miranol~, Steinapon~), preferably the sodium salt of
1-((3-carboxymethyloxyethyl)-1-(carboxymethyl)-2-laurylimidazolinium;
amine oxides, e.g. (Ct2-Cog)-alkyldimethylamine oxide or fatty acid
amidoalkyldimethylamine oxide.
Suitable nonionic surfactants which can be employed as detergent sub-
stances are, for example: fatty alcohol ethoxylates (alkyl polyethylene
glycols); alkylphenol polyethylene glycols; alkylmercaptan polyethylene
glycols; fatty amine ethoxylates (alkylamino polyethylene glycols); fatty acid
ethoxylates (acyl polyethylene glycols)) polypropylene glycol ethoxylates
(Pluronic~); fatty acid alkylolamides (fatty acid amide polyethylene glycols);
sucrose esters; alkyl polyglucosides; sorbitol esters and polyglycol ether.
Suitable cationic surfactants are, for example, quaternary ammonium salts
such as di-((C~ p-C24)-alkyl)dimethylammonium chloride or bromide,
preferably di-((C~ 2-C~ g)-alkyl)dimethylammonium choride or bromide;
(Cep-C24)-alkyldimethylethylammonium chloride or bromide; (C1p-C24)-
alkyltrimethylammonium chloride or bromide, preferably cetyltrimethyl-
ammonium chloride or bromide and (C2p-C22)-alkyltrimethylammonium
chloride or bromide; (Cep-C24)-alkyldimethylbenzylammonium chloride or
bromide; preferably (C~ 2-C1 g)-alkyldimethylbenzylammonium chloride;
N-((C~ p-C~ g)-alkyl)pyridinium chloride or bromide, preferably N-((C12-C~ 6)-
alkyl)pyridinium chloride or bromide; N-((C1 p-C1 g)-alkyl)isoquinolinium
chloride, bromide or monoalkylsulfate; N-((Ct 2-C~ g)-alkylcolaminoformyl-
methyl)pyridinium chloride; N-((C~ 2-C~ g)-alkyl)-N-methylmorpholinium
chloride, bromide or monoalkylsulfate, N-((C~ 2-C~ $)-alkyl)-N-ethyl-
morpholinium chloride, bromide or monoalkylsulfate; (C~ g-C~ g)-alkyl-
pentaoxethylammonium chloride; diisobutylphenoxyethoxyethyldimethyl-
benzylammonium chloride; salts of N,N-diethylaminoethylstearylamide and
-oleylamide with hydrochloric acid, acetic acid, lactic acid, citric acid,
phosphoric acid; N-acyfamidoethyl-N,N-diethyl-N-methylammonium
chloride, bromide or monoalkylsulfate and N-acylamidoethyl-N,N-diethyl-
N-benzylammonium chloride, bromide or monoalkylsulfate, where aryl is
preferably stearyl or oleyl.
The preparations according to the invention can additionally contain further
additives, e.g. aromatic substances, colorants, opacifiers and pearl luster
CA 02267165 1999-03-26
agents, for example esters of fatty acids and polyols, magnesium and zinc
salts of fatty acids, dispersions based on copolymers, thickeners such as
sodium, potassium or ammonium chloride, sodium sulfate, fatty acid
alkylolamides, cellulose derivatives of natural gums, collagen hydrolyzates,
furthermore fats, oils, fatty alcohols, silicones, substances having a kerato-
lytic and keratoplastic action, for example sulfur, salicylic acid or enzymes.
The shampoos are prepared in a manner known per se by mixing together
of the individual components and a further processing - if necessary -
suited to the particular type of preparation. Some of these various possible
preparations are described by way of example in the working examples.
The preparations according to the invention can also be present in the form
of aqueous and aqueous-alcoholic hair lotions, and also those in gel form
and in aerosol form as spray or foam. Alcohols employed are preferably
ethanol and isopropyl alcohol.
Further preparations which may be mentioned in which the 1-hydroxy-
2-pyridones can be used according to the invention are, for example,
cream and ointment preparations, products which are primarily used for the
treatment of hairless head and body parts.
The preparation of all these preparations is also carried out - as already
mentioned in the case of shampoo - in a manner known per se with
addition of the active compound employed according to the invention. Of
the abovementioned 1-hydroxy-2-pyridones, the preparations according to
the invention can contain one compound or even several in combination.
The pH of the preparations is in the skin-physiological range of
approximately pH 4.5 to 6.5. Whereas, when using the compounds in salt
form, the adjustment of the pH range mentioned has to be carried out
using organic acids, this measure is not necessary when using the free
compounds.
In the preparations according to the invention, the active compounds is
incorporated in amounts which are customarily between approximately
0.05 and approximately 10%. W ithin this range, the concentrations of the
specific preparations depend on their intended use. Certain preparation
CA 02267165 1999-03-26
9
forms such as concentrates, which are to be diluted before use, can have
considerably higher concentrations.
If they are preparations which remain on the skin and on the scalp, for
example gel preparations, ointments, creams or hair lotions, lower concen-
trations will be employed, for example from about 0.05% to about 1 %,
preferably from 0.1 to 0.5%. In higher concentrations, they will expediently
be used when they are preparations which, optionally after dilution, only act
on the scalp for a short time, for example shampoos or liquid soaps. In
these cases, for example, concentrations of approximately 0.2 to approxi-
mately 10%, preferably from approximately 0.5% to approximately 2%, can
be expedient.
The following quantitative data relate to the weight, if not stated otherwise.
Example 1
A preparation according to the invention has the following composition:
Shampoo
(based on anionic detergent substances)
1-Hydroxy-4-methyl-6-cyclohexyl-2(1 H)pyridone 1.00%
Sodium lauryl diglycol ether sulfate (27% strength solution) 40.00%
Disodium lauryl polyglycol ether sulfosuccinate (33% strength
solution) 10.00%
Sodium chloride 2.50%
Water 46.50%
CA 02267165 1999-03-26
Example 2
A preparation according to the invention has the following composition:
Shampoo
5 (based on anionic detergent substance with amphoteric surfactant as
cosurfactant)
1-Hydroxy-4-methyl-6-cyclohexyl-2(1 H)pyridone 1.00%
Sodium lauryl diglycol ether sulfate (27% strength36.00%
solution)
Cocamidopropylbetaine (30% strength solution) 6.00%
Sodium chloride __ 3.30%
Water 53.70%
10 Example 3
A preparation according to the invention has the following composition:
Shampoo
(based on anionic detergent substance with nonionic surfactant as
cosurfactant)
1-Hydroxy-4-methyl-6-cyclohexyl-2(1 H)pyridone 1.50%
Sodium lauryl diglycol ether sulfate (27% strength solution) 30.00%
Lauryl alcohol polyglucoside 8.00%
Sodium chloride 2.00%
Water 58.50%
CA 02267165 1999-03-26
11
Example 4
A preparation according to the invention has the following composition:
Liquid soap
1-Hydroxy-4-methyl-6-cyclohexyl-2(1 H)pyridone 1.00%
Sodium lauryl diglycol ether sulfate (27% strength35.00%
solution)
Cocamidopolyglycol ether sulfate magnesium salt
(30% strength
solution) 8.00%
Cocamidopropylbetaine (30% strength solution) 10.00%
Lauryl alcohol glycol ether _ 2.00%
Sodium chloride 2.00%
Water 42.00%
CA 02267165 1999-03-26
12
Example 5
A preparation according to the invention has the following composition:
Hair lotion
1-Hydroxy-4-methyl-6-[4-(4-chlorophenoxy)phenoxymethyl]-
2(1 H)pyridone 0.05%
2-Propanol 60.00%
Water 39.95%
Example 6
A preparation according to the invention has the following composition:
Gel preparation
1-Hydroxy-4-methylcyclohexyl-2(1 H)pyridone0.75%
2-Propanol 15.0Q%
2-Octyldodecanol 7.50%
Carbomer 4,000,000 0.50%
Polysorbate 60 1.50%
Sodium hydroxide 0.18%
Water 74.57%
Example 7
A preparation according to the invention has the following composition:
Cream preparation
1-Hydroxy-4-methyl-6-cyclohexyl-2(1 H)-pyridone, aminoethanol
salt 1:1 1.00%
2-Octyldodecanol 7.50%
Liquid paraffin 7.50%
Stearyl alcohol 7.50%
Cetyl alcohol 7.50%
Polysorbate 60 3.00%
Sorbitan monostearate 2.00%
Lactic acid, 90% strength 0.51
CA 02267165 1999-03-26
13
Water 63.49%
Example 8
In a clinical study with a total of 180 patients, it was possible to show that
the symptoms of seborrheic dermatitis of the scalp (severe scaling, inflam-
mation, itching) can be effectively treated by a 1-2 x weekly treatment with
a 1 % strength ciclopirox shampoo preparation over a period of 4 weeks.
Example 9 __
In a clinical study, it was possible to successfully treat 180 patients with
seborrheic dermatitis of the scalp, of the face and of the upper body by
application of a 0.77% strength ciclopirox gel preparation over a period of
4 weeks.