Note: Descriptions are shown in the official language in which they were submitted.
FUEL COMPOSITION
The invention relates to a fuel composition and in particular to such a liquid
composition
to be burned in an engine such as an internal combustion engine, e.g. a petrol
or Diesel
S engine or any engines designed to perform with liquid fuels.
It is well known that liquid fuels when burned in an internal combustion
engine can give
rise to pollution and other undesired side effects. Numerous proposals have
been
advanced to reduce these side effects and enhance efficiency, e.g. miles per
gallon. It has
been realised that surfactants can play a useful role in this context but so
far as we are
aware none has satisfied the modern commercial criteria. It is one object of
this
invention to meet the need.
According to one aspect of the present invention, we provide a fuel
composition
comprising in combination fuel and a fuel additive wherein the additive
comprises a fatty
acid diethanolamide, an alcohol ethoxylate and an ethoxylate of a fatty acid,
the degree
of ethoxylation being selected so that a long term stable fuel composition is
formed
wherein the amounts by volume of fatty acid diethanolamide and ethoxylated
fatty acid
are substantially the same.
According to another aspect of the invention, we provide a fuel composition
comprising
in combination fuel and a fuel additive wherein the additive comprises a fatty
acid
diethanolamide, an alcohol ethoxylate and an ethoxylate of a fatty acid, the
degree of
ethoxylation being selected so that a long term stable fuel composition is
formed,
wherein the ethoxylate of the fatty acid makes up about 25% by volume of the
additive.
Also according to the invention, we provide a fuel composition comprising in
combination fuel and a fuel additive wherein the additive comprises a fatty
acid
diethanolamide, an alcohol ethoxylate and an ethoxylate of a fatty acid, the
degree of
ethoxylation being selected so that a long term stable fuel composition is
formed,
wherein the additive is present in an additive to fuel ratio of from 0.5:1200
to 1:1200 by
volume.
CA 02267864 2001-02-06
-2-
The preferred additive of this invention is a non-ionic surfactant and
preferably a blend
of surfactants, which are preferably selected by their nature and
concentration so that the
additive (as well as any water or other non-fuel liquid present) is
solubilised within the
fuel. For this purpose it is convenient to have regard to the hydrophilic-
lipophilic (HLB)
of the surfactant, the value being calculated according to the expression:-
HLB = mol.wt of hydrophilic chain x 20
total mol. wt
The values will depend on the length of the hydrophilic chain, typically an
ethoxylate
chain. The length of the chain will increase the extent of solubilisation
because of a
greater ability to solubilise.
Normally a blend of surfactants is preferred, preferably by selecting one
appropriate to
the fuel, say 10 to 18 for hydrocarbon fuel, most preferably 13. In the case
of an alcohol
the HLB value of the surfactant is between 3 and 8, most preferably about 4.
But the
additions of surfactants normally create ratios of 1:1 for high volume
emulsions or 5:1
ratios when the solubilisation is required at 1:100.
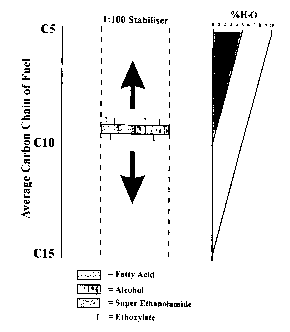
The invention has the ability to unify the HLB requirements of any liquid fuel
which in
turn allows for one dose to be used in any fuel from DS carbon chains up, the
benefit
being the amount of treatment directly related to the co-solvency ability (as
per enclosed
charts). The charts show three different combinations of additive, allowing a
cost
comparison to performance required.
The monolayer aspect of the invention requires the concentration of the
additive to be
very low, typically of the order of 0.5 -1 :1200, preferably about 1 :1000,
most preferably
1 :1200. There appears to be no technical or economic benefit in adding more
unless a
co-solvent dual action is required, when the priority will be dosage against
performance.
The additive preferably comprises of the following:
CA 02267864 2001-02-06
-2a-
- an oil soluble ethoxylated alcohol
- a super diethanolamide
S - a 7 chain ethoxylated fatty acid
The three ingredients must be added as per fuel and molecule production
process.
Preferably the ethoxylate of the fatty acid makes up about 25% by volume of
the additive
and further preferably the alcohol ethoxylate comprises 50% by volume of the
additive.
An additive of the invention may be added to a hydrocarbon fuel, e.g. Diesel
or petrol
or alcohol, which may or may not be contaminated with water. The invention is
seen to
particularly good effect when added to synthetic fuels based on low fraction
oils.
In another aspect the invention provides a fuel composition comprising a light
weight
fraction and including an additive miscible with the fuel selected to
solubilise the fuel and
the additive and any water present to form a clear homogenous composition.
The light weight fraction may be an oil, such as gasoline, alcohol, an
aromatic
hydrocarbon, or a CS to C15, CS to C20, C10 to C25, C10 to C20, CS to C30 or
C15 to
C30 carbon chain.
The presence of the additive of the invention ensures that the fuel
composition forms a
consistent stable homogenous composition and creates a monolayer
simultaneously, a
result of which leads to a better more complete bum which reduces pollution
and
increases miles per gallon.
As a result a blended fuel, particularly alcohol based, is able to combust
more precisely
with a cooler charge to reduce the iron-formates present from the aldehyde
peracids and
peroxide reactions normally attributable to engine degradation.
In another aspect the invention provides a method of forming a stable
composition
comprising adding the three specified ingredients, e.g. as an additive as
defined, to a fuel
CA 02267864 2001-02-06
- 2b-
in volume ratio of about 0.5 - 1 : 1200. Preferably the addition ratio is
about 1:1000, most
preferably about 1 :1200.
In another aspect of the invention we provide a fuel composition comprising in
combination fuel and a fuel additive wherein the additive comprises a fatty
acid
diethanolamide, an alcohol ethoxylate and an ethoxylate of a fatty acid, the
degree of
ethoxylation being selected so that a long term stable fuel composition is
formed, wherein
the additive is present in an additive to fuel weight ratio of from 1:500 to
1:1000.
Alternatively, the additive to fuel ratio by weight may be 1 : 500, preferably
1 : 400, more
preferably 1 : 300, most preferably 1 : 200 and especially 1 : 100.
The present invention further provides a method of running an engine adapted
to use an
alcohol-based fuel, comprising adding to the fuel a miscible additive
according to the
invention selected to solubilise the fuel and additive to counteract the
deposit of a by-
product formed during the combustion of the fuel.
CA 02267864 2001-02-06
CA 02267864 1999-04-O1
WO 98/17745 PCT/GB97/027G3
3
A method of running an Cngine adapted to use a alcohol-based fuel, comprising
adding
to the fuel a miscible additive selected to solubilise the fuel and the
additive so
eliminating the deposit of by-products formed during the combustion of the
fuel.
Fuel Production Process
1. Check water contamination by Karl Fischer and estimate volume of H20 in
enduser
tank.
2. Select from Stabiliser Charts the correct formula taking into consideration
costs and
treatment ratios.
3. When percentage of stabiliser is assessed blend necessary components as per
chart
and dose accordingly blending the molecule into the fuel and not mixing it.
Molecule Production Process
1. After correct selection of Super Amide blend at P.LT. {Phase Inverse
Tension) (55-
58°C) the Alcohol, the Ethylene Oxide.
2. Blend 1 with the *Super Amide chosen at P.LT.
3. Blend Fatty Acid with Ethylene Oxide and blend with 2 at P.LT.
4. Resulting in a total blend of Alcohol Ethoxylate. Which must at least be
50% of the
total weight of the molecule with equal parts of Super Amide and Fatty Acid
Ethoxylate to achieve 100%.
* Although a 50/25/25 blend in theory may not be the correct balance for a
polymer,
margins have to be taken into consideration for alien components such as Free
Amines,
Free PEG's, Free Esters and Isomers which are all present during this process.
The
molecular weight of the two tails invariably balance at this procedure.
Although the example stock solution is suitable for minimal water
contamination
problems the preferred alcohol ethoxylate will be straight chained primary
linear and
3 mols of EO per.mol of alcohol as the precision in calculation is much more
precise
and the absorbant powers of the micelle is increased with the extra additions
of
ethoxylates. The primary and linear alcohol must be a minimum of 80% wlw as
the
balance of predominantly isomers are considered a contaminant and not helpful!
to
the ethoxylation process.
The diethonanolimide should be a super amide which is identifyable as having a
ratio
of !:l fatty acid to diethanolamine as the 2:1 ratio contain 10% free amine
esters and
the nature of process allows this contamination which is not helpful! to the
balancing
of the polymer.
* Super Amide MUST be blended with either Fariy Acid Ethoxylate or Alcohol
Ethoxylate.
CA 02267864 1999-04-O1
WO 98/17745 PCTIGB97/02763
4
The fatty acid is preferrubly a Cl4 acid and is not manufactured by
polyethylene
glycol method as the free PEGS inhibit the ethoxylation process and upset the
HLB
balance.
In order that the invention may be well understood it will now be described by
way of
illustration only with reference to the following example.
Example I
oil soluble primary alcohol ethoxylate (mean 2.75mols ethylene
oxide; mol alcohol) available as NEODOL 91/2.5,
predominatley C9-C~1; mol.wt about 270 1 litre
lauric diethanolamide 500 ml
a fatty acid with 7 ethoxylates per mol of fatty acid
(available as ATLAS 65507) mol.wt about 506 500 ml
The stock was heated to 55 to 58°C as per the diagram to form a 2 litre
stock solution.
Different used vehicles, having Diesel and petrol engines, were tested at a
local
Ministry of Transport test house. The fuel tank of each was filled, and the
vehicle
driven for about 112 Km at an average speed of 96 Kph. A dose of the stock
solution
was added to the tank of each vehicle in a volume ratio of 1:1000. Visual
inspection
showed that a clear homogeneous solution was formed. The tank was refilled and
the
vehicle then driven again over the same journey. The MOT test was repeated.
The results showed a decrease in fuel consumption ranging from 11 to 20%, the
greater
savings being obtained in the case of the larger sized engines.
The tests showed the following reductions in emissions:
Petrol Engine
CO reduced by a mean 80%
hydrocarbon reduced by a mean 40%
Diesel engine
Diesel smoke reduction by a mean 50%
CA 02267864 1999-04-O1
WO 98/I7745 PCT/GB97l02763
S
Example II
A Mercedes Mi 11 basic test engine was cleaned and prepared for testing to
record any
changes in reference gasoline without additive and with additive at a
treatment rate of
1:1000.
The standard methods of measurement were used in accordance with NAMAS
specifications, particular interest was paid to LAMBDA as the
leaning/richening of the
engine would not encourage comparable results. LAMBDA was set at 1=0.05
The basic test was started and the engine was run hot and then dropped from
4,500
r.p.m. WOT to 1,800 r.p.m. PT stopping at different conditions to enable
comparisions.
LAMBDA performed at 1=0.05. At the end of the first test the head was cleaned
and
once again the test was repeated with additive at 1:1,000. C02 was reduced on
average
by 14.08% at 2,500 rpm PT and 20.64% Maximum.
Example III
A Bench Test was carried out under controlled laboratory conditions to
ascertain Fuel
Consumption and Emission Performance at 1,800 r.p.m. and 2,500 r.p.m. part
throttle
and also measuring Power Curve and Torque Curve Performance, using RF83
reference
European non-additised fuel, with all measurements recorded to NAMAS Criteria.
The
engine was a MERCEDES 2 liter M111 Bench Engine suitable for unleaded fuel,
fitted
with a Catalytic Converter. (All figures quoted are on measurements prior to
Catalytic
Converter). The results showed CO reduced on average by 11.3% at 2,500 r.p.m.
PT
and 14.34% Maximum.
Example IV
A test was carried out to measure any reduction in Nox as Nox is directly
related to
combustionability and is a hazard that is impossible to negate in engines as
Air/Fuel
Ratio will always contain Nitrogen. The results showed that Nox reduced on
average by
38.2% at 2,500 r.p.m. PT and 39% Maximum.
There are three ways to reduce Nox:
a) The less air the less nitrogen
b) The lower the temperature of the charge the less Nox
c) The better the delivery of fuel the less Nox
Attached are graphs showing the beneficial effect of adding the additive of
the
invention.
CA 02267864 1999-04-O1
WO 98/17745 PCT/GB97/02763
6
Power Curve shows a power curve measuring within repeatability the same
power with less fuel and less air which reduces C02 and Nox.
Torgue Curve shows a torque curve measuring within repeatability the same
power with less fuel and less air which reduces C02 and Nox.
CA 02267864 1999-04-O1
WO 98/17745 PCT/GB97/02763
7
Co-Solvency Tests
Ezamples
A specific variety of fuels from premium grade gasoline, industry standard
diesel and various alcohol
blended fuels were selected and from each 100 ml were transferred to each of
twelve 200 ml measuring
cylinders for reference to the phase separation caused by saturation of water
to the polymer. The optimal
being two titrations previous to the phase.
E:ample 1.
N~uel No Water ContentAdditive Comments
Gasoline 1 0% 0% Clear Liquid
Gasoline 2 10% 0% Pbase separation
Gasoline 3 10% 10% Clear Liquid
Gasoline 4 10% 9% Clear Liquid
Gasoline 5 10% 8% Clear Liquid
Gasoline 6 10% 7% Clear Liquid
Gasoline 7 10% 6% Clear Liquid
Gasoline 8 10% 5% Clear Liquid
Gasoline 9 10% 4% Phase Separation
Gasoline 10 10% 3% Phase Separation
Gasoline 11 10% Z% Phase Separation
Gasoline 12 10% 1% Phase Separation
After solution
the introduction was gently
of each stirred
titration for twenty
the seconds.
The resultant
effect minutes to
was left settle before
for ten visible
results
were recorded.
Ezample 2. - Gasohol
Consisting of 90% regular unleaded gasoline with 10% denatured ethanol
Fwel No Water ContentAdditive Comments
Gasohol1 0% 0% Clear Liquid
Gasohol2 10% 0% Phase separation
Gasohol3 10% 10% Clear Liquid
Gasohol4 10% 9% Clear Liquid
Gasohol5 10% 8% Clear Liquid
Gasohol6 10% 7% Clear Liquid
Gasohol7 10% 6% Clear Liquid
Gasohol8 10% 5% Clear Liquid
Gasohol9 !0% 4% Clear Liquid
Gasohol10 10% 3% Phase Separation
Gasohol11 10% 2% Phase Separation
Gasohol12 10% 1% Phase Separation
After of each solution
the titration was gently
introduction the stirred
for twenty
seconds.
The resultant
effect re visible
was results
left were recorded.
for
ten
minutes
to
settle
befo
CA 02267864 1999-04-O1
WO 98/17745 PCT/GB97102763
8
E=ample 3. - Diesel
Fuel No Water ContentAdditive Comments
Diesel1 0% 0% Clear Liquid
Diesel2 10% 0% Phase separation
Diesel3 10% 10% Clear Liquid
Diesel4 10% 9% Clear Liquid
Diesel5 10% 8% Clear Liquid
Diesel6 10% 7% Phase Separation
Diesel7 10% 6% Phase Separation
Diesel8 10% 5% Phase Separation
Diesel9 10% 4% Phase Separation
Diesel10 10% 3% Phase Separation
Diesel11 10% 2% Phase Separation
Diesel12 10% 1% Phase Separation
After of each
the titration
introduction the solution
was gently
stirred
for twenty
seconds.
The resultant
efTect
was
left
for
ten
minutes
to
settle
before
visible
results
were
recorded.
Example 4. - Alternative Gasoline
Consisting of Alcohol and a blend of bydro carbons the majority percentage
being alcohol
Fuel No Water ContentAdditive Comments
Alt 1 0% 0% Clear Liquid
Gas
Alt 2 10% 0% Phase separation
Gas
Alt 3 10% 10% Clear Liquid
Gas
Alt 4 10% 9% Clear Liquid
Gas
Alt 5 10% 8% Clear Liquid
Gas
Alt 6 10% 7% Clear Liquid
Gas
Alt 7 10% 6% Clear Liquid
Gas
Alt 8 10% 5% Clear Liquid
Gas
Alt 9 10% 4% Clear Liquid
Gas
Alt 10 10% 3% Clear Liquid
Gas
Alt 11 10% 2% Phase Separation
Gas
Alt 12 10% 1% Phase Separation
Gas
After solution
the was gently
introduction stirred
of for twenty
each seconds.
titration The resultant
the
effect minutes re visible
was to settle results
left befo were recorded.
for
ten
CA 02267864 1999-04-O1
WO 98/17745 PCT/GB97/02763
9
Ezample 5.
Fael No W ater ContentAdditive Comments
Gasoline1 0% 0 % Clear Liquid
Gasoline2 5% 0 % Phase separation
Gasoline3 5% 5 % Clear Liquid
Gasoline4 5% 4.5% Clear Liquid
Gasoline5 5% 4 % Clear Liquid
Gasoline6 5% 3.5% Clear Liquid
Gasoline7 5% 3 % Clear Liquid
Gasoline8 5% 2.5% Clear Liquid
Gasoline9 5% 2 % Phase Separation
Gasoline10 5% 1.5% Pbase Separation
Gasoline11 5% 1 % Phase Separation
Gasoline12 5% 0.5% Phase Separation
After roductionof each
the titration
int the solution
was gently
stirred
for twenty
seconds.
The resultant
effect
was
left
for
ten
minutes
to
settle
before
visible
results
were
recorded.
Ezampie 6. - Gasohol
Consisting of 90% regular unleaded gasoline with 10% denatured ethanol
Feel No Water ContentAdditive Comments
Gasohol1 0% 0 % Clear Liquid
Gasohol2 5% 0 % Phase separation
Gasohol3 5% 5 % Clear Liquid
Gasohol4 5% 4.5% Clear Liquid
Gasohol5 5% 4 % Clear Liquid
Gasohol6 5% 3.5% Clear Liquid
Gasohol7 5% 3 % Clear Liquid
Gasohol8 5% 2.5% Clear Liquid
Gasohol9 5% 2 % Clear Liquid
Gasohol10 5% 1.5% Phase Separation
Gasohol11 5% 1 % Phase Separation
Gasohol12 5% 0.5% Phase Separation
After roductionof each solution
the titration was gently
int the stirred
for twenty
seconds.
The resultant
effect
was
left
for
ten
minutes
to
settle
before
visible
results
were
recorded.
CA 02267864 1999-04-O1
WO 98/17745 PCT/GB97/02?63
Example 7. - Diesel
FY~eI No Water ContentAdditive Comments
DieselI 0% 0 % Clear Liquid
Diesel2 5% 0 % Phase separation
Diesel3 5% 5 % Clear Liquid
Diesel4 5% 4.5% Clear Liquid
Diescl5 5% 4 % Clear Liquid
Diesel6 5% 3.5% Phase Separation
Diesel7 5% 3 % Phase Separation
Diesel8 5% Z.5% Phase Separation
Diesel9 5% 2 % Phase Separation
Diesel10 5% 1.5% Phase Separation
Diesel11 5% 1 % Phase Separation
Diesel12 5% 0.5% Phase Separation
After of each
the titration
introduction the solution
was gently
stirred
for twenty
seconds.
The resultant
effect
was
Icft
for
ten
minutes
to
settle
before
visible
results
were
recorded.
Example 8. - Alternative Gasoline
Consisting of Alcohol and a blend of hydra carbons the majority percentage
being alcohol
Feel No Water ContentAdditive Comments
Alt I 0% 0 % Clear Liquid
Gas
Alt 2 S% 0 % Phase separation
Gas '
Alt 3 5% 5 % Clear Liquid
Gas
Alt 4 5% 4.5% Clear Liquid
Gas
Alt 5 5% 4 % Clear Liquid
Gas
Alt 6 5% 3.5% Clear Liquid
Gas
Alt 7 5% 3 % Clear Liquid
Gas
Alt 8 5% 2.5% Clear Liquid
Gas
Alt 9 5% 2 % Clear Liquid
Gas
Alt 10 5% 1.5% Clear Liquid
Gas
Alt 11 5% 1 % Phase Separation
Gas
Alt 12 5% 0.5% Phase Separation
Gas
After solution
the was gently
introduction stirred
of for twenty
each seconds.
titration The resultant
the
effect minutes
was to settle
left before
for visible
tcn results
were recorded.
CA 02267864 1999-04-O1
WO 98/17745 PCT/GB97/02763
To record the usual aspects of phase separation for the one percent water and
0.1 percent titratians it
was decided to scale up the volumes tenfold to enable accurate readings,
therefore 1 litre of each fuel
was transferred to each of twelve 2 litre measuring cylinders.
E=ample 9.
Fuel No Water ContentAdditive Comments
GasolineI 0% 0% Clear Liquid
Gasoline2 1% 0% Phase separation
Gasoline3 I% 1 % Clear Liquid
Gasoline4 1% 0.9% Clear Liquid
Gasoline5 1% 0.8% Clear Liquid
Gasoline6 1% 0.7% Clear Liquid
Gasoline7 I% 0.6% Clear Liquid
Gasoline8 1% 0.5% Phase Separation
Gasoline9 1% 0.4% Phase Separation
Gasoline10 I% 0.3% Phase Separation
Gasoline11 I% 0.2% Phase Separation
Gasoline12 1% 0.1% Phase Separation
After the introduction of each titration the solution was gently stirred for
twenty seconds. The resultant
effect was left for ten minutes to settle before visible results were
recorded.
Ezample 10. - Gasohol
Consisting of 90% regular unleaded gasoline with 10% denatured ethanol
Fuel No Water ContentAdditive Comments
GasoholI 0% 0% Clear Liquid
Gasohol2 1% 0% Phase separation
Gasohol3 1% 1 % Clear Liquid
Gasohol4 1% 0.9% Clear Liquid
Gasohol5 1% 0.8% Clear Liquid
Gasohol6 I% 0.7% Clear Liquid
Gasohol7 1% 0.6% Clear Liquid
Gasohol8 I% 0.5% Clear Liquid
Gasohol9 1% 0.4% Phase Separation
Gasohol10 1% 0.3% Phase Separation
GasoholI1 I% 0.2% Phase Separation
Gasohol12 I% 0.1% Phase Separation
After the introduction of each titration the solution was gently stirred for
twenty seconds. The resultaet
effect was left for ten minutes to settle before visible results were
recorded.
CA 02267864 1999-04-O1
WO 98/17745 PCT/GB97/02?63
12
Ezample 11. - Diesel
Fuel No Water ContentAdditive Comments
Diesel1 0% 0% Clear Liquid
Diesel2 1% 0% Pbase separation
Diesel3 1% 1 % Clear Liquid
Dieseld 1% 0.9% Clear Liquid
Diesel5 I% 0.8% Clear Liquid
Diesel6 1% 0.7% Phase Separation
Diesel7 1% 0.6% Phase Separation
Diesel8 i% 0.5% Phase Separation
Diesel9 i% 0.4% Phase Separation
Diesel10 1% 0.3% Phase Separation
Diesel11 1% 0.2% Phase Separation
Diesel12 i% 0.1% Phase Separation
After of each solution
the titration was gently
introduction the stirred
for twenty
seconds.
The resultant
effect
was
left
for
ten
minutes
to
settle
before
visible
results
were
recorded.
Ezample 12. - Alternative Gasoline
Consisting of Alcohol and a blend of hydro carbons the majority percentage
being alcohol
Fuel No Water ContentAdditive Comments
Alt 1 0% 0% Clear Liquid
Gas
Alt 2 1% 0% Phase separation
Gas
Alt 3 1% 1 % Clear Liquid
Gas
Alt 4 1% 0.9% Clear Liquid
Gas
Alt 5 1% 0.8% Clear Liquid
Gas
Alt 6 1% 0.7% Clear Liquid
Gas
Alt 7 1% 0.6% Clear Liquid
Gas
Alt 8 1% 0.5% Clear Liquid
Gas
Alt 9 I% 0.4% Clear Liquid
Gas
Alt 10 i% 0.3% Clear Liquid
Gas
Alt 11 1% 0.2% Phase Separation
Gas
Alt 12 i% 0.1% Phase Separation
Gas
After solution
the was gently
introduction stirred
of for twenty
each seconds.
titration The resultant
the
effect minutes re visible
was to settle results
left befo were recorded.
for
ten
CA 02267864 1999-04-O1
WO 98/17745 PCT/GB97/027b3
13
TESTING - USA
Testing exhaust emissions using indoline fuel
with a treatment of an additive known to be a
maj or component for stabilising fuel.
Introduction
With the phase out of leaded fuel it has become imperative to allow the
maximum combustion from the available fuel to maximise performance and
minimise pollution by burning as much fuel as possible completely. The tests
set
out to compare results of treated and un-treated fuel were performed under
extreme controls and indoline was used as the carbon balance of this fuel is
much
more repeatable than un-leaded gasoline.
Experimental Details
The vehicle used was a 1993 California certified Mercury Cougar with 26,333
Miles on the odometer. This vehicle is equipped with a 3.8 litre engine with
an
SFI fuel system and has an inertia weight of 38,875 Ibs. this vehicle was
supplied
by the test laboratories at Roush Laboratiries, Los Angeles, California and
was
prepared by them for the test.
A chassis dynamometer similar to a Clayton Water Break model was used in
accordance with Federal Test Procedure CFR40 also known as the LA4 test.
Firstly the vehicle was pre-conditioned with indoline and this
sequence follows these steps:-
1/ Drain and fill the tank to 40% capacity with indoline.
2/ Disconnect the vehicles battery to eliminate and mis-reading by a fuel
computer.
3/ Drive vehicle for a period of 10 miles on the dynamometer in the specified
controlled conditions and allow to soak for a minimum of 12 hours to a
maximum of 24 hours.
CA 02267864 1999-04-O1
WO 98/17745 PCT/GB97/02763
Specified control conditions:-
The test of additised fuel against base fuel was run with base fuel first.
The soak time from pre-condition to test was 15 hours, the soak temperature
was
76° F and the barometer H.g. was 29.85 .
Additised control conditions:-
The additised test did not take place until another pre-conditioning test was
complete.
The soak time from pre-condition to test was 20.5 hours, the soak temperature
was 76° F and the barometer H.g. was 29.82 .
As the results of interest were potential reduction in Hydrocarbon and Carbon
Monoxide emissions a flame ionization detection system was used after
collecting the diluted exhaust gases in Tedlar Bags these background bags were
analysed within 1 hour of testing so as not to lose any sensitive constituents
necessary for a total HC count.
As a more complete combustion was expected the CO detection was in
accordance with the LA4 - CVS11 test as per recommendations from the
California Air Resources Board.
Test Criteria:-
The pre-conditioning consisted of a LA4 test drive lasting 505 seconds plus
873
seconds.
The base fuel test consisted of a cold start for 505 seconds, a cold transient
for
873 seconds, a soak for 10 minutes and a hot transient for 505 seconds. Total
time = 1883 seconds.
The additised fuel test consisted of a cold start for 505 seconds, a cold
transient
for 873 seconds, a soak for 10 minutes and a hot transient for 505 seconds.
Total
time = 1883 seconds.
CA 02267864 1999-04-O1
WO 98/17745 PCT/GB97/02763
is
Results and Discussion:-
HC HC CO CO
Base Fuel Additised Base Fuel Additised
BAG 1 53228 47832 212617 160S91
BAG 2 0641 0549 24888 22699
BAG 3 4356 2842 39765 14449
All fiQUresppm '.s
in
AVERAGE % HC CO
-
IMPROVEMENT 271 3907
As can be seen a reduction in Hydrocarbons and Carbon
Monoxide was achieved. Although this was encouraging the fact
that the control conditions did not allow for any ambient
temperature activities proved the theory that by creating a
Monolayer enables the fuel to be delivered in a better condition
with less resistance.
The major improvements were on BAG 3. This confirms that the
hot transient phase of the test did allow for some temperature
difference to enable a co-solvent reaction as well.
The encouragement of these results led us to continue testing but
be more precise with the measurements and create a fuel tank as
per normal ambient conditions.
The venue for this was the Associated Octel Co. Milton Keynes,
England.
CA 02267864 1999-04-O1
WO 98J17745 PCT/GB97/02763
16
TESTING - UK
Generating more miles per gallon of an un-
leaded reference gasoline additised with a fuel
component at a treatment ratio of 1:1,000. The
fuel component is a maj or contributing factor
to the stabilisation of fuel. The reduction in
C02 proved the measurement of fuel
consumed by weight to be in accordance with
our claims.
Introduction
With the phase out of leaded fuel it has become imperative to allow the
maximum combustion from the available fuel to maximise performance and
minimise pollution by burning as much fuel as possible completely. The tests
set
out to compare results of treated and un-treated fuel were performed under
controls and reference RF-08 gasoline was used on a Mercedes Ml l 1 bench test
engine these results were achieved prior to catalytic converter.
Experimental Details
The engine used was a Mercedes MI 1 I and was supplied by the test
laboratories
at the Associated Octel Co. and details were recorded to N.A.M.A.S. standards.
Firstly the vehicle was pre-conditioned with base fuel and these
steps were followed:-
I/ Prepare 55 litre drum of RF08 gasoline and leave external to test shop as
per
simulation of regular fuel tank.
2/ Clean and polish head of engine and run base test programme from full
throttle
4,500 rpm down to idle.
CA 02267864 1999-04-O1
WO 98/17745 PCT/GB97/027b3
(7
After the base run additise the fuel at 1:1,000 and prepare and
test as for base fuel.
Specified control conditions:-
The test of additised fuel against base fuel was run with base fuel first.
Additised control conditions:-
The additised test did not take place until another pre-conditioning test was
complete.
As the results of interest were potential reduction in Hydrocarbon and Carbon
Monoxide emissions a flame ionization detection system was used after
collecting the diluted exhaust gases in Tedlar Bags these background bags were
analysed within 1 hour of testing so as not to lose any sensitive constituents
necessary for a total HC count.
As a more complete combustion was expected the CO detection was in
accordance with the N.A.M.A.S. recommendations.
Fuel consumption was measured by weighted control which was fed by the
simulated fuel tank and was accurate to 100 ml's.
The results shown are for testing at 2,500 rpm in August 1995 and at a
complete retest in November 1995 the results shown are at 1,800 rpm using
RF83 fuel which is of a tighter specification than RF08.
CA 02267864 1999-04-O1
WO 98/I7745 PCT/GB97/02763
l$
RESULTS DATA (MERCEDES MIII Bench Test)
MAXIMUM RESULTS
Units l
-
CO CO~ HC Nor SFC
B
1,800 RPM
P.T.
Base Fuel 487 162036 920 1011 S50O1
2,500 RPM
P.T.
Base Fuel 413 12216 54 1299 * 40378
1,800 RPM
P.T.
Additised 3301 117974 702 591 38191
Fuel
2,500 RPM
P.T.
Additised 3612 10126 553 8096 * 3374
Fuel
* Denotes
WOT
Units - glh
CO CO~ HC Nox MFC
-
1,800 RPM P.T.
Base Fuel 2185 72252 4103 4505 2453
2,500 RPM P.T.
Base Fuel 4503 142679 6315 3496 4716
1,800 RPM P.T.
Additised Fuel14718 52628 3122 2631 170372
~ 2,500 RPM
P.T.
Additised Fuel4162 116686 6376 13726 3888
CA 02267864 1999-04-O1
WO 98/17745 PCT/GB97/02~63
t9
RESULTS DATA (MERCEDES MIII Bench Test
AVERAGE RESULTS
Units lg Kwh
-
CO CO, HC No,~ BSFC
1,800 RPM P.T.
Base Fuel 481 15658 881 915 5275
1,800 RPM P.T.
Additised Fuel372 12853 781 714 42359
2,500 RPM P.T.
Base Fuel 402 11549 5465 13045 38474
2,500 RPM P.T.
Additised Fuel3613 10126 491 810 33746
Units - glh
CO C0, HC No,~ MFC
1,800 RPM P.T.
Base Fuel 21453 696781 3920 4072 234738
1,800 RPM P.T.
Additised Fuel16554 571959 3475 3177 188458
2,500 RPM P.T.
Base Fuel 46298 1330098 6294 15024 443105
2,500 RPM P.T.
Additised Fuel41599 1166/61 5609 9324 388584
CA 02267864 1999-04-O1
WO 98/17745 PCT/GB97/02763
TESTING - UK Diesel
Due to the success the above results we took a diesel vehicle at random and
used
a dosage of 1:1,000 for a before and after smoke test.
The results are extremely encouraging and once again confirm the two aspects
of
the invention with the treatment ratio at 1:1,000 the predominant force is
monolayer construction.
The two graphs show overall percentage black smoke reduction of 66% and
using a smoke unit conversion chart the particulate matter reduction equals
71.7%.
DIESEL TEST
Vehicle: FORD "Fiesta" Diesel
Test: As per M.O.T. Standards
Criteria: Exhaust Emissions Diesel, Pass Beiow 2~5m -t(k)
Method: Pre - Condition (Oil Temperature Check)
Fast Idle Test N°- 1
Fast Idle Test I~ 2
Fast Idle Test 1~ 3
Fast Idle Test N'-' 4
Idle up to Governor "Cuts In" then reading is taken. Computer
decides how many readings necessary prior to averaging "k".