Note: Descriptions are shown in the official language in which they were submitted.
. .
CA 02268684 2003-03-24
65920-34
_1_
PROCESS FOR ALKYtJITING HINDERED SULFONAMIDES
Background of the Invention
The present invention relates to a process for alkylating hindered
sulfonamides by
Michael addition to propiolates and to novel intermediates prepared in said
process. The
products of the aforesaid reaction can be converted into matrix
metalloproteinase inhibitors.
Inhibitors of matrix metalloproteinase (MMP) are known to be useful for the
treatment
of a condition selected from the group consisting of arthritis (including
osteoarthritis and
rheumatoid arthritis), inflammatory bowel disease, Crohn's disease, emphysema,
acute
respiratory distress syndrome, asthma, chronic obstructive pulmonary disease,
Alzheimer's
disease, organ transplant toxicity, cachexia, allergic reactions, allergic
contact
hypersensitivity, cancer, tissue ulceration, restenosis, periodontal disease,
epidermolysis
bullosa, osteoporosis, loosening of artficial joint implants, atherosclerosis
(including
atherosclerotic plaque rupture), aortic aneurysm (including abdominal aortic
aneurysm and
brain aortic aneurysm), congestive heart failure, myocardial infarction,
stroke, cerebral
ischemia, head trauma, spinal cord injury, neuro-degenerative disorders (acute
and chronic),
autoimmune disorders, Huntington's disease, Parkinson's disease, migraine,
depression,
peripheral neuropathy, pain, cerebral amytoid angiopathy, nootropic or
cognition
enhancement, amyotrophic lateral sclerosis, multiple sclerosis, ocular
angiogenesis, corneal
injury, macular degeneration, abnormal wound healing, bums, diabetes, tumor
invasion, tumor
growth, tumor metastasis, corneal scarring, scleritis, AIDS, sepsis, septic
shock and other
diseases characterized by inhibition of metaAoproteinase or ADAM (including
TNF-a)
expression. In addition, the products which can be prepared from the compounds
and
processes of the present invention may be used in combination therapy with
standard non-
steroidal anti-inflammatory drugs (hereinafter NSAIO'S), COX-2 inhibitors and
analgesics for
the treatment of arthritis, and in combination with cytotoxic drugs such as
adriamycin,
daunomycin, cis-platinum, etoposide, taxol, taxotere and alkaloids, such as
vincristine, in the
treatment of cancer.
The alkylsulfonamides that can be prepared by the methods of the present
invention
are described in the literature. PCT Publications WO 96127583 and WO 98107697,
published
March 7, 1996 and February 26, 1998, respectively, refer to arylsuffonyl
hydroxamic acids.
The above references refer to methods of preparing sulfonamides using methods
other than
those described in the present invention.
Summan,~ of the Invention
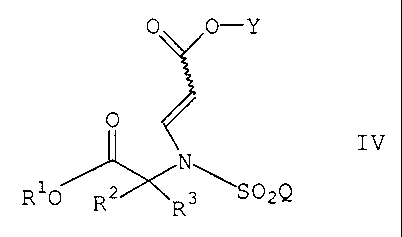
The present invention relates to a compound of the formula
CA 02268684 2003-10-02
65920-34
2
0 0-Y
0
IV
N
R10 R2 R \S02Q
wherein R1 is (C1-C6) alkyl or optionally
substituted benzyl;
RZ and R3 are independently (C1-C6) alkyl or Rz and
R3 are taken together to form a three to seven membered
cycloalkyl, a tetrahydropyran-4-yl ring or a bicyclo ring of
the formula
~0~
wherein the asterisk indicates the carbon atom
common to RZ and R3;
Q is (C1-C6) alkyl, (C6-Clo) aryl, (C1-Clz) heteroaryl,
(C6-Clo) aryl (C1-C6) alkyl, (C1-C1z) heteroaryl (C1-C6) alkyl,
(Cs-C1o) aryloxy (C1-C6) alkyl, (C6-CZO) arYloxy (C6-Cio) aryl.
(C6-Clo) aryloxy (C1-C12) heteroaryl, (C6-Clo) aryl (C6-Clo) aryl,
(C6-Clo) aryl (C1-C1z) heteroaryl, (C6-Clo) aryl (C6-Clo) aryl-
(C1-C6) alkyl, (C6-Clo) aryl (C6-Clo) aryl (C6-Cio) aryl. (C6-Cio) aryl-
(C6-Clo) aryl (C1-C12) heteroaryl, (C1-C12) heteroaryl (C6-Clo) aryl,
(C1-C12) heteroaryl (C1-C12) heteroaryl, (C6-Clo) aryl (C1-C6) alkoxy-
(C1-C6) alkyl, (C6-Clo) aryl (Ci-Cs) alkoxy (C6-Clo) aryl, (C6-Cio) arYl-
(C1-C6) alkoxy (C1-C12) heteroaryl, (C1-C12) heteroaryloxy-
(C1-C6) alkyl, (C1-C12) heteroaryloxy (C6-Clo) aryl,
(C1-C12) heteroaryloxy (C1-C12) heteroaryl, (C1-C12) heteroaryl-
(C1-C6) alkoxy (C1-C6) alkyl, (C1-C12) heteroaryl (C1-C6) alkoxy-
CA 02268684 2003-10-02
65920-34
2a
(C6-Clo) aryl or (C1-C1z) heteroaryl (C1-C6) alkoxy-
(C1-C1z) heteroaryl;
wherein each (C6-Clo) aryl or (C1-C12) heteroaryl
moieties of said (C6-Clo) aryl, (C1-C1z) heteroaryl,
(C6-Clo) aryl (C1-C6) alkyl, (C1-C12) heteroaryl (C1-C6) alkyl,
(C6-Cio) aryloxy (C1-C6) alkyl, (C6-Cio) aryloxy (C6-Cio) arYl~
(C6-Clo) aryloxy (C1-C12) heteroaryl, (C6-Clo) aryl (C6-Clo) aryl,
(C6-Clo) aryl (C1-C12) heteroaryl, (C6-Clo) aryl (C6-Clo) aryl-
(C1-C6) alkyl, (Cs-Cio) aryl (Cs-Clo) aryl (Cs-Cio) arYl~
(C6-Clo) aryl (C6-Clo) aryl (C1-C12) heteroaryl, (C1-C12) heteroaryl-
(C6-Clo) aryl, (C1-C12) heteroaryl (C1-Clz) heteroaryl,
(C6-Clo) aryl (C1-C6) alkoxy (C1-C6) alkyl, (C6-Clo) aryl-
(C1-C6) alkoxy (C6-Clo) aryl, (C6-Clo) aryl (C1-C6) alkoxy-
(C1-C12) heteroaryl, (C1-Clz) heteroaryloxy (C1-C6) alkyl,
(C1-C1z) heteroaryloxy (C6-Clo) aryl, (C1-C12) heteroaryloxy-
(C1-C12) heteroaryl, (C1-C12) heteroaryl (C1-C6) alkoxy (C1-C6) alkyl,
(C1-C12) heteroaryl (C1-C6) alkoxy (C6-Clo) aryl or
(C1-Clz) heteroaryl (C1-C6) alkoxy (C1-C12) heteroaryl is optionally
substituted on any of the ring carbon atoms capable of
forming an additional bond by one or more substituents per
ring independently
CA 02268684 1999-04-08
-3-
selected from fluoro, chloro, bromo, (C,-C6)alkyl, (C,-C6)alkoxy, perfluoro(C,-
C3)alkyl,
perfluoro(C~-C3)alkoxy and (Cs-C,o)aryloxy;
and Y is hydrogen, (C,-Cs)alkyl or a suitable protecting group.
Preferred compounds of formula IV are those wherein RZ and R3 are taken
together to
form a cyclobutyl, cyclopentyl, pyran-4-yl ring or a bicyclo ring of the
formula
O
wherein the asterisk indicates the carbon atom common to RZ and R3;
and wherein Q is 4-(4-fluorophenoxy)phenyl.
The present invention also relates to a process for preparing a compound of
the
formula
O O-Y
O IV
N
R'O \S02Q
R2 ~ Rs
wherein R', Rz, R3, Q and Y are as defined above;
comprising, reacting a compound of the formula
O
H
Ri0 N-SOZQ V
R2 R3
wherein R' is optionally substituted benzyl; and R2, R3, R4 and Q are as
defined
above;
with a compound of the formula
VI
O Y
wherein Y is (C~-Cs)alkyl;
CA 02268684 1999-04-08
-4-
in the presence of a base, such as tetrabutylammonium fluoride, potassium
carbonate,
tertiary amines and cesium carbonate, preferably tetrabutylammonium fluoride,
and a polar
solvent, such as tetrahydrofuran, acetonitrile, tert-butanol, t-amyl alcohols
and N,N-
dimethylformamide, preferably tetrahydrofuran.
The present invention also relates to a process comprising reducing said
compound
of the formula
O~~ O Y
O
IV '
N
R'O \SOzQ
RZ ~ Rs
wherein R', RZ, R3, Y and Q are as defined above;
with a reducing agent, such as palladium catalysts and a source of hydrogen,
preferably hydrogen over palladium on carbon, in a solvent, such as alcohols
or
tetrahydrofuran, preferably ethanol, to form a compound of the formula
O
O Y
O
III
N
R O ~SOZQ
R2 ~Rs
wherein R5 is hydrogen; and
R2, R3, Y and Q are as defined above.
The present invention also relates to a process further comprising reacting
said
compound of formula III, wherein R5 is hydrogen, with amines such as
dicyclohexylamine to
form the amine salts such as dicyclohexylammonium salt of the compound of
formula III.
The term "protecting group" as a substituent for Y is as described in Greene
and
Wuts, Protective Groups in Organic Synthesis, (John Wiley & Sons, Inc., Wiley
Interscience
Second Edition, 1991 ).
CA 02268684 2003-10-02
w 65920-34
-5-
The term "alkyl", as used herein, unless otherwise indicated, includes
saturated
monovalent hydrocarbon radicals having straight, branched or cyclic moieties
or combinations
thereof.
The term "alkoxy", as used herein, includes O-alkyl groups wherein "alkyl" is
defined
above.
The term "aryl", as used herein, unless otherwise indicated, includes an
organic radical
derived from an aromatic hydrocarbon by removal of one hydrogen, such as
phenyl or naphthyl.
The term "heteroaryl", as used herein, unless otherwise indicated, includes an
organic
radical derived from an aromatic heterocyclic compound by removal of one
hydrogen, such as
pyridyl, furyl, PYn~lyh thienyl, isothiazolyl, imidazolyl, benzimidazolyl,
tetrazolyl, pyrazinyl,
pyrimidyl, quinolyl, isoquinolyl, benzofuryl, isobenzofuryl, benzothienyl,
pyrazolyl, indolyl,
isoindolyl, purinyl, carbazolyl, isoxazolyl, thiazolyl, oxazolyl,
benzthiazolyl or benzoxazolyl.
Preferred heteroaryls include pyridyl, furyl, thienyl, isothiazolyl,
pyrazinyl, pyrimidyl, pyrazolyl,
isoxazolyl, thiazolyl or oxazolyl. Most preferred heteroaryls include pyridyl,
furyl or thienyl.
The term "aryl", as used herein, unless otherwise indicated, includes a
radical of the
general formula R-(C=O)- wherein R is alkyl, alkoxy, aryl, arylalkyl or
arylalkoxy and the terms
"alkyl" or "aryl" are as defined above.
The term "acyloxy", as used herein, includes O-acyi groups wherein "aryl" is
defined
above.
The squiggly line (i.e. " ~ ") in formula IV indicates that the carboxy group
can exist in
either a cis or traps configuration.
The compounds of formulae I-V may have chiral centers and therefore exist in
different
diasteriomeric or enantiomeric forms. This invention relates to all optical
isomers, tautomers and
stereoisomers of the compounds of formula I-V and mixtures thereof.
Preferably, compounds of the formula I' exist as the exo isomer of the formula
O
HOHN- J- Z S02Q
of
CA 02268684 1999-04-08
-6-
Detailed Description
The following reaction Schemes illustrate the preparation of the compounds of
the
present invention. Unless otherwise indicated n, R', R2, R3, Q and Z in the
reaction Schemes
and the discussion that follow are defined as above.
CA 02268684 1999-04-08
_7_
SCHEME1
O
H
R'O N-S02 Q
Rz R3
V
O\ O-Y
O
R' O N-SOZ Q
R2 R3
IV
O O-Y
O
R50 N-S02 Q
R2 R3
CA 02268684 1999-04-08
_g_
SCHEME 1 continued
O O-Y
O O
CI NHS-Q
R2 R3 O
O O-Y
O
HON
H R2 \ R3 O
CA 02268684 1999-04-08
_g_
Scheme 1 refers to the preparation of matrix metalloproteinase inhibiting
compounds of
formula I.
Referring to Scheme 1, compounds of said formula I are prepared from compounds
of
formula II by reaction with an in situ formed silyated hydroxylamine followed
by treatment with an
acid. Specifically, in situ formed silyated hydroxylamine compounds are
prepared by reaction of
hydroxylamine hydrochloride or hydroxylamine sulfate, preferably hydroxylamine
hydrochloride,
with a ((C~-C4)alkyl)3silyl halide in the presence of a base to form O-
trimethylsilylhydroxylamine,
N,O-bistrimethylsilylhydroxylamine or combinations thereof. Suitable bases
include pyridine,
2,6-lutidine or diisopropylethylamine, preferably pyridine. The reaction is
performed at a
temperature of about 0° to about 22°C (i.e., room temperature)
for about 1 to about 12 hours,
preferably about 1 hour. Suitable acids include hydrochloric or sulfuric,
preferably hydrochloric.
Compounds of said formula II, preferably not isolated, are prepared from
compounds of
formula III, wherein R5 is hydrogen, by reaction with oxalyl chloride or
thionyl chloride, preferably
oxalyl chloride, and a catalyst, preferably about 2% of N,N-dimethylformamide,
in an inert
solvent such as methylene chloride or toluene. The reaction is performed at a
temperature of
about 0° to about 22°C (i.e., room temperature) for about 1 to
about 12 hours, preferably about 1
hour.
Compounds of the formula III, wherein R5 is hydrogen, can be prepared from
compounds of the formula IV, wherein R' is optionally substituted benzyl, by
reduction in a polar
solvent. Suitable reducing agents include palladium catalysts with a source of
hydrogen, such
as hydrogen over palladium, hydrogen over palladium on carbon or palladium
hydroxide on
carbon, preferably hydrogen over palladium on carbon. Suitable solvents
include
tetrahydrofuran, methanol, ethanol and isopropanol and mixtures thereof,
preferably ethanol.
The aforesaid reaction is performed at a temperature of about 22°C
(i.e., room temperature) for a
period of 1 to 7 days, preferably about 2 days.
Compounds of the formula III, wherein RS is other than hydrogen, such as a
protonated
amine (such as protonated primary amine, secondary amine or tertiary amine),
alkali metal or
alkaline earth metal, can be prepared from compounds of the formula III,
wherein R5 is
hydrogen, by treatment with an aqueous or alkanolic solution containing an
acceptable cation
(e.g., sodium, potassium, dicyclohexylamine, calcium and magnesium, preferably
dicyclohexylamine), and then evaporating the resulting solution to dryness,
preferably under
reduced pressure or filtering the precipitate, preferably the
dicyclohexylamine salt precipate.
Compounds of the formula IV, wherein R' is (C~-Cs)alkyl or optionally
substituted
benzyl, can be prepared from compounds of the formula V, wherein R' is
optionally substituted
benzyl, by Michael addition to a propiolate ester in the presence of a base in
a polar solvent.
Suitable propiolates are of the formula H-C---C-COZY, wherein Y is (C~-
C6)alkyl. Compounds of
4 . r
65920-34
CA 02268684 2003-03-24
-10-
the fomwla H-C---C-C02Y are commercially available or can be made by methods
well known to
those of ordinary skill in the art. Suitable bases include tetrabutylammonium
fluoride, potassium
carbonate, tertiary amines and cesium carbonate, preferably tetrabutylammonium
fluoride.
Suitable solvents include tetrahydrofuran, acetonitrile, tert-bukanol, t-amyl
alcohols and N,N-
dimethylformamide, preferably tetrahydrofuran. The aforesaid reaction is
performed at a
temperature of about -10°C to about 60°C, preferably ranging
behnreen 0°C and about 22°C (i.e.,
room temperature). The compounds of formula IV are obtained as mixtures of
geometric
isomers about the olefinic double bond (i.e. cis and traps isomers);
separation of the isomers is
not necessary.
Compounds of said formula I, wherein Y is (C,-Cg)alkyl, can be saponfied to
the free
acid (i.e. Y is hydrogen) using a base such as sodium hydroxide in a protic
solvent such as
ethanol, methanol or water or a mixture such as water and ethanol, water and
toluene, or water
and THF. The preferred solvent system is water and toluene. The reaction is
conducted for a
period of 30 minutes to 24 hours, preferably about 2 hours.
Compounds of the formula V, wherein R' is optionally substituted benryl can be
prepared according to methods known in the art. The atkylsutfonamides that can
be prepared
by the methods of the present invention and the starting materials of formula
V are also
described in the literature. PCT Publications WO 96127583 and WO 98107697,
published
March 7, 1996 and February 26, 1998, respectively, refer to aryisulfonyi
hydroxamic acids.
Compounds of the formula V wherein RZ and R' are tetrahydropyran-4-yl or a
bicyclo
ring of the formula
O
wherein the asterisk indicates the carbon atom common to RZ and R' can be
prepared
according to methods analogous to those of Examples 2 and 3.
The compounds of the formula I which are basic in nature are capable of
forming a
wide variety of different salts with various inorganic and organic acids.
Although such salts
must be pharmaceutically acceptable for administration to animals, it is often
desirable in
practice to initially isolate a compound of the formula i from the reaction
mixture as a
pharmaceutically unacceptable salt and then simply convert the latter back to
the free base
compound by treatment with an alkaline reagent, and subsequentfyr convert the
free base to a
pharmaceutically acceptable acid addition salt. The acid addition salts of the
base
compounds of this invention are readily prepared by treating the base compound
with a
CA 02268684 1999-04-08
-11-
substantially equivalent amount of the chosen mineral or organic acid in an
aqueous solvent
medium or in a suitable organic solveht such as methanol or ethanol. Upon
careful
evaporation of the solvent, the desired solid salt is obtained.
The acids which are used to prepare the pharmaceutically acceptable acid
addition
salts of the base compounds of this invention are those which form non-toxic
acid addition
salts, i.e., salts containing pharmacologically acceptable anions, such as
hydrochloride,
hydrobromide, hydroiodide, nitrate, sulfate or bisulfate, phosphate or acid
phosphate, acetate,
lactate, citrate or acid citrate, tartrate or bitartrate, succinate, maleate,
fumarate, gluconate,
saccharate, benzoate, methanesulfonate and pamoate [i.e., 1,1'-methylene-bis-
(2-hydroxy-3-
naphthoate)] salts.
Those compounds of the formula I which are also acidic in nature, are capable
of
forming base salts with various pharmacologically acceptable cations. Examples
of such salts
include the alkali metal or alkaline-earth metal salts and particularly, the
sodium and
potassium salts. These salts are all prepared by conventional techniques. The
chemical
bases which are used as reagents to prepare the pharmaceutically acceptable
base salts of
this invention are those which form non-toxic base salts with the herein
described acidic
compounds of formula I. These non-toxic base salts include those derived from
such
pharmacologically acceptable cations as sodium, potassium, calcium and
magnesium, etc.
These salts can easily be prepared by treating the corresponding acidic
compounds with an
aqueous solution containing the desired pharmacologically acceptable cations,
and then
evaporating the resulting solution to dryness, preferably under reduced
pressure.
Alternatively, they may also be prepared by mixing lower alkanolic solutions
of the acidic
compounds and the desired alkali metal alkoxide together, and then evaporating
the resulting
solution to dryness in the same manner as before. In either case,
stoichiometric quantities of
reagents are preferably employed in order to ensure completeness of reaction
and maximum
product yields.
The ability of the compounds of formula I or their pharmaceutically acceptable
salts
(hereinafter also referred to as the active compounds) to inhibit matrix
metalloproteinases or
ADAMs (such as inhibiting the production of tumor necrosis factor (TNF)) and,
consequently,
demonstrate their effectiveness for treating diseases characterized by matrix
metalloproteinase
or ADAM (such as the production of tumor necrosis factor) can be determined
according to in
vitro assay tests well known to those of ordinary skill in the art. One
example of an assay
recognized as demonstrating that the final products produced by the methods of
the invention is
the following Inhibition of Human Collagenase Assay.
CA 02268684 1999-04-08
-12-
Biological Assay
Inhibition of Human Collagenase (MMP-1 )
Human recombinant collagenase is activated with trypsin using the following
ratio: 10
~g trypsin per 100 ~g of collagenase. The trypsin and collagenase are
incubated at room
temperature for 10 minutes then a five fold excess (50 ~g/10 ~g trypsin) of
soybean trypsin
inhibitor is added.
10 mM stock solutions of inhibitors are made up in dimethyl sulfoxide and then
diluted
using the following Scheme:
10mM >120~M >12pM------>1.2pM >0.12~M
Twenty-five microliters of each concentration is then added in triplicate to
appropriate
wells of a 96 well microfluor plate. The final concentration of inhibitor will
be a 1:4 dilution after
addition of enzyme and substrate. Positive controls (enzyme, no inhibitor) are
set up in wells
D1-D6 and blanks (no enzyme, no inhibitors) are set in wells D7-D12.
Collagenase is diluted to 400 ng/ml and 25 ~I is then added to appropriate
wells of the
microfluor plate. Final concentration of collagenase in the assay is 100
ng/ml.
Substrate (DNP-Pro-Cha-Gly-Cys(Me)-His-Ala-Lys(NMA)-NHZ) is made as a 5 mM
stock in dimethyl sulfoxide and then diluted to 20 mM in assay buffer. The
assay is initiated by
the addition of 50 ~I substrate per well of the microfluor plate to give a
final concentration of 10
~M.
Fluorescence readings (360 nM excitation, 460 nm emission) were taken at time
0 and
then at 20 minute intervals. The assay is conducted at room temperature with a
typical assay
time of 3 hours.
Fluorescence vs time is then plotted for both the blank and collagenase
containing
samples (data from triplicate determinations is averaged). A time point that
provides a good
signal (the blank) and that is on a linear part of the curve (usually around
120 minutes) is chosen
to determine ICS values. The zero time is used as a blank for each compound at
each
concentration and these values are subtracted from the 120 minute data. Data
is plotted as
inhibitor concentration vs % control (inhibitor fluorescence divided by
fluorescence of
collagenase alone x 100). ICS s are determined from the concentration of
inhibitor that gives a
signal that is 50% of the control.
If ICS s are reported to be <0.03 ~M then the inhibitors are assayed at
concentrations of
0.3 ~M, 0.03 ~M, 0.03 ~M and 0.003 ~M.
The following Examples illustrate the preparation of the compounds of the
present
invention. Melting points are uncorrected. NMR data are reported in parts per
million (b) and
are referenced to the deuterium lock signal from the sample solvent
(deuteriochloroform
CA 02268684 1999-04-08
-13-
unless otherwise specified). Commercial reagents were utilized without further
purification.
THF refers to tetrahydrofuran. DMF refers to N,N-dimethylformamide.
Chromatography
refers to column chromatography performed using 32-63 mm silica gel and
executed under
nitrogen pressure (flash chromatography) conditions. Room or ambient
temperature refers to
20-25°C. All non-aqueous reactions were run under a nitrogen atmosphere
for convenience
and to maximize yields. Concentration at reduced pressure means that a rotary
evaporator
was used.
EXAMPLE 1
3-[(4-(4-FLUOROPHENOXY)BENZENESULFONYL]-(1-HYDROXYCARBAMOYL-
CYCLOPENTYL)AMINO]PROPIONIC ACID
A) 1-[4-(4-Fluorophenoxy)benzenesulfonylamino]cyclopentanecarboxylic
Acid Benzyl Ester
To a mixture of 12.41 g (0.032 mol) of 1-aminocyclopentanecarboxylic acid
benzyl
ester, toluene-4-sulfonic acid salt (can be prepared according to literature
methods such as
those described in United States Patent 4,745,124), and 10.0 g (0.035 mol, 1.1
equivalents)
of 4-(4-fluorophenoxy)benzenesulfonyl chloride (prepared according to
Preparation 3) in 113
mL of toluene was added 11.0 mL (0.079 mol, 2.5 equivalents) of triethylamine.
The resulting
mixture was stirred at ambient temperature overnight, washed with 2N
hydrochloric acid (2 x
100 mL) and brine (100 mL), dried over sodium sulfate, and concentrated to 30
mL. Hexane,
149 mL, was added drop-wise over three hours giving a solid precipitate which
was
granulated at 0 °C for one hour and filtered yielding 12.598 (85%) of 1-
[4-(4-
fluorophenoxy)benzenesulfonylamino]cyclopentane-carboxylic acid benzyl ester.
1 H NMR (CDC13) 8 7.78-7.82 (m, 2H), 7.30-7.39 (m, 5H), 7.06-7.12 (m, 2H),
6.99-
7.04 (m, 2H), 6.93-6.97 (m, 2H), 5.15 (s, 1 H), 5.02 (s, 2H), 2.04-2.13 (m,
2H), 1.92-1.98 (m,
2H), 1.62-1.69 (m, 4H).
A 4.0 g sample was granulated in a mixture of 4 mL of ethyl acetate and 40 mL
of
hexanes overnight giving 3.72 g (93% recovery) of 1-[4-(4-
fluorophenoxy)benzenesulfonyl-
amino]-cyclopentanecarboxylic acid benzyl ester as light tan solids, mp 97.0-
97.5°C.
B) 1-{(2-Ethoxycarbonylvinyl)-[4-(4-fluorophenoxy)benzenesulfonyl]-
amino}cyclopentanecarboxylic Acid Benzyl Ester
A solution of 25.0 g (53,~ mmol) of 1-[4-(4-
fluorophenoxy)benzenesulfonylamino]-
cyclopentanecarboxylic acid benzyl ester and 10.8 mL (106 mmol, 2 equivalents)
of ethyl
propiolate in 200 mL of dry tetrahydrofuran at 1 °C was treated with
53.2 mL (53.2 mmol, 1
equivalent) of a solution of tetrabutylammonium fluoride in tetrahydrofuran (1
M) over 45
minutes. The resulting solution was allowed to warm slowly to ambient
temperature and
CA 02268684 1999-04-08
-14-
stirred overnight. The tetrahydrofuran was displaced with toluene at reduced
pressure, and
the toluene solution was washed with water and brine, diluted to 600 mL with
toluene, stirred
with 90 g of silica gel for three hours, filtered, and concentrated to 25.14 g
(83%) of 1-{(2-
ethoxycarbonylvinyl)-[4-(4-fluorophenoxy)benzenesulfonyl]amino}-
cyclopentanecarboxylic
acid benzyl ester as an orange oil. 1 H NMR (CDCI3) indicated a 1.5:1
translcis ratio.
Trans 8 7.74-7.78 (m, 2H), 7.72 (d, J=14 Hz, 1 H), 7.26-7.36 (m, 5H), 6.96-
7.12 (m,
4H), 6.78-6.84 (m, 2H), 5.44 (d, J=14 Hz, 1 H), 5.11 (s, 2H), 4.12 (q, J=7.1
Hz, 2H), 2.08-
2.43 (m, 4H), 1.63-1.80 (m, 4H), 1.24 (t, J=7.1 Hz, 3H). Cis 8 7.68-7.72 (m,
2H), 7.26-7.36
(m, 5H), 6.96-7.12 (m, 4H), 6.86-6.91 (m, 2H), 6.47 (d, J=8.1 Hz, 1 H), 5.90
(d, J=8.1 Hz, 1 H),
5.11 (s, 2H), 3.93 (q, J=7.2 Hz, 2H), 2.08-2.43 (m, 4H), 1.63-1.80 (m, 4H),
1.17 (t, J=7.2 Hz,
3H).
C) 1-{(2-Ethoxycarbonylethyl)-(4-(4-fluorophenoxy)benzenesulfonyl]-
amino}-cyclopentanecarboxylic Acid
A solution of 2.50 g (4.4 mmol) of 1-{(2-ethoxycarbonylvinyl)-[4-(4
fluorophenoxy)benzenesulfonyl)amino}cyclopentanecarboxylic acid benzyl ester
in 25 mL of
ethanol was treated with 2.5 g of 50% water wet 10% palladium on carbon
catalyst and
shaken under 53 psi of hydrogen for 21 hours. The catalyst was removed by
filtration and
washed with ethanol (4 x 25 mL). The filtrate and washings were combined and
concentrated
under vacuum to 1.74 g (82%) of crude 1-{(2-ethoxycarbonylethyl)-[4-(4-
fluorophenoxy)benzenesulfonyl)amino}cyclopentanecarboxylic acid as a viscous
oil.
1 H NMR (CDC13) 8 7.78-7.82 (m, 2H), 6.94-7.09 (m, 6H), 4.09 (q, J=7.2 Hz,
2H),
3.56-3.60 (m, 2H), 2.75-2.79 (m, 2H), 2.33-2.39 (m, 2H), 1.93-2.03 (m, 2H),
1.69-1.76 (m,
2H), 1.56-1.63 (m, 2H), 1.22 (t, J=7.2 Hz, 3H).
D) 1-{(2-Ethoxycarbonylethyl)-(4-(4-fluorophenoxy)benzenesulfonyl]-
amino}-cyclopentanecarboxylic Acid, Dicyclohexylaminium Salt
A solution of 3.10 g (6.5 mmol) of crude 1-{(2-ethoxycarbonylethyl)-[4-(4-
fluorophenoxy)benzenesulfonyl]amino}cyclopentanecarboxylic acid in 30 mL of
ethanol was
treated with 1.28 mL (6.5 mmol, 1 equivalent) of dicyclohexylamine at ambient
temperature
producing solids within five minutes. This mixture was stirred at ambient
temperature
overnight and then at 0°C for five hours. White solids were isolated by
filtration, washed with
10 mL of cold ethanol, and air dried giving 2.89 g (67%) of 1-{(2-
ethoxycarbonylethyl)-[4-(4-
fluorophenoxy)benzenesulfonyl]amino}cyclopentanecarboxylic acid,
dicyclohexylaminium
salt.
1 H NMR (CDC13) 8 7.86-7.91 (m, 2H), 6.99-7.09 (m, 4H), 6.90-6.94 (m, 2H), 5.3
(br
s, 2H), 4.07 (q, J=7.1 Hz, 2H), 3.54-3.59 (m, 2H), 2.88-2.95 (m, 4H), 2.31-
2.38 (m, 2H), 1.95-
CA 02268684 1999-04-08
-15-
2.22 (m, 6H), 1.68-1.77 (m, 6H), 1.53-1.60 (m, 4H), 1.40-1.50 (m, 4H), 1.21
(t, J=7.1 Hz, 3H),
1.14-1.22 (m, 6H). Mp 164.5-165.9 °C.
E) 1-{(2-Ethoxycarbonylethyl)-[4-(4-fluorophenoxy)benzenesulfonyl]-
amino}-cyclopentanecarboxylic Acid
A solution of 3.0 g (4.5 mmol) of 1-{(2-ethoxycarbonylethyl)-[4-(4
fluorophenoxy)benzenesulfonyl]amino}cyclopentanecarboxylic acid,
dicyclohexylaminium salt
in 30 mL of dichloromethane was treated with 30 mL of 2N hydrochloric acid at
ambient
temperature causing immediate precipitation of solids. This mixture was
stirred at ambient
temperature for three hours. The solids were filtered, the aqueous phase was
extracted with
dichloromethane, and the combined organic phases were washed with water, dried
over
sodium sulfate, and concentrated under vacuum to 2.2 g (100%) of 1-{(2-
ethoxycarbonylethyl)-[4-(4-
fluorophenoxy)benzenesulfonyl]amino}cyclopentanecarboxylic
acid as a clear oil.
1 H NMR (DMSO-dg) 8 12.68 (bs, 1 H), 7.76-7.80 (m, 2H), 7.25-7.31 (m, 2H),
7.16
7.21 (m, 2H), 7.03-7.08 (m, 2H), 4.01 (q, J=7.1 Hz, 2H), 3.48-3.54 (m, 2H),
2.64-2.70 (m, 2H),
2.13-2.21 (m, 2H), 1.90-1.98 (m, 2H), 1.52-1.59 (m, 4H), 1.14 (t, J=7.1 Hz,
3H).
F) 3-{(1-Chlorocarbonylcyclopentyl)-[4-(4-fluorophenoxy)benzene-
sulfonyl]amino}propionic Acid Ethyl Ester
A solution of 7.26 g (15.1 mmol) of 1-{(2-ethoxycarbonylethyl)-[4-(4
fluorophenoxy)benzenesulfonyl]amino}cyclopentanecarboxylic acid in 73 mL of
dichloromethane was treated with 1.4 mL (17 mmol, 1.1 equivalents) of oxalyl
chloride and
0.02 mL (0.3 mmol, 0.02 equivalents) of dimethylformamide at ambient
temperature, causing
some bubbling, and was stirred overnight. The resulting solution of 3-{(1
chlorocarbonylcyclopentyl)-[4-(4-fluorophenoxy)benzenesulfonyl]amino}propionic
acid ethyl
ester was used for the preparation of 3-[[4-(4-fluorophenoxy)benzenesulfonyl]-
(1
hydroxycarbamoylcyclopentyl)amino]propionic acid ethyl ester without
isolation.
A similarly prepared solution of 3-{(1-chlorocarbonylcyclopentyl)-[4-(4-
fluorophenoxy)benzenesulfonyl]amino}propionic acid ethyl ester was
concentrated under
vacuum to an oil.
1 H NMR (CDCI3) b 7.84-7.87 (m, 2H), 6.97-7.12 (m, 6H), 4.10 (q, J=7.2 Hz,
2H),
3.55-3.59 (m, 2H), 2.68-2.72 (m, 2H), 2.47-2.53 (m, 2H), 1.95-2.02 (m, 2H),
1.71-1.76 (m,
4H), 1.24 (t, J=7.2 Hz, 3H).
CA 02268684 1999-04-08
-16-
G) 3-[[4-(4-Fluorophenoxy)benzenesulfonyl]-(1-hydroxycarbamoylcyclo-
pentyl)amino]propionic Acid Ethyl Ester
A solution of 1.37 g (19.7 mmol, 1.3 equivalents) of hydroxylamine
hydrochloride in
9.2 mL (114 mmol, 7.5 equivalents) of dry pyridine at 0 °C was treated
with 5.8 mL (45 mmol,
3.0 equivalents) of trimethylsilyl chloride, causing white solids to
precipitate. The mixture was
allowed to warm to ambient temperature overnight. This mixture was then cooled
to 0°C and
treated with a solution of 7.54 g (15.1 mmol) of 3-{(1-
chlorocarbonylcyclopentyl)-[4-(4-
fluorophenoxy)-benzenesulfonyl]amino}propionic acid ethyl ester in 73 mL of
dichloromethane, prepared as described above, without isolation, causing an
exotherm to
about 8°C. This mixture was stirred at 0°C for 30 minutes and at
ambient temperature for
about one hour. The reaction was then treated with 50 mL of 2N aqueous
hydrochloric acid
and was stirred at ambient temperature for one hour. The aqueous phase was
extracted with
dichloromethane and the combined organic phases were washed with 2N aqueous
hydrochloric acid (2 x 50 mL) and water (50 mL). This solution of 3-[[4-(4-
fluorophenoxy)benzenesulfonyl]-(1-hydroxycarbamoylcyclopentyl)amino]propionic
acid ethyl
ester in dichloromethane was used for the preparation of 3-[[4-(4-fluoro-
phenoxy)benzenesulfonyl]-(1-hydroxycarbamoylcyclopentyl)amino]propionic acid
without
isolation. An aliquot was concentrated to a foam.
1 H NMR (DMSO-dg) b 10.37 (s, 1 H), 8.76 (s, 1 H), 7.74-7.79 (m, 2H), 7.24-
7.30 (m,
2H), 7.14-7.20 (m, 2H), 7.01-7.05 (m, 2H), 3.99 (q, J=7.1 Hz, 2H), 3.42-3.47
(m, 2H), 2.62
2.67 (m, 2H), 2.16-2.23 (m, 2H), 1.77-1.85 (m, 2H), 1.43-1.52 (m, 4H), 1.13
(t, J=7.1 Hz, 3H).
A similarly prepared solution was concentrated under vacuum to 6.71 g (89%) of
3-
[[4-(4-fluorophenoxy)benzenesulfonyl)-(1-
hydroxycarbamoylcyclopentyl)amino)propionic acid
ethyl ester as a hard dry foam.
H) 3-((4-(4-Fluorophenoxy)benzenesulfonyl]-(1-hydroxycarbamoylcyclo-
pentyl)amino]propionic Acid
A solution of 7.48 g (15.1 mmol) of 3-[[4-(4-fluorophenoxy)benzenesulfonyl]-(1-
hydroxycarbamoylcyclopentyl)amino]propionic acid ethyl ester in
dichloromethane was
concentrated by rotary evaporation with the addition of 75 mL of toluene. This
solution was
treated with 75 mL of water, cooled to 0°C, and treated with 6.05 g
(151 mmol, 10
equivalents) of sodium hydroxide pellets over 10 minutes with vigorous
stirring. This mixture
was stirred for 15 minutes at 0°C and warmed to ambient temperature
over one hour. The
aqueous phase was separated, diluted with 7.5 mL of tetrahydrofuran, cooled to
0°C, and
treated with 33 mL of 6N aqueous hydrochloric acid over 20 minutes. This
mixture was
stirred with 75 mL of ethyl acetate at 0°C to ambient temperature, and
the ethyl acetate phase
CA 02268684 1999-04-08
_17_
was separated and washed with water. The ethyl acetate solution was slowly
treated with
150 mL of hexanes at ambient temperature causing solids to precipitate, and
was stirred
overnight. Filtration yielded 5.01 g of 3-[[4-(4-
fluorophenoxy)benzenesulfonyl]-(1-
hydroxycarbamoylcyclopentyl)amino]propionic acid as a white solid (71% yield
from 1-{(2-
ethoxycarbonylethyl)-[4-(4-
fluorophenoxy)benzenesulfonyl]amino}cyclopentanecarboxylic
acid).
1 H NMR (DMSO-d6) 8 12.32 (s, 1 H), 10.43 (s, 1 H), 8.80 (s, 1 H), 7.82 (d,
J=8.6 Hz,
2H), 7.28-7.35 (m, 2H), 7.20-7.26 (m, 2H), 7.08 (d, J=8.9 Hz, 2H), 3.44-3.49
(m, 2H), 2.61-
2.66 (m, 2H), 2.24-2.29 (m, 2H), 1.86-1.90 (m, 2H), 1.54-1.55 (m, 4H). mp
162.9-163.5 °C
(dec).
EXAMPLE 2
3-([4-(4-Fluoro-phenoxy)-benzenesulfonyl]-(4-hydroxycarbamoyl-tetrahydro-
pyran-4 -yl)-amino]-propionic acid
A) 4-[N-(Diphenylmethylene)amino]tetrahydropyran-4-carboxylic acid
benzyl ester
To a suspension of sodium hydride (6.56 grams. 0.164 mole) in ethylene glycol
dimethyl ether (150 mL) at 0°C is added a solution of the N-
(diphenylmethylene)glycine benzyl
ester (0.07398 mole) in ethylene glycol dimethyl ether (50 mL) dropwise via
addition funnel. A
solution of 2-bromoethyl ether (23.21 grams, 0.090 mole) in ethylene glycol
dimethyl ether (50
mL) is then added, in 10 mL portions over approximately 5 minutes, to the
ethylene glycol
dimethyl ether solution. The ice bath is removed and the reaction is stirred
at room
temperature for 16 hours. The mixture is diluted with diethyl ether and washed
with water.
The aqueous layer is extracted with diethyl ether. The combined organic
extracts are washed
with brine, dried over magnesium sulfate, and concentrated to afford crude
product.
Chromatography on silica gel eluting first with 4 L of 5% ethyl acetate/hexane
followed by 4
liters of 10% ethyl acetate/hexane provides 4-[N-
(diphenylmethylene)amino]tetrahydropyran-
4-carboxylic acid benzyl ester as a clear yellow oil.
B) 4-Aminotetrahydropyran-4-carboxylic acid benzyl ester
To a solution of 4-[N-(diphenylmethylene)amino]tetrahydropyran-4-carboxylic
acid
benzyl ester (0.047 mole) in diethyl ether (120 mL) is added 1M aqueous
hydrochloric acid
solution (100 mL). The mixture is stirred vigorously at room temperature for
16 hours. The
layers are separated and the aqueous layer washed with diethyl ether. The
aqueous layer is
brought to pH 10 with dilute aqueous ammonium hydroxide solution and extracted
with
dichloromethane. The organic extract is dried over sodium sulfate and
concentrated to give 4-
aminotetrahydropyran-4-carboxylic acid benzyl ester.
CA 02268684 1999-04-08
-18-
C) 4-[4-(4-Fluorophenoxy)benzenesulfonylamino]tetrahydropyran-4-
carboxylic acid benzyl ester
To a solution of 4-aminotetrahydropyran-4-carboxylic acid benzyl ester (0.0404
mole)
in N,N-dimethylformamide (40 mL) is added triethylamine (5.94 mL, 0.043 mole).
Solid 4-(4-
fluorophenoxy)benzenesulfonyl chloride (12.165 grams, 0.0424 mole) is added to
the above
solution in portions. The resulting mixture is stirred at room temperature for
16 hours and
then most of the solvent is removed by evaporation under vacuum. The residue
was
partitioned between saturated sodium bicarbonate solution and dichloromethane.
The
aqueous layer is separated and extracted with dichloromethane. The combined
organic
layers are washed with brine and dried over sodium sulfate. Evaporation of the
solvent under
vacuum provided crude 4-[4-{4-
fluorophenoxy)benzenesulfonylamino)tetrahydropyran-4-
carboxylic acid benzyl ester. Flash chromatography on silica gel eluting with
25% ethyl
acetate / hexane followed by 50% ethyl acetate / hexane provided 4-[4-(4-
fluorophenoxy)benzenesulfonylamino]tetrahydropyran-4-carboxylic acid benzyl
ester.
D) 4-{(2-Ethoxycarbonyl-vinyl)-[4-(4-fluoro-phenoxy)-benzenesulfonyl]-
amino}-tetrahydro-pyran-4-carboxylic acid benzyl ester
A solution of (53.2 mmol) of the product of the previous step and 10.8 mL (106
mmol,
2 equivalents) of ethyl propiolate in 200 mL of dry tetrahydrofuran at 1
°C is treated with 53.2
mL (53.2 mmol, 1 equivalent) of a solution of tetrabutylammonium fluoride in
tetrahydrofuran
(1M) over 45 minutes. The resulting solution is allowed to warm slowly to
ambient
temperature and stirred overnight. The tetrahydrofuran is displaced with
toluene at reduced
pressure, and the toluene solution is washed with water and brine, diluted to
600 mL with
toluene, stirred with 90 g of silica gel for three hours, filtered, and
concentrated to the title
compound.
E) 4-~(2-Ethoxycarbonyl-ethyl)-[4-(4-fluoro-phenoxy)-benzenesulfonyl]-
amino}-tetrahydro-pyran-4-carboxylic acid
A solution of (4.4 mmol) of the product of step D in 25 mL of ethanol is
treated with
2.5 g of 50% water wet 10% palladium on carbon catalyst and shaken under 53
psi of
hydrogen for 21 hours. The catalyst is removed by filtration and washed with
ethanol (4 x 25
mL). The filtrate and washings are combined and concentrated under vacuum to
crude
product.
F) ~(4-Chlorocarbonyl-tetrahydro-pyran-4-yl)-[4-(4-fluoro-phenoxy)-
benzenesulfonyl]-amino}-propionic acid ethyl ester
A solution of (15.1 mmol) of the product from Step E in 73 mL of
dichloromethane is
treated with 1.4 mL (17 mmol, 1.1 equivalents) of oxalyl chloride and 0.02 mL
(0.3 mmol, 0.02
CA 02268684 1999-04-08
-19-
equivalents) of dimethylformamide at ambient temperature, causing some
bubbling, and is
stirred overnight. The resulting solution of the title compound is used in
step G without
isolation.
G) 3-[(4-(4-Fluoro-phenoxy)-benzenesulfonyl]-(4-hydroxycarbamoyl-
tetrahydro-pyran-4-yl)-amino]-propionic acid ethyl ester
A solution of (19.7 mmol, 1.3 equivalents) of hydroxylamine hydrochloride in
9.2 mL
(114 mmol, 7.5 equivalents) of dry pyridine at 0 °C is treated with 5.8
mL (45 mmol, 3.0
equivalents) of trimethylsilyl chloride, causing white solids to precipitate.
The mixture is
allowed to warm to ambient temperature overnight. This mixture is then cooled
to 0°C and
treated with a solution of (15.1 mmol) of the product from Step F in 73 mL of
dichloromethane
causing an exotherm to about 8°C. This mixture is stirred at 0°C
for 30 minutes and at
ambient temperature for about one hour. The reaction is then treated with 50
mL of 2N
aqueous hydrochloric acid and was stirred at ambient temperature for one hour.
The
aqueous phase is extracted with dichloromethane and the combined organic
phases are
washed with 2N aqueous hydrochloric acid (2 x 50 mL) and water (50 mL). This
solution of
the title compound in dichloromethane is used in the next step.
(H) 3I(4-(4-Fluoro-phenoxy)-benzenesulfonyl]-(4-hydroxycarbamoyl-
tetrahydro-pyran-4-yl)-amino]-propionic acid
A solution of 15.1 mmoles of the product from Step G in dichloromethane is
concentrated by rotary evaporation with the addition of 75 mL of toluene. This
solution is
treated with 75 mL of water, cooled to 0°C, and treated with 6.05 g
(151 mmol, 10 equivalents)
of sodium hydroxide pellets over 10 minutes with vigorous stirring. This
mixture is stirred for
15 minutes at 0°C and warmed to ambient temperature over one hour. The
aqueous phase is
separated, diluted with 7.5 mL of tetrahydrofuran, cooled to 0°C, and
treated with 33 mL of 6N
aqueous hydrochloric acid over 20 minutes. This mixture is stirred with 75 mL
of ethyl acetate
at 0°C to ambient temperature, and the ethyl acetate phase is separated
and washed with
water. The ethyl acetate solution was concentrated to yield the title
compound.
EXAMPLE 3
3-[[4-(4-Fluoro-phenoxy)-benzenesulfonyl]-(3-hydroxycarbamoyl-8-oxa-
bicyclo(3.2.1]oct-3-yl)-amino]-propionic acid
A) 3-(Benzhydrylideneamino)-8-oxabicyclo[3.2.1]octane-3-carboxylic acid
benzyl ester
To a suspension of sodium hydride (0.41 grams, 17.1 mmole) in N,N-
dimethylformamide (50 mL) at 0°C is added dropwise a solution of N-
diphenylmethylene
glycine benzyl ester (7.8 mmole) in N,N-dimethylformamide (50 mL). After
stirring for 30
minutes at room temperature, a solution of cis-2,5-bis(hydroxymethyl)-
tetrahydrofuran
CA 02268684 1999-04-08
-20-
ditosylate (4.1 grams, 9.3 mmole)( prepared by literature methods such as
those described in
JOC, 47, 2429-2435 (1982)) in N,N-dimethylformamide (50 mL) is added dropwise.
The
reaction mixture is gradually heated to 100°C in an oil bath and
stirred at this temperature
overnight. The solvent is evaporated under vacuum and the residue is taken up
in water and
extracted twice with diethyl ether. The combined organic extracts are washed
with brine,
dried over magnesium sulfate and concentrated to a crude product.
B) 3-Amino-8-oxabicyclo[3.2.1]octane-3-carboxylic acid benzyl ester
hydrochloride
A two-phase mixture of 3-(benzhydrylideneamino)-8-oxabicyclo[3.2.1]octane-3-
carboxylic acid benzyl ester (3.9 mmole) in aqueous 1N hydrochloric acid
solution (100 mL)
and diethyl ether (100 mL) is stirred at room temperature overnight. The
aqueous layer is
concentrated to provide the title compound.
C) 3-exo-[4-(4-Fluorophenoxy)benzenesulfonylamino]-8-oxabicyclo[3.2.1]-
octane-3-carboxylic acid benzyl ester
A solution of 3-amino-8-oxabicyclo[3.2.1]octane-3-carboxylic acid benzyl ester
hydrochloride (2.9 mmole), 4-(4-fluorophenoxy)benzenesulfonylchloride (923 mg,
3.2 mmole)
and triethylamine (0.9 mL, 6.5 mmole) in N,N-dimethylformamide (45 mL) is
stirred at room
temperature overnight. The solvent is removed under vacuum and the residue is
taken up in
saturated aqueous sodium bicarbonate solution. After extracting twice with
methylene
chloride, the combined organic layers are washed with brine, dried over
magnesium sulfate
and concentrated to a brown oil. The title compound is isolated by
chromatography on silica
using 1 % methanol in methylene chloride as eluant.
D) 3-{(2-Ethoxycarbonyl-vinyl)-[4-(4-fluoro-phenoxy)-benzenesulfonyl]-
amino}-8-oxa- bicyclo[3.2.1]octane-3-carboxylic acid benzyl ester
A solution of (53.2 mmol) of the product of the previous step and 10.8 mL (106
mmol,
2 equivalents) of ethyl propiolate in 200 mL of dry tetrahydrofuran at 1
°C is treated with 53.2
mL (53.2 mmol, 1 equivalent) of a solution of tetrabutylammonium fluoride in
tetrahydrofuran
(1M) over 45 minutes. The resulting solution is allowed to warm slowly to
ambient
temperature and stirred overnight. The tetrahydrofuran is displaced with
toluene at reduced
pressure, and the toluene solution is washed with water and brine, diluted to
600 mL with
toluene, stirred with 90 g of silica gel for three hours, filtered, and
concentrated to the title
compound.
CA 02268684 1999-04-08
-21-
E) 3-{(2-Ethoxycarbonyl-ethyl)-[4-(4-fluoro-phenoxy)-benzenesulfonyl]-
amino}-8-oxa- bicyclo[3.2.1]octane-3-carboxylic acid
A solution of (4.4 mmol) of the product of step D in 25 mL of ethanol is
treated with
2.5 g of 50% water wet 10% palladium on carbon catalyst and shaken under 53
psi of
hydrogen for 48 hours. The catalyst is removed by filtration and washed with
ethanol (4 x 25
mL). The filtrate and washings are combined and concentrated under vacuum to
crude
product.
F) 3-{(3-Chlorocarbonyl-8-oxa-bicyclo[3.2.1]oct-3-yl)-[4-(4-fluoro-phenoxy)-
benzene sulfonyl]-amino}-propionic acid ethyl ester
A solution of 15.1 mmoles of the product from Step E in 73 mL of
dichloromethane is
treated with 1.4 mL (17 mmol, 1.1 equivalents) of oxalyl chloride and 0.02 mL
(0.3 mmol, 0.02
equivalents) of dimethylformamide at ambient temperature, causing some
bubbling, and is
stirred overnight. The resulting solution of the title compound is used in
step G without
isolation.
G) 3-([4-(4-Fluoro-phenoxy)-benzenesulfonyl]-(3-hydroxycarbamoyl-8-oxa-
bicyclo[3.2.1]oct-3-yl)-amino]-propionic acid ethyl ester
A solution of (19.7 mmol, 1.3 equivalents) of hydroxylamine hydrochloride in
9.2 mL
(114 mmol, 7.5 equivalents) of dry pyridine at 0 °C is treated with 5.8
mL (45 mmol, 3.0
equivalents) of trimethylsilyl chloride, causing white solids to precipitate.
The mixture is
allowed to warm to ambient temperature overnight. This mixture is then cooled
to 0°C and
treated with a solution of (15.1 mmol) of the product from Step F in 73 mL of
dichloromethane
causing an exotherm to about 8°C. This mixture is stirred at 0°C
for 30 minutes and at
ambient temperature for about one hour. The reaction is then treated with 50
mL of 2N
aqueous hydrochloric acid and was stirred at ambient temperature for one hour.
The
aqueous phase is extracted with dichloromethane and the combined organic
phases are
washed with 2N aqueous hydrochloric acid (2 x 50 mL) and water (50 mL). This
solution of
the title compound in dichloromethane is used in the next step.
(H) 3-[[4-(4-Fluoro-phenoxy)-benzenesulfonyl]-(3-hydroxycarbamoyl-8-oxa-
bicyclo[3.2. 1]oct-3-yl)-amino]-propionic acid
A solution of 15.1 mmoles of the product from Step G in dichloromethane is
concentrated by rotary evaporation with the addition of 75 mL of toluene. This
solution is
treated with 75 mL of water, cooled to 0°C, and treated with 6.05 g
(151 mmol, 10 equivalents)
of sodium hydroxide pellets over 10 minutes with vigorous stirring. This
mixture is stirred for
15 minutes at 0°C and warmed to ambient temperature over one hour. The
aqueous phase is
separated, diluted with 7.5 mL of tetrahydrofuran, cooled to 0°C, and
treated with 33 mL of 6N
aqueous hydrochloric acid over 20 minutes. This mixture is stirred with 75 mL
of ethyl acetate
CA 02268684 1999-04-08
-22-
at 0°C to ambient temperature, and the ethyl acetate phase is separated
and washed with
water. The ethyl acetate solution was concentrated to yield the title
compound.
PREPARATION 1
4-/4-Fluorophenoxy)benzenesulfonic Acid 4-Fluorophenyl Ester
A solution of 14.68 g (0.131 mol, 2.0 equivalents) of potassium tert-butoxide
in 27 mL
of dry N-methylpyrrolidinone was treated with a solution of 15.39 g (0.137
mol, 2.1
equivalents) of 4-fluorophenol in 27 mL of dry N-methylpyrrolidinone at
ambient temperature
causing a mild exotherm to 45°C. A solution of 13.81 g (0.065 mol) of 4-
chlorobenzenesulfonyl chloride in 27 mL of dry N-methylpyrrolidinone was
slowly added to the
dark reaction mixture causing a mild exotherm to 44°C. The resulting
mixture was stirred at
room temperature for one hour and then at 130°C for 11 hours. The
cooled reaction mixture
was treated with 162 mL of water, seeded with a trace of 4-(4-
fluorophenoxy)benzenesulfonic
acid 4-fluorophenyl ester, and granulated at room temperature overnight. The
resulting solids
were filtered yielding 20.24 g (85%) of 4-(4-fluorophenoxy)benzenesulfonic
acid 4-
fluorophenyl ester.
1 H NMR (CDCI3) b 7.74 (dd, J=7.0, 2.0 Hz, 2H), 7.14-6.97 (m, 10H). mp 78-83
°C.
PREPARATION 2
4-~4-Fluorophenoxy)benzenesulfonic Acid , Sodium Salt
To a slurry of 47.43 g (0.131 mol) of 4-(4-fluorophenoxy)benzenesulfonic acid
4-
fluorophenyl ester in 475 mL of ethanol was added 13.09 g (0.327 mol, 2.5
equivalents) of
sodium hydroxide pellets. This mixture was heated at reflux for three hours
and stirred
overnight at room temperature. The resulting solids were filtered yielding
37.16 g (98%) of 4-
(4-fluorophenoxy)benzenesulfonic acid, sodium salt.
1 H NMR (CD30D) b 7.73-7.78 (m, 2H), 7.05-7.13 (m, 2H), 6.99-7.05 (m, 2H),
6.90-
6.95 (m, 2H).
PREPARATION 3
4-/4-Fluorophenoxy)benzenesulfonyl Chloride
To a slurry of 15.0 g (0.052 mol) of 4-(4-fluorophenoxy)benzenesulfonic acid ,
sodium
salt, in 150 mL of dry toluene was added 11.3 mL (0.155 mol, 3 equivalents) of
thionyl chloride
and 0.04 mL (0.5 mmol, 0.01 equivalents) of dimethylformamide. The resulting
mixture was
stirred at room temperature for 48 hours, filtered through diatomaceous earth,
and
concentrated under reduced pressure to 40 mL. This solution was used without
further
purification to prepare 1-[4-(4-fluorophenoxy)benzenesulfonylamino]
cyclopentanecarboxylic
acid benzyl ester.
CA 02268684 1999-04-08
-23-
5 A 5.0 mL portion of this solution was concentrated to 1.77 g of 4-(4-
fluorophenoxy)benzenesulfonyl chloride as an oil, corresponding to a 96%
yield.
1 H NMR (CDC13) b 7.92-7.97 (m, 2H), 7.01-7.13 (m, 6H). A portion of similarly
prepared oil was crystallized from hexane, mp 80°C.