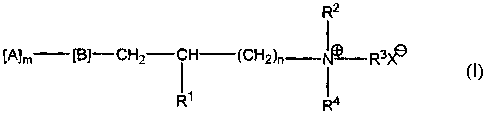Note: Descriptions are shown in the official language in which they were submitted.
CA 02268734 1999-04-07
GIUATERNARY NITROGEN CONTAINING AMPHOTERIC WATER
BOLUBLE POLYMERS AND THEIR USE IN DRILUNG FLUIDS
Field of The Invention
The present invention generally relates to quatemary nitrogen
containing amphoteric polymers and their use in drilling fluids.
Backaround of the Invention
The success of a well-drilling operation depends on many factors, none
of which is more important than the drilling fluid or mud. Drilling fluids
perform
a variety of functions which influence the drilling rate, cost, efficiency and
safety of the operation. More specifically, drilling muds prevent the influx
of
formation fluids into the wellbore, seal exposed permeable formations to
prevent leakage of the drilling fluid into the formation, maintain the
stability of
exposed formulations, cool and lubricate the bit and drill string, hold back
pressure and stabilize the formation, e.g., shale inhibition. How well the
drilling fluid fuffills these requirements greatly affects the cost of the
operation
and the productivity of the well.
During operation, drilling fluids are pumped down a hollow drill string,
through nozzles in the bit at the bottom of the weil, and back up the annulus
formed by the hole or casing and drill string to the surface. Once reaching
the
surface, the drilling fluid is passed through a series of vibrating screens,
settling tanks, hydrocyclones and centrifuges to remove formation debris
brought to the surface. It is thereafter treated with additives to obtain the
desired set of properties; pumped back into the well and the cycle is
repeated.
Drilling fluids are generally composed of liquids, e.g., water, petroleum -
oils, synthetic oils and other organic liquids; dissolved inorganic and
organic
additives; and suspended, finely divided solids of various types. Drilling
fluids
are classified as to the nature of the continuous phase; thus there are four
main divisions: gaseous (including foam), water-base, oil-base, or synthetic.
Growing concern among govemment and environmental agencies over the
environmental impact of drilling fluids has led to a significant increase in
the
CA 02268734 2007-12-07
industry's reliance on water-based muds. In fact, about 85% of all drilling
fluids
used today are water-based systems. The types depend on the composition of
the water phase (pH, ionic content, etc), viscosity builders (clays, polymers
or a
combination), filtration control agents (clays, polymers or a combination) and
other rheological control agents (deflocculants or dispersants (qv)).
Generally,
there are six main categories or types of water-based muds:
Fresh Water. Fresh water fluids range from clear water having no
additives to high density muds containing clays, barite, and various organic
additives. The composition of the mud is determined by the type of formation
to
be drilled. When a viscous fluid is required, clays and / or water-soluble
polymers (qv) are added. Fresh water is ideal for formulating stable drilling
fluids as many mud additives are most effective in a system of low ionic
strength. Inorganic and / or organic additives control the rheological
behavior of
the clays, particularly at elevated temperatures. Water swellable and water
soluble polymers and / or clays may be used for filtration control. Mud pH is
generally alkaline and, in fact, viscosity control agents like the
montmorillonite
clays are more efficient at a pH >9. Sodium hydroxide is by far the most
widely
used alkalinity control agent. Freshwater muds can be weighted with insoluble
agents to desired density required to control formation pressures.
Seawater. Many offshore wells are drilled using a seawater system
because of ready availability. Seawater muds generally are formulated and
maintained in the same way that a freshwater mud is used. However, because
of the presence of dissolved salts in seawater, more electrolyte stable
additives
are needed to achieve the desired flow and filtration (qv) properties.
Salt Water. In many drilling areas both onshore and offshore, salt beds or
salt domes are penetrated. Saturated salt muds are used to reduce the hole
enlargement that would result from formation-salt dissolution by contact with
an
undersaturated liquid. In the United States, the salt formations are
2
DOCSMTL: 2470480\1
CA 02268734 1999-04-07
primariiy made up of sodium chioride. In other areas, e.g., northem Europe,
the salt may be composed of mixed saits, predominantly magnesium and
potassium chlorides. It has become quite common to use high (20-23 wt%
NaCI) salt muds in wells being driiied in deep (>500-m water depth) water
regions of the Gulf of Mexico. The reasons are twofold: stabilization of water-
sensitive shales and inhibition of the formation of gas hydrates. The high
salinity of salt water muds may require different clays and organic additives
than those used in fresh- or seawater muds. Salt water clays and organic
polymers contribute to viscosity. Filtration properties are adjusted using
starch (qv) or cellulosic polymers. The pH ranges from that of the makeup
brine which may be somewhat acidic, to 9-11 through use of sodium
hydroxide or lime.
Calcium Treated. Fresh- or seawater muds may be treated with
gypsum or lime to alleviate drilling problems that may ariso from drilling
water-
sensitive shale or clay-bearing formations. Gyp muds (gypsum added) are
generally maintained at a pH of 9-10, whereas lime muds (lime added) are in
the 12-13 pH range. Calcium-treated muds generally require more additives
to control flow and filtration properties than those without gypsum or lime.
Potassium Treated. Generally potassium treated systems combine
one or more polymers and a potassium ion source, primariiy potassium
chloride, In order to prevent problems associated with drilling certain water-
sensitive shales. The flow and filtration properties may be quite different
from
those of the other water-base fluids. Potassium muds have been applied in
most active drilling regions around the world. Environmental regulations in
the United States have limited the use of potassium muds in offshore drilling
owing to the apparent toxicity of high potassium levels in the bioassay test
required by discharge permits.
Low Solids. Fresh water, clay, and polymers for viscosity
enhancement and filtration control make up low solid and so called non-
dispersed polymer muds. Low solids muds are maintained using minimal
amounts of clay and require removal of all but modest quantities of drill
solids.
3
CA 02268734 2007-12-07
Low solid muds can be weighted to high densities, but are used primarily in
the
unweighted state. The main advantage of these systems is the high drilling
rate
that can be achieved because of the lower colloidal solids content. Polymers
are used in these systems to provide the desired rheology, especially xanthan
has proven to be an effective solids suspending agent. These low solid muds
are normally applied in hard formations where increasing the penetration rate
can reduce drilling costs significantly and the tendency for solids buildup is
minimal.
Bentonite is by far the most commonly used clay in drilling muds because it
provides excellent rheological and filtration properties to the mud,
especially in
combination with polyelectrolytes like CMC. Bentonite clay which mainly is
montmorillonite (a smectite type of clay) exists as very thin platelets
(sheets). A
number of attempts have been made to determine the particle size of sodium
montmorillonite but this is rather difficult because of the flat, thin
irregular shape
of the platelets and because of the wide range of sizes. The clay platelets
exhibit a superior ability to swell uniformly in fresh water upon shear
application.
The swelling of the dehydrated agglomerated bentonite clay when it is
contacted with water is caused by a penetration of water molecules in between
the clay platelets. The swelling pressure is so strong that the layers
separate
into smaller aggregates and even into individual unit layers with a thickness
of
10 A. Thus, a relatively stable suspension of the hydrated clay can be
obtained.
In aqueous suspensions, the edges of the platelets of smectite type clays,
such
as bentonite, are positively charged while the faces are negatively charged.
Because of these opposite charges there is an interaction with the positive
edges and negative faces. However in a fresh water hydrated clay suspension
(without electrolytes) these electrostatic interactions are rather weak
because of
the thick bounded water layer around the clay platelets. This thick water
layer
keeps the particles that far from each other that the clay is almost
completely
dispersed but still a very weak flocculation remains as is shown by the gel
properties and yield stress.
4
DOCSMTL: 2470480\1
CA 02268734 1999-04-07
A wide variety of organic polymers also serve a number of useful
purposes In drilling fluids such as increasing viscosity and controlling
filtration
rates, which often are directly related to the degree of flocculation and
aggregation of the bentonite clay particles in the drilling mud. The ability
to
reduce fluid loss is also influenced by these properties, i.e., in order to
build
up a good filtercake to minimize filtrate loss into the formation, the clay
suspension should be in a deflocculated condition. These polymers are either
natural polysaccharides, e.g., starch, guar gum, xanthan gum, and other
biopolymers; or derivatives of natural polymers, e.g., derivatives of
cellulose,
starch, guar and other biopolymers; or lignosutfonate, lignite and synthetic
polymers, e.g., polymers and copolymers of acrylic acid, acrylonitrile,
acrylamide, and 2-acrylamido-2-methylpropanesulfonic acid (AMPS). The
most commonly used polymeric viscosity builders are the cellulosics,
starches, xanthan gum, and polyacrylamides.
Sodium carboxymethyl cellulose (CMC) and polyanionic
cellulose(PAC) are two of the more widely used anionic polymers in drilling
fluids In order to control viscosity and filtration rates. The effectiveness
of
CMC, being a polyelectrolyte, as a viscosity builder has its limitations,
however, as its effectiveness decreases with increasing electrolyte
concentration_ In fresh water low DS CMC's adsorbs on bentonite while
higher DS CMC's (e.g. PAC's) shows a decreased amount of adsorption. Only
a very small amount of a low DS CMC (DS - 0.7) is sufficient to realize
complete dispersion of the bentonite as it adsorbs on the positive edges of
the
platelets. This complete dispersion results in a reduction of the gel-strength
to
almost zero. In general a good dispersed bentonite/CMC suspension gives a
good build-up of the filter-cake and an excellent fluid loss reduction
performance Is obtained. Such a system does not, however, demonstrate
significant gel-strength and yield point.
It is clear that the rheological properties of a drilling fluid determines
very much the success of the overall drilling operation. The rheological
properties of the mud determine very much (1) the hole cleaning efficiency
and hole stability, (2) cuttings suspension efficiency, (3) mud hydraulics
5
CA 02268734 1999-04-07
performance, (4) ease of mud handling operations, and (5) rate of penetration.
The rheological requirements for these diverse purposes may often conflict,
so it is necessary to optimize the mud properties in order to obtain the best
overall performance. Optimal rheological properties are necessary in order to
carry the drilled cuttings efficiently to the surface. While drilling, the
high shear
viscosity should not be too high to allow an efficient transmission of the
hydraulic horsepower of the drilling fluid to the drill bit. But when the
circulation is slow or interupted the viscosity should be high and the gel-
strength should be sufficient to prevent / minimize settling of the cuttings.
Such rheology profile can be obtained by using a thixotropic system, which in
case of a bentonite system can be realized with a somewhat flocculated
system at which the particle links are temporarily broken by/during stirring
and
are only restored during rest.
On the other hand the drilling fluid should cover the wall of the borehole
with a
thin filtercake in order to stabilize the borehole and to prevent loss of the
circulation fluid into the driiled porous formation. In general this is done
most
efficiently by a well dispersed clay suspension.
The rheology and thus the flow properties of a drilling mud is Influenced
by:
1. The state of hydration and dispersion of bentonite or other clay particles
in
the aqueous phase and the'amount of them.
2. The rheology of the aqueous phase as it might be modified by additives
(viscosifiers) e.g. by the polymers mentioned earlier.
3. The clay interparticle forces as well as by the interaction of the polymers
with the clay and / or with each other.
Thus the solids content is related to the mud rheology as well as to the mud
density. And in general the higher the solid content of a mud (added clays and
driiled cuttings which might become dispersed) the more difficult and time
consuming the mud cleaning will be. in this respect low-solid mud systems
can improve the rate of penetration.To maintain the desired rheological
profile
for such low-solid mud systems viscosifying polymers are added. Examples of
such polymers are xanthan, high molecular weight CMC's and acrylic
polymers.
6
CA 02268734 2008-01-09
The claimed invention relates to the use of quaternary nitrogen containing
amphoteric water-soluble polymers which proved to be very efficient drilling
fluid
rheology modifiers especially for bentonite containing system while it allows
very low
uses of bentonite while providing the desired drilling fluid characteristics.
The additives
of the invention improve the overall performance of the mud by enhancing the
following
properties:
= a pseudoplastic rheology behaviour
= a sufficient Yield Point and gel-strength with a fast build up of the gel-
strength in the
first 10 sec
= a low to moderate Plastic Viscosity (PV)
= sufficient fluid loss reduction
Summary of the Invention
The present invention generally relates to a water-based drilling fluid
composition
which comprises water, at least one viscosity builder, and at least one
rheological control
agent. The rheological control agent is preferably an amphoteric polymer
containing
both cationic groups and anionic groups, wherein said cationic groups comprise
quaternary ammonium groups. Preferred amphoteric polymers are quaternary
nitrogen
containing water soluble polymers (QN-WSP's) such as quatemary nitrogen
containing
amphoteric carboxymethyl cellulose: The viscosity builder is preferably a
smectite type
of clay (e.g. bentonite).
The invention also contemplates a water-based drilling fluid system which
comprises, as a rheological control agent, a combination of a cationic polymer
wherein
the cationic groups comprise quatemary ammonium groups and an anionic polymer,
with
or without the aforementioned amphoteric polymer.
Thus, in one aspect of the invention, there is provided a water-based drilling
fluid
composition which comprises water, at least one clay viscosity builder and at
least one
7
DOCSMTL: 2573180\1
CA 02268734 2008-04-16
rheological control agent, wherein said at least one rheological control agent
is an
amphoteric polymer of formula (I):
R2
[A]m [B]-CH2-CH (CHZ)n I R3X11
11 1
R R4
wherein R' is H or OH, R2, R3 and R4 are the same or different and are
selected from the
group consisting of Cl -C24 alkyl, C6 -C24 aryl, C7 -C24 aralkyl, C7 -C24
alkaryl, C3 -C24
cycloalkyl, C2 -C24 alkoxyalkyl and C7 -C 24 alkoxyaryl groups, or R2, R3, R4,
and the
quatemary nitrogen atom form an aliphatic or aromatic heterocyclic ring; n is
an integer
of I to 4, B is 0, OC(O), C(O)O, C(O)-NH, NHC(O), S, OS03, OP03, NH, or NR5
where
R5 is a C2 -C6 acyl, or a C, -C4 alkyl radical, (A)m is an anionic water-
soluble
polysaccharide or substituted polysaccharide, m is greater than 10 and X- is
an anion.
In another aspect of the invention, there is provided a water-based drilling
fluid
composition which comprises water, at least one clay viscosity builder and at
least one
rheological control agent, wherein said at least one rheological control agent
is an
amphoteric synthetic polymer derived from copolymerizing an anionic synthetic
monomer, a quatemary nitrogen atom containing cationic monomer, and optionally
at
least one nonionic monomer, said amphoteric polymer being selected from the
group
consisting of (partially) hydrolyzed polyacrylamide containing quatemary
ammonium
groups, polyacrylate containing quatemary ammonium groups, and (partially)
hydrolyzed
polyacrylamide containing quatemary ammonium groups copolymerized with
acrylate
monomers and mixtures thereof.
7a
DOCSMTL: 2695555\l
CA 02268734 2008-01-09
7b
In still another aspect of the invention, there is provided a water-based
drilling
fluid composition which comprises water, at least one clay viscosity builder
and at least
one rheological control agent wherein said rheological control agent comprises
a
combination of at least one cationic polymer having cationic groups which are
quaternary
ammonium groups and an anionic polymer.
Detailed Description of the Invention
The present invention generally relates to a water based drilling fluid
composition
which comprises water, at least one viscosity builder, and at least
7b
DOCSMTL: 2573180\1
CA 02268734 1999-04-07
one rheological control agent. The rheological control agent Is preferable an
amphoteric polymer containing both cationic groups and anionic groups,
wherein said cationic groups comprise quaternary ammonium groups, i.e.,
amphoteric quatemary nitrogen containing water soluble polymers (QN-
WSP's). In another embodiment, the invention contemplates a water-based
drilling fluid system which comprises, as a rheological control agent, a
combination of a cationic polymer wherein the cationic groups comprise
quaternary ammonium groups and an anionic polymer, with or without the
aforementioned amphoteric polymer. The drilling fluids of the present
invention can comprise QN-WSP's either alone, or in combination with
conventional polymer additives.
As a viscosity builder, the drilling fluid compositions of the present
Invention can contain any commercial clay used for increasing the viscosity of
drilling fluids. Preferably, smectite type clays such as montmorillonites
(bentonite) are employed, as well as mixed layer types and attapulgite and
sepiolite. Bentonite is the most preferred viscosity builder either alone or
in
combination with other commercial clays. Without wishing to be bound by any
particular theory, it is believed that because of the strong interaction of
the
cationic groups of the QN-WSP's of the present invention with the bentonite
particles of the drilling mud, the QN-WSP's of the present Invention are very
efficient in providing sufficient gel-strength and yield stress to a fresh
water
bentonite based mud.
The QN-WSP's of the present invention can be divided into two broad
types: those containing synthetically derived water soluble polymers, and
those containing semi-synthetically or natural water soluble polymers, e.g.,
polysaccharide derivatives.
The amphoteric QN-WSP's of the present invention comprising a
polysaccharide derivative have the following general formula:
8
CA 02268734 2007-12-07
R2
[A], -[B]-CH2-CH-(CH2)õ - N+ - R3 X- (I)
R~ R4
wherein R' is H or OH, R2, R3 and R4are the same or different and are selected
from Cl-C24 alkyl, C6-C24 aryl, C7-C24 aralkyl, C7-C24 alkaryl, C3 -C24
cycloalkyl,
C2-C24 alkoxyalkyl and C7-C24 alkoxyaryl groups, or R2, R3 , R4 and the
quaternary nitrogen atom form an aliphatic or aromatic heterocyclic ring like
a
pyridinium ring and the like; n is an integer of 1 to 4, B is selected from 0,
OC(O), C(O)O, C(O)-NH, NHC(O), S, OSO3, OPO3, NH, or NR5 where R5 is a
C2-C6 acyl, or a Cl-C4 alkyl radical, [A]r,, is a anionic water-soluble
polysaccharide or polysaccharide derivative, m is greater than 10, preferably
10-50,000 and still more preferably 10-30,000, and X- is an anion.
By way of example, X- may be selected from the group consisting of
chloride, bromide, iodide, sulphate, methylsulphate, nitrate, phosphate,
acetate
and mixtures thereof.
Preferred polysaccharide starting materials for the amphoteric QN-WSP
include but are not limited to members of the starch and cellulose families,
the
natural gums such as guar, bio-derived gums such as xanthan, and the like.
Typically, the amphoteric polysaccharides contain or have been provided with
an anionic and a cationic group and may contain also a non-ionic group or
substituent. The most preferred polysaccharide starting materials include but
are not limited to water soluble or swellable anionic cellulose ethers or
esters,
starch or starch derivatives, and/or anionic guar or guar derivatives. It
should
also be understood that substituted polysaccharides (e.g., carboxymethylated
polysaccharides) are within the scope and meaning of polysaccharide starting
materials.
The anionic group of the amphoteric polysaccharide preferably is a
carboxylate (e.g. carboxymethyl), sulphonate (e.g. sulphoethyl), phosphate or
phosphonate group, although one of ordinary skill in the art will recognize
that
other anionic groups can be readily employed. The most preferred anionic
polysaccharide starting materials include, but are not limited to anionic
group
9
DOCSMTL: 2470480\1
CA 02268734 2007-12-07
containing polysaccharides or polysaccharide derivatives including but not
limited to carboxymethyl cellulose, sulphoethyl carboxymethyl cellulose,
carboxymethyl hydroxyethyl cellulose (CM-HEC), carboxymethyl cellulose
wherein the cellulose is substituted with one or more nonionic substituents,
carboxymethyl starch, carboxymethyl starch wherein the starch is substituted
with one or more nonionic substituents, carboxymethyl guar, carboxymethyl
guar wherein the guar is substituted with one or more nonionic substituents,
xanthan gum, and mixtures thereof. A particularly preferred anionic
polysaccharide starting material is carboxymethyl cellulose.
The amphoteric polysaccharide starting materials are provided with a
quaternary nitrogen-containing group through various methods known to those
of ordinary skill in the art. For example, the polysaccharide starting
material
can be quaternized with a quaternization agent which are quaternary
ammonium salts to effect substitution of the polysaccharide chain with a
quaternary nitrogen-containing group. In this regard, applicants refer to U.S.
Patent No. 4,940,785.
Typical quaternary ammonium salts which can be utilized include quaternary
nitrogen-containing halides, halohydrins and epoxides. The quaternary
ammonium salt may contain hydrophobes. Exemplary ammonium salts include
one or more of the following:
3-chloro-2-hydroxypropyl dimethyldodecyl ammonium chloride; 3-chloro-2-
hydroxypropyl dimethyloctadecyl ammonium chloride; 3-chloro-2-hydroxypropyl-
dimethyloctyl ammonium chloride; 3-chloro-2-hydroxypropyl trimethyl
ammonium chloride; 2-chloroethyl trimethyl ammonium chloride; and the like.
Preferred quaternization agents include 2, 3-epoxypropyl trimethyl
ammonium chloride , 3-chloro-2-hydroxypropyl trimethyl ammonium chloride;
3-chloro-2-hydroxypropyl dimethyldodecyl ammonium
DOCSMTL: 2470480\1
CA 02268734 1999-04-07
chioride; 3-chloro-2-hydroxypropyl dimethyltetradecyl ammonium
chioride;3-chloro-2-hydroxypropyl dimethylhexadecyl ammonium
chloride and 3-chloro-2-hydroxypropyl dimethyioctadecyl
ammonium chloride.
Quaternization can also be achieved using a two-step synthesis of
aminating the polysaccharide by reaction with an aminating agent, such as an
amine halide, halohydrin or epoxide followed by quatemizing the product by
reaction with quatemizing agents or mixtures thereof, containing a functional
group which forms a salt with the amine. Preferred quatemizing agents
Include but are not limited to alkyl halides such as methyl-, ethyl-, propyl-,
and
benzyl- halides. The alkylation with the quatemizing agent to form the
quatemized polysaccharide ethers may be conducted in a separate reaction
step or may be combined in the etherification with the other alkylating
agents.
The polysaccharides may also contain non-ionic groups or substituents
to assist in providing the requisite hydrophilicity and / or hydrophobicity
and /
or the requisite electrolyte stability. To provide the polysaccharide with
such a
non-ionic substituent the polysaccharide may be alkylated with suitable
alkylating agents or mixtures thereof using processes which are known in the
art. Preferred non-ionic substituents include but are not limited to methyl,
ethyl, hydroxyethyl, hydroxypropyl, dihydroxypropyl, benzyl, and hydrophobic
groups such as 3-(Cl-C24 aikoxy)-2-hydroxypropyl, 3-(C6-C24 aryloxy)-2-
hydroxypropyl, 3-(CrC24 aralkoxy)-2-hydroxypropyl, 3-(C7-C24 alkaryloxy)-2-
hydroxypropyl groups, and halides, epoxides and/or glycidylethers of Cl-C24
alkyl, C1-024 alkoxy, C6-C24 aryloxy, CrC24 aralkoxy, C7-C24 alkaryloxy and
mixtures thereof. Substitution with these non-ionic substituents may be
conducted in a separate reaction step or may be combined in the
etherification with the other alkylating agents. The several used substituents
may therefore be provided as substituents connected directly to the
polysaccharide chain, the quatemary nitrogen or connected to other available
ether substitutents. Alkylation is achieved by reacting an alkylating agent or
mixtures thereof containing at least one functional group which is reactive
(1)
with the hydroxyl groups on the polysaccharide chain or ether substituents, or
11
CA 02268734 1999-04-07
(2) with a tertiary nitrogen atom, producing a quatemary substituent, or (3)
both.
The most preferred amphoteric QN-WSP's comprising a
polysaccharide derivative include, but is not limited to any quaternary
ammonium group (QN) containing polysaccharide derivatives such as, for
example, QN-CMC, QN-sulphoethyl CMC, QN-CM-hydroxyethylcellulose, QN-
CM cellulose (mixed) ether, ON-CM starch and ON-CM starch derivatives,
ON-CM guar and ON-CM guar derivatives, QN phosphate or phosphonate
containing polysaccharide derivatives and the like.
In another embodiment, the present invention contemplates water-
based drilling fluids which utilize, as a rheological control agent, an
amphoteric QN-WSP's comprising a synthetic polymer derivative. These QN-
WSP's comprise synthetic polymer derivatives which are prepared by
copolymerizing anionic synthetic monomers (e.g., acrylates and/or
acrylamides) with quaternary nitrogen containing cationic monomers (e.g.,
diallyl-dimethyl-ammonium chloride (DADMAC)) and which also may be
copolymerized with nonionic monomers. Instead of copolymerization with an
anionic monomer the amphoteric synthetic QN-WSP may also be derived by
copolymerization of a cationic monomer with a non-Ionic monomer which is at
least partly hydrolyzed in a post-polymerization step resulting in an
amphoteric polymer. In the QN-WSP the different monomers can be
randomly distributed as well as block structures can be used in the context of
the invention. The quaternary ammonium groups are incorporated in the
polymer by using cationic monomers in the polymerization process or by a
cationization reaction afterwards.
Suitable cationic starting monomers include but are not limited to diallyl
dimethyl ammonium chloride (DADMAC) and its derivatives and those of the
formula:
12
CA 02268734 2008-04-16
R6
I
H2C CI
C O (II)
R7
wherein R6 is selected from H, OH or a CI-C4 alkyl group, or a hydroxyethyl-
group; Z is
NH or 0 and R7 is a group of formula III:
R8
O
LYI-N R9 X" (III)
I10
wherein Y is a linear or branched CI-C6 alkylene, R8, R9 and R10" are the same
or different
and are selected from CI-C24 alkyl, C6-C24 aryl, C7-C24 aralkyl, C7-C24
alkaryl, C3-C24
cycloalkyl, C2-C24 alkoxyalkyl, and C7-C24 alkoxyaryl groups, or R8, R9 and
R10 and the
quaternary nitrogen atom form an aliphatic or aromatic heterocyclic ring such
as a
pyridinium and the like, and X is an anion like chloride, bromide, iodide,
sulphate,
methylsulphate, nitrate, phosphate, acetate and the like.
Preferred quaternary ammonium containing monomers include diallyl dimethyl
ammonium chloride (DADMAC), acryloyloxyethyl-trimethyl ammonium chloride
AETAC), methacrylamido propyl trimethyl ammonium chloride (MAPTAC), 3-
acrylamido-3-methyl-butyl-trimethyl ammonium chloride and the like.
Suitable anionic groups for the amphoteric synthetic QN-WSP include, but are
not
limited to carboxylate, sulphonate, phosphate and phosphonate groups. A
suitable way to
introduce these groups into the amphoteric polymer is by copolymerization of
these
anionic monomers or monomers which yield an anionic group by partial or
complete
hydrolyses in a post-polymerization step with cationic monomers and possibly
non-ionic
monomers.
13
DOCSMTL: 2695555\1
CA 02268734 1999-04-07
Preferred anionic monomers include but are not Iimited to acrylic acid esters,
methacrylic acid esters, acrylamide, methacrylamide, and maleic acid
anhydride, wherein said synthetic monomers yield an anionic group in a post
polymerization step, acrylic acid, acrylates, methacrylic acid, 2-acrylamido-2-
methyi-propane sulphonic acid, vinyisulphonate, vinyisulphonic acid,
styrenesulphonate, styrene sulphonic acid, and mixtures thereof. The
different monomers can be randomly distributed as well as block structures
can be used in the context of the invention.
To regulate the charge density and the hydrophilic / hydrophobic
balance of the amphoteric synthetic polymer, non-ionic monomers are
optionally copolymerized with said cationic monomers and anionic monomers.
Some non-ionic monomers such as acrylamide and maleic acid anhydride
may yield anionic groups as they may be partly or completely hydrolized
during or after the polymerization. Suitable non-ionic monomers are
acrylamide and its derivatives, acrylates such as acrylic acid esters or
methacrylic acid esters, maieic acid anhydride and its derivatives and other
non-ionic vinyimonomers. These monomers may also contain hydrophobic
groups such as alkyl, aryl, aralkyl and alkaryl groups containing 1- 24 carbon
atoms. Preferred non-ionic monomers are acrylamide, methyl acrylate, methyl
methacrylate, ethyl acrylate, ethyl methacrylate, hydroxyethyl acrylate,
hydroxyethyl methacrylate, hydroxypropyl acrylate, hydroxypropyl
methacrylate, butyl acrylate, butyl methacrylate, vinylacetate, styrene and
mixtures thereof.
The synthetic polymer containing QN-WSP's of the present invention
can be prepared by various means which is readily apparent to one of
ordinary skill in the art. For example, in order to produce high molecular
weight amphoteric or cationic polymers, acrylamide and / or acrylic acid is
copolymerized with varying proportions of amino derivatives of acrylamide,
acrylic acid esters or methacrylic acid esters. The cationic charge is present
in the form of a mineral acid or quatemary ammonium salt. Typical
quaternary ammonium containing co-monomers include diallyl dimethyl
14
CA 02268734 1999-04-07
ammonium chloride (DADMAC), acryloyloxyethyl-trimethyl ammonium
chloride (AETAC), methacrylamido propyl trimethyl ammonium chloride
(MAPTAC), 3-acrylamido-3-methyl-butyl-trimethyl ammonium chloride and the
like.
In a most preferred embodiment of the invention, the amphoteric QN-
WSP's comprising a synthetic polymer derivative preferable contain on
average 1 to 500 quatemary ammonium containing monomers on every 1000
monomer units, or more preferably 10 to 300 on every 1000 monomer units
and most preferably 50 to 200 quaternary ammonium containing monomer
units per total of 1000 monomer units, and it contains preferably 1 to 900
anionic group containing monomer units on every 1000 monomer units and
most preferably 100 to 600 anionic group containing monomer units on every
-
1000 monomer units, and the polymer preferably has a average molecular
weight of > 50,000, more preferably > 250,000 and most preferably >
500,000.
In a most preferred embodiment, the present invention contemplates a
quatemary nitrogen containing carboxymethyl cellulose (QN-CMC). ON-CMC
Is an amphoteric, water-soluble polymer containing both anionic and cationic
charges. But also other zwitterionic polymers (polymers with the positive and
negative charges on the same pendant groups, e.g. betaines) or on the same
backbone (ampholytes) can be used. The betaines necessarily have an equal
balance of anionic and cationic groups. Amphoteric polymers such as QN-
CMC which actually is a polyampholyte (having the positive and negative
charge on the same polymer backbone) can be charge-balanced or charge-
imbalanced. Also mixtures can be used of cationic- and anionic polymers
(e.g. ON-Starch, QN-HEC and QN-Polyacryfamides with CMC or PAC) which
form an amphoteric solution and Interpolymer complexes can be formed.
When the stoichiometry of the charges is 1:1 or close to, a water insoluble
poly-salt can be formed, dependant on the presence and concentration of
other electrolytes. In the context of the invention charge-ratio's and
electrolyte
concentrations are chosen in such a way that a soluble system is obtained.
CA 02268734 1999-04-07
Amphoteric polymers show unusual solution properties. Charge
balanced poly-ampholytes often are more soluble and show higher viscosities
in salt than in pure water soiution. Therefore amphoteric polymers have found
utility as water and brine viscosifiers and as brine drag reduction agents. In
all of these applications unusual interplay of positive and negative charges
on
the same group or backbone, between chains and/or between chains and
extemal electrolytes play an important role. These charge interactions in the
different (electrolyte) environments determine very much resulted viscosity of
the solutions.
The most important commercial clays used for increasing the viscosity
of drilling fluids are the smectite type of clays such as montmorillonites
(bentonite), as well as mixed layer types and attapulgite and sepiolite.
Although the QN-WSP's of the present invention can usefully be employed as
rheology modifiers in most water-based drilling fluids, they are most useful
in
those systems which contain bentonite either alone or in combination with
other commercial clays.
As previously mentioned, the state of flocculation and aggregation are
the most important factors in determining the rheology of a drilling mud. The
ability to reduce the fluid loss is also determined by these properties. CMC
is
one of the most popular polymers in use to disperse the bentonite in drilling
muds which improves fluid loss reduction and to a lesser extent, to modify the
viscosity of the mud. CMC does not, however, provide the desired yield point
and gel-strength to the mud. Because of the strong interaction of the cationic
groups of QN-CMC with the bentonite particles, QN-CMC is a very efficient
material to provide sufficient gel-strength and yield stress to a fresh water
bentonite based mud. More particularly, the strong interaction of QN-CMC
with the bentonite particles causes bridging flocculation which gives the
desired rheology. Additionaliy, the extent of flocculation can be regulated by
the DS(QN), i.e., the more cationic groups attached to the cellulose backbone
the more pronounced the flocculation. However, while increased flocculation
is beneficial for rheology It is not for the fluid loss reducing capability
because
the more the system is flocculated, the less It provides sufficient fluid loss
16
CA 02268734 2007-12-07
reduction. For example, a QN-CMC with a rather low MS(QN) which gives a
weakly flocculated system which gives the desired gel-strength and yield
stress
and also gives sufficient fluid loss reduction. It also has been found that by
using a high molecular weight QN-CMC the amount of smectite type clay used
can be reduced. Another advantage of the QN-CMC is that it is compatible with
commonly used (anionic) polymers like CMC, PAC and starch or other generally
used fluid loss reducing and viscosifying polymers, which means that in cases
where the fluid loss should be reduced further such materials can be used
together with QN-CMC.
To prepare QN-CMC one can start either with cellulose or with the cellulose
already reacted with monochloro-acetic acid or its (sodium) salt. To provide
the
cellulose with the cationic group an etherification reaction in the presence
of a
small amount of caustic soda is performed with either CHPTAC or GTAC.
CH2 - CH - CH2 - N+ -(CH3)3 CI- CH2 - CH - CH2 - N+ -(CH3)3 CI-
~
CI OH 0
CHPTAC Glycidyl trimethyl ammonium chloride (GTAC)
(chloro-hydroxy compound) (epoxide compound)
Using CHPTAC the etherification reaction goes also via the epoxide.
The difference with GTAC is that one equivalent caustic soda will be consumed
to form the epoxide and one equivalent NaCl will be produced. The reaction
of GTAC with CMC needs only a catalytic amount of caustic soda. In case
the etherification is performed in the sequence of first reacting the
alkalicellulose with monochloroaceticacid (MCA) after which the etherification
with CHPTAC or GTAC is performed without a purification of the CMC, some
of the CHPTAC or GTAC might also react with the byproducts of the MCA-
etherification. This is also the case when the etherification with MCA and
17
DOCSMTL: 2470480U
CA 02268734 1999-04-07
CHPTAC or GTAC are performed at the same time. Other QN-(CM)-
Polysaccharides can be derived in the same way as QN-CMC is prepared and
processes to do so are known in the art.
Production of water swellable and water soluble polysaccharide ethers
in general is done by suspending the polysaccharide in a diluent . When the
polysaccharide is cellulose it may be used as milled cellulose or cutted
cellulose sheets. Suitable and readily available cellulose starting materials
include cotton linters and pur'rfied wood pulp celluloses. Suitable diluents
include ethanol, isopropyl alcohol, tert-butyl alcohol, acetone, water,
methylethyl ketone and mixtures thereof. The reactions may be conducted in
a relatively large amount of diluent or with a minimal amount of diluent as
desired, i.e., using either a so called slurry or a so called dry process.
Typically, the polysaccharide is reacted with an alkali metal hydroxide
to prepare an alkali metal polysaccharide. The amount of alkali metal
hydroxide per saccharide repeating unit may vary, depending on the type and
amount of alkylating agents used. Typically, a molar ratio of between 0.001
and 5, respectively, is used. If desired, during the alkylation additional
alkali
metal hydroxide can be added or excess of the alkali metal hydroxide can be
neutralized. To prevent uncontrolled degradation of the (alkalized)
polysaccharide polymer, it is preferred to exclude oxygen from the reaction
vessel during the alkalization and alkylation.
After reaction of the polysaccharide with a suitable amount of the alkali
metal hydroxide, for the production of an amphoteric polysaccharide the alkali
metal polysaccharide may be, reacted first with the anionic alkylating
reagent,
e.g. monochloroacetic acid or its alkali metal salt, followed by the reaction
with
the cationic alkylating reagent, e.g. CHPTAC or GTAC, at a suitable
temperature and for a time sufficient to provide the desired level of
substitution. Altematively, the cationic alkylating reagent may be added
first,
after which the anionic alkylating reagent is allowed to react, or the alkali
metal polysaccharide may be simultaneously reacted with the different
alkylating reagents. A further altemative reaction path is to purify the
18
CA 02268734 1999-04-07
anionically alkylated polysaccharide before the cationic alkylating reagent is
added. This generally does increase the reaction selectivity and/or yield of
the
cationic alkylating reagent.
In another preferred embodiment of the present invention non-ionic
alkylating reagents are incorporated in the reaction step either added before,
after or together with the anionic and cationic alkylating reagents. Also here
the cationization may be performed after the already alkylated polysaccharide
has been purified.
In the context of the present invention the quatemary ammonium
modified polysaccharide derivatives may be applied either as purified or as
unpurified materials. Of the purified materials the byproducts have been
removed, e.g. by extraction with an alcohol/water mixture.
When the quaternary ammonium modified polysaccharide is an
amphoteric or cationic cellulose ether the material useful for the present
invention typically have an average degree of polymerization (MWpd~,ef =
MWmonome- unit ' DP) of greater than 30, preferably greater than 100 and
typically in the range of 30 to 6,000, preferably 100 to 5,000, an average
degree of substitution of (i.e. DS) of the anionic substituent in the range of
0.05 to 1.4, preferably 0.3 to 1.4, and a molar substitution (i.e. MS) of the
non-
ionic substituent in the range of 0.05 to 5, preferably 0.1 to 3.5, and a
molar
substitution of the cationic group In the range of 0.005 to 1.0, preferably
0.01
to 0.6.
In a preferred embodiment of the present invention the ON-WSP is an
amphoteric carboxymethyl cellulose (QN-CMC). The QN-CMC typically have
an average degree of polymerization of greater than 30, preferably greater
than 100 and typically in the range of 30 to 6,000, preferably 100 to 5,000,
more preferably 1000 to 5000, an average degree of substitution of (i.e. DS)
of the anionic carboxymethyl substituent in the range of 0.2 to 1.4,
preferably
0.5 to 1.4, more preferably 0.7 to 1.3, and a molar substitution of the
cationic
19
CA 02268734 1999-04-07
group in the range of 0.005 to 1.0, preferably 0.01 to 0.6, more preferably
0.01
to 0.3.
Finally, the claimed invention contemplates a water-based drilling mud
formulation which comprises, as a rheological control agent, a combination of
a cationic poiymer wherein the cationic groups comprise quaternary
ammonium groups and an anionic polymer, either alone or in combination
with the aforementioned amphoteric polymers.
The cationic polymers employable in the context of the present
invention include, but are not limited to cationic group containing
polysaccharides or polysaccharide derivatives, cationic group containing
synthetic polymers and mixtures thereof. Examples of cationic group
containing polysaccharides or polysaccharide derilvatives include but are not
limited to such as quaternary (ON) -HEC, QN-cellulose (mix d) ether, QN-
starch, QN-starch derivatives, QN-guar, ON-guar derivatives, mixtures thereof
and the like. Examples of cationic group containing synthetic polymers
include but are not limited to QN-polyacrylamide, polyacrylate homo- and
copolymers containing cationic groups, QN-polyacrylamide copolymerized
with non-ionic acrylate monomers, QN-polyamines, QN-polyethyleneimines,
mixtures thereof and the like.
These cationic polymers are utilized in combination with the anionic
polymers previously exemplified in this application, including the anionic
group
containing polysaccharides and the anionic group containing synthetic
polymers which are the building blocks of the amphoteric polymers of the
present invention.
Finally, the drilling fluids of the present invention can optionally contain
various other ingredients conventionally employed in water-based fluids. For
example, such additional ingredients can include conventional organic
polymers additives such as CMC, PAC, starch, modified starch, xanthan, etc.
Additionally, the drilling fluids of the present invention can contain
weighing
agents, thinners, inhibiting agents, electrolytes, pH adjusting agents, etc.
The
CA 02268734 1999-04-07
pH of the drilling fluids of the present invention are preferably maintained
at a
pH of from 8-11, preferable 8.5-10.5 and still more preferably 9-10.
Although there is a wide variety of water-based drilling fluids (muds)
employable in the context of the present invention, a typical mud in
accordance with the present invention contains the following ingredients:
= water as the main component;
= viscosity builder In an amount of from 5-100, more preferably 10-60
and most preferred 20-40 kg/m3 ;
= amphoteric rheological control agent in an amount of from 0_01 -
10, more preferably 0.05 - 5, and still more preferably 0.1 - 3 kg/m3
= alkaline agent(s) in an amount effective to adjust pH to about 9-10;
and
= various other additives/polymers including, but not limited to CMC,
PAC, starch, modified starch, xanthan, weighing agents, thinners,
inhibiting agents such as glycol derivatives and the like, electrolytes
and the like.
For a QN-CMC based drilling fluid, the drilling fluid would ideally have a
yield point of between 5 and 15 pascals, not develop progressive gel-
strengths over time, plastic viscosity of 10-25 mPa.s and an API RP42 fluid
loss of 5-10mL. A QN-CMC based drilling fluid having these properties would
typically be comprised of fresh water, '0.2-0.6 kg/m3 of QN-CMC, 20-40 kg/m3
unpeptized sodium bentonite and sufficient caustic soda to adjust the pH of
the drilling fluid to a pH of from 8.5 to 10,5.
When a combination of a quatemary ammonium containing cationic
polymer and a anionic polymer is used the QN-WSP preferably has on
average a low cationic charge density of about 1 to 300 cationic groups per
1000 monomer or polysaccharide units, more preferably about 1 to 100
cationic groups per 1000 monomer or polysaccharide units. Suitable ratio's in
which the cationic polymer may be used with an anionic and /or amphoteric
21
------------------ - - -------- - -
CA 02268734 1999-04-07
polymer are 1:20 to 1:1, preferably 1:10 to 1:5. Typically, the total amount
of
the cationic polymer, the anionic polymer and /or the amphoteric polymer
added to a mud system is in the range of 0.1 to 4 kg/m3.
The invention will now be iilustrated by means of the following non-
limiting examples.
Examgle 1 - Use in QN-CMC's in Drilling operations.
The following provides examples of the effectiveness and advantages
of QN-CMC in actual downhole drilling situations. Included in the example is
a general description of the wells drilled with ON-CMC fluids, usage/cost
comparisons of QN-CMC fluids versus gel-chemical fluids, rheological
comparison of QN-CMC fluids versus gel-chemical fluids and advantages
noted for the QN-CMC materials.
General Well Descriation:
Three wells were drilled using the QN-CMC drilling fluid in Alberta, Canada
at locations 08-33-39-20 W4, 11-09-40-19 W4 and 05-35-39-21 W4. All wells
were drilled to a final depth of between 1407 m and 1422 m in the same
general manner:
= Wells were started by rotary drilling a hole with a 311 mm diameter drill
bit
from surface ground location to a depth of approximately 200 m. Upon
reaching this approximate 200 m depth, surface casing of 219 mm size
was run into the bottom of the hole and subsequently cemented into place
in the usual manner. The drilling fluid used to help achieve the drilling
process was a mixture of sodium bentonite and caustic as commonly
practiced in the industry.
= Rotary drilling of the well continued using a 200 mm diameter bit and -
proceeded to a final and total depth of between 1407 m and 1422 m. The
drilling fluid used from the surface casing depth to between 1225 m and
1269 m was water treated with calcium and a partially hydrolyzed
polyacrylamide, as often practiced in the industry. Drilled solids contained
22
CA 02268734 1999-04-07
within the calcium water were removed from the drilling fluid in the usual
manner using shaker screens and centrifuges.
= From the depths of 1225 m to 1269 m to between the final total depth, a
QN-CMC based drilling fluid was used. The QN-CMC fluid is referred to
as the main hole mud system. The principle constituents in the QN-CMC
main hole mud were bentonite at a concentration of between 20 - 35
kg/m3, QN-CMC at a concentration of between 0.3 - 0.6 kg/rn3, caustic
soda to provide a pH in the range of 8.5 to 10.0, and 0- 0.2 kg/m3 of a low
viscosity polyanionic cellulosic polymer (PAC). The total circulating main
hole mud system of between 80 - 100 m3 was designed to provide an API
fluid loss of between 7 - 8 mL under standard API test conditions and
sufficient viscosity to remove the drilled cuttings generated in the drilling
of
the well.
Usaae/Cost Comoarisons
A comparison of material usage and costs of the wells drilled with the
QN-CMC drilling fluids and a group of nearby wells drilled with a gel-chemical
fluid is given in Table 1. The gel-chemical wells were drilled in a similar
manner to those previously described for the QN-CMC fluids, except for the
use of get-chemical drilling fluid in place of the QN-CMC fluid. The general
composition of the gel-chemical fluid is sodium bentonite at a concentration
of
- 60 kg/m3, polyanionic cellulosic polymer at a concentration of 0.5 - 2.0
kg/m3 and caustic soda to provide a pH of 8.0 - 10Ø
23
CA 02268734 1999-04-07
Table 1: Material Usage and Cost Comparison of Wells Drilled with QN-CMC
and Ge{-chemical Drilling Fluids
Well Fluid System Well Sxs bentonhe Sxs oN- Sxs PAC Mud material
Identification Used Depth - main hole CMC - main main hole cost - main
hole hole
08-33-39- ON-CMC 1407 m 76 8 0 5 3134 Can
20W4
11-09-40- ON-CMC 1422 m 106 3 - 1 S 2305 Can
19W4
05-35-39- ON-CMC 1415 m 90 3 1 $2526 Can
21W4
AVERAGES 1415 m 91 4.7 0.7 $2655
03-01-40- Gel- 1429 m 161 0 4 $ 2665 Can
21W4 chemical
13-26-39- Gel- 1425 m 166 0 9 $ 4848 Can
21W4 chemical
15-34-39- Gel- 1471 m 160 0 18 $ 5973 Can
21W4 chemical
05-30-39- Gel- 1351 m 153 0 14 $ 4987 Can
20 4 chemical
AVERAGES 1419 m 160 0 11.3 4618
PAC - Polyanionic cellulose
The data in Table 1 clearfy indicates that, on average, less bentonite is
being
used on the wells drilled with QN-CMC as compared to the wells drilled with
gel-chemical drilling fluid. Usage of CMC derived materials (QN-CMC and
PAC) was also clearly less on average for the QN-CMC weils. The lower
material usage on the wells drilled with QN-CMC is reflected on the lower.
average mud material costs in the main hole as compared to the wells drilled
with a gel-chemical fluid.
heoloaical Comparison:
Rheological properties of a drilling fluid are important to the drilling
operation in order to efficiently clean drilled cuttings from the wellbore. In
simple terms, the ideal drilling fluid is shear thinning or thixotropic in
nature:
having low viscosity at high shear rates (as seen at the drill bit) and high
viscosities at lower shear rates (as seen in the annular space between the_
drilistring and wellbore wall).
Table 2 provides rheological comparisons of the wells described above and in
Table 1. In all cases, the rheologies reported in Table 2 were in the main
hole
24
------~
CA 02268734 1999-04-07
section where either QN-CMC fluid or gel-chemical fluid was being used in the
rotary drilling operation.
Table 2: Rheolo ical Com arison of QN-CMC fluids vs. e1-chemical fluids
WELL ID DEPTN PLASTIC YIELD YP/PV n K 10 sec. GEL 10 min. GEL
(mud) AT MUD VISCOSITY POINT RATIO VALUE VALUE STRENGTH STRENGTH
CHECK (mPa.s) (Pa) (no (poise) (Pa) (Pa)
units)
08-33 1266 m 16 7 0.44 0.62 3.21 7 10
(ON-CMC) 1267 m 16 14 0.8e 0.45 13.83 6 9
11=09 1280 m 8 5.5 0.92 0.44 5.73 3 3
(flN=CMC) 1282 m 29 10 0.34 0.67 3.83 4.5 7
1418 m 20 11 0.55 0.56 6.47 4 7
05-35 1355 m 14 11 0.79 0.47 9.59 6 8
(a1V=CMC)
AML 17 10 0.65 0.54 7.11 5 7
03.01 (gc") 1343 m 12 2.5 0.21 0.77 0.71 1 3
13-26 (9c") 1200 m 9 3 0.33 0.68 1.12 2 7
1400 m 14 3 0.21 0.77 0.64 2 14
18-34 ( o") 1352 m 12 4 0.33 0.68 1.40 4 12
0&30 (9c'-) 1171 m 8 3.5 0.44 0.82 1.60 2 B
1371 m 18 8 0.32 0.69 2.10 a 13
AVERAGE 12 4 0.31 0.70 1.29 3 9
YP/PV Ratio = yield pointlplastic viscosity ratio
- gc = gel-chemical drilling fluid
Plastic viscosities and yield points are derived from the Bingham Plastic
rheological model, a model commonly used to describe drilling fluid
rheological properties. The higher the YP/PV (yield point/plastic viscosity)
ratio, the more thixotropic is the nature of the drilling fluid and the better
the
drilling fluid is to remove drilled cuttings from the wellbore. The average
YP/PV ratio for the QN-CMC drilling fluids is approximately twice that of the
average gel-chemical fluids.
The "n" and "K" values, laminar flow index and laminar flow consistency
factor, respectively, are factors derived from the Power Law rheological
model, another model commonly used to characterize drilling fluid rheological
properties. Shear thinning behavior is described by the "n" value, where the
lower the "n" values the more shear thinning or thixotropic is the fluid.
Similar
to the Bingham Plastic model, the QN-CMC fluids indicate better shear
thinning characteristics by nature of their lower "n" values than the offset
wells
drilled with gel-chemical fluids.
CA 02268734 1999-04-07
Gel strengths, although not derived from any rheological model, are
commonly used to describe the ability of drilling fluids to hold and suspend
solids when the drilling fluid is subjected to low shear rates. An "ideal"
drilling
fluid will have good initial gel strength (typically 5 - 12 Pa for drilling
operations) and not develop progressive gel strengths over time. The
average gel strengths of the QN-CMC fluids are better than the average gel
strengths of the gel-chemical fluids.
Advantages ofIheQN-CMC System
In summary, the advantages of the QN-CMC drilling fluid system in
accordance with the present invention, as compared to the commonly used
gel-chemical fluid, for the wells drilled in above cited example include:
= Less material usage of sodium bentonite
= Less material usage of CMC derived materials
= Lower total material cost for the main hole mud system
= Better rheological profile
26