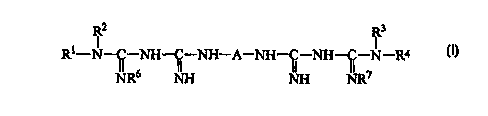Note: Descriptions are shown in the official language in which they were submitted.
CA 02269764 1999-04-22
WO 98/20738 PCT/US97/20087
COMPOSITIONS AND METHODS IN WHICH BIS(BIGUANIDES)
PROVIDE ENHANCED ANTIMICROBIAL EFFICACY
FOR DISINFECTING CONTACT LENSES
Field of the Invention
This invention relates to new and improved solutions for the treatment of
contact
lenses and to methods for treating contact lenses with such solutions. In
particular, the
present invention is directed to disinfecting solutions comprising certain
bis(biguanides)
and to their use in improved regimens for treating contact lenses.
Background of the lnvention
Generally, contact lenses in wide use fall into three categories: ( 1 ) hard
lenses
formed from materials prepared by polymerization of acrylic esters, such as
polymethyl
methacrylate (PMMA), (2) rigid gas permeable (RGP) lenses formed from silicone
acrylates and fluorosilicone methacrylates, and (3) gel, hydrogel or soft type
lenses made
of polymerized hydrophilic or hydrophobic monomers, such as 2-hydroxyethyl
methacrylate (HEMA). The hard acrylic type contact lenses are characterized by
low
water vapor diffusion constants, resistance to the effects of light, oxygen
and hydrolysis
and absorb only minor amounts of aqueous fluids. Because of the durability of
hard
contact lenses, coupled with their tendency not to absorb appreciable amounts
of water,
the selection of suitable disinfecting agents, cleaning agents or other lens
care compounds
is relatively non-critical.
However, unlike hard lenses, soft type contact lenses and certain of the newer
rigid gas permeable contact lenses have a tendency to bind and concentrate
significantly
more fluids, environmental pollutants, water impurities, as well as
antimicrobial agents
and other active ingredients commonly found in lens care solutions. In most
instances, the
low levels of the ingredients in lens care solutions do not lead to eye tissue
irritation when
used properly. Nevertheless, because of the inherent binding action of protein
deposits to
soft lens materials, some disinfecting agents and preservatives tend to build
up on lens
*rB
CA 02269764 1999-04-22
WO 98I20738 PCT/US97/20087
surfaces and may become concentrated to potentially hazardous levels, such
that when
released could cause corneal inflammation and other eye tissue irritation.
Previous ei~orts to alleviate the problem of binding and concentrating
disinfectants
and preservatives onto contact lens surfaces, and reducing the potential for
eye tissue
irritation have not been totally satisfactory. For example, in spite of low
toxicity levels,
not a11 disinfectants are compatible for use with all types of contact lenses.
Although they
are effective antibacterial agents, their use can result in a loss of lens
hydrophilic
properties, cause solution instability or may even lack compatibility with
certain types of
hard lenses, e.g., high silicon content.
Certain antibacterial agents were found to be more compatible with contact
lenses
and exhibit less binding on lens surfaces. In one case, it was found that
chlorhexidine, a
biguanide, binds to soft lens material seven times less than benzalkonium
chloride, but the
presence of proteinaceous oily tear-film deposits on a lens can double the
amount of
chlorhexidine absorbed on the lens compared to a clean lens. U.S. patent
4,354,952
discloses very dilute disinfecting and cleaning solutions containing
chlorhexidine or its
salts in combination with certain amphoteric and non-ionic surfactants. These
solutions
were found to reduce the amount of binding of chlorhexidine on hydrophilic
soft contact
lenses. Notwithstanding the reduction in binding achieved by this invention,
the use of
chlorhexidine did result in certain tradeoffs. The antimicrobial activity of
the
chlorhexidine may be diminished when used with certain amphoteric surfactants.
Furthermore, it was reported that if not used in proper ratio, the surfactant
and
disinfectant will precipitate unless a non-ionic type surfactant is also
employed.
U.S. patent 4,361,548 discloses a contact lens disinfectant and preservative
containing dilute aqueous solutions of a polymer; namely,
polydimethyldiallylammonium
chloride (DMDAAC) having molecular weights ranging from about 10,000 to
1,000,000.
Amounts of DMDAAC homopolymer as low as 0.00001 percent by weight may be
employed when an enhancer, such as thimerosal, sorbic acid or phenylmercuric
salt is used
therewith. Although lens binding and concomitant eye tissue irritation with
DMDAAC
were reduced; it was found in some users to be above desirable clinical
levels.
-2-
CA 02269764 1999-04-22
WO 98/20738 PCT/US97/20087
British Patent 1,432,34S discloses contact lens disinfecting compositions
containing a polymeric biguanide and a mixed phosphate buffer. The products
embraced
by this patent have not found acceptance by the consumer. Corneal staining is
an
indication of patient acceptability and compositions as disclosed by this
patent have
staining values of 17% or more present) far above that which is desirable for
patient
acceptability.
Other efforts to reduce or eliminate binding of disinfectants to contact
lenses have
led to the use of anti-binding or detoxifying agents, like polyvinyl
pyrrolidone (PVP) and
polyvinyl alcohol (PVA). For the most part, however, these polymers alone were
found
to be ineffective in reducing lens binding and eye tissue irritation.
U.S. Patent 4,758,595 to Ogunbiyi et al. disclosed that a contact-lens
solution
containing a polyaminopropyl biguanide (PA.PB) has enhanced efficacy when
combined
with a borate buffer. Such solutions are compatible with both non-soft and
soft-type
lenses, and are adaptable for use with virtually any of the commonly known
disinfecting
techniques, including "cold" soaking under ambient temperature conditions, as
well as
with high temperature disinfecting methods. These disinfecting and
preservative solutions
are especially noteworthy for their broad spectrum of bactericidal and
fungicidal activity
at low concentrations coupled with very low toxicity when used with soft-type
contact
lenses. Ogunbiyi et al. stated that biguanide polymers in the higher molecular
weight
ranges usually demonstrate lower toxicity levels than corresponding lower
molecular
weight materials.
Compositions containing PAPB and borate, or other non-phosphate buffers, have
been commercialized in various products, but at levels of about 1 ppm or less
for use with
soft contact lenses. It is generally desirable to provide the lowest level of
a bactericide
possible, while maintaining the desirable level of disinfection efFcacy, in
order to provide
a generous margin for safety and comfort.
Some of the most popular products for disinfecting lenses are multipurpose
solutions that can be used to clean, disinfect and wet contact lenses,
followed by direct
insertion (placement on the eye) without rinsing. Obviously, the ability to
use a single
solution for contact-lens care is an advantage. Such a solution, however, must
be
-3-
CA 02269764 1999-04-22
WO 98/20738 PCT/US97/20087 - -
particularly gentle to the eye, since, as indicated above, some of the
solution will be on the
lens when inserted and will come into contact with the eye.
With conventional contact-lens cleaners or disinfectants, including mufti-
purpose
solutions, lens wearers typically need to digitally or manually rub the
contact lenses
(typically between a finger and palm or between fingers) during daily cleaning
and/or
disinfecting of contact lenses. The necessity for frequent "rubbing" of
contact lenses adds
to the time and effort involved in the daily care of contact lenses. Many
contact-lens
wearers dislike having to perform a daily "rubbing" regimen or consider it to
be an
inconvenience. Some wearers may be negligent in the proper "rubbing" regimen,
which
may result in contact-lens discomfort and other problems. Sometimes rubbing,
if
performed too rigorously, which is particularly apt to occur with beginning
lens wearers,
may damage the lenses. This can be very problematic or inconvenient when a
replacement
lens is not immediately available.
A contact lens solution that does not require rubbing would generally require
a
more efficacious or stronger disinfectant than a solution that does not
require rubbing.
The stronger the bactericidal effect of a solution, however, the more likely
that it may
exhibit toxic effects or adversely effect lens-wearer comfort. In fact, many
very
efficacious bactericides used in other contexts, such as mouthwashes,
cosmetics, or
shampoos, while being sufficiently safe for use in such products, would be too
toxic for
ophthalmic use, especially for use with soft lenses because of the above-
mentioned
tendency of soft lenses to bind chemicals. Similarly, the concentrations of
certain
bactericides may need to be within lower limits in solutions for use with soft
contact
lenses than in other products or in solutions for other types of lenses,
especially when
such solutions are for use in the eye.
It would be desirable to obtain a contact-lens solution that would
simultaneously
provide both ( 1 ) an increased level of antibacterial activity, and (2) a low
order of toxicity
to eye tissue, such that the solution can be used to treat contact lenses
without rinsing,
despite any tendency of a disinfectant to bind onto lens surfaces. While
challenging to
develop, it would be especially desirable to obtain such a disinfecting
solution that could
be used as a mufti-purpose solution for soft contact lenses, which would allow
direct
-4-
CA 02269764 1999-04-22
WO 98I20738 PCT/US97/20087
placement of a contact lens on an eye following soaking in the solution and/or
rinsing and
rewetting with the solution. Finally, it would be additionally desirable for
the antibacterial
efficacy of the disinfecting solution to be sufficiently high that rubbing
would not be
required to achieve effectively complete disinfection of a contact lens.
S
Brief Description of the Invention
The present invention is directed to an ophthalmically safe disinfecting
solution for
contact lenses comprising about 2.0 to about 8.0 ppm of a bis(biguanide) in
the form of a
dihydrochloride salt, or a corresponding concentration of the same
bis(biguanide) in the
form of the free base or a different water-soluble salt, which bis(biguanide)
has the
following general formula:
R2 R3
R~-N-C-NH-C-NH-A-NH-C-NH-C-N-R4
NR6 II~-I I~ 117
(I)
wherein R' and R' are independently selected (i.e., the same or different)
from the group
consisting of branched or unbranched alkyl having 4-12 carbon atoms,
alkoxyalkyl or
alkylsulfide radical having 4-12 carbon atoms, or cycloalkyl or cycloalkyl-
alkyl radical
having 5-12 carbon atoms; R2 and R3 are independently selected from the group
consisting of hydrogen, alkyl having 1-12 carbon atoms, alkoxyalkyl having 1-
12 carbon
atoms, or cycloalkyl or cycioalkyl-alkyl having 5-12 carbon atoms; R6 and R'
are
independently selected from the group consisting of hydrogen and alkyl radical
having 1-6
carbon atoms, and A in the above formula is a divalent group having 4-16
carbon atoms
and is selected from the group consisting of an alkylene, alkyloxyalkyl, and
alkylsufide
radical, wherein the aforesaid alkyoxyalkyl or alkylsulfide radicals are
either a
polymethylene chain interrupted with one or more oxygen and/or sulfur atoms or
a
polymethylene chain substituted with an alkoxy (-ORg) or alkylthio {-SR9)
group, wherein
Rg and R9 are independently selected from the group consisting of alkyl having
1-12
carbon atoms or a cycloalkyl or cycloaikyl-alkyl having 5-12 carbons, or
wherein A is a
-5-
*rB
CA 02269764 1999-04-22
WO 98/20738 PCT/US97/2008T
divalent polymethylene group having 8 to 16 carbon atoms interrupted with a
divalent
radical of cyclohexane or 1,4-diazacyclohexane.
The solutions of the present invention also comprise an effective amount of
one or
more buffering agents, preferably in the amount of from about 0.01 to 5% by
weight of
the composition (solution), an effective amount of one or more surfactants,
preferably in
an amount of about 0.01% to 5% by weight, and water in an amount of at least
about
90% by weight. In one embodiment of the invention, the surfactant is a neutral
or non-
ionic surfactant.
The invention is also directed to a method of cleaning and/or disinfecting a
contact
lens comprising soaking the lens for a given period of time in an aqueous
solution
comprising a microbiocidally effective amount, within the range from about 2.0
to about
8.0 ppm, preferably 2.0 to 6.0 ppm, of a bis(biguanide) of Formula (I) in the
form of the
hydrochloride salt (or a corresponding concentration or molar amount of the
same
bis(biguanide) in the form of the free base or a salt other than the
hydrochloride), an
effective amount of a buffering agent, and an effective amount of a
surfactant, and
subsequently directly placing the treated lens on an eye of the wearer. In a
preferred
embodiment of this method, the contact lens does not require rubbing with the
solution to
achieve the necessary disinfection.
In yet another embodiment of the present invention, 0.1 to 4.0 ppm of a
bis(biguanide) of Formula (I) has been shown to provide synergistic efficacy
in
combination with a polymeric biguanide in the amount of 0.1 to 3.0 ppm.
Detailed Descriution of the Invention
As indicated above, the present invention is directed to a composition, in the
form
of an aqueous solution containing a compound of Formula (I) above, and a
method of
using the solution for disinfecting and/or preserving contact lenses,
especially soft contact
lenses. The disinfecting solutions of the present invention are effective at
low
concentrations against a wide spectrum of microorganisms, including but not
limited to
Staphylacoccus aureus, Pseudomanas aeruginosa, Serratia marcescens, Candida
albicans, and Fusarium solani. A disinfecting solution is generally defined as
a contact-
-6-
CA 02269764 1999-04-22
WO 98/20738 PCT/US97/20087
lens care product containing one or more active ingredients (for example, anti-
microbial
agents and/or preservatives) in sufficient concentrations to destroy harmful
microorganisms on the surface of a contact lens within the recommended minimum
soaking time. The recommended minimum soaking time is included in the package
instructions for use of the disinfecting solution. The present solution, in
combination with
its container or bottle and packaging, including instructions for use in
accordance with a
specified regimen, may be considered a novel and improved kit, package, or
system for
the care of contact lenses.
By the term "soft lens" is meant a lens having a proportion of hydrophilic
repeat
units such that the water content of the lens during use is at least 20% by
weight. The
term "soft contact lens" as used herein generally refers to those contact
lenses which
readily flex under small amounts of force. Typically, soft contact lenses are
formulated
from polymers having a certain proportion of repeat units derived from
hydroxyethyl
methacrylate and/or other hydrophilic monomers, typically crosslinked with a
crosslinking
1 S agent. In contrast, conventional "hard contact lenses", which cover only a
part of the
cornea of the eye, usually consist of poly(methyl methacrylate) crosslinked
with ethylene
glycol dimethacrylate or the like, and conventional rigid gas permeable lenses
(RGP)
typically comprises monomers containing silicon that result in a more oxygen-
permeable
material.
By the term "disinfecting solution" is meant a solution containing one or more
microbiocidal compounds, that is effective for reducing or substantially
eliminating the
presence of an array of microorganisms present on a contact lens, which can be
tested by
challenging the solution, or a contact lens that has been immersed in the
solution, with
specified inoculums of such microorganisms. The term "disinfecting solution"
does not
exclude the possibility that the solution may also be useful as a preserving
solution, or that
the disinfecting solution may also be useful for other purposes such as daily
cleaning,
rinsing and storage of contact lenses, depending on the particular
formulation.
A solution that is useful for cleaning, chemical disinfection, storing, and
rinsing a
contact lens is referred to herein as a "multi-purpose solution." Such
solutions may be
part of a "mufti-purpose solution system" or "mufti-purpose solution package."
The
CA 02269764 1999-04-22
WO 98/20738 PCT/US97/20U8T
procedure for using a mufti-purpose solution, system or package is referred to
as a "multi-
functional disinfection regimen." Mufti-purpose solutions do not exclude the
possibility
that some wearers, for example, wearers particularly sensitive to chemical
disinfectants or
other chemical agents, may prefer to rinse or wet a contact lens with another
solution, for
example, a sterile saline solution prior to insertion of the lens. The term
"mufti-purpose
solution" also does not exclude the possibility of periodic cleaners not used
on a daily
basis or supplemental cleaners for removing proteins, for example enzyme
cleaners, which
are typically used on a weekly basis. By the term "cleaning" is meant that the
solution
contains one or more cleaning agents in su~cient concentrations to loosen and
remove
loosely held lens deposits and other contaminants on the surface of a contact
lens,
especially if used in conjunction with digital manipulation (for example,
manual rubbing of
the lens with a solution) or with an accessory device that agitates the
solution in contact
with the lens, for example, a mechanical cleaning aid. The critical micelle
concentration
of a surfactant-containing solution is one way to evaluate its cleaning
effectiveness.
The term "effective mufti-purpose solution," "effective mufti-purpose solution
system," "effective mufti-purpose solution package" and "effective mufti-
functional
disinfection regimen" analogously refers to a solution useful for daily
chemical
disinfection, storing, and rinsing a contact lens, which solution does not
require cleaning
that involves rubbing, but which solution still obviates the need for any
other solution for
daily cleaning, that is, no other solution must be used in conjunction or
combination with
the solution on a daily basis. Such solutions may comprise a surfactant or
other agent for
loosening or preventing lens deposits to some extent. Such a solution does not
require
digital rubbing or similar manual or mechanical means that provide a rubbing
action (a
means for providing pressure and friction along the surface of the lens) to
remove
deposits from a lens. A no-rub regimen may be especially applicable to a
frequent
replacement lens, a lens which is recommended for replacement after a limited
number of
days in the eye of the wearer, for example, after being worn about 30 days or
less,
preferably after being worn about 14 days or less.
By the term "ophthalmically safe" with respect to a contact-lens solution is
meant
that a contact lens treated with the solution is safe for direct placement on
the eye without
_g_
CA 02269764 1999-04-22
WO 98/20738 PCT/US97/20087
rinsing, that is, the solution is safe and comfortable for daily contact with
the eye via a
contact lens that has been wetted with the solution. An ophthalmically safe
solution has a
tonicity and pH that is compatible with the eye and comprises materials, and
amounts
thereof, that are non-cytotoxic according to ISO standards and U.S. FDA (Food
& Drug
Administration) regulations.
According to the present invention, the bis(biguanide) germicides employed in
the
present solutions include compounds, and their water-soluble salts, having
following
formula:
R2 R3
R~-N-C-NH-C-NH-A-NH-C-NH-C-N-R4
NR6 NH NH NR7
(I)
wherein R' and R4 are independently selected (i.e., the same or different)
from the group
consisting of branched or unbranched alkyl having 4-12, preferably 6-10,
carbon atoms,
alkoxyalkyl (i.e., ether) or alkylsulfide (thioether or dialkylsulfide)
radical having 4-12,
preferably 6-10, carbon atoms, or cycloalkyl or cycIoaikyl-alkyl radical
having 5-12,
preferably 7-10, carbon atoms; RZ and R3 are independently selected from the
group
consisting of hydrogen, alkyl having 1-12, preferably 1-6, carbon atoms,
alkoxyalkyl
having 1-12, preferably 1-6, carbon atoms, or cycloalkyl or cycloalkyl-alkyl
having 5-I2,
preferably 6-10, carbon atoms; R6 and R' are independently selected from the
group
consisting of hydrogen and alkyl radical having 1-6 carbon atoms, and A in the
above
formula is a divalent group having 4-16 carbon atoms, preferably 6-10, carbon
atoms and
is selected from the group consisting of an alkylene (a divalent radical of an
aliphatic
hydrocarbon), alkyloxyalkyl, and alkylsufide radical, wherein the aforesaid
alkyoxyalkyl or
alkylsulfide radicals are either a polymethylene chain interrupted with one or
more oxygen
and/or sulfur atoms or a polymethylene chain substituted with an alkoxy (-ORg)
or
alkylsulfide (-SR9) group, wherein Rg and R9 are independently selected from
the group
consisting of alkyl having 1-12 carbon atoms, or a cycloalkyl or cycloalkyl-
alkyl having S-
12 carbon, or wherein A is a divalent polymethylene group having 8 to 16
carbon atoms,
preferably 6 to 12 carbon atoms interrupted with a divalent radical of a
cyclohexane or
-9-
CA 02269764 1999-04-22
WO 98/20738 PCT/US97/20087
diazacyclohexane ring (a 1,4-diazacyclohexane or piperazine connected to the
polymethylene ring via the nitrogen atoms). By the term "cycloalkyl," either
in cycloalkyl
or cycloalkyl-alkyl, is meant unsubstituted or substituted cycloalkyl, where
the
substituents are one or more alkyl, alkoxy (-OR) , or alkylthio (-SR) groups
having I -6
carbon atoms.
In one embodiment of the present invention, the biguanides of Formula (I) are
suitably used in the total amount (with respect to the weight of the
hydrochloride salt,
often containing two hydrochlorides as in alexidine) of 2.0 to 8.0 ppm,
preferably 2.5 to
6.0 ppm based on the total aqueous solution. More preferably, the
bis(biguanides) are
used in the amount of 3.0 to 5.0, most preferably 3.5 to 4.5 ppm, with respect
to the
hydrochloride salt, or a corresponding molar amount of another salt or its
free base.
When the bis(biguanide) that is used in the present invention is in the form
of the free base
or a salt other than the hydrochloride, then the above-indicated ppm ranges
must be
adjusted to obtain the corresponding ppm ranges such that the ranges are the
same on a
1 S molar basis as for the hydrochloride salt of the bis(biguanide). The
concentration of the
bis(biguanide) in solution is directly related to its bactericidal efficacy.
The corresponding
weight of an equal molar amount or equal concentration of a different salt
form of a
bis(biguanide) can be readily calculated, for use determining a suitable
amount of the
ingredient. The term "ppm" refers to "parts per million" and 1.0 ppm
corresponds to
0.000l percent by weight. It is based on the total weight of the composition
or, in this
case, the total weight of the aqueous disinfecting solution.
In the present application, the amount of the bis(biguanide) or other
components
in a solution according to the present invention refers to the amount
formulated and
introduced into the solution at the time the solution is made. Over time {for
example,
over a storage period of 3 8 months), the assayed amount of a bis(biguanide)
in solution
may decrease somewhat.
Preferably, the bis(biguanide) compounds have the following formula:
R~-NH-C-NH- i -NH-(CH2 p NH- i -NH- i -NH-R4
NH NH NH NH {II)
-10-
CA 02269764 1999-04-22
WO 98/20738 PCT/US97/20087
or their water-soluble salt form, wherein R' and R" are independently selected
from the
group consisting of branched or unbranched alkyl, alkoxyalkyl (i.e., ether) or
alkylsulfide
(ihioether) radical, and n is S to 7.
Each of R' and R4 in Formulas (I) or (II) above may be, for example, an n-
butyl,
isobutyl, sec-butyl, tert-butyl, pentyl, neopentyl, octyl, 2-ethylhexyl,
dodecyl, cyclobutyl,
cyclopentyl, cyclohexyl, cycloheptyl, cyclopentylmethyl or cyclohexylmethyl
radical.
Preferred are 2-ethylhexyl (alexidine), 1,5-dimethylhexyl, 1-methylhexyl, 1,3-
dimethylpentyl, 1,4-dimethylpentyl, cyclohexylmethyl, 2-norbornyl,
propyloxyoctyl, and
propyloxybutyl.
Each of RZ and R3 in Formula (I) above may be, for example, a methyl, ethyl, n-
propyl, isopropyl, n-butyl, isobuty(, sec-butyl, tert-butyl, pentyl,
neopentyl, octyl, 2-
ethylhexyl, dodecyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl,
cyclopentylmethyl
or cyclohexylmethyl radical. When one or more of RZ and R3 is an alkoxyalkyl
radical, it
may be, for example, a 2-methoxyethyl.
Each of R6 and R' in Formula (I) may be, for example, a methyl, ethyl, n-
propyl,
isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, pentyl, or neopentyl
radical.
Preferred A groups in Formula (I) include hexamethylene and a divalent
hexamethylene interrupted with a divalent radical derived from N,N'-(2-
aminoethyl)-1,4-
piperadine or the like in which each of the nitrogen atoms are connected to a
polymethylene group having 1 to 4 carbon atoms.
The acid-addition salts of the invention may be derived from an inorganic or
organic acid. In most circumstances it is preferable that the salts be derived
from an acid
which is readily water soluble and which affords an anion which is suitable
for human
usage, for example a pharmaceutically-acceptable anion. Examples of such acids
are
hydrochloric, hydrobromic, phosphoric, sulphuric, acetic, D-gluconic, 2-
pyrrolidino-5-
carboxylic, methanesulphonic, carbonic, lactic and glutamic acids. The
hydrochloride salt
is preferred.
The bis(biguanides) of Formula (I) preferably have relatively hydrophobic end
groups. Preferably, the Log P of the compounds is 5 to 10, preferably 6 to 8,
wherein P
CA 02269764 1999-04-22
WO 98I20738 PCTlUS97/20087
is the partition coefficient of the free base, using the following equation,
wherein a is the
degree of ionization:
P-
Cb,~r~( 1-a)
To obtain the partition coefficient of a bis(biguanide), the compound is
partitioned
between a 0.05 M phosphate buffer (pH 11 ) saturated with octanol and octanol
saturated
with phosphate buffer after gentle shaking at room temperature (26 ~C). The
volume
ratio of these two phases and the amount of sample are chosen so that the
absorbance of
the sample from the buffered layer after partitioning has a value between 0.2
and 0.9,
using a 1-cm cell and buffer solution as a blank. By working at a fixed pH and
knowing
or calculating the pK,, the P value can be determined using the above formula.
See
"Quantitative Structure-Activity Relationships for Biguanides, Carbamidates,
and
Bisbiguanides as Inhibitors of Streptococcus mutans NO. 6715", and Warner, V.
and
Lynch, D., J. Med. Chem, l979, Vol. 22, no. 4 at 359, 365; and Albert, and
Serjeant, E.,
"Determination of Ionization and Stability Constants," Butler and Tanner Ltd.,
London,
England, l962, both references hereby incorporated by reference.
Particularly preferred bis(biguanide) compounds of this invention are 2-
(decylthiomethyl)-pentane-1,5-bis(S-isopropylbiguanide), 2-(decylthio-
methyl)pentane-
1,5-bis(5,5-diethylbiguanide), and hexane-1,6-bis(2-ethylhexylbiguanide), the
latter also
known as alexidine ar 1,1'-hexamethylenebis(5-(2-ethylhexyl)-biguanide)
dihydrochloride.
Other preferred bis(biguanides) include l,1'-hexamethylenebis(5-heptyl-
biguanide)
dihydrochloride, 1,1'-hexamethylenebis(5-octyl-biguanide) dihydrochloride, and
1,1'-
hexamethylenebis(5-hexyl-biguanide) dihydrochloride.
The biguanide compounds of Formula (T) wherein R6 and R' are each hydrogen
may be made by reacting a bis-cyanoguanidine of the formula:
NC-NH-C-NH-A-NH-C-NH-CN
II 41
NH NH
with an amine R'R2NH, or with two different amines R'RZNH and R3R4NH, in the
form
of an acid addition salt thereof, wherein A, R', RZ, R3 and R4 have the
meanings stated
-12-
CA 02269764 1999-04-22
WO 98/Z0738 PCTlUS97/20087
above, at a temperature of l00~C to 170~C. A preferred amine salt is the
hydrochloride.
Most diamines are commercially available from a variety of sources.
The reactants are heated together until the reaction is complete. The reaction
proceeds fastest at higher temperatures, but if thermal stability is a
problem, the reaction
should be carried out at lower temperature for a longer period. The reactants
are most
conveniently melted together in the absence of a solvent, but if desired an
inert solvent
such as DMSO, 2-methoxyethanol, 2-ethoxyethanol, nitrobenzene, sulpholane,
isopropanol, n-butanol, ethylene glycol dimethyl ether or water, or a mixture
of such
solvents, may be used.
The bis-cyanoguanidine of the Formula (VI) may be manufactured from known
starting materials such as hexamethylenedinitrile which is reduced, for
example, with
hydrogen and Raney nickel or with borane in dimethyl sulphide to the
corresponding
diamine (VIII), and the diamine in the form of an acid-addition salt,
conveniently the
dihydrochloride, is reacted with sodium dicyanamide or other suitable salt to
form the
required starting material (VI), as depicted below.
NC-A-CN -~s~ NHZ A-NH2
(VII) (VIII)
VIII ~ 2-MN(CN)2-ANC-NH-C-NH-A-NH- i-NH-CN
NH NH
wherein M is a sodium, potassium, zinc or other suitable salt. The sodium salt
is
commercially available.
The compounds of the present invention can also be made by reacting a diamine
of
the Formula (VIII) in the form of an acid addition salt, with a cyanoguanidine
of the
formula:
R' R2N-C-NH-CN
NR6
(IX)
-13-
*rB
CA 02269764 1999-04-22
WO 98/20738 PCT/US97/20087
' or with a cyanoguanidine of the Formula (IX) and a cyanoguanidine of the
formula:
R3 R4 N -C-NH-CN
NR7
(X)
wherein A, R', RZ, R3, R4, R6 and R' have the meanings stated above, at a
temperature of
l00~ to I70~C.
A suitable salt of the diamine is, for example, the dihydrochloride. The
reactants
are heated together until the reaction is complete. The reaction proceeds
fastest at higher
temperature, but if thermal stability is a problem, the reaction should be
carried out at
lower temperature over a longer period. If a melt can be formed at those
temperatures
the reactants are conveniently melted together in the absence of a solvent. If
not, or
alternatively, the reactants are heated together in a suitable inert solvent,
for example
those mentioned above. The acid-addition salts of the invention are obtained
by
conventional means.
The cyanoguanidines of the Formulae (IX) and (X) wherein R~ and R' are
hydrogen, which may be used as starting materials in the above process, may be
obtained
by reacting sodium dicyanamide with an appropriate amine R'R2NH or R3R4NH, in
the
form of an acid-addition salt, conveniently the dihydrochloride, in a suitable
inert solvent.
The cyanoguanidines of the Formulae (IX) and (X) wherein R6 and R' are other
than hydrogen, which may be used as starting materials in the above process,
may be
obtained by reacting a dialkyl (cyanoimido)dithio-carbonate, for example
dimethyl
(cyanoimido)dithio-carbonate, with appropriate amines R'RZNH and R''NHz or
R3R4NH
and R'NHz.
For example, the bis(biguanide) known as alexidine is produced from the
following sequence of reactions.
a
. 2 Cl
~ Na~
~ ~ N
H3N-(CH2)6--NH3 + 2 NC CN
(~I)
-14-
CA 02269764 1999-04-22
WO 98/20738 PCT/US97/20087
NC-NH-C-NH-(CH2)6-NH- i -NH-CN
NH NH
(XIII)
C~
2 H3N~
(XIV)
-NH-i -NH--C-NH-(CHz)6-NH--i -NH-- i-NH ~ 2 HCl
NH NH NH NH
(
Compound {XI) is hexamethylenediamine dihydrochloride (MW l89), Compound (XII)
is sodium dicyanamide, Compound XIII is HMBDA, hexamethylene
bis(cyanoguanido),
Compound (XIV) is 2-ethyl-hexylamine hydrochloride (MW 165.7), and Compound
(XV) is alexidine dihydrochloride a.k.a. [ 1,6-bis-(2-
ethylhexylbiguanido]hexane
dihydrochloride a.k.a. hexane-1,6-bis(2-ethylhexyl biguanide) dihydrochloride.
This
compound has a molecular weight in g/mole (MW) of 58l .7 and empirical formula
C26Hs6N~o~2HCl. The Compound (XV) is commercially available from various
sources,
including Sigma Chemical Co. (St Louis, Missouri).
Various compounds can be made by appropriate starting materials, for example,
where "A" in Formula (I) above is an alkoxy or thioalkyl-substituted alkylene,
a
compound of Formula (VII) may be made from hexenedinitrile by reaction with
RSYH,
where Y is an oxygen or sulfur atom as a strong base. Various compounds of
Formula
(I), where R2 and R' are H, can be readily obtained as N derivatives of 1,6-
bis(biguanido)hexane.
-15-
CA 02269764 1999-04-22
WO 98I20738 PCT/US97/20087
The methods for synthesized compounds of the present invention are also
disclosed in European Patent Application Publication No. 0 125 092 (published
14.11.84); Rose, F.L. and Swain, G., "Bisdiguanide Having Antibacterial
Activity," J.
Chem. Soc., p. 4422-4425 ( 1956); Warner, Victor D. and Lynch, Donald,
"Quantitative
Structure-Activity Relationships of Biguanide, Carbamimidates, and
Bisdiguanides as
Inhibitors of Streptococcus Mutans No. 6715, "J. Med Chem., Vol. 22, No. 6, p.
359-
366 ( 1979).
A second disinfectant/germicide can be employed as a solution preservative,
but it
may also function to potentiate, compliment or broaden the spectrum of
microbiocidal
activity of Formula (I). This includes microbiocidally effective amounts of
germicides
which are compatible with and do not precipitate in the solution, in
concentrations
ranging from about 0.00001 to about 0.5 weight percent, and more preferably,
from about
0.0001 to about 0.1 weight percent. Suitable complementary germicidal agents
include,
but are not limited to thimerosol, sorbic acid, alkyl triethanolamines,
phenylmercuric salts,
e.g. nitrate, borate, acetate, chloride and mixtures thereof. Suitable salts
are soluble in
water at ambient temperature to the extent of at least 0. S weight percent.
These salts
include the gluconate, isethionate, (2-hydroxyethanesulfonate), formate,
acetate,
glutamate, succinanate, monodiglycollate, methanesulfonate, lactate,
isobutyrate and
glucoheptonate.
Further embodiments of potentiating or complementary disinfecting agents for
use
in the present invention also include certain quaternary ammonium compounds
which
possess a generally wide spectrum of bacteriocidal activity and wetting
properties.
Representative examples of the quaternary ammonium compounds are compositions
comprised of balanced mixtures of n-alkyl dimethyl benzyl ammonium chlorides.
Other
examples include polymeric quaternary ammonium salts used in ophthalmic
applications
such as polyquaternium 1 ~ (chemical registry number 75345-27-6) available
from Onyx
corporation.
In another embodiment of the present invention, the bis(biguanides) defined
above
may be used in combination with lesser amounts of other bis(biguanides), for
example
-16-
*rB
CA 02269764 1999-04-22
WO 98/20738 PCT/US97I20087
chlorhexidine. Such compounds have been disclosed in greater detail in United
Kingdom
patent specification No. 705,838.
Finally, the bis(biguanides) of the present invention (Formula I or II) may be
used
in combination with one or more polymeric biguanides. In particular, one
embodiment of
the present invention is directed to an ophthalmically safe disinfecting
solution for contact
lenses comprising: about 0.10 to about 4.0 ppm of a bis(biguanide), as defined
herein, in
combination with 0.10 to 3.0 ppm of a polymeric biguanide. Preferably, the
bisbiguanide
has Formula (II) provided above, wherein R' and R4 are independently selected
from the
group consisting of branched or unbranched alkyl having 4-12, preferably 6-10,
carbon
atoms, alkoxyalkylor alkylsulfide radical having 4-12, preferably 6-10, carbon
atoms, or
cycloalkyl or cycloalkyl-alkyl radical having 5-12, preferably 7-10, carbon
atoms; and n is
4 to 16, preferably 4 to 10.
In this embodiment, in which a bis(biguanide) is used in a synergistic
combination
with a polymeric biguanide, the bis(biguanides) of Formula (I) are suitably
used (with
respect to the weight of the hydrochloride salt, often containing two
hydrochlorides as in
alexidine) in the amount of 0. I to 4.0 ppm, preferably I .0 to 3.0 ppm based
on the total
aqueous solution. More preferably, the bis(biguanides) are used in the amount
of 1.5 to
2.5, most preferably about 2.0 ppm, with respect to the hydrochloride salt, or
a
corresponding molar amount of another salt or its free base. When the
bis(biguanide) that
is used in the present invention is in the form of the free base or a salt
other than the
hydrochloride, then the above-indicated ppm ranges must be adjusted to obtain
the
corresponding ppm ranges such that the ranges are the same on a molar basis as
for the
hydrochloride salt of the bis(biguanide).
In this embodiment, 0.10 to 4.0 ppm of the bis(biguanides) of the present
invention (Formula I or II) may be used in combination with one or more
polymeric
biguanides, and water-soluble salts thereof, having the following formula:
Xl-f -Z-NH- I -NH-C-NH~--n -X2
NH NH
(IV)
-1?-
CA 02269764 1999-04-22
WO 98I20738 PCT/US97l20087
wherein Z is an organic divalent bridging group which may be the same or
different
throughout the polymer, n is on average at least 3, preferably on average 5 to
20, and X'
and XZ are independently selected from the groups -NHZ and -NH - C - NH - CN.
If
One preferred group of water-soluble polymeric biguanides will have number
average
molecular weights of at least 1,000 and more preferably will have number
average
molecular weights from 1,000 to 5Q,000. Suitable water-soluble salts of the
free bases
include, but are not limited to hydrochloride, borate, acetate, gluconate,
sulfonate, tartrate
and citrate salts.
The above-disclosed biguanides and methods of preparation are described in the
literature. For example, U.S. patent 3,428,576 describes the preparation of
polymeric
biguanides from a diamine and salts thereof and a diamine salt of dicyanimide.
The polymeric biguanides, in combination with the bisbiguanides of the present
invention, are effective in concentrations as low as 0.0000l weight percent
(0.1 ppm). It
has also been found that the bactericidal activity of the solutions may be
enhanced or the
spectrum of activity broadened through the use of a combination of such
polymeric
biguanides with the compounds of Formula (I) above. The effective amount of
the
polymeric biguanides (irrespective of the particular salt form or whether the
free base is
used ) may in total be as low as about 0.000010 weight percent (0. I O ppm)
and up to
about 0.00030 weight percent (3.0 ppm) in the present invention, whether in
the form of a
water-soluble salt or the free base. Preferabiy, the total amount of polymeric
biguanide, in
combination with the total amount of compounds of Formula (I) above is about
0.3 to
2.0 ppm, more preferably about 0.4 to 1.0, most preferably about 0.5 to 0.8
ppm.
Most preferred are the polymeric hexamethyiene biguanides, commercially
available, for example, as the hydrochloride salt from Zeneca (Wilmington, DE)
under the
trademark CosmocilTM CQ. Such polymers and water-soluble salts are referred to
as
PI-iMB or polyaminopropyl biguanide (PAPB). The term polyhexamethylene
biguanide,
as used herein, is meant to encompass one or more biguanides have the
following
formula:
-18-
CA 02269764 1999-04-22
WO 98/20738 PCT/I1S97/20087
X'-(CH2~ (CH2)3-NH-C-NH-C-NH---(CHZ)3 (CH2)3-X2
NH NH n
(V)
wherein X' and XZ are as defined above and n is from 1 to S00.
Depending on the manner in which the biguanides are prepared, the predominant
compound falling within the above formula may have different X' and XZ groups
or the
same groups, with lesser amounts of other compounds within the formula. Such
compounds are known and are disclosed in US Patent No. 4,7S8,S9S and British
Patent
1,432,345, which patents are hereby incorporated herein by reference.
Preferably, the
water-soluble salts are compounds where n has an average value of 2 to I S,
most
preferably 3 to 12.
The present solution comprises at least one surfactant. Suitable surfactants
can be
either amphoteric, cationic, anionic, or nonionic which may be present
(individually or in
combination) in amounts up to 1 S percent, preferably up to S percent by
weight of the
composition or solution. Preferred surfactants are amphoteric or nonionic
surfactants,
1 S which when used impart cleaning and conditioning properties. The
surfactant should be
soluble in the lens care solution and non-irntating to eye tissues. Many
nonionic
surfactants comprise one or more chains or polymeric components having
oxyalkylene (-
O-R-) repeats units wherein R has 2 to 6 carbon atoms. Preferred non-ionic
surfactants
comprise block polymers of two or more different kinds of oxyalkylene repeat
units,
which ratio of different repeat units determined the HLB of the surfactant.
Satisfactory
non-ionic surfactants include polyethylene glycol esters of fatty acids, e.g.
coconut,
polysorbate, polyoxyethylene or polyoxypropylene ethers of higher alkanes (C
12-C 18).
Examples of the preferred class include polysorbate 20 (available under the
trademark
Tween~ 20), polyoxyethylene (23) lauryl ether (Brij~ 3S), polyoxyethyene (40)
stearate
2S (Myrj~ S2), polyoxyethylene (2S) propylene glycol stearate (Atlas~ G 2612).
One non-
ionic surfactant in particular consisting of a poly(oxypropylene)-
poly(oxyethylene) adduct
of ethylene diamine having a molecular weight from about 7,S00 to about 27,000
wherein
at least 40 weight percent of said adduct is poly(oxyethylene) has been found
to be
particularly advantageous for use in cleaning and conditioning both soft and
hard contact
-19-
CA 02269764 1999-04-22
WO 98I20738 PCT/US97/20087
lenses when used in amounts from about 0,01 to about 15 weight percent. The
CTFA
Cosmetic Ingredient Dictionary's adopted name for this group of surfactants is
poloxamine. Such surfactants are available from BASF Wyandotte Corp.,
Wyandotte,
Michigan, under the registered trademark "Tetronic". An analogous of series of
surfactants, suitable for use in the present invention, is the poloxamer
series which is a
poly(oxyethylene) poly(oxypropyiene) block polymers available under the
trademark
"Pluronic" (commercially available form BASF).
Various other ionic as well as amphoteric and anionic surfactants suitable for
in
the invention can be readily ascertained, in view of the foregoing
description, from
McCutcheon's Detergents and EmNlsifiers, North American Edition, McCutcheon
Division, MC Publishing Co., Glen Rock, NJ 07452 and the CTFA International
Cosmetic
Ingredient Handbook, Published by The Cosmetic, Toiletry, and Fragrance
Association,
Washington, D.C.
Amphoteric surfactants suitable for use in a composition according to the
present
invention include materials of the type are offered commercially under the
trade name
"Miranol." Another useful class of amphoteric surfactants is exemplified by
cocoamidopropyl betaine, commercially available from various sources.
The foregoing surfactants when employed with a buffer enhancer will generally
be
present in an amount from 0.01 to 5.0 percent (w/w), preferably 1.0 to 5.0
percent.
Typically, the aqueous solutions of the present invention for treating contact
lenses are also adjusted with tonicity agents, to approximate the osmotic
pressure of
normal lacrimal fluids which is equivalent to a 0.9 percent solution of sodium
chloride or
2.5 percent of glycerol solution. The solutions are made substantially
isotonic with
physiological saline used alone or in combination, otherwise if simply blended
with sterile
water and made hypotonic or made hypertonic the lenses will lose their
desirable optical
parameters. Correspondingly, excess saline may result in the formation of a
hypertonic
solution which will cause stinging and eye irritation.
The pH of the present solutions should be maintained within the range of 5.0
to
8.0, more preferably about 6.0 to 8.0, most preferably about 6. S to 7.8,
suitable buffers
may be added, such as boric acid, sodium borate, potassium citrate, citric
acid, sodium
-20-
CA 02269764 1999-04-22
WO 98/20738 PCT/US97/2008T
bicarbonate, TRIS, and various mixed phosphate buffers (including combinations
of
Na2HP04, NaH2P04 and KHZP04) and mixtures thereof. Borate buffers are
preferred,
particularly for enhancing the efficacy of biguanides. Generally, buffers will
be used in
amounts ranging from about 0.0S to 2.5 percent by weight, and preferably, from
0.1 to
1.5 percent. The disinfecting/preserving solutions of this invention
preferably contain a
borate or mixed phosphate buffer, containing one or more of boric acid, sodium
borate,
potassium tetraborate, potassium metaborate or mixtures of the same. In
addition to
buffering agents, in some instances it may be desirable to include
sequestering agents in
the present solutions in order to bind metal ions which might otherwise react
with the lens
and/or protein deposits and collect on the lens. Ethylene-diaminetetraacetic
acid (EDTA)
and its salts (disodium) are preferred examples. They are usually added in
amounts
ranging from about 0.0l to about 0.2 weight percent. Other suitable
sequestering agents
include gluconic acid, citric acid, tartaric acid and their salts, e.g. sodium
salts.
The aqueous isotonic solutions of the bis(biguanides) of Formula (I) with
optional
1 S other germicidal agents are especially useful for soft contact lenses,
with or without
further additives. Nevertheless, the solutions of the present invention may be
formulated
into specific contact lens care products, such as wetting solutions, soaking
solutions,
cleaning and conditioning solutions, as well as multi-purpose type lens care
solutions, etc.
and mixtures thereof. Such additives make the solutions more acceptable to the
user in
terms of greater comfort. However, the additives must be non-toxic and
compatible with
contact lenses.
It may also be desirable to include water-soluble viscosity builders in the
solutions
of the present invention. Because of their demulcent effect, viscosity
builders have a
tendency to enhance the lens wearer's comfort by means of a film on the lens
surface
cushioning impact against the eye. Included among the water-soluble viscosity
builders
are the cellulose polymers like hydroxyethyl or hydroxypropyl cellulose,
carboxymethyl
cellulose and the like. Such viscosity builders may be employed in amounts
ranging from
about 0.01 to about 4.0 weight percent or less. The present solutions may also
include
optional demulcents.
-21-
CA 02269764 1999-04-22
WO 98/20738 PCT/IJS97/20087
The aqueous solutions according to the present invention can be effectively
used
in disinfecting contact lenses by any of the well recognized methods. The
lenses may be
treated by the "cold" soaking method at room temperature for a period ranging
from
about 20 minutes to about 12 hours. The lenses are then removed from the
solution,
rinsed with the same or a different solution, for example a preserved isotonic
saline
solution and then replaced on the eye.
As indicated above, contact-lens wearers are commonly required to digitally or
manually rub the contact lenses (typically between a finger and palm or
between fingers)
during daily cleaning and/or disinfecting of contact lenses. In one embodiment
of the
present invention, a method is provided in which rubbing is not required
during treatment
with the claimed specified solution, between removal from the eye and
replacement of the
lens following lens care. In a preferred embodiment of such a method, a soft
lens is
disinfected or both disinfected and cleaned with a multipurpose solution or an
effective
multipurpose solution that is the only daily solution needed for treating the
lens outside
the eye. Thus, in one embodiment of a method according to the invention, the
described
solution is used to treat a contact lens without rubbing, by a method
comprising:
(a) soaking the contact lens that has not been rubbed with the solution
for a specified time period, and
(b) direct placement of the treated contact lens on the eye of the
wearer.
Typically, step (a) may involve immersing the contact lens in the solution.
Soaking
may optionally comprise shaking or similarly agitating a container of the
solution by
manual means. Preferably, step (a) involves a period of soaking the contact
lens in a
container wherein the contact lens is completely immersed in the solution. By
the term
"direct placement" is herein meant that the solution is not diluted or rinsed
off the lens
with a different contact-lens solution prior to "insertion" or placement on
the eye. In a
particularly preferred embodiment, the method uses a no-rub multi-purpose or
effective
multi-purpose solution, wherein no other solution or product is required for
daily cleaning
of the lens, with the possible exception of an enzyme cleaner.
-22-
CA 02269764 1999-04-22
WO 98/20738 PCT/US97120087
Because of the increased microbial efllcacy of the solution according to the
present invention, the solution may be used to treat a lens, without rubbing
the lens, in a
regimen comprising a soak period that is less than four hours, for example,
where
sufficient disinfection can be obtained within a period that is in the range
of about 20
S minutes to about 90 minutes, wherein the recommended minimum soaking period
is a
given time within the range of about 20 minutes to about 90 minutes.
Preferably, the
solution may be formulated to provide the necessary disinfection, without
rubbing, by
soaking the lens in the solution for a given period within the range of about
50 minutes to
about 70 minutes, preferably about 60 minutes, corresponding to a minimum
soaking
period of about 50 to about 70 minutes, preferably a minimum soaking period of
about 60
minutes.
In yet another embodiment of a method according to the present invention, the
claimed solution is used to clean a frequent replacement lens (FRL) that is
planned for
replacement after not more than about three months of use in the eye, or that
is planned
for replacement after not more than about 30 days of use in the eye, or that
is planned for
replacement after not more than about two weeks in the eye. Preferably, the
lens is made
from a polymer comprising about 0.0 to 5 mole percent repeat units derived
from
methacrylic acid (MAA), 10 to 99 mole percent of repeat units derived from
hydroxyethyl
methacrylate, and about 0.5 to 5 mole percent of cross-linking repeat units.
Cross-linking
repeat units may be derived, for example, from such monomers as ethyleneglycol
dimethacrylate, divinylbenzene, and trimethylpropane trimethacrylate.
The following Examples illustrate the compositions and methods of the instant
invention.
EXAMPLE 1
This Example illustrates the preparation of 1,6 bis(cyanoguanidino) hexane,
used
as a starting material for bis{biguanides) of the present invention. In the
amount of 35.80
g (0.402 mole), sodium dicyanamide (NaC2N3) is suspended in 400 mL of 1-
butanol.
Then, 23.60 g (0.204 mole) of 1,6-hexanediamine were added as well as 33.0 mL
of conc.
aqueous hydrochloric acid (0.400 mole). A milky white precipitate appeared
immediately
-23-
CA 02269764 1999-04-22
WO 98l20738 PCT/US97120087
which was probably the amine hydrochloride. The mixture was then refluxed for
3.5 hr.
The suspension was then cooled to room temperature and filtered. The white
solid was
then washed well with distilled water before drying under vacuum. Yield 46.38
g; 93.1%.
C 10 H 18 N 8 calc'd: C 48.0%; H 7.20%; N 44.80%; found: C 47.7%; H 7.40%; N
45.12%. 300 MHz'H NMR (d6-DMSO) 6.60 ppm (6p, br m); 2.93 ppm (4p, m); 1.34
ppm (4p, br s); 1.15 ppm (4p, br s). IR (KBr pellet, cm-') 3142 (m); 2943;
2912; 2862
(w); 2179 (s); 1658; l609 (s).
EXAMPLE 2
This Example illustrates the preparation of alexidine for use in the present
invention. Hexamethylene bis(cyanoguanido) in the amount of 1.003g (0.004498
moles)
was placed into a flask. To this was added 1.474 mL ( 1.163g; 0.008996 moles)
of 2-
ethylhexylamine. Then, 0.74 mL (0.008996 moles) of concentrated HCl was added.
The
mixture was heated in a flask to boil away the H20. After the H20 was gone,
the
temperature of the melt had risen to 195~C. The temperature was decreased to
150-
160~C and maintained for 1 hour. The material was cooled to room temperature.
The
solid can be dissolved in hot water and allowed to crystallize.
EXAMPLE 3
This Example illustrates the preparation of poly(hexamethylene biguanide),
also
referred to as PAPB or PHMB, for use in combination with bis(biguanides) in
the present
invention. In 500 mL of distilled water was suspended 25.08 g (0.100 mole) of
1,6-
bis(cyanoguanidino)hexane and 18.99 g (0.100 mole) of 1,6-hexanediamine
dihydrochloride. The pH of this mixture was then brought down to 6.8 with
dilute
hydrochloric acid. The water was then removed by distillation under reduced
pressure.
The white solid was then transferred to a three-necked flask fitted with a
mechanical
stirrer and heating mantle. The intimate mixture of solids was then placed
under nitrogen
and the temperature of the mixture was raised to 1 SO-55~C. The molten
reaction mixture
possessed the consistency of honey. The mixture was stirred at 150-55~C for 1 -
1.5 hr.
before cooling to room temperature. The resulting poly(hexamethylene
biguanide) is
-24-
CA 02269764 1999-04-22
WO 98/20738 PCT/US97/20087
obtained as a glassy solid. The yield is essentially quantitative. Melting
range 105-l25~C.
300 MHz 'H NMR (DZO) 3.13 ppm (21.1 p, br t); 2.93 ppm (2p, t); 1.49 ppm (21.1
p, br
s); 1.28 ppm (21.1p, br s). IR (KBr pellet, crri') 3325; 3201 (s); 2931; 2858
(m); 2175
(m-w); 1631; 1589; 1550(s).
EXAMPLE 4
This Example illustrates the preparation of an aqueous contact-lens
disinfectant
solution according to the present invention.
TABLE 1
_ ..Percent (w/v)
Alexidine2HCl 0.0004
Poloxamine 1107 * * 1.0
I S Na2EDTA 0.11
Boric Acid 0.66
Sodium Borate 0.10
Sodium Chloride 0.54
Distilled Water qs 100.0
** molecular weight I4,500) 70% (w/v) Tetronic~ 1107, a poly(oxypropylene)
poly(oxyethylene) block
copolymer adduct of ethylene diamine, a trademark of BASF Wyandotte Corp.,
Wyandotte) MI.
The solution is prepared by gradually heating 80 percent of the water to 80~C
while dissolving the disodium EDTA therein. The boric acid and sodium borate
are added
to the heated solution of disodium EDTA and dissolved. The sodium chloride is
then
added to the solution and dissolved, followed by the addition of surfactants.
The solution
is sterilized by autoclaving to 120~C for 45 minute. After the solution is
cooled to room
temperature, the bis(biguanide) is added through a sterile filter, followed by
the balance of
distilled water. The solution is packaged in sterilized plastic containers.
-25-
CA 02269764 1999-04-22
WO 98/20738 PCT/US97/20087
EXAMPLE S
This Example illustrates the microbiocidal efficacy of solutions according to
the
present invention. The antimicrobial efficacy of each of various compositions
for the
chemical disinfection of contact lenses was evaluated. Microbial challenge
inoculums
were prepared using Pseudomonas aeruginosa (ATCC 9027), Staphylococcus aureus
(ATCC 6538), Serratia marcescens (ATCC 13880), Candida albicans (ATCC 10231),
and Fusarium solani (ATCC 36031 ). The test organisms were cultured on
appropriate
agar and the cultures were harvested using sterile DPBST (Dulbecco's Phosphate
Buffered Saline plus 0.05% w/v polysorbate 80) or a suitable diluent and
transferred to a
suitable vessel. Spore suspensions were filtered through sterile glass wool to
remove
hyphal fragments. Serratia marcescens, as appropriate, was filtered (eg.,
through a 1.2t
filter) to clarify the suspension. After harvesting, the suspension was
centrifuged at no
more than S000 x g for a maximum of 30 minutes at 20-25~C. The supernatant was
poured off and resuspended in DPBST or other suitable diluent. The suspension
was
centrifuged a second time, and resuspended in DPBST or other suitable diluent.
All
challenge bacterial and fungal cell suspensions were adjusted with DPBST or
other
suitable diluent to 1 x 10'-10g cfu/mL. The appropriate cell concentration may
be
estimated by measuring the turbidity of the suspension, for example using a
spectrophotometer at a preselected wavelength, for example 490 nm. One tube
was
prepared containing a minimum of 10 mL of test solution per challenge
organism. Each
tube of the solution to be tested was inoculated with a suspension of the test
organism
su~cient to provide a final count of 1.0 x 105-106 cfu/mL, the volume of the
inoculum not
exceeding 1 % of the sample volume. Dispersion of the inoculum was ensured by
vortexing the sample for at least 1 S seconds. The inoculated product was
stored at 10-
25~C. Aliquots in the amount of 1.0 mL were taken of the inoculated product
for
determination of viable counts after certain time periods of disinfection. The
time paints
for the bacteria were, for example, 1, 2, 3, and 4 hours when the proposed
regimen
soaking time was 4 hours. Yeast and mold were tested at an additional
timepoint of >_ 16
hours (4 times the regimen time). The suspension was mixed well by vortexing
vigorously for at least 5 second. The 1.0 mL aliquots removed at the specified
time
-26-
CA 02269764 1999-04-22
WO 98/20738 PCT/EJS97/20087 -
intervals were subjected to a suitable series of decimal dilutions in
validated neutralizing
media. The suspensions were mixed vigorously and incubated for a suitable
period of
time to allow for neutralization of the microbial agent. The viable count of
organisms was
determined in appropriate dilutions by preparation of triplicate plates of
trypticase soy
(TSA) agar for bacteria and Sabouraud dextrose agar (SDA) for mold and yeast.
The
bacterial recovery plates were incubated at 30-35~C for 2-4 days. The yeast
was
incubated at 20-30~C for 2-4 days and mold recovery plates at 20-25~C for 3-7
days. The
average number of colony forming units was determined on countable plates.
Countable
plates refer to 30-300 cfu/plates for bacteria and yeast, and 8 to 80
cfu/plate for mold
except when colonies are observed only for the 10~ or 10'' dilution plates.
The microbial
reduction was then calculated at the specified time points. In order to
demonstrate the
suitability of the medium used for growth of the test organisms and to provide
an
estimation of the initial inoculum concentration, inoculum controls were made
by
dispersing an identical aliquot of the inoculum into a suitable diluent, for
example
DPBST, using the same volume of diluent used to suspend the organism as listed
above.
Following inoculation in a validated neutralizing broth and incubation for an
appropriate
period of time, the inoculum control must be between 1.0 x 1 OS - 1.0 x 106
cfu/mL
The solutions were evaluated based on the performance requirement referred to
as
the "Stand-Alone Procedure for Disinfecting Products" (hereafter the "stand-
alone test")
and is based on the Disinfection Efficacy Testing for contact lens care
products under the
Draft Premarket Notification (510(k)) Guidance Document For Contact Lens Care
Products dated April 1, 1996, prepared by the U.S. Food and Drug
Administration,
Division of Ophthalmic Devices. This performance requirement is comparable to
current
ISO standards for disinfection of contact lenses (revised 1995). The stand-
alone test
challenges a disinfecting product with a standard inoculum of a representative
range of
microorganisms and establishes the extent of viability loss at pre-determined
time intervals
comparable with those during which the product may be used.. There is a
primary
performance criteria and secondary performance criteria. The primary criteria
for a given
disinfection period (corresponding to a potential minimum recommended
disinfection
period) is that the number of bacteria recovered per mL must be reduced by a
mean value
-27-
CA 02269764 1999-04-22
WO 98/20738 PCT/US97/20087
of not less than 3.0 logs within the given disinfection period. The number of
mold and
yeast recovered per mL must be reduced by a mean value of not less than 1.0
log within
the minimum recommended disinfection time with no increase at four times the
minimum
recommended disinfection time. If failing to pass this primary performance
criteria, the
secondary performance criteria exists which if passed qualifies the solution
for the so-
called "regimen test procedure" described in the FDA's Draft for Premarket
Notification
(510(k)) Guidance Document For Contact Lens Care Product, dated April 1, 1996
or
similarly described in the ISO/CEN regimen test procedure. Under the secondary
performance criteria, there must be a combined log reduction for the mean
values of all
three bacteria of not less than 5.0 logs within the recommended given
disinfection period.
The minimum acceptable mean log reduction for any single bacterial type is 1.0
log.
Stasis for the yeast and mold must be observed for the recommended
disinfection period.
If passing the secondary performance criteria (also referred to as minimum
antimicrobial
activity by the Stand-Alone Procedure), the regimen test challenges the
proposed
disinfection regimen (typically involving rubbing) with a standard inoculum of
a
representative range of microorganisms, in which test the inoculum is carried
through the
various stages of regimen by preliminary application to contact lenses.
The above testing procedures were used for evaluating the antimicrobial
efficacy
of disinfecting solutions such as prepared in Example 4, but which contain the
bis(biguanide) alexidine at various concentrations extending from 1 ppm to 5
ppm for a
testing period of one hour. The results are shown in Table 2 below
-28-
CA 02269764 1999-04-22
WO 98I20738 PCT/US97/20087
' TABLE 2
Alexidine2HC1
Microo anism Concentration Log Reduction
m 1 hr
S. aureus 1 1.6
S. aureus 2 4.3
S. aureus 3 >4.8
S. aureus 4 >4.8
S. aureus S >4.8
P. aeruginosa 1 >5.0
P. aeruginosa 2 >5.0
P. aeruginosa 3 >5.0
P. aeruginosa 4 >5.0
P. aeruginosa 5 >S.0
S. marcescens 1 3.5
S. marcescens 2 >4.8
S. marcescens 3 >4.8
S. marcescens 4 >4.8
S marcescens 5 >4.8
The above results show that at 2.0 ppm the bis(biguanide) alexidine solution
passes the one hour stand-alone test for bacteria, by which a 3 log reduction
of all three
bacteria is needed in the given disinfection time. According to the above
results, at 1.0
ppm, alexidine will not pass the one hour stand-alone test for bacteria
(failing with respect
to Serratia marcescens). In order to pass the 1 hour stand-alone test, the
fungi must also
be tested, as in the next example.
EXAMPLE 6
This Example shows the antimicrobial efficacy of the solution of Example 4
according to the present invention using the testing procedures described in
Example 5
above, but formulated at a concentration of 4.0 ppm alexidine and at time
intervals of 15
-29-
*rB
CA 02269764 1999-04-22
WO 98/20738 PCT/US97/20087
' minutes, 30 minutes, 45 minutes, and 60 minutes, which would represent 25%,
50%, 75%
and 100% of a one hour minimum recommended disinfection time. The solution had
been
aged for 19 months at 25~C. The results are shown in Table 3 below.
TABLE 3
Microorganism Soak Period Log Reduction
15 min. 2.5
Staphylococcus 30 min. 4.4
aureus 45 min. >4.9
60 min. >4.9
15 min. 4.0
Pseudomoas 30 min. >4.8
aerugiosa 45 min. >4.8
60 min. >4.8
15 min. 3.2
Serratia 30 min. >4.7
marcesces 45 min. >4.7
60 min. >4.7
15 min. 1.9
Candida 30 min. 2.5
albicas 45 min. 2.7
60 min. 2.8
4 hr. 3.4
15 min. 3.4
Fusarium 30 min. 4.3
Bolai 45 min. 4.4
60 min. 4.2
4 hr. >4.4
Based on the same criteria as described in Example 5, the alexidine solution
formulated at 4.0 ppm is able to pass a stand-alone test (with no rub or
rinse) at the 30
minute time point, 45 time point and the 60 minute time point. With respect to
Staphylococcus aureus the 1 S minute time point failed.
-30-
CA 02269764 1999-04-22
WO 98/20738 PCTIUS97I20087
' EXAMPLE 7
This Example shows the antimicrobial eiI'lcacy of a solution according to the
present invention using the testing procedures described in Example S above.
These tests
were similar to Example 6 except with a different lot that had been aged for
18 months.
The concentration of alexidine was formulated at 4.0 ppm and the time
intervals were 15
minutes, 30 minutes, 45 minutes, and 60 minutes. The results are shown in
Table 4
below.
TABLE 4
Microorganism Soak Period Log Reduction
1 S min. 3.7
Staphylococcus 30 min. 4.7
aureus 45 min. >4.8
60 min. >4.8
I S min. 4.2
Pseudomonas 30 min. >4.8
aeruginosa 45 min. >4.8
60 min. >4.8
15 min. 3.0
Serratia 30 min. 4.7
marcescens 45 min. >4.8
60 min. >4.8
15 min. 1.0
Candida 30 min. 1.7
albicans 45 min. 1.9
60 min. 2.1
4 hr. 3.1
15 min. 2.4
Fusarium 30 min. >4.5
solani 45 min. >4.5
60 min. >4.5
4 hr. >4. 5
In the above testing, the alexidine passed the stand alone test at 30 minutes,
45 minutes,
and 60 minutes, but only just passed the 15 minute stand-alone test with
respect to
Serratia marcescens and Candida albicans. It is clear that at 30 minutes, 45
minutes, and
one hour stand-alone showed greater disinfection, easily passing the
acceptance criteria.
-31-
CA 02269764 1999-04-22
WO 98/20738 PCTlUS97/20087
EXAMPLE 8
This Example shows the antimicrobial efficacy of a solution according to the
present invention using the testing procedures described in Example 5 above.
For a final
time, these tests were similar to Example 6 except with a different batch. The
concentration of alexidine was formulated at 4.0 ppm and the time intervals
were 15
minutes, 30 minutes, 45 minutes, and 60 minutes, which would represent 25%,
50% and
100% of a one hour minimum recommended disinfection period. The solution had
been
aged 19 months at 25~C. The results are shown in Table 5 below.
TABLE 5
Microorganism Soak Period Log Reduction
15 min. . 3.5
Staphylococcus 30 min. >4.9
aureus 45 min. >4.9
60 min. >4.9
15 min. 3.3
Pseudomonas 30 min. >4.8
aerugiosa 45 min. >4.8
60 min. >4.8
15 min. 2.5
Serratia 30 min. 4.6
marcescens 45 min. >4.7
60 min. >4.7
15 min. 0.9
Candida 30 min. 1.7
albicans 45 min. 1.8
60 min. 1.9
4 hr. 2.6
15 min. 3.6
Fusarium 30 min. >4.6
solani 45 min. >4.6
60 min. >4.6
4 hr. >4.6
-32-
CA 02269764 1999-04-22
WO 98I20738 PCT/US97/20087
' In the above test, the alexidine solution passed the stand-alone test
(without
rubbing) at 30 minutes, 45 minutes, and 60 minutes) but did not pass the test
at 15
minutes, since at 15 minutes the log reduction for Serratia marcescens was
less than 3
logs and the number of the yeast Candida albicans was not reduced by at least
1 log. The
results in Tables 3, 4, and 5 above show some variation due to the testing of
different lots
of the solutions described in Example 4 above, but that a 4.0 ppm alexidine
solution
would require at least about 30 minutes in order to pass a stand-alone
procedure without
rubbing.
COMPARATIVE EXAMPLE 9
This Example compares the antimicrobial efficacy of a solution according to
the
present invention (such as formulated in Example 4) compared to an analogous
solution
containing only PInVIB as the germicide. Both solutions were borate buffered
to enhance
the efficacy of the biguanide. The testing procedures used for evaluating the
antimicrobial
efficacy of disinfecting solutions was the same as in Example 5 above. The
results are
shown in Table 6 below.
-33-
CA 02269764 1999-04-22
WO 98/20738 PCT/US97120087
TABLE 6
Microorganism 4.0 ppm
Tested Time Tested 1.0 ppm PHMB Alexidine2HCI
1 hr 3.5 >4.9
Staphylococcus2 hr 3.9 >4.9
aureus 3 hr >4.9 >4.9
4 hr >4.9 >4.9
1 hr 3.5 >4.8
Pseudomonas 2 hr 4.0 >4.8
aeruginosa 3 hr 4.1 >4.8
4 hr >4.8 >4.8
1 hr 3.2 3.7
Serratia 2 hr 4.2 >4.7
marcescens 3 hr >4.7 >4.7
4 hr >4.7 >4.7
1 hr 2.9 2.9
Candida 2 hr 3.0 4.3
albicans 3 hr 3.4 >4.7
4 hr 3.4 3.9
24 hr 4.3 4.6
1 hr 0.4 3.1
Fusarium 2 hr 0.5 4.1
solani 3 hr 0.4 4.5
4 hr 0.4 4.6
24 hr 1.3 >4.6
As indicated in Example S, the acceptance criteria was that the number of
bacteria
recovered per mL must be reduced by a factor of not less than 99.9% (3 logs)
with the
minimum disinfection period (which would be recommended with the product, in
label,
package, and/or package insert instructions), and the number of mold and yeast
recovered
per mL shall be reduced by a factor of not less than 90% ( 1 log) within the
minimum
recommended disinfection time, with no increase at four times the minimum
recommended disinfection time. The above results show that the 1.0 ppm PHIvlB
did not
pass the one hour stand-alone test, since the Fusarium solani, a mold, was not
sufficiently
reduced. In contrast, the 4.0 ppm alexidine solution according to the present
invention
passed the one hour stand-alone test, showing a reduction in the number of
mold by 3.1
log after 1 hour. Presently, Pl-I1VVIB is used in an amount of about 1.0 ppm
in commercial
-34-
CA 02269764 1999-04-22
W0 98/20738 PCT/tTS97/20087
solutions for disinfecting soft lenses. It has been found that increasing the
level of P1~IB
to 3.0 ppm results in clinical findings that suggest PHNIB may be less safe
than desirable,
for use with soft contact lens. In contrast, as shown in the next example,
based on rabbit
studies, it has been found that a 4.0 ppm solution of alexidine would be very
safe for
human use in disinfecting soft lenses.
EXAMPLE 10
This example demonstrates the ocular toxicology of alexidine in solutions such
as
described in Example 4 above, but at various concentrations of the
bis{biguanide). A
series of formulations were prepared in which the amount of alexidine were,
respectively
4 ppm (test I ), 6 ppm (test 2), 8 ppm (test 3), 10 ppm (test 4), 12 ppm (test
5), I S ppm
{test 6), 18 ppm (test 7) , 22 ppm (test 8), 27 ppm (test 9), 33 ppm {test
10), 40 ppm (test
11 ) and 50 ppm (test 12). These test solutions of alexidine were used in
soaking J&J
SureVue~ (etafilcon A) lenses (soft lenses) in 2.5 mL of solution for 1 week
or more in
order to reach maximal alexidine uptake.
An ocular irritation screening study was conducted in the rabbit to determine
the
threshold for ocular irntation. Eyes treated with lenses were compared to a
contralateral
control eye. Treated lenses were placed on the right eye of rabbits; control
lenses were
placed on the left eye. Any lenses displaced from the eyes during the lens
wear day were
reinserted after rinsing with 0.9% sodium chioride USP solution (SC). Eyes
were
examined with the aid of a direct light source before lens placement, at 50-70
minutes, 7-8
hours, 23-24 hours, and 30-32 hours after placement. Macroscopic observations
were
recorded in accordance with criteria in the Draize score system. The maximum
total
score is the sum of all scores obtained for the cornea, iris, and
conjunctivae. Total
maximum score possible is 110 per eye, 80 with respect to the cornea, 10 with
respect to
the iris, and 20 with respect to the conjunctivae.
The results of macroscopic ocular examinations for each animal appear in Table
7
below (weighted totals present).
* Draize, J.H., G. Woodward and H.O. Calvery. l944. Methods for the study of
irritation and
toxicity of substances applied topically to the skin and mucous membranes. J.
Pharmacol. Exp. Ther.
82:377-390.
-35-
CA 02269764 1999-04-22
WO 98/20738 PCT/US97120087
TABLE 7
POS T PPLICATION
LENS
A
ppm
Test No. Alexidine Prior 50-70 7-8 23-24 30-32
2HC1 to Minutes Hours Hours Hours
Lens
A
lication
R L R L R L R L R L
1 4 0 0 2 2 2 0 2 0 2 2
2 4 0 0 2 2 2 2 2 2 2 2
3 4 0 0 2 2 2 2 2 2 2 2
4 6 2 0 2 2 2 0 2 0 2 0
6 2 2 2 2 2 2 2 2 2 2
6 6 2 2 2 2 2 0 2 0 2 0
7 8 0 0 2 0 2 0 2 2 2 2
8 8 2 2 2 2 2 2 2 0 2 2
9 8 2 2 2 2 2 2 2 2 2 2
10 2 0 2 0 4 0 2 0 2 0
11 10 2 2 2 0 2 0 2 2 2 2
12 10 0 0 2 0 4 2 0 0 0 0
13 12 2 0 2 2 2 2 2 2 2 2
14 12 2 2 2 2 2 2 2 2 2 2
12 2 2 2 2 2 2 2 2 2 2
16 15 0 0 2 2 4 0 2 0 2 0
17 15 2 2 4 2 4 0 2 2 2 2
18 15 0 0 2 0 4 0 0 0 2 0
19 18 2 0 2 2 6 2 2 0 2 0
18 2 2 2 2 6 2 2 2 2 2
21 18 2 2 2 2 6 2 2 2 2 2
22 22 2 0 2 2 11 0 2 0 2 2
23 22 2 2 2 2 I1 0 2 0 2 2
24 22 0 0 2 0 I1 0 2 0 2 0
27 2 0 2 2 13 2 2 2 2 0
26 27 2 0 2 2 11 2 2 2 2 0
27 27 2 2 2 2 6 2 2 2 2 0
28 27 2 0 2 2 11 2 7 2 ? 2
29 27 2 0 2 2 11 2 7 2 7 2
27 2 0 2 2 9 0 2 0 2 0
R = Right (Test) Eye
L = Left (Control) Eye
-36-
CA 02269764 1999-04-22
WO 98I20738 PCT/US97/20087
When worn by rabbits, lenses soaked in 10 ppm Alexidine showed the first signs
of
increased ocular toxicity, with conjunctiva) reactions observed after 7-8
hours of lens
wear. This sporadic conjunctiva) reaction was clearly defined at a dose of 15
ppm
alexidine in solution and continued to become more severe as the dosage was
elevated.
Inidial involvement was observed at 22 ppm and corneal opacity was observed at
27 ppm.
Conjunctiva) redness, chemosis, and discharge were the sole basis of the
Draize findings
for lens numbers until 22 ppm and 27 ppm (test nos. 8 and 9). Iritis, in
addition to
previously described findings were observed for lens number 8 and 9. The above
results
indicate that a formulation with 10 ppm of alexidine or more is not
recommended for
disinfecting soft contact lenses. The preferred formulation with 4 ppm
alexidine,
represents a 2.5-fold safety margin relative to the initial onset of any
possible abnormal
ocular health in rabbits.
EXAMPLE i 1
This example illustrates the preparation of aqueous disinfecting solutions
according to the present invention comprising a combination of alexidine and
polyhexamtheylene biguanide (also referred to as PAPB or PHMB), The following
components are used, in the indicated percent weight per total volume of the
solution:
Percent (w/v)
PHMB HCl 0.00008
Alexidine2HCI 0.0002
Poloxamine 1107** 1.0
NazEDTA 0.1l
Boric Acid 0.66
Sodium Borate 0.10
Sodium Chloride 0..54
Distilled Water (qs) 100.0
** molecular weight 14,500, 70% (w/v) Tetronic~ 1107, a poly(oxypropylene)
poly(oxyethylene) block
copolymer adduct of ethylene diamine) a trademark of BASF Wyandotte Corp.,
Wyandotte, MI.
-37-
CA 02269764 1999-04-22
WO 98/20738 PCT/US97/20087
The solution is prepared by gradually heating 80 percent of the water to 80~C
while dissolving the disodium EDTA therein. The boric acid and sodium borate
are added
to the heated solution of disodium EDTA and dissolved. The sodium chloride is
then
added to the solution and dissolved, followed by the addition of surfactants.
The solution
is sterilized by autoclaving to 120~C for 45 minute. After the solution is
cooled to room
temperature, the bis(biguanide) and the PAPB are added through a sterile
filter, followed
by the balance of distilled water. The solution is packaged in sterilized
plastic containers.
EXAMPLE 12
This Example illustrates the improved antimicrobial efficacy of a combination
of
alexidine with polyhexamtheylene biguanide (PAPB) in an aqueous disinfecting
solution
for contact lenses. The testing procedures described in Example 5 above were
followed
to determine whether the primary performance criteria would be passed at time
intervals
of 5 minutes, 15 minutes, 30 minutes, and 4 hours. The concentration of
alexidine in this
set of tests was formulated in amounts ranging from 0.0 to 4.0 ppm in
combination with
either 0.0 (control) or 0.8 ppm alexidine, the latter the amount of PAPB
currently used in
commercial multi-purpose solutions for soft contact lenses. The results are
shown in
Table 8 below.
-38-
CA 02269764 1999-04-22
WO 98I20738 PCT/US97/20087
TABLE 8
Alexidine PAPB Log ReductionLog Reduction
2HC1 HCl Soak Period ,~ marcescensG albicans
~PPm) ~PPm)
S min. - 0.6
0.5 0.8 1 S min. 2.0 1.1
30 min. 3.2 1.8
60 min. 4.3 3.5
5 min. - 0.5
1.0 0. 8 15 min. 2.6 1.1
30 min. 3.7 2.2
60 min. >4.3 4.2
S min. - 0.7
2.6 0.8 15 min. 2.7 2.2
30 min. >4.3 3.3
60 min. >4.3 >4.2
5 min. - 1.1
4.0 0.8 1 S min. >4.3 3.0
30 min. >4.3 >4.2
60 min. >4.3 >4.2
5 min. - 0.3
0.5 0.0 I S min. 0.6 1.0
30 min. 1.6 -0.2
60 min. 2.5 -0.1
5 min. - 0.4
2.6 0.0 15 min. 2.2 0. S
30 min. >4.3 0.5
60 min. >4.3 I .4
15 min. - 0.6
0.0 0.8 30 min. 1.3 0.8
45 min. 2.0 1.4
60 min. 3.1 2.9
The results show that the addition of alexidine to the polyhexametheylene
biguanide very significantly improved antimicrobial efficacy, with the
improved efficacy
reaching a trade-off, for practical purposes, at about 4.0 ppm, such that any
improved
antimicrobial efficacy would be unlikely to be justified by the increased
potential for
toxicity at higher concentrations of the antimicrobial agents. With respect to
C. albicans,
there appears to be a synergistic effect at time periods of 15 minutes and 30
minutes, with
-39-
CA 02269764 1999-04-22
WO 98l20738 PCT/US97/20(187
' the combination of 2.6 ppm alexidine and 0.8 ppm PAPB showing greater
ei~icacy than
the sum of 2.6 ppm alexidine by itself and 0.8 ppm PAPB by itself.
EXAMPLE 13
This example illustrates the preparation of an aqueous disinfecting solution
according to the present invention comprising a combination of alexidine and
polyhexamtheylene biguanide (also referred to as PHMB). The following
components are
used, in the indicated percent weight per total volume of the solution:
TABLE 9
Percent w/v
PHMB 0.00008
Alexidine2HC1 0.0002
Poloxamine 1l07** 1.0
Na2EDTA 0.11
Boric Acid 0.66
Sodium Borate 0.10
Sodium Chloride 0
.54
Distilled Water (qs) - _. _
I -100.0
** molecular weight I4,500) 70% (w/v) Tetronic~ 1107, a poly(oxypropylene)
poly(oxyethylene) block
copolymer adduct of ethylene diamine, a trademark of BASF Wyandotte Corp.,
Wyandotte) MI.
The solution is prepared by gradually heating 80 percent of the water to 80~C
while dissolving the disodium EDTA therein. The boric acid and sodium borate
are added
to the heated solution of disodium EDTA and dissolved. The sodium chloride is
then
added to the solution and dissolved, followed by the addition of the
surfactant. The
solution is sterilized by autoclaving to 120~C for 45 minute. After the
solution is cooled
to room temperature, the alexidine bis(biguanide) and the PHMB are added
through a
sterile filter, followed by the balance of distilled water. The solution is
packaged in
sterilized plastic containers.
-40-
CA 02269764 1999-04-22
WO 98/20738 PCT/US97/20087
EXAMPLE 14
This Example illustrates the microbiocidal efficacy of solutions according to
the
present invention. The above testing procedures were used for evaluating the
antimicrobial e~cacy against C. albicans of disinfecting solutions such as
prepared in
S Example 13, but which contain the bis(biguanide) alexidine at various
concentrations
extending from 1 ppm to 4 ppm and the biguanide polymer at various
concentrations
extending from 0.3 to 1.5 ppm. The results are shown in Table 10 after a 5
minute soak,
Table 11 after a 15 minute soak, Table 12 after a 30 minute soak, and Table 13
after a 45
minute soak. Tables 14 to 17, corresponding respectively to Tables 10 to 13,
compares
the theoretical kill, based on the sum of individual disinfecting agents
versus actual kill
using the combination of disinfecting agents.
TABLE 10
(8 Minutes)
PHMB
0 ppm 0.3 ppm 0.8 ppm 1.5 ppm
0 ppm 0.4 0.4 0.7 1.0
1 ppm 0.5 0.6 0.9 1.1
Alexidine 2 ppm 0.7 0.8 0.9 1.5
3 ppm 0.8 1.1 1.6 2.6
4 ppm 1.1 1.8 2.8 3.4
-41-
CA 02269764 1999-04-22
WO 98/20738 PCT/IJS97/20087
TABLE 11
(15 Minutes)
PHMB
0 ppm 0.3 ppm 0.8 ppm 1.5 ppm
0 ppm 0.4 0. 7 1. S 1.9
1 ppm 0.4 I .1 2.2 2.9
Alexidine 2 ppm 0.8 1.7 2.8 3.5
3 ppm 1.6 2.7 3.7 4.5
4 ppm 2.6 3.8 4.8 >4.8
TABLE 12
(30 Minutes)
PHMB
0 ppm 0.3 ppm 0.8 ppm 1.5 ppm
0 ppm 0.4 1.0 2.1 3.1
I ppm 0.5 1.8 3.3 4.4
Alexidine 2 ppm 1.2 2.1 3.7 4.5
3 ppm 1.9 3.5 4.8 >4.8
4 ppm 3.4 >4.8 >4.8 >4.8
-42-
CA 02269764 1999-04-22
WO 98/20738 PCT/US97/20087
TABLE 13
(45 Minutes)
PHMB
0 ppm 0.3 ppm 0.8 ppm 1.5 ppm
0 ppm 0.4 1.1 3.0 4.1
1 ppm 0.4 2.1 3.7 >4.8
Alexidine 2 ppm 0.9 2.6 4.5 >4.8
3 ppm 2.1 4.5 >4.8 >4.8
4 ppm 3.7 4.8 >4.8 >4.8
TABLE 14
(5 Minutes)
Log Kill
Formulation Theoretical Actual
1 ppm Alex/0.3 ppm P 0.9 0.6
2 ppm Alexl0.3 ppm P~ 1.1 0.8
3 ppm Alex/0.3 ppm PHNIB1.2 1.1
4 ppm Alex/0.3 ppm PHMIi1.5 1.8
1 ppm Alex/0.8 ppm PH1VIB1.2 0.9
2 ppm Alex/0.8 ppm PHM131.4 0.9
3 ppm Alex/0.8 ppm PH1V1>B1.5 1.6
4 ppm Alex/0.8 ppm PHMB 1.8 2.8
1 ppm Alex/1.5 ppm PHMA 1.5 1.1
2 ppm Alex/1.5 ppm P~ 1.7 1.5
3 ppm Alex/1.5 ppm PH1V1131.8 2.6
4 ppm Alex/1.5 ppm PHIVViB2.1 3.4
-43-
CA 02269764 1999-04-22
WO 98I20738 PCT/US97/20087
TABLE 15
(15 Minutes)
~~i
. Log Kill
Formulation Theoretical Actual
I ppm Alex/0.3 ppm PHN>B I .1 I . I
2 ppm Alex/0.3 ppm Pl-llVIB1.5 I .7
3 ppm Alex/0.3 ppm PI-nViB2.3 2.7
4 ppm Alex/0.3 ppm PHIVVIB3.3 3.8
I ppm Alex/0.8 ppm PHMB 1.9 2.2
2 ppm Alex/0.8 ppm PH1VI82.3 -. 2.8
3 ppm Alex/0.8 ppm PHIVVIB3. I 3.7
4 ppm Alex/0.8 ppm PI-IIVVIB4. I 4.8
I ppm Alex/1.5 ppm PH1VI82.3 2.9
2 ppm Alex/1.5 ppm PHNIB 2.7 3.5
3 ppm Alex/ I . 5 ppm 3 . S 4.5
PHIvlB
4 ppm Alex/1.5 ppm PHIVIB4.5 4.8
-44-
CA 02269764 1999-04-22
WO 98/20738 PCT/US97/20087
TABLE 16
(30 Minutes)
Log Kill
Formulation Theoretical Actual
1 ppm Alex/0.3 ppm PHIvvIB1.5 - I , g
2 ppm Alex/0.3 ppm PI-~IB2.2 2. I
3 ppm Alex/0.3 ppm PHMI3 2.9 3.5
4 ppm Alex/0.3 ppm PI-SIB4.4 4.8
I ppm Alex/0.8 ppm PIE 2.6 3.3
2 ppm Alex/0.8 ppm PHMB 3.3 _ - 3 .7
3 ppm Alex/0.8 ppm PHNIB 4.0 4.8 _ _
4 ppm Alex/0.8 ppm PHIvvlB4.8 4.8
1 ppm Alex/I .5 ppm PHNiB3.6 4.4
2 ppm Alex/I.5 ppm PHIvviB4.3 4.5
3 ppm Alex/1.5 ppm PH1VVI84.8 4.8
4 ppm Alex/1.5 ppm PHN18 4.8 4.8
-45-
CA 02269764 1999-04-22
WO 98I20738 PCT/LTS97/20(187
TABLE 17
(45 Minutes)
Log Kill
Formulation Theoretical Actual
1 ppm Alex/0.3 ppm PH1VVIB1.5 2.1
2 ppm Alexl0.3 ppm PHN18 2.0 2,6
3 ppm Alex/0.3 ppm PHIVVIB3.2 4.5
4 ppm Alex/0.3 ppm PIE 4.8 4, 8
1 ppm Alex/0.8 ppm PH1VV)B3.4 3.7
2 ppm Alex/0.8 ppm PI-~4B 3.9 4.5
3 ppm Alex/0.8 ppm PfiNlB 4.8 4.8
4 ppm Alex/0.8 ppm PH1VVIB4.8 4.8
1 ppm Alex/1.5 ppm PHlVlB 4.5 4.8
2 ppm Alex/1.5 ppm PI-~B 4.8 4.8
3 ppm Alexll.5 ppm Pl-1:MB4.8 4.8
4 ppm Alex/1.5 ppm PI-~1~B4.8 4.8
The above results show synergistic microbicidal effects against C. albicans,
in
which the log kill from the combination of both alexidine and PHIVIB, in a
good
proportion of cases, is higher than the sum of the individual disinfecting
agents, which
synergistic effects are evident, beginning with the results after 15 minutes.
At 45 minutes,
these synergistic effects may become less evident, when a higher proportion of
the
microorganisms have already been killed.
While the invention has been described in conjunction with specific examples
thereof, this is illustrative only. Accordingly, many alternatives,
modifications, and
variations will be apparent to those skilled in the art in light of the
foregoing description
and it is, therefore, intended to embrace all such alternatives,
modifications, and variations
as to fall within the spirit and scope of the appended claims.
-46-