Note: Descriptions are shown in the official language in which they were submitted.
CA 02270056 1999-04-27
WO 98/18778 PCT/DK97/00485
1
Title
Novel cis-3,4-chroman derivatives useful in the prevention or treatment of
estrogen related
diseases or syndromes.
Field of the invention
The present invention relates to new cis-3,4-chroman derivatives and the use
of such
compounds in the prevention or treatment of estrogen related diseases or
syndromes,
preferably diseases or syndromes caused by an estrogen-deficient state in a
mammal, in
particular bone loss, osteoporosis, cardiovascular diseases, cognitive
disorders, senile
dementia-Alzheimer's type, menopausal symptoms, including flushing and
urogenital
atrophy, dysmenorrhea, threatened or habitual abortion, dysfunctional uterine
bleeding;
acne, hirsutism, prostatic carcinoma, post-partum lactation, and the use of
such com-
pounds in a contraceptive method or as an aid in ovarian development.
Background of the Invention
The osteopenia that accompanies the menopause continues to represent a major
public
health problem. Left unchecked, the cumulative loss of bone can potentially
compromise
the skeleton's structural integrity, resulting in painful and debilitating
fractures of the wrist,
spine and femur. Efforts to reduce the risk and incidence of fractures have
focused on the
development of therapies that conserve skeletal mass by inhibiting bone
resorption.
Among various treatment modalities, estrogen replacement therapy remains the
preferred
means to prevent the development of post menopausal osteoporosis (Lindsey R,
Hart DM)
MacClean A 1978, "The role of estrogen/progestogen in the management of the
meno-
pause", Cooke ID, ed, Proceedings of University of Sheffield symposium on the
rote of
estrogen and progestogen in the management of the menopause, Lancaster, UK:
MTP
Press Ltd. pp. 9-25; Marshall DH, Horsmann A, Nordin BEC 1977, "The prevention
and
management of post-menopausal osteoporosis.", Acta Obstet Gynecol Scand
(Supply
65:49-56; Recker RR, Saville PD, Heaney RP 1977, "Effect of estrogen and
calcium
carbonate on bone loss in post-menopausal women", Ann Intern Med. 87:649-655;
CA 02270056 1999-04-27
WO 98/18778 PCT/DK97/00485
2
Nachtigall LE, Nachtigall RH, Nachtigall RD, Beckman EM 1979) "Estrogen
replacement
therapy", Obstet Gynecol. 53:277-281 ) and it is now accepted that estrogens
significantly
decrease fracture incidence and risk (Krieger N, Kelsey JL, Holford TR,
O'Connor T 1982,
"An epidemiological study of hip fracture in postmenopausal women", Am J
Epidemiol.
116:141-148; Hutchinson TA, Polansky SM, Feinstein AR 1979, "Post-menopausal
estrogens protect against fractures of hip and distal radius: A case-control
study", Lancet
2:705-709; Paginini-Hill A, Ross RK, Gerkins VR, Henderson BE, Arthur M, Mack
TM
1981, "Menopausal oestrogen therapy and hip fractures", Ann Intern Med. 95:28-
31;
Weiss NS, Ure CL, Ballard JH, Williams AR, Daling JR 1980, "Decreased risk of
fractures
on the hip and lower forearm with post-menopausal use of estrogen", N Eng J
Med.
303:1195-1198).
While the beneficial actions of estrogen replacement therapy on the skeleton
are clearly
significant, there is also considerable evidence for a positive effect of
estrogen on the
cardiovascular system. Previous studies have attributed these actions to
estrogen's
effects on serum lipids, but recent data has now shown that in addition to the
effects on
the lipid profile, estrogen can also directly influence vessel wall
compliance, reduce
peripheral resistance and prevent atherosclerosis (Lobo RA 1990)
"Cardiovascular
implication of estrogen replacement therapy", Obstetrics and Gynaecology,
75:18S-24S;
Mendelson ME, Karas RH 1994, "Estrogen and the blood vessel wall", Current
Opinion in
Cardiology) 1994(9):619-626). Based on available epidemiological data, the
overall impact
of these physiological and pharmacological actions of estrogen is an age
independent
reduction in cardiovascular mortality and morbidity in women (Kannel WH,
Hjortland M,
McNamara PM 1976 "Menopause and risk of cardiovascular disease: The Framingham
Study", Ann Int Med, 85:447-552). Furthermore, a more recent analysis has
concluded
that post-menopausal estrogen replacement therapy reduces the risk of
cardiovascular
disease by approximately 50 percent (Stampfer MJ, Colditz GA 1991, "Estrogen
repiace-
rnent therapy and coronary heart disease: a quantitative assessment of the
epidemiologi-
cal evidence", Preventive Medicine, 20:47-63.}.
In addition to the positive effects of estrogen on bone and cardiovascular
system, there
are now data which indicate that the central nervous system can benefit from
estrogen
replacement therapy. Short term studies in human subjects have shown that
increased
CA 02270056 1999-04-27
WO 98/18778 PCT/DK97/00485
3
levels of estrogen are associated with higher memory scores in post menopausal
women
(Kampen DL, Sherwin BB 1994, "Estrogen use and verbal memory in healthy
postmeno-
pausaf women", Obstetrics and Gynecology, 83(6):979-983). Furthermore, the
administra-
tion of exogenous estrogen to surgically post menopausal women specifically
enhances
short-term memory. Moreover, the effects of estrogen on cognition do not
appear confined
to short-term effects as epidemiological findings indicate that estrogen
treatment signifi-
cantly decreases the risk of senile dementia-Alzheimer's type in women
(Paganini-Hill A,
Henderson VW) 1994, "Estrogen deficiency and risk of Alzheimer's disease in
women",
Am J Epidemiol, 140:256-261; Ohkura T, Isse K, Akazawa K, Hamamoto M,
Yoshimasa Y,
Hagino N, 1995, "Long-term estrogen replacement therapy in female patients
with
dementia of the Alzheimer Type: 7 case reports", Dementia, 6:99-107). While
the
mechanism whereby estrogens enhance cognitive function is unknown, it is
possible to
speculate that the direct effects of estrogen on cerebral blood flow (Goldman
H, Skelley
Eb, Sandman CA, Kastin AJ, Murphy S, 1976, "Hormones and regional brain blood
flow",
Pharmacol Biochem Rev. 5(suppl 1 ):165-169; Ohkura T, Teshima Y, Isse K,
Matsuda H,
Inoue T, Sakai Y, Iwasaki N, Yaoi Y, 1995, "Estrogen increases cerebral and
cerebellar
blood flows in postmenopausal women", Menopause: J North Am Menopause Soc.
2(1 ):13-18) and neuronal cell activities (Singh M, Meyer EM, Simpkins JW,
1995, "The
effect of ovariectomy and estradiol replacement on brain-derived neurotrophic
factor
messenger ribonucleic acid expression in cortical and hippocampal brain
regions of female
Sprague-Dawley rats", Endocrinology, 136:2320-2324; McMillan PJ, Singer CA,
Dorsa
DM, 1996, "The effects of ovariectomy and estrogen replacement on trkA and
choline
acetyltransferase mRNA expression in the basal forebrain of the adult female
Sprague
Dawley rat", J Neurosci., 16(5):1860-1865) are potential effectors for these
beneficial
actions.
The therapeutic applications of naturally occurring estrogens and synthetic
compositions
demonstrating estrogenic activity alone or in combination are not limited to
the chronic
conditions described above. Indeed, the more traditional applications of
estrogen
therapies would include the following: relief of menopausal symptoms (i.e.
flushing and
urogenital atrophy); oral contraception; prevention of threatened or habitual
abortion, relief
of dysmenorrhea; relief of dysfunctional uterine bleeding; an aid in ovarian
development;
CA 02270056 1999-04-27
WO 98/18778 PCT/DK97/00485
4
treatment of acne; diminution of excessive growth of body hair in women
(hirsutism);
treatment of prostatic carcinoma: and suppression of post-partum lactation
[Goodman and
Gilman, The Pharmacological Basis of Therapeutics (Seventh Edition) Macmillan
Publishing Company, 1985) pages 1421-1423j.
Even though the beneficial effects of estrogen replacement on a wide variety
of organ
systems and tissues appear indisputable, the dose and duration of estrogen
therapy is
also associated with an increased risk of endometrial hyperplasia and
carcinoma. The use
of concomitant cyclic progestins does reduce the risk of endometrial
pathology, but this is
achieved at the expense of the return of regular uterine bleeding, a result
that is objection-
able to many patients. In addition to estrogen's stimulatory effect on the
endometrium,
there remains considerable controversy regarding reports of an association
between long-
term estrogen replacement and an increased risk of breast cancer (Bergkvist L,
Adami
HO, Persson I, Hoover R, Schairer C, 1989, "The risk of breast cancer after
estrogen and
estrogen-progestin replacement", N Eng J Med, 321:293-297; Colditz GA,
Hankinson SE,
Hunter DJ, Willett WC, Manson JE, Stampfer MJ, Hennekens C, Rosner B, Speizer
FE,
1995, "The use of estrogens and progestins and the risk of breast cancer in
postmeno-
pausal women") N Eng J Med, 332(24):1589-1593). Furthermore, there are other
side
effects of estrogen replacement which, while they may not be life threatening)
contraindi-
cate estrogen's use and reduce patient compliance.
From the foregoing discussion it would appear that the availability of
therapies which could
mimic the beneficial actions of estrogen on the bone, cardiovascular system)
and central
nervous system without the undesirable side effects on uterus and breast,
would essen-
tially provide a "safe estrogen" which could dramatically influence the number
of patients
that would be able to benefit from estrogen replacement therapy. Therefore, in
recognition
of estrogen's beneficial effects on a number of body systems and disease
conditions,
there is a continuing need for the development of potent estrogen agonists
which can
selectively target different body tissues.
Description of the invention
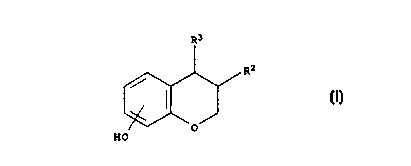
The present invention provides compounds of the formula I in which
substituents RZ and R'
CA 02270056 1999-04-27
wo 98n8778 rcTmx97iooass
are arranged in cis-configuration:
R3
R2
(I)
O
HO
5 wherein:
RZ is phenyl optionally substituted with 1 to 5 substituents independently
selected from
the group consisting of OH, halogen, vitro, cyano, SH, SR4, trihalo-C,-C6-
alkyl, C,-Cs-
alkyl, C,-C6-alkoxy and phenyl;
R3 is phenyl substituted with -X-(CHZ)~-Y, wherein:
X is a valency bond, O or S)
n is an integer in the range of 4 to 12, preferably 6 to 12,
Y is H, halogen, OH, OR4, NHR4, NR z , NHCOR4, NHS02R", CONHR4, CONR 2 ,
COOH, COOK", S02R4, SOR4, SONHR", SONR z , a C3-C, heterocyclic ring, satu-
rated or unsaturated, containing one or two heteroatoms independently selected
from the group consisting of O, S and N, optionally being substituted with 1
to 3
substituents independently selected from the group consisting of H, OH,
halogen,
vitro, cyano, SH, SR~, trihalo-C,-Cs-alkyl, C,-C6 alkyl and C,-C6 alkoxy;
R4 is C,-C6-alkyl;
and optical and geometrical isomers, pharmaceutically acceptable esters,
ethers and
salts thereof.
CA 02270056 1999-04-27
WO 98/18778 PCT/DK97/00485
6
The general chemical terms used in the above formula have their usual
meanings.
For example the term C,-Cs-alkyl includes straight-chained as well as branched
alkyl groups
such as methyl, ethyl, propyl, isopropyl, butyl, s-butyl and isobutyl.
The term halogen means chloro, bromo, iodo and fluoro.
The term C3-C,-heterocyclic ring include groups such as pyrrolidinyl,
pyrrolinyl, imidazolyl,
imidazolidinyl, pyrazolyl, pyrazolidinyi, pyrazolinyl, piperidyl, piperazinyl,
pyrrol, 2H-pyrrol,
triazolyl, pyridyl, pyrazinyl, pyrimidinyl, pyridazinyl, morpholino,
thiomorpholino) isothiazolyl,
isoxazolyl, oxazolyl, oxadiazolyl, thiadiazolyl and thiazolyl.
The compounds of this invention are new estrogen agonists and are useful for
prevention
and treatment of bone loss, prevention and treatment of osteoporosis; the
prevention and
treatment of cardiovascular disease; treatment and prevention of physiological
disorders
associated with an excess of neuropeptide Y (e.g. obesity, depression, etc.);
and for
regulation of glucose metabolism in e.g. non-insulin dependent diabetes
melitus; and the
prevention and treatment of senile dementia-Alzheimer's type in women. In
addition, these
estrogen agonists are useful for oral contraception; relief of menopausal
symptoms (e.g.
hot flushes, urogenital atrophy, depression, mania, schizophrenia, etc.);
incontinence;
prevention of threatened or habitual abortion; relief of dysmenorrhea; relief
of dysfunc-
tional uterine bleeding; an aid in ovarian development; treatment of acne;
diminution of
excessive growth of body hair is women (hirsutism); treatment of prostatic
carcinoma; and
the suppression of post-partum lactation. These agents also lower serum
cholesterol and
have a beneficial effect on plasma lipid profiles.
While the compounds of this invention are estrogen agonists in bone and
cardiovascular
tissues, they are also capable of acting as antiestrogens in other estrogen
target organs.
For example, these compounds can act as antiestrogens in breast tissue and the
colon
and therefore would be useful for the prevention and treatment of estrogen-
dependent
cancers such as breast cancers and colon cancers.
The hydroxy substituent on the phenyl ring in formula I is preferably attached
to the phenyl
ring at the 6- or 7-position. Accordingly, compounds of the invention having
one of the
CA 02270056 1999-04-27
WO 98/18778 PCT/DK97/00485
7
following formulae la or Ib are preferred:
R3
Rz
(Ia)
Ho o , or
R3
HO R2
(Ib)
O
wherein R', Rz and R3 are as defined above.
In a preferred embodiment, the present invention is concerned with cis-forms
of the
compounds of the following formula:
0~ (CHZ)",' \
HO
0
wherein m is an integer from 2 to 10, preferably 4 to 10.
In another preferred embodiment, the present invention is concerned with cis-
forms of the
compounds of the following formula:
CA 02270056 1999-04-27
WO 98I18778 PCT/DK97/00485
8
O~ (CH2)",~
N
/
/
HO
wherein m is as defined above.
In another preferred embodiment, the present invention is concerned with cis-
forms of the
compounds of the following formula:
p~ (CHZ)m~
N
S V
wherein m is as defined above.
In another preferred embodiment, the present invention is concerned with cis-
forms of the
compounds of the following formula:
CA 02270056 1999-04-27
WO 98/18778 PCT/DK97/00485
9
/ R4
N
R4
wherein m is as defined above and both R" independently are as defined above.
The most preferred compounds are the following:
{-)-cis-7-Hydroxy-3-phenyl-4-(4-(5-pyrrolidinopentoxy)phenyl)chromane,
(-)-cis-7-Hydroxy-3-phenyl-4-(4-(6-pyrrolidinohexyloxy)phenyl)chromane,
(-)-cis-7-Hydroxy-3-phenyl-4-(4-(7-pyrrolidinoheptyloxy)phenyl)chromane,
(-)-cis-7-Hydroxy-3-phenyl-4-(4-(8-pyrroiidinooctyloxy)phenyl)chromane,
(-)-cis-7-Hydroxy-3-phenyl-4-(4-(9-pyrrolidinononyloxy)phenyl)chromane,
(-)-cis-7-Hydroxy-3-phenyl-4-(4-(10-pyrrolidinodecyloxy)phenyl)chromane,
(-)-cis-7-Hydroxy-3-phenyl-4-(4-(11-pyrrolidinoundecyloxy)phenyl)chromane,
(-)-cis-7-Hydroxy-3-phenyl-4-(4-(12-pyrrolidinododecyloxy)phenyl)chromane,
(-)-cis-6-Hydroxy-3-phenyl-4-(4-(5-pyrrolidinopentoxy)phenyl)chromane,
(-)-cis-6-Hydroxy-3-phenyl-4-(4-(6-pyrrolidinohexyloxy)phenyl)chromane,
(-)-cis-6-Hydroxy-3-phenyl-4-(4-(7-pyrrolidinoheptyloxy)phenyt)chromane,
(-)-cis-6-Hydroxy-3-phenyl-4-(4-(8-pyrrolidinooctyloxy)phenyl)chromane,
(-}-cis-6-Hydroxy-3-phenyl-4-(4-(9-pyrrolidinononyioxy)phenyl)chromane)
(-)-cis-6-Hydroxy-3-phenyl-4-(4-{10-pyrrolidinodecyloxy)phenyl)chromane,
(-)-cis-6-Hydroxy-3-phenyl-4-(4-(11-pyrrolidinoundecyloxy)phenyl)chromane,
(-)-cis-6-Hydroxy-3-phenyl-4-(4-(12-pyrrolidinododecyloxy)phenyl)chromane,
(+}-cis-7-Hydroxy-3-phenyl-4-(4-(5-pyrrolidinopentoxy)phenyl)chromane,
(+)-cis-7-Hydroxy-3-phenyl-4-(4-{6-pyrrolidinohexyloxy)phenyl)chromane,
(+)-cis-7-Hydroxy-3-phenyl-4-(4-(7-pyrrolidinoheptyloxy)phenyl)chromane,
(+)-cis-7-Hydroxy-3-phenyl-4-(4-{8-pyrrolidinooctyloxy)phenyl)chromane,
CA 02270056 1999-04-27
WO 98I18778 PCT/DK97/00485
(+)-cis-7-Hydroxy-3-phenyl-4-(4-(9-pyrrolidinononyloxy)phenyt)chromane,
(+)-cis-7-Hydroxy-3-phenyl-4-(4-( 10-pyrrolidinodecyloxy)phenyl)chromane,
(+)-cis-7-Hydroxy-3-phenyl-4-(4-(11-pyrrolidinoundecyloxy)phenyl)chromane,
(+)-cis-7-Hydroxy-3-phenyl-4-(4-(12-pyrrolidinododecyloxy)phenyl)chromane,
5 (+)-cis-6-Hydroxy-3-phenyl-4-(4-(5-pyrrolidinopentoxy)phenyl)chromane,
(+)-cis-6-Hydroxy-3-phenyl-4-{4-(6-pyrrolidinohexyloxy)phenyl)chromane,
(+)-cis-6-Hydroxy-3-phenyl-4-(4-(7-pyrrolidinoheptyloxy)phenyl)chromane,
(+)-cis-6-Hydroxy-3-phenyl-4-(4-(8-pyrrolidinooctyloxy)phenyl)chromane,
(+)-cis-8-Hydroxy-3-phenyl-4-(4-(9-pyrrolidinononyloxy)phenyl)chromane,
10 (+)-cis-6-Hydroxy-3-phenyl-4-(4-(10-pyrrolidinodecyloxy)phenyl)chromane,
(+)-cis-6-Hydroxy-3-phenyl-4.-(4-(11-pyrrolidinoundecyloxy)phenyl)chromane,
(+)-cis-6-Hydroxy-3-phenyl-4-(4-(12-pyrrolidinododecyloxy)phenyl)chromane)
and any mixture thereof including racemic mixtures.
The following compounds form part of the disclosure of the present invention:
(t)-cis-4-(4-Hexyfphenyl)-7-hydroxy-3-phenylchromane,
(+,-) cis 7-hydroxy-4-[4-(6-morpholinohexyloxy)-phenyl]-3-phenyl-chroman,
(+,-) cis 4-[4-(6-Dibutylaminohexyloxy)-phenyl]-7-hydroxy-3-phenyl-chroman,
(+,-) cis 7-Hydroxy 4-[4-(morpholinobutyloxy)-phenylj-3-phenyl-chroman,
(+,-) cis 7-Hydroxy 4-[4-(morpholinodecyloxy)-phenyl]-3-phenyl-chroman
including the pure enantiomers thereof.
The compounds of the invention may be prepared by resorting to the chroman
chemistry
which is well-known in the art) for example in P.K. Arora, P.L. Kole and S.
Ray, Indian J.
Chem. 20 B, 41-5, 1981; S. Ray, P.K. Grover and N. Anand, Indian J. Chem. 9,
727-8, 1971;
S. Ray, P.K. Grover, V.P. Kamboj, S.B. Betty, A.B. Kar and N. Anand, J. Med.
Chem. 19,
276-9, 1976; Md. Salman, S. Ray) A.K. Agarwal, S. Durani, B.S. Betty) V.P.
Kamboj and N.
Anand, J. Med. Chem. 26, 592-5, 1983; Teo, C., Sim, K., BuII. Singapore Natl.
Inst. Chem.
22, 69-74, 1994.
CA 02270056 1999-04-27
WO 98/18778 PCT/DK97/00485
11
However, the invention is furthermore concerned with a general method for the
preparation
of compounds of formula (I) comprising the steps of:
a) reacting a compound of the formula (II)
0
0
\ \ cm
OH OH
with a compound of the formula (III)
~ COOH
(III)
RS
wherein RS represents 1 to 3 substituents independently selected from the
group
consisting of H, OH, halogen, nitro, cyano, SH, SR4, trihalo-C,-Cs-alkyl, C,-
Cs-alkyl and
C,-C6-alkoxy, and R4 is as defined above,
in the presence of triethylamine and acetic anhydride to form a compound of
the formula
(IV)
CA 02270056 1999-04-27
WO 98/18778 PCT/DK97/00485
12
R5
O
(IV)
wherein R5 is as defned above,
b) reducing a compound of the formula (IV) with a suitable hydride reducing
agent to form
a compound of formula (V}
R5
(V)
v
wherein RS is as defined above,
c) hydrogenating a compound of the formula (V) in the presence of a suitable
catalyst to
form a compound of the formula (VI) with a 3,4-cis configuration
CA 02270056 1999-04-27
WO 98/18778
PCT/DK97/00485
13
R5
O
(VI)
v
wherein R5 is as defined above)
d) alkylating a compound of the formula (VI) with an appropriate electrophile
to form a
compound of the formula (VI I)
R5
(VII)
wherein R5, Y and n are as defined above,
e) deprotecting a compound of formula (VII) with a suitable deprotection
agent, preferably
by pyridine hydrochloride fusion, to form a compound of the formula (I); or
f) reacting a compound of formula (VI) with trifluoromethane sulphonic acid
anhydride to
form a compound of the formula (XI)
/ (CH~)n-Y
CA 02270056 1999-04-27
WO 98I18778 PCT/DK97/00485
14
oso,cF,
RS
O
(XI)
wherein R$ is as defined above,
g) cross-coupling a compound of the formula (XI) with the appropriate cross-
coupling
partner to form a compound of the formula (XIl)
Y
(CH.,)~
RS
O
(XII)
wherein R5, Y and n are as defined above,
h) deprotecting a compound of the formula (XII) with a suitable deprotection
agent,
preferably by pyridine hydrochloride fusion, to form a compound of the formula
(I),
i) reacting a compound of the formula (VI) with methanesulfonylchloride to
form a
compound of the formula (X111)
CA 02270056 1999-04-27
WO 98/18778 PCT/DK97/00485
O
S
O
Rs
5
(X111)
wherein R5 is defined as above,
j) deprotecting a compound of the formula (X111) with a suitable deprotection
agent, such
as pyridine hydrochloride fusion or boron tribromide, to form a compound of
the for-
mula (XIV)
10 p
o. S.
0
lw
i i
Rs
HO
O
(XIV)
15 wherein R5 is defined as above,
k) reacting a compound of the formula (XIV) with a suitable protection agent,
such as
benzyl bromide or 4-methoxybenzyl bromide, to form a compound of formula (XV)
CA 02270056 1999-04-27
WO 98/18778 PCT/DK97/00485
16
O
O. S~
R~
R
O
5 (XV)
wherein R5 is defined as above, and R6 is H or methoxy,
I) deprotecting a compound of the formula (XV) with a suitable deprotection
agent, such
as sodium or potassium hydroxide in alcohol, to form a compound of formula
(XVi)
OH
F
\ R5
O
(XVI)
wherein R5 is defined as above, and R6 is H or methoxy,
m) alkylating a compound of the formula {XVI) with an appropriate efectrophile
to form a
compound of the formula (XViI)
CA 02270056 1999-04-27
WO 98/18778 PCT/DK97/00485
17
O~(CHZ)~ Y
F
\ Rs
O
(XVI I)
wherein n, Rs and Y is defined as above, and R6 is H or methoxy,
n) deprotecting a compound of the formula (XVII) with a suitable deprotection
agent,
preferably catalytic hydrogenation for R6 equals H or a strong acid for R6
equals
methoxy, to form a compound of the formula (XVIII)
O~(CHZ)~ Y
Rs
HO
(XVlll)
wherein n, Rs and Y is defined as above,
o) Alkylating a compound of the formula (XVI) with an appropriate
dihalogenated alkane
such as 1,4-dibromobutane, 1,6-dibromohexane) 1,8-dibromooctane, 1,10-
dibromodecane, preferably catalysed by potassium iodide, to form a compound of
the
formula (XIX)
CA 02270056 1999-04-27
WO 98/I8778 PCT/DK97/00485
18
O~(CHZ)~ Hal
F
\ Rs
O
(XIX)
wherein n and Rs is defined as above, R6 is H or methoxy, and Hal is chloro,
bromo,
or iodo,
p) reacting a compound of the formula (XtX) with an appropriate nucleophile,
preferably
an amine, to form a compound of the formula (XX)
O/ (CHZ)n Z
F
\ Rs
O
(XX)
wherein R6 is H or methoxy, and Z is NHR4, NR Z , or a C3-C, heterocyclic
amine
optionally containing oxygen or nitrogen, optionally being substituted with 1
to 3 sub-
stituents independently selected from the group consisting of H, OH, halogen,
vitro,
cyano, trihalo-C,-C6-alkyl, C,-Cs-alkyl and C,-C6-alkoxy) and n, R4, and Rs is
defined
as above,
CA 02270056 1999-04-27
WO 98I18778 PCT/DK97/00485
19
q) deprotecting a compound of the formula (XX) with a suitable deprotection
agent,
preferably catalytic hydrogenation for R6 equals H or a strong acid for R6
equals
methoxy, to form a compound of the formula (XXI)
(CH~)-Z
Rs
HO
(XXI)
wherein R6 is H or methoxy, and Z is NHR~, NR 2 , or a C3-C, heterocycfic
amine optionally containing oxygen or nitrogen, optionally being substituted
with 1 to 3
substituents independently selected from the group consisting of H, OH,
halogen, vitro,
cyano, trihalo-C,-C6-alkyl, C,-Cs-alkyl and C,-C6-alkoxy, and n, R4 and RS is
defined as
above.
The starting benzophenones of the formula (II) are easily prepared via Friedel-
Craft acylation
of the appropriate dimethyl ether with p-hydroxybenzoic acid followed by
selective monode-
methylation with hydrobromic acid in acetic acid.
Optical pure compounds of formula (I) can be obtained by introducing in the
above method a
resolution step. The resolution can be carried out after any step of the
process which results
in a racemic mixture of enantiomers. Any resolution technique may be used to
separate a
(-)-enantiomer and/or a (+)-enantiomer from a racemic mixture, including
diastereomeric salt
formation and chiral HPLC.
The expression "appropriate electrophile" typically means an alkylhalogenide
of the formula
Y-(CHz )n-Hlg, wherein Y is as defined above and Hlg is CI, Br or I.
The expression "appropriate cross-coupling partner" typically means an
organometallic
CA 02270056 1999-04-27
WO 98/18778 PCT/DK97/00485
reagent together with a transition metal catalyst, for example a Grignard
reagent with a Ni(0)
catalyst.
The present invention also relates to pharmaceutical compositions comprising
an effective
amount of a compound according to the invention and a pharmaceutical carrier
or diluent.
5 Such compositions are preferably in the form of an oral dosage unit or
parenteral dosage
unit.
Furthermore, the invention is concerned with a method of treating or
preventing estrogen
related diseases or syndromes, preferably diseases or syndromes caused by an
estrogen-
deficient state in a mammal, comprising administering to a subject in need
thereof an
10 effective amount of a compound according to the invention.
The compounds of this invention are new estrogen agonists and are useful for
prevention
and treatment of bone loss, prevention and treatment of osteoporosis; the
prevention and
treatment of cardiovascular disease; treatment and prevention of physiological
disorders
associated with an excess of neuropeptide Y (e.g. obesity, depression, etc.);
and for
15 regulation of glucose metabolism in e.g. non-insulin dependent diabetes
melitus; and the
prevention and treatment of senile dementia-Alzheimer's type in women. In
addition, these
estrogen agonists are useful for oral contraception; relief of menopausal
symptoms (e.g.
hot flushes, urogenital atrophy, depression, mania, schizophrenia, etc.);
incontinence;
prevention of threatened or habitual abortion; relief of dysmenorrhea; relief
of dysfunc-
20 tional uterine bleeding; an aid in ovarian development; treatment of acne;
diminution of
excessive growth of body hair is women {hirsutism); treatment of prostatic
carcinoma; and
the suppression of post-partum lactation. These agents also lower serum
cholesterol and
have a beneficial effect on plasma lipid profiles.
While the compounds of this invention are estrogen agonists in bone and
cardiovascular
tissues, they are also capable of acting as antiestrogens in other estrogen
target organs.
For example, these compounds can act as antiestrogens in breast tissue and the
colon
and therefore would be useful for the prevention and treatment of estrogen-
dependent
cancers such as breast cancers and colon cancers.
CA 02270056 1999-04-27
WO 98/18778 PCT/DK97/00485
21
In vitro estrogen receptor bindin assay
An in vitro receptor binding assay was used to determine the estrogen receptor
binding
affinity of the compounds of this invention. This assay measures the ability
of the
compounds of this invention to displace 3H-17f3-estradiol (17f3-E2), from
estrogen receptor
(ER) obtained from rabbit uterus. Experimentally, the ER rich cytosol from
rabbit uterine
tissue is diluted with ER poor cytosol isolated from rabbit muscle to achieve
approximately
20 - 25% maximal binding of 0.5 nM 'H-17f3-E2. For each assay, fresh aliquots
of cytosol
are thawed on the day of analysis and diluted with assay buffer to ca. 3 mg
cytosol
protein/ml. The assay buffer (PB) is as follows: 10 mM KzHP04/KHZP04, 1.5 mM
KZEDTA,
10 mM monothioglycerol, 10 mM NazMo04.2H20, 10 % glycerol (v/v); pH 7.5. Radio-
inert
17f3-E2 is obtained from Sigma.
Test solutions are prepared in appropriate solvents (ethanol or DMSO) at a
concentration
of 8 x 10-3M and serial dilutions prepared with PB or DMSO. Aliquots of 10 NI
are
incubated in duplicate for each concentration tested in microtitre plates to
which have
been added 20 NI 3H-17f3-E2 (assay concentration equals 0.4 nM) and 50 NI
cytosol. For
control samples as well as maximal binding sample) 10 NI PB is added in lieu
of test
compound.
Following an 18 - 20 hr incubation at 4~C the reaction is terminated with 100
NI DCC slurry
[0.5% activated charcoal (Sigma) and 0.005% Dextran T70 (Pharmacia) in PB)
added to
each sample and incubated with continuous shaking for 15 min at 4~C. DCC
background
counts are assessed using 50 Ni of 0.3% BSA in PB in lieu of cytosol.
To separate bound and free 3H-17(3-E2) Titertek plates are centrifuged for 10
min (800 x
g) at 4~C and aliquots of 100 NI are removed from each sample for
scintillation counting
using Optiflour scintillation liquid. Standard and control samples are
incubated in quadru-
plicate, while test compounds are incubated in duplicate. The mean counts per
minute
(cpm) in each sample is calculated, background (DCC) is subtracted, and the
percent of
maximal 3H-17f3-E2 binding is determined. Individual cpm's are plotted against
their
respective concentrations of test compound (logarithmic scale), and the IC50
expressed
as the compound concentration required to displace 50% of the maximal binding.
CA 02270056 1999-04-27 _
WO 98/18778 PCT/DK97/00485
22
Bone Mineral Density
Bone mineral density (BMD) as a measure of bone mineral content (BMC) accounts
for
greater than 80% of a bone's strength. The loss of BMD with ageing and the
accelerated
loss following the menopause reduce the strength of the skeleton and render
specific sites
more susceptible to fracture; i.e. most notably the spine, wrist and hip. True
bone density
can be measured gravimetrically using Archimede's Principle (an invasive
technique). The
BMD can also be measured non-invasively using dual energy x-ray absorptiometry
(DEXA). In our laboratory, we have utilized a gravimetric method to evaluate
changes in
BMD due to estrogen deficiency in ovariectomized rodents. Following
ovariectomy (the
surgical removal of the ovaries), the animals are treated with vehicle, 17f3-
E2 as a positive
control, and/or other estrogen agonists. The objective of these investigations
is to evaluate
the ability of the compounds of this invention to prevent bone loss in rodent
models of
human disease.
Female Sprague-Dawley rats (ca. 3 to 5 months old), or female Swiss-Webster
mice (ca.
3 to 5 months old) underwent bilateral ovariectomy or sham surgery. Following
recovery
from anesthesia the animals are randomized to the following groups, minimum of
8
animals per group:
sham animals treated with vehicle;
ovariectomized animals treated with vehicle;
ovariectomized animals treated with 25 Ng estradiollkg; and
ovariectomized animals treated with 200 Nglkg of test compound.
All compounds are weighed and dissolved in vehicle solvent in sterile saline
and the
animals are treated daily via subcutaneous injections for 35 days. At the
conclusion of the
35 day protocol, the animals are sacrificed and the femora are excised and
cleaned of
adherent soft tissue. In rats, the distal 1 cm of the defleshed femora are
removed with a
diamond wheel cut-off saw and fixed in 70% ethyl alcohol (in mice the distal
.5 cm are
removed and fixed). Following fixation in 70% ethyl alcohol (EtOH) an
automated tissue
processor was used to dehydrate the bone specimens in an ascending series of
alcohol to
100%. The dehydration program was followed by defatting in chloroform and
rehydration
in distilled water. All automated tissue processing occurred under vacuum. The
hydrated
CA 02270056 1999-04-27
WO 98/18778 PCT/DK97/00485
23
bones were weighed in air and weighed while suspended in water on a Mettler
balance
equipped with a density measurement kit. The weight of each sample in air is
divided by
the difference between the air weight and the weight in water to determine
total bone
density; i.e. organic matrix plus mineral per unit volume of tissue. After the
determination
of total bone density the samples are ashed overnight in a muffle furnace at
600 ~C. The
mineral density can then be determined by dividing the ash weight of each
sample by the
tissue volume (i.e. air weight - weight suspended in water). The mean bone
densities (total
and mineral bone densities) are calculated for each group and statistical
differences from
the vehicle-treated and estrogen-treated controls are determined using
computerized
statistical programs.
Cholesterol lowering activity
The effects of the compounds of the present invention on the serum levels of
total
cholesterol were measured either in blood samples taken from the animals in
the bone
density studies described above or from ovariectomized female rats or mice
that had been
treated with compound for a period of not less than 28 days. In each type of
experiment,
blood from treated animals was collected via cardiac puncture and placed in a
tube
containing 30 NI of 5% EDTA/1 ml of blood. Following centrifugation at 2500
rpm for 10
minutes at 20~ C the plasma was removed and stored at -20~ C until assayed.
Cholesterol
was measured using a standard enzymatic determination kit purchased from Sigma
Diagnostics (Kit No. 352).
Pharmaceutical preparations
The compounds of the invention, together with a conventional adjuvant, carrier
or diluent,
and if desired in the form of a pharmaceutically acceptable acid addition salt
thereof, may
be placed into the form of pharmaceutical compositions and unit dosages
thereof, and in
such form may be employed as solids, such as tablets or filled capsules, or
liquids, such
as solutions, suspensions, emulsions, elixirs, or capsules filled with the
same, all for oral
use; in the form of suppositories for rectal administration; or in the form of
sterile injectable
solutions for parenteral use (including subcutaneous administration and
infusion). Such
pharmaceutical compositions and unit dosage forms thereof may comprise
conventional
CA 02270056 1999-04-27
WO 98I18778 PCT/DK97/00485
24
ingredients in conventional proportions, with or without additional active
compounds or
principles, and such unit dosage forms may contain any suitable effective
amount of a
compound of the invention commensurate with the intended daily dosage range to
be
employed. Tablets containing ten {10) milligrams of active ingredient or, more
broadly, ten
(10) to hundred (100) milligrams, per tablet, are accordingly suitable
representative unit
dosage forms.
The compounds of this invention can thus be used for the formulation of
pharmaceutical
preparation, e.g. for oral and parenteral administration to mammals including
humans, in
accordance with conventional methods of galenic pharmacy.
Conventional excipients are such pharmaceutically acceptable organic or
inorganic carrier
substances suitable for parenteral or enteral application which do not
deleteriously react
with the active compounds.
Examples of such carriers are water, salt solutions, alcohols, polyethylene
glycols,
polyhydroxyethoxylated castor oil, gelatine, lactose amylose, magnesium
stearate, talc,
1 S silicic acid, fatty acid monoglycerides and diglycerides, pentaerythritol
fatty acid esters,
hydroxymethylcellulose and polyvinylpyrrolidone.
The pharmaceutical preparations can be sterilized and mixed, if desired, with
auxiliary
agents, emulsifiers, salt for influencing osmotic pressure, buffers and/or
colouring
substances and the like, which do not deleteriously react with the active
compounds.
For parenteral application, particularly suitable are injectable solutions or
suspensions,
preferably aqueous solutions with the active compound dissolved in
polyhydroxylated
castor oil.
Ampoules are convenient unit dosage forms.
Tablets, dragees, or capsules having talc andlor carbohydrate carrier or
binder or the like,
the carrier preferably being lactose and/or corn starch and/or potato starch,
are particu-
larly suitable for oral application. A syrup, elixir or the like can be used
in cases where a
sweetened vehicle can be employed.
CA 02270056 1999-04-27
WO 98/18778 PCT/DK97/00485
Generally, the compounds of this invention are dispensed in unit form
comprising 0.05-100
mg in a pharmaceutically acceptable carrier per unit dosage.
- The dosage of the compounds according to this invention is 0.1-300 mg/day,
preferably
10-100 mg/day, when administered to patients, e.g. humans, as a drug.
5 A typical tablet which may be prepared by conventional tabletting techniques
contains:
Active compound 5.0 mg
Lactosum 67.0 mg Ph.Eur.
IO AvicelT"~ 31.4 mg
AmberliteT""IRP 88 1.0 mg
Magnesii stearas 0.25 mg Ph.Eur.
The compounds of the invention may be administered to a subject, e.g., a
living animal
I S body, including a human, in need of a compound of the invention, and if
desired in the
form of a pharmaceutically acceptable acid addition salt thereof (such as the
hydrobro-
mide, hydrochloride, or sulphate, in any event prepared in the usual or
conventional
manner, e.g., evaporation to dryness of the free base in solution together
with the acid),
ordinarily concurrently, simultaneously, or together with a pharmaceutically
acceptable
20 carrier or diluent, especially and preferably in the form of a
pharmaceutical composition
thereof, whether by oral, rectal, or parenteral (including subcutaneous)
route, in an
amount which is effective for the treatment of the disease. Suitable dosage
ranges are 1-
200 milligrams daily, 10-100 milligrams daily, and especially 30-70 milligrams
daily,
depending as usual upon the exact mode of administration, form in which
administered)
25 the indication toward which the administration is directed, the subject
involved and the
body weight of the subject involved, and the preference and experience of the
physician or
veterinarian in charge.
CA 02270056 1999-04-27
WO 98/18778 PCT/DK97/00485
26
EXAMPLE 1
(~)-cis-4-(4-Hexylphenyl -~ydroxy-3-~henylchromane
Step 1:
(~)-(cis-4-((7-methoxy-3-phenyl)chroman-4-yl)phenyl trifluoromethanesulfonic
acid ester
A stirred suspension of (t}-cis-4-(4-hydroxyphenyl)-7-methoxy-3-phenylchromane
(5.0 g,
mmol} in a mixture of dichloromethane (50 ml) and triethylamine (2.9 ml, 21
mmol) was
treated dropwise at 0~C, under a nitrogen atmosphere) with
trifluoromethanesulfonic
anhydride (5.0 g, 17 mmof), and the mixture warmed slowly to room temperature.
The
10 resulting rather viscous solution was diluted with tetrahydrofuran (100
ml), and stirred for a
further 24 hours. The reaction mixture was filtered, the solvents evaporated,
and the
residue partitioned between dichloromethane {200 ml) and 10% aqueous acetic
acid (200
ml). The organic layer was separated, washed with 10% aqueous acetic acid (100
ml),
sodium hydrogen carbonate solution (3x100 ml), brine (100 ml), dried over
sodium sulfate,
15 and evaporated. The product was purified by column chromatography on silica
gel 60, with
dichloromethane as the eluent. This gave the product as a clear gum, which was
crystal-
lised from diethylether and petrol, to afford the title product as colourless
crystals.
Yield 2.88 g (41 %) of (t)-{cis-4-({7-methoxy-3-phenyl)chroman-4-yl)phenyl
trifluoromethanesulfonic acid ester. M.p. 98-99~C. 'H-NMR (CDC13, 300MHz) 8:
3.65 (m,
1 H), 3.81 (s, 3H), 4.24-4.40 (m, 3H), 6.49 (dd, 1 H), 6.53 (d, 1 H), 6.61-
6.69 {m, 4H)) 6.82
(d, 1 H), 6.96 (d, 2H), 7.11-7.20 (m, 3H). LRMS(E1): 464 (M')
Step 2:
(~)-cis-4-(4-Hexylphenyl)-7-methoxy-3-phenylchromane
An oven dried 2-necked flask fitted with a reflex condenser and an inlet
septum, was
charged, under a nitrogen atmosphere, with 9-borabicylo[3.3.1 ]nonane) (0.5M
in tetrahy-
CA 02270056 1999-04-27
WO 98I18778 PCT/DK97/00485
27
drofuran solution) 4.74 ml, 2.37 mmol), which was cooled to 0~C. 1-Hexene (199
mg, 2.37
mmol) was added, and the mixture slowly warmed to room temperature over 7
hours.
Anhydrous dioxane (20 ml), caesium carbonate (1.05 g, 3.23 mmol),
tetrakis(triphenylphosphine)palladium (0) (62 mg, 0.05 mmol) and (t)-(cis-4-
((7-methoxy-
3-phenyl)chroman-4-yi)phenyl trifluoromethanesulfonic acid ester (1.0 g, 2.15
mmol) were
added, and the mixture heated to gentle reflux for 24 h. The mixture was
cooled to room
temperature and diluted with hexane (20 ml), then 2M sodium hydroxide (1.6 ml)
and 30%
hydrogen peroxide (1 ml) were added, and the mixture stirred for 2 hours to
destroy the
excess borane. The resulting mixture was diluted with ethyl acetate (200 ml)
and water
(200 ml), and the organic layer separated, washed with water (100 ml), brine
(100 ml),
dried over sodium sulfate, and evaporated. The crude product was purified by
column
chromatography on silica gel 60, with 2% methanol in dichloromethane as the
eluent. This
gave the title compound as a clear oil.
Yield 0.758 g (88%) of (t)-cis-4-(4-hexylphenyl)-7-methoxy-3-phenylchromane.
'H-NMR
(CDCl3,, 300 MHz) b: 0.88 (t, 3H), 1.20-1.32 (m, 6H), 1.53 (m, 2H), 2.50 (t,
2H), 3.59 (m,
1 H), 3.80 (s, 3H), 4.20-4.29 (m, 2H), 4.45 (t, 1 H), 6.42-6.53 (m, 4H)) 6.61-
6.68 (m, 2H),
6.82-6.89 (m, 3H), 7.08-7.19 (m, 3H). LRMS (EI): 400 (M').
Step 3:
(~)-cis-4-(4-Hexylphenyl)-7-hydroxy-3-phenylchromane
A mixture of (t)-cis-4-(4-hexyipheny!)-7-methoxy-3-phenylchromane (0.46 g,
1.15 mmol)
and anhydrous pyridine hydrochloride (8.60 g, 57.4 mmol) was heated to 150-
155~C as a
melt for 18 hours. The mixture was cooled to room temperature, and the
resulting orange
coloured wax dissolved in a mixture of water (100 ml), hot ethanol (10 ml) and
di-
chloromethane (100 ml). The organic layer was separated and the aqueous layer
further
extracted with dichloromethane (3 x 50 ml). The combined organic layers were
washed
with saturated sodium chloride, dried over sodium sulfate and evaporated to a
dark
coloured oil, which was purified by column chromatography on silica gel 60,
with 2%
methanol in dichloromethane as eluent; giving the title compound as a red oil.
CA 02270056 1999-04-27
WO 98/18778 PCT/DK97/00485
28
Yield 158 mg (35%) of (t)-cis-4-(4-Hexylphenyl)-7-hydroxy-3-phenylchromane. 'H-
NMR
(CDC13, 300MHz) 8: 0.87 (t, 3H), 1.20-1.32 (m, 6H), 1.51 (m, 2H), 2.49 (t,
2H), 3.57
(m,1 H), 4.18-4.25 {m, 2H}, 4.43(t, 1 H), 4.78 (s, 1 H), 6.36 (dd, 1 H), 6.46
(d, 1 H), 6.49 (d)
2H), 6.62 (dd, 2H), 6.79-6.88 (m, 3H), 7.08-7.20 (m, 3H). Elemental analysis:
calculated
for C2,H3oOZ: C, 83.91; H, 7.82%; Found: C, 83.45; H, 7.78%. LRMS (EI): 386
(M').
EXAMPLE 2
(+.-) cis 7-hydroxy-4-[~6-morpholinohexyloxy)-phenyl]-3-phenyl-chroman
Step 1: -
(+,-) cis 7-8enzyloxy-4-j4-(6-morpholinohexyloxy)-phenyl)-3-phenyl-chroman
7-Benzyloxy-4-[4-(6-bromohexyloxy)-phenyl]-3-phenyl-chroman (300 mg, 0.53
mmol),
morpholine (229 mg, 2.63 mmol), potassium carbonate (145 mg, 1.05 mmol}) and
potassium iodide (17 mg, 0.11 mmol) was refluxed in toluene (5 ml) for 3 days.
The
reaction mixture was cooled, filtered, and the precipitate washed with
toluene. The
combined filtrate and washings were evaporated, triturated with ethanol, and
recrystallised
from ethanol.
Yield 229 mg (75 %) of (+,-) cis 7-Benzyloxy-4-[4-(6-morpholinohexyloxy)-
phenyl]-3-
phenyl-chroman, m.p. 85.7-87.5~C.
The product was identified by'H-NMR.
Step 2:
(+,-) cis 7-hydroxy-4-j4-(6-morpholinohexyloxy)-phenyl)-3-phenyl-chroman
CA 02270056 1999-04-27
WO 98/18778 PCT/DK97/00485
29
(+,-) cis 7-Benzyloxy-4-[4-(6-morpholinohexyloxy)-phenyl]-3-phenyl-chroman
(150 mg,
0.26 mmol) was dissolved in a solution of 1 % Hydrochloride in ethanol (5 ml).
The
solution was added to a 10 % palladium on carbon catalyst, and hydrogenated (1
atm.)
overnight. The reaction mixture was filtered and evaporated to give an oil
which solidified.
Yield 117 mg (86 %) of (+,-) cis 7-hydroxy-4-[4-(6-morpholinohexyloxy)-phenyl]-
3-phenyl-
chroman.HCl m.p. 97-99~C.
The product was identified by'H-NMR.
The following example was performed essentially as described above.
EXAMPLE 3
(+.-) cis 4-f4-(6-DibutylaminohexyloxyLphenyl]-7-hydroxy-3-phenyl-chroman
Step 1:
(+,-) cis 7-benzyloxy 4-(4-(6-dibutylamino-hexyloxy)-phenyl]-3-phenyl-chroman
From 7-Benzyloxy-4-[4-(6-bromohexyloxy)-phenyl)-3-phenyl-chroman (300 mg, 0.53
mmol) and N,N-dibutylamine (339 mg, 2.63 mmol).
Yield 184 mg (56 %) of (+,-) cis 7-Benzyloxy-4-(4-(6-dibutylaminohexyloxy)-
phenyl]-3-
phenyl-chroman, m.p. 72-73.5~C.
The product was identified by'H-NMR.
Step 2:
(+,-) cis 4-[4-(6-Dibutylaminohexyloxy)-phenyl]-7-hydroxy-3-phenyl chroman
From (+,-) cis 7-Benzyloxy-4-[4-(6-dibutylaminohexyloxy)-phenyl]-3-phenyl-
chroman (150
mg, 0.24 mmol).
CA 02270056 1999-04-27
WO 98I18778 PCT/DK97/00485
Yield 135 mg (98 %) of (+,-) cis 4-(4-(6-Dibutylaminohexyloxy)-phenyl]-7-
hydroxy-3-
phenyl-chroman, m.p. 85-87~C.
The product was identified by'H-NMR.
5 EXAMPLE 4
(~+_-~cis 7-Hydroxy 4-[4-(morpholinodecyloxyL~yl]-3-phenyl-chroman
Step 1:
10 (+,-) cis 7-Benzyloxy 4-j4-(morpholinodecyloxy)-phenylj-3-phenyl-chroman
From (+,-) cis 7-Hydroxy 4-{4-(10-Bromodecyloxy)-phenyl]-3-phenyl-chroman
(300mg,
0.48 mmol) and morpholine (208 mg, 2.4 mmol).
Yield 122 mg (40 %} of (+,-) cis 7-Benzyl 4-[4-(morpholinodecyloxy)-phenyl]-3-
phenyl-
chroman. Oil 'H -NMR (CDC13, 200 MHz) 8:1.3 (m, 14H}; 1.7 (m) 2H); 2.31 (t,
2H}; 2.40
15 (m, 4H); 3.55 (m, 1 H}; 3.70 (m, 4H); 3.85 (t, 2H); 4.22 (m, 2H); 4.43 (t,
1 H); 5.05 (s, 2H);
6.58 (m, 8H); 6.85 (d, 1 H); 7.15 (m, 3H); 7.38 (m, 5H).
Step 2:
(+,-) cis 7-Hydroxy 4-j4-(morpholinodecyloxy)-phenyl]-3-phenyl-chroman
20 From (+,-) cis 7-Benzyl 4-[4-(morpholinodecyloxy)-phenyl]-3-phenyl-
chroman.HCl (122
mg, 0.19 mmol). The evaporated product was evaporated from acetone (x 3) to
give a
solid.
Yield 64 mg (61 %) of (+,-) cis 7-Hydroxy-4[4-(morpholinodecyloxy)-phenyl]-3-
CA 02270056 1999-04-27
WO 98I18778 PCT/DK97/00485
31
phenylchroman.HCl. 'H -NMR (CDC13, 200 MHz) b: 1.35 (m, 14H); 1.7 (m, 2H);
2.85 (m,
4H); 3.1 (m, 2H); 3.55 (m, 1 H); 3.82 (m, 4H); 3.85 (t, 2H); 4.22 (m, 2H);
4.43 (t, 1 H); 5.05
(s, 2H); 6.3 (m, 2H); 6.65 (m, 7H); 7.1 (m, 3H).
EXAMPLE 6
,(+,-) cis 7-Hydroxy 4-[~morpholinobutyloxy~ phenyl-3-phenyl-chroman
Step 1:
(+,-) cis 7-Benzyloxy 4-[4-(morpholinobutyloxy)-phenyl)-3-phenyl-chroman
(+,-) cis 7-Benzyioxy-4-[4-(4-chlorobutyloxy)-phenyl]-3-phenyl-chroman (300
mg, 0.60
mmol), morpholine (240 mg, 3 mmol), potassium carbonate 165 mg, 1.2 mmol), and
potassium iodide (19 mg, 0.12 mmol) in dimethylformamide (5 ml) was stirred at
60~C for
4 days. The reaction mixture was cooled and ether and water was added. The
aquous
phase was extracted with ether, and the combined organic phases were washed
water
1 S and brine, dried (magnesium sulphate), and evaporated. The evaporated
product was
crystallised from ether - petrolether, and further purified by silica column
chromatography
using methanol - methylene chloride (1+19) as eluent.
Yield 160 mg (47 %).of (+,-) cis 7-Benzyloxy 4-[4-(morphoiinobutyloxy)-phenyl]-
3-phenyl-
chroman Oii. 'H -NMR (CDCI3, 200 MHz) b: 1.88 (m,4H); 2.38 (m, 6H); 3.55 (m, 1
H); 3.70
(t, 4H); 3.88 (t, 2H); 4.2 {m, 2H); 4.40 (t, 1 H); 5.03 (s, 2H); 6.56 (m, 8H);
6.83 {d, 1 H); 7.15
(m, 3H); 7.38 (m, 5H).
Step 2:
(+,-) cis 7-Hydroxy-4-(4-(morpholinobutyloxy)-phenyl)-3-phenyl-chroman
CA 02270056 1999-04-27
WO 98I18778 PCTIDK97l00485
32
(+,-) cis 7-Benzyloxy-4-[4-(morpholinobutyfoxy)-phenyl]-3-phenyl-chroman (160
mg, 0.29
mmol) was dissolved in a solution of 1 % hydrochloride in ethanol (5 ml). The
solution was
added to a 10 % palladium on carbon catalyst, and hydrogenated (1 atm.)
overnight. The
reaction mixture was filtered and evaporated, The evaporated product
crystallised.
Yield 130 mg (92 %) of (+,-) cis 7-Hydroxy-4-[4-(morpholinobutyloxy)-phenyl]-3-
phenyl-
chroman. HCI m.p. 79.8 - 81.8~C
The product was identified by'H-NMR.