Note: Descriptions are shown in the official language in which they were submitted.
CA 02270385 1999-04-29
WO 98/19727 PCT/US97/19681
TITLE
BUILD-UP RESISTANT SPACERS FOR
METERED DOSE INHALERS
RELATED APPLICATION
This application claims the benefit of provisional application Serial No.
60/030,152, filed November 1, l996.
FIELD OF THE INVENTION
The instant invention relates to a new spacer design for metered dose
inhalers by using effective amounts of a suitable fluoropolymer which prevents
build-up of the drug on the inside walls of the spacer and improves delivery
to
patients.
BACKGROUND OF THE INVENTION
Spacers are attachments used with metered dose inhalers (MDIs) primarily
to maximize transport of a drug into the respiratory airways and minimize
1 S oropharyngeal deposition. These attachments are typically made of a
plastic
material such as polycarbonate or polystyrene and are available in many
different
designs having an annular cross-section and being open at each end, such
designs
including hollow cylinder and hollow cone . The spacer is attached to the
metered
dose inhaler in a way that the spacer receives the metered dose of the drug.
The
patient using the inhaler then inhales the drug from the interior of the
spacer.
While spacers are effective in improving the depth of drug penetration,
thereby minimizing the amount of esophageal impaction (and, therefore, product
swallowing) and increasing the total amount of drug deposited in the lungs,
the
primary disadvantage of a spacer is the potential build-up of the drug (active
ingredient possibly accompanied by other ingredients present in the drug, e.g.
carriers, surfactant) that can occur within the spacer. Build-up can occur for
several reasons, but the most predominant is electrostatic activity. The
consequences of drug build up in the spacer include a higher possibility of
microbial growth within the spacer, increased difficulty cleaning the spacer
and
premature disposal of the metered dose inhaler, among others.
1
._...~.. _.___...~._. __ __,..-.,..._a....~~_. ._.~-,.,__.._...__..~_.___._-
~........_..r _ ~..._..
CA 02270385 1999-04-29
WO 98I19727 PCT/US97119681
SUMMARY OF THE INVENTION
The present invention solves problems associated with the use of a spacer
attached to a metered dose inhaler by reducing the undesired build-up of the
drug
dispensed by the inhaler. The solution provided by the present invention
relates to
the development of spacers for metered dose inhalers that are resistant to
build-up
of the drug (active ingredients) upon the inside wall (interior surface) of
the
spacer.
It has been found that when the interior surface of the spacer is comprised
of fluoropolymer, the drug does not build up on such surface, whereby the
patient
inhaling the drug receives the full metered dose of the drug and the other
problems
associated with drug build-up do not arise.
Build-up resistance by the spacer of the present 'invention can be achieved
by a number of different ways of obtaining the fluoropolymer interior surface,
such as by ( I ) coating the interior surface with fluoropolymer, (2) applying
a
1 S fluoropolymer lining to the interior surface by using an adhesive, or
(3) manufacturing the spacer entirely out of a fluoropolymer.
Preferably, method ( 1 ) is used for reasons of economics, in that the
structural integrity of the spacer can be obtained by the spacer being first
made of
conventional plastics (for that purpose), which are less expensive than
fluoropolymer, and using the more expensive fluoropolymer as a relatively thin
coating only for rendering the interior surface of the spacer non-attractive
to the
dispensed drug.
BRIEF DESCRIPTION OF THE DRAWINGS
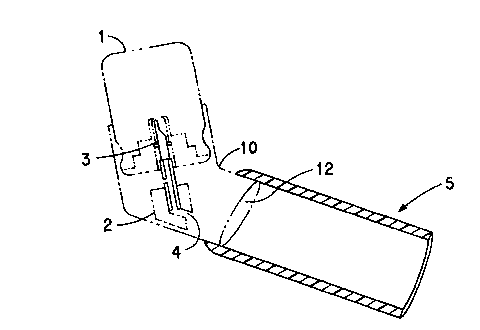
Fig. 1 shows schematically and side view cross-section a portion of a
metered dose inhaler incorporating a spacer of the present invention. The
metered
dose inhaler is shown in phantom lines.
Fig. 2 shows schematically and in side view cross-sectional enlargement
another embodiment of a spacer of annular design of the present invention.
DETAILED DESCRIPTION
The present invention relates to the development of a build-up resistant
spacer for use in delivering medicaments by any metered dose inhaler (MDI).
MDIs are used to deliver dermal, pulmonary or mucosal (e.g., buccal or nasal)
CA 02270385 1999-04-29
WO 98I19727 PCT/US97/19681
administration of such drugs as antiallergic agents. analgesics,
bronchodilators,
antihistamines, antitussives, antianginal agent, antibiotics, anti-
inflammatory
agents, hormones, peptides, steroids, enzymes, sulphonamides, among others. A
typical metered dose inhaler is illustrated in Fig. 1. In Fig. 1, the MDI
comprises
an aerosol container 1 which holds the active ingredient (drug) to be
delivered and
a propellant such as 1,1,1,2-tetrafluoroethane (HFC-134a) or
1,1,l,2,3,3,3-heptafluoropropane (HFC-227ea) or one of the older propellants
such as CFC 11, 12, or 114. The MDI also has an outer sleeve 10 which is
slidably mounted on the exterior of the container 1, and the sleeve 10 has an
outlet
spout 12. Other components of the MDI include an actuator 2, a metering valve
3,
a means defining an actuator orifice 4 which faces the spout 12, and a spacer
S slip
fit over the exterior of the spout. The actuator 2 and metering valve 3
operate
conventionally upon application of external force on the sleeve 10 to cause it
to
slide along (into) the container 1 to operate the actuator 2 to deliver a
metered
dose amount of the drug through the orifice 4 into the interior of the spacer
5 via
spout 12, from which the dispensed drug can then be inhaled. The drug may be
dissolved or dispersed in the propellent, and at least in the latter case,
would be in
the form of a powder as dispensed by the aerosol container into the spacer.
The dimensions of spacer S can be tailored to control the delivery pattern
of the medicament to improve the depth of drug penetration, minimize the
amount
of esophageal impaction (and, therefore, product swallowing) and increase the
total amount of drug deposited into the lungs. Typically, the spacer will have
an
annular cross-sectional such as a hollow cylinder which has an outlet having a
shape which can be tolerated by the mouth of the patient. In the case of the
spacer
being a hollow cylinder, dimensions of the cylinder will generally fall within
a
cylinder length of 50 to 300 mm millimeters and an inner diameter of 24 to 40
mm. More often the cylinder length and inner diameter dimensions will be from
50-100 mm and 24 to 32 mm, respectively. Similar spacer lengths will be used
when the spacer has a different design. In the case of the spacer having the
design
of hollow cones connected together at their bases, to provide an expansive
interior
volume, the largest interior diameter of the spacer (at the base of the
cones), can
be e.g. 100 to 150 mm.
3
CA 02270385 1999-04-29
WO 98/19727 PCT1US97/19681
The effectiveness of the MDI is improved by employing one or more of
the previously identified fluoropolymer embodiments. In the embodiment of Fig.
I, the entire spacer is made of fluoropolymer: In the embodiment of Fig. 2,
wherein the spacer 20 of cylindrical design is shown to comprise an outer
layer 22
and an inner layer 24, the outer layer forms the structural component of the
spacer,
of such plastic as polycarbonate or polystyrene, and the inner layer 24
comprises
fluoropolymer. The fluoropolymer is preferably coated onto the inner surface
of
the cylinder of plastic, and the coating can be very thin depending on the
fluoropolymer used and the method of application. For example, fluoropolymers
which are soluble in solvents which do not dissolve the plastic cylinder can
form
coatings as thin as 0.5 to 5 micrometers, as compared to the thickness of the
plastic making up the outer layer of the spacer, which will be on the order of
1 to
5 mm. The spacer 20 has openings at opposite ends, opening 26 being the inlet
for the dispensed drug and opening 28 being the outlet into the mouth of the
patient.
The fluoropolymer will generally comprises at least 35 wt% fluorine.
Examples of fluoropolymers include polytetrafluoroethylene (PTFE), melt-
fabricable tetrafluoroethylene copolymers such as fluorinated ethylene-
propylene
(FEP), perfluoroalkoxy polymer (PFA), also known as copolymer of
tetrafluoroethylene and perfluoro(alkyl vinyl ether) wherein the alkyl group
contains 1 to 6 carbon atoms, preferably 2 or 3 carbon atoms, ethylene-
tetrafluoroethylene copolymer (ETFE) polyvinylidene fluoride (PVDF), ethylene-
chlorotrifluoroethylene copolymer (ECTFE), mixtures thereof, among others
(some of these fluoropolymers are sold by the DuPont Company, Wilmington,
Delaware under the trademark TEFLON~). As is well known, small amounts of
additional comonomer can be present to improve properties of the copolymer.
Additional examples of fluoropolymers are copolymers of perfluoro-2,2-dimethyl-
1,3-dioxoie with comonomer such as tetrafluoroethylene or
chlorotrifluoroethylene such as disclosed in U.S. Patent 4,754,009 and
copolymers
of tetrafluoroetheylene with a sufficient amount of other monomer than the
resultant copolymer has increased solubility and is preferably even amorphous.
Examples of such copolymers include partially crystalline copolymers of
4
CA 02270385 1999-04-29
WO 98/19727 PCT/L1S97/19681
tetrafluoroethylene with hexafluoropropylene (HFP) wherein the HFP content is
characterized by an HFPI of 6.4 to 9 as disclosed in U.S. Patent 5,266,639,
and
amorphous copolymers wherein the HFP content is even higher, e.g. at least 20
mol% as disclosed in U.S. Patents 5,543,217 and 5,478,905.
Preferably the spacer is transparent, so that the user of the MDI can see the
cleanliness and lack of drug buildup on the interior surface of the spacer.
The
fluoropolymer can be transparent in thick sections, e.g. the thickness of the
cylinder wall forming the spacer when the fluoropolymer is amorphous. Even
partially crystalline fluoropolymers will be sufficiently transparent when
used as
very thin coatings on the interior surface of a cylinder of transparent
plastic, e.g.
polycarbonate or polystyrene.
Thin films of fluoropolymer can be adhered to the outer layer of plastic
forming the structural portion of the spacer to form a coating of
fluoropolymer on
the interior surface of the spacer by first etching the film with sodium
naphthalene
1 S and then using an epoxy adhesive to adhere the etched surface of the film
to the
spacer interior surface.
Alternatively, the fluoropolymer coating can be applied to the interior
surface of the plastic cylinder by coating the interior surface with a
solution of
fluoropolymer. Examples of solvents include perfluorooctane, perfluoro(2-butyl
tetrahydrofizran), and perfluorinated cycloalkane as disclosed in U. S. Patent
5,459,191. The fluoropolymer is selected so that it has solubility in the
solvent.
After application of the solution to the interior surface, the coating
is~dried to
remove the solvent, leaving a fluoropolymer coating on the surface.
Another method for coating the interior surface with fluoropolymer
involves plasma deposition, wherein the surface is exposed to a plasma in the
presence of fluoromonomer vapor, to cause the deposition of the vapor on the
surface where the fluoromonomer polymerizes to form the coating. For example,
the coating can be formed using hexafluoropropylene as the vaporized
fluoromonomer and RF discharge operating at 100 watts, 50 mTorr and 5 min.
exposure.
Fluoropolymers tend not to attract the spray of drug entering the spacer
from the MDI and therefor tend to avoid buildup of any of the drug on the
interior
5
m_. _ . ~_._. ~ ~.~. __..._ ...._._ _. ___..~.., _...-..~._._._...~ __ . _ . _
__.T _
CA 02270385 1999-04-29
WO 98/I9727 PCT/US97/19681
surface of the spacer when the interior surface comprises fluoropolymer. This
lack of buildup is believed to come from the spray of drug and the
fluoropolymer
spacer interior surface both being negatively electrically charged. To insure
that
opposite electrostatic charge between the sprayed drug and the interior
surface of
the spacer does not arise, the fluoropolymer forming the interior surface can
also
contain electrically conductive carbon particles sufficient to dissipate any
opposite
electrical charge while not destroying transparency.
Examples
The inside walls of a plastic spacer for a metered dose inhaler are coated
with polytetrafluoroethylene (PTFE) or other fluoropolymer polymer or
copolymer including, but not limited to FEP, ETFE, or PFA. by spraying,
dipping, brushing or any suitable application method. This creates a nonstick
surface onto which the pharmaceutical agents and other components of the
formulated drug product will not adhere or accumulate.
Two commercially available spacers in cylindrical form believed to made
of polycarbonate are tested for drug buildup as follows:
Allen & Hansburys Volumatic~ - 23 cm long and 9 cm double cone
annular design; inlet and outlet openings of about 25 mm
Forest Pharmaceuticals Aerochamber~ - 15 cm long and 4.5 cm in inner
diameter
The interior surfaces of both spacers are partially coated with
fluoropolymer solution ( one wt% fluoropolymer) and dried, with the resultant
fluoropolymer coating being visible so as to be distinguishable from the
uncoated
portion. The fluoropolymer is tetrafluoroetheylene/hexafluoropropylene
copolymer having a weight average molecular weight of 450,000 and containing
50 wt% of each monomer, and the solvent is perfluorooctane. The fluoropolymer
is made essentially by the process of Examples 15 and 17 of U.S. Patent
5,478,905. An MDI containing a drug/propellent mixture is sprayed for 5
seconds
into each spacer. It is observed that drug buildup occurs on the uncoated
areas of
the interior surface of the spacers, while no buildup is observed on the
coated area.
This test is repeated except that the contents of the MDI are sprayed directly
on
the interior surface instead of aiming through the spacer so that buildup
occurs on
6
CA 02270385 1999-04-29
WO 98/19727 PCT/US97/19681
both coated and uncoated areas. The buildup is allowed to dry and then the
spacer
is subjected to rinsing in water. The buildup on the coated area washes away
in
15 seconds, while the buildup on the uncoated area requires 40 seconds of
rinsing
to wash away the buildup.
S Spacers like the two spacers described above are cleaned by rinsing in
acetone and drying and then tested for adhesion of the fluoropolymer coating
when applied by soaking in a solution rather than by a spray. The same
fluoropolymer as described above is used except that the solution is 3 wt% of
the
fluoropolymer. A primer solution of 3 wt%
tetrafluoroethylene/hexafluoropropylene/maleic anhydride copolymer in acetone
is prepared. In one series of experiments, just the
fluoropolymer/perfluorooctane
solution is used, and the coating is carned out as follov~rs: One end of each
of the
two different spacers is closed by pressing against a smooth sheet of
polyethylene.
Each spacer is then filled with the fluoropolymer solution. After waiting one
minute, the spacers are drained, air dried for one hour and then oven dried at
50~C
for 4 hours. The resultant fluoropolymer coating rinses well to discharge any
buildup of drug, but is subject to removal from the interior surface of the
spacer
by scratching with a fingernail. Repetition of this experiment using clean
spacers
and application of the primer to the interior surface by the same procedure as
the
fluoropolymer solution is applied, followed by application of the
fluoropolymer
solution in perfluorooctane to the dried primer layer, whereby the primer
forms an
intermediate layer, gives a layer that both resists drug buildup and removal
by
scratching with a fingernail. The composite layer is also not removed by
rubbing
with a No. 10l eraser from Eberhard Faber. Thus the composite fluoropolymer
coating can be cleaned by scrubbing as well as by rinsing if the need arises.
The tetrafluoroethylene/hexafluoropropylene/maleic anhydride copolymer
is made by the following procedure: A mixture of 2000 g of
hexafluoropropylene,
110 g of tetrafluoroethylene and 5 g of malefic anhydride dissolve'g in 10 ml
of
trifluoracetic acid, and 1.1 g of nitrogen trifluoride is made in a one gallon
reservoir. About 1120 g of this mixture are added to a polymerization
autoclave
which is shaken and heated at 250~C for 125 min. The contents of the autoclave
are then removed and dried under vacuum, yielding 80 g of yellow colored
7
CA 02270385 1999-04-29
WO 98/19727 PCT/US97/19b81
polymer, which analyzes as follows: wt. average molecular weight of 88,600,
61.4
mol% of TFE (carbon-I3 NMR), 37.7 mol% HFP (carbon-13NMR) and 0.9 mol%
malefic anhydride (carbon-13 NMR in hexafluorobenzene at 60~C, absorption @
161ppm).
Similar results on buildup resistance are obtained when the fluoropolymer
coating on the interior surface of the spacer is a fluoropolymer film
laminated to
the surface and when the entire spacer is made of fluoropolymer.
A formulation of fluoropolymer along with an electrically conductive
material is applied to the inside of an MDI spacer. The conductive material
eliminates the buildup of static electricity during the ingestion of the
medication,
thereby eliminating the resultant loss of active ingredient (powder) by either
random powder repulsion or by electrostatic adhesion of the powder on the MDI
spacer. The electrically conductive material is in the form of carbon, or
other anti-
static material.
20
8