Note: Descriptions are shown in the official language in which they were submitted.
CA 02271031 1999-OS-07
WO 98/31311 PCT/iJ897/21013
NEAR HYPERTHERMIC HEATER WOiJND COVERING
TECHNICAL FIELD
This invention relates to a wound covering for wound treatment and, in
particular,
wound covers having a substantial portion of the wound cover in non-contact
with the
wound and capable of delivering heat to the wound. The wound covering
preferably
controls the temperature, humidity and other aspects of the environment at the
wound site.
BACKGROUND ART
Wounds in general, as used in this context, are breaks in the integrity of the
skin of
- a patient. Wounds may occur by several different mechanisms. One such
mechanism is
through mechanical traumatic means such as cuts, tears, and abrasions. There
are many
instruments of causality for mechanical wounds, including a kitchen bread
knife, broken
glass, gravel on the street, or a surgeon's scalpel. A different mechanism
cause for
mechanical wounds is the variable combination of heat and pressure, when the
heat alone
is insufficient to cause an outright burn. Such wounds that result are
collectively referred
to as pressure sores, decubitus ulcers, or bed sores, and reflect a mechanical
injury that is
more chronic in nature.
Another type of mechanism causing a wound is vascular in origin, either
arterial or
venous. 'the blood flow through the affected region is altered sufficiently to
cause
secondary weakening of the tissues which eventually disrupt, forming a wound.
In the case
of arterial causes, the primary difficulty is getting oxygenated blood to the
affected area.
For venous causes, the primary difficulty is fluid congestion to the affected
area which
backs up, decreasing the flow of oxygenated blood. Because these wounds
represent the
skin manifestation of other underlying chronic disease processes, for example,
atherosclerotic vascular disease, congestive heart failure, and diabetes,
these vascular
injuries also are chronic in nature, forming wounds with ulcerated bases.
Traditional wound coverings, such as bandages, are used to mechanically cover
and
assist in closing wounds. Such bandages typically cover the wound in direct
contact with
the wound. This may be acceptable for acute, non-infected traumatic wounds,
but it must
Kl \ . W V : 1:1'.\ -:w O..wltt..v W . . - _-;f;f ~ m . - t ~ m i i ~ .:~ W t
t;f ;~:f _,saa-t-L.~i,~ ~ N' is
1 LL . v .. - V~ ~ a..~~ t t
t . V
y .:.~ CA 02271031 1999-OS-07
rr.
'~ ~~~TITL'TE PAGES 2
. .... . ,
be kept in mind that direct bandag;. contact with a wound can interfere with
the
v Healing process. This interference is particularly prevalent for ch; onic
ulcerated
wounds because of the repeated trechanical impact and interactier. of the
bandage
-_.~uit ~ sst:re-ser~sitis.~~issues~itbin~~a~'ovund. __._____ _
The ber.etits of application of nezt to a wour_d are known. and documented
. (_
benefits include: increased cutaneous and subcutaneous blood flow; incr:ased
oxygen
w partial pressure at the wound site; and increased immune system fanc~ons,
bath
h~unoral and cell rsediated, including increased mi exation of v~~hite blood
cells and
,:
-.:~~ HbTob~sts to the si~.e.
. .~Yv i~ ., a..j
w:.- ' However, heat therapy for th_ trea;tnent of wo~,mda, either irr~cted or
clean,
r ; . , . .
has bees: rzr~'ictxlt to achieve :n practice. For instance, hea?ang iari!ps
have berz: used.
bat than resulted L~ dryinn of woiznus, and in some cases, e-. en burning
tissue frera
' ~ the hig.~: heat. Due to these and other dif~.culties, and since m cst
acute w'cunds
'''~'~ usually hzal ever tirr.e, physicians no longs: oonsidzr the arplication
of heat tc the
wot:nd as par. of the trearnent process. The thirldrg among medical personnel
is
that any inter ference in a r_atu.~l prwess should be tninimize3 url~l it is
probably that
the nansal process a going :o fai'. additionally, the availrbiliry eT
ar~ibiotics ier use
in association wit'? into ted waun ds has tale precedent a a~~e: other the:
apses for ih a
= ~ treatment c~f chronic wo'ands and topical i.~.iections.
In PCT application CVO 93F19~06, a heat dressing for treat:ner.t of a cvound
ir_cludes a heat~r and a liquid-absorbing adhesive layer that attaches to the
s>~in, i.-t
cor~taet with the wound.
,,.: a.,:
R .w''~ Ir: L.~. Patent No. 5,OS3.02~4. warts are ?.rooted by applicatio: of
an
exothermic pad in direct contact with. ~:he s?cir_ whic't hears the skin iota!
l; ~ >_o a
temperature of about :~? ~~'-a3 ~C.
. . ~ . :.~ : :. : :_:a
' x ~ Ln Frenc:~ patent number 1.527, 887 _asued April 29, 196g to Veilhaa
tllera is
'~ disclosed a e:overing with a rigid oval dorr.e, its edge resting directly
on t,'te patient's
skin. Qne aspect of the Veilhan wotuid protecmr is a single oval heati.~ig
element
y restin; cn the cure: surface oFthe ri5 d dome, positioned at the ;ae.~iphery
cf the rigid
;.
dora. Veilhan does not discuss the heaHrg aspect other than to otate that it
is a
.. . : ,; .~:, ~"lnponent.
-' 1:4208.A.~S:
l11'vtLivi:~~D SNE~T
IW . . . v.. vl~ '~. vyv v n y v w.fJvJ' W 1 I I . . ~ ... n ~ .. ~ , . v. v .
I I L.\ t ~ i , n m~i W '1"t'v.)sj ~ If W
CA 02271031 1999-OS-07
SUHSTITL'TE PAGES ZA
The benefits of controlling other environmental parameters around the ~~vu3d
site are not as well Down. Controllutg the hu,~-nidity at the wound site and
the
benefits of isolating the wound have not been erensiveiy studied and
documented.
~~hile the benefit of applying heat to v:ounds is generally knowr~, the manner
of how tha: h:.at sk~ould ~Oe used er agplitd is not known. Historically, heat
was
applied at kigher tempe:-afiires watt: the goal ~~f tr_alczna the wou.W
'ttyperth~ic.
'I~ese higher temYe.Tat~-es of'en res4l:ed ui increasing t;ssue damage ratl:zr
thzn ta'~eir
intended pu.~ose of wound therapy and healing. Ty=re is a need for approp: ate
wound care management
P:IIAL~GUS rrni.~aoaa,.~t
AArIFiJGcJ SHEET
CA 02271031 1999-OS-07
WO 98/31311 PCT/US97/21013
-3-
incorporating a heating regimen that is conducive to wound healing, yet safe
and cost
effective.
DISCLOSURE OF INVENTION
The present invention disclosed herein approaches the treatment of wounds with
heat based on an understanding of physiology. 'fhe normal core temperature of
the human
body, defined herein for purposes of this disclosure, is 37~ C ~ 1 ~ C (36~ -
38~ C), which
represents the normal range of core temperatures for the human population. For
proposes
of discussion and this disclosure, normal core temperature is the same as
normothermia.
Depending on the environmental ambient temperature, insulative clothing and
location on
the body, skin temperature typically ranges between about 32~ C and about 37~
C. From
a physiologic point of view, a 32~ C skin temperature of the healthy distal
leg is moderate
hypothermia. The skin of the distal leg of a patient with vascular
insufficiency may be as
low as 25~ C under normal conditions, which is severe hypothermia.
A fundamental physiologic premise is that all cellular physiologic functions,
1 S biochemical and enzymatic reactions in the human body are optimal at
normal body core
temperature. The importance of this premise is seen in how tightly core
temperature is
regulated. Normal thermoregulatory responses occur when the core temperature
changes
as little as ~ 0.1 ~ C. However, the skin, as noted above, is usually
hypothermic to varying
degrees. For example, the skin of the torso is usually only slightly
hypothermic, whereas
the skin of the lower legs is always hypothermic. Consequently, wounds and
ulcers of the
skin, regardless ohlocation, are usually hypothermic. This skin hypothermia
slows cellular
functions and biochemical reactions, inhibiting wound healing.
The effects of hypothermia on healing are well known. A number of regulatory
systems within a human are affected, such as the immune system and
coagulation, with
both platelet function as well as the clotting cascade affected. Patients with
hypothermic
wounds experience more infections which are more difficult to treat, have
increased
bleeding times and have been shown to require more transfusions of blood. A11
of these
complications increase morbidity and the cost of patient care and, to a lesser
extent,
increase the likelihood of mortality.
CA 02271031 1999-OS-07
WO 98/31311 PCT/US97/21013
-4-
One purpose of the present invention is to raise the wound tissue and/or
periwound
tissue temperatures toward normothermia to promote a more optimal healing
environment.
The present invention is not a "heating therapy", per se, where it is the
intent of "heating
therapy" to heat the tissue above normothermia to hyperthermia levels. Rather,
the present
invention is intended to bring the wound and periwound tissues toward
normothermia
without exceeding normothermia.
The medical community has not historically considered normothermic heating to
be therapeutic. Many physicians feel that hypothermia is protective and,
therefore,
desirable. Studies with the present invention would indicate that this widely
held belief that
hypothermia is at least benign or possibly beneficial is incorrect with regard
to wound
healing.
The present invention is a wound covering for application to a selected
treatment
area of a patient's body that includes, at least as a portion of the selected
treatment area, a
selected wound area. The selected treatment area may also include a portion of
the area
immediately proximate to the wound area referred to as the periwound area. The
wound
covering comprises a heater suitable for providing heat to the selected
treatment area, an
attachment for attaching the heater in a non-contact position proximate the
selected
treatment area, and a heater controller, connected to the heater and including
a power source
for the heater, for controlling the temperature of the heater in a temperature
range from 38~
C temperature to about 46 ~ C. Ambient temperature is that temperature of the
environment
immediately around the selected treatment area not a part of the patient's
body, i.e., the bed,
the air in the room, the patient's clothing.
The heater is selectable from among several types of heat sources such as
warmed
gases directed over the selected treatment area and electrical heater arrays
placed proximate
the selected treatment area. Electrical heater arrays are adaptable for
construction into a
layer of variable proportion and geometry or as a point source. The present
invention
anticipates the ability to provide several different sizes and geometric
configurations for the
heater. The present invention is flexible in being able to provide uniform
heating over the
entire selected treatment area or provide a non-uniform heating distribution
over selected
portions of the selected treatment area. Alternate heat source embodiments
could include
CA 02271031 1999-OS-07
WO 98/31311 PCT/US97/21013
-5-
warm water pads, exothermic chemical heating pads, phase-change salt pads, or
other heat
source materials.
The present invention anticipates that the controller is able to control both
the
temperature and the duration of the application of heat. This control may
extend from
manual to fully automatic. Manual control anticipates the controller
maintaining the heater
temperature at an operator-selected temperature for as long as the operator
leaves the heater
on. More automatic modes provide the operator an ability to enter duty cycles,
to set
operating temperatures, as well as to define therapy cycles and therapeutic
sequences. As
used herein, a duty cycle is a single on cycle when heating of the heater is
occurring,
measured from the beginning of the on cycle to the end of that on cycle. A
heater cycle is
a single complete on/off cycle measured from the beginning of a duty cycle to
the
beginning of the next duty cycle. Consequently, a duty cycle may also be
represented in
a percentage of, or as a ratio of the time on over the time off. A plurality
of heater cycles
are used to maintain heater temperature around a selectable temperature set
point during a
therapy cycle which is defined as an "on" period, composed of a plurality of
heater cycles,
and an "off' period equivalent to remaining off for an extended period of
time. A
therapeutic sequence, as used herein, is a longer period of time usually
involving a plurality
of therapy cycles spread out over an extended period of time, the most obvious
being a day
in length. The present invention anticipates the use of any period of time as
a therapeutic
sequence and involving one, or more than one therapy cycles.
The present invention also anticipates programmability for a number of
modalities
including peak heater temperature for a duty cycle and/ar therapy cycle,
average heater
temperature for a duty cycle and/or therapy cycle, minimum heater temperature
for a heater
cycle and/or therapy cycle, ratio of duty cycle, length of therapy cycle,
number of duty
cycles within a therapy cycle, and number of therapy cycles in a therapeutic
sequence.
Different duty cycles within a therapy cycle may be programmed to have
different peak
heater temperatures and/or heater cycles may have average heater temperatures
over that
therapy cycle. Different therapy cycles within a therapeutic seguence may be
programmed
to have different peak heater temperatures and/or average heater temperatures
over each
therapy cycle. The wound covering control is operator-programmable or may have
CA 02271031 1999-OS-07
WO 98/31311 PCT/US97/21013
_b_
preprogrammed duty cycles, therapy cycles, and therapeutic sequences
selectable by the
operator.
A preferred form of the wound covering includes an attachment as a peripheral
sealing ring which, in use, completely surrounds the area of the wound and
periwound, i.c.,
the selected treatment area. The upper surface of the peripheral sealing ring
is spanned by
a continuous layer which is preferably transparent and substantially
impermeable, although
the present invention also anticipates the use of a gas permeable layer
suitable for some
applications. Once in position, the sealing ring and the layer define a wound
treatment
volume which sulTOUnds the wound. Additional ly, the layer spanning the
peripheral sealing
ring may be sealed about the periphery of the sealing ring and act as a
barrier layer over the
wound treatment volume. Optionally, the heater may be incorporated into the
barrier layer
or the barrier layer may be incorporated into the heater. An adhesive and a
suitable release
liner is applied to the lower surf ace of the peripheral sealing ring to
facilitate the application
of the wound covering to the patient's skin.
The barrier layer may include a pocket adapted to receive an active heater. An
alternate form of the invention provides for the transport of heated air from
a remote heat
source to the wound treatment volume. In the active heater embodiments a
thermostat
and/or a pressure-activated switch may be used to control the heating effects
of the heater.
Each of these heated embodiments promote wound healing by maintaining the
wound site
at a generally elevated, but controlled, temperature.
In general, the peripheral sealing ring is made from an absorbent material
which
may act as a reservoir to retain and/or dispense moisture into the treatment
volume
increasing the humidity at the wound site. The reservoir may also contain and
deliver
medicaments and the like to promote healing.
The present invention is designed to directly elevate the temperature of the
hypothermic skin and subcutaneous tissue of the selected wound area to a
temperature
which is close to or at normothermia. The purpose of this device is to create
within the
wound and periwound tissues of the selected treatment area a more normal
physiologic
condition, specifically a more normothermic condition, which is conducive to
better wound
healing. The present invention anticipates the use of an active heater that
creates small heat
gradient from heater to wound and periwound tissues. The usual temperature
gradient for
CA 02271031 1999-OS-07
WO 98/31311 PCT/US97/21013
_7_
tissues goes from about 37 ~ C deep in the body core down to about 32 ~ C at
the skin
surface of the leg. The heater of the present invention operates in the range
above 38 ~ C.'
to about 46 ~ C which is slightly above the body core temperature. Since heat
must flow
down a temperature gradient, heat will flow directly from the near
hyperthermic heater to
the wound and periwound tissues. The thermal mass of the body, the blood flow
and the
inefficiencies of heat transfer will result in tissue temperatures that arc
less than 38~ C.
In contrast, typical local heating therapy (e.g. hot water bottles, hot water
pads,
chemical warmers, infrared lamps) deliver temperatures greater than 46 ~ C to
the skin. The
goal of traditional heating therapy is to heat the tissue above normal, to
hyperthermic
temperatures.
The present invention differs from infrared lamps in two ways. First, the
present
invention includes a dome over the wound that is relatively impermeable to
water vapor
transmission. After application of the bandage, moisture from the intact skin
or wound
evaporates, and air within the dome quickly reaches 100% relative humidity.
The interior
of the present invention is now warm and humid. For example, a 2.5 square inch
bandage
at 28 ~ C requires only 0.0014 g of water to reach saturation. When the air is
thus saturated,
no further evaporation can occur and, therefore, no drying of the wound can
occur. This
equilibrium will be maintained as long as the bandage is attached to the
patient.
When heat is provided by the preferred embodiment of the present invention,
the
absolute amount of water needed to reach 100% relative humidity is slightly
increased since
warm air has a greater capacity for holding moisture. I-Iowever, the air
within the dome of
the bandage still reaches water vapor saturation-very quickly, and no further
evaporation
occurs. For example, a 2.5 square inch bandage of the present invention at 38
~ C requires
only 0.0024 g of water to reach saturation. Excess moisture is absorbed by the
foam ring,
but still is retained within the bandage. The enclosed dome design maintains
100%
humidity over the wound which also prevents evaporation due to the heat. As
long as the
humidity is retained within the bandage, heating therapy could theoretically
be continued
indefinitely without causing the wound to dry. In contrast, when using
infrared lamps, the
wounds are open and exposed to the environment. The result is excessive drying
of the
wound, increasing tissue damage.
CA 02271031 1999-OS-07
WO 98/31311 PCT/US97/21013
_g_
Secondly, the present invention operates at low temperatures, from 38 ~ C to
about
46 ~ C. This causes only minimal heating of the skin. In contrast, infrared
lamps operate
at temperatures in excess of 200 ~ C. These lamps heal the wound to
hyperthermic
temperatures which can cause thermal damage to the tissue of the wound.
At the low (normothermic) operating temperatures of the present invention, the
heat
transfer to the skin is minimal. The low wattage heater, the inefficiencies of
the heat
transfer into the tissue, the thermal mass of the tissue and the blood flow
(even if markedly
reduced), all prevent the wound temperature from reaching the heater
temperature.
Hypothermic wound tissue is warmed as a result of "migration" of the body's
core
temperature zone toward the local wound area.
The following data document the tissue temperatures resulting from a 38 ~ C
heater
of the present invention on:
Averaee Maximum
Normally perfused human skin 36 C 36 C
Arterial/diabetic foot ulcers 32 C 35 C
I S Venous/arterial leg/foot ulcers 33 C 35 C
Non-perfused human model 35 C 35 C
When warmed with a 38~ C heater, wounds on poorly perfused legs reach stable
average temperatures of 32-33 ~ C. In contrast, normally perfused skin reaches
36~ C. It
is important to note that these data are contradictory to the assumption that
poorly perfused
tissue would reach a higher temperature than normally perfused tissue. This
result
substantiates the physiologic f nding that the "migration" of the core
temperature zone
toward the local wound zone, decreasing the gradient difference between the
core and
surface temperatures, is the cause for the observed increased wound
temperatures. Core
temperature regulation is heavily dependent on perfusion, and migration of the
core
temperature zone is also heavily dependent on perfusion. At no point in time
did the poorly
perfused tissue reach normothermia. Consequently, poorly perfused legs are
much colder
than normally perfused legs, and, thus, poorly perfused legs constitute a
substantially
deeper heat-sink.
A wound-healing pilot study is under way) studying patients with chronic
arterial
and/or venous ulcers of the lower leg. These patients have suffered from these
ulcers for
CA 02271031 1999-OS-07
WO 98/31311 PCT/US97/21013
_c)_
many 1110I1thS and, in some cases, even years, despite aggressive medical and
surgical
therapy. Of 29 patients enrolled, 24 have completed the study protocol or are
still being
treated. Of these 24 patients, 29% are completely healed, and 38% show a
significant
reduction of the wound size within 2-5 weeks of receiving therapy with the
present
invention.
A known consequence of restoring normothermia to tissues is to induce some
degree of vasodilatation which increases local blood flow. Preliminary data
collected
during trials of the present invention, studying the effects of the present
invention on
normal subjects and on wound healing, has borne this out. An added effect has
been to
increase the partial pressure of oxygen in the subcutaneous tissues (PS~O,),
which is an
indirect indicator of the status of the tissue. The higher the PS~O~, the
greater the likelihood
the tissue will benefit and improve the healing process. T'he results of some
ofthese studies
are presented in Tables I-4.
In conducting the studies presented in Tables I -4, a wound covering according
to
I 5 the present invention is placed over the skin. The temperature of the
subcutaneous tissue
is then measured over time. From -60 minutes to the 0 minute mark, the heater
is off in
order to obtain a baseline temperature. At the 0 minute mark the heater is
activated and its
temperature kept constant over the next 120 minutes when it is turned off.
Temperature
measurements were taken during this 120 minute period and for an additional
180 minutes
after turning the heater off. As shown in Table 1, with activation of the
heater to 38~ C, the
subcutaneous tissue temperature rapidly rose from about 34.3~ C to about 36~ C
over the
first 30 minutes. The temperature of the subcutaneous tissue continued to
slowly raise over
the next 90 minutes to a temperature of about 36.7~ C. After turning the
heater off, the
temperature of the subcutaneous tissue fell to about 35.9~ C and held this
temperature fairly
uniformly for at least the next 120 minutes.
Table 2 presents the skin temperature data collected from within the wound
cover
of the present invention for the same periods as those in Table 1. The general
curve shape
is similar to the subcutaneous tissue temperature curve. The baseline
temperature at the 0
minute mark was about 33.5~ C. After turning the heater on to 38~ C, the skin
temperature
rose rapidly to about 35.8~ C in the first 30 minutes, then slowly rose to
about 36.2~ C by
CA 02271031 1999-OS-07
WO 98/31311 PCT/US97/21013
_ l 0_
the end of the I 20 minute heating period. After turning the heater off; the
skin temperature
fell to about 35~ C and held there for at least the next two hours.
Table 3 represents laser Doppler data collected from the tissue during the
experiments and correlates to blood flow through the local area being treated
with heat.
The baseline flow is approximately 80 ml/100g/lnin and rises to about 200
ml/IOOg/nnn
at its peak, half way through the heating period. The flow "normalizes" back
to baseline
during the last half of the heating period and remains at about baseline for
the remainder
of the measuring period.
The change in P502 is followed in Table 4. The baseline PSq02 is about 75 when
heating begins and rises steadily to about 130 by the end of the heating
period. The P50
remains at this level for tile remainder of the measuring period despite the
lack of heating
for the last 180 minutes. The added benefit of increased Ps~O~ by heating
continues well
into the period of time after active heating has ceased. Wounds will continue
to benefit
from the effects of heating for substantial periods of time after the heating
is turned off.
The consequences of this study with the present invention is that the heating
need not be
constant, but deliverable over a heater therapy cycle or cycles that may or
may not be part
of a larger therapeutic sequence.
Similar trials were conducted using a heater temperature of 46~ C. This data
is
presented in tables 5-8. Only slight additional benefits were found in any of
the four
measured parameters when studied at this higher temperature. The benefits
imparted by
active heating according to the present invention seem to peak at about 46 ~
C. In many
instances, 43 ~ C appears to be the optimal temperature for maximal efficiency
in terms of
least energy required for the greatest therapeutic gain.
Our initial human clinical data shows that the beneficial effects of heating
on blood
flow and PS~OZ last at least one hour longer than the actual duration of heat
application.
Further, we have noted that cycled heating seems to be more effective for
wound healing
than continuous heating. ~fherefore, the data recommends cycling the heater in
a therapy
cycle (e.g. I hour "on" and 1 hour "off') for a total heating time of 2-8
hours per day as a
therapeutic sequence.
None of the 29 patients with compromised circulation treated to date have
shown
any indication of skin damage due to 38 ~ C heat. Furthermore, none of these
wounds have
CA 02271031 1999-OS-07
WO 98/31311 PCT/LJS97/Z1013
-11-
exceeded 35 ~ C tissue temperature, with an average wound temperature of 32-33
~ C. The
present invention raises the wound temperature toward normothermia, but even
on a poorly
perfused leg, the tissue does not reach normothermia.
BRIEF DES('.RIPTION OF DRAWING
The objects, advantages and features of this invention will be more readily
appreciated from the following detailed description, when read in conjunction
with the
accompanying drawing, in which:
Figure 1 A is an exploded view of a wound covering according to the present
invention;
Figure 1 B illustrates an assembled view of the wound covering of Figure 1 A;
Figure 2A is a view of an alternate wound covering;
Figure 2B is a view of an alternate wound covering of Figure 2A with passive
heating card inserted in the wound covering;
Figure 3A is an exploded view of an additional alternate wound covering;
Figure 3B is an assembled view of the wound covering of Figure 3A;
Figure 4 is a side elevation view of a wound covering;
Figure 5 is an enlarged top plan view of a wound covering;
Figure 6 is an enlarged sectional view taken along line 6-6 of Figure 5;
Figure 7 is a bottom view of the wound covering of Figure 4;
Figure 8A is an exploded view of an alternate wound covering:
Figure 8B is an assembly view showing the air flow through the wound covering;
Figure 9A is a perspective view oi~ an alternate wound covering;
Figure 9B is a side view of the wound covering of Figure 9A;
Figure 10 is a perspective view of an alternate wound covering;
Figure 1 lA is a perspective view of an alternate wound covering;
Figure 11 B is a side elevational view of the wound covering of Figure 11 A;
Figure 11 C is a view of the wound covering of Figure 1 lA;
Figure 12 is a perspective view of an alternate connector apparatus for the
wound
covering;
Figure 13A is an alternate connector arrangement for the wound covering;
CA 02271031 1999-OS-07
WO 98/31311 PCT/US97/21013
-12-
Figure 13B is a side sectional view of the wound covering of~ Figure 13A;
Figure 14 is a view of a rigid connector for engagement with a wound covering;
Figure 15 is an alternate fluid inlet line for the wound covering;
Figure 16A is a view of a two ply barrier layer wound covering;
S Figure 16B is a side elevational view of the wound covering of Figure 16A;
Figurc 17 is an alternate wound covering;
Figure 18A is an alternate wound covering;
Figure I 8B is a side sectional view of the wound covering of Figure 18A;
Figure 1 J is a side elevational view of an alternate wound covering
Figure 20 is a schematic diagram of an embodiment of the present invention;
and
Figure 21 A is a schematic representation of an alternate embodiment of the
heater
array distribution shown in Figure 20;
Figure 21 B is a schematic representation of an alternate embodiment of the
heater
array distribution shown in Figures 20 and 21 A;
figure 21 C is a schematic representation of an alternate embodiment of the
heater
array distribution shown in Figures 20, 21 A, and 21 B;
Figure 21 D is a schematic representation of an alternate embodiment of the
heater
array distribution shown in Figures 20, 21 A, 21 B, and 21 C;
Figure 22 is a graphical representative sample of an operational scheme for an
embodiment of the present invention, such as the embodiment shown in Figure
20;
Figure 23 is a graphical representative sample of an additional operational
scheme
for an embodiment of the present invention, such as the embodiment shown in
Figure 20
using the scheme depicted in Figure 22; and
Figure 24 is a graphical representative sample of another additional
operational
scheme for an embodiment of the present invention, such as the embodiment
shown in
Figure 20 using the schemes depicted in Figures 22 and 23.
BEST MODE FOR CARRYING OL1T THE INVENTION
The present invention is directed to a non-contact wound covering for
controlling
the local environment at a wound site on a patient. A wound site includes
those portions
of the patient's skin obviously definable as the wound area and the
immediately adjacent
CA 02271031 1999-OS-07
WO 98I31311 PCT/US97/21013
_13_
periwound area as the selected treatment area of the wound site. 'the wound
covering
protects the wound from contamination by materials from the outside
environment and also
prevents the wound site from shedding contaminants into the local environment
of the
patient, i.e. the hospital room. The treatment volume formed over the wound
site can be
controlled to create an optimal healing environment. The word "wound" as used
herein
refers generically to surgical incisions, ulcers, or other lesions or breaks
in the skin.
First, a substantially vertical wall is provided to encircle the selected
treatment area
on the surface of the patient's skin. This vertical wall provides an upper
surface to support
a layer spanning this structure above the level of the wound and a lower
surface suitable for
attachment to the patient's skin. This structure is referred to throughout as
an attachment
or a peripheral sealing ring. Together these elements form a wound treatment
volume
- between the layer and the surface of the selected treatment area. The fact
that the layer does
not contact the wound itself promotes healing by minimizing mechanical
stresses on the
tissues. The lower surface suitable for attaching to the skin may include an
adhesive and
I 5 a complimentary release liner assembly to facilitate the attachment of the
wound covering
to the skin of the patient. The present invention anticipates using a heater
such that the
layer may comprise the heater formed as the layer or as a layer which includes
a heater
within some portion of the layer. The layer may also include functioning as a
barrier layer
completely enclosing the wound treatment volume.
In accordance with the present invention, the climate within the wound
treatment
volume may be controlled. Typically the temperature, humidity, and gas
composition, for
example adding oxygen, nitric oxide or ozone, are controlled. Also,
aerosolized
medications or compounds may be released into this volume as well. The above
list is
exemplary of the climate controls which may promote healing of the wound, and
is not
intended to limit the scope of the present invention. It will be understood by
those skilled
in the art that numerous other climate factors can be controlled within the
treatment volume
of the present wound covering system without departing from the scope of the
invention.
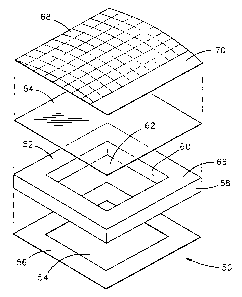
Figure lA illustrates an exploded view of a wound covering 50. In this
embodiment, a peripheral sealing ring 52 is substantially square in outline.
Peripheral
sealing ring 52 is intended to be attached to uninjured skin surrounding a
selected treatment
area 54 using an adhesive 56. In this embodiment, a layer of adhesive hydrogel
is shown
CA 02271031 1999-OS-07
WO 98/31311 PCT/US97/2I013
-14-
as the adhesive 56. Additionally, peripheral sealing ring 52 is preferably
constructed of an
open cell hydrophilic foam plastic having a sealed outer surface 58 which
isolates the
wound from the e17v11'Olllllent. ~I~11C: peripheral sealing ring is fabricated
from a material
which may conform to the curved surface of the patient's body. An imer surface
60 of
sealing ring 52 is preferably porous or absorbent so chat it can form a
reservoir to contain
and release moisture or water vapor into the air within a treatment volume 62
to create a
high humidity environment if desired. Additionally, the hydrophilic absorbent
nature of
peripheral sealing ring 52 absorbs fluids and blood weeping from the wound.
A layer 64 is preferably attached to an upper surface 66 of peripheral sealing
ring 52
as a barrier layer to seal treatment volume 62. Layer 64 is preferably
constructed of a
flexible synthetic polymeric film, such as polyethylene, polyvinyl chloride,
polyurethane,
or polypropylene. Additionally, other polymeric films, natural and semi-
synthetic, that are
suitable for use in medical applications such as cellulose and cellulose
acetate, may be used.
A wound tracing grid 68, also constructed of a substantially clear flexible
material, may
optionally be used as, or attached to, layer 64 to facilitate wound care
management so that
the physician can draw an outline of the wound as an aid to tracking the
healing process of
the wound. The wound tracing grid preferably contains a labeling area 70 for
identifying
the patient, date when the wound was traced, and other patient medical data.
It will be understood by those skilled in the art that the volume of
peripheral sealing
ring 52 will depend on the structural strength of the support material and the
amount of
fluid absorption desired. Additionally, the total area of peripheral sealing
ring 52 is
dependent on the sire of the wound. For example, larger wounds and more
flexible covers
will require a thicker sealing ring so that the center of the cover does not
touch the wound.
Upper surface 66 of peripheral sealing ring 52 is preferably sealed by
extending
barrier layer 64 over the entire area of upper surface 66 as shown in Figures
1 A and I B.
Adhesive 56 for attaching peripheral sealing ring 52 to uninjured skin
surrounding selected
treatment area 54 may take any form, however, the preferred adhesive is
preferably a two-
faced hydrogcl which attaches to a lower surface 72 of peripheral sealing rmg
S2. This
adhesive 56 permits the attachment of peripheral sealing ring 52 to the
patient's skin.
Finally, peripheral sealing- ring 52 may serve as a reservoir for retaining
water or
CA 02271031 1999-OS-07
WO 98/31311 PCT/US97/21013
-I 5-
medicaments in treatment volume 62 in order to maintain a high humidity in the
air within
the volume. Water may be added to peripheral sealing ring 52 at any time
during treatment.
It will be understood by those skilled in the art that peripheral sealing ring
52 can
be supplied in a variety of shapes and sizes to accommodate various wounds.
fhe shapes
may include circles, squares, or rectangles. Although it is preferred to
dispense the wound
covering as a unitary assembly it should be apparent that individual segments
of peripheral
ring material could be assembled into any shape necessary to form a perimeter
around the
wound area. Likewise, barrier layer 64 and wound tracing grid 68 could be
provided in
large sheets which may be cut to size and then attached to the peripheral
sealing ring.
I 0 Figure I B is an assembled view of wound covering 50 of Figure I A. To
dispense
the assembled product, a release liner 74 of Figure 1 B is applied to adhesive
56 in
Figure 1 A. Release liner 74 may span the entire lower surface of the covering
to maintain
the sterility of treatment volume 62. Release liner 74 preferably has a grip
tab 76 to
facilitate removal of release liner 74 from wound covering 50 immediately
prior to
application of wound covering 50 to the skin of a patient.
Figures 2A and 2B illustrate an alternate embodiment of the present invention
as
a wound covering 80 utilizing passive heating of the treatment volume 62.
Because heat
is constantly being radiated from the patient's skin surface, the insulation
properties of the
trapped air within treatment volume 62 will reduce this heat loss. By adding
an infrared
reflector 82 over treatment volume 62, the infrared heat from the body can be
reflected back
to the skin for added passive heating.
An edge 84 of wound tracing grid 86 is preferably not attached to the barrier
layer
to form an envelope or a pocket 94 between the wound tracing grid 86 and the
barrier layer.
A piece of reflective foil material 88 may be inserted into pocket 94. A thin
layer of
insulating material 90 may be optionally attached to foil layer 88 to enhance
heat retention
and to provide foil layer 88 with additional resiliency. A tab 92 is
preferably attached to
infrared reflector 82 to allow easy insertion and removal from pocket 94 and
wound
covering 80.
Figures 3A and 3B illustrate a preferred alternate embodiment of a non-contact
wound covering 108 utilizing active heating of a treatment volume 112. Wounds
may be
CA 02271031 1999-OS-07
WO 98I31311 PCT/US97121013
-16-
safely and easily heated utilizing a heater assembly 100. Heater assembly I 00
alternatively
comprises a pressure-sensitive switch 102, an insulating layer 104, and a foil
heater 106.
Pressure-sensitive switch 102 is optionally laminated to the upper surface of
heater
assembly 100. The purpose of switch 102 is to shut off power to foil heater
106 in the
event that external pressure is applied to wound covering 108 with sufficient
force to cause
foil heater 106 to contact the skin or wound below. This feature prevents the
possibility of
applying heat and pressure to the skin at the same time. The combination of
heat and
pressure is known to cause burns even at low temperatures (40~ C) because the
pressure
prevents blood flow in the skin making it susceptible to thermal injury.
Pressure-sensitive
switch 102 preferably covers the entire area of heater assembly I 00 so that
pressure applied
anywhere to the surface of heater assembly 100 will deactivate foil heater
106.
It will be understood by those skilled in the art that a variety of devices
are suitable
for use as pressure-sensitive switch I 02. I~ orce sensing resistors,
resembling a membrane
switch, which change resistance inversely with applied force are one such
example of a
I 5 pressure sensitive switch. Devices of this type offer the substantial
advantage of being low
cost, flexible, and durable. A variety of other force sensing switch devices
may be utilized
as well.
An alternative safety feature anticipated by the present invention is a
monitoring
function for detecting dramatic increases in power utilization by the heater
trying to
maintain an operating temperature. Under normal operation, the heater is in a
non-contact
position proximate the selected treatment area and the heater will have been
programmed
to operate at a temperature that may be either a straight temperature value or
an averaged
value for either a duty cycle, therapy cycle or therapeutic sequence. If
physical pressure
is placed on the heater and it comes into contact with the patient's body,
there will be a
considerable increase in the rate of heat loss from the heater because of the
body's greater
heat sink capacity. The heater controller would sense this drop in temperature
and initially
adjust either the duty cycle ratio or power output, or both, in an attempt to
compensate for
the increase rate of loss. The safety aspect of this monitoring function would
be to override
this increase and turn off the device, thus preventing heating the tissue
while in direct
contact with, and under pressure from, the heater.
CA 02271031 1999-OS-07
WO 98/31311 PCT/US97/21013
_17_
Heater element 106 is preferably a thin film typc resistance heater which is
commercially available. Such thin film resistance heaters utilize low voltage,
minimizing
the electrical risk to the patient and allowing for battery-powered mobility.
Foil heater l06
is preferably sized for each wound covering 108. In actual use, foil heater
l06 is preferably
provided in sheets with a pair of electrical leads 110 along one edge. While
an electrical
resistance heater is the preferred embodiment of the invention, other heating
devices are
anticipated such as warm water pads, exothermic chemical heating pads, and
phase-change
salt pads.
Heater assembly I00 is preferably insertable into a pocket 114 formed between
wound tracing grid 86 and the barrier layer as discussed above. Finally, a
temperature
monitoring device, such as a liquid crystal temperature monitor, may be
applied to an upper
- surface of heater assembly 100 or within treatment volume I 12 to monitor
the temperature
within treatment volume 112.
Figures 4-7 illustrate an alternate embodiment of wound covering 10. In this
embodiment, wound covering 10 includes a generally circular head, designated
generally
at 12, which transitions to an elongated non-kinking, collapsible air supply
or hose 14
The apparatus, as illustrated in Figure 4, is connected by suitable supply
line or
tube 16 to a source 18 of thermally controlled air which is schematically
illustrated. The
term air as used herein is intended to encompass mixtures of gases of
controlled
composition. The apparatus is constructed to apply a continuous stream of
thermally
controlled air to a wound treatment volume.
The specific form of the apparatus aid details of construction can best be
understood by reference to the various figures. The. overall appearance of the
wound
covering is best seen in Figure 4 and Figure 5. It is preferred to construct
the apparatus
from top and bottom sheets of thin heat-sealable polymer film which overlay
one another.
A top sheet or membrane 20 overlies a bottom sheet or membrane 22 which are
heat sealed
together along a plurality of seal lines, including a continuous outer seam
24, which extends
in a circle around head 12 and continues in a sinusoidal or convoluted fashion
along and
forming hose 14. An inner continuous circular seam 26 is provided as best seen
in
Figures 6 and 7. This inner-seam 26 secures the sheets together along a
continuous circle
to form the inner wall of a torus defining a supply volume 28.
CA 02271031 1999-OS-07
WO 98I31311 PCT/US97/21013
- I 8-
The inner circular portion of the two sheets 20, 22 lying in the plane within
the
center of the supply volume 28 forms a wall 30 separating a lower wound
treatment
volume 32 from an upper insulation chamber 34. Wall 30 includes a plurality of
apertures 3f formed by making small circular seals 38 and cutting and removing
circular
portions within the circular seals 38. Thus, wall 30, with a plurality of
apertures 36, is
formed between the wound treatment volume 32 and insulation chamber 34. A
plurality
of apertures 40 are formed in the common circular wall surrounding treatment
volume 32
for distributing and conveying heated air or gases from supply volume 28 into
wound
treatment volume 32.
The heated air flowing into treatment volume 32 bathes the wound surface of a
patient's body 42. The air circulates throughout wound treatment volume 32,
and then
passes through apertures 36 into the upper or insulating chamber 34, where it
then passes
through a filter 44 forming an outer wall of insulation chamber 34. Filter 44
filters the air
leaving wound treatment volume 32, trapping contaminants shed from the wound.
Filter 44
I 5 may be constructed of a filter paper bonded along its periphery to the
outer tangential walls
of head 12 forming the torus. The filter paper also provides an insulating
layer which
suppresses loss of heat by radiation through upper wall 30.
The lower surface of the head 12 as shown in Figures 6 and 7 is provided with
a
peripheral sealing ring 46 made of an absorbent material such as foam and
bonded by a
suitable adhesive to the walls of head 12 and skin 42 of the patient around
the wound.
Preferably, foam or cotton peripheral sealing ring 46 is provided with a peel-
off tape so that
it adheres to the wall of the housing and on the other side to the skin of the
patient. The
adhesive or tape holds the apparatus in place and prevents airflow escape
between the
device and the skin of the patient. The absorbent material of the ring absorbs
weeping
blood and fluids and insulates the skin from direct conduction of heat from
head 12.
Hose 14 is designed to be non-kinking by forming it of symmetrically
convoluted
flexible material. The hose and housing are integrally formed essentially of a
unitary
structure, such as a thin film membrane. Hose 14 is inflatable upon the
application of
heated air through supply line 16. The indentations in hose 14 permit it to
bend without
kinking and, thus, different-fate from a straight tubular hose which may kink
when bent.
CA 02271031 1999-OS-07
WO 98!31311 PCT/U597l21013
-19-
Since the thermal body treatment apparatus of the invention and the supply
hose
section are formed from two, thin, sealed-together membranes, the hose, and in
fact the
entire apparatus, is collapsible. This prevents the possibility of applying
heat and pressure
to the skin as might happen if a patient rolled over on the device. Instead,
the weight of the
patient's body collapses the device, obstructing the flow of air, and
preventing the
application of heat.
The film membrane may preferably be transparent to enable viewing the wound
without removal. However for cosmetic reasons the layer may be opaque. Filter
paper 44
is attached across the tangential surfaces of the toroidal housing, thus
providing a large area
of filter for the escaping air. Head 12 of the apparatus may be about one foot
in diameter
for most applications. However, it may be made smaller for certain other
applications.
Figure 8A illustrates an exploded view of an alternate embodiment of a non-
contact
wound covering 120 with climate control within a treatment volume 122 as shown
in
Figure 8B. An inflatable structure 124 is preferably attached to a fluid inlet
line l26 at a
fluid inlet port l29 on the perimeter of inflatable structure I24. Inflatable
structure 124 is
preferably attached to an absorbent peripheral sealing ring 128, which is in
turn attached
to a wound area 54 by a suitable adhesive 56. Peripheral sealing ring 128
preferably has
a sealed outer surface and a porous inner surface which performs the same
function as
peripheral sealing ring 52 discussed above. A barrier layer 130 having an
exhaust filter 132
is attached to a top surface 134 on inflatable structure l24.
Turning now to the assembly illustrated in Figure 8B, a gas, illustrated by
direction
arrows "A", is introduced into inflatable structure 124 from an external
source (not shown)
through inlet line l26. The gas pressurizes inflatable structure 124 in order
to maintain
barrier layer 130 and exhaust filter 132 in an elevated position relative to
wound area 54.
An inner surface 136 of inflatable structure l24 preferably has a plurality of
apertures 138
through which the fluid is introduced into wound treatment volume l22. As
pressure
within the treatment chamber increases, excess pressure is relieved through
exhaust filter
132. In this fashion, various fluids or gases can be introduced into wound
treatment
volume 122.
The use of the term "fluid" in the context of this application refers to both
liquid and
gaseous materials, and combinations thereof. In one embodiment, oxygen may be
CA 02271031 1999-OS-07
WO 98/31311 PCT/US97/21013
-20-
introduced into treatment volume l22 through apertures 138 of inflatable
structure 124.
The presence of oxygen within wound treatment volume 122 may increase the
oxygen
available to the superficial layer of growing cells in wound area 54. Nitric
oxide
alternatively may be infused into treatment volume 122. Nitric oxide (NO) is a
potent
vasodilator which in theory may be absorbed across the wound surface and
increase
localized blood flow. A very small concentration of NO (parts per million) may
provide
this effect. NO may also be pre-absorbed into absorbent peripheral sealing
ring l28 and
then allowed to passively diffuse into the volume once ii is applied to the
wound. Finally,
gaseous or aerosolized medications or compounds may be introduced into the gas
flow
entering treatment volume l22.
Figures 9A and 9B illustrate an alternate embodiment of the climate control
system
discussed above wherein a fluid inlet line 140 may form part of a barrier
layer l 42. Barrier
layer l42 is unitary with fluid inlet line 140 and is preferably attached to
an exhaust filter
media 144 to allow excess pressure to be released from a wound treatment
volume 146.
In this embodiment, filter media 144 forms part of barrier layer l42. The
arrows "A" in
Figure 9B illustrate the movement of the fluid through fluid inlet line 140,
treatment
volume 146, and exhaust filter 144.
Figure 10 illustrates an alternate embodiment wherein an exhaust filter 154 is
retained in a recess 150 formed in one side of a peripheral sealing ring 152.
This structure
allows excess fluid to be exhausted through the side of peripheral sealing
ring 1 ~2, rather
than through the top, as illustrated in Figures 9A and 9B.
Figure 11 A is a perspective view of the embodiment illustrated in Figure 9A,
wherein a connector 160 on the end of a fluid supply line 162 engages with an
opening 164
on fluid inlet line 140. Figure 1 1 B illustrates a side view of fluid supply
line 162 as it
engages with fluid inlet line 140. Figure 11 C illustrates the embodiment in
Figures 1 1 A
and I 1B where fluid inlet line 140 is folded over the top of peripheral
sealing ring 152 to
seal treatment volume l46 when supply line l62 is uncoupled.
Figure 12 illustrates an alternate embodiment in which a fluid inlet slot l 70
engages
with a rigid connector l 72 on a fluid inlet line l 74. Fluid inlet slot l70
forms an opening
in one portion of a peripheral sealing ring 176. The opening is in fluid
communication with
CA 02271031 1999-OS-07
WO 98I31311 PCT/US97/21013
-2 I -
a treatment volume l78. This configuration allows for quick disconnection of
fluid inlet
line l 74 from wound covering l 80 providing the patient with additional
mobility.
Figure 13A is a perspective view of an alternate non-contact wound covering
190
having a fluid inlet connector l92 attached to a top surface 194 of a
peripheral sealing
ring 196. Fluid inlet connector 192 preferably contains an inlet filter media
198. A rigid
com~ector 200 on a fluid inlet line 202 mates with fluid inlet connector 192.
As illustrated
in Figure 13B, a cover 204 extends from the top of fluid inlet connector 192
across the top
of peripheral sealing ring 196 where it engages with an exhaust filter media
206. Figure 14
illustrates the embodiment of~Figures 13A and 13B utilizing a non-disposable
fluid supply
line 210.
Figure 15 illustrates an alternate embodiment which utilizes a manifold
structure 220 as part of a fluid inlet line 222 to provide even distribuiion
of the fluid being
introduced into a treatment volume 224. Fluid inlet line 222 preferably has a
series of
seals 226 along its edge which are interrupted by a plurality of side openings
228 from
which the fluid can be transmitted into treatment volume 224. The embodiment
disclosed
in Figure 1 S illustrates an exhaust filter 230 recessed into the side of
peripheral sealing
ring 232. However, it will be understood that a variety of exhaust filter
configurations are
possible with the disclosed manifold structure 220.
Figures 16A and 16B illustrate an alternate wound covering 240 with a top
barrier
layer 242 and a lower layer 244 having a plurality of holes 24G. As is
illustrated in
Figure 16B, a top cover 243 forms the barrier layer 242 and it extends
substantially across
the area of the peripheral sealing ring 248. Louver layer 244 likewise extends
across the
peripheral sealing ring 248. Thus, an upper insulating layer 2S0 is formed
between lower
layer 244 and the top of barrier layer 242. Fluid in a fluid inlet line 252 is
directed into
2S upper insulating layer 2S0. The pressurized fluid in upper insulating layer
2S0 passes
through holes 246 into a treatment volume 254. Holes 246 in lower layer 244
provide a
generally even distribution of the fluid within wound treatment volume 2S4. An
optional
seal 258 may be formed in the center portion of barrier layer 242 and lower
layer 244 to
provide these layers with additional structural support. An exhaust filter
medium 2S6 is
provided in a recess along one side of peripheral sealing ring 248 to relieve
pressure in
treatment volume 2S4.
CA 02271031 1999-OS-07
WO 98I31311 PCT/US97/21013
-22-
Figure 17 illustrates an alternate embodiment of a non-contact wound covering
260
utilizing semi-rigid supports 262 to retain a barrier layer 264 above a wound
area. It will
be understood by those skilled in the art that a variety of semi-rigid
supports 262 may be
utilized for this application. For example, plastic or resilient rubber
materials may provide
sufficient support to barrier layer 264 with a minimum risk of injuring the
patient.
Figures 18A and 1813 illustrate an alternate exhaust filter medium 270 with an
enlarged surface area to accommodate larger volumes of air flow through a non-
contact
wound covering 280. Exhaust filter 270 is incorporated into a fluid inlet line
272. Fluid
inlet line 272 also forms a portion of a barrier layer 274, which is in turn
attached to a
peripheral sealing ring 276. As is best shown in Figure I 813, fluid
illustrated as the arrows
"A" is introduced into a fluid inlet line 272, where it is directed into a
wound treatment
volume 278, past the wound area and out through exhaust filter medium 270.
Figure 19 illustrates a bi-directional line 290 with a center divider 292.
Fluid is
introduced into a fluid inlet line 294 where it proceeds through a fluid inlet
port 296 into
a treatment volume 298. The fluid then is forced through a fluid outlet port
300 where it
is driven away from treatment volume 298 in a fluid outlet line 302. It will
be understood
by those skilled in the art that it would be possible to utilize separate
fluid inlet and outlet
lines to achieve the same result.
A schematic diagram of an embodiment of the present invention using active
heating and control is depicted in Figure 20 as an active heater assembly 310
including a
heater 312, a heater filament 3l4 within heater 312, a controller 316,
electrically coupled
between heater filament 3I4 and a power source 318 by electrical connectors
315, and
using a heater temperature sensor 320, and an operator interface 322 suitable
for an operator
to input programming parameters into controller 316. Heater assembly 3 I 0 is
useful in
several different configurations, for example, as providing a heater layer for
use directly in
a pocket such as that depicted by heater l00 inserted into pocket 1 14 shown
in Figures 3A
and 3B or as a heat source for warming air that is circulated proximate the
wound as is
depicted in the several embodiments of Figures 4 through 19.
In addition to the various suggested fluid delivered heater "geometries"
depicted in
Figures 4-19, the present invention anticipates numerous possible heater
electrical resistive
filament 314 geometries. Examples of four such geometries are shown in Figures
2I A, B,
CA 02271031 1999-OS-07
WO 98/3131I PCT/US97/21013
_23_
C, and D wherein there is depicted additional alternate heater array
geometries for heating
filament 314 within heater 312. In Figure 21 A, there is depicted a linear
geometry for
heater filament 3I4. This geometry is suitable for non-uniform heating where
maximum
heating is desired over a linear area, such as a linear surgical wound without
direct heating
over adjacent periwound areas. Figure 21 B depicts a geometry for heater
filament 314
consistent more as a point source. Figure 21 C depicts an ovoid geometry for
filament 314
suitable for non-uniform heating of selected periwound area. Alternatively,
this non-
uniform heating tray be achievable with circular, square, rectangular,
triangular or other
such geometries depending on the type and shape of wound encountered.
In operation, heater assembly 3 I 0 is programmable, controlling several
parameters,
such as heater temperature, duty cycle, therapy cycle, number of duty cycles
per therapy
cycle, average heater temperature per duty cycle, average heater temperature
per therapy
cycle, peak and minimum heater temperatures per heater cycle, and peak and
minimum
heater temperatures for a therapy cycle. The programming may be preset at time
of
manufacture and may provide a menu of several treatment scenarios.
Additionally, the
parameter programmability may be entirely under the control of an operator and
suitable
for inputting any number of custom treatment regimens.
By way of example, and not limiting in scope of treatment versatility, Figure
22 is
a graphic representation of one such therapy cycle represented by several
heater and duty
cycles. In Figure 22, several duty cycles have been defined within a therapy
cycle where
time (t) is represented along the abscissa and heater temperature (T) along
the ordinate. At
t" 330, the heater is at ambient temperature Ta";,) 332 and the first of
several duty cycles
begins at to 330 by turning on the heater to heat up to a temperature at about
T~,~"k 334.
Upon reaching Teak 334 by t, 336, the heater power is turned off and the
heater cools to
T",;" 338. The first duty cycle is completed at t, 336 when the heater is
turned off. The first
heater cycle is completed at tz 340 when the heater is turned back on to begin
the next duty
and heater cycle. This first heater cycle and subsequent heater cycles
maintain an average
heater temperature TS~, 342. The duty cycle is given by the ratio of the
duration of t"-t, over
to-t2. Those familiar with the art of heater activity control will appreciate
there are a variety
of methods for manipulating heater activity, including proportional action
controllers using
processor logic to maximize heater action and control. A therapy cycle for
this example
CA 02271031 1999-OS-07
WO 98/31311 PCT/US97/21013
-24-
is the time duration from t" 330 to t, 344 during which time the heater
temperature has been
allowed to fall to T~",,, 3 32, where at t, 344 the heating regimen begins
again starting the
next therapy cycle.
For the above example, a peak temperature, T~,~ak 334 may be the parameter
inputted
into the program. For the present invention anticipating a guard heater
function, this range
is preferably ii~om above 38~ C to about 46~ C. The present invention
anticipates
alternative selections of temperatures inputted for the operating temperature.
A first
alternative is to establish an average heater cycle temperature. In Figure 22,
this concept
is represented by Ts~t 342. For the present invention this average heater
cycle temperature
would have the same range, from above 38~ C to about 46~ C. Alternatively, the
temperature selection may be inputted as a T~,~ak 334 and a T",;" 338, both
temperatures
- selected from the same range.
By way of another example, and not limiting in scope of treatment versatility,
a
plurality of therapy cycles are depicted in Figure 23, wherein individual
heater cycles
within each therapy cycle have been averaged out for purposes of clarification
and for
purposes herein are treated as the heater being "on". A first therapy cycle
begins at t~ 350
as depicted by the heater turning "on", i.e., a series of heater cycles is
begun, and the heater
heats to T,~~ 3S2. This "on" segment goes until t, 354 at which time the
heater is turned
"off' and allowed to cool to Ta",b 356. This first therapy cycle ends at tz
358 when a second
therapy begins by turning "on" the heater again. As in the first therapy
cycle, this second
therapy cycle heats to TS~, 352 and has a duration from tz 358 to t3 360. A
third therapy
cycle begins at t3 360 turning "on" the heater. For purposes of example to
depict
anticipated versatility of the present invention, this third therapy cycle is
given a different
TS~, 362. The heater is turned "off' at t~ 364. This entire period of multiple
therapy cycles
may also be part of a therapeutic sequence, depicted in Figure 23 as that
period of time
from t~ 350 to t, 366 encompassing three therapy cycles. The present invention
anticipates
the use of any number of therapy cycles having any length or duration per
cycle and
different set temperatures.
Another aspect of heater therapy control, averaging therapy cycle and
therapeutic
sequence temperatures, is depicted in Figure 24 as an additional example, the
example not
intended to be limiting in scope of treatment versatility. In Figure 24, a
therapy cycle starts
CA 02271031 1999-OS-07
WO 98/313I1 PCT/US97/21013
-25-
at t" 370 and ends at t, 372. The overall heater temperature average Ta~,r 374
for this
therapy cycle may be pre-selected or programmed. The heater, begim~ing at an
ambient
temperature Tp",,, 376, heats to an appropriate temperature for the "on" phase
and then is
"off" for an additional appropriate time such that the total period of time is
equivalent to
the period t" 370 to t, 372 and the average temperature for this period is
equivalent to
Ta,,~ 374.
An alternative approach, depicted in Figure 24, anticipates the programming of
a
number of therapy cycles as elements of a therapeutic sequence, in this
example there being
three therapy cycles of varying time and heater temperature. The present
IIlVeI1t10I1
versatility provides for the inputting of an average temperature Tae 378 for
the therapeutic
sequence. The therapeutic sequence begins at time t" 370 and ends at time t,
380. The
heater temperatures and durations of the therapy cycles within the therapeutic
sequence are
averaged by the controller over the entire period of time from t" 370 to t,
380 so as to
achieve the therapeutic average temperature Ta~~ 378. Each of these average
temperatures,
whether an average over a therapy cycle or over a therapeutic sequence, is
intended to have
the same temperature range from above 38~ C to about 46~ C. A secondary
consequence
of this controller regimen is that if average temperatures are used, either
over the therapy
cycle andlor therapeutic sequence, then the resultant peak temperatures
achieved by the
heater may be substantially higher than the 38~ C to about 46~ C range. These
peak
temperatures are short lived by comparison and do not represent a safety
concern.
The present invention is the development of a safe, efficacious non-contact
heater
wound covering providing heat to a patient's wortnd from the heater that is in
a temperature
range from above 38~ C to about 46~ C or controlled to an average temperature
range from
above 38~ C to about 46~ C. While the invention has been illustrated by means
of specific
embodiments and examples of use, it will be evident to those skilled in the
art that many
variations and modifications may be made therein without deviating from the
scope and
spirit of the invention. However, it is to be understood that the scope of the
present
invention is to be limited only by the appended claims.
CA 02271031 1999-OS-07
WO 98I31311 PCT/US97/21013
-26-
Table 1:
Time Subcutaneous Temperature
(in minutes) (~C mean ~= S.D.j
-60 33.9 ~ 1.1
0 34.7 -~ 1.2
30 36.9 ~ 0.6
60 37.1 ~ 0.4
90 37.4 t 0.5
I20 37.G ~ 0.5
180 36.0 ~ 0.4
240 36.1 ~ 0.2
300 35.80.6
- Table 2:
Time Skin Temperature Inside Covering
(in minutes] (~C mean ~ S.D.)
-60 33.2 ~ 1.1
0 33.9 ~ 1.1
30 36.7 t 0.5
60 37.0 ~ 0.4
90 37.1 ~ 0.4
120 37.2 ~ 0.4
I 80 35.2 ~ 0.5
240 3 5.2 ~ 0.4
300 35.l ~ 0.5
CA 02271031 1999-OS-07
WO 98/31311 PCT/US97/21a13
-27-
Table 3:
Time Laser Doppler Flow
(in minutes) (rnl/100g/min mean t S.D.)
-60 G4 ~ 42
0 110t 100
30 199 ~ l91
60 178 t 131
90 222 t 143
l20 271 t 235
180 158 ~ l46
240 14G ~ l25
300 173 ~ I58
Table 4:
Time Subcutaneous Oxygen Tension (YS~O,)
(in minutes) (mm ~-lg mean ~ S.D.)
-60 54 ~ 10
0 11060
30 109 ~ 58
60 122 ~ 59
90 136 t 57
I20 159 ~ 55
180 153 t 60
240 156 t 61
300 ~ 148 ~ 52
CA 02271031 1999-OS-07
WO 98/31311 PCT/US97/21013
-28-
Table 5:
Time Subcutaneous Temperature
(in minutes) (~C)
-60 33.8 ~ 1.5
0 35.2 t 1.5
30 37.l t 1.1
60 37.3 ~ 0.8
90 37.4 ~ 0.7
l20 37.3 ~ 0.8
180 35.8 ~ 1.2
240 35.8 ~ 1.0
300 36.l ~ 0.9
Table 6:
Time Skin 'Temperature Inside Covering
(in minutes) (~C)
-60 32.9 t 1.5
0 34.5 t 1.0
30 37.1 ~ 0.6
60 37.5 ~ 0.5
90 37.6 ~ 0.5
120 37.6 ~ 0.5
180 34.9 ~ 0.8
240 3 5.0 t 0.7
300 - 35.2 f 0.6
CA 02271031 1999-OS-07
WO 98I31311 PCT/CTS97/21013
-29-
Table 7:
Time Laser Doppler Flow
(in minutes) (ml/100g/min)
-60 44 t 22
0 95 ~ 132
30 142 ~ I55
60 19l ~ I50
90 207 ~ 209
120 161 t 91
I 80 73 ~ 29
240 70 t 30
300 71.5 ~ 25
Table 8:
Time Subcutaneous Oxygen Tension (YSqOz)
(in minutes) (mm Hg)
-60 58 ~ 11
0 123 ~ 73
30 128 ~ 67
60 l45 ~ 69
90 157 t 73
l20 l53 ~ SS
l80 148 ~ 73
240 l42 ~ 73
300 ~ l43 ~ 76