Note: Descriptions are shown in the official language in which they were submitted.
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
GROUNDWATER REMEDIATION WITH MICROPOROUS DIFFUSOR
BACKGROUND OF THE INVENTION
FIELD OF INVENTION (Technical Field)
The present invention relates to sparging apparatus, systems and methods of in-
situ
groundwater remediation for removal of contamination including dissolved
chlorinated
hydrocarbons and dissolved hydrocarbon petroleum products. In particular, the
present invention
is directed to the use in injection wells of microfine bubble generators,
matched to substrates of
selected aquifer regions, for injection and distribution of said microfine
bubbles containing
oxidizing gas through said aquifer. Further, the present invention relates to
selectively
encapsulating multiple gases including oxygen and ozone in said microfine
bubbles to form
"gas-gas" bubbles which, in the presence of co-reactant substrate material
acting as a catalyst, are
effective to encourage biodegradation of leachate plumes which contain
biodegradable organics,
or Criegee decomposition of leachate plumes containing dissolved chlorinated
hydrocarbons.
BACKGROUND PRIOR ART
The introduction of air bubbles into aquifers for the purpose of remediation
is a recent
advancement in in-situ treatment of groundwater (Marley, et al., 1992; Brown
et al., 1991 ).
Contained air entrainment has been used for many years to provide vertical
movement of water in
low-head aquariums and in the development of public well supplies (Johnson,
1975). Aeration of
aquifers for plume management was suggested to accelerate bacterial
degradation of dissolved
organic compounds (JRB, 1985). As bubble volume increases in density above re-
aeration needs
by approaching ratios beyond 1 to 10 ( 1 water to 10 air), gas transfer begins
to dominate. In this
case, volatile organics may be physically transported from the saturated
aquifer to the overlying
unsaturated zone (vadose zone).
Also there is a well recognized need for a simple test to evaluate a potential
site to assist
with design of sparging apparatus, systems and methods deployed on a
remediation site. Whereas
hydraulic tests have been performed for some period of time based upon the
well known Theis
equation, the introduction of air bubbles (particularly microscopic bubbles)
is new. Also, whereas
the introduction of air to the pressure vessel is continuous, the production
of bubbles, particularly
a
the microscopic variety, is a discrete discontinuous process. Bubbles, once
generated, may take
preferential pathways, determined largely by the substratum and, secondarily,
by the introduction
of pressure (Ji, et al, 1993).
Applicant is aware of prior art devices that have used injection of air to
facilitate
biodegradation of plumes. U. S. Patent No. 5,221,159 to Billings shows
injection of air into
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
aquifer regions to encourage biodegradation of leachate plumes which contain
biodegradable
organics together with simultaneous soil vacuum extraction.
In U.S. Patent No. 5,167,806 to Wang et a!. there is disclosed apparatus for
treatment of
a contaminated liquid stream comprising generating extremely fine gas bubbles
through porous
diffusers, wherein the gas may be a combination of air and ozone. One process
disclosed by Wang
involves removing dissolved organics from contaminated groundwater by means of
generating
micro gas bubbles. In the first stage of the process for removing dissolved
organics, which
involves generating bubbles, no vacuum is employed, as gas bubbles are
completely dissolved by
the method. Wang teaches an enhanced dissolved aqueous reaction. No
particulate or substrate
mechanics are discussed.
In U.S. Patent No. 4,832,122 to Corey et al. is disclosed an in-situ method
for removing
contamination from groundwater comprising a horizontal well positioned in the
saturated zone
which has multiple apertures for injecting gas. The apertures are shown in the
figures to be
sequentially arranged and closely spaced so that the bubble zones produced
from each one would
overlap with the adjacent zones. Corey et al. teaches that the configuration
of the injection system
is dictated by the size and shape of the plume, drilling economics, and the
subsurface geology
(column 1, lines 4-9, 41-43, 64-68; column 2, lines 1-8, 43-48). Corey also
teaches an enhanced
dissolved aqueous reaction.
U.S. Patent No. 4,614,596 to Wyness discloses a method for dissolving a gas in
an
aqueous stream which comprises diffusing a gas in an aqueous stream to produce
small gas
bubbles which are rotated to provide a long flow distance over which the
bubbles have increased
contact time. The figures show that the bubbles are dispersed within and
outward from a vessel, or
well casing, by maximizing the dispersal of bubbles from a well casing and
maximizing contact
with the bubbles. Wyness also teaches an enhanced dissolved aqueous reaction.
Notwithstanding the teachings of Wang et al., Corey et al., and Wyness, there
has not
been shown sparging apparatus, system or methods for remediating a site in a
controlled manner
of poorly biodegradable organics, employing oxidizing gas encapsulated in
microbubbles
generated from microporous diffusors wherein said bubbles are matched to soil
porosity pulsed in
a wave form for even distribution through the substrate (aquifer structure)
employing a
co-reactant in the form of substrate material. Further, the prior art fails to
show matching of
micron sized bubble formation with substrate material of a selected aquifer or
to show the
beneficial effect of uniform distribution of sized bubbles through such a
formation by means of a
pulsed wave form without fracturing said substrate. The present invention
accomplishes this by
2
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
injecting micron size bubbles containing an ozone oxidizing agent by means of
microporous
difiusors into aquifer regions, in combination with substrate materials acting
as a catalyst to
encourage biodegradation of leachate plumes which contain biodegradable
organics by means of a
' gas/gas/water reaction which overcomes at least some of the disadvantages of
prior art.
SLTMMARY OF THE INVENTION
The present invention relates to injection of oxidizing gas in the form of
microfine bubbles
into aquifer regions by means of a sparging apparatus, systems and methods
which includes one or
more injection wells to encourage in-situ remediation of subsurface leachate
plumes by means of a
gas-gas-water reaction. The present invention is directed to sparging
apparatus, systems and
methods of in-situ groundwater remediation in combination with co-reactant
substrate materials
acting as a catalyst to encourage biodegradation of leachate plumes for
removal of dissolved
chlorinated hydrocarbons and dissolved hydrocarbon petroleum products.
Remediation of
saturated soils may also be obtained by employment of the present invention.
The sparging system
of the present invention encourages biodegradation of leachate plumes which
contain
biodegradable organics or Criegee decomposition of leachate plumes containing
dissolved
chlorinated hydrocarbons.
Referring to the sparging apparatus of the present invention, said apparatus
includes
microporous bubble generators adapted to generate micron sized gas-gas bubbles
for injection into
aquifer regions by means of one or more vertically arranged injection wells
having a bubble
chamber configured to control the size of said bubbles. In particular the
present invention is
directed to sparging apparatuses for employing microporous diffusers for
injecting micro-fine
bubbles containing encapsulated gas bubbles into aquifer regions to encourage
biodegradation of
leachate plumes which contain biodegradable organics, or Criegee decomposition
of leachate
plumes containing dissolved chlorinated hydrocarbons. The sparging apparatuses
of the present
invention, employing microporous diffusers for injecting an encapsulated mufti-
gas oxidizing
agent, are particularly useful in that the apparatuses promote extremely
efficient removal of poorly
biodegradable organics, particularly dissolved chlorinated solvents, without
vacuum extraction of
undesirable by-products of remediation and wherein remediation occurs by
employing
encapsulated mufti-gas oxidizing agents for destroying organic and hydrocarbon
material in place
without release of contaminating vapors.
In the present invention the groundwater and soil remediation system comprises
oxidizing
gas encapsulated in microbubbles generated from microporous diffusers matched
to soil porosity.
A unique bubble size range is matched to underground formation porosity and
achieves dual
3
CA 02271844 1999-OS-11
WO 98/21152 PCT/I1S97/19907
properties of fluid-like transmission and rapid extraction of selected
volatile gases, said size being
selected so as to not to be so small as to lose vertical mobility. In order to
accomplish a proper
matching, a prior site evaluation test procedure is devised to test
effectiveness of fluid
transmission at the site to be remediated. The advantage of controlled
selection of small bubble
S size is the promotion of rapid extraction of selected volatile organic
compounds, such as PCE,
TCE, or DCE by incorporating the exceptionally high surface to gas volume
ratio. The dual
capacity of the small production and rise time is matched to the short
lifetime of an oxidative gas,
such as ozone, to allow rapid dispersion into water saturated geological
formations, and extraction
and rapid decomposition of the volatile organic material. The unique apparatus
of the present
invention provides for extraction efficiency with resulting economy of
operation by maximizing
contact with oxidant by selective rapid extraction providing for optimum
fluidity to permit bubbles
to move like a fluid through media which can be monitored.
The use of microporous bubble generators provides a more even distribution of
air into a
saturated formation than the use of pressurized wells. A microfine spurge
system installed to
1 S remediate contaminated groundwater is made more cost-effective by sparging
different parts of
the plume area at sequenced times. Through the proper placement of bubble
generator locations
and sequence control, any possible off site migration of floating product is
eliminated. With
closely spaced bubble generators, water mounding is used to advantage in
preventing any off site
escape of contaminant. The mounding is used to herd floating product toward
extraction sites.
In the present invention, the concept of microflne spurge system manipulation
is predicated
upon a thorough knowledge of the features of the groundwater or saturated
zones on a site
selected for remediation. Balancing the volume of air to the microfine system
spurge loci enables
control of sparging efficiency and balancing of any downgradient movement of a
contaminated
plume while remediation is accomplished. Critical to microfine spurge system
design and
2S accomplishment of any of the above points is the initial performance of a
"spurge point test" for
the purpose of evaluating the characteristics of the site for matching
purposes.
The invention employs the well recognized Criegee mechanism which describes
the
gaseous reaction of ozone with the incoming PCE, TCE, DCE, and vinyl chloride
into
microbubbles produced by bubble generators with the resultant products then
hydrolysed, i.e.,
reacted with water to decompose into HCI and COz. It is this physical/chemical
reaction which
produces the rapid removal rate employed by the present invention (see
reference Maston S 198b,
"Mechanisms and Kinetics of Ozone Hydroxal Radical Reactions with Model
Alafadic and
Olanfadic Compounds", Ph.D. Thesis, Harvard University, Cambridge, MA). Unlike
the prior art,
4
CA 02271844 1999-OS-11
WO 98/21152 PCTlUS97/19907
the contaminated groundwater is injected with an air/ozone mixture wherein
microfine air bubbles
strip the solvents from the groundwater and the encapsulated ozone acts as an
oxidizing agent to
break down the contaminants into carbon dioxide, very dilute HCi and water.
This process is also
known as the C-SpargerTM system, which is directed to low-cost removal of
dissolved chlorinated
hydrocarbon solvents such as percolate from contaminated soil and groundwater
aquifers.
The C-SpargerTM system employs microporous diffusers, hereinafter
Spargepoints~ for
producing micro-fine bubbles containing an oxidizing agent that decomposes
chlorinated
hydrocarbons into harmless byproducts. The C-SpargerTM system comprise pumps
for pumping a
mufti-gas oxidizing mixture through a) the Spargepoint diffusors into
groundwater in a soil
formation; b) a bubble production chamber to generate bubbles of differing
size, c) a timer to
delay pumping until large bubbles have segregated from small bubbles by rise
time, wherein fine
bubbles and liquid are forced into the formation. The pump intermittently
agitates the water in the
well in which the C-Sparger is installed. This is effective to disturb the
normal inverted
cone-shaped path of the bubbles injected by the Spargepoint through the soil
formation and
disperses them in a random manner, ensuring improved contact between the
oxidizing agent
(contained in each bubble) by stripping the pollutant from solution in the
water into the
mini-atmosphere contained in each bubble. The pulsing action promotes movement
of the bubbles
through the porous formation. It is the in-situ stripping action and
maintenance of low solvent gas
concentration in the bubbles which improves the efficacy and speed (and
resulting cost) of
remediation of a site. In the present invention a unique bubble size range is
matched to
underground formation porosity and achieves dual properties of fluid-like
transmission and rapid
extraction of selected volatile gases, said size being so selected so as to
not to be so small as to
lose vertical mobility. In order to accomplish a proper matching, a prior site
evaluation test
procedure is devised to test effectiveness of fluid transmission at the site
to be remediated. The
dual capacity of the small bubble production pulsed injection and rise time is
matched to the short
lifetime of an oxidative gas, such as ozone to allow rapid dispersion into
predominantly
water-saturated geological formations, and extraction and rapid decomposition
of the volatile
organic material. The unique apparatus of the present invention provides for
extraction efficiency
with resulting economy of operation by maximizing contact with oxidant by
selective rapid
extraction providing for optimum fluidity to permit bubbles to move like a
fluid through media
which can be monitored. The micro-fine bubbles produced by the Spargepoint
diffusors contain
oxygen and ozone which oxidize the chlorinated hydrocarbons to harmless gases
and weak acids.
High initial concentrations of these dissolved organics have been, under some
specific
5
CA 02271844 1999-OS-11
WO 98/21152 PCT/LTS97/19907
circumstances, reduced to levels of 1 ppb or less in periods of a few weeks.
None of the models to
date are designed for explosive environments.
The use of microporous diffusor points provides a more even distribution of
air into a
saturated formation than the use of pressurized wells. A spurge system
installed to remediate
contaminated groundwater is made more cost-effective by sparging different
parts of the plume
area at sequenced times. Through the proper placement of spurge locations and
sequence control,
any possible off site migration of floating product is eliminated. With
closely spaced spurge points,
water mounding is used to advantage in preventing any off site escape of
contaminant. The
mounding is used to herd floating product toward extraction sites.
The present invention employs a plurality of configurations consisting of
Series 3500 and
Series 3600 C-Spurge models. The 3600 Series is larger and has more capacity.
Specifically, the
3600 Series has a better compressor rated for continuous use, a larger ozone
generator, a second
spargepoint below the first in each well, and larger diameter gas tubing. Both
model series have
control units that can support: one (Models 3501 & 3601), two (Models 3502 &
3602) and three
separate wells (Models 3503 & 3603). The differences between the one, two, and
three well
models are in the numbers of relays, internal piping, external ports and
programming of the
timer/controller.
Normal operation for C-SpargerTM systems includes carrying out, in series for
each well,
the following functions on a timed basis: pumping air & ozone through
Spargepoint diffusers into
the soil formation, pumping aerated/ozonated water in the well into the soils,
and recovering
treated water above. Treatment is followed by a programmable period of no
external treatment
and multiple wells are sequenced in turn. Agitation with pumped water disturbs
the usually
inverted cone-shaped path of bubbles through the soils and disperses them much
more widely
This increases contact and greatly improves efficiency and speed of
remediation. Vapor capture is
not normally necessary.
Series 3500 and 3600 systems include a control Module (Box), one to three well
assemblies depending on specific model selected, a submersible pump power-gas
line for each
well, and a flow meter (to check spargepoint flow rates). Model Series 3500 &
3600 Control
Modules have been successfizlly deployed outdoors in benign and moderate
environments for
prolonged periods of time. The Control Module must be firmly mounted
vertically on 4 x 4 posts
or a building wall near the wells.
The actual placement depths, separations, number/size of wells and overall
remediation
system geometry are highly variable. Differences in specific pollutant, spill,
soil, groundwater and
6
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
climate characteristics can greatly influence the design and geometry of the
overall remediation
system. Monitoring wells are usually also needed. In short, specific
circumstances and conditions
are often critical. However, a generic or typical overall system is shown on
Figure 1.
The unique use of Microfine Bubbles for simultaneous extraction/decomposition:
The use of microporous Spargepoint diffusers to create fine bubbles, which
easily
penetrate sandy formations to allow fluid flow, has unexpected benefits when
used with multiple
gas systems. Microfine bubbles accelerate the transfer rate of PCE from
aqueous to gaseous state.
The bubble rise transfers the PCE to the vadose zone. The ten-fold difference
in
surface-to-volume ratio of Spargepoint diffusor microbubbles compared to
bubbles from well
screens results in a four-fold improvement in transfer rates. To block the
gaseous state from
reverting to surface dissolved state in the vadose (unsaturated) zone, a
microprocessor system
shuttles an oxidizing gas through the vadose zone to chemically degrade the
transported PCE.
Elimination rate of PCE Relative to Ozone Content:
The reaction of ozone with tetrachIoroethane (PCE) will produce degradation
products of
hydrochloric acid, carbon dioxide, and water. By adjusting the ozone
concentration to match the
dissolved PCE level, the PCE can be removed rapidly without excess ozone
release to the air or
release of PCE vapor into the unsaturated zone.
Accordingly, the object and purpose of the present invention is to provide
microporous
diffusors for in-situ removal of contaminants from soil and associated
subsurface ground water
aquifer, without requiring applying a vacuum for extraction biodegradation by-
products.
Another object is to provide mufti-gas systems to be used in combination with
the
microporous diffusors to promote an efficient removal of poorly biodegradable
organics,
particularly dissolved chlorinated solvents, without vacuum extraction.
A further object is to provide that remediation occurs by destroying organic
and
hydrocarbon material in place without release of contaminating vapors to the
atmosphere.
A fizrther object is to provide for economical and efficient remediation of
contaminated
groundwater by providing a calculated plan of sparging different parts of a
plume area at
sequenced times.
Yet a further object is to control off site migration of floating product by
employing a
water mounding technique which effectively herds floating product to
extraction sites.
Another object is to provide microfine sparge system manipulation predicated
on
performance of a site evaluation test.
7
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
A further object is to provide that remediation occurs by destroying organic
and
hydrocarbon material in place without release of contaminating vapors to the
atmosphere.
Another object is to provide a microfine sparge system providing for optimum
fluidity to
permit bubbles to move like a fluid through media.
The invention will be described for the purposes of illustration only in
connection with
certain embodiments; however, it is recognized that those persons skilled in
the art may make
various changes, modifications, improvements and additions on the illustrated
embodiments all
without departing from the spirit and scope of the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
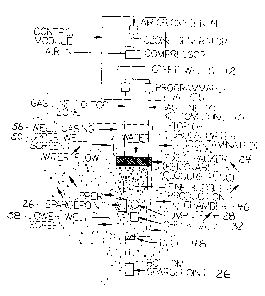
Figure I. is a cross sectional schematic illustration of a soil formation
showing the system of the
present invention.
Figure 2. shows an enlarged piping schematic of the present invention of
Figure 1 showing the
unique fine bubble production chamber;
Figure 3. is an electrical schematic for a 3 well system of the present
invention of Figure 1;
I S Figure 4. shows an internal layout of the Control Module box for a three
well system of the
present invention of Figure 1;
Figure SA. shows the geometry of the bottom panel on the Control Module
identifying the
external connections and ports for three well units of the invention of Figure
1;
Figure SB. is the left side view of Figure SA;.
Figure 6. is a schematic illustration of a soil formation showing the method
for the present
invention.
Figure 7. is a graph illustrating pore size compared with air bubble size.
Figure 8. is an illustration of radiation of bubbles from standard .010 (10
Slot) well screen
compared to microporous diffusor.
Figure 9. is an illustration of permeability of glass beads compared with
permeability of soil
fractions.
Figure 10. is a plan view of three different types of bubble generators and
installations of the
present invention.
Figure 11. is an illustration of flow chart for a sparge test according to the
present invention.
Figure 12. is a schematic illustration of apparatus used in in-situ sparge
test according to the
present invention.
Figure 13. is a graph illustrating pressure/flow relationship observed in
different formations.
Figure 14. is a graph illustrating influence of depth and pressure on radius
of bubble zone.
8
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
Figure 15. is a graphical illustration of PCE removal rate as function of
bubble size.
Figure 16. is an illustration of flushmount wellhead assembly in roadbox
according to the present
invention.
Figure 17. is a schematic illustration of the use of zone control in the
present invention.
Figure 18. is a schematic illustration of depiction of bubble zone and
mounding.
Figure 19. is a schematic illustration of bubble zone and mounded area above
the active aeration
according to the present invention.
Figure 20. is an illustration of sequential rise in water table from bubbling
and concentric zones
permitting containment of any floating contaminant - side view.
Figure 21. is schematic illustration of sequential rise in water table from
bubbling and concentric
zones permitting containment of any floating contaminant - top view.
Figure 22A. is a schematic illustration of contrast between aeration gaps with
non-overlapping and
thirty percent (30%) overlapping sparged zones.
Figure 22B. is a cross sectional schematic illustration of bubble generator
(Spargepoint~)
apparatus well pumping above.
Figure 23. is a plan view of a "C-SpargerTM system.
Figure 24. is a cross sectional schematic illustration of an inwell assembly.
Figure 25. is a top view of a ten point diffusor installation.
Figure 26. is a cross sectional schematic illustration of deep slant-well
installations to create
selective bubble fence using equal spacing of ten diffusors.
Figure 27. is a graphical illustration of PCE concentrations.
Figure 28. shows movement of microbubbles through saturated pores as diameter
of bubble
increases, showing coalescing
Figure 29. is a graphical illustration of rapid reaction of gas/gas mixture
when passing through
moistened sand.
Figure 30. is a schematic diagram of gas/gas/water reactions contrasted with
previous known
ozone reactions.
Figure 31. is a diagram of ozone reactions illustrating Criegee mechanism for
gas/gas/water
reaction with tetrachloroethene (PCE).
Figure 32. shows a microbubble generator column chamber and process.
Figure 33. shows pressure waves created by C-Sparger'r'M unit during
operation.
Figure 34. is a graphical illustration of frequency of microbubbles entering
monitoring well screen
at 15 ft. distance compared to pressure wave from C-SpargerTM unit during
water pumpage
9
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
(pump), lower bubble generator (Spargepoint~) operation (lover SP) and in-well
bubble
generator (Spargepoint~) operation (in-well SP).
Figure 3S. shows induced recirculation from bubble distribution.
Figure 36. is a graphical illustration of expanding zone of influence when air
and then air/ozone
mixtures are injected with C-SpargerTM recirculation system.
Figure 37. shows remote C-SpargerTM process interrogator and controller.
Figure 38. shows a pass-through threaded microporous spargepoint assembly.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
Referring to the figures I-38 there is shown a microfine spurge apparatus,
system and
method employing oxidizing gas encapsulated in microbubbles generated from
microporous
diffusors matched to soil porosity in a wave form employing a co-reactant in
the form of substrate
material for use with injection wells known as the C-SpargerTM system. Said
system consists of the
following components: referring to the Figures 1 through 6 there is shown a
Spurge System 10
consisting of multiple microporous difl'usors in combination with an
encapsulated mufti-gas
1 S system, the system I 0 consists of a master unit 12 and one or more in-
well sparging units 14.
Each master unit 12 can operate up to a total of three wells simultaneously
and treat an area up to
SO feet wide and 100 feet song. Actual performance depends upon site
conditions. Vapor capture
is not normally necessary. In the preferred embodiment, as shown in Fig. 1 and
Fig. 2, master unit
12 consists of the following: a gas generator 16, a gas feed line I5, a
compressor 18, a power
source 19, a pump control unit 20, a timer 22. The master unit 12 must be
firmly mounted on 4 x
4 posts or a building wall near in-well sparging units 14. A heavy-duty power
cable 44, not over
50 feet in length, may be used to run from the power source to the master unit
12.
Referring to Figs. l and 2, the in-well sparging unit 14 consists of a casing
S6, an inlet
screen SO an expandable packer 52, an upper site grout 54, an outlet screen
S8, and lower grout
62. Each in-well unit 14 includes a fixed packer 24, at least two diffusors 26
hereinafter
"SpargepointTM dii~'usors" 26, a water pump 28, ozone line 30, check valve 32,
and fittings.
As is shown in Figs. 1 and 2 the diffusor 26 employs a microporous diffusor in
place of
standard slotted well screen to improve dispersion of bubbles 60 through soil
shown at 84 and
improve rate of gaseous exchange. A normal 10-slot PVC well screen contains
roughly twelve
percent (12%) open area. Under pressure most air exits the top slits and
radiates outward in a
starlike fracture pattern, evidencing fracturing of the formation.
CA 02271844 1999-05-11
WO 98/21152 PCTIUS97/19907
Referring to Fig. 2 there is shown a fine bubble production chamber 46
positioned in the
well casing 56 between the upper well screen 50 positioned inunediately below
fixed packer 24
consisting of a removable closure plug and the lower plug 48 consisting of the
fine bubble
production chamber 46 containing bubbles 60 including upper SpargepointTM 26
positioned above
lower well screen 58 including pump 28 and check valve 32. Referring to Fig. 4
there is shown the
internal layout of the control module box 12 including an AC/DC power
converter 71, and ozone
generator 72, well gas relays 73 (three wells shown), a compressor 74, a
master relay 75, a main
fuse 76. There is also shown a programmable timer controller 77, a power strip
78, a gas regulator
and pressure gauge 79, together with a solenoid manifold 80, a ground fall
intercvpter 81 and a
cooling fan 82.
NEW EQUIPMENT DESIGNS
In the present invention several new equipment designs associated with the
spargepoint
diffusors have been implemented. Most important is the incorporation of I~PE
porous material
with well fittings and pass-through design which allows individual pressure
and flow control.
Secondarily, the push-probe points have been developed for use with pneumatic
tools, instead of a
drilling auger, for controlled insertion, and the right-angle mirror of the
wellhead assembly is
provided with a protective shield. Also one of the major pass-through
spargepoints problems in
horizontal sparging is even distribution of air bubbles. If inflow is attached
to the end of a screen,
the pressure drops continuously as air is released from the screen. The
resulting distribution of
flow causes most bubbles to be produced where the connection occurs with flow
alternating
outwards. The end of the screen products little or no bubbles. To allow even
distribution of
bubbles, either individual spargepoints are bundled (spaghetti tube approach)
or the spargepoints
are constructed in a unique way which allows interval tubing connections with
flow and pressure
control for each spargepoint region. With the proposed arrangement for
connecting tubing, the
spargepoints pass through the spargepoint internally without interfering with
function of
producing small bubbles on a smooth external surface. The tubing penetration
reduces the internal
gas volume of the spargepoint, thereby reducing residence time for oxidative
gases (important
since ozone has only a certain lifetime before decomposition), and allows 3 to
4 spargepoints to be
operated simultaneously with equal flow and pressure. Each spargepoint can
also be programmed
to pulse on a timed sequencer, saving electrical costs and allowing certain
unique vertical and
horizontal bubble patterns. Spargepoint diffusors can be fitted with F480
Thread with internal
bypass and compression fittings. This arrangement has the advantages that it
fits standard well
11
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
screen; allows individual flow/pressure control; reduces residence time; and
allows casing/sparge
instead of continuous bubbler.
Use of Injectable Points configured as Molded: 18 Inch 40 inch HDPE molded
into 1 /4
inch pp tubing or HDPE tubing allows smooth tube to be inserted into push
probe with detachable
point. Use of "Bullet" prepacked Spargepoint diffusers: with KVA "hefty
system" prepacked sand
cylinder and bentonite cylinder placed over tubing and porous point. Also use
of a porous point
reinforced with inner metal tube (perforated) to allow strength throughout
tubing, resisting
disintegration of plastic during insertion.
Use of Pressure/flow headers: Rotometer/mirror: Mirror assembly for flush-
mounted
rotometer (flowmeter) allows reading from vertical down, and controls flow off
lateral lines to
adjust to back pressure from varying types of formations (silt, sand, gravel)
below.
It is well recognized that the effectiveness of treatment is dependent upon
uniformity of
dispersion of the gas as it travels through the formation. A porous structure
with appropriate
packing matches the condition of the pores of the soil with thirty percent
(30%) pore distribution.
The dispersion of bubbles as a fluid can be checked with Darcy's equation.
The use of microporous materials in the "SpargepointTM~ 26 to inject gases
into
groundwater saturated formations has special advantages for the following
reasons:
1. Matching permeability and channel size; 2. Matching porosity; and 3.
Enhancing fluidity, which
can be determined in-situ. The most effective range of pore space for the
diffusor material selected
depends upon the nature of the unconsolidated formation to be injected into,
but the following
serves as a general guide: 1. Porosity of porous material: thirty percent
(30%); 2. Pore space:
5-200 microns; a. 5-20 very fine silty sand; b. 20-SO medium sand; and c. 50-
200 coarse sand and
gravel. The surrounding sand pack placed between the spargepoint 26 and
natural material to fill
the zone of drilling excavation should also be compatible in channel size to
reduce coalescing of
the produced bubbles.
The permeability range for fluid injection function without fracturing would
follow: ( 1 )
10'Z to 10~ cm/sec, corresponding to 2 to 2000 Darcy's; (2) 20'2 to 10~
cm/sec; or (3) 100 to .O1
ft/day hydraulic conductivity.
Permeability is the measure of the ease of movement of a gas through the soil.
The ability
of a porous soil to pass any fluid, including gas, depends upon its internal
resistance to flow,
dictated largely by the forces of attraction, adhesion, cohesion, and
viscosity. Because the ratio of
surface area to porosity increases as particle size decreases, permeability is
often related to particle
size.
12
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
I. EVALUATION TEST
The first step in preparing a site for treatment is to conduct an evaluation
test to determine
whether or not an aquifer has characteristics which make it suitable for
treatment by the
microbubble sparging system of the present invention. The test employs one or
more microporous
bubble generators known as Spargepoints'~'M which produce extremely fine
bubbles and are sized
to penetrate fine sands by matching the bubble size to the soil porosity. The
bubble generator
(Spargepoint~) may be injected with a hydraulic or pneumatic hammer into the
aquifer or inserted
through a hollow stem auger usually up to 10 feet below static water level.
(See Figure 11 for
flow chart of spurge test.) Prior to conducting an evaluation test,
reconnaissance steps normally
performed at a site include 1 ) soil coring to establish the extent of
volatile organic carbon (vocn)
contamination and 2) soil types with depth and hydrocarbon content. Monitoring
wells are usually
installed for later observation points, typically having well screens which
extend five to seven feet
below static water with one to three feet above, depending upon historic
record of water level
changes for the area. If floating nonaqueous liquid petroleum is observed
(greater than sheen
1 S thickness), efforts to remove the product should be undertaken prior to
evaluation testing. Oil
corings are commonly scanned with a PID detector to establish the three-
dimensional extent of
petroleum contamination. Subsamples can be forwarded to a laboratory to
determine precise
chemical composition.
The next step is to prepare a site map, noting the distances between the test
point and
adjacent wells. Immediately prior to conducting the test, check water
elevation in monitoring wells
and/or point piezometers. The following is a list of the materials and a
stepwise procedure (see
Figure I 1 ) for conducting a spurge test with the micro-bubbler generator:
3/4-inch OD x 18 inch.
(for .5 inch ID schedule 90 PVC); Wellhead surface assembly, ( 1 /4 inch
connections, 0-2. S cfm);
Gas tank regulator, (acetylene torch type, zero air or nitrogen, 0-100 psi
adjustable, 0-3 cfm flow
capacity, male 1/4 inch NPT connector); Zero air tank (medium, 500 cf, 15600
to 2000 psi) 90
Ibs.; I/4 inch compression fittings, 1/4 inch copper tube. (See Figure 12 for
assembly of parts.)
Suggested well locations are at 5, I0, 1 S, and 20 feet from point of bubble
injection. The
screen should be five feet, with two feet placed into the unsaturated (vadose)
zone and three feet
below static water level. Note: Initially water may move out of wellpoint
causing a period of time
(1-2 minutes) before bubbling begins. A step-wise procedure is as follows:
A. Connect I/4 inch NPT of flowmeter assembly to regulator output;
B. Test before connection to wellhead to check flow to 2/3 cfrn, with tubing
wide open:
(1) Leave 3/4-inch NPT weIlhead connector off; (2) Shut valve b on regulator,
open valve on
13
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
flowmeter; (3) Adjust pressure to 20 psi; (4) Slowly open valve b; (5) Briefly
check flow up to
2-3 cfm. Shut down by turning valve b off
C. Connect 1/4 inch compression fitting to wellhead stickup 0.5 inch PVC
pressure cap
{either glue or screw top to casing, leaving enough for later completion);
S D. Bring pressure down to 10 psi; (1) Slowly open valve b; (2) Check flow
(yield) on
flowmeter, in cfph (cubic ft. per hour). Divide by 60 to get cfm. (3 ) If
yield is less than .3 cfm,
increase pressure valve to 15 psi; maintain for 5 minutes, opening valve b to
maximum flow. (4)
Maintain for 30 minutes if flow is near .S cfm. (5) Check observation wells
with electronic dip
meter to record water levels at 15 minute intervals. Check surface with
flashlight for bubbles
reaching surface. Verify with transparent bailers. It normally takes 30 to 40
minutes for bubbles to
appear. (6) After one hour, increase pressure by another 5 psi, again opening
valve b to maximum.
(a). Record maximum yield from flowmeter. (b) Repeat procedure 1-6. (7) Record
pressure and
maximum flow, and confirm distance of bubbling out from the injection
location. (8) Continue
with stepwise procedure recording pressure and yield; plot on graph paper.
Record water
1 S elevations in wells and time of onset of bubbling. A test is usually
conducted for a period of three
hours, using about 150 to 200 cubic feet of gas. (9) After onset of bubbling,
insert a bubble trap
into the well. This allows quantification of the volume of gas being evolved
into the unsaturated
zone. A sample of the gas can be analyzed later to determine volatile mass
transfer. As a
substitute, count the number of bubbles present in a volume of water obtained
with a bailer or
peristaltic pump. ( 10) If you are dealing with silt or clay you may want to
modify the procedure to
increase pressure at 10 psi intervals up to 50 psi. Check for fracturing by
sudden change in slope
upwards (increase in permeability}. If bentonite or grout seal fails, flow
also increases suddenly
with a noticeable drop in water elevation in monitoring wells. Normally test
is completed when 25
or 30 psi is reached or non-linear conditions are encountered. ( 11 ) In clay
soils there may be
substantial back pressure following cessation of test. Be careful unhooking
lines. Wait until
pressure reads below 20 psi before disengaging line or use a t-valve in line
for venting.
iI. INTERPRETATION OF RESULTS
Upon completion of a qualifying spurge test, there should be sufficient data
to plot curves
for the relationship between pressure and gas yield, zone of influence and
bubble region. These
plots will determine whether the area is amenable for use of the sparging
system of the present
invention.
The injection of air into an aquifer closely approaches Darcian flow, as long
as fracturing
pressures are not exceeded. With microporous materials, the initial bubble can
be sized below or
14
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
matching the interparticle pore space, allowing gas conductivity to more
approximate fluid
conditions (Kerfoot, 1993). The injection of air then approximates the more
familiar injection of
water, exhibiting mounding and outward movement until equilibrium is reached.
The creation of bubbling occurs when the gas pressure overcomes the hydraulic
head
(depth of water from static elevations to bottom of bubbler), the line
friction, the membrane
resistance of the bubbler wall, and the back pressure of the formation. The
hydraulic head is
converted to psi equivalents by multiplying depth of water by .43. The
resistance of a half (1/2)
inch tube is negligible under ten feet. The membrane resistance of a three
quarter (3/4) inch bubble
generator is roughly two psi. For a ten (10) foot installation, the critical
bubbling pressure would
be the following:
Hydraulic Head: 10 ft. x .43 - 4.3
Line Friction: Negligible - .0
Bubbler Wall Resistance: - _2.0
Critical Bubbling Pressure 6.3 psi
The most crucial pressure to overcome is the formation back pressure which
varies with
the surface to volume relationship of the pore spaces and the extent of their
occlusion by fines.
For a rough approximation, previous field tests have shown the following
ranges:
Gravel .2 to 2 psi
Coarse sand .3 to 4 psi
Medium sand .5 to 6 psi
Fine sand 1.0 to 10 psi
Silty sands 3.0 to 30 psi
III. INTERSTITIAL GAS: VELOCITY AND SOIL CONDUCTIVITY- DARCY'S LAW
( 1 ) Gas is a fluid that, unlike water, is compressible. Vapor flow rates
through porous
material, such as soil, are affected by the material's porosity and
permeability, as well as the
viscosity, density, and pressure gradient of the gas. The movement of gas
through soil can be
approximated by Darcy's law. A simple formulation of Darcy's law for saturated
gas flow in one
dimension is:
V q k(dP/dm)
V = ____ _ _____ - _______________
n An un
where:
V - seepage velocity (cm/sec)
V - gas yield (cm3)/ (cmz)(sec))
q - flow rate (cm3/sec)
k - gas permeability (cm2) (Darcies)
A - cross-sectional area (cmz)
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
a - viscosity (g/(cm)(sec))
dP/dm - pressure gradient (g/cm)(sec2)/cm
n - specific porosity (i.e., void nonwetted volume)
(2) The simplified Darcy equation can be used in conjunction with simple
vadose-zone well
S tests to directly relate soil permeability to gas viscosity, flow rate, and
pressure gradient. By using
direct gas velocity and rearranging the Darcy equation to solve for gas
permeability (k), the
following equation is derived and compared with its groundwater equivalent
(Masserman, 1989):
Gas flow: k = _Vn
dP/dm
Groundwater equivalent: k - Vn
dh/dl
(3) The slope (dh/dl) change in water head with change in distance (dI) is
replaced by the
pressure gradient (dp/dm) in the soil gas equivalent. The solution for k can
be found for a known
gradient and porosity. Effective porosity (n) remains unchanged, except that
moisture content
must be considered with gas movement. The viscosity of air (u) is estimated
from Table 1.
TABLE 1
VISCOSITY OF AIR
Temperature (°C) Viscosity (g/(cm)(sec))*
0 0.00017
9 0.000176
18 0.000182
29 0.000186
40 0.00019
*The Units are called Poises.
Source: CRC 1972
(4) Petroleum engineers have defined the Darcy as a unit of permeability.
Technically, one
Darcy is defined as the permeability that will lead to a specific discharge
(v) of 1 cm/sec for a fluid
with a viscosity of 1 centipoise under a pressure gradient that makes the term
pg/u (dp/dl) equal to
1 atmosphere, where p is density, a viscosity, and g is the force of gravity.
To convert Darcies to
cm2, multiply by 9.88 x 10-9. To convert Darcies to gas conductivity in
cm/sec, multiply by 9.11 x
10'. To convert Darcies to cm/sec, divide by 10'.
The differential equations that govern pressure flow of gas and vapor in soil
are non-linear
since gas density depends upon gas pressure. Masserman 1989 has pointed out,
however, that if
the maximum pressure difference between any two points in the flow field is
less than
16
CA 02271844 1999-OS-11
WO 9$/21152 PCT/US97/19907
approximately 0.5 atmospheres, the differential equations developed to model
groundwater flow
provide good approximations of gas flow. Analytical models used to evaluate
groundwater flow
can then be designed to estimate gas flow in sandy soils.
(5) Following Darcy's Law, the rate of gas discharge from the bubble generator
increases
proportionately to the pressure (head) applied above critical bubbling
pressure. The outflow
through the aquifer can be predicted by an analogy to the Darcy equation:
Qo = K8A _____________
where:
x
Qo - gas flow (cfm)
K8 - bubble conductivity
A - cross-sectional flow area (ftz)
(hm-hx) - pressure head (ft)
x - distance from source (ft)
Since the area of a 3/4 inch bubble generator (Spargepoint~) is fixed at .29
square feet,
the gas yield is directly proportional to pressure. (A plot of pressure versus
gas flow should be a
straight line.) If, however, sufficient excessive pressure is applied to
fracture the formation,
thereby increasing its conductivity, the line will bend in the direction of
more flow with less
pressure. This creates an undesirable condition where a greater air volume can
bypass soil without
permeating through it. As a result, extraction efficiency drops rapidly as
large channels are
formed. Secondly, within confined aquifers or semi-confined aquifers, the
cross-sectional area
through which the air bubbles (fluid) is being injected may be limited having
a ceiling or floor, and
thereby limit the volume which can be injected. See Figure 13 for depiction of
the pressure/flow
relationship in different formations.
(6) Referring to the drawings there is shown use of unique microporous
diffusors in place
of standard slotted well screen to improve bubble dispersion through soil and
improve rate of
gaseous exchange. A normal 10-slot PVC well screen contains roughly twelve
percent (12%)
open area. Under pressure most air exits the top slits and radiates outwards
in a starlike fracture
pattern, evidencing fracturing of the formation.
The effectiveness of treatment is dependent upon uniformity of dispersion of
the gas as it
travels through the formation. A porous structure with appropriate packing
matches the condition
of the pores of the soil with thirty percent (30%) pore distribution. The
dispersion of bubbles as a
fluid can be checked with Darcy's equation. The use of microporous materials
to inject gases into
groundwater saturated formations has special advantages for the following
reasons: ( 1 ) Matching
17
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
permeability and channel size: (2) Matching porosity; (3) Enhancing fluidity,
which can be
determined in-situ.
The most effective range of pore space for the diffusor depends upon the
nature of the
unconsolidated formation to be injected into, but the following serves as a
general guide: ( 1 )
S Porosity of porous material: thirty percent (30%); (2) Pore Space: S-200
microns: (a) S-20 very
fine silty sand; (b) 20-SO medium sand; (c) SO-200 coarse sand and gravel.
The surrounding sand pack placed between the bubble generator and natural
material to fill
the zone of drilling excavation should also be compatible in channel size to
reduce coalescing of
the produced bubbles.
The permeability range for fluid injection function without fracturing would
follow: (1)
10'z to 10'~ cm/sec, corresponding to 2 to 2000 Darcies ; or (2) 20'Z to 10~
cm/sec: or (3) 100 to
.O1 ft/day hydraulic conductivity.
(7) Permeability is the measure of the ease of movement of a gas through the
soil. The
ability of a porous soil to pass any fluid, including gas, depends upon its
internal resistance to
flow, dictated largely by the forces of attraction, adhesion, cohesion, and
viscosity. Because the
ratio of surface area to porosity increases as particle size decreases,
permeability is often related to
particle size, see Figure 9.
An estimate of the permeability of a soil can be obtained by comparing its
grain size in
millimeters with glass beads of a similar size, see Figure 9. This method is
generally limited to
uniformly graded sands, i. e., sands with a uniformity coefficient of less
than 5Ø Permeability (k)
is a function only of the soil medium and is expressed as an area (cm2).
Reference is made to
Figure 8 and Figure 9.
IV. EQUIPMENT
1. Unique Microporous Diffusors - types
a. Direct substitute for well screen, 30% porosity S-50 micron channel size
resistance to flow only 1 to 3 psi, can take high volume flow, need selective
annular pack (sized to
formation). High density polyethylene or polypropylene is light weight,
inexpensive.
b. Dif~usor on end of narrow diameter pipe riser KVA 14-291. This reduces the
residence time in the riser volume.
c. Shielded microporous diffusor which is injected with a hand-held or
hydraulic
vibratory hammer. The microporous material is molded around an internal metal
(copper)
perforated tubing and attached to an anchor which pulls the bubble generator
out when the
18
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
protective insertion shaft is retracted. Unit is connected to surface with 3/
16 or 1 /4 inch
polypropylene tubing with a compression fitting.
d. Thin Spargepoint bubble generators with molded tubing can be inserted down
narrow shaft for use with push or vibratory tools with detachable points. The
shaft is pushed to
the depth desired, then the bubble generator inserted, the shaft is pulled
upwards, pulling off the
detachable drive point and exposing the bubble generator.
e. Microporous dif~usor/pump combination placed within a well screen in such a
manner that bubble production and pumping is sequenced with a delay to allow
separation of large
bubbles from the desired fine "champagne" bubbles. The pressure from the pump
is allowed to
offset the formation back pressure to allow injection of the remaining fine
bubbles into the
formation.
V. BUBBLING RADIUS AND BUBBLE CONDUCTIVITY OF AN AQUIFER
The back pressure from the aquifer and radius of bubbling represent some of
the major
unknowns in the sparging system field design. The following test was designed
and field tested to
I S evaluate the capacity of the aquifer for sparging and to provide critical
design information. A
microporous bubbler of known characteristics is placed by injection or hollow
stem auger a fixed
distance below static water. A gas tank (zero air or nitrogen) with unlimited
pressure and outfitted
with a flowmeter provides the source of gas. The pressure is increased in a
stepwise manner while
observing flow. The yield versus pressure is then recorded. The shape of the
curve indicates the
pressure range of normal formation acceptance of flow under Darcian conditions
and non
-Darcian fracturing pressures.
Observation points away from the source use water table levels in both well
screens and
point piezometers. The rise in water level is recorded and the presence of
bubbles noted. There is
always a lag in time between bubble injection at depth and arrival at the
surface. The yield curves
and bubble zones are compared against theoretical and other curves observed
for known
formations.
VI. MOUNDING
The phenomenon of groundwater mounding occurs when a fluid is introduced into
soil in
unconfined sandy aquifers. Small bubbles displace an equivalent volume of
water creating a
movement of water horizontally and vertically. Hantush ( 1976) and Fielding (
I 981 ) have
developed equations to depict two-dimensional behavior of groundwater in a
constant-recharging
system. Assuming a radial flow of bubbles in an aquifer of thickness D, the
head distribution can
be represented as:
19
CA 02271844 1999-OS-11
WO 98/21152 PCT/LTS97/19907
(~_l~) _
Qo
2n KB (D+hx)
where:
S Kg - bubble conductivity of aquifer
(hm-hx) - pressure head (ft)
hm - maximum (head) water rise (ft)
D - depth of aquifer
n - pi, a constant (3.14...)
Qo - gas outflow (cfd)
x - distance from source (ft)
In a theoretical depiction, the introduced bubbles exit the sparge bubble
generator and
migrate vertically resulting in a symmetrical spheroid shape. In reality,
circular regions rarely are
found. More commonly, an elliptical region is found, reflecting higher
hydraulic conductivity in
one axis than another, inherent with the depositional history of the
formation. (See Figure 19 for a
depiction of groundwater mounding caused by sparging.)
VII. BUBBLE RADIUS AND DISTRIBUTION
As with mounding, it is often convenient to think of bubble movement as being
symmetrical and circular. In reality, it is rarely so uniform. However, there
are some general finds
which can serve as guidelines in interpreting results of the bubble tests.
First of all, bubbles in a
more uniform sandy deposit move upwards at about a 45° angle when
released at critical bubbling
pressure. Doubling the depth doubles the radius. Unfortunately, stratified
deposits may also be
encountered which may divert bubble vertical movement.
For every doubling of pressure above critical bubbling pressure, the radius of
influence will
expand 1.42 times its original radius. This approximation is based upon
maintaining a fixed
thickness of aquifer while doubling the volume of the cylinder. An
approximation of the
relationship between depth, radius and pressure for a medium to fine sand is
presented in Figure
14.
The relationship is observed between depth of the bubbler and radius of the
bubble zone
with air pressure set to only 10 psi above critical bubbling pressure with a
three quarter (3/4) inch
diameter bubble generator. The diameter observed for bubbling was noticeably
less than the
measured zone of influence of the displaced water. At ten feet below static
water, a 10-foot
pressure radius was observed at the top of the water when operated at critical
bubbling. The
radius of the observed bubble zone fit closely the relationship predicted by
Repa and Kufs, 1985.
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
At a fixed pressure set at 10 psi above critical bubbling, the radius expands
linearly, (directly
proportional), to increasing depth.
VIII. PRESSURE INFLUENCE
A second test was conducted on an 18-inch bubble generator (Spargepoint~)
located five
(5) feet below static water. Pressure was increased in increments (5.0 psi)
well above critical
bubbling pressure (see Figure 13). Although the bubbling zone radius was five
(5) feet at critical
bubbling pressure, it expanded with increased pressure to approximately the
square root of the
pressure increase:
Pressure
IO r - ____________________ ~0.5 do
where:
Critical Pressure
r - radius of bubbling zone
do = depth of installation
IX. PRESSURE VERSUS FLOW
As pressure increases, the gas flow to the bubble generator (Spargepoint~)
also increases
(see Figure 13). For comparison, the gas yield (flow) was measured with the
bubbler in air, the
main resistance being through the porous sidewalls of the cylinder. The bubble
generator was also
place in medium sand with less than one foot of waterhead. The same pressure
was applied.
If the critical bubbling pressure is subtracted, the sand and water curve will
show the
expected flow in medium sand. For example, at a 10-foot depth (critical
bubbling = 7.8 psi) and
1 S psi pressure, about 1.2 cfrn would be expected.
If a fine porous diffusor (/O micron) is used with a highly permeable deposit
(medium
sand, 100 fllday hydraulic conductivity), the resistance to flow may be so low
that a shallow curve
of pressure versus flow occurs. If so, assume that the radius of bubbling will
increase by the
square root of 2 {i.e., 1.4) times each time the flow volume is doubled.
X. DEGREE OF OVERLAP OF BUBBLE ZONES
It is important to achieve overlap of the zones of aeration. To achieve thirty
percent (30%)
overlap, the distance between aeration zones should be set at 3/4 d6 (db = the
diameter of bubble
zone).
Critical bubbling pressure (pressure to initiate bubbling) is defined as: Pc
(psi) _ [0.43 x
depth below water (ft)] + 3.5 (psi).
21
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
The diameter of the bubbling zone produced by the bubble generator when
supplied with
the critical bubbling pressure is equal to the installation depth of the
bubble generator below the
static groundwater surface: Dc (ft) = Installation Depth below Water (ft).
The increase in the radius of the bubbling zone produced by the bubble
generator when
supplied with greater than the critical bubbling pressure is defined as:
R = [(Pressure / Pc) ~0.5] x Dc;
Input bubble generator (Spargepoint~) depth below static groundwater level.
10.0 (ft);
Critical bubbling pressure is calculated as . . . . . . . . . . . . . . . . .
. . . . . . . . . . 6.0 (psi);
Critical bubbling radius is calculated as . . . . . . . . . . . . . . . . . .
. . . . . . . . . . 20.0 (ft);
Input proposed delivery pressure to spargepoints . . . . . . . . . . . . . . .
. . . . . . . 12 (psi);
Bubbling zone radius based on input pressure 12.0 and volume (20 scfin) . . .
. 30 (ft);
Recommended horizontal spacing between bubble generators. . . . . . . . . . .
. 22. 0 (ft);
Estimated air flow through Spargepoint~ based on input pressure . . . . . . .
. . .20 (cfin);
Correction for vertical/horizontal (V/H) permeability if ratio of V/H is:
1:1 multiply R by 1
1:10 multiply R by 1.5
1:100 multiply R by 2Ø
XI. GAS/GAS/WATER REACTIONS DURING MICROSPARGING
DETAIL ON PROCESS AND DELIVERY SYSTEM
Microsparging: The unique use of Microfine Bubble injection for simultaneous
extraction/decomposition reactions in saturated and partially-saturated
capillary zones (soil and
geological formations).
As opposed to simply creating smaller and smaller sized bubbles for the
purpose of
injecting into free water, the microsparge process involves generation of fine
bubbles which can
enter and pass through the torturous pathways of the substrate (aquifer
structure) and promote
rapid gas/gas/water reactions with volatile organic compounds which the
substrate participates in,
instead of solely enhancing dissolved (aqueous) disassociations and reactions.
The microsparging
process encompasses the following unique aspects:
(1) The production of microbubbles and selection of appropriate size
distribution for
optimizing gaseous exchange in sandy aquifers (i.e., passage through
interconnected fine
capillary-sized passageways), using microporous materials, bubble chamber, and
pulsed gas/water
injection.
(2) Physical methodology and equipment for promoting the continuous movement
of
microbubbles through porous aquifers without coalescing or adhesion (i.e.,
small bubbles will not
move through fine channels without assistance, otherwise they accumulate,
coalesce, or
immobilize). The injected air/water combination moves as a fluid through the
aquifer without
22
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
fracturing or channeling, which interfere with even distribution and
efficiency of exchange. The
injected gas/water combination is pulsed in such a way to move the bubbles on
a pressure wave
for lateral distribution. The wave form has an amplitude which falls above
critical bubbling
pressure but below fracturing pressure for formation. The pulsing is done to
create short-term
tidal waves in three dimensions. The combination of recirculating the water
also assists in creating
and promoting vertical airlift which induces the generation of a three-
dimensional eddy current
adjacent to the spargewell, greatly assisting in evening the reaction rate
throughout a broad
aquifer region.
(3) The use of microencapsulated ozone to enhance and promote in-situ
stripping of
volatile organics and simultaneously terminate the normal reversible Henry's
reaction.
(4) The demonstration and enhancement of unique gas/gas/water reactions for
the rapid
decomposition of HVOCs and petroleum products (BTEX-related compounds). In her
doctoral
thesis, Masten (1986) identified a particular chemical pathway by which ozone
can react with
chlorinated olefinic VOCs (PCE, TCE, DCE) to decompose the molecule by direct
rather than
i5 indirect means (i.e., hydroxide or super oxide formation). Heretofore, the
reaction had not been
demonstrated to be significant in aqueous remediation processes, since the
reaction progresses
very slowly with PCE. For instance, if a HVOC/ozone gaseous mixture or
microbubble injection
occurs into free water alone, forming superhydroxides as the primary reactive
agents (Masten and
Hoigne (1992). The process described here is called C-Sparging and involves
promoting
simultaneous VOC in-situ stripping and gaseous decomposition, with moisture
(water) and
substrate as co-reactants in the later stages. This is not a dissolved aqueous
reaction. The
following text elaborates on this by demonstrating that the reaction kinetics
are entirely different
from existing aqueous literature values. Bench scale and f eld testing
demonstrate the facilitating
role of the mineral substrate as part of the reaction process.
(S) Remote Process Controller and Monitor: This allows for the capacity for
sensor
feedback and remote communication to the Timer/Sequencer ozone (or oxygen or
both) generator
to achieve a certain level of gaseous content (e.g., dissolved oxygen, ozone,
or other gas) and rate
of mixing to promote ef~tcient reactions. This is done by sensors placed in
monitoring wells at
certain distances from the central spargewell. A groundwater flow meter and
pressure sensor
monitors rate and direction of rotation of a three-dimensional gyre (or eddy)
produced by pulsing
the unit. The unique combination of pressure and flow allows a quick
determination of where and
how fast mixing will occur. Oxygen content, redox potential, and dissolved VOC
concentration of
the water can be monitored at a nearby monitoring well or top well screen of
the spargewell. The
23
CA 02271844 1999-OS-11
WO 98/21152 PCT/ITS97119907
operator can access the information, modify operations and diagnose the
condition of the unit by
telephone modem or satellite cell phone. This provides on-site process
evaluation and adjustment
without operator presence.
Appropriately-sized micro-fine bubbles, generated in a pulsing manner, which
easily
penetrate sandy formations, and/or bubble generation and selection chambers
which allow
alternating water/bubble/water/bubble fluid flow, have unexpected benefits
when used with
multiple gas systems.
Firstly, microfine bubbles substantially accelerate the transfer rate of
volatile organic
compounds like PCE from aqueous to gaseous state. The bubble rise has the
potential to transfer
the PCE to the watertable surface and above (vadose zone). The ten-fold
difference in
surface-to-volume ratio of bubble generator (Spargepoint~) microbubbles
compared to bubbles
from well screens results in at least four-fold improvement in transfer rates.
Further reducing the size of the bubbles to microfine sizes, from 1/10 to 1/2
mean pore
size, appears to boost extraction rates between 4 and 20 fold. These sizes
boost exchange rates
but do not tend to be retarded in rise time by too small a size.
Secondly, when an oxidizing gas (ozone) is added into the microbubbles, the
rate of
extraction is enhanced further by maintaining a low interior (intrabubble)
concentration of PCE,
while simultaneously degrading the PCE by a gas/gas/water reaction. The
combination of both
processes acting simultaneously provides a unique rapid removal system which
is identified in the
field by a logarithmic rate of removal of PCE, and a characteristic ratio of
efficiency quite different
from dissolved (aqueous) ozone reactions. The compounds commonly treated are
HVOCs
(halogenated volatile organic compounds), PCE, TCE, DCE, vinyl chloride (VC),
petroleum
compounds (BTEX: benzene, toluene, ethylbenzene, xylenes). The rapid removal
in saturated soils
or unsaturated but wet soils can be so complete as to not require any vacuum
extraction to
recover the remaining solvents.
XII. GASEOUS EXCHANGE AND PARTITIONING ENHANCEMENT
If gaseous exchange is proportional to available surface area, with partial
pressures and
mixtures of volatile gases being held constant, a halving of the radius of
bubbles would quadruple
(i.e., 4X) the exchange rate. If, in the best case, a standard well screen
creates air bubbles 200
times the size of a medium sand porosity, a microporous diffusor of 5 to 20
micron size creates a
bubble 1/10 the diameter and six to ten times the volume/surface ratio.
24
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
TABLE 2
Diameter Surface Area Volume Surface Areal
(microns) 4~ 1z 4/3n r3 Volume
200 124600 4186666 0.03
20 1256 4186 0.3
Theoretically, the microporous bubbles exhibit an exchange rate of ten times
the rate of a
comparable bubble from a standard ten slot well screen.
The relationship between exchange efficiency and bubble configuration can be
further
explained by the surface to volume change between spheres (unconfined
microbubbles) and
cylinders (confined bubbles within capillary tubes). The injection of air into
geological formations
without concern for volume/pore size relationships will result in elongate
cylinders of gas
("microchannels") as observed by the University of Connecticut (1995). The
effect of changing
from spherical or small cylinder (radius 2x length) to elongate cylinder
dramatically affects the
ratio of exchange surface area to volume.
To illustrate, the loss of efficiency from spherical to elongate cylinder can
be shown by
contrasting the ratios of transforming from one quarter ( 1 /4) pore size
(given as 1.0) to ten ( 10)
times pore size for a constrained gas bubble. As a micron-sized unconstrained
bubble enters the
channel, it retains a spherical shape and an A/V ratio of 24. As the volume
expands to pore size,
the ratio decreases to an AN ratio of six (6). As the bubble volume becomes
larger, it is forced to
elongate into a cylinder. When the cylinder elongates, the A/V ratio shrinks
further and begins to
converge between 2.0 and 4Ø The surface to volume ratio has reduced to about
one-twelfth
(spheroid) or one-sixth {cylinder) of that found with spherical (or mini-
cylinders) of one quarter
( 1 /4) pore size.
TABLE 3. SURFACE TO VOLUME
(A/u) RATIO CHANGES AS FUNCTION OF PORE SIZE
AS BUBBLE VOLUME INCREASES
D (i.e., 2r) or h as 0.1 0.25 0.5 1 2 5 10 20
Fraction of Pore
Size
SPHERE SPHERO1D
Area = 4~ r2 0.03140.196250.7853.14 18.837.7 69 131
Volume = 4/3n 0.00050.008170.0650.53 6.3 15.7 31 62
r~
Ratio 62 24 12 5.9 3 2.4 2.2 2.1
CYLINDER (diameter for reater )
is constant h than
at 1Ø g 1
Area 2~ r(r+h)0.04710.2944 1.174.71 7.9 17.2 33 64
Volume n rzh 0.00080.0123 0.0980.78 1.6 3.9 7.9 16
Ratio 59 24 12 6 4.9 4.4 4.2 4
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
In wastewater treatment, the two-film theory of gas transfer (Metcalf and
Eddy, Inc.,
1991 ) states the rate of transfer between gas and liquid phases is generally
proportional to the
surface area of contact and the difference between the existing concentration
and the equilibrium
concentration of the gas in solution. Simply stated, if we increase the
surface to volume ratio of
contact, we increase the rate of exchange. If, secondly, we consume the gas
(VOC) entering the
bubble (or micropore space bounded by a liquid film), the difference is
maintained at a higher
entry rate than if the VOC is allowed to reach saturation equilibrium. In the
case of a halogenated
volatile organic carbon compound (HVOC), PCE, gas/gas reaction of PCE to by-
products of HCI,
COZ and HZO accomplishes this. in the case of petroleum products like BTEX
(benzene, toluene,
ethylbenzene, and xylenes), the benzene entering the bubbles reacts to
decompose to COZ and H20.
The normal equation for the two-film theory of gas transfer is stated (Metcalf
and Eddy, 1991 ):
rm = Kg A (CS-C)
where:
rm = rate of mass transfer
K8 = coefficient of diffusion for gas
A = area through which gas is diffusing
Cs = saturation concentration of gas in solution
C = concentration of gas in solution.
The restatement of the equation to consider the inward transfer of phase
change from
dissolved HVOC to gaseous HVOC in the inside of the bubble would be:
CS = Saturation concentration of gas phase of HVOC or VOC in bubble
C = Initial concentration of gas phase of HVOC or VOC in bubble volume.
XIII. PARTITIONING ENHANCEMENT
Soil vapor concentrations are related to two governing systems: water phase
and
(non-aqueous) product phase. Henry's. and Raoult's Laws (DiGiulio, 1990) are
commonly used to
understand equilibrium-vapor concentrations governing volatization from
liquids. When soils are
moist, the relative volatility is dependent upon Henry's Law. Under normal
conditions (free from
product) where volatile organic carbons (VOCs) are relatively low, an
equilibrium of soil, water,
and air is assumed to exist. The compound tetrachloroethene (PCE), has a high
exchange capacity
from dissolved form to gaseous form. If the surface/volume ratio is modified
at least ten fold, the
rate of removal should be accelerated substantially.
Figure 15 plots a curve of the removal rate of PCE for an aqueous solution
equivalent to
120 ppb, subjected to differing bubble sizes. The air volume and water volume
was held constant.
The only change was the diameter of bubbles passed through the liquid from air
released from a
diffusor.
26
CA 02271844 1999-OS-11
WO 98/21152 PCT/tTS97119907
XIV. PCE REMOVAL RATE AS FUNCTION OF BUBBLE SIZE
OZONE ENCAPSULATION - C-SPARGINGTM
Ozone is an effective oxidant used for the breakdown of organic compounds in
water
treatment. The major problem in effectiveness is a short lifetime. If ozone is
mixed with sewage
containing water above ground, the half life is normally minutes. Ozone reacts
quantitatively with
PCE to yield breakdown products of hydrochloric acid, carbon dioxide, and
water.
To offset the short life span, the ozone could be injected with microporous
diffusors,
enhancing the selectiveness of action of the ozone. By encapsulating the ozone
in fine bubbles, the
bubbles would preferentially extract volatile compounds like PCE from the
mixtures of soluble
organic compounds they encountered. The ozone destruction of organics would
then target
volatile organics selectively pulled into the fine air bubbles. Even in a
groundwater mixture of high
organic content like diluted sewage, PCE removal could be rapid. Gas entering
a small bubble of
volume (4n r3) increases until reaching an asymptotic value of saturation. If
we consider the
surface of the bubble to be a membrane, a first order equation can be written
for the
monomolecular reaction of the first order. The reaction can be written as
follows:
dx
______ - K (Q_X)
dt
where X is the time varying concentration of the substance in the bubble, Q is
the external
concentration of the substance, and K is the absorption constant.
If at time t = O, X = O,
then:
X=Q (1-e"')
The constant K is found to be:
dx/dt
K = ___________
Q-X
By multiplying both numerator and denominator by V, the volume of the bubble,
we obtain
vdx/dt
IC = ___________
v (Q-~
which is the ratio between the amount of substance entering the given volume
per unit time and
quantity V (Q-X) needed to reach the asymptotic value. By (1) analyzing the
concentration
change within the fine bubbles sent through a porous matrix with saturated
(water filled) solution
interacting with the matrix (sand), and (2) determining the rate of
decomposition of the products
27
CA 02271844 1999-OS-11
WO 98!21152 PCT/LTS97/19907
[TCE + ozone = COZ+ HCI] and [Benzene + ozone = COZ+ HOH], we can characterize
the kinetic
rates of reaction.
The rate which the quantity K,QV of the substance flows in one unit of time
from aqueous
solution into the bubble is proportional to Henry's Constant. The second rate
of decomposition
within the bubble can be considered as k, , a second rate of reaction (-kzX),
where
dx
_______ _ k~Q_~X
dt
and, at equilibrium, as dx/dt = 0, we would have
k,
X = ____ Q
k2
However, if the reaction to decompose is very rapid, so -kzX goes to zero, the
rate of reaction
would maximize k,Q; i.e., be proportional to Henry's Constant and maximize the
rate of
extraction since VOC saturation is not occurring within the bubbles.
The unique combination of microbubble extraction and ozone degradation can be
generalized to predict the volatile organic compounds amenable to rapid
removal. The efficiency
of extraction is directly proportional to Henry's Constant. Multiplying the
Henry's Constant (the
partitioning of VOCs from water to gas phase) times the reactivity rate
constant of ozone for a
particular VOC yields the rate of decomposition expected by the microbubble
process.
The concentration of HVOC expected in the bubble is a consequence of rate of
invasion
and rate of removal. In practice, the ozone concentration is adjusted to yield
0 concentration at
the time of arrival at the surface.
rvoc = 'KL avoc (C-C~
where:
rv~ = rate of VOC mass transfer, ~g/ft'~h (~g/m3~h)
(KLa)v~ = overall VOC mass transfer coefficient, !/h
C = concentration of VOC in liquid
C, = saturation concentration of VOC in liquid ~g/ft3 (ltg/m')
The saturation concentration of a VOC in wastewater is a function of the
partial pressure
of the VOC in the atmosphere in contact with the wastewater.
cg
-- - H~ therefore, C8= H~~ CS (equation 1 )
C,
CB = concentration of VOC in gas phase p.g/ft3 (~tg/m'~
CS = saturation concentration of VOC in liquid ~tg/ft3 (pg/m3)
H~ = Henry's Constant
28
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
The rate of decomposition of an organic compound Cg , (when present at a
concentration
[C]) by ozone can be formulated by the equation:
d[Cg]
- ..-__ = Kp c [~3][Cg]
dt O3
or, after integration for the case of a batch reactor:
[Cg]end
-In (---------) = ICo ~ [03]t (equation 2)
[Cg]O
[Cg]ma
_______ - a k0 c [O3]t
[Cg]o
Cend Co a k0 c [O3]t
[03] = concentration of ozone averaged over the reaction time (t)
[Cg]o = halocarbon initial concentration
[Cg]~ = halocarbon final concentration
Substituting:
rm = KgA (Cg C) From Henry's Law:
rm = KgA ([H~~C,]-C) Cg = H~~Cg (equation 3)
rm = KgZ ([H~~C,]-C) With ozone
rm K (L'ic~Cs]-C-~ c[03][Cg])
[H~~C,)-ICo ~ [03][Cg] = 0 (equation 4)
Rate of decomposition is now adjusted to equal the total HVOC entering the
bubble.
SET: [H~~CS] = ICfl ~ [03][Cg] (equation 5)
therefore surface concentration = 0
This condition has not been formulated before. It speeds up the rate of
extraction because
the VOC never reaches equilibrium or saturation in the bubble.
Table 4 gives the Henry's Constants (H4) for a selected number of organic
compounds and
the second rate constants (R~) for the ozone radical rate of reaction observed
in solely aqueous
reactions where superoxide and hydroxide reactions dominate. The third column
presents the
observed rates of removal in field trials with the C-SpargerTM process.
TABLE 4
REMOVAL RATE COEFFICIENTS FOR THE
MICROBUBBLE/OZONE PROCESS - C-SPARGERTM
29
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
Ozone Aqueous C-SpargerTM
Second Order Rate
Organic Rate Constants Henry's Removal
Compound (M-' SEC-') Constantb Coet~lcient(tl'-
Benzene 2 5.59 x 10'3 0.06
Toluene 14 6.37 x 10'3 0.07
Chlorobenzene 0.75 3.72 x 10'3 0.013
Dichloroethylene 110 7.60 x 10-' 0.035
Trichloroethylene17 9.10 x 10'' 0.05
Tetrachloroethylene0.1 25.9 x 10-3 0.06
Ethanol 0.02 .04 x 10~' 0.0008
a. From Hoigne and Bader, 1983
b. From EPA 540/1-86/060, Superfund Public Health Evaluation Manual, presented
as x 10'3
c. From Site Tests (KVA,1995,1996,1997)
The C-SpargerTM process rapid removal rate clearly does not follow Hoigne and
Bader
(1983) rate constants. There is a close correlation to Henry's Constant as
would be expected from
equation 5. The presence of the substrate (sand) and moisture is necessary to
complete the
reaction. The active ingredient in the sand matrix appears to be an iron
silicate. The breakdown
products include COZ and dilute HCI.
Two sets of equations are involved in the reactions:
Dissolved Halogenated Compounds
Cl C1
C = C + 03 + HOH ----------> 3HC1 + 2C0~
Cl C1 Substrate
Dissolved Petroleum Distillates
HOH
C6Hi2 + 603 ____________> 6C0~ + 6HOH
Substrate
The eligible compounds for the C-SpargerTM process are normally unsaturated
(double
bond), halogenated compounds like PCE, TCE, DCE, Vinyl Chloride, EDB; or
aromatic ring
compounds like benzene derivatives (benzene, toluene, ethylbenzene, xylenes).
However, pseudo
Criegee reactions with the substrate and ozone appear effective in reducing
certain saturated
olefins like trichloro alkanes (1,1-TCA), carbon tetrachloride (CC14), and
chlorobenzene, for
instance.
The following characteristics appear desirable for reaction:
Henry's Constant: 0-Z to 10" m' .atm/mol
Solubility: 10 to 20,000 mg/1
Vapor pressure: 1 to 3000 mmhg
Saturation concentration: 5 to 9000 g/m'
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
XV. TREATMENT SYSTEM EXAMPLE
The following report describes a pilot test of the C-SpargerTM process for
remediation of
dissolved chlorinated solvents from groundwater. The test was conducted by
Mateboer
Milieutechniek B. V. for the Provincial Government of Utrecht at Rembrandt
Street in Bilthoven,
The Netherlands, from March 27, 1997 through April 4, 1997. The test involved
installation of a
C-SpargerTM well (TW), some additional monitoring wells (four 2 inch ff~), the
use of previously
existing miniwells, and a fire well across the site.
XVI. SITE DESCRIPTION
The field test is positioned in a small park area midway on a long plume of
predominantly
trichloroethene (TCE) originating at a commercial building and traveling over
800 ft. across a
predominantly commercial and residential area. The plume region lies in a
thick fine sand deposit
which contains gravel (streambed) deposits. Groundwater exists at a depth of
7.5 ft. (2.5 m)
below grade. About one half of the area of groundwater overlying the TCE plume
is contaminated
with dissolved hydrocarbons (BTEX) from a nearby commercial fizel spill.
Soil borings taken by Tauw Engineering in the vicinity of the plume showed a
shallow
surface loam extending to 6 feet (2 m) deep. Groundwater occurred at 7.5 ft.
(2.5 m). Fine sand
occurred in many wells to over 18 ft. (6 m) deep. Often gravel layers were
intercepted at 12 ft. (4
m) to 18 ft. (6 m) deep. A thick clay layer, which probably serves as a bottom
confining layer, was
found at (110-120 ft. (38-40m) depth. A hydraulic conductivity (K) of 7.5 x
10'z cm/sec has been
estimated for the sand deposits.
Previous groundwater sampling had identified a narrow, long I-iVOC plume on
two
transects A-A' and B-B' extending from a source near wells 120 and 121 at a
commercial facility
to under Rembrandt Street and ending under another commercial complex beyond
well 140 near
Rembrandt Street. The distance was about 765 ft. (225 m) long. The top of the
plume was at
about 30 ft. (10 m) below grade. The highest total HVOC content was expected
to be about 790
ppb combined PCE and TCE (miniscreen 129).
The location of the monitoring wells were varied in distance and depth from
the test
spargewell (TW) to be able to give a 3-dimensional picture of the test
results. The larger diameter
(2-inch ID) wells allowed groundwater flow measurements as well as pressure
change to be
monitored during treatment. A variety of physical and chemical measurements
were performed
during the test.
31
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
TABLE 5. GROUNDWATER MONITORING DURING PILOT TEST
Physical Measurement Chemical Monitoring
Temperature, turbidity pH, Fe, redox potential
Static Water Elevation dissolved oxygen (DO)
Groundwater Flow HVOCs including PCE, TCE, DCE, Vc, DCA
Head Pressure change VOCs including benzene, toluene, xylenes,
ethylbenzene, ozone concentration
XVII. C-SPARGER'~'M WELL INSTALLATION
The C-SpargerTM double-screen well with lower bubble generator was installed
with a
recirculating water system and casing. A small flow (2 gal/min) was obtained
from a shallow fire
well for makeup water. The lower bubble generator was set at a depth of 7.8
ft. (2.6 m). A
one-half inch tubing extended to the surface from a compression fitting on the
bubble generator. A
four inch ID triple-screened well extended from 69 ft. (23 m) to one foot
above grade. 6 ft. long
(2 m) screens were placed with bottom edges at 69 ft. (23 m), 39 ft. ( 13 m),
and 7.5 ft. (2.5 m).
The middle casing between the two lower screens received 3 ft ( 1 m) of
bentonite grout, 3 ft. ( I
m) of cement/bentonite, and 3 ft. ( 1 m) of bentonite to seal the annular
space to prevent
"short-circuiting" of water. Water and fine bubbles are injected into the
formation from the lowest
screen and return water enters the middle screen. The uppermost screen
collects gases from just
above the water table (2.5 m) to assure vapor control
XVIII. RADIUS OF INFLUENCE
The C-SpargerTM system is designed to achieve the injection and distribution
of
microbubbles into the aquifer to be treated. The pressure of the gas
injection, use of microporous
bubblers, and a recirculating well system, all function to distribute fine
bubbles, containing
air/ozone gas through the fine sands under Darcian flow approximating fluid
flow. The injection of
the air/ozone approximates the injection of water, exhibiting mounding and
outward movement
until equilibrium is reached.
Despite numerous monitoring well corings depicting uniform fine sand formation
with
occasional gravel deposits, the immediate injection pressures and distribution
suggested hydraulic
conductivities consistent with semi-confined conditions. The presence of
microbubbles, gas
release, and dissolved oxygen changes normally demark the expansion of the
treatment zone. On
April 1 st, operation of the C-SpargerTM unit began at about 2:00 PM. By the
afternoon of April
2nd, gas bubbles were found discharging at minipoint 129, over 51 ft. (17.1 m)
from the
spargewell (TW) installation (Table 6). Well D, only about 7 ft. (2.2 m) from
the injection area
showed almost immediate oxygen changes and water which was effervescent with
fine bubbles.
32
CA 02271844 1999-OS-11
WO 98/21152 PCT/US971I9907
However, the lateral spread from this long axis progressed siowly (Well A and
C), as if the wells
were in tight (silty) material.
Tables 6 and 7 give the results of field measurements taken during sampling.
The bubble
zone was still expanding during the ten day test. Based upon the time
sequence, a long axis
extending outwards about 100 ft. (30 m) in a westerly and easterly direction
would be reached,
with a minor axis (at right angles) of about 56 ft. ( 18 m). Each well would
then treat a region 200
ft. long by about 100 ft. wide and 90 ft. (30 m) deep. Although an elliptic
zone is considered here,
the occurrence of bubbles at miniwell 126 about day 5 complicates the picture.
We must assume
that a buried gravel streambed, about 30 feet wide was originally intercepted
and either small
streamlets (braided streams) intercepted it or some secondary fracturing by
air pockets was
occurring to create the offshoot to miniwell 126. A geological basis exists
for assuming gravel
streambeds originating east-west across the region from glacial streams. Side
connections could
occur.
TABLE 6. DISSOLVED OXYGEN (DØ) CHANGES OBSERVED IN
MONITORING WELLS NEAR THE SPARGEWELL.
WELL DAY
1 2 3 4 5 6 7 8 9 10 11
2.8 11.5 ___ ___ ___ ___ ___ __ ___ ___ ___
D 1.2 14.2 12.2 11.8 8.9 --- 12 12.3 13 9.2 15.8
129 0 1.6 9.3 9.2 7.4 --- 7.9 8.6 9.1 8.5 9.4
C 0 0.7 1.4 0.9 1.1 --- 3.5 3.4 5.7 5.2 8.2
A 0 0 0.1 3.1 8.6 --- 8.6 8.8 11.6 11.7 9.3
B* 0 0 0 0.2 0--- 0 0 0 0 0
126
(14-15) 0 0 0 3.5 3.7 --- 5.9 6.3 6.8 6.7 6
*Well B showed a continual increase in redox potential despite exhibiting no
oxygen increase. A
hydrocarbon plume with high oxygen demand existed in the region.
XIX. GROUNDWATER FLOW MEASUREMENTS
CIRCULATION PATTERN DEFINITION
Direct groundwater flow measurements were performed with a KVA Model 40 GeoFlo
Meter prior to the startup and during the operation to determine background
velocity and
changes. Initial measurements indicated a flow near the spargewell (TW) in a
north westerly
direction at a velocity of between .6 and .8 ft./d, coinciding with the
direction of movement of the
plume.
33
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
Additional measurements were taken after beginning injection to determine the
velocity of
groundwater eddies created by the double-screen well system and rising bubbles
which expand the
treatment zone both vertically and horizontally across the site. The observed
change in direction
and rate coincided with a slow vertical mixing rate (wtlich is 5.4 m. east of
TV~. The change was
measured at about 3 ft!d in an easterly direction. At well B (which is 11 m.
west of TV~ a velocity
change of .5 ft/d occurred towards the TW well. The A well is shallow {37 ft.
below grade) and
the B well significantly lower {49 ft. below grade). Groundwater moving in
towards the
spargewelt would reach a maximum at about 71 ft. (27 m) below grade.
The outward gyre would reach a maximum velocity at about 36 ft. (12 m). The
vertical
eddy for mixing appeared to reach a velocity with a diameter of about 60 ft.
(20 m) by day 10 of
the test, with an estimated velocity of about 10 ft/day (3.3 m/d). This is
stow by normal standards
and probably the result of loss of pressure along the narrow gravel streambed,
intercepted
between 60 ft. and 75 ft. deep (20 and 25 m).
XX. CHEMICAL RESULTS
VOC REMOVAL - CHLORINATED AND PETROLEUM COMPOUNDS
The site held a combination of a lower dissolved chlorinated solvent plume,
dominated by
PCE and TCE, and an upper dissolved fuel spill, dominated by BTEX compounds. A
large region
of the wells exlubited elevated HVOCs in groundwater, with initial samples
from wells D, B, C
and miniweil 129 (14-15 m) showing concentrations of 2,100 ppb; 14,500 ppb;
12,500 ppb; and
1,450 ppb, respectively, well above the 800 ppb originally expected.
Groundwater from the
spargewell ('TV~ and wells A, B, C, and D exhibited total BTEX concentrations
ranging from 62
to 95 p.g/1-ppb.
Concentration of HVOCs (VOCs) located in the gravel zone underwent immediate
rapid
reduction (wells TM, D, 129). Nearby wells located at right angles {probably
in fine sands) to the
buried gravel streambed, showed a slower removal, converging on a logarithmic
decay rate. Those
in the outlying wells, mainly requiring recirculation to treat the
groundwater, tended to show
decaying oscillating concentrations with time, reflecting the circular water
movement.
The process of removal of dissolved volatiles is similar to detoxification in
human bodies.
Elimination and detoxification processes correspond to first order reactions
where the rate of
decrease in concentration of the toxic substance is directly proportional to
the concentration of the
substance. The following differential equation expresses the direct
relationship between the rate of
elimination and the concentration of the dissolved volatile compound:
dc/dt = -be
34
CA 02271844 1999-OS-11
WO 9$/21152 PCT/US97/19907
where:
do = change in concentration of dissolved volatile
dt = change in time
b = fraction of volatile substance that leaves groundwater in one unit of time
(day)
c = concentration of volatile compound
If b = 0.20, 20 percent of the volatile substance present at any given time is
eliminated per unit of
time (day). To determine the actual amount of substance eliminated per unit of
time, the initial
concentration was compared to later concentrations at increasing time
intervals from start of
operation. The amount of material elinunated is obtained from:
Bc (dc/dt) = fc
where:
Bc = volume of groundwater block (prism) containing the chlorinated substance
C - concentration of volatile substance in groundwater, in pg/i-ppb
f = a constant
The following equation oilers the simple solution:
C = Coe~'
where:
a = an exponent
Co = Initial concentration of volatile organic compound in groundwater (~tg/1-
ppb)
The equation shows the behavior of the phenomenon of "exponential decay",
since the exponential
term e'~' appears in it. The curve starts from a known groundwater
concentration and decreases in
proportion to the remaining concentration, thus
log C/Co = bt/2.303
or log C = log Co - btJ2.303
for C = Co/2, b = 0.693/t~
The constant t,n corresponds to the length of time needed for the
concentration of Co to decrease
by 50% ; i.e.. t,n is the half life of the substance in the groundwater.
In figures 27 and 29, the mean -iogC/Co is plotted versus time to derive the
approximate
decay rate observed for the site conditions. Note that because the y-axis is
the negative logarithm,
a positive (upwards) slope indicates proportional reduction. The decay
constants for both HVOCs
and BTEX compounds were computed. In some cases, a linear mean value was
clearly being fitted
to a dampened oscillating decay as a first approximation.
HVOC removal rates fell between .09 and .14t. BTEX removal rates fell between
.07 and
20t. This corresponds to a steady rate of reduction to 1/2 value every 7 to 11
days. In a
conservative estimate it would take slightly less than 100 days to reduce the
core region (SOfr
CA 02271844 1999-OS-11
WO 98121152 PCT/US97/19907
wide by 30 ft deep by 200 ft long) to below 5 ug/1-ppb, assuming no other
sources invade the
eddies with the treatment volume.
With HVOCs, the time to bring core region concentrations to 1 pg/1-ppb ranged
from 50 to
100 days. For BTEX compounds, the level ranged between 20 to 60 days. Please
note that the
HVOC removal rate is somewhat slower since the beginning concentration of 2000
ppb total
HVOC is higher than the starting point of the BTEX compounds (50-70 ppb)
XXI. BUBBLE CHAMBER (SELECTOR AND INJECTOR)
To generate a higher proportion of micron-sized bubbles, a recirculating
liquid flow
system under pressure was combined with a porous cylinder, with counter-
gravity flow (for
segregating bubble size) to create a micro-bubble production chamber. The
combination of flow
across porous plates has been known to fractionate bubbles to produce small
bubbles (Adler,
Bourbigot, and M. Faivre, 1985). To increase the number of fine ozoneated
bubbles and decrease
their size, the partial water flow was pressurized, saturated with ozone and
then released,
producing fine bubbles with a size between 50 to 200 pm (Boisoon, Faivere, and
Martin, 1995).
The concept of mounting a porous plate with vigorous mixing vertically below a
pulsing
water pump, then allowing a time delay or low volume flow to allow segregation
of small bubbies
from large bubbles, can produce 10 to 100 pm bubbles suitable for injection
into finely porous
geological formations (fine silts and sands to medium sands). Groundwaters are
naturally lower
temperatures (40°-50°F), which allow low loss of ozone (2% to
5%) during compression.
The process can be visualized in three steps. Firstly (step A) generation of a
large range of
bubble sizes at one time, secondly (step B) the segregation step when larger
bubbles segregate out
and form a gas space at the top, and thirdly (step C) the fine bubbles
remaining are then pumped
out the lower well screen as water under pressure is introduced from the top.
XXII. INDUCTION OF MICROBUBBLE MOVEMENT
The induction of microbubble flow through a sandy saturated deposit (aquifer)
can be
compared to that of transferring electron movement through alternating
current. An alternating
wave of pressure is created where the amplitude varies continuously.
p = P~. ~ sin A
where:
p = the instantaneous pressure amplitude in inches (cm) of water
P~ = the maximum pressure in inches (cm) of water
8 = the angle at which pressure is being calculated
36
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
These values are repeated during the remainder of the first alternation, but
in reverse order
if a modal value is chosen as reference. The magnitude of P""x lies above
critical bubbling pressure
but below fracturing pressure of formation.
The pulsing pressure wave can be seen at distance from the C-SpargerTM well
(figure 33).
Cycle time can vary from 30 minutes to 10 minutes.
The movement of microbubbles and inbetween water occurs as a response to the
pressure
difference, and has a similar equation:
v = V""x ~ sinA
where:
v = the instantaneous velocity in ft/day
Vm"~ = the maximum velocity in ft/day
A = the angle at which instantaneous velocity is being calculated
Because of the nature of resistance and storage capacity in an aquifer,
pressure change
(inductance) and bubble flow (velocity) may have a phase difference
The alternating pressure of the C-SpargerTM unit creates a wavefront which
pushes the
microbubbles along. By alternating water injection with microbubble
production, a continuous
flow of microbubbles is produced. When a monitoring well is encountered, the
fine bubbles can be
seen to enter the screens in spurts (figure 34).
Microbubbles, being less dense than water, will tend to rise, resulting in a
parabolic
upwards pathway. The rising rate (velocity) produces a displacement of water
upwards which
creates an inflow of lower water, inducing an eddy and mixing with a
particular radius of the
installed spargewell.
XXIII. RECIRCULATION MECHANICS
The use of the bubble chamber, composed of vertically offset well screens,
creates large
circular eddies on each side of the C-SpargerTM unit when it is placed in an
unconfined aquifer.
The rise of bubbles, combined with pulsed liquid recirculation between the
screens, drives a mass
of groundwater vertically which then moves along the surface region before
diving below. The net
effect is to act as a big vacuum cleaner, sucking water from below and lateral
to it and exposing
the circulating water to continual treatment and removal of VOCs. The
advantages are several:
(1) Rates of reaction within the circulating cell do not diminish
(exponentially decay) with
distance from the bubble emission source as would be the case if reaction were
bubble density
dependent. Instead the recirculating water creates a rate of removal which is
uniform within the
circulating cell of water. Concentration level then is only affected by
initial rate of removal and the
absence of influx from another source or upgradient groundwater.
37
CA 02271844 1999-OS-11
WO 98/21152 PCTlUS97/19907
(2) The system can expand its region of erect beyond the upper regions which
the bubbles
transmit through. It can "vacuum" concentrations along a bottom confining
layer and circulate
them within the region of exposure to the bubbles.
(3) The lateral extent of treatment depends upon the distance between the
lower bubble
generator and the topmost well screen. Generally, the cross-sectional area of
influence is about 2.5
times the vertical distance between the lower sparge bubble generator and the
upper screen.
(4) The mixing capacity of recirculation allows mechanics to be used
equivalent to
Di$'used-Air Aeration Process Mechanics. The three-dimensional recirculation
cell can be
considered similar to the boundaries of a tank (figure 35). If groundwater
flow is very slow, the
cell is considered a fixed reactor with only circulation and no inflow. If
groundwater movement is
significant, the transfer into the cell is equivalent to inflow and the loss
of groundwater
downgradient, the discharge efrluent.
The tremendous advantage that the microbubbie injection has over slotted well
screen
injection can be shown in Figure 36. A flow of 5 CFM to a five-foot long, two-
inch slotted PVC
monitoring well screen placed 10 feet below static water results in a measured
rise in dissolved
oxygen at distances of 15 feet. With the use of a microporous bubble generator
(Spargepoint~)
under pulsed operation at the same position, the radius increases to over 20
feet. If the
C-SpargerTM unit is used with microbubble production chamber and pulsed
recirculation, the zone
expands to beyond a 50-foot radius. Enlarging the distance between
recirculating well screens can
even further enlarge the radius.
XXIV. PROCESS CONTROL, MONITORING, COMMUNICATOR UNIT
The C-SpargerTM unit is equipped with a telephone modem diagnostic sensor unit
and
monitoring well sensor which feed back to the sequencer to control the
groundwater/soil
remediation process. A remote unit can then monitor the extent of treatment
and induced
groundwater mixing and determine when to move to another spargewell. An
operator can dial the
unit and receive past and ongoing data on groundwater condition and machine
operation. The
recorded data can be dumped for graphic presentation.
XXV. ELIMINATION OF THE NEED FOR VAPOR EXTRACTION
The need for vapor control exists when vapors of VOCs, partitioned from
dissolved form
into the microbubbles, reach the unsaturated zone, releasing vapors. Without
reaction with a
decomposing gas, such as ozone, a large mass can be transmitted in a short
time, creating
potential health problems near residential basement areas.
38
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
The combined extraction/decomposition process has the capacity to eliminate
the need for
vapor capture. If the decomposition rate with ozone exceeds the vertical time-
of travel, vapors
will not be produced or their concentration will be so low as to not require
capture. By controlling
the size of microbubbles and matching them to suitable slow rise times, the
need for vapor control
is eliminated.
The rise time of bubbles of different sizes was computed for water, giving the
upwards
gravitational velocity. The upwards velocity provides the positive pressure to
push the bubbles
through the porous media, following Darcy's equation. The actual rise time is
dependent upon the
size of the bubble, the frequency of agritation (pulsing) and pressure
differential during pulses. By
timing the rise rate in the field, the rise time, proportional to upwards
pressure, can be calculated.
Following is rise time in medium to coarse sand, based upon 15 minute pulse
cycles of generation
with an equivalent pressure differential of 20 psi at the source, .5 ft.
change at 30 ft. radius from
generation (Table 7).
TABLE 7
T)ZuvIE (MINUTES) FOR
BUBBLE UPWARD VELOCITY UPWARDS MIGRATION
DIAMETER 1N WATER
(3 METERS)
lOmm .25m/s 19 min.
2mm .16m/s 30 min.
.2mm .018m1s 240 min.
.02mm .OOSm/s 600 min.
Local recirculation of the water by a vertical bubble production chamber
(double-screen
well), greatly increases the rate of reaction by circulating water through the
bubble pulses.
XXVI. ELIMINATION RATE OF PCE RELATIVE TO OZONE CONTENT
The reaction of ozone with tetrachloroethene (PCE) in the presence of
substrate (sub)
sand will produce degradation products of hydrochloric acid and carbon
dioxide. By adjusting the
ozone concentration to match the dissolved PCE level, the PCE can be removed
rapidly without
excess ozone release to the air or release of PCE vapor into the unsaturated
zone.
TCE
C1 C1
C = C + O, + HOH - 2C02 + 3HC1
sub
C1 H
39
CA 02271844 1999-OS-11
WO 98/21152 PCT/US97/19907
CI Cl
C = C + 03 + HOH - 2C02 + 2H* + 4C1-
sub
C1 CI
The reaction of ozone and PCE in the air bubbles is a gas reaction. The
molecular weight
of PCE is 168 gm/mole; ozone is 48 gm/mole. A mass of 3.5 grams of PCE reacts
with one gram
of 03 needed to react with 1 mole PCE. To calculate the concentration of
gms/day ozone to match
the removal need, the total mass of dissolved PCE in the treated water column
is computed.
Assuming a porous cylinder of 8 meters radius and 2 meters deep (contaminated
zone), the liquid
volume of medium sand (.30 porosity) is about 60,000 liters. If the mean PCE
concentration is
100 ppb, 6.0 gm of PCE are contained within the cylindrical water column. From
a mass balance
standpoint, about 2 grams of ozone would be sufl=icient to remove the 100 ppb
PCE concentration
if both could be instantaneously brought into contact.
If the ozone generating unit produces 5 g/1440 minutes and it is operated 200
min/day
then .69 gms/day would be released. Dividing the grams of PCE by 3.5 yields
the ozone needed,
and then dividing by the rate of production of ozone gives an approximation of
removal rate,
assuming good distribution of bubbles throughout the medium sand contaminated
area. For 1000
ppb PCE, a production rate of 50 gms/day ozone is used.
2 gms ozone needed
--------------------------- = 30 days for complete removal, assuming 100%
extraction
.674 gms/day produced and no ozone decay.
XXVII. BUBBLE MECHANICS
In reality, the reaction rate is dependent upon the total number of bubbles
(area of
extraction), the eflxciency of distribution of the bubbles, and the rate of
transfer into the bubbles.
The rate of decomposition within the bubbles is a ratio proportional to
concentration, i.e., it slows
as concentration decreases.
XXVIII. USE OF SPECIALLY-DESIGNED WELLHEADS
(1) The hydraulic conductivity of saturated sandy formations may vary over a
range of
1000 fold. In glacial outwash, a 50 fold range may be common in a short
distance. If a series of
spargepoints are placed at a fixed depth across formations of varying
resistances, like fine sand
(k= 5 ft/d), medium sand (k=100 ft/d), and coarse gravel (k=1000 ft/d), the
point in coarse gravel
would steal all the flow. To compensate for this, a resistance element, like a
needle valve, may be
placed inline with a flowmeter to allow the flow to be equalized to each
point. The capacity to
CA 02271844 1999-OS-11
WO 98121152 PCT/US97/19907
maintain pressure at the wellhead is simultaneousiy measured by a pressure
gauge. (2) By
comparing flow and pressure, the performance can be checked with the original
site test
procedure. The wellheads are often installed at the top of the bubble
generator to limit the number
of individual lines back to the compressor/ozonator. Placed in a wellhead, a
vertical mount block
flow meter cannot be easily read. To allow easy reading, a 45 degrees angle
mirror was installed
and the scale printed in mirror image to allow for easy reading.
XXIX. ZONE SPARGING - MULTIPLE ZONES WITH ONE SYSTEM
The simplest sparging system attaches ten or twenty sparging points to one gas
supply.
The individual flow controllers adjust each sparging point for even air flow
and sparging. A zone
control system adds an electronic or mechanical programmable timer that opens
and closes valves
to direct the air supply to the appropriate manifold. The zone control is
added to the system to
expand the system and improve control of the sparging. Sequential periods of
aeration improve
the sparging action and expand the capabilities of a single air source for the
system. If, for
example, one microfine sparge system can provide adequate gas supply to 10
sparge bubblers,
zone control can increase this to 20, 30 or more.
XXX. PRODUCT MIGRATION CONTROL THROUGH.ZONE SPARGING
If the potential of product migration exists, a design for controlling the
movement of
floating product off site is accomplished by sequential sparging using
discrete zones of sparge
bubble generators. Stylized illustration of such a system shows that an outer
ring of sparge
201ocations provides a barrier for outward migration of contaminants by
concentric mounding
focused toward the center. Control of the height of water mounding through the
length of time of
sparging or pressure/air volume control per sparge locus serves to push any
floating product in a
predictable direction, toward extraction wells. Using sequential timing and
air volume control is an
effective strategy for product migration abatement.
Concentric zones permit containing any floating contaminant. Concentric zones
of
sparging centers, activated for different lengths of time and volumes of air,
will form a barrier to
off site product migration. A contaminated region with overlapping zones of
sparging contains a
plume. The midpoint of Region A is located just outside the contaminated zone.
The sequence of
sparging involves first zone A, then zone B, and finally zone C. Greater
volume and/or duration of
sparging in zone A forms a barrier ridge, forcing product toward the center of
zone C.(Figure 21)
Individual sparge bubble generator effects are shown graphically as the
location of
introduced bubbles in the saturated zone. The shape of the bubbled zone is
composed of the
original groundwater zone plus an area above static water level where water is
mounded and is
41
CA 02271844 1999-05-11
WO 98/21152 PCT/US97/19907
governed by the air pressure and volume. Higher pressure and greater volume
gives a wider
diameter of influence while lower pressure and lower volume influence a
smaller diameter area.
Overlap of these affected areas increases the thickness of the uniformly
sparged areas, decreasing
the areas missing the introduction of air. If there is a natural groundwater
flow and directional
transmissivity in an aquifer, then the sparged zone becomes distorted
downgradient and
non-uniform in diameter. The more knowledge available of the water bearing
zone, the more likely
it is to predict the effects of sparging and control them. The sparged area
then actually becomes a
barrier inhibiting contaminant migration.(Figure 20) '
~. DEGREE OF OVERLAP OF BUBBLE ZONES
Two important reasons exist which support overlapping spurge bubble generator
zones of
influence: (1) even distribution ofthe aeration and gas transfer in sparging,
and (2) elimination of
vertical gaps in the treated areas. While a two-dimensional set of circles can
be arranged in a
triangular or rhomboid configuration with circumferences touching, the region
within is not
equally saturated with air bubbles. From a single source, bubbles are ejected
outwards. Their
density decreases exponentially with distance in a uniform medium. Overlapping
sparging centers
compensate by increasing the bubble density in the outer regions of influence
where the number of
bubbles are smaller.
In a three-dimensional perspective, the spacing of the sparging points leaves
gaps between
the conical zones where the bubbles rise. The closer the points, the smaller
the stagnant zones
become. Overlap in the vertical as well as horizontal dimension tends to
create eddies of
groundwater as well as promote gaseous transfer from entrapment in the
saturated formation to
rising bubbles of introduced air.(Figure 22A)
~I. THE USE OF ALTERNATING (PULSE) PUMPAGE AND BUBBLE INJECTION
Purpose: If a bubble generator is placed within a well, the microbubbles will
not penetrate
into the formation. Installation of an inverted submersible pump with a
pneumatic packer to
alternately pump the well volume water containing the microbubbles out into
the formation allows
the bubble generator to be installed in an existing elongate well screen.
The function of the inverted pump also adds two additional advantages to
normal
microbubble production: ( 1 ) the periodic outwards pressure enlarges the
bubble radius over that
of a microporous point alone and, (2) the alternating of water pulsing after
bubble production
decreases the formation of air channels which tend to enlarge with continual
air injection.
Plugging the forming channels with water resists the re-entry of air,
producing far more channels,
the pathways varying in time.
42
CA 02271844 1999-OS-11
WO 98/21152 PCT/LTS97/19907
III. USE OF A PHYSICAL ARRANGEMENT OF SEQUENTIALLY
ARRANGED SPARGEPOINTS INSTALLED AT AN ANGLE.
The use of angled straight boring for sparging allows unique effects ideal for
treatment of
groundwater plumes of petroleum based volatile organics or volatile solvents.
Increasing the depth
below static water directly increases the radius of bubbling, creating a
natural widening of the
bubble zone. With an inclined well, multiple bubblers can create a broadening
pattern from dense
to diffuse with distance. By overlapping the slanted installation, a three
dimensional bubble "fence"
is created by the staggered placement of bubble emitters.(Figure 26)
What is claimed is
43