Note: Descriptions are shown in the official language in which they were submitted.
CA 02272138 1999-06-O1
-1-
PYRAZOLOPYRTMIDINE AND PYRAZOLOPYRIDINE COMPOUNDS
HAVING CRF ANTAGONIST ACTIVITY
This is a divisional application of Canadian Patent
Application Serial No. 2,150,483 filed November 3, 1993.
The subject matter of this divisional application is
restricted to pyrazolopyrimidine and pyrazolopyridine compounds
of the formula I shown hereinunder in which A and R1 are taken
together, and to pharmaceutical compositions containing them.
These compounds have corticotropin-releasing factor (CRF)
antagonist activity.
It should be understood that the expression "this
invention" or the like found in this specification encompasses
the subject matter of both this divisional and parent
applications.
CRF antagonists are mentioned in U. S. Patents Nos.
4,605,642 and 5,063,245 referring to peptides and pyrazolinones,
respectively. The importance of CRF antagonists is set out in
the literature, e. g. as discussed in U. S. Patent No.
5,063,245. A recent outline of the different activities
possessed by CRF antagonists is found in M. J. Owens et al.,
Pharm. Rev., Vol. 43, pages 425 to 473 (1991). Based on the
research described in these two and other references, CRF
antagonists are effective in the treatment of a wide range of
diseases including stress-related illnesses, such as stress-
induced depression, anxiety, and headache; abdominal bowel
syndrome; inflammatory diseases; immune suppression, human
immunodeficiency virus (HIV) infections; Alzheimer's disease;
gastrointestinal diseases; anorexia nervosa; hemorrhagic stress;
drug and alcohol withdrawal symptoms; drug addiction, and
fertility problems.
The compound of formula I below wherein A is C=O,
Rl is amino, R2 is methylthio, R3 is 2-chlorophenyl, and R4 is
2,4,6-trichlorophenyl is a commercial compound of no known
utility.
The present invention relates to compounds of the
formula:
64680-810E
CA 02272138 1999-06-O1
-la-
R.,
I
r,l
R4
and the acid addition salts thereof, wherein A is C=0 or S02,
or A and Rl together with the carbons to which they are
attached form pyrimidinyl or 5-pyridyl, each of which may be
substituted by R5 which is hydrogen, C1-C6 alkyl, fluoro,
chloro, bromo, hydroxy, amino, O(C1-C6 alkyl), NH(Cl-C6 alkyl),
N(C1-C6 alkyl)(C1-C6 alkyl), 4-ethoxycarbonylpiperazinyl, SH or
S(O)n(C1-C6 alkyl) wherein n=0, 1 or 2, wherein each C1-C6 alkyl
may be substituted by from 1 to 3 substituents R6 which is
hydroxyl,
64680-810E
CA 02272138 1999-06-O1
-2-
amino) C,-C3 alkoxy, dimethyfamino, diethylamino, methylamino, ethylamino,
NH(C=O)CH3, fluoro, chloro, bromo or C,-C3 thioalkyl;
R, is hydrogen, C,-Ce alkyl, amino, O(C,-Ce alkyl), NH(C,-Ca alkyl), N(C,-Ce
aikyl)(C,-Ce alkyl), wherein said C,-Ce alkyl may be substituted by from 1 to
3
substituents Re as defined above;
RZ is hydrogen, C,-Ce alkyl, hydroxyl amino, O(C,-Ce alkyl), NH(C,-CQ alkyl),
N(C,-Cs alkyl)(C,-Ca alkyl), SH, S(O)"(C,-Ce alkyl) wherein n = 0, 1, or 2,
cyano,
hydroxyl carboxyl or amido) wherein said alkyls may be substituted by one to
three of
hydroxyl amino, carboxyl amido, NH(C=O)(C,-Ce alkyl), N(C,-Cs alkyl)(C,-Ce
alkyl},
(C=O)O(C,-Ca alkyl), C,-C, alkoxy, C,-C, thioalkyl, fluoro, bromo, chloro,
iodo, cyano
or vitro;
R, is phenyl, naphthyf, thienyl, benzothienyi, pyridyl) quinofyf, pyrazinoiyl)
pyrimidyl, imidazolyl) furanyl, benzofuranyl, benzothiazolyl, isothiazolyi)
benzoisothiazolyl) thiazolyl, isoxazolyi, benzisoxazolyi, benzimidazolyl,
triazolyl)
pyrazolyl, pyrrolyl) indolyi, azaindolyl, benzoxazolyi) oxazolyl)
pyrrolidinyl) thiazolidinyl)
morpholinyl, pyridinyl, tetrazolyl, or 9 to 12 membered bicycloalkyl,
optionally containing
one to three of O, S or N-Z wherein Z is hydrogen, C,-C4 alkyl, C,-C4
alkanoyl, phenyl
or phenyfmethyi) wherein each one of the above groups may be substituted
independently by from one to three of fluoro, chloro, bromo, C,-Ce alkyl, C,-
Cs alkoxy,
or tr'rfiuoromethyl, or one of cyano, vitro) amino, NH(C,-Ce alkyl), N(C,-C4
alkyl)(C,-C2
alkyl), COO(C,-C~ alkyl), CO(C,-C4 alkyl), SO=NH(C,-C, alkyl), SOzN(C,-C4
alkyl)(C,-Cz
alkyl), SO=NHS, NHSOz(C,-C, alkyl)) S(C,-Ce alkyl}) SOz(C,-Ce alkyl)) wherein
said C,-C~
alkyl and C,-Ce alkyl may be substituted by one or two of fluoro, chloro,
hydroxy,
amino, methylamino, dimethyiamino or acetyl; and
R4 is phenyl, naphthyl, thienyl, benzothienyl, pyridyl) quinolyl, pyn3zinolyl,
pyrimidyl, imidazoiyl, furanyl, benzofuranyl, benzothiazolyt, isothiazolyl,
benzoisothiazolyl, thiazolyi) isoxazolyl, benzisoxazolyl, benzimidazolyl,
triazolyf,
pyrazolyi, pyrrolyl, indolyl, azaindoiyl, benzoxazolyl, oxazolyl,
pyrrolidinyl, thiazolidinyl,
morpholinyi, pyridinyl) tetrazolyl, or 3 to 8-membered cycloalkyi or 9 to 12-
membered
bicycloalkyl, optionally containing one to three of O, S or N-Z wherein Z is
hydrogen)
C,-C4 alkyl, C,-C,~ alkanoyl) phenyl or phenyimethyi, wherein each of the
above groups
may be substituted independently by from one to three of fluoro, chloro,
bromo,
trifluoromethyl, C,-Ce alkyl or C,-Ca alkoxy, or one of cyano) vitro, amino,
NH(C,-C6
CA 02272138 1999-06-O1
alkyl), N(C,-C4 alkyl)(C,-Cz alkyl), COO(C,-C4 alkyl), CO(C,-C~ alkyl),
S02NH(C,-C4
alkyl), S02N(C,-C4 alkyf)(C,-C~ alkyl), SOZNHz, NH2SOs(C,-C,~ alkyl), S(C,-CQ
alkyl),
S02(C,-Ce alkyl), wherein said C,-C, alkyl and C,-CQ alkyl may be su5stituted
by one
or two of fluoro, chloro, hydroxy, amino, methytamino, dimethytamino or
acetyl;
provided that (1 ) R~ is not unsubstituted phenyl; (2) when R, is amino, R2 is
methytthio,
R4 is 2,4,6-trichiorophenyl, and A is C=O, then R, is not 2-chiorophenyf; and
(3) R, and
R~ are not both hydrogen.
More spedfic compounds of the formula I include those wherein R3 is phenyl
substituted independently with one or two of fluoro, chloro, bromo, methyl,
trifluoromethyl, vitro, C,-CQ alkyl, C,-Ce alkyioxy, S02NHz, SOZNH(C,-CQ
alkyl), SOZN(C,
CQ alkyf)Z, or R3 is primary) secondary or tertiary alkyl of from 4-9 carbon
atoms wherein
said C4 Ce alkyl may contain from one to two double or triple bonds and may be
substituted by from 1 to 3 substituents Ra which is hydroxy, amino, C,-C3
alkoxy)
dimethytamino) diethylamino, methylamino, ethylamino, NH(C=O)CH3, fluoro,
chloro,
bromo, or C,-C, thioalkyl.
More specific compounds of the formula t are those wherein A is C=O, those
wherein R, is amino) methytamino or dimethytaminQ; those wherein RZ is ethyl
or
methytthio and those wherein R4 is 2,4,6-trichlorophenyt,
2,4,&trimethylphenyl, 2,6-
dichforo-4-trifluoromethyiphenyl or 4-bromo-2,6-dimethyiphenyl.
More specfic compounds of formula 1 further include those wherein R, is phenyl
which may be substituted at positions 2 or 5 with one ar two of methyl, C2 Ce
straight-
chain or branched alkyl, trifluoromethyl, fluoro, chloro, bromo or vitro,
those wherein
A and R, together form a pyrimidine ring, such that the bicydic structure
formed is
pyrazolo[3,4-d]pyrimidine, and R5 is substituted at the 6 position; and those
wherein R3
is phenyl substituted independently with one or two of fluoro) d~toro, bromo,
methyl,
trifluoromethyl, vitro, C,-Ce alkyl, C,-Ce alkyloxy) SOzNHZ, SOZNH(C,-Ce
alkyl), or
SOZN(C,-Caslkyl)Z, R, is 2,4,trtrichlorophenyl, 2,4,6-trtmethytptrenyl, 2.6-
dichloro-4-
trifluoromethyfphenyl or 4-bromo-2,6-dimethylphenyl, and RZ is methytthio,
methyl or
ethyl.
. More sped6c compounds of formula I also include those wherein R, is phenyl
substituted independently with one or two of fluoro, chloro, bromo, methyl)
trifluoromethyl, vitro, C,-Ca alkyl, C,-Ca alkyloxy, SOzNHZ, SOzNH(C,-CQ
alkyl),
S02N(C,-CQ alkyl)Z, or R3 is primary, secondary or tertiary alkyl of firom 4-9
carbon
CA 02272138 1999-06-O1
-4 -
atoms, wherein the C4-C9 alkyl may contain from one to two
double or triple bonds and may be substituted by from 1 to 3
substituents R6 which is hydroxyl, amino C1-C3 alkoxy,
dimethylamino, diethylamino, methylamino, ethylamino,
NH(C=O)CH3, fluoro, chloro, bromo or C1-C3 thioalkyl; R4 is
2,4,6-trichlorophenyl, 2,4,6-trimethylphenyl, 2,6-dichloro-4-
trifluoromethylphenyl or 4-bromo-2,6-dimethylphenyl; R1 is
amino, methylamino or dimethylamino; and R2 is methylthio or
ethyl.
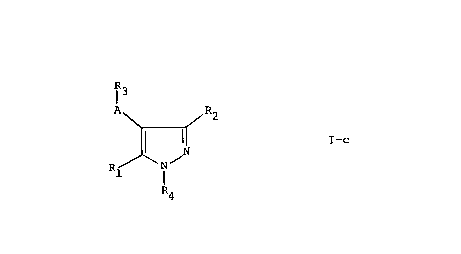
Claimed in this divisional application are those
compounds of the formula:
R3
I
A R2
I-c
\N/
Rl I
R4
and pharmaceutically acceptable salts thereof, wherein A and Rl
together with the carbons to which they are attached form
pyrimidinyl or 5-pyridyl which may be substituted as defined
above; and R2, R3 and R4 are as defined above.
The invention also relates to a composition for the
treatment of illnesses induced or facilitated by corticotropin
releasing factor which comprises a compound of the formula I
as defined above or the known compound of formula I wherein A
is C=O, R1 is amino, R2 is methylthio, R3 is 2-chlorophenyl,
and R4 is 2,4,6-trichlorophenyl, in an amount effective in the
treatment of said illnesses, and a pharmaceutically acceptable
carrier, and to a composition for the treatment of inflammatory
disorders, stress and anxiety related disorders including
stress-induced depression and headache, abdominal bowel
syndrome, immune suppression, HIV infections, Alzheimer's
disease, gastrointestinal diseases, anorexia nervosa,
hemorrhagic stress, drug and alochol withdrawal symptoms, drug
64680-810E
CA 02272138 1999-06-O1
-4a-
addiction, and fertility problems, which comprises a compound
of the formula I as well as the known compound, both as defined
above in an amount effective in the treatment of said disorders,
and a pharmaceutically acceptable carrier. More specific and
most preferred compositions for the treatment of such illnesses
and disorders comprise a more specific and most preferred
compound of formula I as defined above.
64680-810E
CA 02272138 1999-06-O1
The invention further includes a method for the treatment of illnesses induced
or facilitated by corticotropin releasing factor by administering to a subject
in need of
such treatment a compound of formula I or the known compound, both as defined
above, and a method for the treatment of stress and anxiety related disorders,
including
stress-induced depression and headache, abdominal bowel syndrome, inflammatory
disorders, immune suppression, HIV infections) Alzheimer's disease,
gastrointestinal
diseases, anorexia nervosa) hemorrhagic stress, drug and alcohol withdrawal
symptoms, drug addiction, and fertility problems) particularly depression, by
administering to a subject in need of such treatment a compound of formula I
as well
as the known compound, both as defined above. More spedfic and most preferred
methods for the treatment of such illnesses and disorders comprise a more
specfic and
most preferred compound of formula I as described above.
Whenever reference is made herein to C,-Ce alkyl) a straight or branched chain
alkyl of one to six carbon atoms is meant, such as methyl, ethyl) isopropyl or
hexyl.
Whenever reference is made herein to C,-Ce alkyl, in the definition of R5 and
R"
this indudes unsaturated Ci Ce alkyl, such as Ci Ce alkyl having one double or
triple
bond, C3 Ca alkyl having two double bonds, and C4 Ca alkyl having two triple
bonds.
Whenever reference is made hereafter to a compound of formula I, this includes
the known compound of formula I as described above.
Whenever R3 is a heterocyclic group, the attachment to A, defined above, is
through one of the carbons in the heterocyciic group. Similarly, when R4 is a
heterocydic group, the attachment to the nitrogen in the pyrazofe ring is
through one
of the carbons in the heterocyclic group.
Whenever reference is made herein to 3- to &membered cydoalkyl or 9- to 12
membered bicydoalkyl containing one to three of O, S or N-Z, It is understood
that the
oxygen and sulfur ring atoms are not adjacent to each other.
The compounds of the formula 1 wherein R, is amino or C,-Ce alkyl, and RZ is
methytthio, having the formula II .(not shown), may be prepared from a
compound of
the formula
CA 02272138 1999-06-O1
-6-
R3- q -C -Rio
II III
C
I
<SCH3)2
wherein R,o is cyano or C(O)(C,-CQ alkyl) and A and R3 are as defined above
with
reference to formula I, by reaction with a compound of the formula
R4-NHNH2 I V
wherein R4 is as defined with reference to formula 1. This reaction is
generally carried
out in a polar solvent, such as a C,-Ca alcohol. The reaction temperature
generally
ranges from about 20 ° C to about 160 ° C, and is conveniently
the refiux temperature of
the reaction mixture.
The compounds of formula III may be prepared by treating a compound of the
formula
Ra_R-CH2Rio V
with a base such as sodium hydride, in the presence of carbon disulfide
followed by
reaction of the formed intermediate with methyl iodide in a reaction solvent
such as
dimethylsuffoxide.
The compounds of formula IV are readily available or may be obtained by
methods known in the art.
The compounds of formula V may be prepared by known methods.
The compounds of the formula I wherein Rz is alkoxy) amino, or mono- or
disubstituted amino may be prepared by using the above procedure with R4NHNH2
from the con-esponding compounds of the formula
Ra-A-C-Rlo R3-p-C-Rio
IC 0 R
. (OR) . (NR~R~~)2
2
CA 02272138 1999-06-O1
_7_
wherein A and R, are as defined with reference to formula I, R, o is as
defined with
reference to formula III, and R, R' and R" are each hydrogen or C,-CQ alkyl in
accordance with the definition of Rz above.
The compounds of the formula I wherein R, is C,-Ce alkoxy or C,-CQ alkylthio
and RZ is C,-Ce alkyl may be prepared from a compound of the formula
R3
z
IX
R
i
Ra
wherein RX is chloro or bromo, RZ is C,-CQ alkyl, and R,, R4 and A are as
defined above
with reference to formula l) with a C,-Ce alcohol or C,-Cs mercaptan in the
presence of
a base. The reaction is generally carried out in a polar solvent such as
ethanol or t-
butanol at temperatures from about 20°C to about 160°C and
conveniently room
temperature.
The compounds of the formula IX may be prepared by treating a compound of
the formula
R
~3
R R2
~N X
H 0 ~N~
Ra
with a halogenating agent such as thiortyl chloride or bromide, or phosphorous
oxychloride or pentachloride, or phosphorous oxybromide or pentabromide. The
reaction may be carried out without a solvent or in an aprotic solvent such as
methylene chloride, or dichloroethane at temperatures of about 0 ° C to
about 100 ° C.
Compounds of formula X may be prepared by treating compounds of the
formula
CA 02272138 1999-06-O1
-8-
R2
II XI
/N
N
R4
with an activated derivative of a carboxylic or sulfonic acid of the formula
R,AOH, such
as an acid chloride of the formula R3ACl wherein R, and A are as defined with
reference
to fom~uia i, in the presence of calcium hydroxide in an aprotic solvent such
as dioxane
as described in ,lensen, Acta Chem. Scand., 13, 1668-1670 (1959) at
temperatures of
from about 20°C to about 100°C. Compounds of the formula XI are
known in the art.
The compounds of the formuta f wherein R, is C,-Ce alkoxy or C,-CQ alkyithio
and R2 is C,-Ce alkylthio may be prepared from a compound of the formula
R3
A R2
XII
R Y \N/
Ra
wherein R" is chloro or bromo and R3, R4 and A are as defined above with
reference
to formula I, with n C,-CQ alcohol or C,-Ce mercaptan in the presence of a
base. The
reaction is generally carried out in a polar organic solvent such as ethanol-
or t-butanol
at temperatures firom about 20 ° C to about 160 ° C,
conveniently room temperature.
The compounds of the formula Xil may be prepared from a compound of the
formula
R3
R2
so XIII
H
i
Ra
CA 02272138 1999-06-O1
-g-
by reaction with a hafogenating agent such as thionyl chloride or bromide, or
phosphorous oxychloride or pentachloride, or phosphorous oxybromide or
pentabromide. The reaction may be carried out with a solvent or in ari aprotic
solvent
such as methylene chloride or dichloroethane at temperatures of about
0°C to about
100°C.
The compounds of the formula Xiil may be prepared by treating a compound
of the formula
R2
ll xIV
/N
N
R4
with activated benzoic or sutfonic acid derivatives) conveniently an acid
chloride, in the
presence of calcium hydroxide in an aprotic solvent such as dioxane as
described in
the above reference by Jensen.
The compounds of the formula XIV may be prepared by treating a compound
of the formula
R
z
II XV
/N
N
R4
wherein Rz is chloro, bromo with a C,-Ce mercaptan in the presence of a base.
The
reaction is generally carried out in a potar organic solvent such as t-butanol
at
temperatures from about 20 ° C to about .160 ° C, convenierttiy
the reflux temperatures
of the reaction mixture.
The compounds of the formula XV may be prepared from compounds of the
formula
CA 02272138 1999-06-O1
-10-
OH
II XV I
/N
N
R4
with a halogenating agent such a thionyl chloride or bromide, or phosphorous
oxychloride or pentachioride, or phosphorous oxybromide or pentabromide. The
reaction may be carried out without a solvent or in an aprotic solvent such as
methylene chloride or dichioroethane at temperatures of about 0 ° C to
about 100 ° C.
The compounds of the formula I wherein R, is C,-Ca alkoxy or C,-Ca alkylthio
and R2 is C,-CQ alkoxy may be prepared from a compound of the formula
R3
A Rz
XVII
Rxx \N/
Ra
wherein R,~,~ is chloro or bromo, R2 is C,-CQ alkoxy and R,, R4 and A are as
defined
above with reference to formula I, with a C,-Ca alcohol or C,-CQ mercaptan in
the
presence of a base. The reaction is generally carried out in a polar organic
solvent
such as ethanol or t-butanol at temperatures from about 20°C to about
160C)
conveniently the refiux temperature of the reaction mixture.
The compounds of the formula XVII may be prepared from a compound of the
formula
R3
A R2
~ IN X V I I I
H 0 \N/
i
Ra
CA 02272138 1999-06-O1
-11-
by reaction with a hslogenating agent such as thionyl chloride or bromide, or
phosphorous oxychloride or pentachloride, or phosphorous oxybromide or
pentabromide. The reaction may be carried out without a solvent or in an
aprotic
solvent such as methylene chloride, or dichloroethane at temperatures of about
0 ° C
to about 100°C.
The compounds of the formula XVIII may be prepared by treating a compound
of the formula
R2
1o II XIX
/N
N
Ra
with an activated derivative of a carboxylic or sulfonic acid of the fomnula
R3AOH,
conveniently an acid chloride of the formula R3ACI wherein R, and A are as
defined
above with reference to formula I) in the presence of calcium hydroxide in an
aprotic
solvent such a dioxane as described by Jensen in the reference cited above.
The compounds of the formula XIX may be prepared by treating a compound
of the above formula XV with an alcohol in the presence of a base. The
reaction is
generally carried out in a polar organic solvent such as ethanol at
temperatures from
about 20 ° C to about 160 ° C, conveniently the reflux
temperatures of the reaction
mixture.
The compounds of the formula I wherein R, is amino and R= is O(C,-Caalkyl)
may be prepared by reacting a compound of the formula
R 3
A 0(C1-C6 alkyl)
0 I I~ XX
/N
. / ~ R
4
wherein R,, R4 and A are as defined above with reference to formula i, with
hydrazine
in a solvent such as a C,-CQ alcohol, conveniently at the boiling point of the
solvent.
CA 02272138 1999-06-O1
-12-
The compounds of the formula XX may be prepared by treating a compound
of the formula
R3
A OH
0 I IN X X I
R
4
wherein R3, R4 and A are as defined above with reference to formula I, with an
alkylating
agent such as di(C,-Ce alkyl) sulfate, and a base such as sodium hydride, in a
solvent
such as dimethylsutfoxide.
The compounds of the formula XXI may be prepared by treating a compound
of the formula
OH
0
XXII
I
Ra
'0
with an activated derivative of a carboxylic or sutfonic acid of the formula
R,AOH such
as an acid chloride of the formula R3ACI, wherein R3 and A are as defined with
reference to formula I, in the presence of a lewis acid such as aluminum
chloride in an
aprotic solvent such as methylene chloride, dichloroethane, or
tetrachloroethane, at
temperatures of about 0°C to about 150°C.
The compounds of the formula XXII may be prepared by treatment of a
compound of the formula
CA 02272138 1999-06-O1
-13-
OH
XXIII
H 2 N \N/
R4
with phthalic anhydride in acetic acid at the boiling point of the solvent.
The compounds of the formula XXlll may be prepared by contacting cyanoacetyl
chloride with R4NHNHZ in the presence of a base followed by heating the
resulting
hydrazide at refiux in alcoholic solution in the presence of a base.
The compounds of formula I wherein A and R, are taken together to form
pyrimidinyl have the fomnula
R3
,, r ,R2
iN XXIV
R 5 \N N~
Ra
wherein Rz, R3, R4, and RS are as defined above with reference to formula I.
These
compounds may be prepared by cyclization of a compound of the above formula I
wherein A is C=O and R, is amino with a compound of the formula
0
I t
R5-C-NH2 XXV
wherein RS is as defined with reference to formula I. This reaction is
generally carried
out at 100 to 250°C, and conveniently at the reflux temperature of the
compound XXV.
The compounds of formula I wherein A and R, are taken together to form 5-
pyridyl have the formula
CA 02272138 1999-06-O1
-14-
R3
R2
I IN XXV I
\N/
R5 I
Ra
wherein R2, R3, R, and RS are as defined with reference to tonnula 1. These
compounds
may be prepared as shown in Reaction Scheme 1.
Reaction Scheme 1
0
0 0
R3-C-CH3 + CzH50C-CH2-CH-
R5
XXVII XXVIII
R3-C-CH2-C-CHz-CHRS-
0 0
XXIX
CA 02272138 1999-06-O1
-15-
13 ~ 15
_/G ~ ,CH
~~C~CH2 ~N --.
I I
,,,.~~ o'
H3C0 R2
XxX
Ra R2 Ra
~C ~ Re
H
0 R5'N'- CH2 N/ ~ R5 /N XXVI
0 R< II
Ra
XXXI XXXII
The compounds of formula XXIX are prepared by reacting a ketone of the
formula XXVII with a compound of the formula XXVIII in a suitable solvent such
as
tetrahydrofurane in the presence of a base such as sodium hydride. The
reaction is
conveniently carried out at the refiux temperature of the reaction mixture.
The compound XXIX is reacted with a compound of the formula RzC(OCH3), to
form the compound XXX. The reaction is carried out in a suitable solvent such
as ethyl
acetate, conveniently at the reflex temperature of the reaction mixture. The
wavy line
--- in formula XXX indicates that either isomer of this compound is included,
in
accordance with accepted convention for indicating stereoisomers.
The compound XXXI is prepared by reacting compound XXX with a hydrazine
of the formula H2NNHR~ wherein R, is as defined with reference to formula I.
The
reaction is carried out in a suitable solvent such as ethanol, conveniently at
the reflex
temperature of the reaction mixture.
The compounds of formula XXVI wherein R5 is linked to position 6 is formed by
first reacting compound XXXI with hydrazine hydrate in a suitable solvent such
as
ethanol, conveniently at the reflex temperature of the reaction mixture. The
compound
XXXII is separated from precipitated phthalhydrazide and taken up in an
organic solvent
CA 02272138 1999-06-O1
-16-
such as toluene. The compound XXVI is formed by dehydrogenation of compound
X)OCII with palladium over carbon.
Reaction Scheme 1 shows the preparation of compounds XXVl~wherein R5 is in
the 6-position. A similar reaction sequence may be followed to prepare
compounds
XXVI wherein RS is in the 7-position by replacing compound XXVIII by a
compound of
the formula
0
0
C2H50C-CH-CH2-N XXX I I I
0
The compounds of formula I wherein A is C=O and R, and Rz are the same
group R, may be prepared by reacting a B-ketone of the formula
0 0
II II
R7 C~CH2~C~R7
with the hydrazine of the formula IV as defined above to form a pyrazole
compound of
the formula
R7
R 7 \N/
Ra
The reaction proceeds at reflux in an appropriate solvent such as ethanol.
After
bromination of the pyrazole compound, e.g. with bromine in acetic acid, to
fom~ the
corresponding 4-bromo derivative and conventional metallation, e.g, with t-
butyl lithium,
at -78°C in tetrahydrofuran, a suitably activated R3 carboxylic acid
such as the acid
chloride R,C(O)CI is added to give the desired compound I.
CA 02272138 1999-06-O1
7-
The compounds of formula 1 wherein A is C=O and R, and RZ are not the same)
and wherein R, or Rs is attached through a CZH4 fragment) may be prepared from
a
pyranone of the fomnuia
0 0
ii
,,/~,, xxxtv
Rii ~z
wherein R, is as defined above, and Rz is as defined above when R" is C; Ca
alkyl
which may be substituted by 1 to 3 of Re, or R2 is R, when R" is C; Ca alkyl
which may
be substituted by one to three of hydroxy, amino) carboxy) amido, NH(C=O)(C,-
Ca
alkyl), N(C,-Ce alkyl)(C,-Ca alkyl), (C=O)O(C,-Ca), C,-C3 alkoxy, C,-C3
thioslkyl) fluoro)
bromo, chloro, iodo, cyano or vitro. The compound XXXIV is reacted with a
hydrazine
of the formula HZNNHR, wherein R4 is as defined above to form compounds of the
formulae
~J~ ~ 3 ~ 3
~C CH2CH(OH)R11 ~C Rz
and ~ IN
R~ N/ R11-CH- N/
H2
Ra OH Ra
which on dehydration and hydrogenation result in compounds of the formulae
3 ~ 3
~C C2H4R11 ~C Rz
and
N N
R2 N/ R11H4C2 N/
Ra R4
The compounds of the formula I wherein A is C=O and R2 is O(C,-CQ alkyl) may
be prepared by reacting a hydrazine of the formula R4NHNHZ with a compound of
the
fomlula (A) in n suitable solvent
CA 02272138 1999-06-O1
-18-
OH
~0
C H 3 ~ IN
CH Ri N~
3
Ra
(R) (B>
such as THF or methylene chloride and cyclization of the resulting hydrazide
with heat
to give the intermediate (B). This compound may be reacted with an activated
carboxyclic acid derivative such as the acid chloride R3(C=O)CI in the
presence of a
Lewis acid such as aluminum trichloride in a solvent such as ethylene
dichloride at
temperatures of from about -10 ° C to about 80 ° C. The formed
compound of formula
I wherein RZ is hydroxy may be reacted with (C,-Ce alkyl)L wherein L is a
leaving group
such as chloro) bromo, or tosylate and C,-Ca alkyl may be substituted in
accordance
with the substituents in the definition of R2.
Those compounds of formula I wherein R, is C,-Ce alkylamino or di(C,-Ce
alkyl)amino may be prepared from corresponding compounds of fomnula I wherein
R,
is amino. When R) is me~thylamino or dimethylamino, reaction is with a
methyfating
agent such as methyl iodide. When R, is Cz-Ce alkylamino or di(CZ Ce
alkyl)amino,
reaction is with an alkylating agent such as Cz-Ca alkyl-L wherein L is a
leaving group
such as chloro, bromo) tosyiate, or mesylate. Both the methyiation and the Ci
Ce
alkylation is in the presence of a base such as sodium hydride and a solvent
such as
tetrahydrofuran, dimethyl fonnamide or dimethyl suifoxide.
The acid addition salts are prepared in a conventional manner by treating a
solution or suspension of the free base of formula I with one chemical
equivalent of a
pharmaceutically acceptable acid. Conventional concentration or
crystallization
techniques are employed in isolating the salts. Illustrative of suitable acids
are acetic,
lactic, succinic, malefic) tartaric, citric, giuconic, ascorbic, benzoic)
cinnamic, fumaric,
sulfuric, phosphoric, hydrochloric, hydrobromic, hydroiodic, sulfamic,
sutfonic acids
such as methanesutfonic, benzene suffonic, p-toluenesutfonic, and related
acids.
CA 02272138 1999-06-O1
-19-
The compound of the invention may be administered alone or in combination
with pharmaceutically acceptable carriers, in either single or multiple doses.
Suitable
pharmaceutical carriers include inert solid diluents or fillers, sterile
aqueous solution and
various organic solvents. The pharmaceutical compositions formed by combining
the
novel compounds of formula I and the pharmaceutically acceptable carriers are
then
readily administered in a variety of dosage forms such as tablets, powders,
lozenges,
syrups, injectable solutions and the like. These pharmaceutical compositions
can, if
desired, contain additional ingredients such as flavorings, binders,
excipients and the
like. Thus, for purposes of oral administration, tablets containing various
excipients
such as sodium citrate, calcium carbonate and calcium phosphate may be
employed
along with various disintegn~nts such as starch, alginic acid and certain
complex
silicates, together with binding agents such as poiyvinyipyrrolidone, sucrose,
gelatin
and acacia. Additionally, lubricating agents such as magnesium stesrate,
sodium fauryi
sulfate and talc are often useful for tabletting purposes. Solid compositions
of a similar
type may also be employed as fillers in soft and hard filled gelatin capsules.
Preferred
materials for this include lactose or milk sugar and high molecular weight
polyethylene
glycols. When aqueous suspensions or elixirs are desired for oral
administration, the
essential active ingredient therein may be combined with various sweetening or
flavoring agents, coloring matter or dyes and, if desired, emulsifying or
suspending
agents, together with diluents such as water, ethanol, propylene glycol,
glycerin and
combinations thereof.
For parenteral administration, solutions of the novel compound of formula ! in
sesame or peanut oil, aqueous propylene glycol, or in sterile aqueous solution
may be
employed. Such aqueous solutions should be suitably buffered if necessary and
the
liquid diluent first rendered isotonic with sufficient saline or glucose.
These particular
aqueous solutions are especially suitable for intravenous, intramuscular,
subcutaneous
and intraperitoneal administration. The sterile aqueous media employed are all
readily
available by standard techniques known to those skilled in the art.
Additionally, it is possible to administer the compounds of the present
invention
topically when treating inflammatory conditions of the skin $nd this may be
done by
way of creams, jellies, gels, pastes and ointments, in accordance with
standard
pharmaceutical practice.
CA 02272138 1999-06-O1
The effective dosage for the compound of formula I
depends on the intended route of administration and other
factors such as age and weight of the patient, as generally
known to a physician. The dosage also depends on the illness
to be treated, although in general the daily dosage will range
from about 0.1 to 50 mg/kg of the body weight of the patient.
More specifically, the daily dosage for stress-induced
illnesses will generally range from about 0.1 to 50 mg/kg of
the body weight of the patient to be treated, for treatment of
inflammatory diseases aboutØ1 to about 100 mg/kg will be
needed, for Alzheimer's disease, about 0.1 to about 50 mg/kg,
as well as for gastrointestinal diseases, anorexia nervosa,
hemorrhagic stress, and drug and alcohol withdrawal symptoms.
The pharmaceutical composition may be put in a
commercial package for practical use. Such commercial package
normally comprises a container in which the composition is
contained. The commercial package usually also comprises a
written matter which says that the pharmaceutical composition
can or should be used for the treatment described in this
specification.
The methods far testing the compounds for formula I
for their CRF antagonist activity are according to the
procedures of Endocrinology, 116, 1653-1659(1985) and
Peptides, 10,179-188(1985) which determine the binding
activity of a test compound to a CRF receptor. The binding
activity for the compounds of formula I generally ranges from
about 0.2 nanomolar to about 10 micromolar.
64680-810
CA 02272138 1999-06-O1
The following abbreviations are used in the Examples=
Ph=phenyl; iPr-isopropyl; HRMS=high resolution mass spectrum.
Example 1
A. 2-Bromo-2',5'-dimethylacetonhenone.
A mixture of 10.60 g (0.10 mol) of para-xylene and
16.53 g (0.105 mol) of a-bromoacetyl chloride 1n 300 mL of
1,2-dichloroethane was cooled in an ice bath under an
atmosphere of dry N2 and treated portion wise with 14.15 g
(0.106 mol) of aluminum chloride. The reaction mixture was
stirred for 30 minutes at 0-5°C and then for 2.5 hours at room
temperature. The mixture was then poured onto ice and the
aqueous layer was acidified with concentrated HC1. The
organic layer was separated and the aqueous layer was
extracted twice with methylene dichloride. The combined
organic extracts were dried with brine solution and With
magnesium sulfate. The solvent was evaporated to give 23.87 g
of an amber oil which was for use in the next reaction without
further purification.
B. 2-Cvano-2',5'-dimethy_lacetophenone.
The product of the above reaction (approximately 0.10
mol) was dissolved in 300 mL of ethanol and was treated with a
solution of 16.25 g (0.25 mol) of potassium cyanide in 30 mL
of water and the resulting mixture was refluxed for 90
minutes. After
-20a-
64680-810
CA 02272138 1999-06-O1
-21-
cooling) the ethanol was stripped from the mixture on the rotary evaporator
and the
residues were made slightly acidic with concentrated hydrogen chloride. The
product
was extracted into ethyl acetate using precautions to avoid escape of hydrogen
cyanide. The organic extracts were dried with brine and with magnesium sulfate
and
evaporated to a gummy semi-solid. This was triturated repeatedly with hot
hexane
which, on cooling, deposited needles to give the desired product, 8.50 g (4996
for the
two reactions), m.p. 75 - 76°C.
C. 3,3-Bis-methytthio-2-(2.5-dimethylbenzoyll-acrylonitrile.
A solution of 4.96 g (28.6 mmol) of 2-cyano-2',5'-dimethylacetophenone in 120
mL of dry dimethyl sulfoxide and 3.43 mL (57.3 mmol) of carbon disulfide in a
flame-
dried 3-neck round bottom flask under dry nitrogen was stirred at 15 -18
° C while 1.41
g (58.7 mmoi) of oil free sodium hydride was added in 5 portions. The
resulting deep
red solution was stirred for 1 hour at 18 ° C and then cooled to 15
° C whereupon 3.92
mL (8.95 g, 63.0 mmol) of methyl iodide was added dropwise. The temperature
rose
to about 22 ° C during the addition. After stirring for 2 hours at room
temperature, the
reaction mixture was poured into cold water and the aqueous layer was
extracted three
times with ethyl acetate. The combined extracts were washed three times with
water
and then dried with brine and magnesium sulfate) Evaporation gave 8.96 g of
the title
compound as a heavy on3nge oil which crystallized in the refrigerator. The
analytical
sample crystallized from ethanol, m.p. 74.5 - 75.5°C.
D. 5-Amino-1-(2.6~iichloro-4-trilluoromethylahenH)-4-12.5~iimethyfbenzoyil~-
methvithioavrazole.
A suspension of 7.94 g (28.6 mmol) of the product of step C and 7.01 g (28.6
mmol) of 2,6-dichioro-4-trifluoromethylphenylhydn3zine in 100 mL of ethanol
was heated
at reflux for 2 hours, solution occurring as the reactjon mixture was warmed:
Then the
ethanol was mostly removed on the rotary evaporator and the residues were
partitioned
between dilute aqueous hydrogen chloride solution and ethyl acetate. The
organic
phase was washed once with water and with brine, then dried with magnesium
sulfate
and treated with decoforizing carbon. The flftered solution was evaporated and
the
residues crystallized from 10:1 hexane/ethyl acetate to give 12.00 g (8896) of
the title
product in two crops, m.p. 130 - 132°C.
CA 02272138 1999-06-O1
' -22-
Example 2
5-Methyiamino-4.-t2-chlorobenzoyl)-3-methylthio-l-(2.4.6-
trichlorophenyl)pYrazole
and 5-dime~thytamino-4-(2-chtorobenzoyll-3-methvtthio-1-(2.4.6-
trichlor~phenyf)-
pyrazole.
A mixture of 0.50 g (1.16 mmol) of 5-amino-4-(2-chlorobenzoyl}-3-methytthio-1-
(2,4,6-trichlorophenyi)pyrazole in 5 mL of tetrahydrofuran was treated with 50
mg (1.16
mmol} of sodium hydride and stirred at room temperature for 30 minutes. Then
0.75
ml (1.71 g, 12.0 mmol) of methyl iodide was added dropwise and the reaction
mixture
was stirred for 60 minutes at room temperature. The reaction mixture was then
quenched with water and the products were extracted into ethyl acetate.
Concentration
of the dried solution and chromatography on silica gel with mixture of hexane
and ethyl
acetate gave the less polar dimethylamino title compound (300 mg, 5496) as a
white
foam. Anal. Calcd. for C,eH~50N3SCl,: C, 48.02; H, 3.18; N, 8.88. Found: C,
47.84;
H, 3.09; N, 9.01.
The more polar monomethyl title compound was isolated from the column in like
manner as a white foam (34 mg, 696). Anal. Calcd. for C,BH"ON3SCI4: C, 46.88;
H,
2.84; N, 9.11. Found: C, 46.54; H, 2.89; N, 9.07.
Example 3
5-Amino-4-(2-methoxybenzoyl)-3-methytthio-1-(2.4.6-trichloroahenyi pyrazole.
To a solution of 2-methoxyphenylmagnesium bromide prepared from 18.7 g
(0.10 mol) of 2-bromoanisole and 2.43 g (0.10 mol) of magnesium turnings in
ether
under dry nitrogen was added 1.6 g (5.0 mmol) of 5-amino-4-cyano-3-methytthio-
1-
(2,4,6-trichlorophenyl)pyrazoie and the resulting mixture was stirred and
refluxed for 16
hours. Upon cooling, the reaction was decomposed with 50 mL of saturated
ammonium chloride solution. The organic phase was extracted with aqueous
hydrogen
chloride and the acidic extract was treated with 10 mL of concentrated
hydrogen
chloride and heated at 80 - 90 ° C for 10 minutes after which the
mixture was cooled and
made alkaline. Extraction with methylene dichloride and chromatography of the
extracts with mixtures of hexane and ethyl acetate gave 313 mg (1496) of the
title
compound, m.p. 200-202 ° C. Anal. Calcd, for C, ~H,4OzN,SCI,: C) 48.82;
H, 3.18; N,
9.49. Found: C, 48.54; H, 3.32; N, 9.09.
CA 02272138 1999-06-O1
-23-
Example 4
A. 5-Amino-3-methyl-1-(2.4,6-trichloroohenvi)p~razole.
To a solution of 0.51 g (22.0 mmol) of sodium in methanol was added 1.66 g
(20.0 mmol) of 5-methylisoxazofe. The reaction mixture as refiuxed for 8 hours
and
then stirred overnight at room temperature. Then 4.23 g (20.0 mmol) of 2,4,6
trichlorophenylhydrazine was added and the reaction mixture was again refluxed
for 4
hours. A second portion of sodium in methanol was added and reflux was
continued
for 24 hours. The reaction mixture was taken up with ether and dilute hydrogen
chloride. The organic extracts were washed with dilute hydrogen chloride and
brine,
and then dried with magnesium sulfate and evaporated to give crystals, m.p.
132-
134 ° C. Analysis of this material, particularly two CN bands in the IR
spectrum at 2190
cm-' and 2250 cm'' , revealed it to be a mixture of the cis- and tn3ns-isomers
of 1-
cyanoacetone-2,4,6-trichiorophenyfhydrazone. This material was suspended in
methanol and treated with 10.0 mmol of sodium methoxide in 5 mL of methanol.
After
5 minutes at room temperature, water was added to crystallize the product
which was
filtered off and washed well with water. After sir drying, the product weighed
2.21 g
(4096) and melted at 134.0-135.5 ° C. Despite the similarity in melting
points, the latter
material was distinctly different from the former, having an R, of 0.67 vs.
0.78 for the
intermediate on silica get TLC plates developed with 1:1 hexane ethyl acetate
and a
distinctly different 300 MHz proton NMR spectrum.
B. 5-(2-Chlorobenzamido)-4-(2-chlorobenzoyl~-3-methyl-1-(2.4.6-
trichlorophenyl)pyrazofe.
A suspension of 2.34 g (17.50 mmol) of aluminum trichloride in 20 mL of
1,1,2,2-
tetrachloroethane was treated with 2.02 mL (2.78 g, 15.9 mmol) of 2-
chlorobenzoyl
chloride and the resulting solution was stirred for 20 minutes at room
temperature.
Then 2.00 g (7.23 mmol) of the product of Step A was added and the reaction
mixture
was reiiuxed for 16 hours. The cooled reaction mixture was poured over ice and
the
insolubles were littered off and washed with ethyl acetate. The organic layer
was
separated and the aqueous was washed twice with ethyl acetate. The combined
organic layers were dried over magnesium sulfate and evaporated. The residues
were
chromatographed on silica gel, eluting with 4:1 hexane ethyl acetate to give
2.05 g
(5196) of the title product, an amorphous foam. Anal. Calcd. for
C~'H,,~O~N3Cl5: C,
52.06; H, 2.55; N, 7.59. Found: C, 52.11; H, 2.57; N, 7.27.
CA 02272138 1999-06-O1
-24-
C. 5-Amino-4-(2-chforobenzoyll~-methyl-1-(2.4.6-trichlorophenyilpyrazole.
A solution of 1.94 g (3.50 mmol of the product of Step B in 20 mL of glacial
acetic acid was treated with 20 mL of 4896 hydrogen bromide and stirred at
reflux for
8 hours. The cooled reaction mixture was treated with water to crystallize the
product
which was separated by flttration, washed with water and air dried to give the
title
product, 1.45 g (10096)) m.p. softens about 210°C and melts at
222°C. Anal. Calcd.
for C"H" ONsCl4: 412.9656. Found: 412.9722.
F~cample 5
5-Methylamino~-(2-chlorobenzoyll~-methyl-1-(2.4.6-trichtorophenyl)pyrazafe and
5-dimethyiamino-4.-!2-chlorobenzo~rl)-3-methyl-1-l2.4,6-
trichlorophe~l)pyrazole.
A solution of 0.208 g (0.5 mmol) of the compound of Example 4C in 20 mL of
tetrahydrofuran (THF) was stirred in an ice/water bath while 5.0 mL of 1.0 M
sodium
hexamethyfdisilazide in THF was added followed by 0.5 mL (1.14 g, 8 mmol) of
methyl
iodide. The reaction mixture was then stirred overnight at room temperature.
The
reaction mixture was poured into water and the products were extracted into
ethyl
acetate, dried and concentrated. The residues were chromatographed on silica
gel
using 5:1 hexan%thyl acetate as eluent to give the less polar dimethyfamino
title
compound, 52 mg (2396)) m.p. 10&109°C (ether/pentane).
The more polar product likewise crystallized from etheNpentane to give 39 mg
(1896) of the monomethylamino title compound, m.p. 174-175°C.
Example 6
5-amino-1-12.6-dichloro-4-trifluoromethylohenvll-4-(2.5-dimethylbenzoyll-3-(n-
propyl~pYrBZOIe.
A solution of 0.52 g (3.0 mmol) of 2,5-dimethylbenzoylacetonitrile, 0.45 g
(3.0
mmol) of trimethylorthobutyrate and 0.632 g (0.58 mh 6.2 mmol) of acetic
anhydride
in 5.0 mL of ethyl acetate was refluxed overnight and then cooled. The
solvents were
removed in vacuo and the residues were dissolved in 10 mL of ethanol. One-half
of this
solution, containing 1.5 mmol of 1-cyano-1-(2,5-dimethylbenxoyl)-3-methoxy-1-
pentane
was mixed with 0.7 mL (0.51 g, 5.0 mmol) of triethylamine and 0.37 g (1.50
mmol) of
2,6-dichioro-4.-triftuoromethyiphenylhydrazine and refluxed for 2.5 hours. The
reaction
mixture was cooled and partitioned between dilute hydrogen chloride and efhyl
acetate.
The organic phase was washed with water and then dried with brine and with
magnesium sulfate. The solvent was evaporated to give an oil which was
CA 02272138 1999-06-O1
-25-
chromatographed on silica gel by the flash method eluting with 4:1
hexane/ethyl acetate
to give the title compound as an amorphous foam. Anal. Calcd. for
Cz2H2oON3CIzFj,
469.0935. Found: 469.0889.
Example 7
A. 5-Amino-3-hvdroxy-1-12.4.6-trichloroahenvl)-cvrazole.
Cyanoacetic acid (8.5 g, 0.10 mol) in 200 mL of dry ether was treated with
20.8
g (0.10 mol) of phosphorous pentachloride, warmed briefly to reflux and let
cool to
room tempen3ture at which time all of the phosphorous pentnchloride, had
dissolved.
After a small amount of insoluble material was removed by filtration, the
ether was
removed on the rotary evaporator. Then 100 mL of toluene was added and
stripped
to remove phosphorous oxytrichloride. The residual pale yellow oil was
immediately
dissolved in 50 mL of cold methylene dichloride and added to a cold suspension
of
21.15 g (0.10 mol) of 2,4,6-trichlorophenylhydrazine in 14.0 mL of
triethylamine and 100
mL of methyiene dichloride, keeping the temperature below 20 ° C by use
of an ice bath.
The reaction mixture was allowed to warm to room temperature and stirred for
one
hour. Then 500 mL of cold water was added. The precipitated solid was filtered
and
washed with water and with a little methylene dichloride to give the
intermediate 2-
cyano-N-(2,4,6-trichlorophenyl)acethydn3zide, 14.92 g (5496)) m.p. 166-
168°C. Anal.
Calcd. for CeHaON3: C, 38.81; H, 2.17; N, 15.09. Found: C, 38.83; H, 2.06; N,
14.81.
This material (14.92 g, 53 mmol) was dissolved in a solution of 2.80 g (0.12
mol)
of sodium in 200 mL of methanol and refluxed for 4 hours. After stirring
overnight at
room temperature, the methanol was mostly evaporated and the residues were
poured
into water. The aqueous layer was extracted with ether and was then acidfied
with
concentrated hydrogen chloride. The product was extracted into ethyl acetate.
The
extracts were dried with brine and magnesium sulfate and evaporated to give a
foam
which crystallized from ether to give 12.28 g (9396) of the title product,
m.p. 221 -
223 ° C. Anal. Calcd. for CeHeON,: C, 38.81; H, 2.17; N, 15.09. .Found:
C, 38.81; H,
2.16; N, 14.84.
B. 3-Hydroxy-5-ahthalimido-1-12,4.6-trichlorophenyl)pvn~zole.
A mixture of 4.50 g (18.0 mmol) of the compound of Step A and 2.81 g (19.0
mmol) of phthalic anhydride in 40 mL of glacial acetic acid was refluxed for 4
hours and
stirred overnight at room temperature. About two volumes of water were added
dropwise and the resulting solid was filtered and washed with water. The damp
solid
CA 02272138 1999-06-O1
-26-
was taken up in a little ethanol) filtered and washed with a little ethanol
and ether) and
air dried to give the title compound, 5.11 g (6996), m.p. 295 - 298 °
C(dec). Anal. Calcd.
for C"H803N,C1,: C, 49.97; H, 1.97; N, 10.28. Found: C) 49.28; H,=1.95; N,
10.06.
C. 4-(2-Chlorobenzoyll-3-hydroxy-5-phthalimido-1-(2.4.6-
trichlorophenYl, pyrazole.
Aluminum trichloride (2.34 g) 17.6 mmol) was added to a solution of 2-
chlorobenzoy) chloride in 60 mL of 1 ( 1,2,2-tetrachloroethane and the
resulting mixture
was stir-ed for 30 minutes at room temperature. Then 2.87 g of the compound of
Step
B was added all at once and the reaction mixture was refluxed overnight. The
cooled
mixture was poured into water and the aqueous phase was extracted three times
with
ethyl acetate. The organic extracts were dried with brine and magnesium
sulfate and
evaporated to give a red oil which was taken up in methanol and crystallized
to give the
title compound, 2.97 g (7796), m.p. 245 - 246°C.
D. 4-(2-Chlorobenzoyl)-3-ethoxy-5-phthalimido-1-(2.4.6-
trichloroahenyl)pyrazole.
A solution of 0.55 g (1.0 mmol) of the compound of Step C in 10 mL of dry
dimethyl sutfoxide was treated portionwise with 36 mg (1.5 mmol) of sodium
hydride
and the resulting mixture was stirred for 30 minutes at room temperature. Then
0.21
mL (0.25 g, 1.61 mmol) of diethyl sulfate was added and the reaction mixture
was
stirred for one hour at room temperature. The reaction mixture was poured into
water
and the product was extracted into ethyl acetate. The extracts were washed
with water
and dried with brine and magnesium sulfate, and evaporated to a gum. The
product
was crystallized from boiling ethanol to give the product (230 mg, 4096) as
fine crystals,
m.p. 215 - 216°C.
E. 5-Amino-4-~2-chlorobenzoyl)-3-ethoxy-1-(2.4.&trichlorophenyl)pvrazole.
A suspension of 184 mg of the compound of Step D in 10 mL of ethanol was
treated with 0.5 mL of 5596 hydrazine hydrate and refiuxed for 1 hour. Solids
in the
cooled reaction mixture were filtered off and discarded and the filtrate was
evaporated
to a gum which was triturated with ether and filtered. The filtrate was again
evaporated
to a foam which was shown to be 104 mg of the analytically pure title
compound. Anal.
Calcd. for C,sH,3O2N,Cl4: C) 48.57; H, 2.94; N, 9.44. Found, C) 48.41; H,
2.52; N, 9.43.
CA 02272138 1999-06-O1
-27-
Example 8
5-Dimethrfamino-4-l2~hlorobenzovi)~-methoxy-1-(2.4,6~richlorophenyi)pyrazole.
A solution of 60 mg (0.14 mmol) of 5-amino-4-(2-chlorobenzoyl)~-methoxy-1-
(2,4,6-trichlorophenyl) pyrazole prepared according to Example 7 in 5 mL of
dry
dimethyt sutfoxide was treated with 22 mg (0.88 mmol) of oil-free sodium
hydride to
give 8 yellow solution. After i hour at room temperature, 0.2 mL (0.46 g) 3.21
mmot)
of methyl iodide was added. After stirring for 5 hours, the reaction mixture
was poured
into water and the product was extracted into ethyl acetate. After drying with
brine and
magnesium sulfate, the solvent was removed to give the title product as a one-
spot
foam. ' H-NMR (COCt,): 2.77 (6H, s), 3.63 (3H, s)) 7.24 - 7.42 (4H, m), 7.48
(2H) s).
Example 9
A. 3.3-Bis-ethoxy-2-j3-trifluoromethylbenzoyl)-acrylonitrile.
Sodium (0.126 g, 5.5 mmol) was dissolved in 15 mL of ethanol and 20 mL of
dioxane was added followed by 1.59 g (5.0 mmol) of 3,3-bis-methytthio-2-(3-
trifluoromethylbenzoyl)-acryfonitrile and the reaction mixture was refluxed
for 4 hours
and let stir overnight at room temperature. This compound was relatively
unstable to
aqueous conditions and was not isolated as such. Instead, an aliquot of the
mixture
was stripped and the product was identified by 300 MHz proton NMR: NMR (DMSO-
dQ): 1.14 (6H, tJ = 7), 3.45 (4H, q, J = 7)) 7.44 - 8.16 (4H) m).
8. 5-Amino-1-12.6-dichloro-4-trifluoromethylphenyl)-3-ethoxy-4-(3-
trifiuoromethylbenzoy~l pyrazole.
An aliquot of the above solution of step A containing approximately two
mifiimofes of 3,3-bis-ethoxy-2-(3-trifluoromethyfbenzoyf)acrylorirtrile was
reacted with
0.49 g (2.0 mmol) of 2,6-dichforo-4.-trifluoromethylphenylhydrazine in 10 mL
of ethanol
under reflux overnight. The cooled mixture was poured into dilute hydrogen
chloride
(HCI) and the product was extracted into ethyl acetate (EtOAc), washed with
water and
brine, and dried over magnesium sulfate (MgS04). Chromatography on silica gel
with
4:1 hexane/EtOAc gave the title product, 320 mg (3196), m.p. 77 - 78 °
C from pentane.
Example 10
_ 5-Amino-1-(2.6-dichloro-4-trifluoromethyfphenyl)-4-(2,5-dimethylbenzoyl)-3-
ethoxypyrazole.
A solution of 0.26 g (1.5 mmol) of 2,5-dimethylbenzoyiacetonitrile, 0.34 mL
(0.31
g, 1.60 mmol) of tetraethylorthocarbonate and 0.30 mL (0.33 g, 3.20 mmol) of
acetic
CA 02272138 1999-06-O1
-28-
anhydride in 10 mL of EtOAc was refluxed overnight. The solvent was evaporated
and
mL of absolute ethanol was added and then stripped. The residues were
dissolved
in 10 mL of ethanol, 368 mg (1.5 mmol) of 2,6-dichloro-4-trifluofomethyiphenyl-
hydrazine and 0.7 mL (0.51 g, 5.0 mmol) of triethylamine were added, and the
mixture
5 was refluxed for 90 minutes. The mixture was poured into water, extracted
with EtOAc
and the organic extracts were washed with dilute HCI and brine, and dried over
MgS04.
Evaporation gave a gum which was chromatographed on silica gel with 4:1
hexane/EtOAc to give the title product which crystallized from pentane, 15 mg
(296),
m.p. 99 - 101 °C.
10 Example 11
1-(2.6-Dichloro-4-trifluoromethylphen~)-5-methyl-4-(3-methylbenzoyl)-3-
methylthiopvrazole.
A solution of 2.97 g (16.9 mmol) of 4-(3-methylphenyi)butane-2,4-dione and
4.04
mL (5.14 g, 67.6 mmol) of carbon disulfide in 60 mL of dry dimethy) sutfoxide
was
treated portionwise with 0.89 g (37.1 mmol) of oil free sodium hydride at 15 -
18 ° C.
After stirring 30 minutes, 2.31 mL (5.27 g) 37.1 mmol) of methyl iodide was
added
dropwise and the reaction mixture was allowed to stir at room temperature for
1 hour.
It was then poured into water and the product was extracted into ether,
backwashed
with water and dried over MgS04 to give 4.30 g (9196) of an oil which
crystallized in the
refrigerator overnight, m.p. 44 - 46 ° C. ' H-NMR (CDCl3): 2.16 (3H,
s), 2.38 (6H, s), 2.72
(3H, s), 7.26 - 7.38 (2H, m), T.58 - 7.T4 (2H, m). A mixture of 1.95 g (6.96
mmol) of 3,3-
bismethytthio-2-(3-methylbenzoyl)-2-acetyiethene and 1.71 g of 2,6-dichloro-4-
trifluoromethylphenylhydn3zine in 20 mL of ethanol was refluxed for 6 hours
and then
stirred at room temperature for 48 hours. The reaction mixture was poured into
dilute
HCI solution and the product was extracted into EtOAc. The solution was dried
and
concentrated and the residues were chromatographed on silica gel with 10:1
hexane/EtOAc to give the title product which crystallized from pentane, 1.67 g
(5296),
m.p. 103 - 104°C.
Example 12
. 5-Amino-1-(2.6-dichloro-4-trifluorometylphenyll-4-(5-f3-h~droxyaropyll-2-
methylbenzoyl)-3-methyfthiopyn3zole.
A solution of 0.530 g (1.0 mmol) of 5-amino-1-(2,6-dichloro~-
trifluoromethylphenyl)-4-(5-[t3-methoxycarbonylethylJ-2-methylbenzoyl)-3-
CA 02272138 1999-06-O1
-29-
methytthiopyrazole in 10 mL of THF was cooled in an ice bath while 1.33 mL of
a 1.5
M solution of DIBAL in THF was added. The reaction mixture was warmed to room
temperature and then quenched with water. The product was extracted into
EtOAc,
dried and concentrated. The residues were chromatognsphed on silica gel using
mixtures of hexane/EtOAc to elute the title product which was isolated as an
amorphous foam) 174 mg (3496). Anal. Calcd. for CZ2HZ°O=N3SCI2F3: C,
50.97; H,
3.88; N, 8.10. Found, C, 51.10; H, 3.96; N, 7.60.
Example 13
f5-Amino-l-(2.6-dichloro-4-trifluoromethylphenyil-3-methyisulfonyl-1 H~yrazol-
4-
yl]-(2,5-dimethylphenvl)methanone.
To a solution of 200 mg (0.42 mmol) of [5-amino-1-(2,6-dichloro-4-
trifluoromethylphenyl~3-methylsulfanyl-1 H-pyrazol-4-yl]-(2,5-
dimethylphenyl)methanone
in 10 mL of THF was added 0.176 g (2.10 mmol) of anhydrous sodium bicarbonate
followed by a solution of 145 mg (0.42 mmol) of 3-chloroperoxybenzoic acid in
8 mL
of THF. After two hours at room temperature, 0.5 g of sodium bicarbonate and
an
additional 290 mg (0.84 mmol) of 3-chioroperoxybenzoic acid was added. The
reaction
mixture was heated briefly to 50 ° C, let cool and stirred overnight at
room temperature.
The reaction mixture was added to water and the product was extracted into
ethyl
acetate. The organic extracts were washed with dilute sodium bicarbonate
solution and
then dried and evaporated. The title compound was crystallized from ether to
give 150
mg (7096 yield) of colorless crystals, m.p. 193.5 - 194.5°C
Example 14
The following compounds in Tables 1 and 2 were prepared according to the
indicated Example.
CA 02272138 1999-06-O1
-30-
TABLE 1
Ri2 Rli
0=C
1o R1 N/N
Ris Ri3
Process
R, RZ R, 3 R, 4 R, 6 R" R, ~ Physical
Data of
(m.p. in C) Example
amino SCH3 2-CI 4-CI 6-CI 2-CI H m.p.178-180
1 ,
amino SCH3 2-CI 4-CI 6-CI H H m.p.178-180
1
amino SCH, 4-CF3 H H 2-CI H m.p.150-153
1
n
amino SCH, 2-CI 4-CI 6-CI 2-F H m.p.204-206
1 0
NHCH3 SCH, 2-CI 4-CI 6-CI 2-CI H m.p.135-137
2
N
N(CH3)z SCH3 2-CI 4-CI 6-CI 2-CI H m
2
194-197
.p.
N(CH3)Z CH3 2-CI 4-CI 6-CI 2-CI H m.p.220-222
6
i '
amino SCH3 2-CF, H H 2-CI H m.p.186-188
1
amino SCH3 2-CI 4-CI 6-CI 2-cyclopropylH m.p.165-
167 1
amino SCH3 2-CI 4-CI 6-CI 4-CH, H m.p.230-232
1
amino SCH3 2-CI 4-CI 6-CI 2-CI 4-CI m.p.
Amorphous. 1
Anal. Calc'd
for
C17H10N30SCI5:
C, 42.39; H,
2.09; N,
8.72. Found:
C,41.94; H, 2.06;
N,
8.07.
N(CH3)Z CH3 2-CI 4-CI 6-CI 2-CI H m.p.108-109
6
NHZ SCH3 2-F 4-F 6-F 2-CI H m.p.156-158
1
I
Process
R, Rz R,3 R,4 R,s R" R,z , Physical
Data of
(m.p. in C) Example
N(CH3)z SCH, 2-CI 4-CI 6-CI 2-F H m.p.
Amorphous. 2
HRMS for
C19H15N30SC13F
.
Caic'd: 466.9984
Found: 456.9990 n
NH2 SCH, 2-CI 6-CI H 2-F H m.p
1
182-184
.
NHZ SCH, 2-F 4-F H 2-CI H m.p.147-150
1
N(CH3)z SCH, 2-CI 4-CI 6-CI 3-CI H m.p.121-125
2 (
w
NHZ NHCH, 2-CI 4-CI 6-CI 2-CI H m
p
86-90
.
.
NHz SCH, 2-CI 4-CI 6-CI 2-OCH, H m.p.200-202
1
0
NH2 SCH, 2-CI 6-CI 4-CF3 2-CI H m.p.207-210
1
NH(CH2)30CeH5SCH3 2-CI 6-CI 4-CI 2-CI H m.p.
Ambrphous. 5
Anal. Calcd.
for
C26H21 N3O2SCI4:
C, 53.71; H,
3.64; N,
7.22. Found:
C,
53.57; H, 3.41;
N,
7.46.
NHZ SCH, 2-CI 6-CI 4-CI 3-CH3 H m.p.178-180
~ i
NHZ SCH3 2-CI 6-CI 4-CF3 3-CI H m.p.149-151
1
NHz SCH, 2-CI 6-CI 4-CF3 3-CH3 H m.p.150-153
1
i
Process
.R, Rz R,3 R)4 R,s R" R,2 Physical
Data of
(m.p. In C) Example
I N(CH3)z SCH3 2-CI 6-CI 4-CF3 3-CI H m.p. 120-
122 2 ,
NHS SCH3 2-Cl 6-CI 4-CI 3-CF, H m.p.158-160
1
NHCHZCOOH SCH, 2-CI 6-CI 4-CI 2-CI H m.p. > 250
1
n
HRMS for
C19H13N3O3SC14:
Calcd: 525.9930
Found: 525.9348
NHZ SCH3 2-CI 6-CF3 4-CI 3-CF3 H m.p.
Amorphous. 1 ,
Anal. Calcd.
for
C19H11 N30SCI2F6: ~ o
C, 44.30; H, o
2.15; N)
8.15. Found:
C,
44.51; H, 2.29;
N,
, 7.98
NHz SCH3 2-CH3 4-CI H 2-CI H m.p.125-129
1
NHZ SCH3 2-CH3 4-CI H 3-CI H m.p.112-115
1
NHS SCH3 2-CI 4-CF3 6-CI 2-CH3 5-CH3 m.p.130-132
1
NHZ SCH3 2-CH3 6-CH3 H 3-C! H m.p.148-151
4 1
i
Process
R, Rz R" R,4 R,5 R" R,z Physical
Data of
(m.p. In C) Example
N(CH3)~ SCH3 2-CI 4-CF3 6-CI 3-CF3 H m.p.
Amorphous. 2
Anal. Calcd. for
C21 H15N30SCI2F6:
C) 46.50; H, 2.78;
N,
7.74. Found: C,
46.75; H, 2.93;
N,
o
7.58 N
N
J
NH2 SCH3 2-CI 4-CF3 H 3-CI H m.p. t24-
127 1
__
NHZ SCH3 2-CH3 6-CH3 H 3-CI H m.p.139-142
1
w ~o
NHZ SCH3 2-CI 4-CI 6-CI 2-CH 5-CH m.p.182-184
1 ~
3 3
0
CH3 SCH3 2-CI 4-CI 6-CI 3-CH3 H m.p.116-117
0
NHZ SCH3 2-CH3 4-CI 6-CH3 3-CF, H m.p.
Amorphous. 1
Anal. Calcd. for
C20H17N30SCIF3:
C, 54.48; H) 3.88;
N,
9.53. Found: C)
54.60; H) 3.91;
N)
9.08
Process
R, Rz R,3 R,4 R,6 R" R,z Physical
Data of
(m.p. In C) Example
NHz SCH, 2-CH3 4-CI 6-CH, 3-CI H m.p.
Amorphous. 1
Anal. Calcd.
for
C19H17N30SC12:
C, 56.16; H,
4.21; N,
10.34. Found:
C,
55.93; H, 4.21;
N,
10.01
NHz SCH3 2-CH3 4-CH3 6-CH3 3-CI H m.p.163-165
1
NHS SCH, 2-CH3 4-CH3 6-CH3 3-CH, H m.p.124-127
~ 1
w
NH2 SCH3 2-CH3 4-CH3 6-CH3 3-CF3 H m.p.143-146
1 'i
NHz SCH, 2-CH, 4-Br 6-CH, 3-CF3 H m.p.
Amorphous. 1
Anal. Calcd.
for
C20H17N30SBrF3:
C, 49.69; H)
3.53; N,
8.67. Found:
C)
49.45; H, 3.49;
N,
8.37
NHZ SCH, 2-CI 4-CF3 6-CI 2-CH3 3-CH3 m.p.196-198
1
Process
R, RZ R" R,4 R,5 R" R,z Physical
Data of
(m.p. in C) Example
NHz SCH, 2-CH, 4-Br 6-CH3 3-CI H m.p.
Amotphous. 1
Anal. Calcd. for
C19H17N30SBrCl:
C)
50.63; H, 3.80;
N,
9.32. Found: C,
50.40; H, 3.77;
N,
8.94
N
N
J
NHz SCH, 2-CI 4-CF3 6-CI 2-CH, 5-(CH~)3 m.p.
Amorphous. 1
COzCH3 Anai. Calcd. for
C24H22N3O3SCI2F3:
C, 51.43; H, 3.96;
N,
7.50. Found: C, o
51.59; H, 4.10;
N,
7.17
NHz SCH3 2-CH, 6-CH, 4-Br 2-CH3 5-CH3 m.p.
Amorphous. 1
Anal. Calcd. for
~'21 H~2NJOCJ:
C,
56.76; H, 4.99;
N,
9.45. Found: C,
56.37; H, 5.01;
N,
9.04
NHZ SCH3 2-C) 4-CF3 6-CI 2-OCH3 H m.p.172-174
. 1
Process
i Rz R,3 R" R,5 R" R,Z Physical
Data of
R,
(m.p. in C) Example
NHZ OCH, 2-CI 4-CI 6-CI 2-CI H m.p.
Amorphous. 7
' H-NMR(CDCI,):3.68
(3H) s), 5.86(2H,
broad s)) 7.26-
7.44(4H, m) 7.51 y
(2H,
s). o
N
NH2 OCH2CH3 2-CI 4-CI 6-CI 2-CI H m.p.
Amorphous. 7
Anal. Calcd. for
C18H13N3O2CI4:
C)
48.5?; H ) 2.94; ~
N ) w ~o
9.44. Found: C, ;
48.41; H, 2.52;
N)
9.43 0
N(CH3)z OCH3 2-CI 4-CI 6-CI 2-CI H m.p.
Amorphous. 8
HRMS for
C19H15N3O2CI4:
Calcd: 456.9913
Found: 456.9960
NHZ OCH2CH3 2-CI 4-CF3 6-CI 3-CF3 5-CF3 m.p.
Amorphous. 1
Anal. Calcd. for
,
C20H10N30SCI2F9:
C, 41.25; H) 1.73;
N,
7.21. Found: C,
41.49; H, 2.01;
N,
7.0t
Process
R, R~ R" R,4 R,5 R" R,I Physical
Data of
(m.p. in C) Example
NHS OCHzCH, 2-CI 4-CF3 6-CI 2-CH, 5-CH3 m.p.
Amorphous. 1
High Resolution
Mass Spectrum
Caicd. for
C22H20N30CI2F3:
472.0808. Found,
472.0817
NH2 CHzCH2 2-CI 4-CF3 6-CI 2-CH3 5-CH3 m.p.
Amorphous. 6
CH High Resolution
Mass Spectrum
Calcd. for ,
Cz, HzN3~C, zFa~
469.0935. Found:
469.0889
NHS CHzCHz- 2-CI 4-CI 6-CI. 2-CH, 5-CH3 m.p.
Amorphous. 6
CH, High Resolution
Mass Spectrum
Calcd. for
C22H20N30CI2F3:
436.0761. Found:
436.0764
Process
i
R, Rz R" R,4 R,5 R" R,2 Physical
Data of
(m.p. In C) Example
NHZ SCH3 2-CI 4-CF3 6-CI 3-OCH3 H m.p.
Amorphous. 3
'H-NMR(CDCI,)
d
2.38(3H) s))
3.90(3H)
s), 5.79(2H)
broad
s)) 7.07(1 H,
d, J=7),
7.16(1 H, s),
7.23(1 H,
d, J=7), 7.39(1
H, t,
7.79(2H
J=7)
s)
,
)
W
NHz SCH3 2-CI 4-CF3 6-C1 2-CH, 5-(CH2)40Hm.p.
Amorphous. i2
'
High Resolution t
,,
Mass Spectrum ' '
Calcd, for
C23H22N302SCI2F3 0
. 532.0840. Found:
532.0871
NH2 CH2CH3 2-CI 4-CF, 6-CI 2-CH3 5-CH3 m.p.
Amorphous. 6
Anal. Calcd.
for
C21 H18N30CI2F3:
C, 55.29; H,
3.97; N,
8.21. Found:
C)
55.62; H, 4.35;
N,
8.15
Process
R, Rz R, 3 R, 4 R, 5 R" R, Z Physical
Data of
(m.p. in C) Example
I NHz CH2CH3 2-CI 4-CI 6-CI 2-CH3 5-CH3 m.p.
Amorphous. 6
Anal. Caicd.
for
C20H18N30CI3:
C,
56.81; H) 4.29;
N,
9.94. Found:
C,
56.88; H, 4.09;
N,
9.62
o
NHS SCH3 2-CI 4-CF3 6-CI 2-CF3 5-CF3 m.p.
Amorphous. 1
Anal. Calcd.
for
C20H20N30SCI2F9:
C)41.25; H,1.71; ,
N)
7.21. Found:
C)
41.20; H, 1.87;
N,
6.89
0
NHz SCH3 2-CI 4-CF3 6-CI 2-CH3 5-CH2COz m.p.
Amorphous. 1
C2H5 Anai. Caicd.
for
C23H20N3O3SCI2F3
C, 50.55; H,
3.68;
N, 7.69. Found:
C,
50.50; H) 3.50;
N,
7.29
Process
R) Rz R,3 R,4 R,6 R" R,z Physical
Data of
(m.p. in C) Example
NHz SCH3 2-CI 4-CF, 6-CI 2-CH, 5-(CHz)zOHm.p.
Amorphous. 12
HRMS for -
C21 H18N3O2SCI2F3
Calcd: b03.0646
Found: 503.0147
NHz SCH, 2-CI 4-CF3 6-CI 2-OCH3 5-OCH, m.p.
Amorphous. 1
'H-NMR(CDCI3)
d
2.30(3H, s),
3.60(6H,s), 6.85(2H,
broad s), 6.80-
6.96(3H) m),
7.74(2H, s).
NHZ OCH~CH, 2-CI 4-CF3 6-CI 3-CF, H m.p.112-114
9
NH2 OCHzCH3 2-CI 4-CF, 6-C~ 3-CF3 H m.p.77-78
9
NHz OCH~CH3 2-CI 4-CF, 6-CI 3-CH, H m.p.103-104
9
NHz OCHzCH3 2-CH3 4-Br 6-CH, 3-CF3 H ~ m.p.158-
160 9
NHS SCH3 2-CI 4-CF, 6-CI 2-CH3 5-isopropylm.p.
Amorphous. 1
- Anal.
Calcd.
for
C22H20N30SCI2F'3:
- C, 52.59; H)
3.98; N,
. 8.36. Found:
C,
52.39; H, 4.01;
N,
8.08
Process
R, Rz R,3 R,~ R,5 R" R,~ Physical
Data of
(m.p. in C) Example
NH2 SCH, 2-CI 4-CF3 6-CI 2-CH3 5-(CHz)30Hm.p.
Amorphous. 12
Anal. Calcd. for
C22H20N3O2SCI2F3:
C, 50.97; H, 3.88;
N,
8.10. Found: C,
51.10; H, 3.96;
N,
7.60
_--- _ _.
NHz SCH3 2-CI 4-CF3 6-CI 2-COOC~HS H m.p.211-216
1
NHz SCH, 2-CI 4-CF3 6-CI R" and R,2 H m.p. 115-118
1 ,
with the
phenyl to
which they
0
are attached
form 1-
naphthyl
NHS SCH3 2-CI 4-CF, 6-CI 3-Br H m.p.
Amorphous. 1
Calcd. for
C18H11 N30SBrC12F3
C41.16,H2.11,N
8.00.
Found C 41.37,
H
1.92, N 7.85.
NH2 SCH3 2-CI 4-SO~ 6-CI 3-CF3 H , m.p.272-
274 1
NHz
Process
R, R2 R" R" R,6 R" R,2 physical
Data of
(m.p. In C) Example
NHz SCH3 2-CI 4-CF3 6-CI 3-S02NHz H m.p.99-100
1 ,
NH2 SCH3 2-CH, 4-Br 6-CH3 3-SOZNHZ H m.p.242-24-
4 1
NHS SCH3 2-CI 4-CF3 6-CI 3-SOZN(CH3)zH m.p.
Amorphous. 1
Anal. Caicd.
for
C20H17N4O3S2CI2F
3/1/4C4H100:
C,
44.10; H, 3.44;
N,
9.80. Found:
C,
43.88; H, 3.29;
N,
9.68 w '
i o
NHz SCH3 2-CH3 4-Br 6-CH3 3-SOZN(CH3)2H m.p.
Amorphous. 1 0
Anal. Caicd.
for
C21 H23N4O3S28r:
C, 48.18; H,
4.43; N,
10.70. Found:
C,
48.42; H, 4.24;
N)
10.52
NHz SCH3 2-CI 4-CF3 6-CI 3-SOzN(CH3)ZH m.p.192-
194 1
NHz SCH3 2-CI 4-C) 6-C) 2-(2-thienyi)H m.p. t
71.5-172.5'1
NHz SCH, 2-CI 6-CI 4-CF3 H H m.p.192-194
1
NHz CH, 2-CI 6-CI 4-CF3 2-CH3 5-CH, m.p.175-176
6
NHS CH3 2-CH3 6-CH3 4-Br 2-CH3 5-CH3 m.p.158-160
6
ii
Process
R,~ R~ R,3 R,4 R,5 R" R,2 Physical
Data of
(m.p. in C) Example
NHz SCH, 2-CI 6-CI 4-CF3 3-N(CH3)z H HRMS for
1
C2o11mON4SCizF3
Calcd: 488.0452
Found: 488.0408
NH(C3H~) SCH3 2-CI 6-CI 4-CI 2-CI H Calcd. C,
49.10;5
H,
3.50; N, 8.54;
Found: C) 5o.37;
H,
3.28; N, 8.47.
N
H
NHz SOzCH3 2-CI 6-CI 4-CF3 2-CH3 5-CH3 m.p.193.5-
194.5 13
NH SCH 2-CI 6-CI 4-CF3 3-phenyl H m.p.124-127
1
Z 3
NHz SCH3 2-Ci 6-CI 4-CF3 2-pyrrolyl H HRMS Calcd.
1 0
510.0295
0
Found 510.0455
N(CH3)(CzHS) SCH, 2-CI 6-CI 4-CI 2-CI H FAB Mass
5
Spectrum: 490
NHS SCH, 2-CI 6-CI 4-CI 2-CH, 5-iPr HRMS Calcd.
1
467.0393
Found 457.0395
NH2 SCH, 2-CI 6-CI 4-CF3 2-CH3 5-n-butyl HRMS
Cal,cd. 1
515.0809
Found 515.0892
'
Process
R, R~ R,3 R,4 R,5 R" R,Z Physical
Data of
(m.p. in C) Example
N(CzH5)2 SCH, 2-CI 6-CI 4-CF, 2-CH, 5-iPr HRMS Calcd.
5
657.127y
Found 557.1287
NHz CH3 2-CI 6-CI 4-CI 2-CH3 5-iPr HRMS Calcd.
1
435.066
Found 435.0682
N(CzHS)(C3H,) SCH3 2-CI 6-CI 4-CF, 2-CH, 5-iPr MS parent
571, 5
529,
494, 360, 160
(10096)
N(CzHS) (sec- SCH3 2-CI 6-CI 4-CF3 2-CH3 5-iPr HRMS Caicd.
5 i
butyl) 585.1589
Found 585.1500
N(CH3)(CZH5) SCH3 2-CI 6-CI 4-CF3 2-CH3 5-iPr HRMS Calcd.
5
543.1121
Found 543.1166
N(CzHS)(C3H,) SCH3 2-CI 6-CI 4-CF3 2-CH3 5-iPr HRMS Calcd.
5
569.1277
Found 569.1433
NHz SCH, 2-CI 6-CI 4-CF, 2-n-propyl 5-CH3 HRMS Calcd.
1
501.os53
Found 501.0551
Process
R, a R, 4 R, s R" R, z Physical Data of
(m.p. in C) Example
NHz SCH3 2-CI 4-CF3 6-CI 2-Br 5-Br m.p.172-174
1
NHZ SCH3 2-CH3 4-Br 6-CH3 2-Br 5-Br m.p.179-181
1
NHZ SCH3 2-CH, 4-CH3 6-CH3 2-Br 5-Br m.p.195-197
1
NHZ SCH3 2-CH, 4-Br 6-CH3 2-CH3 5-N02 m.p.118-119
1
NH2 SCH3 2-CI 4-CI 6-CI 2-C~HS H m.p.146-150
1
NHZ SCH3 2-CI 4-CI 6-CI 2-CF3 H .p. 175-177
1
m
NHZ SCH3 2-CI 4-CF3 6-CI 2-CI 5-NOZ m.p.98.0-
98.5 1
NHZ SCH3 2-CH3 4-Br 6-CH3 2-CI 5-NO~ m.p.116-118
1
NHZ SCH3 2-CI 4-CF3 6-CI 2-CH3 5-NO~ m.p.99-100
NHZ SCH3 2-CH, 4-CH3 6-CH3 2-CH3 5-NOz ~ m.p.193-
195 1
NH2 SCH3 2-CI 4-CI 6-Cl 2-OC3H, H 'H-NMR
(CDCI,) 1
d
o.87 (3N, t, J=7),
1.68(2H, m))
2.32(3H, s), 3.96(2H,
q) J=7), 5.80
(1 H)
broad s), 6.93
(1 H~
d) J=7), 7.03
1 H, t,
J=7), 7.27 (1
H, d,
J=7), 7.42 (1
H, t,
J=7), 7.52 (2H,
s)
CA 02272138 1999-06-O1
-48-
TABLE 2
R3
0=C SCH3
N
1o H2N N/
Ra
Phys. Data Process of
R3 RQ (m.p. in C) Example
15 CZH5CHC4H9 2,6-CI2-4-CF3Ph HRMS Calcd. 1
467.0809.
Found
467.0913.
C3H,CH=CH-CZHS 2,6-CIZ-4-CF3Ph FAB Mass 1
Spectrum:
466
20 2-methylcyclopentyl2,4,6-CI3Ph HRMS Calcd. 1
417.0236.
Found -
417.0328.
2-OCzHS-1-naphthyi2,6-CIZ-4-CF3Ph m.p.149-151 1
2-OC2H5-1-naphthy)2,4,6-CI3 Ph m.p. 125-128 1
25 3_CF3-Ph 1,3-(CH3)2-4-NOZ-Calcd. for
pyrazol-5-yl C"H,503NBSF,:
C, 46.36;
H,
3.43; N, 19.08.
Found: C,
46.51; H,
3.37;
N) 18.10.
30
CA 02272138 1999-06-O1
-49-
Example 15
A. 3-Trifluoromethylphenyithioacetonitrile.
To sodium (0.62 g, 27.0 mmol) dissolved in 40 mL of ethanol was added 4.79
g (26.9 mmol) of 3-trrfiuoromethytthiophenol and 2.04 g (27.0 mmol) of
chloroacetonitrile. The reaction mixture was heated at reflux for 1 hour and
then
stin-ed overnight at room temperature. To the cooled reaction mixture was
added one
volume of ether and the precipitated solids were removed by filtration. The
filtrate was
evaporated on the rotary evaporator to give the product as an oil in
essentially
quantitative yield. This material was used in the following reaction without
further
purification.
B. 3-Trifluoromethylphenylcyanomethyisulfoxide.
A solution of 3.00 g (13.8 mmol) of 3-trifluoromethylphenylthioacetonitrile in
130
mL of methylene chloride was cooled to 5 ° C under dry NZ and treated
with 4.89 g
(28.35 mmol) of m-chloroperbenzoic acid. The reaction mixture was stirred for
48
hours at room temperature and then cooled in ice, after which the insolubles
were
removed by filtration. The filtrate was washed with 10~°sodium sulfate
solution until
all traces of peroxides had been removed and then dried over magnesium sulfate
and
evaporated to a pale yellow oil which was used in the subsequent reaction
without
further purification.
C. 3.3-Bis-methyfthio-2-(3-tr'rfiuoromethylphenylsulfonyl)acrylonitrile.
A solution of 13.82 mmol (crude product) of 3-
trifluoromethyiphenyicyanomethylsulfoxide in 30 mL of dry dimethylsutfoxide
and 1.25
mL (1.58 g, 20.7 mmol) of CSz was cooled to about 15 ° C in an ice bath
under dry
nitrogen. Then 0.99 g (41.5 mmol) of oil-free sodium hydride was added
portionwise
below 20 ° C and the deep red solution was let stir at room temperature
for 75
minutes. The reaction mixture was cooled to 15 ° C, quenched with 2.58
mL (5.89 g,
41.5 mmol) of methyl iodide and let stir overnight at room temperature. The
reaction
mixture was poured into ice/water and let granulate for 3.5 hours. The product
was
filtered and air dried to give 3.51 g (72%) of the product. The analytical
sample was
crystallized from EtOH/HZO, m.p. 109-110°C.
CA 02272138 1999-06-O1
-50-
D. 5-Amino-1-(2,6-dichloro-4-trifluorometh~lphenyl)-4-(3-
trifluoromethylbenzenesulfon~ -3-met(~Ithiopyrazole.
A suspension of 0.50 g (1.42 mmol) of 3,3-bis-methylthio-2-(3-
trifiuoromethyiphenylsulfonyl)acrylonitrile and 0.35 g (1.42 mmol) of 2,6-
dichloro-4-
trifiuoromethylphenylhydrazine in 10 mL of ethanol was heated at refiux for
2.5 hours,
solution occurring as the reaction mixture was warmed. The mixture was stirred
overnight at room temperature and was then poured into cold water. The product
was extracted into ethyl acetate and the extracts were dried with magnesium
sulfate
and evaporated. The residues were crystallized from ether, and the product was
filtered off and air dried to give 314 mg (40°.6) of the desired
product, m.p. 201 -
203°C. Anal. Calcd. for C,eH"OZN3SZClZFe: C, 39.28; H, 2.02; N, 7.64.
Found: C,
39.35; H, 2.19; N, 7.48.
E. 5-Dimethylamino-1-12.6-dichloro-4-trifluorometh~rlphenyll-4-
~benzenesulfonyl)-3-meth5rlthioayrazole.
A solution of 0.241 g (0.5 mmol) of 5-amino-1-(2,6-dichloro-4-
trifiuoromethylphenyl)-4-(benzenesulfonyl)-3-methylthiopyrazole in 5 mL of dry
dimethylsulfoxide was treated with 36 mg (1.5 mmol) of oil-free sodium hydride
under
dry nitrogen in a flame-dried flask at room temperature. After 30 minutes, a
clear, pale
yellow solution had formed. This solution was treated with 0.5 mL (8.0 mmoi)
of
methyl iodide and stirred for 1 hour. Then the reaction mixture was poured
into water
and extracted twice with ethyl acetate. The combined extracts were dried with
brine
and with magnesium sulfate and evaporated. The residues crystallized from
ether to
give the desired product in 819'° yield, m.p. 163 - 164°C.
Example 16 '
The foNowing compounds were prepared in accordance with Example 15.
CA 02272138 1999-06-O1
-51-
R11
S02 SCH3
I IN
~N~
R1
R13 R15
R14
- -
R,3,R,4,R,5 R, M.P. (C) or
Analysis
H 2,4,6-trichloroNHZ 161-162
H 2,4,6-trichloroN(CH3)2 200-202
2-(i-propyl)2,4,6-trichloroNH2 180-182
2-OCH3 2,4,6-trichforoNHZ 212-215
2-Ci 2,4,6-trichloroNHz Anal. Calcd. for
C,BH"OzN3SZCl,: C, 39.60;
H, 2.39; N, 8.33. Found:
C,39.76; H, 229; N, 8.69
H 2,6-Clz-4-CF3NHz 209.5-210.5
H 2,6-C12-4-CF3N(CH3)Z 163-164
3-CF3 2,6-CIZ-4-CF3NH2 201-203
3-CF3 2,6-CIZ-4-CF,N(CH3)Z 137-138
Example 17
4-I2-Chloronhenvl)-1-12.6-dichloro-4-trifluoromethLlphenyl)-3-
methylthiopyrazolo f 3.4-dl pyrimidine.
A suspension of 669 mg (1.39 mmol) of 5-amino-1-(2,6-dichloro-4
trifluoromethylphenyl)-4-(2-chlorobenzoyl}-3-methylthiopyrazole in 5 mL of
formamide
was heated at 150 ° C overnight. A pale yellow solid precipitated upon
cooling of the
CA 02272138 1999-06-O1
-52-
reaction mixture. A total of 50 mL of water was added to the stirred
suspension to
complete the precipitation of the product which was filtered off and washed
with water.
An inseparable trace of starting material was observed in the product by thin
layer
chromatography (TLC) and so the above procedure was repeated on the mixture
giving a brown solid containing no trace of starting material. Trituration of
this solid
with methyiene chloride gave a pale yellow solution which was concentrated to
give
the desired product as a white crystalline solid, m.p. 156-158 ° C.
Example 18
The following compounds were prepared in accordance with Example 17.
R .~
SCH3
R
R13
R3 Rs R,3 m.p.(C) or HRMS
2-CI-Ph H CI 193-195
3-CI-Ph H CI 171-173
2-CI-Ph H CF3 156-158
2-CI-Ph OH CI 313-316
2-CI-Ph CI CI ~ 193-195
2-CI-Ph 4-ethoxycarbonyl-CI 222-225
piperazinyl
1-naphthyl H CF3 171-173
2-CI-Ph CH, Cl 210-212
2-CH3_5-iPrPhCH3 CI 141-142
CA 02272138 1999-06-O1
-53-
R3 R9 R,3 m.p.(C) or Hi~MS
2,6-(CH,)Z CH3 CI HRMS Calcd. 462.0239.
Ph
Found, 462.0369.
2-(OCzHs)-Ph CH, CI 189.192
2-(OCZH5)-1- CH, CI HRMS: Calcd.: 528.0345
naphthyi Found: 528.0226
2-OCH3-Ph CH3 C1 214-216
2-CzH5 Ph CH3 CI HRMS: Calcd. 462.0239
Found: 462.0219
Ph CH3 CF3 114-116
2,5-(CH3)Z-PhCH3 CF3 HRMS: Calcd. 497.0579
Found: 497.0602
2-CF3-Ph CH3 CI HRMS: Caicd. 501.9800
Found: 501.9778