Note: Descriptions are shown in the official language in which they were submitted.
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
2-IMIDAZOLINYLAMINOBENZOXAZOLE COMPOUNDS
USEFUL AS ALPHA-2 ADRENOCEPTOR AGONISTS
TECHNICAL FIELD
This invention relates to certain substituted (2-
imidazolinylamino)benzoxazole compounds. The compounds have been found
to be alpha-2 adrenoceptor agonists and are useful for treatment of disorders
modulated by alpha-2 adrenoceptors.
BACKGROUND OF THE INVENTION
Therapeutic indications of alpha-2 adrenoceptor agonists have been
discussed in the literature: Ruffolo, R.R., A.J. Nichols, J.M. Stadel, & J.P.
Hieble, "Pharmacologic and Therapeutic Applications of Alpha-2 Adrenoceptor
Subtypes", Annual Review of Pharmacolog~i & Toxicoloay Vol. 32 (.1993) pp.
243-279.
Information regarding alpha adrenergic receptors, agonists and
antagonists) in general) and regarding compounds related in structure to those
of this invention are disclosed in the following references: Timmermans,
P.B.M.W.M., A.T. Chiu 8 M.J.M.C. Thoolen, "12.1 a-Adrenergic Receptors",
Comprehensive Medicinal Chemistry, Vol. 3, Membranes & Receptors, P. G.
Sammes & J. B. Taylor, eds., Pergamon Press (1990), pp. 133-185;
Timmermans, P.B.M.W.M. & P.A. van Zwieten, "a-Adrenoceptor Agonists and
Antagonists", Drugs of the Future, Vol. 9, No. 1, (January) 1984), pp. 41-55;
Megens) A.A.H.P., J.E. Leysen, F.H.L. Awouters 8~ C.J.E. Niemegeers, "Further
Validation of in vivo and in vitro Pharmacological Procedures for Assessing
the
a1 and a2-Selectivity of Test Compounds: (2) a-Adrenoceptor Agonists",
European Journal of Pharmacology) Vol. 129 (1986), pp. 57-64; Timmermans,
P.B.M.W.M., A. de Jonge, M.J.M.C. Thoolen, B. Wilffert) H. Batink & P.A.
van Zwieten, "Quantitative Relationships between a-Adrenergic Activity and
Binding Affinity of a-Adrenoceptor Agonists and Antagonists", Journal of
Medicinal Chemistry, Vol. 27 (1984) pp. 495-503; van Meel, J.C.A., A. de
Jonge,
P.B.M.W.M. Timmermans & P.A. van Zwieten, "Selectivity of Some Alpha
Adrenoceptor Agonists for Peripheral Alpha-1 and Alpha-2 Adrenoceptors in the
Normotensive Rat", The Journal of Pharmacoiog~ and EJ~erimental
Therapeutics, Vol. 219, No. 3 (1981), pp. 760-767; Chapieo) C.B., J.C. Doxey,
P.L. Myers, M. Myers, C.F.C. Smith 8~ M. R. Stillings, "Effect of 1,4-Dioxanyl
Substitution on the Adrenergic Activity of Some Standard a-Adrenoreceptor
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
2
Agents", Euroaean Journal of Medicinal Chemistry, Vol. 24 (1989), pp. 619-622;
Chapleo, C.B., R.C.M. Butler, D.C. England, P.L. Myers, A.G. Roach) C.F.C.
Smith, M.R. Stillings & I.F. Tulloch, "Heteroaromatic Analogues of the a2-
Adrenoreceptor Partial Agonist Clonidine", Journal of Medicinal Chemistry,
Vol.
32 (1989), pp. 1627-1630; Clare, K.A., M.C. Scrutton & N.T. Thompson, "Effects
of a2-Adrenoceptor Agonists and of Related Compounds on Aggregation of, and
on Adenylate Cyclase Activity in, Human Platelets", British Journal of
Pharmacology) Vol. 82 (1984), pp. 467-476; U.S. Patent No. 3,890,319 issued to
Danielewicz, Snarey 8~ Thomas on June 17, 1975; and U.S. Patent No.
5,091,528 issued to Gluchowski on February 25, 1992. _ However, many
compounds related in structure to those of this invention do not provide the
activity and specificity desirable when treating disorders modulated by alpha-
2
adrenoceptors.
For example, many compounds found to be effective nasal
decongestants are frequently found to have undesirable side effects, such as
causing hypertension and insomnia at systemically effective doses. There is a
need for new drugs which provide relief from nasal congestion without causing
these undesirable side effects.
OBJECTS OF THE INVENTION
It is an object of the invention to provide compounds and compositions
useful in treating disorders modulated by alpha-2 adrenoceptors.
It is an object of this invention to provide novel compounds having
substantial activity in preventing or treating nasal congestion, otitis media,
and
sinusitis, without undesired side effects.
It is also an object of this invention to provide novel compounds for
treating cough, chronic obstructive pulmonary disease (COPD) andlor asthma.
It is also an object of this invention to provide novel compounds for
treating diseases and disorders associated with sympathetic nervous system
activity, including benign prostatic hypertrophy, cardiovascular disorders
comprising myocardial ischemia) cardiac reperfusion injury, angina, cardiac
arrhythmia, heart failure and hypertension.
It is also an object of this invention to provide novel compounds for
treating ocular disorders) such as ocular hypertension) glaucoma, hyperemia,
conjunctivitis and uveitis.
_. r._._.. ~___.._.._....
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
3
It is also an object of this invention to provide novel compounds for
treating gastrointestinal disorders, such as diarrhea, irritable bowel
syndrome,
hyperchlorhydria (hyperacidity) and peptic ulcer (ulcer).
It is also an object of this invention to provide novel compounds for
treating migraine.
It is also an object of this invention to provide novel compounds for
treating pain, substance abuse and/or withdrawal.
It is a still further object of this invention to provide such compounds
which have good activity from peroral, parenteral, intranasal and/or topical
dosing.
SUMMARY OF THE INVENTION
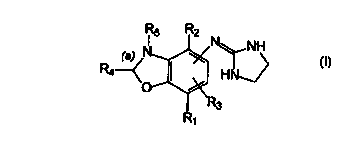
This invention relates to compounds having the following structure:
R4
Formula I
wherein: --
a) R~ ) R2 and R3 are each independently selected from hydrogen;
unsubstituted C~-Cg alkanyl, aikenyl or alkynyl; cycloalkanyl,
cycloalkenyl; unsubstituted C~-C3 aikylthio or alkoxy; hydroxy; thio;
nitro; cyano; amino; C~-Cg alkylamino or C~-C3 dialkylamino and
halo;
b) R4 is selected from hydrogen; unsubstituted C~-C3 alkanyl; amino,
hydroxy, mercapto; C~-C3 alkylthio or alkoxy; C~-C3 alkylarnino or
C'-Cg diaikylamino and halo;
c) R5 is hydrogen; or alkyl or nil;
d) where R5 is nil, bond (a) is a double bond; and
e) the imidazolinylamino moiety is attached to the 5- or 6- position of the
benzoxazole ring;
and enantiomers) optical isomers, stereoisomers, diastereomers, tautomers,
addition salts, biohydrolyzable amides and esters, and pharmaceutical
compositions containing such novel compounds, and the use of such
R~ R~
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
4
compounds for preventing or treating disorders modulated by aipha-2
adrenoceptors.
- DETAILED DESCRIPTION OF THE INVENTION
As used herein, "alkanyl" means a saturated hydrocarbon substituent,
straight or branched chain) unsubstituted or substituted.
As used herein, "alkenyl" means a hydrocarbon substituent with one
double bond, straight or branched chain, unsubstituted or substituted.
As used herein, "alkylthio" means a substituent having the structure Q-S-,
where Q is alkanyl or alkenyl.
As used herein, "alkoxy" means a substituent having the structure Q-O-,
where Q is alkanyl or alkenyl.
As used herein, "alkylamino" means a substituent having the structure
Q-NH-) where Q is alkanyl or alkenyl.
As used herein, "dialkylamino" means a substituent having the structure
Q1-N(Q2)-, where each Q is independently alkanyl or alkenyl.
"Halo", "halogen", or "halide" is a chloro, bromo, fluoro or iodo.
A "pharmaceutically-acceptable salt" is a cationic salt formed at any
acidic (e.g., carboxyl) group, or an anionic salt formed at any basic (e.g.,
amino) group. Many such salts are known in the art) as described in World
Patent Publication 87/05297, Johnston et al.) published September 11, 1987,
incorporated by reference herein. Preferred cationic salts include the alkali
metal salts (such as sodium and potassium), alkaline earth metal salts (such
as magnesium and calcium) and organic salts. Preferred anionic salts
include halides, sulfonates) carboxylates, phosphates, and the like. Clearly
contemplated in such salts are addition salts that may provide an optical
center, where once there was none. For example, a chiral tartrate salt may
be prepared from the compounds of the invention, and this definition
includes such chiral salts.
The compounds of the invention are sufficiently basic to form acid-
addition salts. The compounds are useful both in the free base form and the
form of acid-addition salts, and both forms are within the purview of the
invention. The acid-addition salts are in some cases a more convenient form
for
use. In practice, the use of the salt form inherently amounts to the use of
the
base form of the active. Acids used to prepare acid-addition salts include
preferably those which produce, when combined with the free base) medicinally
acceptable salts. These salts have anions that are relatively innocuous to the
_ . .__... _..~_. ... __ _.. T __
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
animal organism) such as a mammal, in medicinal doses of the salts so that the
beneficial property inherent in the free base are not vitiated by any side
effects
ascribable to the acid's anions.
Examples of appropriate acid-addition salts include, but at not limited to
hydrochloride, hydrobromide, hydroiodiode, sulfate, hydrogensulfate, acetate,
trifluoroacetate, nitrate, maleate, citrate) fumarate, formate, stearate,
succinate,
mallate, malonate, adipate, glutarate) lactate) propionate, butyrate,
tartrate,
methanesulfonate, trifluoromethanesulfonate, p-toluenesulfonate, dodecyl
sulfate, cyclohexanesulfamate, and the like. However, other appropriate
medicinally acceptable salts within the scope of the invention are those
derived
from other mineral acids and organic acids. The acid-addition salts of the
basic
compounds are prepared by several methods. For example the free base can
be dissolved in an aqueous alcohol solution containing the appropriate acid
and
the salt is isolated by evaporation of the solution. Alternatively, they may
be
prepared by reacting the free base with an acid in an organic solvent so that
the
salt separates directly. Where separation of the salt is difficult, it can be
precipitated with a second organic solvent, or can be obtained by
concentration
of the solution.
Although medicinally acceptable salts of the basic compounds are
preferred, all acid-addition salts are within the scope of the present
invention.
All acid-addition salts are useful as sources of the free base form, even if
the
particular salt per se is desired only as an intermediate product. For
example,
when the salt is formed only for purposes of purification or identification,
or when
it is used as an intermediate in preparing a medicinally acceptable salt by
ion
exchange procedures, these salts are clearly contemplated to be a part of this
invention.
"Biohydrolyzable amide" refers to an amide of the compound of the
invention that is readily converted in vivo by a mammal subject to yield an
active compound of the invention.
A "biohydrolyzable ester" refers to an ester of the compound of the
invention that is readily converted by a mammal subject to yield an active
compound of the invention.
"Optical isomer") "stereoisomer", "enantiomer," "diastereomer," as
referred to herein have the standard art recognized meanings (Cf., Hawleys
Condensed Chemical Dictionary, 11 th Ed.). Of course, an addition salt may
provide an optical center) where once there was none. For example, a chiral
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
6
tartrate salt may be prepared from the compounds of the invention, and this
definition includes such chiral salts. It will be apparent to the skilled
artisan
that disclosure of the racemic mixture alone discloses any enantiomers
therein. Thus by one disclosure, more than one compound is taught.
As used herein "animal" includes "mammals" which includes
"humans".
The skilled artisan will appreciate that tautomeric forms will exist in
certain
compounds of the invention. For example, when R4 is hydroxy and bond (a) is
a double bond, it is understood to include the keto form of that molecule,
where
R4 is oxo, and bond (a) is a single bond, though not specifically described.
Thus, in this description the disclosure of one tautomeric form discloses each
and all of the tautomers. Similarly, when the 2-iminoimidazolidinyl form of
the
molecule is shown, it is understood to include the 2-imidazoiinylamino form of
that molecule although not specifically depicted.
The illustration of specific protected forms and other derivatives of the
Formula (I) compounds is not intended to be limiting. The application of
other useful protecting groups, salt forms, etc. is within the ability of the
skilled artisan.
As defined above and as used herein, substituent groups may
themselves be substituted. Such substitution may be with one or more
substituents. Such substituents include those listed in C. Hansch and
A. Leo, Substituent Constants for Correlation Analysis in ChemistrSr and
Biolo4v (1979), incorporated by reference herein. Preferred substituents
include (for example) alkyl, alkenyl, alkoxy, hydroxy, oxo, vitro, amino,
aminoalkyl {e.g., aminomethyl, etc.), cyano, halo, carboxy, alkoxyacetyl
(e.g.,
carboethoxy) etc.)) thiol) aryl, cycloalkyl, heteroaryl, heterocycloalkyl
(e.g.,
piperidinyl) morpholinyl, pyrroiidinyl, etc.), imino, thioxo) hydroxyalkyl,
aryloxy, aryialkyl, and combinations thereof.
For the purposes of nomenclature, the numbering of the benzoxazole
follows the IUPAC convention. Thus, as shown in the following example, the
location of the imidazolinylamino radicals are denoted:
O , 6 ~NH N 4\ NH
~2 3
HNJ ~ , ~ ~ 6 HN
N ~ J
0
6-(2-IMIDAZOLINYLAMINO)- 5-(2-IMIDAZOLINYLAMINO)-
__.__~..._~_..._._ _._.__. T .~..~_.._. __-._~_.. _._
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
7
Compounds
This invention includes compounds having the following structure:
R5 R2
~NH
~a J N
Raw' I HN J
O
R3
R~
wherein:
a) R1, R2 and R3 are each independently selected from hydrogen;
unsubstituted C1-Cg alkanyl, alkenyl or alkynyl; cycloalkanyl,
cycloalkenyl; unsubstituted C1-C3 alkylthio or alkoxy; hydroxy; thio;
nitro; cyano; amino; C1-C3 alkylamino or C1-C3 dialkylamino and
halo;
b)_ R4 is selected from hydrogen; unsubstituted C1-C3 aikanyl; amino)
hydroxy, mercapto; C1-C3 alkylthio or alkoxy; C1-Cg alkylamino or
C1-C3 dialkylamino and halo;
c) R5 is hydrogen; or alkyl or nil;
d) where R5 is nil, bond (a) is a double bond; and
e) the imidazofinylamino moiety is attached to the 5- or 6- position of the
benzoxazole ring;
and enantiomers, optical isomers, stereoisomers, diastereomers, tautomers,
addition salts) biohydrolyzable amides and esters thereof.
In the above structure, when the imidazolinylamino is at the 6-position,
preferably R1 is unsubstituted alkanyl or aikenyl having from 1 to about 3
carbon
atoms and R2 is preferably alkanyl or halo. R1 and R2 are most preferably
methyl.
In the above structure, when the imidazolinylamino is at the 5-position,
preferably R2 is selected from unsubstituted alkanyl or alkenyl having from 1
to
about 3 , carbon atoms; cycloalkyl and cycloalkenyl; unsubstituted alkylthio
or
alkoxy having from 1 to about 3 carbon atoms; hydroxy; thiol; cyano; and halo.
R1 is preferably hydrogen, cyano, halo or methyl. R2 is also preferably
alkanyl,
more preferably methyl or ethyl, most preferably ethyl. R2 is also preferably
cycloalkyl, more preferably cyclopropyl; R2 which is alkenyl is preferably
ethenyl.
R2 which is alkylthio or alkoxy is preferably saturated, also preferably C1 or
C2,
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
8
most preferably methylthio or methoxy. R2 which is halo is preferably chloro
or
bromo.
Preferred compounds of this invention have the following structure:
R~ R2
O ~ ~NH / NH
HNJ ~ J
N
R2 OR
6-substituted 5-substituted
where R1 and R2 are as indicated in the following table:
Compound No. R_1- R_2 5-or 6- substitution
1 H CHg 5-
2 H CH2 CHg 5-
Methods of making the compounds of the invention
The compounds of this invention are synthesized using the following
procedures. The R1 - R~ radicals are omitted for clarity, unless they are
prepared in that specific scheme. The skilled artisan will appreciate that the
radicals omitted are added using techniques known in the art. For purposes of
this description, structure II below represents two types of benzoxazoles.
These
are represented by lil, where X is nitrogen and Y is sulfur, and- t-V, where X
is
sulfur and Y is nitrogen. Thus, for the purposes of this description these
structures are shown below;
A A A
II III IV
Imidazolinylamino groups are conveniently prepared from vitro and amino
compounds via the following example synthetic sequence:
CA 02272683 1999-OS-25
WO 98/23611 PCT/LTS97/20803
9
~ N02 SnCI X \ NHZ 1 ) CI2CS X ~ NCS
<~ ~ 2 ' ~~ ' I~i -- <,~ I
Y Y 2 NaOH Yi V
H2NCH2CH2NH2
X ~ N~NH Hg(OAc)2 X ~ NH NH~NH
I i HN
Y
Preferably these compounds are made from vitro or amino compounds, for
example those described above. The above starting vitro and amino
compounds are obtained via one or more synthetic steps comprising alkylations,
halogenations (usually brominations), and halogen displacement reactions.
These reaction types are summarized below;
ALKYLATION REACTION:
R
CY I j N02 RMgX CX I % N02
DD4 Y
FLUORINATION:
NOZ Pd(0) N02 Npz
,N I R3SnSnR3 ~N I CH- 3CO~F
O Br O - O ,
SnR3 F
HALOGENATION, PREFERABLY BROMINATION:
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
Br
X I w N02 SnCl2 X I w NH2 Br2 X I w NH2
i
Y Y
Br Br
X ~ NH2 Br2 X ~ NHZ
<Y I ~ ~Y
Br
R~ R~
X w NH2 Br2 X ~ NHz
~Y I i ~Y I i
Br
R2 R2
X w NOz Br2 X I w NOZ
i
Br
Preferably, chlorination is accomplished using C12, and iodination, by ICI
using the same reactions.
HALOGEN DISPLACEMENT REACTIONS:
Br Br Ra
R3SnL3
X w NH2 1 ) HNOz X ~ NO2 Fd (O~ X ~ N02
i 2) NaN02 y
CuCN R4ZH
Base
CN ZR4
I w NOZ ~ I w Npz
rY i Y i
Z = O, S, NR
_.._._~_____..._.. _.._~.~. J
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
11
R
R5 RsSnL3 s
X I w NOZ p~ X ~ NOz
Y ~ ~Y I i
Bf
CuCN R~ZH
Base
R R
~X I w N02 ~ I w N02
Y i Y i
CN ZR~
Z = O, S, N R
X ~ N RsSnL3 X ~ N
I ~ Pd (off
r
Y ~ Br Y ~ Rs
CuCN R~oZH
Base
Rs R$
~X w N02 X I w NOZ
r
Y CN <Y ~~o
Z = O, S, NR
INCORPORATION OF 2-SUBSTITUENTS:
HO N~ RCOX N N~
,, --~ R ~ /~
H2N ~ dehydrating agent O
( X = CI or alkoxy)
It will be apparent to the skilled artisan that the reactions illustrated
above
are known reactions. Furthermore) it is within the purview of the skilled
artisan
to vary these reactions to prepare compounds within the scope of the claims.
CA 02272683 1999-OS-25
WO 98/23611 PCT/CTS97/20803
12
In the above schemes, where an R is alkoxy or alkylthio, the
corresponding hydroxy or thiol compounds are derived from the final compounds
by using a standard dealkylating procedure (Bhatt, et al.) "Cleavage of
Ethers",
Synthesis, 1983, pp. 249-281 ).
The starting materials used in preparing the compounds of the invention
are known, made by known methods, or are commercially available as a starting
material.
It is recognized that the skilled artisan in the art of organic chemistry can
readily carry out manipulations without further direction, that is, it is well
within
the scope and practice of the skilled artisan to carry out these
manipulations.
These include reduction of carbonyl compounds to their corresponding alcohols,
oxidations, acylations) aromatic substitutions, both electrophilic and
nucleophilic,
etherifications, esterifications and saponifications and the like. These
manipulations are discussed in standard texts such as March, Advanced
Organic Chemistry (Wiley), Carey and Sundberg, Advanced Organic Chemistry
(2 vol.) and Trost and Fleming Comprehensive Organic S nthesis (6 vol.). The
skilled artisan will readily appreciate that certain reactions are best
carried out
when other functionality is masked or protected in the molecule, thus avoiding
any undesirable side reactions and/or increasing the yield of the reaction.
Often
the skilled artisan utilizes protecting groups to accomplish such increased
yields
or to avoid the undesired reactions. These reactions are found in the
literature
and are also well within the scope of the skilled artisan. Examples of many of
these manipulations are found, for example, in T. Greene, Protecting Groups in
Organic Synthesis.
Compound Examples
The following non-limiting examples provide details for the synthesis of 5-
imidazolinyiaminobenzoxazoles:
Example 1
5-f2-Imidazolinylamino)-4-methylbenzoxazoie.
A. 2.4-Dinitro-3-methyianisole. A solution of 3-methyl-2-nitroanisole (15.0 g,
89.73 mmol) in acetic acid (200 mL) is treated dropwise with a mixture of
concentrated nitric acid (5.70 mL, 89.73 mmol, d = 1.41 g/mL) and acetic
acid (40 mL). After addition of the nitric acid solution) concentrated
sulfuric acid (40 mL) is added dropwise. The reaction is poured over
crushed ice (500 g) and stirred for 1 hour while a white precipitate forms.
The precipitate is filtered and dried in a vacuum to afford a white solid
_ __.~_~._._..... __~.. _... r .._.._____ . __._...~~.m..._...
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
13
(15.1 g). This material is purified by chromatography (silica-gel, 20%
ethyl acetate/hexanes) to afford 9.20 g of 2,4-dinitro-3-methylanisole.
B. 4-Amino-3-methyl-2-nitroanisoie. A solution of 2,4-dinitro-3-methylanisole
(5.03 g, 23.7 mmol) in methanol (200 mL) is treated with stannous
chloride (18.72 g, 83.0 mmol). A reflux condenser is attached and the
mixture is heated with an oil bath to 45 oC for two hours. The mixture is
cooled to room temperature and poured over a saturated sodium
carbonate solution {300 mL). The mixture is extracted with methylene
chloride (400 mL). The methylene chloride layer is dried over magnesium
sulfate and concentrated under reduced pressure to yield- a dark orange
solid (3.53 g). This material is purified by chromatography (silica-gel,
30% ethyl acetate/hexanes) to afford 2.70 g of 4-amino-3-methyl-2-
nitroanisole as an orange solid.
C. 3-Methyl-2-vitro-4-trifluoroacetamidoanisole. A solution of 4-amino-3-
methyl-2-nitroanisole (3.53 g, 19.37 mmol) and triethylamine (2.83 mL,
20.34 mmol) in dichloromethane (25 mL) is treated with trifluoroacetic
anhydride (2.87 mL, 20.34 mmol). The mixture is stirred for one hour.
The reaction mixture is distributed between methylene chloride (50 mL)
and hydrochloric acid (50 rfiL of a 1.0 M solution). The methylene
chloride layer is dried over magnesium sulfate and concentrated under
reduced pressure to yield 5.37 g of 3-methyl-2-vitro-4-
trifluoroacetamidoanisole as a yellow solid.
D. 3-Methy(-2-vitro-4-trifluoroacetamidophenol. A solution of 3-methyl-2-
nitro-4-trifluoroacetamidoanisole (5.37 g, 19.3 mmol) in dry methylene
chloride (250 mL) at -78 oC under nitrogen atmosphere is treated
dropwise with a solution of boron tribromide (9.16 mL, 96.9 mmol) in dry
methylene chloride (50 mL). After complete addition, the mixture is
allowed to warm to room temperature and stirring is maintained for one
hour. The mixture is cooled to -78 oC and methanol (100 mL) is added
dropwise. After complete addition, the solution is allowed to warm to
room temperature. The reaction mixture is distributed between water
(100 mL) and methylene chloride (200 mL). The methylene chloride layer
is dried over magnesium sulfate and concentrated under reduced
pressure to yield 4.81 g of 3-methyl-2-vitro-4-trifluoroacetamidophenol.
E. 2-Amino-3-methyl-4-trifluoroacetamido~henol. A mixture of 3-methyl-2-
nitro-4-trifluoroacetamidophenol (4.81 g, 18.21 mmol) and 10%
CA 02272683 1999-OS-25
WO 98/23611 PCT/L1S97/20803
14
palladium-on-carbon (0.45 g) in methanol (100 mL) is shaken under an
atmosphere of hydrogen (50 psi) for three hours. The mixture is filtered
through Celite and concentrated under reduced pressure to yield 4.25 g
of 2-amino-3-methyl-4-trifluoroacetamidophenol as a red solid.
F. 4-Methyl-5-trifluoroacetamidobenzoxazole. A solution of 2-amino-3-
methyl-4-trifluoroacetamidophenol (4.25 g, 18.15 mmol) and concentrated
hydrochloric acid (45 mL) in methanol (5.2 mL) is treated with triethyl
orthoformate (2.98 mL, 27.2 mmol). A simple distillation apparatus is
attached and the mixture is heated with an oil bath to 90 oC and
methanol is distilled from the reaction. The reaction mixture is cooled to
room temperature and distributed between ethyl ether (200 mL) and
water (20 mL). The ether layer is washed with a 0.1 M aqueous solution
of sodium hydroxide (20 mL), dried over magnesium sulfate and
concentrated under reduced pressure to yield 3.40 g of 4-methyl-5-
trifluoroacetamidobenzoxazole as an orange solid.
G. 5-Amino-4-methylbenzoxazofe. A solution of 4-methyl-5-
trifluoroacetamidobenzoxazole (3.45 g, 14.13 mmol) in a 20% mixture of
water in methanol (20 mL) is treated with anhydrous potassium carbonate
(7.80 g) 56.52 mmol). A reflux condenser is attached and the mixture is
heated to 90 oC with an oil bath for five hours. The mixture is cooled to
room temperature and distributed between ethyl acetate (100 mL) and
water (100 mL). The ethyl acetate layer is dried over magnesium sulfate
and concentrated under reduced pressure to yield a black residue. This
material is purified by chromatography (silica gel, 50% ethyl
acetate/hexanes) to afford 2.30 g of 5-amino-4-methylbenzoxazole as an
off white solid.
H. 5-Isothiocyanato-4-methylbenzoxazole. A solution of 5-amino-4-
methylbenzoxazole (0.59 g, 3.98 mmol) in dichloromethane (100 mL) is
treated with di-2-pyridyl thionocarbonate (1.02 g, 4.39 mmol). The
mixture is stirred for two hours. The reaction mixture is concentrated
under reduced pressure to yield a brown residue. This material is purified
by chromatography (silica-gel) 20% ethyl acetate/hexanes) to afford 0.68
g of 5-isothiocyanato-4-methylbenzoxazole as a white solid.
I. 5-fN'-(2-AminoethYl?thioureido]-4-methylbenzoxazole. A solution of 5-
isothiocyanato-4-methylbenzoxazole (0.68 g, 3.58 mmol} in toluene (75
mL) is treated with ethylenediamine (0.84 mL, 12.53 mmol). The reaction
.~__~._.____.. _ .__ _.._ _.
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
is stirred for 30 minutes as the product precipitates. The precipitate is
filtered and dried in an oven to yield 0.85 g of 5-[N'-(2-
aminoethyl)thioureidoj-4-methylbenzoxazole as a white solid.
J. 5-(2-Imidazolinylamino)-4-methylbenzoxazole A solution of 5-[N'-(2-
aminoethyl)thioureidoj-4-methylbenzoxazole (0.85 g, 3.40 mmol) in
ethanol (75 mL) is treated with mercuric acetate (1.08 g, 3.40 mmol). The
color changes from light yellow to black. After four hours of stirring, the
reaction mixture is filtered through Celite and concentrated under reduced
pressure to yield a viscous oil. This material is purified by
chromatography (silica gel, 1 % saturated ammonium hydroxide
solution/20% methanol/chloroform) to afford 0.69 g of 5-(2-
imidazolinylamino)-4-methylbenzoxazole as the acetic acid salt, as a
white solid.
Example 2
4-Ethvl-5-(2-imidazolinylamino)benzoxazole
A. 2,4-Dinitro-3-ethvlanisole. A solution of 3-ethyl-2-nitroanisole (89.73
mmol) in acetic acid (200 mL) is treated dropwise with a mixture of
concentrated nitric acid (89.73 mmol, d = 1.41 g/mL) and acetic _acid (40
mL). After addition of the nitric acid solution, concentrated sulfuric acid
(40 mL) is added dropwise. The reaction is poured over crushed ice (500
g) and stirred for 1 hour while a white precipitate forms. The precipitate is
filtered and dried in a vacuum to afford a white solid. This material is
purified by chromatography {silica gel, 20% ethyl acetate/hexanes) to
afford 2,4-dinitro-3-ethylanisole.
B. 4-Amino-3-ethyl-2-nitroanisole. A solution of 2,4-dinitro-3-ethyianisole
(23.7 mmol) in methanol (200 mL) is treated with stannous chloride (83.0
_ mmol). A reffux condenser is attached and the mixture is heated with an
oil bath to 45 oC for two hours. The mixture is cooled to room
temperature and poured over a saturated sodium carbonate solution (300
mL). The mixture is extracted with methylene chloride (400 mL). The
methylene chloride layer is dried over magnesium sulfate and
concentrated under reduced pressure to yield the crude product. This
material is purified by chromatography (silica gel, 30% ethyl
acetate/hexanes) to afford 4-amino-3-ethyl-2-nitroanisoie.
CA 02272683 1999-OS-25
WO 98/23611 PCTIUS97/20803
16
C.3-Ethyl-2-nitro-4-trifluoroacetamidoanisole. A solution of 4-amino-3-ethyl-
2-nitroanisole (19.37 mmol) and triethylamine (20.34 mmol) in
dichloromethane (25 mL) is treated with trifluoroacetic anhydride (20.34
mmol). The mixture is stirred for one hour. The reaction mixture is
distributed between methylene chloride (50 mL) and hydrochloric acid (50
mL of a 1.0 M solution). The methylene chloride layer is dried over
magnesium sulfate and concentrated under reduced pressure to yield 3-
ethyl-2-vitro-4-trifluoroacetamidoanisole as a solid.
D. 3-Ethyl-2-vitro-4-trifluoroacetamidophenol. A soEution of 3-ethyl-2-vitro-4-
trifluoroacetamidoanisole (19.3 mmol) in dry methylene chloride (250 mL)
at -78 oC under nitrogen atmosphere is treated dropwise with a solution
of boron tribromide (96.9 mmol) in dry methylene chloride (50 mL). After
complete addition, the mixture is allowed to warm to room temperature
and stirring is maintained for one hour. The mixture is cooled to -78 oC
and methanol (100 mL) is added dropwise. After complete addition, the
solution is allowed to warm to room temperature. The reaction mixture is
distributed between water (100 mL) and methylene chloride (200 mL).
The methylene chloride layer is dried over magnesium sulfate and
concentrated under reduced pressure to yield 3-ethyl-2-vitro-4-
trifluoroacetamidophenol.
E. 2-Amino-3-ethyl-4-trifluoroacetamido~henol. To a solution of 3-ethyl-2-
nitro-4-trifluoroacetamidophenol (18.21 mmol) and 10% palladium-on-
carbon (0.45 g) in methanol (100 mL) is shaken under an atmosphere of
hydrogen (50 psi} for three hours. The mixture is filtered through Celite
and concentrated under reduced pressure to yield 2-amino-3-ethyl-4-
trifluoroacetamidophenol.
F. 4-Ethy-5-trifluoroacetamidobenzoxazole. A mixture of 2-amino-3-ethyl-4-
trifluoroacetamidophenol (18.15 mmol) and concentrated hydrochloric
acid (45 mL) in methanol (5.2 mL) is treated with triethyl orthoformate
(27.2 mmol). A simple distillation apparatus is attached and the mixture is
heated with an oil bath to 90 oC and methanol is distilled from the
reaction. The reaction mixture is cooled to room temperature and
distributed between ethyl ether (200 mL) and water (20 mL). The ether
layer is washed with a 0.1 M aqueous solution of sodium hydroxide (20
mL), dried over magnesium sulfate and concentrated under reduced
pressure to yield 4-ethyl-5-trifluoroacetamidobenzoxazole.
_._. _..._..
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
17
G. 5-Amino-4-ethylbenzoxazoie A solution of 4-ethyl-5-
trifluoroacetamidobenzoxazole (14.13 mmol) in a 20% mixture of water in
methanol (20 mL) is treated with anhydrous potassium carbonate (56.52
mmol). A reflux condenser is attached and the mixture is heated to 90 oC
with an oil bath for five hours. The mixture is cooled to room temperature
and distributed between ethyl acetate (100 mL) and water (100 mL). The
ethyl acetate layer is dried over magnesium sulfate and concentrated
- under reduced pressure to yield a black residue. This material is purified
by chromatography (silica gel, 50% ethyl acetate/hexanes) to afford 5-
amino-4-ethylbenzoxazole.
H. 4-Ethvl-5-isothiocyanatobenzoxazole A solution of 5-amino-4-
ethylbenzoxazole (3.98 mmol) in dichioromethane (100 mL) is treated
with di-2-pyridyl thionocarbonate {4.39 mmol). The mixture is stirred for
two hours. The reaction mixture is concentrated under reduced pressure
to yield a brown residue. This material is purified by chromatography
(silica gel, 20% ethyl acetate/hexanes) to afford 4-ethyl-5-
isothiocyanatobenzoxazole.
I. 5-(N-(2-Aminoethyl)thioureido~-4-ethylbenzoxazole A solution of 4-ethyl-
5-isothiocyanatobenzoxazole (3.58 mmol) in toluene (75 mL) is treated
with ethylenediamine (12.53 mmol). The reaction is stirred for 30
minutes as the product precipitates. The precipitate is filtered and dried
in an oven to yield 5-[N-(2-aminoethyl)thioureidoj-4-ethylbenzoxazole as
a white solid.
J. 4-Ethvl-5-(2-imidazolinylamino~enzoxazole A solution of 5-[N'-(2-
aminoethyl)thioureidoj-4-ethylbenzoxazole (3.40 mmol) in ethanol (75
mLj is treated with mercuric acetate (3.40 mmol). The color changes
from light yellow to black. After four hours of stirring, the reaction mixture
is filtered through Celite and concentrated under reduced pressure to
yield a viscous oil. This material is purified by chromatography (silica gel,
1 % saturated ammonium hydroxide solution/20% methanol/chloroform) to
afford 5-{2-imidazolinylamino)-4-ethylbenzoxazole as an acetic acid salt.
Alternative Imidazolinylamine Formation from Aryl Amines
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
18
H O N
N Abs. EtOH N ~ KZC03 ~ ~-SCH3
~S + CH31 ----~ ~ ~--SCH3 + CI OCH3 ~ N
30 - 35 oC N 30 - 35 oC
H H O
CN
Me ~--SCH3 Me
<N I \ N~ ~OCH3 ~N ~ ~ N~NH HOAc
O / ~ O / HNJ
AcOH, Reflux
A. 2-Methylthio-2-imidazoline. 2-Imidazolidinethione (5.0 g) is added to
absolute ethanol (40 mL) while stirring. Methyl iodide (4.3 mL) is rapidly
added. The reaction mixture is warmed to 30-35 °C for 45 minutes. This
solution is used directly in the next reaction.
B. N-Carbomethoxy-2-thiomethyl-2-imidazoline. Potassium carbonate (10.1
g) is added to the mixture in (A) above, followed by addition of methyl
chloroformate (4.2 mL) while stirring. After 45 minutes, the reaction
mixture is heated to 55 °C and the insoluble salts are filtered off.
These
salts are washed with 10 mL of absolute ethanol. The filtrate (and
ethanol wash) is cooled to -20 °C and the recrystallized product is
isolated on a Buchner funnel. The product is washed with 10 mL cold (-
20 °C) absolute ethanol. The product is dried overnight under vacuum at
room temperature, yielding N-carbomethoxy-2-thiomethyl-2-imidazoline.
C. 5-(2-Imidazolinylamino)-4-methylbenzoxazole. The N carbomethoxy-2-
thiomethyl-2-imidazoiine is combined with the amine (1 G) of Example 1 in
10% acetic acid in ethanol and heated to reflux. After the starting amine
is consumed, the mixture is decolorized with carbon. The mixture is
cooled, filtered and rotary evaporated. Upon recrystallization and drying,
the compound (1J) of Example 1 is obtained as an acetic acid salt.
Using the methodologies outlined and exemplified above the following
compounds are made;
_._ __ ___ ____ _ ._.~ _.r.~._____. __
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
19
R2
N ~ ~NH
CO I / NH.~
~Rs
R~
Example R R R
3 H OMe H
4 H SMe H
H cyclopropyl H
6 H -CH=CH H
7 H CI H
8 H Br H
H I H
Me Me H
11 OMe Me H
12 F Me H
13 CI Me H
14 Br Me H
I Me H
16 Me Et H
17 CN Et _ H
18 OMe Et H
19 F Et H
CI Et H
21 Br Et H
22 I Et H
23 Me OMe H
24 CN OMe H
OMe OMe H
28 F OMe H
27 CI OMe H
28 Br OMe H
29 I OMe H
F -CH=CH H
31 CI -CH=CH H
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
32 Br -CH=CH H
33 I -CH=CH H
34 Me -CH=CH H
35 OMe -CH=CH H
36 CN -CH=CH H
37 F Cyciopropyl H
38 CI Cyclopropyl H
39 Br Cyclopropyl H
40 I Cyciopropyl H
41 Me Cyclopropyl H
42 OMe Cyclopropyl H
43 CN Cyclopropyl H
44 Me Br H
45 CN Br H
46 OMe Br H
47 F Br H
48 CI Br H
49 Br Br H
50 I Br H
51 H Br Me
52 H Br OMe
53 H Br Br
54 H OMe Me
55 H OMe OMe
56 H _OMe _ ~ - B
R1
NYNH
HNJ
N R3
R2
Example R~ R R
57 Me -CH=CH H
58 Me OMe H
59 CI Me H
_ _._ _ _._..___. _ ~_.
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
21
60 CI CI H
61 CI Br H
62 CI i H
63 CI OMe H
64 Br Me H
65 Br CI H
66 Br Br H
67 Br I H
68 Br OMe H
69 I Me H
70 I CI H
71 I Br
72 I I H
73 I OMe H
74 Et Me
75 Et CI H
76 Et Br H
77 Et I H
78 Et OMe H __-
79 CN -CH=CH H
80 Me H CI
81 Me H Br
82 Me H I
83 CI H CI
OMe -CH=CH H
85 Me Me CI
86 Me Me Br
87 Me Me I
88 Et Me H
89 Et CI H
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
22
N R N
Rs
R~
Exam le R R R R
90 H Me H Me
91 H Et H Me
92 H OMe H Me
Ri
N
~R3
2
Exam le R R R R
93 Me Me H Me
COmpOSItIOrIS
Another aspect of this invention is compositions which comprise a safe
and effective amount of a subject compound, or a pharmaceutically-acceptable
salt thereof, and a pharmaceutically-acceptable carrier.
As used herein, "safe and effective amount" means an amount of the
subject compound sufficient to significantly induce a positive modification in
the
condition to be treated, but low enough to avoid serious side effects (at a
reasonable benefitlrisk ratio), within the scope of sound medical judgment. A
safe and effective amount of the subject compound will vary with the age and
physical condition of the patient being treated, the severity of the
condition, the
duration of the treatment) the nature of concurrent therapy, the particular
pharmaceutically-acceptable carrier utilized, and like factors within the
knowledge and expertise of the attending physician.
Preparing a dosage form is within the purview of the skilled artisan.
Examples are provided for the skilled artisan, but are non-limiting) and it is
contemplated that the skilled artisan can prepare variations of the
compositions
claimed.
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
23
Compositions of this invention preferably comprise from about 0.0001
to about 99% by weight of the subject compound) more prEferably from about
0.01 % to about 90% of the compound of the invention. Depending upon the
route of administration and attendant bioavailability, solubility or
dissolution
characteristics of the dosage form, the dosage form has preferably from about
10% to about 50%, also preferably from about 5% to about 10%, also preferably
from about 1 % to about 5%, and also preferably from about 0.01 % to about 1
of the subject compound. The frequency of dosing of the subject compound is
dependent upon the pharmacokinetic properties of each specific agent (for
example, biological half-life) and can be determined by the skilled artisan.
In addition to the subject compound, the compositions of this invention
contain a pharmaceutically-acceptable carrier. The term "pharmaceutically-
acceptable carrier", as used herein, means one or more compatible solid or
liquid filler diluents or encapsulating substances which are suitable for
administration to a mammal. The term "compatible", as used herein, means that
the components of the composition are capable of being commingled with the
subject compound, and with each other, in a manner such that there is no
interaction which would substantially reduce the pharmaceutical efficacy of
the
composition under ordinary use situations. Preferably when liquid dose forms
are used, the compounds of the invention are soluble in the components of the
composition. Pharmaceutically-acceptable carriers must, of course, be of
sufficiently high purity and sufficiently low toxicity to render them suitable
for
administration to the mammal being treated.
Some examples of substances which can serve as pharmaceutically-
acceptable carriers or components thereof are sugars, such as lactose, glucose
and sucrose; starches, such as corn starch and potato starch; cellulose and
its
derivatives, such as sodium carboxymethyl cellulose, ethyl cellulose, and
methyl
cellulose; powdered tragacanth; malt; gelatin; talc; solid lubricants, such as
stearic acid and magnesium stearate; calcium sulfate; vegetable oils, such as
peanut oil, cottonseed oil, sesame oil, olive oil, corn oil and oil of
theobroma;
polyols such as propylene glycol, glycerine, sorbitol, mannitol) and
polyethylene
glycol; alginic acid; emulsifiers) such as the Tweens~; wetting agents, such
sodium lauryl sulfate; coloring agents; flavoring agents; tableting agents,
stabilizers; antioxidants; preservatives; pyrogen-free water; isotonic saline;
and
phosphate buffer solutions.The choice of a pharmaceutically-acceptable carrier
to be used in conjunction with the subject compound is basically determined by
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97120803
24
the way the compound is to be administered. If the subject compound is to be
injected, the preferred pharmaceutically-acceptable carrier is sterile,
physiological saline, with a blood-compatible suspending agent, the pH of
which
has been adjusted to about 7.4.
If the preferred mode of administering the subject compound is perorally)
the preferred unit dosage form is therefore tablets, capsules, lozenges,
chewable tablets, and the like. Such unit dosage forms comprise a safe and
effective amount of the subject compound, which is preferably from about 0.01
mg to about 350 mg) more preferably from about 0.1 mg to about 35 mg, based
on a 70 kg person. The pharmaceutically-acceptable carrier suitable for the
preparation of unit dosage forms for peroral administration are well-known in
the
art. Tablets typically comprise conventional pharmaceutically-compatible
adjuvants as inert diluents, such as calcium carbonate, sodium carbonate,
mannitol) lactose and cellulose; binders such as starch, gelatin and sucrose;
disintegrants such as starch, alginic acid and croscarmeiose; lubricants such
as
magnesium stearate, stearic acid and talc. Glidants such as silicon dioxide
can
be used to improve flow characteristics of the powder mixture. Coloring
agents,
such as the FD&C dyes, can be added for appearance. Sweeteners and
flavoring agents, such as aspartame, saccharin, menthol) peppermint, and fruit
flavors, are useful adjuvants for chewable tablets. Capsules typically
comprise
one or more solid diluents disclosed above. The selection of carrier
components
depends on secondary considerations like taste) cost, and shelf stability,
which
are not critical for the purposes of this invention, and can be readily made
by a
person skilled in the art.
Peroral compositions also include liquid solutions, emulsions)
suspensions, and the like. The pharmaceutically-acceptable carriers suitable
for
preparation of such compositions are well known in the art. Such liquid oral
compositions preferably comprise from about 0.001 % to about 5% of the subject
compound, more preferably from about 0.01 % to about 0.5%. Typical
components of carriers for syrups, elixirs) emulsions and suspensions include
ethanol, glycerol) propylene glycol, polyethylene glycol, liquid sucrose,
sorbitol
and water. For a suspension, typical suspending agents include methyl
cellulose) sodium carboxymethyi cellulose, Avicel~ RC-591 ) tragacanth and
sodium alginate; typical wetting agents include lecithin and polysorbate 80;
and
typical preservatives include methyl paraben and sodium benzoate. Peroral
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
liquid compositions may also contain one or more components such as
sweeteners) flavoring agents and colorants disclosed above.
Other compositions useful for attaining systemic delivery of the subject
compounds include sublingual and buccal dosage forms. Such compositions
typically comprise one or more of soluble filler substances such as sucrose)
sorbitol and mannitol; and binders such as acacia, microcrystalline cellulose,
carboxymethyl cellulose and hydroxypropyl methyl cellulose. Glidants,
lubricants, sweeteners, colorants, antioxidants and flavoring agents disclosed
above may also be included.
Compositions can also be used to deliver the compound to the site where
activity is desired: intranasal doses for nasal decongestion, inhalants for
asthma,
and eye drops, gels and creams for ocular disorders.
Preferred compositions of this invention include solutions or emulsions,
preferably aqueous solutions or emulsions comprising a safe and effective
amount of a subject compound intended for topical intranasal administration.
Such compositions preferably comprise from about 0.001 % to about 25% of a
subject compound, more preferably from about 0.07 % to about 10%. Similar
compositions are preferred for systemic delivery of subject compounds by the
intranasal route. Compositions intended to deliver the compound systemically
by intranasal dosing preferably comprise sirniiar amounts of a subject
compound
as are determined to be safe and effective by peroral or parenterai
administration. Such compositions used for intranasal dosing also typically
include safe and effective amounts of preservatives, such as benzalkonium
chloride and thimerosal and the like; chelating agents) such as edetate sodium
and others; buffers such as phosphate, citrate and acetate; tonicity agents
such
as sodium chloride, potassium chloride, glycerin, mannitol and others;
antioxidants such as ascorbic acid) acetylcystine, sodium metabisulfate and
others; aromatic agents; viscosity adjustors, such as polymers, including
cellulose and derivatives thereof, and polyvinyl alcohol and acids and bases
to
adjust the pH of these aqueous compositions as needed. The compositions may
also comprise local anesthetics or other actives. These compositions can be
used as sprays, mists, drops) and the like.
Other preferred compositions of this invention include aqueous solutions,
suspensions, and dry powders comprising a safe and effective amount of a
subject compound intended for atomization and inhalation administration. Such
compositions preferably comprise from about 0.1 % to about 50% of a subject
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
26
compound, more preferably from about 1 % to about 20%; of course, the amount
can be altered to fit the circumstance of the patient contemplated and the
package. Such compositions are typically contained in a container with
attached
atomizing means. Such compositions also typically include propellants such as
chiorofluorocarbons 12/11 and 12/114, and more environmentally friendly
fluorocarbons, or other nontoxic volatiles; solvents such as water, glycerol
and
ethanol, these include cosolvents as needed to solvate or suspend the active;
stabilizers such as ascorbic acid, sodium metabisulfite; preservatives such as
cetylpyridinium chloride and benzalkonium chloride; tonicity adjustors such as
sodium chloride; buffers; and flavoring agents such as sodium saccharin. Such
compositions are useful for treating respiratory disorders, such as asthma and
the like.
Other preferred compositions of this invention include aqueous solutions
comprising a safe and effective amount of a subject compound intended for
topical intraocular administration. Such compositions preferably comprise from
about 0.0001 % to about 5% of a subject compound) more preferably from about
0.01 % to about 0.5%. Such compositions also typically include one ~r more of
preservatives) such as benzalkonium chloride, thimerosal, phenylmercuric
acetate; vehicles, such as poloxamers) modified celluloses, povidone and
purified water; tonicity adjustors, such as sodium chloride, mannitol and
glycerin;
buffers such as acetate, citrate, phosphate and borate; antioxidants such as
sodium metabisulfite, butylated hydroxy toluene and acetyl cysteine; acids and
bases may be used to adjust the pH of these formulations as needed.
Other preferred compositions of this invention useful for peroral
administration include solids, such as tablets and capsules, and liquids, such
as
solutions, suspensions and emulsions (preferably in soft gelatin capsules))
comprising a safe and effective amount of a subject compound. Such
compositions preferably comprise from about 0.01 mg to about 350 mg per
dose, more preferably from about 0.1 mg to about 35 mg per dose. Such
compositions can be coated by conventional methods, typically with pH or time-
dependent coatings, such that the subject compound is released in the
gastrointestinal tract at various times to extend the desired action. Such
dosage
forms typically include, but are not limited to) one or more of cellulose
acetate
phthalate, polyvinylacetate phthalate, hydroxypropyl methyl cellulose
phthalate,
ethyl cellulose) Eudragit~ coatings, waxes and shellac.
_ __... ._. _ T .~~__. ..__.
CA 02272683 1999-OS-25
WO 98/23611 PCT/(TS97/20803
27
Any of the compositions of this invention may optionally include other
drug actives. Non-limiting examples of drug actives which may be incorporated
- in these compositions, include:
Antihistamines, including'
Hydroxyzine, preferably at a dosage range of from about 25 to about 400 mg;
Doxylamine, preferably at a dosage range of from about 3 to about 75 mg;
Pyrilamine, preferably at a dosage range of from about 6.25 to about 200 mg;
Chlorpheniramine, preferably at a dosage range of from about 1 to about 24 mg;
Phenindamine, preferably at a dosage range of from about 6.25 to about 150
mg; Dexchlorpheniramine, preferably at a dosage range of from about 0.5 to
about 12 rng; Dexbrompheniramine, preferably at a dosage range of from about
0.5 to about 12 mg; Ciemastine, preferably at a dosage range of from about 1
to
about 9 mg; Diphenhydramine, preferably at a dosage range of from about 6.25
to about 300 mg; Azelastine, preferably at a dosage range of from about 140 to
about 1,680 ~g (when dosed intranasally); 1 to about 8 mg (when dosed orally);
Acrivastine, preferably at a dosage range of from about 7 to about 24 mg;
Levocarbastine (which can be dosed as an intranasal or ocular medicament))
preferably at a dosage range of from about 100 to about 800 mg; Mequitazine,
preferably at a dosage range of from about 5 to about 20 mg; Astemizole)
preferably at a dosage range of from about 5 to about 20 mg; Ebastine,
preferably at a dosage range of from about 5 to about 20 mg; Loratadine,
preferably at a dosage range of from about 5 to about 40 mg; Cetirizine,
preferably at a dosage range of from about 5 to about 20 mg; Terfenadine,
preferably at a dosage range of from about 30 to about 480 mg; Terfenadine
metabolites; Promethazine, preferably at a dosage range of from about 6.25 to
about 50 mg; Dimenhydrinate, preferably at a dosage range of from about 12.5
to about 400 mg; Meclizine, preferably at a dosage range of from about 6.25 to
about 50 mg; Tripelennamine, preferably at a dosage range of from about 6.25
to about 300 mg; Carbinoxamine, preferably at a dosage range of from about
0.5 to about 16 mg; Cyproheptadine, preferably at a dosage range of from about
2 to about 20 mg; Azatadine) preferably at a dosage range of from about 0.25
to
about 2 mg; Brompheniramine, preferably at a dosage range of from about 1 to
about 24 mg; Triprolidine, preferably at a dosage range of from about 0.25 to
about 10 mg; Cyclizine, preferably at a dosage range of from about 12.5 to
about 200 mg; Thonzylamine, preferably at a dosage range of from about 12.5
to about 600 mg; Pheniramine, preferably at a dosage range of from about 3 to
CA 02272683 1999-OS-25
WO 98/Z3611 PCT/US97/20803
28
about 75 mg; Cyclizine, preferably at a dosage range of from about 12.5 to
about 200 mg and others;
Antitussives, including;
Codeine, preferably at a dosage range of from about 2.5 to about 120 mg;
Hydrocodone, preferably at a dosage range of from about 2.5 to about 40 mg;
Dextromethorphan, preferably at a dosage range of from about 2.5 to about 120
mg; Noscapine, preferably at a dosage range of from about 3 to about 180 mg;
Benzonatate) preferably at a dosage range of from about 100 to about 600 mg;
Diphenhydramine, preferably at a dosage range of from about 12.5 to about 150
mg; Chlophedianol, preferably at a dosage range of from about 12.5 to about
100 mg; Clobutinol, preferably at a dosage range of from about 20 to about 240
mg; Fominoben, preferably at a dosage range of from about 80 to about 480
mg; Glaucine; Pholcodine, preferably at a dosage range of from about 1 to
about 40 mg; Zipeprol, preferably at a dosage range of from about 75 to about
300 mg; Hydromorphone) preferably at a dosage range of from about 0.5 to
about 8 mg; Carbetapentane, preferably at a dosage range of from about 15 to
about 240 mg; Caramiphen, preferably at a dosage range of from about 10 to
about 100 mg; Levopropoxyphene, preferably at a dosage range of from about
25 to about 200 mg and others; -
Antiinflammatories, preferably Non-Steroidal Anti-inflammatories tNSAIDS~
including:
Ibuprofen) preferably at a dosage range of from about 50 to about 3,200 mg;
Naproxen, preferably at a dosage range of from about 62.5 to about 1,500 mg;
Sodium naproxen, preferably at a dosage range of from about 110 to about
1,650 mg; Ketoprofen, preferably at a dosage range of from about 25 to about
300 mg; Indoprofen) preferably at a dosage range of from about 25 to about 200
mg; Indomethacin, preferably at a dosage range of from about 25 to about 200
mg; Sulindac) preferably at a dosage range of from about 75 to about 400 mg;
Diflunisal, preferably at a dosage range of from about 125 to about 1,500 mg;
Ketorolac) preferably at a dosage range of from about 10 to about 120 mg;
Piroxicam) preferably at a dosage range of from about 10 to about 40 mg;
Aspirin) preferably at a dosage range of from about 80 to about 4,000 mg;
Meclofenamate, preferably at a dosage range of from about 25 to about 400 mg;
Benzydamine) preferably at a dosage range of from about 25 to about 200 mg;
Carprofen, preferably at a dosage range of from about 75 to about 300 mg;
Diclofenac, preferably at a dosage range of from about 25 to about 200 mg;
_._. ~_~ _. __ T
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
29
Etodoiac, preferably at a dosage range of from about 200 to about 1,200 mg;
Fenbufen, preferably at a dosage range of from about 300 to about 900 mg;
Fenoprofen, preferably at a dosage range of from about 200 to about 3,200 mg;
Flurbiprofen, preferably at a dosage range of from about 50 to about 300 mg;
Mefenamic acid, preferably at a dosage range of from about 250 to about 1,500
mg; Nabumetone, preferably at a dosage range of from about 250 to about
2,000 mg; Phenylbutazone, preferably at a dosage range of from about 100 to
about 400 mg; Pirprofen, preferably at a dosage range of from about 100 to
about 800 mg; Tolmetin) preferably at a dosage range of from about 200 to
about 1,800 mg and others;
Analgesics, includina~
Acetaminophen, preferably at a dosage range of from about 80 to about 4,000
mg; and others:
Expectorants/Mucolytics including;
Guaifenesin, preferably at a dosage range of from about 50 to about 2,400 mg;
N-Acetylcysteine, preferably at a dosage range of from about 100 to about 600
mg; Ambroxol, preferably at a dosage range of from about 15 to about 120 mg;
Bromhexine) preferably at a dosage range of from about 4 to about 64 mg;
Terpin hydrate, preferably at a dosage range of from about 100 to about 1,200
mg; Potassium iodide, preferably at a dosage range of from about 50 to about
250 mg and others;
Anticholineraics (e a Atroainics) preferably intranasally or orally
administered
anticholineraics includina~
Ipratroprium (preferably intranasally), preferably at a dosage range of from
about 42 to about 252 pg; Atropine sulfate (preferably oral), preferably at a
dosage range of from about 10 to about 1,000 pg; Belladonna (preferably as an
extract)) preferably at a dosage range of from about 15 to about 45 mg
equivalents; Scopolamine, preferably at a dosage range of from about 400 to
about 3,200 fig; Scopolamine methobromide, preferably at a dosage range of
from about 2.5 to about 20 mg; Homatropine methobromide) preferably at a
dosage range of from about 2.5 to about 40 mg; Hyoscyamine (preferably oral),
preferably at a dosage range of from about 125 to about 1,000 ~.g;
Isopropramide (preferably oral), preferably at a dosage range of from about 5
to
about 20 mg; Orphenadrine (preferably oral), preferably at a dosage range of
from about 50 to about 400 mg; Benzalkonium chloride (preferably intranasally)
preferably a 0.005 to about 0.1 % solution and others;
CA 02272683 1999-OS-25
WO 98/23611 PCT/(1S97/20803
Mast Cell Stabilizers, preferably intranasally or orally administered mast
cell
stabilizers, including:
Cromalyn, preferably at a dosage range of from about 10 to about 60 mg;
Nedocromil, preferably at a dosage range of from about 10 to about 60 mg;
Oxatamide, preferably at a dosage range of from about 15 to about 120 mg;
Ketotifen, preferably at a dosage range of from about 1 to about 4 mg;
Lodoxamide, preferably at a dosage range of from about 100 to about 3,000 ~g
and others;
Leukotriene Antagonists. includinc~Zileuton and others;
Meth_yfxanthines, including;
Caffeine, preferably at a dosage range of from about 65 to about 600 mg;
Theophylline) preferably at a dosage range of from about 25 to about 1,200 mg;
Enprofylline; Pentoxifylline) preferably at a dosage range of from about 400
to
about 3,600 mg; Aminophylline, preferably at a dosage range of from about 50
to about 800 mg; Dyphylline, preferably at a dosage range of from about 200 to
about 1,600 mg and others;
Antioxidants or radical inhibitors, including;
Ascorbic acid, preferably at a dosage range of from about 50 to about 10,000
mg; Tocopherol, preferably at a dosage range of from about 50 to about 2,000
mg; Ethanol, preferably at a dosage range of from about 500 to about 10,000
mg and others;
Steroids, preferaby intranasally administered steroids including
Beclomethasone, preferably at a dosage range of from about 84 to about 336 ~
g; Fluticasone, preferably at a dosage range of from about 50 to about 400
fig;
Budesonide, preferably at a dosage range of from about 64 to about 256 fig;
Mometasone, preferably at a dosage range of from about 50 to about 300 mg;
Triamcinolone, preferably at a dosage range of from about 110 to about 440
fig;
Dexamethasone, preferably at a dosage range of from about 168 to about 1,008
p,g; Flunisolide, preferably at a dosage range of from about 50 to about 300
pg;
Prednisone (preferably oral), preferably at a dosage range of from about 5 to
about fi0 mg; Hydrocortisone (preferably oral), preferably at a dosage range
of
from about 20 to about 300 mg and others;
Bronchodilators, preferably for inhalation, includingi
Albuterol, preferably at a dosage range of from about 90 to about 1,080 pg; 2
to
about 16 mg (if dosed orally); Epinephrine, preferably at a dosage range of
from
about 220 to about 1,320 fig; Ephedrine, preferably at a dosage range of from
._~._.__. .. ._ . _ 1 ._ _.___.__ ._~.._.._~._. ._
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
31
about 15 to about 240 mg (if dosed orally); 250 to about 1,000 p,g (if dosed
intranasally); Metaproterenol, preferably at a dosage range of from about 65
to
about 780 p.g or 10 to about 80 mg if dosed orally; Terbutaline) preferably at
a
dosage range of from about 200 to about 2,400 p.g; 2.5 to about 20 mg (if
dosed
orally); Isoetharine, preferably at a dosage range of from about 340 to about
1,360 p,g; Pirbuterol) preferably at a dosage range of from about 200 to about
2,400 fig; Bitolterol) preferably at a dosage range of from about 370 to about
2,220 pg; Fenoterol, preferably at a dosage range of from about 100 to about
1,200 ~,g; 2.5 to about 20 mg (if dosed orally); Rimeterol, preferably at a
dosage
range of from about 200 to about 1,600 p,g; Ipratroprium, preferably at a
dosage
range of from about 18 to about 216 ~g (inhalation) and others; and
Antivirals. includin4:
Amantadine, preferably at a dosage range of from about 50 to about 200 mg;
Rimantadine, preferably at a dosage range of from about 50 to about 200 mg;
Enviroxime; Nonoxinols, preferably at a dosage range of from about 2 to about
20 mg (preferably an intranasal form); Acyclovir, preferably at a dosage range
of
from about 200 to about 2,000 mg (oral); 1 to about 10 mg (preferably an
intranasal form); Alpha-Interferon, preferably at a dosage range of from about
3
to about 36~ MIU; Beta-Interferon, preferably at a dosage range of from about
3
to about 36 MIU and others;
Ocular Drug actives: acetylcholinesterase inhibitors, e.g., echothiophate from
about 0.03% to about 0.25% in topical solution and others; and
Gastrointestinal actives: antidiarrheals, e.g., loperamide from about 0.1 mg
to
about 1.0 mg per dose, and bismuth subsalicylate from about 25 mg to about
300 mg per dose and others.
Of course) clearly contemplated and included in the description above are
the acid or base addition salts, esters, metabolites, stereoisomers and
enantiomers of these preferred combination actives, as well as their analogues
of these actives that are safe and effective. It is also recognized that an
active
may be useful for more than one of the above uses, and these uses are clearly
contemplated as well. This overlap is recognized in the art and adjusting
dosages and the like to fit the indication is well within the purview of the
skilled
medical practitioner.
Methods of use
Without being bound by theory, it is contemplated that the primary
mechanism by which alpha-2 agonists provide efficacy is by intervening in the
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
32
biological cascade responsible for disorders) and/or manifestations) thereof.
It
may be that there is no deficit in alpha-2 adrenoceptor activity: such
activity may
be normal. However, administration of an alpha-2 agonist may be a useful way
of rectifying a disorder, condition or manifestation thereof.
Thus as used herein, the terms "disease," "disorder" and "condition" are
used interchangeably to refer to maladies related to or modulated by alpha-2
adrenoceptor activity.
As used herein, a disorder described by the terms "modulated by alpha-2
adrenoceptors," or "modulated by alpha-2 adrenoceptor activity" refers to a
disorder) condition or disease where alpha-2 adrenoceptor activity is an
effective
means of alleviating the disorder or one or more of the biological
manifestations
of the disease or disorder; or interferes with one or more points in the
biological
cascade either leading to the disorder or responsible for the underlying
disorder;
or alleviates one or more symptoms of the disorder. Thus, disorders subject to
"modulation" include those for which:
~ The lack of alpha-2 activity is a "cause" of the disorder or one or more of
the
biological manifestations, whether the activity was altered genetically, by
infection, by irritation) by internal stimulus or by some other cause;
~ The disease or disorder or the observable manifestation or manifestations of
the disease or disorder are alleviated by alpha-2 activity. The lack of alpha-
2
activity need not be causally related to the disease or disorder or the
observable manifestations thereof;
~ Alpha-2 activity interferes with part of the biochemical or cellular cascade
that
results in or relates to the disease or disorder. In this respect, the alpha-2
activity alters the cascade, and thus controls the disease, condition or
disorder.
The compounds of this invention are particularly useful for the treatment
of nasal congestion associated with allergies, colds, and other nasal
disorders,
as well as the sequeiae of congestion of the mucous membranes (for example,
sinusitis and otitis media). At effective doses, it has been found that
undesired
side effedts can be avoided.
While not limited to a particular mechanism of action, the subject
compounds are believed to provide advantages in the treatment of nasal
decongestion over related compounds through their ability to interact with
alpha-
2 adrenoceptors. The subject compounds have been found to be alpha-2
~~_.._._..._.._ _..~~. T
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
33
adrenoceptor agonists which cause constriction of peripheral vascular beds in
the nasal turbinates.
Alpha-2 adrenoceptors are distributed both inside and outside of the
central nervous system. Thus) though not essential for activity or efficacy,
certain disorders preferably are treated with compounds that act on alpha-2
adrenoceptors in only one of these regions. Compounds of this invention vary
in
their ability to penetrate into the central nervous system and, thus, to
produce
effects mediated through central alpha-2 adrenoceptors. Thus, for example, a
compound which displays a higher degree of central nervous system activity is
preferred for central nervous system indications over other compounds as
described below. However, even for compounds that exhibit primarily peripheral
activity, central nervous system actions can be evoked by an increase in the
dose of the compound. Further specifcity of action of these compounds can be
achieved by delivering the agent to the region where activity is desired (for
example, topical administration to the eye, nasal mucosa or respiratory
tract).
Compounds preferred for, but not limited to, the treatment of certain
cardiovascular disorders, pain, substance abuse and/or withdrawal, ulcer and
hyperacidity include those compounds that are centrally acting. By centrally
acting what is meant is that they have some action on the _alpha-2
adrenoceptors in the central nervous system in addition to their action at
peripheral alpha-2 adrenoceptors.
Compounds preferred for, but not limited to, the treatment of respiratory
disorders, ocular disorders, migraine, certain cardiovascular disorders, and
certain other gastrointestinal disorders are peripherally acting. By
peripherally
acting) what is meant is that these compounds act primarily on alpha-2
adrenoceptors in the periphery, rather than those in the central nervous
system.
Methods are available in the art to determine which compounds are primarily
peripherally acting and which are primarily centrally acting.
Thus, compounds of the subject invention are also useful for the
treatment of ocular disorders such as ocular hypertension) glaucoma,
hyperemia) conjunctivitis, and uveitis. The compounds are administered either
perorally, or topically as drops, sprays, mists, gels or creams directly to
the
surface of the mammalian eye.
The compounds of this invention are also useful for controlling
gastrointestinal disorders) such as diarrhea, irritable bowel syndrome,
hyperchlorhydria and peptic ulcer.
CA 02272683 1999-OS-25
WO 98/23611 PCT/ITS97/20803
34
The compounds of this invention are also useful for diseases and
disorders associated with sympathetic nervous system activity, including
hypertension, myocardial ischemia, cardiac reperfusion injury, angina, cardiac
arrhythmia, heart failure and benign prostatic hypertrophy. Due to their
sympatholytic effect, compounds are also useful as an adjunct to anesthesia
during surgical procedures.
The compounds of this invention are also useful for relieving pain
associated with various disorders. The compounds are administered perorally,
parenteraily) and/or by direct injection into the cerebrospinal fluid.
The compounds of this invention are also useful for the prophylactic or
acute treatment of migraine. The compounds are administered perorally,
parenterally or intranasally.
The compounds of this invention are also useful for treatment of
substance abuse, in particular abuse of alcohol and opiates, and alleviating
the
abstinence syndromes evoked by withdrawal of these substances.
The compounds of this invention are also useful for other diseases and
disorders where vasoconstriction) particularly of veins, would provide a
benefit,
including septic or cardiogenic shock) elevated intracranial pressure,
hemmorhoids, venous insufficiency, varicose veins, and menopausal flushing.
The compounds of this invention are also useful for neurologic diseases
and disorders, including spasticity, epilepsy, attention deficit hyperactive
disorder, Tourette's syndrome) and cognitive disorders.
The pharmacological activity and selectivity of these compounds can be
determined using published test procedures. The alpha-2 selectivity of the
compounds is determined by measuring receptor binding affinities and in vitro
functional potencies in a variety of tissues known to possess alpha-2 and/or
alpha-1 receptors. (See, e.g., The Alpha-2 Adrenergic Receptors, L.E. Limbird,
ed., Humana Press, Clifton, NJ.) The following in vivo assays are typically
conducted in rodents or other species. Central nervous system activity is
determined by measuring locomotor activity as an index of sedation. (See,
e.g.)
Spyraki, C. & H. Fibiger) "Clonidine-induced Sedation in Rats: Evidence for
Mediation by Postsynaptic Alpha-2 Adrenoreceptors", Journal of Neural
Transmission, Vol. 54 (1982), pp. 153-163). Nasal decongestant activity is
measured using rhinomanometry as an estimate of nasal airway resistance.
(See, e.g., Salem, S. & E. Clemente, "A New Experimental Method for
Evaluating Drugs in the Nasal Cavity", Archives of Otolarvnqology, Vol. 96
_ _ _._ _.__ . .
CA 02272683 1999-OS-25
WO 98/23611 PCT/LTS97/20803
(1972), pp. 524-529). Antiglaucoma activity is determined by measuring
intraocular pressure. (See) e.g., Potter) D., "Adrenergic Pharmacology of
Aqueous Human Dynamics", Pharmacological Reviews, Vol. 13 (1981 ), pp. 133_
153). Antidiarrheal activity is determined by measuring the ability of the
compounds to inhibit prostaglandin-induced diarrhea. (See, e.g., Thollander,
M.,
P. Hellstrom 8~ T. Svensson, "Suppression of Castor Oil-Induced Diarrhea by
Alpha-2 Adrenoceptor Agonists", Alimentary Pharmacology and Theraaeutics,
Vol. 5 (1991 ), pp. 255-262). Efficacy in treating irritable bowel syndrome is
determined by measuring the ability of compounds to reduce the stress-induced
increase in fecal output. (See, e.g.) Barone, F., J. Deegan, W. Price, P.
Fowler,
J. Fondacaro & H. Ormsbee III, "Cold-restraint stress increases rat fecal
pellet
output and colonic transit", American Journal of Physiology, Vol. 258 (1990))
pp.
6329-G337). Antiulcer and reduction of hyperchlorhydria efficacy is determined
by measuring the reduction in gastric acid secretion produced by these
compounds (See, e.g., Tazi-Saad, K., J. Chariot) M. Del Tacca & C. Roze,
"Effect of a2-adrenoceptor agonists on gastric pepsin and acid secretion in
the
rat", British Journal of Pharmacoloav, Vol. 106 (1992), pp. 790-796).
Antiasthma
activity is determined by measuring the effect of the compound on
bronchoconstriction associated with pulmonary challenges such as inhaled
antigens. (See, e.g., Chang, J. J. Musser & J. Hand, "Effects of a Novel
Leukotriene D4 Antagonist with 5-Lipoxygenase and Cyclooxygenase Inhibitory
Activity, Wy-45,911 ) on Leukotriene-D4- and Antigen-Induced
Bronchoconstriction in Guinea Pig", International Archives of Alierqy and
Applied
Immunolo4y, Vol. 86 (1988), pp. 48-54; and Delehunt, J., A. Perruchound, L.
Yerger, B. Marchette, J. Stevenson & W. Abraham, "The Role of Slow-Reacting
Substance of Anaphylaxis in the Late Bronchial Response After Antigen
Challenge in Allergic Sheep", American Reviews of Res~~iratory Disease, Vol.
130 (1984), pp. 748-754). Activity in cough is determined by measuring the
number and latency of the cough response to respiratory challenges such as
inhaled citric acid. (See, e.g., Callaway, J. & R. King, "Effects of Inhaled
a2-
Adrenoceptor and GABAB Receptor Agonists on Citric Acid-Induced Cough and
Tidal Volume Changes in Guinea Pigs", Euroaean Journal of Pharmacoloqv_,
Vol. 220 (1992), pp. 187-195). The sympatholytic activity of these compounds
is
determined by measuring the reduction of plasma catecholamines (See, e.g., R.
Urban, B. Szabo & K. Starke "involvement of peripheral presynaptic inhibition
in
the reduction of sympathetic tone by moxonidine) rilmenidine and UK 14,304",
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
36
European Journal of Pharmacology, Vol. 282 (1995), pp. 29-37) or the reduction
in renal sympathetic nerve activity (See, e.g., Feng, Q., S. Carlsson, P.
Thoren &
T. Hedner, "Effects of clonidine on renal sympathetic nerve activity,
natriuresis
and diuresis in chronic congestive heart failure rats", Journal of
Pharmacolopy
and Experimental Therapeutics Vol. 261 (1992), pp. 1129-1135), providing the
basis for their benefrt in heart failure and benign prostatic hypertrophy. The
hypotensive effect of these compounds is measure directly as a reduction in
mean blood pressure (See, e.g., Timmermans, P. & P. Van Zwieten, "Central
and peripheral a-adrenergic effects of some imidazolidines", European Journal
of Pharmacolo4y, Vol. 45 (1977), pp. 229-236). Clinical studies have
demonstrated the beneficial effect of alpha-2 agonists in the prevention of
myocardial ischemia during surgery (See, e.g., Talke, P., J. Li, U. Jain, J.
Leung,
K. Drasner, M. Hollenberg & D. Mangano, "Effects of Perioperative
Dexmedetomidine Infusion in Patients Undergoing Vascular Surgery",
Anesthesiology, Vol. 82 (1995), pp. 620-633) and in the prevention of angina
(See, e.g., Wright, R.A., P. Decroly, T. Kharkevitch & M. Oliver) "Exercise
Tolerance in Angina is Improved by Mivazerol--an a2-Adrenoceptor Agonist",
Cardiovascular Drugs and Thera~,y, Vol. 7 (1993), pp. 929-934). The efficacy
of
these compounds in cardiac reperfusion injury is demonstrated by measuring
the reduction of cardiac necrosis and neutrophil infiltration (See, e.g.)
Weyrich,
A., X. Ma, & A. Lefer, "The Role of L-Arginine in Ameliorating Reperfusion
Injury
After Myocardial Ischemia in the Cat", Circulation, Vol. 86 (1992)) pp. 279-
288).
The cardiac antiarrhythmic effect of these compounds is demonstrated by
measuring the inhibition of ouabain induced arrhythmias (See, e.g., Thomas, G.
8~ P. Stephen, "Effects of Two Imidazolines (ST-91 and ST-93) on the Cardiac
Arrhythmias and Lethality Induced by Ouabain in Guinea-Pig", Asia-Pacific
Journal of Pharmacoloay, Vol. 8 (1993), pp.109-113; and Samson, R., J. Cai, E.
Shibata) J. Martins & H. Lee, "Electrophysioiogical effects of a2-adrenergic
stimulation in canine cardiac Purkinje fibers", American Journal of
Physiology,
Vol. 268 (1995), pp. H2024-H2035). The vasoconstrictor activity of these
compounds is demonstrated by measuring the contractile properties on isolated
arteries and veins in vitro (See, e.g., Flavahan, N., T. Rimele, J. Cooke & M.
Vanhoutte, "Characterization of Postjunctional Alpha-1 and Alpha-2
Adrenoceptors Activated by Exogenous or Nerve-Released Norepinephrine in
the Canine Saphenous Vein", Journal of Pharmacology and Experimental
Therapeutics, Vol. 230 (1984)) pp. 699-705). The effectiveness of these
._._~~_ .. _ t _... _
CA 02272683 1999-OS-25
WO 98/23611 PCT/ITS97/20803
37
compounds at reducing intracranial pressure is demonstrated by measurement
of this property in a canine model of subarachnoid hemorrhage (See, e.g.,
McCormick, J., P. McCormick, J. Zabramski & R. Spetzler) "Intracranial
pressure
reduction by a central alpha-2 adrenoreceptor agonist after subarachnoid
hemorrhage", Neurosuraery, Vol. 32 (1993), pp. 974-979). The inhibition of
menopausal flushing is demonstrated by measuring the reduction of facial blood
flow in the rat (See) e.g., Escott, K., D. Beattie, H. Connor & S. Brain, "The
modulation of the increase in rat facial skin blood flow observed after
trigeminal
ganglion stimulation", European Journal of Pharmacoloay, Vol. 284 (1995), pp.
69-76) as demonstrated for alpha-2 adrenergic agonists on cutaneous blood
flow in the tail (See, e.g., Redfern, W.) M. MacLean, R. Clague & J. McGrath,
"The role of alpha-2 adrenoceptors in the vasculature of the rat tail",
British
Journal of Pharmacoloay, Vol. 114 (1995), pp. 1724-1730). The antinociceptive
and pain reducing properties of these compounds is demonstrated by measuring
the increase in pain threshold in the rodent writhing and hot plate
antinociceptive
models (See) e.g., Millan, M., K. Bervoets) J. Rivet, R. Widdowson, A.
Renouard, S, Le Marouille-Girardon & A. Gobert, "Multiple Alpha-2 Adrenergic
Receptor Subtypes. II. Evidence for a Role of Rat Alpha-2A Adrenergic
Receptors in the Control of Nociception, Motor Behavior and Hippocampai
Synthesis of Noradrenaline", Journal of Pharmacoloqy and Experimental
Therapeutics, Vol. 270 (1994), pp. 958-972). The antimigraine effect of these
compounds is demonstrated by measuring the reduction of durst neurogenic
inflammation to trigeminal ganglion stimulation in the rat (See, e.g.,
Matsubara,
T., M. Moskowitz & Z. Huang, "UK-14,304, R(-)-alpha-methyl-histamine and
SMS 201-995 block plasma protein leakage within dura mater by prejunctionai
mechanisms", European Journal of Pharmacology, Vol. 224 (1992), pp. 145-
150). The ability of these compounds to suppress opiate withdrawal is
demonstrated by measuring the suppression of enhanced sympathetic nerve
activity (See, e.g., Franz, D., D. Hare & K. McCloskey, "Spinal sympathetic
neurons: possible sites of opiate-withdrawal suppression by clonidine",
Science,
VoI. 215' (1982), pp. 1643-1645). Antiepileptic activity of these compounds is
demonstrated by measuring the inhibition of the kindling response (See, e.g.,
Shouse, M., M. Bier, J. Langer, O. Alcalde, M. Richkind ~ R. Szymusiak, "The
a2-agonist cionidine suppresses seizures, whereas the alpha-2 antagonist
idazoxan promotes seizures--a microinfusion study in amygdala-kindled
kittens",
Brain Research, Vol. 648 (1994), pp. 352-356). The effectiveness of other
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
38
alpha-2 agonists in the management of neurologic disorders has been
demonstrated, including attention-deficit hyperactive disorder and Tourette's
syndrome (See) e.g., Chappell P., M. Riddle, L. Scahill, K. Lynch, R. Schultz,
A.
Arnsten, J. Leckman & D. Cohen, "Guanfacine treatment of comorbid attention-
deficit hyperactivity disorder and Tourette's syndrome: preliminary clinical
experience", Journal of American Academy of Child and Adolescent Psychiatry,
Vol. 34 (1995), pp. 1140-1146), cognitive disorders (See, e.g., Coull, J.,
- "Pharmacological manipulations of the a2-noradrenergic system. Effects on
cognition", Dru4s and Aging, Vol. 5 (1994), pp. 116-126), and spasticity (See,
e.g.) Eyssette, M., F. Rohmer, G. Serratrice) J. Waiter & D. Boisson,
"Multicenter, double-blind trial of a novel antispastic agent, tizanidine) in
spasticity associated with multiple sclerosis") Current Medical Research 8~
Opinion) Vol. 10 (1988)) pp. 699-708).
Another aspect of this invention involves methods for preventing or
treating nasal congestion by administering a safe and effective amount of a
subject compound to a mammal experiencing or at risk of experiencing nasal
congestion. Such nasal congestion may be associated with human diseases or
disorders which include, but are not limited to, seasonal allergic rhinitis,
acute
upper respiratory viral infections) sinusitis, perennial rhinitis, and
vasomotor
rhinitis. In addition) other disorders can be generally associated with mucous
membrane congestion (for example, otitis media and sinusitis.) Each
administration of a dose of the subject compound preferably administers a dose
within the range of from about 0.0001 mg/kg to about 5 mg/kg of a compound,
more preferably from about 0.001 mg/kg to about 0.5 mg/kg. Peroral
administration of such doses is preferred. The frequency of administration of
a
subject compound according to this invention is preferably from about once to
about six times daily, more preferably from about once to about 4 times daily.
Such doses and frequencies are also preferred for treating other respiratory
_ conditions, such as, cough, chronic obstructive pulmonary disease (COPD) and
asthma. Such doses and frequencies are also preferred for treating conditions
that are associated with mucous membrane congestion (for example, sinusitis
and otitis media).
Another aspect of this invention involves methods for preventing or
treating glaucoma by administering a safe and effective amount of a subject
compound to a mammal experiencing or at risk of experiencing glaucoma. If
administered systemically, each administration of a dose of the subject
___ ._. __._ _._. _T.. ~~._ . ._.__._
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
39
compound preferably administers a dose within the range of from about 0.0001
mg/kg to about 5 mg/kg of a compound, more preferably from about
-0.001 mg/kg to about 0.5 mg/kg. If intraocular dosing is used then preferably
one administers a typical volume (for example, 1 or 2 drops) of a liquid
composition, comprising from about 0.0001 % to about 5% of a subject
compound, more preferably from about 0.01 % to about 0.5% of the compound.
Determination of the exact dosage and regimen is within the purview of the
skilled artisan. Intraocular administration of such doses is preferred. The
frequency of administration of a subject compound according to this invention
is
preferably from about once to about six times daily, more preferably from
about
once to about 4 times daily.
Another aspect of this invention involves methods for preventing or
treating gastrointestinal disorders) such as diarrhea) irritable bowel
syndrome,
and peptic ulcer by administering a safe and effective amount of a subject
compound to a mammal experiencing or at risk of experiencing gastrointestinal
disorders. Each administration of a dose of the subject compound preferably
administers a dose within the range of from about 0.0001 mg/kg to about
mg/kg of a compound, more preferably from about 0.001 mg/kg to about
0.5 mg/kg. Peroral administration of such doses is preferred. The frequency of
administration of a subject compound according to this invention is preferably
from about once to about six times daily, more preferably from about once to
about 4 times daily.
Another aspect of this invention involves methods for preventing or
treating migraine, by administering a safe and effective amount of a subject
compound to a mammal experiencing or at risk of experiencing migraine. Each
administration of a dose of the subject compound preferably administers a dose
within the range of from about 0.0001 mglkg to about 5 mg/kg of a compound,
more preferably from about 0.001 mg/kg to about 0.5 mg/kg. Peroral, parenteral
or intranasal administration of such doses is preferred. The frequency of
peroral
administration of a subject compound according to this invention is preferably
from about once to about six times daily, more preferably from about once to
about 4 times daily. The frequency of parenteral dosing of a subject compound
according to this invention is preferably from about once to about six times
daily,
more preferably from about once to about 4 times daily or by infusion to the
desired effect. The frequency of intranasal dosing of a subject compound
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
according to this invention is preferably from about once to about six times
daily,
more preferably from about once to about 4 times daily.
Another aspect of this invention involves methods for preventing or
treating disorders related to sympathetic nervous system activity) such as
hypertension, myocardial ischemia, cardiac reperfusion injury, angina, cardiac
arrhythmia, and benign prostatic hypertrophy, by administering a safe and
effective amount of a subject compound to a mammal experiencing or at risk of
experiencing these diseases or disorders. Each administration of a dose of the
subject compound preferably administers a dose within the range of from about
0.0001 mg/kg to about 5 mg/kg of a compound, more preferably from about
0.001 mg/kg to about 0.5 mg/kg. Peroral and parenteral administration of such
doses are preferred. The frequency of peroral administration of a subject
compound according to this invention is preferably from about once to about
six
times daily, more preferably from about once to about 4 times daily. The
frequency of parenteral dosing of a subject compound according to this
invention is preferably from about once to about six times daily, more
preferably
from about once to about 4 times daily or by infusion to the desired effect.
Another aspect- of this invention involves methods for preventing or
treating pain, by administering a safe and effective amount of a subject
compound to a mammal experiencing or at risk of experiencing pain. Each
administration of a dose of the subject compound preferably administers a dose
within the range of from about 0.0001 mg/kg to about 5 mg/kg of a compound,
more preferably from about 0.001 mglkg to about 0.5 mg/kg. Peroral or
parenteral administration of such doses is preferred. The frequency of peroral
administration of a subject compound according to this invention is preferably
from about once to about six times daily, more preferably from about once to
about 4 times daily. The frequency of parenteral dosing of a subject compound
according to this invention is preferably from about once to about six times
daily,
more preferably from about once to about 4 times daily or by infusion to the
desired effect.
Another aspect of this invention involves methods for preventing or
treating substance abuse and the abstinence syndrome resulting from
withdrawal of these substances, such as alcohol and opiates, by administering
a
safe and effective amount of a subject compound to a mammal experiencing or
at risk of experiencing substance abuse or withdrawal symptoms. Each
administration of a dose of the subject compound preferably administers a dose
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
41
within the range of from about 0.0001 mg/kg to about 5 mg/kg of a compound,
mare preferably from about 0.001 mg/kg to about 0.5 mg/kg. Peroral
administration of such doses is preferred. The frequency of administration of
a
subject compound according to this invention is preferably from about once to
about six times daily, more preferably from about once to about 4 times daily.
Composition and Method Examples
The following non-limiting examples illustrate the compositions and
methods of use of this invention.
Exam~~le A
Oral Tablet Composition
Inaredient Amount per tablet (mg)
Subject Compound 2 20.0
Microcrystalline cellulose (Avicel PH 102~) 80.0
Dicalcium phosphate 96.0
Pyrogenic silica (Cab-O-Sil~) 1.0
Magnesium stearate _3.0
Total = 200.0 mg
One tablet is swallowed by a patient with nasal congestion. The congestion is
substantially diminished.
Other compounds having a structure according to Formula I are used with
substantially similar results.
Example B
Chewable Tablet Com position
Ingredient Amount per tablet (ma)
Subject Compound 1 15.0
Mannitol 255.6
Microcrystalline cellulose (Avicel 100.8
PH 101 ~)
DextriniZed sucrose (Di-Pac~) 199.5
Imitation orange flavor 4.2
Sodium saccharin 1.2
Stearic acid 15.0
Magnesium stearate 3.0
FD8~C Yellow #6 dye 3.0
Pyrogenic silica (Cab-O-Sil~) _2,7
Total = 600.0 mg
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97120803
42
One tablet is chewed and swallowed by a patient with nasal congestion. The
congestion is substantially reduced.
Other compounds having a structure according to Formula I are used with
substantially similar results.
Example C
Sublingual Tablet Composition
In redient Amount per tabletlmp)
Subject Compound 2 2.00
Mannitol 2.00
Microcrystalline cellulose (Avicel PH 101~) 29.00
Mint flavorants 0.25
Sodium saccharin 0.08
Total = 33.33 mg
One tablet is placed under the tongue of a patient with nasal congestion and
allowed to dissolve. The congestion is rapidly and substantially diminished.
Other compounds having a structure according to Formula I are used with
substantially similar results.
Exam~fe D
Intranasal Solution Composition
In4redient Composition (% w/v)
Subject Compound 1 0.20
Benzalkonium chloride 0.02
Thimerosai 0.002
d-Sorbitol 5.00
Glycine 0.35
Aromatics 0.075
Purified water cp.s.
Total = 100.00
One-tenth of a mL of the composition is sprayed from a pump actuator into each
nostril of a patient with nasal congestion. The congestion is substantially
diminished.
Other compounds having a structure according to Formula I are used with
substantially similar results.
Examgle E
Intranasal Gel Composition
Ingredient Comeosition (% wlv~
_.~_. _ _. T . _ .._
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
43
Subject Compound 2 0.10
~Benzalkonium chloride 0.02
Thimerosal 0.002
Hydroxypropyl methylcellulose 1.00
(Metolose 65SH4000~)
Aromatics 0.06
Sodium chloride (0.65%)
- Total = 100.00
One-fifth of a mL of the composition is applied as drops from a dropper into
each
nostril of a patient with nasal congestion. The congestion is substantially
red uced.
Other compounds having a structure according to Formula I are used with
substantially similar results.
Example F
Inhalation Aerosol Composition
inctredient Composition (% w/v)
Subject Compound 5.0
2
Alcohol 33.0
Ascorbic acid 0.1
Menthol 0.1
Sodium Saccharin 0.2
Propellant (F12,
F114)
Total = 100.0
Two-puffs of the aerosol composition is inhaled from a metered-dose inhaler by
a patient with asthma. The asthmatic condition is effectively relieved.
Other compounds having a structure according to Formula I are used with
substantially similar results.
Exam~~le G -
To~ical Ophthalmic Composition
Ingredient Composition % w/v)
Subject Compound 2 0.10
Benzalkonium chloride 0.01
EDTA 0.05
Hydroxyethylcellulose (Natrosol M~) 0.50
Sodium metabisulfite 0.10
Sodium chloride (0.9%) , ,mss,
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
44
Total = 100.0
One-tenth of a mL of the composition is administered directly into each eye of
a
patient with glaucoma. The intraocular pressure is substantially reduced.
Other compounds having a structure according to Formula I are used with
substantially similar results.
Example H
Oral Liguid Composition
Ingredient Amount/15 mL
Dose
Subject Compound 2 15 mg
Chlorpheniramine maleate 4 mg
Propylene glycol 1.8 g
Ethanol (95%) 1.5 mL
Methanol 12.5 mg
Eucalyptus oil 7.55 mg
Flavorants 0.05 mL
Sucrose 7.65 g
Carboxymethyicellulose (CMC) 7.5 mg
Microcrystalline cellulose and 187.5 mg
Sodium CMC (Avicel RC 591 ~)
Polysorbate 80 3.0 mg
Glycerin 300 mg
Sorbitol 300 mg
FD&C Red #40 dye 3 mg
Sodium saccharin 22.5 mg
Sodium phosphate monobasic 44 mg
Sodium citrate monohydrate 28 mg
Purifred Water g; s.
Total = 15 mL
One 15 mL dose of the liquid composition is swallowed by a patient with nasal
congestion and runny nose due to allergic rhinitis. The congestion and runny
nose are effectively reduced.
Other compounds having a structure according to Formula I are used with
substantially similar results.
Example J
Oral Liquid Composition
Ingredient Amount/15 mL aose
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
Subject Compound 2 30 mg
Sucrose 8.16 g
-Glycerin 300 mg
Sorbitol 300 mg
Methylparaben 19.5 mg
Propylparaben 4.5 mg
Menthol 22.5 mg
Eucalyptus oil 7.5 mg
Flavorants 0.07 mL
FD&C Red #40 dye 3.0 mg
Sodium saccharin 30 mg
Purified water q,s.
Total = 15 mL
One 15 mL dose of the alcohol-free liquid medication is swallowed by a patient
with nasal congestion. The congestion is substantially diminished.
Other compounds having a structure according to Formula I are used with
substantially similar results.
Example K
Oral Tablet Comaosition
Ingredient Amount per tablet (mp)
Subject Compound 2 4
Microcrystalline cellulose, NF 130
Starch 1500, NF 100
Magnesium stearate, USP _2
Total = 236 mg
One tablet is swallowed by a patient with migraine. The pain and aura of
migraine is substantially diminished.
Other compounds having a structure according to Formula I are used with
substantially similar results.
Example L
Oral Tablet Composition
Ingredient Amount per tablet (mq~
Subject Compound 2 12
Hydroxypropyl methylcellulose, USP 12
Magnesium stearate) USP 2
Lactose anhydrous, USP 200
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
46
Total= 226 mg
For the relief of pain. Adults 12 and over take one tablet every twelve hours.
Other compounds having a structure according to Formula I are used with
substantially similar results.
Example M
Oral Caplet Composition
Ingredient Amount per tablet (mg)
Naproxen sodium anhydrous, USP 220
Subject Compound 2 6
Hydroxypropyl methylcellulose) USP 6
Magnesium stearate, USP
Povidone K-30, USP 10
Talc, USP 12
MicrocrystaNine cellulose, NF _44
Total= 300 mg
For relief of symptoms associated with the common cold, sinusitis, or flu
including nasal congestion, headache, fever, body aches, and pains. Adults 12
and over take two caplets every twelve hours.
Other compounds having a structure according to Formula I are used with
substantially similar results.
Example N
Oral Tablet Comiuosition
Ingredient Amount per tablet (mg)
Subject Compound 1 6
Hydroxypropyl methylcellulose, USP 6
Silicon dioxide, colloidal, NF 30
Pregelatinized starch, NF 60
Magnesium stearate, USP 4
Total = 96 mg
For treatment of benign prostatic hypertrophy. Take one tablet per day.
Other compounds having a structure according to Formula i are used with
substantially similar results.
Exam
Oral Tablet Composition
Ingredient Amount per caplet lmg)
Subject Compound 1 6
_ ._ _.__ _ ____ .~_ - . _ T .. _ .. _ _.
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
47
Hydroxypropyl methylcellulose, USP g
Magnesium stearate, USP 2
Povidone K-30, USP 10
Talc, USP 12
Microcrystaliine cellulose, NF _44
Total = 80 mg
For the use in the treatment of alcoholism or opiate addiction. Adults 12 and
over take two caplets every twelve hours.
Other compounds having a structure according to Formula I are used with
substantially similar results.
Example P
Oral Tablet Composition
In redient Amount per tablet lma_)
Subject Compound 2 g
Hydroxypropyl methylcellulose, USP 12
Magnesium stearate, USP 2
Povidone K-30) USP 10
Talc, USP 12
Microcrystalline cellulose) NF _44
Total = 86 mg
For the treatment of ulcer and hyperacidity. Take two tablets as appropriate.
Other compounds having a structure according to Formula I are used with
substantially similar results.
Example Q
Oral Tablet Comp osition
Ingredient Amount per tablet
lmg,)
Component Amount
Subject Compound 2 10 mg/ml carrier
Carrier:
Sodium citrate buffer with (percent
by weight of carrier):
Lecithin 0.48%
Carboxymethylcellulose 0.53
Povidone 0.50
Methyl paraben 0.11
Propyl paraben 0.011
CA 02272683 1999-OS-25 -
WO 98/23611 PCT/IJS97/20803
48
For the reduction of cardiac reperfusion injury.
Other compounds having a structure according to Formula I are used with
substantially similar results.
Example R
Oral Liguid Comp osition
Ingredient Amount/fl oz Dose
~mg)
Acetaminophen, USP 1000
Doxylamine succinate, USP 12.5
Dextromethorphan hydrobromide, USP 30
Subject Compound 1 6
Dow XYS-40010.00 resin 3
High fructose corn syrup 16000
Polyethylene glycol, NF 3000
Propylene glycol, USP 3000
Alcohol, USP 2500
Sodium citrate dihydrate, USP 150
Citric acid) anhydrous, USP 50
Saccharin sodium, USP 20
Flavor 3.5
Purified water, USP 3500
Total = 29275 mg/fl oz
For the relief of minor aches) pains, headache) muscular aches, sore throat
pain, and fever associated with a cold or flu. Relieves nasal congestion,
cough
due to minor throat and bronchial irritations, runny nose, and sneezing
associated with the common cold. Adults 12 and over take one fluid ounce
every six hours.
Other compounds having a structure according to Formula I are used with
substantially similar results.
Examale S
Oral Liquid Composition
Ingiredient Amount/fl oz Dose (mgt
Naproxen sodium anhydrous, USP 220
Doxylamine succinate, USP 12.5
Dextr omethorphan hydrobromide, USP 30
Subject Compound 1 6
Dow XYS-40010.00 resin 3
__~_~ ~ __ _ _
CA 02272683 1999-OS-25
WO 98/23611 PCT/US97/20803
49
High fructose corn syrup 16000
Polyethylene glycol, NF 3000
Propylene glycol, USP 3000
Alcohol, USP 2500
Sodium citrate dehydrate, USP 150
Citric acid, anhydrous, USP 50
Saccharin sodium, USP 20
Flavor 3.5
Purifred water, USP 3800
Total = 28795 mg/fl oz
For the relief of minor aches, pains, headache, muscular aches, sore throat
pain) and fever associated with a cold or flu. Relieves nasal congestion,
cough
due to minor throat and bronchial irritations, runny nose, and sneezing
associated with the common cold. Adults 12 and over take one fluid ounce
every six hours.
Other compounds having a structure according to Formula I are used with
substantially similar results.
COMPOSITION EXAMPLE T
A composition for parenteral administration, according to this invention) is
made comprising:
Component Amount
Subject Compound 2 10 mg/ml carrier
Carrier:
Sodium citrate buffer with (percent
by weight of carrier):
Lecithin 0.48%
Carboxymethylcellulose 0.53
Povidone 0.50
Methyl paraben 0.11
Propyl paraben 0.011
The above ingredients are mixed, forming a solution. Approximately
2.0 ml of the solution is administered, intravenously, to a human subject
suffering from septic or cardiogenic shock. The symptoms subside.
Other compounds having a structure according to Formula I are used with
substantially similar results.
Example U
CA 02272683 1999-OS-25
WO 98/23611 PCT/IJS97/20803
50 .
Oral Tablet Composition
Ingredient Amount per tablet (ma)
Subject Compound 1 10
Hydroxypropyl methylcellulose) USP 12
Magnesium stearate, USP 2
Povidone K-30, USP 10
Talc, USP 12
- Microcrystalline cellulose, NF 44
Total = 90 mg
For the treatment of cardiac arrhythmia. Take as prescribed.
Other compounds having a structure according to Formula I are used with
substantially similar results.
Example V
Oral Tablet Composition
In redient Amount per tablet (ma)
Subject Compound 1 4
Microcrystalline cellulose, NF 130
Starch 1500, N F 100
Magnesium stearate) USP 2
Total = 236 mg
For the treatment of congestive heart failure. Take as prescribed.
Other compounds having a structure according to Formula I are used with
substantially similar results.
Modification of the preceding embodiments is within the scope of the
skilled artisan in formulation, given the guidance of the specification in
light of
the state of the art.
Other examples of combination actives are contemplated. Examples of
medicaments which can be combined with the primary active are included in
U.S. Patent No. 4,552,899 to Sunshine, et al., hereby incorporated by
reference.
All other references referred to throughout this specification are hereby
incorporated by reference.
While particular embodiments of this invention have been described, it will
be obvious to those skilled in the art that various changes and modifications
of
this invention can be made without departing from the spirit and scope of the
invention. It is intended to cover, in the appended claims, all such
modifications
that are within the scope of this invention.
__~~_.__ ._ ___... T ___