Note: Descriptions are shown in the official language in which they were submitted.
CA 02272857 1999-05-05
WO 98/19779 PCT/AU97/00751
IMPROVED LEACHING OF MINERAL ORES
FIELD OF THE INVENTION
The present invention relates to a process for improved base
metal and/or uranium leaching and relates particularly,
though not exclusively, to a process involving oxidation of
ferrous ions to ferric ions and recirculation of the ferric
ions for reuse in the leaching process.
BACKGROUND TO THE INVENTION
It is well known in the mineral processing arts that ferric
ion either as ferric sulphate (Fe2(SO4)3) or ferric chloride
(FeC13), etc. can be employed for the leaching of copper and
other base metals from sulphide ores or concentrate in
accordance with the following typical reactions (chalcocite
is used as a typical example of copper/base metal sulphides) :
Cu2S + Fe2(SO4)3 -4 CuS + CuSO4 + 2FeSO4 (a)
Chalcocite + Ferric sulphate -4 Covellite + Copper sulphate + Ferrous Sulphate
CuS + Fe2(SO4)3 --* CuSO4 + 2FeSO4 + S (b)
Covellite + Ferric sulphate -> Copper sulphate + Ferrous Sulphate + Sulphur
If ferric chloride is employed, hydrochloric acid will
substitute sulphuric acid in reactions (a) and (b) resulting
in reactions (c) and (d):
Cu2S + 2FeCI3 --> CuS + CuCI2 + 2FeCi2 (c)
Chalcocite + Ferric Chloride -4 Covellite + Copper sulphate + Ferrous Sulphate
CuS + 2FeCI3 -> CuCi2 + 2FeCI2 + S (d)
Covellite + Ferric Chloride -4 Copper Chloride + Ferrous Chloride + Sulphur
The dissolved copper sulphate can then be recovered by
extracting it into an organic solvent such as kerosene with
the use of a suitable extractant. This is followed by back
extracting the copper into a sulphuric acid solution. The
CA 02272857 1999-05-05
WO 98/19779 PCT/AU97/00751
- 2 -
resulting copper containing sulphuric acid solution can then
be subjected to an electrowinning process to produce pure
cathodic copper metal.
However, hitherto direct use of ferric sulphate for the
leaching of copper and base metal sulphides has not been
commercially and technically feasible due to:
(i) the high cost of ferric sulphate/ferric chloride
that would be required for the dissolution of copper (the
stoichiometric requirement is 6.30 tonnes of ferric sulphate
per tonne of copper from chalcocite (Cu2S)) and
(ii) problems due to the presence of an excessive
quantity of iron (in either ferric or ferrous state) which
would interfere with the solvent extraction/electrowinning
process for the purification and production -of cathodic
copper metal.
In order to make the process commercially and technically
feasible, one must be able to effect high copper recovery
with the use of a relatively small quantity of ferric
sulphate in the leach solution. Once the ferric sulphate is
consumed and converted to ferrous state, it must be oxidised
back to the ferric state (in accordance with reaction (e)).
2FeSO4 + 0.502 + H2S04 -4 Fe2(SO4)3 + H20 (e)
Ferrous sulphate + Oxygen + sulphuric acid -a Ferric Sulphate
However, direct sparging of oxygen containing gases into
solution is uneconomic due to low solubility of oxygen in
solution resulting in high wastage of oxygen and long
residence time. This problem is exacerbated because of the
fact that an elevated temperature (eg 80-100 C) is often
required to achieve appreciable ferrous oxidation and copper
sulphide dissolution reactions.
Various approaches have therefore been investigated
including:
CA 02272857 1999-05-05
WO 98/19779 PCT/AU97/00751
- 3 -
(i) using bacteria such as thiobacillus ferrioxidant
for the oxidation of ferrous sulphate to ferric sulphate. In
fact, without bacterial activities, economic heap leaching of
copper sulphide bearing ores would not be feasible;
(ii) using a pressure vessel (autoclave) at an elevated
temperature to oxidise ferrous sulphate to ferric sulphate
during which time direct oxidation of some sulphide also
occurs to a certain extent.
In each case the object is to re-oxidise the ferrous sulphate
back to the ferric sulphate state in accordance with reaction
(e) in order to reutilise the ferric ion for the copper
leaching duty (reactions (a) and (b)) without the need for
excessive supply of ferric sulphate into the leach slurry.
In such operations, oxygen essentially plays the role of an
oxidising agent whilst the ferric ion acts as an electron
carrier for the copper sulphide oxidation process.
However, a major disadvantage with bacterial oxidation of
ferrous ion is that it is very slow and normally takes many
days to perform the task. It has therefore been found only
suitable for heap leaching practice when the leaching time
can be as long as 12 months or more. On the other hand,
although pressure oxidation requires shorter residence time
(in the order of a few hours) it incurs a high capital
investment and high operating costs.
Ferric ion is also useful for the leaching of uranium from
uranium ores due to its high oxidative property. In general,
uranium occurs in nature as oxides in different oxidation
states: U6+, Us', U4' and U3+. Whilst U6' is readily soluble in
sulphuric acid, other forms of uranium minerals are either
sparingly soluble or not soluble at all.
In order to solubilize the uranium bearing minerals, it is
CA 02272857 2005-03-15
-4-
SUMMARY OF THE INVENTION
The present invention was developed with a view to providing a more efficient
process for oxidising ferrous ion back to ferric ion so that it can be reused
in the
leaching of base metals and/or uranium.
According to one aspect of the present invention there is provided a process
for
oxidation of ferrous ions in solution, the process comprising: forcing the
ferrous
ion-containing solution through an in-line mixer under the influence of a
controlled
pressure differential between an inlet and an outlet of the mixer; and
injecting
oxygen or an oxygen-containing chemical reagent directly into the in-line
mixer to
facilitate oxidation of said ferrous ions (Fe2{) to form ferric ions (Fe3+).
According to another aspect of the present invention there is provided a
process
for improved base metal and/or uranium leaching from ores, concentrates or
tailings using ferric ions as an oxidising agent, the process comprising:
dissolution
of an insoluble base metal compound or uranium into a soluble metal compound
in a leach slurry or solution by chemical oxidation with ferric ions (Fe3+) so
as to
produce a byproduct ferrous ion (Fez+); recovering the base metal or uranium
by
extracting the soluble metal compound and subjecting it to a suitable winning
process; recirculating the leach slurry or solution with the byproduct ferrous
ion
through an in-line mixer by forcing the leach slurry or solution through the
in-line
mixer under the influence of a controlled pressure differential between an
inlet and
an outlet of the mixer; and, converting the ferrous ion back to ferric ion by
oxidation, wherein said oxidation is facilitated by injecting oxygen or an
oxygen-
containing chemical reagent directly into the in-line mixer.
CA 02272857 2005-03-15
-5-
Preferably said in-line mixer is a static reactor operated so as to subject
the leach
slurry or solution to elevated pressures and/or high shear whilst injecting
said
oxygen or oxygen-containing chemical reagent.
CA 02272857 1999-05-05
WO 98/19779 PCT/AU97/00751
- 6 -
BRIEF DESCRIPTION OF THE DRAWINGS
In order to facilitate a more comprehensive understanding of
the nature of the invention, preferred embodiments of the
process in accordance with the invention will now be
described in detail, by way of example only, with reference
to the accompanying drawings, in which:
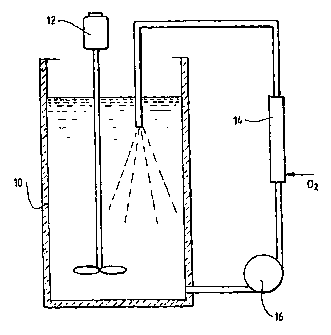
Figure 1 is a schematic diagram of a possible embodiment of
the process for ferrous oxidation and recirculation in a base
metal leaching process;
Figures 2(a), 2(b) and 2(c) illustrate schematically three
alternative embodiments for a typical base metal/uranium
leaching installation utilising the process -of ferrous
oxidation and recirculation in accordance with the present
invention;
Figure 3 is a schematic diagram of an embodiment of a typical
base metal/uranium heap leaching process utilising the
process of ferrous oxidation and recirculation in accordance
with the present invention;
Figure 4 is a graph illustrating % recovery of copper from a
concentrate sample (51% Cu) during a pilot leaching test;
and,
Figure 5 is a flowsheet illustrating a possible process for
recovery of copper from sulphides which incorporates a
preferred leaching process utilizing recirculation of ferrous
ion and oxidation in accordance with the present invention.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
The present invention is based on the discovery by the
inventors that continuous ferrous to ferric ion conversion
and copper/uranium leaching can be facilitated by
recirculating a ferrous ion-containing leach slurry or a
CA 02272857 2005-11-30
-7-
ferrous ion-containing leach solution through a pressurised in-line mixer
which is
injected with oxygen or an oxygen-containing chemical reagent gas. In the
following described embodiments the in-line mixer employed is a static
reactor,
such as the Applicant's FILBLASTTM Gas Shear Reactor (GSR), which is operated
so as to subject the leach slurry or solution to elevated pressures and/or
high
shear whilst injecting the oxygen or oxygen-containing chemical reagent gas.
The
FILBLAST GSR is itself the subject of U.S. Patent No. 5,741,466. However, it
is to
be understood that any suitable in-line mixer may be employed which allows the
injection of oxygen or an oxygen-containing chemical reagent into the mixer to
facilitate oxidation of Fe2+ ions to Fe3+ ions under the influence of a
controlled
pressure differential between the inlet and the outlet of the mixer. Figure 1
illustrates schematically one possible embodiment of the process for ferrous
to
ferric conversion in accordance with the invention.
In FIG. 1 a reaction vessel 10 holds a ferrous ion-containing solution, for
example,
a copper sulphide leach slurry or concentrate. Optionally, an agitator 12 may
be
provided to promote leaching of the base metal into solution. Some of the
ferrous
ion containing solution is drawn off from the reaction vessel 10 and pumped
through an in-line mixer, in this case a FILBLAST reactor 14 via a feed pump
16.
In this embodiment, pure oxygen is injected into the FILBLAST reactor 14 to
facilitate oxidation of the ferrous sulphate to form ferric sulphate in
accordance
with reaction (e) noted above. The ferric ion-containing solution is then
recirculated back to the reaction vessel 10 where the ferric ions are reused
in the
dissolution of the copper sulphide into soluble copper sulphate in accordance
with
reactions (a) and (b) noted above. That is, the FILBLASTTM reactor 14 provides
the means for saturating the liquor/slurry with oxygen, while the reactor
vessel 10
provides the residence
CA 02272857 1999-05-05
WO 98/19779 PCT/AU97/00751
- 8 -
time for the oxygen/ferrous to ferric reaction to occur.
The two processes of ferrous oxidation and metal leaching can
be conducted either simultaneously or sequentially to effect
recovery of copper, other base metals or uranium from ores,
concentrates or tailings. Simultaneous ferrous oxidation and
metal leaching is suitable for leaching of feed materials
which contain high concentrations of valuable metals and low
concentration of soluble metal ion. On the other hand,
sequential operation (ferrous oxidation followed by metal
leaching) is a preferred option for leaching of feed
materials which contain low concentrations of valuable metals
and high concentration of soluble metal ion.
The leach slurry or solution in reaction vessel 10 is treated
at atmospheric pressure whereas the ferrous ion-containing
slurry or solution is forced through the in-line mixer 14
under the influence of a controlled pressure differential
between the liquid inlet and outlet of the mixer. This
pressure differential is generated by feed pump 16, and in
the experimental installation pressures in the range of 400-
950 kPa gauge were applied. However the required pressure
will vary depending on the nature of the ore, concentrate or
tailings to be leached, and the desired leaching time. The
rate of ferrous to ferric conversion is increased with an
increase in solution temperature. Generally temperatures
between 50-95 C have been found sufficient for ferrous
oxidation and leaching of copper from some ores and tailings.
The rate of ferrous oxidation increases with decrease in acid
concentration and the oxidation is most favourable at
sulphuric concentrations below 1N (or 49 g/L). However it
was observed that at sulphuric acid concentrations below 10
g/l, precipitation of jarosite occurred resulting in
significant loss of ferric sulphate. Since the precipitation
of jarosite is irreversible, initial sulphuric acid
concentration of less than 30 g/L is preferably avoided
CA 02272857 1999-05-05
WO 98/19779 PCT/AU97/00751
- 9 -
particularly when the ferrous concentration is higher than 30
g/L in order to make sufficient allowance for the acid
consumption during ferrous oxidation [reaction (a)].
For example, if the ferrous sulphate solution contained 40
g/L of ferrous ion and the initial acid concentration was 30
g/L, a 60% ferrous oxidation would consume 21 g/L of
sulphuric acid resulting in the final acid concentration of
9 g/L, the threshold below which precipitation of jarosite
occurs. Therefore the most preferred range of acid
concentration is between 30-50 g/L. The lower end of such a
range corresponds to low ferrous concentration (20 g/L) and
the higher end would be suitable for high ferrous
concentration (40-50 g/L).
Alternatively, the acid content of the solution should be
continuously controlled at 20-30 g/L in order to maximise
ferrous oxidation without the danger of jarosite formation.
However controlled acid addition by direct pH reading from
the hot solution can be unreliable. This can be overcome by
cooling a small stream of the solution where the pH is
continuously measured for a proportionally controlled acid
addition.
Because the ferrous sulphate is continuously re-oxidised to
the ferric state, which is simultaneously or sequentially
employed in the copper leaching process, there is no need for
2S the addition of excessive quantities of ferrous or ferric
sulphate into the leach slurry or solution. A very moderate
requirement of approximately 20 -50g/L Fe7+ has been found
sufficient for near complete dissolution of copper sulphides
in ores/concentrates. The Fe2+ ion can be added in any of the
following forms:
I. Ferrous sulphate in either a hydrated or non-hydrated
state
II. Ferric sulphate (in either a hydrated or non-hydrated
CA 02272857 1999-05-05
WO 98/19779 PCT/AU97/00751
- - 10 -
state) which would be reduced to ferrous sulphate upon
reaction with copper sulphide (reactions (a) & (b))
III. Ferrous or ferric chloride in either hydrated or non-
hydrated state
IV. Iron sulphides such as pyrite (FeS2) or pyrrhotite (FeS)
or chalcopyrite (CuFeS2), etc. which are often present
naturally in copper sulphide ores/concentrates and would
react with oxygen and sulphuric acid to form ferrous
sulphate (or hydrochloric acid to form ferrous chloride)
V. Metallic iron which would dissolve in acid to form
ferrous and/or ferric ions
VI. Any other iron containing chemical compounds or minerals
that release ferrous or ferric ions in the leach
solution.
Since most copper/base metal sulphide ores, concentrates and
tailings normally contain certain quantities of partially
soluble iron bearing minerals such as pyrite (FeS2) and
pyrrhotite (FeS), addition of ferrous compounds is not always
required.
Any suitable oxygen-containing chemical reagent may be
injected into the in-line mixer to effect the ferrous to
ferric conversion. However, since oxygen is probably the
least expensive and the most environmentally friendly
oxidising chemical reagent, its use as the reagent would
undoubtedly attract the greatest interest amongst mining
companies. Only 0.14 kg of oxygen is required to oxidise 1
Kg of ferrous ion or 2.71 Kg of ferrous sulphate. As a
result, the stoichiometric requirement of oxygen is about lkg
for the dissolution of 5 Kg of chalcocite (which contains
3.97Kg of copper). Assuming an oxygen utilisation rate of
80%, only 0.32Kg of oxygen would be required to extract 1 Kg
of copper from chalcocite. Currently the cost of oxygen is
in the proximity of $ 0.10 to $ 0.18 / kg, depending on
whether it is generated on site or is purchased as liquefied
CA 02272857 1999-05-05
WO 98/19779 PCT/AU97/00751
- 11 -
oxygen, and depending on power, transport costs, etc.
Therefore the potential cost savings of the process are
enormous as the cost of oxygen consumption would amount to
only 3.2 to 5.8 cents per kilogram of copper, which is
currently worth around $ 2.5/kg.
To achieve effective ferrous oxidation and base metal
recovery with maximum oxygen utilisation and minimal short
circuiting, a multiple stage installation such as that
illustrated in Figure 2 would preferably be employed. The
same installation and operating conditions have also been
found to be effective for recovery of uranium from ores,
concentrates or tailings. Figure 2(a) illustrates
schematically a typical multiple stage installation for base
metal/uranium leaching which employs simple in-line mixers to
effect recirculation and ferrous oxidation.
The leach solution is made up in surge tank 20 by the
addition of ferric sulphate and sulphuric acid to the ore
slurry. The slurry/liquor from surge tank 20 is then fed
into the recirculation circuit of an adjacent reaction vessel
22. Reaction vessel 22 is provided with a simple in-line
mixer 24, in this case a FILBLAST GSR, through which the
ferrous sulphate-containing solution from reaction vessel 22
and surge tank 20 is forced by a feed pump 26. The overflow
from reaction vessel 22 can be fed into the recirculation
circuit of a substantially identical stage in the
installation cascade. Bypass lines 28 allow one or more
stages in the cascade to be bypassed if desired. The number
of stages will vary depending on the characteristics of the
ore/concentrate/tailings. The overflow from the final stage
is fed into a collector tank 30 which discharges to a
filter/thickener for recovery of the dissolved base metal or
uranium. The collector tank allows completion of the
leaching reaction without the addition of further oxygen,
CA 02272857 1999-05-05
WO 98/19779 PCT/AU97/00751
- 12 -
thereby resulting in a reduction of the ferric ion
concentrate. This is of benefit when the next step following
solid/liquid separation is solvent extraction/electrowinning.
Figure 2(b) illustrates an alternative embodiment of a
multistage installation in accordance with the present
invention, in which the in-line FILBLAST GSR 24 in the
recirculation circuit of each stage is replaced with a partly
or fully submerged in-line mixer 32. Oxygen or a suitable
oxygen containing reagent is injected into the in-line mixer
32 to facilitate oxidation of the ferrous sulphate (and
copper sulphate) under elevated pressures and/or high shear
conditions. The in-line mixer 32 may be any suitable static
mixer.
Figure 2(c) illustrates a still further embodiment of a base
metal/uranium leaching process which incorporates ferrous to
ferric conversion in accordance with the present invention.
In this embodiment, the reaction vessels 22 are replaced with
Pachuca tanks 34 which are each provided with a recirculation
circuit similar to the embodiment of Figure 2(a). A simple
in-line mixer 36 provided in each recirculation circuit may
be a FILBLAST GSR or other suitable static mixer.
The ferrous to ferric conversion process of the present
invention is also applicable to a base metal/uranium heap
leaching process. A schematic diagram of a possible
embodiment of a heap leaching process is illustrated in
Figure 3. A heap of ore 40, such as in a tailings dump at a
mine site, may be subjected to leaching by a reticulation
system 42. The leaching solution with mineral values and
ferrous ions contained therein is collected and subjected to
the ferrous to ferric conversion process in accordance with
the invention in a similar manner to that described in
relation to Figure 1. The ferrous ion-containing solution is
forced by a recirculating pump 44 through an in-line mixer 46
which is injected with an oxygen containing chemical reagent
CA 02272857 1999-05-05
WO 98/19779 PCT/AU97/00751
- - 13 -
to facilitate oxidation of the ferrous ions (Fe2+) to form
ferric ions (Fe3+)
In order to further illustrate the present invention the
following examples are given for illustrative purposes only,
and are not to be taken as limiting the process according to
the invention in any way.
Example 1
Table 1 below summarises the typical chemical and physical
properties of a plant solution tested for ferrous/ferric
conversion in accordance with the present invention.
Analysis Values Unit
Cu 1,577. ppm
Co 7.1 ppm
Fe (mainly Fe2+) 20-22 g/L
Cl- 1.75 g/L
Free Acid (as H2SO4) 28 to 48 g/L
SO42- 65.5 g/L
pH 1.08
SG 1.10
Ternperature 50-60 C
Table 1
It was found that at solution temperatures within the range
80-95 C a ferrous (ion) conversion rate of approximately 30-
40 g/L was recorded during the first 10-15 minutes, but this
rate decreased as the reaction proceeded towards equilibrium.
This slowing of the reaction occurs because the reaction rate
is second order to ferrous concentration (proportional to
[Fez+] Z) . After 90 minutes, up to 70% conversion of the total
ferrous concentrations had been achieved. Under conditions
of simultaneous ferrous oxidation and copper leaching, the
ferric ion is consumed by the oxidation process soon after it
_..._.__._.R.._........~,.~.~-.._ .~ _ __.... ____________-~..d_.. . .
._.._.._a....,_.,,_... _ _ . _.._.._.....__. _ _ .
CA 02272857 1999-05-05
WO 98/19779 PCT/AU97/00751
- 14 -
forms and higher reaction rates are expected in practice.
Example 2
Leaching of copper and uranium from a flotation tailing
sample was conducted using oxidised concentrate leach
thickener overflow in lieu of sodium chlorate. The
laboratory test results (conducted in stirred beaker), as
tabulated in Table 2, indicate that uranium and copper can be
effectively dissolved to equivalent plant level by using the
oxidised ferrous sulphate solution. This is in despite of
the fact that the ferric content of the thickener overflow
was significantly lower then the equivalent value required to
wholly substitute the total quantity of sodium chlorate
currently employed. This can be explained by the fact that
although appreciable sodium chlorate was added, the
concentration of ferric iron in the plant leaching circuit
seldom exceed 1-2 g/L, similar to what was found in the final
laboratory leach solution. It is thought the ferric iron
formed by sodium chlorate addition has been largely consumed
by the formation of jarosite due to localised high oxidation
potential at the point of chlorate addition.
Appreciable improvement in uranium dissolution can be
achieved with the FILBLAST leach process. The results as
tabulated in Table 2 indicate that in general the FILBLAST
leach process resulted in better copper and uranium recovery.
This was evident by comparison of the FILBLAST leach residue
with those of laboratory leach and plant leach. On average
dissolution of an additional 1 Kg/t of copper and 100 g/t of
uranium have been achieved by the FILBLAST leach process.
PROCESS AVERAGE Cu AVERAGE U308
Mg/t) (g/t)
Unleached 4.1 823
Plant Leach 3.3 204
Lab Leach (with Fe3+ ) 2.1 222
Filblast leach (with Fe3+) 1.6 128
Table 2
CA 02272857 1999-05-05
WO 98/19779 PCT/AU97/00751
- 15 -
High dissolution of the valuable minerals may be explained by
the following factors:
= Maintenance of high ferric concentration throughout the
FILBLAST leach. Ferric sulphate reduced by the uranium
and copper sulphide was effectively regenerated by the
FILBLAST leach process.
= High shearing effect on the mineral particles,
= High mixing intensity of the FILBLAST process.
However, there was an adverse effect of the FILBLAST leach
process in regard to the level of silica in the leach
solution. The silica concentration in the FILBLAST leach
solution consistently ranged from 7-10 g/L which was twice
those found in the plant and laboratory leach liquors. It
was not possible to determine accurately the actual
concentration in the leach liquors because filtration
necessary for liquor analysis might have removed a certain
quantity of the silica that was larger than the 8 m pore
size of the filter paper. Such a high silica level exceeds
the limit preferred by plant operators particularly in regard
to the solvent extraction stage. The problem was exacerbated
by the fact that the dissolved silica appeared to be well
polymerised into colloidal form that cause emulsification of
the leach pulp. This would be likely to result in difficult
solids - liquid separation process. It is thought that high
shear rate is the main reason for such an excessive
dissolution and polymerisation of silica.
Another test was conducted to investigate the effect of shear
reduction by leaching at low pulp density. The flotation
tailing's pulp density was reduced to approximately 20%
solids by dilution with process water prior to a FILBLAST
leaching experiment. The results as tabulated in Table 3
indicate that excessive silica concentration was still
observed. However, the pulp viscosity did not seem to be
greatly affected and the resulting pulp settled down as
readily as the unleached pulp.
CA 02272857 1999-05-05
WO 98/19779 PCT/AU97/00751
- 16 -
LEACH TIME (H) Cu GRADE (kg/t) U308 GRADE (g/t)
0 22.1 759
2 11.1 130
Table 3
It is significant to note that the uranium concentration in
the low pulp density leach residue was found to be quite low
(130 g/t) although a residence time of only 2 hours was
employed. While such a single test may not be sufficient to
provide conclusive results, it is quite probable that low
pulp density leach would result in improved uranium recovery.
Such a high uranium recovery could be accounted for by a
better diffusion of ferric ions toward the uranium bearing
mineral particles.
Example 3
Exploratory tests have been conducted using a chalcocite
filter cake sample with particle size P80=53gm, taken from a
flotation plant. The tests were conducted in the temperature
range of 85-90 C. A pulp density of approximately 8% was
employed and flotation concentrate leach thickener overflow
was used as a source of ferrous sulphate (approximately 30
g/L). A Sulphuric acid content of approximately 50 g/L was
employed. The results as presented graphically in Figure 4
indicate that the FILBLAST reactor can be employed for
effective leaching of copper from concentrate which contains
mainly chalcocite (51% Cu) and covellite. A final recovery
of 97% could be achieved within a residence time of
approximately 2 hours. During the leaching process, ferrous
sulphate was oxidised to ferric state which in turn reacted
with chalcocite and covellite to form copper sulphate and
elemental sulphur. Spent ferric sulphate was continuously
re-oxidised to ferric state resulting in an increasing ferric
concentration in the solution from an initial value of
approximately 1 g/L to approximately 20 - 30 g/L when copper
sulphide dissolution approached completion. Such a final
CA 02272857 1999-05-05
WO 98/19779 PCT/AU97/00751
- - 17 -
ferric concentration can be controlled at a lower level by
appropriate control of oxygen injection.
Example 4
Tests have also been conducted using a bornite filter cake
sample with particle size P80=45 .m, taken from a flotation
plant. The tests were conducted on 1000L of slurry in the
temperature range of 85-90 C. A pulp density of
approximately 8% was employed and a ferrous sulphate solution
containing sulphuric acid was used as a source of ferrous
sulphate (approximately 30 g/L). A Sulphuric acid content of
approximately 50 g/L was employed. The results as presented
graphically in Figure 4 indicate that the FILBLAST reactor
can be employed for effective leaching of copper from
concentrate which contains mainly bornite and.chalcopyrite
(29.8% Cu). A final recovery of 95% could be achieved with
a FILBLAST reactor operating time of approximately 4 hours
followed by 2 hours of agitation without the FILBLAST reactor
operating. During the leaching process, ferrous sulphate was
oxidised to ferric state which in turn reacted with bornite
and chalcopyrite to form copper sulphate and elemental
sulphur. After 4 hours the FILBLAST reactor was turned off
and the concentrate was allowed to react with the excess
ferric sulphate.
RECTIFIED SHEET (RULE 91)
CA 02272857 1999-05-05
WO 98/19779 PCT/AU97/00751
- 18 -
A major advantage of the FILBLAST copper leaching process is
that a high concentration of copper in the leach can be
achieved particularly when leaching a high grade flotation
concentrate. On the other hand because the copper
concentration in the feed material is not a critical factor,
as long as the gangue minerals do not interfere with the
leaching process, the flotation concentration stage can be
designed on the basis of high recovery instead of product
grade. An overall high recovery would be quite probable.
In Figure 5 a preferred process for recovery of cathodic
copper from sulphides is illustrated in flow sheet form. The
recovery process incorporates a leaching step which utilises
recirculation and oxidation of ferrous ion using a FILBLAST
GSR similar to that described above. Following the steps of
milling 100 and flotation 101, the copper flotation
concentrate is subject to leaching 102 using a FILBLAST GSR
for recirculation and oxidation of ferrous ion in the leach
liquor. Sulphuric acid (H2SO4) is added to the slurry prior
to leaching. Remilling 103 of the flotation concentrate may
also be performed prior to leaching if required. A
flocculant is added to the leached liquor as it is fed to
thickener 104. Thickener overflow is subject to solvent
extraction 105 and the mineral values obtained are subject to
stripping 106 and electrowinning 107 to produce high grade
cathodic copper. Raffinate from the solvent extraction stage
105 is returned to the leach circuit 102. Underflow from the
thickener 104 is filtered 108 and the filter cake neutralised
109 prior to carbon in leach (CIL) processing 110. Carbon
from the CIL process 110 is stripped 111 and any precious
metals (gold, silver) are recovered by electrowinning 112.
Stripped carbon is reactivated 113 and
RECTIFIED SHEET (RULE 91)
CA 02272857 1999-05-05
WO 98/19779 PCT/AU97/00751
- 19 -
returned to the CIL process 110. The process illustrated in
Figure 5 is made feasible by the high concentration of copper
that can be achieved in the FILBLAST leach 102.
From the above description of preferred embodiments of the
process of ferrous oxidation and base metal/uranium leaching
in accordance with the invention, it will be evident that the
process has significant advantages compared to the prior art
processes, including the following advantages:
(1) The process has a much shorter residence time of around
a few hours that would be required for near complete base
metal recovery, depending on the nature of the ore or
concentrate, compared to at least several days for a
bacterial oxidation process.
(2) Although the residence time is comparable to that of a
conventional pressure oxidation (autoclave) process,
applicant's process achieves equivalent ferrous oxidation and
base metal/uranium dissolution without the application of
pressure over the bulk volume of the ore/concentrate/tailings
slurry or solution for the duration of the leaching process.
Instead, the ore/concentrate/slurry or solution is preferably
recirculated through a pressurised in-line mixer and
discharged back to an unpressurised tank or reaction vessel.
Hence, during leaching the slurry or solution spends much of
the time in the unpressurised reaction vessel. Typically,
during the leaching process each ore particle/unit solution
volume passes through the higher pressure zone within the in-
line mixer for less than a minute. That is, the present
invention eliminates the need for an autoclave with its high
capital cost. If desired autoclaves can still be used, but
significantly, autoclave performance will be enhanced when
used in embodiments of the present invention due to more
efficient oxygen dissolution.
(3) Substantial savings both in capital investment and
CA 02272857 1999-05-05
WO 98/19779 PCT/AU97/00751
- 20 -
operating costs compared to bacterial leaching and pressure
oxidation processes can be achieved.
(4) Substantial savings in chemical reagents are possible by
continuously reoxidising the ferrous ions to the ferric
state, as well as using low-cost oxygen as the oxidising
agent.
Numerous variations and modifications to the process
according to the invention will suggest themselves to persons
skilled in the chemical engineering arts, in addition to
those already described, without departing from the basic
inventive concepts. For example, separate oxidation of
ferrous solution prior to delivery to a leaching circuit may
be preferable in some applications. All such variations and
modifications are to be considered within the scope of the
present invention, the nature of which is to be determined
from the foregoing description.