Note: Descriptions are shown in the official language in which they were submitted.
CA 02273097 1999-OS-27
DESCRIPTION
SUGAR COMPOUND, GELLING AGENT, GELLING AGENT COMPOSITION,
PROCESSES FOR THEIR PREPARATION AND GEL COMPOSITION
Field of the Invention
The present invention relates to a novel useful
sugar compound, a process for preparing the sugar
compound, an organic gelling agent containing the sugar
compound, a heat-reversible gel prepared using the
organic gelling agent, a process for preparing the gel
and its use.
The present invention also concerns with a
novel useful gelling agent composition which can be used
for an aqueous medium, a process for preparing the
gelling agent composition, and a gel composition prepared
using the gelling agent composition.
The term "gel" used herein refers, from the
viewpoint of industrial meaning, to a state of a
substance wherein the substance is non-flowable and has a
yield value of at least 60 g/cm2 although it contains a
large amount of a medium, or to a substance in such
state. Further, the term "gelling agent composition" is
used herein to mean a composition capable of gelling a
medium when dissolved or dispersed in the medium. The
term "gel composition" used herein means a gel comprising
CA 02273097 1999-OS-27
-2-
at least a gelling agent composition and a medium as
constituent elements.
Background Art
Dibenzylidene sorbitol derivatives or
dibenzylidene xylitol derivatives even used in a small
amount can gel a wide range of organic solvents or
polymers (Kobayashi et al, Journal of Japan Rheology
Society, vol. 17, p.104 (1989) and Kobayashi et al,
Journal of Japan Rheology Society, vol. 17, p.112
(1989)). These derivatives are used in a wide variety of
applications, chiefly as solid marking agents, solid
adhesives, effluent oil gelling agents, sol-gel
transition type clarifying nucleating agents for
polyolefins and so on. However, said derivatives are
sparingly dissolved in water and can not be used as a
gelling agent alone for water.
Various high molecular weight gelling agents
useful in forming a heat-reversible water-containing gel
are known and include non-electrolytic polymers such as
natural high molecular weight starch or cellulose
derivatives, electrolytic polymers such as alginic acid,
agar and carrageenan, and protein-containing polymers
such as gelatin, collagen and casein. Synthetic polymers
such as copolymers of poval and sodium polyacrylate,
semi-synthetic polymers such as starch-acrylic acid graft
CA 02273097 1999-OS-27
-3-
polymers, and the like are widely used as a water-
absorbing polymer in the manufacture of paper diapers.
However, natural materials have a drawback of
decomposing. The gel-forming ability of electrolytic
polymers is seriously affected by a salt and is markedly
impaired in the presence of a salt. These polymers pose
a problem of operational efficiency because they are
dissolved in water usually at a low rate and a sol system
is given an elevated viscosity.
Sericin, which is expensive and a specific
species of amino acid, has an ability to gel water, but
its gelling ability is markedly affected by the acidity
of a system. Further, sericin involves a problem in
terms of preservation. For these reasons, sericin has
not been put to practical use.
When an aqueous medium is gelled using a
conventional low molecular weight gelling agent,
typically a dibenzylidene sorbitol derivative, an organic
solvent is essentially used in combination.
In the above situation, there is an earnest
desire for neutral low molecular weight organic gelling
agents free from said defects, namely, those which can be
stored without decomposition, do not adversely affect the
environment, show high efficiency in a gel-forming
process from a stage of sol formed on dissolution and are
CA 02273097 1999-OS-27
-4-
not affected by the presence of an inorganic salt.
Disclosure of the Invention
An object of the present invention is to
provide a novel useful low molecular weight organic
gelling agent which is usable for gelling an aqueous
medium.
Another object of the invention is to provide a
gelling agent composition capable of easily gelling an
aqueous medium even in the environment wherein a heat
source can not be used.
In view of the foregoing situation, the
inventors conducted extensive research on the aqueous
medium-gelling properties of various low molecular weight
organic compounds, and found that a sugar compound having
a specific structure which is undisclosed in literature
has a remarkable ability to specifically gel an aqueous
medium. A further finding was that the organic gelling
agent containing the sugar compound is a neutral low
molecular weight organic gelling agent which not only has
a gelling ability insusceptible to influence from the
presence of an inorganic salt in a system but also can be
stored without decomposition, and is unlikely to
adversely affect the environment and capable of achieving
a gel-forming process with high efficiency without
elevation of viscosity in a sol stage. The present
CA 02273097 1999-OS-27
-5-
invention was completed based on these novel findings.
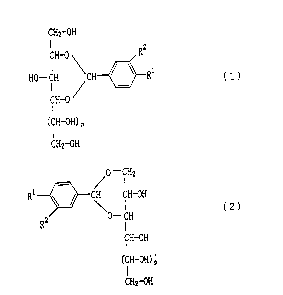
The sugar compound of the present invention is
characterized by being represented by the formula (1) or
the formula (2)
CHZ-OH
CH-0 Rz
HO-CH 'CH ~ ~ Rl
(1)
CH-0
(CH-OH) p
CHZ-OH
~0- ~H2
R1 ~ ~ CH CH-OH
\0- CH ( 2 )
RZ
CH-OH
(CH-OH) p
CHZ-OH
wherein R1 and R2 are the same or different and each
represents an alkyl group having 1 to 3 carbon atoms, an
alkoxyl group having 1 to 3 carbon atoms or a halogen
atom, and p is 0 or 1.
The present inventors conducted extensive
research on the aqueous medium-gelling properties of
CA 02273097 1999-OS-27
-6-
various low molecular weight organic compounds, and found
that a composition of specific formula comprising a sugar
compound of specific structure and a dispersant can form
a gel when dissolved in an aqueous medium with heating
and cooled, and also can easily form a gel approximately
at room temperature without heating. Based on this novel
finding, the present invention was accomplished.
The gelling agent composition of the invention
comprises 100 parts by weight of at least one sugar
compound selected from the group consisting of sugar
compounds represented by the formula (1) and sugar
compounds represented by the formula (2), and 1 to 1000
parts by weight of at least one dispersant selected from
the group consisting of nonionic surfactants, anionic
surfactants, cationic surfactants, amphoteric surfactants
and hydrophilic organic solvents
CH2-OH
CH-0 RZ
HO-CH 'CH ~ ~ Rl
(1)
CH-0
(CH-OH) p
CHz-OH
CA 02273097 1999-OS-27
~0- ~H2
CH iH-OH
\0- CH ( 2 )
CH-OH
(CH-OH) P
CH2-OH
wherein R1 and R2 are the same or different and each
represents an alkyl group having 1 to 3 carbon atoms, an
alkoxyl group having 1 to 3 carbon atoms or a halogen
atom, and p is 0 or 1.
Examples of the sugar compounds of the formula
(1) or the formula (2) are mono(3-chloro-4-methylbenzy-
lidene)-D-sorbitol, mono(3,4-dimethoxybenzylidene)-D-
sorbitol, mono(3-ethyl-4-methylbenzylidene)-D-sorbitol,
mono(3-methyl-4-ethylbenzylidene)-D-sorbitol, mono(3-
propyl-4-methylbenzylidene)-D-sorbitol, mono(3,4-
diethylbenzylidene)-D-sorbitol, mono(3,4-dichloro-
benzylidene)-D-sorbitol, mono(3,4-dimethylbenzylidene)-D-
sorbitol, mono(3,4-dipropylbenzylidene)-D-sorbitol,
mono(3,4-diethoxybenzylidene)-D-sorbitol, mono(3,4-
diisopropoxybenzylidene)-D-sorbitol and like sorbitol
derivatives; and mono(3-ethyl-4-methylbenzylidene)-
xylitol, mono(3-methyl-4-ethylbenzylidene)-xylitol,
mono(3-propyl-4-methylbenzylidene)-xylitol, mono(3,4-
CA 02273097 1999-OS-27
_g_
diethylbenzylidene)-xylitol, mono(3,4-dichloro-
benzylidene)-xylitol, mono(3,4-dimethylbenzylidene)-
xylitol, mono(3,4-dipropylbenzylidene)-xylitol, mono(3-
chloro-4-methylbenzylidene)-xylitol, mono(3,4-dimethoxy-
benzylidene)-D-xylitol and like xylitol derivatives.
The sugar compounds of the present invention
can be prepared by the following process.
A process for preparing the contemplated sugar
compound comprises the step of subjecting a sugar alcohol
(A) and a benzaldehyde derivative (B) to dehydration
condensation in water or in an organic solvent, said
sugar alcohol (A) being at least one member selected from
the group consisting of sorbitols and xylitols, and said
benzaldehyde derivative (B) being at least one member
selected from the group consisting of 3,4-disubstituted
benzaldehyde represented by the formula (3) and dialkyl
ether of 3,4-disubstituted benzaldehyde represented by
the formula (4), the molar ratio (A/B) of the sugar
alcohol (A) to the benzaldehyde derivative (B) being 2/1
to 1/2, thereby producing the contemplated sugar compound
at a high reactivity and a high selectivity:
1
R ~ ~ CHO
R2
CA 02273097 1999-OS-27
_g_
/oR~
R1 ~ ~ ~H ~ 4 )
RZ \oR3
wherein R1 and R2 are the same or different and each
represents an alkyl group having 1 to 3 carbon atoms, an
alkoxyl group having 1 to 3 carbon atoms, or a halogen
atom, and R3 represents an alkyl group having 1 to 4
carbon atoms.
The sugar alcohol (A) is used preferably in the
form of an aqueous solution in a concentration of 5 to
95~ by weight, mare preferably in the form of an aqueous
solution in a concentration of 50 to 80~ by weight.
Examples of the benzaldehyde derivative (B) are
3-chloro-4-methylbenzaldehyde, 3,4-dimethoxybenzaldehyde,
3-ethyl-4-methylbenzaldehyde, 3-methyl-4-ethyl-
benzaldehyde, 3-propyl-4-methylbenzaldehyde, 3,4-
diethylbenzaldehyde, 3,4-dichlorobenzaldehyde, 3,4-
dimethylbenzaldehyde, 3,4-dipropylbenzaldehyde, 3,4-
diethoxybenzaldehyde, 3,4-diisopropoxybenzaldehyde,
dimethyl ethers thereof, diethyl ethers thereof, dipropyl
ethers thereof and dibutyl ethers thereof. Among them,
3,4-dimethylbenzaldehyde and 3,4-dichlorobenzaldehyde are
CA 02273097 1999-OS-27
-10-
preferred.
The molar ratio (A/B) of the reaction substrate
to be used is in the range of 2/1 to 1/2. The
concentration of the reaction substrate is preferably 5
to 90~ by weight, more preferably 15 to 80~ by weight.
The dispersing medium to be used in the
reaction can be water or organic solvents such as
cyclohexane, methyl cyclohexane, xylene, toluene or the
like. It is suitable to use water from the viewpoints of
yield, economy and the like. A powder of sorbitol or
xylitol can be used as a reaction substrate in a reaction
system wherein water is used as a dispersing medium.
Preferably the reaction is carried out in the
presence of an acid catalyst. The acid catalyst to be
used can be any of strongly acidic catalysts. Preferred
strongly acidic catalysts are sulfuric acid, hydrochloric
acid, ortho-, meta- or para-toluenesulfonic acid, alkyl
(2 to 18 carbon atoms) benzenesulfonic acid, phosphoric
acid and cationic exchange resin.
The amount of the catalyst to be used is not
specifically limited, but is preferably in the range of
0.05 to 20~ by weight, more preferably 1 to 10~ by
weight, based on the total amount of the reaction
substrate (components (A)+(B)).
The reaction temperature is preferably 80°C or
~
CA 02273097 1999-OS-27
-11-
lower, more preferably 15 to 40°C.
It is desirable to replace the air in the
reaction system by a gas such as nitrogen or carbon
dioxide gas which is inert to the benzaldehyde derivative
to be used as the starting material.
The reaction time is not specifically limited,
but usually 1 to 50 hours, preferably 3 to 10 hours.
After completion of the reaction, the reaction
mixture is neutralized with an alkali while being
stirred. The neutralized mixture is filtered, washed and
dried. Useful alkalis are, for example, sodium
hydroxide, potassium hydroxide, sodium carbonate,
potassium carbonate, sodium hydrogencarbonate and sodium
silicate. The alkalis can be used in the form of any of
powders, granules and solutions. When the alkali is used
in the form of a solution, the concentration thereof is
not specifically limited, but is preferably 5 to 10~ by
weight. The solvent to be used for washing can be any of
water, a solvent mixture of water and lower alcohol of 1
to 3 carbon atoms, hexane, cyclohexane, toluene, xylene
and like organic solvents. Optionally, recrystallization
can be further conducted.
The sugar compound of the formula (1) or the
formula (2) prepared as above is very useful as a gelling
agent for gelling an aqueous medium. The organic gelling
CA 02273097 1999-OS-27
-12-
agent of the present invention has a feature of
containing at least one of said sugar compounds.
The sugar compounds of the present invention
includes 1,3-O-isomer (corresponding to the compound of
the formula (2)) and 2,4-0-isomer (corresponding to the
compound of the formula (1)) as position isomers of 6-
membered dioxane-type acetal ring. Any of these position
isomers and mixtures thereof is effectively used as a
gelling agent for gelling an aqueous medium. Among them,
2,4-0-(3,4-disubstituted benzylidene)-D-sorbitol and 2,4-
O-(3,4-disubstituted benzylidene)-xylitol as 2,4-O-
isomers are preferred from the viewpoints of the ability
to gel an aqueous medium, and 2,4-O-(3,4-
dimethylbenzylidene)-D-sorbitol and 2,4-0-(3,4-
dimethylbenzylidene)-xylitol are more preferred.
The gel of the invention can be prepared by
adding the organic gelling agent of the invention to the
specified aqueous medium, heating the mixture to dissolve
the gelling agent in the medium and cooling the solution.
The amount of the gelling agent to be used is
not specifically limited. Although suitably selected
according to the use of the gel, the amount is 0.02 to
10~ by weight, preferably 0.4 to 5$ by weight, based on
the aqueous medium. Generally as the amount of the
gelling agent increases, the hardness of the gel rises.
CA 02273097 1999-OS-27
-13-
When 1.8~ by weight or less of the gelling agent is
present, a clear gel is easily formed.
The heating temperature is not specifically
limited but it is, at normal pressure, 100°C or lower,
preferably 50 to 100°C. At an elevated pressure
(specifically, up to 50 kg/cm2G), it is 200°C or lower,
preferably 100 to 150°C.
In forming a gel, any desired cooling
procedure, either slow cooling or rapid cooling, can be
effected. Stated more specifically, when a sol (flowable
solution) is cooled, a sol-gel transition occurs to give
a non-flowable gel. The cooling temperature is 10 to
50°C, preferably 20 to 40°C.
The aqueous media to be gelled with the organic
gelling agent of the invention include, for example,
water, aqueous solutions, aqueous emulsions and aqueous
dispersions. These media include aqueous solutions,
aqueous emulsions and aqueous dispersions all of which
contain a water-soluble polymer and/or inorganic salt.
Also included are aqueous emulsions containing an
ethylene-vinyl acetate copolymer. The aqueous media
further include, for example, medium mixtures of water
and a water-soluble medium (such as lower alcohol,
dioxane, THF and the like). The concentration of the
water-soluble polymer, inorganic salt or ethylene-vinyl
CA 02273097 1999-OS-27
-14-
acetate copolymer in the aqueous media is preferably 0.05
to 20$ by weight.
When a water-soluble polymer exists as
dissolved in an aqueous medium, the obtained gel has a
higher gel hardness. Water-soluble polymers which can
effectively increase the gel hardness include, for
example, hydroxypropyl cellulose (number average
molecular weight 10,000 to 500,000), hydroxyethyl
cellulose (number average molecular weight 10,000 to
500,000), polyvinyl alcohol (number average molecular
weight 400 to 100,000), polymethyl vinyl ether (number
average molecular weight 400 to 100,000) and polyvinyl
pyrrolidone (number average molecular weight 400 to
100,000).
The gel hardness can be increased when
combinedly using conventional high molecular weight
gelling agents such as starch, cellulose derivatives,
alginic acid or agar.
One of the features of the gelling agent
according to the invention is that the stability of the
obtained gel and the hardness thereof are insusceptible
to adverse influence from inorganic salts, alkalis, urea
and surfactants present in the aqueous medium. Stated
more specifically, a gel can be easily formed with
substantially no influence on the gel hardness, when any
CA 02273097 1999-OS-27
-15-
of the following media is used, as when water is used: an
aqueous medium having dissolved or dispersed therein a
salt containing nitrogen, phosphorus, potassium (such as
calcium nitrate, sodium nitrate, calcium phosphate,
ammonium phosphate and potassium phosphate), urea or a
salt thereof or the like; a 1~ by weight aqueous solution
of sodium chloride such as sea water; or an aqueous
solution of a surfactant such as alkyl sulfate, alkyl
ether sulfate, polyoxyethylene alkyl ether, quaternary
ammonium salt, amphoteric surfactants and the like. It
is seen from the above that the gelling agent of the
invention can be used as a material for a water-retaining
sanitary napkin as effectively as a water-absorbing
polymer and as a water-holding agent in the field of
civil engineering.
An aqueous emulsion of an ethylene-vinyl
acetate copolymer, an aqueous solution of polyvinyl
alcohol or an aqueous solution of polyvinyl pyrrolidone
can easily form a gel in the presence of the gelling
agent of the invention. A gel can be prepared in the
form of a stick and can be filled in a container as such.
The gel is useful as a solid bonding agent or solid
adhesive.
A gel which is used for a medical purpose such
as a poultice can be produced by gelling an aqueous
CA 02273097 1999-OS-27
-16-
medium containing a percutaneous drug, e.g. an
antiphlogistic analgesic. Useful antiphlogistic
analgesics are, for example, methyl salicylate, glycol
salicylate and others such as alkyl ester of salicylic
acid, hydroxyalkyl ester of salicylic acid, menthol or
camphor. The amount of these antiphlogistic analgesics
in the aqueous medium is 0.5 to 30$ by weight, preferably
3 to 15~ by weight.
A gel which is useful for a cosmetic purpose
can be prepared by gelling an aqueous medium containing a
surfactant, a humectant, a ultraviolet absorber or like
active components for cosmetic compositions. Cosmetic
gels can be used as a hair styling agent, makeup remover,
facial cleaning composition, facial pack auxiliary, nail
polish, nail polish remover, lipstick, deodorant
(including a stick-shaped product), etc.
The gelling agent of the invention can be
widely used in other applications, e.g. as artificial
snow, low-temperature preservatives (materials for
keeping cool), culture media, solid flavoring agents,
solid agricultural insecticides, solid fertilizers,
water-retaining gels for transformation of dessert into
green land, aromatics, deodorants, artificial gel feeds,
coagulants, gel flame retardants, battery electrolyte
gels, gel marking agents, inks, coating compositions and
CA 02273097 1999-OS-27
-17-
so on.
The novel sugar compounds of the invention are
very useful as a gelling agent for aqueous media.
The gelling agent composition of the invention
comprises 100 parts by weight of said sugar compound and
1 to 1000 parts by weight of the dispersant.
The dispersant accelerates dispersing the sugar
compound as a gelling component in the medium, promotes
the formation of a gel, enhances a gel-forming rate and
increases the gel strength.
Dispersants to be used in the invention are,
for example, nonionic surfactants, anionic surfactants,
cationic surfactants, amphoteric surfactants and
hydrophilic organic solvents. These dispersants can be
used in the invention either alone or in combination.
Examples of nonionic surfactants to be used as
the dispersant are polyoxyalkylene alkyl ether,
polyoxyalkylene alkenyl ether, polyoxyalkylene aryl
ether, polyoxyalkylene polyol ether, polyoxyalkylene
fatty acid ester, fatty acid amide, polyol ester, alkyl
polyglycoside, amine oxide, etc.
Among the nonionic surfactants to be used in
the invention, polyoxyalkylene alkyl ether,
polyoxyalkylene alkenyl ether and polyoxyalkylene aryl
ether are compounds of the formula (5) prepared by an
CA 02273097 1999-OS-27
-18-
addition reaction wherein an alcohol is reacted with an
alkylene oxide:
R4-O-(AO)n-H (5)
wherein R4 is a straight-chain or a branched-chain alkyl
group having 6 to 20 carbon atoms, an alkenyl group
having 6 to 20 carbon atoms or an aryl group, A is an
alkylene group having 2 to 4 carbon atoms, and n is an
average molar number of added oxyalkylene groups and is
0.1 to 40.
Specific examples of the polyoxyalkylene alkyl
ether having a straight-chain alkyl group are
polyoxyethylene hexyl ether, polyoxypropylene hexyl
ether, polyoxybutylene hexyl ether, polyoxyethylene
heptyl ether, polyoxypropylene heptyl ether,
polyoxybutylene heptyl ether, polyoxyethylene octyl
ether, polyoxypropylene octyl ether, polyoxybutylene
octyl ether, polyoxyethylene nonyl ether,
polyoxypropylene nonyl ether, polyoxybutylene nonyl
ether, polyoxyethylene decyl ether, polyoxypropylene
decyl ether, polyoxybutylene decyl ether, polyoxyethylene
undecyl ether, polyoxypropylene undecyl ether,
polyoxybutylene undecyl ether, polyoxyethylene dodecyl
ether, polyoxypropylene dodecyl ether, polyoxybutylene
dodecyl ether, polyoxyethylene tridecyl ether, polyoxy-
propylene tridecyl ether, polyoxybutylene tridecyl ether,
CA 02273097 1999-OS-27
-19-
polyoxyethylene tetradecyl ether, polyoxypropylene
tetradecyl ether, polyoxybutylene tetradecyl ether,
polyoxyethylene pentadecyl ether, polyoxypropylene
pentadecyl ether, polyoxybutylene pentadecyl ether,
polyoxyethylene hexadecyl ether, polyoxypropylene
hexadecyl ether, polyoxybutylene hexadecyl ether,
polyoxyethylene heptadecyl ether, polyoxypropylene
heptadecyl ether, polyoxybutylene heptadecyl ether,
polyoxyethylene octadecyl ether, polyoxypropylene
octadecyl ether and polyoxybutylene octadecyl ether.
Specific examples of the polyoxyalkylene alkyl
ether having a branched-chain alkyl group are
polyoxyethylene-(2-ethylhexyl) ether, polyoxypropylene-
(2-ethylhexyl) ether, polyoxybutylene-(2-ethylhexyl)
ether, polyoxyethylene isooctyl ether, polyoxypropylene
isooctyl ether, polyoxybutylene isooctyl ether,
polyoxyethylene isoheptyl ether, polyoxypropylene
isoheptyl ether, polyoxybutylene isoheptyl ether,
polyoxyethylene isononyl ether, polyoxypropylene isononyl
ether, polyoxybutylene isononyl ether, polyoxyethylene
isodecyl ether, polyoxypropylene isodecyl ether,
polyoxybutylene isodecyl ether, polyoxyethylene
isoundecyl ether, polyoxypropylene isoundecyl ether,
polyoxybutylene isoundecyl ether, polyoxyethylene
isododecyl ether, polyoxypropylene isododecyl ether,
CA 02273097 1999-OS-27
-20-
polyoxybutylene isododecyl ether, polyoxyethylene
isotridecyl ether, polyoxypropylene isotridecyl ether,
polyoxybutylene isotridecyl ether, polyoxyethylene
isotetradecyl ether, polyoxypropylene isotetradecyl
ether, polyoxybutylene isotetradecyl ether,
polyoxyethylene isopentadecyl ether, polyoxypropylene
isopentadecyl ether, polyoxybutylene isopentadecyl ether,
polyoxyethylene isohexadecyl ether, polyoxypropylene
isohexadecyl ether, polyoxybutylene isohexadecyl ether,
polyoxyethylene isoheptadecyl ether, polyoxypropylene
isoheptadecyl ether, polyoxybutylene isoheptadecyl ether,
polyoxyethylene isooctadecyl ether, polyoxypropylene
isooctadecyl ether, polyoxybutylene isooctadecyl ether,
polyoxyethylene-(2-hexyldecyl) ether, polyoxypropylene-
(2-hexyldecyl) ether, polyoxybutylene-(2-hexyldecyl)
ether, polyoxyethylene-(2-octyldodecyl) ether,
polyoxypropylene-(2-octyldodecyl) ether and
polyoxybutylene-(2-octyldodecyl) ether.
Specific examples of the polyoxyalkylene
alkenyl ether are polyoxyethylene oleyl ether,
polyoxypropylene oleyl ether and polyoxybutylene oleyl
ether.
Specific examples of the polyoxyalkylene aryl
ether are polyoxyethylene-p-octylphenyl ether,
polyoxypropylene-p-octylphenyl ether, polyoxybutylene-p-
CA 02273097 1999-OS-27
-21-
octylphenyl ether, polyoxyethylene-p-nonylphenyl ether,
polyoxypropylene-p-nonylphenyl ether, polyoxybutylene-p-
nonylphenyl ether, polyoxyethylene-p-dodecylphenyl ether,
polyoxypropylene-p-dodecylphenyl ether and
polyoxybutylene-p-dodecylphenyl ether.
The molar number of the alkylene oxide reacted
in the addition reaction is generally expressed in terms
of an average value. An average molar number thereof is
0.1 to 40 moles, preferably 2 to 20 moles.
Among said polyoxyalkylene alkyl ethers and
polyoxyalkylene alkenyl ethers, those which are about 5
to about 20 in HLB are preferred. Of such polyoxy-
alkylene alkyl ethers and polyoxyalkylene alkenyl ethers,
preferred is an addition reaction product of saturated or
unsaturated alcohol of 10 to 20 carbon atoms with an
average of 2 to 20 moles of alkylene oxide.
Examples of the polyoxyalkylene polyol ether to
be used as the dispersant are compounds prepared by an
addition reaction wherein an alkylene oxides such as
ethylene oxide, propylene oxide or butylene oxide is
reacted with a saccharide such as glucose, fructose or
sucrose, a sugar alcohol such as sorbitol, xylitol or
maltitol, a diol such as ethylene glycol or propylene
glycol, a polyol such as glycerin, pentaerythritol or
trimethylolpropane. Among them, preferred is a compound
CA 02273097 1999-OS-27
-22-
prepared by an addition reaction wherein an average of
about 1 to about 50 moles of alkylene oxide is reacted.
Examples of the polyoxyalkylene fatty acid
ester to be used as the dispersant include a compound
prepared by an addition reaction wherein saturated or
unsaturated fatty acid of 8 to 20 carbon atoms is reacted
with an average of 0.1 to 40 moles of alkylene oxide of 2
to 4 carbon atoms. Preferred is a compound prepared by
an addition reaction wherein saturated or unsaturated
fatty acid of 8 to l8 carbon atoms is reacted with an
average of 2 to 20 moles of alkylene oxide, such as
polyoxyethylene oleyl ester.
Fatty acid amides useful as the dispersant
include, for example, alkanolamide (alkanol of 1 to 6
carbon atoms) of saturated or unsaturated fatty acid of 8
to 20 carbon atoms. Preferred is lower alkanolamide
(alkanol of 2 to 4 carbon atoms) of saturated or
unsaturated fatty acid of 12 to 18 carbon atoms.
Further usable as the dispersant is a
derivative prepared by an addition reaction wherein said
fatty acid amide is reacted with an average of 1 to 40
moles of alkylene oxide.
Examples of the polyol ester to be used as the
dispersant are esters of fatty acids of 2 to 18 carbon
atoms with a saccharide such as glucose, fructose or
CA 02273097 1999-OS-27
-23-
sucrose, a sugar alcohol such as sorbitol, xylitol or
maltitol, a diol such as ethylene glycol or propylene
glycol, or a polyol such as glycerin, pentaerythritol or
trimethylolpropane. Preferred is a polyol ester of fatty
acid of 12 to 18 carbon atoms.
Further usable as the dispersant is a fatty
acid ester of polyol prepared by an addition reaction
wherein said polyol is reacted with an average of 1 to 40
moles of alkylene oxide.
An alkyl polyglycoside useful as the dispersant
is represented by the formula (6)
(G)m_O_R5 (6)
wherein G is a pentose or a hexose, m is an average
polymerization degree of the sugar, and 1 to 10, and R5
is an alkyl group or an alkenyl group each having 6 to 20
carbon atoms.
Examples of the alkyl polyglycoside are decyl
polyglucoside, dodecyl polyglucoside, tetradecyl
polyglucoside, decyl polygalactoside, dodecyl
polygalactoside, tetradecyl polygalactoside, decyl
polyfructoside, dodecyl polyfructoside and tetradecyl
polyfructoside.
The average polymerization degree of the alkyl
polyglycoside is 1 to 10 moles, preferably 1 to 3.0
moles. A preferred HLB of the alkyl polyglycosides is
CA 02273097 1999-OS-27
-24-
selected from the range of about 10 to about 15.
Further usable as the dispersant is a
derivative prepared by an addition reaction wherein said
alkyl polyglycoside is reacted with an average of 1 to 40
moles of alkylene oxide.
The amine oxide to be used as the dispersant is
represented by the formula (7)
R6-N~R7)~R8)-~ U )
wherein R6 is an alkyl group or an alkenyl group each
having 6 to 18 carbon atoms and each of R~ and R8 is a
methyl group or an ethyl group.
Among the amine oxides, an amine oxide having
an alkyl group of 10 to 12 carbon atoms is preferred from
the viewpoint of dispersibility.
Examples of the anionic surfactant to be used
as the dispersant are alkyl or alkenyl sulfate, alkyl
ether sulfate, alkyl phenyl ether sulfate, alkyl
benzenesulfonic acid salt, sulfosuccinic acid ester salt,
metal salt of fatty acid and alkyl or alkenyl ether
carboxylic acid salt.
Among them, preferred from the viewpoint of
dispersibility are alkyl or alkenyl sulfate having about
12 to about 20 carbon atoms, alkyl ether sulfate having
about 12 to about 20 carbon atoms, and sodium salt of
fatty acid having an alkyl group or an alkenyl group each
CA 02273097 1999-OS-27
-25-
having about 12 to about 20 carbon atoms.
Examples of the cationic surfactant to be used
as the dispersant are quaternary ammonium salt,
especially tetraalkyl ammonium salt, and ammonium salt
prepared by an addition reaction of alkylene oxide.
Among them, preferred from the viewpoint of
dispersibility are trimethyl alkyl ammonium chloride
having an alkyl group of 12 to 18 carbon atoms, dimethyl
dialkyl ammonium chloride having two alkyl groups of 12
to 18 carbon atoms, and N,N-bis(polyoxyethylene)alkyl
methyl ammonium chloride having an alkyl group of 12 to
18 carbon atoms.
Amphoteric surfactants useful as the dispersant
include, for example, amino acid-based or betaine-based
surfactants. Among them, preferred is alkyl dimethyl
aminoacetic acid betaine having about 8 to about 20
carbon atoms.
The hydrophilic organic solvent to be used as
the dispersant is a solvent which is homogenous when
mixed with water in a desired ratio. Specific examples
are polyalkylene glycol and polyhydric alcohol.
The polyalkylene glycol useful as the
dispersant includes, for example, polyethylene glycol,
polypropylene glycol, etc. A preferred polyalkylene
glycol is a compound prepared by an addition reaction
CA 02273097 1999-OS-27
-26-
wherein about 0.1 to about 150 moles, preferably about 1
to about 100 moles, of alkylene oxide is reacted.
Polyhydric alcohols useful as the dispersant
are, for example, saccharides such as glucose, fructose
and sucrose, sugar alcohols such as sorbitol, xylitol and
maltitol, diols such as ethylene glycol and propylene
glycol, glycerin, pentaerythritol and trimethylolpropane.
The dispersant to be used in the invention can
be in various forms such as crystals, powders, paste and
solutions, and can be in any form insofar as the
dispersant can be easily mixed with or dispersed in a
medium (especially an aqueous medium) in a gelling agent
composition. Essentially the dispersant is easily
dissolved in an aqueous medium. For this reason, the
dispersant in the form of fine crystals, powders, paste
or solutions are preferred. More preferred is a
dispersant as a solution.
The amount of the dispersant to be used in the
invention is determined in a specific weight ratio
relative to at least one sugar compound selected from the
group consisting of sugar compounds of the formula (1)
and sugar compounds of the formula (2) so that the amount
of the dispersant is 1 to 1000 parts by weight,
preferably 10 to 500 parts by weight, per 100 parts by
weight of the sugar compound. When the dispersant is
CA 02273097 1999-OS-27
-27-
excessively used, the gelling ability is lowered in a
non-heated system. On the other hand, a lesser amount of
the dispersant tends to lower the dispersibility of the
sugar compound in which case it is difficult to form a
homogeneous gel.
To disperse the fine solids in a liquid, the
dispersant has been conventionally used in a
concentration in the vicinity of a critical micelle
concentration (C.M.C.) (e. g. about 500 ppm or less)
("Surfactant", edited by Kodan Sha Scientific, published
in 1979, pp.69-82). However, the dispersant is used in
the invention to disperse the sugar compound in a
concentration higher by one order of magnitude than
C.M.C., namely in a high concentration of about 5000 ppm
to about 15~ by weight in which the dispersant can
exhibit the dispersibility well.
If an aqueous medium is gelled using a sugar
compound as a gelling agent without use of a dispersant,
heating is required in gelling process or a prolonged
period of time, e.g. at least 48 hours is involved in
gelling at room temperature. On the other hand, since
the gelling agent composition of the invention contains a
large amount of the dispersant, namely at least 5000 ppm,
a far larger amount than the conventional amount, the
gelling time can be controlled so that the medium is
CA 02273097 1999-OS-27
-28-
gelled immediately or about 20 minutes after mixing the
gelling agent composition with the aqueous medium in case
of short time, or 120 minutes to 24 hours thereafter in
case of deferred time.
On the other hand, the obtained gel composition
has a markedly high gel hardness when formed at room
temperature, compared with a gel obtained without use of
a dispersant. Moreover, the above feature produces a
surprising effect of extending the concentration range of
the gelling agent to be used in gelling process.
This advantage may be presumably derived from
the following phenomenon. When the gelling agent forms a
three-dimensional network in the medium, the dispersant
lodges in the structure of the network in a complicatedly
entangled manner. Yet the detailed reason remains to be
clarified.
Of the dispersants usable in the invention, a
hydrophilic organic solvent is mixed with the sugar
compound to make a composition. In this case, the
hydrophilicity of the composition is enhanced, thereby
causing the composition to become dispersed better in the
aqueous medium.
The sugar compound dispersed by the dispersant
and made easily wettable is combined in a complicated
form with near-by water molecules due to the hydrogen
CA 02273097 1999-OS-27
_29_
bond to thereby enlarge the three-dimensional network
throughout the system, so that the entire aqueous medium
is gelled.
The gelling agent composition of the invention
can be prepared by merely mixing the sugar compound and
the dispersant.
The gelling agent composition of the invention
can also be prepared by suspending the sugar compound and
the dispersant in a small amount of a medium (in an
amount substantially equal to the total amount of the
sugar compound and the dispersant), aging the suspension
at a temperature of 30 to 80°C, preferably 40 to 70°C for
10 to 300 hours, preferably 24 to 200 hours, and
optionally drying the obtained product.
The gelling agent composition of the invention
can also be prepared merely by dissolving the sugar com-
pound and the dispersant in a medium such as water with '
heating to give a sol, cooling the sol to a temperature
ranging from room temperature to about 40°C to produce a
gel, and removing the medium from the gel at a
temperature ranging from room temperature to 90°C under a
reduced pressure or normal pressure. The medium may be
removed from the gel by freezing the gel and eliminating
(lyophilizing) the medium from the frozen product under a
pressure reduced below the vapor pressure of the frozen
CA 02273097 1999-OS-27
-30-
medium, e.g., at a pressure reduction degree of 10 2
mmHg.
The medium in which the sugar compound and the
dispersant are suspended or dissolved includes, for
example, aqueous media such as water, an aqueous solution
of a salt and the like, lower alcohols having 1 to 4
carbon atoms such as methanol, ethanol, propanol and the
like, dioxane and so on. Aqueous media are preferred
among them.
A preferred process for preparing the gelling
agent composition is, for example, a process comprising
the steps of dissolving 100 parts by weight of at least
one sugar compound selected from the group consisting of
the sugar compound of the formula (1) and the sugar
compound of the formula (2) and 10 to 500 parts by weight
(preferably 20 to 400 parts by weight) of the dispersant
in 5000 to 20000 parts by weight of water with heating,
cooling the solution to 25°C to form a gel, freezing the
gel at a temperature of about -78°C and removing the
water under a reduced pressure (lyophilizing).
The form of the sugar compound to be used as
the starting material in preparing the gelling agent
composition of the invention is not specifically limited
and includes, for example, granules, powders, fine
particles, xerogel powders and so on. When the sugar
CA 02273097 1999-OS-27
-31-
compound and the dispersant are merely mixed together to
give a gelling agent composition, xerogel powders are
preferably adopted as the form of the sugar compound.
The term "xerogel powder" used herein refers to a powder
obtained by drying or lyophilizing the gel to remove the
medium from the gel consisting of the sugar compound and
the medium.
The gel composition can be prepared without
heating the gelling agent composition of the invention.
That is, the gel composition can be easily
prepared by adding an aqueous medium to the gelling agent
composition of the invention.
In this case, while usually the gel composition
can be easily prepared approximately at room temperature,
it can be prepared by means of heating, ultrasonic wave
irradiation or physical agitation by a homomixer or the
like.
The aqueous media to be used in preparing the
gel composition include water, aqueous solutions, aqueous
emulsions and aqueous dispersions. A mixture of water
and at least one medium other than water can be used.
Examples of such media other than water are methanol,
ethanol, propanol and like lower alcohols of about 1 to
about 4 carbon atoms, dioxane, THF, cellosolve, etc.
The amount of the aqueous medium to be
CA 02273097 1999-OS-27
-32-
incorporated in the gel composition is 5 to 200 parts by
weight, preferably 5 to 150 parts by weight, per part by
weight of the gelling agent composition. If less than 5
parts by weight of the aqueous medium is used, there is a
tendency to give a gel with the gelling agent
precipitated. If over 200 parts by weight of the aqueous
medium is used, the obtained gel is likely to become
unstable because of its low strength.
The gelling agent composition or the gel
composition of the invention may contain at least one
additive selected from antioxidants, stabilizers and the
like which do not impair the ability to form a gel.
Examples of the antioxidant are phenolic
compounds, phosphite, sulfur compounds, etc. The amount
of the antioxidant to be used is, for example, 0.001 to
3$ by weight based on the gelling agent composition.
The stabilizer to be used is classified into
two types, a stabilizer for the sugar compound and a
stabilizer for the gel structure.
Examples of useful stabilizers for the sugar
compound are alkaline compounds including inorganic
alkali salts such as sodium hydroxide, potassium
hydroxide, sodium carbonate, potassium carbonate or
sodium salt of ethylenediaminetetraacetic acid (EDTA),
salts of alkali metals such as potassium or sodium of
CA 02273097 1999-OS-27
-33-
organic acids such as citric acid, succinic acid, lactic
acid, benzoic acid or fatty acid, and salts of alkaline
earth metals such as magnesium or calcium of said organic
acids. The amount of the stabilizer to be used is, for
example, 0.001 to 3~ by weight, based on the gelling
agent composition.
The stabilizer for the gel structure to be used
includes, for example, a chelating agent such as EDTA.
The amount of the stabilizer to be used is, for example,
0.001 to 3~ by weight based on the gelling agent
composition.
The gelling agent composition or the gel
composition of the invention may further contain at least
one additive other than said additives such as inorganic
salts, organic salts, flavoring agents, anticeptics,
pigments, lower alcohols, surfactants other than the
dispersants of the invention, polymers and so on.
The gelling agent composition or the gel
composition of the invention may be used in combination
with conventional other gelling agents including low
molecular weight gelling agents such as dibenzylidene
sorbitol, hydroxy-fatty acid derivatives, alkylamide
derivatives or cholesterol derivatives, or high molecular
weight gelling agents such as agar, gelatin or
carageenan.
CA 02273097 1999-OS-27
-34-
One of the features of the gelling agent
composition in the present invention is that a gel can be
easily formed in the invention without heating.
When a gel is formed using a gelling agent
(i.e. sugar compound) and water as a solvent, the sugar
compound is used in a concentration of up to about 2~ by
weight in view of the solubility of the sugar compound.
If the concentration of the sugar compound is in excess
of 1.8~ by weight, a system containing the gel and
crystals as mixed is produced. However, when a gel is
formed using the gelling agent composition of the present
invention, a gelling agent can be used in a high
concentration and the obtained gel is stable in shape and
higher in gel hardness.
Because the gelling agent composition of the
invention can be easily gelled at room temperature, it
can be used in applications as replacement products made
of water-absorbing resins such as throw-away diapers and
sanitary napkins, as products in the fields of water gel
mats and cooling pillows, as coagulants for water
treatment, as water-holding agents in civil engineering
field, and as supports of cut flowers.
The gelling agent composition of the invention
can easily gel an aqueous medium at room temperature, can
easily form a gel in the environment lacking a heat
CA 02273097 1999-OS-27
-35-
source and can be used for various purposes.
Best Mode for Carrying Out the Invention
The present invention will be described below
in more detail with reference to the following Examples.
The properties were determined according to the
following methods.
Measurement of gel hardness
Measured with an instrumenir for measuring the
agar jelly strength (manufactured by Kiya Seisakusho,
Ltd.)
Measurement of melting point
Measured at a temperature-elevating rate of
1°C/min using an instrument for measuring the melting
point of a minute amount of sample (manufactured by
Yanagimoto Seisaku Sho Co., Ltd.)
Measurement of purity by gas chromatography (GC)
After pretreatment by reaction for conversion
to trimethylsilyl derivative, the purity was measured by
gas chromatography using columns of silicone fillers.
Example 1
A 500 ml four-necked flask was charged with a
70 wt~ aqueous solution of 143 g (0.55 mole) of D-
sorbitol, 73.7 g (0.55 mole) of 3,4-dimethylbenzaldehyde
and 12 g of 50 wt~ sulfuric acid. The air inside the
system was replaced with nitrogen gas. The contents of
CA 02273097 1999-OS-27
-36-
the flask were stirred at a temperature of 21°C for 6
hours. The obtained reaction mixture was analyzed by GC.
It was found that mono(3,4-dimethylbenzylidene)-D-
sorbitol was produced in a yield of 70~ and 0.5~ or less
of bis(3,4-dimethylbenzylidene)-D-sorbitol was also
produced.
After standing for 24 hours, the reaction
mixture was neutralized for 2 hours using 8.6 g of
potassium hydroxide, and filtered to obtain cake. The
cake was dispersed in 150 ml of toluene and was filtered
again. After drying at 100°C under a pressure ranging
from normal pressure to 5 mmHg for 7 hours, 65 g of 2,4-
O-(3,4-dimethylbenzylidene)-D-sorbitol was obtained with
a purity of 99.5 as measured by GC (yield 39.70 . The
product had a melting point of 199 to 200°C.
A 300 ml beaker was charged with 99 g of water
and 1 g of the 2,4-O-(3,4-dimethylbenzylidene)-D-sorbitol
obtained above. The mixture was heated to 96°C with
stirring. Two minutes later, a transparent homogenous
solution (sol) was obtained. The solution (20 ml) was
poured into a 300 ml sample beaker, and quenched in a
water bath at 20°C, giving a transparent gel one minute
later. In measurement three hours later, the gel
hardness was 270 g/cm2. The gel heated again to 96°C
heat-reversibly became a homogenous solution. When the
CA 02273097 1999-OS-27
-37-
solution was quenched in the same manner as above, the
same gel was obtained again.
Example 2
A 300 ml beaker was charged with 99 g of a 1
wt~ aqueous solution of sodium chloride and 1 g of
the 2,4-O-(3,4-dimethylbenzylidene)-D-sorbitol obtained
in Example 1. The contents of the beaker were heated to
96°C with stirring. Two minutes later, a transparent
homogenous solution was obtained. The solution (20 ml)
was poured into a 300 ml sample beaker, and quenched in a
water bath at 20°C, giving a transparent gel one minute
later. When measured three hours later, the gel hardness
was 280 g/cm2.
Example 3
The same procedure as in Example 2 was repeated
with the exception of using 99 g of a mixture of a 1 wt~
aqueous solution of sodium nitrate, a 1 wt~ aqueous
solution of ammonium phosphate and a 1 wt~ aqueous
solution of potassium phosphate in equal amounts in place
of the 1 wt~ aqueous solution of sodium chloride, whereby
a gel was formed. When measured three hours later, the
gel hardness was 270 g/cm2.
Example 4
The same procedure as in Example 1 was repeated
with the exception of using a 1 wt~ aqueous solution of
CA 02273097 1999-OS-27
-38-
polyvinyl alcohol (number average molecular weight
(Mn)=700) in place of water, whereby a gel was formed.
In measurement three hours later, the gel hardness was
470 g/cm2.
Example 5
The same procedure as in Example 1 was repeated
with the exception of using a 25 wt$ emulsion of a
ethylene-vinyl acetate copolymer in place of water,
whereby a gel was formed. In measurement three hours
later, the gel hardness was 300 g/cm2.
Example 6
A 500 ml four-necked flask was charged with a
70 wtg aqueous solution of 120 g (0.55 mole) of D-
xylitol, 73.7 g (0.55 mole) of 3,4-dimethylbenzaldehyde
and 12 g of 50 wt~ sulfuric acid. The air inside the
system was replaced with nitrogen gas. The contents of
the flask were stirred at a temperature of 21°C for 6
hours. The obtained reaction mixture was analyzed by
GC. It was found that mono(3,4-dimethylbenzylidene)-D-
xylitol was produced in a yield of 70~ and 0.5~ or less
of bis(3,4-dimethylbenzylidene)-D-xylitol was also
produced. After standing 24 hours, the reaction mixture
was neutralized for 2 hours using 8.6 g of potassium
hydroxide, and filtered to obtain cake. The cake was
dispersed in 150 ml of toluene and was filtered again.
CA 02273097 1999-OS-27
-39-
After drying at 100°C under a pressure ranging from
normal pressure to 5 mmHg for 7 hours, 44.5 g of 2,4-0-
(3,4-dimethylbenzylidene)-D-xylitol was obtained with a
purity of 99.2 as measured by GC (yield 30.10 . The
product had a melting point of 179.5 to 180.0°C.
The same subsequent procedure as in Example 1
was repeated with the exception of using 2,4-O-(3,4-
dimethylbenzylidene)-xylitol obtained above in place of
2,4-O-(3,4-dimethylbenzylidene)-D-sorbitol, giving a gel.
When measured three hours later, the gel hardness was 250
g/cm2.
Example 7
The same procedure as in Example 2 was repeated
with the exception of using 2,4-O-(3,4-dimethylbenzy-
lidene)-xylitol in place of 2,4-O-(3,4-dimethyl-
benzylidene)-D-sorbitol, whereby a gel was formed. In
measurement three hours later, the gel hardness was 240
g/cm2.
Example 8
A 500 ml four-necked flask was charged with a
70 wt~ aqueous solution of 52 g (0.20 mole) of D-
sorbitol, 35.0 g (0.20 mole) of 3,4-dichlorobenzaldehyde
and 6 g of 50 wt~ sulfuric acid. The air inside the
system was replaced with nitrogen gas. The contents of
the flask were stirred at a temperature of 21°C for 6
CA 02273097 1999-OS-27
-40-
hours. The obtained reaction mixture was analyzed by GC.
It was found that mono(3,4-dichlorobenzylidene)-D-
sorbitol was produced in a yield of 70~ and 0.5$ or less
of bis(3,4-dichlorobenzylidene)-D-sorbitol was also
produced. After standing for 24 hours, the reaction
mixture was neutralized for 2 hours using 8.6 g of
potassium hydroxide, and filtered to obtain cake. The
cake was dispersed in 150 ml of toluene and was filtered
again. After drying at 100°C under a pressure ranging
from normal pressure to 5 mmHg for 7 hours, 19.7 g of
2,4-O-(3,4-dichlorobenzylidene)-D-sorbitol was obtained
with a purity of 99.2 as measured by GC (yield 29.20 .
The product had a melting point of 202 to 205°C.
The same procedure as in Example 2 was repeated
with the exception of using the above-obtained 2,4-O-
(3,4-dichlorobenzylidene)-D-sorbitol in place of 2,4-O-
(3,4-dimethylbenzylidene)-D-sorbitol, whereby a gel was
formed. When measured three hours later, the gel
hardness was 250 g/cm2.
Example 9
A 300 ml beaker was charged with 99.5 g of
water and 0.5 g of the 2,4-O-(3,4-dimethylbenzylidene)-D-
sorbitol obtained in Example 1. The contents of the
beaker were heated to 90°C with stirring. One minute
later, a transparent homogenous solution was obtained.
CA 02273097 1999-OS-27
-41-
The solution (20 ml) was poured into a 300 ml sample
beaker, and quenched in a water bath at 20°C, giving a
transparent gel 8 hours later. Twenty four hours later,
the gel hardness was 100 g/cm2. The gel heated again to
90°C heat-reversibly became a homogenous solution. When
the solution was cooled, the same gel was formed 8 hours
later.
Example 10
A 300 ml four-necked flask was charged with 65
g of water, 20 g of glycerin, 5 g of glycol salicylate, 5
g of methyl salycylate and 5 g of L-menthol. The
contents of the flask were stirred at room temperature to
give a homogeneous solution. Added thereto was 1.5 g of
a powder of the gelling agent obtained in Example 1 [2,4-
O-(3,4-dimethylbenzylidene)-D-sorbitol]. The mixture was
heated to 90°C with stirring to obtain a solution. After
cooling to 80°C, the solution was poured into a vat of
stainless steel wherein it was further cooled to room
temperature. Two minutes later, a gel of 2 mm thickness
was formed. After the gel was cut to a sheet, 5 X 5 cm,
the sheet was applied over the skin of bruised arm. Then
the wounded part was covered with a wrapping sheet. Two
minutes later, manifest antiphlogistic and analgesic
effects were confirmed.
Example 11
CA 02273097 1999-OS-27
-42-
A 0.8 g quantity of the gelling agent powder
obtained in Example 1 [2,4-0-(3,4-dimethylbenzylidene)-D-
sorbitol] was added to 100 g of a 8 wt$ aqueous solution
of polyvinyl alcohol (Mn=10,000). The mixture was heated
to 90°C with stirring to obtain a sol. After cooling to
40°C, the sol was applied over human skin. Five minutes
later, the sol coat was transformed into a gel coat.
When the gel coat was peeled off, the soil over the skin
was removed.
Comparative Example 1
A sol was prepared in the same manner as in
Example 11 with the exception of not using a gelling
agent. The sol was applied over human skin, but the
formation of a film took 30 minutes.
The properties in the following Examples and
Reference Examples were determined by the methods
described below.
Measurement of gel hardness
The predetermined amount of a gel was prepared.
The gel hardness of the gel 1 day or 1 month later was
measured in a beaker, 4 cm in diameter and 6 cm in height
using an instrument for measuring the agar jelly strength
(manufactured by Kiya Seisakusho, Ltd.). Unless
otherwise indicated, the concentration of the sugar
compound of the gel compositions in the following
CA 02273097 1999-OS-27
-43-
Examples was 1~ by weight. The gel hardness thus
obtained was taken as a yield value. The higher the
yield value was, the more stable the gel was.
HLB value
The HLB value of polyoxyalkylene alkyl ether
was calculated by the equation of Griffin (W. C. Griffin,
J. Soc. Cosmetic, Chemists, 1, 1180 (1949). The HLB
values of nonionic surfactants other than polyoxyethylene
alkyl ether were those shown in a brochure offered for
sales.
Equation of Griffin
HLB=20 X (molecular weight of hydrophilic
groups in surfactant/molecular weight of surfactant)
If the gel retains the original shape one month
later, it was taken as stable.
Example 12
Added to 100 ml of water were 1.0 g of 2,4-O-
(3,4-dimethylbenzylidene)-D-sorbitol, 200 mg of CONION
275 (90) (trade name, product of New Japan Chemical Co.,
Ltd., an addition reaction product of an alcohol
predominantly containing 12 carbon atoms with an average
of 9 moles of ethylene oxide, HLB=13.5) and 10 mg of
K2C03. The three components were dissolved in water with
heating at 98°C (sugar compound/dispersant=1/0.2 (weight
ratio)). The solution was cooled to 25°C, giving a gel
CA 02273097 1999-OS-27
-44-
composition. One day later, the gel composition had a
gel hardness of 600 g/cm2.
Example 13
The gel composition obtained in Example 12 was
frozen at -78°C for 2 hours in a dry ice-acetone bath.
Then the composition was dried under reduced pressure
(pressure reduction degree of 10 4 mmHg) (the procedure
being hereinafter referred to as "lyophilized") with a
vacuum pump for 24 hours to produce 1.2 g of a gelling
agent composition as white powder.
Example 14
When 10 ml of water was added to 140 mg of the
gelling agent composition obtained in Example 13
(concentration of 1~ by weight calculated as the sugar
compound), the water was immediately gelled. One day
later, the obtained gel composition had a gel hardness of
350 g/cm2. One month later, the gel remained stable and
showed a gel hardness of 380 g/cm2.
Example 15
When 10 ml of water was added to 400 mg of the
gelling agent composition obtained in Example 13
(concentration of 3~ by weight calculated as the sugar
compound), the water was immediately gelled. One day
later, the obtained gel composition had a gel hardness of
380 g/cm2. One month later, the gel remained stable and
CA 02273097 1999-OS-27
-45-
showed a gel hardness of 400 g/cm2
Example 16
Artificial urine (10 ml) was added to 140 mg of
the gelling agent composition obtained in Example 13
(concentration of 1$ by weight calculated as the sugar
compound). The artificial urine was gelled in about 30
minutes. One day later, the obtained gel composition had
a gel hardness of 260 g/cm2. {Make-up of artificial
urine: 1.94$ by weight of urea, 0.8~ by weight of sodium
chloride, 0.06 by weight of calcium chloride, 0.11 by
weight of magnesium sulfate and 97.09$ by weight of
water).
Example 17
Added to 100 ml of water were 1.0 g of 2,4-O-
(3,4-dimethylbenzylidene)-D-sorbitol, 1.0 g of CONION 275
(60) (trade name, product of New Japan Chemical Co.,
Ltd., an addition reaction product of an alcohol
predominantly containing 12 carbon atoms with an average
of 6 moles of ethylene oxide, HLB=11.5) and 10 mg of
K2C03. The three components were dissolved in water with
heating at 98°C (sugar compound/dispersant=1/1 (weight
ratio)). The solution was cooled to 25°C, giving a gel
composition. One day later, the gel composition had a
gel hardness of 550 g/cm2. The gel composition was
frozen at -78°C in a dry ice-acetone bath. Then the
CA 02273097 1999-OS-27
-46-
composition was lyophilized with a vacuum pump for 24
hours to produce 2.0 g of a gelling agent composition as
white powder.
Example 18
When 1.0 g of sand and 10 ml of water were
added to 120 mg of the gelling agent composition obtained
in Example 17, the water was immediately gelled. One day
later, the obtained gel composition had a gel hardness of
350 g/cm2. The same gel was formed in a pipe (made of
acryl, 35 mm in inner diameter and 100 mm in length)
which was erectly placed. Water was dropped into the gel
from the top of the pipe but was unable to pass through
the gel which showed a water-holding effect.
Example 19
Added to 100 ml of water were 1.0 g of 2,4-O-
(3,4-dimethylbenzylidene)-D-sorbitol, 2.0 g of CONION 275
(60) and 10 mg of K2C03. The three components were
dissolved in water with heating at 98°C (sugar
compound/dispersant=1/2 (weight ratio)). The solution
was cooled to 25°C, giving a gel composition. One day
later, the gel composition had a gel hardness of 600
g/cm2. The gel composition was frozen at -78°C in a dry
ice-acetone bath. Then the composition was lyophilized
with a vacuum pump for 24 hours to produce 2.98 g of a
gelling agent composition as white powder.
CA 02273097 1999-OS-27
-47-
Example 20
When 10 ml of water was added to 300 mg of the
gelling agent composition obtained in Example 19, the
water was immediately gelled. One day later, the
obtained gel composition had a gel hardness of 300 g/cm2.
One month later, the gel remained stable and showed a gel
hardness of 350 g/cm2.
Example 21
Added to 100 ml of water were 1.0 g of 2,4-O-
(3,4-dimethylbenzylidene)-D-sorbitol, 600 mg of sodium
dodecylbenzene sulfonate (product of Nacalai Tesque Co.,
Ltd.) and 10 mg of K2C03. The three components were
dissolved in water with heating at 98°C (sugar compound/
dispersant=1/0.6 (weight ratio)). The solution was
cooled to 25°C, giving a gel composition. One day later,
the gel composition had a gel hardness of 450 g/cm2. The
gel composition was frozen at -78°C in a dry ice-acetone
bath. Then the composition was lyophilized with a vacuum
pump for 24 hours to produce 1.6 g of a gelling agent
composition as white powder.
Example 22
When 10 ml of water was added to 120 mg of the
gelling agent composition obtained in Example 21, the
water was immediately gelled. One day later, the
obtained gel composition had a gel hardness of 320 g/cm2.
CA 02273097 1999-OS-27
-48-
One month later, the gel remained stable and showed a gel
hardness of 320 g/cm2.
Example 23
Added to 100 ml of water were 1.0 g of 2,4-O-
(3,4-dimethylbenzylidene)-D-sorbitol, 3.0 g of N-NON A
2020A5S (trade name, product of New Japan Chemical Co.,
Ltd., polyoxyethylene (20 moles reacted)-2-hexyldodecyl
ether, HLB=15.2) and 10 mg of K2C03. The three
components were dissolved in water with heating at 98°C
(sugar compound/dispersant=1/3 (weight ratio)). The
solution was cooled to 25°C, giving a gel composition.
One day later, the gel composition had a gel hardness of
600 g/cm2. The gel composition was frozen at -78°C in a
dry ice-acetone bath. Then the composition was
lyophilized with a vacuum pump for 24 hours to produce
4.0 g of a gelling agent composition as white powder.
Example 24
When 10 ml of water was added to 120 mg of the
gelling agent composition obtained in Example 23, the.
water was immediately gelled. One day later, the
obtained gel composition had a gel hardness of 280 g/cm2
One month later, the gel remained stable and showed a gel
hardness of 300 g/cm2.
Example 25
Added to 100 ml of water were 1.0 g of 2,4-O-
CA 02273097 1999-OS-27
-49-
(3,4-dimethylbenzylidene)-D-sorbitol, 200 mg of Riponox
100 (trade name, product of Lion Co., Ltd.,
polyoxyethylene nonyl phenol, HLB (value shown in a
brochure)=13.3) and 10 mg of K2C03. The three components
were dissolved in water with heating at 98°C (sugar
compound/dispersant=1/0.2 (weight ratio)). The solution
was cooled to 25°C, giving a gel composition. The gel
composition was frozen at -78°C in a dry ice-acetone
bath. Then the composition was lyophilized with a vacuum
pump for 24 hours to produce 1.2 g of a gelling agent
composition as white powder.
Example 26
When 10 ml of water was added to 120 mg of the
gelling agent composition obtained in Example 25, the
water was immediately gelled. One day later, the
obtained gel composition had a gel hardness of 320 g/cm2.
One month later, the gel remained stable and showed a gel
hardness of 330 g/cm2.
Example 27
Added to 100 ml of water were 1.0 g of 2,4-O-
(3,4-dimethylbenzylidene)-D-sorbitol, 700 mg of APG-600
(trade name, product of Henkel Hakusui Co., Ltd., the
alkyl chain length being that of alkyl polyglucoside
predominantly containing 12 carbon atoms, average
polymerization degree 1.4, HLB (value shown in a
CA 02273097 1999-OS-27
-50-
brochure)=13.5) and 10 mg of K2C03. The three components
were dissolved in water with heating at 98°C (sugar
compound/dispersant= 1/0.7 (weight ratio)). The solution
was cooled to 25°C, giving a gel composition. The gel
composition was frozen at -78°C in a dry ice-acetone
bath. Then the composition was lyophilized with a vacuum
pump for 24 hours to produce 1.7 g of a gelling agent
composition as white powder.
Example 28
When 10 ml of water was added to 120 mg of the
gelling agent composition obtained in Example 27, the
water was immediately gelled. One day later, the
obtained gel composition had a gel hardness of 320 g/cm2.
One month later, the gel remained stable and showed a gel
hardness of 350 g/cm2.
Example 29
Added to 100 ml of water were 1.0 g of 2,4-O-
(3,4-dimethylbenzylidene)-D-sorbitol, 200 mg of
monolaurin (glycerin monolauric acid ester, product of
Nacalai Tesque Co., Ltd.) and 10 mg of K2C03. The three
components were dissolved in water with heating at 98°C
(sugar compound/dispersant=1/0.2 (weight ratio)). The
solution was cooled to 25°C, giving a gel composition.
The gel composition was frozen at -78°C in a dry ice-
acetone bath. Then the composition was lyophilized with
CA 02273097 1999-OS-27
-51-
a vacuum pump for 24 hours to produce 1.2 g of a gelling
agent composition as white powder.
Example 30
When 10 ml of water was added to 120 mg of the
gelling agent composition obtained in Example 29, the
water was gelled in about 60 minutes. One day later, the
obtained gel composition had a gel hardness of 220 g/cm2.
One month later, the gel remained stable and showed a gel
hardness of 200 g/cm2.
Example 31
Added to 100 ml of water were 1.0 g of 2,4-O-
(3,4-dimethylbenzylidene)-D-sorbitol, 200 mg of Amizol
CDE (trade name, product of Kawaken Fine Chemical Co.,
Ltd., coconut oil fatty acid diethanolamide) and 10 mg of
K2C03. The three components were dissolved in water with
heating at 98°C (sugar compound/dispersant=1 /0.2 (weight
ratio)). The solution was cooled to 25°C, giving a gel
composition. The gel composition was frozen at -78°C in
a dry ice-acetone bath. Then the composition was
lyophilized with a vacuum pump for 24 hours to produce
1.2 g of a gelling agent composition as white powder.
Example 32
When 10 ml of water was added to 120 mg of the
gelling agent composition obtained in Example 31, the
water was immediately gelled. One day later, the
CA 02273097 1999-OS-27
-52-
obtained gel composition had a gel hardness of 300 g/cm2
One month later, the gel remained stable and showed a gel
hardness of 300 g/cm2.
Example 33
Added to 100 ml of water were 1.0 g of 2,4-O-
(3,4-dimethylbenzylidene)-D-sorbitol and 500 mg of sodium
oleate (product of Nacalai Tesque Co., Ltd.). The two
components were dissolved in water with heating at 98°C
(sugar compound/dispersant=1/0.5 (weight ratio)). The
solution was cooled to 25°C, giving a gel composition.
The gel composition was frozen at -78°C in a dry ice-
acetone bath. Then the composition was lyophilized with
a vacuum pump for 24 hours to produce 1.5 g of a gelling
agent composition as white powder.
Example 34
When 10 ml of water was added to 150 mg of the
gelling agent composition obtained in Example 33, the
water was gelled in about 60 minutes. One day later, the
obtained gel composition had. a gel hardness of 170 g/cm2.
Example 35
Added to 100 ml of water were 1.0 g of 2,4-O-
(3,4-dimethylbenzylidene)-D-sorbitol and 200 mg of Arcard
T-800 (trade name, product of Lion Co., Ltd., stearyl
trimethyl ammonium chloride). The two components were
dissolved in water with heating at 98°C (sugar
CA 02273097 1999-OS-27
-53-
compound/dispersant=1/0.2 (weight ratio)). The solution
was cooled to 25°C, giving a gel composition. The gel
composition was frozen at -78°C in a dry ice-acetone
bath. Then the composition was lyophilized with a vacuum
pump for 24 hours to produce 1.2 g of a gelling agent
composition as white powder.
Example 36
When 10 ml of water was added to 200 mg of the
gelling agent composition obtained in Example 35, the
water was gelled in about 60 minutes. One day later, the
obtained gel composition had a gel hardness of 100 g/cm2.
Example 37
Added to 100 ml of water were 1.0 g of 2,4-0-
(3,4-dimethylbenzylidene)-D-sorbitol and 200 mg of
RIKABION A 100 (trade name, product of New Japan Chemical
Co., Ltd., lauryl dimethyl aminoacetic acid betaine).
The two components were dissolved in water with heating
at 98°C (sugar compound/dispersant=1/0.2 (weight ratio)).
The solution was cooled to 25°C, giving a gel
composition. The gel composition was frozen at -78°C in
a dry ice-acetone bath. Then the composition was
lyophilized with a vacuum pump for 24 hours to produce
1.2 g of a gelling agent composition as white powder.
Example 38
When 10 ml of water was added to 150 mg of the
CA 02273097 1999-OS-27
-54-
gelling agent composition obtained in Example 37, the
water was gelled in about 60 minutes. One day later, the
obtained gel composition had a gel hardness of 100 g/cm2.
Example 39
Added to 100 ml of water were 1.0 g of 2,4-O-
(3,4-dimethylbenzylidene)-D-sorbitol, 4.0 g of glycerin
(product of Nacalai Tesque Co., Ltd.) and 10 mg of K2C03.
The three components were dissolved in water with heating
at 98°C (sugar compound/dispersant=1/4 (weight ratio)).
The solution was cooled to 25°C, giving a gel
composition. The obtained gel composition had a gel
hardness of 240 g/cm2. The gel composition was frozen at
-78°C in a dry ice-acetone bath. Then the composition
was lyophilized with a vacuum pump for 24 hours to
produce 5.0 g of a gelling agent composition as white
powder.
Example 40
When 10 ml of water was added to 500 mg of the
gelling agent composition obtained in Example 39, the
water was gelled in about 120 minutes. One day later,
the obtained gel composition had a gel hardness of 80
g/cm2.
Example 41
Added to 100 ml of water were 1.0 g of 2,4-O-
(3,4-dimethylbenzylidene)-D-sorbitol, 200 mg of
CA 02273097 1999-OS-27
-55-
polyethylene glycol #6000 (trade name, product of Nacalai
Tesque Co., Ltd.) and 10 mg of K2C03. The three
components were dissolved in water with heating at 98°C
(sugar compound/dispersant=1/0.2 (weight ratio)). The
solution was cooled to 25°C, giving a gel composition.
The obtained gel composition had a gel hardness of 280
g/cm2. Then the gel composition was dried under reduced
pressure (2 mmHg) at 90°C to produce 1.2 g of a gelling
agent composition as white powder.
Example 42
When 10 ml of water was added to 120 mg of the
gelling agent composition obtained in Example 41, the
water was gelled in about 360 minutes. One day later,
the obtained gel composition had a gel hardness of 210
g/cm2. One month later, the gel remained stable and
showed a gel hardness of 200 g/cm2.
Example 43
After crushing 1.0 g of 2,4-O-(3,4-dimethyl-
benzylidene)-D-sorbitol into particles, the particles
were mixed with 2.0 g of CONION 2P (50) (trade name,
product of New Japan Chemical Co., Ltd., an addition
reaction product of an alcohol predominantly containing
12 carbon atoms with an average of 5 moles of ethylene
oxide, HLB=12.8) and 10 mg of K2C03. After 4.0 ml of
water was fully mixed with the mixture, the opening of
CA 02273097 1999-OS-27
-56-
the reactor was closed. The mixture was aged for 7 days
at 50°C, whereby a gelling agent composition in the form
of cake was obtained (sugar compound/dispersant=1/2
(weight ratio)).
Example 44
When 10 ml of water was added to 1.0 g of the
gelling agent composition obtained in Example 43, the
water was gelled in about 60 minutes. One day later, the
obtained gel composition had a gel hardness of 100 g/cm2.
Reference Example 1
A 10 ml quantity of water was added to a
mixture of 100 mg of 2,4-O-(3,4-dimethylbenzylidene)-D-
sorbitol with 1 mg of K2C03. The mixture was stirred at
room temperature. After 24 hours, the mixture remained
a suspension without forming a gel. The suspension, when
heated to 98°C, was transformed into a sol. The sol was
quenched to 25°C to give a gel. One day later, the gel
had a gel hardness of 280 g/cm2.
Reference Example 2
The gel obtained in Reference Example 1 was
cooled to -78°C to freeze the gel. The water was removed
by lyophilizing for 24 hours with a vacuum pump to
produce a gelling agent in the form of powdery crystals
(xerogel powder). The gelling agent thus obtained was
suspended in 10 ml of water, whereby the particles were
CA 02273097 1999-OS-27
-57-
agglomerated. The agglomerate became a soft gel (sol-
gel) in its periphery in about 48 hours so that the gel
hardness was_non-measurable (less than 60 g/cm2).
Example 45
Added to 1.0 g of the gelling agent in the form
of xerogel powder obtained in Reference Example 2 were
1.0 g of Wandamine OX-300 (trade name, New Japan Chemical
Co., Ltd., lauryl dimethylamine oxide) and 10 mg of
K2C03. A gelling agent composition (2.0 g) was obtained
in the form of cake by mixing them (sugar compound/
dispersant= 1/1 (weight ratio)).
Example 46
When 5.0 ml of water was added to 1.0 g of the
gelling agent composition obtained in Example 45, the
water was gelled in about 120 minutes. One day later,
the obtained gel composition had a gel hardness of 80
g/cm2.
Reference Example 3
Added to 10 ml of water were 300 mg of 2,4-0-
(3,4-dimethylbenzylidene)-D-sorbitol and 10 mg of K2C03
(concentration of the sugar compound: 3~ by weight). The
two components were dissolved in water with heating at
98°C. The solution was cooled to 25°C, whereby crystals
were precipitated, resulting in failure to become a gel.
Reference Example 4
CA 02273097 1999-OS-27
-58-
Added to 100 ml of water were 1.0 g of 2,4-O-
(3,4-dimethylbenzylidene)-D-sorbitol, 4 mg of CONION 275
(90) and 10 mg of K2C03. The three components were
dissolved in water with heating at 98°C (sugar
compound/dispersant=1/0.004 (weight ratio)). The
solution was cooled to 25°C, giving a gel composition.
The gel composition was frozen at -78°C'.in a dry ice-
acetone bath for 2 hours. Then the composition was
lyophilized with a vacuum pump for 24 hours to produce
1000 mg of a gelling agent composition as white powder.
A 10 ml quantity of water was added to 100 mg of the
gelling agent composition thus obtained, whereby the
particles were agglomerated. The agglomerate became a
soft gel (sol-gel) in its periphery in about 48 hours so
that the gel hardness was non-measurable (less than 60
g/cm2).
Reference Example 5
Added to 100 ml of water were 1.0 g of 2,4-O-
(3,4-dimethylbenzylidene)-D-sorbitol, 11 g of CONION 275
(90) and 10 mg of K2C03. The three components were
dissolved in water with heating at 98°C (sugar
compound/dispersant=1/11 (weight ratio)). The solution
was cooled to 25°C, but a gel was not formed.