Note: Descriptions are shown in the official language in which they were submitted.
CA 02273436 1999-06-O1
ANTIFUNGAL AND ANTIMYCOBACTERIAL BASILISh:AMIDES
Field of the Invention
This invention relates to polyketide amides having antibiotic activity.
Background of the Invention
There is an urgent need for new antibiotics to treat pathogens that have
developed
resistance to antibiotics currently in use. Further, compounds that have
antimycobacterial activity are rare. Compounds produced by marine
microorganisms
are being screened for antibiotic activity.
Japanese patent application 06-27802 published September 12, 1995 under No.
07238018 and entitled "Antimycotic Antibiotic Substance and its Production"
discloses
an antifungal compound YL-03709B-A obtained by fermentation of Bacillus sp. YL-
03709B (FERM P-14126). The Bacillus was isolated from soils near Okinawa,
Japan.
The compound was reported as having antifungal activity against several
organisms but
low activity against Candida albicarrs, Candida parapellosis; Saccharomyces
cerevisiae; Saccharomyces sake; and Aspergillus niger on Sabouraud/dextrose
Agar
medium.
An unidentified Bacillus sp. (MK-PNG-276A) was isolated from the tissues of a
tubeworm collected in the tropical waters off Papau, New Guinea. Extracts from
laboratory cultures of the latter organism exhibited broad spectrum antibiotic
activity
against a panel of antibiotic-resistant pathogens. Initial bioassay guided
fractionation
of crude extracts resulted in the isolation of the loloatins, a family of
novel cyclic
decapeptides (see PCT/CA97/00529). More recently, a class of novel polyketide
amides were isolated from MK-PNG-276A cultures, which are termed
basiliskamides
herein. The basiliskamides have antibiotic activity.
1
' ' CA 02273436 1999-06-O1
Summary of the Invention
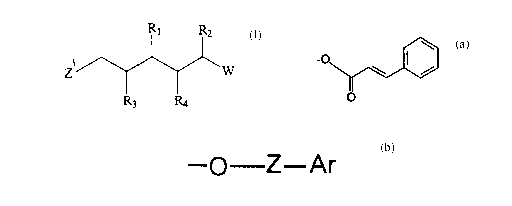
This invention provides a compound or a physiologically acceptable salt
thereof, wherein the compound has the formula:
R~ RZ
t
Z
R3 R4
wherein:
R~ and R2 are the same or different and are independently H or R;
R is a structural fragment having a saturated or unsaturated linear, branched,
or cyclic,
skeleton containing one to ten carbon atoms in which the carbon atoms may be
optionally substituted with a substituent selected from the group consisting
o~ -OH;
=O; -ORS; -OzCRS, -SH; -SRS; -SOCRS; -NH2; -NHRS; -NH(RS)2; -NHCOR;;
NRCORS; -I; -Br; -Cl; -F; -CN; -C02H; -C02R5; -CHO; -CORS; -CONH2; -CONHR;;
-CON(RS)2; -COSH; -COSRS; -N02; -S03H; -SOR;; and -S02R;, wherein RS is a
linear, branched or cyclic, one to ten carbon saturated or unsaturated alkyl
group;
R3 and R4 are different and are independently selected from the groups
consisting of
OH,
(a) _O
\ /
O
2
' ' CA 02273436 1999-06-O1
and
(b) -p-Z-Ar
wherein,
Z 1 and Z are linear or branched, saturated or unsaturated, one to ten carbon
fragments
optionally substituted with Y;
Ar is a monocyclic, bicyclic or tricyclic, fully or partially aromatic system
containing
five or six membered carbocyclic or, oxygen, nitrogen or sulphur containing
heterocyclic rings, optionally substituted with R or Y;
Y is selected from the group consisting of: H; =O, -OH; -OR; -02CR; -SH; -SR; -
SOCR; -NH2; -NHR; -NH(R)~; -NHCOR; NRCOR; -I; -Br; -Cl; -F; -CN- -CO~H: -
CO~R; -CHO; -COR; -CONH2; -CONHR; -CON(R)2; -COSH; -COSR; -N02; -SO;H;
-SOR; -S02R; and, -O- (epoxide);
WisHorR;
with the provisos that when W is H, R2 is not H; when R2 is CH3, W is not n-
propyl;
and, one of R3 and R4 is (a) or (b) and another of R3 and R4 is OH.
This invention also provides a compound or a physiologically acceptable salt
thereof, wherein the compound has the formula:
Ii I I I 2
/C l~ /C4~ /C6~ /C8~ j 10~ /C l2
X C2-C3 Cg 17 I9 C I I
R3 R4
3
' ' CA 02273436 1999-06-O1
wherein:
a single, double or triple bond exists between one or more of: C-2 and C-3; C-
3 and
C-4; C-4 and C-5; and, C-5 and C-6;
X is NH2, NHR, NR2, OH, OR, SH, SR, H, or CF3;
R is a structural fragment having a saturated or unsaturated linear, branched,
or cyclic,
skeleton containing one to ten carbon atoms in which the carbon atoms may be
optionally substituted with a substituent selected from the group consisting
of: -OH;
=O; -ORS; -02CR5, -SH; -SRS; -SOCRS; -NH2; -NHRS; -NH(R;)2; -NHCOR;;
NRCORS; -I; -Br; -Cl; -F; -CN; -C02H; -C02R5; -CHO; -CORS; -CONH2; -CONHR;;
-CON(RS)2; -COSH; -COSR;; -N02; -S03H; -SORS; and -S02R5, wherein R; is a
linear, branched or cyclic, one to ten carbon saturated or unsaturated alkyl
group;
Ri and R2 are the same or different and are independently H or R;
R3 and R4 are different and are selected from the group consisting of: OH,
(a)
O
and
(b) -O -Z-Ar
4
CA 02273436 1999-06-O1
wherein, Z is a linear or branched, saturated or unsaturated, one to ten
carbon
fragment optionally substituted with Y;
Ar is a monocyclic, bicyclic or tricyclic, fully or partially aromatic system
containing
five or six membered carbocyclic or, oxygen, nitrogen or sulphur containing
heterocyclic rings, optionally substituted with R or Y;
Y is selected from the group consisting of: H; =O, -OH; -OR; -02CR; -SH; -SR; -
SOCR; -NH2; -NHR; -NH(R)~; -NHCOR; NRCOR; -I; -Br; -Cl; -F; -CN- -COzH; -
C02R; -CHO; -COR; -CONH~; -CONHR; -CON(R)2; -COSH; -COSR; -N02; -S03H;
1 ~ -SOR; -SOzR; and, -O- (epoxide);
with the proviso that one of R~ and R4 is (a) or (b), and another of R3 and R4
is OH.
If a compound of this invention is naturally occurring (such as basiliskamide
A or B as
described herein) such a compound may be obtained from a natural source or may
be
sythnesized as described herein. In cases where such a naturally occurring
compound
is obtained from a natural source, the compound of this invention is
characterized as
being purified or partially purified. Thus, any compound of this invention
that is
naturally occurring will be substantially free of cellular contaminants.
Cellular
contaminants are defined as any component of a living cell (eg. proteins,
nucleic acids,
cell wall fragments, etc.) or a naturally occurring compound that is not a
compound of
this invention. The term "substantially free of cellular contaminants" means
that a
compound or a mixture of compounds of this invention, whether or not present
in a
pharmaceutical composition, will be present at a ratio of at least 3:1 (w/w)
of the total
amount of a compound or compounds of this invention to total amount of
cellular
contaminants present.
This invention also provides pharmaceutical compositions comprising a compound
of
this invention and a pharmaceutically acceptable carrier selected for the
particular
indication and mode of treatment in which the compound is to be used.
5
CA 02273436 1999-06-O1
Compounds of this invention that are capable of forming salts may be in the
form of a
physiologically acceptable salt. Such a salt is any salt that is acceptable
for use in
pharmaceutical formulations. For example, where a compound of this invention
has a
carboxyl or sulphonic acid moiety, the counterion may be Na, K, Mg or Zn.
Where
the compound comprises a basic moiety such as an amine, the salt may be
hydrochloride.
Pharmaceutical compositions of this invention will contain a compound or
physiologically acceptable salt thereof in admixture with any Garner,
excipient,
dilutant, filler, thickener, etc., or in combination with any drug delivery
moiety or
device selected as suitable for a particular indication and mode of treatment
desired.
For example, pharmaceutical compositions of this invention may be formulated
for
injection (intravenous or otherwise), topical application, oral dosage (eg.
tablets,
capsules, powders), eye drops, aerosol delivery or cosmetic/cleansing
formulations
such as shampoos or skin cleansers. Selection of pharmaceutically acceptable
carriers
for compounds of this invention are within the knowledge of those of skill in
the art.
An example of a formulation for topical use is a creme-based formulation or
carrier in
which a compound of this invention or a pharmaceutically acceptable thereof is
dissolved or emulsified.
This invention also provides a method for treatment of a patient (animal or
human)
afflicted with a fungal or mycobacterial infection comprising the
administration to said
patient of a therapeutically effective amount or a compound of pharmaceutical
composition of this invention. This invention provides the use of a compound
or
pharmaceutical composition of this invention as an antifungal agent or as an
antimycobacterial agent. The dose range of a compound of this invention will
be
selected in accordance with a particular indication or mode of treatment.
Generally,
the dose range will be between about 50 and about 500 mg/day for oral and
intravenous applications and between about 0.5 and about 5 gram for topical
applications.
6
CA 02273436 1999-06-O1
Detailed Description of Embodiments of the Invention
Marine Bacterium MK-PNG-276A: The marine bacterium MK-PNG-276A was
isolated during a collecting expedition off of Loloata Island, Papua New
Guinea.
MIDI analysis of cellular fatty acids indicated that MK-PNG-276A was an
unknown
species possibly within the genus Bacillus. MK-PNG-276A was deposited July 2,
1996 at the American Type Culture Collection (ATCC) under No. 55797.
Isolation of the Basiliskamides: The marine bacterium MK-PNG-276A was grown
in moderate scale culture as confluent lawns for 5 days at 16 ~C on trays of
solid
trypticase soy agar supplemented with NaCI to a final concentration of I %.
The
cultures were harvested by gently scraping the cells from the agar surface.
Bacterial
cells (21.5 g dry weight) were immersed in and subsequently extracted with
MeOH (3
X 250 mL) over a period of six days. Crude MeOH extracts showed broad spectrum
antimicrobial activity against a variety of human pathogens, including
methicillin
resistant Staphylococcus aureus. Eschericia coli, Candida albicans and
Mycobacterium tuberculosis.
The combined MeOH extracts were concentrated in vacuo and then partitioned
between EtOAc (3 x 100 mL) and H20/MeOH (10:1 200 mL). The EtOAc extract
was dried over anhydrous Na2S04, filtered and reduced to dryness in vacuo to
give
6.5 g of a gum. The gum was fractionated by Sephadex LH-20 chromatography
(eluent MeOH) to give 226 mg of a fraction containing strongly UV absorbing
compounds. This fraction was subsequently subjected to step gradient reversed-
phase
chromatography (eluent: 1:1 MeOH/H20 to 100% MeOH) on a lOg Waters Sep-Pak.
A strongly UV absorbing fraction (82 mg) was further separated into crude
basiliskamide A and crude basiliskamide B (28 mg total) by a normal-phase
silica gel
flash chromatography (4:1 EtOAC 'CH2C12). Final purification was accomplished
by
reversed-phase HPLC (7:3 MeOHi'H20), yielding pure basiliskamides A (l, 14 mg)
and B (2, 9 mg) as clear solids.
Structure Elucidation of Basiliskamides A (1) and B (2): Basiliskamide A (1)
was
isolated as a clear solid that gave a [M + H)+ ion at m/z 386.23358 in the
high
resolution fast atom bombardment mass spectrum appropriate for a molecular
formula
7
CA 02273436 1999-06-O1
of C23H31NO4. The ~'C NMR spectrum (Table I) of basiliskamide A (1) showed
only
21 well resolved resor~ances, indicating that there was an element of symmetry
in the
molecule. Resonances in the ~H NMR spectrum of basiliskamide A were all well
dispersed, which facilitated identification of the two major substructures.
A broad three proton 1H NMR resonance at 8 7.40-7.41, that showed HMQC
correlations to carbon resonances at S 128.9 and 130.4, along with a broad two
proton
~H NMR resonance at 8 7.71, that showed HMQC correlations to a carbon
resonance
at 8 128.4, were all assigned to a monosubstituted phenyl ring. The phenyl
ring
accounted for the element of symmetry required by the ~3C NMR data. A one
proton
doublet at b 7.65 in the ~H NMR spectrum showed COSY correlations to the
phenyl
I 5 multiplet at 8 7.71 and to another one proton doublet at 8 6.61. The two
doublets were
assigned to a vinyl group that was the only substituent on the phenyl ring.
HMBC
correlations observed between the vinyl doublet resonance at 8 7.65 and the
phenyl
carbon resonance at 8 128.4 confirmed the attachment of the vinyl group to the
phenyl
ring. HMBC correlations observed between both of the vinyl proton resonances
at 8
7.65 and 6.61 and a carbon resonance at 8 166.0, showed that the phenyl and
vinyl
fragments were part of a cinnamoyl residue. The vinyl protons had a vicinal
scalar
coupling of 16 Hz demonstrating the cinnamoyl residue had the E configuration.
Analysis of COSY, HMQC, and HMBC data collected for basiliskamide A (1)
routinely identified the linear carbon chain extending from C-2 to C-12,
including the
positions of the D2°3 and D4°' olefins, the methyl branches at C-
8 and C-10, and the
presence of -OR substituents at C-7 and C-9. HMBC correlations observed
between
both the H-2 and H-3 resonances at 8 5.55 and 6.31, respectively, and a carbon
resonance at 8 167.5, showed that C-2 was attached to a carbonyl carbon. Only
one
nitrogen and two hydrogen atoms remained unaccounted for by the cinnamoyl and
linear C-1 to C-12 chain fragments, suggesting that the C-1 carbonyl was a
primary
amide. A pair of broad one proton resonances at 8 6.82 and 7.32, that showed
COSY
correlations to each other but did not show HMQC correlations to carbon
resonances,
were assigned to the primary amide NH protons. The NH resonance at 8 6.82
showed
an HMBC correlation to the C-2 resonance at 8 119.3, confirming the presence
of the
primary amide at the terminus of the linear C-1 to C-12 carbon chain. A COSY
8
CA 02273436 1999-06-O1
correlation observed between an OH proton resonance at 8 4.57 and the H-7
resonance at b 3.55 showed that there was an alcohol functionality at H-7 and,
therefore, the cinnamoyl fragment had to be attached to the linear carbon
chain via an
ester linkage at C-9. An HMBC correlation observed between the H-9 methine
resonance at a 4.92 and the cinnamoyl carbonyl resonance at 8 166.0 confirmed
the
presence of the C-9 ester linkage.
H-2 and H-3 had a vicinal scalar coupling constant of 11 Hz typical of Z
olefins, while H-4 and H-5 showed a 15 Hz vicinal coupling typical of E
olefins.
Difference nOe experiments confirmed the assigned olefinic configurations.
Irradiation of the H-3 resonance at 8 6.31 induced an nOe in the H-2 resonance
at 8
5.55 in agreement with the Z configuration for the 02°3 olefin.
Similarly, irradiation of
the H-5 resonance at 8 5.91 induced a strong nOe in the H-3 resonance at 8
6.31
supporting the E configuration for the 0'~~' olefin.
The relative stereochemistry at C-7 and C-9 was determined by converting
basiliskamide A (1) to the acetonide derivative 7. Analysis of the HMQC data
for 7,
showed that the acetonide methyl carbon resonances had chemical shifts of 19.8
and
30.4 ppm, typical of acetonides formed from syn-1,3-diols. Further analysis of
the 1H
NMR data for the acetonide 7 showed that the dioxane ring existed in a chair
conformation with the C-6 and C-10 carbons equatorial. A vicinal coupling
constant
of 10 Hz was observed between H-9 and H-8 indicating that H-8 was axial and.
therefore, the C-14 methyl had to be equatorial, establishing the relative
stereochemistries at C-7, C-8, and C-9 as shown in 7. Standard Mosher ester
methodology was used to show that C-7 in basiliskamide A (1) had the S
configuration. The configuration of C-10 in 1 was not determined. However,
basiliskamide A (1) is a homolog of YM47522 (5) and the absolute configuration
at
C-10 in 5 has been determined by synthesis to be R. Since the other chiral
centers in 1
and 5 have identical configurations, and the and the ~H and ~3C NMR data for 1
and 5
are nearly identical for the C-7, C-8, C-9 and C-10 centers, it is indicated
that 1 also
has the R configuration at C-10.
Basiliskamide B (2) was also isolated as a clear solid that gave a [M + H~+
ion
at m/z 386.23358 in the high resolution fast atom bombardment mass spectrum
appropriate for a molecular formula of C23H31NOq, identical to the formula of
9
CA 02273436 1999-06-O1
basiliskamide A (1). Analysis of the 1D and 2D NMR data obtained for
basiliskamide
B (2) showed that it was simply an isomer of basiliskamide A, in which the
cinnamoyl
ester was at C-7 instead of C-9. Basiliskamide B (2) and basiliskamide A (1)
were
both converted to the same diol 6 by DIBAL reduction, demonstrating that both
molecules had identical absolute configurations.
Basiliskamide A (1): isolated as a clear solid: 1H NMR, see Table 1; 1'C
NMR, see Table 1; IR (film) umax~ 3348, 3205, 2966, 2934, 1705, 1697, 1635,
1595,
1450 cm-1; UV (MeOH) ~,max: 262 nm (s 41 000); [a]'-SD (MeOH) _ -78 ; pos
itive-
ion HRFABMS [M+ H]+ m/z 386.23358 (C23H32NO4, calcd 386.23313).
Basiliskamide B (2): isolated as a clear solid: 1H NMR, see Table 1; aC
I S NMR, see Table 1; IR (film) u,,.,ax~ 3348, 3205, 2962, 2926, 1702, 1664,
1637, 1595,
1450 cm-i; UV (MeOH) 7~max~ 262 nm (E 43 000); [a]25D (MeOH) _ -12 ;
HREIMS [M]+ m/z 385.22531 (C23H31NO4, calcd 38.22531).
Reduction of Basiliskamides. To basiliskamides B ( 2" 4.7 mg) in 1 mL
THF under Ar (g), at -78 , 4 equivalents of diisobutylaluminum hydride (DIBAL-
H)
were added. The reaction was stirred overnight then diluted with EtOAc (3 mL)
and
quenched by the addition of 2 mL NH4C1 (aq), stirring until the reaction
mixture
turned cloudy (10 min). The mixture was extracted thrice with EtOAc, and the
combined organics were reduced to dryness in vacuo. Preparative normal-phase
TLC
(100 % EtOAc) followed by reversed-phas HPLC (70/30 MeOH/H20, 280 nm) gave
2 mg of 6. 1 H NMR (500 MHz, DMSO-d6) 8 7.44 ( 1 H, dd, J = 15 Hz, 11 Hz),
7.35
( 1 H, br s, NH), 6. 86 ( 1 H, br s, NH), 6.36 ( 1 H, dd, J = 11 Hz), 6.02 ( 1
H, dt, J = 15 Hz,
7 Hz), 5.57 ( 1 H, d, J = 11 Hz), 4.48 ( 1 H, d, J = 5.4 Hz, OH). 4.69 ( 1 H,
d, J = 4.4 Hz,
OH), 3 . 80 ( 1 H, m), 3 .23 ( 1 H, m), 2.26 ( 1 H, m), 2.07 ( 1 H, m), 1.61 (
1 H, m), 1.3 6 ( 1 H,
m), 1.3 5 ( 1 H, m), 1.18 ( 1 H, m), 0.84 (3 H, t, J = 7 Hz), 0,72 (3H, d, J =
7 Hz), 0.66
(3H, d, J = 7 Hz); positive-ion HRFABMS [M+ H]+ m/z 256.19211 (C 14H26N03,
calcd 256.19127).
Formation of Acetonide (7). To 1.5 mg of 6 in 0.5 mL 2,2-
dimethoxypropane, pyridinium p-toluenesulfonate (5 wt% diolbasiliskamide) was
added. The reaction mixture was stirred under Ar (g) and heated at 60 C for 1
h.
CA 02273436 1999-06-O1
The reaction mixture was filtered through silica (rinsed with EtoAc) and the
solvents
removed in vacuo. Reversed-phase HPLC (80/20 MeOH/H20) yielded 1 mg of 7.
1 H NMR (400 MHz, DMSO-d6) 8 7.46 ( I H, dd, J = 15 Hz, 11 Hz, H-4), 7.3 5 ( 1
H. br
s, NH), 6.85 ( 1 H, br s, NH), 6.37 ( I H, dd, J = I 1 Hz, I 1 Hz, H-3), 5.94
( 1 H, dt, J = 15
Hz, 7 Hz, H-S), ~.~8 (1H, d, J= 11 Hz, H-2), 3.57 (1H, m, H-7), 3.48 (1H, dd,
J= I0
Hz, 2 Hz, H-9), 2.44 ( 1 H, m, H-6), 2. I 9 ( 1 H, m, H-6'), 1.54 ( 1 H, m, H-
10), I .3 6 ( : H.
s, Me-17), 1.33 (1H, m, H-8), 1.30 (1H, m, H-11), 1.25 (IH, m, H-I1'), 1.23
(3H. s.
Me-16), 0.83 (3H, t, J= 7 Hz, Me-12), 0.75 (3H, d, J= 7 Hz, Me-13), 0.71 (3H,
d, J=
7 Hz, Me-14); 13C NMR (DMSO-d6, 100 MHz) b 167.8 (C-I), 140.6 (C-3), 138.5
(C-5), 128.9 (C-4), 120.2 (C-2), 97.6 (C-IS), 74.9 (C-9), 74.0 (C-7), 36.5 (C-
6), 3s.1
(C-8), 34.6 (C-10), 30.4 (C-16), 26.7 (C-11), 19.8 (C-17), 12.7 (C-13), 12.1
(C-12),
11.6 (C-14); positive-ion HRFABMS [M+ H]+ m/z 296.22198, C17H3pN03, calcd
296.2257.
Reaction of 1 with (R)-MTPA Acid. To a solution of 1 (1.5 mg) in 0.5 mL
dry CH2Cl2 were added DMAP (I mg), a drop of triethylamine and (R)-MTPA acid
(4 mg) and the solution stirred for 16 h. Removal of solvent in vacuo,
followed by
preparative reversed-phase TLC (100% MeOH), then reversed-phase HPLC
(MeOH/H20 4:1) gave the (R)-MTPA ester la (0.8 mg). 1H NMR (500 MHz,
DMSO-d6) 8 7.72 (3H, br envelope), 7.43 (9H, br envelope), 7.35 (1H, br s),
6.84
( 1 H, br s), 6.70 ( 1 H, d, J = 16 Hz), 6.20 ( 1 H, dd, J = 11 Hz, I 1 Hz),
5.5 8 (2 H, m),
5.17 ( 1 H, m), 4.98 ( 1 H, m), 3 .43 (3 H, s), 2.60 ( 1 H, m), 2.26 (2H, br
m), 1.72 ( 1 H, m),
1.29 (2H, br m), I .17 (I H, m), 0.95 (3H, d, J = 7 Hz), 0.93 (3H, d, J = 7
Hz), 0.88
(3H, t, J= 7 Hz); positive-ion HRFABMS [M+ H]+ m/z 602.27148, C33H39N06F3,
calcd 602.272950.
Reaction of 1 with (.S~-MTPA Acid. To a solution of 1 ( 1.5 mg) in 0.5 mL
dry CH2C12 were added DMAP (I mg), a drop of triethylamine and (S~-MTPA acid
(4 mg) and the solution stirred for 16 h. The reaction was quenched and
purified as
above, yielding the (S~-MTPA ester lb (0.4 mg). IH NMR (500 MHz, DMSO-d6) b
7.72 (3H, br envelope), 7.54 ( 1 H, m), 7.43 (8H, br envelope), 7.38 ( I H, br
s), 6.91
( 1 H, br s), 6.72 ( 1 H, d, J = 16 Hz), 6.36 ( 1 H, dd, J = 11 Hz, 1 I Hz),
5.78 ( 1 H, m),
11
CA 02273436 1999-06-O1
S .64 ( 1 H, d, J = 11 Hz), 5.13 ( 1 H, m), 4.94 ( 1 H, m), 3.42 (3 H, s),
2.63 ( 1 H, br m),
2.35 ( 1 H, m), 2.17 ( 1 H, m), 1.65 ( 1 H, m), 1.27 (2H, br m), 1.15 ( 1 H,
m), 0.89 (3H, d,
J= 7 Hz), 0.87 (3H, t, J= 7 Hz), 0.70 (3H, d, J = 7 Hz); positive-ion HRFABMS
[M+
H]+ m/z 602.27352, C33H39N06F3, calcd 602.272950.
12
CA 02273436 1999-06-O1
Table 1. ~3C (100 MHz) and'H (500 MHz) NMR Spectal Data for Basiliskamides A
and B in DMSO-db
Basiliskamide A Basiliskamide B
(1) (2)
Atom g g 1H (intgrn, b 8 1H (intgrn,
13C m, 13C m,
J(Hz)) J(Hz))
I 167.5 167.4
2 119.35.55 (lH, d, I 5.57 (1H, d,
11) 19.9 11)
3 140.56.31 (1H, dd, 140.06.33 (1H, dd,
11, 1 I) 11, 11)
4 128.27.40 (1H, m) 128.87.51 (1H, dd,
15, 11)
S 140.55.91 ( 1 H, 13 5.87 ( 1 H,
dt, 15, 7) 8.0 dt, 1 S, 7)
6 34.7 2.28 (1H, m) 31.8 2.53 (IH, m)
6' 1.99 (1H, m) 2.36 (1H, m)
7 69.6 3.49 ( 1 H, 73.0 5.40 ( 1 H,
m) dt, 10.5, 3)
8 40.7-2.03 ( 1 H, 39.4 1.92 ( 1 H,
m) m)
9 76.3 4.92 ( 1 H, 74.0 3.26 ( 1 H,
dd, 9.5, 2) m)
35.5 1.67 (IH, m) 36.3 1.40 (1H, m)
11 26.4 1.25 (1H, m) 26.5 1.38 (1H, m)
11' 1.11 ( 1 H, 1.21 ( 1 H,
m) m)
12 10.1 0.87 (3H, t, 11.8 0.85 (3H, t,
7.5) 7)
13 I 0.84 (3H, d, 10.7 0.83 (3H, d,
1.6 7) 7)
14 12.8 0.90 (3H, d, 12.1 0.74 (3H, d,
7) 7)
166.0 165.5
16 118.06.61 ( I H, 118.56.59 ( i H,
d, 16) d, 16)
17 144.67.65 ( 1 H, 144.17.60 ( 1 H,
d, 16) d, 16)
18 134.0 134.0
19 128.47.71 (2H, m) 128.27.70 (2H, m)
128.97.41 (2H, m) 129.07.40 (2H, m)
21 130.47.40 (1H, m) 130.27.40 (1H, m)
NH2 7.31, 6.83 7.34, 6.86 (2H,
(2H, s) s)
OH 4.57 (1H, d, 4.48 (1H, m)
5)
13
CA 02273436 1999-06-O1
Structures of Basilikamides and Derivatives
13 14
O
H N 1 \ ~ 9 11 \
3 5
OH O \ / 20
16 " 18
O
Basiliskamide ~
O
H2N \
O OH
Basiliskamide B
O
HZN \
OH OH
O
HZN \
O O
14
CA 02273436 1999-06-O1
Preparation of Basiliskamides: Compounds of this invention may be prepared
from
a natural source by fermentation as described above, or by total synthesis,
for example
by modification of the total synthesis of YM47522 that was described in
Ermokenko,
M.S. Tetrahedron Letters, 1996, 37, 6711-12 (as exemplified in the scheme
below for
basiliskamide A).
O p O O
H O a HO b,c d,e,f
O' ----~ O --~. O. --s p --
O
OH OH OH
10 11 12 13
S
9,h,i,l
k I
OAc ~ : : I ---.~ / I
8 ~ O O
~ 16
14
O NHZ O NHz
m,n,o
/ / -
O ~ OH
17
Basiliskamide A ( 1 )
a) (CH3CH2)2Mg/Et20, ~, 0.5 h;
a) NMO-Pr4NRu04 (0.02 eq), MS 4 ~/ MeCN, rt, 0.~ h;
15 b) NaBH4-CeC13~7H20/MeOH, -20 C, 0.5 h;
c) HSCH2CH2SH, BF3.Et20/CH2C12, rt, 2 h;
d) Ac20/Py, -10 C, 2h;
e) Me2C0-Me2C(OMe)2, TsOH (cat);
fj Ra-Ni/EtOH, O, 0.5 h;
CA 02273436 1999-06-O1
g) K2C03/MeOH, rt, 0.5 h
h) TsCI/Py;
i) LiI-HMPA/PhMe, 0, 0.5 h;
j) Me2CuLi-LiCN, 2 eq (E)-Bu3SnCH=CHSnBu3/THF-Et20, rt, 2 h, then 15, -78 C
to rt, then NIS, rt;
k) (Z)-Bu3SnCH=CHCONH~. (MeCN)2PdCl2 (0.05 eq)/DMF, rt, 24 h;
1) AcOH-H20 (4:1), 60 C, 6 h;
m) 1.2 eq Et3SiC1/Py, 0 C, then PhCH=CHCOCI, DMAP/CHZC12, rt;
n) HF (aq)-MeCN, rt, 1 h.
Conversion of compound 10 to compound 11 is as described in Sviridov, A. F. et
al.
Izv. Akad. Nauk SSR, Ser. Khim. 1982, 2572-2574.
Analogs can be prepared by modification of the total synthesis shown above or
by
semisynthesis from a product of the total synthesis or a naturally derived
product. An
example employing basiliskamide A (1) is shown in the scheme below.
16
CA 02273436 1999-06-O1
O NHZ
O NH2
/ /
O O OH a / / b
OH OH
Basiliskamide A ( 1 )
O NHZ O NHZ
c d
/ / ----~ / /
OH OSi(Et)3 OR3 OSi(Et)3
O NH2
/ /
OR3 OH
a) DIBAL (4 eq), THF, -78 C , 1 Sh
b) 1.2 eq Et3SiC1/Py, 0 C
c) alkylate or acylate alcohol (i.e for acylation ArCH=CHCOCI, DMAP/CH2C12,
rt)
d) HF (aq)-MeCN, rt, 1 h
17
CA 02273436 1999-06-O1
Antifungal Activity of the Basiliskamides: The antifungal activity of
basiliskamides
A and B was compared to that of the known antifungal agent, amphotericin B. A
standardized macrobroth dilution method as used which was developed and
published
by the National Committee for Clinical Laboratory Standards (Reference Method
for
Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard
1996
M27-A, Vol. 15, No.lO). An agar method based on the standardized broth method
was also used. The results are presented in Tables 2-5 below.
Table 2. Antifungal Activity of Basiliskamide A and B
1 o Tested by Macrobroth Dilution
Minimal Inhibitory Concentration ~,1~/ml)
Basiliskamide Candida albicans Trichophyton rubrum Aspergillus fumigatus
A 0.5 1.0 4.0
B >_ 32 4.0 >_ 32
Tabte 3. Antifungal Activity of Basiliskamide A and B
Tested by Agar Dilution
Minimal Inhibitory Concentration (~g/ml)
Basiliskamide Candida albicans Trichophyton rubrum Aspergillus fumigatus
A 1.0 0.5 2.5
B 3.1 5.0 5.0
18
CA 02273436 1999-06-O1
10
Table 4. Activity of Basiliskamide A Compared to Amphotericin B
Against 7 Clinical Isolates of Candida albicans as
Determined by i~iacrobroth Dilution
Minimal Inhibitory Concentration (ug/ml)
Isolate Number Basiliskamide A Amphotericin B
8167 0.5 0.5
8362 0.5 0.5
8363 0.5 0.5
8364 0.5 0.5
8365 0.5 0.5
8366 0.5 0.5
8367 0.5 0.5
25
35
Table 5. Comparative Activity of Basiliskamides A, B and YL-03709B-A
as Determined by Agar Dilution
Minimal Inhibitory Concentration (,ug/ml)
Target Organism Basiliskamide A Basiliskamide B Reported Value for YL-03709B-A
Candida albicans 1.0 3.1 25
Aspergillus fumigatus 2.5 5.0 >_ 50
These findings demonstrate that the basiliskamides have superior antifungal
activities and are active against the dermatophyte, Trichophyton rubrum, the
yeast,
Candida albicans, and the opportunistic fungus, Aspergillus fumigatus.
Basiliskamide
A activity against clinical isolates of the yeast, Candida albicans when
tested by the
4 o broth dilution method is comparable to that of amphotericin B, a commonly
used
antifungal agent. The data presented in Table 5 demonstrates that
Basiliskamide A
is about 25 x more active than YL-03709B-A against the yeast Candida albicans
and
the ftlamentous fungus Aspergillus fumigatus, in view of the information for
YL-
19
CA 02273436 1999-06-O1
03709B-A reported in Japanese patent application O6-27802. Basiliskamide B
also is
significantly more active against these organisms than YL-03709B-A.
Antimycobacterial Acti~~ity of the Basiliskamides: The Basiliskamides were
tested
for antimycobacterial activity using a standardized agar dilution method
(Underlied,
CB and Salfinger, S. 1995. In Manual of Clinical Microbiolocy, Murray, Baron,
Pfaller, Tenover, Yolken (Eds.), ASM Press, page 1395-1404). Activity of
basiliskamide A, basiliskamide B, and acylated derivatives of the two
compounds
were tested for activity against Mycobacterium tuberculosis, the cause of
tuberculosis,
and Mycobacterium avium-intracellulare, an important cause of mycobacterial
infections in immunocompromized patients such as those with AIDS. The results
are
shown in Table 6.
Table 6. Antimycobacterial activity of the Basiliskamides
Minimal Inhibitory Concentration (u~/ml)
Compound M. tuberculosis M. avium-intracellulare
Basiliskamide A 25 100
Basiliskamide B 50 > 100
Acylated A (2-16) > 100 > 100
Acylated B (2-16) > 100 >_ 50
These results indicate that basiliskamide A and B each have activity against
M. tuberculosis. Basiliskamide A has activity against M. avium-intracellulare,
but
3 0 basiliskamide B appears to be relatively inactive against this organism.
Decreasing
the number of carbons in the backbone of the molecule may increase activity.
Such
is the case with the increased antifungal activity of the basiliskamides (A
and B) that
have one less carbon in the molecule's backbone than YL-03709B-A.
20
CA 02273436 1999-06-O1
Cytotoxicity Testing of Basiliskamide A: Serial dilutions of basiliskamide A
were
prepared in cell culture medium and tested for toxicity for normal human
fibroblast
cells and for human tumor cell line. The effect of basiliskamide (basil) was
compared
to that of the known antifungal compound amphotericin B (ampho). The
appearance
of the cells was assessed after 48 hours exposure to the compounds. The
results are
shown in Table 7. Basiliskamide produced no cytotoxicity for normal human
fibroblast cells at concentrations less than 100 ~,g/ml compared to
amphotericin B
(ampho) which was toxic at concentrations as low as 25 ~g/ml. Against human
tumor
cells, basiliskamide showed minor toxicity at concentrations above 3 ~g/ml.
These
findings suggested that basiliskamide is less toxic for normal human cells
than the
widely used amphotericin B.
Table 7 Basiliskamide Cytoxicity Testing
Cytopathic
Effect (48
hours)
Human Fibroblast
Human Tumor
Conc (~g/ml Basil Ampho Basil Ampho
100 2 4 4 4
2 50 0 2 1 3
0
0 2 1 1
12.5 0 1 1 0
6.25 0 0 2 0
3.12 0 0 2 0
2 1. 57 0 0 0 0
5
0.78 0 0 0 0
0 0 0 0 0
1 - slight change in morphology vs. control
2 - occasional rounding, vacuolization, or granularity
3 - rounding, vacuolization, detachment of 50% of cells
3 5 4 - destruction of monolayer
21
CA 02273436 1999-06-O1
All publications, patents and patent applications referred to herein are
hereby
incorporated by reference. While this invention has been described according
to
particular embodiments and by reference to certain examples, it will be
apparent to
those of skill in the art that variations and modifications of the invention
as described
herein fall within the spirit and scope of the attached claims.
22