Note: Descriptions are shown in the official language in which they were submitted.
CA 02273958 1999-03-15
WO 97/27168 PCT/GB97/00104
PRODUCTION OF AROMATIC CARBOXYLIC ACIDS
This invention relates to a process for the production of an aromatic
carboxylic
acid, especially dicarboxylic acids such as terephthalic acid and isophthalic
acid, by
the liquid-phase oxidation of a precursor (e.g. p-xylene) of said carboxylic
acid.
In a widely practised method of producing terephthalic acid, p-xylene is
oxidised under elevated temperature and pressure conditions in a liquid phase
reaction using air or other source of oxygen, the oxidation being carried out
in a
reaction solvent comprising a C2 - C6 monocarboxylic acid) such as acetic
acid, in
the presence of a catalyst system comprising one or more heavy metal compounds
and one or more promoter compounds including bromine. Water is present in the
reaction solvent and is formed as a result of the oxidation reaction. The
oxidation
reaction is accompanied by evolution of a reaction offgas which generally
comprises
inter alia nitrogen, unreacted oxygen, carbon dioxide, carbon monoxide and
methyl
bromide.
Because the reaction is exothermic, usual practice is to remove the heat of
reaction by allowing the monocarboxylic acid solvent to vaporise resulting in
a
gaseous reactor overheads stream containing monocarboxylic acid and water. The
overheads stream may be processed in various ways. For instance, one method
involves passing the overheads stream to a condensing system in which a large
proportion of the monocarboxylic acid and water is condensed, the condensate
in
part being returned to the reactor as reflux and in part processed further for
recycle
of monocarboxylic acid to the oxidation reaction - see for example US Patent
No.
4777287. The non-condensed components of the gaseous overheads stream are
vented from the condensing system.
In another method, the gaseous overheads stream may be passed directly to a
distillation column in which substantially all of the monocarboxylic acid is
separated
and recovered as a bottoms product while the tops product comprises steam and
other components - see for example GB Patent No. 1373230. In both methods, an
offgas is obtained which may be processed further to recover energy for use in
the
production of the aromatic carboxylic acid. JP-A-55-9517 discloses a method
for
processing the offgas to recover energy.
The present invention is concerned with the treatment of the offgas derived
from the oxidation reactor.
According to a first aspect of the present invention there is provided a
process
for the production of an aromatic carboxylic acid comprising:
oxidising a precursor of the aromatic carboxylic acid in a liquid-phase C2 -
C6
monocarboxylic acid solvent containing water and in the presence of a catalyst
system containing one or more heavy metals and bromine;
1
CA 02273958 1999-03-15
WO 97/27168 PCT/GB97/00104
withdrawing from the reaction an overheads gaseous stream at elevated pressure
containing inter alia water, monocarboxylic acid and gaseous by-products
including
methyl bromide and feeding the high pressure gaseous stream to means for
removing said monocarboxylic acid from the overheads stream to produce a high
pressure monocarboxylic acid-depleted gaseous stream containing inter alia
water
and methyl bromide;
effecting high temperature combustion of the high pressure monocarboxylic
acid-depleted gaseous stream so as to convert the methyl bromide to bromine
and/or
hydrogen bromide: and
passing the treated gas containing bromine and/or hydrogen bromide to an
energy
recovery system.
Preferably the pressure and temperature conditions are controlled so as to
prevent condensation of bromine and/or hydrogen bromide on passage through the
energy recovery system.
Conveniently following combustion, preferably by catalytic combustion, the gas
stream is passed to the energy recovery system without scrubbing the gas
stream.
Thus, instead of removing the potentially corrosive bromine compounds prior
to passage of the treated gas stream through the energy recovery system e.g.
by
scrubbing the gas stream, the temperature of the treated gas stream is
maintained
and corrosion is suppressed by control of the temperature and pressure
conditions to
ensure the potentially corrosive bromine compounds) remain in the gaseous
phase
during passage through the energy recovery system. In this way, full advantage
is
taken of the increase in temperature imparted to the gas stream in the course
of
high temperature combustion without the necessity of fabricating the energy
recovery system using expensive highly corrosion-resistant materials. Thus,
for
example, the energy recovery system (eg a gas turbine) may be fabricated using
more conventional materials such as high chrome or austenitic stainless
steels.
Prior to introduction into the energy recovery system, at least part of the
gas
stream may be used to preheat the gas stream upstream of the combustion step.
Preferably following passage through the energy recovery system, the treated
gas is treated to remove substantially all of the bromine species and/or
hydrogen
bromide.
Preferably the monocarboxylic acid is removed from the overheads gas stream
to such an extent that the water content of the resulting gas stream exceeds
its
monocarboxylic acid content.
Typically at least 90 wt%, more preferably at least 95 wt%, of said
monocarboxylic acid is removed from the overheads stream.
2
CA 02273958 1999-03-15
WO 97/27168 PCTlGB97/00104
The means for removing monocarboxylic acid from the overheads stream
conveniently includes a separation column in which water/monocarboxyfic acid
distillation is effected, e.g a packed or trayed distillation or rectification
column
capable of effecting a separation whereby at least 95%, more preferably about
98%
and most preferably at least about 99%, by weight of the monocarboxylic acid
solvent is removed from the gaseous overheads stream from the oxidation
reaction.
The separation column is preferably operated at the same, or close to the,
pressure at which the oxidation reaction is conducted. The separation column
may
be mounted separately from the oxidation reactor with suitable pipework
connecting
the reactor to the column for the supply of the overheads stream to the
latter.
Alternatively the separation column may be mounted directly above and
integrated
with the oxidation reactor.
Thus, in a preferred embodiment of the present invention, the high pressure
gaseous overheads stream derived from the oxidation reactor vapor (which
typically
contains, in addition to water and monocarboxylic acid, residual oxgyen, by-
product
gases such as methyl bromide and methyl acetate formed as a result of the
oxidation, carbon dioxide, carbon monoxide and nitrogen) is passed to the
separation column to remove most of the monocarboxylic acid solvent.
Consequently, the offgas stream discharged from the separation column will be
at
high pressure and will contain, in addition to a significant amount of water
in the
form of steam, residual oxgyen, by-product gases such as such as methyl
bromide
(typically present in an amount in the range of 25 to 125 ppm) and methyl
acetate
formed as a result of the oxidation, carbon dioxide, carbon monoxide and
nitrogen.
As such therefore, the high pressure offgas stream discharged from the
separation
column constitutes a significant source of energy which can be extracted by
suitable
means such as an expander.
Typically the residual oxygen content of gaseous overheads stream withdrawn
from the oxidation reactor constitutes about 3 to 8% by volume of the
non-condensibles present in the overheads stream. Preferably the liquid phase
oxidation process is carried out in such a way that the amount of residual
oxygen in
the offgas is sufficient under normal operating conditions to avoid the need
to supply
further oxygen under pressure to the offgas treatment process while ensuring
that
there is substantially no risk of oxygen starvation in the offgas treatment
without
giving rise to potentially hazardous conditions that can obtain if the gaseous
overheads stream contains excessive oxygen.
Where necessary, scrubbing of the high pressure monocarboxylic
acid-depleted gaseous stream with liquor may be effected upstream of the
combustion zone in order to recover at least in part any volatile precursor or
3
CA 02273958 1999-03-15
WO 97127168 PCTIGB97/00104
precursors of said aromatic carboxylic acid which would otherwise be entrained
in
the gaseous stream passing to the combustion step. Where the means for
removing
said monocarboxylic acid from the overheads stream comprises a separation
column, such scrubbing may be effected within the separation column,
conveniently
using water which has been recovered from the treated gas after passage
through
the energy recovery system and may optionally be preheated upstream of said
scrubbing.
The combustion step (preferably catalytic combustion) of the present invention
serves to eliminate combustible components and carbon monoxide present in the
high pressure offgas stream before it is directed to the energy recovery means
and
contributes to elevation of the offgas temperature prior to the energy
recovery
system, thereby increasing power recovery and avoiding dewing of corrosive
bromine species e.g. HBr in the system. Additional temperature elevation may
be
provided by indirect heating via steam or a fired heater, direct firing of
fuel and/or
injection of a support fuel such as methyl acetate, methanol, methane,
propane,
butane, etc.
In the combustion step, the offgas stream is preferably contacted with a
suitable catalyst so that at least a substantial proportion of the combustible
components and carbon monoxide is converted to environmentally acceptable
forms.
The combustion step also results in conversion of methyl bromide to bromine
and/or
hydrogen bromide which can be highly corrosive but, in accordance with the
invention, are passed through the energy recovery system under controlled
conditions instead of being scrubbed from the offgas thus avoiding the
inevitable
substantial reduction in the temperature which would be necessary if scrubbing
of
the offgas is to be undertaken before passage through the energy recovery
means
coupled with the necessary reheating prior to passing the scrubbed offgas
stream to
the energy recovery system. Moreover, in the process of the present invention,
it is
possible to avoid pre-heating of the combusted offgas that might otherwise be
necessary in order to revaporise any condensed water to steam before passage
through the energy recovery means.
After passage through the energy recovery means and conveniently after
removal of substantially all of the bromine (preferably in such a way as to
retain all
or at least a substantial part of the steam content in the offgas stream), the
offgas
stream may be condensed to recover water for recycle within the overall
process for
the production of the aromatic carboxylic acid. The recovered water may for
instance be used as:
a reflux in the separation column for separating the monocarboxylic acid from
the
high pressure gaseous overheads stream; and/or
4
CA 02273958 1999-03-15
WO 97/27168 PCT/GB97/00104
solvent for dissolution of crude carboxylic acid in preparation for
purification of the
aromatic carboxylic acid by hydrogenation, for example in the manner disclosed
in
our prior EP-A-498591 and/or EP-A-502628.
Thus, for example. following passage through the energy recovery system, the
offgas stream may be desuperheated to a temperature corresponding to or close
to
the dewpoint of the stream and scrubbed to remove the bromine and HBr
components (e.g. using an aqueus caustic soda) while retaining the water
content
within the offgas stream. The scrubbed offgas stream at the dewpoint
temperature
may then be condensed to remove its water content.
Although it is preferred to remove the bromine and HBr components from the
offgas stream before recovery of the water vapour content of the gas stream,
we do
not exclude the possibility of recovering the water by condensation followed
by
subsequent removal of the bromine and HBr components by scrubbing.
Typically the offgas stream obtained following separation of the
monocarboxylic acid in said separation column is at a pressure in the range of
5 to
tiara (for instance between 10 and 16 tiara) and a temperature of the order of
160
to 200°C (e.g. about 177°C and 14 tiara). Prior to combustion
thereof, the offgas
stream from the separation column is conveniently heated directly or
indirectly (eg
by means of high pressure steam, heating oil, heat exchange between the offgas
20 stream upstream and downstream of the combustion step, passage through a
fuel-fired heater or by direct firing of fuel into the gas stream) to an
elevated
temperature, usually in the range of 250 to 450°C (typically about
300°C).
Depending on the exotherm available from the combustion step, it may be
appropriate to introduce a combustion assistant into the combustion zone. The
25 combustion assistant is preferably pre-mixed with the gas stream prior to
entry into
the combustion zone. One form of device for effecting good mixing of the
combustion assistant with the offgas stream is disclosed in our prior PCT
Published
Patent Application No. WO 94/23813, the disclosure of which is incorporated
herein
by this reference.
The combustion assistant is preferably, but need not necessarily be, one
including one or more oxygen atoms per molecule. Various assistants may be
used,
eg methanol, methyl acetate, hydrogen, natural gas, methane, propane, butane
or
mixtures thereof. Where methyl acetate is used, it is conveniently derived
from the
terephthalic acid production process as it is generated as a by-product of the
liquid
phase oxidation of p-xylene in acetic acid solvent. Where methane is used) it
may
be derived from an anaerobic process far the treatment of effluent produced in
the
manufacture of the aromatic carboxylic acid, e.g. terephthalic acid. if
desired,
additional air may be introduced into the combustion zone to promote
oxidation.
5
CA 02273958 1999-03-15
WO 97/27168 PCT/GB97/00104
The combustion step is carried out with regard to ensuring that, during the
subsequent expansion on passage through the energy recovery system, bromine
and
HBr derived from the methyl bromide constituent of the effluent gas stream
remain
in the gas phase thereby avoiding dew point corrosion conditions in the energy
recovery system. Usually the combustion step is carried out in the presence of
a
catalyst and the temperature of the treated offgas stream exiting the
oxidation zone
will be in the range from about 250 to about 700°C, e.g. 350 to
700°C, and will
depend on whether or not the gas stream is preheated before introduction into
the
catalytic oxidation zone and whether or not a combustion assistant is
employed. For
instance, the catalytic oxidation may be conducted in such a way that the
temperature of the treated gas exiting the catalytic oxidation zone is of the
order of
400°C or greater. Where no combustion assistant is used, or where the
combustion
assistant is one which is easily o«elatively easily oxidised (e.g. methanol,
methyl
acetate or hydrogen) the exit temperature may be in the range of about 250 to
about
550°C, typically about 350 to 500°C (e.g. about 480°C).
With a combustion assistant
which is less readily oxidisable (e.g. methane, propane or butane) the exit
temperature will usually be higher, i.e. about 400 to about 700°C,
typically 550 to
700°C, e.g. of the order of 630°C.
Although catalytic combustion is preferred, we do not exclude the possibility
of
carrying out the combustion step in the absence of a catalyst and using a
supply of
fuel. In this case, the temperature of the gas stream following combustion
will
typically be in excess of 700°C, usually in excess of 800°C, and
conveniently the
liquid phase oxidation process is operated so that the oxygen content of the
gaseous
overheads stream withdrawn from the oxidation reactor is increased. eg in
excess of
5% by volume relative to the non-condensible components in the overheads
stream,
thereby avoiding the need for separate supply of pressurised oxygen to the
combustion step under normal operating conditions of the liquid phase
oxidation
process.
In general, the combustion process will be carried out using operating
conditions (eg. temperature, space velocity, catalyst composition) selected to
ensure
that methyl bromide is substantially completely converted to HBr and Br2, the
aim
being to minimise or avoid the production of underconversion brominated
aromatic
compounds which have high dew points. In addition, pressure and temperature
conditions are controlled so as to prevent condensation of the HBr and/or Br2
compound (s) on passage through the energy recovery system.
The energy recovery system may produce an output in mechanical or electrical
form and may for instance be used to power other equipment in the production
plant
such as a compressor forming part of the system for feeding air, oxygen-
enriched
6
r
CA 02273958 1999-03-15
WO 97/27168 PCT/GB97/00104
air, oxygen-containing gas or oxygen to the reactor in which the liquid phase
oxidation is carried out.
According to a further aspect of the present invention there is provided in a
process for the production of an aromatic carboxylic acid such as terephthalic
acid,
which process comprises oxidising a precursor of said aromatic carboxylic acid
(e.g.
paraxylene) in a reaction medium comprising a monocarboxylic acid (e.g. acetic
acid) to produce a slurry of crude aromatic carboxylic acid in said aliphatic
acid,
recovering the crude acid from said slurry, dissolving the recovered crude
acid in
water, purifying the crude acid by a reaction comprising contacting the
solution with
hydrogen, and separating the purified acid from the mother liquor component of
said
solution,
the steps of withdrawing from the reaction zone a high pressure gaseous
overheads
stream containing inter olio monocarboxyiic acid, water and methyl bromide,
separating substantially all of the monocarboxylic acid from said overheads
stream
in a separation column to which aqueous mother liquor obtained from said
purification reaction is also supplied whereby a high pressure offgas stream
is
obtained which contains inter olio water derived from both the oxidation
reaction and
the purification reaction and methyl bromide obtained from the oxidation
reaction,
oxidising said high pressure offgas stream, optionally in the presence of a
catalyst
andlor a combustion assistant) whereby the methyl bromide is converted to
bromine
andlor hydrogen bromide, passing the treated gas containing bromine and/or HBr
in
the vapour phase through an energy recovery system under temperature and
pressure conditions such that condensation of the bromine andlor HBr is
substantially prevented, and removing substantially all of the bromine from
the
treated gas following passage through the energy recovery system.
Usually following such treatment to remove bromine, the treated gas has a
bromine content of less than 4 ppm vol/vol.
The aqueous mother liquor separated from the purified aromatic acid, e.g by
means of filtration equipment such as that disclosed in our prior
International Patent
Application No. WO 93/24440, contains various impurities, reaction
intermediates
and also said aromatic carboxylic acid in suspended and dissolved forms. By
recycling the aqueous mother liquor to the separation column, such impurities,
reaction intermediates and dissolved aromatic carboxylic acid content can be
separated from the water content of the aqueous mother liquor and returned to
the
oxidation reactor as bottoms product from the column along with the
monocarboxylic
acid recovered by means of the column.
Usually a major fraction of the aqueous mother liquor will be recycled to the
separation column in this way, preferably as reflux.
7
CA 02273958 1999-03-15
WO 97/27168 PCT/GB97/00104
The mother liquor recovered from the purification reaction may be treated,
e.g.
by cooling or evaporation, to recover therefrom crystals of less pure aromatic
carboxylic acid, and at least part of the mother liquor supplied to the
separation
column may comprise the secondary mother liquor obtained following such
treatment. Alternatively, since the separation will tend to be carried out at
relatively
high temperature, primary mother liquor recovered from the purification
reaction
may be recycled to the separation column without cooling the same to any
signficant
extent although it may be filtered before introduction into the separation
column in
order to remove any suspended fines.
Conveniently the crude acid is separated from said monocarboxylic acid by
replacing the monocarboxylic acid in said slurry with water to produce a wet
deposit
of crude acid containing water for use in the subsequent purification of the
crude
acid (i.e. by hydrogenation of an aqueous solution formed from said wet
deposit),
replacement of the monocarboxylic acid with water being effected by means of
an
integrated separation and water washing filter preferably operating under
elevated
pressure conditions.
The integrated separation and water washing filter may comprise a gas
pressurised belt filter, a gas pressurised rotary cylindrical filter, a
hydraulically
pressurised multi-celled pressure drum filter or a centrifuge provided with
washing
facilities. In each instance, the washing operation may be carried out in
stages,
preferably in countercurrent fashion so that the filter cake is washed with
water of
increasing purity as it advances downstream from the location at which
separation of
the crystals from the mother liquor takes place.
Typically the filter operates wish a pressure differential in the range of 0.1
to
15 tiara (preferably between 0.3 and 7 tiara), preferably such that the
pressure on
the lower pressure side thereof is no less than one tiara although we do not
exclude
the possibility of the lower pressure side being at sub-atmospheric pressure.
Another aspect of the invention is concerned with the removal from the gas
stream of bromine and/or hydrogen bromide following high temperature
particularly
with the aim of processing the gas stream to remove the bromine components so
that any discharge to atmosphere is substantially free of such components.
Such
processing may for instance be effected by desuperheating the gas stream using
water and contacting the gas stream with a suitable aqueous scrubbing media in
a
scrubbing section to remove the Br~ and HBr. HBr for instance may be removed
by
countercurrent contact with HBr solution or it may be removed simply by
contact with
water, e.g. a water spray, white at the same time desuperheating the treated
gas.
Contacting the treated gas with HBr solution is for instance appropriate where
the
aim is to recover HBr for reuse as part of the catalyst system employed in the
8
I ._
CA 02273958 1999-03-15
WO 97/27168 PCT/GB97/00104
oxidation reactor. Where this is not required, water may be used. If used, it
is
preferred that sufficient water is employed to irrigate the pipeline
transporting the
water treated gas downstream and thereby prevent corrosion problems. Br, may
be
removed by countercurrent contact with an aqueous solution of components such
as
sodium hydroxide, potassium hydroxide, sodium carbonate, sodium bicarbonate,
sodium bromide, sodium formate, sodium sulphite, urea or mixtures containing
any
combination of two or more of these compounds (e.g. sodium hydroxide and
sodium
sulphite).
The oxidation reaction for the production of the aromatic carboxylic acid and
its subsequent purification may be carried out in accordance with the
teachings in
our prior EP-A-498591 and EP-A-502628, the entire disclosures of which are
incorporated herein by this reference.
The aromatic carboxylic acid may be terephthalic acid, in which the case the
precursor thereof which is oxidised to produce terephthalic acid will be p-
xylene.
Alternatively the aromatic carboxylic acid may be isophthalic acid (precursor
m-xylene) or 2,6-naphthalene-dicarboxylic acid (precursor 2,6-
dimethylnaphthalene).
The oxidation reaction is typically carried out at a temperature in the range
of
about 120°C to about 240°C and a pressure which is at least
sufficient to maintain
the reaction mixture under liquid phase conditions, typically 5 tiara to 30
tiara.
The promoter component of the catalyst system contains bromine and is
typically in the form of hydrogen bromide, molecular bromine, sodium bromide
and/or suitable organic bromide compounds well known to those in the art. The
heavy metal components of the catalyst system usually in the form of cobalt
and/or
manganese compounds, for example cobalt and/or manganese carbonate.
According to a further aspect of the present invention there is provided in a
process for the production of an aromatic carboxylic acid such as terephthalic
acid,
which process comprises oxidising a precursor of said aromatic carboxylic acid
(e.g.
paraxylene) in a reaction medium comprising an aliphatic monocarboxylic acid
(e.g.
acetic acid) to produce a slurry of crude aromatic carboxylic acid in said
aliphatic
acid, and replacing the aliphatic monocarboxylic acid in said slurry with
water to
produce a wet deposit of crude aromatic carboxylic acid containing water for
use in
the subsequent purification of the crude carboxylic acid (e.g. by
hydrogenation of an
aqueous solution formed from said wet deposit), replacement of the aliphatic
monocarboxylic acid with water being effected by means of an integrated
separation
and water washing filter operating under elevated pressure conditions,
the steps of processing a high pressure gaseous overheads stream comprising
water, non-condensible gases such as nitrogen and carbon oxides, and organic
compounds including aliphatic monocarboxylic acid and methyl bromide,
evaporated
9
CA 02273958 1999-03-15
WO 97127168 PCT/GB97/00104
in the course of the oxidation reaction to produce a high pressure gaseous
effluent
stream containing water and organic compounds such that the water content of
the
effluent stream exceeds the organic compound content, subjecting the high
pressure
gaseous effluent stream to high temperature combustion, optionally in the
presence
of a catalyst andlor a combustion assistant, with accompanying conversion of
methyl
bromide to bromine and/or hydrogen bromide, and removing bromine and/or HBr
from the treated gaseous effluent.
Preferably the high pressure gaseous effluent stream is substantially free of
said aliphatic monocarboxylic acid. Thus, for example, processing of the
overheads
stream conveniently includes the step of separating water and aliphatic
monocarboxylic acid in a separation column to allow recovered aliphatic acid
to be
recycled to the reactor. Conveniently, aqueous mother liquor derived from
purification of the crude aromatic carboxylic acid is also supplied to the
distillation
column, preferably as reflex, whereby impurities, reaction intermediates and
aromatic carboxylic acid present in the aqueous mother liquor are passed to
the
oxidation reaction together with aliphatic monocarboxylic acid and the water
content
of the gaseous effluent stream includes water derived from said aqueous mother
liquor.
It is to be understood that where the context admits features of the invention
as defined in the consistory clauses which follow said first aspect of the
invention
are also applicable to the other aspects of the invention defined herein.
The invention will now be described by way of example only with reference to
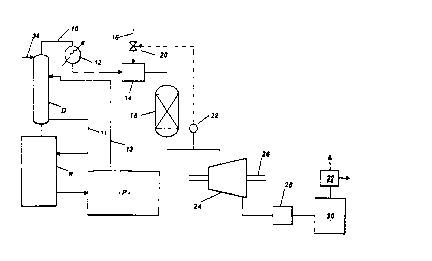
the accompanying drawings, in which:
Figure 1 is a schematic flow diagram illustrating one embodiment of the
invention as
applied to the treatment of an effluent gas stream derived from plant for the
production of terephthalic acid: and
Figure 2 illustrates a scrubbing unit for reducing the brominelhydrogen
bromide
content of the effluent gas.
Referring to Figure 1 , the effluent gas stream entering the treatment plant
via
line 10 is derived from a distillation column or rectifier D associated with a
reactor R
for the production of terephthalic acid by liquid phase oxidation of p-xylene,
for
example by means of the process disclosed in our prior EP-A-498591 and/or
EP-A-502628. In the process disclosed in these patent applications, catalysed
liquid
phase oxidation of paraxylene is carried out in a solvent comprising acetic
acid to
produce terephthalic acid) the catalyst system comprising heavy metals such as
cobalt and manganese and bromine promoter. The temperature of the liquid phase
reaction is controlled by withdrawing a vapour phase overheads stream from the
reactor comprising the acetic acid, water, gaseous by-products including
methyl
CA 02273958 1999-03-15
WO 97/27168 PCT/GB97/00104
bromide and methyl acetate, and gases such as nitrogen, carbon monoxide)
carbon
dioxide and unreacted oxygen. Following processing involving removal of a
large
proportion of the acetic acid, an offgas or gaseous effluent stream is
obtained which
is at elevated pressure and typically contains 20 to 125 ppm methyl bromide,
depending on the reactor operating temperature and pressure.
Processing of the overheads stream in the illustrated embodiment comprises
passing the gaseous stream to the column D which serves to effect the
separation of
acetic acid from water. Column D is operated so that the heavy components such
as
acetic acid are recovered as a liquid phase bottoms product while the light
components such as water, gaseous by-products such as methyl bromide and
methyl
acetate and gases such as nitrogen, carbon monoxide, carbon dioxide and
oxygen,
are recovered as a gaseous phase tops product constituting the high pressure
gaseous offgas stream which is to be treated. Water in the resulting offgas
stream is
in the form of steam. Thus, for example, while the overheads stream withdrawn
from
the oxidation reactor may contain acetic acid and water in the proportion of
70 to
95% acetic acid to 30 to 5% water (on a weighs to weight basis) , the high
pressure
offgas stream recovered from the column D will typically contain acetic acid
and
water in the proportion of 0.5 to 1.0 wt% acetic to 99.5 to 99.0 wt% water as
steam.
The steam content will typically be in the range of about 40 to 70% by weight
of total
offgas.
The acetic acid-rich bottoms product from the column D is returned to the
oxidation reactor R via line 11. This bottoms product will comprise acetic
acid and
some water and may also contain other organics such as precursors, e.g.
paratoluic
acid, of terephthalic acid routed to the column D in the manner described
below. The
tops product) namely the high pressure offgas comprising steam as a primary
component, is removed via line 10.
A feature of the process disclosed in EP-A-498591 and EP-A-502628 is the
handling of the aqueous mother liquor produced in the purification process.
The
purification process involves the hydrogenation of an aqueous solution of
crude
terephthalic acid obtained from the oxidation of paraxylene, crystallisation
of
purified terephthalic acid and separation of the purified crystals from the
aqueous
mother liquor. The resulting aqueous mother liquor contains impurities and
intermediates such as paratofuic acid and in prior processes was treated as a
waste.
EP-A-498591 and EP-A-502628 teach recycle of at least part of this primary
mother
liquor to the distillation column associated with the oxidation reactor,
conveniently
as reflux, in such a way that high boiling point impurities such as paratoluic
acid are
recovered in the acetic acid-rich bottom product withdrawn from the column for
recycle to the oxidation reactor. The mother liquor recovered from the
purification
11
CA 02273958 1999-03-15
WO 97/27168 PCT/GB97/00104
process may be recycled to the distillation column with or without treatment.
Such
treatment, where employed, may comprise subjecting it to cooling or
evaporation to
precipitate further, but less pure, terephthalic acid and feeding the
resulting
secondary mother liquor (as a reflux feed) to the distillation column for
separating
water and acetic acid. The less pure terephthalic acid precipitate is also
recycled to
the oxidation reactor, e.g. by slurrying it in acetic acid derived from the
distillation
column. Use of the purification mother liquor in this way may also be employed
in
the present invention, with the mother liquor (treated or untreated) being
supplied to
the distillation column D, preferably as reflux.
The effluent treatment disclosed herein may for instance be used in
conjunction with aromatic polycarboxylic acid production plant wherein the
crude
acid crystals and the purified acid crystals are separated from the primarily
aliphatic
monocarboxylic acid mother liquor and primarily aqueous mother liquor
respectively
and are subjected to washing with water by means of an integrated solids
separation
and water washing apparatus such as those described in our prior published
International Patent Applications Nos. WO 93124440 and WO 94/17982 (the entire
disclosures of which are incorporated herein by this reference) so that the
mother
liquor is replaced by water as a result of washing. Thus, for example the
integrated
solids separation and water washing apparatus may comprise a belt filter unit
operated with the slurry side under superatmospheric conditions, or a
pressurised
rotary cylindrical filter unit operated with the slurry side under
superatmospheric
conditions, or a pressure drum filter unit (e.g. a BHS-Fest pressure filter
drum
formed with a plurality of slurry receiving cells in which the mother liquor
is
displaced from filter cake by water under hydraulic pressure supplied to the
cells).
The processes for the filtration and washing of crude terephthalic acid, its
subsequent purification) recovery and washing and the recycle of the
purification
mother liquor are fully described in EP-A-498591 , EP-A-502628, WO 93/24440
and
WO 94117982. Detailed description herein is consequently unnecessary and these
processes are represented in Figure 1 by reference P with the recycled mother
liquor
(treated or untreated) being depicted by reference 13.
The effluent gas stream is typically at a pressure of the order of 10 to 16
tiara
and a temperature of the order of 170 to 190°C. The gas stream is
preheated in heat
exchanger 12 using for example high pressure steam as the heat source.
Typically
the temperature of the gas stream following such heat exchange is of the order
of
250 to 450°C, preferably 300 to 400°C. The gas stream then
enters a mixer 14 into
which a combustion assistant is also introduced via line 16, the combined gas
stream and combustion assistant then being fed to a catalytic combustion unit
18
12
CA 02273958 1999-03-15
WO 97/27168 PCT/GB97I00104
with a space velocity of the order of 10' to 5 x 10° h~', preferably 5
x 103 to
2 x 10° h '.
A convenient combustion assistant is methyl acetate which is produced as a
by-product in the terephthalic acid production process. Various other
combustion
assistants may be used instead or in addition, especially those which contain
oxygen
- eg methanol. The amount of combustion assistant introduced is cnrh fh~t rho
temperature of the combusted gas stream exiting the catalytic combustion unit
18 is
of the order of 400°C or greater, typically of the order of
480°C. A feedback
arrangement comprising valve 20 in line 16, temperature sensor 22 and
appropriate
control equipment is used to regulate the supply of combustion assistant to
the
mixer 14 so that the desired temperature is maintained at the exit of the unit
18.
The catalyst employed in the catalytic combustion unit 18 may comprise any
suitable oxidation catalyst, usually in solid form, to secure substantially
total
conversion of methyl bromide to bromine and HBr while also securing, in
combination with the combustion assistant (where needed), substantially total
oxidation of other organics such as acetic acid, elimination of carbon
monoxide and
production of heat to produce the desired exit temperature. Typically the
catalyst
employed comprises a noble metal catalyst such as platinum and/or palladium
supported on a suitable support which may be inert. The support may be ceramic
or
metallic in the form of a monolith or pellets. Suitable commercial catalysts
are
available from catalyst manufacturers such as Johnson Matthey (e.g. Halocat
AH/HTB-10 or LHC catalyst), Allied Signal/Degussa (e.g. HDC-2 or T2-HDC
catalyst)
and Engelhard (e.g. VOCAT 300H or VOCAT 450H catalyst).
Following catalytic combustion, the treated gas stream typically has a
temperature of the order of 400 to 700°C and a pressure only marginally
lower than
the untreated gas stream, ie about 9.5 to 15.5 tiara in the case where the
untreated
gas stream has a pressure of the order of 10 to 16 tiara. The treated gas is
then
passed through expander 24 in which the energy content of the gas stream is
converted into mechanical power which, via shaft 26, can be employed
appropriately
within the terephthafic acid production process, for instance as power input
for an air
compressor for feeding air under pressure to the oxidation reactor of the
production
process or for generation of electric power for distribution either within the
plant or
to other users. At the exit side of the expander 24, the gas stream
temperature is
typically of the order of 140 to 220°C (eg about 170 to 200°C)
and its pressure is
near atmospheric, eg about 1.2 tiara. The temperature and pressure conditions
employed are such that the bromine and HBr derived from methyl bromide in the
course of the catalytic combustion remain in the gas phase thereby avoiding
any risk
of dew point corrosion. In this way, cost penalties otherwise incurred through
the use
13
CA 02273958 1999-03-15
WO 97/27168 PCTIGB97/00104
of scrubbing plant upstream of the expander 24 (with consequent reduction in
energy
available for extraction by means of the expander) or through the use of
expensive
materials of construction for the expander 24, are avoided.
Following energy recovery, the gas stream is processed to remove the bromine
components so that any discharge to atmosphere is substantially free of such
components. Such processing may for instance be effected by desuperheating the
gas stream in unit 28 using water and contacting the gas stream with a
suitable
aqueous scrubbing media in a scrubbing section 30 to remove the Brz and HBr.
HBr
for instance may be removed by countercurrent contact with HBr solution and
Br2
may be removed by countercu«ent contact with an aqueous solution of components
such as sodium hydroxide, sodium carbonate, sodium bicarbonate, sodium
bromide,
sodium formate, sodium sulphite or mixtures containing any combination of two
or
more of these compounds (e.g. sodium hydroxide and sodium sulphite). The water
used for desuperheating may also be employed in the scrubbing section. The
cleaned offgas (typically containing 40 to 70% by weight water vapour) is then
cooled, in a cooling water exchanger 32 for example, to recover a major
proportion
and preferably substantially all of the water therein for re-use on the
oxidation
andlor purification stages of the plant. Following condensation of the bulk of
the
water, the offgas stream comprises mainly nitrogen and may be discharged to
atmosphere andlor used elsewhere in the production process, eg for inerting
duties.
Although not shown in Figure 1 , the column D may include a scrubbing section
to which water derived for example from the water recovered from the cleaned
offgas is supplied via line 34 in order to improve the recovery of the more
volatile
precursors of terephthalic acid such as paratoiuic acid which may otherwise
pass out
of the columm with the offgas removed as tops product from the column. This
scrubbing section is located above the point of introduction of the reflux
stream
(pure plant mother liquor) into the column D and the water supplied to this
scrubbing
section may be preheated in order to increase the steam content of the tops
product.
Figure 2 illustrates one form of scrubbing unit 50 for use in scrubbing the
effluent gas in order to achieve a bromine content in the discharged gas of
less than
4 ppm vollvol, more preferably less than 2 ppm vollvol, with 1 ppm vollvol
being
readily achievable. The unit 50 comprises a vessel having two packed sections
52
and 54. The packings employed may be any suitable type, e.g. Raschig rings,
Pal(
rings etc. A liquid collection tray 56 is located between the two sections 52,
54. The
effluent gas (together with water employed to irrigate the pipeline),
following
treatment to remove HBr, is fed to an inlet 58 at the base of the vessel 50
where the
gas and liquid entering the vessel impinge on a plate (not shown) within the
vessel
base to prevent the gaslliquid mixture impinging on that part of the vessel
wall
14
CA 02273958 1999-03-15
WO 97/27168 PCT/GB97/00104
opposite the inlet 58. The gas rises through the vessel, traversing the packed
sections 52, 54, and leaves the vessel via outlet 60 which may be a discharge
to
atmosphere.
The scrubbing liquid employed may be any sui#able liquid capable of removing
bromine from the effluent gas, including the chemicals specified above. The
scrubbing liquid is circulated around a loop including the upper section 52,
exit line
62, pump 64 and inlet line 66 so that the liquid flows countercurrent to the
direction
of gas flow passing up through the vessel 50. A second recirculatory flow of
scrubbing liquid is established in the lower part of the vessel 50, again in
countercurrent relation to the gas flow, by means of outlet line 68, pump 70
and
return line 72. Spent scrubbing liquid is purged from the system via line 74
and
make-up liquid is supplied via line 76. The amount of scrubbing liquid pumped
through the vessel per unit time will generally be far in excess of that being
purged)
e.g. a ratio of at least 20:1 , e.g. at least 30:1 (typically of the order of
40:1 }. A purge
line 78 interconnects the outlet of pump 64 and line 72 so that scrubbing
liquid
collecting in the collection tray 56 is passed to the lower recirculatory
liquid flow
loop. In a modification, the purge line 78 may be omitted and transfer of the
scrubbing liquid from the upper section 52 to the lower section 54 and hence
into the
lower recirculatory loop may be implemented by allowing overflow of the liquor
collecting in the tray 56. A small amount of the scrubbing liquid is routed to
the inlet
58 via line 80, for example from the pump 70, in order to prevent any risk of
corrosion in the region of the inlet.
In a further modification, the HBr scrubbing process may be integrated with
the
Br2 process by incorporating a further packing section into the scrubbing
tower
beneath the sections 52 and 54 so that the gas stream initially passes through
the
HBr scrubbing section. In the HBr scrubbing section, the scrubbing liquor may
be an
aqueous solution of HBr flowing in a recirculatory loop as described above in
relation to sections 52 and 54 with suitable purging and make-up of the loop.
The
HBr purge may for instance be supplied to catalyst make up.
From the foregoing, it will be seen that the bromine containing gas is
subjected
to a two stage scrubbing treatment allowing the bromine to be substantially
completely removed before the gas is discharged from the vessel. As mentioned
previously, the scrubbing liquid may be any suitable liquid for effecting
bromine
removal, with alkali metal compounds being preferred. Where for example the
liquid
is caustic soda, this is converted to sodium carbonate and bicarbonate in the
scrubbing vessel as a result of absorption into the hydroxide of carbon
dioxide
contained in the effluent gas. Instead of, or in addition to, caustic soda,
the
scrubbing liquid may comprise one or more of the chemicals previously
mentioned,
CA 02273958 1999-03-15
WO 97/27168 PCT/GB97/00104
e.g. sodium sulphite or sodium formate or other suitable reducing agent, or
other
compounds such as potassium hydroxide or urea.
16