Note: Descriptions are shown in the official language in which they were submitted.
CA 02274132 2007-07-11
= _ 1 t.. '
1
Macrocyclic Metal Complex Carboxylic Acids,. Use And Method
For The Production Thereof
The invention relates to the subject characterized in the
claims, i.e., new macrocyclic metal complex carboxylic acids,
their use as well as process for their production.
Regarding the prior art, the following pages 1-23 from
WO 97/02051 are cited:
The contrast media that are now used in clinical practice
for the modern imaging processes of nuclear spin tomography (MRI)
and computer tomography (CT) (Magnevist(R), Pro Hance(R),
Ultravist(R) and Omniscan(R)J are dispersed in the entire
extracellular space of the body (intravascular space and
interstitium). This dispersion space comprises about 20% of the
volume of the body.
In clinical practice, extracellular MRI contrast media were
first used successfully. in the diagnosis'of cerebral and spinal
disease processes since here a quite special situation exists
with respect to the regional dispersion space. In the brain and
spinal cord, extracellular contrast media in healthy tissue do
not leave the intravascular space because of the blood-brain.
barrier. In the case of pathological processes with disruption
of the blood-brain barrier (e.g., malignant tumors,
inflammations, demyelinating diseases, etc.), regions with
elevated blood-vessel permeability then develop inside the brain
CA 02274132 1999-10-21
2
for these extracellular contrast media (Schmiedl et al., MRI of
Blood-Brain Barrier Permeability in Astrocytic Gliomas:
Application of Small and Large Molecular Weight Contrast Media,
Magn. Reson. Med. 22: 288, 1991). Affected tissue can be
identified with high contrast relative to healthy tissue by
exploiting this disruption of vascular permeability.
Outside of the brain and the spinal cord, however, no such
permeability barrier exists for the above-mentioned contrast
media (Canty et al., First-Pass Entry of Nonionic Contrast Agent
into the Myocardial Extravascular Space. Effects on Radiographic
Estimate of Transit Time and Blood Volume. Circulation 84:
2071, 1991). Thus, the concentration of the contrast medium is
no longer dependent on vascular permeability, but only on the
size of the extracellular space in the corresponding tissue.
Delimitation of the vessels relative to the surrounding
interstitial space using this contrast medium is not possible.
A contrast medium that is dispersed exclusively in the
vascular space would be desirable, particularly for the
visualization of vessels. The purpose of such a blood-pool agent
is to make it possible, with the aid of nuclear spin tomography,
to delimit tissue with sufficient blood supply from tissue with
insufficient blood supply, and thus to diagnose an ischemia.
Infarcted tissue can also be delimited, based on its anemia, from
surrounding healthy or ischemic tissue if a vasal contrast medium
is used. This is of special importance if, e.g., the point is to
distinguish a myocardial infarction from an ischemia.
CA 02274132 1999-10-21
3
To date, most of the patients in whom there is suspicion of
cardiovascular disease (this disease is the most frequent cause
of death in Western industrialized countries) have to undergo
invasive diagnostic tests. In angiography at present, diagnostic
radiology with the aid of iodine-containing contrast media is
used in particular. These tests suffer from various drawbacks:
they are associated with the risk of radiation exposure, as well
as with difficulties and stresses, which therefore particularly
have the effect that the iodine-containing contrast media, as
compared with NMR contrast media, have to be used in much higher
concentrations.
There is therefore a need for NMR contrast media which can
mark the vascular space (blood-pool agents). These compounds are
to be distinguished by good compatibility and by high
effectiveness (high increase of signal intensity with MRI).
Thus far, the attempt to solve at least a part of this
problem by using complexes that are bonded to macromolecules or
biomolecules has been successful only to a very limited extent.
Thus, for example, the number of paramagnetic centers in the
complexes that are described in European Patent Applications No.
0 088 695 and No. 0 150 844 is not sufficient for satisfactory
imaging.
If the number of metal ions required is increased by
repeated introduction of complexing units into a macromolecular
biomolecule, this is associated with an intolerable impairment of
the affinity and/or specificity of this biomolecule [J. Nucl.
Med. 24, 1158 (1983)].
CA 02274132 1999-10-21
4
Macromolecules can generally be suitable as contrast media
for angiography. Twenty-four hours after intravenous injection
in rats, however, albumin-GdDTPA (Radiology 1987; 162: 205),
e.g., shows a concentration in the liver tissue that constitutes
almost 30% of the dose. In addition, only 20% of the dose is
eliminated in 24 hours.
The macromolecule polylysine-GdDTPA (European Patent
Application, Publication No. 0 233 619) has also proved suitable
as a blood-pool agent. Because of production, however, this
compound consists of a mixture of molecules of different sizes.
In excretion tests in rats, it was shown that this macromolecule
is excreted unchanged by glomerular filtration through the
kidneys. Due to factors related to synthesis, however,
polylysine-GdDTPA may also contain macromolecules that are so
large that they cannot pass through the capillaries of the
kidneys in the case of glomerular filtration and thus remain in
the body.
Also, macromolecular contrast media based on carbohydrates,
e.g., dextran, have been described (European Patent Application,
Publication No. 0 326 226). The drawback of these compounds lies
in the fact that the latter generally carry only about 5% of the
signal-enhancing paramagnetic cation.
The polymers described in European Patent Application No. 0
430 863 already represent a step toward blood-pool agents since
they no longer exhibit the size and molecular weight relative to
heterogeneity that are characteristic of the previously mentioned
polymers. They leave something to be desired, however, as
CA 02274132 2006-08-04
regards complete elimination, compatibility, and/or
effectiveness.
As described in WO 97/02051, it has been found that
complexes which consist of nitrogen-containing cascade polymers
that are provided with complexing ligands, at least 16 ions of an
element of atomic numbers 20-29, 39, 42, 44 or 57-83, and
optionally cations of inorganic and/or organic bases, amino acids
or amino acid amides, and which optionally contain acylated amino
groups are surprisingly very well suited for the production of
NMR and x-ray diagnostic agents without exhibiting the mentioned
drawbacks.
The complexing cascade polymers that are described in the
indicated patent application can be described by general formula
I
A-{X-[Y-(Z-( W-K) Z)ylx}a (I) r
in which
A stands for a nitrogen-containing cascade nucleus of
base multiplicity a,
X and Y, independently of one another, stand for a direct
bond or a cascade reproduction unit of reproduction
multiplicity x or y,
Z and W, independently of one another, stand for a cascade
reproduction unit of reproduction multiplicity z or w,
K stands for the radical of a complexing a'gent,
a stands for numbers 2 to 12,
x, y, z and w, independently of one another, stand for
numbers 1 to 4,
CA 02274132 1999-10-21
6
provided that at least two reproduction units are different
and that
16 < a" x* y, z* w< 64
holds true for the product of the multiplicities.
As cascade nucleus A, the following are suitable:
nitrogen atom,
1
u
/N -CHZ CHZ N CH2 CH2 N,
u2 11 P U
u
u uz
N--CH= CHz N
I I
C
I H2 I H2
IiH,)m (iHs)m
~N--CHz CHz N
s p U~
U
CA 02274132 1999-10-21
7
Z U2
U 1
CH2CH2 _'N '_'CHZ-EH2 -N -CH2CH2
N -CHZCHZ -N =CH2-~HZ N -CH2CH2 N
U U
1 2 IZ
\CH2CH2 -'N --CH2EH2 _"N -CH2CHZ
1 2 Iz
U U
1 2
UU
M
";Z~
U N ~M M \N
12 12
U u
1 1
U U
N---M M--N
. 2
U ' U
0 CH2--CH2-0 Ui
P ~ ~
N _.,pg 2
M -NU
U ,
CA 02274132 1999-10-21
8
V~
R CfM N
=
3
2
U
U N N
N .N N
Uz~ ~U2
,
E
I
0 N,%E
O
E~
N N E
E E
C..r O
N -E
in which
m and n stand for numbers 1 to 10,
p stands for numbers 0 to 10,
Ul stands for Ql or E,
UZ stands for Q2 or E with
E meaning the group Q
-- CHZ--N 2
4,
9
whereby
o stands for numbers 1 to 6,
Q~ stands for a hydrogen atom or Q2 and
Q2 stands for a direct bond,
M stands for a Ci-Cio alkylene chain which optionally is
interrupted by 1 to 3 oxygen atoms and/or optionally is
substituted with 1 to 2 oxo groups,
R stands for a branched or unbranched Ci-Cio alkyl
radical, a nitro, amino, carboxylic acid group or for
1
U
M--N~
, 2
U
whereby number Q2 corresponds to base multiplicity a.
The nitrogen atom, whose three bonds (base multiplicity a
3) in a first "inner layer" (generation 1) are occupied by three
reproduction units X or Y (if X stands for a direct bond) or Z
(if X and Y in each case stand for a direct bond), represents the
simplest case of a cascade nucleus; in other words: the three
hydrogen atoms of the basic cascade starter ammonia A(H)a = NH3
have been substituted by three reproduction units X or Y or Z.
In this case, number Q2 contained in cascade nucleus A represents
base multiplicity a.
Reproduction units X, Y, Z and W contain -NQ'Q2 groups, in
which Ql means a hydrogen atom or Q2 and Q2 means a direct bond.
The number Q2 contained in the respective reproduction unit
(e.g., X) corresponds to the reproduction multiplicity of this
unit (e.g., x in the case of X). The product of all
CA 02274132 1999-10-21
10
multiplicities a- x- y- z- w indicates the number of
complexing agent radicals K bonded in the cascade polymers. The
polymers according to the invention contain at least 16 and at
most 64 radicals K in the molecule, which in each case can bond
one to a maximum of three (in the case of divalent ions),
preferably one ion, to an element of the above-mentioned atomic
numbers.
The last generation, i.e., reproduction unit W bonded to
complexing agent radical K, is bonded to K by NH groups (-NQlQZ
with Ql meaning a hydrogen atom and Q2 = direct bond), while the
preceding reproduction units can be linked together both by NHQ2
groups (e.g., by acylation reactions) and by NQ2Q2 groups (e.g.,
by aikylation reactions).
The cascade polymer complexes exhibit a maximum of 10
generations (i.e., more than just one of reproduction units X, Y
and Z can also be present in the molecule in each case), but
preferably 2 to 4 generations, whereby at least two of the
reproduction units in the molecule are different.
As preferred cascade nuclei A, those are indicated which
fall under the above-mentioned general formulas if
m stands for numbers 1-3, especially preferably for
number 1,
n stands for numbers 1-3, especially preferably for
number 1,
p stands for numbers 0-3, especially preferably for
number 1,
o stands for number 1,
CA 02274132 1999-10-21
11
M stands for a-CH2, -CO or -CH2CO group and
R stands for a -CHZNU' U2 , CH3 or NO 2 group.
As further preferred cascade starters A(H)e, there can be
listed by way of example:
(In the parentheses, base multiplicity a is indicated for
the case where subsequent mono- or disubstitution is used in
building the next generation)
Tris(aminoethyl)amine (a = 6 or 3);
tris(aminopropyl)amine (a = 6 or 3);
diethylenetriamine (a = 5 or 3);
triethylenetetramine (a = 6 or 4);
tetraethylenepentamine (a = 7 or 5);
1,3,5-tris(aminomethyl)benzene (a = 6 or 3);
trimesic acid triamide (a = 6 or 3);
1,4,7-triazacyclononane
(a = 3);
1,4,7,10-tetraazacyclododecane (a = 4);
1,4,7,10,13-pentaazacyclopentadecane (a = 5);
1,4,8,11-tetraazacyclotetradecane (a = 4);
1,4,7,10,13,16-hexaazacyclooctadecane (a = 6);
1,4,7,10,13,16,19,22,25,28-decaazacyclotriacontane (a = 10);
tetrakis(aminomethyl)methane (a = 8 or 4);
1,1,1-tris(aminomethyl)ethane (a = 6 or 3);
tris(aminopropyl)-nitromethane (a = 6 or 3);
2,4,6-triamino-1,3,5-triazine (a = 6 or 3);
1,3,5,7-adamantanetetracarboxylic acid amide (a = 8 or 4);
3,3',5,5'-diphenylether-tetracarboxylic acid amide (a = 8 or 4);
1,2-bis[phenoxyethane]-3',3",5',5"-tetracarboxylic
acid amide (a = 8 or 4);
1,4,7,10,13,16,21,24-octaazabicyclo[8.8.8]hexacosane(a = 6).
CA 02274132 1999-10-21
12
It can be pointed out that the definition as cascade nucleus
A and thus the separation of cascade nucleus and first
reproduction unit can be selected by purely formal means and thus
independently of the actual synthesis of the desired cascade
polymer complexes. Thus, e.g., the tris(aminoethyl)amine used in
Example 4 can be considered as cascade nucleus A itself (compare
the general formula, indicated first for A, with m = n = p = 1,
Ul = E with o meaning number 1 and Ul = U2 = Q2) but also as a
nitrogen atom (= cascade nucleus A), which as a first generation
i
Q
/
exhibits three reproduction units - -CH'-CHZ----N
(compare the definition of E).
Cascade reproduction units X, Y, Z and W are determined,
independently of one another, by
E,
3 U
U-N \ 2
U ,
/U
U4. N U2
3
U-V ~US U1
--~ 2
CA 02274132 1999-10-21
13
3 U
U ---N
~ ~U2
-CO q L
U
3
U -N
U 2
in which
Ul stands for Ql or E,
U2 stands for Q2 or E with
E meaning the group -~Hz~ -CHZ--N Z
\Q~
whereby
o stands for numbers 1 to 6,
Q1 stands for a hydrogen atom or Q2,
Q 2 stands for a direct bond,
U3 stands for a Cl-C20 alkylene chain, which optionally is
interrupted by 1 to 10 oxygen atoms and/or 1 to 2
-N(CO)q-RZ radicals, 1 to 2 phenylene radicals and/or 1
to 2 phenylenoxy radicals and/or optionally is
substituted by 1 to 2 oxo, thioxo, carboxy, Ci-C5
alkylcarboxy, C1-C5 alkoxy, hydroxy, Ci-C5 alkyl groups,
whereby
q stands for numbers 0 or 1 and
R2 stands for a hydrogen atom, a methyl or an ethyl
radical, which optionally is substituted with 1-2
hydroxy or 1 carboxy group(s),
CA 02274132 1999-10-21
14
L stands for a hydrogen atom or the group
1
U
3 ~
U-N
\ U2 I
V stands for methine group if at the
same time U4 means a direct bond or group M
and U5 has one of the meanings of U3
or Co-
-NH
V stands for group if at the
CO
same time U4 and U5 are identical and mean the
direct bond or group M.
Preferred cascade reproduction units X, Y, Z and W are those
in which in the above-mentioned general formulas, radical U3
stands for -CO-, -COCH2OCHZCO-, -COCH2-1 -CH2CH2-1 -CONHC6H4-1
-COCH2CHZCO-, -COCHZ-CH2CHZCO-, -COCH2CH2CH2CH2CO-,
radical U4 stands for a direct bond, for -CHZCO-,
radical U5 stands for a direct bond, for -(CH2) 4-1 -CHZCO-,
-CH (COOH) -, CH2OCHZCH2-, -CH2C6H4-, CH2-C6H4OCH2CH2-,
1
radical E stands for a group 'CH2 "CH2--N
\ Q 2
Cascade reproduction units X, Y, Z and W that are mentioned
CA 02274132 1999-10-21
15
as examples can be cited:
-CH2CH2NH-; -CH2CH2N< ;
-COCH(NH-)(CH2)4NH-; -COCH(N< )(CHZ)aN< ;
=COCHZOCHZCON(CH2CHZNH-)2; -COCH2OCH2CON(CH2CH2N< )2 ;
-COCHZN(CHZCH2NH-)Z; -COCH2N(CH2CH2N< )2;
-COCHZNH-; -COCH2N< ;
-COCHZCH2CON(CH2CHZNH )Z; -COCH2CH2CON(CH2CH2Ng )2;
-COCH2OCH2CONH-C6H,i-CH[CH2CON(CH2CH2NH-)2]2;
-COCH2OCH2CONH-C6H4-CH[CH2CON(CH2CH2N< )2]Z ;
-COCHZCH2CO-NH-C6Ha=CH[CH2CON(CHz CHZNH-)2]2 ;
-COCHZCH2CO-NH-C6H4-CH[CHZCON(CH2CHZN<)2]Z ;
-CONH-C6H,4-CH[CH2CON(CH2CH2NH-)2
,]2 ;
-CONH-C6H4-CH(CH-)CON(CH2CH2N<)2]2;
-COCH(NH-)CH(COOH)NH-; -COCH(N< )CH(COOH)N< ;
~CON J:H2CH2NH-~
~OCH,OCH2CONH
CON GH2CHZNH-~
CON GH2CH2N< h
-COCHZOCH,CONH
CON CHZCH.,N<~
CA 02274132 1999-10-21
16
~CON ~H2CH2NH-~
-CO
-EON q CON rHZCHzNH-~
CON CH2CH2N< ~
-EONH
CON GH2CHZN<~
CON CH2CH2NH-~
-COCHZCH2CONH
CON CH2CH2NH-~
CON CH2CH2N< ~
fOCH2CH2CONH
CON GHzCH2N<~
CA 02274132 1999-10-21
17
OCH2CH2NH- OCH2CH2N
-C0 c -Ep
OCH2CH2NH- OCH2CHZN<
;
OCH2CH2NH- OCE=ZCH2N<
-Co OCH=CH2NH- .CO ( OCH2CH2N<
OCH~CHZNH. OCHCHZN<
0 CHZCH-'0)~CH2CH,NH- 0 CH2C1'20)zCH2CH2N<
-C0 0 CH,CH_0 ~CH2CH:NH- -e0 0 CH'CH2O ~CH2CH_N<
0 CH.CH.OhCHZCHzNH- 0 CH2CH2O)2CH2CH2N<
In the case of a macrocycle, complexing agent radical K is
described by general formula IA:
CA 02274132 1999-10-21
18
1 2 3
R OOC-R HC R
N -CH2 -CH2 -N
I I
CHZ CH2
I I
CH2 CHZ
I I
N-CH2--CHZ - N
CHR2-CO ORl CHR2-CO OR1 (IA
))
in which
R', independently of one another, stand for a hydrogen atom
or a metal ion equivalent of atomic numbers 20-29, 39,
42-44 or 57-83,
R2 stands for a hydrogen atom, a methyl or an ethyl
radical which optionally is substituted with 1-2
hydroxy or 1 carboxy group(s),
R3 stands for a
R4 R2
i 1
-CH -CO -N -Ug-T-i group,
R4 stands for a straight-chain, branched, saturated or
unsaturated CI-C30 alkyl chain, which optionally is
interrupted by 1-10 oxygen atoms, 1 phenylene group, 1
phenylenoxy group and/or optionally is substituted by
1-5 hydroxy, 1-3 carboxy, 1-phenyl group(s),
CA 02274132 1999-10-21
19
tJ6 stands for a straight-chain, branched, saturated or
unsaturated CI-C20 alkylene group optionally containing
1-5 imino, 1-3 phenylene, 1-3 phenylenoxy, 1-3
phenylenimino, 1-5 amide, 1-2 hydrazide, 1-5 carbonyl,
1-5 ethylenoxy, 1 urea, 1 thiourea, 1-2
carboxyalkylimino, 1-2 ester groups; 1-10 oxygen, 1-5
sulfur and/or 1-5 nitrogen atom(s) and/or optionally
substituted by 1-5 hydroxy, 1-2 mercapto, 1-5 oxo, 1-5
thioxo, 1-3 carboxy, 1-5 carboxyalkyl, 1-5 ester and/or
1-3 amino group(s), whereby the phenylene groups that
optionally can be contained can be substituted by 1-2
carboxy, 1-2 sulfo or 1-2 hydroxy groups,
T stands for a -CO-a, -NHCO-a or -NHCS-a group, and
a stands for the binding site to the terminal nitrogen
atoms of the last generation, of reproduction W.
As preferred complexing agent radicals K, those can be
mentioned in which in above-indicated formula IA, the Cl-CZO1 and
preferably C1-C,Z alkylene chain that stands for U6 contains the
groups
-CH2, -CH2NHCO, -NHCOCH2O1 -NHCOCH2OC6H4, -N(CHZCOZH) ,
-NHCOCHZC6H4, -NHCSNHC6H4, -CH20C6H4, -CH2CH2O and/or is
substituted by groups -COOH, -CH2COOH.
As examples of i16, the following groups can be cited:
CA 02274132 1999-10-21
20
-CH2-, -CH2CHZ-, -CH2CH2CH2-, -C6Ha-, -C6Hlo=, -CH2C6H5-,
-CHZNHCOCHZCH(CHZCOZM-C6H4-,
-CH2NHCOCH2OCH2-,
-CH2NHCOCHZC6H4-,
-CH2NHCOCH2p___.
COzH
-CH2NHC SNH-C6H4-CH(CHZCOOH)CH2-,
-CH,OCsH4-N(CHZCOOH)CHZ-,
-CHZNHC OCHZ 0 (CHz CHZ 0)4-C5H4-,
-CHZO-C6H4-,
-CH2CHZ-O-CHZCH2-, -CHZCHZ-0-CH2CHZ-0-CH2CH2-,
Q " . ~ ~
~
S03H COZH
As examples of R4, the following groups can be indicated:
-CH3, -C6H51 -CHZ-COOH,
-CHZ-C6H5, -CHZ-O- (CH2CH2-O-) 6CH3, -CH2-OH.
If the agent is intended for use in NMR diagnosis, the
central ion of the complex salt must be paramagnetic. These are
especially the divalent and trivalent ions of the elements of
atomic numbers 21-29, 42, 44, and 58-70. Suitable ions are, for
example, the chromium(III), iron(II), cobalt(II), nickel(II),
copper(II), praseodymium(III), neodymium(III), samarium(III), and
CA 02274132 1999-10-21
21
ytterbium(III) ions. Because of their very strong magnetic
moment, the gadolinium(III), terbium(III), dysprosium(III),
holmium(III), erbium(III), manganese(II), and iron(III) ions are
especially preferred.
If the described agent is intended for use in diagnostic
radiology, the central ion has to be derived from an element of
higher atomic number in order to achieve sufficient absorption of
the x rays. It has been found that for this purpose, diagnostic
agents which contain a physiologically compatible complex salt
with central ions of elements of atomic numbers of between 21-29,
39, 42, 44 and 57-83 are suitable; these are, for example, the
lanthanum(III) ion and the above-mentioned ions of the lanthanide
series.
The cascade polymer complexes contain at least 16 ions of an
element of the above-mentioned atomic numbers.
The remaining acid hydrogen atoms, i.e., those which were
not substituted by the central ion, optionally can be replaced
completely or partially by cations of inorganic and/or organic
bases, amino acids, or amino acid amides.
Suitable inorganic cations are, for example, the lithium
ion, the potassium ion, the calcium ion, the magnesium ion, and
especially the sodium ion. Suitable cations of organic bases
are, i.a., those of primary, secondary, or tertiary amines, such
as, for example, ethanolamine, diethanolamine, morpholine,
glucamine, N,N-dimethylglucamine, and especially N-
methylglucamine. Suitable cations of amino acids are, for
CA 02274132 1999-10-21
22
example, those of lysine, arginine, and ornithine, as well as the
amides of otherwise acidic or neutral amino acids.
The compounds which have a molecular weight of 10,000-80,000
D, preferably 15,000-40,000 D, exhibit the desired properties
described above. They contain the large number, required for
their use, of metal ions bonded in a stable manner in the
complex. They accumulate in regions with high vascular
permeability, such as, e.g., in tumors, they make it possible to
make statements regarding the perfusion of tissues, and they
provide the possibility of determining the blood volume in
tissues, of shortening selectively the relaxation times or
densities of the blood, and of graphically representing the
permeability of blood vessels. Such physiological data cannot be
obtained through the use of extracellular contrast media, such
as, e.g., Gd-DTPA [Magnevist(R) ]. From these standpoints, there
also follow the uses in the modern imaging processes of nuclear
spin tomography and computer tomography: more specific diagnoses
of malignant tumors, early therapy monitoring in cases where
cytostatic, antiphlogistic, or vasodilative therapy is used,
early identification of underperfused regions (e.g., in the
myocardium), angiography in vascular diseases, and identification
and diagnosis of (sterile or infectious) inflammations.
The described cascade polymer complexes are also extremely
well suited for (interstitial and i.v.) lymphography.
As further advantages relative to extracellular contrast
media, such as, e.g., Gd-DTPA [Magnevist(R)], the greater
effectiveness as contrast media for nuclear spin tomography
CA 02274132 1999-10-21
23
(higher relaxivity) must be emphasized; this ensures a marked
reduction of the diagnostically required dose. At the same time,
the described contrast media can be formulated as solutions in an
isoosmolar manner in the blood and thus reduce the osmotic stress
of the body, which is reflected in a reduced toxicity on the part
of the substance (higher toxic threshold). Smaller doses and
higher toxic thresholds result in a significant increase of the
reliability of contrast medium uses in modern imaging processes.
In comparison with macromolecular contrast media based on
carbohydrates, e.g., dextran (European Patent Application,
Publication No. 0 326 226), which carry -- as mentioned --
generally only about 5% of the signal-enhancing paramagnetic
cation, the polymer complexes exhibit a content of the
paramagnetic cation of generally about 20%. Thus, the described
macromolecules produce much better signal enhancement per
molecule, which simultaneously has the effect that the dose
necessary for nuclear spin tomography is considerably smaller
relative to macromolecular contrast media based on carbohydrates.
These polymer complexes are large enough to be able to leave
the vascular space only slowly, but at the same time small enough
to be able to pass through the capillaries of the kidneys, which
are 300-800 A in size.
In comparison to the other mentioned polymer compounds of
the prior art, the described cascade polymer complexes are
distinguished by improved excretion behavior, greater
effectiveness, greater stability, and/or better compatibility.
CA 02274132 1999-10-21
24
The production of the macrocyclic cascade polymer complexes
is carried out in that compounds of general formula I'
A-{X-[Y-(Z-(W-B~ =)y)X}a (I' ) ,
in which
A stands for a nitrogen-containing cascade nucleus of
base multiplicity a; X and Y, independently of one
another, stand for a direct bond or a cascade
reproduction unit of reproduction multiplicity x or y,
Z and W, independently of one another, stand for a cascade
reproduction unit of reproduction multiplicity z or w,
a stands for numbers 2 to 12,
x, y, z and w, independently of one another, stand for
numbers 1 to 4 and
8 stands for the binding site of the terminal NH groups
of the last generation, of reproduction unit W
provided that at least two reproduction units are different,
and that for the product of multiplicities,
16 < a * x* y* z= w<_ 64,
holds true,
are reacted with a complex or complexing agent K' of general
CA 02274132 1999-10-21
25
formula I'A
3.
.
R~ OOC-R=HC R
N --C H , - CH -- 2 N
I 1
CH~ CH,
I ~ CH, CH,
I - I _
N--CHz--CHZ-N (I
12 1,
CHR -CO OR CHRZ-CO ORls
whereby
RIindependently of one another, stand for a hydrogen
atom, a metal ion equivalent of atomic numbers 20-29,
39, 42-44, or 57-83 or an acid protective group,
R2 stands for a hydrogen atom, a methyl or an ethyl
radical which optionally is substituted with 1-2
hydroxy or 1 carboxy group(s),
R3' stands for a
R4 Rz
-CH-CO-N -U6--T- group,
R4 stands for a straight-chain, branched, saturated or
unsaturated Cl-C30 alkyl chain, which optionally is
interrupted by 1-10 oxygen atoms, 1 phenylene group, 1
CA 02274132 1999-10-21
26
phenylenoxy group and/or optionally substituted by 1-5
hydroxy, 1-3 carboxy, 1-phenyl group(s),
U6 stands for a straight-chain, branched, saturated or
unsaturated C1-C20 alkylene group optionally containing
1-5 imino, 1-3 phenylene, 1-3 phenylenoxy, 1-3
phenylenimino, 1-5 amide, 1-2 hydrazide, 1-5 carbonyl,
1-5 ethylenoxy, 1 urea, 1 thiourea, 1-2
carboxyalkylimino, 1-2 ester groups; 1-10 oxygen, 1-5
sulfur and/or 1-5 nitrogen atom(s) and/or optionally is
substituted by 1-5 hydroxy, 1-2 mercapto, 1-5 oxo, 1-5
thioxo, 1-3 carboxy, 1-5 carboxyalkyl, 1-5 ester and/or
1-3 amino group(s), whereby the phenylene groups that
are optionally contained can be substituted by 1-2
carboxy, 1-2 sulfo or 1-2 hydroxy groups,
T' stands for a -C*O, -COOH, -N=C=O or -N=C=S group, and
C*O stands for an activated carboxyl group,
provided that -- if K' stands for a complex -- at least two
(in the case of divalent metals) or three (in the case of
trivalent metals) of substituents R' stand for a metal ion
equivalent of the above-mentioned elements and that
optionally other carboxyl groups are present in the form of
their salts with inorganic and/or organic bases, amino acids
or amino acid amides,
optionally present protective groups are cleaved, the cascade
polymers that are thus obtained -- if K' stands for a complexing
agent -- are reacted in a way known in the art with at least one
metal oxide or metal salt of an element of atomic numbers 20-29,
CA 02274132 1999-10-21
27
39, 42, 44, or 57-83 and then optionally in the cascade polymer
complexes that are thus obtained, acid hydrogen atoms that are
still present are completely or partially substituted by cations
of inorganic and/or organic bases, amino acids, or amino acid
amides, and optionally still present free terminal amino groups
are optionally acylated -- before or after the metal complexing.
The reaction with complexing agents of general formula I'A,
where Rl' = t-butyl, is disclosed.
The production of the complexes and complexing agents of
general formula I'A is carried out according to or analogously to
the instructions described in the experimental part or according
to methods known in the literature (see, e.g., European Patent
Applications Nos. 0 512 661, 0 430 863, 0 255 471 and 0 565 930.
Thus, the production of compounds of general formula I'A can
be carried out, e.g., in that a group T" is used as a precursor
of functional group T', either in the meaning of a protected acid
function, which can be converted to the free acid function
independently of acid protective groups R" according to the
above-indicated process, or in the meaning of a protected amine
function, which unblocks according to processes known in the
literature [Th. W. Greene, P. G. M. Wuts, Protective Groups in
Organic Synthesis, 2nd Edition, John Wiley & Sons (1991), pp.
309-385) and then can be converted into the isocyanates or
isothiocyanates (Methoden der Org. Chemie (Methods of Organic
Chemistry] (Houben-Weyl), E 4, pp. 742-749, 837-843, Georg Thieme
Verlag, Stuttgart, New York (1983)]. Such compounds can be
produced according to or analogously to the instructions that are
CA 02274132 1999-10-21
28
described in the experimental part by monoalkylation of cyclene
with suitable a-halogenated acid amides [in aprotic solvents,
such as, e.g., chloroform].
For further details on coupling reactions, starting
substances, introduction of the desired metal ions, production
and administration of pharmaceutical agents, etc., please refer
to WO 96/01655, especially pages 22 to 33.
CA 02274132 1999-10-21
CA 02274132 2006-11-07
28a
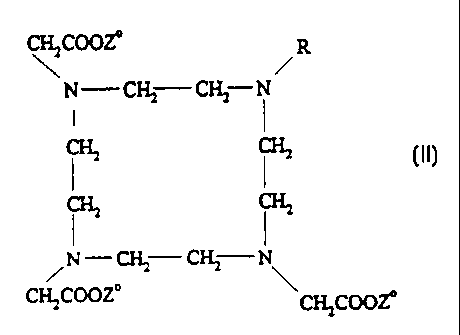
This invention relates to new macrocyclic metal complex
carboxylic acids of formula II
CH.,COOZ
R
~N_~ CH2 N
I I
{II},
CIL
/N-CH,-Cfi: N
C.HCOOZ
~Q3.,COOZ
whereby
Z stands for a metal ion equivalent of atomic numbers 58-
71 and
R stands for a CHX'-CO-NH-CHY'-(CH2)f-COOH group, in which
Xl and Y', independently of one another, mean a
straight-chain or branched C1-C7 alkyl radical, a
phenyl or benzyl group and Y' in addition means a
hydrogen atom and f means numbers 0 to 9,
which can be used as intermediate products for the synthesis of
cascade polymer complexes of general formula I of WO 97/02051.
None of the syntheses of cascade polymer complexes that are
described in EP-A-430863, DE-A-19549286, DE-A-19525924 and DE-A-
4344460 uses the metal complex carboxylic acids of Formula II
according to the invention.
CA 02274132 2006-11-07
28b
For radical XI or YI, methyl, ethyl, propyl, butyl or
hydrogen, methyl, isopropyl, phenyl and benzyl can be mentioned
by way of.example. Methyl or hydrogen is preferred.
Index f preferably stands for numbers 0, 1 or 2.
Of the above-mentioned lanthanides, gadolinium, dysprosium
and ytterbium are preferred.
The reaction of the new macrocyclic metal complex carboxylic
acids of formula II to the desired cascade polymer complexes is
carried out analogously to methods that are known in the
literature, e.g., B. Belleau, G. Malek, J. Amer. Chem. Soc. 90,
1651 (1968).
29
The products that are thus obtained exhibit less
by-product dispersion than the cascade polymer complexes that are
synthesized without using the metal complex carboxylic acids of
formula II according to the invention.
The synthesis of the compounds of general formula II
according to the invention is carried out in that compounds of
general formula III
CH,COOZ' R,
N CH2 CH= i
ct; i CHI
:
H
C 2
/N-CFiz CI;- N
CHCOOZ' \ CH.COOZ'
CA 02274132 1999-10-21
30
in which
R' has the meaning of R, whereby the carboxyl group
contained therein is optionally present in protected
form and
Zl stands for a hydrogen atom or a carboxyl protective
group, after cleavage of the optionally present
carboxyl protective groups, are reacted in a way known
in the art with a metal oxide or metal salt of an
element of atomic numbers 58-71.
The introduction of the desired metal ions is carried out in
the way in which it was disclosed in, e.g., Patents EP 71564, EP
130934 and DE-3401052, by the metal oxide or a metal salt (for
example, the nitrate, acetate, carbonate, chloride or sulfate) of
the element of atomic numbers 58-71 being dissolved or suspended
in water and/or a lower alcohol (such as methanol, ethanol or
isopropanol) and being reacted with a solution or suspension of
the equivalent amount of complexing agent of general formula III.
If Z' stands for an acid protective group, e.g., straight-
chain or branched C1-C6 alkyl, aryl and aralkyl groups, for
example, the methyl, ethyl, propyl, butyl, phenyl, benzyl,
diphenylmethyl, triphenylmethyl, bis(p-nitrophenyl)-methyl
groups, as well as trialkylsilyl groups, are suitable. The t-
butyl group is preferred.
The cleavage of the protective groups is carried out
according to the processes known to one skilled in the art, by,
for example, hydrolysis, hydrogenolysis, alkaline saponification
of esters with alkali in aqueous-alcoholic solution at
CA 02274132 1999-10-21
31
temperatures of 0 C to 50 C, acid saponification with mineral
acids or in the case of, e.g., tert-butyl esters with the aid of
trifluoroacetic acid. [Protective Groups in Organic Synthesis,
2nd Edition, T. W. Greene and P. G. M. Wuts, John Wiley and Sons,
Inc., New York, 1991].
Compounds of general formula III can be obtained by reaction
of a-halocarboxylic acid esters or a-haloacids of general formula
IV
Hal-CHZ-COZZi (IV),
in which
Zi has the above-mentioned meaning and Hal stands for
chlorine, bromine or iodine,
with compounds of general formula V
N~ Xi Y
(C~)r -COORS
H N N ~ M,
N 0
H
in which
R5 stands for a hydrogen atom, or an acid protective group
and
X1, Y' and f have the above-mentioned meaning.
If Z' and R5 in each case stand for an acid protective
group, the latter can have varying meanings, so that Z' (e.g.,
benzyl) can be cleaved optionally selectively (e.g., by
CA 02274132 1999-10-21
32
hydrolysis) in the presence of R5 protective groups (e.g., t-
buty l ) .
If Z' stands for an acid protective group, the reaction is
carried out preferably in solvents such as methylene chloride,
dimethylformamide, acetonitrile, tetrahydrofuran, dioxane,
chloroform, lower alcohols such as methanol, ethanol and
isopropanol, as well as mixtures of the above-mentioned solvents
with water.
When a haloacid is used as an educt, water is the preferred
working medium.
As acid traps, organic bases such as pyridine, triethylamine
or diisopropylethylamine or inorganic bases such as sodium
hydroxide, lithium hydroxide, potassium hydroxide, calcium
hydroxide or sodium carbonate, potassium carbonate, sodium
bicarbonate or lithium carbonate are used. Alkylation is
performed at temperatures of between 0 - 100 C, but preferably at
20 - 80 C.
Compounds of general formula V are obtained by reaction of
cyclene (formula VI)
H
N
HN NH (VI),
L~J
with compounds of general formula VII
X' Y1
Nu~~~ COORS
O
CA 02274132 1999-10-21
33
in which
Xl, Y', R5 and f have the above-mentioned meaning and Nu
stands for a nucleofuge. As nucleofuges, chloride, bromide,
iodide, mesylate, tosylate or triflate can be mentioned.
The reaction is carried out in solvents, such as chloroform,
methylene chloride, tetrahydrofuran, dioxane, dimethylformamide,
dimethyl sulfoxide or else in water at temperatures of 0 C to
100 C, but preferably at 20 - 60 . If desired, an organic or
inorganic base can be added. Triethylamine, pyridine, sodium
carbonate, sodium hydroxide or potassium hydroxide can be
mentioned by way of example.
Compounds of general formula VII are obtained by reaction of
compounds of general formula VIII
x'
Nu 'J~1' COOH
in which
Nu and Xl have the above-mentioned meaning, with compounds
of general formula IX
Y
111'~ (CFi2)r - CO R3
2
~
in which
Y1, f and R5 have the above-indicated meaning.
The reaction is carried out according to peptide-chemistry
methods that are known to one skilled in the art. Thus, for
example, a derivative, such as, e.g., an acid chloride, acid
CA 02274132 1999-10-21
34
bromide or active ester (such as, e.g., NHS-ester) can be
produced, for example, from the acid of general formula VIII,
whereby said derivative is condensed with an amino acid
(optionally terminally-protected).
Compounds of general formula VIII, as well as their acid
chlorides and acid bromides are commercially available.
Compounds of general formula IX are also commercially available
as free amino acids or in protected form.
As an alternative, compounds of general formula III can be
obtained by reaction of compounds of general formula X
rC02Z'
~N N--H
C02Z' ~N J (X)~
CO=Z'
in which Z' has the above-mentioned meaning, with compounds of
general formula VII, after cleavage of the optionally present
acid protective groups.
The reaction is carried out in solvents, such as, for
example, acetonitrile, dimethylformamide, tetrahydrofuran,
dioxane or lower alcohols such as methanol, ethanol or i-propanol
as well as mixtures of the latter with water; but the reaction
can also be performed in pure water. The work is generally done
at temperatures of 20 C - 100 C.
As acid traps, organic or inorganic bases are used.
Triethylamine, pyridine, 4-dimethylaminopyridine, sodium
CA 02274132 1999-10-21
35
hydroxide, potassium hydroxide, potassium carbonate and sodium
carbonate can be mentioned by way of example. Metal hydrides
such as sodium hydride and calcium hydride can also be used, but
only in the case of aprotic solvents.
The addition of a catalytic amount of an iodide has proven
advantageous. Sodium iodide, potassium iodide, lithium iodide or
tetrabutylammonium iodide can be mentioned by way of example.
The purification of the metal complexes of general formula
II according to the invention is carried out by, for example,
chromatography on silica gel or RP-18.
Most of the metal complexes of general formula II according
to the invention can be crystallized from alcohols such as
methanol, ethanol or isopropanol or else from their mixtures with
water.
It has also proven advantageous to dissolve the metal
complexes according to the invention in alcohols or mixtures of
alcohols with water and to precipitate them by adding acetone in
drops.
The drying of metal complex carboxylic acids according to
the invention takes place advantageously in a vacuum at
temperatures of 20 - 200 C, preferably 50 - 130 C, within about
6 hours to 3 days.
The metal complex carboxylic acids of general formula II
that are thus obtained are stored in a moisture-free environment
and can be used directly in a coupling reaction.
Overall, it has been possible with the metal complex
carboxylic acids of general formula II according to the invention
CA 02274132 1999-10-21
CA 02274132 2006-08-04
36
to make available important intermediate products, which make it
possible to synthesize cascade polymer complexes with a small
portion of by-products.
Examples 1 to 3 below are used to explain the synthesis of
polymer complexes by means of coupling macrocyclic ligands, as
described in WO 97/02051.
37
WO 98/24774 PCT/EP97/06593
Example 1
a) Bis[2-(benzyloxycarbonylamino)-ethyl]-amine
51.5 g (500 mmol) of diethylenetriamine and 139 ml (1 mol)
of triethylamine are dissolved in dichloromethane and mixed at
-20 C with 161 g of benzyl cyanoformate (Fluka) in
dichloromethane and then stirred overnight at room temperature.
After the reaction is completed, concentration by evaporation is
performed during draw-off, the residue is taken up in diethyl
ether, the organic phase is washed with sodium carbonate solution
and dried with sodium sulfate. The filtrate is mixed with
hexane, the precipitate is filtered off and dried.
Yield: 163.4 g (88% of theory)
Elementary analysis:
Cld: C 64.67 H 6.78 N 11.31
Fnd: C 64.58 H 6.83 N 11.28
b) N,N,N',N',N",N"-Hexakis[2-(benzyloxycarbonylamino)-ethyl]-
trimesic acid triamide
13.27 g (50 mmol) of trimesic acid trichloride (Aldrich) and
34.7 ml (250 mmol) of triethylamine are dissolved in
dimethylformamide (DMF) and mixed at 0 C with 65.0 g (175 mmol)
of the amine described in Example la) and then stirred overnight
at room temperature. The solution is concentrated by evaporation
CA 02274132 1999-10-21
38
in a vacuum, and the residue is chromatographed on silica gel
with ethyl acetate.
Yield: 39.4 g (62% of theory)
Elementary analysis:
Cld: C 65.24 H 5.95 N 9.92
Fnd: C 65.54 H 5.95 N 9.87
c) Ne,N~-Bis(N,N'-dibenzyloxycarbonyl-lysyl)-lysine, protected
"tri-lysine"
3.6 g (20 mmol) of lysine-hydrochloride and 6.95 ml (50
mmol) of triethylamine are dissolved in DMF, mixed with 26.8 g
(50 mmol) of Ne,N~-dibenzyloxycarbonyl-lysine-p-nitrophenylester
(Bachem) and stirred for 2 days at room temperature. After the
reaction is completed, it is concentrated by evaporation in a
vacuum, the residue is taken up in ethyl acetate and shaken out
with dilute hydrochloric acid. The organic phase is dried with
sodium sulfate, the solvent is concentrated by evaporation, and
the residue is chromatographed with ethyl acetate/ethanol in a
step gradient.
Yield: 10.7 g(57$ of theory)
Elementary analysis:
Cld: C 63.95 H 6.65 N 8.95
Fnd: C 63.63 H 6.69 N 8.93
CA 02274132 1999-10-21
39
d) Completely protected benzyloxycarbonyl-24mer-polyamine based
on N,N,N',N',N",N"-hexakis[2-(trilysyl-amino)-ethyl]-
trimesic acid triamide
1.27 g (1 mmol) of the hexa-benzyloxycarbonylamine described
in Example lb) is dissolved in glacial acetic acid and mixed with
33% hydrogen bromide in glacial acetic acid while being stirred.
After 60 minutes, the incipient precipitation is completed with
diethyl ether, the hexaamine-hydrobromide produced is washed with
ether, dried in a vacuum and used in the subsequent reaction
described below without further purification.
Yield: 0.95 g (quantitative)
7.0 g (7.5 mmol) of the protected "tri-lysine" described in
Example ic), 1.2 g (7.5 mmol) of 1-hydroxybenzotriazole and 2.4 g
(7.5 mmol) of 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium
tetrafluoroborate (TBTU; Peboc Limited, UK) are dissolved in DMF
and stirred for 15 minutes. This solution is then mixed with
5.16 ml (30 mmol) of N-ethyldiisopropylamine and with 0.95 g(1
mmol) of the hexaamine-hydrobromide described above, and it is
stirred overnight at room temperature. After the reaction is
completed, it is concentrated by evaporation in a vacuum, and the
residue is chromatographed on silica gel with ethyl
acetate/ethanol (2:1).
Yield: 4.55 g(76$ of theory)
CA 02274132 1999-10-21
40
Elementary analysis:
Cld: C 64.35 H 6.71 N 10.52
Fnd: C 64.08 H 6.57 N 10.29
e) N-(2-Bromopropionyl)glycine-benzyl ester
55.9 g (326.1 mmol) of 2-bromopropionic acid chloride is
added in drops at 0 C to 100 g (296.4 mmol) of glycine benzyl
ester-p-toluenesulfonic acid salt and 33.0 g (326.1 mmol) of
triethylamine in 400 ml of methylene chloride. The temperature
is not allowed to exceed 5 C. After addition is completed, it is
stirred for one hour at 0 C, then for 2 hours at room
temperature. 500 ml of ice water is added, and the water phase
is set at pH 2 with 10t aqueous hydrochloric acid. The organic
phase is separated, washed once each with 300 ml of 5% aqueous
soda solution and 400 ml of water. The organic phase is dried on
magnesium sulfate and evaporated to the dry state in a vacuum.
The residue is recrystallized from diisopropyl ether.
Yield: 68.51 g (75% of theory) of a colorless, crystalline
powder
Melting point: 69 - 70 C
Elementary analysis:
Cld: C 48.02 H 4.70 N 4.67 Br 26.62
Fnd: C 47.91 H 4.82 N 4.51 Br 26.47
CA 02274132 1999-10-21
41
f) 1-[4-(Benzyloxycarbonyl)-1-methyl-2-oxo-3-azabutyl]-
1,4,7,10-tetraazacyclododecane
50 g (162.2 mmol) of the title compound of Example le) is
added to 55.8 g (324.4 mmol) of 1,4,7,10-tetraazacyclododecane,
dissolved in 600 ml of chloroform, and it is stirred overnight at
room temperature. 500 ml of water is added, the organic phase is
separated and in each case washed twice with 400 ml of water.
The organic phase is dried on magnesium sulfate and evaporated to
the dry state in a vacuum. The residue is chromatographed on
silica gel (mobile solvent: chloroform/methanol/aqueous 25%
ammonia = 10/5/1).
Yield: 40.0 g(63% of theory relative to the le) used] of a
slightly yellowish viscous oil.
Elementary analysis:
Cld: C 61.36 H 8.50 N 17.89
Fnd: C 61.54 H 8.68 N 17.68
g) 10-[4-(Benzyloxycarbonyl)-1-methyl-2-oxo-3-azabutyl]-1,4,7-
tris(tert-butoxycarbonylmethyl)-1,4,7,10-
tetraazacyclododecane (sodium bromide complex)
33 g (169 mmol) of bromoacetic acid-tert-butyl ester is
added to 20 g (51.08 mmol) of the title compound of Example if)
and 17.91 (169 mmol) of sodium carbonate in 300 ml of
acetonitrile, and it is stirred for 24 hours at 60 C. It is
cooled to 0 C, the salts are filtered out, and the filtrate is
evaporated to the dry state. The residue is chromatographed on
CA 02274132 1999-10-21
42
silica gel (mobile solvent: ethyl acetate/ethanol: 15/1). The
fractions that contain the product are concentrated by
evaporation, and the residue is recrystallized from diisopropyl
ether.
Yield: 34.62 g (81% of theory) of a colorless, crystalline
powder
Melting point: 116-117 C
Elementary analysis:
Cid: C 54.54 H 7.59 N 8.37 Na 2.74 Br 9.56
Fnd: C 54.70 H 7.65 N 8.24 Na 2.60 Br 9.37
h) 10-(4-Carboxy-l-methyl-2-oxo-3-azabutyl)-1,4,7-tris(tert-
butoxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane (sodium
bromide complex)
30 g (35.85 mmol) of the title compound of Example lg) is
dissolved in 500 ml of isopropanol, and 3 g of palladium catalyst
(10% Pd/C) is added. It is hydrogenated overnight at room
temperature. Catalyst is filtered out, the filtrate is
evaporated to the dry state in a vacuum and recrystallized from
acetone.
Yield: 22.75 g (85% of theory) of a colorless, crystalline
powder
Melting point: 225 C (decomposition)
CA 02274132 1999-10-21
43
Elementary analysis:
Cld: C 49.86 H 7.69 N 9.38 Na 3.07 Br 10.71
Fnd: C 49.75 H 7.81 N 9.25 Na 2.94 Br 10.58
i) 24-mer N-(5-DO3A-yl-4-oxo-3-azahexanoyl)-cascade polyamide
based on N,N,N',N',N",N"-hexakis[2-(trilysylamino)-ethyl]-
trimesic acid triamide*)
6.0 g(1 mmol) of the poly-benzyloxycarbonylamine described
in Example id) is dissolved in glacial acetic acid and mixed with
33% hydrogen bromide in glacial acetic acid while being stirred.
After 3 hours, the incipient precipitation is completed with
diethyl ether, the 24-amine-hydrobromide produced is washed with
ether and dried in a vacuum.
35.84 g (48 mmol) of the acid described in Example lh) above
is dissolved in DMF, mixed with 7.35 g (48 mmol) of 1-
hydroxybenzotriazole, with 15.41 g (48 mmol) of TBTU (Peboc
Limited, UK) and with 49.3 ml (288 mmol) of N-
ethyldiisopropylamine, and it is stirred for 20 minutes at room
temperature. This solution is then mixed with the (1 mmol) 24-
amine-hydrobromide described above, and it is stirred for 4 days
at room temperature. The solution is concentrated by evaporation
in a vacuum, the remaining oil is cooled in an ice bath and mixed
with trifluoroacetic acid, stirred overnight at room temperature
DO3A = 1,4,7-tris(carboxymethyl)-1,4,7,10-
tetraazacyclododecane
CA 02274132 1999-10-21
44
and then precipitated with diethyl ether. The precipitate is
dried in a vacuum, taken up in water, set at pH 7, a
YM3 Amicon(R)-ultrafiltration membrane is used to remove low-
molecular portions, and the retentate is ultimately membrane-
filtered and freeze-dried.
Yield: 13.5 g (83% of theory)
H20 content (Karl-Fischer): 6.2%
Elementary analysis (relative to anhydrous substance):
Cld: C 45.82 H 6.09 N 15.07 Na 10.79
Fnd: C 45.56 H 6.15 N 14.80 Na 10.52
k) 24-mer-Gd-complex of N-(5-DO3A-yl-4-oxo-3-azahexanoyl)-
cascade polyamide based on N,N,N',N',N",N"-hexakis[2-
(trilysylamino)-ethyl]-trimesic acid triamide
8.13 g (0.5 mmol) of the complexing agent acid described in
Example ii) above is set at pH 3 in water with dilute
hydrochloric acid, mixed with 2.17 g (6 mmol) of Gd2031 stirred
for 30 minutes at 80 C, set at pH 7 after cooling and desalinated
with a YM3 AMICON(R) ultrafiltration membrane. The retentate is
ultimately membrane-filtered and freeze-dried.
Yield: 8.89 g(92.1$ of theory)
H20 content (Karl-Fischer): 9.6%
Gd determination (AAS): 19.6%
CA 02274132 1999-10-21
45
Elementary analysis (relative to anhydrous substance):
Cld: C 40.26 H 5.35 N 13.24 Gd 21.62
Fnd: C 39.98 H 5.51 N 13.42 Gd 21.37
Example 2
a) 2-Bromopropionyl-B-alanine-benzyl ester
53.65 g (313 mmol) of 2-bromopropionic acid chloride is
added in drops at 0 C to 100 g (285 mmol) of B-alanine-benzyl
ester-p-toluenesulfonic acid salt and 31.67 g (313 mmol) of
triethylamine in 400 ml of methylene chloride. The temperature
is not allowed to exceed 5 C. After addition is completed, it is
stirred for 1 hour at 0 C, then for 2 hours at room temperature.
500 ml of ice water is added, and the water phase is set at pH 2
with 10% aqueous hydrochloric acid. The organic phase is
separated, washed once each with 300 ml of 5% aqueous
hydrochloric acid, 300 ml of 5% aqueous soda solution and 400 ml
of water. The organic phase is dried on magnesium sulfate and
evaporated to the dry state in a vacuum. The residue is
recrystallized from diisopropyl ether.
Yield: 71.36 g (78% of theory) of a colorless, crystalline
powder
Elementary analysis:
Cld: C 48.46 H 7.51 N 4.35 Br 24.80
Fnd: C 48.29 H 7.65 N 4.25 Br 24.61
CA 02274132 1999-10-21
46
b) 1-[5-(Benzyloxycarbonyl)-1-methyl-2-oxo-3-azapentyl]-
1,4,7,10-tetraazacyclododecane
50 g (155.2 mmol) of the title compound of Example 2a) is
added to 53.32 g (310 mmol) of 1,4,7,10-tetraazacyclododecane
dissolved in 600 ml of chloroform, and it is stirred overnight at
room temperature. 500 ml of water is added, the organic phase is
separated, and it is washed twice in each case with 400 ml of
water. The organic phase is dried on magnesium sulfate and
evaporated to the dry state in a vacuum. The residue is
chromatographed on silica gel (mobile solvent:
chloroform/methanol/aqueous 25% ammonia: 10/5/1).
Yield: 38.39 g(61% of theory relative to the 2a) used] of
a light yellowish viscous oil.
Elementary analysis:
Cld: C 62.20 H 8.70 N 17.27
Fnd: C 62.05 H 8.81 N 17.15
C) 10-[5-(Benzyloxycarbonyl)-1-methyl-2-oxo-3-azapentyl]-1,4,7-
tris(tert-butoxycarbonylmethyl)-1,4,7,10-
tetraazacyclododecane (sodium bromide complex)
31.8 g (163 mmol) of bromoacetic acid-tert-butyl ester is
added to 20 g (49.32 mmol) of the title compound of Example 2b)
and 17.28 g (163 mmol) of sodium carbonate in 300 ml of
acetonitrile, and it is stirred for 24 hours at 60 C. It is
cooled to 0 C, salts are filtered out, and the filtrate is
evaporated to the dry state. The residue is chromatographed on
CA 02274132 1999-10-21
47
silica gel (mobile solvent: ethyl acetate/ethanol = 10/1). The
fractions that contain the product are concentrated by
evaporation, and the residue is recrystallized from diisopropyl
ether.
Yield: 31.89 g(76$ of theory) of a colorless, crystalline
powder
Elementary analysis:
Cld: C 55.05 H 7.70 N 8.23 Na 2.69 Br 9.40
Fnd: C 55.17 H 7.85 N 8.10 Na 2.51 Br 9.30
d) 10-[5-(Carboxy)-1-methyl-2-oxo-3-azapentyl]-1,4,7-tris(tert-
butoxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane (sodium
bromide complex)
30 g (35.26 mmol) of the title compound of Example 2c) is
dissolved in 500 ml of isopropanol, and 3 g of palladium catalyst
(10% Pd/C) is added. It is hydrogenated overnight at room
temperature. Catalyst is filtered out, the filtrate is
evaporated to the dry state in a vacuum and recrystallized from
acetone.
Yield: 24.41 g (91% of theory) of a colorless, crystalline
powder
Elementary analysis:
Cld: C 50.52 H 7.82 N 9.21 Na 3.01 Br 10.52
Fnd: C 50.41 H 7.95 N 9.10 Na 2.91 Br 10.37
CA 02274132 1999-10-21
48
e) 24-mer N-(6-DO3A-yl-5-oxo-4-azaheptanoyl)-cascade polyamide
based on N,N,N',N',N",N"-hexakis[2-(trilysylamino)-ethyl)-
trimesic acid triamide
6.0 g(1 mmol) of the poly-benzyloxycarbonylamine described
in Example id) is dissolved in glacial acetic acid and mixed with
33% hydrogen bromide in glacial acetic acid while being stirred.
After 3 hours, the incipient precipitation is completed with
diethyl ether, the 24-amine-hydrobromide produced is washed with
ether and dried in a vacuum.
36.52 g (48 mmol) of the acid described in Example 2d) above
is dissolved in DMF, mixed with 7.35 g (48 mmol) of 1-
hydroxybenzotriazole, with 15.41 g (48 mmol) of TBTU (Peboc
Limited, UK) and with 49.3 ml (288 mmol) of N-
ethyldiisopropylamine, and it is stirred for 20 minutes at room
temperature. This solution is then mixed with the (1 mmol) 24-
amine-hydrobromide described above and stirred for 4 days at room
temperature. The solution is concentrated by evaporation in a
vacuum, the remaining oil is cooled in an ice bath and mixed with
trifluoroacetic acid, stirred overnight at room temperature and
then precipitated with diethyl ether. The precipitate is dried
in a vacuum, taken up in water, set at pH 7; a YM3 Amicon(R)
ultrafiltration membrane is used to remove low-molecular
portions, and the retentate is ultimately membrane-filtered and
freeze-dried.
Yield: 14.4 g (85% of theory)
H20 content (Karl-Fischer): 8.7%
CA 02274132 1999-10-21
49
Elementary analysis (relative to anhydrous substance):
Cld: C 46.82 H 5.98 N 14.79 Na 10.59
Fnd: C 47.04 H 6.23 N 14.96 Na 10.26
f) 24-mer-Gd Complex of N-(6-DO3A-yl-5-oxo-4-azaheptanoyl)-
cascade polyamide based on N,N,N',N',N",N"-hexakis[2-
(trilysylamino)-ethyl]-trimesic acid triamide
8:5 g (0.5 mmol) of the complexing agent acid described in
Example 2e) above is set at pH 3 in water with dilute
hydrochloric acid, mixed with 2.17 g (6 mmol) of Gdz03, stirred
for 30 minutes at 80 C, set at pH 7 after cooling and desalinated
with a YM3 AMICON(R) ultrafiltration membrane. The retentate is
ultimately membrane-filtered and freeze-dried.
Yield: 8.50 g (88% of theory)
H20 content (Karl-Fischer): 7.9%
Gd determination (AAS): 19.4%
Elementary analysis (relative to anhydrous substance):
Cld: C 41.12 H 5.52 N 12.99 Gd 21.21
Fnd: C 40.86 H 5.34 N 13.25 Gd 20.95
Example 3
a) N,N'-Bis(benzyloxycarbonyl)-3-[carboxymethoxyacetyl]-3-
azapentane-1,5-diamine
37.14 g (100 mmol) of the bis(benzyloxycarbonyl-aminoethyl)-
amine described in Example la) is dissolved in DMF, mixed in an
ice bath with 17.4 g (150 mmol) of diglycolic anhydride (Janssen
CA 02274132 1999-10-21
50
Chimica) and 21 ml (150 mmol) of triethylamine and then stirred
overnight at room temperature. The solution is concentrated by
evaporation in a vacuum, the residue is taken up in ethyl acetate
and shaken out with dilute hydrochloric acid. The organic phase
is dried with sodium sulfate and after the drying agent is
filtered, it is crystallized by adding hexane.
Yield: 41.4 g (85% of theory)
Elementary analysis:
Cld: C 59.13 H 6.00 N 8.62
Fnd: C 58.99 H 5.93 N 8.70
b) N,N',N",N " '-Tetrakis{8-(benzyloxycarbonylamino)-6-[2-
(benzyloxycarbonylaminoethyl]-5-oxo-3-oxaoctanoyl}cyclene
345 mg (2 mmol) of 1,4,7,10-tetraazacyclododecane (cyclene;
Fluka) is azeotropically dehydrated with toluene. A solution of
4.88 g (10 mmol) of N,N'-bis(benzyloxycarbonyl)-3-
[carboxymethoxyacetyl]-3-azapentane-1,5-diamine [Example 3a)] in
tetrahydrofuran (THF) as well as 2.47 g (10 mmol) of 2-ethoxy-l-
ethoxycarbonyl-1,2-dihydroquinoline (EEDQ; Fluka) are added to
the cooled solution of cyclene in toluene at room temperature,
and it is stirred overnight. After the reaction is completed,
the product is precipitated by adding hexane, decanted from
solvent and reprecipitated once more from THF/hexane and then
from THF/toluene. After drying in a vacuum, 2.78 g (68% of
theory) of a pale yellow solid is obtained.
CA 02274132 1999-10-21
51
Elementary analysis:
Cld: C 60.93 H 6.29 N 10.93
Fnd: C 60.68 H 6.40 N 10.97
C) Completely protected benzyloxycarbonyl-32-polyamine based on
32-amine condensed with Na,Nf-bis(lysyl)-lysine ("tri-
lysine) from N,N',N",N " '-tetrakis{8-
benzyloxycarbonylamino)-6-[2-(benzyloxycarbonylamino)-
ethyl]-5-oxo-3-oxaoctanoyl}cyclene
2.05 g (1 mmol) of the octa-benzyloxycarbonylamine described
in Example 3b) is dissolved in glacial acetic acid and mixed with
33% hydrogen bromide in glacial acetic acid while being stirred.
After 90 minutes, the incipient precipitation is completed with
diethyl ether, the octa-amine-hydrobromide produced is washed
with ether, dried in a vacuum and used in the subsequent reaction
described below without further purification.
Yield: 1.6 g (quantitative)
9.4 g (10 mmol) of the protected "tri-lysine" described in
Example ic), 1.5 g (10 mmol) of 1-hydroxybenzotriazole and 3.2 g
(10 mmol) of 2-(1H-benzotriazol-l-yl)-1,1,3,3-tetramethyluronium
tetrafluoroborate (TBTU; Peboc Limited, UK) are dissolved in DMF
and stirred for 15 minutes. This solution is then mixed with
5.16 ml (30 mmol) of N-ethyldiisopropylamine and with 1.6 g (1
mmol) of the octaamine-hydrobromide described above, and it is
stirred overnight at room temperature. After the reaction is
CA 02274132 1999-10-21
52
completed, it is concentrated by evaporation in a vacuum, and the
residue is chromatographed on silica gel with dichloromethane/
methanol (10:1).
Yield: 6.0 g (72% of theory)
Elementary analysis:
Cld: C 63.32 H 6.76 N 10.74
Fnd: C 62.98 H 6.91 N 10.43
d) 32-mer N-(5-DO3A-yl-4-oxo-3-azahexanoyl)-cascade polyamide
based on the 32-mer amine described in Example 3c) above
8.35 g (1 mmol) of the 32-mer-benzyloxycarbonylamine
described in Example 3c) is dissolved in glacial acetic acid and
mixed with 33% hydrogen bromide in glacial acetic acid while
being stirred. After 3 hours, the incipient precipitation is
completed with diethyl ether; the 32-amine-hydrobromide produced
is washed with ether and dried in a vacuum.
47.8 g (64 mmol) of the acid described in Example ih) is
dissolved in DMF, mixed with 9.8 g (64 mmol) of 1-
hydroxybenzotriazole, with 20.5 g (64 mmol) of TBTU (Peboc
Limited, UK) and with 65.7 ml (384 mmol) of
N-ethyldiisopropylamine, and it is stirred for 20 minutes at room
temperature. This solution is then mixed with the (1 mmol) 32-
amine-hydrobromide described above and stirred for 4 days at room
temperature. The solution is concentrated by evaporation in a
vacuum, the remaining oil is cooled in an ice bath and mixed with
trifluoroacetic acid, stirred overnight at room temperature and
CA 02274132 1999-10-21
53
then precipitated with diethyl ether. The precipitate is dried
in a vacuum, taken up in water, set at pH 7, a YM3 Amicon(R)
ultrafiltration membrane is used to remove low-molecular
portions, and the retentate is ultimately membrane-filtered and
freeze-dried.
Yield: 17.2 g (76.4% of theory)
H20 content (Karl-Fischer): 7.6%
Elementary analysis (relative to anhydrous substance):
Cld: C 45.73 H 6.12 N 15.08 Na 10.61
Fnd: C 45.89 H 6.30 N 14.84 Na 10.31
e) 32-mer-Gd Complex of N-(5-DO3A-yl-4-oxo-3-azahexanoyl)-
cascade polyamide based on the 32-mer amine described in
Example 3c)
10.4 g (0.5 mmol) of the complexing agent acid described in
Example 3d) above is set at pH 3 in water with dilute
hydrochloric acid, mixed with 2.89 g (8 mmol) of Gd2031 stirred
for 30 minutes at 80 C, set at pH 7 after cooling and desalinated
with a YM3 AMICON(R) ultrafiltration membrane. The retentate is
ultimately membrane-filtered and freeze-dried.
Yield: 12.1 g(91.1; of theory)
H20 content (Karl-Fischer): 11.0%
Gd determination (AAS): 18.6%
CA 02274132 1999-10-21
54
Elementary analysis (relative to anhydrous substance):
Cld: C 40.26 H 5.39 N 13.28 Gd 21.30
Fnd: C 40.10 H 5.21 N 13.04 Gd 21.03
The ytterbium complex is obtained analogously with Yb2(C03)3:
Elementary analysis (relative to anhydrous substance):
Cld: C 39.42 H 5.28 N 13.00 Yb 22.94
Fnd: C 39.29 H 5.40 N 12.81 Yb 22.65
Examples 4 to 14 below are used to explain the subject of
the invention:
Example 4
a) 10-[4-Carboxy-l-methyl-2-oxo-3-azabutylJ-1,4,7,10-
tetraazacyclododecane-1,4,7-triacetic acid
77 g (103.1 mmol) of the title compound of Example lh is
dissolved in 500 ml of trifluoroacetic acid and stirred for 3
hours at room temperature. It is evaporated to the dry state,
the residue is taken up in 300 ml of water and the solution is
added to a column, filled with Reillex(R) 425 PVP. It is eluted
with water. The product-containing fractions are combined and
evaporated to the dry state, and the residue is recrystallized
from methanol/acetone.
Yield: 44.04 g (84% of theory) of a colorless, hygroscopic
solid
Water content: 6.5%
CA 02274132 1999-10-21
55
Elementary analysis (relative to anhydrous substance):
Cld: C 47.99 H 6.99 N 14.73
Fnd: C 47.83 H 7.12 N 14.55
b) Gadolinium complex of 10-[4-carboxy-l-methyl-2-oxo-3-
azabutyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triacetic
acid
15.27 g (42.06 mmol) of gadolinium oxide is added to 40 g
(84.12 mmol) of the title compound of Example 4a, dissolved in
400 ml of water, and it is heated for 3 hours to 90 C. It is
evaporated to the dry state (vacuum), and the residue is
recrystallized from 90% aqueous ethanol. The crystals are
suctioned off, washed once with ethanol, then with acetone and
finally with diethyl ether and dried in a vacuum furnace at 130 C
(24 hours).
Yield: 50.53 g (93% of theory) of a colorless, crystalline
powder
Water content: 2.5%
Elementary analysis (relative to anhydrous substance):
Cld: C 36.24 H 4.80 N 11.12 Gd 24.97
Fnd: C 36.35 H 4.95 N 10.98 Gd 24.80
CA 02274132 1999-10-21
56
Example 5
Dysprosium complex of 10-[4-carboxy-l-methyl-2-oxo-3-azabutyl]-
1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid
7.84 g (21.03 mmol) of dysprosium oxide is added to 20 g
(42.06 mmol) of the title compound of Example 4a, dissolved in
200 ml of water, and it is heated for 3 hours to 90 C. it is
evaporated to the dry state (vacuum), and the residue is
recrystallized from 90% aqueous ethanol. The crystals are
suctioned off, washed once with ethanol, then with acetone and
finally with diethyl ether and dried in a vacuum furnace at 130 C
(24 hours).
Yield: 24.98 (91% of theory) of a colorless, crystalline
powder
Water content: 2.7%
Elementary analysis (relative to anhydrous substance):
Cld: C 35.94 H 4.76 N 11.03 Dy 25.59
Fnd: C 35.85 H 4.91 N 10.90 Dy 25.42
Example 6
Ytterbium complex of 10-[4-carboxy-l-methyl-2-oxo-3-azabutyl]-
1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid
8.29 g (21.03 mmol) of ytterbium oxide is added to 20 g
(42.06 mmol) of the title compound of Example 4a, dissolved in
200 ml of water, and it is heated for 3 days to 90 C. It is
evaporated to the dry state (vacuum), and the residue is
recrystallized from 90% aqueous ethanol. The crystals are
CA 02274132 1999-10-21
57
suctioned off, washed once with ethanol, then with acetone and
finally with diethyl ether and dried in a vacuum furnace at 130 C
(24 hours).
Yield: 21.79 (78% of theory) of a colorless, crystalline
powder
Water content: 2.8%
Elementary analysis (relative to anhydrous substance):
Cld: C 35.35 H 4.68 N 10.85 Yb 26.81
Fnd: C 35.25 H 4.79 N 10.68 Yb 26.61
Example 7
a) N-(2-Bromobutyryl)-glycine benzyl ester
65.96 g (355.7 mmol) of a-bromobutyric acid chloride is
added in drops at 0 C to 100 g (296.4 mmol) of glycine benzyl
ester p-toluenesulfonic acid salt and 89.98 g (889.2 mmol) of
triethylamine in 500 ml of methylene chloride. In this case, the
temperature remains between 0 C - 5 C. 1000 ml of 5% aqueous
hydrochloric acid is added, and the organic phase is separated.
The organic phase is extracted once more with 500 ml of 5%
aqueous hydrochloric acid, dried on magnesium sulfate and
evaporated to the dry state in a vacuum. The residue is
recrystallized from diisopropyl ether.
Yield: 75.43 g (81% of theory) of a colorless, crystalline
powder
CA 02274132 1999-10-21
58
Elementary analysis:
Cld: C 49.70 H 5.13 N 4.46 Br 25.43
Fnd: C 49.51 H 5.27 N 4.31 Br 25.28
b) 10-[4-(Benzyloxycarbonyl)-1-ethyl-2-oxo-3-azabutyl]-
1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid-tri-
tert-butyl ester
500 ml of acetonitrile is added to 50 g (159.14 mmol) of the
title compound of Example 7a, 36.98 g (79.6 mmol) of 1,4,7-
tris(tert-butoxy-carbonylmethyl)-1,4,7,10-tetraazacyclododecane
(= D03A-tri-tert-butyl ester), 44 g (318.4 mmol) of potassium
carbonate and 1 g (60 mmol) of potassium iodide, and it is
refluxed for 12 hours. Salts are filtered out, and the filtrate
is evaporated to the dry state in a vacuum. The residue is
dissolved in 800 ml of dichloromethane and extracted twice with
300 ml of 5% aqueous sodium carbonate solution each. The organic
phase is dried on magnesium sulfate and concentrated by
evaporation. After chromatography on silica gel (mobile solvent:
dichloromethane/methanol = 20:1), 19.11 g of the title compound
(32.1% of theory) is obtained as a colorless foam.
Elementary analysis:
Cld: C 62.63 H 8.76 N 9.36
Fnd: C 62.51 H 8.91 N 9.18
CA 02274132 1999-10-21
59
C) 10-(4-Carboxy-l-ethyl-2-oxo-3-azabutyl)-1,4,7,10-
tetraazacyclododecane-1,4,7-triacetic acid-tri-tert-butyl
ester
19 g (25.40 mmol) of the title compound of Example 7b is
dissolved in 300 ml of isopropanol, and 2 g of palladium catalyst
(10% Pd/C) is added. It is hydrogenated overnight at room
temperature. Catalyst is filtered out, and the filtrate is
evaporated to the dry state.
Yield: 16.54 g (99% of theory) of a viscous oil
Elementary analysis:
Cld: C 58.43 H 9.04 N 10.65
Fnd: C 58.65 H 9.27 N 10.47
d) 10-(4-Carboxy-l-ethyl-2-oxo-3-azabutyl)-1,4,7,10-
tetraazacyclododecane-1,4,7-triacetic acid
16 g (24.32 mmol) of the title compound of Example 7c is
dissolved in 100 ml of trifluoroacetic acid, and it is stirred
for 3 hours at room temperature. It is evaporated to the dry
state, the residue is taken up in 50 ml of water, and the
solution is added to a column, filled with Reillex(R) 425 PVP. It
is eluted with water. The product-containing fractions are
combined and evaporated to the dry state, and the residue is
recrystallized from methanol/acetone.
Yield: 10.10 g (79% of theory) of a colorless, hygroscopic
solid
Water content: 6.9%
CA 02274132 1999-10-21
60
Elementary analysis (relative to anhydrous substance):
Cld: C 49.07 H 7.21 N 14.31
Fnd: C 49.28 H 7.39 N 14.15
e) Gadolinium complex of 10-(4-carboxy-l-ethyl-2-oxo-3-
azabutyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triacetic
acid
3.33 g (9.19 mmol) of gadolinium oxide is added to 9 g
(18.38 mmol) of the title compound of Example 7d, dissolved in 70
ml of water, and it is heated for 3 hours to 90 C. It is
evaporated to the dry state (vacuum), and the residue is
recrystallized from 90% aqueous ethanol. The crystals are
suctioned off, washed once with ethanol, then with acetone and
finally with diethyl ether and dried in a vacuum furnace at 130 C
(24 hours).
Yield: 11.44 g (94% of theory) of a colorless, crystalline
powder
Water content: 2.8%
Elementary analysis (relative to anhydrous substance):
Cld: C 37.32 H 5.01 N 10.88 Gd 24.43
Fnd: C 37.15 H 5.21 N 10.65 Gd 24.25
CA 02274132 1999-10-21
61
Example 8
Dysprosium complex of 10-(4-carboxy-l-ethyl-2-oxo-3-azabutyl)-
1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid
3.81 g (10.21 mmol) of dysprosium oxide is added to 10 g
(20.43 mmol) of the title compound of Example 7d, dissolved in 80
ml of water, and it is heated for 3 hours to 90 C. It is
evaporated to the dry state (vacuum), and the residue is
recrystallized from 90% aqueous ethanol. The crystals are
suctioned off, washed once with ethanol, then with acetone and
finally with diethyl ether, and it is dried in a vacuum furnace
at 130 C (24 hours).
Yield: 12.40 g (91% of theory) of a colorless, crystalline
powder
Water content: 2.7%
Elementary analysis (relative to anhydrous substance):
Cld: C 37.01 H 4.97 N 10.79 Dy 25.04
Fnd: C 36.85 H 5.13 N 10.61 Dy 24.87
Example 9
a) N-[2-Bromo-2-phenyl-acetyl]-glycolic acid-tert-butyl ester
72.69 g (311.3 mmol) of a-bromophenylacetic acid chloride is
added in drops at 0 C to 50 g (296.5 mmol) of glycine-tert-butyl
ester hydrochloride salt and 90 g (889.5 mmol) of triethylamine
in 500 ml of methylene chloride. In this case, the temperature
remains between 0 C - 5 C. 1000 ml of 5% aqueous hydrochloric
acid is added, and the organic phase is separated. The organic
CA 02274132 1999-10-21
62
phase is extracted once more with 500 ml of 5% aqueous
hydrochloric acid, dried on magnesium sulfate and evaporated to
the dry state in a vacuum. The residue is recrystallized from
diisopropyl ether/n-hexane.
Yield: 78.8 g(81$ of theory)
Elementary analysis:
Cld: C 51.23 H 5.53 N 4.27 Br 24.35
Fnd: C 51.15 H 5.66 N 4.11 Br 24.18
b) 1-[4-(tert-Butoxycarbonyl)-oxo-l-phenyl-3-azabutyl]-4,7,10-
tris(tert-butoxycarbonylmethyl)-1,4,7,10-
tetraazacyclododecane
500 ml of acetonitrile is added to 50 g (159.14 mmol) of the
title compound of Example 9a, 53.12 g (114.3 mmol) of 1,4,7-
tris(tert-butoxy-carboxymethyl)-1,4,7,10-tetraazacyclododecane
(= D03A-tri-tert-butyl ester), 63.16 g (457.0 mmol) of potassium
carbonate and 1 g (6 mmol) of potassium iodide, and it is
refluxed for 12 hours. Salts are filtered out, and the filtrate
is evaporated to the dry state in a vacuum. The residue is
dissolved in 1000 ml of dichloromethane, and it is extracted
twice with 400 ml of 5% aqueous sodium carbonate solution each.
The combined organic phases are dried on magnesium sulfate and
concentrated by evaporation. After chromatography on silica gel
(mobile solvent: dichloromethane/methanol = 20:1), 27 g of the
title compound (31% of theory) is obtained as a colorless foam.
CA 02274132 1999-10-21
63
Elementary analysis:
Cld: C 63.05 H 8.86 N 9.19
Fnd: C 62.91 H 8.98 N 9.02
C) 1-(4-Carboxy-2-oxo-l-phenyl-3-azabutyl)-4,7,10-
tris(carboxymethyl)-1,4,7,10-tetraazacyclododecane
26 g (34.12 mmol) of the title compound of Example 9b is
dissolved in 300 ml of trifluoroacetic acid, and it is stirred
for 3 hours at room temperature. It is evaporated to the dry
state, the residue is taken up in 80 ml.of water, and the
solution is added to a column, filled with ReillexIR) 425 PVP. It
is eluted with water. The product-containing fractions are
combined and evaporated to the dry state, and the residue is
recrystallized from methanol/acetone.
Yield: 16.22 g (81% of theory) of a colorless, hygroscopic
solid
Water content: 8.4%
Elementary analysis (relative to anhydrous substance):
Cld: C 53.62 H 6.56 N 13.03
Fnd: C 53.48 H 6.71 N 12.87
d) Gadolinium complex of 1-(4-carboxy-2-oxo-l-phenyl-3-
azabutyl)-4,7,10-tris(carboxymethyl)-1,4,7,10-
tetraazacyclododecane
5.06 g (13.95 mmol) of gadolinium oxide is added to 15 g
(27.90 mmol) of the title compound of Example 9c, dissolved in
CA 02274132 1999-10-21
64
200 ml of water, and it is heated for 3 hours to 90 C. It is
evaporated to the dry state (vacuum), and the residue is
recrystallized from 90% aqueous ethanol. The crystals are
suctioned off, washed once with ethanol, then with acetone and
finally with diethyl ether, and it is dried in a vacuum furnace
at 130 C (24 hours).
Yield: 18.27 g (92% of theory) of a colorless, crystalline
powder
Water content: 2.8%
Elementary analysis (relative to anhydrous substance):
Cld: C 41.67 H 4.66 N 10.12 Gd 22.73
Fnd: C 41.40 H 4.80 N 9.95 Gd 22.51
Example 10
a) N-(2-Bromopropionyl)-8-alanine
72.69 g (311.3 mmol) of a-bromopropionic acid chloride is
added in drops at 0 C to 40 g (448.98 mmol) of b-alanine and 90 g
(889.5 mmol) of triethylamine in 500 ml of methylene chloride.
In this case, the temperature remains between 0 C - 5 C. 1000 ml
of 5% aqueous hydrochloric acid is added, and the organic phase
is separated. The organic phase is extracted once more with 500
ml of 5% aqueous hydrochloric acid, dried on magnesium sulfate
and evaporated to the dry state in a vacuum. The residue is
.recrystallized from acetone/diisopropyl ether.
Yield: 62.37 g (62% of theory)
CA 02274132 1999-10-21
65
Elementary analysis:
Cld: C 32.16 H 4.50 N 6.25 Br 35.66
Fnd: C 32.02 H 4.65 N 6.13 Br 35.74
b) 10-(5-Carboxy-l-methyl-2-oxo-3-azapentyl)-1,4,7,10-
tetraazacyclododecane-1,4,7-triacetic acid
46.38 g (133.9 mmol) of 1,4,7-tris(carboxymethyl)-1,4,7,10-
tetraazacyclododecane (= D03A), 129.54 g (937.3 mmol) of
potassium carbonate and 1 g (6 mmol) of potassium iodide are
added to 60 g (267.80 mmol) of the title compound of Example 10a,
dissolved in 300 ml of acetonitrile/200 ml of water. It is
refluxed for 12 hours. It is evaporated to the dry state in a
vacuum, the residue is taken up in 500 ml of methanol, and then
salts are filtered out. The filtrate is evaporated to the dry
state, the residue is taken up in 300 ml of water and set at pH 1
with 5N hydrochloric acid. Then purification is done on a column
filled with Reillex(R) 425 PVP. It is eluted with water. The
product-containing fractions are evaporated to the dry state in a
vacuum, and the residue is recrystallized from methanol/acetone.
Yield: 19.19 g (27% of theory) of a colorless solid
Water content: 7.8%
Elementary analysis (relative to anhydrous substance):
Cld: C 49.07 H 7.21 N 14.31
Fnd: C 48.85 H 7.31 N 14.19
CA 02274132 1999-10-21
66
C) Gadolinium complex of 10-(5-carboxy-l-methyl-2-oxo-3-
azapentyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triacetic
acid
6.66 g (18.38 mmol) of gadolinium oxide is added to 18 g
(36.77 mmol) of the title compound of Example lOb, dissolved in
300 ml of water, and it is heated for 3 hours to 90 C. It is
evaporated to the dry state (vacuum), and the residue is
recrystallized from 90% aqueous ethanol. The crystals are
suctioned off, washed once with ethanol, then with acetone and
finally with diethyl ether and dried in a vacuum furnace at 130 C
(24 hours).
Yield: 21.6 g (89% of theory) of a colorless, crystalline
powder
Water content: 2.5%
Elementary analysis (relative to anhydrous substance):
Cld: C 37.32 H 5.01 N 10.88 Gd 24.43
Fnd: C 37.15 H 5.21 N 10.67 Gd 24.25
Example 11
a) N-(2-Bromopropionyl)-ii-aminoundecanoic acid
30.65 g (178.8 mmol) of a-bromopropionic acid chloride is
added in drops at 0 C to 30 g (149 mmol) of 11-aminoundecanoic
acid and 45.24 g (447.1 mmol) of triethylamine in 600 ml of
methylene chloride. In this case, the temperature remains
between 0 C - 5 C. 800 ml of 5% aqueous hydrochloric acid is
added, and the organic phase is separated. The organic phase is
CA 02274132 1999-10-21
67
extracted once more with 300 ml of 5% aqueous hydrochloric acid,
dried on magnesium sulfate and evaporated to the dry state in a
vacuum. The residue is recrystallized from acetone/diisopropyl
ether.
Yield: 25.55 g(51$ of theory)
Elementary analysis:
Cid: C 50.01 H 7.79 N 4.17 Br 23.76
Fnd: C 49.82 H 7.95 N 4.03 Br 23.59
b) 10-(13-Carboxy-l-methyl-2-oxo-3-aza-tridecyl)-1,4,7,10-
tetraazacyclododecane-1,4,7-triacetic acid
12.88 g (37.18 mmol) of 1,4,7-triscarboxymethyl-1,4,7,10-
tetraazacyclododecane (= D03A), 35.97 g (260.3 mmol) of potassium
carbonate and 1 g (6 mmol) of potassium iodide are added to 25 g
(74.35 mmol) of the title compound of Example ila, dissolved in
250 ml of acetonitrile/150 ml of water. It is refluxed for 12
hours. It is evaporated to the dry state in a vacuum, the
residue is taken up in 300 ml of methanol, and then salts are
filtered out. The filtrate is evaporated to the dry state, the
residue is taken up in 300 ml of water, and it is set at pH 1
with 5N hydrochloric acid. Then purification is done on a column
filled with Reillex(R) 425 PVP. It is eluted with water. The
product-containing fractions are evaporated to the dry state in a
vacuum, and the residue is recrystallized from methanol/acetone.
Yield: 6.63 g (27% of theory) of a colorless solid
Water content: 8.9%
CA 02274132 1999-10-21
68
Elementary analysis (relative to anhydrous substance):
Cld:. C 55.89 H 8.54 N 11.64
Fnd: C 55.71 H 8.70 N 11.57
C) Gadolinium complex of 10-(13-carboxy-l-methyl-2-oxo-3-aza-
tridecyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triacetic
acid
1.81 g (10.21 mmol) of gadolinium oxide is added to 6 g
(9.97 mmol) of the title compound of Example lib, dissolved in 80
ml of water, and it is heated for 3 hours to 90 C. It is
evaporated to the dry state (vacuum), and the residue is
recrystallized from 90% aqueous 2-propanol. The crystals are
suctioned off, washed once with ethanol, then with acetone and
finally with diethyl ether and dried in a vacuum furnace at 130 C
(24 hours).
Yield: 6.75 g (87% of theory) of a colorless, crystalline
powder
Water content: 2.9%
Elementary analysis (relative to anhydrous substance):
Cld: C 44.49 H 6.40 N 9.26 Gd 20.80
Fnd: C 44.28 H 6.55 N 9.11 Gd 20.63
Example 12
a) N-(2-Bromopropionyl)-alanine
69.26 g (404 mmol) of a-bromopropionic acid chloride is
added in drops at 0 C to 30 g (336.7 mmol) of alanine and 102.2 g
CA 02274132 1999-10-21
69
(1010.2 mmol) of triethylamine in 600 ml of methylene chloride.
In this case, the temperature remains between 0 C - 5 C. 1000 ml
of 5% aqueous hydrochloric acid is added, and the organic phase
is separated. The organic phase is extracted once more with 400
ml of 5% aqueous hydrochloric acid, dried on magnesium sulfate
and evaporated to the dry state in a vacuum. The residue is
recrystallized from acetone/diisopropyl ether.
Yield: 52.05 g (69% of theory)
Elementary analysis (relative to anhydrous substance):
Cld: C 32.16 H 4.50 N 6.25 Br 35.66
Fnd: C 32.33 H 4.70 N 6.13 Br 35.41
b) 10-(4-Carboxy-l-methyl-2-oxo-3-azapentyl)-1,4,7,10-
tetraazacyclododecane-1,4,7-triacetic acid
38.65 g (111.6 mmol) of 1,4,7-triscarboxymethyl-1,4,7,10-
tetraazacyclododecane (= D03A), 108 g (781.2 mmol) of potassium
carbonate and 1 g (6 mmol) of potassium iodide are added to 50 g
(223.2 mmol) of the title compound of Example 12a, dissolved in
300 ml of acetonitrile/200 ml of water. It is refluxed for 12
hours. It is evaporated to the dry state in a vacuum, the
residue is taken up in 500 ml of methanol, and then salts are
filtered out. The filtrate is evaporated to the dry state, the
residue is taken up in 300 ml of water and set at pH 1 with 5N
hydrochloric acid. Then purification is done on a column filled
with Reillex(R) 425 PVP. It is eluted with water. The product-
CA 02274132 1999-10-21
70
containing fractions are evaporated to the dry state in a vacuum,
and the residue is recrystallized from methanol/acetone.
Yield: 17.72 g (30% of theory) of a colorless solid
Water content: 7.5%
Elementary analysis (relative to anhydrous substance):
Cld: C 49.07 H 7.21 N 14.31
Fnd: C 49.23 H 7.38 N 14.15
C) Gadolinium complex of 10-(4-carboxy-l-methyl-2-oxo-3-
azapentyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triacetic
acid
5.55 g (15.32 mmol) of gadolinium oxide is added to 15 g
(30.64 mmol) of the title compound of Example 12b, dissolved in
150 ml of water, and it is heated for 3 hours to 90 C. It is
evaporated to the dry state (vacuum), and the residue is
recrystallized from 90% aqueous ethanol. The crystals are
suctioned off, washed once with ethanol, then with acetone and
finally with diethyl ether and dried in a vacuum furnace at 130 C
(24 hours).
Yield: 18.22 g (90% of theory) of a colorless, crystalline
powder
Water content: 2.6%
Elementary analysis (relative to anhydrous substance):
Cld: C 37.32 H 5.01 N 10.88 Gd 24.43
Fnd: C 37.13 H 5.20 N 10.61 Gd 24.41
CA 02274132 1999-10-21
71
Example 13
a) N-(2-Bromopropionyl)-valine
70.2 g (409.7 mmol) of a-bromopropionic acid chloride is
added in drops at 0 C to 40 g (341.4 mmol) of valine and 103.7 g
(1024 mmol) of triethylamine in 600 ml of inethylene chloride. In
this case, the temperature remains between 0 C - 5 C. 1000 ml of
5% aqueous hydrochloric acid is added, and the organic phase is
separated. The organic phase is extracted once more with 500 ml
of 5% aqueous hydrochloric acid, dried on magnesium sulfate and
evaporated to the dry state in a vacuum. The residue is
recrystallized from acetone/diisopropyl ether.
Yield: 59.39 g (69% of theory)
Elementary analysis (relative to anhydrous substance):
Cld: C 38.11 H 5.60 N 5.56 Br 31.69
Fnd: C 38.01 H 5.75 N 5.41 Br 31.48
b) 10-(4-Carboxy-1,5-dimethyl-2-oxo-3-azahexyl)-1,4,7,10-
tetraazacyclododecane-1,4,7-triacetic acid
37.8 g (109.7 mmol) of 1,4,7-triscarboxymethyl-1,4,7,10-
tetraazacyclododecane (= D03A), 106.13 g (767.9 mmol) of
potassium carbonate and 1 g (6 mmol) of potassium iodide are
added to 55 g (218.2 mmol) of the title compound of Example 13a,
dissolved in 200 ml of acetonitrile/200 ml of water. It is
refluxed for 12 hours. It is evaporated to the dry state in a
vacuum, the residue is taken up in 500 ml of methanol, and then
salts are filtered out. The filtrate is evaporated to the dry
CA 02274132 1999-10-21
72
state, and the residue is taken up in 300 ml of water and set at
pH 1 with 5N hydrochloric acid. Then purification is done on a
column filled with Reillex(R) 425 PVP. It is eluted with water.
The product-containing fractions are evaporated to the dry state
in a vacuum, and the residue is recrystallized from
methanol/acetone.
Yield: 17.57 g (29% of theory) of a colorless solid
Water content: 6.3%
Elementary analysis (relative to anhydrous substance):
Cld: C 51.05 H 7.59 N 13.53
Fnd: C 51.18 H 7.70 N 13.39
c) Gadolinium complex of 10-(4-carboxy-1,5-dimethyl-2-oxo-3-
azahexyl]-1,4,7,10-tetraazacyclododecane-1,4,7-triacetic
acid
5.25 g (14.49 mmol) of gadolinium oxide is added to 15 g
(28.98 mmol) of the title compound of Example 13b, dissolved in
150 ml of water, and it is heated for 3 hours to 90 C. It is
evaporated to the dry state (vacuum), and the residue is
recrystallized from 90% aqueous ethanol. The crystals are
suctioned off, washed once with ethanol, then with acetone and
finally with diethyl ether and dried in a vacuum furnace at 130 C
(24 hours).
Yield: 18.57 g (93% of theory) of a colorless, crystalline
powder
Water content: 2.5%
CA 02274132 1999-10-21
73
Elementary analysis (relative to anhydrous substance):
Cld: C 39.33 H 5.40 N 10.42 Gd 23.41
Fnd: C 39.17 H 5.55 N 10.31 Gd 23.27
Example 14
a) N-(2-Bromoacetyl)-glycine-tert-butyl ester
77.8 g (385.5 mmol) of a-bromoacetic acid bromide is added
in drops at 0 C to 50 g (296.5 mmol) of glycine-tert-butyl ester
hydrochloride salt and 90 g (889.5 mmol) of triethylamine in 500
ml of methylene chloride. In this case, the temperature remains
between 0 C - 5 C. 1000 ml of 5% aqueous hydrochloric acid is
added, and the organic phase is separated. The organic phase is
extracted once more with 500 ml of 5% aqueous hydrochloric acid,
dried on magnesium sulfate and evaporated to the dry state in a
vacuum. The residue is recrystallized from diisopropyl ether/n-
hexane.
Yield: 30.5 g (61% of theory)
Elementary analysis:
Cld: C 38.11 H 5.60 N 5.65 Br 31.69
Fnd: C 37.92 H 5.76 N 5.38 Br 31.42
b) 10-[4-(tert-Butoxycarbonyl)-2-oxo-3-azabutyl]-1,4,7,10-
tetraazacyclododecane-1,4,7-triacetic acid-tri-tert-butyl
ester
200 ml of acetonitrile is added to 20.35 g (80.70 mmol) of
the title compound of Example 14a, 25 g (53.8 mmol) of 1,4,7-
CA 02274132 1999-10-21
74
tris(tert-butoxy-carboxymethyl)-1,4,7,10-tetraazacyclododecane
D03A-tri-tert-butyl ester), 29.74 g (215.8 mmol) of potassium
carbonate and 1 g (6 mmol) of potassium iodide, and it is
refluxed for 12 hours. Salts are filtered out, and the filtrate
is evaporated to the dry state in a vacuum. The residue is
dissolved in 800 ml of dichloromethane and extracted twice with
200 ml of 5% aqueous sodium carbonate solution. The organic
phase is dried on magnesium sulfate and concentrated by
evaporation. After chromatography on silica gel (mobile solvent:
dichloromethane/methanol = 20:1), 25.09 g of the title compound
(68% of theory) is obtained as a colorless foam.
Elementary analysis:
Cld: C 59.54 H 9.26 N 10.21
Fnd: C 59.35 H 9.42 N 10.03
c) 10-[4-Carboxy-2-oxo-3-azabutyl]-1,4,7,10-
tetraazacyclododecane-1,4,7-triacetic acid
25 g (36.45 mmol) of the title compound of Example 14b is
dissolved in 300 ml of trifluoroacetic acid, and it is stirred
for 3 hours at room temperature. It is evaporated to the dry
state, the residue is taken up in 80 ml of water, and the
solution is added to a column, filled with Reillex(R) 425 PVP. It
is eluted with water. The product-containing fractions are
combined and evaporated to the dry state, and the residue is
recrystallized from methanol/acetone.
CA 02274132 1999-10-21
75
Yield: 15.24 g (84% of theory) of a colorless, hygroscopic
solid
Water content: 7.3%
Elementary analysis (relative to anhydrous substance):
Cld: C 46.85 H 6.77 N 15.18
Fnd: C 46.61 H 6.95 N 15.02
d) Gadolinium complex of 10-[4-carboxy-2-oxo-3-azabutyl]-
1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid
5.86 g (16.25 mmol) of gadolinium oxide is added to 15 g
(32.50 mmol) of the title compound of Example 14c, dissolved in
200 ml of water, and it is heated for 3 hours to 90 C. It is
evaporated to the dry state (vacuum), and the residue is
recrystallized from 90% aqueous ethanol. The crystals are
suctioned off, washed once with ethanol, then with acetone and
finally with diethyl ether and dried in a vacuum furnace at 130 C
(24 hours).
Yield: 18.92 g (92% of theory) of a colorless, crystalline
powder
Water content: 2.7%
Elementary analysis (relative to anhydrous substance):
Cld: C 35.11 H 4.58 N 11.37 Gd 25.54
Fnd: C 34.92 H 4.71 N 11.14 Gd 25.33
CA 02274132 1999-10-21
76
The examples below are used to explain the use of the
macrocyclic metal complex carboxylic acids according to the
invention:
Example 15
24-mer-Gd Complex of N-(5-D03A-yl-4-oxo-3-azahexanoyl)-cascade
polyamide based on N,N,N',N',N",N"-hexakis[2-(trilysylamino)-
ethyl]-trimesic acid triamide
4.2 g (0.7 mmol) of the benzyloxycarbonyl-24mer-polyamine
based on N,N,N',N',N",N"-hexakis[2-(trilysylamino)-ethyl]-
trimesic acid triamide, described in Example id, is dissolved in
glacial acetic acid and mixed with 33% hydrogen bromide in
glacial acetic acid while being stirred. After 3 hours, the
incipient precipitation is completed with diethyl ether, the
24mer-amine-hydrobromide produced is washed with ether, dried in
a vacuum (3.3 g, quantitative) and used in the following reaction
.without further purification.
31.74 g (50.4 mmol, 3X excess) of the Gd-complex of 10-[4-
carboxy-l-methyl-2-oxo-3-azabutyl]-1,4,7,10-
tetraazacyclododecane-1,4,7-triacetic acid described in Example
4b is dissolved in 250 ml of formamide under heat. After cooling
to room temperature, 13.69 g (55.4 mmol) of 2-ethoxy-l-
ethoxycarbonyl-1,2-dihydroquinoline (EEDQ, Fluka), 3.3 g (0.7
mmol) of the above-described tetracosahydrobromide and 1.70 g
(16.8 mmol) of triethylamine are added, and it is stirred
overnight at room temperature. The solution is then mixed with
acetone, the precipitate is suctioned off, dried, taken up in
CA 02274132 1999-10-21
77
water, insoluble portions are filtered out, and the filtrate is
desalinated with an Amicon(" YM3 ultrafiltration membrane (cut
off 3,000 Da) and low-molecular components are removed. The
retentate is then freeze-dried.
Yield: 10.46 g(78$ of theory)
H2O content (Karl Fischer): 9%
Gd determination (AAS): 18.8%
Elementary analysis (relative to anhydrous substance):
Cld: C 40.26 H 5.35 N 13.24 Gd 21.62
Fnd: C 40.07 H 5.32 N 13.14 Gd 21.43
The.MALDI-MS (Matrix Assisted Laser Desorption/Ionization
Mass Spectrometry: e.g.: F. Hillenkamp, M. Karas, R. Beavis, B.
T. Chait, Anal. Chem. 63, 1193A (1991)) shows signals at m/z =
about 17,470 (24mer), about 16,960 (23mer) and about 16,480
(22mer) and thus confirms less by-product dispersion than the
product that is obtained according to Example 1k, which has the
following dispersion: Signals at m/z = about 17,450 (24mer),
about 16,830 (23mer), about 16,230 (22mer), about 15,680 (21mer),
about 15,070 (20mer) and 14,450 (19mer).
CA 02274132 1999-10-21
78
Example 16
24mer-Gd-complex of N-(6-D03A-yl-5-oxo-4-azaheptanoyl)-cascade
polyamide based on N,N,N',N',N",N"-hexakis[2-(trilysylamino)-
ethyl]-trimesic acid triamide
4.2 g (0.7 mmol) of the completely protected
benzyloxycarbonyl-24mer-polyamine based on N,N,N',N',N",N"-
hexakis[2-(trilysylamino)-ethylJ-trimesic acid triamide described
in Example id is dissolved in glacial acetic acid and mixed with
33% hydrogen bromide in glacial acetic acid while being stirred.
After 3 hours, the incipient precipitation is completed with
diethyl ether, the 24mer-amine-hydrobromide produced is washed
with ether, dried in a vacuum (3.3 g, quantitative) and used in
the following reaction without further purification.
32.45 g (50.4 mmol, 3X excess) of the Gd complex of 10-(5-
carboxy-l-methyl-2-oxo-3-aza-pentyl)-1,4,7,10-
tetraazacyclododecane-1,4,7-triacetic acid described in Example
lOc is dissolved in 250 ml of formamide under heat. After
cooling to room temperature, 13.69 g (55.4 mmol) of 2-ethoxy-l-
ethoxycarbonyl-1,2-dihydroquinoline (EEDQ, Fluka), 3.3 g (0.7
mmol) of the above-described tetracosahydrobromide and 1.70 g
(16.8 mmol) of triethylamine are added, and it is stirred
overnight at room temperature. The solution is then mixed with
acetone, the precipitate is suctioned off, dried, taken up in
water, insoluble portions are filtered out, and the filtrate is
desalinated with an Amicon(R) YM3 ultrafiltration membrane (cut
off 3,000 Da) and low-molecular components are removed. The
retentate is then freeze-dried.
CA 02274132 1999-10-21
79
Yield: 10.53 g(77$ of theory)
H20 content (Karl Fischer): 9%
Gd determination (AAS): 18.5%
Elementary analysis (relative to anhydrous substance):
Cld: C 41.12 H 5.52 N 12.99 Gd 21.21
Fnd: C 40.95 H 5.62 N 12.78 Gd 21.01
The MALDI-MS shows signals at m/z = about 17,790 (24mer),
about 17,180 (23mer) and about 16,540 (22mer).
Example 17
32-mer-Dysprosium complex of N-(5-D03A-yl-4-oxo-3-azahexanoyl)-
cascade polyamide based on the 32mer amine is described in
Example 3c)
8.35 g (1 mmol) of the 32-mer-benzyloxycarbonylamine
described in Example 3c) is dissolved in glacial acetic acid and
mixed with 33% hydrogen bromide in glacial acetic acid while
being stirred. After 3 hours, the incipient precipitation is
completed with diethyl ether, the 32-amine-hydrobromide produced
is washed with ether, dried in a vacuum (quantitative yield) and
used in the following reaction without further purification.
60.96 g (96 mmol, 3X excess) of the Dy complex of 10-(4-carboxy-
1-methyl-2-oxo-3-azabutyl)-1,4,7,10-tetraazacyclododecane-1,4,7-
triacetic acid described in Example 5 is dissolved in 500 ml of
formamide under heat. After cooling to room temperature, 26.1 g
(105.6 mmol) of 2-ethoxy-l-ethoxycarbonyl-1,2-dihydroquinoline
CA 02274132 1999-10-21
80
(EEDQ, Fluka), 1 mmol of the above-described dotriaconta-
hydrobromide and 3.24 g (32 mmol) of triethylamine are added, and
it is stirred overnight at room temperature. The solution is
then mixed with acetone, the precipitate is suctioned off, dried,
taken up in water, insoluble portions are filtered out, and the
filtrate is desalinated with an Amicon(R) YM3 ultrafiltration
membrane (cut off 3,000 Da) and low-molecular components are
removed. The retentate is then freeze-dried.
Yield: 19.0 g (75% of theory)
H 20 content (Karl Fischer): 6%
Dy determination (AAS): 19.7%
Elementary analysis (relative to anhydrous substance):
Cld: C 39.98 H 5.35 N 13.19 Dy 21.85
Fnd: C 39.83 H 5.26 N 13.28 Dy 21.51
The MALDI-MS shows signals at m/z = about 23,800 (32mer),
about 23,200 (31mer) and about 22,600 (30mer).
CA 02274132 1999-10-21
81
Example of an In Vivo Comparison with an Extracellular Contrast
Medium
The suitability of the compound described in Example lk) as
a blood-pool-agent is shown in the following test.
As test animals, five male (Schering-SPF) rats that are 300-
350 g in weight are used. Before the test, the abdomen is
opened, the intestines are shifted and then the renal vessels
(arterial + venous) of both sides are ligated through the rear
peritoneum with a surgical needle. Then, the abdominal cavity is
closed again. 0.3 ml (respectively 50 mmol/L) of the following
contrast medium solution per animal is then administered
intravenously: mixture of 1 part each of the compound of Example
lk), named compound 1 below, and the dysprosium complex of 10-(1-
hydroxymethyl-2,3-dihydroxypropyl)-1,4,7-tris(carboxymethyl)-
1,4,7,10-tetraazacyclododecane, produced analogously to the
instructions in European Patent Application EP 448 191, named
compound 2 below. Blood samples are taken with a catheter in the
common carotid artery at the following times: 15, 30, 45, 60, 90
seconds, 3, 5, 10, 15 minutes p.i. In the blood samples
obtained, the concentrations of gadolinium (Gd) and dysprosium
(Dy) are measured with the aid of atomic emission spectrometry
(ICP-AES) in each case in a parallel manner. The portion of the
injected contrast medium of compound 1 (Gd) and compound 2 (Dy,
comparison substance), remaining in the blood space, can be
compared in the same animals by the different marking. Since
renal excretion is not possible, the decrease of the blood
CA 02274132 1999-10-21
82
concentration can be attributed only to a dispersion in the blood
spaces and to the diffusion in the interstitial tissue.
Results: The diffusion of compound 1 in the interstitium is
considerably slowed-down in comparison to an extracellular
contrast medium compound 2 (see Figure 1).
The extracellular contrast medium (compound 2) diffuses
quickly into the interstitial spaces of the body, so that as
early as after 3-5 minutes p.i., an equilibrium is reached
(displayed by constant blood level). In contrast to this, not
only are constantly higher blood concentrations measured with the
cascade polymer (compound 1) (reference to smaller volume of
distribution), in addition no equilibrium is reached over the
entire examination period of 15 minutes (reference to diffusion
into interstitial tissue proceeding only very slowly). This
means that compound 1 behaves as a blood-pool contrast medium.
Example of a Lymph Node Concentration in Guinea Pigs
The compound according to the invention that was mentioned
under Example lk was studied 30 minutes to 24 hours after
subcutaneous administration (10 mol of gadolinium/kg of body
weight, hind paw s.c.) to stimulated guinea pigs (complete
Freund's adjunct; in each case 0.1 ml i.m. in the right and left
upper and lower legs; 2 weeks before administration of test
substances) with respect to their lymph node concentration in
three successive lymph node stations (popliteal, inguinal,
CA 02274132 1999-10-21
83
iliac). In this connection, the results listed below
(determination of the gadolinium concentration by means of ICP-
AES) were obtained:
Time of Gadolinium Concentration in Three Successive
Lymph Node Lymph Node Stations
Removal [ mol/l]
[% dose/g of tissue]
Popliteal Inguinal Iliac Ratio
30 min p.i. 921 Amol/l 387 Amol/l 215 Amol/l 10:4.2:2.3
20.1% 8.5% 4.7%
90 min p.i. 659 Amol/l 120 Amol/l 68 mol/1 10:1.8:1.0
14.4% 2.6% 1.5%
4 h p.i. 176 Amol/l 79 Amol/l 47 Amol/l 10:4.5:2.7
3.9% 1.7% 1.0%
24 h p.i. 62 mol/l 13 Amol/l 28 Amol/l 10:2.1:4.5
1.4% 0.3% 0.6%
CA 02274132 1999-10-21