Note: Descriptions are shown in the official language in which they were submitted.
CA 02274478 1999-06-03
CVO ~12?01$ PCTIUS97/21W33
ORGANOMETALLICS FOR LIGHTWAVE
OPTICAL CIRCUIT APPLICATIONS
FIELD OF THE INVENTION
This invention relates generally to conversion systems such as flame
hydrolysis,
and to the use of organometallic sources for depositing thin uniform oxide
soot or pre-
sintered glass layers on planar substrates. The deposited soot is consolidated
into glass
1o layers. The glass layers form the core and cladding glasses that make up
the optical
waveguides in integrated optical circuits.
BACKGROUND OF THE INVENTION
1s Applicants have developed flame hydrolysis systems which deposit thin oxide
soot layers on planar surfaces for Lightwave Optical Circuits (LOC)
applications and the
making of integrated optical waveguide devices such as integrated optical
circuits.
These soot layers are consolidated into glass layers which form optical
waveguide cores
and cladding.
2o U.S. Patent Application No. 08/581,186 filed 12729/95, Bandwidth-Adjusted
Wavelength Demultiplexer, by Denis M. Trouchet, discloses such an optical
circuit
which functions as a wavelength demultiplexer.
CA 02274478 1999-06-03
WO 98n70ig PCTfUS971Z0433
2
Conventional methods have relied on the combustion of halide compounds which
are advantageous for certain applications, but which also have serious
drawbacks.
Halogens such as chlorine will strip some of the oxides from the source
material which
will result in nonuniformity in the glass composition resulting from the
deposited soot
layer. This nonuniformity translates into a degradation of the optical
properties of the
resulting waveguiding glass layers made by these conventional halide processes
In addition to the above, the end product of the combustion reaction of the
halides is chlorine, which can react with moisture in the air to form HCI,
which is highly
corrosive and toxic and requires equipment capable of containing it.
to It can therefore be seen that there is a need for a system for forming
oxide soot
layers on planar surfaces or substrates which provides for improved uniformity
in the
glass composition, better optical performance of waveguiding glass layers
formed by the
flame hydrolysis technique, and an elimination of toxic or harmful combustion
by-
products.
SUMMARY OF THE INVENTION
The invention is directed to the use of organometallic sources for passive
planar
waveguide applications and making integrated optical waveguide devices such as
2o integrated optical circuits. The advantages of using these materials is the
elimination of
chlorine from the system. Chlorine will strip some of the laydown oxides from
these
source materials via reaction and presents problems in obtaining the desired
concentration of desired oxides) in the deposited layer. Another advantage of
using
organometallic sources is the ease of delivering materials to a conversion
site, such as a
burner, and the added safety that the elimination of chlorine provides.
A soot layer is deposited using improved flame hydrolysis deposition (FHD)
techniques of the present invention. FHD is capable of depositing thin,
uniform oxide
soot or pre-sintered' glass layers on planar substrates. In one embodiment,
waveguiding
layers are made using planar substrates made from fused silica, 100 mm in
diameter, 1
3o mm thick. Waveguiding soot glass core compositions within the Ge02-B203-
P205-Si02
system are made and cladded with a glass cladding layer within the B2O3-P2O5-
SiO2
CA 02274478 1999-06-03
WO 9B/~7818 PGT11JS97120433
3
ternary system. The glass compositions are chosen to yield targeted refractive
indices.
(For example, increasing Ge02 increases the %n.)
The FHD system of the present invention consists of a mixture of fuel gases
and
organometallic vapors that are blended and fed into a common stream within a
flame
which comprises a conversion site that is aimed directly at the fused silica
planar target
substrate. Within the methane/oxygen flame, the organometallic vapors (from at
least
two materials selected from octamethylcyclotetrasiloxane, trimethylphosphate,
triethylborate, titanium isopropoxide and germanium ethoxide) are combusted to
yield
multicomponent oxide soot particles.
1o The velocity of the flame, the ratios of the component gases in the flame,
and the
rate of vapor delivery control the final soot particle size and the degree to
which they are
sintered. The height of the target can be changed and the target can be
traversed and/or
rotated to control the temperature and distribution of the deposited
particles.
Once the,soot layer is deposited to the desired thickness, the sample may be
given a heat treatment in order to sinter and fully densify the glass. The
sintering or
consolidation depends on the glass composition and thickness. The thickness of
the
resulting waveguiding layer is typically S-9 ,um thick. The range of
consolidation
temperatures is from about 1150-1340° C and the hold time in this
temperature range is
between about 1-7 hours.
2o To make an integrated optical waveguide device, a soot layer is deposited
and
sintered on a planar substrate as described above to form a core layer. A
waveguide
circuit is etched into this core layer using photolithographic and reactive
ion etching
(RIE) techniques. A cladding layer is then deposited over the etched layer and
sintered.
2s BRIEF DESCRIPTION OF THE DRAWINGS
For a fuller understanding of the nature and objects of the invention,
reference
should be made to the following detailed description of a preferred mode of
practicing
the invention, read in connection with the accompanying drawings, in which:
3o FIG. 1 is a schematic diagram of the flame hydrolysis system for the
present
invention.
CA 02274478 1999-06-03
WO 98127018 PCT/US97/20433
4
FIG. 2 is an enlarged perspective view of the burner, showing the flame front
and tails, and how the burner-to-sample height is measured.
FIG. 3 is an exploded perspective view of the burner assembly.
FIG. 3a is a cross-sectional view through the center of the burner shown in
FIG.
3.
FIG. 4 is a side sectional view of one bubbles assembly.
FIG. 5 is a plan view of the substrate holder vacuum chuck assembly.
FIG. Sa is a bottom view of the chuck bottom.
FIG. 6 is an exploded perspective view of a second embodiment of a burner
1o assembly.
FIG. 7 illustrates a plot of consolidated glass roughness as a function of
burner
to sample height and the number of burner rows.
FIG. 8 illustrates a plot of change in composition as a fiinction of sample to
burner height.
DETAIZ,ED DESCRIPTION OF THE INVENTION
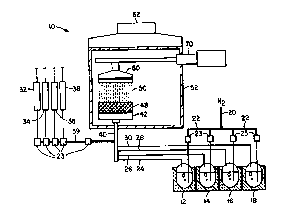
The present invention is best illustrated by FIG. 1 which is a schematic
diagram
of the flame hydrolysis system 10 of the present invention which is suitable
for use in
2o producing oxide soot layers on planar surfaces for use in lightwave optical
circuit (LOC)
applications and for making integrated optical waveguide devices such as
integrated
optical circuits. Selected organometallic liquids are stored in bubblers 12,
14, 16, and
18, respectively. The organometallic vapors generated by the system are
carried by a
nitrogen source 20 which passes N2 through heated pipes 22 into each bubbles.
The
organometallic liquid is vaporized within the bubbles and carried by the
nitrogen vapor
through heated lines 24, 26, 28 and 30, respectively. The organometallic
vapors are
mixed with a preselected mixture of fuel gases which include air 32, nitrogen
34, oxygen
36 and methane 38, and delivered from pipe 39 through pipe 40 to a burner
assembly 42
as a single stream. Oxide soot 50 is generated by combustion of the vapors in
3o conversion site flame 48 (see Fig. 2). The soot is deposited on a substrate
53 that is
held in place by a vacuum chuck assembly 60 (Figs. 5 and Sa). Optionally, the
chuck
CA 02274478 1999-06-03
WO 98/29018 PCT/US11712A~433
can be traversed and/or rotated by a conventional transverse planetary
mechanism 70
well known to the art. The exhaust from combustion, not shown, travels through
a
hood 62 to a scrubber (not shown). The burner assembly 42 and the vacuum chuck
assembly 60 are surrounded by a filtered enclosure 52.
5 As shown in Figs. 3 and 3 a, the conversion site burner assembly 42 consists
of a
housing 43 with a single row of 0.30" diameter holes 44. The housing contains
a glass-
ceramic insert 45 which contains parallel rows of holes 41, and fine mesh
stainless steel
screen wrap 46 that is inserted into a cylindrical manifolding chamber 47. In
a preferred
embodiment, which provides for a more even distribution of the vapor mixture,
insert 84
of Fig. 6 replaces screen wrap 46. The fuel gas-organometallic vapor mixture
enters the
burner through pipe 48 which is threaded into housing 43 and which is
connected to
pipe 40. The manifold chamber is sealed with threaded nut 49.
A second embodiment of a burner assembly which provides for maintaining the
flame points at equal height across the entire burner face is illustrated in
Fig. 6. In this
embodiment, the burner assembly 70 consists of a housing 72 and a burner slot
74. The
burner baffle consists of two inserts that are placed inside cylindrical
manifolding
chamber 76. A ceramic insert 80 has two parallel rows of hole ~ 82. A
stainless steel
insert 84 is constructed in such a manner that when butted against the ceramic
insert
provides a path for the vapors to be distributed evenly, while maintaining the
flame
2o points at equal height across the entire burner face. The vapor mixture
enters the burner
through pipe 86 and the chamber is sealed with threaded nut 78.
In order to ensure a layer of uniform thickness, and avoid rastering, the
length
"L" of the burner face or top surface which contains the burner slot or holes
should be at
least equal to or longer than the diameter or width of the planar substrate
being coated.
The vacuum chuck assembly 60 which holds the substrate in place is shown more
clearly in Figs. S and Sa. The chuck contains a vertical shaft 61, a rotation
collar 62, an
inner shielding ring 63 and an outer ring 67. The chuck bottom face 64 (Fig.
5a) shows
the placement of the vacuum holes 65 that hold the sample 53 in place.
In operation, organometallic liquid components such as
octamethylcyclotetrasiloxane, trimethylphosphate, triethylborate, and
germanium
ethoxide are separately placed in bubblers 12, 14, 16 and 18, respectively.
The bubblers
CA 02274478 1999-06-03
WO 98/27018 PGT/U897/Z0433
6
are connected to a nitrogen carrier gas inlet valve with an aerator in its
base and a vapor
output valve for each bubbler. As more clearly shown in FIG. 4, which is an
enlarged
view of an individual bubbler as shown in FIG. 1, each bubbler chamber 12 is
made of
stainless steel and is roughly cylindrical with a round bottom and top. The
bubbler
contains ports for the inlet of nitrogen and out-flowing vapor output 13 to
the burner
42. The nitrogen flows into the bubbler through input 15 at a given rate and
delivered
by an aerator tube that is submerged in the liquid and positioned at the
bottom of the
bubbler. For temperature control, the bubblers may optionally be wrapped in
heat tape
which are controlled by temperature controllers. The bubblers are then
insulated with an
1o appropriate insulator. The outlet lines are also heated by wrapped heat-
tape to
temperatures in excess of the boiling point of the organometallics in order to
ensure that
the vapor remains gaseous. All of the outlet lines lead to a single common
pipe 40
which acts to mix the vapors before reaching the burner. The temperature of
the pipe
must be at least as high as the highest boiling point of the components.
15 Thermocouples may be used to monitor the temperature of the organometallic
liquids. Thermocouples 17 and 18 are placed inside of each bubbler so that
they are
submerged in the liquid. An additional thermocouple 19 is attached to the
outside of
each of the bubblers, along the inlet and outlet pipes.
The bubblers also have ports for filling and draining. A drain 21 is contained
in
2o the bottom of each bubbler which is capped offunless the bubbler is being
drained.
The delivery rates of the vapor and gases are governed by mass flow
controllers
(mfcs) 23 available under the trade name TYLAN and provide a volumetric flow
rate.
The organometallics in vapor form are delivered by the nitrogen stream. The
methane
and oxygen gases are delivered separately. The system also has the capability
for
25 nitrogen, air and hydrogen as part of the fuel premix. For one embodiment
of the
invention, the ranges of the mfcs are as follows:
CITd and 02 = 10 sLpm (standard Iiter per minute)
N2 for premix = 10 sLpm
OMCTS N2 = 100 sccm (standard cc per minute)
3o TMP N2 = 200 sccm
TEB NZ = 50 sccm
GeE = 1000 sccm
CA 02274478 1999-06-03
W0 98127018 PCTIUS97IZ~.33
7
Secondary regulator (going to the mfcs):
N2 = 15 psi
OZ = 17 psi
Only the nitrogen line is filtered to trap moisture.
The lines from the mfcs are stainless steel, and lead either to the burner
manifold
directly, or to the bubblers. The lines are preheated and temperature
controlled by heat-
tape and commercially available temperature controllers. The temperature of
each line is
to equal to its own bubbler temperature.
Alternatively, the bubblers may be replaced with a vaporizer system which are
known to the art, as shown in U.S. Patent No. 4,529,427 and JP 60-108338 and
which
are incorporated herein by reference.
In one embodiment of the present invention, a soot core and a soot clad layer
are
1s formed as follows:
The core soot glass composition #5 (see Table 3 is within the GeOz-B203-P20s-
Si02 system ( 15 .79, 3.86, 2.19, and 78.16% by weight) and the clad layer
glass # 10 (see
Table 1 ) has a composition within the B2O3-PzOs-S1O2 ternary (7.95, 3.25, and
88.8%
by weight). The compositions were analyzed by standard electron probe
microanalysis
20 (EPMA) techniques.
A soot layer is deposited using standard flame hydrolysis deposition (FHD)
techniques as described for Figs. 1-S. The FHD system basically consists of
fuel gases
and organometallic vapors that are blended and fed into a common stream within
a
conversion site flame that is aimed directly at a planar target substrate.
Within the
2s methane/oxygen flame, the organometallic vapors (from
octamethylcyclotetrasiloxane
OMCTS, trimethylphosphate TMP, triethylborate TEB, and germanium ethoxide TEOG
or GE) ire combusted and converted into multicomponent oxide soot particles.
The
delivery system uses bubblers, one for each component. The bubblers are 100-
400 mL
in volume and the liquid is kept at a constant level. The carrier gas is
nitrogen, and the
30 lines into the bubblers are preheated to the same temperature as the
bubbler in order to
avoid cooling the liquid as the N2 flows. The temperatures for the bubblers
were chosen
to be as low as possible while still high enough to produce adequate vapor for
a given
N2 flow. The outlet lines need to exceed the boiling point in order to keep
the vapors of
CA 02274478 1999-06-03
wo ~s~z~ois rc°r~s9~rio~3
s
the liquids from condensing. The temperature controllers for the carrier gas
inlet lines,
bubblers, and outlet lines are preheated to the following temperatures:
Temperature (° C)
81 Inlet to the OMCTS bubbler and the bubbler
itself (22 + 18)
70 Inlet to TMP and TMP bubbler (22 + 16)
60 Inlet to TEB and TEB bubbler (22 + 14)
51 Inlet to GE and GE bubbler (22 + 12)
>176 Outlet line from OMCTS bubbler (30)
> 197 Outlet tine from TMP bubbIer (28)
> 117 Outlet line from TEB bubbler (26)
>185 Outlet line from GE bubbler (24)
r 197 Common port below the burner
A fused silica substrate (100 mm diameter, 1 mm thick) is cleaned and weighed
before placing on the chuck 60 which holds the sample above the flame by
vacuum. The
mass flow controllers are turned on to flow the carrier gas into the bubblers
to deliver a
given volume flow rate of vapor (sccm), as well as to control the flow rate of
the
2o methane and oxygen (sLpm). The delivery rates for the materials for the
core and clad
glass are as follows:
OMCTS TMP TEB GE CH., Oz
core #5 0.023 0.0002 0.009 0.006 5.85 5.6
clad #10 0.025 0.0040 0.007 0 5.85 5.6
The magnehelic below the burner monitors the backpressure, usually at 1.0 in.
H20. The height of the substrate above the flame is set accordingly to a
predetermined
distance and the substrate is traversed at a constant speed and simultaneously
rotated by
mechanism 70 to control the temperature and distribution of the deposited
particles.
The thickness of the glass soot is controlled by how many times the substrate
traverses
above the flame. Typically, the thickness of the core layer is from about 5 to
7 microns
and the thickness of the clad layer is from about 4 to 20 microns.
3o Once the soot layer is deposited to the desired thickness, the sample is
given a
heat treatment in order to sinter and fully densify the glass. The
consolidation schedule
depends on the glass composition and thickness. The thickness of the core
layer #S is 5
,um thick; the clad layer # 10 is about 4 ,cun thick. The top consolidation
temperature for
CA 02274478 1999-06-03
wo ~snao~s rcrrt~ssr~ruw~3
9
the core was 1290° C, and for the clad was 1200° C. The hold
times at those
temperatures were 2 and I hours, respectively.
The step of forming an integrated optical waveguide circuit device includes:
1. A device circuitry is etched into the core layer using photolithographic
and reactive ion etching (ItIE) techniques.
2. A cladding layer is deposited and sintered, blanketing this device
circuitry.
3. The device is pigtailed, packaged, and connectorized.
Tables 1 and 2 show a comparison of standard deviations of oxide weight
1o percentages from cross-section EPMA data for organometallic-generated
glasses of this
invention and for traditionally deposited halide-generated cladding glasses.
(The lowest
values for the halide-generated glasses (0.47 for SiOz, 0.39 for B203, and 0.
I3 P205)
were used as upper values for the degree of compositional contaol needed for
the
organometallic-generated glasses of this invention.) The glasses of this
invention show
is significantly less variation for each oxide throughout the deposited glass
layer.
25
CA 02274478 1999-06-03
WO 981270!8 PCT/US97l2A433
Table 1
- Organometallic
Clad
Example P205 stdev B203 stdev Si02 stdev
#
1 .09 0.23 0.21
2 0.10 0.19 0.23
3 0.13 0.19 0.28
4 0.11 O.I3 0.16
5 0.03 0.28 0.21
6 0.04 0.22 0.16
7 0.04 0.18 0.12
8 0.03 0.23 0.05
9 0.05 0.15 0.37
10 0.06 0.13 0.02
11 0.06 0.13 0.09
~2 0.06 0.21 0.41
13 0.09 0.13 0.10
14 0.08 0.26 0.10
0. i 1 0.31 0.02
stdev max 0.13 0.31 0.41
stdev min 0.03 0.13 0.02
Table 2
- Halide
Clad
'
I
Example P205 stdev B203 stdev SiOz stdev I
#
1 0.16 1.50 1.44
2 0.13 0.56 0.51
3 0.41 0.96 0.77
4 0.67 1.38 1.11
5 0.22 0.6I 0.47
6 0.13 0.64 0.83
7 0.15 0.55 0.51
8 0.21 0.72 0.74
stdev max 0.67 1.50 1.44
stdev min 0.13 0.5 5 0.47
CA 02274478 1999-06-03
wo 9snms rcrmss~ruw33
The following Tables 3 and 4 show comparisons of standard deviations of oxide
weight percentages for cross-section EPMA data for halide-generated core-type
glasses
and for organometallio-generated glasses of this invention. The glasses of
this invention
show significantly less variation for Ge02 and Si02 throughout the deposited
core glass
layer.
Table 3
- Organometallic
Core
Example P20s stdevB2O3 stdev Si02 stdev Ge02 stdev
#
1 0.22 0.19 0.66 0.30
2 0.02 0.16 0.59 0.45
3 0.28 0.13 0.42 0.65
4 0.21 0.24 0.17 0.26
0.21 0.13 0.21 0.16
6 0.19 0.13 0.20 0.28
7 0.15 0.23 0.55 0.56
8 0.13 0.20 0.05 0.08
9 0.36 0.20 0.31 0.30
0.14 0.12 0.01 0.21
11 0.15 0.10 0.24 0.40
stdev max 0.36 0.24 0.66 0.65
stdev min 0.02 0.01 0.01 0.08
CA 02274478 1999-06-03
WO ~2?plg PCT/US97/Z0433
12
Table 4
- Halide
Core
Example P20s stdevBz03 stdev Si02 stdevGe02 stdev
#
1 0.21 0.70 0.98 0.79
2 0.17 0.49 1.17 1.12
3 0.41 0.73 2.3 9 2.25
4 0.15 0.27 1.78 1.46
0.26 0.29 1.09 1.13
6 0.29 0.34 1.05 0.72
7 0.10 0.26 0.96 0.66
8 0.16 0.44 2.13 1.55
9_ 0.12 0.18 _ 1.74 1.45
stdev max 0.41 0.73 2.39 2.25
stdev min 0.10 0.18 0.96 0.66
The following formulas illustrate the combustion products of typical
organometallics which may be used in the present invention.
s
Table 5
Combustion Products of Organometallics
OMCTS : Octamethylcylcotetrasiloxane
CgH2aOaSi4 + 16 02 = 4 Si02 + 8 COZ + 12 H20
to
TMP: Trimethylphosphate
2(CH30)3P0 + 9 02 = P20s + 6 C02 + 9 H20
TEB: Triethylborate
2B(OCZHs)3 + 18 02 = B203 + 12 C02 + 15 H20
GeE: Germanium ethoxide
CaHzoOaGe + 12 O2 = GeO2 + 8 COi + 10 H20
CA 02274478 1999-06-03
WO 98l270I8 PCTJUS97IZA~33
13
Titanium isopropoxide
Ti(OC3H~)4 + 18 Oa = TiOa + 12 COa + 14 Ha0
In a further embodiment of the present invention, the oxide soot particles can
be
concurrently deposited and sintered into a uniform glass layer on the planar
substrate
without melting or softening the substrate. This embodiment provides the
advantage of
eliminating a separate consolidation step, avoids wafer warpage, and provides
a glass
surface which is smooth with few or no defects.
The methods by which this sintering in situ can be accomplished is by raising
the
1o substrate temperature as follows:
1. increase the methane concentration (hotter flame)
2. insulate or heat the sample holder
3. lower sample height (as close as possible to burner to obtain hotter and
smaller soot particles)
i5 4. alter the composition to a lower sintering temperature (e.g., increase
B2O3/P205)
5. use a high velocity burner (single row vs. triple row).
The first method consists of increasing the methane to oxygen ratio, while
holding all other conditions constant. The level of the oxygen is high enough
to result in
2o stoichiometric reactions while the higher methane produces a hotter flame.
Samples 10
cm in diameter are used, and the pre-sintered area is circular and clear
glass. The
boundaries of this region are measured in terms of outer diameter (cm) (see
Table 6).
When the CHI /OZ ratio increases, the diameter increases from 4.8 to 6.0 cm.
When the
ratio is about the same but the CHa is increased, the diameter increases from
6.0 to 8.8
25 cm.
Table 6
Sample # 02 (sLpm)CH4 (sLpm) CH4 /Oa Pre-sintered diameter
95-276 5.06 5.20 1.03 6.0 cm
95-278 6.00 5.20 0.87 4.8 cm
95-279 5.60 5.90 1.05 8.8 cm
CA 02274478 1999-06-03
WO 98n'r018 PCT/US97120433
14
Another method is to insulate the sample holder. This is accomplished by
covering the vacuum chuck with a thick layer of form-fitting fiberfrax. The
pre-sintered
area increased from 6.0 to 6.5 when the chuck was kept hotter through the run
(see
Table 7).
Table 7
Sample 02 (sLpm)CH4 (sLpm) CH4 /02 Pre-sintered diameter
#
95-276 5.06 5.20 1.03 6.0 cm : No insulation
95-277 5.06 5.20 1.03 6.5 cm : With insulation
A third method consists of bringing the substrate holder close to the flame
front.
The optimum burner-to-sample height is directly above the points of the flame.
A fourth method consists of modifying the composition of the glass. Fig. 8
to shows a change in composition in the consolidated glass with a ratio of
B203lPZOs
decreasing as a function of sample-to-burner height. For higher B203/P205
ratios, the
snots are more easily sintered.
A fifth method consists of increasing the velocity of the flame, such as by
using a
single row of burner holes rather than three rows of holes. The roughness of
the soot as
15 a function of coarseness of the soot can be correlated with the number of
rows of burner
holes.
Consolidated samples were measured for roughness using a profilometer,
averaging 3 traces at 3 different sites over a distance of 4 mm. The roughness
average
as a function of sample-to-burner height is shown in Fig. 7 for samples
generated using a
2o single row burner and a triple row burner. The glass surface is smoother
for the single
row burner and roughens for both cases with increasing height above the
burner.
While the present invention has been particularly shown and described with
reference to the preferred mode as illustrated in the drawing, it will be
understood by
one skilled in the art that various changes in detail may be effected therein
without
25 departing from the spirit and scope of the invention as defined by the
claims.