Note: Descriptions are shown in the official language in which they were submitted.
CA 02275535 1999-06-25
WO 98/20125 PCT/US97/14649 _
SECRETED PROTEINS AND POLYNUCLEOTIDES ENCODING THEM
This application is a continuation-in-part of application Ser. No. 08/702,420,
filed
August 14, 1996.
FIELD OF THE INVENTION
The present invention provides novel polynucleotides and proteins encoded by
such
polynucleotides, along with therapeutic, diagnostic and research utilities for
these
polynucleotides and proteins.
BACKGROUND OF THE INVENTION
Technology aimed at the discovery of protein factors (including e.g.,
cytokines, such
as lymphokines, interferons, CSFs and interleukins) has matured rapidly over
the past decade.
The now routine hybridization cloning and expression cloning techniques clone
novel
polynucleotides "directly" in the sense that they rely on information directly
related to the
discovered protein (i.e., partial DNA/amino acid sequence of the protein in
the case of
hybridization cloning; activity of the protein in the case of expression
cloning). More recent
2 0 "indirect" cloning techniques such as signal sequence cloning, which
isolates DNA sequences
based on the presence of a now well-recognized secretory leader sequence
motif, as well as
various PCR-based.or low stringency hybridization cloning techniques, have
advanced the state
of the art by making available large numbers of DNA/amino acid sequences for
proteins that
are known to have biological activity by virtue of their secreted nature in
the case of leader
2 5 sequence cloning, or by virtue of the cell or tissue source in the case of
PCR-based techniques.
It is to these proteins and the polynucleotides encoding them that the present
invention is
directed.
SUMMARY OF THE INVENTION
3 0 In one embodiment, the present invention provides a composition comprising
an
isolated polynucleotide selected from the group consisting of:
4 (a) a polynucleotide comprising the nucleotide sequence of SEQ ID
NO:1;
(b) a polynucleotide comprising the nucleotide sequence of SEQ ID NO:1
3 S from nucleotide 89 to nucleotide 421;
CA 02275535 1999-06-25
WO 98/20125 PCT/US97/14649 _ '
(c) a polynucleotide comprising the nucleotide sequence of the full length
protein coding sequence of clone H438_1 deposited under accession number ATCC
98140;
(d) a polynucleotide encoding the full length protein encoded by the
cDNA insert of clone H438_1 deposited under accession number ATCC 98140;
(e) a polynucleotide comprising the nucleotide sequence of the mature
protein coding sequence of clone H438_1 deposited under accession number ATCC
98140;
(f) a polynucleotide encoding the mature protein encoded by the cDNA
insert of clone H438_1 deposited under accession number ATCC 98140;
(g) a polynucleotide encoding a protein comprising the amino acid
sequence of SEQ ID N0:2;
(h) a polynucleotide encoding a protein comprising a fragment of the
amino acid sequence of SEQ ID N0:2 having biological activity;
(i) a polynucleotide which is an allelic variant of a polynucleotide of (a)-
(d) above;
(j) a polynucleotide which encodes a species homologue of the protein
of (g) or (h) above.
Preferably, such polynucleotide comprises the nucleotide sequence of SEQ ID
NO:1
2 0 from nucleotide 89 to nucleotide 421; the nucleotide sequence of the full
length protein coding
sequence of clone H438_1 deposited under accession number ATCC 98140; or the
nucleotide
sequence of the mature protein coding sequence of clone H438_1 deposited under
accession
number ATCC 98140. In other preferred embodiments, the polynucleotide encodes
the full
length or mature protein encoded by the cDNA insert of clone H438_1 deposited
under
2 5 accession number ATCC 98140. In yet other preferred embodiments, the
present invention
provides a polynucleotide encoding a protein comprising the amino acid
sequence of SEQ ID
N0:2 from amino acid 1 to amino acid 76.
Other embodiments provide the gene corresponding to the cDNA sequence of SEQ
ID NO:1 or SEQ ID N0:3.
3 0 In other embodiments, the present invention provides a composition
comprising a
protein, wherein said protein comprises an amino acid sequence selected from
the group
consisting of:
(a) the amino acid sequence of SEQ ID N0:2;
(b) the amino acid sequence of SEQ )D N0:2 from amino acid 1 to amino
3 5 acid 76;
2
___._.._. __ ____- ~___ _._
CA 02275535 1999-06-25
WO 98/20125 PCT/US97/14649 _
(c) fragments of the amino acid sequence of SEQ iD N0:2; and
- (d) the amino acid sequence encoded by the cDNA insert of clone
H438_1 deposited under accession number ATCC 98140;
the protein being substantially free from other mammalian proteins. Preferably
such protein
comprises the amino acid sequence of SEQ ID N0:2 or the amino acid sequence of
SEQ ID
N0:2 from amino acid 1 to amino acid 76.
- In certain preferred embodiments, the polynucleotide is operably linked to
an
expression control sequence. The invention also provides a host cell,
including bacterial, yeast,
insect and mammalian cells, transformed with such polynucleotide compositions.
Processes are also provided for producing a protein, which comprise:
(a) growing a culture of the host cell transformed with such
polynucleotide compositions in a suitable culture medium; and
(b) purifying the protein from the culture.
The protein produced according to such methods is also provided by the present
invention.
Preferred embodiments include those in which the protein produced by such
process is a
mature form of the protein.
Protein compositions of the present invention may further comprise a
pharmaceutically
acceptable carrier. Compositions comprising an antibody which specifically
reacts with such
protein are also provided by the present invention.
2 0 Methods are also provided for preventing, treating or ameliorating a
medical condition
which comprises administering to a mammalian subject a therapeutically
effective amount of
a composition comprising a protein of the present invention and a
pharmaceutically acceptable
carver.
2 5 BRIEF DESCRIPTION OF FIGURES
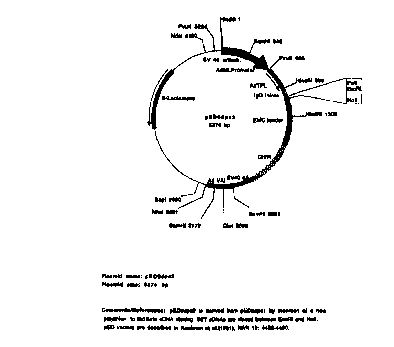
Fig. 1 is a schematic representation of the pED6 and pNotS vectors used for
deposit
of clones disclosed herein.
Fig. 2 is an autoradiograph evidencing the expression of H438_1 in COS cells.
3 0 DETAILED DESCRIPTION
ISOLATED PROTEINS AND POLYNUCLEOTIDES
Nucleotide and amino acid sequences are reported below for each clone and
protein
disclosed in the present application. In some instances the sequences are
preliminary and may
include some incorrect or ambiguous bases or amino acids. The actual
nucleotide sequence
3 5 of each clone can readily be determined by sequencing of the deposited
clone in accordance
3
CA 02275535 1999-06-25
WO 98/20125 PCT/US97/14649 _
with known methods. The predicted amino acid sequence (both full length and
mature) can
- then be determined from such nucleotide sequence. The amino acid sequence of
the protein
encoded by a particular clone can also be determined by expression of the
clone in a suitable
host cell, collecting the protein and determining its sequence.
For each disclosed protein applicants have identified what they have
determined to be
the reading frame best identifiable with sequence information available at the
time of filing.
Because of the partial ambiguity in reported sequence information, reported
protein sequences
include "Xaa" designators. These "Xaa" designators indicate either (1 ) a
residue which cannot
be identified because of nucleotide sequence ambiguity or (2) a stop codon in
the determined
2 0 nucleotide sequence where applicants believe one should not exist (if the
nucleotide sequence
were determined more accurately).
As used herein a "secreted" protein is one which, when expressed in a suitable
host
cell, is transported across or through a membrane, including transport as a
result of signal
sequences in its amino acid sequence. "Secreted" proteins include without
limitation proteins
secreted wholly (e.g., soluble proteins) or partially (e.g. , receptors) from
the cell in which they
are expressed. "Secreted" proteins also include without limitation proteins
which are
transported across the membrane of the endoplpasmic reticulum.
Clone "H438 1 "
2 0 A polynucleotide of the present invention has been identified as clone
"H438 1 ".
H438_1 was isolated from a human PBMC cDNA library using methods which are
selective
for cDNAs encoding secreted proteins. H438_1 is a full-length clone, including
the entire
coding sequence of a secreted protein (also referred to herein as "H438_1
protein")
The nucleotide sequence of the 5' portion of H438_I as presently determined is
2 5 reported in SEQ ID NO:1. What applicants believe is the proper reading
frame and the
predicted amino acid sequence encoded by such internal sequence is reported in
SEQ >D N0:2.
Additional nucleotide sequence from the 3' portion of H438_1, including the
polyA tail, is
reported in SEQ ID N0:3.
The nucleotide sequence disclosed herein for H438_1 was searched against the
3 0 GenBank database using BLASTA/BLASTX and FASTA search protocols. H438_1
demonstrated at least some identity with an EST identified as "y178e09.r1 Homo
sapiens
cDNA clone 44074 5"' (H06234, BlastN). Based upon identity) H438_1 proteins
and each
identical protein or peptide may share at least some activity.
3 5 Deposit of Clones
4
CA 02275535 1999-06-25
WO 98/20125 PCT/US97/14649 _ '
Clone H438_1 was deposited on August 14,,1996 with the American TypaCulture
Collection under accession number ATCC 98140, from which the clone comprising
a
particular polynucleotide is obtainable. Cells containing H438_1 have been
deposited as a
composite deposit with cells containing other clones. Each clone has been
transfected into
separate bacterial cells (E. coli) in this composite deposit.
Each clone can be removed from the vector in which it was deposited by
performing
- an EcoRI/NotI digestion (5' cite, EcoRI; 3' cite, NotI) to produce the
appropriate fragment for
such clone. Each clone was deposited in either the pED6 or pNotS vector
depicted in Fig. 1.
The cDNA may also be expressed from the vectors in which they were deposited.
Bacterial cells containing a particular clone can be obtained from the
composite
deposit as follows:
An oligonucleotide probe or probes should be designed to the sequence that is
known
for that particular clone. This sequence can be derived from the sequences
provided herein,
or from a combination of those sequences. The sequence of the oligonucleotide
probe that was
used to isolate each full-length clone is identified below, and should be most
reliable in
isolating the clone of interest.
Clone Probe Sequence
H438_I SEQ ID N0:4
In the sequences listed above which include an N at position 2, that position
is occupied in
preferred probes/primers by a biotinylated phosphoaramidite residue rather
than a nucleotide
(such as , for example, that produced by use of biotin phosphoramidite (1-
dimethoxytrityloxy
2-(N-biotinyl-4-aminobutyl)-propyl-3-O-(2-cyanoethyl)-(N,N-diisopropyl)-
phosphoramadite)
2 5 {Glen Research, cat. no. 10-1953)).
The design of the oligonucleotide probe should preferably follow these
parameters:
(a) It should be designed to an area of the sequence which has the fewest
ambiguous bases ("N's"), if any;
3 0 (b) It should be designed to have a Tm of approx. 80 ° C (assuming
2° for each A
or T and 4 degrees for each G or C).
The oligonucleotide should preferably be labeled with g 3zP ATP (specific
activity 6000
Ci/mmole) and T4 polynucleotide kinase using commonly employed techniques for
labeling
oligonucleotides. Other labeling techniques can also be used. Unincorporated
label should
3 5 preferably be removed by gel filtration chromatography or other
established methods. The
5
CA 02275535 1999-06-25
WO 98/20125 PCT/US97/14649 _
amount of radioactivity incorporated into the probe should be quantitated by
measurement an
- a scintillation counter. Preferably, specific activity of the resulting
probe should be
approximately 4e+6 dpm/pmole.
The bacterial culture containing the pool of full-length clones should
preferably be
thawed and 100 pI of the stock used to inoculate a sterile culture flask
containing 25 ml of
sterile L-broth containing ampicillin at 100 pg/ml. The culture should
preferably be grown to
saturation at 37°C, and the saturated culture should preferably be
diluted in fresh L-broth.
Aliquots of these dilutions should preferably be plated to determine the
dilution and volume
which will yield approximately 5000 distinct and well-separated colonies on
solid
bacteriological media containing L-broth containing ampicillin at 100 pg/ml
and agar at 1.5%
in a 150 mm petri dish when grown overnight at 37°C. Other known
methods of obtaining
distinct, well-separated colonies can also be employed.
Standard colony hybridization procedures should then be used to transfer the
colonies
to nitrocellulose filters and lyse, denature and bake them.
The filter is then preferably incubated at 65°C for 1 hour with gentle
agitation in 6X
SSC (20X stock is 175.3 g NaCI/liter, 88.2 g Na citrate/liter, adjusted to pH
7.0 with NaOH}
containing 0.5% SDS, 100 pg/ml of yeast RNA, and 10 mM EDTA (approximately 10
mL per
150 mm filter). Preferably, the probe is then added to the hybridization mix
at a concentration
greater than or equal to le+6 dpm/mL. The filter is then preferably incubated
at 65°C with
2 0 gentle agitation overnight. The filter is then preferably washed in 500 mL
of 2X SSC/0.5 %
SDS at room temperature without agitation, preferably followed by 500 mL of 2X
SSC/0.1 %
SDS at room temperature with gentle shaking for 15 minutes. A third wash with
O.1X
SSC/0.5% SDS at 65°C for 30 minutes to 1 hour is optional. The filter
is then preferably dried
and subjected to autoradiography for sufficient time to visualize the
positives on the X-ray
2 5 film. Other known hybridization methods can also be employed.
The positive colonies are picked, grown in culture, and plasmid DNA isolated
using
standard procedures. The clones can then be verified by restriction analysis,
hybridization
analysis, or DNA sequencing.
Fragments of the proteins of the present invention which are capable of
exhibiting
3 0 biological activity are also encompassed by the present invention.
Fragments of the protein
may be in linear form or they may be cyclized using known methods, for
example, as described
in H.U. Saragovi, et al., Bio/Technology 10, 773-778 (1992) and in R.S.
McDowell, et al., J.
Amer. Chem. Soc. 1 I 4, 9245-9253 ( 1992), both of which are incorporated
herein by reference.
Such fragments may be fused to earner molecules such as immunoglobulins for
many
3 5 purposes, including increasing the valency of protein binding sites. For
example, fragments
6
CA 02275535 1999-06-25
WO 98/20125 PCT/US97/14649 _ '
of the protein may be fused through "linker" sequences to the Fc portion of _
an
immunoglobulin. For a bivalent form of the protein, such a fusion could be to
the Fc portion
of an IgG molecule. Other immunoglobulin isotypes may also be used to generate
such
fusions. For example, a protein - IgM fusion would generate a decavalent form
of the protein
of the invention.
The present invention also provides both full-length and mature forms of the
disclosed
. proteins. The full-length form of the such proteins is identified in the
sequence listing by
translation of the nucleotide sequence of each disclosed clone. The mature
form of such
protein may be obtained by expression of the disclosed full-length
polynucleotide (preferably
those deposited with ATCC) in a suitable mammalian cell or other host cell.
The sequence of
the mature form of the protein may also be determinable from the amino acid
sequence of the
full-length form.
The present invention also provides genes corresponding to the cDNA sequences
disclosed herein. The corresponding genes can be isolated in accordance with
known methods
using the sequence information disclosed herein. Such methods include the
preparation of
probes or primers from the disclosed sequence information for identification
and/or
amplification of genes in appropriate genomic libraries or other sources of
genomic materials.
Where the protein of the present invention is membrane-bound (e.g., is a
receptor), the
present invention also provides for soluble forms of such protein. In such
forms part or all of
2 0 the intracellular and transmembrane domains of the protein are deleted
such that the protein
is fully secreted from the cell in which it is expressed. The
intraceilular_and transmembrane
domains of proteins of the invention can be identified in accordance with
known techniques
for determination of such domains from sequence information.
Species homologs of the disclosed polynucleotides and proteins are also
provided by
2 5 the present invention. Species homologs may be isolated and identified by
making suitable
probes or primers from the sequences provided herein and screening a suitable
nucleic acid
source from the desired species.
The invention also encompasses allelic variants of the disclosed
polynucleotides or
proteins; that is, naturally-occurring alternative forms of the isolated
polynucleotide which also
3 0 encode proteins which are identical, homologous or related to that encoded
by the
polynucleotides.
The isolated polynucleotide of the invention may be operably linked to an
expression
control sequence such as the pMT2 or pED expression vectors disclosed in
Kaufman et al.,
Nucleic Acids Res. 19, 4485-4490 ( 1991 ), in order to produce the protein-
recombinantly.
3 5 Many suitable expression control sequences are known in the art. General
methods of
7
CA 02275535 1999-06-25
WO 98/20125 PCT/US97/14649 _
expressing recombinant proteins are also known and are exemplified in R.
Kaufman; Methods
- in Enzymology 185, 537-566 ( 1990). As defined herein"operably linked" means
that the
isolated polynucleotide of the invention and an expression control sequence
are situated within
a vector or cell in such a way that the protein is expressed by a host cell
which has been
transformed (transfected) with the ligated polynucleotide/expression control
sequence.
A number of types of cells may act as suitable host cells for expression of
the protein.
Mammalian host cells include) for example, monkey COS cells, Chinese Hamster
Ovary
(CHO) cells, human kidney 293 cells, human epidermal A431 cells, human Co1o205
cells, 3T3
cells, CV-1 cells, other transformed primate cell lines) normal diploid cells,
cell strains derived
from in vitro culture of primary tissue, primary explants, HeLa cells, mouse L
cells, BHK, HL-
60, U937, HaK or Jurkat cells.
Alternatively, it may be possible to produce the protein in lower eukaryotes
such as
yeast or in prokaryotes such as bacteria. Potentially suitable yeast strains
include
Saccharomyces cerevisiae, Schizosaccharomyces pombe, Kluyveromyces strains,
Candida, or
any yeast strain capable of expressing heterologous proteins. Potentially
suitable bacterial
strains include Escherichia coli, Bacillus subtilis, Salmonella typhimurium)
or any bacterial
strain capable of expressing heterologous proteins. If the protein is made in
yeast or bacteria,
it may be necessary to modify the protein produced therein, for example by
phosphorylation
or glycosylation of the appropriate sites, in order to obtain the functional
protein. Such
2 0 covalent attachments may be accomplished using known chemical or enzymatic
methods.
The protein may also be produced by operably linking the isolated
polynucleotide of
the invention to suitable control sequences in one or more insect expression
vectors, and
employing an insect expression system. Materials and methods for
baculovirus/insect cell
expression systems are commercially available in kit form from, e.g.,
Invitrogen, San Diego,
2 5 California, U.S.A. (the MaxBacO kit), and such methods are well known in
the art, as
described in Summers and Smith) Texas Aericultural Experiment Station Bulletin
No 1555
1( 987), incorporated herein by reference. As used herein, an insect cell
capable of expressing
a polynucleotide of the present invention is "transformed."
The protein of the invention may be prepared by culturing transformed host
cells under
3 0 culture conditions suitable to express the recombinant protein. The
resulting expressed protein
may then be purified from such culture (i.e.) from culture medium or cell
extracts) using known
purification processes, such as gel filtration and ion exchange
chromatography. The
purification of the protein may also include an affinity column containing
agents which will
bind to the protein; one or more column steps over such affinity resins as
concanavalin A
3 5 agarose, heparin-toyopearl~ or Cibacrom blue 3GA Sepharose~; one or more
steps involving
8
_~.__ _ _._______ .~_._ T.____r.~.~__ _ _ .~__ 1.
CA 02275535 1999-06-25
WO 98/20125 PCT/US97/14649 _ '
hydrophobic interaction chromatography using such resins as phenyl ether,
butyl.ether,. or
propyl ether; or immunoaffinity chromatography.
Alternatively, the protein of the invention may also be expressed in a form
which will
facilitate purification. For example, it may be expressed as a fusion protein,
such as those of
maltose binding protein (MBP), glutathione-S-transferase (GST) or thioredoxin
(TRX). Kits
for expression and purification of such fusion proteins are commercially
available from New
England BioLab (Beverly, MA), Pharmacia (Piscataway, NJ) and InVitrogen,
respectively.
The protein can also be tagged with an epitope and subsequently purified by
using a specific
antibody directed to such epitope. One such epitope ("Flag") is commercially
available from
Kodak (New Haven, CT).
Finally, one or more reverse-phase high performance liquid chromatography (RP-
HPLC) steps employing hydrophobic RP-HPLC media, e.g., silica gel having
pendant methyl
or other aliphatic groups, can be employed to further purify the protein. Some
or all of the
foregoing purification steps, in various combinations, can also be employed to
provide a
1 S substantially homogeneous isolated recombinant protein. The protein thus
purified is
substantially free of other mammalian proteins and is defined in accordance
with the present
invention as an "isolated protein."
The protein of the invention may also be expressed as a product of transgenic
animals,
e.g., as a component of the milk of transgenic cows, goats, pigs, or sheep
which are
2 0 characterized by somatic or germ cells containing a nucleotide sequence
encoding the protein.
The protein may also be produced by known conventional chemical synthesis.
Methods for constructing the proteins of the present invention by synthetic
means are known
to those skilled in the art. The synthetically-constructed protein sequences,
by virtue of sharing
primary, secondary or tertiary structural and/or conformational
characteristics with proteins
2 5 may possess biological properties in common therewith, including protein
activity. Thus, they
may be employed as biologically active or immunological substitutes for
natural, purified
proteins in screening of therapeutic compounds and in immunological processes
for the
development of antibodies.
The proteins provided herein also include proteins characterized by amino acid
3 0 sequences similar to those of purified proteins but into which
modification are naturally
provided or deliberately engineered. For example, modifications in the peptide
or DNA
sequences can be made by those skilled in the art using known techniques.
Modifications of
interest in the protein sequences may include the alteration, substitution,
replacement, insertion
or deletion of a selected amino acid residue in the coding sequence. For
example, one or more
3 5 of the cysteine residues may be deleted or replaced with another amino
acid to alter the
9
CA 02275535 1999-06-25
WO 98/20125 PCT/US97/14649 _
conformation of the molecule. Techniques for such alteration, substitution,
replacemept,
insertion or deletion are well known to those skilled in the art (see, e.g.,
U.S. Patent No.
4,518,584). Preferably, such alteration, substitution, replacement, insertion
or deletion retains
the desired activity of the protein.
Other fragments and derivatives of the sequences of proteins which would be
expected
to retain protein activity in whole or in part and may thus be useful for
screening or other
immunological methodologies may also be easily made by those skilled in the
art given the
disclosures herein. Such modifications are believed to be encompassed by the
present
invention.
USES AND BIOLOGICAL ACTIVITY
The polynucleotides and proteins of the present invention are expected to
exhibit one
or more of the uses or biological activities (including those associated with
assays cited herein)
identified below. Uses or activities described for proteins of the present
invention may be
provided by administration or use of such proteins or by administration or use
of
polynucleotides encoding such proteins (such as, for example, in gene
therapies or vectors
suitable for introduction of DNA).
Research Uses and Utilities
2 0 The polynucleotides provided by the present invention can be used by the
research
community for various purposes. The polynucleotides can be used to express
recombinant
protein for analysis, characterization or therapeutic use; as markers for
tissues in which the
corresponding protein is preferentially expressed (either constitutively or at
a particular stage
of tissue differentiation or development or in disease states); as molecular
weight markers on
2 5 Southern gels; as chromosome markers or tags (when labeled) to identify
chromosomes or to
map reiated gene positions; to compare with endogenous DNA sequences in
patients to identify
potential genetic disorders; as probes to hybridize and thus discover novel,
related DNA
sequences; as a source of information to derive PCR primers for genetic
fingerprinting; as a
probe to "subtract-out" known sequences in the process of discovering other
novel
3 0 polynucleotides; for selecting and making oligomers for attachment to a
"gene chip" or other
support, including for examination of expression patterns; to raise anti-
protein antibodies using
DNA immunization techniques; and as an antigen to raise anti-DNA antibodies or
elicit
another immune response. Where the polynucleotide encodes a protein which
binds or
potentially binds to another protein (such as, for example, in a receptor-
ligand interaction), the
3 5 polynucleotide can also be used in interaction trap assays (such as, for
example, that described
__ ___. __T T _.__..._ . 1.
CA 02275535 1999-06-25
WO 98/20125 PCT/US97/14649 _
in Gyuris et al., Cell 75:791-803 ( 1993)) to identify polynucleotides
encoding the other protein
- with which binding occurs or to identify inhibitors of the binding
interaction.
The proteins provided by the present invention can similarly be used in assay
to
determine biological activity, including in a panel of multiple proteins for
high-throughput
screening; to raise antibodies or to elicit another immune response; as a
reagent (including the
labeled reagent) in assays designed to quantitatively determine levels of the
protein (or its
receptor) in biological fluids; as markers for tissues in which the
corresponding protein is
preferentially expressed (either constitutively or at a particular stage of
tissue differentiation
or development or in a disease state); and, of course, to isolate correlative
receptors or ligands.
Where the protein binds or potentially binds to another protein (such as, for
example, in a
receptor-ligand interaction)) the protein can be used to identify the other
protein with which
binding occurs or to identify inhibitors of the binding interaction. Proteins
involved in these
binding interactions can also be used to screen for peptide or small molecule
inhibitors or
agonists of the binding interaction.
Any or all of these research utilities are capable of being developed into
reagent grade
or kit format for commercialization as research products.
Methods for performing the uses listed above are well known to those skilled
in the
art. References disclosing such methods include without limitation "Molecular
Cloning: A
Laboratory Manual", 2d ed., Cold Spring Harbor Laboratory Press) Sambrook, J.,
E.F. Fritsch
2 0 and T. Maniatis eds.) 1989, and "Methods in Enzymology: Guide to Molecular
Cloning
Techniques", Academic Press, Berger, S.L. and A.R. Kimmel eds.) 1987.
Nutritional Uses
Polynucleotides and proteins of the present invention can also be used as
nutritional
2 5 sources or supplements. Such uses include without limitation use as a
protein or amino acid
supplement, use as a carbon source, use as a nitrogen source and use as a
source of
carbohydrate. In such cases the protein or polynucleotide of the invention can
be added to the
feed of a particular organism or can be administered as a separate solid or
liquid preparation,
such as in the form of powder, pills, solutions, suspensions or capsules. In
the case of
3 0 microorganisms, the protein or polynucleotide of the invention can be
added to the medium
in or on which the microorganism is cultured.
Cytokine and Cell Proliferation/Differentiation Activi
A protein of the present invention may exhibit cytokine) cell proliferation
(either
3 5 inducing or inhibiting) or cell differentiation (either inducing or
inhibiting) activity or may
11
CA 02275535 1999-06-25
WO 98/20125 PCT/L1S97/14649 _ '
induce production of other cytokines in certain cell populations. Many protein
factors
discovered to date, including all known cytokines, have exhibited activity in
one or more factor
dependent cell proliferation assays, and hence the assays serve as a
convenient confirmation
of cytokine activity. The activity of a protein of the present invention is
evidenced by any one
of a number of routine factor dependent cell proliferation assays for cell
lines including,
without limitation, 32D, DA2, DA1G, T10, B9, B9/11, BaF3, MC9/G) M+ (preB M+))
2E8,
RBS, DA1, 123, T1165, HT2, CTLL2, TF-l, Mo7e and CMK.
The activity of a protein of the invention may, among other means, be measured
by the
following methods:
Assays for T-cell or thymocyte proliferation include without limitation those
described
in: Current Protocols in Immunology, Ed by J. E. Coligan, A.M. Kruisbeek, D.H.
Margulies,
E.M. Shevach, W Strober, Pub. Greene Publishing Associates and Wiley-
Interscience (Chapter
3, In Vitro assays for Mouse Lymphocyte Function 3.1-3.19; Chapter 7,
Immunologic studies
in Humans); Takai et aL, J. Immunol. 137:3494-3500, 1986; Bertagnolli et al.)
J. Immunol.
145:1706-1712, 1990; Bertagnolli et al., Cellular Immunology 133:327-341,
1991;
Bertagnolli, et al., J. Immunol. 149:3778-3783, 1992; Bowman et al., J.
Immunol. 152: 1756-
1761, 1994.
Assays for cytokine production and/or proliferation of spleen cells) lymph
node cells
or thymocytes include, without limitation, those described in: Polyclonal T
cell stimulation,
2 0 Kruisbeek, A.M. and Shevach, E.M. In Current Protocols in Immunology.
J.E.e.a. Coligan
eds. Vol 1 pp. 3.12.1-3.12.14, John Wiley and Sons, Toronto. 1994; and
Measurement of
mouse and human Interferon y, Schreiber) R.D. In Current Protocols in
Immunology. J.E.e.a.
Coligan eds. Vol I pp. 6.8.1-6.8.8, John Wiley and Sons, Toronto. 1994.
Assays for proliferation and differentiation of hematopoietic and
lymphopoietic cells
2 5 include, without limitation, those described in: Measurement of Human and
Murine Interleukin
2 and Interleukin 4, Bottomiy, K., Davis, L.S. and Lipsky, P.E. In Current
Protocols in
Immunology. J.E.e.a. Coligan eds. Vol I pp. 6.3.1-6.3.12, John Wiley and Sons,
Toronto.
1991; deVries et al., J. Exp. Med. 173:1205-1211, 1991; Moreau et al., Nature
336:690-692,
1988; Greenberger et al., Proc. Natl. Acad. Sci. U.S.A. 80:2931-2938, 1983;
Measurement of
3 0 mouse and human interleukin 6 - Nordan, R. In Current Protocols in
Immunology. J.E.e.a.
Coligan eds. Vol I pp. 6.6.1-6.6.5, John Wiley and Sons, Toronto. 1991; Smith
et al., Proc.
Natl. Acad. Sci. U.S.A. 83:1857-1861, 1986; Measurement of human Interleukin
11 - Bennett,
F., Giannotti, J., Clark, S.C. and Turner, K. J. In Current Protocols in
Immunology. J.E.e.a.
Coligan eds. Vol 1 pp. 6.15.1 John Wiley and Sons, Toronto. 1991; Measurement
of mouse
3 5 and human Interleukin 9 - Ciarletta, A., Giannotti, J., Clark, S.C. and
Turner, K.J. In Current
12
_ _ _ _r___..~__ _ __
CA 02275535 1999-06-25
WO 98/20125 PCT/ITS97/14649 _ '
Protocols in Immunology. J.E.e.a. Coligan eds. Vol 1 pp. 6.13.1, John Wiley
and Sons,
- Toronto. 1991.
Assays for T-cell clone responses to antigens (which will identify, among
others,
proteins that affect APC-T cell interactions as well as direct T-cell effects
by measuring
proliferation and cytokine production) include, without limitation, those
described in: Current
Protocols in Immunology, Ed by J. E. Coligan, A.M. Kruisbeek, D.H. Margulies,
E.M.
Shevach, W Strober, Pub. Greene Publishing Associates and Wiley-Interscience
(Chapter 3)
In Vitro assays for Mouse Lymphocyte Function; Chapter 6, Cytokines and their
cellular
receptors; Chapter 7, Immunologic studies in Humans); Weinberger et al., Proc.
Natl. Acad.
Sci. USA 77:6091-6095, 1980; Weinberger et al., Eur. J. Immun. 11:405-411,
1981; Takai
et al., J. Immunol. 137:3494-3500, 1986; Takai et al., J. Immunol. 140:508-
512, 1988.
Immune Stimulating or Suppressing Activity
A protein of the present invention may also exhibit immune stimulating or
immune
suppressing activity) including without limitation the activities for which
assays are described
herein. A protein may be useful in the treatment of various immune
deficiencies and disorders
(including severe combined immunodeficiency (SCID)), e.g., in regulating (up
or down)
growth and proliferation of T and/or B lymphocytes, as well as effecting the
cytolytic activity
of NK cells and other cell populations. These immune deficiencies may be
genetic or be
2 0 caused by viral (e.g., HIV) as well as bacterial or fungal infections, or
may result from
autoimmune disorders. More specifically) infectious diseases causes by viral,
bacterial) fungal
or other infection may be treatable using a protein of the present invention,
including
infections by HIV, hepatitis viruses, herpesviruses, mycobacteria, Leishmania
spp., malaria
spp. and various fungal infections such as candidiasis. Of course, in this
regard, a protein of
2 5 the present invention may also be useful where a boost to the immune
system generally may
be desirable, i.e.) in the treatment of cancer.
Autoimmune disorders which may be treated using a protein of the present
invention
include, for example, connective tissue disease, multiple sclerosis, systemic
lupus
erythematosus, rheumatoid arthritis, autoimmune pulmonary inflammation,
Guillain-Bane
3 0 syndrome, autoimmune thyroiditis, insulin dependent diabetes mellitis,
myasthenia gravis,
graft-versus-host disease and autoimmune inflammatory eye disease. Such a
protein of the
present invention may also to be useful in the treatment of allergic reactions
and conditions,
such as asthma (particularly allergic asthma) or other respiratory problems.
Other conditions,
in which immune suppression is desired (including, for example, organ
transplantation), may
3 5 also be treatable using a protein of the present invention.
13
CA 02275535 1999-06-25
WO 98/20125 PCT/IJS97/14649 _ '
Using the proteins of the invention it may also be possible to immune
responses) in_a
number of ways. Down regulation may be in the form of inhibiting or blocking
an immune
response already in progress or may involve preventing the induction of an
immune response.
The functions of activated T cells may be inhibited by suppressing T cell
responses or by
inducing specific tolerance in T cells, or both. Immunosuppression of T cell
responses is
generally an active, non-antigen-specific, process which requires continuous
exposure of the
T cells to the suppressive agent. Tolerance, which involves inducing non-
responsiveness or
anergy in T cells, is distinguishable from immunosuppression in that it is
generally antigen-
specific and persists after exposure to the tolerizing agent has ceased.
Operationally, tolerance
can be demonstrated by the lack of a T cell response upon reexposure to
specific antigen in the
absence of the tolerizing agent.
Down regulating or preventing one or more antigen functions (including without
limitation B lymphocyte antigen functions (such as , for example, B7)), e.g.,
preventing high
level lymphokine synthesis by activated T cells, will be useful in situations
of tissue, skin and
organ transplantation and in graft-versus-host disease (GVHD). For example,
blockage of T
cell function should result in reduced tissue destruction in tissue
transplantation. Typically,
in tissue transplants, rejection of the transplant is initiated through its
recognition as foreign
by T cells, followed by an immune reaction that destroys the transplant. The
administration
of a molecule which inhibits or blocks interaction of a B7 lymphocyte antigen
with its natural
2 0 ligand(s) on immune cells (such as a soluble, monomeric form of a peptide
having B7-2
activity alone or in conjunction with a monomeric form of a peptide having an
activity of
another B lymphocyte antigen (e.g., B7-l, B7-3) or blocking antibody), prior
to transplantation
can lead to the binding of the molecule to the natural ligand(s) on the immune
cells without
transmitting the corresponding costimulatory signal. Blocking B lymphocyte
antigen function
2 5 in this matter prevents cytokine synthesis by immune cells, such as T
cells, and thus acts as an
immunosuppressant. Moreover, the lack of costimulation may also be sufficient
to anergize
the T cells, thereby inducing tolerance in a subject. Induction of long-term
tolerance by B
lymphocyte antigen-blocking reagents may avoid the necessity of repeated
administration of
these blocking reagents. To achieve sufficient immunosuppression or tolerance
in a subject,
3 0 it may also be necessary to block the function of a combination of B
lymphocyte antigens.
The efficacy of particular blocking reagents in preventing organ transplant
rejection
or GVHD can be assessed using animal models that are predictive of efficacy in
humans.
Examples of appropriate systems which can be used include allogeneic cardiac
grafts in rats
and xenogeneic pancreatic islet cell grafts in mice, both of which have been
used to examine
3 5 the immunosuppressive effects of CTLA4Ig fusion proteins in vivo as
described in Lenschow
14
_.... _....__.______ T~.___ _ _ _ _ .__.~__
CA 02275535 1999-06-25
WO 98120125 PCT/US97/14649 _ '
et al., Science 257:789-792 ( 1992) and Turka et al.) Proc. Natl. Acad. Sci
USA, 89:11102-
11105 (1992). In addition, murine models of GVI-ID (see Paul ed., Fundamental
Immunology,
Raven Press, New York, 1989, pp. 846-847) can be used to determine the effect
of blocking
B lymphocyte antigen function in vivo on the development of that disease.
Blocking antigen function may also be therapeutically useful for treating
autoimmune_
diseases. Many autoimmune disorders are the result of inappropriate activation
of T cells that
are reactive against self tissue and which promote the production of cytokines
and
autoantibodies involved in the pathology of the diseases. Preventing the
activation of
autoreactive T cells may reduce or eliminate disease symptoms. Administration
of reagents
which block costimulation of T cells by disrupting receptor:ligand
interactions of B
lymphocyte antigens can be used to inhibit T cell activation and prevent
production of
autoantibodies or T cell-derived cytokines which may be involved in the
disease process.
Additionally) blocking reagents may induce antigen-specific tolerance of
autoreactive T cells
which could lead to long-term relief from the disease. The efficacy of
blocking reagents in
preventing or alleviating autoimmune disorders can be determined using a
number of well-
characterized animal models of human autoimmune diseases. Examples include
murine
experimental autoimmune encephalitis, systemic lupus erythmatosis in
MRLllprllpr mice or
NZB hybrid mice, murine autoimmune collagen arthritis, diabetes mellitus in
NOD mice and
BB rats, and murine experimental myasthenia gravis (see Paul ed., Fundamental
Immunology,
2 0 Raven Press, New York, 1989, pp. 840-856).
Upregulation of an antigen function (preferably a B lymphocyte antigen
function), as
a means of up regulating immune responses) may also be useful in therapy.
Upregulation of
immune responses may be in the form of enhancing an existing immune response
or eliciting
an initial immune response. For example, enhancing an immune response through
stimulating
2 5 B lymphocyte antigen function may be useful in cases of viral infection.
In addition, systemic
viral diseases such as influenza, the common cold) and encephalitis might be
alleviated by the
administration of stimulatory forms of B lymphocyte antigens systemically.
Alternatively, anti-viral immune responses may be enhanced in an infected
patient by
removing T cells from the patient, costimulating the T cells in vitro with
viral antigen-pulsed
3 0 APCs either expressing a peptide of the present invention or together with
a stimulatory form
of a soluble peptide of the present invention and reintroducing the in vitro
activated T cells into
the patient. Another method of enhancing anti-viral immune responses would be
to isolate
infected cells from a patient, transfect them with a nucleic acid encoding a
protein of the
present invention as described herein such that the cells express all or a
portion of the protein
CA 02275535 1999-06-25
WO 98/20125 PCT/US97/14649 _ '
on their surface, and reintroduce the transfected cells into the patient. The
infected cells would
now be capable of delivering a costimulatory signal to, and thereby activate,
T cells in vivo.
In another application, up regulation or enhancement of antigen function
(preferably
B lymphocyte antigen function) may be useful in the induction of tumor
immunity. Tumor
cells (e.g., sarcoma, melanoma, lymphoma, leukemia, neuroblastoma, carcinoma)
transfected
with a nucleic acid encoding at least one peptide of the present invention can
be administered
to a subject to overcome tumor-specific tolerance in the subject. If desired)
the tumor cell can
be transfected to express a combination of peptides. For example> tumor cells
obtained from
a patient can be transfected ex vivo with an expression vector directing the
expression of a
peptide having B7-2-like activity alone, or in conjunction with a peptide
having B7-1-like
activity and/or B7-3-like activity. The transfected tumor cells are returned
to the patient to
result in expression of the peptides on the surface of the transfected cell.
Alternatively, gene
therapy techniques can be used to target a tumor cell for transfection in
vivo.
The presence of the peptide of the present invention having the activity of a
B
lymphocyte antigens) on the surface of the tumor cell provides the necessary
costimulation
signal to T cells to induce a T cell mediated immune response against the
transfected tumor
cells. In addition) tumor cells which lack MHC class I or MHC class II
molecules, or which
fail to reexpress sufficient amounts of MHC class I or MHC class II molecules,
can be
transfected with nucleic acid encoding all or a portion of (e.g., a
cytoplasmic-domain truncated
2 0 portion) of an MHC class I a chain protein and ~3, microglobulin protein
or an MHC class II
a chain protein and an MHC class II ~i chain protein to thereby express MHC
class I or MHC
class II proteins on the cell surface. Expression of the appropriate class I
or class II MHC in
conjunction with a peptide having the activity of a B lymphocyte antigen
(e.g., B7-l, B7-2, B7-
3) induces a T cell mediated immune response against the transfected tumor
cell. Optionally,
2 5 a gene encoding an antisense construct which blocks expression of an MHC
class II associated
protein, such as the invariant chain, can also be cotransfected with a DNA
encoding a peptide
having the activity of a B lymphocyte antigen to promote presentation of tumor
associated
antigens and induce tumor specific immunity. Thus, the induction of a T cell
mediated
immune response in a human subject may be sufficient to overcome tumor-
specific tolerance
3 0 in the subject.
The activity of a protein of the invention may, among other means, be measured
by the
following methods:
Suitable assays for thymocyte or splenocyte cytotoxicity include, without
limitation,
those described in: Current Protocols in Immunology, Ed by J. E. Coligan, A.M.
Kruisbeek,
3 5 D.H. Margulies, E.M. Shevach, W Strober, Pub. Greene Publishing Associates
and Wiley-
16
__ _____ _ T ~ _
CA 02275535 1999-06-25
WO 98/20125 PCT/US97I14649 _
Interscience (Chapter 3, In Vitro assays for Mouse Lymphocyte Function 3.1-
3.19; Chapter_7,
Immunologic studies in Humans); Hemnann et al., Proc. Natl. Acad. Sci. USA
78:2488-2492,
1981; Herrmann et al., J. Immunol. 128:1968-1974, 1982; Handa et al., J.
Immunol.
135:1564-1572, 1985; Takai et ai., J. Immunol. 137:3494-3500, 1986; Takai et
al., J.
Immunol. 140:508-512, 1988; Herrmann et al., Proc. Natl. Acad. Sci. USA
78:2488-2492,
1981; Hemnann et al., J. Immunol. I 28:1968-1974, 1982; Handa et al., J.
Immunol.
135:1564-1572, 1985; Takai et al., J. Immunol. 137:3494-3500, 1986; Bowmanet
al., J.
Virology 61:1992-1998; Takai et al., J. Immunol. 140:508-512, 1988;
Bertagnolli et al.,
Cellular Immunology 133:327-341, 1991; Brown et al., J. Immunol. 153:3079-
3092) 1994.
Assays for T-cell-dependent immunoglobulin responses and isotype switching
(which
will identify) among others, proteins that modulate T-cell dependent antibody
responses and
that affect Th 1 /Th2 profiles) include, without limitation, those described
in: Maliszewski, J.
Immunol. 144:3028-3033, 1990; and Assays for B cell function: In vitro
antibody production,
Mond, J.J. and Brunswick) M. In Current Protocols in Immunology. J.E.e.a.
Coligan eds. Vol
1 pp. 3.8.1-3.8.16, John Wiley and Sons, Toronto. 1994.
Mixed lymphocyte reaction (MLR) assays (which will identify, among others,
proteins
that generate predominantly Th I and CTL responses) include) without
limitation, those
described in: Current Protocols in Immunology, Ed by J. E. Coligan, A.M.
Kruisbeek, D.H.
Margulies, E.M. Shevach, W Strober, Pub. Greene Publishing Associates and
Wiley-
2 0 Interscience (Chapter 3, In Vitro assays for Mouse Lymphocyte Function 3.1-
3.19; Chapter 7,
Immunologic studies in Humans); Takai et al., J. Immunol. 137:3494-3500, 1986;
Takai et al.,
J. Immunol. 140:508-512, 1988; Bertagnolli et al.) J. Immunol. 149:3778-3783,
1992.
Dendritic cell-dependent assays (which will identify, among others, proteins
expressed
by dendritic cells that activate naive T-cells) include, without limitation,
those described in:
2 5 Guery et al., J. Immunol. 134:536-544, 1995; Inaba et al.) Journal of
Experimental Medicine
173:549-559, 1991; Macatonia et al., Journal of Immunology 154:5071-5079,
1995; Porgador
et al., Journal of Experimental Medicine 182:255-260, 1995; Nair et al.)
Journal of Virology
67:4062-4069, 1993; Huang et al., Science 264:961-965, 1994; Macatonia et al.,
Journal of
Experimental Medicine 169:1255-1264, 1989; Bhardwaj et al., Journal of
Clinical
3 0 Investigation 94:797-807, 1994; and Inaba et al., Journal of Experimental
Medicine 172:631-
640, 1990.
Assays for lymphocyte survivaUapoptosis (which will identify, among others,
proteins
that prevent apoptosis after superantigen induction and proteins that regulate
lymphocyte
homeostasis) include, without limitation, those described in: Darzynkiewicz et
al., Cytometry
3 5 13:795-808) 1992; Gorczyca et al.) Leukemia 7:659-670, 1993; Gorczyca et
al., Cancer
17
CA 02275535 1999-06-25
WO 98/20125 PCT/US97/14649 _ '
Research 53:1945-1951, 1993; Itoh et al., Cell 66:233-243, 1991; Zacharchuk,
Journal of
Immunology 145:4037-4045, 1990; Zamai et al., Cytometry 14:891-897, 1993;
Gorczyca et
al., International Journal of Oncology 1:639-648, 1992.
Assays for proteins that influence early steps of T-cell commitment and
development
include) without limitation, those described in: Antica et al., Blood 84:111-
117, 1994; Fine
et al., Cellular Immunology 155:111-122, 1994; Galy et al., Blood 85:2770-
2778, 1995; Toki
et al., Proc. Nat. Acad Sci. USA 88:7548-7551, 1991.
Hematopoiesis Re ulg arin~ Activitx
A protein of the present invention may be useful in regulation of
hematopoiesis and,
consequently> in the treatment of myeloid or lymphoid cell deficiencies. Even
marginal
biological activity in support of colony forming cells or of factor-dependent
cell lines indicates
involvement in regulating hematopoiesis, e.g. in supporting the growth and
proliferation of
erythroid progenitor cells alone or in combination with other cytokines,
thereby indicating
utility) for example, in treating various anemias or for use in conjunction
with
irradiation/chemotherapy to stimulate the production of erythroid precursors
and/or erythroid
cells; in supporting the growth and proliferation of myeloid cells such as
granulocytes and
monocytes/macrophages (i.e., traditional CSF activity) useful, for example, in
conjunction with
chemotherapy to prevent or treat consequent myelo-suppression; in supporting
the growth and
2 0 proliferation of megakaryocytes and consequently of platelets thereby
allowing prevention or
treatment of various platelet disorders such as thrombocytopenia, and
generally for use in place
of or complimentary to platelet transfusions; and/or in supporting the growth
and proliferation
of hematopoietic stem cells which are capable of maturing to any and all of
the above-
mentioned hematopoietic cells and therefore find therapeutic utility in
various stem cell
2 5 disorders (such as those usually treated with transplantation) including,
without limitation,
aplastic anemia and paroxysmal nocturnal hemoglobinuria), as well as in
repopulating the stem
cell compartment post irradiation/chemotherapy, either in-vivo or ex-vivo
(i.e., in conjunction
with bone marrow transplantation or with peripheral progenitor cell
transplantation
(homologous or heterologous)) as normal cells or genetically manipulated for
gene therapy.
3 0 The activity of a protein of the invention may, among other means, be
measured by the
following methods:
Suitable assays for proliferation and differentiation of various hematopoietic
lines are
cited above.
Assays for embryonic stem cell differentiation (which will identify, among
others,
3 5 proteins that influence embryonic differentiation hematopoiesis) include)
without limitation,
18
_ ___ . ..T ~
CA 02275535 1999-06-25
WO 98/20125 PCT/US97/14649 _
those described in: Johansson et al. Cellular Biology 15:141-151, I 995;
Keller et al., Molecular
and Cellular Biology 13:473-486, 1993; McClanahan et al., Blood 81:2903-2915,
1993.
Assays for stem cell survival and differentiation (which will identify, among
others)
proteins that regulate lympho-hematopoiesis) include, without limitation,
those described in:
Methylcellulose colony forming assays, Freshney, M.G. In Culture of
Hematopoietic Cells. R.I.
Freshney, et al. eds. Vol pp. 265-268, Wiley-Liss, Inc., New York, NY. 1994;
Hirayama et
al., Proc. Natl. Acad. Sci. USA 89:5907-5911, I 992; Primitive hematopoietic
colony forming
cells with high proliferative potential, McNiece, LK. and Briddell, R.A. In
Culture of
Hematopoietic Cells. R.I. Freshney, et al. eds. Vol pp. 23-39, Wiley-Liss,
Inc., New York,
NY. 1994; Neben et al., Experimental Hematology 22:353-359) 1994; Cobblestone
area
forming cell assay, Ploemacher, R.E. In Culture of Hematopoietic Cells. R.I.
Freshney) et al.
eds: Vol pp. 1-21, Wiley-Liss, Inc.., New York, NY. 1994; Long term bone
marrow cultures
in the presence of stromal cells) Spooncer, E., Dexter, M. and Allen, T. In
Cultccre of
Hematopoietic Cells. R.I. Freshney, et al. eds. Vol pp. 163-179, Wiley-Liss)
Inc., New York,
NY. 1994; Long term culture initiating cell assay, Sutherland, H.J. In Culture
of Hematopoietic
Cells. R.I. Freshney, et al. eds. Vol pp. 139-162, Wiley-Liss, Inc., New York,
NY. 1994.
Tissue Growth Activity
A protein of the present invention also may have utility in compositions used
for bone,
2 0 cartilage, tendon) ligament and/or nerve tissue growth or regeneration, as
well as for wound
healing and tissue repair and replacement, and in the treatment of burns,
incisions and ulcers.
A protein of the present invention, which induces cartilage and/or bone growth
in
circumstances where bone is not normally formed, has application in the
healing of bone
fractures and cartilage damage or defects in humans and other animals. Such a
preparation
2 5 employing a protein of the invention may have prophylactic use in closed
as well as open
fracture reduction and also in the improved fixation of artificial joints. De
novo bone
formation induced by an osteogenic agent contributes to the repair of
congenital, trauma
induced, or oncologic resection induced craniofacial defects, and also is
useful in cosmetic
plastic surgery.
3 0 A protein of this invention may also be used in the treatment of
periodontal disease,
and in other tooth repair processes. Such agents may provide an environment to
attract bone-
forming cells) stimulate growth of bone-forming cells or induce
differentiation of progenitors
of bane-forming cells. A protein of the invention may also be useful in the
treatment of
osteoporosis or osteoarthritis, such as through stimulation of bone and/or
cartilage repair or by
19
CA 02275535 1999-06-25
WO 98/20125 PCT/US97114649 _
blocking inflammation or processes of tissue destruction (collagenase
activity, osteoclast
activity, etc.) mediated by inflammatory processes.
Another category of tissue regeneration activity that may be attributable to
the protein
of the present invention is tendon/ligament formation. A protein of the
present invention,
which induces tendon/ligament-like tissue or other tissue formation in
circumstances where
such tissue is not normally formed, has application in the healing of tendon
or ligament tears,
deformities and other tendon or ligament defects in humans and other animals.
Such a
preparation employing a tendon/ligament-like tissue inducing protein may have
prophylactic
use in preventing damage to tendon or ligament tissue, as well as use in the
improved fixation
of tendon or ligament to bone or other tissues, and in repairing defects to
tendon or ligament
tissue. De novo tendon/ligament-like tissue formation induced by a composition
of the present
invention contributes to the repair of congenital, trauma induced, or other
tendon or ligament
defects of other origin, and is also useful in cosmetic plastic surgery for
attachment or repair
of tendons or ligaments. The compositions of the present invention may provide
an
environment to attract tendon- or ligament-forming cells, stimulate growth of
tendon- or
ligament-forming cells, induce differentiation of progenitors of tendon- or
ligament-forming
cells, or induce growth of tendon/ligament cells or progenitors ex vivo for
return in vivo to
effect tissue repair. The compositions of the invention may also be useful in
the treatment of
tendinitis, carpal tunnel syndrome and other tendon or ligament defects. The
compositions
2 0 may also include an appropriate matrix and/or sequestering agent as a
carrier as is well known
in the art.
The protein of the present invention may also be useful for proliferation of
neural cells
and for regeneration of nerve and brain tissue, i.e. for the treatment of
central and peripheral
nervous system diseases and neuropathies, as well as mechanical and traumatic
disorders,
2 5 which involve degeneration, death or trauma to neural cells or nerve
tissue. More specifically,
a protein may be used in the treatment of diseases of the peripheral nervous
system, such as
peripheral nerve injuries, peripheral neuropathy and localized neuropathies,
and central
nervous system diseases, such as Alzheimer's, Parkinson's disease,
Huntington's disease,
amyotrophic lateral sclerosis, and Shy-Drager syndrome. Further conditions
which may be
3 0 treated in accordance with the present invention include mechanical and
traumatic disorders,
such as spinal cord disorders, head trauma and cerebrovascular diseases such
as stroke.
Peripheral neuropathies resulting from chemotherapy or other medical therapies
may also be
treatable using a protein of the invention.
~_ _. .~.~____ __~.. _ i
CA 02275535 1999-06-25
WO 98/20125 PCT/US97/14649 _
Proteins of the invention may also be useful to promote better or faster
closure of non-
- healing wounds, including without limitation pressure ulcers, ulcers
associated with vascular
insufficiency, surgical and traumatic wounds, and the like.
It is expected that a protein of the present invention may also exhibit
activity for
generation or regeneration of other tissues, such as organs (including, for
example, pancreas,,
liver, intestine, kidney) skin) endothelium), muscle (smooth, skeletal or
cardiac) and vascular
(including vascular endothelium) tissue, or for promoting the growth of cells
comprising such
tissues. Part of the desired effects may be by inhibition or modulation of
fibrotic scarring to
allow normal tissue to regenerate. A protein of the invention may also exhibit
angiogenic
activity.
A protein of the present invention may also be useful for gut protection or
regeneration
and treatment of lung or liver fibrosis, reperfusion injury in various
tissues, and conditions
resulting from systemic cytokine damage.
A protein of the present invention may also be useful for promoting or
inhibiting
differentiation of tissues described above from precursor tissues or cells; or
for inhibiting the
growth of tissues described above.
The activity of a protein of the invention may, among other means) be measured
by the
following methods:
Assays for tissue generation activity include, without limitation, those
described in:
2 0 International Patent Publication No. W095/16035 (bone) cartilage, tendon);
International
Patent Publication No. W095/05846 (nerve, neuronal); International Patent
Publication No.
W091 /07491 {skin, endothelium ).
Assays for wound healing activity include, without limitation, those described
in:
Winter, Epidermal Wound Healing, pps. 71-i 12 (Maibach, HI and Rovee, DT,
eds.), Year
2 5 Book Medical Publishers) Inc., Chicago, as modified by Eaglstein and
Mertz, J. Invest.
Dermatol 71:382-84 (1978).
Activin/Inhibin Activity
A protein of the present invention may also exhibit activin- or inhibin-
related
3 0 activities. Inhibins are characterized by their ability to inhibit the
release of follicle stimulating
hormone (FSH), while activins and are characterized by their ability to
stimulate the release
of follicle stimulating hormone (FSH). Thus) a protein of the present
invention, alone or in
heterodimers with a member of the inhibin a family, may be useful as a
contraceptive based
on the ability of inhibins to decrease fertility in female mammals and
decrease spermatogenesis
3 5 in male mammals. Administration of sufficient amounts of other inhibins
can induce infertility
21
CA 02275535 1999-06-25
WO 98/20125 PCT/US97/14649 _
in these mammals. Alternatively, the protein of the invention) as a homodimer
or as a
- heterodimer with other protein subunits of the inhibin-~3 group, may be
useful as a fertility
inducing therapeutic, based upon the ability of activin molecules in
stimulating FSH release
from cells of the anterior pituitary. See, for example, United States Patent
4,798,885. A
protein of the invention may also be useful for advancement of the onset of
fertility in sexually
immature mammals, so as to increase the lifetime reproductive performance of
domestic
animals such as cows, sheep and pigs.
The activity of a protein of the invention may, among other means, be measured
by the
following methods:
Assays for activin/inhibin activity include, without limitation, those
described in: Vale
et al., Endocrinology 91:562-572, 1972; Ling et al., Nature 321:779-782, 1986;
Vale et al.,
Nature 321:776-779, 1986; Mason et al., Nature 318:659-663, 1985; Forage et
at., Proc. Natl.
Acad. Sci. USA 83:3091-3095, 1986.
Chemotactic/Chemokinetic Activity
A protein of the present invention may have chemotactic or chemokinetic
activity (e.g.,
act as a chemokine) for mammalian cells, including, for example, monocytes,
fibroblasts,
neutrophils, T-cells, mast cells, eosinophils, epithelial and/or endothelial
cells. Chemotactic
and chemokinetic proteins can be used to mobilize or attract a desired cell
population to a
2 0 desired site of action. Chemotactic or chemokinetic proteins provide
particular advantages in
treatment of wounds and other trauma to tissues, as well as in treatment of
localized infections.
For example, attraction of lymphocytes) monocytes or neutrophils to tumors or
sites of
infection may result in improved immune responses against the tumor or
infecting agent.
A protein or peptide has chemotactic activity for a particular cell population
if it can
2 5 stimulate, directly or indirectly, the directed orientation or movement of
such cell population.
Preferably, the protein or peptide has the ability to directly stimulate
directed movement of
cells. Whether a particular protein has chemotactic activity for a population
of cells can be
readily determined by employing such protein or peptide in any known assay for
cell
chemotaxis.
3 0 The activity of a protein of the invention may, among other means, be
measured by the
following methods:
Assays for chemotactic activity (which will identify proteins that induce or
prevent
chemotaxis) consist of assays that measure the ability of a protein to induce
the migration of
cells across a membrane as well as the ability of a protein to induce the
adhesion of one cell
3 5 population to another cell population. Suitable assays for movement and
adhesion include,
22
CA 02275535 1999-06-25
WO 98/20125 PCT/US97/14649 _ '
without limitation, those described in: Current Protocols in Immunology, Ed by
J.E. Coligan,
A.M. Kruisbeek, D.H. Margulies, E.M. Shevach, W.Strober, Pub. Greene
Publishing
Associates and Wiley-Interscience (Chapter 6.12, Measurement of alpha and beta
Chemokines
6.12.1-6.12.28; Taub et al. J. Clin. Invest. 95:1370-1376, 1995; Lind et al.
APMIS
103:140-146, 1995; Muller et al Eur. J. Immunol. 25: 1744-1748; Gruber et al.
J. of Immunol.
152:5860-5867, 1994; Johnston et al. J. of Immunol. 153: 1762-1768, 1994.
Hemostatic and Thrombolytic Activity
A protein of the invention may also exhibit hemostatic or thrombolytic
activity. As
a result, such a protein is expected to be useful in treatment of various
coagulation disorders
(including hereditary disorders) such as hemophilias) or to enhance
coagulation and other
hemostatic events in treating wounds resulting from trauma) surgery or other
causes. A protein
of the invention may also be useful for dissolving or inhibiting formation of
thromboses and
for treatment and prevention of conditions resulting therefrom (such as, for
example, infarction
of cardiac and central nervous system vessels (e.g., stroke).
The activity of a protein of the invention may, among other means, be measured
by the
following methods:
Assay for hemostatic and thrombolytic activity include, without limitation,
those
described in: Linet et al., J. Clin. Pharmacol. 26:131-140, 1986; Burdick et
al., Thrombosis
2 0 Res. 45:413-419, I 987; Humphrey et al., Fibrinolysis 5:71-79 ( I 991 );
Schaub, Prostaglandins
35:467-474, 1988.
Receptor/Ligand ActivitX
A protein of the present invention may also demonstrate activity as receptors,
receptor
2 5 ligands or inhibitors or agonists of receptor/ligand interactions.
Examples of such receptors
and ligands include, without limitation, cytokine receptors and their ligands,
receptor kinases
and their ligands, receptor phosphatases and their ligands, receptors involved
in cell-cell
interactions and their ligands (including without limitation, cellular
adhesion molecules (such
as selectins, integrins and their ligands) and receptor/ligand pairs involved
in antigen
3 0 presentation, antigen recognition and development of cellular and humoral
immune responses).
Receptors and ligands are also useful for screening of potential peptide or
small molecule
inhibitors of the relevant receptor/ligand interaction. A protein of the
present invention
(including, without limitation, fragments of receptors and ligands) may
themselves be useful
as inhibitors of receptor/ligand interactions.
23
CA 02275535 1999-06-25
WO 98/20125 PCT/US97/14649 _
The activity of a protein of the invention may, among other means, be measured
by the
- following methods:
Suitable assays for receptor-ligand activity include without limitation those
described
in:Current Protocols in Immunology, Ed by J.E. Coligan, A.M. Kruisbeek, D.H.
Margulies,
E.M. Shevach, W.Strober, Pub. Greene Publishing Associates and Wiley-
Interscience
(Chapter 7.28, Measurement of Cellular Adhesion under static conditions 7.28.1-
7.28.22),
Takai et al., Proc. Natl. Acad. Sci. USA 84:6864-6868, 1987; Bierer et al., J.
Exp. Med.
168:1145-I 156, 1988; Rosenstein et al., J. Exp. Med. 169:149-160 1989;
Stoltenborg et
al., J. Immunol. Methods 175:59-68, 1994; Stitt et al., Cell 80:661-670, 1995.
Anti-Inflammatory Activity
Proteins of the present invention may also exhibit anti-inflammatory activity.
The anti-
inflammatory activity may be achieved by providing a stimulus to cells
involved in the
inflammatory response, by inhibiting or promoting cell-cell interactions (such
as, for example,
cell adhesion), by inhibiting or promoting chemotaxis of cells involved in the
inflammatory
process, inhibiting or promoting cell extravasation, or by stimulating or
suppressing production
of other factors which more directly inhibit or promote an inflammatory
response. Proteins
exhibiting such activities can be used to treat inflammatory conditions
including chronic or
acute conditions), including without limitation inflammation associated with
infection (such
2 0 as septic shock, sepsis or systemic inflammatory response syndrome
(SIRS)), ischemia
reperfusion injury, endotoxin lethality, arthritis, complement-mediated
hyperacute rejection,
nephritis, cytokine or chemokine-induced lung injury, inflammatory bowel
disease, Crohn's
disease or resulting from over production of cytokines such as TNF or IL,-1.
Proteins of the
invention may also be useful to treat anaphylaxis and hypersensitivity to an
antigenic substance
2 5 or material.
Tumor Inhibition Activity
In addition to the activities described above for immunological treatment or
prevention
of tumors, a protein of the invention may exhibit other anti-tumor activities.
A protein may
3 0 inhibit tumor growth directly or indirectly (such as, for example, via
ADCC). A protein may
exhibit its tumor inhibitory activity by acting on tumor tissue or tumor
precursor tissue, by
inhibiting formation of tissues necessary to support tumor growth (such as,
for example, by
inhibiting angiogenesis), by causing production of other factors, agents or
cell types which
inhibit tumor growth, or by suppressing) eliminating or inhibiting factors,
agents or cell types
3 5 which promote tumor growth.
24
._____. _ __ ~~.~.__.. __
CA 02275535 1999-06-25
WO 98/20125 PCT/LTS97/14649 _ '
Other Activities
A protein of the invention may also exhibit one or more of the following
additional
activities or effects: inhibiting the growth, infection or function of, or
killing, infectious agents)
including, without limitation, bacteria, viruses, fungi and other parasites;
effecting (suppressing
or enhancing) bodily characteristics, including, without limitation, height,
weight, hair color,
eye color, skin, fat to lean ratio or other tissue pigmentation, or organ or
body part size or shape
(such as, for example, breast augmentation or diminution) change in bone form
or shape);
effecting biorhythms or caricadic cycles or rhythms; effecting the fertility
of male or female
subjects; effecting the metabolism, catabolism, anabolism) processing,
utilization, storage or
elimination of dietary fat, lipid, protein, carbohydrate, vitamins, minerals,
cofactors or other
nutritional factors or component(s); effecting behavioral characteristics,
including, without
limitation, appetite, libido, stress, cognition (including cognitive
disorders), depression
(including depressive disorders) and violent behaviors; providing analgesic
effects or other
pain reducing effects; promoting differentiation and growth of embryonic stem
cells in lineages
other than hematopoietic lineages; hormonal or endocrine activity; in the case
of enzymes,
correcting deficiencies of the enzyme and treating deficiency-related
diseases; treatment of
hyperproliferative disorders (such as, for example, psoriasis); immunoglobulin-
like activity
2 0 (such as, for example, the ability to bind antigens or complement); and
the ability to act as an
antigen in a vaccine composition to raise an immune response against such
protein or another
material or entity which is cross-reactive with such protein.
2 5 ADMINISTRATION AND DOSING
A protein of the present invention (from whatever source derived, including
without
limitation from recombinant and non-recombinant sources) may be used in a
pharmaceutical
composition when combined with a pharmaceutically acceptable carrier. Such a
composition
may also contain (in addition to protein and a carrier) diluents, fillers,
salts, buffers, stabilizers,
3 0 solubilizers, and other materials well known in the art. The term
"pharmaceutically
acceptable" means a non-toxic material that does not interfere with the
effectiveness of the
biological activity of the active ingredient(s). The characteristics of the
carrier will depend on
the route of administration. The pharmaceutical composition of the invention
may also contain
cytokines, lymphokines, or other hematopoietic factors such as M-CSF, GM-CSF,
TNF, IL-1,
35 IL-2, IL-3, IL-4, IL-5) IL-6, IL,-7, IL,-8, IL,-9, 1L-10, IL-11, IL-12, IL-
13, IL-14, IL-15, IFIV,
CA 02275535 1999-06-25
WO 98/20125 PCT/ITS97/14649 _ '
TNFO, TNF1) TNF2, G-CSF, Meg-CSF, thrombopoietin, stem cell factor, and
erythropoietin.
The pharmaceutical composition may further contain other agents which either
enhance the
activity of the protein or compliment its activity or use in treatment. Such
additional factors
and/or agents may be included in the pharmaceutical composition to produce a
synergistic
effect with protein of the invention, or to minimize side effects. Conversely,
protein of the
present invention may be included in formulations of the particular cytokine,
lymphokine, other
hematopoietic factor, thrombolytic or anti-thrombotic factor, or anti-
inflammatory agent to
minimize side effects of the cytokine, lymphokine, other hematopoietic factor,
thrombolytic
or anti-thrombotic factor, or anti-inflammatory agent.
A protein of the present invention may be active in multimers (e.g.,
heterodimers or
homodimers) or complexes with itself or other proteins. As a result,
pharmaceutical
compositions of the invention may comprise a protein of the invention in such
multimeric or
complexed form.
The pharmaceutical composition of the invention may be in the form of a
complex of
the proteins) of present invention along with protein or peptide antigens. The
protein and/or
peptide antigen will deliver a stimulatory signal to both B and T lymphocytes.
B lymphocytes
will respond to antigen through their surface immunoglobulin receptor. T
lymphocytes will
respond to antigen through the T cell receptor (TCR) following presentation of
the antigen by
w MHC proteins. MHC and structurally related proteins including those encoded
by class I and
2 0 class II MHC genes on host cells will serve to present the peptide
antigens) to T lymphocytes.
The antigen components could also be supplied as purified MHC-peptide
complexes alone or
with co-stimulatory molecules that can directly signal T cells. Alternatively
antibodies able
to bind surface immunolgobulin and other molecules on B cells as well as
antibodies able to
bind the TCR and other molecules on T cells can be combined with the
pharmaceutical
2 5 composition of the invention.
The pharmaceutical composition of the invention may be in the form of a
liposome in
which protein of the present invention is combined, in addition to other
pharmaceutically
acceptable carriers, with amphipathic agents such as lipids which exist in
aggregated form as
micelles, insoluble monoiayers, liquid crystals, or lamellar layers in aqueous
solution. Suitable
3 0 lipids for liposomal formulation include, without limitation,
monoglycerides, diglycerides,
sulfatides, lysolecithin, phospholipids, saponin, bile acids, and the like.
Preparation of such
liposomal formulations is within the level of skill in the art, as disclosed,
for example, in U.S.
Patent No. 4,235,871; U.S. Patent No. 4,501,728; U.S. Patent No. 4,837,028;
and U.S. Patent
No. 4,737,323, all of which are incorporated herein by reference.
26
CA 02275535 1999-06-25
WO 9$/20125 PCT/US97/14649 _
As used herein, the term "therapeutically effective amount" means the total
amount of
each active component of the pharmaceutical composition or method that is
sufficient to show
a meaningful patient benefit, i.e.) treatment) healing) prevention or
amelioration of the relevant
medical condition, or an increase in rate of treatment, healing, prevention or
amelioration of
such conditions. When applied to an individual active ingredient, administered
alone, the term
refers to that ingredient alone. When applied to a combination, the term
refers to combined
amounts of the active ingredients that result in the therapeutic effect,
whether administered in
combination) serially or simultaneously.
In practicing the method of treatment or use of the present invention, a
therapeutically
effective amount of protein of the present invention is administered to a
mammal having a
condition to be treated. Protein of the present invention may be administered
in accordance
with the method of the invention either alone or in combination with other
therapies such as
treatments employing cytokines, lymphokines or other hematopoietic factors.
When co-
administered with one or more cytokines, lymphokines or other hematopoietic
factors, protein
of the present invention may be administered either simultaneously with the
cytokine(s),
lymphokine(s), other hematopoietic factor(s), thrombolytic or anti-thrombotic
factors, or
sequentially. If administered sequentially, the attending physician will
decide on the
appropriate sequence of administering protein of the present invention in
combination with
cytokine(s), lymphokine(s), other hematopoietic factor(s), thrombolytic or
anti-thrombotic
2 0 factors.
Administration of protein of the present invention used in the pharmaceutical
composition or to practice the method of the present invention can be carried
out in a variety
of conventional ways, such as oral ingestion, inhalation) topical application
or cutaneous,
subcutaneous) intraperitoneai, parenteral or intravenous injection.
Intravenous administration
2 5 to the patient is preferred.
When a therapeutically effective amount of protein of the present invention is
administered orally, protein of the present invention will be in the form of a
tablet, capsule,
powder, solution or elixir. When administered in tablet form, the
pharmaceutical composition
of the invention may additionally contain a solid carrier such as a gelatin or
an adjuvant. The
3 0 tablet, capsule, and powder contain from about 5 to 95% protein of the
present invention, and
preferably from about 25 to 90% protein of the present invention. When
administered in liquid
form, a liquid carrier such as water, petroleum, oils of animal or plant
origin such as peanut oil,
mineral oil, soybean oil, or sesame oil, or synthetic oils may be added. The
liquid form of the
pharmaceutical composition may further contain physiological saline solution,
dextrose or
3 5 other saccharide solution, or glycols such as ethylene glycol, propylene
glycol or polyethylene
27
CA 02275535 1999-06-25
WO 98/20125 PCT/US97/14649 _
glycol. When administered in liquid form) the pharmaceutical composition
contains from
- about 0.5 to 90% by weight of protein of the present invention, and
preferably from about 1
to 50% protein of the present invention.
When a therapeutically effective amount of protein of the present invention is
administered by intravenous, cutaneous or subcutaneous injection, protein of
the present
invention will be in the form of a pyrogen-free, parenterally acceptable
aqueous solution. The
preparation of such parenterally acceptable protein solutions, having due
regard to pH,
isotonicity, stability, and the like, is within the skill in the art. A
preferred pharmaceutical
composition for intravenous, cutaneous, or subcutaneous injection should
contain, in addition
to protein of the present invention, an isotonic vehicle such as Sodium
Chloride Injection,
Ringer's Injection, Dextrose Injection, Dextrose and Sodium Chloride
Injection, Lactated
Ringer's Injection, or other vehicle as known in the art. The pharmaceutical
composition of
the present invention may also contain stabilizers, preservatives, buffers)
antioxidants, or other
additives known to those of skill in the art.
The amount of protein of the present invention in the pharmaceutical
composition of
the present invention will depend upon the nature and severity of the
condition being treated,
and on the nature of prior treatments which the patient has undergone.
Ultimately) the
attending physician will decide the amount of protein of the present invention
with which to
treat each individual patient. Initially, the attending physician will
administer low doses of
2 0 protein of the present invention and observe the patient's response.
Larger doses of protein of
the present invention may be administered until the optimal therapeutic effect
is obtained for
the patient, and at that point the dosage is not increased further. It is
contemplated that the
various pharmaceutical compositions used to practice the method of the present
invention
should contain about 0.01 pg to about 100 mg (preferably about 0.1 pg to about
10 mg, more
2 5 preferably about 0.1 pg to about 1 mg) of protein of the present invention
per kg body weight.
The duration of intravenous therapy using the pharmaceutical composition of
the
present invention will vary, depending on the severity of the disease being
treated and the
condition and potential idiosyncratic response of each individual patient. It
is contemplated
that the duration of each application of the protein of the present invention
will be in the range
3 0 of 12 to 24 hours of continuous intravenous administration. Ultimately the
attending physician
will decide on the appropriate duration of intravenous therapy using the
pharmaceutical
composition of the present invention.
Protein of the invention may also be used to immunize animals to obtain
polyclonal
and monoclonal antibodies which specifically react with the protein. Such
antibodies may be
3 5 obtained using either the entire protein or fragments thereof as an
immunogen. The peptide
28
CA 02275535 1999-06-25
WO 98/20125 PCTlUS97/14649 _
immunogens additionally may contain a cysteine residue at the carboxyl
terminus, and are
conjugated to a hapten such as keyhole limpet hemocyanin (KLH). Methods for
synthesizing
such peptides are known in the art, for example, as in R.P. Merrifield, J.
Amer.Chem.Soc. 85,
2149-2154 (I963); J.L. Krstenansky, et al., FEBS Lett. 211, 10 (1987).
Monoclonal
antibodies binding to the protein of the invention may be useful diagnostic
agents for the
immunodetection of the protein. Neutralizing monoclonal antibodies binding to
the protein
may also be useful therapeutics for both conditions associated with the
protein and also in the
treatment of some forms of cancer where abnormal expression of the protein is
involved. In
the case of cancerous cells or leukemic cells, neutralizing monoclonal
antibodies against the
protein may be useful in detecting and preventing the metastatic spread of the
cancerous cells)
which may be mediated by the protein.
For compositions of the present invention which are useful for bone,
cartilage, tendon
or ligament regeneration) the therapeutic method includes administering the
composition
topically) systematically, or locally as an implant or device. When
administered, the
therapeutic composition for use in this invention is, of course, in a pyrogen-
free,
physiologically acceptable form. Further, the composition may desirably be
encapsulated or
injected in a viscous form for delivery to the site of bone, cartilage or
tissue damage. Topical
administration may be suitable for wound healing and tissue repair.
Therapeutically useful
agents other than a protein of the invention which may also optionally be
included in the
2 0 composition as described above, may alternatively or additionally, be
administered
simultaneously or sequentially with the composition in the methods of the
invention.
Preferably for bone and/or cartilage formation, the composition would include
a matrix capable
of delivering the protein-containing composition to the site of bone and/or
cartilage damage,
providing a structure for the developing bone and cartilage and optimally
capable of being
2 5 resorbed into the body. Such matrices may be formed of materials presently
in use for other
implanted medical applications.
The choice of matrix material is based on biocompatibility, biodegradability,
mechanical properties, cosmetic appearance and interface properties. The
particular
application of the compositions will define the appropriate formulation.
Potential matrices for
3 0 the compositions may be biodegradable and chemically defined calcium
sulfate,
tricalciumphosphate, hydroxyapatite, polylactic acid, polyglycolic acid and
polyanhydrides.
Other potential materials are biodegradable and biologically well-defined,
such as bone or
dermal collagen. Further matrices are comprised of pure proteins or
extracellular matrix
components. Other potential matrices are nonbiodegradable and chemically
defined, such as
3 5 sintered hydroxapatite, bioglass, aluminates, or other ceramics. Matrices
may be comprised
29
CA 02275535 1999-06-25
WO 98/20125 PCT/US97/14649 _
of combinations of any of the above mentioned types of material, such as
polylactic acid and
hydroxyapatite or collagen and tricalciumphosphate. The bioceramics may be
altered in
composition, such as in calcium-aluminate-phosphate and processing to alter
pore size, particle
size) particle shape, and biodegradability.
Presently preferred is a 50:50 (mole weight) copolymer of lactic acid and
glycolic acid
in the form of porous particles having diameters ranging from 150 to 800
microns. In some
applications, it will be useful to utilize a sequestering agent, such as
carboxymethyl cellulose
or autologous blood clot, to prevent the protein compositions from
disassociating from the
matnx.
A preferred family of sequestering agents is cellulosic materials such as
alkyIcelluloses
(including hydroxyalkylcelluloses)) including methylcellulose, ethylcellulose,
hydroxyethylcellulose, hydroxypropylcellulose, hydroxypropyl-methylcellulose,
and
carboxymethylcellulose, the most preferred being cationic salts of
carboxymethylcellulose
(CMC). Other preferred sequestering agents include hyaluronic acid, sodium
alginate,
polyethylene glycol), polyoxyethylene oxide, carboxyvinyl polymer and
polyvinyl alcohol).
The amount of sequestering agent useful herein is 0.5-20 wt%, preferably 1-10
wt% based on
total formulation weight, which represents the amount necessary to prevent
desorbtion of the
protein from the polymer matrix and to provide appropriate handling of the
composition, yet
not so much that the progenitor cells are prevented from infiltrating the
matrix, thereby
2 0 providing the protein the opportunity to assist the osteogenic activity of
the progenitor cells.
In further compositions, proteins of the invention may be combined with other
agents
beneficial to the treatment of the bone and/or cartilage defect, wound, or
tissue in question.
These agents include various growth factors such as epidermal growth factor
(EGF), platelet
derived growth factor (PDGF), transforming growth factors (TGF-a and TGF-(3),
and insulin-
2 5 like growth factor (IGF).
The therapeutic compositions are also presently valuable for veterinary
applications.
Particularly domestic animals and thoroughbred horses, in addition to humans,
are desired
patients for such treatment with proteins of the present invention.
The dosage regimen of a protein-containing pharmaceutical composition to be
used
3 0 in tissue regeneration will be determined by the attending physician
considering various factors
which modify the action of the proteins, e.g., amount of tissue weight desired
to be formed, the
site of damage, the condition of the damaged tissue, the size of a wound, type
of damaged
tissue (e.g., bone), the patient's age, sex, and diet, the severity of any
infection, time of
administration and other clinical factors. The dosage may vary with the type
of matrix used
3 5 in the reconstitution and with inclusion of other proteins in the
pharmaceutical composition.
_._.____ __. ~~_~...__._-~ ..~ _..__._1.
CA 02275535 1999-06-25
WO 98/20125 PCT/LTS97/14649 _ '
For example, the addition of other known growth factors, such as IGF I
(insulin like growth
factor I), to the final composition, may also effect the dosage. Progress can
be monitored by
periodic assessment of tissue/bone growth and/or repair, for example, X-rays,
histomorphometric determinations and tetracycline labeling.
Polynucleotides of the present invention can also be used for gene therapy.
Such
polynucleotides can be introduced either in vivo or ex vivo into cells for
expression in a
- mammalian subject. Polynucleotides of the invention may also be administered
by other
known methods for introduction of nucleic acid into a cell or organism
(including, without
limitation, in the form of viral vectors or naked DNA).
Cells may also be cultured ex vivo in the presence of proteins of the present
invention
in order to proliferate or to produce a desired effect on or activity in such
cells. Treated cells
can then be introduced in vivo for therapeutic purposes.
Patent and literature references cited herein are incorporated by reference as
if fully
set forth.
31
CA 02275535 1999-06-25
WO 98/20125 PCT/US97114649 _ '
SEQUENCE LISTING . _
(1) GENERAL INFORMATION:
(i) APPLICANT: Jacobs, Kenneth
McCoy, John
LaVallie, Edward
Racie, Lisa
Merberg, David
Treacy, Maurice
Spaulding, Vikki
(ii) TITLE OF INVENTION: SECRETED PROTEINS AND POLYNUCLEOTIDES
ENCODING THEM
(iii) NUMBER OF SEQUENCES: 4
(iv) CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: Genetics Institute, Inc.
(B) STREET: 87 CambridgePark Drive
{C) CITY: Cambridge
(D) STATE. Massachusetts
(E) COUNTRY: U.S.A.
(F) ZIP: 02140
(v) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Floppy disk
(B) COMPUTER: IBM PC compatible
(C) OPERATING SYSTEM: PC-DOS/MS-DOS
(D) SOFTWARE: PatentIn Release #1.0, Version #1.30
(vi) CURRENT APPLICATION DATA:
{A) APPLICATION NUMBER:
(B) FILING DATE:
(C) CLASSIFICATION:
(viii) ATTORNEY/AGENT INFORMATION:
(A) NAME: Brown, Scott A.
(B) REGISTRATION NUMBER: 32,724
(ix) TELECOMMUNICATION INFORMATION:
(A) TELEPHONE: (617) 498-8224
(B) TELEFAX: (617) 876-5851
(2) INFORMATION FOR SEQ ID N0:1:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 676 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
32
____ ~_-_..__- ~~ _.
CA 02275535 1999-06-25
WO 98/20125 PCT/US97/14649 _
(xi) SEQUENCE DESCRIPTION:
SEQ ID N0:1:
GAACCTAAGT TACACAGTGG TTGCTTTCACCAAACAGACCATGGGCTTCT TGGAAGAGGC60
CCTGAAGCTG TATTTCCCAT AGCTGCACATGGTACTTTTGGAGAGCCTGG TGGAAATCAT120
-
TTTGGTTGCT GTTCAGCATG TGGATTATAGTCTTCGATGTGAGCAGGATC CAGAGAAGAA180
AGCTTTTATC AGACAGAATG CATCCTTTTTATATGAAACAGTCCTCCCTG TGGTGGAGAA240
AAGGTTTGAA GAAGGTGTGG GGAAACCTGCCAAGCAACTCCAAGATCTGA GGAATGCATC300
TAGACTTATT CGTGTGAATC CTGGAAAGTACAACATCWGTGGTCTAATGC TTGGGTCTGT360
YTATATGTGT ATATATGCAG ANAGAGAGCTTTATATTATTTATATTTATA TTAAGTTGTA420
TTAGCATACT CTATAGTTTC AAACACAACTTGAAAATTAAAAGTGCCCTC TTAAAAATAC480
AAAAATCAAA AAGAGGAAAA TAAGTTAAATTAAGCCCAAGTAACAAAAAT ACTGGAATTA540
TTAAAACGTA TAGTATGCTA GCTATCCTTTTAAATTATGCTAATTCTCTT CTTCTGAAAT600
TATGGTCACA CTATATACTA TAGCATTTCGGTTTTATCCTTTGATAAAAC TTTTCTTTTT660
TCTTTTTTTT TTTTGA 676
(2) INFORMATION FOR SEQ
ID N0:2:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 111 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: linear ,
(ii) MOLECULE TYPE: protein
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:2:
Met Val Leu Leu Glu Ser Leu Val Glu Ile Ile Leu Val Ala Val Gln
1 5 10 15
His Val Asp Tyr Ser Leu Arg Cys Glu Gln Asp Pro Glu Lys Lys Ala
20 25 30
Phe Ile Arg Gln Asn Ala Ser Phe Leu Tyr Glu Thr Val Leu Pro Val
35 40 45
Val Glu Lys Arg Phe Glu Glu Gly Val Gly Lys Pro Ala Lys Gln Leu
50 55 60
33
CA 02275535 19~99-06-25
WO 98/20125 PCT/US97/14649 _
Gln Asp Leu Arg Asn Ala Ser Arg Leu Ile Arg Val Asn Pro Gly~Lys _
_ 65 70 75 80
Tyr Asn Ile Xaa Gly Leu Met Leu Gly Ser Val Tyr Met Cys Ile Tyr
85 90 95
Ala Xaa Arg Glu Leu Tyr Ile Ile Tyr Ile Tyr Ile Lys Leu Tyr
100 105 110
(2) INFORMATION FOR SEQ ID N0:3:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 311 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:3:
TCAAAACGTT GGACTATTTG GGAAAAGGAA AAGAGTTGGG AAAATTGGTT TTAAGGTAAG 60
TTTTAGTCAA AAGAATTCTT TNTTGAAACT AGCTGGTTTG TGGATTCAGA TACTCTGATC 120
CTTACAGAAT CCAAGAGGAA GCTTTCATAA AAACAATTCA GCAAATATTT CCAATATAAT 180
TTGAATGGCT AATTTTCAGT TGNTAATTAA TTAGCAGCTT TGTAATACTT GATTTGGGAG 240
CATTTACTTG GAAATCCTAA GGACTATAAT AAAAGTTTTC AACATATTTC TF~AAAAAAAA 300
311
(2) INFORMATION FOR SEQ ID N0:4:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 28 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: other nucleic acid
(A) DESCRIPTION: /desc = "oligonucleotide"
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:4:
TGTGGATTAT AGTCTTCGAT GTGAGCAG 28
34
_._.__._..._.~____.._..__._.~~___. . ~