Note: Descriptions are shown in the official language in which they were submitted.
CA 02276128 1999-06-24
WO 98/29079 PCT/US97/24247
INTERNALLY HEATED ABSORBENT ARTICLE
FIELD OF THE INVENTION
The present invention relates generally to an absorbent article and
particularly to
an absorbent article having a mixture that generates heat when exposed to air
and/or
moisture. ,
BACKGROUND OF THE INVENTION
All manner and variety of devices or applliances are configured for absorption
of
body fluids, such as menses, are well known. Sanitary napkins are the most
frequently
used of these devices. The prior art is replete with patents relating to
protective pads
and sanitary napkins for the absorption of body fluids and protecting the
undergarment
from staining. It has been suggested that from :?0-25 percent of alt sanitary
napkins leak.
A contributing factor to the leakage is that menses is a viscous fluid having
aqueous and
mucus-like components.
A problem in the pertormance of the absorbent is that the more viscous the
material the slower the rate of absorption. Basically, the low viscous
materials readily
pass through the cover of the sanitary napkin and are absorbed by the
absorbent. The
higher viscosity materials in the menses may not be absorbed and can remain on
the
cover. Alternatively, the higher viscosity materials may be absorbed but
remain at or
near the point of insult occluding absorption of the lower viscosity
materials. This limits
the effectiveness of the absorbent and the utilization of the absorbent
capacity of the
sanitary napkin. Moreover, the absorbent may contain superabsorbent materials
which
preferentially absorb the aqueous constituents from low viscous materials
thereby
increasing the viscosity of the remaining materiel. This exacerbates the
problem of the
absorbent to absorb the viscous components of the menses.
Until now, surfactants have been used to improve the absorption of body
fluids.
One or more of the materials used in constructing the sanitary napkin, such as
the cover
and/or absorbent, have been treated to improves the material wetability. The
problem of
using a surfactant is that the surface energy of the coated material is
modified but the
surfactant does appreciably modify, if at all, the viscosity of the body
fluid. Thus, the
higher viscosity materials are still not efficiently absorbed.
Accordingly, there is a need for an absorbent article, such as a sanitary
napkin,
which can modify the viscosity of a viscous material so that it can be
absorbed.
CA 02276128 1999-06-24
WO 98129079 PCT/US97/24247
SUMMARY OF THE INVENTION
Briefly, the present invention is an absorbent article adapted for absorbing
body
fluids. The absorbent article has a cover, a baffle, an absorbent enclosed
between the
cover and the baffle and a heating means for heating the absorbent. The
heating means
can, when activated by exposure to air and/or moisture, generate a temperature
of from
about 22°C to about 55°C.
It is an object of the invention to provide an absorbent article having a
heating
means for producing heat when activated. It is another object of the invention
to provide
a sanitary napkin having a heating means within the sanitary napkin that
produces heat
when activated.
It is another object of the invention to provide a sanitary napkin having a
heating
means and improved absorbent utilization.
It is another object of the invention to provide a sanitary napkin having a
chemical
mixture that will generate heat so that the viscosity of the more viscous
component of
menses can be lowered by heating the fluid.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a top view of one embodiment of the absorbent article of the present
invention.
Fig. 2 is a transverse cross-sectional view of Fig. 1 along lines 2--2.
Fig. 3 is a transverse cross-sectional view of an alternative embodiment of
the
present invention.
Fig. 4 is a transverse cross-sectional view of an alternative embodiment of
the
present invention.
Fig. 5 is a transverse cross-sectional view of an alternative embodiment of
the
present invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
While the specification concludes with claims particularly pointing out and
distinctly claiming that which is regarded as the invention, it is believed
that the invention
2
CA 02276128 1999-06-24
WO 98/29079 PCT/US97/24247
can be more readily understood with reference to the accompanying drawings of
figures
in conjunction with the following detailed description of the invention.
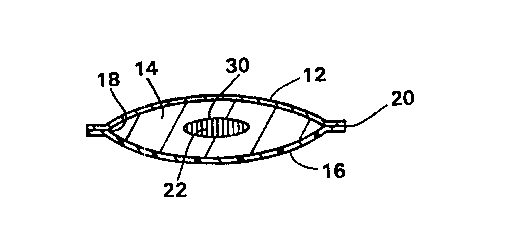
Referring to Figs. 1-5 of the drawings, in which like parts are identified
with like
reference characters, Fig. 1 illustrates a top view of a sanitary napkin 10 in
accordance
with this invention. As shown in these drawing; and viewed from the top, i.e.,
that side
which would normally be placed adjacent to the wearer during use, the sanitary
napkin
is comprised of a fluid permeable cover 12; an absorbent layer 14 which is
shorter
and narrower than the cover 12; and a liquid-impervious backing or baffle 16.
As seen in
Figs. 2-4, the cover 12 and baffle 16 extend beyond an edge 18 of the
absorbent 14 to
enclose the absorbent 14 and to define the perimeter 20 of the sanitary napkin
10. As
used herein "edge" or "edge of the absorbent" are equivalent and encompasses
the
border at which the absorbent 14 terminates) without limitation to
longitudinal sides or
transverse ends of the absorbent 14 unless specifically so stated. Although
not shown,
one skilled in the art would understand that the cover 12, absorbent 14 and
baffle 16 can
have a coterminous edge, but this is not preferred. The cover 12 and the
baffle 16 may
be sealed together using any suitable means that will not leave a hard,
uncomfortable
residue that may be annoying to the wearer. A;s used herein, the term "sealed"
encompasses configurations whereby the cover 12 is directly joined to the
baffle 16 or,
alternatively, by affixing the cover 12 to an intermediate member, not shown,
which may
in turn be affixed to the baffle 16. Methods for attaching the cover 12 and
the baffle 16
are well known to those skilled in the art and in~:lude the use of hot melt
adhesive,
pressure sensitive adhesive, construction adhesive) double-sided tape, heat
sealing and
ultrasonic bonding.
As used herein, the term "sanitary napkin" refers to an article which is worn
by
females adjacent to the pudendal region and which is intended to absorb and
contain
various exudates which are discharged from thn body such as blood, menses, and
urine,
and which is intended to be discarded when soiled, not laundered and reused.
Interlabial
devices which reside partially within and partialliy external of the female
wearer's
vestibule are also within the scope of this invention.
The sanitary napkin 10 further includes a heating means 22 positioned below
the
cover 12 and preferably adjacent to the absorbent 14. The heating means 22 can
be
comprised of one or more cells containing exothermic or electrochemical
reactants that
will produce heat when activated by oxygen or moisture or can comprise a
material that
will effectively distribute heat from the wearer's own body. Desirably, the
heating means
22 lowers the viscosity of the more viscous components of the menses allowing
greater
3
CA 02276128 1999-06-24
WO 98/29079 PCT/US97124247
fluid mobility and thereby obtaining greater utilization of the absorbent
capacity. When
exothermic or electrochemical reactants are used, the heating means 22 should
generate
enough heat to produce a temperature of from about 22°C to about
55°C. More
preferably, the heating means 22 generates enough heat to produce a
temperature of
from about 27.5°C to about 46°C and most preferably , the
heating means 22 generates
enough heat to produce a temperature of from about 27.5°C to about
37°C.
Looking at the sanitary napkin 10 in greater detail, the sanitary napkin 10 is
illustrated as having an oval shape, but is not limited thereto. The sanitary
napkin 10 can
have an hourglass, racetrack or any other shape or configuration that will
allow the
sanitary napkin 10 to come into intimate contact with the wearer.
The sanitary napkin 10 of the present invention may be any thickness and may
cover
periods for light to heavy flow and can have a thickness of a few millimeters
to about 15
millimeters.
Referring to Fig. 3, the sanitary napkin 10 can further include one or more
additional layers 24 that are designed to enhance, modify or transfer fluid in
a
preferential manner. Such layers include cellulosic and polymeric materials
such as
tissue) superabsorbents and melt blown materials. Such layers and materials
are
commercially available from several sources and are well known to those skill
in
construction of disposable absorbent articles, such as sanitary napkins,
diapers and
incontinent devices. The sanitary napkin 10 can also include shape conforming
members adapted to contort and conform the sanitary napkin 10 to a wearer's
anatomy
during use.
The cover 12 is designed to contact the body of the wearer and therefore
should
be easily penetrated by body fluids. The cover 12 should also be non-
irritating to the
wearer's skin and preferably, will not absorb an appreciable amount of fluid
insulting its
surface. The cover 12 can be constructed of a woven or nonwoven, natural or
synthetic
material. Suitable materials include bonded carded webs of polyester,
polypropylene,
polyethylene, nylon, or other heat-bondable fibers. Other polyolefins, such as
copolymers of polypropylene and polyethylene, linear low-density polyethylene,
finely-
perforated film webs and net material, also work well. Particularly preferred
are
composite materials of a polymer and a nonwoven fabric material. Still another
cover
material is a spunbond web of polypropylene. The web can contain about 1 % to
about
6% titanium dioxide pigment to give it a clean, white appearance. A uniform
spunbond
material is desirable because it has sufficient strength in the longitudinal
direction, even
after being perforated, to resist being torn or pulled apart during use. The
most preferred
4
CA 02276128 1999-06-24
WO 98/29079 PCTIUS97124247
polypropylene webs have a basis weight of befinreen about 10 and 40 grams per
square
meter. An optimum basis weight is between about 12 and about 30 grams per
square
meter.
To aid in the penetration of the liquid through the web, the cover 12 can also
be
treated with a surtactant to improve its hydrophilic characteristics. The
surfactant can
include topical additions or internally applied materials like polysiloxanes.
Positioned adjacent to the cover 12 is the absorbent 14. The materials used in
the absorbent 14 are designed to absorb body ~exudates, including menstrual
fluids,
blood and urine. Suitable materials include wood pulp fluff, rayon, cotton and
meltblown
polymer, such as polyester) polypropylene or coform. Coform is an air-formed
combination of meltblown polymers, such as polypropylene, and absorbent
fibers. The
absorbent 14 may be a composite comprised of a hydrophilic material that can
be formed
from various natural or synthetic fibers, wood pulp fibers) regenerated
cellulose or cotton
fibers, an airlaid tissue or a blend of pulp and other fibers. The absorbent
14 can be
made from other well known materials used in absorbent articles, including
multiple
layers of cellulose wadding, cellulose sponge, hydrophilic synthetic sponge,
such as
polyurethane, and the like. The capacity of the absorbent 14 may be varied
depending
upon the intended usage of the final product.
The baffle 16 acts as a barrier between the absorbed body fluids contained in
the
absorbent 14 and the person wearing the sanitary napkin 10. Accordingly, the
baffle 16
is nonabsorbent and impervious to liquids. The: baffle 16 should be soft and
compliant
since a portion of the baffle 16 may reside adjacent the thigh region of the
wearer. As
used herein, the term "compliant" refers to mat~~r7als which will readily
conform to the
general external shape and contours of the human anatomy. In a preferred
embodiment,
the baffle 16 may permit the passage of air or vapor out of the sanitary
napkin 10 while
blocking the passage of liquids from the absorbent 14. A good material for the
baffle 16
is a micro-embossed, polymeric film) such as polyethylene or polypropylene
having a
thickness in the range of from about 0.012 mm to about 1.0 mm. Bicomponent
films can
also be used as well as woven and nonwoven fabrics which have been treated to
render
them liquid-impermeable.
Referring to Figs. 1 and 2, the heating means 22 is positioned beneath the
cover
12 and intermediate the absorbent 14. The heating means 22 can include a
chemical
mixture enveloped in an appropriate air and moisture-permeable material 30.
The
material 30 can be a synthetic or natural, woven or nonwoven material.
Desirably, the
material 30 is capable of permitting air and moisture to pass while retaining
the
CA 02276128 1999-06-24
WO 98/29079 PCT/US97/24247
particulate chemical mixture. Non-limiting examples of such materials 30
include a
polyester nonwoven and a nonwoven spunbond polypropylene. Natural materials
are
also suitable for use in containing the chemical mixture. Cotton is an example
of a
natural material suitable for enveloping the chemical mixture.
The exothermic agents which can be utilized in the present invention may be a
material which easily reacts with oxygen in the air) water from absorbed
menses or both
to generate heat at the time of reaction. When the heating means 22 generates
heat by
an exothermic reaction with oxygen, it is necessary that the reactants have an
exchange
of air. Although not particularly limited hereto, the reactants can be a
mixture of an
oxidizable substances such as iron, reduced iron, nickel, sodium sulfide
andlor sodium
sulfite; an oxidation accelerator and catalyst such as sodium chloride,
calcium chloride,
magnesium chloride, activated carbon, carbon powder and a mixture consisting
of copper
compound, manganese as well as a water retaining agent such as woodmeal or
pulp
powder. Other exothermic reactants are described in U.S. Patents 4,331,731 and
4,573,447 the entire disclosures of which are incorporated herein by
reference.
For example, the chemical mixture of the heating means 22 can include an
intermediate having 30 weight percent vermiculite, 55 weight percent of an
aqueous
solution having 10 weight percent sodium chloride and 15 weight percent carbon
of fine
particle size. The intermediate is combined with a fine iron powder at a ratio
of about
1:1. Iron powder is preferred because it reacts readily with the oxygen in the
air in the
presence of moisture to generate heat. Moreover, the material is a good
thermal
conductor allowing for uniformity of temperature distribution and avoiding
localized areas
of sensible heat. The fineness of the powder can be varied to change the rate
of the
reaction and thereby the amount of heat generated. As a general rule, the
greater the
amount of metal powder, the hotter the reaction.
Sodium chloride is used to catalyze the oxidation of the iron. It is
particularly
desirable in that it is readily available and inexpensive. However, the sodium
chloride
can be replaced with other suitable chlorides and sulfates, such as potassium
chloride,
calcium chloride, magnesium chloride, ferric sulfate, potassium sulfate,
sodium sulfate,
and magnesium sulfate.
Those skilled in the art will understand that the ratios of the components,
particle
size) and ingredient quality of the chemical mixture can be varied
substantially to make
either a hotter or cooler reaction mixture.
When the heating means 22 used in the present invention is an electrochemical
reaction that is activated by water, the electrochemical reaction generates
heat by using
6
CA 02276128 1999-06-24
WO 98/29079 PCT/US97/24247
an electrochemically active reducible element and an electrochemically active
oxidizable
element. The reducible element can be formed from an air depolarized cathode
on
which another material such as oxygen is reduced. The oxidizable element can
be a foil
material made from aluminum or magnesium or an alloy of both. The reducible
element
and oxidizable element are separated by a watE:r absorbing material such as
felt.
Preferably) an electrolyte forming salt is incorporated in a dry granulated
form with or
adjacent to the reducible element to thereby avoid the need to impregnate the
water
absorbing material. This allows the heating means 22 to have an extended
storage life.
Desirably, the electrolyte salt is applied uniformly into the cathode or
reducible element.
tn the case of an air depolarized cathode using activated carbon or manganese
dioxide)
the table salt is originally uniformly dry mixed wiith the activated carbon or
manganese
dioxide in the range of about one to two an a half grams of salt to about one
gram of
carbon. Preferably, the ratio is from about one and a half grams of salt to
one gram of
carbon or manganese dioxide.
The heating means 22 may be composed of a material that will effectively
distribute heat from the wearer's body in the absorbent 14. Desirably, such
materials
have a high thermal conductivity relative to the absorbent 14. Thermal
conductivity of a
substance is readily ascertainable using well known techniques.
Referring to Fig. 3) a transverse cross-section of an alternative embodiment
of the
invention is illustrated. The heating means 22 its positioned between the
absorbent 14
and the baffle 16 so that the top of the heating means 22 is adjacent to the
absorbent 14
and the bottom of the heating means is adjacent to the baffle 16. The heating
means 22
is similar to that described above for Figs. 1 and 2, in that the chemical
mixture
comprising the heating means 22 is surrounded by an enveloping material 30.
Referring to Fig. 4, a transverse cross-section of an alternative embodiment
of the
invention is illustrated. The heating means 22 is positioned between the
baffle 16 and a
retaining layer 28 positioned below the baffle 16. The baffle 16 and retaining
layer 28
are secured together to enclose the heating means 22. In this embodiment,
although it is
preferred for the chemical mixture to be enveloped in the material 30 it is
not necessary.
The retaining layer 28 can be composed of a material similar to the baffle 16.
Desirably,
the retaining layer 28 is composed of an air permeable material 30, such as
spunbond,
an apertured film and the like. The retaining layer 28 can include one or more
apertures
or perforations 32 that will enhance the exchange of oxygen irrespective of
the air
permeability of the retaining layer 28 compositiion. Desirably, the apertures
or
perforations 32 are positioned on the garment-facing surface of the retaining
layer 28.
7
CA 02276128 1999-06-24
WO 98/29079 PCT/US97/24247
The apertures or perforations 32 should be appropriately sized so that the
chemical
mixture does not escape if an enveloping material 30 is not used but still
permit the
interchange of air through the material 30. Perforations 32 are necessary when
the
material 30 is impermeable to the exchange of air such as when a polyolefin
film is used.
Perforations can be made in many different ways) including cutting) needling,
punching and the like. In some cases the perforations 32 can be arranged in a
narrow
area or strip of the retaining layer 28 as opposed to having the perforations
distributed
throughout the retaining layer 28. By having the perforations 32 arranged
along a narrow
strip, it is possible to slow the dissipation of moisture through the material
30 and out of
the chemical mixture. This can provide for a conservation of moisture within
the chemical
mixture, thereby increasing the life of the chemical mixture.
Referring to Fig. 5, a transverse cross-section of an alternative embodiment
of the
invention is illustrated. The chemical mixture of the heating means 22 can be
randomly
or evenly distributed or located in discrete identifiable pockets or areas
similar to that
described above for Figs. 1-4, but with lesser amounts of the chemical mixture
contained
in each pocket.
While the invention has been described with reference to several preferred
embodiments and illustrated with regard to a range of optional features, those
skilled in
the art will appreciate that various substitutions, omissions, modifications,
and changes
may be made without departing from the spirit hereof. Accordingly, it is
intended that the
foregoing description be deemed exemplary of the preferred scope of the
present
invention and not deemed a limitation thereof.
8