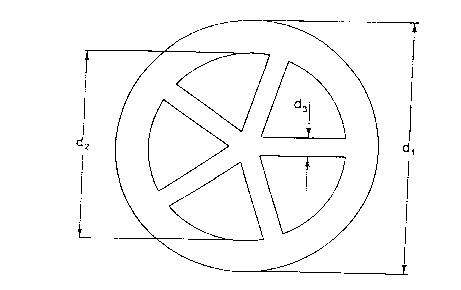Note: Descriptions are shown in the official language in which they were submitted.
CA 02276476 1999-06-25
1
Mouldings based on silica
This invention relates to mouldings based on silica, to a
process for their production, and their use as a catalyst
for the acetoxylation of olefines.
Silicas, particularly pyrogenic silicas, are characterised
by their extremely finely divided state and their
correspondingly high specific surface, by their very high
purity, by their spherical particles and by the absence of
pores. Due to these properties, there is an increasing
interest in pyrogenic silicas as supports for catalysts (D.
Koth, H. Ferch, Chem. Ing. Techn. 52, 628 (1980)).
It is known from DE-B 21 00 778 that granular materials
based on pyrogenic silicas can be used as catalyst supports
for the production of vinyl acetate monomer.
It is known from DE-A 38 03 900 that cylindrical particles
which have arched end faces and which are based on
pyrogenic silicas can be used as catalyst supports for the
production of vinyl acetate monomer.
A process for the production of pressed parts is known from
DE-A 39 12 504 in which aluminium stearate, magnesium
stearate and/or graphite are used as a lubricant, and in
which urea as well as methyl cellulose are used as pore
forming agents.
These known pressed parts which are produced with magnesium
stearate are commercially available as Aerosil Tablets
No. 350, as supplied by Degussa. They contain about 0.4 0
by weight Mg.
Catalyst supports for catalysts for the synthesis of vinyl
acetate monomer are known from EP 0 004 079 which consist
of extruded sections with a star-shaped cross-section or
which consist of ribbed lengths.
CA 02276476 1999-06-25
2
Catalysts for the synthesis of vinyl acetate monomer, which
comprise at least one passageway channel with an inside
diameter of at least 1 mm, are known from EP-B 464 633.
DE-A 195 38 799 describes a catalyst support in the shape
of a honeycomb which predominantly consists of Si02.
According to Example 1 of said patent, this support has a
diameter of 25 mm, a stay width of 1 mm, a stay spacing of
2 mm, and a length of 150 mm. After it has been coated with
catalytically active elements, the resulting catalyst can
be used for the production of unsaturated esters from
olefines, acids and oxygen in the gas phase, for the
purification of off-gas contaminated by organic substances,
and for the alkylation of aromatic compounds.
WO 97/36679 also describes a catalyst support in the shape
of a honeycomb, which is coated with Si02 and which after
impregnation with palladium and gold and after activation
with potassium acetate can be used for the production of
unsaturated esters.
Honeycomb-shaped catalysts are characterised by a very low
pressure drop. However, the use of honeycomb-shaped
catalysts in industrial reactors, particularly in tube
bundle reactors, results in problems which are not
inconsiderable, particularly with regard to packing the
reactor tubes. With tube reactors, it is sometimes
necessary to pack several thousand tubes of an industrial
installation with honeycomb catalysts. In the course of
this procedure, it has to be ensured that the honeycomb
bodies do not break down during filling. Considerable
emphasis has to be placed on the avoidance of edge flow
effects, since otherwise the catalysts are not capable of
contributing their full effect. Moreover, honeycomb-shaped
catalyst materials exhibit poor thermal condutivity in a
radial direction. This is particularly disadvantageous in
reactions in which there is considerable evolution of heat,
CA 02276476 1999-06-25
3
as in oxidation reactions for example. For the
aforementioned reasons, there is currently no known
industrial application in which a tube bundle reactor or
thousands of tubes are operated with honeycomb-shaped
catalyst materials. For this reason, reactors are packed
with mouldings in the form of pellets, which likewise
exhibit a low pressure drop.
It is known from EP-B 0 519 435 that Si02 can be pressed by
means of binders to produce supports, followed by calcining
the supports obtained and washing the calcined support
particles with acid until cations from the binder are no
longer released. In addition, a supported catalyst, a
process for the production thereof, and the use thereof for
the production of vinyl acetate are also described.
EP-A 0 807 615 describes pressed parts based on pyrogenic
silica. These pressed parts can be used as a catalyst or
catalyst support for the production of vinyl acetate
monomer and for the hydration of ethylene. The pressed
parts may be of different shapes, e.g. cylindrical,
spherical or annular, with an outside diameter of 0.8 to 20
mm.
The present invention relates to mouldings based on silica,
which are characterised in that that the supporting
geometry consists of a hollow cylindrical configuration
with internal reinforcing stays or spokes leading from the
inner wall of the hollow cylinder to the centre the
moulding, or is characterised by a multiplicity of
passageway channels.
The mouldings according to the invention can have an
outside diameter from 1 to 25 mm and a ratio of height to
diameter of 0.2 to 5. Furthermore, they can have a total
pore volume of 0.3 to 1,8 ml/g and a BET specific surface
of 5 to 400 m2/g.
CA 02276476 1999-06-25
4
The Si02 content of the mouldings according to the
invention is preferably more than 99.0 o by weight. The
proportion of other constituents can be less than 0.2 s by
weight. The mouldings according to the invention can
therefore be characterised as being free from binders. The
fines can amount to less than 5 % by weight. The bulk
density can range from 100 to 700 g/1.
The present invention further relates to a process for the
production of mouldings based on silica, which is
characterised in that silica is subjected to a kneading and
working process, is extruded, the extrudate is optionally
cut to the desired length by means of a cutting device, is
dried at a temperature of 20 to 150°C, and is annealed for
a period from 0.5 to 10 hours at a temperature from 400 to
1200°C.
In one particular embodiment of the invention, the silica
can be a pyrogenic silica. Silica which can be used
according to the invention, which thus includes pyrogenic
silica, is described in Ullmanns Enzyklopadie der
technischen Chemie, 4th Edition, Volume 21, pages 451 to
476 (1982.
One preferred embodiment of the invention is a process for
the production of mouldings based on silica, which is
characterised in that silica is homogenised with methyl
hydroxyethyl cellulose, wax and/or polyethylene glycol with
the addition of water and optionally with the addition of
an aqueous alkaline ammonia solution, is subjected to a
kneading and working process, is shaped and/or extruded,
the mouldings are optionally cut to the desired length by
means of a cutting device, are dried at a temperature from
10 to 150°C and are annealed for a period from 30 minutes
to 10 hours at a temperature from 400 to 1200°C.
CA 02276476 1999-06-25
Kneaders, mixers or mills which enable good homogenisation
and compaction of the mixed material to be effected, such
as blade, fluidised bed, rotary or air jet mixers for
example, can be used for carrying out the process
5 according to the invention. In particular, mixers can be
used which make it possible to effect additional compaction
of the mixed material, such as plough mixers, kneaders, pan
grinders or ball mills. Mixing and kneading can also be
effected directly in the extruder, however. The production
the mouldings can be effected in single- or twin-screw
extruders, in extrusion presses or in tabletting machines
also. The mouldings according to the invention are
preferably producted by means of extruders.
In one particular embodiment of the invention, the mixture
can have the following composition before it is shaped:
50 - 90 % by weight silica, preferably 65-85 % by
weight;
0.1 - 20 % by weight methyl hydroxyethyl cellulose,
preferably 5-15 % by weight;
0.1 - 15 % wax, preferably 5-12 % by weight;
0.1 - 15 % polyethylene glycol, preferably 5-
10 % by weight.
The mouldings can be annealed at 400 - 1200 °C for 30
minutes to 10 hours. The fracture strength, total specific
surface and the pore volume can be varied within certain
limits by varying the amounts of substances used and by
varying the pressing pressure.
The mouldings according to the invention can be used either
directly as a catalyst or as a catalyst support.
For use as a catalyst support, the mouldings can be-brought
into contact with a catalytically active substance after
CA 02276476 1999-06-25
6
their production and can be activated, if necessary, by
suitable further treatment.
In particular, mouldings made from pyrogenic silica can be
used as a support for the catalyst for the production of
vinyl acetate monomer from ethylene, acetic acid and
oxygen.
The mouldings according to the invention comprise the
following properties or enable such properties to be
obtained:
- low pressure drops
- low bulk density
- relatively large external surface per unit volume of a
reaction vessel
- improved mass and heat transfer
- comparatively simple packing and emptying of industrial
tube bundle reactors, particularly by comparison with
known honeycomb-shaped catalysts.
The low pressure drop across mouldings according to the
invention results, amongst other causes, from their
geometric dimensions, due to which there is an extremely
high free surface area over the cross-section of the
mouldings and/or a very high voids fraction in the catalyst
packing.
Catalysts can be produced based on mouldings according to
the invention which enable higher which enable higher
space-time yields and selectivities to be achieved.
The invention is explained in greater detail below with
reference to the drawings.
The drawings are as follows:
CA 02276476 1999-06-25
7
Figure 1 is a cross-section through a pressed part or
supported catalyst in the form of a cart
wheel;
Figure 2 is a perspective illustration of the cart
wheel shape shown in Figure 2;
Figure 3 shows a pressed part or supported catalyst in
the form of what is termed a minilith; and
Figure 4 is a perspective illustration of the minilith
shown in Figure 3.
The Figures relate to embodiments of the invention. The
maximum outside diameter dl of the cart wheels and of what
are termed miniliths is preferably 25 mm, wherein the ratio
of height h to outside diameter (h/dl) can range from 0.2
to 5. The inside diameter of the mouldings is denoted by
d2. The wall thickness the mouldings ( (dl - d2) X 0.5) can
fall within the range from 0.05 to 0.3 times the outside
diameter. The stay or spoke thickness of the mouldings is
denoted by d3 and can fall within the range from 0.05 to
0.3 times the outside diameter. The number of internal
reinforcing stays or spokes or passageway channels can
amount to at least 3.
The present invention also relates to a supported catalyst
for the production of vinyl acetate monomer (VAM), which
catalyst contains, as catalytically active components on a
support (moulding), palladium and/or compounds thereof and
alkali compounds, and which additionally contains gold
and/or compounds thereof (Pd/alkali/Au system) or cadmium
and/or compounds thereof (Pd/alkali/Cd system) or barium
and/or compounds thereof (Pd/alkali/Ba system) or
palladium, alkali compounds and mixtures of gold and/or
cadmium and/or barium, and which is characterised in that
the support is a moulding according to the invention.
CA 02276476 1999-06-25
8
Potassium compounds, such as potassium acetate for example,
are preferably used as alkali compounds.
The catalytically active components can be present in the
following systems:
Pd/Au/alkali compounds
Pd/Cd/alkali compounds
Pd/Ba/alkali compounds
The supported catalysts according to the invention can be
used for the production of vinyl acetate monomer. For this
purpose, ethene, acetic acid and molecular oxygen or air
are reacted in the gas phase, optionally with the addition
of inert gases, at temperatures between 100 and 250°C and
at normal or elevated pressure in the presence of the
supported catalysts according to the invention.
A production process of this type is known from the
documents DE 16 68 088, US 4,048,096, EP-A 0 519 435, EP-A
0 634 208, EP-A 0 723 810. EP-A 0 634 209, EP-A 0 632 214,
EP-A 0 654 301 and EP-A 0 0807 615.
The poresent invention also relates to a process for the
production of the supported catalyst for the production of
vinyl acetate monomer by depositing Pd, Au, Cd or Ba metal
compounds by impregnation, spraying, evaporation, immersion
or precipitation, optionally reducing the reducible metal
compounds which are deposited on the support, optionally
washing in order to remove chloride fractions which may be
present, impregnating with alkali acetates or with alkali
compounds which under the reaction conditions for the
production of vinyl acetate monomer are completely or
partially converted into alkali acetates, in a suitable
sequence, which is characterised in that the support is a
moulding according to the present invention.
CA 02276476 1999-06-25
9
The present invention further relates to a process for the
production of the supported catalyst for the production of
vinyl acetate monomer by impregnating the support with a
basic solution and a solution which contains gold and
palladium salts, wherein impregnation is effected
simultaneously or in succession, with or without
intermediate drying, optionally washing the support in
order to remove chloride fractions which may be present and
reducing the insoluble compounds which are precipitated on
the support before or after washing, drying the catalyst
precursor which is thus obtained, and impregnating with
alkali acetates or with alkali compounds which under the
reaction conditions for the production of vinyl acetate
monomer are completely or partially converted into alkali
acetates, which is characterised in that the support is a
moulding according to claim 1.
The supported catalysts according to the invention can be
used for the production of unsaturated esters from
olefines, organic acids and oxygen in the gas phase.
The catalysts of the Pd/alkali/Au system according to the
invention can be obtained by impregnating the support with
a basic solution and with a solution which contains gold
and palladium salts, wherein the impregnation steps can be
carried out simultaneously or in succession, with or
without intermediate drying. The support is subsequently
washed in order to remove chloride fractions which may be
present. The insoluble metal compounds which are
precipitated on the support can be reduced before or after
washing. The catalyst precursor which is thus obtained can
be dried in order to activate the catalyst can be
impregnated with alkali acetates or with alkali compounds
which under the reactions conditions for the production of
vinyl acetate monomer are completely or partially converted
inton alkali acetates. In general, the noble metals of
CA 02276476 1999-06-25
Pd/Au catalysts can be present in the form of a shell on
the support.
For Pd/alkali/Ba catalysts, the metal salts can be
deposited by impregnationg, spraying, immersion or
5 precipitation (EP 0 519 436). The same methods are known
for Pd/alkali/Cd catalysts (US-PS 4,902,823, US-PS
3,393,199, US-PS 4,668,819).
Depending on the catalyst system, the the supported
catalyst can be reduced.
10 Reduction of the catalyst can be effected in an aqueous
phase or in the gas phase. Formaldehyde or hydrazine are
suitable for reduction in an aqueous phase, for example.
Reduction in the gas phase can be effected with hydrogen or
forming gas (95 ~ by volume NZ + 5 $ by volume HZ), ethene
or ethene diluted with nitrogen. Reduction with hydrogen
can be conducted at temperatures between 40 and 260 °C,
preferably between 70 and 200 °C. Reduction with forming
gas (95 ~ by volume N2 and 5 $ by volume H2) can be
conducted at temperatures between 300 and 550 °C,
preferably between 350 and 500 °C. The catalyst can also be
reduced with ethene in the production reactor, after
activation with alkali acetate.
The catalyst supports according to the invention
advantageously retain their mechanical strength under the
reaction conditions of the catalytic process, particularly
under the effect of acetic acid.
The production of supported catalysts of the Pd/alkali/Au
system on mouldings according to the invention is described
in greater detail below.
The mouldings according to the invention are impregnated
with a solution which contains palladium and gold.
Simultaneously with the solution which contains noble
CA 02276476 1999-06-25
11
metals, or in any desired sequence in succession, the
mouldings according to the invention are impregnated with a
basic solution which may contain one or more basic
compounds. The basic compound or compounds serve to convert
palladium and gold into their hydroxides.
The compounds in the basic solution may consist of alkali
hydroxides, alkali bicarbonates, alkali carbonates, alkali
silicates or mixtures of these substances. Potassium
hydroxide and/or sodium hydroxide are preferably used.
Palladium chloride, sodium or potassium palladium chloride
or palladium nitrate, for example, can be used as palladium
salts for the production of the solution which contains
noble metals. Gold(III) chloride and tetrachloroauric(III)
acid can be used as gold salts. Potassium palladium
chloride, sodium palladium chloride and/or tetrachloroauric
acid are preferably used.
Impregnation of the mouldings according to the invention
with the basic solution has an effect on the deposition of
the noble metals in the support material. The basic
solution can be used either simultaneously with the
solution of noble metal or can be used in any desired
sequence with this solution. The mouldings according to the
invention are brought into contact with the basic solution
and with the solution of noble either simultaneously or in
any desired sequence in succession. When the mouldings
according to the invention are impregnated in succession
with the two solutions, an intermediate drying step can be
carried out after the first impregnation step.
The pressed parts according to the invention are
preferably first impregnated with the basic compound.
Subsequent impregnation with the solution which contains
palladium and gold results in the precipitation of
palladium and gold in a surface shell on the moulding. The
reverse sequence of impregnation generally results in a
CA 02276476 1999-06-25
12
more or less homogeneous distribution of the noble metals
over the cross-section of the moulding used. When the
process is conducted appropriately, however, catalysts
comprising a defined shell can also be obtained when
employing the reverse sequence of impregnation (see
US 4,048,096 for example). Catalysts which comprise a
homogeneous or almost homogeneous distribution of noble
metal generally exhibit reduced activity and selectivity.
Catalysts with shell thicknesses less than 1 mm are
particularly suitable. The shell thickness is influenced by
the amount of basic compound which is deposited on the
moulding in relation to the desired amount of noble metal.
The higher this ratio is, the lower is the thickness of the
shell which is formed. The quantitative ratio of basic
compound to noble metal compounds which is necessary for a
desired shell thickness depends on the nature of the
moulding and on the basic compound and noble metal
compounds which are selected. The requisite quantitative
ratio is advisedly determined by a few preliminary tests.
The shell thickness which is present can easily be
determined in such tests by cutting open the catalyst
particles.
The minimum necessary amount of basic compound results from
the stoichiometrically calculated amount of hydroxide ions
which are necessary for the conversion of the palladium and
gold in the hydroxide. As an approximate value, the basic
compound should be used in a 1 to 10-fold stoichiometric
excess for a shell thickness of 0.5 mm.
After the pore volume impregnation process, the mouldings
according to the invention can be coated with the basic
compounds and with noble metal salts. If intermediate
drying is employed, the volume of both solutions is
selected so that they each correspond to about 90 to 100 0
of the absorption capacity of the moulding used. If
CA 02276476 1999-06-25
13
intermediate drying is omitted, the sum of the individual
volumes of the two impregnation solutions has to correspond
to the above condition, wherein the individual volumes can
be in a ratio of 1 . 9 to 9 . 1 to each other. A volume
ratio from 3 . 7 to 7 . 3 is preferably employed,
particularly a ratio of 1 . 1. Water is preferably used as
the solvent in both these cases. Suitable organic or
aqueous-organic solvents can also be used, however.
The reaction of the noble metal salt solution with the
basic solution to form insoluble noble metal compounds
occurs slowly and is generally complete after 1 to 24
hours, depending on the method of preparation. Thereafter,
the water-insoluble noble metal compounds are treated with
reducing agents. Wet reduction can be effected, with
aqueous hydrazine hydrate for example, or a gas phase
reduction can be effected with hydrogen, ethene, forming
gas or methanol vapour. Reduction can be effected at normal
temperature or at an elevated temperature, and at normal
pressure or under an elevated pressure, optionally with the
addition of inert gases also.
Before and/or after the reduction ofthe noble metal
compounds, any chloride which may be present on the
moulding is removed by thoroughly washing the latter. After
washing, the moulding should contain less than 500 ppm,
preferably less than 200 ppm, of chloride.
The moulding which is obtained after reduction as a
catalyst precursor is dried and is subsequently impregnated
with alkali acetates or with alkali compounds which under
the reaction conditions for the production of vinyl acetate
monomer are completely or partially converted into alkali
acetates. The moulding is preferably impregnated with
potassium acetate. A pore volume impregnation method is
preferably used again here. This means that the requisite
amount of potassium acetate is dissolved in a solvent,
CA 02276476 1999-06-25
14
preferably water, the volume of which approximately
corresponds to the absorption capacity of the moulding for
the selected solvent. This volume is approximately equal to
the total pore volume of the mouldings.
The finished, coated moulding is subsequently dried to a
residual moisture content of less than 2 %. Drying can be
effected in air, or can also optionally be effected under
nitrogen as an inert gas.
The production of supported catalysts of the Pd/alkali/Cd
or Pd/alkali/Ba systems on mouldings according to the
invention is effected in a known manner according to the
patent specifications cited above.
For the synthesis of vinyl acetate monomer it is advisable
to coat the mouldings with 0.2 to 4, preferably 0.3 to
3 % by weight palladium, 0.1 to 2, preferably 0.15 to
1.5 % by weight gold and 1 to 10, preferably 1.5 to
9 % by weight potassium acetate, with respect to the weight
of the moulding used in each case. These data are
applicable to the Pd/alkali/Au system. In the case of
mouldings with a bulk density of 500 g/1, these
concentration data correspond to volume-based
concentrations of 1.0 to 20 g/1 palladium, 0.5 to 10 g/1
gold and 5 to 50 g/1 potassium acetate. In order to prepare
the impregnation solution, the corresponding amounts of
palladium and gold compounds are dissolved in a volume of
water which approximately corresponds to 90 - 100 % of the
water absorption capacity of the moulding in question. The
same procedure is employed for the preparation of the basic
solution.
The cadmium content of the Pd/alkali/Cd catalyst (finished,
coated moulding) can generally range from 0.1 to 2.5 % by
weight, preferably 0.4 to 2.0 % by weight.
CA 02276476 1999-06-25
The barium content of the Pd/alkali/Ba catalyst (finished,
coated moulding) can generally range from 0.1 to 2.0 % by
weight, preferably 0.4 to 1.8 % by weight.
The palladium content of the Pd/alkali/Cd or Pd/alkali/Ba
5 catalyst (finished, coated moulding) can generally range
from 0.2 to 4 % by weight, preferably 0.3 to 3 % by weight
palladium.
The potassium acetate content of the Pd/alkali/Cd or
Pd/alkali/Ba catalyst (finished, coated moulding) can
10 generally range from 1 to 10 % by weight, preferably 1,5 to
9 % by weight.
Silicas which have the following physical and chemical
properties, and which are also known by the name Aerosil~
of the Degussa company, can be used as pyrogenic silicas:
CA 02276476 1999-06-25
O O M
M ~ OD~ p M
~ u1 c~ O O O
000 +~r ~' ~ N i o~ U
, a~o 0 0
0 0 0
0 0
V V ~ n ~ V V U o
M ro .~ v ~ 0 0
~, 0
m ~ o
O O M O r-I
M tI) , OD~f7p M N W -1 .yJ
~ ~ O O O (a -rl
p 't~ +-~ N 01 ~ ~
(n ~ Q .-~ V 1 ~ O O O O O ~ U7
O fa V ~ n V V V
M , ~ V ~
ro
y.~ O
u7 O M O
N u'7 aou7M M N u7 .~ N U1
'
u c~ O O O N O U7
7
o +IN +.~ ' '-a o~ o N 1-~ O
a '~ V ~ o~o ~ O , O O .-I
o
O O
V
O ,S1 ~ n V V V V V
N ro M
4-1 O
M
O
M ~ "[f -rl
4-1
m n ~ ~,: 0 0 o N o N U O
+Ia +~ ' '_'~ o~ o o -r-I .-1
O ~ o~o o O o o f-~ ~ -IJ
V 'U U
~ V ~ n ~ v ~ v ~
ro
U U
M ~ ~ -rl
~P O M u7 (~ ~ J~
N ~I7 ~ ~, O O O ~ C ~ ~ ~ U1
7
~ 010 Q 0 0 O .C
.Z
O
V ~
~
M ~ V ~ n V V V V V U7 U1
ro
a~ a~
0
00 ~, 00tf)~ M N U7
O ~ '-V r-i 01~ O ~ O O O
O ~ N J.- ~ o~0 0 0 0 0
N ~
o~
V ~
n V V V V V
M U U
N ~ O
tl. ~1
U
o ao
rl u7 M ODaor-1M ~ N N y-I
-~ ,-r ,-m n ~ 0 0 0 0 o Sa Sa U
o
i ' ~ ~
+~~r ~ '- ~ ovo 0 0 0 0
v
Cf 0 o v o0
o n v V V V ~ .~ N
~ -r-1 -~
ro M 3 3 H
3 3 3 3 3 3 3
as ow dpdPda dpas
m
N
U ~' '-I
O O
-,1 ~ N O CO
+ G f-I 01
~ N ~
i-i O
ro -'~ M tn M
M
Q' ~ u~ umn
u7
a~ zzzz
ro O H H H H
~ u, b LILIC~O
- _ U
s-a U
p, O N ~n O O O O
H _ - ~ ~ ~ +~ J-1
~ O ~ JJ
W W '~' O O
~ O
Pa~ ~'1 ~ 'r-1N N
O O
U N v~ >, G ro
+~ ~
ro..~c ~ ~ c '~ b b
ro ro ~ -~
~ ~n W v ~.~ ow O S-~ S-1
S-a S-I
~ ~ ~ ~ O O o O
O ~ ' ~ ~ a, ~ ~ U U U U
y
v tr~o o O ro ~ W ~ U U U U
roro a~ ~ ~ ~ v
o O o
.x
w is f1. cn ~n ~, p p w ~ rtS b
-C .~ ~ a b
N N ~ u n G O O -~ cn
.~ o
~ ro\ ON ON xw ~ ~ a~ w U a~~
~l7~I,'H ~-7 ~7 C1 Cn~(,'Cr.1E x CL'.-1 N M
CT " " " -' c
Ln
CA 02276476 1999-06-25
17
In order to produce AEROSIL°, a volatile silicon compound
is injected through a nozzle into an oxyhydrogen gas flame
formed from hydrogen and air. Silicon tetrachloride is used
in most cases. This substance hydrolyses to form silica and
hydrochloric acid under the effect of the water formed
during the oxyhydrogen gas reaction. After leaving the
flame, the silica enters what is termed a coagulation zone,
in which the AEROSIL° primary particles and primary
aggregates agglomerate. The product, which at this stage is
present as a kind of aerosol, is separated from the
accompanying gaseous substances in cyclones, and is
subsequently post-treated with moist hot air. The residual
content of hydrochoric acid can be reduced to less than
0.025 % by this procedure. Since at the end of this process
the AEROSIL° only has a bulk density of about 15 g/1, is is
subjected to a subsequent vacuum compaction step by means
of which tamped densities of about 50 g/1 or more can be
achieved.
The particle size of products obtained in this manner can
be varied by varying the reaction conditions, such as the
flame temperature, the content of hydrogen or oxygen, the
amount of silicon tetrachloride, the residence time in the
flame or the length of the coagulation section, for
example.
The BET specific surface is determined using nitrogen
according to DIN 66 131. The pore volume is determined by
calculation from the sum of the micro-, meso- and macropore
volumes.
Determination of the micro- and mesopores is effected by
plotting an NZ isotherm and evaluating the latter by the
BET, de Boer and Barret, Joyner or Halenda methods.
The macropores are determined by the Hg penetration method.
The invention is explained by the Examples given below.
CA 02276476 1999-06-25
18
Example 1
85 o by weight Aerosil~ 200
o by weight methyl hydroxyethyl cellulose
5 o by weight wax
5 5 o by weight polyethylene glycol
were compacted in a kneader, with the addition of water
which had been made slightly alkaline with an aqueous
alkaline ammonia solution (15 ml of a 32~ solution for a 2
kg batch). The kneaded material was shaped in a single-
screw extruder to form hollow cylindrical extrudates in the
form of so-called cart wheels comprising five internal
reinforcing spokes or stays leading from the inner wall of
the hollow cylinder to the centre of the moulding, and was
cut to the desired length of 3.5 to 5.5 mm by a cutting
device. The mouldings were dried on a belt drier at 90°C
and were subsequently calcined for 6 hours at 900°C.
The mouldings obtained had the following physical and
chemical properties:
Moulding dimensions:
outside diameter (mm) 7.5 0.5
height (mm) 4 . 5 1
Wall thickness: 1.3 0.05
Stay width: 1.3 0.05
BET specific surface (m2/g) 79
Pore volume (ml/g) 0.69
Bulk density (g/1) 398
Si02 content (o by weight) 99.9
Height/diameter ratio 0.6
CA 02276476 1999-06-25
19
Example 2
85 % by weight Aerosil~ 200
o by weight methyl hydroxyethyl cellulose
5 % by weight wax
5 5 % by weight polyethylene glycol
were compacted in a kneader, with the addition of water
which had been made slightly alkaline with an aqueous
alkaline ammonia solution (15 ml of a 32$ solution for a 2
kg batch). The kneaded material was shaped in a single-
screw extruder to form hollow cylindrical extrudates in the
form of so-called cart wheels comprising five internal
reinforcing spokes or stays leading from the inner wall of
the hollow cylinder to the centre of the moulding, and was
cut to the desired length of 5.5 to 6.5 mm by a cutting
device. The mouldings were dried on a belt drier at 90°C
and were subsequently calcined for 6 hours at 850°C.
The mouldings obtained had the following physical and
chemical properties:
Moulding dimensions:
outside diameter (mm) 6.0 0.2
height (mm) 6. 0 0. 5
Wall thickness: 0.95 0.05
Stay width: 0.95 0.05
BET specific surface (m2/g) 148
Pore volume (ml/g) 0.75
Bulk density (g/1) 390
Si02 content (o by weight) 99.9
Height/diameter ratio 1.0
CA 02276476 1999-06-25
Example 3
85 o by weight Aerosil~ 200
5 ~ by weight methyl hydroxyethyl cellulose
5 o by weight wax
5 5 o by weight polyethylene glycol
were compacted in a kneader, with the addition of water
which had been made slightly alkaline with an aqueous
alkaline ammonia solution (15 ml of a 32% solution for a 2
kg batch). The kneaded material was shaped in a single-
10 screw extruder to form hollow cylindrical extrudates in the
form of so-called cart wheels comprising five internal
reinforcing spokes or stays leading from the inner wall of
the hollow cylinder to the centre of the moulding, and was
cut to the desired length of 3.5 to 5.5 mm by a cutting
15 device. The mouldings were dried on a belt drier at 90°C
and were subsequently calcined for 6 hours at 800°C.
The mouldings obtained had the following physical and
chemical properties:
Moulding dimensions:
outside diameter (mm) 7.5 0.5
height (mm) 4 . 5 1
Wall thickness: 1.3 0.05
Stay width: 1.3 0.05
BET specific surface (m2/g) 170
Pore volume (ml/g) 0.9
Bulk density (g/1) 360
Si02 content (o by weight) 99.9
Height /diameter ratio 0.6
CA 02276476 1999-06-25
21
Example 4
85 o by weight Aerosil~ 200
o by weight methyl hydroxyethyl cellulose
5 o by weight wax
5 5 o by weight polyethylene glycol
were compacted in a kneader, with the addition of water
which had been made slightly alkaline with an aqueous
alkaline ammonia solution (15 ml of a 32~ solution for a 2
kg batch). The kneaded material was shaped in a single-
screw extruder to form hollow cylindrical extrudates in the
form of so-called cart wheels comprising five internal
reinforcing spokes or stays leading from the inner wall of
the hollow cylinder to the centre of the moulding, and was
cut to the desired length of 5.5 to 6.5 mm by a cutting
device. The mouldings were dried on a belt drier at 90°C
and were subsequently calcined for 6 hours at 800°C.
The mouldings obtained had the following physical and
chemical properties:
Moulding dimensions:
outside diameter (mm) 6.0 0.2
height (mm) 6. 0 0. 5
Wall thickness: 0.95 0.05
Stay width: 0.95 0.05
BET specific surface (m2/g) 170
Pore volume (ml/g) 0.9
Bulk density (g/1) 350
SiOz content (o by weight) 99.9
Height/diameter ratio 1.0
CA 02276476 1999-06-25
22
Example 5
85 o by weight Aerosil° 200
o by weight methyl hydroxyethyl cellulose
5 o by weight wax
5 5 o by weight polyethylene glycol
were compacted in a kneader, with the addition of water
which had been made slightly alkaline with an aqueous
alkaline ammonia solution (15 ml of a 32s solution for a 2
kg batch). The kneaded material was shaped in a single-
screw extruder to form hollow cylindrical extrudates in the
form of what are termed miniliths as shown in Figure 3 and
4, comprising nine passageway channels, and was cut to the
desired length of 4 to 5 mm by a cutting device. The
mouldings were dried on a belt drier at 90°C and were
subsequently calcined at 800°C.
The mouldings obtained had the following physical and
chemical properties:
Moulding dimensions:
outside diameter (mm) 5.8 0.2
height (mm) 4 . 5 0.
5
Wall thickness: 0.8 0.05
Stay width: 0.8 0.05
BET specific surface (m2/g) 170
Pore volume (ml/g) 0.9
Bulk density (g/1) 350
Si02 content (o by weight) 99.9
Height/diameter ratio 0.78
CA 02276476 1999-06-25
23
Comparative examle 1
A palladium-gold-potassium acetate catalyst was prepared
according to Example 11 of EP 0 807 615 A1. The catalyst
support which was used was a moulding according to Example
5 of EP 0 807 615 A1, but which had the dimensions 8 x 5 x
3 mm (outside diameter x height x inside diameter) and
which had faceted edges.
The concentrations of the impregnation solutions were
selected so that the finished catalyst contained a
concentration of 0.55 o by weight palladium, 0.25 s by
weight gold and 5.0 a by weight potassium acetate.
In a first step, the support was first of all impregnated
with a basic solution of sodium hydroxide in water. The
volume of the aqueous NaOH solution corresponded to 50
percent of the water absorption capacity of the dry
support. After impregnation with sodium hydroxide, the
support was immediately impregnated, without intermediate
drying, with an aqueous solution of noble metals comprising
sodium palladium chloride and tetrachloroauric acid, the
volume of which likewise corresponded to 50 percent of the
water absorption capacity of the dry support.
After a holding time of 1.5 hours, during which the noble
metal compounds hydrolysed, the support particles were
washed until they were free from chloride. The support
particles were dried and were reduced at 450°C in the gas
phase with forming gas (95 ~ by volume N2, 5 ~ by volume
H2). Thereafter, the catalyst was impregnated with an
aqueous solution of potassium acetate and was dried again.
Drying was effected in the gas phase with nitrogen.
The sodium hydroxide concentration of the basic solution
was calculated so that a shell which contained noble metals
and which was formed on the support particles had a
thickness < 1.0 mm.
CA 02276476 1999-06-25
24
Example 6
A palladium-gold-potassium acetate catalyst as described in
comparative example 1 was produced on the moulding
according to the invention according to Example 1.
Example 7
A palladium-gold-potassium acetate catalyst as described in
comparative example 1 was produced on the moulding
according to the invention according to Example 2.
Example of use 1
The activity and selektivity of the catalysts from
comparative example 1 and from Examples 6 and 7 were
measured during a test procedure which had a duration of up
to 24 hours.
The catalysts were tested with the following gas
composition: 75 % by volume ethene, 16.6 o by volume acetic
acid, 8.3 ~ by volume oxygen, in an oil-heated tubular flow
reactor (reactor length 710 mm, inside diameter 23.7 mm) at
normal pressure and at a space velocity (GHSV) of 400 h-1.
The catalysts were investigated over the temperature range
from 120 to 165°C, as measured in the catalyst bed.
The reaction products were analysed at the outlet of the
reactor by means of on-line gas chromatography. The space-
time yield of the catalyst in grams of vinyl acetate
monomer per hour per kilogram of catalyst (g VAM/(h x
kgcat.) was determined as a measure of the catalyst
activity.
The carbon dioxide which was formed by the combustion of
ethene was also determined and was employed for assessing
the selectivity of the catalyst.
CA 02276476 1999-06-25
The test results on catalysts from comparative example 1
and from Examples 6 and 7 are presented in Table 1. The
catalyst activity and the catalyst selectivity of the
catalyst according to comparative example 2 were taken as
5 100 percent.
CA 02276476 1999-06-25
N
.U
w N
O
O
x
+~
+~ -
O G
rb b
~f'o~ u~01 N l~O ~ J-)
p U lp f-1M a1 N Wit'N ~ N
'GJ o ~' M d'~' M Wit'~ ,~ ~ N
,
.-~'--Iv--I'-W-1 ~--Iv--I -,_.I
N
S-1 J-~O
c~ U
rt
U S-1-rl
U
3
U
O ?~
-W -i u)
do
~ ~ ~ N
U ra .--I
U
G -IIb
-r s
4
-r-1 -~ .~
N
N
~ t~ ~ M C'tn l0 O
S.~
~
-
I
4-1 l~ ~rI'~~ ~ M p ~1 U
~ O u~ 0000 to t1
W
.1 O +~ rtirti
f~ o
_
W '-1 t +~
U 7i
~
o ~ -r
7 .~ ~
-r-I ~ -~i
't~ -r-1J~
O x .CZ
U ~ -rl
N U
O
U
v~ O
+~ -~-IN
m +~ .
m
.-1 ~ -r-I
N ~N ~ ~ 3
U rb -r-I
U
~ +~
Q tnW -1 M f~ N .C fl)
- '
~ x U o n
O 00M ~' ~ M
4-I rl ~ O N O M u~ O
p rlrl f-Iv--I.-i+~ +~ +~
~ \ rtf
b~ U
'--' -r-Ip
3 'U D
is ~ O s~ -~I
-,-~ .C O
u1 U b
U
+-~ ~ +~
.-1 cn ~ ~r1
.. ~ x ~ is o 5
u1 ~ tn ~ U ~.-~I
N -r-i +~
Q ,~ . 1- Wp ~ U
ca C2, ~ 3 Q~
t~ p ~ O ~
r~ O -~ O G p
E-~ U U f~ Cc7 H ~ .~Gcn