Note: Descriptions are shown in the official language in which they were submitted.
CA 02276569 2005-O1-17
1
MECHANICAL AND THERMAL IMPROVEMENTS
IN METAL HYDRIDE BATTERIES, BATTERY MODULES
AND BATTERY PACKS
FIELD OF THE INVENTION
The present invention relates generally to improvements for metal hydride
batteries, battery modules made therefrom and battery packs made from the
modules. More specifically, this invention relates to mechanical and thermal
improvements in battery design, battery module design, and battery pack
design.
BACKGROUND OF THE INVENTION
Rechargeable prismatic batteries are used in a variety of industrial and
commercial applications such as fork lifts, golf carts, uninterruptable power
supplies, and electric vehicles.
Rechargeable lead-acid batteries are presently the most widely used type
of battery. Lead-acid batteries are a useful power source for starter motors
for
internal combustion engines. However, their low energy density, about 30
Wh/kg,
and their inability to reject heat adequately, makes them an impractical power
source for an electric vehicle. An electric vehicle using lead-acid batteries
has a
short range before requiring recharge, require about 6 to 12 hours to recharge
and contain toxic materials. In addition, electric vehicles using lead-acid
batteries
have sluggish acceleration, poor tolerance to deep discharge, and a battery
lifetime of only about 20,000 miles.
Nickel metal hydride batteries ("Ni-MH batteries") are far superior to lead-
acid batteries, and Ni-MH batteries are the most promising type of battery
available for electric vehicles. For example, Ni-MH batteries, such as those
described in copending U.S. Patent No. 5,277,999 to Ovshinsky and Fetcenko,
have a much better energy density than lead-acid batteries, can power an
electric
CA 02276569 2005-O1-17
2
vehicle over 250 miles before requiring recharge, can be recharged in 15
minutes,
and contain no toxic materials. Electric vehicles using Ni-MH batteries will
have
exceptional acceleration, and a battery lifetime of more than about 100,000
miles.
Extensive research has been conducted in the past into improving the
electrochemical aspects of the power and charge capacity of Ni-MH batteries,
which is discussed in detail in U.S. Patent Nos 5,096,667, 5,104,617,
5,238,756
and 5,277,999.
Initially Ovshinsky and his team focused on metal hydride alloys that form
the negative electrode. As a result of their efforts, they were able to
greatly
increase the reversible hydrogen storage characteristics required for
efficient and
economical battery applications, and produce batteries capable of high density
energy storage, efficient reversibility, high electrical efficiency, efficient
bulk
hydrogen storage without structural changes or poisoning, long cycle life, and
repeated deep discharge. The improved characteristics of these "Ovonic"
alloys,
as they are now called, results from tailoring the local chemical order and
hence
the local structural order by the incorporation of selected modifier elements
into a
host matrix. Disordered metal hydride alloys have a substantially increased
density of catalytically active sites and storage sites compared to single or
multi-phase crystalline materials. These additional sites are responsible for
improved efficiency of electrochemical charging/discharging and an increase in
electrical energy storage capacity. The nature and number of storage sites can
even be designed independently of the catalytically active sites. More
specifically,
these alloys are tailored to allow bulk storage of the dissociated hydrogen
atoms
at bonding strengths within the range of reversibility suitable for use in
secondary
battery applications.
Some extremely efficient electrochemical hydrogen storage materials were
formulated, based on the disordered materials described above. These are the
Ti-V-Zr-Ni type active materials such as disclosed in U.S. Patent No.
4,551,400
("the '400 Patent") to Sapru, Hong, Fetcenko, and Venkatesan. These materials
reversibly form hydrides in order to store hydrogen. All the materials used in
the
'400 Patent utilize a generic Ti-V-Ni composition, where at least Ti, V, and
Ni are
present and may be modified with Cr, Zr, and AI. The materials of the '400
Patent
CA 02276569 2005-O1-17
3
are multiphase materials, which may contain, but are not limited to, one or
more
phases with C,4 and C,Stype crystal structures.
Other Ti-V-Zr-Ni alloys are also used for rechargeable hydrogen storage
negative electrodes. One such family of materials are those described in U.S.
Patent No. 4,728,586 ("the '586 Patent") to Venkatesan, Reichman, and
Fetcenko. The '586 Patent describes a specific sub-class of these Ti-V-Ni-Zr
alloys comprising Ti, V, Zr, Ni, and a fifth component, Cr. The '586 Patent,
mentions the possibility of additives and modifiers beyond the Ti, V, Zr, Ni,
and Cr
components of the alloys, and generally discusses specific additives and
modifiers, the amounts and interactions of these modifiers, and the particular
benefits that could be expected from them.
In contrast to the Ovonic alloys described above, the older alloys were
generally considered "ordered" materials that had different chemistry,
microstructure, and electrochemical characteristics. The performance of the
early
ordered materials was poor, but in the early 1980's, as the degree of
modification
increased (that is as the number and amount of elemental modifiers increased),
their performance began to improve significantly. This is due as much to the
disorder contributed by the modifiers as it is to their electrical and
chemical
properties. This evolution of alloys from a specific class of "ordered"
materials to
the current multicomponent, multiphase "disordered" alloys is shown in the
following patents: (i) U.S. Patent No. 3,874,928; (ii) U.S. Patent No.
4,214,043;
(iii) U.S. Patent No. 4,107,395; (iv) U.S. Patent No. 4,107,405; (v) U.S.
Patent No.
4,112,199; (vi) U.S. Patent No. 4,125,688 (vii) U.S. Patent No. 4,214,043;
(viii)
U.S. Patent No.4,216,274; (ix) U.S. Patent No. 4,487,817; (x) U.S. Patent No.
4,605,603; (xi) U.S. Patent No. 4,696,873; and (xii) U.S. Patent No.
4,699,856.
(These references are discussed extensively in U.S. Patent No. 5,096,667).
CA 02276569 1999-07-02
WO 98/31059 PGT/US97/0~05
4
Simply stated, in all metal-hydride alloys, as the degree of modification
increases, the role of the initially ordered base alloy is of minor importance
compared to the properties and disorder attributable to the particular
modifiers.
In addition, analysis of the present multiple component alloys available on
the
market and produced by a variety of manufactures indicates that these alloys
are modified following the guidelines established for Ovonic alloy systems.
Thus, as stated above, all highly modified alloys are disordered materials
characterized by multiple components and multiple phases, i.e. Ovonic alloys.
Clearly, the introduction of Ovonic alloying techniques has made
significant improvements in the active electrochemical aspects of Ni-MH
batteries. However, it should be noted that until recently the mechanical and
thermal aspects of the pertormance of Ni-MH batteries have been neglected.
For example, in electric vehicles, the weight of the batteries is a
significant factor because battery weight is the largest component of the
weight
of the vehicle. For this reason, reducing the weight of individual batteries
is a
significant consideration in designing batteries for electric powered
vehicles. In
addition to reducing the weight of the batteries, the weight of battery
modules
must be reduced, while still affording the necessary mechanical requirements
of
a module (i.e. ease of transport, ruggedness, etc.). Also, when these battery
modules are incorporated into battery pack systems (such as for use in
electric
vehicles) the battery pack components must be as light weight as possible.
It should be particularly noted that electric vehicle applications introduce
a critical requirement for thermal management. This is because individual
cells
are bundled together in close proximity and many cells are electrically and
thermally connected together. Therefore, since there is an inherent tendency
to
generate significant heat during charge and discharge, a workable battery
design for electric vehicles is judged by whether or not the generated heat is
sufficiently controlled.
Sources of heat are primarily threefold. First, ambient heat due to the
operation of the vehicle in hot climates. Second, resistive or IZR heating on
charge and discharge, where I represents the current flowing into or out of
the
CA 02276569 2005-O1-17
battery and R is the resistance of the battery. Third, a tremendous amount of
heat
is generated during overcharge due to gas recombination. While the above
parameters are generally common to all electrical battery systems, they are
particularly important to nickel-metal hydride battery systems. This is
because
5 Ni-MH has such a high specific energy and the charge and discharge currents
are
also high. For example, to charge a lead-acid battery in one hour, a current
of 35
Amps may be used while recharge of a Ni-MH battery may utilize 100 Amps for
the same one-hour recharge. Second, because Ni-MH has an exceptional energy
density (i.e. the energy is stored very compactly) heat dissipation is more
difficult
than lead-acid batteries. This is because the surface-area to volume ratio is
much
smaller than lead-acid, which means that while the heat being generated is 2.5-
times greater for Ni-MH batteries than for lead-acid, the heat dissipation
surface is
reduced. The following illustrative example is useful in understanding the
thermal
management problems faced when designing Ni-MH battery packs for electric
vehicles. In U.S. Patent No. 5,378,555 to General Motors, an electric vehicle
battery pack using lead-acid batteries is described. The battery pack system,
utilizing lead-acid batteries, has a capacity of about 13 kWh, weighs about
800
pounds, and has a vehicle range of about 90 miles. By replacing the lead-acid
battery pack by an Ovonic battery pack of the same size, the capacity is
increased to 35 kWh and vehicle range is extended to about 250 miles. One
implication of this comparison is that in a 15 minute recharge, the power
supplied
to the Ni-MH battery pack is 2.7 times greater than that supplied to the lead-
acid
battery pack, with its commensurate added heat. However, the situation is
somewhat different during discharge. To power a vehicle on the highway at
constant speed, the current draw upon the battery is the same whether it is a
Ni-MH battery or a lead-acid battery (or any other power source for that
matter).
Essentially the electric motor which drives the vehicle does not know or care
where it gets the energy or what type of battery supplies the power. The
difference between the heating of the Ni-MH battery and the lead-acid battery
upon discharge is the length of discharge.
CA 02276569 1999-07-02
WO 98131059 PCT/US97100805
6
That is, since the Ni-MH battery will drive the vehicle 2.7 times farther than
the
lead-acid, it has a much longer time before it has a chance to "cool-off'.
Further, while the heat generated during charging and discharging Ni-MH
batteries is normally not a problem in small consumer batteries or even in
larger
batteries when they are used singly for a limited period of time, large
batteries
that serve as a continual power source, particularly when more than one is
used
in series or in parallel, such as in a satellite or an electric vehicle, do
generate
sufficient heat on charging and discharging to affect the ultimate performance
of
the battery modules or battery pack systems.
1o Thus, there exists a need in the art for battery, battery module, and
battery pack system designs which reduces the overall weight thereof and
incorporates the necessary thermal management needed for successful
operation in electric vehicles, without reducing its energy storage capacity
or
power output, increases the batteries' reliability, and decreases the cost.
DEFICIENCIES OF THE PRIOR ART
Thermal management of an electric vehicle battery system using a high
energy battery technology has never before been demonstrated. Some
technologies, such as Na-S, which operate at elevated temperatures are heavily
insulated to maintain a specific operating temperature. This arrangement is
undesirable due to a heavy penalty in overall energy density due to the
excessive weight of the thermal management, high complexity and excessive
cost. In other systems, such as Ni-Cd, attempts at thermal management have
utilized a water cooling system. Again this type of thermal management system
adds weight, complexity and cost to the battery pack.
Simply stated, the prior art does not teach an integrated battery
configuration/internal design, battery module, and thermally managed battery
pack system which is light weight, simple, inexpensive, and combines the
structural support of the batteries, modules and packs with an air-cooled
thermal management system.
CA 02276569 1999-07-02
WO 98131059 PCT/US9'7/0~5
7
SUMMARY OF THE INVENTION
One aspect of the instant invention provides for a mechanically improved
rechargeable battery. The battery includes: 1 ) a battery case which includes
a
positive battery electrode terminal and a negative battery electrode terminal;
2)
at least one positive battery electrode disposed within the battery case and
electrically connected to the positive battery electrode terminal; 3) at least
one
negative battery electrode disposed within the battery case and electrically
connected to the negative battery electrode terminal; 4) at least one battery
electrode separator disposed between the positive and negative electrodes
within the battery case to electrically insulate the positive electrode from
the
negative electrode, but still allow for chemical interaction thereof; and 5)
battery
electrolyte surrounding and wetting the positive electrode, the negative
electrode, and the separator. The battery case is prismatic in shape and has
an optimized thickness to width to height aspect ratio.
Another aspect of the present invention includes an improved, high-
power battery module. The battery module of the instant invention includes: 1
)
a plurality of individual batteries; 2) a plurality of electrical
interconnects
connecting the individual batteries of the module to one another and providing
means for electrically interconnecting separate battery modules to one
another;
and 3) a battery module bundiing/compression means. The batteries are bound
within the module bundlinglcompression means under external mechanical
compression which is optimized to balance outward pressure due to expansion
of the battery components and provide additional inward compression on the
battery electrodes within each cell to reduce the distance between the
positive
and negative electrodes, thereby increasing overalE cell power.
The module bundling/compression means is designed to: 1 ) allow for
application of the required battery compression; 2) perform the requires
mechanical function of vibration resistant module bundler; and 3) be as light
weight as possible.
Yet another aspect of the present invention is the mechanical design of
light-weight, fluid-cooled, battery pack systems. In its most basic form the
instant fluid-cooled battery pack system includes: 1 ) a battery-pack case
having
CA 02276569 1999-07-02
WO 98/31059 pCT~~~
8
at least one coolant inlet and at least one coolant outlet; 2) at least one
battery
module disposed and positioned within the case such that the battery module is
spaced from the case walls and from any other battery modules within the case
to form coolant flow channels along at least one surtace of the bundled
batteries, the width of the coolant flow channels is optimally sized to allow
for
maximum heat transfer, through convective, conductive and radiative heat
transfer mechanisms, from the batteries to the coolant; and 3) at least one
coolant transport means which causes the coolant to enter the coolant inlet
means of the case, to flow through the coolant flow channels and to exit
through the coolant outlet means of the case. In a preferred embodiment, the
battery pack system is air-cooled.
En still another aspect of the present invention, the above described
mechanical design of the battery, module, and battery pack system is
integrated
electronically through a charger algorithm designed to charge the battery pack
system quickly while extending the battery life through minimized overcharge
and heat generation management.
Finally the batteries, modules and packs can also include means for
providing variable thermal insulation to at least that portion of the
rechargeable
battery system which is most directly exposed to said ambient thermal
condition, so as to maintain the temperature of the rechargeable battery
system
within the desired operating range thereof under variable ambient conditions.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a highly stylized depiction of a cross-sectional view of the
mechanically improved rechargeable battery of the invention, specifically
illustrating the battery electrodes, separator, battery case, and the battery
electrical terminals;
Figure 2 is a stylized depiction of an exploded, cross-sectional view of
the mechanically improved rechargeable battery, specifically illustrating how
many of the battery components interact when assembled;
Figure 3 is a blow-up of the terminal, can top, terminal seal and electrode
comb depicted in Figure 2;
CA 02276569 1999-07-02
WO 98/31059 PGT/US97100805
9
Figure 4 is a stylized depiction of a cross-sectional view of the crimp seal
formed to seal the battery terminal to the battery can top;
Figure 5 is a stylized depiction of a cross-sectional view of one
embodiment of the battery terminal, specifically illustrating how a pressure
vent
can be incorporated into the terminal;
Figure 6 is a stylized depiction of a cross-sectional view of another
embodiment of the battery terminal, specifically illustrating how a socket
type
electrical lead connector can be incorporated into the terminal;
Figure 7 is a stylized depiction of an electrode comb;
1o Figure 8 is a stylized depiction of a top view of a battery module of the
instant invention, specifically illustrated is the manner in which the
batteries are
bundled, including their orientation, the bars and end-plates which hold the
batteries under external mechanical compression, and the axis of compression;
Figure 9 is a stylized depiction of a side view of the battery module of
Figure 8, specifically illustrated is the manner in which the metal bars are
set
into slots in the ribs of the end-plates;
Figure 10 is a stylized depiction of an end view of the battery module of
Figures 8 and 9, specifically shown is the manner in which the end plates and
the compression bars interact;
2o Figure 11 is a stylized depiction of a top view of a battery module of the
instant invention, specifically illustrating the module spacers of the instant
invention and the spacer tabs attached thereto;
Figure 12 is a stylized depiction of a side view of the battery module of
Figure 11, specifically illustrating the manner in which the module spacers
are
placed on the top and bottom of the battery module;
Figure 13a is a stylized depiction of one embodiment of the end ptates of
the instant battery modules, specifically illustrated is a ribbed end plate;
Figure 13b is a stylized depiction of a cross-sectional view of the ribbed
end plate of Figure 13a;
Figure 14 is a stylized depiction of one embodiment of the braided cable
interconnect useful in the modules and battery packs of the instant invention;
specifically shown is a flat braided cable electrical interconnect;
CA 02276569 1999-07-02
WO 98/31059 PCT/ITS97/OOS05
Figure 15 is a stylized depiction of a top view of one embodiment of the
fluid-cooled battery pack of the present invention, specifically illustrated
is the
matrix placement of the battery modules into the pack case, the manner in
which the module spacers form coolant flow channels, the fluid inlet and
outlet
5 ports, and the fluid transport means;
Figure 16 is plot of battery temperature versus stand time indicating the
manner in which temperature controlled fan algorithms affect the battery
temperature during pack self discharge;
Figure 17 is a plot of battery resistance and battery thickness versus
1o external compression pressure, optimal and functional ranges are clearly
present;
Figure 18 illustrates the effect of temperature upon the battery's specific
energy, plotting battery temperature versus specific energy in Wh/Kg;
Figure 19 illustrates the effect of temperature upon the battery's specific
power, plotting battery temperature versus specific power in W/Kg;
Figure 20 is a plot of coolant volumetric flow rate and the percentage of
maximum heat transfer and coolant velocity versus centerline spacing (related
to average coolant channel width) for vertical coolant flow through the
coolant
flow channels;
2o Figure 21 is a plot of coolant volumetric flow rate and the percentage of
maximum heat transfer and coolant velocity versus centerline spacing (related
to average coolant channel width) for horizontal coolant flow through the
coolant
flow channels;
Figure 22 is a plot of temperature rise from ambient and pack voltage
versus time during charge and discharge cycles using a "temperature
compensated voltage !id" charging method;
Figure 23 is a plot of temperature rise from ambient and pack voltage
versus time during charge and discharge cycles using a "fixed voltage lid"
charging method;
3o Figure 24 is a plot of battery capacity measured in Ah verses battery type
for the M series batteries;
CA 02276569 1999-07-02
wo ~icis9 rcTms~roosos
11
Figure 25 is a plot of battery power measured in W verses battery type
for the M series batteries;
Figure 26 is a plot of normalized battery capacity measured in mAhlcm2
verses battery type for the M series batteries;
Figure 27 is a plot of normalized battery power measured in mWlcm2
verses battery type for the M series batteries;
Figure 28 is a plot of specific battery power measured in WIKg verses
battery type for the M series batteries; and
Figure 29 is a plot of specific battery energy measured in Wh/Kg verses
battery type for the M series batteries.
DETAILED DESCRIPTION OF THE INVENTION
One aspect of the instant invention provides for a mechanically improved
rechargeable battery, shown generically in Figure 1. Typically in the field of
rechargeable batteries, such as the nickel-metal hydride battery system much
emphasis is placed upon the electrochemical aspects of the batteries, while
much less time and energy are spent in improving the mechanical aspects of
battery, module and pack design.
The instant inventors have investigated improvements in the mechanical
design of rechargeable battery systems, looking at aspects such as energy
density (both volumetric and gravimetric), strength, durability, mechanical
aspects of battery pertormance, and thermal management.
In response to these investigations, the instant inventors have designed a
mechanically improved rechargeable battery 1 which includes: 1 ) a battery
case
2 which includes a positive battery electrode terminal 7 and a negative
battery
electrode terminal 8; 2) at least one positive battery electrode 5 disposed
within
the battery case 2 and electrically connected to the positive battery
electrode
terminal 7; 3) at least one negative battery electrode 4 disposed within the
battery case 2 and electrically connected to the negative battery electrode
terminal 8; 4) at least one battery electrode separator 6 disposed between the
positive and negative electrodes within the battery case 2 to electrically
insulate
the positive electrode from the negative electrode, but still allow for
chemical
CA 02276569 1999-07-02
WO 98131059 pCT~S~
12
interaction thereof; and 5) battery electrolyte (not shown) surrounding and
wetting the positive electrode 5, the negative electrode 4, and the separator
6.
The battery case 2 is prismatic in shape and has an optimized thickness to
width to height aspect ratio.
As used herein, the term "battery" specifically refers to electrochemical
cells which include a plurality of positive and negative electrodes separated
by
separators, sealed in a case having positive and negative terminal on its
exterior, where the appropriate electrodes are all connected to their
respective
terminals.
This optimized aspect ratio, as described below, allows the battery to
have balanced optimal properties when compared with prismatic batteries which
do not have this optimized aspect ratio. Specifically the thickness, width and
height are all optimized to allow for maximum capacity and power output, while
eliminating deleterious side effects. Additionally, this particular case
design
allows for unidirectional expansion which can readily be compensated for by
applying external mechanical compression in that one direction. The instant
inventors have found that the optimal electrode thickness to width ratio to be
between about 0.1 to 0.75 and the optimal height to width ratio to be between
0.75 and 2.1. Specific examples of batteries and their electrode height to
width
ratio is given in Table 1.
TABLE 1
attery ype eig t mm i mm abo
1_87 g 1 2.06 -. _
-2 16 1 1. 4
M-40 147 91 1.62
1~ ~1_ -40
3o It should be noted that even within the optimal range of ratios, there are
suboptimal ranges depending upon the desired properties of the batteries. For
example, Figures 24-29 show how the different height to width aspect ratios of
the M series of batteries (shown in Table 1 ) give different optimums
depending
upon the specific properties desired. Figures 24 and 25, which are plots of
SUBSTITUTE SHEET (RULE 26)
CA 02276569 1999-07-02
WO 9~8J31059 PCT/US97/OOS05
13
capacity in Ah and power in W verses battery type, respectively, indicate that
for maximum capacity and power, the M cell is best. However, as can be seen
from Figures 26 and 27, which are plots of normalized capacity in mAh/cm2 and
power in mW/cmz verses battery type, respectively, if the capacity and power
are normalized to the area of the electrodes, the M-40 cell is the best.
Additionally, if the specific power of the batteries are determined, the M-40
cell
is also the best, as shown by Figure 28 which plots the specific power of the
batteries in W/Kg verses battery type. Finally, if the specific energy of the
batteries is important, the M-20 cell is the best, as shown by Figure 29 which
plots the specii'IC energy of the batteries in Wh/Kg verses battery type.
In determination of the optimal ratios, the instant inventors have noted
that if the batteries are too high (tall) there is an increased tendency for
the
electrodes to crack upon expansion and contraction. There is also problems
with increased internal electrical resistance of the electrodes, and
gravimetric
segregation of the electrolyte to the bottom of the battery leaving the upper
portions of the electrodes dry. Both of these later problems reduce the
capacity
and power output of the batteries. If, on the other hand, the electrodes are
too
short, the capacity and power of the battery are reduced due to lowered
inclusions of the electrochemically active materials and the specific energy
density of the battery is reduced due to the change in the ratios of dead
weight
battery components to electrochemically active components.
Also, if the batteries are too wide, there is an increased tendency for the
electrodes to crack upon expansion and contraction. There is also a problem
with increased internal electrical resistance which reduces the capacity and
power output of the batteries. But, if the electrodes are too narrow, the
capacity
and power of the battery are reduced due to lowered inclusion of the
electrochemically active materials and the specific energy density of the
battery
is reduced due to the change in the ratios of dead weight battery components
to
electrochemically active components.
Finally, if the battery is too thick there are problems with improper
thermal dissipation from the central electrodes which reduces battery capacity
and power. Also, there is an increased overall electrode bundle expansion in
CA 02276569 2005-O1-17
14
the thickness direction which causes warpage and damage to the battery case
and creates gaps between the positive and negative electrodes thereby reducing
battery power and capacity. This excessive electrode bundle expansion must be
compensated for by external mechanical compression. However, when the
battery is too thick, an excessive amount of external force is required to
compensate for the expansion and cracking of the electrodes occurs. On the
other hand, if the battery is too thin, fewer electrodes will fit in the
battery and
therefore the capacity and power of the battery are reduced due to lowered
inclusion of the electrochemically active materials and the specific energy
density
of the battery is reduced due to the change in the ratios of dead weight
battery
components to electrochemically active components.
Within this application the term "expansion" includes both thermal and
electrochemical expansion. The thermal expansion is due to heating of the
battery
components by the mechanisms described above and the electrochemical
expansion is due to a changing between different lattice structures in the
charged
and discharged states of the electrochemically active materials of the
battery.
The battery case 2 is preferably formed from any material which is
thermally conductive, mechanically strong and rigid, and is chemically inert
to the
battery chemistry, such as a metal. Alternatively, a polymer or composite
material
may be used as the material for the battery case. In choosing such a material,
consideration must be given to thermal heat transfer. As detailed in U.S.
Patent
No. 5,598,950, filed May 5, 1995, experiments with plastic cases show that the
internal temperature of a plastic cased metal-hydride battery rises to about
80°C
from ambient after cycling at C110 to 120% of capacity, while a stainless
steel
case rises to only 32°C. Thus, thermally conductive polymer or
composite
material cases are preferred. Most preferably the case is formed from
stainless
steel. It is advantageous to electrically insulate the exterior of the metal
case from
the environment by coating it with a non-conductive polymer coating (not
shown).
An example of one such layer is insulating polymer tape layer made from a
polymer such as polyester. The mechanical strength and
CA 02276569 1999-07-02
W0 98/31059 PCT/US97/OOS05
ruggedness of the polymer tape is important as well as its insulating
properties.
Additionally, it is preferably inexpensive, uniform, and thin.
The interior of the battery case 2 must also be electrically insulated from
the battery electrodes. This can be accomplished by coating an electrically
5 insulating polymer (not shown) onto the interior of the battery case, or
alternatively, enclosing the battery electrodes and electrolyte in an
electrically
insulating polymer bag (not shown), which is inert to the battery chemistry.
This
bag is then sealed and inserted into the battery case 2.
In a preferred embodiment, shown in Figure 2, the battery case includes
10 a case top 3 onto which the positive battery electrode terminal 7 and the
negative battery electrode terminal 8 are affixed, and a battery case can 9
into
which the electrodes 4, 5 are disposed. Figure 3 shows that the case top 3
includes openings 13, through which the positive and negative battery
terminals
7, 8 are in electrical communication with the battery electrodes 4, 5. The
15 diameter of the openings 13 is slightly larger than the outer diameter of
the
terminal 7, 8, but smaller than the outer diameter of a seal 10 used to seat
the
terminal 7,8 to the case top 3. The terminals 7, 8 include a sealing lip 11
which
assists in sealing the terminal 7, 8 to the case top 3, using the seal 10. The
seal 10 is typically a sealing ring. The seal 10 includes a sealing lip slot
12 into
which the sealing lip 11 of the terminal 7, 8 is fit. This slot 12 helps to
form a
good pressure seal between the terminal 7, 8 and the case top 3 and to keep
the seal 10 in place when the terminal 7, 8 is crimped into the case top 3.
The
seal 10 is preferably formed of an elastomeric, dielectric, hydrogen
impermeable
material, such as, for example, polysulfone. The case top 3 also inGudes a
shroud 14 surrounding the each of the openings 13 and extending outward from
the case top 3. The shroud 14 has an inner diameter slightly larger than the
outer diameter of the seal 10. The shroud 14 is crimped around the seal 10
and the sealing lip 11 of the battery terminal 7, 8, to form an electrically
non-
conductive pressure seal between the terminal 7, 8 and the case top 3. The
3o crimp terminal seal provides vibration resistance when compared to the
threaded seal of the prior art. The case top 3, case can 9, and annular shroud
14 may be formed from 304L stainless steel.
CA 02276569 1999-07-02
WO 98131059 PCTIUS97/008fl5
16
Figure 4, shows a portion of the battery of the present invention
specifically depicting the fashion in which the battery terminal 7, 8 is crimp
sealed into the case top 3. From this figure, it can be clearly determined how
the shroud 14 of the case top 3 is crimp sealed around the seal 10 which is,
in
turn, sealed around the sealing lip 11 of the battery terminal 7, 8. In this
manner the vibration resistant pressure seal is formed.
The method of attaching the terminal 7, 8 to the case top 3 involves
crimp sealing the terminal 7, 8 to the case top 3. This crimp sealing method
has a number of advantages over the prior art. Crimp sealing can be done
1o rapidly on high speed equipment leading to a direct cost reduction. In
addition,
this method uses less material than the prior art which reduces the weight of
the terminals resulting in an indirect cost reduction. The higher surtace area
of
this design coupled with the decreased weight of the materials also results in
increased heat dissipation from the terminals. Yet another advantage of the
present invention is that it permits forming the battery case and other parts
from
any malleable material and specifically does not require laser sealing,
special
ceramic to metal seals, or special (and thereby expensive) methods of any
kind.
In addition, the overall number of parts and the need for highly machined
precisely fabricated parts are eliminated.
2o The battery terminals 7, 8 are typically formed from a copper or copper
alloy material, preferably nickel plated for corrosion resistance. However,
any
electrically conductive material which is compatible with the battery
chemistry
may be used. It should be noted that the battery terminals 7, 8 described in
context with the present invention are smaller in annular thickness and of a
greater diameter than those of the prior art. As a result, the terminals of
the
present invention are very efficient dissipaters of heat, and thus contribute
significantly to the thermal management of the battery.
The terminals 7, 8 may also include an axially aligned central opening
15. The central opening 15 serves many purposes. One important
consideration is that it serves to reduce the weight of the battery. It can
also
serve as an opening into which an external electrical connector may be
friction
fit. That is a cylindrical or annular battery lead connector may be friction
fitted
CA 02276569 2005-O1-17
17
into the central opening 15 to provide an external electrical connection to
the
battery. Finally, it can serve as the location for a pressure release vent for
venting
excessive pressure from the interior of the battery. The opening 15 can extend
partially through the terminal (if it is intended to serve only as a connector
socket)
or all the way through (if it is intended to contain a pressure vent and serve
as a
connector socket).
When at least one of the terminals 7, 8 includes a pressure vent for
releasing internal pressure of the battery to the surrounding atmosphere, the
vent
can be affixed in the axial opening within the terminal, see Figure 5. Most
preferably the pressure vent 16 includes: 1 ) a vent housing 17 having a
hollow
interior area 21 in gaseous communication with the surrounding atmosphere and
the interior of the battery case via the openings 15, 18 and 23; 2) a pressure
release piston 19 is positioned within the hollow interior area 21, the
pressure
release piston 19 is sized to seal the axial opening 16 and has a seal groove
20
on its surface opposite the axial opening 16; 3) an elastomeric, dielectric
seal (not
shown) is mounted within the seal groove, the seal groove 20 is configured to
encapsulate all but one surface of the seal, thereby leaving the non-
encapsulated
surface of the seal exposed; and 4) a compression spring 22 is positioned to
urge
the pressure release piston 19 to compress the seal in the seal groove 20 and
block the axial opening 18 in the terminal 7, 8. Refer to commonly owned U.S.
Patent No. 5,258,242, issued November 2, 1993, entitled "ELECTROCHEMICAL
CELL HAVING IMPROVED PRESSURE VENT". Again, preferably the
elastomeric, dielectric seal is formed of a hydrogen impermeable polysulfone
material. Additionally it is preferable that the vent be designed to release
internal
pressure in excess of about 120 pounds per square inch to insure battery
integrity, since the battery cans are generally rated for at most about 150
pounds
per square inch.
In addition to the resealable vent described above, other types of vents
may be used in the batteries of the instant invention. Specifically, rupture
disks,
pressure plugs and septum vents may be used. One such septum vent is
described in U.S. Patent No. 5,171,647. Also, while it is preferred that the
pressure vent be located within a hollow battery terminal, the vent can just
as
CA 02276569 2005-O1-17
18
effectively be located elsewhere on the battery top in its own protective
housing or
merely attached to an opening in the top of the battery case.
Another alternative embodiment of the battery terminal is presented in
Figure 6, which shows a terminal 7, 8 into which an external battery lead
connector 24 can be friction fit. The connector 24 is attached to an external
battery lead 25. Lead 25 may be any of the type typically known in the art
such as
a solid bar; a metal ribbon; a single or multi strand wire; or a braided, high
current,
battery cable (as is described hereinbelow). Preferably the lead connector 24
is a
hollow annular barrel connector which is friction fit into the axially aligned
central
opening 15 of the battery terminal 7, 8. The lead connector 24 is held in the
battery terminal 7, 8 via a barrel connector web 26. A solid barrel connector
is
described in U.S. Patent No. 4,657,335, dated April 14, 1987 and 4,734,063,
dated March 29, 1988, each to Koch et al. and entitled "RADIALLY RESILIENT
ELECTRICAL SOCKET".
If desired, the embodiments presented in Figures 5 and 6 may be
combined into a single embodiment which incorporates both the pressure vent 16
and the external battery lead connector 24. In addition, a rupture disk (i.e.
a
non-resealable means of releasing excess pressure) can be included instead of
or in addition to the pressure vent.
While the crimp seal terminals and case top are the preferred embodiment
of the instant invention, other types of terminals and, therefore, other types
of
case tops may be used. Specifically, a screw on terminal incorporating an o-
ring
type of seal may be employed. Generally, any type of known sealed terminal may
be used as long as it can contain the operating pressures of the battery and
is
resistant to the electrochemical environment of the battery.
While any battery system may benefit from the present improvements in
battery, module, and pack configuration, it is preferred that the positive
electrodes
are formed from a nickel hydroxide material and the negative electrodes are
formed from a hydrogen absorbing alloy. Preferably, the negative electrode
material is an Ovonic metal-hydride alloy. (That is, a disordered,
multicomponent
metal hydride alloy as described in U.S. Patent No. 5,506,069, issued April 9,
1996, U.S. Patent No. 5,407,781, issued April 18, 1995, and the applications
and
references that depend from them and are specifically referenced in them.).
Also
CA 02276569 2005-O1-17
19
it is preferable that the electrodes are separated by non-woven, felted, nylon
or
polypropylene separators and the electrolyte is an alkaline electrolyte, for
example, containing 20 to 45 weight percent potassium hydroxide. Such
separators are described in U.S. Patent No. 5,330,861.
Ni-MH batteries for consumer applications on the market used pasted
metal hydride electrodes in order to achieve sufficient gas recombination
rates
and to protect the base alloy from oxidation and corrosion. Such pasted
electrodes typically involved mixing the active material powder with plastic
binders
and other non-conductive hydrophobic materials. An unintended consequence of
this process is a significant reduction in the thermal conductivity of the
electrode
structure as compared to a structure of the present invention which consists
essentially of a 100% conductive active material pressed onto a conductive
substrate.
In a sealed prismatic Ni-MH battery according to the present invention, the
buildup of heat generated during overcharge is avoided by using a cell bundle
of
thermally conductive metal hydride electrode material. This thermally
conductive
metal hydride electrode material contains metal hydride particles in intimate
contact with each other. Oxygen gas generated during overcharge recombines to
form water and heat at the surface of these particles. In the present
invention, this
heat follows the thermally conductive negative electrode material to the
current
collector and then to the surface of the case. The thermal efficiency of the
bundle
of thermally conductive metal hydride electrode material is further improved
if this
electrode bundle is in thermal contact with a battery case that is also
thermally
conductive.
In the present invention, the metal hydride negative electrode material is
preferably a sintered electrode such as described in U.S. Patent Nos.
4,765,598;
4,820,481; 4,915,898, 5,507,761, and 5,506,069 fabricated using sintering so
that
the Ni-MH particles are in intimate thermal contact with each other.
The positive electrode used in the present invention are formed from nickel
hydroxide materials. The positive electrodes may be sintered such as described
in
U.S. Patent No. 5,344,728, as well as pasted into nickel foam or nickel fiber
matte
as described in U.S. Patent No. 5,348,822 and continuations thereof.
CA 02276569 2005-O1-17
One aspect of the present invention recognizes that in sealed Ni-MH
batteries, heat generation is particularly high during overcharge, especially
under
commercially desirable fast charge applications. It is noteworthy that the
heat
generated during overcharge is due to oxygen recombination on the surface of
5 the metal hydride electrode. Consequently, it is possible to utilize a
thermally
conductive metal hydride electrode in conjunction with a pasted positive
electrode. This preferred embodiment is especially useful for optimizing
specific
energy, overall performance, and cost of the battery. For a more detailed
description of the use of sintered electrodes see U.S. Patent No. 5,558,950,
10 entitled "OPTIMIZED CELL PACK FOR LARGE SEALED NICKEL-METAL
HYDRIDE BATTERIES", filed May 5, 1994.
As is shown in Figure 2, each of the electrodes 4, 5 which form an
electrode stack have electrical connector tabs 27 attached to them. These tabs
27
are used to transport the current created in the battery to the battery
terminals 7,
15 8. The tabs 27 are electrically connected to the terminals 7, 8 which may
include
a protrusion 28 for just such an attachment. Alternatively this protrusion 28
can be
used to electrically and physically connect the terminal 7, 8 to the electrode
tab
collector comb 29. As shown in Figure 7 the comb 29 is typically an
electrically
conductive bar which includes a plurality of parallel electrode tab collecting
slots
20 30 which hold the electrode tabs 27 by friction,
CA 02276569 1999-07-02
WO 98131059 PGT/US97/00805
21
welding, or brazing. Figure 7 also shows the battery terminal connector
opening 31 in the tab collecting comb 29. The battery terminal welding/brazing
lip 28 is press fit into the opening 31, and may thereafter be brazed or
welded
into place if needed or desired.
The comb 29 provides a vibration resistant connector for transferring
electrical energy from the electrodes 4, 5 to the terminals 7, 8. The comb 29
provides greater vibration resistance compared to the prior art method of
bolting
the collected tabs 27 to the bottom protrusion 28 of the terminal 7, 8. The
prior
art method of connecting the tabs 27 to the terminal 7, 8 also requires longer
tabs and a longer case (a case having a greater head space). This adds to the
total weight and volume of the batteries. The absence of bolts significantly
reduces the head space of the battery resulting in an increase in the
volumetric
energy density. The comb 29 and battery terminals 7, 8 are preferably formed
from copper or a copper alloy, which is more preferably nickel coated for
corrosion resistance. However, they may be formed from any electrically
conductive material which is compatible with the chemistry of the battery.
While
the electrode tab collector comb is the preferred means of attaching the
electrode tabs to the battery terminals, other prior art means such as bolts,
screws, welding or brazing may be used as well, and therefore the instant
inventions is not seen to be limited to the preferred embodiment.
The positive and negative battery electrodes 4, 5 can be disposed in the
battery case 2, such that their respective electrical collection tabs 27 are
disposed opposite one another at the top of the case. That is, all of the
negative electrode electrical collection tabs are positioned on one side of
the
battery and all of the positive electrode electrical collection tabs are
positioned
on the opposite side of the battery. Preferably the positive and negative
battery
electrodes have notched corners (not shown) where the opposite polarity
electrode electrical collection tabs are located, thereby avoiding shorts
between
the electrodes and eliminating unused, dead-weight electrode material. Shorts
can occur when the electrical collection tabs of one electrode become twisted
or
have sharp protrusions which then can pierce the electrode separator and short
to the adjacent, opposite polarity electrode. The dead weight electrode
material
CA 02276569 1999-07-02
WO 98/31059 PCT/US97I00805
22
is caused by incorporation of active material into electrodes which are
inactive
because they are not adjacent to their counter electrode materials.
Although the batteries can have any number of electrodes, depending
upon their thickness, preferably the battery includes 19 positive electrodes
and
20 negative electrodes alternatingly disposed within said case. That is, the
electrodes are alternated with negatives on the outside with alternating
positive
and negatives throughout the electrode stack. This configuration avoids
possible shorts when the batteries are under external mechanical compression.
That is, if there were a positive and a negative electrode at the outside of
the
electrode stack, there would be a possibility that the electrodes would form
an
electrical short path through the metal battery case when the battery is
exposed
to external mechanical compression.
While it is only necessary to have electrode separators 6 surrounding
one set of the battery electrodes (i.e. separators around only the negative or
only the positive electrodes) it may be advantageous to include separators 6
surrounding each set of electrodes. Data indicates that the use of double
separators can reduce the self discharge level of the batteries. Specifically,
charge retention increased from about 80°~ after two days for batteries
with a
single separator to about 93°~ after two days for batteries having
double
2o separators. The separators 6 are typical polypropylene separator materials
well
known in the prior art. They have an oriented grain or groove structure
thought
to be caused by the machine formation thereof and it is preferred that the
grains
or grooves of the polypropylene separator material are aligned lengthwise
along
the electrodes. This orientation lowers friction and prevents catching and
sticking of the grains or grooves of one separator with those of an adjacent
separator during mechanical compression and/or expansion of the electrodes
because the sticking and catching can cause cracking of the electrodes.
Another aspect of the present invention includes an improved, high
power battery module (a "battery module" or "module" as used herein is defined
as two or more electrically interconnected cells), specifically shown in
Figures 8-
12. To be useful, the batteries in a module must be densely packed, portable,
and mechanically stable in use. Additionally, the materials used in
construction
CA 02276569 1999-07-02
WO 98/31059 PCT/US97/00805
23
of the battery modules (aside tram the batteries themselves) must not add
excessive dead weight to the module or the energy densities of the modules
will
suffer. Also, since the batteries generate large amounts of heat during
cycling,
the materials of construction should be thermally conductive and small enough
not to intertere with heat transfer away from the batteries or to act as a
heat
sink, trapping heat within the batteries and modules. In order to meet these
and other requirements the instant inventors have designed the improved, high-
power battery module of the instant invention.
The battery module 32 of the instant invention includes: 1 ) a plurality of
individual batteries 1; 2) a plurality of electrical interconnects 25
connecting the
individual batteries 1 of the module 32 to one another and providing means for
electrically interconnecting separate battery modules 32 to one another; and
3)
a battery module bundling/compression means (described below). The batteries
are bound together under external mechanical compression (the benefits of
which are described below) within the module bundling/compression means
such that they are secure and do not move around or dislodge when subjected
to the mechanical vibrations of transport or use.
While any number of batteries may be bundled into a module, 2-15
batteries per bundle is typical. The battery modules 32 are typically bundles
of
prismatic batteries of the instant invention. Preferably they are bundled such
that they are all oriented in the same fashion with each battery having its
electrical terminals located on top (see Figures 9 and 12). The batteries are
oriented within the module such that their narrowest sides face the sides of
the
module and their wider sides (those which, on expansion of the batteries, will
warp) are placed adjacent to other batteries in the module. This arrangement
permits expansion in only one direction within the module, which is desirable.
The batteries 1 are bound within the module bundling/compression
means under external mechanical compression which is optimized to balance
outward pressure due to expansion of the battery components and provide
3o additional inward compression on the battery electrodes within each battery
to
reduce the distance between the positive and negative electrodes, thereby
increasing overall battery power.
CA 02276569 1999-07-02
wo mos9 rcTrtrs9~roosos
24
As discussed above, the expansion of prismatic batteries preferably used
in the instant modules has been tailored to be unidirectional, therefore,
compression to offset the expansion is only required in this one direction
(see
arrow 33 for compression direction). If not offset, this expansion will cause
bowing and warpage of the battery's external case and larger separation gaps
between the electrodes than optimal, thereby reducing the power of the
batteries. Also, it has been found that overcompensation for the expansion is
useful to a point. That is, up to a certain point, excess compression actually
increases the power output (reduces the internal resistance) of the bundled
batteries. However, extremely excessive compression leads to cracking and
shorting of the electrodes within the batteries. The mechanism for this
increased power on overcompression is believed to result from compression of
the positive electrode, which lowers the resistance by reducing the contact
resistance between the particles of the active material in the electrode and
the
electrode current collector. Also, compression of the separator results in
decreased interplate spacing between the positive and negative electrodes of
the battery which allows for shorter ion travel paths between the electrodes,
thus reducing the electrolyte resistance therebetween.
Figure 17 shows the correlation of module compression to battery
resistance. Modules having end plates (described below) were compressed
using differing amounts of force and the internal battery resistance (related
to
total power output and charging efficiency) and battery thickness were
measured. As can be seen from a perusal of figure 17, there is an optimal
compression range for these modules between of between about 70 and 170
psi (about 1100-2600 pounds force over an area of about 100 cm2) and a
functional range of between about 50 to about 180 psi (about 800 to about 2800
over an area of about 100 cm2). Clearly it can be seen that for these
particular
batteries used in this module, compression above than the upper limit and
compression below the tower limit of the functional range causes an increase
in
internal resistance of the batteries and therefore reduced power. It should be
noted that, while the optimal and functional compression ranges are different
for
different size batteries, the resistance versus compression plots for these
CA 02276569 1999-07-02
WO 98J'31059 PCT/IJS97/00805
different size batteries are all similar in that there are functional and
optimal
ranges of compression for proper cell pertormance.
To find a designlmaterials configuration which: 1 ) allows for application of
the required compression, 2) pertorms the required mechanical function of
5 vibration resistant module bundling/compression means; and 3) is as light
weight as possible, is a formidable task. The instant inventors have found
that
the battery modules can be bound together under high mechanical compression
using metal bars 34 (preferably stainless steel) which are positioned along
all
four sides of the battery module 32 and are welded at the four corners of the
10 module where the bars meet, thereby forming a band around the periphery of
the battery module. Preferably the welded metal bars 34 are centrally
positioned between the top and bottom of the battery module, which is where
the expansion is most severe. Compression of the batteries in areas not
containing the electrode stack is not useful since it does not compress the
15 electrodes. In fact, it can be detrimental, since it results in shorting of
the
electrodes to the metal can, through the interior insulator.
It should be noted that, although it is not readily observable in the
figures, the thickness and width dimensions at the top and bottom perimeter of
the battery cases are between 0.5 and 1.0 mm smaller than the overall
20 thickness and width dimensions. These reduced dimensions insure that all of
the compressive force is translated to the electrode plate stack and
separators
only.
It is more preferred that the welded metal bars 34 include two or three
sets of bars centrally positioned between the top and bottom of the battery
25 module. If three sets of bars are used, a first set of bars should be
disposed
half way between the top and bottom of the battery module, a second set of
bars is then positioned between the first set of bars and the top of the
battery
module, and the third set of bars is positioned between the first set of bars
and
the bottom of the battery module. This allows for uniform compression
distribution and eases the stress on any one set of bars. This compression
distribution also permits use of the smallest, lightest metal bars, thereby
reducing module dead weight.
CA 02276569 2005-O1-17
26
Another preferred design uses metal end plates 35 at the ends of the
module. The stainless steel bars are positioned along the sides of the battery
module and are welded at the corners of the module to rectangular metal tubing
(45 in Figure 9) which replaces the end bars and holds the end plates 35 in
position. This design allows for an even better distribution of the
compressive
forces. The end plates 35 are preferably formed from aluminum and may include
ribs 36 protruding perpendicular to the plane of the end plates 35, thereby
providing added strength to the plates 35 and allowing for lighter materials
to be
used. (One embodiment of the end plates is shown in Figures 13a and 13b. Other
embodiments are described in U.S. Patent No. 5,598,950 filed May 5, 1995.).
When the end plates 35 have such ribbing 36, it is necessary that there are
slots
(not shown, but see Figure 9) in the ribbing to accommodate the rectangular
metal tubing 45. The end plates 35 may preferably be thermally isolated or
insulated from the batteries bundled within the module 32 by a thermally
insulating material such as a thermally insulating layer of polymer or polymer
foam. This insulation prevents uneven battery temperature distribution within
the
module which may be caused by the cooling fin action of the ribs 36 of the end
plates 35. However, the ribs 36 can provide added thermal dissipation for the
batteries 1 within the module 32, if needed, by thermally sinking the end
plates 35
to the adjacent batteries 1.
Each of the modules 32 may additionally include module spacers 37 (see
Figures 11 and 12) which hold the modules 32 at a distance from any other
modules 32 and from a battery pack case. These module spacers 37 are placed
on the top and bottom of the module 32 to provide protection to the corners of
the
batteries 1 within the module 32 and the electrical interconnects 25 and
terminals
7, 8 of the batteries 1. More importantly, tabs 38 on the sides of the spacers
37
hold the modules 32 at the optimal distance apart. The spacers 37 are
preferably
formed from a light weight, electrically non-conductive material, such as a
durable
polymer. Also, it is important to the overall pack energy density that the
spacers
include as little total material as possible to perform their required
function and
still be as light as possible.
CA 02276569 1999-07-02
wo mos9 rcTms9~roosos
27
The batteries and modules of the present invention are preferably
electrically interconnected by conductive leads 25 (see Figures 8 and 9) which
provide a low resistance pathway therebetween. The total resistance, including
the lead resistance and the contact resistance should preferably not exceed
0.1
mohm. The leads are fastened to the terminals by a screw or bolt or preferably
the socket barrel connector 24 discussed hereinabove. The electrical
interconnects 25 of the battery module 32 of the instant invention are
preferably
braided cable interconnects (see Figure 14), which provide for high thermal
dissipation and flexibility of module design/configuration. That is, the
braided
cable interconnects 25 serve two important functions within the battery
modules
of the present invention (besides their normal function of transporting the
electrical energy out of the batteries). First, the braided cable 25 is
flexible
which accommodates expansion and contraction of the individual batteries 1
that results in a change of distance between the terminals 7, 8 of the
individual
batteries within the module 32. Second, the braided cable interconnect 25 has
a significantly higher surtace area than a solid cable or bar. This is
important to
the thermal management of the batteries, modules and packs of the instant
invention because the electrical interconnect is part of a thermal pathway
which
begins within the interior of the battery, passes up through the electrodes 4,
5,
through the electrode tab 27, through the battery terminal 7, 8 and out to the
electrical interconnect 25. Therefore, the higher the surtace area of the
electrical interconnect 25, the greater the thermal dissipation and the better
the
thermal management of the batteries 1. The braided cable electrical
interconnects 25 are preferably formed from copper or a copper alloy which is
preferably coated with nickel for corrosion resistance.
Yet another aspect of the present invention (shown in Figure 15) is the
mechanical design of fluid-cooled battery pack systems (as used herein the
terms "battery pack" or "pack" refer to two or more electrically
interconnected
battery modules). Again, it should be noted that during cycling of the
batteries
3o they generate large amounts of waste heat. This is particularly true during
charging of the batteries. This excess heat can be deleterious and even
catastrophic to the battery system. Some of the negative characteristics which
CA 02276569 1999-07-02
WO 98/31059 PGT/US97/00805
28
are encountered when the battery pack systems have no or improper thermal
management include: 1 ) substantially lower capacity and power; 2)
substantially
increased self discharge; 3) imbatanced temperatures between batteries and
modules leading to battery abuse; and 4) towered cycle life of the batteries.
Therefore, it is clear that to be optimally useful the battery pack systems
need
proper thermal management.
Some of the factors to be considered in the thermal management of
battery pack systems are 1 ) all batteries and modules must be kept cooler
than
65°C to avoid permanent damage to the batteries; 2) all batteries and
modules
must be kept cooler than 55°C to get at least 80% of the battery's
rated
pertormance; 3) all batteries and modules must be kept cooler than 45°C
to
achieve maximum cycle life; and 4) the temperature difference between
individual batteries and battery modules must be kept below 8°C for
optimal
performance. It should be noted that the improvements in the instant invention
regulate the temperature difference between batteries to less than about
2°C.
The thermal management of the battery pack system must provide
adequate cooling to insure optimal pertormance and durability of the Ni-MH
batteries in a wide variety of operating conditions. Ambient temperatures in
the
U.S. lie in a wide range from at least -30°C to 43°C in the
lower 49 states. It is
2o necessary to achieve operational usefulness of the battery packs under this
ambient temperature range while maintaining the batteries in their optimal
pertormance range of about -1 °C to 38°C.
Nickel-metal hydride batteries show charge efficiency performance
degradation at extreme high temperatures over 43°C due to problems
resulting
from oxygen evolution at the nickel positive electrode. To avoid these
inefficiencies the battery temperature during charge should ideally be held
below 43°C. Nickel-metal hydride batteries also show power performance
degradation at temperatures below about -1°C due to degraded
pertormance in
the negative electrode. To avoid, low power, the battery temperature should be
held above about -1°C during discharge.
As alluded to above, in addition to degraded performance at high and low
temperatures, detrimental effects can occur as a result of temperature
CA 02276569 1999-07-02
wo ~~os9 rcr~rs9~roosos
29
differentials between batteries within a module during charge. Large
temperature differentials cause imbalances in charge efficiencies of the
batteries, which, in turn, can produce state-of-charge imbalances resulting in
lowered capacity performance and potentially leading to significant overcharge
and overdischarge abuse. To avoid these problems the temperature differential
between the batteries should be controlled to less than 8°C and
preferably less
than 5°C.
Figure 18 shows the relationship between battery specific energy
measured in Wh/Kg and the battery temperature for nickel-metal hydride
batteries of the instant invention. As can be seen, the specific energy of the
battery starts to fall off beyond about 20°C or so and drops
drastically beyond
about 40°C. Figure 19 shows the relationship between battery specific
power
measured in W/Kg and the battery temperature for nickel-metal hydride
batteries of the instant invention. As can be seen, the specific power of the
battery risis with temperature but levels off above about 40°C.
Other factors in the design of a fluid-cooled battery pack system include
mechanical considerations. For instance, battery and module packing densities
must be as high as possible to conserve space in the end product.
Additionally,
anything added to the battery pack system to provide for thermal management
ultimately reduces the overall energy density of the battery system since it
does
not contribute directly to the electrochemical capacity of the batteries
themselves. In order to meet these and other requirements the instant
inventors have designed the fluid-cooled battery pack system of the instant
invention.
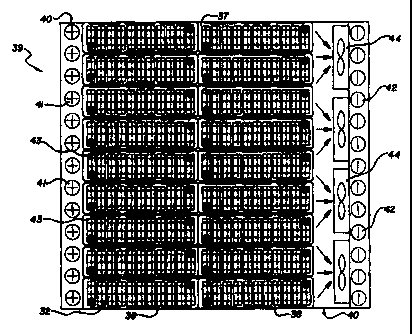
In its most basic form (an embodiment shown in figure 15) the instant
fluid-cooled battery pack system 39 includes: 1 ) a battery-pack case 40
having
at least one coolant inlet 41 and at least one coolant outlet 42; 2) at least
one
battery module 32 disposed and positioned within the case 40 such that the
battery module 32 is spaced from the case walls and from any other battery
modules 32 within the case 40 to form coolant flow channels 43 along at least
one surface of the bundled batteries, the width of the coolant flow channels
43
is optimally sized to allow for maximum heat transfer, through convective,
CA 02276569 1999-07-02
WO 98/31059 PCT/US97/00805
conductive and radiative heat transfer mechanisms, from the batteries to the
coolant; and 3) at least one coolant transport means 44 which causes the
coolant to enter the coolant inlet means 41 of the case 40, to flow through
the
coolant flow channels 43 and to exit through the coolant outlet means 42 of
the
5 case 40. Preferably, and more realistically, the battery pack system 39
includes
a plurality of battery modules 32, typically from 2 to 100 modules, arranged
in a
2 or 3 dimensional matrix configuration within the case. The matrix
configuration allows for high packing density while still allowing coolant to
flow
across at least one surtace of each of the battery modules 32.
1o The battery-pack case 40 is preferably formed from an electrically
insulating material. More preferably the case 40 is formed from a light
weight,
durable, electrically insulating polymer material. The material should be
electrically insulating so that the batteries and modules do not short if the
case
touches them. Also, the material should be light weight to increase overall
pack
15 energy density. Finally, the material should be durable and capable of
withstanding the rigors of the battery pack's ultimate use. The battery pack
case 40 includes one or more coolant inlets 41 and outlets 42, which may be
specialized fluid ports, where required, but are preferably merely holes in
the
battery pack case 40 through which cooling-air enters and exits the battery
2o pack.
The fluid cooled battery-pack system 39 is designed to use electrically-
insulating coolant, which may be either gaseous or liquid. Preferably the
coolant is gaseous and more preferably the coolant is air. When air is used as
the coolant, the coolant transport means 44 is preferably a forced-air blower,
25 and more preferably a blower which provides an air flow rate of between 1-3
SCFM of air per cell in the pack.
The blowers do not need to continuously force cooling air into the battery
pack, but may be controlled so as to maintain the battery pack temperatures
within the optimal levels. Fan control to turn the fan on and off and
preferably
30 to control the speed of the fan is needed to provide for efficient cooling
during
charging, driving, and idle stands. Typically, cooling is most critical during
charge, but is also needed during aggressive driving. Fan speed is controlled
CA 02276569 1999-07-02
WO 98131059 PCT/US9'7100805
31
on the basis of the temperature differential between the battery pack and
ambient, as well as on the basis of absolute temperature, the latter so as not
to
cool the battery when already it is already cold or so as to provide extra
cooling
when the battery nears the top of its ideal temperature range. For nickel-
metal
hydride batteries, fans are also needed in idle periods after charge.
Intermittent
cooling is needed to provide for efficient cooling under this condition and
results
in net energy savings by keeping self discharge rates below fan power
consumption. A typical result (Figure 16) shows a fan on time of 2.4 hours
after
the initial post charge cooldown. Typically the normal fan control procedure
(described below) works welt in this scenario. Fan control allows for the use
of
powertul fans for efficient cooling when needed without the consumption of
full
fan power at all times, thus keeping energy efficiency high. The use of more
powertul fans is beneficial in terms of maintaining optimal pack temperature
which aids in optimization of pack performance and life.
One example of a fan control procedure provides that, if the maximum
battery temperature is over 30°C and the ambient temperature is lower
(preferably 5°C or more lower) than the maximum battery temperature
then the
fans will turn on and circulate cooler air into the coolant channels.
Another useful fan control algorithm operates the fans at variable rates
depending upon certain criterion. These criterion include 1 ) maximum battery
temperature; 2) ambient temperature; 3) present battery usage (i.e. charging,
charge waiting, high temperature, high depth-of-discharge (dod} while driving,
standing, etc.); 4) voltage of any auxiliary battery which powers the coolant
fans. This algorithm is shown in Table 2.
CA 02276569 1999-07-02
WO 98/31059 PCT/US97/00805
32
Table 2
THEN
PWM = Minspeed + 5*Delta
PWM = MIN(PWM,Maxspeed)
ELSE PWM = Minspeed
IF PWM < 30 THEN PWM=0
1o IF (Vauxbat < 13) and (PWM>=30)
THEN PWM = 30
in the Algorithm of Table 2:
"Tbatmax" is the maximum module temperature;
"Tamb" is the ambient air temperature;
"Delta" is Tbatmax-Tamb (with negative values taken as zero)
"PWM" is the fan percentage pulse width modulation (PWM) control signal
(0=OFF, 100=FULL POWER);
"Vauxbat" is the Auxiliary fan battery voltage;
"Minspeed" is the minimum fan speed,
30%PWM if charging, charge waiting, high temperature, high
depth of discharge (dod) while driving; or
0%PWM otherwise; and
"Maxspeed" is the maximum fan speed,
100%PMW if charging or charge waiting, or
65%PMW otherwise.
The flow rate and pressure of the cooling fluid needs to be sufficient to
provide sufficient heat capacity and heat transfer to cool the pack. The flow
rate of the fluid needs to be sufficient to provide for steady state removal
of
heat at the maximum anticipated sustained heat generation rate to result in an
acceptable temperature rise. In typical Ni-MH battery packs, with 5-10 W per
cell generated during overcharge (maximum heat generation), a flow rate of 1-3
CFM of air per cell is needed to provide adequate cooling simply on the basis
of
the heat capacity of air and achieving an acceptable temperature rise. Radial
blower type fans may be used to provide the most effective airflow for thermal
CA 02276569 1999-07-02
WO 98/31059 PGT/US9~100805
33
management. This is due to the higher air pressure generated by these tan
types as contrasted with that generated by axial fans. Generally, a pressure
drop of at least 0.5" of water is required at the operating point of the fan
as
installed in the pack. To produce this pressure drop at high flow rates
generally
requires a fan static pressure capability of 1.5" to 3" of water.
In addition to using the fans to cool the battery pack when it is hot, the
fans can heat the battery pack when it is too cold. That is, if the battery
pack is
below its minimum optimal temperature, and the ambient air is warmer than the
battery pack, the fans may be turned on to draw warmer ambient air into the
1o battery pack. The warmer air then transfers its thermal energy to the
battery
pack and warms it to at least the low end of the optimal range of temperature.
One or more coolant transport means 44 can be positioned at the coolant
inlet 41 to force fresh coolant into the battery pack case 40, through coolant
flow channels 43, and out of the coolant outlet 42. Alternatively, one or more
coolant transport means 44 can be positioned at the coolant outlet 42 to draw
heated coolant out of the battery pack case 40, causing fresh coolant to be
drawn into the battery pack case 40 via the coolant inlet 41, and to flow
through
the coolant flow channels 43.
The coolant may flow parallel to the longest dimension of the coolant flow
channels 43 (i.e. in the direction of the length of the battery modules) or,
alternatively, it may flow perpendicular to the longest dimension of said
coolant
flow channels 43, (i.e. in the direction of the height of the battery module).
It
should be noted that since the coolant withdraws the waste heat from the
batteries as it flows through the cooling channels 43, the coolant heats up.
Therefore, it is preferable that the fluid flow perpendicular to the longest
dimension of the cooling channels 43. This is because as the coolant heats up,
the temperature difference between the batteries and the coolant decreases and
therefore, the cooling rate also decreases. Thus the total heat dissipation is
lowered. To minimize this effect, the coolant flow path should be the shorter
of
the two, i.e. along the height of the batteries.
While air is the most preferred coolant (since it is readily available and
easy to transport into and out of the case) other gases and even liquids may
be
CA 02276569 1999-07-02
WO 98131059 PCTlUS97/00805
34
used. Particularly, liquid coolants such as freon or ethylene glycol, as welt
as
other commercially available fluorocarbon and non-fluorocarbon based materials
may be used. When these other gases or liquids are used as the coolant, the
coolant transport means 44 may preferably be a pump. When using coolants
other than air, the coolant transport means may preferably include a coolant
return line attached to the coolant outlet 42 which recycles heated coolant to
a
coolant reservoir (not shown) from which it is transferred to a coolant heat
exchanger (not shown) to extract heat therefrom and finally redelivered to the
coolant pump 44 for reuse in the cooling of the battery pack 39.
The optimized coolant flow channel width incorporates many different
factors. Some of these factors include the number of batteries, their energy
density and capacity, their charge and discharge rates, the direction,
velocity
and volumetric flow rate of the coolant, the heat capacity of the coolant and
others. It has been found that independent of most of these factors, it is
important to design the cooling channels 43 to impede or retard the cooling
fluid
flow volume as it passes between the modules. Ideally, the retardation in flow
is predominantly due to friction with the cell cooling surfaces, which results
in a
flow reduction of 5 to 30% in flow volume. When the gaps between modules
form the major flow restriction in the cooling fluid handling system, this
produces
a uniform and roughly equal cooling fluid flow volume in the gaps between all
modules, resulting in even cooling, and reducing the influence of other flow
restrictions (such as inlets or exits) which could otherwise produce
nonuniform
flow between the modules. Furthermore, the same area of each cell is exposed
to cooling fluid with similar velocity and temperature.
Battery modules are arranged for efficient cooling of battery cells by
maximizing the cooling fluid velocity in order to achieve a high heat transfer
coefficient between the cell surtace and the cooling fluid. This is achieved
by
narrowing the intermodule gap to the point that the cooling fluid volumetric
flow
begins to diminish, but the fluid velocity is still increasing. The narrower
gap
also helps raise the heat transfer coefficient as the shorter distance for
heat
transfer in the cooling fluid raises the cell to fluid temperature gradient.
CA 02276569 1999-07-02
WO 98/31059 PCT/US<Yf/00805
The optimal coolant flow channel width depends on the length of the flow
path in the direction of flow as well as on the area of the coolant flow
channel in
the plane perpendicular to the flow of the coolant. There is a weaker
dependence of optimal gap on the fan characteristics. For air, the width of
the
5 coolant flow channels 43 is between about 0.3-12 mm, preferably between 1-9
mm, and most preferably between 3-8 mm. For vertical air flow across a
module 7 inches high, the optimal achievable mean module spacing (width of
the coolant flow channels 43) is about 3-4 mm (105 mm centerline spacing).
For horizontal air flow lengthwise across 4 modules 16 inches long in a row
for
10 a total distance of 64 inches, the optimal achievable mean module spacing
(width of the coolant flow channels 43) is about 7-8 mm (109 mm centerline
spacing). Slightly closer intermodule spacing at the far end of this row will
result in a higher airtlow rate and consequently a higher heat transfer
coefficient, thus compensating for the higher air temperature downstream. A
15 secondary inlet or series of inlets partway along the horizontal coolant
flow path
can also be used as a means of introducing additional coolant, thus making the
heat transfer between the battery cells and the coolant more uniform along the
entire flow path.
In should be noted that the term "centerline spacing" is sometimes used
20 synonymously with coolant flow channel width. The reason for this is that
the
quoted coolant flow channel widths are average numbers. The reason for this
averaging is that the sides of the battery modules which form the flow
channels
43 are not uniformly flat and even, the banding which binds the modules
together and the sides of the batteries themselves cause the actual channel
25 width to vary along its length. Therefore, it is sometimes easier to
describe the
width in terms for the spacing between the centers of the individual modules,
i.e. the centerline width, which changes for batteries of different sizes.
Therefore, it is generically more useful to discuss an average channel width,
which applies to battery modules, regardless of the actual battery size used
3o therein.
Figures 20 and 21 plots the relationship between the coolant flow
channel width (i.e. centerline spacing) verses the coolant volumetric flow
rate,
CA 02276569 1999-07-02
WO 98/31059 PCT/US97/00805
36
percentage of maximum coolant velocity and percentage of maximum heat
transfer for vertical and horizontal coolant flow, respectively. The graphs
are for
air as the coolant and assumes turbulent flow and a 30°~ free air
restriction. As
can be seen there are clearly optimal spacings, which differ dependant upon
the
direction of coolant flow. It is most efficient to operate within a range of t
10°~
of optimal heat transfer, however if needed, the system can be operated
outside
of this range by increasing the volumetric flow rate of the coolant. In the
figures, the curves denoted by the squares() represent the volumetric flow
rate
of the cooiant(air) and are read from the left hand ordinate, while the curves
denoted by the triangles (~) and the diamonds (~) represent the percentage of
maximum heat transfer and percentage of maximum coolant flow velocity,
respectively, and are read from the right hand ordinate.
To assist in achieving and maintaining the proper spacing of the modules
within the pack case and to provide electrical isolation between the modules,
each module includes coolant-flow-channel spacers 37 which hold the modules
32 at the optimal distance from any other modules 32 and from the battery pack
case 40 to form the coolant flow channels 43. As disclosed above, the coolant-
flow-channel spacers 37 are preferably positioned at the top and bottom of the
battery modules 32, providing protection to the corners of the modules 32, the
battery terminals 7, 8 and the electrical interconnects 25. More importantly,
tabs on the sides of the spacers 38 hold the modules at the optimal distance
apart. The spacers 37 are preferably formed from a light weight, electrically
non-conductive material, such as a durable polymer. Also, it is important to
the
overall pack energy density that the spacers include as little total material
as
possible to pertorm the required function and still be as light as possible.
As mentioned above Ni-MH batteries operate best in a specific
temperature range. While the cooling system described above enables the
battery pack systems of the instant invention to maintain operating
temperatures
lower than the high temperature limit of the optimal range (and sometimes to
operate above the lower temperature limit of the optimal range, if the ambient
air temperature is both warmer than the battery and warmer than the lower
temperature limit of the optimal range), there are still times when the
battery
CA 02276569 1999-07-02
WO 98/31059 PCT/IJS97
37
system will be colder than the lower limit of optimal temperature range.
Therefore, there is a need to somehow provide variable thermal insulation to
some or all or of the batteries and modules in the battery pack system.
In addition to the cooling systems described above, another way to
thermally control the battery pack systems of the instant invention is by the
use
of temperature dependant charging regimens. Temperature dependent charge
regimens allow for efficient charging under a variety of ambient temperature
conditions. One method involves charging the batteries to a continuously
updated temperature dependent voltage lid which is held until the current
drops
1o to a specified value after which a specified charge input is applied at
constant
current. Another method involves a series of decreasing constant current or
constant power steps to a temperature compensated voltage limit followed by a
specified charge input applied at a constant current or power. Another method
involves a series of decreasing constant current or constant power steps
terminated by a maximum measured rate of temperature rise followed by a
specified charge input applied at a constant current or power. Use of
temperature dependant voltage lids ensures even capacity over a wide range of
temperatures and ensures that charge completion occurs with minimal
temperature rise. For example, use of fixed voltage charge lids results in an
8°C temperature rise in one case where use of temperature compensated
charging resulted in a 3°C temperature rise under similar conditions.
Absolute
charge temperature limits (60°C) are required for this battery to avoid
severe
overheating which can occur in the case of simultaneous failure of charger and
cooling system. Detection of rate of change of voltage with respect to time
(dV/dt) on a pack or module basis allows a negative value of dV/dt to serve as
a charge terminator. This can prevent excessive overcharge and improves
battery operating efficiency as well as serving as an additional safety limit.
An example of a temperature dependant charging regimen is presented
in Table 3.
CA 02276569 1999-07-02
WO 98/31059 PCT/US97/0o805
38
TAB~E 3
1 arge at maximum power untn voltage na w is reacnea. --w
2) Reduce current by 30~ and charge until voltage lid~° is
reached.~'~2~
3) Repeat step 2) until current is <_ 5A.''~2'3
4) Complete charge at 5A constant current charge for one hour if Ampere-hour
recharge is greater than 5 Ah.''~2.3
5) Restart the charge every 2 hours, or every X hours (see below for an
illustrative equation for X)*5. Alternatively restart the charge if the
battery
module voltage falls below 15V, or alternatively restart the charge if the
battery voltage falls below the voltage lid minus an offset (e.g. 0.5V per
module) or alternatively float the battery at the voltage lid minus the above
offset. In all the above cases, the maximum battery temperature must be
less than 50C prior to restarting charge.
*1 ) Current must be limited to 10A if the maximum battery temperature is
greater
than 40°C.
*2) Halt charge if the maximum battery temperature is greater than 60°C
-- only
restart charge if the maximum battery temperature falls below 50°C.
*3) Limit total charge to a maximum of 95Ah for initial charge or 30Ah for
restarts.
*4)
Voltage lid - ( 1 fi.65 V - [ 0'~C V ] * Max battery temp. (°G~) * No.
of Modules
*5) e.g. X=20*(1-Min. Acceptable State of Charge(%))2*(60-Max. Battery Temp.)
Figures 22 and 23 illustrate how "temperature compensated voltage lid"
charging regimens can reduce temperature rise during charging of the battery
pack systems. These figures plot the temperature rise of a battery pack arid
the pack voltage versus time during charge and discharge of the pack. In
Figure 22 (temperature compensated voltage lid), the upper curve represents
pack voltage and the lower curve represents pack temperature above ambient.
Figure 22 indicates ti~at at the end of the charge cycle, indicated by the
peak of
the voltage curve, the battery pack only experienced a 3°C temperature
rise
above ambient. By contrast, Figure 23 indicates an 8°C temperature rise
from
ambient when employing a "fixed voltage lid" charging method. Here the
dashed curve represents pack voltage and the solid curve represents pack
temperature. Therefore, it can be seen that much of the conventional charge
SUBSTITUTE SHEET (RULE 26)
CA 02276569 1999-07-02
wo 98/31059 PCT/US97/0p8p5
39
generated heat has been eliminated by the use of a "temperature compensated
voltage lid" charging regimen.
As discussed above, in addition to having an upper limit on the
operational temperature range of the instant batteries, there is also a lower
limit.
As also discussed above, when the ambient temperature is above the battery
temperature, the "cooling system" can be used as a heating system. However,
it is much more likely that if the battery pack temperature is low, the
ambient
temperature will also be low, and probably lower than the battery pack
temperature. Therefore, there will be times during operational use of the
battery
1o pack system when it will be advantageous to thermally insulate the
batteries
from the ambient. However, the need for thermal insulation will not be
constant
and may vary dramatically in only a matter of a very short time period.
Therefore, the thermal insulation need will also be variable.
In order to accommodate this variable need for thermal insulation, the
instant inventors have devised a means for providing variable thermal
insulation.
The inventive variable thermal insulation means can be used on individual
batteries, battery modules and battery pack systems alike.
In its most basic form, the means provides variable thermal insulation to
at least that portion of the rechargeable battery system which is most
directly
2o exposed to said ambient thermal condition, so as to maintain the
temperature of
the rechargeable battery system within the desired operating range thereof
under variable ambient conditions.
To provide this variable thermal insulation, the inventors have combined
temperature sensor means, compressible thermal insulation means and a
means to compress the compressible thermal insulation means in response to
the temperature detected by the thermal sensor. When the temperature sensor
indicates that the ambient is cold, the thermal insulation is positioned in
the
needed areas to insulated the affected areas of the battery, module or battery
pack system. When the ambient is warmer, the temperature sensor causes the
thermal insulation to be partly or wholly compressed such that the insulation
factor provided to the battery system by the compressible insulation is
partially
or totally eliminated.
CA 02276569 1999-07-02
WO 98131059 PCT/US97/00805
The thermal sensors may be electronic sensors which feed information to
piston devices which variably increases or decreases the compression upon a
compressible foam or fiber insulation. Alternatively, (and more preferably
from
an electrical energy utilization and mechanical reliability point of view,)
the
5 sensor and compression devices may be combined in a single mechanical
devices which causes variable compression upon the thermal insulation in
direct
reaction to the ambient thermal condition. Such a combined
sensorlcompression device and be formed from a bimetallic material such as
the strips used in thermostats. Under low ambient temperatures, the bimetal
1o device will allow the thermal insulation to expand into place to protect
the
battery system from the cold ambient conditions, but when the temperature of
the battery or ambient rises, the bimetal device compresses the insulation to
remove its insulating effect from the battery system.
While the variable thermal insulation can be used to completely surround
15 the entire battery, module or battery pack system, it is not always
necessary to
do so. The variable thermal insulation can be just as effective when it only
insulates the problems spots of the system. For example, in the battery
modules and pack systems of the instant invention, which employ ribbed end
plates, it may only be necessary to thermally insulate the ends of the modules
2o which are most directly influenced by low temperature ambient conditions.
These ambient conditions may cause large temperature imbalances between
the batteries of the modules) and as a result degrade the pertormance of the
module or pack system. By providing variable insulation to the affected ends)
of the modules) the temperature differential between the batteries can be
25 reduced or eliminated and the overall temperature of the modules) can be
controlled. Finally, it should also be noted that the thermal insulation does
not
necessarily need to touch the batteries or modules but can be spaced apart
from the modules and leave a dead air zone near the battery or module which
acts as an additional thermal insulation.
30 The disclosure set forth herein is presented in the form of detailed
embodiments described for the purpose of making a full and complete
disclosure of the present invention, and such details are not to be
interpreted as
CA 02276569 1999-07-02
wo mos9 rcTrtrs9~ro~os
41
limiting the true scope of the invention as set forth and defined in the
claims
below.