Note: Descriptions are shown in the official language in which they were submitted.
CA 02276728 1999-06-29
Ticona GmbH Tic 98/G 012
Single-component adhesive
The present invention relates to a process for producing a thermoformable
composite film and the use of a solvent-tree adhesive for applying a
thermoplastic film to a film comprising cycloolefin copolymer in order to
produce a thermoformable composite film.
To produce high-performance packaging, flexible films are molded to the
shape of a tray or capsule using the action of heat and pressure and a
mechanical ram at super- and/or subatmospheric pressure. The film serves
firstly to protect the contents. It has to protect the contents from the
effect
of the environment. The film must therefore have a high water-vapor, gas
and UV barrier. It must have mechanical stability to protect the contents
from physical effects and so that it does not itself become damaged by the
contents. The quality of the contents must not be impaired by individual
constituents being released from within the film.
Blister packs are increasingly frequently chosen as packaging for a wide
variety of articles, since this type of pack is available in a wide variety of
forms and meets the requirements of mechanized packaging processes.
The starting materials used for blister packs are thermoformable films.
These are plastic films which when heated can be shaped relatively readily
by applying super- or subatmospheric pressure pneumatically, or using a
ram. Appropriate selection of the molds can thus introduce depressions
(blisters) into the film (base film) and these can be matched to the shape of
the article to be packed. After this shaping step the article to be packed is
introduced into the resulting blister. Once the blister has been filled, a
backing film is applied to the base film and encloses the article to be
packed within its blister.
If all of the requirements cannot be covered by a single material, the
properties required in a film are achieved by combining more than one film
to give a composite film. Films produced from cycloolefin copolymers have
very good impermeability to water vapor. However, these films have poor
resistance to fats. Environmental-stress-cracking corrosion occurs on
exposure to unsaturated fatty acids.
CA 02276728 1999-06-29
2
The film most frequently used in blister packs is polyvinyl chloride (PVC).
To increase its barrier properties with respect to gases, in particular water
vapor, the amorphous PVC base film is frequently coated with PVDC. Films
made from unoriented polypropylene (uPP) give better water-vapor barrier
properties than PVC films and are less questionable from an environmental
point of view. However, the disadvantage is the poorer thermoformability
and higher shrinkage of this partially crystalline material.
The amorphous COC mono- or multilayer films described in EP-A-570 188
and EP-A-631 864, when used as base films, give good processing and
good barrier properties.
Relatively new developments in the area of blister packs for
pharmaceuticals describe the use of amorphous polyolefins with good
processing performance and high water-vapor barriers. For example, EP-A-
570 188 and EP-A-631 864 describe the use of polyolefins with cyclic
olefins as polymeric building block. These applications describe the use of
these polyolefins (cycloolefin copolymers, abbreviated to COC) in the form
of mono- or multilayer films for blister packs.
Alongside automated packing and the presentation of the product protected
within the blister, for example pharmaceuticals in the form of tablets,
capsules or the like, the blister pack can markedly reduce exposure to
atmospheric moisture and oxygen and thus increase shelf life.
The object of the present invention is to provide a cost-effective and
environmentally friendly process for producing a thermoformable composite
film with a high level of barrier properties, very good thermoforming
performance and good resistance to fats.
The object of the present invention is achieved by means of a process for
producing a thermoformable composite film, where a solvent-free adhesive
is used to apply at least one thermoplastic film laminated to a film
comprising cycloolefin copolymer.
In particular, the object is achieved by means of a process for producing a
thermoformable composite film, where a solvent-free single-component
adhesive is used to apply the thermoplastic film laminated to the film
comprising cycloolefin copolymer.
' CA 02276728 1999-09-29
3
The novel feature of the novel process is the use of a solvent-free single-
component adhesive for applying a thermoplastic film to a film comprising
cycloolefin copolymer in order to produce a thermoformable composite film.
The composite film produced according to the invention is particularly
suitable for producing blister packs.
At relative humidity of about 85% and at a temperature of about 23°C,
the
film has water-vapor permeability of < 0.05 g/m2d, a puncture ~es~stance of
< 20 N and a thickness of < 100 Nm.
The films suitable for the purposes of the invention comprise at least one
cycloolefin polymer selected from the class consisting of polymers
containing from 0.1 to 100% by weight, preferably from 10 to 90% by
weight, based on the total weight of the cycloolefin copolymer, of
polymerized units of at least one cyclic olefin of the formulae I, II, II',
III, IV,
V or VI
R~
HC -CN-_ CH~
I (I
R3 C R4
HC ' CH ~ CH \\
CH.
HC 'CH _ CH /
a ~ GH II
R3 C R ( ),
HC CH-_
~ H 2
_CH~
CA 02276728 1999-09-29
4
CHz
HC ICH--_ CH /
R3 C Rd GHz (~
HG CH _
~CH~ CHZ
R~
HC'CH-_ CH-~ ~H'CH/
R3 C R4 R5 C Rs ~ ( W ~,
HC CH ~ I CH
~CH~ CH' \ RI
R~
HC ~CH_ CHI IH _CH H CH
R3 - Ra p5 - C - ps p - C - R (IV),
HC'IH CH~CH~CH\CH~CH~R,
R2
R~
(CH~
n
HC 'C~ _ CH / CH
RS C R4
HC ' I ~CH _ CH
C I~
1 R~
R2
RZ
t
-(CH~ CH
HC ~CH_ CH NCH C /H
R~ C R8 (VII.
Rj C R4
CH
/ ~CH \ ~ ' ~ t
'CH/ CH CH R
I
R2
CA 02276728 1999-06-29
where R1, R2, R3, R~, R5, R6, R~ and R$ are identical or different and are
a hydrogen atom or a C1-CZp-hydrocarbon radical, such as a linear or
branched C1-Cg-alkyl radical or Cg-C1g-aryl radical or C7-C2p-alkylenearyi
5 radical, or a cyclic or acyclic C2-C2p-alkenyl radical, or form a saturated,
unsaturated or aromatic ring, where the same radicals R1 to R8 in the
different formulae I to VI may have different meanings, and n may be from
0 to 5, and
from 0 to 99.9 mol%, based on the total composition of the cycloolefin
copolymer, of polymerized units which derive from one or more acyclic
olefins of the formula VII
g R10
R
C C
(VII),
R11 R12
where R9, Rip, R11 and R12 are identical or different and are a hydrogen
atom, a linear or branched, saturated or unsaturated C1-C2p-hydrocarbon
radical, such as a C1-Cg-alkyl radical, or a Cg-C1g-aryl radical.
The cycloolefin copolymers may also be obtained by ring-opening
polymerization of at least one of the monomers with the formulae I to VI,
followed by hydrogenation of the products obtained.
The novel elastomeric cycloolefin copolymer may also contain from 0 to
45 mol%, based on the total composition of the cycloolefin copolymer, of
polymerized units which derive from one or more monocyclic olefins of the
formula VIII
HC CH
(VIII),
(CH2)m
where m is a number from 2 to 10.
CA 02276728 1999-06-29
6
The proportion of the polymerized units which derive from cyclic, in
particular polycyclic, olefins, is preferably from 3 to 75 mol%, based on the
total composition of the cycloolefin copolymer. The proportion of the
polymerized units which derive from acyclic olefins is preferably from 5 to
80 mol%, based on the total composition of the cycloolefin copolymer.
The cycloolefin copolymers are preferably composed of polymerized units
which derive from one or more polycyclic olefins, in particular from
polycyclic olefins of the formulae I or III, and polymerized units which
derive
from one or more acyclic olefins of the formula VII, in particular a-olefins
having from 2 to 20 carbon atoms. Particular preference is given to
cycloolefin copolymers which are composed of polymerized units which
derive from a polycyclic olefin of the formula I or III and from an acyclic
olefin of the formula VII. Preference is also given to terpolymers which are
composed of polymerized units which derive from a polycyclic monoolefin
of the formula I or III, from an acyclic monoolefin of the formula VII and
from a cyclic or acyclic olefin which contains at least two double bonds
(polyene), in particular cyclic, preferably polycyclic, dienes, such as
norbornadiene, or cyclic, particularly preferably polycyclic, alkenes with a
C2-C2p-alkenyl radical, such as vinylnorbornene.
The novel elastomeric cycloolefin copolymers preferably comprise olefins
with a basic norbornene structure, particularly preferably norbornene,
tetracyclododecene and, if desired, vinylnorbornene or norbornadiene.
Preference is also given to cycloolefin copolymers which contain
polymerized units which derive from acyclic olefins with terminal double
bonds, such as a-olefins having from 2 to 20 carbon atoms, particularly
preferably ethylene or propylene. Particular preference is given to
norbornene-ethylene and tetracyclododecene-ethylene copolymers.
Among the terpolymers, particular preference is given to norbornene-
vinylnorbornene-ethylene, norbornene-norbornadiene-ethylene,
tetracyclododecene-vinylnorbornene-ethylene and tetracyclododecene-
vinyltetracyclododecene-ethylene terpolymers. The proportion of the
polymerized units which derive from a polyene, preferably vinylnorbornene
or norbornadiene, is from 0.1 to 50 mol%, preferably from 0.1 to 20 mol%,
and the proportion of the acyclic monoolefin of the formula VII is from 0 to
99.9 mol%, preferably from 5 to 80 mol%, based on the total composition of
the cycloolefin copolymer. In the terpolymers described, the proportion of
CA 02276728 1999-06-29
7
the polycyclic monoolefin is from 0.1 to 99.9 mol%, preferably from 3 to
75 mol%, based on the total composition of the cycloolefin copolymer.
The cycloolefin copolymer according to the invention preferably comprises
at least one cycloolefin copolymer containing polymerized units which
derive from polycyclic olefins of the formula I and containing polymerized
units which derive from acyclic olefins of the formula VII.
The cycloolefin copolymers according to the invention may be prepared at
temperatures of from -78 to 200°C and at a pressure of from 0.01
to 200 bar, in the presence of one or more catalyst systems which
comprise at least one transition metal compound and, if desired, a
cocatalyst and, if desired, a support. Suitable transition metal compounds
are metallocenes, in particular stereorigid metallocenes. Examples of
catalyst systems suitable for preparing the elastomeric cycloolefin
copolymers according to the invention are described in EP-A-407 870,
EP-A-485 893 and EP-A-503 422. These publications are expressly
incorporated herein by way of reference.
Examples of transition metal compounds used are:
rac-dimethylsilylbis(1-indenyl)zirconium dichloride,
rac-dimethylgermylbis(1-indenyl)zirconium dichloride,
rac-phenylmethylsilylbis(1-indenyl)zirconium dichloride,
rac-phenyivinylsilylbis(1-indenyl)zirconium dichloride,
1-silacyclobutylbis(1-indenyl)zirconium dichloride,
rac-diphenylsilylbis(1-indenyl)hafnium dichloride,
rac-phenylmethylsilylbis(1-indenyl)hafnium dichloride,
rac-diphenylsilylbis(1-indenyl)zirconium dichloride,
rac-ethylene-1,2-bis(1-indenyl)zirconium dichloride,
dimethylsilyl(9-fluorenyl)(cyclopentadienyl)zirconium dichloride,
diphenylsilyl(9-fluorenyl)(cyclopentadienyl)zirconium dichloride,
bis(1-indenyl)zirconium dichloride,
diphenylmethylene(9-fluorenyl)cyclopentadienylzirconium dichloride,
isopropylene(9-fluorenyl)cyclopentadienylzirconium dichloride,
rac-isopropylidenebis(1-indenyl)zirconium dichloride,
phenylmethylmethylene(9-fluorenyl)cyclopentadienylzirconium dichloride,
isopropylene(9-fluorenyl)(1-(3-isopropyl)cyclopentadienyl)zirconium
dichloride,
CA 02276728 1999-06-29
isopropylene(9-fluorenyl)(1-(3-methyl)cyclopentadienyl)zirconium
dichloride,
diphenylmethylene(9-fluorenyl)(1-(3-methyl)cyclopentadienyl)zirconium
dichloride,
methylphenylmethylene(9-fluorenyl)(1-(3-methyl)cyclopentadienyl)-
zirconium dichloride,
dimethylsilyl(9-fluorenyl)(1-(3-methyl)cyclopentadienyl)zirconium dichloride,
diphenylsilyl(9-fluorenyl)(1-(3-methyl)cyclopentadienyl)zirconium dichloride,
diphenylmethylene(9-fluorenyl)(1-(3-tert-butyl)cyclopentadienyl)zirconium
dichloride,
isopropylene(9-fluorenyl)(1-(3-tert-butyl)cyclopentadienyl)zirconium
dichloride,
isopropylene(cyclopentadienyl)(1-indenyl)zirconium dichloride,
diphenylcarbonyl(cyclopentadienyl)(1-indenyl)zirconium dichloride,
dimethylsilyl(cyclopentadienyl)(1-indenyl)zirconium dichloride,
isopropylene(methylcyclopentadienyl)(1-indenyl)zirconium dichloride,
4-( rt5-cyclopentadienyl)-4,7,7-trimethyl(r~5-4,5,6,7-tetrahydroindenyl)-
zirconium dichloride,
[4-( rl5-cyclopentadienyl)-4,7,7-triphenyl( r~5-4,5,6,7-tetrahydroindenyl)]-
zirconium dichloride,
[4-( rl5-cyclopentadienyl)-4,7-dimethyl-7-phenyl( rl5-4,5,6,7-tetrahydro-
indenyl)]zirconium dichloride,
[4-( rl -3'-tert-butyicyclopentadienyl)-4,7,7-triphenyl( rl5-4,5,6,7-
tetrahydro-
indenyl)]zirconium dichloride,
[4-( r~5-3'-tent-butylcyclopentadienyl)-4,7-dimethyl-7-phenyl( rl5-
4,5,6,5 -tetrahydroindenyl)]zirconium dichloride,
[4-( r~ -3'-methylcyclopentadienyl)-4,7,7-trimethyl( r~ -4,5,6,7-tetrahydro-
indenyl)]zirconium dichloride,
[4-( rt -3'-methylcyclopentadienyl)-4,7,7-triphenyl( ~5-4,5,6,7-tetrahydro-
indenyl)]zirconium dichloride,
[4-( rl -3'-methylcyclopentadienyl)-4,7-dimethyl-7-phenyl( rl5-4,5,6,7-tetra-
hydroindenyl)]zirconium dichloride,
[4-( ~5-3'-isopropylcyclopentadienyl)-4,7,7-trimethyl( rt5-4,5,6,7-tetrahydro-
indenyl)]zirconium dichloride,
[4-( r~5-3'-isopropylcyclopentadienyl)-4,7,7-triphenyl( r~5-4,5,6,7-tetrahydro-
indenyl)]zirconium dichloride,
[4-( ri5-3'-isopropylcyclopentadienyl)-4,7-dimethyl-7-phenyl( r~5-
4,5,6,7-tetrahydroindenyl)]zirconium dichloride,
CA 02276728 1999-06-29
9
[4-( r~5-cyclopentadienyl)( r~5-4,5-tetr~hydropentalene)]zirconium dichloride,
[4-( rl -cyclopentadienyl)-4-methyl( r~ -4,5-tetrahydropentalene)]zirconium
dichloride,
[4-( rl5-cyclopentadienyl)-4-phenyl( r~5-4,5-tetrahydropentalene)]zirconium
dichloride,
[4-( rl5-cyclopentadienyl)-4-phenyl( r~5-4,5-tetrahydropentalene)]zirconium
dichloride,
[4-( rl5-3'-methylcyclopentadienyl)( r~5-4,5-tetrahydropentalene)]zirconium
dichloride,
[4-( r~5-3'-isopropylcyclopentadienyl)( ~5-4,5-tetrahydropentaiene)]-
zirconium dichloride,
[4-( rl5-3'-benzylcyclopentadienyl)( r~5-4,5-tetrahydropentalene)]zirconium
dichloride,
[2,2,4-trimethyl-4-( rl5-cyclopentadienyl)( r~5-4,5-tetrahydropentalene)]-
zirconium dichloride,
[2,2,4-trimethyl-4-( rl5-(3,4-diisopropyl)cyclopentadienyl)( r~5-4,5-
tetrahydro-
pentalene)]zirconium dichloride.
The COC films used according to the invention are distinguished by
specific mechanical properties. The films can be processed on the
machinery used and also have low puncture resistance and a high level of
barrier properties, especially to water vapor. Suitable COC films of this type
are oriented. They may be monolayer films or have more than one layer.
The films may comprise organic or inorganic fillers in order to reduce light
transmission so as to render the contents invisible (childproof packaging) or
in order to improve printability or sealing properties.
The cycloolefin copolymers are prepared by heterogeneous or
homogeneous catalysis with organometallic compounds as described in
many patents. Catalyst systems based on mixed catalysts made from
titanium salts and organoaluminum compounds are described in DD-A-109
224 and DD-A-237 070. EP-A-156 464 describes the preparation using
catalysts based on vanadium. EP-A-283 164, EP-A-407 870, EP-A-485 893
and EP-A-503 422 describe the preparation of cycloolefin polymers using
catalysts based on soluble metallocene complexes. The preparation
processes described and the catalyst systems used in these patents for
preparing COCs are incorporated herein by way of reference.
CA 02276728 1999-06-29
Unoriented extruded COC films are brittle, making it difficult to find
appropriate processing conditions, and their processing performance is
poor, cf. DE-A-4304309. They readily split or break during winding-up
and/or unwinding under tension. For this reason their mechanical strength
5 has to be increased. This may be achieved by orientation (mono- or biaxial
stretching) of the films. The films oriented in this way are significantly
easier
to handle and do not have the disadvantages as described in
DE-A-4304309. The puncture resistance of oriented films was studied in
accordance with DIN 53373. One measure of puncture resistance is
10 penetration energy. It has now been established that orientation increases
the puncture resistance of the films. Exceptionally, the values found were
larger than those of unoriented films of comparable thickness.
DE-A-4414669 states that 450 N/mm is excessively high for a film to be
useful as a backing film for blister packs. Significantly lower values are
desirable on blister-packing machinery for fragile pharmaceuticals. The
puncture resistances of aluminum foils can be taken as a preliminary guide:
that of a (16 Nm) aluminum film is 90 N/mm. When oriented COC films are
used, therefore, it is not possible to ensure easy removal of the
pharmaceutical from the blister packs.
Alongside ideal, but not excessively low, puncture resistance, the blister
film must have relatively high toughness. Careful balancing of mechanical
strength (hardness), flexibility and the force required to compress the
compartment is required to enable the contents, such as a tablet, capsule
pastille or dragee are to be capable of release from the pack without
damage and without unreasonable expenditure of effort. Low expenditure
of effort is a particular requirement when elderly people use the product,
and also in the clinical sector where medical personnel are continually
using pressure to remove tablets and can suffer from fatigue or aching in
the fingers.
The water-vapor barrier effectiveness is not significantly affected by organic
or inorganic additives. It is from 0.2 to 0.4 g/mZ~d for a film thickness of
100 Nm. The polarity of the surface can be increased by corona-treating the
film.
The additives may be organic polymers, such as polypropylene or
polyethylene in the form of homo- or copolymers, polyesters, polyamides,
polycarbonate, polyacetals, and acrylate and methacrylate polymers.
CA 02276728 1999-06-29
11
Inorganic pigments which may be used are titanium dioxide, barium sulfate,
calcium sulfate, calcium carbonate and barium carbonate.
One or both sides of the COC film may be laminated with a film which
comprises polymers such as unoriented or oriented polyethylene,
polypropylene or polyvinyl chloride. A solvent-free adhesive is preferably
used in the lamination, particularly preferably a solvent-free single-
component adhesive.
It is preferable to laminate COC with unoriented polypropylene (uPP) or
else PVC on one or both sides. Compared with COC, the films produced
with unoriented polypropylene and PVC are significantly thinner. COC and
uPP, and COC and PVC, give as a laminate a flexible, puncture-resistant
composite film of good appearance. A further advantage of a laminate
made from PVC and COC for the user, possibly a pharmaceutical blister
pack manufacturer, is that it can replace packaging previously composed
exclusively of PVC. The novel laminate made from PVC and COC is of high
quality and has relatively high impermeability to water vapor, retaining
surface contact with the contents via the PVC layer. The approval
procedure is thus simplified.
The thickness of the entire film is from 100 to 550 pm, preferably from
200 to 400 Nm. The thickness of the film laminated to the COC film is from
1 to 150 pm, preferably from 1 to 100 pm and particularly preferably from 4
to 50 pm. The thickness of the COC film is from 50 to 400 Nm, preferably
from 150 to 350 Nm, particularly preferably from 200 to 300 Nm.
The novel process for producing a thermoformable composite film uses a
solvent-free adhesive, preferably a solvent-free, single-component
adhesive.
Particularly suitable for this purpose according to the invention is a solvent-
free polyurethane-based moisture-curing single-component laminating
resin. The laminating resin used differs from conventional laminating
adhesives by being supplied and used with 100% solids content. The
laminating resin is suitable for producing laminates from aluminum and
paper, card or parchment, and for producing laminates from plastic films
and paper or aluminum films. According to the invention the novel adhesive
is very particularly suitable for producing thermoformable composite films
CA 02276728 1999-06-29
12
where the novel adhesive is used to apply at least one thermoplastic film
laminated to a film comprising cycloolefin copolymer.
Processing takes place on LF laminating plants which have heatable
application systems and laminating units. Since the laminating resin is
applied without solvent, no drying tunnels are required. A heatable
laminating unit is advantageous for varying combinations. The laminating
resin has to be heated to 80 - 100°C for application.
The novel thermoformable composite film is used for producing blister
packs. The blister pack or PTP (push-through packaging) produced
therefrom has very good water-vapor barrier properties, thus increasing the
value of the packaged item. It can be used to pack contents such as
pharmaceuticals and foodstuffs, in particular pelletized or capsule
pharmaceuticals, foods containing rice, cookies, snacks, and also
hygroscopic items, such as cigarettes and teabags.
The invention is described in more detail using a drawing and examples.
Drawing
The drawing comprises Figures 1 to 3.
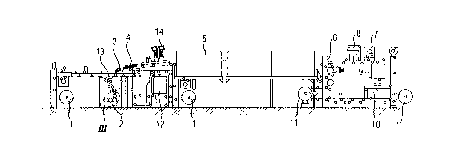
Fig. 1 shows the coating plant
Fig. 2 shows the set-up of the coating plant
Fig. 3 shows the application device in the coating plant.
The coating plant of Fig. 1 comprises an unwind (1 ), application device (2),
short-wavelength infrared source (3), medium-wavelength infrared source
(4), flotation drier (5), dry-lamination unit (6), corona system (7), web edge-
guiding system (8), remoistening equipment (9), cooling unit (10), wind up
(11), electron-beam unit (12), wet-lamination unit (13) and UV source (14).
Fig. 2 shows the set-up of the coating plant for the following procedures:
A) thermal drying or UV curing
B) thermal drying with shock cooling
C) thermal drying with remoistening
D) thermal drying with dry lamination
E) thermal drying with UV curing.
CA 02276728 1999-06-29
13
Fig. 3 shows the application device in the coating plant with application by
smooth rollers using 4 rollers (wet). G here indicates a rubber roll and S a
steel roll. The rolls here are heated and cooled. Application using smooth
rolls is particularly suitable for the lamination of COC with PVC or oPP.
Examples
The solvent-free single-component adhesive used, Herberts GmbH 1 K-~F
190X3, has the following physical properties:
Solids (%) 100,
Viscosity (100 °C) 850 = 150 mPas
Example 1
A COC film 190 Nm thick (194 g/m2) was laminated to both sides of an
unoriented polypropylene film 25 Nm thick (22.5 g/m2) using Herberts
GmbH 1 K-LF 190X3 solvent-free single-component adhesive. Application
was at 1.50 g/m2, with corona treatment at 48 kW. For lamination a width of
810 mm was used, a speed of 50 m/min, and a smooth-roll application
system with four rolls and one pass for each laminated side. The thickness
of the thermoformable composite film was 210 Nm. The bond strength of
the composite film was high, and when this bond strength was measured it
was the composite film which broke.
Example 2
A COC film 190 Nm thick (194 g/m2) was laminated to both sides of an
unoriented PVC film 35 tlm thick (4.6 g/m2) using Herberts GmbH 1 K-LF
190X3 solvent-free single-component adhesive. Application was at
1.50 g/m2, with corona treatment at 48 kW. For lamination a width of
810 mm was used, a speed of 50 m/min, and a smooth-roll application
system with four rolls and one pass for each laminated side. The thickness
of the thermoformable composite film was 260 Nm. The bond strength of
the composite film was high, and when this bond strength was measured it
was the composite film which broke.
CA 02276728 1999-06-29
14
Comparative Example
The procedure of the example was followed except that both sides of the
COC film were laminated to an unoriented polypropylene film. The
thickness of the thermoformable composite film was 210 arm.