Note: Descriptions are shown in the official language in which they were submitted.
CA 02277572 1999-07-13
ELECTROLYTIC GENERATION OF NITROGEN
FIELD OF THE INVENTION
The invention is in the field of methods and
apparatus for electrochemical generation of nitrogen and
hydrogen gases. Particularly generation of nitrogen gas
from organic hydrazides (RCONHNH2) and hydrazino-
carboxylates (RCOONHNH2), and amino guanidine salts,
circuits for spontaneous oxidation of such nitrogen
compounds to generate nitrogen gas and mechanical
transducers actuated by the nitrogen gas so produced,
particularly in the field of fluid dispensers.
BACKGROUND OF THE INVENTION
The controlled electrolytic generation of gases is
useful to convert chemical to mechanical energy in a
variety of applications. For example, a variety of
lubricant or fluid delivery systems driven by the
electrolytic generation of a gas are known. For example,
U.S. Patent No. 4,023,648 to Orlitzky et al. (1977) shows
a lubricant applicator driven by gas generated in an
electrochemical cell and provides a method for the
electrochemical generation of hydrogen gas.
Fluid dispensers driven by electrochemically
generated gases, and other electrochemical transducers
may often be used in circumstances which give rise to
special operational requirements. Typically, components
of any electrolytic cell used in such an application must
be stable over time and over a range of temperatures. In
such devices, it is undesirable to have highly reactive
gases generated, such as hydrogen or oxygen. Once the
circuits are closed to initiate electrolytic gas
-1-
CA 02277572 1999-07-13
generation, it is desirable to have relatively fast
electrode reactions with low overpotential (i.e. a small
difference between the electrode potential under
electrolysis conditions and the thermodynamic value of
the electrode potential in the absence of electrolysis),
small concentration polarisation of solutes across the
cell (i.e. rapid diffusion of reactants to the electrode
surfaces), and small separator resistance effects (i.e.
little resistance caused by solid separators within the
cell. It is also desirable to produce gases from a small
amount of material, i.e. to have efficient gas generation
and high stoichiometric coefficients for gaseous reaction
products.
Hydrogen and oxygen gases are used in a variety of
known electrochemical gas generators. One disadvantage
of such systems is the chemical reactivity of those
gases. Another disadvantage of hydrogen in particular is
that it diffuses relatively rapidly through a variety of
polymeric barriers that might otherwise be used to
contain the electrolytically generated gas in a
mechanical transducer, such as a fluid dispenser.
Nitrogen is a relatively inert gas that may usefully
be produced by electrolytic reactions to provide
controlled amounts of gas. However, existing methods for
the electrolytic generation of nitrogen suffer from a
number of disadvantages.
U.S. Patent No. 5,567,287 issued to Joshi et al.
(1996) discloses a solid state electrochemical nitrogen
gas generator for fluid dispensing applications.
Nitrogen is produced in that system by the electro-
oxidation of a decomposable solid material of the generic
formula AxNy in a divided electrochemical cell, where "A"
-2-
CA 02277572 1999-07-13
is an alkali metal such as sodium or lithium, "N" is
nitrogen, x is 1 to 3 and y is 1 to 3. Example compounds
disclosed therein include LiN3 (lithium nitride) and NaN3
(sodium azide). The azide half cell reaction in such a
system (reaction 1) may however be slow, in part because
of the high overpotential required for the electro-
oxidation of azide.
2N3- -> 3N2 + 2e- (1)
To overcome the problem of the sluggish kinetics of
the azide half-cell, additives such as thiocyanate may be
used to catalyse the iodine mediated formation of
nitrogen from azides, as in reactions 2 and 3:
2I- -4 12 + 2e (2)
12 + 2N3- ScN-> 2I- + 3N2 (3)
However, such systems suffer from the disadvantages
that azides are toxic and the thiocyanate salt catalysts
are also toxic. The presence of toxic compounds may make
it difficult to dispose of a device which generates
nitrogen gas from azides.
SUMMARY OF THE INVENTION
The invention provides methods and devices for the
electrochemical generation of nitrogen from organic
nitrogen compounds, such as hydrazides (RCONHNH2), the
corresponding organic hydrazino-carboxylates (RCOZNHNHz)
and amino-guanidine salts (e.g. aminoguanide bicarbonate
H2NNHC (NH) NHz . H2C03) . A variety of organic hydrazides and
hydrazino-carboxylates may be used, and empirically
tested for performance. For example, in the hydrazides
and hydrazino-carboxylates "R" may be selected from
suitable alkyl, alkenyl, alkynyl or aryl groups, in some
embodiments methyl, ethyl, or benzyl. The alkyl, alkenyl
-3-
CA 02277572 1999-07-13
and alkynyl groups may be branched or unbranched,
substituted or unsubstituted. Some such compounds may not
work in all embodiments, as determined by routine
functional testing. The utility of such compounds may,
for example, be routinely assayed in accordance with the
guidance provided herein, including the Examples set out
herein in which alternative nitrogen compounds may be
substituted for routine test purposes.
The present invention also provides methods and
devices for the auto-electrolytic generation of nitrogen,
using electrochemical cells that comprise both a nitrogen
compound capable of acting as a reductant in an
electrochemical reaction to produce nitrogen gas, and an
electrochemical oxidant capable of driving the oxidation
of the nitrogen compound.
The present invention also provides a housing for
electrochemical gas generating cells. The housing acts to
compress a flexible electrochemical cell to help maintain
electochemical contacts in the cell over a prolonged
period of operation, during which the compositions within
the cell may contract while gas is evolved from the cell.
The housings of the invention may be used with a wide
variety of gas-generating electrochemical cells,
including hydrogen, oxygen and nitrogen generating cells.
The housings of the invention may also be adapted to
enclose a plurality of cells, in which case the cells may
be arranged in series to increase the potential drop
across the cells. There may be advantages associated with
arranging electrochemical gas generating cells in series
to increase the potential of the circuit, particularly
when the cells are to be used in fluid dispensers. A
higher potential difference across the cells allows for
-4-
CA 02277572 1999-07-13
the use of a larger (and in some embodiments variable)
resistance in the circuit of the electrochemical cell.
The larger the resistance, the less sensitive the circuit
is to variations in temperature.
The sensitivity of the circuit (the electrochemical
cell and the external electronic components) to
temperature change generally comes about as a result of
the fact that increasing temperature will generally
decrease the effective resistance of the electrochemical
cell and increase the current in the circuit. However,
increasing temperature will normally increase the
resistance of the electronic components of the circuit
(i.e. the external electronic resistance) and this
partially compensates for the effect of temperature on
the electrochemical cell. In other words, the temperature
coefficient of resistivity of the electrochemical cell,
which is an ionic resistance, is negative, whereas the
temperature coefficient of resistivity of the external
circuit, which is an electronic resistance, is usually
positive (although of a lower order of magnitude than for
the cell). Providing for operation with a greater
potential in the circuit allows the circuit to include a
higher external electronic resistance, and thus makes the
circuit less sensitive to temperature changes. In a fluid
dispenser, it is generally desirable to provide a
constant current that does not fluctuate substantially
with temperature in order to provide a constant flow of
fluid. Of course, if it is desired to make the circuit
temperature sensitive, this may also be accomplished in
accordance with the circuits of the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
-5-
CA 02277572 1999-07-13
Figure 1A is a schematic side elevational view of a
fluid dispenser.
Figure 1B is a schematic cross-sectional view of an
electrical circuit including an electrolytic cell.
Figure 2 is a schematic cross-sectional view of an
electrical circuit including an electrolytic cell.
Figure 3 is a schematic cross-sectional view of an
electrical circuit including an electrolytic cell.
Figure 4 is a schematic cross-sectional view of an
electrical circuit including an electrolytic cell.
Figure 4A is an exploded isometric view of a housing
for an electrolytic cell.
Figure 4B is a side elevational view of a housing
for an electrolytic cell.
Figure 5 is a side elevational view of a housing for
an electrolytic cell.
Figure 6 is a graph showing a plot of the volume of
grease dispensed as a function of time for a lubricant
dispenser driven by the electrochemical nitrogen
generator of Example 9.
DETAILED DESCRIPTION OF THE INVENTION
The generation of nitrogen using the methods of the
present invention may be particularly useful in
electrochemically driven fluid dispensers. For example,
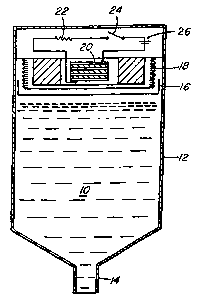
Figure 1 illustrate a dispenser for a fluid 10. The
dispenser has a body 12 and an outlet nozzle 14. There is
-6-
CA 02277572 1999-07-13
a piston 16 and a bellows 18 to force the fluid 10 from
the nozzle 14. The necessary force is generated by an
electrolytic gas generator 20 having an external circuit
that includes a resistor 22, battery 26 and a switch 24.
Figures 4A and 4B illustrate alternative embodiments of
electrochemical cells according to various aspects of the
invention. Such cells may be made of a sandwich
construction comprising an anode 36, such as a gelled
anode, in a conductive cup 38, such as a brass cup,
having a contact 40 to enable wiring t an external
circuit. An electrolyte 42 is contained in a thin-walled
tube 44. A permeable cathode, such as a screen 46 backed
by a graphite felt 48 and a brass disk current collector
50 may be used, with a contact 52 to enable wiring to the
external circuit. The cell may be contained in a
polypropylene cylinder 54. A spring washer 56 on cup 38
may be retained by a lip 60 on cylinder 54. Figure 5
shows the configuration of a bipolar cell, in which cells
such as those illustrated in Figures 4A and 4B are
compressed in series with electronic contact between
adjacent anodes and cathodes, for example by spring
loading of washer 56. Brass plate current collectors 50
and contacts 52 are omitted from all cathodes except the
end cathode.
In the cells shown in Figures 4A, 4B and 5, thin-
walled tube 44 may be selected to be sufficiently
flexible to permit compression of the cells, such as by
spring washer 56, as reactants in electrolyte 42 and
anode 36 are depleted. Such compression may help to
ensure that good electrical contact is maintained, for
example between electrolyte 42 and anode 36. A sealed
connection between tube 44 and cap 38 may be preferred to
avoid loss of electrolyte and short-circuiting of the
bipolar cells. In one aspect, the invention accordingly
-7-
CA 02277572 1999-07-13
provides a housing for an electrolytic cell comprising an
anode, a cathode and an electrolyte biased together in
electrical contact, the electrolyte being contained by a
flexible membrane adapted to accommodate compression of
the electrolyte, the housing having an opening to permit
passage of gas evolved from the electrolyte during
electrolysis.
A permeable cathode, for example comprised as
illustrated of a screen 46 backed by a graphite felt 48,
is useful to permit egress of gas into the space defined
by container 54. Electrolyte 42 may preferably be adapted
to be sufficiently viscous or solid to operate in
combination with a permeable cathode to allow gas to be
evolved from the cathode, but to prevent loss of
electrolyte. The electrolyte should however be
sufficiently liquid to permit adequate mass transfer to
provide for a desired rate of gas evolution. A variety of
absorbent materials or gelling agents may be used to
stabilise the electrolyte against leakage, including
hydrophilic absorbent materials such as cellulose
sponges, cotton wool, synthetic felts, diatomaceous
earth; and gelling agents such as carbopol,
carboxymethylcellulose and others.
The electrolyte solution should contain an ionic
compound (salt, acid or base) capable of mediating
electrical conductivity. An electrolyte compound may also
provide antifreeze properties. In some embodiments,
antifreeze properties may be associated with the use of
inorganic electrolytes such as sodium chloride, calcium
chloride, sulphuric acid or ammonium sulphate. An organic
antifreeze agent may also be added to the electrolyte to
depress its freezing point. In some embodiments, examples
-8-
CA 02277572 1999-07-13
of organic antifreezes may include ethylene glycol,
dimethyl sulphoxide, methanol, ethanol or urea.
As set out particularly in Examples 6 through 9
herein, additives may be used in the electrolyte in
undivided cells to facilitate the generation of nitrogen
at the anode while suppressing the co-generation of
hydrogen on the cathode. A typical cathode reaction in an
undivided cell (such as those shown in Figures 1B, 4A and
4B) is the generation of hydrogen by electro-reduction of
water:
2H20 + 2e- -> 20H- +H2
Hydrogen is however an undesirable product in some
devices, such as certain lubricant dispensers, for the
reasons discussed in the background section herein. It
may accordingly be useful to use additives in an
electrolyte that will react preferentially at the cathode
to suppress the evolution of hydrogen, such compounds are
termed herein 'cathode depolarisers'. Examples 6 through
9 disclose particular embodiments of such compounds. In
some embodiments, preferred cathode depolarisers will not
be reduced to products that suppress the evolution of
nitrogen at the anode.
In various embodiments, the invention provides a
variety of alternative cathode depolarisers, such as
cupric salts, nitroguanidine, nitroethanol and
nitromethane. The performance of candidate cathode
depolarisers may be determined empirically in the context
of a particular electrolytic cell. Preferred depolarisers
may be obtained where the electro-reduction at the
cathode is substantially irreversible. Some depolarisers
may not work well under some conditions, such as low
temperature (for example below -25 C). Some cathode
-9-
CA 02277572 1999-07-13
depolarisers, such as some copper salts, may promote the
spontaneous decomposition of some organic nitrogen
compounds, such as methyl hydrazino-carboxylate, to
nitrogen (a reaction that may compromise the shelf life
of cells containing these reactants). Potential cathode
reactions of exemplified depolarisers are set out below
(although this information may assist others in
identifying other members of this class of compounds,
they do not necessarily represent the true or complete
nature of the cathode reactions - which are not all
known ) :
1. Cupric Salts, i.e. Cu++ (e.g. cupric sulphate, as set
out in Examples 4 and 5):
Cu++ + 2e- -> Cu
2. Nitroguanidine, i.e. NH2(NH)CHNNO2
NH2 (NH) CHNNO2 +6H+ + 6e -> NH2 (NH) CHNNH2 + 2H20
3. Nitroethanol, i.e. OHCH2CH2NO2
OHCH2CH2NO2 + 6H+ +6e- -> OHCH2CH2NH2 + 2H20
4. Nitromethane, i.e. CH3NO2
CH3NO2 + 6H+ + 6e- -> CH3NH2 + 2H20
EXAMPLE 1
In one embodiment, a nitrogen gas generator is
assembled as shown in Figure 1B, comprising:
(a) a circuit comprising an external energy source
26, such as two 1.5 V alkaline batteries
connected in series; a resistor 22, such as a
variable resistor from 1 to 100 kOhm; and a
switch 24;
(c) an undivided electrochemical cell 20
comprising:
-10-
CA 02277572 1999-07-13
i) electrolyte solution 27, comprising an
active nitrogen compound, in one
embodiment, methyl hydrazine carboxylate
(about 0.1 to 4M), urea (about 0.1 to 1M),
ammonium sulphate (about 0.1 to 2M) and
water, all absorbed in a cellulose sponge;
ii) anode 21 and cathode 25, which in various
embodiments may be graphite fibre
impregnated with a polymer such as NylonTM
or polypropylene, GRAFOIL, pyrolytic
carbon, carbon black, platinum or gold.
The probable (but unknown) methyl hydrazino-
carboxylate anode reaction (as in examples 6, 8 and 9)
is:
CH3CO2NHNH2 -> CH3CO2H + N2 + 2H+ + 2e-
In such an embodiment, when switch 24 is closed to
turn the circuit on, with a resistance 12 of 6 kOhm, this
cell generated about 2.5 ml STP of gas per day over a
period of 14 days at 23 C.
EXAMPLE 2
In another embodiment, a nitrogen generator was
assembled according to Figure 2 and consisted of:
(a) an external electronic circuit comprising
switch 24 with a resistor 22, which may be a
variable resistor;
(b) an electrochemical cell 29 divided by a cation
membrane 29 (such as the sulfonated
perfluoroethylene polymer sold under the trade-
mark NAFION 324 by E.I. DuPont & DeNemours Co.,
Wilmington, Delaware, U.S.A., or equivalents
thereof) with:
-11-
CA 02277572 1999-07-13
i) a catholyte 27 of a solution of sodium
bromate in aqueous sulphuric acid;
ii) an anolyte mixture of sodium azide (about
0.1 to 4M), sodium bicarbonate (about 0.1
to 1M), sodium iodide (about 0.1 to 1M)
and sodium thiocyanate (about 0.1 to 1M)
in water;
iii) electrodes of Nylon'" impregnated graphite
fibre and GRAFOIL (such as the product
sold under the trade-mark GRAFOIL GTB by
Union Carbide Corp.).
The putative reaction at the cathode is:
Br03 + 6H+ + 6e -+ Br- + 3 H2O
This cell showed an open circuit (zero current)
voltage of 0.73 volt under ambient conditions (i.e. about
22 C, 101 kPa) and when the circuit was closed through a 2
kOhm resistor the current and voltage varied respectively
from approximately 0.3 to 0.1 mA and 0.6 to 0.2 volt over
a period of 40 days. The azide oxidation reaction is
catalysed by the iodide/thiocyanate system. The putative
net anode reaction for the azide is:
2N3- -> 3N2 + 2e-
EXAMPLE 3
In an alternative embodiment, a nitrogen generator
was assembled according to Figure 2 and consisted of:
(a) an external electronic circuit comprising
switch 24 with a resistor 22, which may be a
variable resistor;
(b) a cathode 25 of graphite in contact with an
oxidant 27 consisting of a paste of manganese
-12-
CA 02277572 1999-07-13
dioxide in aqueous sulphuric acid (about 1 to
4M) ;
(c) an anolyte mixture 31 of oxalic dihydrazide
(about 0.1 to 1M) in aqueous sulphuric acid
(about 0.1 to 1M);
(d) an anode 21 of graphite.
On open circuit, this cell showed a voltage of 0.8
volt and no gas was generated at either electrode over a
period of several days. When the circuit was closed, gas
(nitrogen) was generated at the anode. The probable (but
unknown) electrode reactions are:
Anode : H2NNHCOCONHNH2 -> CO2 + 2N2 + 4H+ + 4e-
Cathode : MnO2 + 4H+ + 2e -> Mn++ + 2HZO
EXAMPLE 4
In an alternative embodiment, a nitrogen generator
was assembled according to Figure 3 and consisted of:
(a) an external electronic circuit with variable
resistance, as in Examples 2 and 3;
(b) a bipolar electrochemical unit with 2 cells:
i) a first cathode 25 of NylonT" impregnated
graphite fibre with an oxidant 33 paste of
manganese dioxide plus carbon powder;
ii) a catholyte 27 of sulphuric acid (about 1
to 4M) in water absorbed in a cellulose
felt;
iii) a bipole electrode 29 of copper sheet;
iv) an anolyte 31 mixture of:
- cupric sulphate (about 0.1 to 1M);
- sulphuric acid (about 0.1 to 1M)
- methyl hydrazino-carboxylate (about
0.1 to 2M);
- water;
-13-
CA 02277572 1999-07-13
v) an anode 21 of graphite.
For which the anode reaction is uncertain and the
putative cathode reaction is:
Mn02 + 4 H+ + 2 e- ~ Mn+2 + 2H20
A bipolar electrode is one without electronic
connection to the current supply, one face of which acts
as an anode surface and the opposite face of which acts
as a cathode surface when an electric current is passed
through the cell.
On open circuit (zero current), this unit produced a
voltage of 0.54 volt. The circuit was closed through a
resistor of 1 kOhm and over a period of 90 days the
current ranged from 0.5 to 0.25 mA while the voltage
dropped from about 0.5 to 0.25 volt and gas was generated
spontaneously at a rate of about 0.15 millimole/day (i.e.
3.4 ml STP/day). This rate of gas generation corresponds
to about 100% currency efficiency for a putative methyl
hydrazino-carboxylate anode reaction 5.
CH3CO2NHNH2 -> CH3CO2H + N2 + 2H+ + 2e- (5)
The cathodic generation of hydrogen is suppressed by
a depolariser for depolarising the bipolar electrode,
such as a copper salt like cupric sulfate, which may
mediate the preferential electrodeposition of copper on
the copper bipole by reaction 6.
Cu++ + 2e- --> Cu (6).
-14-
CA 02277572 1999-07-13
EXAMPLE 5
In a further alternative embodiment, a nitrogen
generator was assembled according to Figure 4 and
consisted of:
(a) an external electronic circuit with variable
resistance, as in examples 2 through 4;
(b) a bipole electrochemical unit with 2 cells:
i) a first anode 21 of "Grafoil" graphite
sheet;
ii) a first electrolyte 31 consisting of a
gelled mixture of:
- methyl hydrazino carboxylate (about
0.1 to 4M);
- cupric sulphate (about 0.1 to 1M);
- acetic acid (about 0.1 to 1M);
- water;
iii) a bipole conductor 29 of Grafoil sheet;
iv) a second anode 37 of gelled zinc particles
in a brass cup 41;
v) a second electrolyte 27 of gel (such as
30% by weight CARBOPOL gelling agent,
potassium hydroxide solution (plus
additives));
vi) an oxidant 39 such as a paste of manganese
dioxide with carbon powder (in about 30%
by weight KOH);
On open circuit at 23 C, this unit gave a voltage of
about 1.2 volt and produced 10 ml STP of gas in 7 days.
When the circuit was closed though a 1 kOhm resistor a
current of 0.01 mA gave 8 ml STP of gas in two days. The
putative electrode reactions in this bipolar unit are:
-15-
CA 02277572 1999-07-13
First anode: CH3CO2NHNH2 -> CH3CO2H + N2 + 2H+ + 2e-
First cathode: Cu++ + 2e- -> Cu
Second anode : Zn -> Zn++ + 2e-
Second cathode: Mn02 + 4H+ + 2e- -> Mn++ + 2H20
EXAMPLE 6
A nitrogen gas generator was assembled as in Figure
1B, comprising:
a. A circuit with two alkaline 1.5 V batteries
connected in series (26), a 3 kOhm resistor (22) and a
switch (24);
b. An undivided electrochemical cell (20) with:
i. about 15 ml of an electrolyte solution
(27) absorbed in a cellulose sponge,
composed of approximately:
- 2.7 grams methyl hydrazino-
carboxylate (anode reactant);
- 3.5 g sodium chloride
(electrolyte);
- 3.9 g nitro-guanidine (cathode
.depolariser); and,
- water;
ii. an anode (21) and cathode (25), each
composed of NylonT" impregnated graphite
fibre.
This nitrogen generating cell was inserted into a
commercial automatic lubricant dispenser (ATS Electro-
Lube MINI-LUBER) as shown in Figure 1A (with 2 1.5V
batteries used in the external circuit). The dispenser
was loaded initially with about 100 grams of grease (10),
with a density of about 900 kg/m3. Switch (24) was closed
to turn the circuit on, and the unit operated at room
temperature and zero kPa(gauge) grease outlet pressure.
The consequent grease dispensing rate averaged about 4
-16-
CA 02277572 1999-07-13
cc/day over a 14 day period. The approximate composition
of total gas produced by the electrochemical cell over 14
days was as shown in Table 1.
Table 1: Gas Production
Component Volume % (dry basis)
hydrogen 0.0
oxygen 1.0
nitrogen 96.0
methane 2.0
carbon monoxide 1.0
EXAMPLE 7
A nitrogen gas generator was assembled as in Figure
1B, comprising:
a. A circuit with two alkaline 1.5 V batteries
connected in series (26), a 3 kOhm resistor
(22) and a switch (24); and,
b. An undivided electrochemical cell (20)
comprising:
i. about 15 ml of an electrolyte solution
(27) absorbed in a cellulose sponge,
composed of approximately:
- 4 grams aminoguanidine bicarbonate
(anode reactant);
- 3.5 g sodium chloride
(electrolyte) ;
- 2.7 g nitroethanol (cathode
depolariser); and,
- water;
ii. an anode (21) and cathode (25), each
composed of Nylont"" impregnated graphite
fibre.
This nitrogen generating cell was inserted into a
commercial automatic lubricant dispenser (ATS Electro-
Lube MINI-LUBER) as shown in Figure 1A (with two, 1.5V
-17-
CA 02277572 1999-07-13
batteries 26 used in the external circuit). The dispenser
was loaded initially with about 100 grams of grease (10),
with a density of about 900 kg/m3. Switch (24) was closed
to turn the circuit on, and the unit operated at room
temperature and zero kPa (gauge) grease outlet pressure.
The consequent grease dispensing rate averaged about 2.8
cc/day over a 14 day period. The approximate composition
of total gas produced by the electrochemical cell over 14
days was as shown in Table 2.
Table 2: Gas Production
Component Volume % (dry basis)
hydrogen 0.0
oxygen 2.0
nitrogen 95.0
methane -
carbon monoxide 2.0
The anode reaction is presumably the electro-
oxidation of amino-guanidine bicarbonate to nitrogen
(with unknown side products).
EXAMPLE 8
A nitrogen gas generator was assembled as in Figure
1B, comprising:
a. A circuit with two alkaline 1.5 V batteries
connected in series (26), a 3 kOhm resistor (22) and a
switch (24);
b. An undivided electrochemical cell (20) with:
i. about 15 ml of an electrolyte solution
(27) absorbed in a cellulose sponge,
composed of approximately:
- 16 wt% methyl hydrazino-carboxylate
(anode reactant);
- 16 wt% sodium chloride
(electrolyte);
-18-
CA 02277572 1999-07-13
- 16 wt% nitroethanol (cathode
depolariser);
- 20 wt% ethylene glycol
(antifreeze); and,
- 32 wt% water;
ii. an anode (21) and cathode (25), each
composed of NylonT" impregnated graphite
fibre.
This nitrogen generating cell was inserted into a
commercial automatic lubricant dispenser (ATS Electro-
Lube MINI-LUBER) as shown in Figure lA (with 2 1.5V
batteries used in the external circuit). The dispenser
was loaded initially with about 100 grams of grease (10),
with a density of about 900 kg/m3. Switch (24) was closed
to turn the circuit on, and the unit operated at room
temperature and zero kPa(gauge) grease outlet pressure.
The consequent grease dispensing rate ranged from about
2.8 cc/day to 0.4 cc/day over a 21 day period. The
approximate composition of total gas produced by the
electrochemical cell over 21 days was as shown in Table
3.
Table 3: Gas Production
Component Volume % (dry basis)
hydrogen 0.0
oxygen 0.7
nitrogen 88.1
methane 0
carbon monoxide 0.9
carbon dioxide 4.6
nitrous oxide 5.8
EXAMPLE 9
A nitrogen gas generator was assembled as in Figure
1B, comprising:
-19-
CA 02277572 1999-07-13
a. A circuit with two alkaline 1.5 V batteries
connected in series (26), a 3 kOhm resistor (22) and a
switch (24);
b. An undivided electrochemical cell (20) with:
i. about 15 ml of an electrolyte solution
(27) absorbed in a cellulose sponge,
composed of approximately:
- 2.7 grams methyl hydrazino-
carboxylate (anode reactant);
- 2.7 g sodium chloride
(electrolyte);
- 2.7 g nitro-methane (cathode
depolariser);
- 3.0 g ethylene glycol (antifreeze);
- 3.0 g dimethyl sulphoxide
(antifreeze); and,
- water;
ii. an anode (21) and cathode (25), each
composed of NylonTM impregnated graphite
fibre.
This nitrogen generating cell was inserted into a
commercial automatic lubricant dispenser (ATS Electro-
Lube MINI-LUBER) as shown in Figure 1A (with 2 1.5V
batteries used in the external circuit). The dispenser
was loaded initially with about 100 grams of grease (10),
with a density of about 900 kg/m3. Switch (24) was closed
to turn the circuit on, and the unit operated at room
temperature and zero kPa(gauge) grease outlet pressure.
Over a period of 49 days, the current in the circuit
ranged from an initial value of 0.49 mA to a final value
of 0.28 mA. The graph of Figure 6 shows a plot of grease
volume dispensed as a function of time over the 49 day
run. The approximate composition of total gas produced by
-20-
CA 02277572 1999-07-13
the electrochemical cell over 49 days was as shown in
Table 4.
Table 4: Gas Production
Component Volume % (dry basis)
hydrogen 5.2
oxygen 0.3
nitrogen 66.4
methane 0.4
carbon monoxide 1.4
carbon dioxide
+ nitrous oxide balance
EXAMPLE 10
The electrolytic cells of the invention may be
housed as shown in Fig. 1A in a dispenser for a fluid 10.
The dispenser has a body 12 and an outlet nozzle 14.
There is a piston 16 and a bellows 18 to force the fluid
10 from the nozzle 14. The necessary force is generated
by a electrolytic gas-generating cell 20 having an
external circuit that includes a resistor 22 and a switch
24.
Figures 4A and 4B illustrate a housing for cells
according to the present invention. The housing is of a
sandwich construction optionally comprising an anode 36
in a conductive cup 38 (such as brass) having a contact
40 to enable wiring to an external circuit. There is an
electrolyte 42 (which may be gelled) contained in a
flexible, thin-walled tube 44 and a cathode 46 (which may
be a screen) backed by a porous member such as graphite
felt 48 to allow gas to escape, and a disk current
collector 50 (which may be brass), with a contact 52 to
enable wiring to the external circuit. The cell is
contained in a an outer wall 54 (such as a polypropylene
cylinder). There is a plastic spring washer 56 on cup 38
retained by a lip 60 on cylinder 54.
-21-
CA 02277572 1999-07-13
The combination of the spring loaded housing and the
flexible, thin-walled electrolyte enclosure 44 allows the
cells to contract over time as gas is evolved, which
helps to ensure that the components of the cell remain in
electrical contact. The use of the flexible, thin-walled
enclosure 44 helps to prevent electrolyte from leaking
from one cell to the next in multi-cell units, which
could short-circuit the units. The electrolyte may also
be gelled or absorbed in a solid to reduce its propensity
to migrate, although the extent to which it is desirable
to 'solidify' the electrolyte is limited by the need to
permit species to migrate through the electrolyte during
electrolysis.
Figures 5 shows the configuration of a bi-cell. In
multiple cell reactors, several of the cells shown in
Figure 4 are compressed in series with intimate
electronic contact between adjacent anodes and cathodes.
This contact is facilitated by the spring loading of the
housing, such as with washer 56. A rigid wall, such as a
polypropylene cylinder, may encompass the composite cell.
Reference numerals are as in Figures 4A and 4B.
-22-